Abstract
The study investigates the effects of the alkaline solution/binder ratio and the curing condition on the mechanical properties of alkali-activated fly ash (AAFA) mortars. Class F fly ash was used as the raw material, and sodium hydroxide and liquid sodium silicate were used for the preparation of alkaline activators. Three alkaline solution-to-binder ratios (0.35, 0.5, and 0.65) and four different initial curing conditions (curing in air at ambient temperature for 24 h, 30°C for 24 h, 65°C for 12 h, and 85°C for 6 h) were considered. Test results show that AAFA mortars with alkaline solution-to-binder ratio of 0.35 had higher compressive strength, lower drying shrinkage, lower water absorption, and lower initial surface absorption rate than the other mortars. Furthermore, the curing condition influenced the compressive strength development and drying shrinkage of AAFA mortars at early ages. AAFA mortars cured at 65°C for 12 h appeared to have superior mechanical properties. XRD demonstrates that the hydration products of AAFA mortars are mainly amorphous alkaline aluminosilicate gel, which attributed to the compressive strength. Consequently, the alkaline solution-to-binder ratio significantly affects more the mechanical properties than the curing condition based on the presented results.
1 Introduction
The use of alkali-activated fly ash (AAFA) instead of ordinary Portland cement (OPC) in concrete manufacturing is environmentally beneficial because the manufacturing process of AAFA requires lesser energy and produces lower CO2 emissions and industrial waste. AAFA is prepared using a chemical process in which fly ash is mixed with strong alkaline solutions and cured at a moderate temperature, and it is in the form of well-compacted cementitious composites [1–3]. In a strongly alkaline environment, silica and alumina in fly ash particles are dissolved, and they form geopolymeric gel [1, 4]. This binder, often called geopolymer, has considerable potential as an alternative to OPC because of its excellent mechanical properties, such as high strength, fire resistance, acid resistance, high thermal stability, and environmental friendliness [4–9]. The formation and properties of a geopolymer are affected by the mix composition and reaction conditions such as Al2O3/SiO2, alkali concentration, curing temperature, curing time, alkaline solution/binder ratio, and the alkaline solution pH value [2, 10–16].
Many studies [17, 18] have addressed the effect of the water/cement ratio on the mechanical properties of OPC. For example, Odler and Röbler [17] indicated that the water/cement ratio affects both the physical properties of OPC and the composition of the calcium silicate hydrate (C-S-H) gel produced during hydration. The findings mentioned above may be extrapolated to alkaline cements. Indeed, the effect of the alkaline solution/binder ratio on alkali-activated systems has been reported in several studies [19–22]. Zuhua et al. [20] found that high liquid/solid ratio could accelerate the dissolution of raw materials. Also, Yao et al. [21] showed that higher liquid/solid ratios increased the geopolymerization period. However, the approach in which it affects the nature of the reaction products has not been studied fully. Moreover, increasing the alkaline solution/binder ratio for these materials not only increases the water content, but also increases the amount of alkaline cations and hydroxide ions (OH-) in the alkali-activated systems [22].
The alkali activation of fly ash requires the supply of external energy in the form of heat for the formation of alkali aluminosilicates because AAFA pastes harden slowly at ambient temperature. Alkali-activated binders with appreciable mechanical properties have been produced at a wide range of temperatures, from 20°C to 90°C [23–25]. The degree of reaction of the fly ash and silica in the aluminosilicates formed increases with the thermal curing time. When the thermal curing time is increased, the gel undergoes polymerization, and its structure becomes highly ordered [26]. Furthermore, curing conditions have a strong effect on both early age and final mechanical properties of geopolymer materials [27–30]. Kovalchuk et al. [28] found that curing in a covered mold at 95°C produced geopolymer materials with the highest compressive strength, and they recommended dry curing at 150°C for NaOH-based systems (low SiO2/Al2O3 ratio); furthermore, they observed that steam curing at 95°C had a moderate effect on strength development. Swanepoel and Strydom [29] conducted a study on geopolymers cured at 40°C, 50°C, 60°C, and 70°C for different durations and found that the optimal curing conditions involved a temperature of 60°C and a duration of 48 h. Chi [30] investigated the physical and mechanical properties and durability of alkali-activated slag concrete (AASC) for three different curing conditions and found that curing at 80% relative humidity (RH) and a temperature of 60°C yielded optimal AASC performance; AASC obtained through air curing and saturated limewater curing showed the next highest performance. Geopolymers formed at room temperature are amorphous. With an increase in the temperature, crystalline phases begin to appear. Most research has been conducted by curing at approximately 95% RH and in the temperature range 30–85°C [1, 28, 31–33]. However, no detailed study has been conducted on the effect of the curing temperature on the geopolymer properties.
It is clear that there is still a large number of influential factors and complicated problems, and particular studies cannot provide all the answers. So then, further investigations regarding alkali-activated binding materials need to be done. The aim of this study is to investigate the effect of the alkaline solution/binder ratio and the curing condition on the mechanical properties of AAFA mortars. Three alkaline solution/binder ratios (0.35, 0.5, and 0.65) and four different initial curing conditions (curing in air at ambient temperature for 24 h, 30°C for 24 h, 65°C for 12 h, and 85°C for 6 h) were considered.
2 Materials and methods
2.1 Materials
Class F fly ash (FA) from Xingda Power Plant in Kaohsiung, Taiwan, was used as the main aluminum and silicate source for synthesizing a geopolymeric binder. The chemical composition of the FA was determined by X-ray fluorescence spectrometry, and it is listed in Table 1. The specific gravity and specific surface area of the FA were 2.06 and 0.237 m2/g, respectively. Standard sand conforming to ASTM C778 [34] was used as the fine aggregate for preparing geopolymer mortars. The maximum grain size and specific gravity of the standard sand were 2.5 mm and 2.65, respectively. The most used alkaline activators were a mixture of sodium hydroxide (NaOH) and sodium silicate (Na2O·γSiO2) [35]. In this study, the alkaline activation of the FA was performed using sodium hydroxide pellets with a density of 2130 kg/m3 and sodium silicate solution (Na2O·γSiO2·nH2O) consisting of 29.2% SiO2, 14.8% Na2O, and 56.0% H2O by mass. Furthermore, Na2SiO3 and NaOH solutions were prepared 1 day before their use.
Chemical compositions of fly ash.
| Chemical compositions (%) | Fly ash |
|---|---|
| Calcium oxide, CaO | 2.82 |
| Silicon dioxide, SiO2 | 56.48 |
| Aluminum oxide, Al2O3 | 20.34 |
| Ferric oxide, Fe2O3 | 6.61 |
| Sulfur trioxide, SO3 | 0.25 |
| Sodium oxide, Na2O | 0.33 |
| Potassium oxide, K2O | 0.8 |
| Magnesium oxide, MgO | 0.93 |
| Loss on ignition, L.O.I. | 2.76 |
| Others | 8.68 |
2.2 Mix design and specimen preparation
AAFA geopolymer specimens were prepared from fly ash, sodium hydroxide, and sodium silicate. Alkaline solution-to-binder ratios of 0.35, 0.5, and 0.65, denoted by M3, M5, and M6, respectively, were selected to produce AAFA mortars. Sodium oxide (Na2O) with a mass of 121 kg per cubic meter of mortar and liquid sodium silicate with a modulus ratio (mass ratio of SiO2 to Na2O) of 1.23 were used as alkaline activators. The sand/binder ratio was constant at 2.75. The mortar mix proportions are presented in Table 2. The mortar was mixed in a mechanical mixer. Subsequently, the mixture was poured into steel molds. The specimens were cast and kept in steel molds for 24 h. The temperature and the curing duration strongly influence the formation of the amorphous aluminosilicate network. To balance the temperature and curing duration to achieve optimal mechanical performance, before demolding, the specimens were subjected to four different curing conditions with temperatures ranging from ambient temperature (23°C) to 85°C and curing times ranging from 6 to 24 h. In other words, the specimens were exposed to air at ambient temperature for 24 h, 30°C for 24 h, 65°C for 12 h, and 85°C for 6 h; these conditions are denoted by A, B, C, and D, respectively. After the initial curing, they were shifted to a curing room with 80% RH and a temperature of 25°C; they were stored in the room until they were tested.
Mix proportions of AAFA mortars.
| Mix no. | Alkaline solution/binder ratio | SiO2/Na2O ratio | Water (kg/m3) | Na2SiO3 (kg/m3) | NaOH (kg/m3) | FA (kg/m3) | Fine Agg. (kg/m3) | Activator (kg/m3) | |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Na2O | ||||||||
| M3 | 0.35 | 1.23 | 107.7 | 78.7 | 14.6 | 574 | 1581 | 28.3 | 23.0 |
| M5 | 0.5 | 1.23 | 178.3 | 72.3 | 13.4 | 528 | 1453 | 26.0 | 21.1 |
| M6 | 0.65 | 1.23 | 241 | 66.9 | 9.6 | 489 | 1345 | 24.0 | 19.5 |
2.3 Methods
2.3.1 Compressive strength test
Compressive strength tests were conducted on the specimens according to ASTM C109 [36]. For each mixture, 50 mm×50 mm×50 mm cubes were prepared, and three specimens of each mixture were tested at the ages of 7, 14, and 28 days to determine the average compressive strength.
2.3.2 Drying shrinkage test
The measurements of length change as scale of the drying shrinkage of mortar were conducted based on ASTM C596 [37]. Prismatic specimens with dimensions of 285 mm×25 mm×25 mm were used for drying shrinkage measurements. After 1 day, the initial lengths (Li) of demolded shrinkage specimens were measured using a digital comparator with an accuracy of 0.001 mm. After curing, the lengths (Lx) of the shrinkage specimens were measured at the ages of 7, 14, and 28 days. The shrinkage value for each age is the average of three readings. During the drying shrinkage test, the specimens were kept at the temperature of 25°C in a relative humidity of 80%. The drying shrinkage was then calculated from the following formula:
where G is the nominal effective length.
2.3.3 Water absorption
Water absorption was determined in accordance with ASTM C642 [38]. The cubes were first placed in an oven at 105±5°C for 24 h and then weighed (Wd). Next, they were immersed in water for 24 h and then weighed again (Ws). The parameter Ws was considered as the saturated weight. It took up to 24 h for the specimens. The water absorption (WA) was then calculated using the following formula [39, 40]:
The porosity (P) was calculated from the expression
where V is the bulk volume of the specimen.
2.3.4 Initial surface absorption test
The initial surface absorption test (ISAT) of concrete is to indicate the water flow into the surface of a dry, flat concrete surface. The principle of test is to determine the time taken for a quantity of water to flow through a standardized glass tube onto a known area of concrete surface. The ISAT was performed according to the test method described in BS 1881-208 [41]. The specimens were cast in 100-mm cubes for measuring the initial surface absorption rate. The specimens were demolded 24 h after casting and then cured for 28 days. At least three specimens were prepared for obtaining the average value of the initial surface absorption test. The specimens were oven dried to constant weight at a temperature of 105±5°C prior to the test. The test consists of the measurement of water flow into the test specimen through a known surface area. The initial surface absorption was measured at intervals of 10, 30, and 60 min after the start of testing. The initial surface absorption rate was expressed in milliliters per square meter per second (ml/(m2·s)).
2.3.5 Mercury intrusion porosimetry
The mercury intrusion porosimetry (MIP) test was performed in accordance with ASTM D4404-10 [42], was and it involved injecting mercury into dried specimens. Specimens aged 28 days were dried in an oven at 105°C for 24 h before testing. Pressure, which was progressively incremented, was applied to mercury, and the intrusion of mercury was monitored at each increment. The intruded volume was used to determine the pore size distribution. Furthermore, the diameter of cylindrical pores was calculated using the Washburn equation [43, 44]:
where d is the cylindrical pore diameter, Φ is the pore shape factor, γ is the surface tension of mercury, θ is the contact angle of mercury, and p is the applied pressure. The contact angle was set at 130°, the surface tension of mercury was assumed to be 0.485 N/m, the measuring pressure ranged from 0.7 to 210 MPa, and the measurable pore size ranged from 0.003 to 360 μm.
2.3.6 X-ray diffraction analysis
X-ray diffraction is a non-destructive technique used to determine crystalline phases present within the investigated material. For X-ray diffraction (XRD) analysis, randomly oriented powder specimens (approximately 1 g in weight) were prepared by grinding small portions of the dried specimens. XRD graphs were obtained using a Siemens D5000 X-ray diffractometer and CuKα radiation at room temperature. The diffractograms were scanned in the 2θ range 10°–80° at 0.05° intervals. The components of the sample were identified by comparing them with standards established by the International Center for diffraction data.
3 Results and discussion
3.1 Compressive strength
The compressive strength developments of AAFA mortars with various alkaline solution-to-binder ratios and cured under four different conditions at the ages of 7, 14, and 28 days are shown in Figures 1–3. As expected, the compressive strength of all AAFA mortars increased with age and decreased with an increase in the alkaline solution-to-binder ratio.

Compressive strength development of AAFA geopolymer with the alkaline solution/binder ratio of 0.35 vs. age.

Compressive strength development of AAFA geopolymer with the alkaline solution/binder ratio of 0.5 vs. age.

Compressive strength development of AAFA geopolymer with the alkaline solution/binder ratio of 0.65 vs. age.
As shown in Figure 1, the compressive strength of AM3 steadily increased with age, and it amounted to 24.9 MPa for the 7-day-old specimen, 30.2 MPa for the 14-day-old specimen, and 37.2 MPa for the 28-day-old specimen. The 28-day compressive strength of AM3 indicated an increase of 50% compared with the 7-day compressive strength. The compressive strength development of DM3 is highly similar to that of AM3. A higher compressive strength was observed for the 7-day-old CM3 (AAFA mortars cured at 65°C). The increase in the compressive strength lasted for 14 days and then began to level off; the compressive strength was 31 MPa at the age of 7 days, 34.8 MPa at the age of 14 days, and 35.2 MPa at the age of 28 days. For AAFA mortars with the alkaline solution-to-binder ratio of 0.35, the curing temperature appeared to have no appreciable influence on compressive strength development, particularly in older specimens. As shown in Figure 2, the compressive strengths of the AM5 and BM5 mortars (AAFA mortars cured at ambient temperature and 30°C, respectively) were lower than those of the CM5 and DM5 mortars at the age of 7 days. At ambient temperature, alkali activation of fly ash increased gradually, and an increase in the temperature accelerated the formation of a hardened structure, particularly in the early stages of the geopolymerization reaction, and enhanced the compressive strength. For example, CM5 mortar reached a compressive strength of 31.5 MPa, which was approximately twice that of AM5 mortars at the age of 7 days. However, the compressive strengths of the AM5 and BM5 mortars steadily increased for 28 days. For example, the compressive strength of AM5 was 18.4 MPa at the age of 7 days, 24 MPa at the age of 14 days, and 32.7 MPa at the age of 28 days. CM5 and DM5 (AAFA mortars cured at 65°C and 85°C) showed a rapid increase in their compressive strength at the age of 7 days; however, the rate of increase in the compressive strength decreased after 14 days. The compressive strength of CM5 was 31.5 MPa at the age of 7 days, 34.8 MPa at the age of 14 days, and 35.2 MPa at the age of 28 days. The 28-day compressive strength of CM5 was only approximately 10% greater than the 7-day compressive strength. The compressive strength development of DM5 was similar to that of CM5. However, in old specimens, the curing temperature has no appreciable influence on the compressive strength. As shown in Figure 3, the compressive strength development could be divided into two groups: one group consisted of AAFA mortars cured at ambient temperature and at 30°C (AM6 and BM6 mortars), and the other group comprised AAFA mortar cured at 65°C and 85°C (CM6 and DM6 mortars). The compressive strength of the CM6 and DM6 mortars was higher than that of the AM6 and BM6 mortars for all ages. The compressive strength of AAFA mortars increased with the temperature because the geopolymerization reaction proceeded faster at higher temperatures, resulting in an increased amount of alkali-activated reaction products [27].
The results showed that both the alkaline solution-to-binder ratio and initial curing conditions appreciably influenced the compressive strength development of AAFA mortars. The lower the alkaline solution-to-binder ratio, the higher the compressive strength. Furthermore, the curing temperature plays a crucial role in determining the compressive strength development of AAFA mortars at early ages, particularly for AAFA mortars with the alkaline solution-to-binder ratios of 0.5 and 0.65. The compressive strength of AAFA mortars cured at 65°C for 12 h was higher than that of AAFA mortars cured at other temperatures.
3.2 Drying shrinkage
Drying shrinkage is the volume change resulting from water loss in a specimen exposed to a dry environment during high-temperature curing. Specifically, it is the shrinkage, including autogenous shrinkage, occurring during the drying phase of the specimens. The drying shrinkage of AAFA mortars with various alkaline solution-to-binder ratios and cured at the four different conditions for 7, 14, and 28 days is shown in Figures 4–6. From these results, it is apparent that the drying shrinkage for all AAFA mortars increased with their age and that the increase lasted for 28 days. The higher the alkaline solution-to-binder ratio, the higher the drying shrinkage.

Drying shrinkage of AAFA geopolymer with the alkaline solution/binder ratio of 0.35 vs. age.
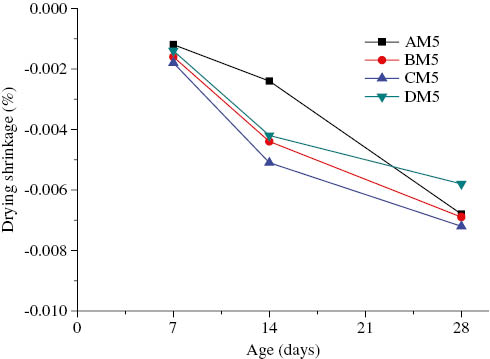
Drying shrinkage of AAFA geopolymer with the alkaline solution/binder ratio of 0.5 vs. age.
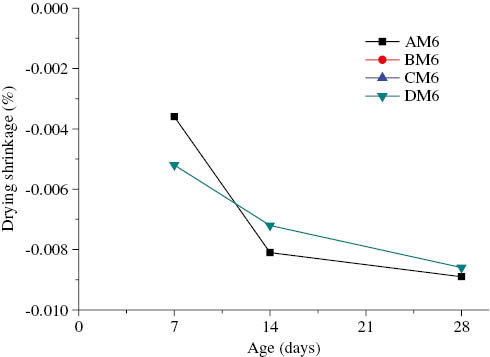
Drying shrinkage of AAFA geopolymer with the alkaline solution/binder ratio of 0.65 vs. age.
As shown in Figure 4, the drying shrinkage of AAFA mortars with the alkaline solution-to-binder ratio of 0.35 showed a rapid increase at early ages. On the basis of the 7-day drying shrinkage, the specimens could be ordered as DM3>CM3>BM3>AM3. However, the rate of increase of drying shrinkage decreased after 14 days. The CM3 mortar (cured at 65°C) had the highest drying shrinkage at the four curing temperatures: 0.0047% at the age of 14 days and 0.0064% at the age of 28 days. At early ages, high-temperature curing has an appreciable influence on the drying shrinkage of AAFA mortars because the high temperature accelerates the alkali-activation reaction of fly ash and increases the drying shrinkage of AAFA mortars. With an increase in age, the effect of curing temperature has a less influence on the drying shrinkage of AAFA mortars. At a late age, the drying shrinkage of the DM3 mortar (cured at 85°C) is almost the same as that of the BM3 mortar (cured at ambient temperature): 0.0042% at the age of 14 days and 0.0052% at the age of 28 days. Figure 5 shows the drying shrinkage of AAFA mortars with the alkaline solution-to-binder ratio of 0.5 and cured at four different temperatures (AM5, BM5, CM5, and DM5). Except for the DM5 mortar, the drying shrinkage of AAFA mortars increased with the curing temperature. On the basis of the drying shrinkage, the specimens aged 7 and 14 days were ordered as CM5>BM5>DM3>AM3. After 14 days, the drying shrinkage of the AM5 mortars began to increase markedly. The 28-day drying shrinkage of the AM5 mortars was close to that of BM5, which had a drying shrinkage of 0.0068%. This is because the alkali activation reaction was incomplete, leading to excess moisture and a high-drying shrinkage. The drying shrinkage of AAFA mortars with the alkaline solution-to-binder ratio of 0.65 and cured at four different temperatures (AM6, BM6, CM6, and DM6) is shown in Figure 6. High-temperature curing for over 12 h for AAFA mortars with the alkaline solution-to-binder ratio of 0.65 (BM6 and CM6) results in crack formation or deterioration, and hence, the drying shrinkages of BM6 and CM6 could not be determined during the curing period. Figure 7A and B show cracks in AAFA mortars with the alkaline solution-to-binder ratio of 0.65 and cured at 30°C for 24 h and at 65°C for 12 h, respectively. At early ages, higher temperatures accelerate the geopolymerization reaction of AAFA mortars, resulting in higher drying shrinkage. However, at late ages, the drying shrinkage of AAFA mortars cured at high temperatures increases gradually because of a reduction in the amount of the alkaline solution.

(A) Crack of AAFA mortars with alkaline solution to binder ratio of 0.65 cured at the temperature of 30°C for 24 h. (B) Crack of AAFA mortars with alkaline solution to binder ratio of 0.65 cured at the temperature of 65°C for 12 h.
3.3 Water absorption and porosity
The water absorption of 28-day-old AAFA mortars cured at different temperatures is listed in Table 3. For the alkaline solution-to-binder ratio of 0.35, the water absorption of AAFA mortars cured at higher temperatures (BM3, CM3, and DM3) varied from 5.2% to 5.9%, whereas the water absorption of the AAFA mortar cured at ambient temperature (AM3) was 5.4%. For the alkaline solution-to-binder ratio of 0.5, the water absorption of AAFA mortars cured at higher temperatures (BM5, CM5, and DM5) ranged from 7.3% to 7.6% and was higher than that of the AAFA mortar cured at ambient temperature (AM5, water absorption=6.7%). Similarly, for the alkaline solution-to-binder ratio of 0.65, the water absorption for AAFA mortars cured at higher temperatures (BM6, CM6, and DM6) was higher (varying between 9.6% and 9.8%) than that for the AAFA mortar cured at ambient temperature (AM6, water absorption=8.9%). Higher temperatures result in increased water absorption. It may be due to the crack formation during higher temperature curing. Furthermore, an increase in the alkaline solution-to-binder ratio increased the porosity and reduced the mechanical strength. As is evident from Table 3, the water absorption and porosity of AAFA mortars increased with the alkaline solution-to-binder ratio. Additionally, an increase in the curing temperature increased the water absorption and porosity of AAFA mortars. Thus, the alkaline solution to binder ratio and curing temperature played a crucial role in determining the water absorption and porosity of AAFA mortars.
Water absorption and porosity of AAFA mortars.
| Mix no. | Water absorption (%) | Relative percentage (%) | Porosity (%) |
|---|---|---|---|
| AM3 | 5.4 | – | 10.4 |
| BM3 | 5.2 | -0.2 | 10.5 |
| CM3 | 5.3 | -0.1 | 11.0 |
| DM3 | 5.9 | 0.5 | 12.1 |
| AM5 | 6.7 | – | 13.4 |
| BM5 | 7.3 | 0.6 | 14.1 |
| CM5 | 7.3 | 0.6 | 14.2 |
| DM5 | 7.6 | 0.9 | 14.7 |
| AM6 | 8.9 | – | 17.2 |
| BM6 | 9.6 | 0.7 | 18.2 |
| CM6 | 9.8 | 0.9 | 18.9 |
| DM6 | 9.7 | 0.8 | 18.4 |
3.4 Initial surface absorption test
Variations in the initial surface absorption for testing times of 10, 30, and 60 min are plotted in Figures 8–10. It can be observed that the initial surface absorption of AAFA mortars decreased with an increase in the testing time. Figure 8 shows that for the alkaline solution-to-binder ratio of 0.35 (M3 mortars), the curing conditions did not appreciably influence the initial surface absorption of AAFA mortars; the reason is that M3 mortars are characterized by more effective alkali activation of fly ash, which results in their having a dense structure. Thus, the difference of initial surface absorption is not obvious for M3 mortars. The initial surface absorption of AAFA mortars with the alkaline solution-to-binder ratio of 0.5 (M5 mortars) is plotted in Figure 9. It can be seen that the CM5 and DM5 mortars had lower initial surface absorption than AM5 and BM5 mortars, indicating that an increase in the curing temperature led to a decrease in the initial surface absorption. Figure 10 shows the initial surface absorption of AAFA mortars with the alkaline solution-to-binder ratio of 0.65 (M6 mortars); AM6 mortars showed the highest initial surface absorption (compared with the BM6, CM6, and DM6 mortars) for a testing time of 30 min. Thus, a high temperature can effectively reduce the initial surface absorption of M6 mortars. The trend of the initial surface absorption for M6 was similar to that for M5. However, the initial surface absorption of M6 was still considerably higher than that of the M3 and M5 mortars. These results indicate that a higher curing temperature and a lower alkaline solution-to-binder ratio lead to a reduction in initial surface absorption.

Initial surface absorption of AAFA geopolymer with the alkaline solution/binder ratio of 0.35 vs. time.

Initial surface absorption of AAFA geopolymer with the alkaline solution/binder ratio of 0.5 vs. time.
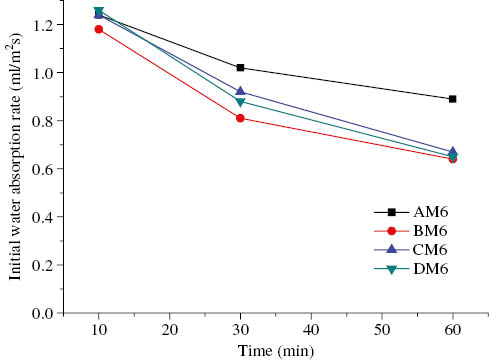
Initial surface absorption of AAFA geopolymer with the alkaline solution/binder ratio of 0.65 vs. time.
3.5 Mercury intrusion porosimetry
The mercury intrusion porosimetry test was performed to determine the pore structure of 28-day-old AAFA mortars. By tracking the applied pressure and intrusion volume at each increment, the cumulative intrusion volume, capillary pore intrusion volume, and gel pore intrusion volume of AAFA geopolymer mortars with the alkaline solution-to-binder ratio of 0.5 were obtained (Table 4). The cumulative intrusion volume of the AAFA mortars slightly increased with the curing temperature. AM5 (AAFA geopolymer mortars cured at ambient temperature) had the smallest cumulative intrusion volume, 0.1003 ml/g (capillary pore intrusion volume: 0.0911 ml/g; gel pore intrusion volume: 0.0092 ml/g). When AAFA mortars were cured at a lower temperature, the geopolymerization products gradually filled the pores in the basic structure, thereby, rendering the structure denser. By contrast, a higher cumulative intrusion volume, 0.1292 ml/g (capillary pore intrusion volume: 0.1198 ml/g; gel pore intrusion volume: 0.0094 ml/g) was obtained for DM5 (AAFA geopolymer mortars cured at 85°C). At higher temperatures, the final pore structure was almost realized within the first 24 h, and it did not change during the aging of geopolymer [27]. The curing of AAFA mortars at high temperatures accelerates the formation of a hardened structure, particularly at early ages. When the process of hardening proceeds too quickly, it results in a less-ordered structure of poorer quality, and larger pores are left in the matrix. The porosimetry measurement results correspond closely with the water absorption and porosity results discussed in Section 3.3.
Intrusion volume of mercury for AAFA mortars.
| Mix no. | Gel pore intrusion volume (ml/g) | Capillary pore intrusion volume (ml/g) | Cumulative pore intrusion volume (ml/g) |
|---|---|---|---|
| AM5 | 0.0092 | 0.0911 | 0.1003 |
| BM5 | 0.0094 | 0.0923 | 0.1017 |
| CM5 | 0.0099 | 0.1023 | 0.1122 |
| DM5 | 0.0094 | 0.1198 | 0.1292 |
3.6 X-ray diffraction analysis
The reaction products were characterized mineralogically by XRD to identify the crystalline components in the system. Figure 11 shows XRD patterns of AAFA mortars (with the alkaline solution-to-binder ratio of 0.5) cured at different temperatures at the age of 28 days. The products of the reaction can be seen to be mostly amorphous, which was confirmed by a wide and diffusive reflection in the 2θ interval 21°–45°. The hydration products of AAFA mortars are mainly amorphous alkaline aluminosilicate gel, which attributed to the compressive strength. The major crystalline phase in all AAFA geopolymer mortars was quartz (SiO2) and zeolite gismondite (CaAl2Si2O8·4H2O). Other phases such as magnesium sulfite (MgSO3), calcium sulfide (CaS), magnesium-aluminum nitrides (Mg3AlN3), anhydrite (CaSO4), magnetite (Fe3O4), leucite structure group (K2MgSi5O12), and iron phosphorus nitride (Fe4P6N12S) were also present. The major identified crystalline peaks corresponded to quartz and zeolite gismondite attributed to the effect of the fly ash components in the crystalline phase were observed at 27°2θ, which are similar to those of most previous research [4, 10, 12, 45, 46]. In addition, DM5 (AAFA mortars cured at 85°C) showed a peak intensity lower than those of the other three AAFA mortars cured at temperatures from ambient temperature to 65°C (AM5, BM5, and CM5). Higher curing temperatures may accelerate the geopolymerization reaction and cause the formation of amorphous alumina-silicate substances at early ages. However, the growth of zeolite crystals is slow at late ages, and therefore, the amorphous products are stabilized at early ages. Thus, the peak intensity for DM5 decreased.

XRD patterns of AAFA geopolymer (alkaline solution/binder ratio of 0.5) with the various curing temperatures at the age 28 days.
4 Conclusions
This study investigated the effect of curing temperature on the properties of AAFA mortars. The main conclusions were as follows:
The lower the alkaline solution-to-binder ratio, the higher the compressive strength and the lower the drying shrinkage. Furthermore, the curing temperature influenced the compressive strength development and drying shrinkage of AAFA mortars at early ages, particularly for AAFA mortars with the alkaline solution-to-binder ratios of 0.5 and 0.65.
A higher temperature led to more effective alkali activation of fly ash, but resulted in increased water absorption. An increase in the alkaline solution-to-binder ratio increased the porosity and degraded the mechanical properties.
A high curing temperature and a low alkaline solution-to-binder ratio led to a decrease in the initial surface absorption.
XRD demonstrates that the hydration products of AAFA mortars are mainly amorphous alkaline aluminosilicate gel, which attributed to the compressive strength. The major crystalline phase of AAFA geopolymer mortars consisted of quartz, zeolite gismondite, mullite, magnesium sulfite, magnetite, anhydrite, and feldspar.
AAFA mortars cured at 65°C for 12 h appeared to have superior mechanical properties.
Acknowledgments:
The author would like to thank the Ministry of Science and Technology (MOST) of Taiwan for Granting the Project under No. NSC-99-2221-E-274-010.
References
[1] Palomo A, Grutzeck MW, Blanco MT. Cem. Concr. Res. 1999, 29, 1323–1329.10.1016/S0008-8846(98)00243-9Search in Google Scholar
[2] Criado M, Fernández-Jiménez A, Palomo A. Fuel 2010, 89, 3185–3192.10.1016/j.fuel.2010.03.051Search in Google Scholar
[3] Vargas ASd, Molin DCCD, Vilela ACF, Silva FJd, Pavão B, Veit H. Cem. Concr. Compos. 2011, 33, 653–660.10.1016/j.cemconcomp.2011.03.006Search in Google Scholar
[4] Jun Y, Oh JE. Cem. Concr. Res. 2014, 52, 396–403.10.1016/j.conbuildmat.2013.11.058Search in Google Scholar
[5] Bakharev, T. Cem. Concr. Res. 2005, 35, 1224–1232.10.1016/j.cemconres.2004.06.031Search in Google Scholar
[6] Němecěk, Jí, Šmilauer V, Kopecký L. Cem. Concr. Compos. 2011, 33, 163–170.10.1016/j.cemconcomp.2010.10.005Search in Google Scholar
[7] Pacheco-Torgal F, Castro-Gomes Jo, Jalali S. Constr. Build. Mater. 2008, 22, 1305–1314.10.1016/j.conbuildmat.2007.10.015Search in Google Scholar
[8] Hu M, Zhu X, Long F. Cem. Concr. Compos. 2009, 31, 762–768.10.1016/j.cemconcomp.2009.07.006Search in Google Scholar
[9] Somaratna J, Ravikumar D, Neithalath N. Cem. Concr. Res. 2010, 40, 1688–1696.10.1016/j.cemconres.2010.08.010Search in Google Scholar
[10] Torres-Carrasco M, Puertas F. J. Clean. Prod. 2015, 90, 397–408.10.1016/j.jclepro.2014.11.074Search in Google Scholar
[11] Chi M, Liu Y, Huang R. IACSIT Inter.J. Eng. Tech. 2015, 7, 59–64.10.7763/IJET.2015.V7.767Search in Google Scholar
[12] Ryu GS, Lee YB, Koh KT, Chung YS. Constr. Build. Mater. 2013, 47, 409–418.10.1016/j.conbuildmat.2013.05.069Search in Google Scholar
[13] Wang J, Wu X-l, Wang J-x, Liu C-z, Lai Ym, Hong Z-K. Micropo. Mesopo. Mat. 2012, 155, 186–191.10.1016/j.micromeso.2012.01.016Search in Google Scholar
[14] Criado M, Jiménez AF, Sobrados I, Palomo A, Sanz J. J. Eur. Ceram. Socy. 2012, 32, 2799–2807.10.1016/j.jeurceramsoc.2011.11.036Search in Google Scholar
[15] Chi M, Huang R. Adv. Sci. Lett. 2012, 16, 7–12.10.1166/asl.2012.3313Search in Google Scholar
[16] Vladimir Z. Constr. Build. Mater. 2007, 21, 1463–1469.10.1016/j.conbuildmat.2006.07.002Search in Google Scholar
[17] Odler I, Röbler M. Cem. Concr. Res. 1985, 15, 401–410.10.1016/0008-8846(85)90113-9Search in Google Scholar
[18] Schulze J. Cem. Concr. Res. 1999, 29, 909–915.10.1016/S0008-8846(99)00060-5Search in Google Scholar
[19] Provis JL, Yong CZ, Duxon P, van Deventer JSJ. Colloids. Surf. A. 2009, 336, 57–63.10.1016/j.colsurfa.2008.11.019Search in Google Scholar
[20] Zuhua Z, Xiao Y, Huajun Z, Yue C. Appl. Clay Sci. 2009, 43, 218–223.10.1016/j.clay.2008.09.003Search in Google Scholar
[21] Xiao Y, Zuhua Z, Huajun Z, Yue C. Thermochim. Acta 2009, 493, 49–54.10.1016/j.tca.2009.04.002Search in Google Scholar
[22] Ruiz-Santaquiteria C, Skibsted J, Fernández-Jiménez A, Palomo A. Cem. Concr. Res. 2012, 42, 1242–1251.10.1016/j.cemconres.2012.05.019Search in Google Scholar
[23] Oh JE, Monteiro PJM, Jun SS, Choi S, Clark SM. Cem. Concr. Res. 2010, 40, 189–196.10.1016/j.cemconres.2009.10.010Search in Google Scholar
[24] Ravikumar D, Peethamparan S, Neithalath N. Cem. Concr. Compos. 2010, 32, 399–410.10.1016/j.cemconcomp.2010.03.007Search in Google Scholar
[25] Khale D, Chaudhary R. J. Mater. Sci. 2007, 42, 729–746.10.1007/s10853-006-0401-4Search in Google Scholar
[26] Criado M, Fernández-Jiménez A, Palomo A. Micropo. Mesopo. Mat. 2007, 106, 180–191.10.1016/j.micromeso.2007.02.055Search in Google Scholar
[27] Rovnaník P. Constr. Build. Mater. 2010, 24, 1176–1183.10.1016/j.conbuildmat.2009.12.023Search in Google Scholar
[28] Kovalchuk G, Fernández-Jiménez A, Palomo A. Fuel 2007, 86, 315–322.10.1016/j.fuel.2006.07.010Search in Google Scholar
[29] Swanepoel J, Strydom C. Appl. Geochem. 2002, 17, 1143–1148.10.1016/S0883-2927(02)00005-7Search in Google Scholar
[30] Chi M. Constr. Build. Mater. 2012, 35, 240–245.10.1016/j.conbuildmat.2012.04.005Search in Google Scholar
[31] Palomo Á, Alonso S, Fernández-Jiménez A, Sobrados I, Sanz J. J. Am. Ceram. Soc. 2004, 87, 1141–1145.10.1111/j.1551-2916.2004.01141.xSearch in Google Scholar
[32] Fernández-Jiménez A, Palomo A. Fuel 2003, 82, 2259–2265.10.1016/S0016-2361(03)00194-7Search in Google Scholar
[33] Jaarsveld Jv, Deventer Jv. Ind. Eng. Chem. Res. 1999, 38, 3932–3941.10.1021/ie980804bSearch in Google Scholar
[34] ASTM C778 Standard Specification for Standard Sand. 2013.Search in Google Scholar
[35] Sievert T, Wolter A, Singh NB. Cem. Concr. Res. 2005, 35, 623–630.10.1016/j.cemconres.2004.02.010Search in Google Scholar
[36] ASTM C109 Standard Test Method for Compressive Strength of Hydraulic Cement Mortars. 2011.Search in Google Scholar
[37] ASTM C596 Standard Test Method for Drying Shrinkage of Mortar containing Hydraulic Cement. 2009.Search in Google Scholar
[38] ASTM C642 Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. 2006.Search in Google Scholar
[39] Collins F, Sanjayan JG. Cem. Concr. Res. 1999, 29, 607–610.10.1016/S0008-8846(98)00203-8Search in Google Scholar
[40] Li H-j, Sun H-H. Int. J. Miner. Metal. Mater. 2009, 16, 482–486.10.1016/S1674-4799(09)60084-4Search in Google Scholar
[41] BS 1881-201 Testing concrete. Method for determination of water absorption. 1986.Search in Google Scholar
[42] ASTM D4404 Standard Test Method for Determination of Pore Volume and Pore Volume Distribution of Soil and Rock by Mercury Intrusion Porosimetry. 2010.Search in Google Scholar
[43] Kumar R, Bhattacharjee B. Cem. Concr. Res. 2003, 33, 417–424.10.1016/S0008-8846(02)00974-2Search in Google Scholar
[44] Fedrizzi L, Azzolini F, Bonora PL. Cem. Concr. Res. 2005, 35, 551–561.10.1016/j.cemconres.2004.05.018Search in Google Scholar
[45] Nath SK, Kumar S. Constr. Build. Mater. 2013, 38, 924–930.10.1016/j.conbuildmat.2012.09.070Search in Google Scholar
[46] Komljenović M, Baščarević Z, Bradić V. J. Hazar. Mater. 2010, 181, 35–42.10.1016/j.jhazmat.2010.04.064Search in Google Scholar PubMed
©2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Review
- Automated profile preforming for structural components
- Original articles
- Effects of surface grafting of copper nanoparticles on the tensile and bonding properties of flax fibers
- Thermomechanical study of polyethylene porous membrane by coating silicon dioxide nanoparticles
- Crystallization, structural and mechanical properties of PA6/PC/NBR ternary blends: effect of NBR-g-GMA compatibilizer and organoclay
- Aging analysis of high voltage silicone rubber/silica nanocomposites under accelerated weathering conditions
- Investigation of viscoelastic properties and thermal behavior of photocurable epoxy acrylate nanocomposites
- The research of soft matter properties by light scattering material adding drop additive
- Microstructure and corrosion properties of SiC/Al-Mg-Cu-Si-Sn composites
- Tribological properties and microstructures of Al2O3-TiC-TiB2 reinforced composites
- Sustained release of OIC-A006 from PLGA microspheres to induce osteogenesis of composite PLGA/β-TCP scaffolds
- Study the effect of fiber loading and alkali treatment on the mechanical and water absorption properties of wheat straw fiber-reinforced epoxy composites
- Effect of mechanical alloying on the synthesis of Fe-TiC nanocomposite
- A comparative finite element analysis of two types of axial and radial functionally graded dental implants with titanium one around implant-bone interface
- Investigation of the effect of inert inclusions on densification during solid-state sintering of metal matrix composites
- Multiscale thermomechanical modeling of short fiber-reinforced composites
- Effects of the alkaline solution/binder ratio and curing condition on the mechanical properties of alkali-activated fly ash mortars
- Failure analysis and strengthening mechanism of Z-pinned composite T-joints under tensile loading
- Characteristic analysis of carbon nanotube thread embedded into three-dimensional braided composite under bending load
Articles in the same Issue
- Frontmatter
- Review
- Automated profile preforming for structural components
- Original articles
- Effects of surface grafting of copper nanoparticles on the tensile and bonding properties of flax fibers
- Thermomechanical study of polyethylene porous membrane by coating silicon dioxide nanoparticles
- Crystallization, structural and mechanical properties of PA6/PC/NBR ternary blends: effect of NBR-g-GMA compatibilizer and organoclay
- Aging analysis of high voltage silicone rubber/silica nanocomposites under accelerated weathering conditions
- Investigation of viscoelastic properties and thermal behavior of photocurable epoxy acrylate nanocomposites
- The research of soft matter properties by light scattering material adding drop additive
- Microstructure and corrosion properties of SiC/Al-Mg-Cu-Si-Sn composites
- Tribological properties and microstructures of Al2O3-TiC-TiB2 reinforced composites
- Sustained release of OIC-A006 from PLGA microspheres to induce osteogenesis of composite PLGA/β-TCP scaffolds
- Study the effect of fiber loading and alkali treatment on the mechanical and water absorption properties of wheat straw fiber-reinforced epoxy composites
- Effect of mechanical alloying on the synthesis of Fe-TiC nanocomposite
- A comparative finite element analysis of two types of axial and radial functionally graded dental implants with titanium one around implant-bone interface
- Investigation of the effect of inert inclusions on densification during solid-state sintering of metal matrix composites
- Multiscale thermomechanical modeling of short fiber-reinforced composites
- Effects of the alkaline solution/binder ratio and curing condition on the mechanical properties of alkali-activated fly ash mortars
- Failure analysis and strengthening mechanism of Z-pinned composite T-joints under tensile loading
- Characteristic analysis of carbon nanotube thread embedded into three-dimensional braided composite under bending load

