Evaluation of glass-fiber grafted by epoxide-terminated hyperbranched polymer on the effect of mechanical characterization of epoxy composites
-
Shuiping Li
, Yanbo Li
Abstract
In this study, glass-fiber, grafted by epoxide-terminated hyperbranched polymer (GF-HBPE), was incorporated into epoxy resins for reinforcement purpose. The effects of GF-HBPE content on mechanical properties of the resulting epoxy-based composites, such as tensile strength, percentage elongation at break, flexural strength, and impact strength, were investigated. The experimental results revealed that GF-HBPE substantially outperformed impact resistance in both tensile and flexural tests. For instance, the tensile strength, percentage elongation at break, flexural strength, and impact strength of the epoxy composite with 1 wt% GF-HBPE increase by about 23.6%, 125%, 26%, and 74.5%, respectively, compared to the unmodified epoxy thermoset.
1 Introduction
Epoxy composite is one of the most common polymer-based composites that is being used in increasing quantities in aerospace, aircraft, wind turbine, automobile, boat, and mechanical and electronic applications [1], [2] owing to their unique properties, such as high thermal stability and low thermal expansivity, good dimensional stability, great modulus, low density, low creep, low dielectric constant, ease of processability, excellent flexible and tensile strength, and relatively low costs [3], [4], [5].
Over the past two decades or more, there have been numerous reports about fiber-reinforced epoxy composites (FRECs) for improving the mechanical properties and comparisons with the non-reinforced or pristine material counterparts [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. The mechanical properties of FRECs are dependent on the mechanical properties of matrix polymer, the ability of fiber, and the strength of interfacial regions between polymer matrix and fibers [20], [21]. Sufficient bonding at the interfaces is required to ensure effective load transmission from the matrix to the fibers [5]. Tehrani et al. [16] investigated the effect of adding multi-walled carbon nanotubes (MWCNTs) to the epoxy matrix of a carbon fiber (SF) reinforced composite and showed that the mechanical properties could be favorably improved. Dong et al. [22] designed a kind of CNT organization form of continuous networks to optimize CNT dispersion state in fiber/epoxy composite and demonstrated that the continuous and porous CNT networks could be assembled in fiber fabric and preserved in composites. Li et al. [23] revealed that the interface and interfacial fracture energy of resin composite with CF, after hygrothermal treatment, could not recover to the level of composite with raw dry fiber, which was assigned to the silicon element on the CF. Liu et al. [24] supported that poly(phthalazinone ether ketone) (PPEK) could be used to size CF without a reduction on the tensile strength, and CF sized by the thermoplastic sizing agent performed better interfacial adhesion. Qi et al. [25] calculated the fiber/matrix interfacial strength in the transverse fiber bundle (TFB) composite and suggested that interfacial debonding was the dominated failure mechanism in the TFB test. Rahmanian et al. [13] evaluated the synergy effects of CNTs and short CFs in the epoxy matrix and demonstrated that the elastic and storage modulus, strength, and impact resistance of the multiscale composites were significantly improved in comparison with CNTs-epoxy or CFs-epoxy composites.
Although the incorporation of fibers into resin matrix offers several advantages over the non-reinforced materials, the notable disadvantage of fiber is its weak interaction with resin matrix [10], [15], [26], [27], [28]. Mercerization and silanized treatments [29], alkaline treatment [15], [30], hygrothermal treatment [23], potassium permanganate treatment [31], and CNTs graft and deposition [20], [22], [27], [32], [33] methods were used to overcome this birth defect of fiber. Improvement of interfacial adhesion between fiber and epoxy matrix through these above methods has not translated directly into sufficient bonding at the interfaces and resulted in favorable mechanical properties. Therefore, research in this area is still in progress.
Hyperbranched polymers (HBPs) are an important area of dendritic polymers, given their potential use in a number of applications such as coating, environment, gene, drug carrier, photosensitive material, printing and dyeing, and nanomaterial owing to their high density and versatile terminal groups, low melting point and viscosity, unique spherical structure, and different phase separation behaviors from linear polymers [34]. A favorable advantage of HBPs is the high density of functional terminal/end groups, which can enhance the compatibility between HBPs and other polymeric matrixes [35], [36], [37]. Of course, HBPs can be used as toughener in epoxy resin thermosets [35], [36], [37]. Here we evaluate the glass-fibers that are grafted by epoxide group terminal HBP on the effect of mechanical properties of epoxy composites, discussing the results from tensile, flexural, and impact tests.
In our former works, we reported the improvement of impact resistance of epoxy thermosets/composites with hydroxyl and amino terminated HBP and core-shell particles [35], [37], [38], [39], [40]. Although the impact resistance was enhanced, other mechanical properties such as tensile strength, percentage elongation at break, and flexural strength were not improved but were even decreased. Recently, glass-fiber was grafted by amino and hydroxyl terminated HBP and used as modifier for epoxy resin [41]. Because the strong polarity of epoxide and excellent compatibility between HBP and epoxy matrix, the aim of this current research was to improve the mechanical properties of epoxy composites with glass-fiber grown by epoxide terminated hyperbranched (HBPE). Glass-fiber was first hydroxylated and silanized by coupling agent and then grown by epoxide-terminated HBP. Then GF-HBPE was incorporated into epoxy resin. The results of tensile, flexural, and impact tests of epoxy composites were discussed.
2 Materials and methods
2.1 Materials
Diglycidyl ether of bisphenol A epoxy resin E51 was purchased from Hangzhou Wuhuigang Adhesive Co., Ltd., Hangzhou, China, which had an epoxide equivalent of 185–208 g/eq. Polyamide 650, with amino value of 220±20 mgKOH/g, was supplied by Wuxi Resin Factory, Wuxi, China. Glass-fibers with an average length of 30 μm and diameter of 10 μm were supplied by Corker Co., Ltd, Taikang, China. Toluene was obtained from Nanjing Chemical Reagent Co., Ltd, Nanjing, China. γ-Aminopropyl triethoxysilane (γ-APS, KH-550) was purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Tetrabutyl ammonium bromide was purchased from Shanghai Dibai Chemical Reagent Co., Ltd. Shanghai, China. N,N-dimethylacetamide (DMA), epichlorohydrin (ECH), succinic anhydride, and diethanolamine were purchased from Chengdu KeLong Chemical Reagent Company, Chengdu, China. Unless especially mentioned, all reagents were of analytic grade and used as received.
2.2 Fabrication of glass-fiber with epoxide-terminated HBP (GF-HBPE)
The typical process is described as follows: 0.5 g of GFs was added into a 25-ml three-neck round-bottom flask with 5 ml of H2O2 and refluxed at 105°C for 4 h under magnetic stirring. The fibers were recovered by centrifugation at 5000 rpm for 10 min and washed with deionized water three times. After being dried under vacuum at 80°C for 12 h, the fibers were added into 5 ml of toluene and sonicated for 30 min, and then 0.25 g of γ-aminopropyl triethoxysilane was added and the mixture was heated to 80°C for 24 h under nitrogen flow and magnetic stirring. The products were recovered by centrifugation at 5000 rpm for 10 min and washed with toluene water three times and dried under vacuum at 80°C for 12 h. The products were named as silane-functionalized GFs.
Then 1.0 g of silane-functionalized GFs was added into the mixture of 1.57 g of succinic anhydride and 1.50 g of diethanolamine, in a 25-ml three-necked round-bottomed flask and stirred for 30 min. The mixture was heated to 70°C and stirred for 4 h. After that, the melting mixture was heated to 120°C and kept under stirring for 6 h. After that, the initial product was dried under vacuum at 80°C for 12 h to eliminate the water formed. Then the dried GF, 3.0 g of ECH, and tetrabutyl ammonium bromide were charged, respectively, in a three-necked round-bottomed flask equipped with stirrer, cooler, and thermometer, respectively. The temperature was raised to 110°C and stirred for 5 h, and then the excessive ECH was removed under a pressure of 3–5 mm Hg at 120°C for 10 min. After that, the temperature was decreased to 25°C and the resulting products were dissolved in DMA to eliminate the indiscerptible materials and dried under vacuum at 80°C for 24 h [42], [43].
2.3 Preparation of epoxy/GF-HBPE composites
For the preparation of epoxy-based composites, GF or GF-HBPE/epoxy=0–5 wt% was mixed with epoxy resins, which was preheated to 60°C and sonicated for 10 min. Then polyamide 650 (epoxy/polyamide 650, 1:1.2 wt/wt), which was also pre-heated up to 60°C, was added, and the resulting mixture was also stirred for 10 min. Finally, the formulations were poured into stainless steel templates (also pre-heated to 60°C) and cured at 60°C for 48 h in an oven.
2.4 Characterization
Fourier transform infrared spectroscopy (FTIR) spectra were conducted with a Tensor 37 instrument (Germany, Bruker) at a resolution of 4 cm−1 and within the range of 400–4000 cm−1 by attenuated total reflection infrared spectroscopy. 1H NMR spectrum was performed on an AVANCE III 500 NMR spectrometer (Switzerland, Bruker) with deuterated dimethyl sulfoxide (DMSO-d6) as the solvent at 293 K. Fifty milligrams of CSPs was added into a 5-ml beaker with 1 ml of DMSO-d6 under magnetic stirring at 300 rpm for 2 h. Then the mixture was centrifuged at 9000 rpm for 10 min. The supernatant liquid was used for 1H NMR spectrum. A SHIMADZU DTG 60 simultaneous measuring instrument (Japan, SHIMADZU) from 30 to 800°C at a heating rate of 20°C min−1 was used to evaluate the thermo-gravity (TGA) of the GFs before and after surface modification. Tensile tests were performed on dog-bone-shaped type according to GB/T 2567–2008 with a SHIMADZU AG-X plus test machine. The flexural strength was characterized by an UTM4000 electromechanical universal testing machine (China, SANS) according to GB/T 2567-2008 at room temperature of dimensions 80×15×4 mm with a loading rate of 2 mm/min. Un-notched Izod tests were performed on a ZBC-50 impact tester (China, SANS). All mechanical properties were performed according to GB/T 2567-2008 of a thickness of 4 mm and a width of 10 mm. All of the presented results were an average of five specimens. FESEM was recorded using a Hitachi SU8000 field-emission scanning electron microscope (Japan, Hitachi).
3 Results and discussion
3.1 Characterization of GF-HBPE
ATR-IR spectra can provide information about the chemical structure of samples. The ATR-IR spectrum of GF-HBPE is listed in Figure 1. The absorption at 3351.7 cm−1 is attributed to the vibration of -OH. The two weak absorptions at 2962.1 and 2888.5 cm−1 are assigned to the a stretching vibration of C-H. The strong peak at 1730.6 cm−1 is attributed to the stretching vibration of C=O. The absorptions at 1629.2 cm−1 and 805 cm−1 belongs to the bending vibration of N-H. The weak absorption at 1406.9 cm−1 is ascribed to the bending vibration of -CH2. The band at 1258.2 cm−1 is ascribed to the stretching vibration of C-O-C. The strong absorption band at 1158.2 cm−1 is ascribed to the stretching vibration of C-C. The strong absorption at 1036.7 cm−1 is attributed to the Si-O symmetrical stretching vibration. And the absorption levels at 910.2 and 849.3 cm−1 confirm the presence of free oxirane ring from the epoxy units.

ATR-IR spectrum of GF-HBPE.
The 1H NMR spectrum of GF-HBPE, as shown in Figure 2, can provide more detailed evidence to prove the successful growing of HBPE on the surface of silane-functionalized GFs. As can be seen in Figure 2, the 1H NMR spectrum clearly displays the characteristic peaks of HBPE, such as 2.49 ppm (CH2CHCH2O-, protons a), 3.52 ppm (CH2CHCH2O-, protons b), 4.03 ppm (CH2CHCH2O-, protons c), 3.63 ppm (N-CH2-CH2-O-, protons d), 3.19 ppm (N-CH2-CH2-O-, protons e), 4.74 ppm (N-CH2-CH2-O-, protons f), 3.48 ppm (N-CH2-CH2-O-, protons g), 2.59 ppm (-OOC-CH2-CH2-, protons h), 4.07 ppm (N-CH2-CH2-O-, protons i), 2.62 ppm (N-CH2-CH2-O-, protons j), 3.90 ppm (N-CH2-CH2-OH, protons k), 3.32 ppm (N-CH2-CH2-OH, protons l), and 4.17 ppm (N-CH2-CH2-OH, protons m). The peaks at 2.49, 3.52, and 4.03 ppm are the characterization of epoxide ring. These peaks can prove the successful grown of HBPE on the surface of GFs. And the spectrum of HBPE shows three kinds of proton peaks, which can be attributed to linear, dendritic, and terminal units. For instance, the peaks at 2.49, 3.19, 3.52, 3.63, and 4.03 ppm are attributed to the protons of the terminal units. The peaks at 2.59, 3.48, 4.07, and 4.74 ppm are ascribed to those of the dendritic units, whereas the peaks at 2.62, 3.32, 3.90, and 4.17 are attributed to the protons of the linear units. The degree of branching (DB) of HBPE, which is the ratio of the sum of the integration of the dendritic (D), linear (L), and terminal (T) units in its structure, i.e. DB=(D+T)/(D+L+T) [44], is 0.63. The 1H NMR result agrees with the ATR-IR spectrum (Figure 1) of HBPE.

1H NMR spectrum of GF-HBPE.
Figure 3 shows the TGA curves of silanized GF and GF-HBPE measured under N2. The temperatures for 5% and 10 wt% weight losses (T5% and T10%) of the silanized GF are 103.8 and 223.2°C, respectively. And the final weight loss is 33.7% at 800°C, which is attributed to the moisture and the decomposition of γ-APS, while the T5% and T10% values of GF-HBPE are 222.8 and 314.4°C, respectively. The high weight loss of GF-HBPE in low temperature may be due to the hygroscopicity of HBPE. Moreover, the GF-HBPE has a higher weight loss (47.6%) than that of the silanized GFs at 800°C. The present hydrocarbon chain in the HBPE frameworks leads to being less stable than silanized under high temperature. The TGA confirms the HBPE molecules being successfully grown to the surface of the silane-functionalized GF.

TGA curves of GF and GF-HBPE.
Figure 4 shows the surface characterizations of the pristine and silanized GFs. The surfaces of pristine GFs are smooth, and the average diameter is about 8–10 μm (Figure 4A). However, the surfaces of the silanized GFs, which are shown in Figure 4B, are rough due to the grafting of γ-APS. The thickness of the grafting γ-APS is about 2 μm, which is calculated from the insetting image in Figure 4B.

FESEM morphologies of the surfaces of the pristine (A) and silane-functionalizated GFs (B).
3.2 Morphologies of epoxy/GF-HBPE composites
The FESEM images of the surface of GF-HBPE and the impact fracture surfaces of the unmodified epoxy thermoset and epoxy composites with 1 wt% GF-HBPE are shown in Figure 5. The GF-HBPE is coated by HBPE in a thin layer and has a rough surface (Figure 5A). The functional group in the framework of HBPE can react with the epoxy matrix and then improve the mechanical properties of epoxy/GF-HBPE composites. The rough surface of GF-HBPE can enhance the friction between GF-HBPE and epoxy matrix and thus also improve the mechanical properties of epoxy/GF-HBPE composites. It can be seen in Figure 5B that the fracture surface of the unmodified epoxy thermoset is relatively smooth and glassy, shows a typical morphology of a brittle thermoset, and results to poor resistance to crack propagation and uninterrupted crack propagation path under impaction. The fracture surfaces of the epoxy composite with 1 wt% GF-HBPE, which are shown in Figure 5C and D, present considerable differences in comparison to the unmodified epoxy thermoset; the surface of the composite is rough. Moreover, the fracture surfaces of the composite have more shearing deformation than that of the unmodified epoxy thermoset. This may demonstrate the favorable compatibility between GF-HBPE and epoxy matrix, and the GF-HBPE and epoxy matrix experience interfacial adhesion due to the grown of HBPE on the surface of silanized GF [12].

FESEM morphologies of the unmodified epoxy thermoset (A), GFs-HBPE (B) and epoxy composites with 1 wt GF-HBPE (C-D).
3.3 Mechanical properties of epoxy/GF-HBPE composites
The influence of GF-HBPE content on the mechanical properties is investigated by discussing the results from the tensile, flexural, and impact tests. The mechanical properties of composites with different amounts of silanized GFs are also tested for comparison. The tensile strength of epoxy composites as function of GF/GF-HBPE content is shown in Figure 6. On the one hand, the tensile strength of composite with different amount of GF decreases with increment of GF except the formulation with 1 wt% GF. On the other hand, the incorporation of GF-HBPH can almost slightly enhance the tensile strength of composites. For instance, the tensile strength of the composite with 1 wt% GF-HBPE increases by about 23.6% than that of the unmodified epoxy thermoset.

Tensile strength of epoxy composites.
The percentage elongations at break of epoxy composites as function of GF-HBPE content are shown in Figure 7. It can be observed that the percentage elongation at break of the composite is independent on the content of GF. In contrast, the percentage elongations of the composites with GF-HBPE are all higher than that of the unmodified epoxy thermoset. The percentage elongation at break of the formulation with 1 wt% GH-HBPE increases by about 125%.
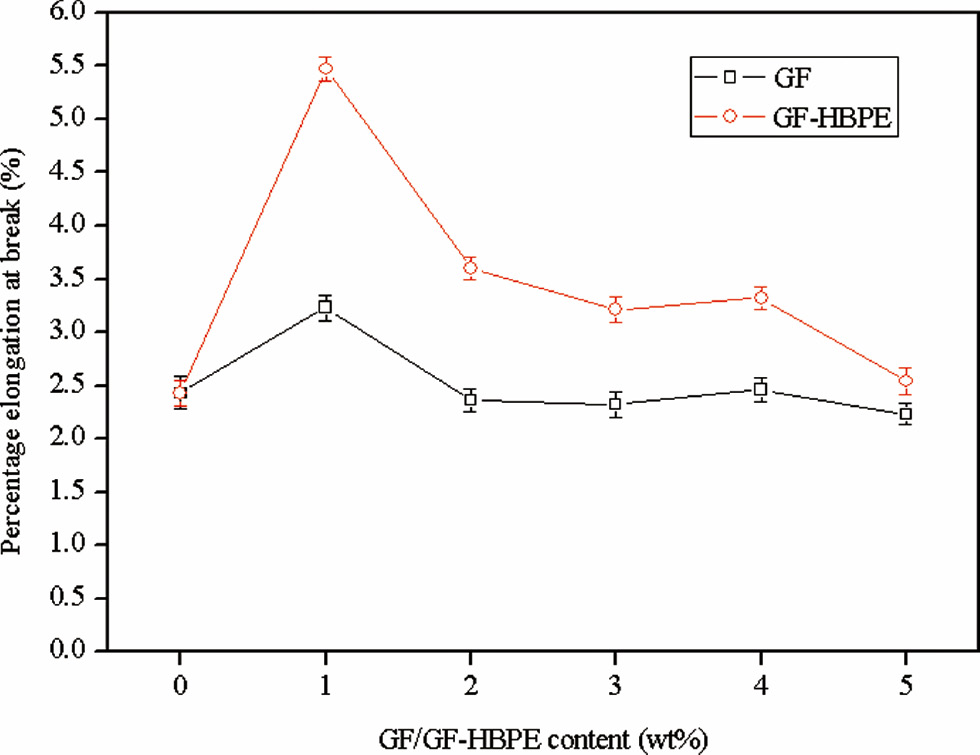
Percentage elongations at break of epoxy composites.
The flexural strength of epoxy composites with different content of GF/GF-HBPE is illustrated in Figure 8. The incorporation of GF just slightly increases the flexural strength, and then the value is lower than the unmodified epoxy thermoset when the content of GF is higher than 2 wt%. The decrease of flexural strength is attributed to the weak bonding between GF and epoxy matrix and also the aggregation of GFs. It is interesting to observe that the addition of a slight content of GF-HBPE, such as 1 wt%, can favorably enhance the flexural value, increasing by about 26% than that of the unmodified epoxy thermoset. However, the increment decreases when the GF-HBPE content is higher than 1 wt%, which may be due to the aggregation of GF-HBPE.

Flexural strength of epoxy composites.
Figure 9 presents the impact strength of epoxy composites with different content of GF/GF-HBPE. The impact strength decreases with increasing the GF content. Generally, the impact strength is more sensitive to the weak compatibility between GF and epoxy matrix than other mechanical properties. The impact testing process is almost instantaneous. The enhancement of GF on impact strength of epoxy composite is even to the point of negligible due to the weak interface adhesive strength of GF and epoxy matrix. It can be seen on Figure 9 that the value increases sharply from 7.667 KJ/m2 for the unmodified epoxy thermoset to 13.375 KJ/m2 for the formulation with 1 wt% GF-HBPE; after that, the trend alters and impact strength decreases to 10.123 KJ/m2 for the composite with 4 wt% GF-HBPE. It also can be observed that the impact strength of composite with 5 wt% GF-HBPE is lower than that of the unmodified epoxy thermoset.

Impact strength of epoxy composites.
On the whole, a slight addition of GF-HBPE can favorably increase the mechanical properties of epoxy-based composites. For instance, the tensile strength, elongation, flexural strength, and impact strength of the composite with 1 wt% GF-HBPE increase by about 23.6%, 125%, 26%, and 74.5%, compared to the unmodified epoxy thermoset, respectively. The strength polarity of the epoxide of HBPE, which is grown on the surface of GF, is attributed to the increment of the mechanical properties of epoxy/GH-HBPE composite. Firstly, the incorporation of GF-HBPE can modify the network of epoxy composite due to the excellent compatibility between GF-HBPE and epoxy unites [41]. Secondly, the dispersion strengthened effect of GF-HBPE can enhance the mechanical properties owing to the good dispersion of GF-HBPE in epoxy matrixes [12]. Lastly, the bridging effect of glass-fiber is also an enhanced mechanism [45]. However, the mechanical properties decrease when GF-HBPE content is higher than a certain threshold due to its aggregation. The enhancements are offset by negative effect, which is caused by the aggregation of GF-HBPE. So there is no obvious change in mechanical properties when GF-HBPE content is higher than a threshold.
4 Conclusions
Glass-fiber (GF) was first hydroxylated and silane-functionalized, and then epoxide-terminated HBPs (HBPE) were grown on their surfaces. The GF-HBPE was incorporated into epoxy resin to fabricate epoxy-based composites. And the effect of GF-HBPE on the mechanical properties of epoxy composites was investigated, discussing the results from tensile, flexural, and impact tests. For comparison, the influence of silanized GFs on the mechanical properties of epoxy composites was also studied.
The results indicated that a slight addition of GF-HBPE can substantially improve the mechanical properties of epoxy composites. For instance, the tensile strength, percentage elongation at break, flexural strength, and impact strength are increased from 37.69 MPa, 2.43%, 70.78 MPa, and 7.667 KJ/m2 for the unmodified epoxy thermoset to 46.58 MPa, 5.47%, 89.16 MPa, and 13.375 KJ/m2 for the epoxy composite with 1 wt% GF-HBPE, respectively.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 51603179
Award Identifier / Grant number: 51402251
Award Identifier / Grant number: 51578289
Award Identifier / Grant number: 51572234
Award Identifier / Grant number: 51502259
Funding statement: The authors gratefully acknowledge the funds from the National Natural Science Foundation of China (51603179, 51402251, 51578289, 51572234, and 51502259), the joint research fund between Collaborative Innovation Center for Ecological Building Materials and Environmental Protection Equipments and Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province (CP201506 and GX2015107), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A025), and the Natural science fund of Jiangsu Province (BK20130428).
Acknowledgments
The authors gratefully acknowledge the funds from the National Natural Science Foundation of China (51603179, 51402251, 51578289, 51572234, and 51502259), the joint research fund between Collaborative Innovation Center for Ecological Building Materials and Environmental Protection Equipments and Key Laboratory for Advanced Technology in Environmental Protection of Jiangsu Province (CP201506 and GX2015107), the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015A025), and the Natural science fund of Jiangsu Province (BK20130428).
References
[1] Eskizeybek V, Avci A, Gulce A, Composites Part A. 2014, 63, 94–102.10.1016/j.compositesa.2014.04.013Suche in Google Scholar
[2] Boon MS, Saw WPS, Mariatti M. J. Magn. Magn. Mater. 2012, 324, 755–760.10.1016/j.jmmm.2011.09.009Suche in Google Scholar
[3] Zhou W, Cai J. J. Appl. Polym. Sci. 2012, 124, 4346–4351.10.1002/app.35417Suche in Google Scholar
[4] Xiong X, Chen P, Zhang J, Yu Q, Wang B. J. Appl. Polym. Sci. 2011, 121, 3122–3130.10.1002/app.33588Suche in Google Scholar
[5] Punchaipetch P, D’Souza NA, Brostow W, Smith JT. Polym. Compos. 2002, 23, 564–573.10.1002/pc.10457Suche in Google Scholar
[6] Bozkurt E, Kaya E, Tanoglu M. Compos. Sci. Technol. 2007, 67, 3394–3403.10.1016/j.compscitech.2007.03.021Suche in Google Scholar
[7] Park JS, Park SS, Lee S. Macromol. Symposia 2007, 249–250, 568–572.10.1002/masy.200750438Suche in Google Scholar
[8] Patnaik A, Kumar P, Biswas S, Kumar M. Comput. Mater. Sci. 2012, 62, 142–151.10.1016/j.commatsci.2012.05.020Suche in Google Scholar
[9] Masoodi R, El-Hajjar RF, Pillai KM, Sabo R. Mater. Des. 2012, 36, 570–576.10.1016/j.matdes.2011.11.042Suche in Google Scholar
[10] Xu Y, Van Hoa S. Compos. Sci. Technol. 2008, 68, 854–861.10.1016/j.compscitech.2007.08.013Suche in Google Scholar
[11] Hameed N, Sreekumar PA, Thomas PS, Jyotishkumar P, Thomas S. J. Appl. Polym. Sci. 2008, 110, 3431–3438.10.1002/app.28207Suche in Google Scholar
[12] Xiao X, Lu S, Qi B, Zeng C, Yuan Z, Yu J. RSC Adv. 2014, 4, 14928–14935.10.1039/c3ra45732jSuche in Google Scholar
[13] Rahmanian S, Suraya AR, Shazed MA, Zahari R, Zainudin ES. Mater. Des. 2014, 60, 34–40.10.1016/j.matdes.2014.03.039Suche in Google Scholar
[14] Hossain MK, Chowdhury MMR, Salam MB, Malone J, Hosur MV, Jeelani S, Bolden NW. J. Appl. Polym. Sci. 2014, 131, 40709 (1–12).10.1002/app.40709Suche in Google Scholar
[15] Venkateshwaran N, Perumal AE, Arunsundaranayagam D. Mater. Des. 2013, 47, 151–159.10.1016/j.matdes.2012.12.001Suche in Google Scholar
[16] Tehrani M, Boroujeni AY, Hartman TB, Haugh TP, Case SW, Al-Haik MS. Compos. Sci. Technol. 2013, 75, 42–48.10.1016/j.compscitech.2012.12.005Suche in Google Scholar
[17] Soykok IF, Sayman O, Ozen M, Korkmaz B. Compos. Part B 2013, 45, 192–199.10.1016/j.compositesb.2012.08.008Suche in Google Scholar
[18] Phong NT, Gabr MH, Okubo K, Chuong B, Fujii T. Compos. Struct. 2013, 99, 380–387.10.1016/j.compstruct.2012.12.018Suche in Google Scholar
[19] Kim D, Chung I, Kim G. Fibers Polym. 2013, 14, 2141–2147.10.1007/s12221-013-2141-9Suche in Google Scholar
[20] An F, Lu CX, Li YH, Guo JH, Lu XX, Lu HB, He SQ. Yang Y. Mater. Des., 2012, 33, 197–202.10.1016/j.matdes.2011.07.027Suche in Google Scholar
[21] Fujiki K, Sakamoto M, Yoshikawa S, Sato T, Tsubokawa N. Compos. Interfaces 1998, 6, 215–226.10.1163/156855499X00044Suche in Google Scholar
[22] Dong LB, Hou F, Li Y, Wang L, Gao HX, Tang YL. Compos. Part A 2014, 56, 248–255.10.1016/j.compositesa.2013.10.016Suche in Google Scholar
[23] Li M, Liu HX, Gu YZ, Li YX, Zhang ZG. Appl. Surf. Sci. 2014, 288, 666–672.10.1016/j.apsusc.2013.10.093Suche in Google Scholar
[24] Liu WB, Zhang S, Li BC, Yang F, Jiao WC, Hao LF, Wang RG. Polym. Compos. 2014, 35, 482–488.10.1002/pc.22685Suche in Google Scholar
[25] Qi GC, Du SY, Zhang BM, Tang ZW, Yu YL. Compos. Sci. Technol. 2014, 105, 1–8.10.1016/j.compscitech.2014.09.014Suche in Google Scholar
[26] Liu TM, Zheng YS, Hu J. J. Appl. Polym. Sci. 2010, 118, 2541–2552.10.1002/app.32478Suche in Google Scholar
[27] Guo JH, Lu CX, An F. J. Mater. Sci. 2012, 47, 2831–2836.10.1007/s10853-011-6112-5Suche in Google Scholar
[28] Ge HY, Ma XL, Liu HS. J. Appl. Polym. Sci. 2015, 132, 41882 (1–9).10.1002/app.41882Suche in Google Scholar
[29] Liu K, Zhang XZ, Takagi H, Yang ZM, Wang D. Compos. Part A 2014, 66, 227–236.10.1016/j.compositesa.2014.07.018Suche in Google Scholar
[30] Pires EN, Merlini C, Al-Qureshi HA, Salmoria GV, Barra GMO. Polimeros-Ciencia E Tecnologia 2012, 22, 339–344.10.1590/S0104-14282012005000053Suche in Google Scholar
[31] Patra A, Bisoyi DK, Manda PK, Singh AK. J. Appl. Polym. Sci. 2013, 128, 1011–1019.10.1002/app.38195Suche in Google Scholar
[32] Zhang C, Xu H, Jiang Z, Zhu F, Huang Y. Polym. Compos. 2012, 33, 927–932.10.1002/pc.22215Suche in Google Scholar
[33] Kamae T, Drzal LT. Compos. Part A 2012, 43, 1569–1577.10.1016/j.compositesa.2012.02.016Suche in Google Scholar
[34] Sato E, Uehara I, Horibe H, Matsumoto A. Macromolecules 2014, 47, 937–943.10.1021/ma402300zSuche in Google Scholar
[35] Li S, Cui C, Hou H, Wu Q, Zhang S. Compos. Part B 2015, 79, 342–350.10.1016/j.compositesb.2015.04.048Suche in Google Scholar
[36] Li S, Cui C, Hou H. Colloid Polym. Sci. 2015, 293, 2681–2688.10.1007/s00396-015-3665-xSuche in Google Scholar
[37] Li S, Cui C, Hou H. Polym. Compos. 2015, doi: 10.1002/pc.23602.Suche in Google Scholar
[38] Li S, Zhu H, Lv T, Lin Q, Hou H, Li Y, Wu Q, Cui C. Colloid Polym. Sci. 2016, 294, 607–615.10.1007/s00396-015-3811-5Suche in Google Scholar
[39] Li S, Lin Q, Cui C. J. Mater. Res. 2016, 31, 1393–1402.10.1557/jmr.2016.174Suche in Google Scholar
[40] Li S, Lin Q, Lv T, Li Y, Hou H, Zhu H, Wu Q, Cui C. Polym. Sci. Series A 2016, 58, 43–50.10.1134/S0965545X16060110Suche in Google Scholar
[41] Li S, Cui C. J. Mater. Sci. 2016, 51, 1829–1837.10.1007/s10853-015-9488-9Suche in Google Scholar
[42] Huang P, Gu A, Liang G, Yuan L. J. Appl. Polym. Sci. 2012, 123, 2351–2359.10.1002/app.34791Suche in Google Scholar
[43] Zhang D, Jia D. Eur. Polym. J. 2006, 42, 711–714.10.1016/j.eurpolymj.2005.09.013Suche in Google Scholar
[44] Zhang D, Liang E, Li T, Chen S, Zhang J, Cheng X, Zhou J, Zhang A. RSC Adv. 2013, 3, 9522–9529.10.1039/c3ra00023kSuche in Google Scholar
[45] Wong S, Shanks RA, Hodzic H. Macromol. Mater. Eng. 2004, 289, 447–456.10.1002/mame.200300366Suche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Review
- A review on the intensification of metal matrix composites and its nonconventional machining
- Original articles
- Optimization of multi-sandwich-panel composite structures for minimum weight with strength and buckling considerations
- An automated portable multiaxial pressure test rig for qualifications of glass/epoxy composite pipes
- Effects of nano-SiO2 on mechanical and hygric behaviors of glass fiber reinforced epoxy composites
- Comparison of the mechanical and wear behaviour of aluminium alloy with homogeneous and functionally graded silicon nitride composites
- Engineering behavior of clay soils stabilized with class C and class F fly ashes
- Preparation and erosion-corrosion behavior of polyetheretherketone (PEEK)/nickel foam co-continuous composites
- Optimization design, manufacturing and mechanical performance of box girder made by carbon fiber-reinforced epoxy composites
- Recent advances in the manufacturing processes of functionally graded materials: a review
- Numerical prediction of thermal conductivity in ZrB2-particulate-reinforced epoxy composites based on finite element models
- High-speed electrical sliding wear behaviors of Cu-WS2-graphite-WS2 nanotubes composite
- Adsorption removal of methylene blue from aqueous solution on carbon-coated Fe3O4 microspheres functionalized with chloroacetic acid
- Thermal degradation of coir fiber reinforced low-density polyethylene composites
- Preparation and analysis of polypropylene composites with maleated tea dust particles
- Predicting the thermal conductivity of polypropylene-multiwall carbon nanotubes using the Krenchel model
- Growth mechanism of 3D graphene-carbon nanotube hybrid structure
- Reinforcing abilities of microfibers and nanofibrillated cellulose in poly(lactic acid) composites
- Sintered TiO2/recycled glass composites designed for the potential degradation of waterborne pollutants
- Evaluation of glass-fiber grafted by epoxide-terminated hyperbranched polymer on the effect of mechanical characterization of epoxy composites
Artikel in diesem Heft
- Frontmatter
- Review
- A review on the intensification of metal matrix composites and its nonconventional machining
- Original articles
- Optimization of multi-sandwich-panel composite structures for minimum weight with strength and buckling considerations
- An automated portable multiaxial pressure test rig for qualifications of glass/epoxy composite pipes
- Effects of nano-SiO2 on mechanical and hygric behaviors of glass fiber reinforced epoxy composites
- Comparison of the mechanical and wear behaviour of aluminium alloy with homogeneous and functionally graded silicon nitride composites
- Engineering behavior of clay soils stabilized with class C and class F fly ashes
- Preparation and erosion-corrosion behavior of polyetheretherketone (PEEK)/nickel foam co-continuous composites
- Optimization design, manufacturing and mechanical performance of box girder made by carbon fiber-reinforced epoxy composites
- Recent advances in the manufacturing processes of functionally graded materials: a review
- Numerical prediction of thermal conductivity in ZrB2-particulate-reinforced epoxy composites based on finite element models
- High-speed electrical sliding wear behaviors of Cu-WS2-graphite-WS2 nanotubes composite
- Adsorption removal of methylene blue from aqueous solution on carbon-coated Fe3O4 microspheres functionalized with chloroacetic acid
- Thermal degradation of coir fiber reinforced low-density polyethylene composites
- Preparation and analysis of polypropylene composites with maleated tea dust particles
- Predicting the thermal conductivity of polypropylene-multiwall carbon nanotubes using the Krenchel model
- Growth mechanism of 3D graphene-carbon nanotube hybrid structure
- Reinforcing abilities of microfibers and nanofibrillated cellulose in poly(lactic acid) composites
- Sintered TiO2/recycled glass composites designed for the potential degradation of waterborne pollutants
- Evaluation of glass-fiber grafted by epoxide-terminated hyperbranched polymer on the effect of mechanical characterization of epoxy composites

