Abstract
The effect of multiwalled carbon nanotubes (MWCNTs)-magnetite (Fe3O4) hybrid content on the thermal, dynamic mechanical, and morphological properties of thermoplastic natural rubber (TPNR) nanocomposites was evaluated. TPNR/filler nanocomposites were prepared using a melt-blending method with a ball-milling technique as a premixed process. The acid treatment successfully shortened the lengths and disentangled the crowds of MWCNTs, which led to a better dispersion of MWCNTs in the polymer matrix, as revealed by optical microscopy and scanning electron microscopy. The improved dispersion of acid-treated MWCNTs-Fe3O4 in the TPNR matrix and the enhanced interfacial adhesion between acid-treated MWCNTs-Fe3O4 and the TPNR matrix increased the thermal stability and dynamic mechanical properties. Acid-treated MWCNTs acted as radical scavengers, which helped delay the onset of thermal degradation and hence improved the thermal stability. As expected, MWCNTs played a role as the nucleating agent in the TPNR matrix; however, the effect was more pronounced for the TPNR matrix containing pristine MWCNTs.
1 Introduction
The research field of carbon nanotubes (CNTs) has received continuous growing interest since they were discovered in 1991 [1]. They have attracted attention as an ideal reinforcement in composites with various matrices owing to their unique structural, extraordinary mechanical, electrical, thermal, and magnetic properties [2–7]. The mixing of polymers and CNTs can open ways of developing engineering-flexible composites that have potential to be used in a number of applications, including electrostatic dissipation, electromagnetic interference shielding, and bipolar plates of fuel cells [2, 8].
In recent years, many efforts have been made toward decorating CNTs with different materials [9–12], especially CNTs coated or filled with magnetic nanoparticles, which have recently attracted considerable interest owing to their excellent microwave-absorbing property. The microwave-absorbing property of CNTs has been limited by its small magnetic loss. To optimize the performance of CNTs as a radar-absorbing material, it is important to hybridize CNTs with magnetic nanoparticles that display both dielectric and magnetic losses.
Many works have been reported on preparing magnetic nanoparticle-filled CNTs. More recently, Lin et al. found that the encapsulation of Fe [10] and Co [13] in CNTs has significant effects on the dielectric and magnetic properties and microwave-absorbing behavior of the CNTs. Zhao et al. [14] reported that the microwave absorption of Ag nanowire-filled MWCNTs results mainly from dielectric loss rather than magnetic loss, whereas the microwave absorption of Ni-coated MWCNTs could be attributed to both dielectric and magnetic losses. Jia et al. [15] prepared self-assembled Fe3O4 beads along MWCNTs, and this kind of heterojunction has potential application in electronic nanodevices such as diodes. However, most of their works focused on the MWCNTs-magnetic materials hybrid, with only few studies reported on the properties of MWCNTs-magnetic materials hybrid reinforced polymer composites.
To benefit from the outstanding intrinsic properties of both CNTs and magnetic nanoparticles, proper dispersion and appropriate interfacial adhesion between the hybrid nanofillers and the polymer matrix have to be guaranteed. Thus, the challenges have been to incorporate the individual nanofillers into the polymer matrix, to deal with the length and size of the CNTs, and also to stabilize them in the polymer matrix to avoid secondary agglomeration.
The carbon atoms on CNT walls are chemically stable because of the aromatic nature of the bond. As a result, the reinforcing CNTs are insoluble in any solvent, they are inert and can interact with the surrounding matrix mainly through van der Waals interactions, unable to provide an efficient load transfer across the CNT/matrix interface [16–18]. Functionalization is one of the effective ways to make CNTs processable and to tune their properties; hence, functionalized CNTs can achieve a better dispersion in a polymer matrix. These methods can be divided into covalent and non-covalent functionalization based on the interactions between the active molecules and carbon atoms on the CNTs. Non-covalent functionalization of CNTs does not create defects on the CNT sidewalls. A large number of defects may result in severe degradation in the properties of CNTs. However, the main drawback of non-covalent functionalization is that the forces between the wrapping molecule and CNTs might be weak; thus, the efficiency of the load transfer across the CNT/matrix interface might be low. The covalent functionalization is based on the covalent linkage of functional entities onto the carbon scaffold of CNTs. It improves the solubility and dispersion in solvents and polymer. The functional groups on the surface of CNTs make the strongest type of interfacial bonding with the polymer matrix. An optimal density of covalently bonded groups on the nanotubes would ensure that sufficient linkages to the matrix are provided without impeding too much the mechanical stability of the nanotubes [19].
Blending using a ball mill is an effective method to help to break up the agglomerates resulting from the strong interactions between neighbor CNTs caused by strong van der Waals forces and to reduce the physical entanglements [20, 21]. It is believed that the centrifugal forces of grinding balls act on the mixture in the grinding bowl, and hence produce high shear forces to separate fine particles in agglomerates of CNTs [22]. In addition, it has been reported that the difficulty of dispersing nanofillers in the polymer matrix can be overcome by premixing the two or more species in the solid state at near room temperature. It has been proven that ball milling is an effective alternative and low-cost technique of producing novel polymer composites with a homogeneous dispersion of nanofillers in the polymer matrix, and improved properties [23–25]. Melt blending is also an interesting processing method, particularly useful in fabricating thermoplastic-based composites. In melt blending, nanofillers are dispersed using high temperature and high shear force into a polymer matrix. This approach is simple and compatible with current industrial practices. Furthermore, no solvent is employed to disperse CNTs in this method [17, 26].
In this article, the thermal, dynamic mechanical, and morphological properties of MWCNTs-Fe3O4 hybrid-filled thermoplastic natural rubber (TPNR) nanocomposites were investigated. The MWCNTs have been functionalized covalently for better dispersion in the matrix. A ball-milling technique was used as a dispersion method to facilitate the mixing of MWCNTs and Fe3O4 nanoparticles, and to premix the MWCNTs-Fe3O4 hybrid and polypropylene (PP) pellets in a solid state, whereas a melt-blending technique was used to prepare the Fe3O4-MWCNTs hybrid-filled TPNR nanocomposites. It is expected that with the combination of functionalization of CNTs and the modified processing methods, the properties of the prepared nanocomposites will be greatly enhanced.
2 Materials and methods
2.1 Materials
Multiwalled CNTs (MWCNTs) (Nanocyl NC7000 series; average diameter, 9.5 nm; average length, 1.5 μm; purity, 90%; and surface area, 250–300 m2 g-1) were provided by Nanocyl SA (Sambreville, Belgium), as shown in Figure 1A. Nanocyl NC7000 is an as-grown material produced in an industrial large-scale chemical vapor deposition process. Fe3O4 nanoparticles, with the particle size ranging from 20 to 30 nm, were obtained from commercial suppliers in powder form (Nanostructured & Amorphous Materials Inc., Houston, TX, USA). Natural rubber (NR) and PP were supplied by Rubber Research Institute of Malaysia (RRIM, Kuala Lumpur, Malaysia) and Polypropylene Malaysia Sdn. Bhd. (Kuantan, Malaysia), respectively. Liquid natural rubber (LNR) was prepared by the photosynthesized degradation of NR in visible light. Nitric acid (HNO3, 60%) was obtained from Merck Sdn. Bhd. (Petaling Jaya, Malaysia).
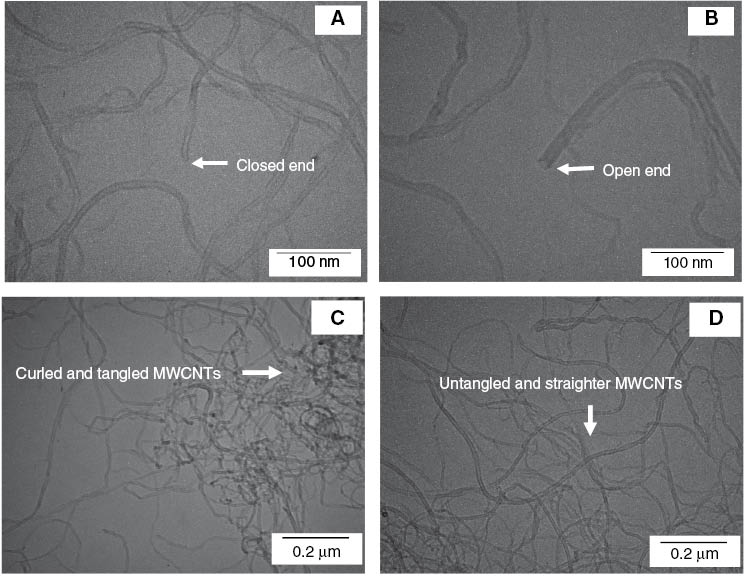
TEM images of fragmented (A) pristine MWCNTs and (B) acid-treated MWCNTs, and complete (C) pristine MWCNTs and (D) acid-treated MWCNTs.
2.2 Preparation of Fe3O4-MWCNTs hybrid filler
MWCNTs were dispersed in a round-bottomed flask containing HNO3 solution with the aid of an ultrasonic water bath for 1 h. The solution was refluxed at 80°C with vigorous mixing for 24 h. The acid-treated MWCNTs were collected by centrifugation, and then they were dried at 80°C for 24 h. From the treatment in boiling nitric acid, the MWCNTs were broken into untangled and straighter forms with open ends, as presented in Figure 1B. Figure 1C and D show the TEM images of complete pristine and acid-treated MWCNTs, respectively, for better comparison. To untie the entanglement of MWCNTs and facilitate the mixing of MWCNTs and Fe3O4 nanoparticles, the MWCNTs were mixed with Fe3O4 nanoparticles at a weight ratio of 1:1 for 1 h in a ball-milling machine. The porcelain balls in the mill were 15 mm in diameter and 11 g in mass. The ball-to-powder weight ratio was 10:1.
2.3 Preparation of Fe3O4-MWCNTs hybrid-filled TPNR nanocomposites
To further disperse the MWCNTs-Fe3O4 hybrid in the polymer matrix, MWCNTs-Fe3O4 hybrid and PP pellets were pretreated before a melt-blending process. The mixture was ground by a ball-milling process for 2 h to allow the MWCNTs-Fe3O4 hybrid to be attached onto the surface of PP pellets. Thereafter, the mixture was melt blended by using a laboratory mixer (Model Thermo Haake 600p). The weight ratio of PP, NR, and LNR was 70:20:10, with LNR being the compatibilizer for the mixture. Blending was carried out at a mixing speed of 100 rpm at 180°C for 13 min. The final nanocomposites contained 2 wt% filler in the TPNR matrix. As a reference, the neat TPNR was also similarly melt blended for thermal and mechanical studies.
2.4 Morphological characterization
The morphology of the MWCNTs before and after acid treatment was observed using a high-resolution transmission electron microscope (TEM; JEOL-1010), with an accelerating voltage of 100 kV. The samples used for TEM observations were prepared by dispersing a small amount of MWCNTs in absolute ethanol followed by ultrasonic vibration for 10 min, then placing a drop of dispersion onto a 200-mesh carbon-coated copper grid, which was then allowed to dry in air. Optical microscopy examinations using Leica DM2500M with a capture camera were carried out on the surface of the prepared nanocomposite samples. A 20× objective was used to examine the samples. The fracture morphology of nanocomposites was examined using a Supra 55VP field emission-scanning electron microscope (FE-SEM). To prepare the specimen for FE-SEM, first, a notch was made in the specimen. Then, the notched specimen was immersed in liquid nitrogen for 10 min and subsequently fractured along the notch. A thin gold layer was then sputter deposited on the fractured surface.
2.5 Wide-angle X-ray scattering
Wide-angle X-ray scattering (WAXS) studies were performed on a Bruker AXS NANOSTAR system operated at 45 kV and 35 mA. The detector was positioned at the smallest possible distance, 4.8 cm, to provide a 2θ range of 30°.
2.6 Differential scanning calorimetry
Thermal analysis of the neat TPNR and its nanocomposites was performed using differential scanning calorimetry (DSC) with Perkin-Elmer Pyris 1, calibrated with indium standard. For each measurement, a sample of about 5 mg was used, placed in a hermetically sealed aluminum pan. Before a cooling scan, the samples were first heated to 250°C and held in the molten state for 2 min to remove the thermal history. The second heating scan measurements were obtained as the melting data. The measurement was carried out at a scanning rate of 10 K·min-1 using N2 as the purging gas.
2.7 Thermogravimetric analysis
Thermal degradation measurements were performed in a Perkin-Elmer TGA-7 thermogravimetric analyzer with a TAC-7/DX thermal analysis controller. Samples of ∼25 mg were heated from 30°C to 850°C at 20 K·min-1. The purging gas (flow rate: 20 ml·min-1) was nitrogen from 30°C to 700°C; then, it was changed to air at 700°C.
2.8 Dynamic mechanical properties
Dynamic mechanical analysis (DMA) was carried out with Perkin-Elmer Pyris Diamond DMA. DMA tests were carried out in tensile mode at a fixed frequency of 1 Hz. A rectangular sample with a dimension of 10 mm×10 mm× 0.7 mm was cut and submitted to a temperature sweep from -120°C to 100°C at 2 K·min-1.
3 Results and discussion
3.1 Morphological characterization
Morphological analysis is very important for the evaluation of the dispersion state of the filler in the polymer matrix. Polarized optical light microscopy allows taking clear images of the fillers embedded in the TPNR matrix. Figure 2 presents the morphologies of TPNR containing 2 wt% filler with different formulations, showing the overall distribution of filler in the TPNR matrix. It can be seen from Figure 2A that the dispersion of pristine MWCNTs was poor in the TPNR matrix. Large MWCNT agglomerates were observed in the TPNR matrix owing to their large length, large surface areas, and their entanglement, which cannot be avoided. Furthermore, the strong intrinsic van der Waals attractions among MWCNTs and the poor interfacial interaction between MWCNTs and the TPNR matrix made the uniform dispersion of MWCNTs in polymer matrix very difficult. The acid-treated MWCNTs formed smaller agglomerates in the TPNR matrix, which can be clearly seen in Figure 2B. During acid treatment, the length of acid-treated MWCNTs was reduced and the entanglement of MWCNTs became loose, and this allowed a finer dispersion in the TPNR matrix compared with the pristine MWCNTs. The existence of functional groups on the surface of MWCNTs after acid treatment might have contributed to the improvement in the interfacial interaction between MWCNTs and the TPNR matrix, which assisted in increasing the dispersion and homogenization of the MWCNTs in the TPNR matrix. Therefore, it is anticipated that the mechanical properties of TPNR with acid-treated MWCNTs as filler would be relatively higher, as strong interfacial interactions between MWCNTs and the TPNR matrix lead to a sufficient load transfer from the polymer to the MWCNTs. Pristine MWCNTs-Fe3O4 and acid-treated MWCNTs-Fe3O4 showed a better dispersion in the TPNR matrix than pristine MWCNTs and acid-treated MWCNTs, as shown in Figure 2C and D. As the filler content in the nanocomposites was fixed at 2 wt%, the nanocomposites reinforced with MWCNTs-Fe3O4 hybrid fillers contained less MWCNTs than those reinforced with a single type of filler, either pristine or acid-treated MWCNTs. This could be the reason for the better dispersion in nanocomposites with hybrid fillers, as higher contents of MWCNTs resulted in a tendency for crowding and entangling. Figure 2E shows the optical microscopic image of the TPNR matrix containing 2 wt% Fe3O4. Fe3O4 nanoparticles formed agglomerates in the TPNR matrix.
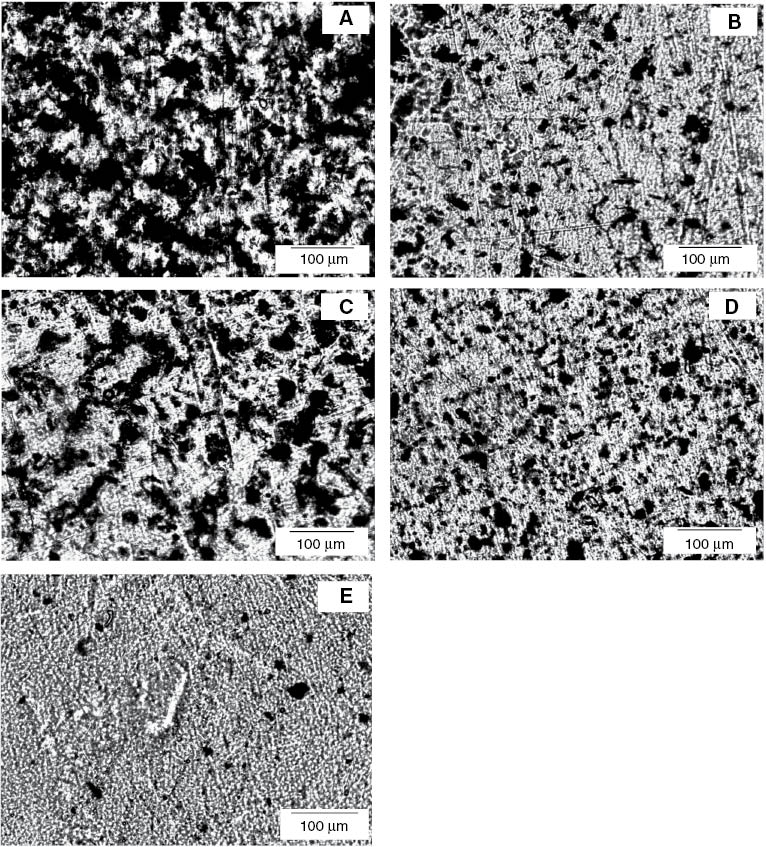
Optical microscopic images of the TPNR matrix containing 2 wt% (A) pristine MWCNTs, (B) acid-treated MWCNTs, (C) pristine MWCNTs-Fe3O4, (D) acid-treated MWCNTs-Fe3O4, and (E) Fe3O4 nanoparticles.
To observe the morphology at the micron and submicron levels, the nanocomposites were further examined using FE-SEM. The FE-SEM images of the cross-sectional fracture of the TPNR matrix with addition of 2 wt% pristine MWCNTs, acid-treated MWCNTs, pristine MWCNTs-Fe3O4, acid-treated MWCNTs-Fe3O4, and Fe3O4 are shown in Figure 3. The dispersed MWCNTs and the Fe3O4 nanoparticles are illustrated as bright dots, and some lines are the ends of the broken MWCNTs. It can be seen from Figure 3A that the dispersion of the pristine MWCNTs was poor in the TPNR matrix. The image revealed the agglomerates of MWCNT bundles, where long MWCNTs are entangled together. After acid treatment, a better dispersion was observed as expected, as shown in Figure 3B. It is very interesting to note that most of the pristine MWCNTs were pulled out during the fracture of the nanocomposites, whereas most of the acid-treated MWCNTs were broken rather than pulled out owing to the strong interfacial bonding between the MWCNTs and the polymer matrix. The acid treatment acted as a “cutting” process for the MWCNTs, shortening their length and disentangling them. The untangled and straighter MWCNTs were much better dispersed in the polymer matrix owing to this disentanglement, in spite of the polar groups introduced onto their surface, considering the non-polar nature of the matrix. These observations were consistent for the TPNR matrix containing pristine MWCNTs-Fe3O4 and acid-treated MWCNTs-Fe3O4, as shown in Figure 3C and D.
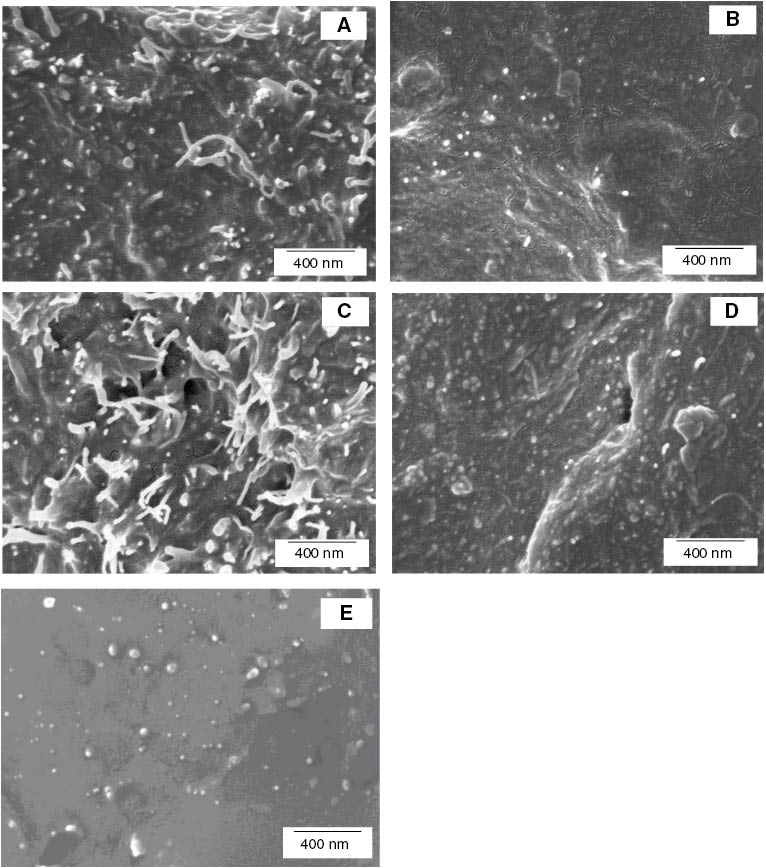
FE-SEM images of the cross section of TPNR containing 2 wt% (A) pristine MWCNTs, (B) acid-treated MWCNTs, (C) pristine MWCNTs-Fe3O4, (D) acid-treated MWCNTs-Fe3O4, and (E) Fe3O4 nanoparticles.
3.2 Structure
The introduction of nanoparticles into the TPNR matrix can cause changes in the crystallization rate of the polymer and its crystal conformation. To examine this possibility, the neat TPNR matrix and its nanocomposites were studied by using WAXS in the range of 2θ=10–30°. Figure 4 shows the WAXS patterns of the neat TPNR and its nanocomposites. Comparison of the WAXS patterns revealed no significant difference between the neat TPNR and its nanocomposites. Both the neat TPNR and its nanocomposites with 2 wt% filler displayed characteristic diffraction peaks at 2θ=14.1°, 16.7°, 18.5°, and 21.8°, which can be assigned to the respective (110), (040), (130), and (111) planes of the α-phase crystals of PP. This implies that the addition of filler in the TPNR matrix does not change the crystal structure of the neat TPNR. Similar results have been reported by other researchers [5].
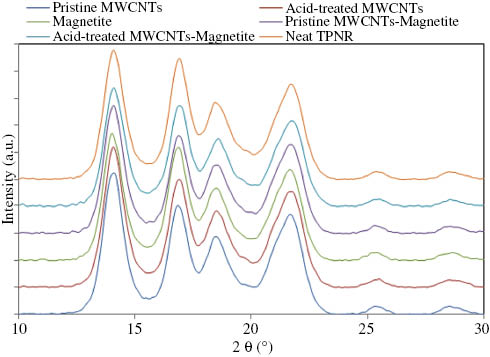
WAXS patterns of the neat TPNR and its nanocomposites.
3.3 Crystallization
The melting temperature (Tm) and heat of fusion (ΔHm) for the neat TPNR and its nanocomposites were determined from the DSC second heating runs of the samples. The crystallization temperature (Tc) and heat of crystallization (ΔHc) were determined from the DSC cooling scans of the samples. Many researchers have reported the effect of addition of MWCNTs on the crystallization behavior of polymer matrices. It has been known that MWCNTs either act as nucleating agents [5, 18, 27–29] or as obstacles to crystallization [30] depending on the types of matrices. The DSC first cooling and second heating curves of the neat TPNR matrix and its nanocomposites at a rate of 10°C·min-1 are presented in Figure 5A and B. The crystallization temperature, crystallization enthalpy, melting temperature, melting enthalpy, and degree of crystallinity of the neat TPNR and its nanocomposites are presented in Table 1. The degree of crystallinity (Xc) was determined from DSC measurements. Xc can be calculated from the heat evolved during crystallization (ΔHc), using the following equation:
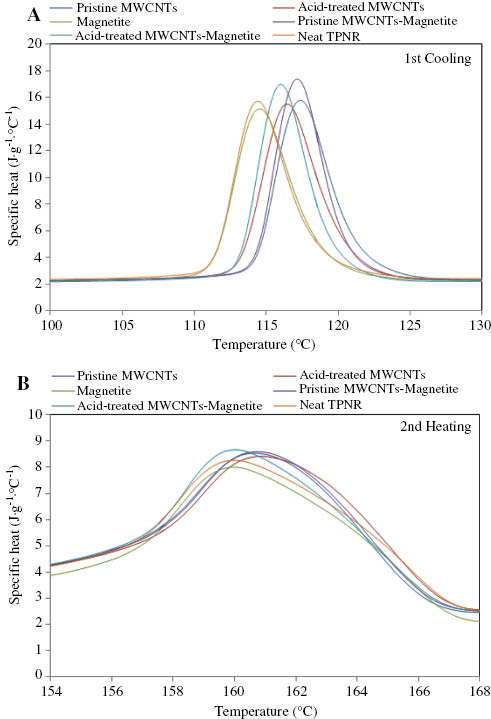
Heat capacity of (A) first cooling and (B) second heating of the neat TPNR and its nanocomposites obtained at a scanning rate of 10°C·min-1.
Crystallization temperature, crystallization enthalpy, melting temperature, and melting enthalpy of the neat TPNR and its nanocomposites.
| Sample | Tc(°C) | ΔHc(J·g-1) | Tm(°C) | ΔHm(J·g-1) | Xc(%) |
|---|---|---|---|---|---|
| Neat TPNR | 114.0 | 67.8 | 160.2 | 71.9 | 32.4 |
| Pristine MWCNTs | 117.0 | 69.3 | 160.8 | 72.5 | 33.8 |
| Acid-treated MWCNTs | 116.2 | 69.5 | 161.0 | 71.4 | 33.9 |
| Fe3O4 | 114.2 | 69.4 | 160.0 | 71.3 | 33.9 |
| Pristine MWCNTs-Fe3O4 | 116.8 | 68.7 | 160.8 | 71.0 | 33.5 |
| Acid-treated MWCNTs-Fe3O4 | 115.6 | 68.1 | 160.2 | 72.0 | 33.2 |
where
From Figure 5A, it can be seen that the neat TPNR showed a crystallization temperature of about 114°C. Apparently, the introduction of MWCNTs into the polymeric matrix led to an increase of the crystallization temperature. Interestingly, the shift of Tc to high temperatures was more prominent for nanocomposites containing 2 wt% MWCNTs compared with those of hybrid fillers. It is well known that MWCNTs have a nucleating effect on the crystallization of semicrystalline polymer matrices in the composites system [5, 18, 28, 29]. The incorporation of MWCNTs into the TPNR matrix helped induce more polymer chains to crystallize and promoted a faster crystal growth, thus resulting in an enhancement of the crystallization process of the TPNR matrix. The nucleation effect was more evident for the TPNR matrix with pristine MWCNTs as compared with other nanocomposites. The crystallization temperature passed from 114°C to 116°C with the addition of acid-treated MWCNTs and to 117°C with the addition of pristine MWCNTs. A similar result was observed by Sahoo et al. [18]. They suggested that the less evident nucleation effect on a polymer matrix with acid-treated MWCNTs as filler is because the functionalized MWCNTs had stronger interactions with the polymer matrix resulting in a better dispersion of MWCNTs, but had a higher barrier for the mobility of polymer chains. It is also very interesting to note that the addition of 2 wt% Fe3O4 nanoparticles did not seem to influence the crystallization temperature of the TPNR matrix. In other words, the nucleation effect promoted by Fe3O4 nanoparticles was far less than that of MWCNTs.
3.4 Thermal stability
The thermal stability of nanocomposites was studied using thermogravimetric analysis (TGA) by determining their mass losses during heating. Thermogravimetry (TG), expanded region of TG, and derivative TG (DTG) curves for the neat TPNR and its nanocomposites are represented in Figure 6A, B, and C, respectively, whereas the characteristic thermal decomposition temperatures of the neat TPNR and its nanocomposites are shown in Table 2. Two weight loss transitions can be observed from the TG and DTG curves of all the samples, which correspond to the NR and PP components. The NR component underwent thermal degradation at 350°C, which is extremely close to the decomposition of the PP component. Therefore, both of the decomposition events overlapped in the DTG curve. However, both of the components can be identified by their composition in the samples, which was about 30 wt% for NR and 70 wt% for PP.
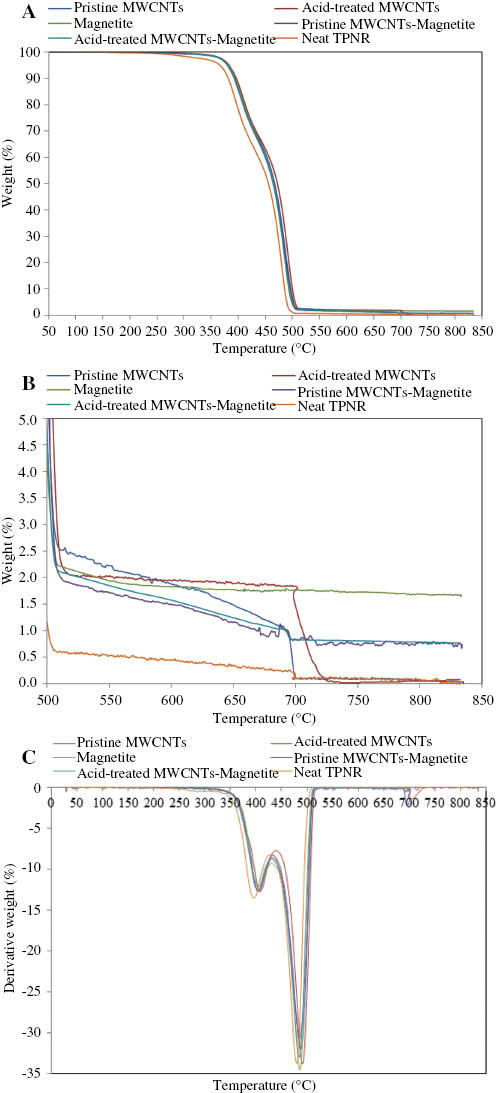
(A) TG, (B) expanded region of TG, and (C) DTG curves of the neat TPNR and its nanocomposites at a heating rate of 20°C·min-1.
Characteristic thermal decomposition temperatures of the neat TPNR and its nanocomposites.
| Sample | Onset (°C) | 5% (°C) | 50% (°C) | Endset (°C) |
|---|---|---|---|---|
| Neat TPNR | 205 | 367 | 456 | 506 |
| Pristine MWCNTs | 214 | 382 | 467 | 508 |
| Acid-treated MWCNTs | 218 | 383 | 472 | 511 |
| Fe3O4 | 207 | 379 | 462 | 506 |
| Pristine MWCNTs-Fe3O4 | 210 | 380 | 464 | 510 |
| Acid-treated MWCNTs-Fe3O4 | 212 | 379 | 465 | 507 |
From the TG curves, it can be seen that the neat TPNR and its nanocomposites showed relatively good thermal stability, as no remarkable weight loss occurred until 250°C. The 5% and 50% decomposition of the neat TPNR occurred at 367°C and 456°C, respectively. Compared with the neat TPNR, the addition of filler led to a remarkable increase of thermal stability. In the case of addition of 2 wt% pristine MWCNTs to the TPNR matrix, the decomposition temperature increased by 15°C and 11°C at 5% and 50% decomposition. The thermal stabilization effect of MWCNTs could be attributed to the enhancement of crystallinity, as presented in Table 1, as well as the restriction of molecular mobility around the MWCNTs. The polymer chains near the MWCNTs may degrade slower, which helps extend the decomposition temperature to the higher side. The thermal stabilization effect of MWCNTs could be explained by the improved thermal conductivity of the composites that facilitates heat dissipation within the composites [18, 31]. The acid-treated MWCNT-filled TPNR showed the highest decomposition temperature. The decomposition temperatures of acid-treated MWCNT-filled TPNR increased by 16°C at both 5% and 50% weight losses. This enhancement is probably due to the finer dispersion of the acid-treated MWCNTs in the TPNR matrix. The polymer chains were interconnected with the acid-treated MWCNTs by the presence of carboxylic groups on the surface of MWCNTs, which gave a strong interfacial interaction between the TPNR matrix and the MWCNTs [18]. As the polymer thermal degradations begin with chain cleavage and radical formation, the acid-treated MWCNTs in the composites acted as radical scavengers and chain traps in both chain cleavage and radical formation, delayed the onset thermal degradation, and hence improved the thermal stability [18, 32, 33].
Incorporation of Fe3O4 nanoparticles increased the decomposition temperature of TPNR. When comparing the decomposition temperatures at which 5% and 50% weight losses occur, Fe3O4-filled TPNR exhibited higher temperatures than the neat TPNR, whereas those temperatures were among the lowest compared with other nanocomposites. The improved thermal stability of TPNR by Fe3O4 nanoparticles is related to the barrier of nanoparticles to heat permeability into the TPNR and the reduction of diffusivity of the volatile decomposition products [34]. The hybrid of pristine MWCNTs-Fe3O4 and acid-treated MWCNTs-Fe3O4 nanocomposites showed slightly delayed decomposition compared with Fe3O4 nanocomposites, indicating that MWCNTs have a more pronounced effect in improving the thermal stability of TPNR.
To determine the content of Fe3O4 nanoparticles and MWCNTs, the purge gas was switched from nitrogen gas to air at 700°C. It can be seen from the Figure 6B that the remaining residue at 520°C for the neat TPNR was due to the formation of carbonaceous products. At 700°C, the carbonaceous products reacted with oxygen and released carbon dioxide. This explains the negligible remaining residue at the maximum temperature. The remaining residue at 520°C for nanocomposites with the addition of 2 wt% pristine MWCNTs, acid-treated MWCNTs, Fe3O4, pristine MWCNTs-Fe3O4, and acid-treated MWCNTs-Fe3O4 was 2.45%, 2.03%, 2.14%, 1.88%, and 2.04%, respectively, which is close to the added filler amount. Analysis of the filler content, carried out after oxidation, showed that the residues of both pristine MWCNT and acid-treated MWCNT nanocomposites continued burning until almost 100% of samples were degraded at maximum temperature, whereas the residues of pristine MWCNTs-Fe3O4 and acid-treated MWCNTs-Fe3O4 nanocomposites, after exposing the sample to oxygen, was maintained at about 1%, which corresponds to the amount of inert Fe3O4 nanoparticles in the TPNR matrix. The remaining residue of TPNR containing Fe3O4 nanoparticles was not affected by the switching of purge gas.
3.5 Dynamic mechanical properties
Nanocomposites with 2 wt% fillers were tested by DMA in a tensile mode at a temperature range from -120°C to 100°C. The same testing was carried out on the neat TPNR samples for comparison purposes. The storage modulus (E′) vs. temperature is shown in Figure 7A. The storage modulus at -90°C obtained from DMA is summarized in Table 3. The value of storage modulus signifies the elastic stiffness of the material. Moving from very low temperature to a higher temperature, all the curves show three distinct regions: a glassy high modulus region where the segmental mobility is restricted, a transition zone where a substantial decrease in the E′ values and the physical properties change drastically with increase of temperature, and a rubbery region where there is a drastic decay in the modulus with temperature. It can be noted that, at low temperature, the modulus of the neat TPNR and its nanocomposites was high. All samples showed transition and a plateau region corresponding to NR and PP. The storage modulus then decreased with increasing temperature, as expected, and finally leveled off at high temperature. The two-step curves are due to the two-phase morphology indicating that the blend between the phases is incompatible. The results are in agreement with those of systems based on blends of nylon copolymer-EPDM rubber by Komalan et al. [35] and blends of PP-EPDM by Karger-Kocsis and Kiss [36].
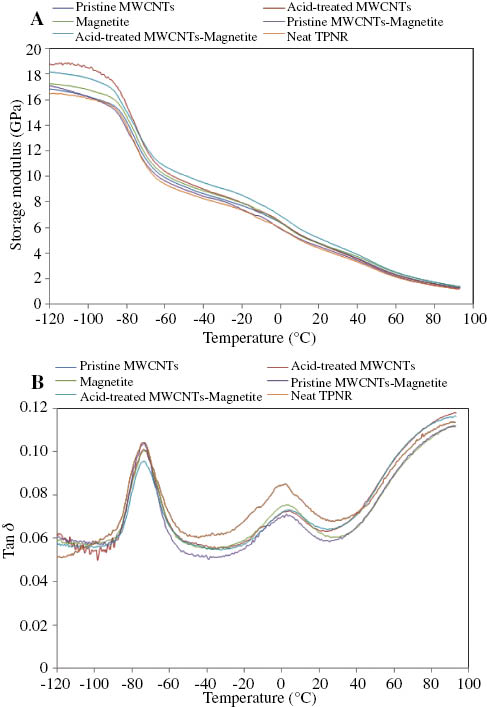
DMA of the neat TPNR and its nanocomposites: (A) storage modulus vs. temperature and (B) tan(δ) vs. temperature.
Dynamic mechanical parameters of the neat TPNR and its nanocomposites at 1 Hz.
| Samples | Tg (°C) | E′ at -90°C (GPa) | |
|---|---|---|---|
| NR phase | PP phase | ||
| Neat TPNR | –73.6 | 1.71 | 16.1 |
| Pristine MWCNTs | –73.5 | 2.11 | 16.3 |
| Acid-treated MWCNTs | –73.7 | 4.22 | 18.5 |
| Fe3O4 | –74.9 | 2.43 | 16.8 |
| Pristine MWCNTs-Fe3O4 | –73.6 | 2.73 | 16.5 |
| Acid-treated MWCNTs-Fe3O4 | –73.9 | 3.51 | 17.7 |
It was observed that at -90°C, the TPNR matrix containing acid-treated MWCNTs had the highest storage modulus, whereas the neat TPNR sample had the lowest. The addition of 2 wt% pristine MWCNTs has no significant effect on the storage modulus of TPNR. This is probably attributed to the poor dispersion and weak interfacial adhesion of MWCNTs in the TPNR matrix. However, the presence of acid-treated MWCNTs in the TPNR matrix increased the storage modulus of the TPNR matrix. The same trends have been observed by Špitalský et al. [6], Pan et al. [8], and Sahoo et al. [18] on the mechanical properties of acid-treated filled PP, epoxy, and nylon-6 composites. They reported that the enhanced storage modulus of the nanocomposites are due to the effect of the fine dispersion of the acid-treated MWCNTs into the polymer matrix, and the enhanced interaction between functional groups of MWCNTs and the polymer chains. This result is consistent with the TGA result as reported above. TPNR filled with acid-treated MWCNTs-Fe3O4 showed a significantly high storage modulus, which is slightly lower than that of acid-treated MWCNT-filled TPNR. The addition of 2 wt% acid-treated MWCNTs-Fe3O4 to TPNR increased its storage modulus from 16.1 to 17.7 GPa, i.e., about 10% improvement over the neat TPNR. However, the storage modulus for TPNR containing Fe3O4 and pristine MWCNTs-Fe3O4 are relatively low. For TPNR containing Fe3O4, the low storage modulus is probably caused by the preferential agglomeration of Fe3O4 nanoparticles due to the strong magneto-dipole interactions between particles [37], which weakens the adhesion of polymer molecules on the nanofiller surface, as shown in Figure 2E and 3E.
Figure 7B shows the variation of loss tangent (tan(δ)) with temperature for the neat TPNR and its nanocomposites. The tan(δ) of each nanocomposite has a wider distribution than that of the neat TPNR because of the incorporation of filler into the TPNR matrix. Examining the tan(δ) variation curves of the TPNR and its nanocomposites, two distinct peaks can be observed where each peak corresponds to the glass transition of NR and PP indicating immiscibility between the phases. The temperature corresponding to the maxima of a tan(δ) peak is normally associated with the glass transition temperature Tg. Table 2 indicates the effect of the addition of filler on the Tg of the TPNR matrix. It was noted that there was no significant change in the Tg values of the NR phase with the addition of fillers into the neat TPNR in the tan(δ) curve. Komalan et al. [35] reported that this phenomenon could be attributed to two types of constraints imposed on the motions of the molecular segments in the vicinity of the interface at the lower Tg. One is due to the presence of another phase, and the other is due to the presence of chemical bonds between the two phases. As the blends of TPNR goes through the lower Tg, one phase, which has a higher Tg, retains its glassiness. The constraints imposed by the glassy phase apparently dominate the presence of chemical bonds between phases. Consequently, addition of fillers makes no difference to the Tg of the NR phase.
From the variation of tan(δ) with temperature curve, the Tg of the neat TPNR appeared at approximately -73.6°C for the NR phase and at 2.73°C for the PP phase. The Tg of the neat TPNR for the PP phase was slightly shifted to the higher temperature by the addition of acid-treated MWCNTs. This phenomenon could be explained in two ways. First, this is due to the interaction between TPNR and acid-treated MWCNTs; the adsorption of the polymer chains on the surface of MWCNTs restricted the mobility of the molecular segment in the zone surrounding the MWCNTs [2, 8, 18]. Second, this effect can be explained in terms of decreasing the free volume of polymer. When MWCNTs are added into the TPNR matrix, absorption of polymer molecules will cause the matrix phase to become more compacted and the free volume will be decreased. Consequently, the Tg will be increased.
4 Conclusions
A method has been developed to disperse different formulations of fillers in TPNR using a combined ball-milling and melt-blending approach, in order to examine their contribution to thermal and mechanical properties. The results from optical microscopy and SEM showed that acid-treated MWCNTs-Fe3O4 were well embedded in the matrix and exhibited an effective dispersion of the nanofillers in the matrix due to the reduction of length and disentanglement of MWCNTs by acid treatment. The finer dispersion of acid-treated MWCNTs in the TPNR matrix helped improve the thermal stability of the TPNR matrix, where the acid-treated MWCNTs acted as radical scavengers and delayed the onset of thermal degradation. From the DMA results, the storage modulus and glass transition temperature were modified by the addition of MWCNTs-Fe3O4 due to uniform dispersion and the strong interaction between acid-treated MWCNTs and polymer chains. A better dispersion of acid-treated MWCNTs that led to a stronger interaction between the MWCNTs and the TPNR matrix set a higher barrier for the mobility of polymer chains. Consequently, less evident nucleation was observed in the TPNR matrix containing acid-treated MWCNT nanocomposites.
Acknowledgments
The authors gratefully acknowledge the financial support 2010 Endeavour Australia Cheung Kong Research Fellowship (to I. Kong).
References
[1] Iijima S. Nature 1991, 354, 56–58.10.1038/354056a0Suche in Google Scholar
[2] Teng C, Ma C, Huang Y, Yuen S, Weng C, Chen C, Su S. Compos. Pt. A Appl. Sci. Manuf. 2008, 39, 1869–1875.10.1016/j.compositesa.2008.09.004Suche in Google Scholar
[3] Abdalla M, Dean D, Theodore M, Fielding J, Nyairo E, Price G. Polymer 2010, 51, 1614–1620.10.1016/j.polymer.2009.05.059Suche in Google Scholar
[4] Sharma A, Tripathi B, Vijay YK. J. Membr. Sci. 2010, 361, 89–95.10.1016/j.memsci.2010.06.005Suche in Google Scholar
[5] Bao S, Tjong S. Mater. Sci. Eng. A 2008, 485, 508–516.10.1016/j.msea.2007.08.050Suche in Google Scholar
[6] Špitalský Z, Krontiras CA, Georga SN, Galiotis C. Compos. Pt. A Appl. Sci. Manuf. 2009, 40, 778–783.10.1016/j.compositesa.2009.03.008Suche in Google Scholar
[7] Xu C, Jia Z, Wu D, Han Q, Meek T. J. Electron. Mater. 2006, 35, 954–957.10.1007/BF02692553Suche in Google Scholar
[8] Pan Y, Li L, Chan SH, Zhao J. Compos. Pt. A Appl. Sci. Manuf. 2010, 41, 419–426.10.1016/j.compositesa.2009.11.009Suche in Google Scholar
[9] Jain D, Wilhelm R. Carbon 2007, 45, 602–606.10.1016/j.carbon.2006.10.012Suche in Google Scholar
[10] Lin H, Zhu H, Guo H, Yu L. Mater. Lett. 2007, 61, 3547–3550.10.1016/j.matlet.2007.01.077Suche in Google Scholar
[11] Zhang L, Zhu H, Song Y, Zhang Y, Huang Y. Mater. Sci. Eng. B 2008, 153, 78–82.10.1016/j.mseb.2008.10.029Suche in Google Scholar
[12] Lv R, Kang F, Cai D, Wang C, Gu J, Wang K, Wu D. J. Phys. Chem. Solids 2008, 69, 1213–1217.10.1016/j.jpcs.2007.10.006Suche in Google Scholar
[13] Lin H, Zhu H, Guo H, Yu L. Mater. Res. Bull. 2008, 43, 2697–2702.10.1016/j.materresbull.2007.10.016Suche in Google Scholar
[14] Zhao D, Li X, Shen Z. Compos. Sci. Technol. 2008, 68, 2902–2908.10.1016/j.compscitech.2007.10.006Suche in Google Scholar
[15] Jia B, Gao L, Sun J. Carbon 2007, 45, 1476–1481.10.1016/j.carbon.2007.03.025Suche in Google Scholar
[16] Kuzmany H, Kukovecz A, Simon F, Holzweber M, Kramberger C, Pichler T. Synth. Met. 2004, 141, 113–122.10.1016/j.synthmet.2003.08.018Suche in Google Scholar
[17] Ma P-C, Siddiqui NA, Marom G, Kim J-K. Compos. Pt. A Appl. Sci. Manuf. 2010, 41, 1345–1367.10.1016/j.compositesa.2010.07.003Suche in Google Scholar
[18] Sahoo NG, Cheng HKF, Cai J, Li L, Chan SH, Zhao J, Yu S. Mater. Chem. Phys. 2009, 117, 313–320.10.1016/j.matchemphys.2009.06.007Suche in Google Scholar
[19] Balasubramanian K, Burghard M. Small 2005, 1, 180–192.10.1002/smll.200400118Suche in Google Scholar PubMed
[20] Krause B, Villmow T, Boldt R, Mende M, Petzold G, Pötschke P. Compos. Sci. Technol. 2011, 71, 1145–1153.10.1016/j.compscitech.2011.04.004Suche in Google Scholar
[21] Kukovecz Á, Kanyó T, Kónya Z, Kiricsi I. Carbon 2005, 43, 994–1000.10.1016/j.carbon.2004.11.030Suche in Google Scholar
[22] Deng S, Zhang J, Ye L. Compos. Sci. Technol. 2009, 69, 2497–2505.10.1016/j.compscitech.2009.07.001Suche in Google Scholar
[23] Gorrasi G, Sarno M, Di Bartolomeo A, Sannino D, Ciambelli P, Vittoria V. J. Polym. Sci. Pt. B Polym. Phys. 2007, 45, 597–606.10.1002/polb.21070Suche in Google Scholar
[24] Guo M, Frechette MF, David E, Couderc H, Savoie S, Bouanga CV, Demarquette NR. Elect. Insul. Conf. (EIC), 2013 IEEE 2013, 444–448.10.1109/EIC.2013.6554285Suche in Google Scholar
[25] Azhdar B, Stenberg B, Kari L. Polym. Compos. 2008, 29, 252–261.10.1002/pc.20353Suche in Google Scholar
[26] Sahoo NG, Rana S, Cho JW, Li L, Chan SH. Prog. Polym. Sci. 2010, 35, 837–867.10.1016/j.progpolymsci.2010.03.002Suche in Google Scholar
[27] Lee G-W, Jagannathan S, Chae HG, Minus ML, Kumar S. Polymer 2008, 49, 1831–1840.10.1016/j.polymer.2008.02.029Suche in Google Scholar
[28] Bikiaris D, Vassiliou A, Chrissafis K, Paraskevopoulos K, Jannakoudakis A, Docoslis A. Polym. Degrad. Stab. 2008, 93, 952–967.10.1016/j.polymdegradstab.2008.01.033Suche in Google Scholar
[29] Fernández M, Landa M, Muñoz ME, Santamaría A. Macromol. Mater. Eng. 2010, 295, 1031–1041.10.1002/mame.201000176Suche in Google Scholar
[30] Sanchez-Garcia MD, Lagaron JM, Hoa SV. Compos. Sci. Technol. 2010, 70, 1095–1105.10.1016/j.compscitech.2010.02.015Suche in Google Scholar
[31] Moniruzzaman M, Winey KI. Macromolecules 2006, 39, 5194–5205.10.1021/ma060733pSuche in Google Scholar
[32] Zeng H, Gao C, Wang Y, Watts P, Kong H, Cui X, Yan D. Polymer 2006, 47, 113–122.10.1016/j.polymer.2005.11.009Suche in Google Scholar
[33] Watts PCP, Fearon PK, Hsu WK, Billingham NC, Kroto HW, Walton DRM. J. Mater. Chem. 2003, 13, 491–495.10.1039/b211328gSuche in Google Scholar
[34] Chae D, Kim B. Compos. Sci. Technol. 2007, 67, 1348–1352.10.1016/j.compscitech.2006.09.018Suche in Google Scholar
[35] Komalan C, George KE, Kumar PAS, Varughese KT, Thomas S. Express Polym. Lett. 2007, 1, 641–653.10.3144/expresspolymlett.2007.88Suche in Google Scholar
[36] Karger-Kocsis J, Kiss L. Polym. Eng. Sci. 1987, 27, 254–262.10.1002/pen.760270404Suche in Google Scholar
[37] Kong I, Hj Ahmad S, Hj Abdullah M, Hui D, Nazlim Yusoff A, Puryanti D. J. Magn. Magn. Mater. 2010, 322, 3401–3409.10.1016/j.jmmm.2010.06.036Suche in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Frontmatter
- Original articles
- In vitro degradation and bioactivity of poly(propylene fumarate)/bioactive glass sintered microsphere scaffolds for bone tissue engineering
- Properties enhancement in multiwalled carbon nanotube-magnetite hybrid-filled polypropylene natural rubber nanocomposites through functionalization and processing methods
- Synthesis of magnetic photocatalyst and sensitization properties of polypyrrole
- Effect of silane coupling agent on the mechanical properties of nitrile butadiene rubber (NBR)/organophilic montmorillonite (OMMT) nanocomposites
- Monitoring of jute/hemp fiber hybrid laminates by nondestructive testing techniques
- Size effects on the in-plane mechanical behavior of hexagonal honeycombs
- Investigation on corrosion performance of multilayer Ni-P/TiO2 composite coating on steel
- Investigation of pozzolanic activity of volcanic rocks from the northeast of the Black Sea
- The effect of delayed ettringite formation on fine grained aerated concrete mechanical properties
- Effect of early-age freeze-thaw exposure on the mechanical performance of self-compacting repair mortars
- Modeling cement hydration by connecting a nucleation and growth mechanism with a diffusion mechanism. Part I: C3S hydration in dilute suspensions
Artikel in diesem Heft
- Frontmatter
- Original articles
- In vitro degradation and bioactivity of poly(propylene fumarate)/bioactive glass sintered microsphere scaffolds for bone tissue engineering
- Properties enhancement in multiwalled carbon nanotube-magnetite hybrid-filled polypropylene natural rubber nanocomposites through functionalization and processing methods
- Synthesis of magnetic photocatalyst and sensitization properties of polypyrrole
- Effect of silane coupling agent on the mechanical properties of nitrile butadiene rubber (NBR)/organophilic montmorillonite (OMMT) nanocomposites
- Monitoring of jute/hemp fiber hybrid laminates by nondestructive testing techniques
- Size effects on the in-plane mechanical behavior of hexagonal honeycombs
- Investigation on corrosion performance of multilayer Ni-P/TiO2 composite coating on steel
- Investigation of pozzolanic activity of volcanic rocks from the northeast of the Black Sea
- The effect of delayed ettringite formation on fine grained aerated concrete mechanical properties
- Effect of early-age freeze-thaw exposure on the mechanical performance of self-compacting repair mortars
- Modeling cement hydration by connecting a nucleation and growth mechanism with a diffusion mechanism. Part I: C3S hydration in dilute suspensions

