Abstract
Objectives
Several clinical and experimental studies reported the anxiety as one of the neuropathic pain comorbidities; however, the mechanisms involved in this comorbidity are incompletely cleared. The current study investigated the consequence of pain induced by peripheral neuropathy on the serotonin (5-HT) level of the CA1 region of the hippocampus, which is known as a potential reason, for anxiety associated with neuropathic pain.
Methods
In this manner, 72 male rats were inconstantly subdivided into three experimental groups as follows: control, sham, and chronic constriction injury (CCI). Neuropathic pain was initiated by the CCI of the sciatic nerve, and then, mechanical allodynia, thermal hyperalgesia, and anxiety-like behavior were evaluated using the von Frey filaments, radiant heat, open field test (OFT), and elevated plus maze (EPM) respectively. To investigate the probable mechanisms, the in vivo extracellular levels of 5-HT were assessed by microdialysis and using reverse-phase high-pressure liquid chromatography (HPLC) in the CA1 region of hippocampus on days 16 and 30 post-CCI.
Results
Our data suggested that CCI caused anxiety-like behavior in OFT and EPM test. 5-HT concentration in the CA1 region of the hippocampus significantly (F=43.8, p=0.000) reduced in CCI rats, when the pain threshold was minimum. Nevertheless, these alterations reversed while the pain threshold innate increased.
Conclusions
Neuropathic pain, initiated by constriction of the sciatic nerve can induce anxiety-like behavior in rats. This effect accompanies the reduction in 5-HT concentration in the CA1 region of the hippocampus. When the pain spontaneously alleviated, 5-HT level increased and anxiety-like behavior relieved.
Introduction
Neuropathic pain caused by lesions or disorders of the somatosensory nervous system is usually comorbid by cognitive impairment such as anxiety and depressive symptoms. Mood and cognition disorders are prevalent among up to 65% of the individuals suffering from neuropathic pain [1], [2], [3]. Although the pathogenesis of neuropathic pain and its comorbidity are mainly unidentified, alterations of the central serotonergic system have entailed in both sensory and mood symptoms of neuropathic pain situation [4], [5], [6].
A review of the literature mentioned that the neuropathic pain mechanisms could imply serotonin (5-HT) receptors and transporters. Alterations in their expression chiefly regulate the pain by affecting the serotonergic transmission in the descending and ascending pathways [4], [7], [8], [9].Previous studies demonstrated that the impairment of descending 5-HT pathways inhibit hyperalgesia. However, nerve injury can induce pain hypersensitivity by stimulating some spinal 5-HT receptors [10], [11], [12]. 5-HT and noradrenaline reuptake inhibitors have determined to generate the analgesia in several animal models of neuropathic pain and can be clinically used for the neuropathic pain treatment [3], [9], [10], [13].
In particular, 5-HT contributes to pain signaling in the brain and nervous system, and is also implicated in anxiety [3], [14], [15]. It was revealed that, anxiety disorders often are accompanied by a decrease in the activity of monoaminergic systems, specifically in the 5-HT system [15], [16].
In recent years, considerable data have disclosed that, the neuropathic pain shares biological mechanisms with anxiety. Such similarities are important in causing and developing neuropathic pain-induced anxiety [17], [18]. Furthermore, same brain regions including the insular cortex, prefrontal cortex, anterior cingulate, thalamus, hippocampus, and amygdala, are responsible for modulating the transmission of pain involved in mood management [19], [20], [21], [22].
The hippocampus plays an independent fundamental role in the development and maintenance of neuropathic pain and abnormal emotionality, including anxiety and depression [2], [23], [24], [25]. Biochemical, physiological, and behavioral findings showed that the hippocampal formation is involved in pain processing. Blockade of neural transmission along with the efferent or afferent hippocampal pathways has reported alleviating pain behaviors [26], [27], [28]. Also, data established hippocampal neuroanatomical and neurochemical changes in neuropathic pain situations [2], [29], [30]. In this regard, human and animal studies determined that neuropathic pain conditions can lead to hippocampal dysfunctions, such as reduced adult hippocampal neurogenesis, changing in synaptic plasticity and resting state connectivity, abnormal cytokine and neurotrophic expression, the decreased glutamate level, the increased gamma-Aminobutyric acid (GABA) level, and the instability of place cells [23], [30], [31], [32], [33].
Previous data demonstrated that the density of 5-HT transporters enhanced and 5-HT ratio decreased in the hippocampus after the peripheral nerve injury [13], [34]. However, another study reported that, the hippocampal messenger RNAs (mRNAs) of 5-HT 1A receptors and 5-HT 2A receptors altered in rats after the chronic constriction injury (CCI) [35].The CA1 region of hippocampus receives a robust serotonergic innervation from the raphe nuclei, and is excited during noxious stimulation and predominantly replies to painful stimuli [36], [37], [38]. Serotonergic receptors of the dorsal hippocampus, in particular, the postsynaptic 5-HT1A receptors, are involved in the mechanisms of controlling the anxiety-related behaviors [39], [40].The results suggested that the ascending serotonergic pathway that connect the median raphe nucleus to the dorsal hippocampus, could facilitate the 5-HT neurotransmission and reduce the anxiety. The activation of dorsal hippocampal 5-HT1A receptors attenuated the anxiogenic effects of stressful stimuli [41], [42]. It was also determined that the anxiety-like behavior significantly increased in 5-HT1A receptor knockout mice [43]. Accordingly, the alteration of serotonergic neuronal excitation in the dorsal hippocampus may be considered as a crucial factor in the mediation of anxiety-like behavior under the neuropathic pain condition [44].
Hence, the goal of this assay was to explicate if the neuropathic pain induced by sciatic nerve injury can alter the hippocampal 5-HT level, and also to determine whether hippocampal 5-HT alteration is related to anxiety following peripheral nerve injury.
Methods
Animals
A total of 72 adult male albino Wistar rats weighing 250–300 g took from the Pasteur Institute of Iran. Rats were kept in a humidity and temperature standard room (22 ± 2 °C) on a 12 h light/dark cycle (lights off at 9:00 a.m.) with food and water accessible ad libitum. All testing performed between 10:00 a.m. and 2:00 p.m. The animals were adapted at least for one week before experiments and then inconstantly were subdivided between three experimental groups (control, sham, and CCI). The different time points of behavioral evaluation tests carried out on independent sets of animals. Therefore, in all experimental groups, rats divided into two subgroups (n=6 per each group), one used for days 14, 15, and 16 and other for days 28, 29, and 30 of the experiment. In anxiety-like behavior assessments, each animal tested only once. The experiments obeyed to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP) and accepted by the Ethical Committee of the University (96-01-30-24473).
Pain induction
Pain initiated by CCI model previously defined by Bennett and Xie [45]. In brief, for animal anesthetic sodium pentobarbital (40 mg/kg intraperitoneally) used. An incision shaped on the left thigh and, the sciatic nerve disclosed. Four loose ligatures 1 mm aside formed around the nerve through the epidural vasculature was not interrupted. Sham surgery achieved while the left sciatic nerve disclosed but not further manipulated. The control group was intact. Behavioral evaluation achieved for all experimental groups. Surgery is considered on the zero-day of the study.
Behavioral evaluation
Assessment of mechanical allodynia
von Frey filaments (Stoelting, Wood Dale, IL, USA) used for mechanical threshold assessment described by Chaplan [46]. Rats put in alone in a Plexiglas box with a wire mesh bottom and allowed to adapt for 30 min. The calibrated von Frey filaments (4.56, 4.74, 4.93, 5.07, 5.18, 5.46, and 5.88 gr) administered to the mid plantar surface of the hind paw using up and down method. Withdrawal threshold measured by subsequently increasing and decreasing the stimulus strength, and mean withdrawal threshold measured by the Dixon nonparametric test [47]. This test was performed for all experimental groups on days 14 and 28 of the study.
Assessment of thermal hyperalgesia
Radiant heat test performed to measure the thermal threshold based on the Hargreaves protocol [48] by using the plantar test (Eugo Basile Co., Italy). Each rat placed in the special enclosure of the device, then thermal radiation achieved to their foot sole after 10 min of environmental adaptation. A stopwatch utilized from the start of radiation, and then, ended as soon as the foot moved or lifted, and the time latency recorded. This test achieved on the surgery foot three times at intervals of at least 2 min, and then its average calculated. This test performed for all experimental groups on days 14 and 28 of the study.
Open field test
According to the methods described previously [49], the locomotor activity and anxiety-like behaviors examined by open field test (OFT). Each rat placed in a test chamber and tested in an arena 40 × 40 × 35 cm with transparent walls for 10 min. The test achieved under infrared beam (ITTCINC life science company) in a brightly illuminated (white light) room. The total distance that the rat traveled in the arena and the number of entries into the central area (16 × 16 cm2) of the OF recorded for 10 min using a computer-based video tracking system (Ethovision 1.6 Noldes, Waninggen) simultaneously for measuring the anxiety-like behavior and locomotor activity. This test, performed one day after pain assessment for all experimental groups, on days 15 and 29 of the study .The different time points of test carried out on independent sets of animals and, each animal tested only once. The test chamber was cleaned with 10% ethanol solution after each trial.
Elevated plus maze (EPM) test
The EPM test operated to check anxiety-like behaviors according to the methods described by Filho [50]. The EPM equipment exists of two closed and two open arms (15 × 50 cm each arm) and elevated 50 cm from the floor. Each rat put in the center of the equipment while its face in front of the open arms. The parameters detected in video records as the number of entries and the time spent in closed and open arms during a 5 min period for each animal (Ethovision1.6 Noldes, Waninggen). The entries considered when all four paws rat were inside the arm. The frequency of entries into open arms was calculated by the percentage of the total number of entries into both open and closed arms. The duration spent in the open arms was measured and reported by a percentage of the total time (300 s) that rats spent in the maze. This test, performed one day after pain assessment for all experimental groups, on days 15 and 29 of the study. The different time points of test carried out on independent sets of animals and, each animal tested only once. The EPM equipment was cleaned after each trial.
In vivo microdialysis & HPLC assessment
Stereotaxic surgery and brain dialysis
Stereotaxic surgery was performed concurrently with CCI or sham surgery on the zero-day of the study. The microdialysis guide cannula(MAB 4.15.IC, Microbiotech/se AB, Stockholm, Sweden) implanted into the hippocampal CA1 area with the following coordinates: 3.8 mm posterior to the bregma, ±2.2 laterals to the midline and 2.7 ventral to the skull surface based on Paxinos Atlas (2001). On the days 16, 23, and 30 of the study, a microdialysis probe (MAB 4.15.1.Cu, b6000 kDa, 1 mm membrane length, Microbiotech/se AB, Stockholm, Sweden) was inserted into the guide cannula. The microdialysis probe was connected to the syringe pump (WPI, SP 210, syringe pump) and pervaded by artificial cerebrospinal fluid (ACSF) at a flow rate of 1 μL/min. ACSF consisted 114 mM NaCl, 3 mM KCl, 1 mM CaCl2, 2 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, 1 mM NaOH, 10 mM glucose and pH = 7.4. After a 30 min equilibration period, the four consecutive samples (40 μL) collected for the determination of the 5-HT level. The intervals between sample collections were 10 min. All rats at least one day prior were habituated, before the microdialysis procedure, to reduce anxiety behavior as a result of handling or the infusion process. The microdialysis procedure performed on days 16 and 30 of the study. The samples immediately put in−80 °C until analysis.
Serotonin determination
The 5-HT level was measured by the reverse-phase high-pressure liquid chromatography (HPLC) method in dialysate samples as previously described by Singewald [51]. Briefly, HPLC column was Shim-pack VP-ODS, 250 L × 4.6 mm, 5μm; Pump: LC-10AD, Shimadzu, Japan that connected to an electrochemical detector (Pharmacia LKB, type-2143 RPE, USA). A glassy carbon electrode arranged at a potential of +750 mV relative to Ag/AgCl reference. The mobile phase existed of 0.35 g EDTA, 100 mg octane sulfonic acid (soap), 3.2 g NaH2PO4, 600 μL triethylamine (TEA), 89 mL methanol and 500 mL distilled H2O (pH 5.40) and pumped (Waters 510, USA) at a rate of 1.0 mL/min. Data acquired on-line and exported to a software system (Autochro 2000, USA) for peak amplification, integration, and analysis. 5-HT peaks identified by comparison to a 5-HT standard (5.07 ± 0.2 moL/40 μL sample). The 3:1 signal to the noise detection limit of this system for 5-HT was 0.24 ± 0.11 pg.
Microdialysis site verification
At the end of the study, animals killed with an overdose of ether; brains rapidly pulled out and kept in 10% formalin for at least 48 h. The location of microdialysis probe tips within the CA1 area of the hippocampus proved from 10 μm coronal sections, and only brains with the correct location added in data analysis.
Statistical analysis
Differences between the groups determined by using SPSS 21(IBM® SPSS® Statistics V21.0). One way ANOVA with a post hoc test (Scheffé’s Method) performed to analyze the behavioral alterations and 5-HT concentration. Pearson correlation test used to evaluate the relationship between variables related to anxiety behaviors and 5-HT concentration in the CA1 region of the hippocampus. The data presented as mean ± SEM and p<0.05 considered significant.
Results
Pain behavioral assessment
Mechanical allodynia
As mentioned in Figure 1, after CCI surgery, the rats progressed mechanical allodynia, which indicated by a decreased withdrawal threshold to tactile stimulation on the injured side. On the 14th postoperative days, the paw withdrawal threshold significantly (F=4.2, p=0.000) decreased as compared with the sham group (3.02 ± 0.4, 14.7 ± 0.31 g respectively). Although on the 28th days after surgery, the mean withdrawal threshold for the CCI group (13.1 ± 0.46 g), was significantly (F=3, p=0.000) more than the 14th day, but lower than the sham group (15 ± 0.35 g). The significant difference in the paw withdrawal threshold did not show between sham and control animals (p=0.108).
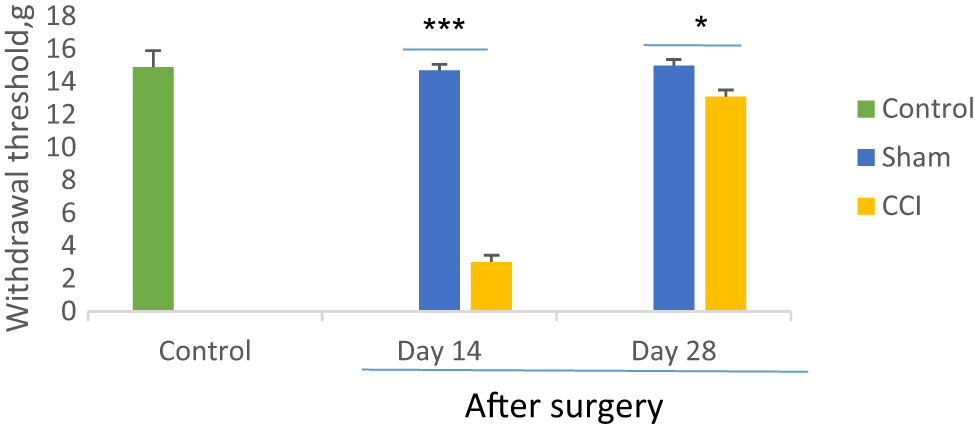
Comparison of mechanical allodynia between CCI and sham groups on days 14 and 28th after sciatic nerve surgery. *p<0.05 and ***p<0.001 indicate the differences between the groups. Data analyzed by one-way ANOVA with a post hoc test (Scheffe’s test) and expressed as mean ± SEM of six animals per each group.
Thermal hyperalgesia
As showed in Figure 2, the one-way ANOVA and post hoc analysis identified that withdrawal latency to noxious heat 14 days after CCI significantly decreased as compared with the sham group (F=2.8, p=0.000). The means of withdrawal latency respectively were 4.38 ± 0.4 s and 15 ± 0.5 s in the CCI and sham groups two weeks after surgery. However, the paw withdrawal latency increased in day 28 after CCI surgery (13.9 ± 0.45 s) but significantly (F=2.8, p=0.03) less than the sham group (16 ± 0.5 s). No statistical differences noticed in the sham group compared to the control group (p=0.261).
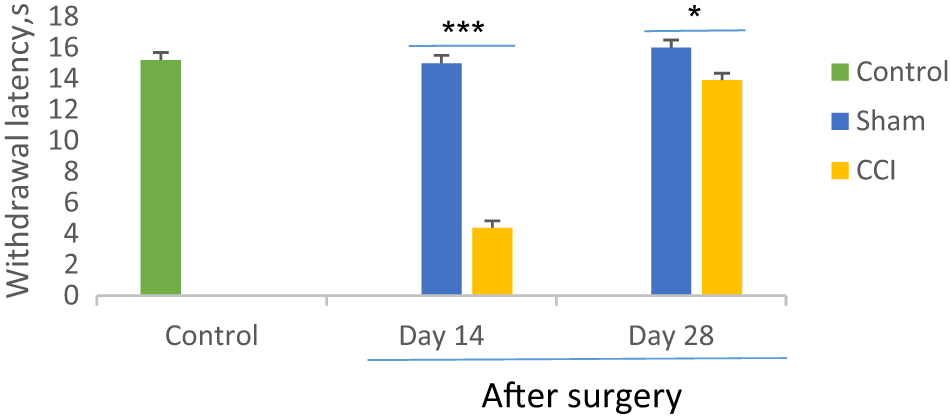
Comparison of thermal hyperalgesia between CCI and sham groups on days 14 and 28th after sciatic nerve surgery. *p<0.05 and ***p<0.001 indicate the differences between the groups. Data analyzed by one-way ANOVA with a post hoc test (Scheffe’s test) and expressed as mean ± SEM of six animals per each group.
The pain behavioral assessment results determined, the neuropathic pain induced by CCI, persisted about the four weeks after sciatic nerve injury and might promote a proper model to study the chronic pain comorbidity.
Anxiety-like behavior assessment
Open field test
The CCI rats exhibited significantly less time spent (F=18.38, p=0.000) and entries (F=69.09, p=0.000) into the central area in OFT that considered anxiety-like behavior in CCI rats (Figure 3). On day 29 of the experiment, the CCI rats more time spent in the center area of the maze (7.23 ± 0.67 s) as compared to day 15 (0.39 ± 0.14 s). At the end of the second, and fourth weeks after surgery, the means number of entered to the center for CCI rats were 1 ± 0.36 and 4.8 ± 0.94 respectively, while these means of the sham group were 14.33 ± 0.91 and 13.6 ± 0.8 respectively. The total distance moved in the OFT is considered the locomotor ability of the rats [52]. Our result showed that there was no significant difference (F=2.5, p=0.28) among experimental groups, so it seems that CCI surgery no effect on the locomotor ability of rats (Figure 3E).
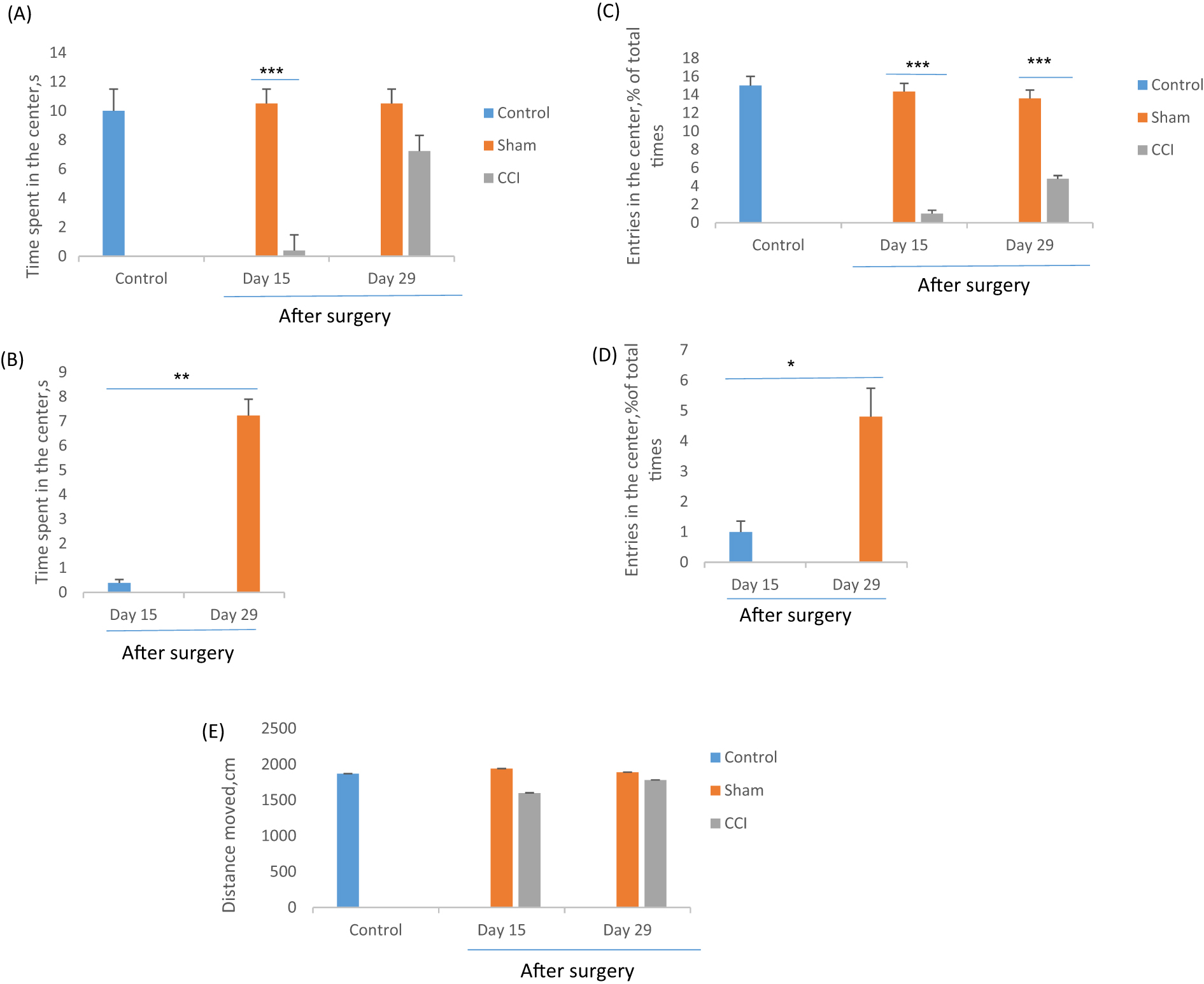
The effect of chronic constriction injury on open field test on days 15 and 29th after surgery. The time spent in the center (A and B), the entries in the center (C and D), and the total distance moved (E) were measured by one-way ANOVA with a post hoc test (Scheffe’s test). *p<0.05, **p<0.01 and ***p<0.001 indicate the differences between the groups. Data are expressed as mean ± SEM of six animals per each group.
Elevated plus maze test
In the EPM test, the CCI group showed a significant (F=5.85, p=0.024) reduction in time spent and the meaningful (F=30.02, p=0.000) entries in open arms that considered anxiety-like behavior in CCI rats (Figure 4). One-way ANOVA analysis determined that on day 15, the CCI rats less time spent in the open arms (5 ± 2.5 s) as compared to day 29 (10.3 ± 0.47 s) and sham groups (13.16 ± 1.19, 13.16 ± 1.37).Additionally, the CCI group on days 15 and 29 entered fewer in the open arms (0.5 ± 0.2, 3 ± 0.47, respectively) compared to the sham groups (9 ± 0.96, 9.1 ± 1.07).
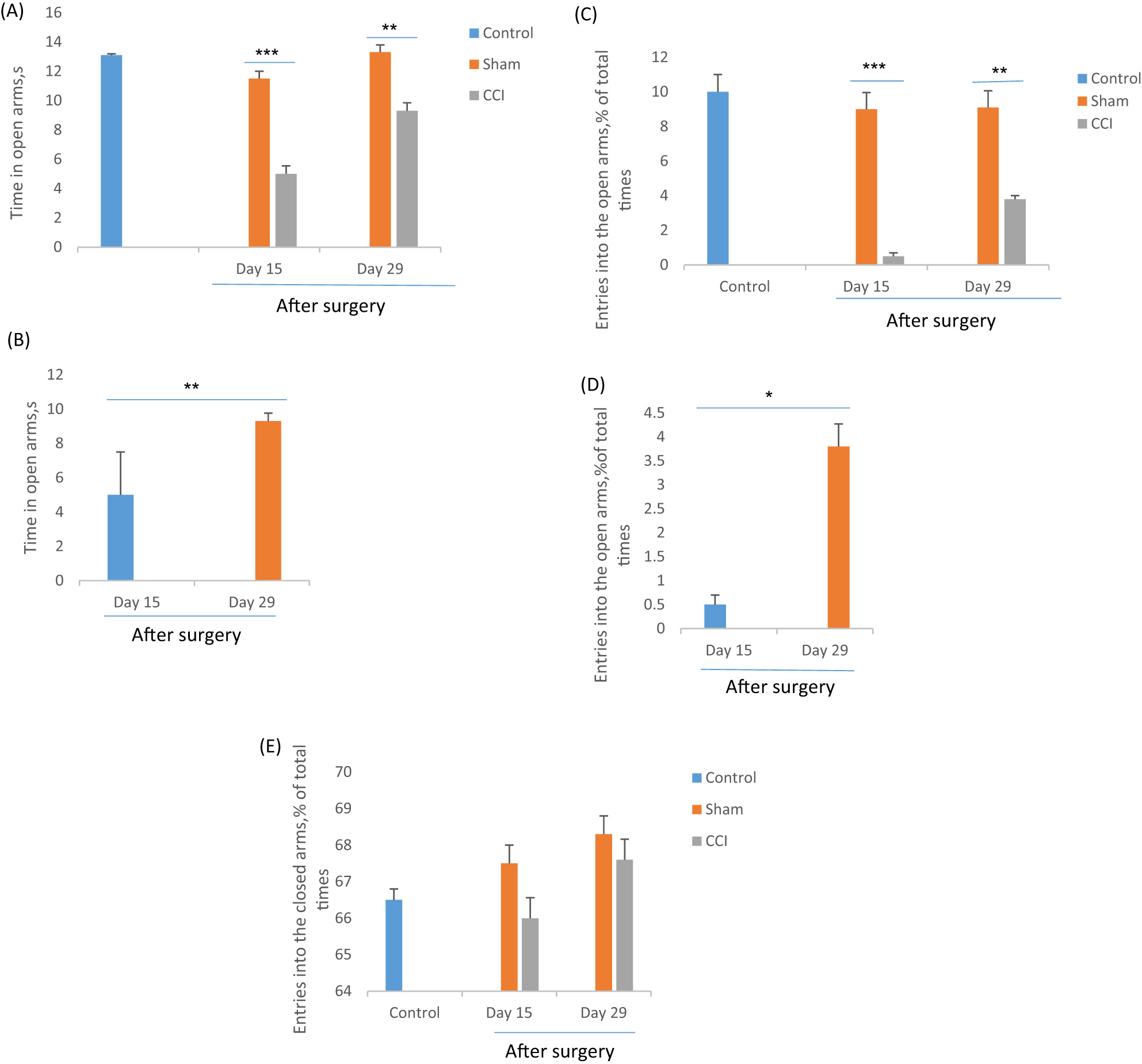
The effect of chronic constriction injury on elevated plus maze test on days 15 and 29th after surgery. The time spent in the open arms (A and B), the entries into the open arms(C and D) and the time spent in the closed arms, were measured by one-way ANOVA with a post hoc test (Scheffe’s test). *p<0.05, **p<0.01 and ***p<0.001 indicate the differences between the groups. Data are expressed as mean ± SEM of six animals per each group.
The total number of entries in closed arms is considered the activity index in the EPM test [53]. Our result showed that there was no significant difference (F=1.123, p=0.31) among experimental groups, so it seems that CCI surgery no effect on the activity rate of rats (Figure 4E).
Microdialysis & HPLC analysis
Based on one-way ANOVA and post hoc analysis, the 5-HT release in the CA1 region of CCI rats significantly (F=43.8, p=0.000) declined at the 10 min interval in four consecutive samples in contrast with the sham groups on days 16 and 30 of the experiment. The results revealed on day 30 the mean 5-HT concentration of CCI rats (36.6 ± 3.39 fmoL/40 μL) significantly (F=40.8, p=0.000) was more than day 16 (11.87 ± 1.75 fmoL/40 μL).No significant differences showed between the control and sham groups (Figure 5).
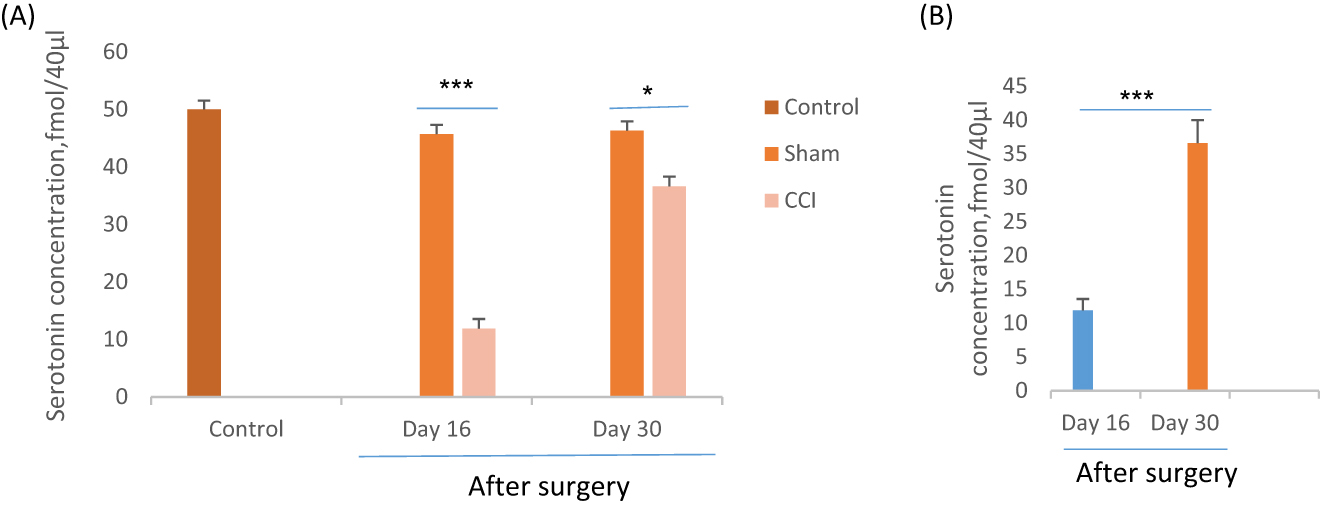
Effect of CCI or sham surgery on extracellular level of serotonin (5-HT) in CA1 region of hippocampus of rats after behavioral assessment. *p<0.05 and ***p<0.001 indicate the differences between the groups on days 16 and 30th after surgery. Data analyzed by one-way ANOVA with a post hoc test (Scheffe’s test) and expressed as mean ± SEM of six animals per each group.
Pearson correlation test showed that 5-HT levels in the hippocampal CA1 region strongly correlated with anxiety-related behavior in OFT and EPM tests in CCI rats. The direction of its correlation was negative. Correlation data between time spent at the center and 5-HT levels was p=0.015, r=0.9 in the OFT test two weeks after CCI surgery. Also, the entries (p=0.001, r=0.97) and time spent (p=0.05, r=0.8) in open arms significantly correlated with the 5-HT levels in the CA1 region of hippocampous in the EPM test two weeks after CCI surgery. The Result showed that CCI-rats with more anxiety-like behavior have lower extracellular 5-HT levels in the CA1 region two weeks after CCI surgery. Pearson correlation test showed no meaningful correlation between 5-HT levels in the hippocampal CA1 region with anxiety-related behavior in OFT and EPM tests in CCI rats four weeks after surgery.
Discussion
Our results show that, the CA1 region of hippocampus was altered in neuropathic pain situation, as determined by a reduction in 5-HT level after the occur of sciatic nerve injury while CCI rats exhibited the inflated anxiety-like behaviors in open field and elevated plus maze (EPM) tests. The anxiety-like behaviors of the CCI rats significantly decreased when the pain threshold and CA1 5-HT level increased by passing almost four weeks from the surgery. Noticeable patients with neuropathic pain endure anxiety, which can significantly decrease their quality of life [2]. In laboratory studies, anxiety-like behaviors were also reported in different animal models of chronic neuropathic pain [54], [55], [56]. However, some experimental studies declined to display the chronic pain-induced anxiety-like behaviors. Although various factors might be involved in this conflicting, it implies that the time of behavioral assessment and chronic pain-induced model to be important for identifying the anxiety-like behaviors [57], [58], [59].
In this assay, we induced neuropathic pain by CCI of the sciatic nerve, which has been frequently described to persuade anxiety-like behaviors by previously performed studies [60], [61]. The current data presented that pain threshold significantly decreased at the second week after CCI, and then it partly backward at the fourth week. Previous results identified that CCI of the sciatic nerve could cause lengthened and notable allodynia and hyperalgesia. These neuropathic pain-related symptoms reached to the top after two weeks, and then decreased gradually up to week seven after sciatic nerve injury. It was defined that the nerve fibers began to rejuvenate from the third week and this rejuvenating was associated with the increasing pain threshold in the CCI model [45], [62], [63], [64]. Thus, it seems that the CCI consequences are in part reversible.
The enduring pain symptoms in this model permitted the incidence of anxiety related to neuropathic pain state. We reported that the CCI rats displayed anxiety-like behaviors in both OF and EPM tests, two classical, and reliable anxiety experiments in rodents [65], [66], by passing two weeks from the sciatic nerve injury. It was thought that these behavioral changes are related to the anxiety-like behaviors, rather than surgery-induced motor disorders, as determined by the unchanged total distance that the CCI rats moved in the OF test.
Although the relationship between neuropathic pain and anxiety was perceived, the factors involved in this interaction are incompletely known yet [54], [61].
The current study found that, the neuropathic rats displayed the anxiety-like symptoms in OF and EPM tests, and concurrently, the CA1 5-HT level has significantly fluctuated when the pain threshold was changed.
The Hippocampus is recognized to contribute to the modulation of anxiety through interaction with the anxiety networks, such as prefrontal cortex, thalamus, and hypothalamus [67], [68].
Many clinical and experimental studies have identified that the hippocampus sustained both structural and functional changes under the chronic pain conditions [22], [25], [26], [31]. These changes may consequently result in pain-related mood disorders like anxiety [23], [32]. The earlier obtained data noted the involvement of the CA1 region of the hippocampus in pain and mood disorders comorbidity [24], [69], [70], [71].
We observed that, rats with lower pain threshold presented the anxiety-like behavior, and their 5-HT levels were also lower in the CA1 region of the hippocampus. When the pain threshold increased, the anxiety-like behavior decreased, as well as the 5-HT levels in the CA1 region of the hippocampus that increased in CCI rats. Accordingly, this suggests that, the pain induced by sciatic nerve injury could be contributed to the anxiety-like behaviors associated with the reduction of CA1 5-HT level, and the elevation in pain threshold improved the anxiety-like behavior, which was accompanied by the lowered the CA1 5-HT concentration in CCI rats. In this regard, our results propose that, anxiety symptoms would be accompanied by a reduction of the 5-HT concentration; conversely, the increase of pain threshold was able to reverse the anxiety-like behaviors and to increase the 5-HT levels in the CA1 region of the hippocampus in the CCI rats.
Correspondingly, previous documents supported that, the reduction of 5-HT level in the hippocampus could possibly promote the anxiety-like behaviors [72], [73]. It was documented that the abnormality in the brain 5-HT system is involved in mood disorders, and alteration in the brain 5-HT level is accompanied by anxiety and depression. Pharmacological inhibition of 5-HT reuptake increases the serotonergic concentration and reduces the symptoms of anxiety [13], [16], [34], [39], [41], [74].
In consistent with our data, recent studies reported that the analgesic effect of 5-HT agonist in other assays revealed that, the adaptive changes in the 5-HT level of the hippocampus could alleviate pain and anxiety comorbidity in the neuropathic pain models [13], [34], [75].
Moreover, it was identified that the CA1 region of the hippocampus can obtain projections from the median raphe nucleus, which were thought to modulate 5-HT releasing, and also to regulate the anxiety-related behaviors [72], [73]. Also, other studies explained that the CA1 region of the hippocampus was altered during neuropathic pain situations [20], [29], [71]. Despite this information, our data supported this idea that, the neuropathic pain induced by sciatic nerve injury could be associated with the related anxiety-like behaviors and also with the alteration of 5-HT concentration of the CA1 region of the hippocampus in CCI rats.
Conclusion
In conclusion, our study determined the association between the 5-HT levels of the CA1 region of the hippocampus and the anxiety-related behaviors in CCI of the sciatic nerve. Our findings suggested that pain alleviation could improve the pain-related anxiety-like behaviors and be accompanied with 5-HT level enhancement in the CA1 region of hippocampus.
Award Identifier / Grant number: 9466
Funding source: Physiology Research Center of Iran University of Medical Science
Award Identifier / Grant number: 96-01-30-24473
-
Research funding: This experiment received grants from the Neuroscience Research Center of Kerman University of Medical Sciences (Grant number: 9466) and the Physiology Research Center of Iran University of Medical Science (Grant number: 96-01-30-24473).
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interest: The authors declare that there are no competing financial interests.
-
Informed consent: Not applicable.
-
Ethical approval: The experiments obeyed to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain (IASP) and accepted by the Ethical Committee of the University (96-01-30-24473).
References
1. Bushnell, MC, Ceko, M, Low, LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;14:502–11. https://doi.org/10.1038/nrn3516.Search in Google Scholar PubMed PubMed Central
2. Yalcin, I, Barthas, F, Barrot, M. Emotional consequences of neuropathic pain: insight from preclinical studies. Neurosci Biobehav Rev 2014;47:154–64. https://doi.org/10.1016/j.neubiorev.2014.08.002.Search in Google Scholar PubMed
3. Wright, ME, Rizzolo, D. An update on the pharmacologic management and treatment of neuropathic pain. Jaapa 2017;30:13–7. https://doi.org/10.1097/01.jaa.0000512228.23432.f7.Search in Google Scholar PubMed
4. Panczyk, K, Golda, S, Waszkielewicz, A, Zelaszczyk, D, Gunia-Krzyzak, A, Marona, H. Serotonergic system and its role in epilepsy and neuropathic pain treatment: a review based on receptor ligands. Curr Pharmaceut Des 2015;21:1723–40. https://doi.org/10.2174/1381612821666141121114917.Search in Google Scholar PubMed
5. Bannister, K, Dickenson, AH. What do monoamines do in pain modulation? Curr Opin Support Palliat Care 2016;10:143–8. https://doi.org/10.1097/spc.0000000000000207.Search in Google Scholar PubMed PubMed Central
6. Lopez-Alvarez, VM, Puigdomenech, M, Navarro, X, Cobianchi, S. Monoaminergic descending pathways contribute to modulation of neuropathic pain by increasing-intensity treadmill exercise after peripheral nerve injury. Exp Neurol 2018;299:42–55. https://doi.org/10.1016/j.expneurol.2017.10.007.Search in Google Scholar PubMed
7. Avila-Rojas, SH, Velazquez-Lagunas, I, Salinas-Abarca, AB, Barragan-Iglesias, P, Pineda-Farias, JB, Granados-Soto, V. Role of spinal 5-HT5A, and 5-HT1A/1B/1D, receptors in neuropathic pain induced by spinal nerve ligation in rats. Brain Res 2015;1622:377–85. https://doi.org/10.1016/j.brainres.2015.06.043.Search in Google Scholar PubMed
8. Munoz-Islas, E, Vidal-Cantú, GC, Bravo-Hernández, M, Cervantes-Durán, C, Quiñonez-Bastidas, GN, Pineda-Farias, JB, et al. Spinal 5-HT(5)A receptors mediate 5-HT-induced antinociception in several pain models in rats. Pharmacol Biochem Behav 2014;120:25–32. https://doi.org/10.1016/j.pbb.2014.02.001.Search in Google Scholar PubMed
9. Bjorkman, R. Central antinociceptive effects of non-steroidal anti-inflammatory drugs and paracetamol. Experimental studies in the rat. Acta Anaesthesiol Scand Suppl 1995;103:1–44. https://doi.org/10.1111/j.1399-6576.1995.tb04249.x.Search in Google Scholar
10. Obata, H. Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 2017;18. https://doi.org/10.3390/ijms18112483.Search in Google Scholar PubMed PubMed Central
11. Hayashida, KI, Obata, H. Strategies to treat chronic pain and strengthen impaired descending noradrenergic inhibitory system. Int J Mol Sci 2019;20. https://doi.org/10.3390/ijms20040822.Search in Google Scholar PubMed PubMed Central
12. Kremer, M, Salvat, E, Muller, A, Yalcin, I, Barrot, M. Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 2016;338:183–206. https://doi.org/10.1016/j.neuroscience.2016.06.057.Search in Google Scholar PubMed
13. Rojo, ML, Rodríguez-Gaztelumendi, A, Pazos, Á, Díaz, Á. Differential adaptive changes on serotonin and noradrenaline transporters in a rat model of peripheral neuropathic pain. Neurosci Lett 2012;515:181–6. https://doi.org/10.1016/j.neulet.2012.03.050.Search in Google Scholar PubMed
14. Zhuo, M. Descending facilitation. Mol Pain 2017;13:1744806917699212.10.1177/1744806917699212Search in Google Scholar PubMed PubMed Central
15. Heinricher, MM, Tavares, I, Leith, JL, Lumb, BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev 2009;60:214–25. https://doi.org/10.1016/j.brainresrev.2008.12.009.Search in Google Scholar PubMed PubMed Central
16. Vahid-Ansari, F, Daigle, M, Manzini, MC, Tanaka, KF, Hen, R, Geddes, SD, et al. Abrogated freud-1/cc2d1a repression of 5-HT1A autoreceptors induces fluoxetine-resistant anxiety/depression-like behavior. J Neurosci 2017;37:11967–78. https://doi.org/10.1523/jneurosci.1668-17.2017.Search in Google Scholar
17. Sheng, J, Liu, S, Wang, Y, Cui, R, Zhang, X. The link between depression and chronic pain: neural mechanisms in the brain. Neural Plast 2017;2017:9724371.10.1155/2017/9724371Search in Google Scholar PubMed PubMed Central
18. Simons, LE, Elman, I, Borsook, D. Psychological processing in chronic pain: a neural systems approach. Neurosci Biobehav Rev 2014;39:61–78. https://doi.org/10.1016/j.neubiorev.2013.12.006.Search in Google Scholar PubMed PubMed Central
19. Sang, K, Bao, C, Xin, Y, Hu, S, Gao, X, Wang, Y, et al. Plastic change of prefrontal cortex mediates anxiety-like behaviors associated with chronic pain in neuropathic rats. Mol Pain 2018;14: https://doi.org/10.1177/1744806918783931.Search in Google Scholar PubMed PubMed Central
20. Descalzi, G, Mitsi, V, Purushothaman, I, Gaspari, S, Avrampou, K, Loh, YH, et al. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci Signal 2017;10. https://doi.org/10.1126/scisignal.aaj1549.Search in Google Scholar PubMed PubMed Central
21. Ong, WY, Stohler, CS, Herr, DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol 2019;56:1137–66. https://doi.org/10.1007/s12035-018-1130-9.Search in Google Scholar PubMed PubMed Central
22. Thompson, JM, Neugebauer, V. Cortico-limbic pain mechanisms. Neurosci Lett 2019;702:15–23. https://doi.org/10.1016/j.neulet.2018.11.037.Search in Google Scholar PubMed PubMed Central
23. Fasick, V, Spengler, RN, Samankan, S, Nader, ND, Ignatowski, TA. The hippocampus and TNF: common links between chronic pain and depression. Neurosci Biobehav Rev 2015;53:139–59. https://doi.org/10.1016/j.neubiorev.2015.03.014.Search in Google Scholar PubMed
24. Saffarpour, S, Shabani, M, Taheripak, G, Esmaeili-Mahani, S, Janzadeh, A, Nasirinezhad, F. The alteration of hippocampal BDNF expression is associated with anxiety-like behavior following the injury to the sciatic nerve. Arch Neurosci 2018;6:e74029.10.5812/ans.74029Search in Google Scholar
25. Apkarian, AV, Mutso, AA, Centeno, MV, Kan, L, Wu, M, Levinstein, M, et al. Role of adult hippocampal neurogenesis in persistent pain. Pain 2016;157:418–28. https://doi.org/10.1097/j.pain.0000000000000332.Search in Google Scholar PubMed PubMed Central
26. Khanna, S, Chang, LS, Jiang, F, Koh, HC. Nociception-driven decreased induction of Fos protein in ventral hippocampus field CA1 of the rat. Brain Res 2004;1004:167–76. https://doi.org/10.1016/j.brainres.2004.01.026.Search in Google Scholar PubMed
27. Soleimannejad, E, Semnanian, S, Fathollahi, Y, Naghdi, N. Microinjection of ritanserin into the dorsal hippocampal CA1 and dentate gyrus decrease nociceptive behavior in adult male rat. Behav Brain Res 2006;168:221–5. https://doi.org/10.1016/j.bbr.2005.11.011.Search in Google Scholar PubMed
28. Khanna, S, Sinclair, JG. Responses in the CA1 region of the rat hippocampus to a noxious stimulus. Exp Neurol 1992;117:28–35. https://doi.org/10.1016/0014-4886(92)90107-2.Search in Google Scholar PubMed
29. Khanna, S, Sinclair, JG. Noxious stimuli produce prolonged changes in the CA1 region of the rat hippocampus. Pain 1989;39:337–43. https://doi.org/10.1016/0304-3959(89)90047-x.Search in Google Scholar PubMed
30. Nakamura, H, Katayama, Y, Kawakami, Y. Hippocampal CA1/subiculum-prefrontal cortical pathways induce plastic changes of nociceptive responses in cingulate and prelimbic areas. BMC Neurosci 2010;11:100. https://doi.org/10.1186/1471-2202-11-100.Search in Google Scholar PubMed PubMed Central
31. Kalman, E, Keay, KA. Different patterns of morphological changes in the hippocampus and dentate gyrus accompany the differential expression of disability following nerve injury. J Anat 2014;225:591–603. https://doi.org/10.1111/joa.12238.Search in Google Scholar PubMed PubMed Central
32. You, Z, Zhang, S, Shen, S, Yang, J, Ding, W, Yang, L, et al. Cognitive impairment in a rat model of neuropathic pain: role of hippocampal microtubule stability. Pain 2018;159:1518–28. https://doi.org/10.1097/j.pain.0000000000001233.Search in Google Scholar PubMed PubMed Central
33. Saffarpour, S, Shaabani, M, Naghdi, N, Farahmandfar, M, Janzadeh, A, Nasirinezhad, F. In vivo evaluation of the hippocampal glutamate, GABA and the BDNF levels associated with spatial memory performance in a rodent model of neuropathic pain. Physiol Behav 2017;175:97–103. https://doi.org/10.1016/j.physbeh.2017.03.025.Search in Google Scholar PubMed
34. Jiang, X, Yan, Q, Liu, F, Jing, C, Ding, L, Zhang, L, et al. Chronic trans-astaxanthin treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with chronic pain. Neurosci Lett 2018;662:36–43. https://doi.org/10.1016/j.neulet.2017.09.064.Search in Google Scholar PubMed
35. Arai, M, Genda, Y, Ishikawa, M, Shunsuke, T, Okabe, T, Sakamoto, A. The miRNA and mRNA changes in rat hippocampi after chronic constriction injury. Pain Med 2013;14:720–9. https://doi.org/10.1111/pme.12066.Search in Google Scholar PubMed
36. McKenna, JT, Vertes, RP. Collateral projections from the median raphe nucleus to the medial septum and hippocampus. Brain Res Bull 2001;54:619–30. https://doi.org/10.1016/s0361-9230(01)00465-8.Search in Google Scholar PubMed
37. Wang, S, Yabuki, Y, Matsuo, K, Xu, J, Izumi, H, Sakimura, K, et al. T-type calcium channel enhancer SAK3 promotes dopamine and serotonin releases in the hippocampus in naive and amyloid precursor protein knock-in mice. PLoS One 2018;13:e0206986. https://doi.org/10.1371/journal.pone.0206986.Search in Google Scholar PubMed PubMed Central
38. Soleimannejad, E, Naghdi, N, Khatami, S, Semnanian, S, Fathollahi, Y. Formalin pain increases the concentration of serotonin and its 5-hydroxyindoleacetic acid metabolite in the CA1 region of hippocampus. Daru 2010;18:29–34. https://doi.org/10.1016/s1090-3801(06)60224-3.Search in Google Scholar
39. File, SE, Kenny, PJ, Cheeta, S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 2000;66:65–72. https://doi.org/10.1016/s0091-3057(00)00198-2.Search in Google Scholar PubMed
40. Liu, KC, Guo, Y, Zhang, J, Chen, L, Liu, YW, Lv, SX, et al. Activation and blockade of dorsal hippocampal serotonin 6 receptors regulate anxiety-like behaviors in a unilateral 6-hydroxydopamine rat model of Parkinson’s disease. Neurol Res 2019:1–11. https://doi.org/10.1080/01616412.2019.1611204. 31056008.Search in Google Scholar PubMed
41. Graeff, FG, Guimarães, FS, De Andrade, TG, Deakin, JF. Role of 5-HT in stress, anxiety, and depression. Pharmacol Biochem Behav 1996;54:129–41. https://doi.org/10.1016/0091-3057(95)02135-3.Search in Google Scholar PubMed
42. Sant’Ana, AB, Vilela-Costa, HH, Vicente, MA, Hernandes, PM, de Andrade, TG, ZangrossiJrH. Role of 5-HT2C receptors of the dorsal hippocampus in the modulation of anxiety- and panic-related defensive responses in rats. Neuropharmacology 2019;148:311–9. https://doi.org/10.1016/j.neuropharm.2019.01.026.Search in Google Scholar PubMed
43. Overstreet, DH, Commissaris, RC, De La Garza, R, File, SE, Knapp, DJ, Seiden, LS. Involvement of 5-HT1A receptors in animal tests of anxiety and depression: evidence from genetic models. Stress 2003;6:101–10. https://doi.org/10.1080/1025389031000111311.Search in Google Scholar PubMed
44. Matsuo, M, Kataoka, Y, Mataki, S, Kato, Y, Oi, K. Conflict situation increases serotonin release in rat dorsal hippocampus: in vivo study with microdialysis and Vogel test. Neurosci Lett 1996;215:197–200. https://doi.org/10.1016/0304-3940(96)12982-7.Search in Google Scholar PubMed
45. Bennett, GJ, Xie, YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87–107. https://doi.org/10.1016/0304-3959(88)90209-6.Search in Google Scholar PubMed
46. Chaplan, SR, Bach, FW, Pogrel, JW, Chung, JM, Yaksh, TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994;53:55–63. https://doi.org/10.1016/0165-0270(94)90144-9.Search in Google Scholar PubMed
47. Dixon, WJ. The up-and-down method for small samples. J Am Stat Assoc 1965;60:967–78. https://doi.org/10.1080/01621459.1965.10480843.Search in Google Scholar
48. Cheah, M, Fawcett, JW, Andrews, MR. Assessment of thermal pain sensation in rats and mice using the Hargreaves test. Bio Protoc 2017;7. https://doi.org/10.21769/bioprotoc.2506.Search in Google Scholar
49. Zhao, Z, Loane, DJ, Murray, MG, Stoica, BA, Faden, AI. Comparing the predictive value of multiple cognitive, affective, and motor tasks after rodent traumatic brain injury. J Neurotrauma 2012;29:2475–89. https://doi.org/10.1089/neu.2012.2511.Search in Google Scholar PubMed PubMed Central
50. Curio, M, Jacone, H, Perrut, J, Pinto, ÂC, Filho, VF, Silva, RC. Acute effect of Copaifera reticulata Ducke copaiba oil in rats tested in the elevated plus-maze: an ethological analysis. J Pharm Pharmacol 2009;61:1105–10. https://doi.org/10.1211/jpp/61.08.0015.Search in Google Scholar PubMed
51. Singewald, N, Kaehler, S, Hemeida, R, Philippu, A. Release of serotonin in the rat locus coeruleus: effects of cardiovascular, stressful and noxious stimuli. Eur J Neurosci 1997;9:556–62. https://doi.org/10.1111/j.1460-9568.1997.tb01632.x.Search in Google Scholar PubMed
52. Seibenhener, ML, Wooten, MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. JoVE 2015:e52434, PMID: 25742564 PMCID: PMC4354627. https://doi.org/10.3791/52434.10.3791/52434.Search in Google Scholar
53. Walf, AA, Frye, CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2007;2:322–8. https://doi.org/10.1038/nprot.2007.44.Search in Google Scholar PubMed PubMed Central
54. Narita, M, Kaneko, C, Miyoshi, K, Nagumo, Y, Kuzumaki, N, Nakajima, M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology 2006;31:739–50. https://doi.org/10.1038/sj.npp.1300858.Search in Google Scholar PubMed
55. Wu, Y, Yao, X, Jiang, Y, He, X, Shao, X, Du, J, et al. Pain aversion and anxiety-like behavior occur at different times during the course of chronic inflammatory pain in rats. J Pain Res 2017;10:2585–93. https://doi.org/10.2147/jpr.s139679.Search in Google Scholar PubMed PubMed Central
56. Minami, M. Neuronal mechanisms for pain-induced aversion behavioral studies using a conditioned place aversion test. Int Rev Neurobiol 2009;85:135–44. https://doi.org/10.1016/s0074-7742(09)85010-1.Search in Google Scholar PubMed
57. Yalcin, I, Bohren, Y, Waltisperger, E, Sage-Ciocca, D, Yin, JC, Freund-Mercier, MJ, et al. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatr 2011;70:946–53. https://doi.org/10.1016/j.biopsych.2011.07.017.Search in Google Scholar PubMed
58. Leite-Almeida, H, Pinto-Ribeiro, F, Almeida, A. Animal models for the study of comorbid pain and psychiatric disorders. Mod Trends Pharmacopsychiatr 2015;30:1–21. https://doi.org/10.1159/000435929.Search in Google Scholar PubMed
59. Urban, R, Scherrer, G, Goulding, EH, Tecott, LH, Basbaum, AI. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011;152:990–1000. https://doi.org/10.1016/j.pain.2010.12.003.Search in Google Scholar PubMed PubMed Central
60. Llorca-Torralba, M, Mico, JA, Berrocoso, E. Behavioral effects of combined morphine and MK-801 administration to the locus coeruleus of a rat neuropathic pain model. Prog Neuro-Psychopharmacol Biol Psychiatr 2018;84:257–66. https://doi.org/10.1016/j.pnpbp.2018.03.007.Search in Google Scholar PubMed
61. Alba-Delgado, C, Cebada-Aleu, A, Mico, JA, Berrocoso, E. Comorbid anxiety-like behavior and locus coeruleus impairment in diabetic peripheral neuropathy: a comparative study with the chronic constriction injury model. Prog Neuro-Psychopharmacol Biol Psychiatr 2016;71:45–56. https://doi.org/10.1016/j.pnpbp.2016.06.007.Search in Google Scholar PubMed
62. Roeska, K, Doods, H, Arndt, K, Treede, RD, Ceci, A. Anxiety-like behaviour in rats with mononeuropathy is reduced by the analgesic drugs morphine and gabapentin. Pain 2008;139:349–57. https://doi.org/10.1016/j.pain.2008.05.003.Search in Google Scholar PubMed
63. Karl, F, Colaço, MB, Schulte, A, Sommer, C, Üçeyler, N. Affective and cognitive behavior is not altered by chronic constriction injury in B7-H1 deficient and wildtype mice. BMC Neurosci 2019;20:16. https://doi.org/10.1186/s12868-019-0498-4.Search in Google Scholar PubMed PubMed Central
64. Attal, N, Filliatreau, G, Perrot, S, Jazat, F, Di Giamberardino, L, Guilbaud, G. Behavioural pain-related disorders and contribution of the saphenous nerve in crush and chronic constriction injury of the rat sciatic nerve. Pain 1994;59:301–12. https://doi.org/10.1016/0304-3959(94)90083-3.Search in Google Scholar PubMed
65. Takahashi, A, Kato, K, Makino, J, Shiroishi, T, Koide, T. Multivariate analysis of temporal descriptions of open-field behavior in wild-derived mouse strains. Behav Genet 2006;36:763–74. https://doi.org/10.1007/s10519-005-9038-3.Search in Google Scholar PubMed
66. Feyissa, DD, Aher, YD, Engidawork, E, Höger, H, Lubec, G, Korz, V. Individual differences in male rats in a behavioral test battery: a multivariate statistical approach. Front Behav Neurosci 2017;11:26. https://doi.org/10.3389/fnbeh.2017.00026.Search in Google Scholar PubMed PubMed Central
67. Bueno-Junior, LS, Leite, JP. Input convergence, synaptic plasticity and functional coupling across hippocampal-prefrontal-thalamic circuits. Front Neural Circ 2018;12:40. https://doi.org/10.3389/fncir.2018.00040.Search in Google Scholar PubMed PubMed Central
68. Jimenez, JC, Su, K, Goldberg, AR, Luna, VM, Biane, JS, Ordek, G, et al. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 2018;97:670–83. https://doi.org/10.1016/j.neuron.2018.01.016.Search in Google Scholar PubMed PubMed Central
69. Tyrtyshnaia, AA, Manzhulo, IV, Sultanov, RM, Ermolenko, EV. Adult hippocampal neurogenesis in neuropathic pain and alkyl glycerol ethers treatment. Acta Histochem 2017;119:812–21. https://doi.org/10.1016/j.acthis.2017.10.007.Search in Google Scholar PubMed
70. Lax, NC, Parker, SA, Hilton, EJ, Seliman, Y, Tidgewell, KJ, Kolber, BJ. Cyanobacterial extract with serotonin receptor subtype 7 (5-HT7 R) affinity modulates depression and anxiety-like behavior in mice. Synapse 2018;72:e22059.10.1002/syn.22059Search in Google Scholar PubMed PubMed Central
71. Lyu, D, Yu, W, Tang, N, Wang, R, Zhao, Z, Xie, F, et al. The mTOR signaling pathway regulates pain-related synaptic plasticity in rat entorhinal-hippocampal pathways. Mol Pain 2013;9:64. https://doi.org/10.1186/1744-8069-9-64.Search in Google Scholar PubMed PubMed Central
72. Cervo, L, Mocaer, E, Bertaglia, A, Samanin, R. Roles of 5-HT(1A) receptors in the dorsal raphe and dorsal hippocampus in anxiety assessed by the behavioral effects of 8-OH-DPAT and S 15535 in a modified Geller-Seifter conflict model. Neuropharmacology 2000;39:1037–43. https://doi.org/10.1016/s0028-3908(99)00189-6.Search in Google Scholar PubMed
73. Mlinar, B, Corradetti, R. Differential modulation of CA1 impulse flow by endogenous serotonin along the hippocampal longitudinal axis. Hippocampus 2018;28:217–25. https://doi.org/10.1002/hipo.22825.Search in Google Scholar PubMed
74. Kaufman, J, DeLorenzo, C, Choudhury, S, Parsey, RV. The 5-HT1A receptor in major depressive disorder. Eur Neuropsychopharmacol 2016;26:397–410. https://doi.org/10.1016/j.euroneuro.2015.12.039.Search in Google Scholar PubMed PubMed Central
75. Zuena, AR, Maftei, D, Alemà, GS, Dal Moro, F, Lattanzi, R, Casolini, P, et al. Multimodal antidepressant vortioxetine causes analgesia in a mouse model of chronic neuropathic pain. Mol Pain 2018;14:1744806918808987.10.1177/1744806918808987Search in Google Scholar PubMed PubMed Central
© 2020 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial Comments
- Patients with shoulder pain referred to specialist care; treatment, predictors of pain and disability, emotional distress, main symptoms and sick-leave: a cohort study with a 6-months follow-up
- Inferring pain from avatars
- Systematic Review
- Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review
- Topical Reviews
- Exploring the underlying mechanism of pain-related disability in hypermobile adolescents with chronic musculoskeletal pain
- Pain management programmes via video conferencing: a rapid review
- Clinical Pain Research
- Prevalence of temporomandibular disorder in adult patients with chronic pain
- A cost-utility analysis of multimodal pain rehabilitation in primary healthcare
- Psychosocial subgroups in high-performance athletes with low back pain: eustress-endurance is most frequent, distress-endurance most problematic!
- Trajectories in severe persistent pain after groin hernia repair: a retrospective analysis
- Involvement of relatives in chronic non-malignant pain rehabilitation at multidisciplinary pain centres: part one – the patient perspective
- Observational Studies
- Recurrent abdominal pain among adolescents: trends and social inequality 1991–2018
- Cross-cultural adaptation and psychometric validation of the Hausa version of Örebro Musculoskeletal Pain Screening Questionnaire in patients with non-specific low back pain
- A proof-of-concept study on the impact of a chronic pain and physical activity training workshop for exercise professionals
- Intravenous patient-controlled analgesia vs nurse administered oral oxycodone after total knee arthroplasty: a retrospective cohort study
- Everyday living with pain – reported by patients with multiple myeloma
- Original Experimental
- The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats
- A single bout of coordination training does not lead to EIH in young healthy men – a RCT
- Think twice before starting a new trial; what is the impact of recommendations to stop doing new trials?
- The association between selected genetic variants and individual differences in experimental pain
- Decoding of facial expressions of pain in avatars: does sex matter?
- Differences in personality, perceived stress and physical activity in women with burning mouth syndrome compared to controls
- Educational Case Reports
- Leiomyosarcoma of the small intestine presenting as abdominal myofascial pain syndrome (AMPS): case report
- Duloxetine for the management of sensory and taste alterations, following iatrogenic damage of the lingual and chorda tympani nerve
- Lead extrusion ten months after spinal cord stimulator implantation: a case report
- Short Communication
- Postoperative opioids and risk of respiratory depression – A cross-sectional evaluation of routines for administration and monitoring in a tertiary hospital
Articles in the same Issue
- Frontmatter
- Editorial Comments
- Patients with shoulder pain referred to specialist care; treatment, predictors of pain and disability, emotional distress, main symptoms and sick-leave: a cohort study with a 6-months follow-up
- Inferring pain from avatars
- Systematic Review
- Repetitive transcranial magnetic stimulation of the primary motor cortex in management of chronic neuropathic pain: a systematic review
- Topical Reviews
- Exploring the underlying mechanism of pain-related disability in hypermobile adolescents with chronic musculoskeletal pain
- Pain management programmes via video conferencing: a rapid review
- Clinical Pain Research
- Prevalence of temporomandibular disorder in adult patients with chronic pain
- A cost-utility analysis of multimodal pain rehabilitation in primary healthcare
- Psychosocial subgroups in high-performance athletes with low back pain: eustress-endurance is most frequent, distress-endurance most problematic!
- Trajectories in severe persistent pain after groin hernia repair: a retrospective analysis
- Involvement of relatives in chronic non-malignant pain rehabilitation at multidisciplinary pain centres: part one – the patient perspective
- Observational Studies
- Recurrent abdominal pain among adolescents: trends and social inequality 1991–2018
- Cross-cultural adaptation and psychometric validation of the Hausa version of Örebro Musculoskeletal Pain Screening Questionnaire in patients with non-specific low back pain
- A proof-of-concept study on the impact of a chronic pain and physical activity training workshop for exercise professionals
- Intravenous patient-controlled analgesia vs nurse administered oral oxycodone after total knee arthroplasty: a retrospective cohort study
- Everyday living with pain – reported by patients with multiple myeloma
- Original Experimental
- The CA1 hippocampal serotonin alterations involved in anxiety-like behavior induced by sciatic nerve injury in rats
- A single bout of coordination training does not lead to EIH in young healthy men – a RCT
- Think twice before starting a new trial; what is the impact of recommendations to stop doing new trials?
- The association between selected genetic variants and individual differences in experimental pain
- Decoding of facial expressions of pain in avatars: does sex matter?
- Differences in personality, perceived stress and physical activity in women with burning mouth syndrome compared to controls
- Educational Case Reports
- Leiomyosarcoma of the small intestine presenting as abdominal myofascial pain syndrome (AMPS): case report
- Duloxetine for the management of sensory and taste alterations, following iatrogenic damage of the lingual and chorda tympani nerve
- Lead extrusion ten months after spinal cord stimulator implantation: a case report
- Short Communication
- Postoperative opioids and risk of respiratory depression – A cross-sectional evaluation of routines for administration and monitoring in a tertiary hospital


