Abstract
Delayed ettringite formation (DEF) is a chemical reaction with proven damaging effects on the mechanical properties of hydrated cementitious composite (concrete). Ettringite crystals can cause cracks and the widening of cracks due to pressure on the crack walls caused by the positive volume difference in the reaction. In this paper, we investigated the potential to utilise the positive volume difference in DEF in order to improve the mechanical properties of hydrated fine grained aerated concrete. Fine dispersed crystallisation nuclei, achieved by adding air-entraining agent (AEA) and by short vibration of specimens, are presented as the main requirement for such improvements. Control tests of expansion and mechanical properties were performed on samples of concrete with and without AEA by inducing DEF. The microstructure of fine grained aerated concrete was examined with an optical microscope and scanning electron microscope. We found that the controlled DEF, which is guaranteed by adding AEA and with the formation of uniformly dispersed air bubbles, which are crystallisation sites for ettringite crystals, improves the mechanical properties. The specimens with induced DEF were measured and found to have a 6.8% increase of compressive strength.
1 Introduction
Delayed ettringite formation (DEF) in cementitious materials is considered as a harmful chemical reaction leading to a variety of damages [1–3]. The volume of the formed DEF crystals in the hardened concrete is larger than the volume of reactants, which results in forces from the growing crystals acting upon the walls of the crack. Consequently, DEF cracks continue getting wider and spread through the concrete structure [4, 5]. In recent years, considerable research has led to a better understanding of the mechanisms of DEF [6]. In general, it is acknowledged that DEF is a result of various factors and conditions including excessive temperatures of above 70°C, the presence of sulphates, existing cracks, moist conditions and so on [7–10]. Ekolu et al. [11] summarise various control measures that could be used for the prevention of DEF including the use of chemical additives. However, preventative measures and improvements in general durability require further attention.
In practice, concrete and mortar mixes are normally based on Portland cement clinker, where the chemical process of hydration of clinker minerals yields hydrates and hydroxides. However, because of the presence of gypsum, the chemical reaction between tricalcium aluminate (C3A), gypsum (CaSO4 2H2O) and water forms ettringite crystals (3CaO Al2O3 3CaOSO4 32H2O). The volume difference in this reaction is positive, and ettringite crystals grow fast, growing quickly on the unhydrated cement particles, which can slow down the hydration [12]. The presence of ettringite in a liquid cementitious system is unproblematic, but its formation or re-formation in already hydrated concrete can lead to extensive damages [6]. Due to the resulting volume difference, particularly in the presence of sulphates, an expansive force within concrete can cause its disintegration (sulphate corrosion). It is well known that cements with low C3A content are more resilient to sulphate corrosion, but that also depends on the form of C3A [13]. For instance, crystalline C3A is more reactive than its amorphous version.
The positive volume difference as a result of early ettringite formation in cementitious materials rich with
The formation of a new phase characterised by substantial volume expansion for the purpose of strength improvement is well known in the mainstream material science. Such strengthening is based upon the creation of the internal compressive stress on the contact between the existing matrix and the new phase particles and depends on their shape, size and their overall dispersion. It is desirable that the newly formed particles are small, spherical and located sufficiently apart from each other to avoid overlapping stress fields. In the case of metallic materials, dispersion strengthening is known of the cooper matrix with zirconium oxide (ZrO2) particles [15, 16]. Strength improvement of the aluminium oxide (Al2O3) ceramics with fine dispersed ZrO2 particles is one example of this kind of internal stresses in the Al2O3 matrix created by applying the external force trigger polymorphic transformation of a tetragonal crystalline structure of ZrO2 into a monoclinic crystalline structure. Increased volume creates substantial compressive stresses in the matrix surrounding the transformed particles leading to a several fold increase in compressive strength as well as resistance to the spreading of cracks. Studies that apply this type of mechanism for strength improvement of concrete are rare.
The investigation of potential strength improvement by controlling delayed formation of ettringite crystals in hydrated fine grained aerated concrete makes three significant research contributions. Firstly, the study describes a DEF-based model of the strengthening of fine grained hydrated aerated concrete and details the mechanism through which the strengthening is achieved. Secondly, it further confirms that controlled formation of ettringite crystals in fine grained hydrated aerated concrete can improve its mechanical properties. Thirdly, the study confirms that an optimum volume of air voids in hydrated fine grained aerated concrete leads to formation of crystallisation nuclei where growth of ettringite crystals may be used for developing chemically prestressed concrete.
2 Materials and methods
Our experimental work was carried out according to an experimental work plan aimed at proving
that the strength of hydrated fine grained aerated concrete can be improved by DEF;
that the addition of an aerant can form uniformly distributed nuclei sites in volume leading to the formation of ettringite and the final microstructure.
The experimental work plan is shown schematically in Figure 1.

Experimental design research.
Concrete is a particular composite with a matrix of hydrated cement paste in which mineral aggregate grains are distributed. For the production of cement paste, from which hydrated paste is formed, we used pure Portland cement (LAFARGE CEMENT d.o.o., Trbovlje, Slovenia), without additives, because additives added by producers of cement during production do not always have equal properties or effect. Part of the cement was replaced by fly ash. With the addition of fly ash into concrete, we lengthened the stabilisation phase of the structure and were assured with reactive components of fly ash, specifically sulphates, conditions for sulphate attack. In the production of cement paste and concrete, we always used clean water because it does not contain noxious admixtures that could influence hydration, sulphate attack and the expected DEF. As it is known that DEF in hydrated concrete is a harmful and unwanted phenomenon, which positively changes the volume, we had to assure a sufficiently porous concrete. The necessary porosity was achieved with the addition of an aerant which increases the quantity of air pores and assures an optimal distribution of air bubbles in the structure of hardened cement composite. Air bubbles in the hardened paste are indispensable nuclei sites for the growth of crystals. With the use of standard sand, we also assured repetition in the production of fine grained aerated concrete samples, because standard sand has a precisely defined grain and mineral composition. The components of concrete were tested and investigated to confirm physical and chemical properties which influence the properties of cement composites.
The cement paste was tested according to building standards and codes, which allowed us to observe the hardening process and determine all required properties of hardened paste:
volume stability and
chemical stability.
We tested fine grained aerated concrete using standard tests for determining physical, mechanical and chemical properties. The growth of ettringite crystals, which result from chemical reactions – DEF and an alkali-silica reaction, was triggered with the Duggan test where conditions for rapid growth of crystals are assured with temperature and moisture.
Besides standard and non-standard tests, we also performed scientific tests on the components used for the production of cement paste and fine grained aerated concrete and on samples of hardened paste and hydrated fine grained concrete:
optical microscopy to analyse the obtained microstructure,
electronic microscopy with the energy-dispersive X-ray and X-ray mapping (XRM) analysis to determine chemical elements in the cement composite, and
differential thermal analysis to control temperatures in the phase of hydration of cement composite because it is known that hydration is an exothermic chemical reaction.
2.1 Materials and mixture design
For sample preparation, the cement CEM I 42.5 R was used. In order to ensure the formation of the alumina-ferric oxide-monosulphate phase (AFm), we used fly ash with defined components and a specific surface area. The role of the fly ash was two-fold – chemical and physical, whereby the chemical components are actively involved in the pozzolanic reaction, while the physical ones work as nuclei sites and filler. We used chemically neutral standard sand with properties according to EN ISO 196-1 and ISO 697 for the aggregate. The purpose of adding a chemically neutral aggregate was to prevent an alkali silica reaction, which would result from reactive minerals in the aggregate. The most important precaution was the use of the petrographic components of aggregates that will not react with alkali [17]. The used additive – an air entraining agent – was the anion type based on abietic acid (TKK d.o.o., Srpenica, Slovenia). The components were mixed with clean drinking water.
2.2 Test procedures
Concrete mixture components were determined on the basis of a standard consistency of cement paste. We prepared three different series of samples. The composition of mixtures is shown in Table 1. Samples were mixed with a laboratory mixer to the requirements of EN 196-1. Fresh concrete was built in standard moulds with dimensions of 40/40/160 mm by a vibrating table with vibration of 5 s, frequency of 50 Hz and amplitude of 0.75 mm. Samples were tended for 28 days in a climate chamber at a temperature of 20±2°C and a relative humidity of 98±2%. Measurements of density, compressive strength and flexural strength were performed on all samples at intervals of 7, 14, 28 and 56 days. The set of samples that contained fly ash were subjected to the Duggan test after 28 days of treatment in the climatic chamber. This test is used to achieve accelerated DEF. The test consists of soaking the samples in demineralised water at 20±2°C and drying them at a temperature of 81±2°C. After the Duggan [18] test, the prisms were laboratory conditioned for 48 h in a desiccator, in-between each of the above phases, and were then once again immersed in demineralised water for 24 h in order to fill the capillaries and voids with water. In these samples, we controlled DEF, by measuring the expansion as a result of ettringite crystal growth.
The composition of fine grained concrete mixtures.
| Specimen | Aggregate (g) | Cement (g) | Fly ash (g) | Water (g) | AEA (g) |
|---|---|---|---|---|---|
| AI | 1350 | 450 | – | 218.2 | 6.8 |
| BI | 1350 | 310 | 140 (type 1) | 218.2 | 6.8 |
| CI | 1350 | 310 | 140 (type 2) | 218.2 | 6.8 |
At the moment when, in micrometres, expansion was no longer observed, we stopped the measurements. We considered that DEF had been completed. Measurements of density and strength were then performed on the samples. These measurements were repeated on the samples on which the Duggan test was not carried out, so we could obtain the comparisons of these values.
3 Results and discussion
3.1 The results of investigations of concrete components
Cement, standard sand and air entraining agent were used as the benchmark for standard-quality results. Therefore, declared properties are not included. Fly ash contains silicates, carbonates and phosphates of calcium, magnesium, iron and aluminium and other elements. Illite-kaolinite clays, apart from illite and kaolinite minerals, also contains a-quartz, Fe2O3 and CaCO3 [19]. The results of the laboratory analysis for fly ash are shown in Table 2.
Results of the laboratory analysis for fly ash.
| Component part | Fly ash type 1 Content (m%) | Fly ash type 2 Content (m%) |
|---|---|---|
| Loss on ignition | 2.63 | 0.41 |
| Insoluble residue | 10.23 | 16.67 |
| SiO2 impure | 5.77 | 13.08 |
| SiO2 pure | 42.82 | 47.62 |
| SiO2 soluble | 0.48 | 0.64 |
| SiO2 total | 43.30 | 48.26 |
| SiO2 active | 37.53 | 35.18 |
| CaO reactive | 8.01 | 7.56 |
| SO3 | 1.88 | 1.88 |
| CaO free | 1.22 | 2.00 |
3.2 Characterisation of the macrostructure
We observed significant break areas of the hydrated concrete using the optical microscope Olympus SZX. The control of dispersion bubbles of the air entraining agent showed that they were mainly uniformly distributed. The bubbles had a diameter between 25 and 50 μm. The average measured distance between the bubbles was 0.1 to 1.2 mm.
3.3 Scanning electron microscopy characterisation
The characteristic fields of air entraining bubbles were observed with a scanning electron microscope (SEM) JEOL JSM 5610 (JEOL Ltd., Tokyo, Japan) and QUANTA 200 3D (FEI, Eindhoven, Netherlands). We discovered ettringite crystals in these bubbles. This proved our assumption that the bubbles of air entraining agents are nucleation sites. The morphology of ettringite crystals is very similar. Ettringite crystals grow in bunches, and all crystals are needle-like and thin. The observation of a large number of bubbles showed that the sample labelled AI had less crystals of ettringite as shown in Figure 2. The samples that were labelled BI and CI showed many more bunches of ettringite crystals. These crystals are mainly grown on porous sites in air bubbles – see Figure 3. More microcracks in bubbles were noticed on the samples labelled BI-DT and CI-DT compared to the samples labelled BI and CI. In these microcracks, we could also observe the presence of very thin and needle-like ettrigite crystals suggesting their rapid growth – see Figure 4.

Micro fractography of the sample AI with ettringite crystals; scanning electron microscope (SEM), secondary electron imaging (SEI).

Micro fractography of the sample BI with ettringite crystals; SEM, SEI.

Micro fractography of the sample BI-DT microcrack on the wall of air bubble with ettringite crystals; SEM, SEI.
The comparison of the microstructures of the specimens from fine grained concrete mixes AI, BI and BI-DT presented in Figures 2–7 clearly demonstrates that, as expected, similar air-entraining agent (AEA)-induced nuclei exist in the AI, BI, CI, BI-DT and CI-DT specimens. Although ettringite crystals can be found in concretes produced by using pure Portland cement, no visible ettringite crystals were detected in any of the large number of prisms from the mix AI. However, ettringite crystals did appear in specimens of all other fine grained concrete mixes. Microstructures of the specimens from the fine grained concrete mix AI show very little ettringite crystals in AEA nuclei themselves, and they were detected in microcracks. Similar to Myneni et al. [20], these crystals have thin, needle-shaped morphology and are approximately 2 μm in length, revealing rapid growth. Fly ash in fine grained concrete mix BI may well be a source of soluble calcium for ettringite formation, as reported by Solem and McCarthy [21], Zhang and Reardon [22] and Chrysochoou and Dermatas [23], because its crystals were found in greater quantities in microcracks and within the AEA-induced nuclei. Figures 2 and 3 show that ettringite crystals have thin, needle-shaped morphology, but those found in microcracks are only approximately 2 μm long as opposed to the 10 μm long crystals found in the nuclei. The microcrack that appeared on the surface of the nucleus in Figure 5 can be associated with the shrinkage of the matrix during hydration [24]. The comparison between various BI and CI specimens shows that ettringite crystals start growing wherever there is enough space for growth before further damaging concrete, which enables further growth. AEA-induced nuclei may, therefore, act as relief reservoirs enabling the growth of substances like ettringite crystals in hardened concrete with minimum or no damaging effects. Hime [25] even recommends air entrainment as a way to prevent DEF and reports on only a single incident where air-entrained concrete suffered from DEF.

Micro fractography of the sample BI-DT air bubble with microcracks and ettringite crystals; SEM, SEI.
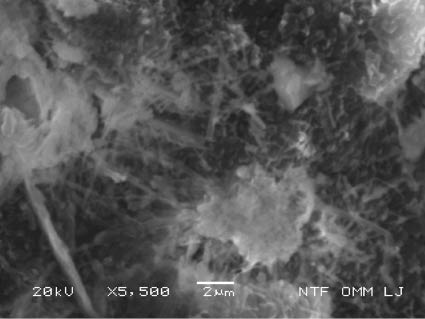
Micro fractography of the sample CI with ettringite crystals on a porous site; SEM, SEI.

Micro fractography of the sample BI-DT microcrack with ettringite crystals; SEM, SEI.
3.4 Thermal gravimetric analysis
Pozzolanic materials can partially substitute Portland cement in order to enhance the properties of concrete and mortars such as durability and mechanical properties. The interaction between additions such as fly ash and Portland cement can be investigated by various techniques such as thermal analysis and X-ray diffraction. Thermal gravimetric analysis (TG), derivative thermogravimetry and differential scanning calorimetry are considered important tools to evaluate the nature of hydrated products according to different cement hydration, in addition to quantifying the different phases.
Simultaneous thermal analysis and TG analysis were performed on one sample of cement paste of concrete samples AI, BI and CI in a static air atmosphere. Measurements were performed with the device Netzsch STA 449C Jupiter (NETZSCH, Selb, Germany). We measured the change in mass of the sample at 25°C. Each measurement lasted 60 h. The maximum change in mass, which was 19.9%, was found in a sample of cement paste of the concrete sample BI, after a time of 446.8 min. The change in mass of the sample of cement paste – the cement sample AI – was lower and amounted to 19.7% at the time of 438.2 min, and it was higher for the sample CI amounting to 19.9 at the time of 462.9 min – see Figure 8. TG measurements have shown that by modifying the mixture of a concrete sample, changes with the fly ash did not transgress the setting time of concrete, which must be >360 and <540 min.

Mass degradation of AI, BI and CI according TGA.
From the thermogravimetric investigations performed, which are shown in the Tg curves, it can be concluded that fly ash and AEA additions have no significant effect on the hydration of cement.
3.5 XRM analysis
XRM analyses were performed with electron scanning microscope JEOL JSM 5610 on samples which are fracture sites of hydrated fine grained concrete labelled BI-DT and CI-DT. We analysed the particular field with crystals of ettringite as shown in Figure 9. Based on the results, it was suggested that in areas of air bubbles in the cement matrix, the sulphate attack is completed. The purpose of this study was to determine the chemical composition of ettringite crystals of the fine grained concrete mixes AI, BI and CI and in the samples BI-DT and CI-DT on which Duggan’s test was performed. These results confirm that the process of DEF is completed too. Chemical elements sulphur and aluminium, which are characterised by a chemical reaction, are present only in sites with ettringite crystals.

XRM analysis of ettringite crystal in the air bubble of AEA: (A) specimen BI-DT; (B) specimen CI-DT.
3.6 pH measurement
The fall of pH usually indicates the occurrence of reaction of fly ash. The pozzolanic activity of fly ash depends on the pH value of the pore solution. Sampling of fine grained hydrated concrete was done by breaking off a piece. This piece of concrete was crushed and sifted through a sieve with an aperture diameter of 0.02 mm. Fifty grams of sample was mixed with a laboratory stirrer with 10 ml of distilled water. The solution was filtered before being tested through a filter paper N°40. The measurement procedure was taken from the literature [26]. Measurements were performed with the pH meter Mettler Toledo S20 (METTLER TOLEDO, Greifensee, Switzerland) on three samples of each type of concrete at intervals of 1, 2, 3 and 4 min. For each sample, we performed three measurements and calculated the mean value. The results of mean values are presented in Table 3. Based on the measurements, we found that the pH of hydrated concrete with fly ash was reduced due to the Duggan test, although this value did not change significantly. It is known that the pH of concrete with fly ash is less than the pH of concretes without it. This is due to the binding of Ca(OH)2 with reactive silicates contained in fly ash [27].
Measurements results of pH value.
| Sample | pH (mean value) |
|---|---|
| AI | 12.33 |
| BI | 11.88 |
| BI-DT | 11.86 |
| CI | 11.88 |
| CI-DT | 11.89 |
3.7 Expansion measurement
After a required 28-day curing period, six prisms of the mix BI and CI were exposed to the Duggan test in order to achieve the accelerated ettringite formation. The prisms were then placed into a standard apparatus for the determination of length change of hardened cement paste, mortar and concrete, constructed according to ASTM C490-86. During these measurements, the apparatus itself was placed in a climatic chamber with a constant temperature of 20±2°C and relative humidity of 98±2% (see Figure 10).

The apparatus for the measurement of expansion of hardened concrete according to ASTM C490-86 placed in a climatic chamber.
Ettringite formation was then monitored by measuring length change (expansion) with the Mahr’s MarCator 1080/12.5/0.005 mm digital micrometer (MAHR, Esslingen, Germany). The results were recorded with an analogue/digital converter connected to a workstation. Developing expansion was measured regularly at 15-min intervals with a measurement accuracy of 0.005 mm, although intervals could well be longer considering the slow pace of DEF.
Figures 11 and 12 show the change of length for six fine grained concrete prisms from the mixes BI and CI that were exposed to the Duggan test and a final 24 h immersion in demineralised water. The change of length of prisms was measured daily and stopped at prisms for the mix BI after 73 days and for the mix CI after 82 days when measurements did not show any further expansions. The results of measurements of the length change (expansion) of prismatic samples were evaluated as an average value, which is equal to 0.0125%. All samples were inspected visually, and no cracks were found.

The results of expansion measurements for samples labelled BI-DT after the Duggan test.

The results of expansion measurements for samples labelled CI-DT after the Duggan test.
3.8 Mechanical properties
The density of hardened fine grained concrete (ρ), its compressive strength (fc) and flexural strength (fm) were measured on six additional prisms for each of the three mixes after standard 7, 14 and 28 days and additionally after 56 and 121 days when compressive and flexural strengths should reach a plateau. The mechanical properties of fine grained concrete were examined with a universal dynamometer Zwick Roell (Zwick/Roell, Ulm, Germany) and a method according to EN 196-1.
Tables 4, 5 and 6 show measured densities (ρ), flexural (fm) and compressive strength (fc) with the value of standard deviation (σ) of hardened fine grained concrete prisms for mixes AI, BI, CI, BI-DT and CI-DT. The compressive strength of (BI) prisms after 121 days is 6.9% lower than that of prisms (BI-DT), and the compressive strength of (CI) prisms after 112 days is 6.8% lower than that of (CI-DT) prisms. The comparison of results showed that concrete with fly ash has a slightly lower early strength and increased final strength [28].
Densities and mechanical properties of hardened fine grained concrete (mix AI).
| Time interval (days) | P (kg/m3) | fm (MPa) | fc (MPa) | σ(fc) (MPa) |
|---|---|---|---|---|
| 7 | 1806 | 2.5 | 18.0 | 0.68 |
| 14 | 1804 | 4.0 | 14.8 | 0.62 |
| 28 | 1883 | 5.2 | 17.3 | 0.67 |
| 56 | 1885 | 5.3 | 19.5 | 0.63 |
| 121 | 1887 | 5.4 | 20.5 | 0.71 |
σ(fc), standard deviation.
Densities and mechanical properties of hardened fine grained concrete (mix BI and BI-DT).
| Time interval (days) | P (kg/m3) | fm (MPa) | fc (MPa) | σ(fc) (MPa) | Mix |
|---|---|---|---|---|---|
| 7 | 1801 | 2.7 | 11.6 | 0.42 | BI |
| 14 | 1807 | 4.1 | 14.3 | 0.81 | BI |
| 28 | 1803 | 5.2 | 17.7 | 0.45 | BI |
| 56 | 1817 | 5.3 | 18.8 | 0.67 | BI |
| 121 | 1818 | 5.6 | 21.0 | 0.48 | BI |
| 121 | 1810 | 6.0 | 22.5 | 0.67 | BI-DT |
σ(fc), standard deviation.
Densities and mechanical properties of hardened fine grained concrete (mix CI and CI-DT).
| Time interval (days) | P (kg/m3) | fm (MPa) | fc (MPa) | σ(fc) (MPa) | Mix |
|---|---|---|---|---|---|
| 7 | 1819 | 3.2 | 13.0 | 0.36 | CI |
| 14 | 1822 | 3.5 | 15.3 | 0.61 | CI |
| 28 | 1804 | 3.9 | 17.3 | 0.64 | CI |
| 56 | 1823 | 4.1 | 18.5 | 0.34 | CI |
| 112 | 1799 | 5.6 | 20.5 | 0.68 | CI |
| 112 | 1781 | 6.0 | 21.9 | 0.62 | CI-DT |
σ(fc), standard deviation.
4 Conclusions
Controlling DEF by using AEA as a nucleation agent results in a slight increase of the compressive strength of fine grained concrete. Small and thin crystals of ettringite, which result from a series of chemical reactions that take place in hydrated concrete, caused swelling of the concrete. Local stress concentration at the nucleation sites, which are air bubbles of air entraining agent, where ettringite crystals grew, did not cause the extension of microcracks which could lower the compressive strength of the concrete.
Ettringite crystal growth in porous parts of the walls with air bubbles caused the change of the microstructure of concrete. This change represents a transformation of the existing porous microstructure in line with tiny crystals condensed in the microstructure.
The result of these changes in the microstructure of the nucleation sites is a reinforced cementitious matrix. Strength improvement is a result of hardening of the cementitious matrix, causing an increase in the compressive strength of the concrete.
References
[1] Diamond S. Cem. Concr. Compos. 1996, 18, 205–215.10.1016/0958-9465(96)00017-0Search in Google Scholar
[2] Thomas MDA. Materials Science of Concrete VI, American Ceramics Society, Westerville, OH, 2001, 435–482.Search in Google Scholar
[3] Barbarulo R, Peycelon H, Prené S, Marchand J. Cem. Concr. Res. 2005, 35, 125–131.10.1016/j.cemconres.2004.05.041Search in Google Scholar
[4] Sahu S, Thaulow N. Cem. Concr. Res. 2004, 34, 1675–1681.10.1016/j.cemconres.2004.01.027Search in Google Scholar
[5] Thomas M, Folliard K, Drimalas T, Ramlochan T. Cem. Concr. Res. 2008, 38, 841–847.10.1016/j.cemconres.2008.01.003Search in Google Scholar
[6] Collepardi MA. Cem. Concr. Compos. 2003, 25, 401–407.10.1016/S0958-9465(02)00080-XSearch in Google Scholar
[7] Taylor, HFW. Cement Chemistry, Academic Press: London, 1990.Search in Google Scholar
[8] Lawrence CD. Cem. Concr. Res., 1995a, 25, 903–914.10.1016/0008-8846(95)00081-MSearch in Google Scholar
[9] Lawrence CD. Materials Science of Concrete, vol. IV, American Ceramic Society, Ohio, USA, 1995b, 113–154.Search in Google Scholar
[10] Ekolu SO, Thomas MDA, Hooton RD. Cem. Concr. Res. 2007a, 37, 942–947.10.1016/j.cemconres.2007.01.014Search in Google Scholar
[11] Ekolu SO, Thomas MDA, Hooton R D. Cem. Concr. Res. 2007, 37, 161–165.10.1016/j.cemconres.2006.10.014Search in Google Scholar
[12] Swaddiwudhipong S, Chen D. Adv. Cem. Res. 2002, 14, 61–69.10.1680/adcr.2002.14.2.61Search in Google Scholar
[13] Mather B. Thorvaldson Symposium Proceedings, University of Toronto Press, Toronto, 1968), 66–76.10.3138/9781487584092-005Search in Google Scholar
[14] Collepardi M. Proceedings of the 5th CANMET/ACI International Conference on Recent Advances in Concrete Technology, Singapore, 2001, 27–39.Search in Google Scholar
[15] Anžel I, Kosec L, Križman A. Kovine, Zlitine, Tehnol. 1996, 30, 225–229.Search in Google Scholar
[16] Anžel I, Kneissl A, Križmnan A, Rudolf R. Z. Met. kd. 2003, 94, 127–133.10.3139/146.030127Search in Google Scholar
[17] Mladenovič A, Vižintin N, Drnovšek S. Mater. Tehnol. 2000, 34, 103–105.Search in Google Scholar
[18] Grabowski E, Czarnecki B, Gillott JE, Duggan CR, Scott JF. ACI Mater. J. 1992, 89, 469–480.Search in Google Scholar
[19] Krgović M, Knežević M, Ivanović M, Bošković I, Vukčević M, Zeljak R, Zlatičanin B, Durković S. Mater. Tehnol 2009, 43, 327–331.Search in Google Scholar
[20] Myneni SCB, Traina SJ, Logan TJ, Waychunas GA. Environ. Sci. Technol. 1997, 31, 1761–1768.10.1021/es9607594Search in Google Scholar
[21] Solem JK, McCarthy GJ. Mater. Res. Soc. Symp. Proc. 1992, 71, 245.10.1557/PROC-245-71Search in Google Scholar
[22] Zhang M, Reardon EJ. Environ. Sci. Technol. 2003, 37, 2947–2952.10.1021/es020969iSearch in Google Scholar
[23] Chrysochoou M, Dermatas D. J. Hazard. Mater. 2006, 136, 20–33.10.1016/j.jhazmat.2005.11.008Search in Google Scholar
[24] Stang H. Adv. Cem. Based Mater. 1996, 4, 106–115.10.1016/S1065-7355(96)90079-6Search in Google Scholar
[25] Hime WG. PCI J. 1996, 26–30.10.15554/pcij.07011996.26.30Search in Google Scholar
[26] Grubb J, Limaye H, Kakade A. Concrete International – ACI publication, 2007.Search in Google Scholar
[27] Koukouzas N, Papayianni I, Tsikardani E, Papanikolaou D, Ketikidis C. World of Coal Ash, Covington, USA, 2007.Search in Google Scholar
[28] Zajc A. Kemijski in mineralni dodatki v tehnologiji betona, 14. slovenski kolokvij o betonih, Posebne lastnosti betonov z dodatki, Zbornik gradiv in referatov, Ljubljana, 2007.Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Original articles
- In vitro degradation and bioactivity of poly(propylene fumarate)/bioactive glass sintered microsphere scaffolds for bone tissue engineering
- Properties enhancement in multiwalled carbon nanotube-magnetite hybrid-filled polypropylene natural rubber nanocomposites through functionalization and processing methods
- Synthesis of magnetic photocatalyst and sensitization properties of polypyrrole
- Effect of silane coupling agent on the mechanical properties of nitrile butadiene rubber (NBR)/organophilic montmorillonite (OMMT) nanocomposites
- Monitoring of jute/hemp fiber hybrid laminates by nondestructive testing techniques
- Size effects on the in-plane mechanical behavior of hexagonal honeycombs
- Investigation on corrosion performance of multilayer Ni-P/TiO2 composite coating on steel
- Investigation of pozzolanic activity of volcanic rocks from the northeast of the Black Sea
- The effect of delayed ettringite formation on fine grained aerated concrete mechanical properties
- Effect of early-age freeze-thaw exposure on the mechanical performance of self-compacting repair mortars
- Modeling cement hydration by connecting a nucleation and growth mechanism with a diffusion mechanism. Part I: C3S hydration in dilute suspensions
Articles in the same Issue
- Frontmatter
- Original articles
- In vitro degradation and bioactivity of poly(propylene fumarate)/bioactive glass sintered microsphere scaffolds for bone tissue engineering
- Properties enhancement in multiwalled carbon nanotube-magnetite hybrid-filled polypropylene natural rubber nanocomposites through functionalization and processing methods
- Synthesis of magnetic photocatalyst and sensitization properties of polypyrrole
- Effect of silane coupling agent on the mechanical properties of nitrile butadiene rubber (NBR)/organophilic montmorillonite (OMMT) nanocomposites
- Monitoring of jute/hemp fiber hybrid laminates by nondestructive testing techniques
- Size effects on the in-plane mechanical behavior of hexagonal honeycombs
- Investigation on corrosion performance of multilayer Ni-P/TiO2 composite coating on steel
- Investigation of pozzolanic activity of volcanic rocks from the northeast of the Black Sea
- The effect of delayed ettringite formation on fine grained aerated concrete mechanical properties
- Effect of early-age freeze-thaw exposure on the mechanical performance of self-compacting repair mortars
- Modeling cement hydration by connecting a nucleation and growth mechanism with a diffusion mechanism. Part I: C3S hydration in dilute suspensions

