Abstract
The present review deeply delves the major methods for the synthesis of organotellurium compounds along with their biological significance. Elaboration of various precursors (ligands) including Schiff bases, naphthoquinones, amino acids, β-hydroxy alkylated compounds, β-phenyltelluro alcohols, β-aryltelluro amines, β-aryl-chalcogenium azides, chalcogenobiotin, benzenesulfonamide, carbonic anhydrase, and Sulpha compounds has been briefly described. Furthermore, the article investigates their possible biological uses, specifically as antioxidant and anticancer agents while comparing their effectiveness with their respective ligands and with the standard medications in terms IC50 values. All types of details are haunted to make the information a priceless resource for researchers who wish to probe deeper into and examine the diverse roles that organotellurium complexes play in biological settings.
1 Introduction
The aging process and age-related diseases such as cancer, Parkinson’s disease, and Alzheimer’s are associated with oxidative stress, which is marked by increased levels of reactive oxygen species (ROS) and decreased antioxidant defenses. As a result, the development of novel antioxidants is urgently needed to protect cells from oxidative damage. Although antioxidant mimetics have been developed, their effectiveness in biological systems has been inconsistent, indicating ongoing challenges in this field. 1 Cancer is a formidable disease that threatens world health. 2 , 3 According to the World Health Organization (WHO), Cancer is the second most common cause of death worldwide, taking around 9.6 million lives annually. This is a concerning figure, since it represents nearly one in six fatalities globally. The aging of the population, genetic predispositions, unhealthy lifestyles, and the influence of environmental variables are some of the causes contributing to the growth in cancer incidence. 4 , 5 The absence of disease-modifying medications, limited variety, and unfavorable combinations with current treatments are causing widespread pandemics and serious harm to economies and public health. Therefore, a great deal of research efforts are concentrated on developing new pharmacological compounds from metals in an effort to overcome these constraints and provide useful remedies that have significant biological benefit for humans. 6 , 7 , 8
Metal-coordinated complexes are the focus of increasing biological research because of their unique properties, which enable precise interactions with biomolecules including DNA and proteins for a range of beneficial bio-functions. These substances are helpful indicators of intricate biological processes and may prove to be helpful in the creation of novel therapeutics. 9 , 10 , 11 , 12 , 13 Better biological performance of these complexes than ligands alone is a crucial feature for therapeutic applications. 14 The important advancement of cisplatin’s discovery as an anticancer medication spurred further investigation and development of other metal complexes for a variety of biological applications. 15 , 16 This trend emphasizes how important metal complexes are to the advancement of biological science’s therapeutic approaches. Coordination chemistry with tellurium (Te) ligands has advanced significantly in the last three decades. Although it was originally closely related to selenium chemistry, it has subsequently grown into its own independent field. 13 , 16 , 17 , 18 , 19 Tellurium (Te), a metalloid found in the periodic table alongside sulfur and selenium (Se), has chemical and biological properties that are remarkably comparable to Se. 20 , 21 , 22 While Se was once deemed hazardous, it has since become an essential element, playing important roles in a variety of therapies. 23 Te, on the other hand, is considered a rare, non-essential trace element. 24 Despite this, investigations have shown that the average human body contains Te level upto 0.5 g, surpassing that of all trace elements except iron, zinc, and rubidium. 25 , 26 Selenium is critical in therapy for cancer, HIV/AIDS, and age-related disorders, providing promising treatment options. 27 , 28 , 29 Te’s toxicity varies based on the chemical form and quantity eaten, with symptoms including nausea, somnolence, and garlic odor caused by dimethyl telluride. 30 Additionally, Te can impair cholesterol synthesis and promote peripheral neuropathy, as evidenced by changes in myelin protein transcription and cognitive abnormalities in rats. 31 , 32 , 33 Te also has hemolytic and genotoxic effects on human blood cells and causes cytotoxicity in rat hippocampus astrocytes. 34 Te’s toxicity, on the other hand, has gotten less attention than Se’s, presumably because humans have less regular interaction with Te compounds. 35 Overall, while Te’s toxicity and biological impacts are significant, they are comparatively underexplored in comparison to Se, emphasizing the need for additional research to explain Te’s modes of action and potential health implications. 30 , 36 Te has become increasingly important and useful in coordination chemistry due to its distinct amphoteric characteristics to react with both acid and bases and its capacity to adopt a wide range of oxidation states, 0 (native tellurium), −2 (tellurides), +4 (tellurites), and +6 (tellurates) both fractional and integral. 37 , 38 , 39 Te’s adaptability has been highlighted by this groundbreaking study, which has made it a notable component in the field. 40 , 41 Organotellurium compounds are strong antioxidants that provide defense against damage caused by reactive oxygen species (ROS). They resemble the enzyme glutathione peroxidase (GPx). Their potency in counteracting oxidative stress in the body is increased by their capacity to oxidize from Te(II) to Te(IV). This is the reason for their antioxidant action. 42 , 43 Tellurium complexes for medicines have been the subject of extensive investigation in recent decades. 44 , 45 Both inorganic and organic Te derivatives have antibacterial, anticancer, and antiepileptogenic effects. Promising discoveries indicate that Te could follow selenium’s trajectory from toxicity to essentiality in medicine. 46 , 47 , 48 This comprehensive review examines synthesis methods for producing organotellurium compounds using various types of ligands, focusing on their emerging biological uses. Comparative investigation, including IC50 values, demonstrates their antioxidant and anticancer properties. Valuable insights are provided, assisting researchers in understanding and investigating their several biological activities.
2 Synthesis of Te complexes and anticancer potential
2.1 Schiff base based Te complexes (1–6)
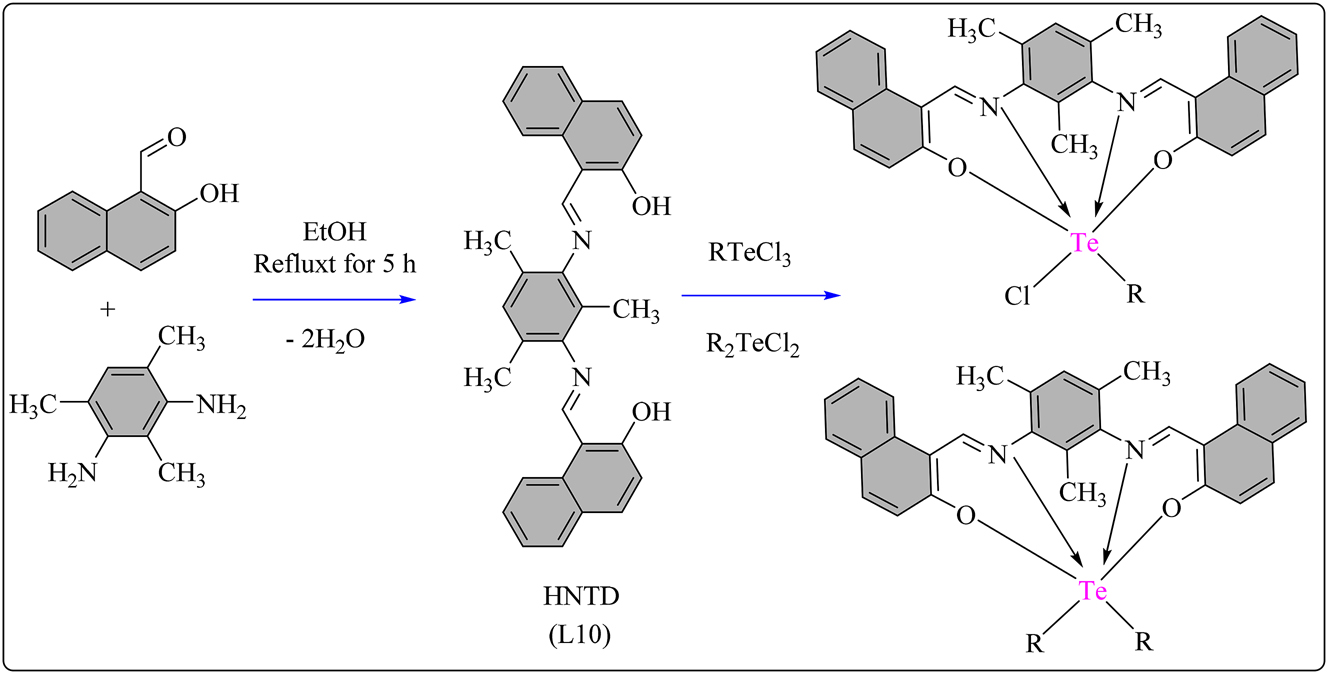
A tetradentate Schiff base ligand, L1 (HNDP), was prepared by refluxing a mixture of 2-Hydroxy-1-naphthaldehyde and 1,3-diaminopropane in absolute ethanol for 5 h at 60–65 °C. The yellow precipitates formed upon condensation were filtered, purified with ethanol, dried, and stored under vacuum (Scheme 1A). Subsequently, various hexa-coordinated Te (IV) complexes (1–6) were synthesized using the HNDP ligand. The method involved refluxing and stirring a 1 mM solution of HNDP with 1 mM of different Te (IV) chlorides (RTeCl3/R2TeCl2) in methanol for 4 h (Scheme 1B). 49 Instant complexation occurred, resulting in the formation of precipitated solids. These solids were filtered, washed with petroleum ether, dried, and then stored under vacuum for further use. The synthesized compounds (1–6) (Figure 1) were evaluated for their cytotoxicity against L929, PC3, and Saos-2 cell lines using the MTT assay (Table 1). The IC50 values were determined, representing the concentration causing 50 % cell death compared to controls. Schiff base HNDP showed no activity against L929 cells but limited activity against PC3 and Saos-2 cells. Most complexes demonstrated enhanced activity compared to HNDP. Complex 1 exhibited the best activity against L929 cells, comparable to doxorubicin. 50 Complex 3 showed promising activity against PC3 cells, 51 and complexes 1, 3, and 5 were most effective against Saos-2 cells, surpassing the performance of doxorubicin used as the standard drug. 52
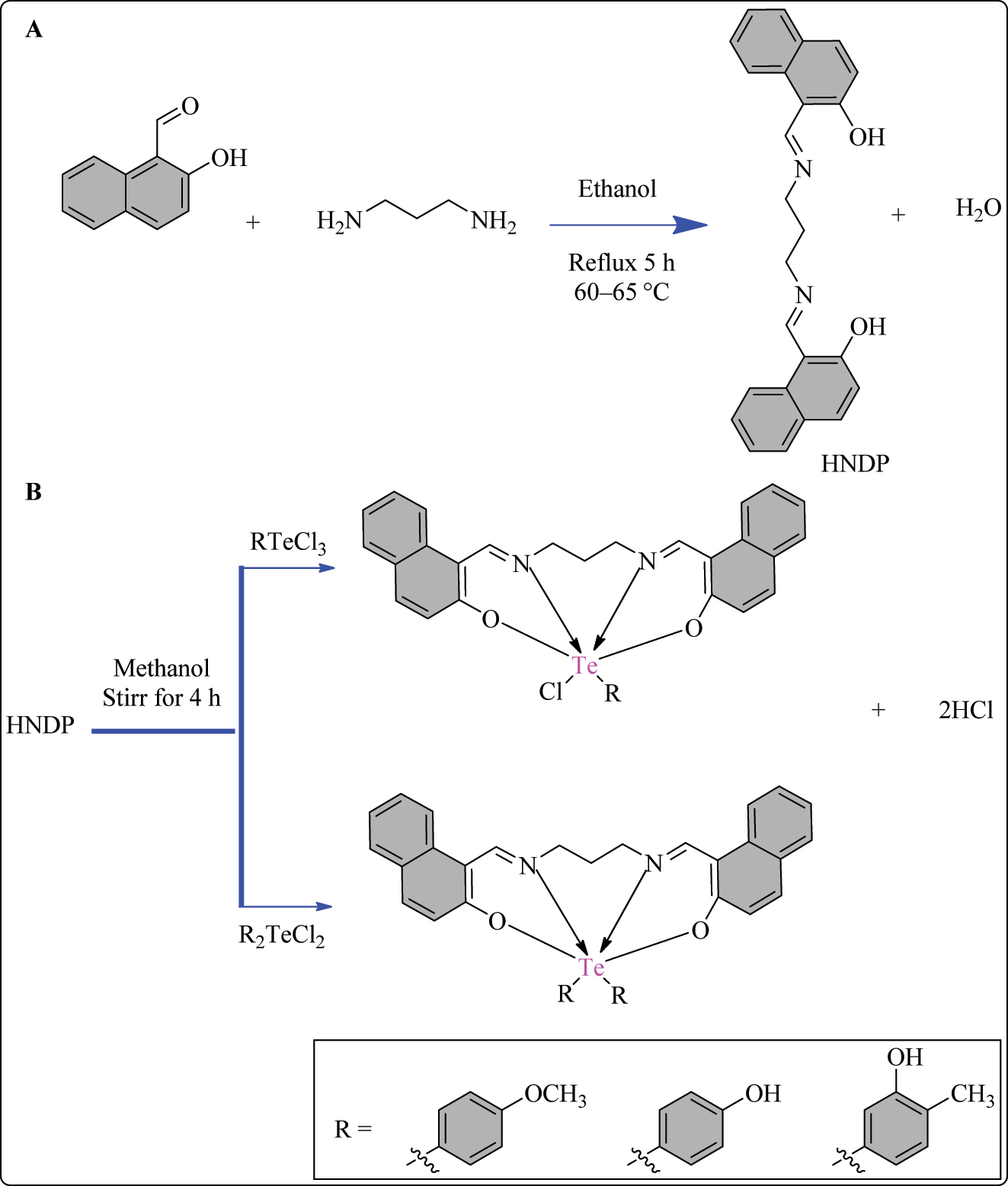
Methodology for the synthesis of A) Schiff base ligand HNDP and B) HNDP based Te complexes.

Structural representation of HNDP based hexa-coordinated Te(IV) complexes.
Visualize the comparison of IC50 values of ligand L1 and its associated complexes.
| Compound | IC50 (μM) | ||
|---|---|---|---|
| L929 | PC3 | Saos-2 | |
| Doxorubicin | 1.08 ± 0.55 | 3.76 ± 1.1 | 37 ± 16 |
| L1 | Nill | 48.26 ± 0.10 | 57.06 ± 0.09 |
| 1 | 3.06 ± 0.02 | 16.19 ± 0.04 | 14.77 ± 0.04 |
| 2 | 6.74 ± 0.01 | 14.86 ± 0.05 | 161.56 ± 0.11 |
| 3 | 8.36 ± 0.04 | 3.14 ± 0.02 | 30.77 ± 0.02 |
| 4 | 10.36 ± 0.08 | 15.01 ± 0.05 | 140.50 ± 0.14 |
| 5 | 7.34 ± 0.07 | 42.48 ± 0.18 | 21.02 ± 0.07 |
| 6 | 9.82 ± 0.03 | 17.58 ± 0.09 | 78.80 ± 0.06 |
2.2 Naphthoquinone based Te complexes (7–9)
The synthetic process was started with conversion of 2-hydroxynaphthalene-1,4-dione, a commercially available starting material, into propargyl alkylated lawsone (L2) (Scheme 2). Rocha et al. reported a 60 % yield utilizing propargyl bromide, potassium carbonate, and dimethylformamide (DMF) as solvents for 24 h at 60 °C. 53 , 54 Additionally, a well-known method that has been documented in the literature might be used to create diorganoyl dichalcogenides (A-C). 55 Using this process, the Grignard reagent was formed by reacting bromobenzene (which may be substituted), which subsequently facilitates the production of the target product. The target molecules were then produced using L2, diphenyl ditelluride (A–C), copper iodide (CuI), and 2.0 mL of DMSO in a traditional process. 56 In order to produce Csp-chalcogen bonds, CuI was used in a chemical reaction. The reaction proceeded effectively, indicating the suitability of the selected materials and circumstances for attaining the desired chemical conversion. 57 Three diorganoyl ditellurides (A–C) were employed in the procedure for evaluating the biological impact of different chalcogens on the target structure as well as the adaptability of the developed technique. In this approach, the compound library was enlarged by synthesizing these chalcogen derivatives. In this case, compounds 7–9 had moderate to good yields, with 9 (chlorine-substituted) having the greatest yield of 60 %. Compound 8 (containing a methyl group) was collected with a yield of 55 %, but the unsubstituted aromatic ring in 7 yielded 49 %, indicating a consistent effect independent of substituents. The cytotoxic activity of the three synthesized samples (7–9) was tested in SCC-9 cells, a type of human tongue cancer cell renowned for its sensitivity to cytotoxic chemicals. 58 , 59 , 60 The normal cell control was made out of NIH3T3 murine fibroblasts. Carboplatin, a conventional chemotherapy agent for oral cancer treatment, and doxorubicin, which is routinely used to treat a variety of cancers, acted as positive controls. 61 , 62 Notably, all drugs showed dose-dependent cytotoxicity in SCC9 cells, outperforming carboplatin in anticancer activity. Their IC50 values are shown in Table 2. Surprisingly, these complexes (7–9) also showed more cytotoxicity in SCC-9 cells than standard drugs as well as derivatives of diorganoyl diselenides or thiophenol. Complexes 7–9 were also tested against carboplatin on the NIH3T3 cell line to establish their selectivity index (S.I.). A high S.I. value (≥ 2) implies selective toxicity against cancer cells, whereas a S.I. < 2 indicates potential general toxicity. 63 Complexes 7–9 showed stronger cytotoxicity in NIH3T3 cells than other diorganoyl diselenide derivatives, with 7 having the highest selectivity index (S.I. = 3.53) of all examined chalcogen-naphthoquinones. All novel compounds demonstrated better selectivity than carboplatin (S.I. = 0.27) and the majority compared to doxorubicin (S.I = 0.61). 64
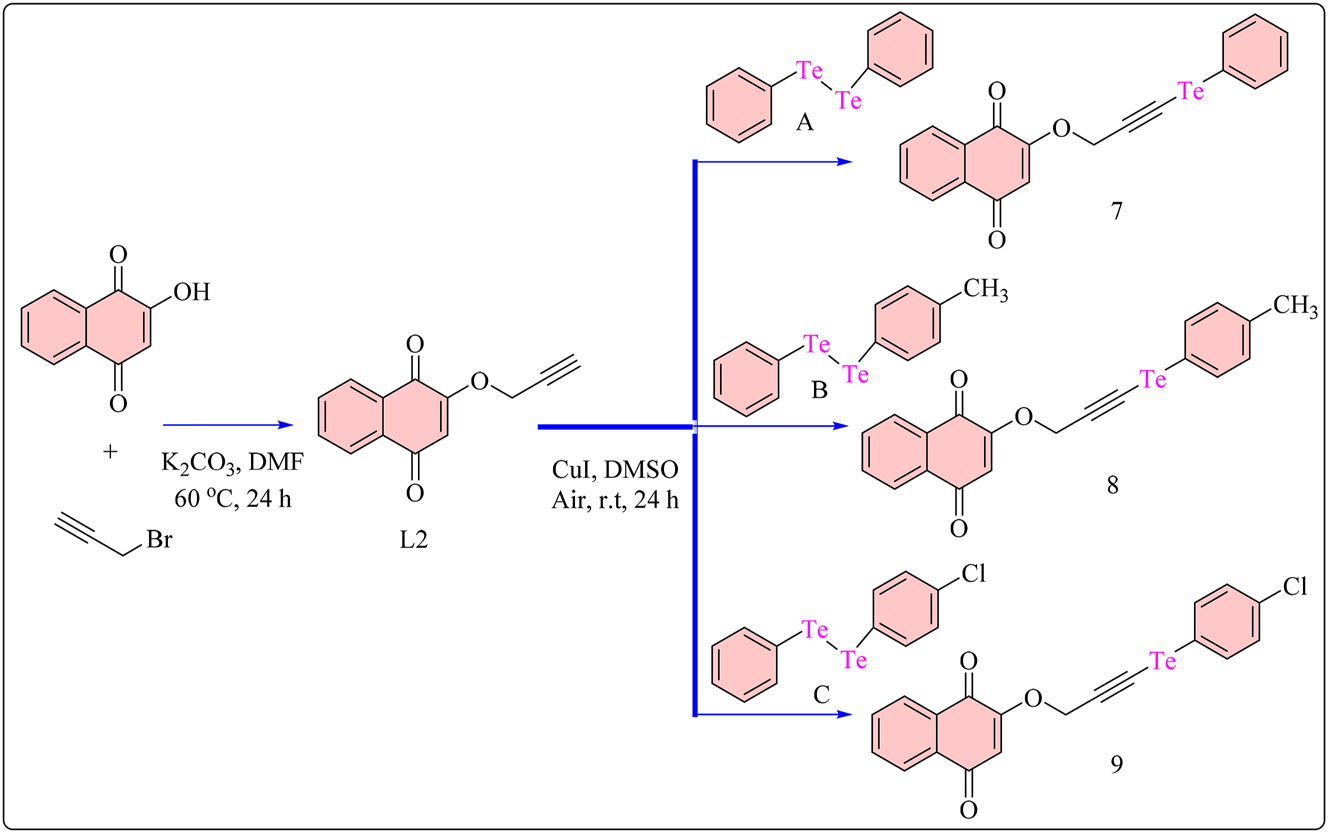
Methodology for the synthesis of naphthoquinone based Te(IV) complexes.
Visualize the comparison of IC50 values and S.I. of L2 complexes with standard drugs.
| Compound | SSC9 | NIH3T3 | |
|---|---|---|---|
| IC50 (μM) | S.I. | ||
| Carboplatin | 571.9 | 155.70 | 0.27 |
| Doxorubicin | 2.705 | 1.66 | 0.61 |
| 7 | 1.648 | 5.83 | 3.53 |
| 8 | 4.001 | 2.27 | 0.57 |
| 9 | 2.885 | 4.90 | 1.70 |
2.3 Amino acid-based Te complexes (10–12)
The synthesis of complex 10 followed conventional protocols, 47 beginning with l-Tellurocystine in a round bottom flask filled with degassed distilled water and N2. After 15 min of stirring with H2O2 and concentrated HCl at 0 °C, the solution became transparent (Scheme 3). The colorless solid complex 10, a zwitterionic Te(IV), was obtained after 16 h of freeze-drying. To avoid breakdown, storage at temperatures below 10 °C was recommended. Complex 10, showed substantial dose-dependent anticancer activity against MCF-7 cells. The maximum efficacy was reported at doses ranging from 2.5 to 20 μg/mL, leading to a considerable reduction in cell viability. Although cell viability decreased less at higher dosages, anticancer activity was consistent throughout the analysis, with good to moderate efficacy observed up to 60 μg/mL. Complex 10 demonstrated effective anticancer action against MFC-7 breast cancer cells, with an IC50 value of 2.86 ± 0.02 μg/mL. 65 The mechanism of action could be attributed to its similarity to AS101, redox-modulating properties, and interaction with cysteine thiol residues, which could inhibit integrin activity and IL-10 secretion, sensitizing tumors to chemotherapeutic treatments and specifically targeting cancerous cells. 30 , 47
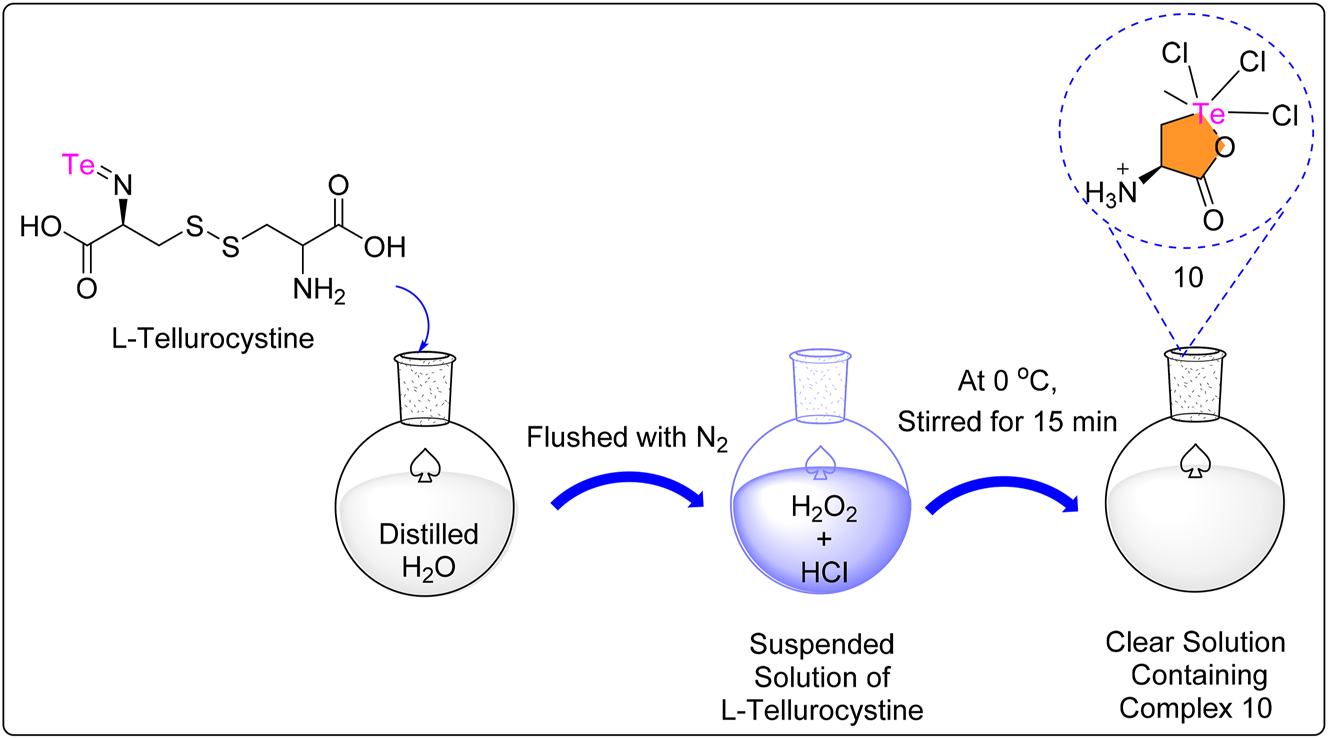
Methodology for the synthesis of zwitterionic Te complex. 10
Complex 11 was produced through the addition of tellurocysteine 66 to a mixture of degassed EtOH and water in a round-bottom flask under a N2 environment at temperatures below 10 °C (Scheme 4A). Sodium borohydride was progressively introduced, yielding a clear solution. Diiodomethane was then gradually added, resulting in the production of a white precipitate. The precipitate was filtered, washed, and dried before being stored. Complex 12 was synthesized from l-methionine using a technique described in the literature for selenohomocystine. 67 First, l-methionine was converted into homoserine using a two-step procedure using iodomethane in methanol and hydrobromic acid (Scheme 4B). Te powder was then suspended in ethanol under N2 and reduced by NaBH4 to get Na2Te2. The solution was then heated for 24 h at 80 °C with 2-amino-4-bromobutanoic acid hydrobromide. Following evaporation and HCl treatment, the resultant mixture was washed with diethyl ether and dried, producing tellurohomocystine 12. Compound 1l and 12 were tested for cytotoxicity on MCF-7 cells at doses of up to 125 μg/mL. Both compounds showed a concentration-dependent decrease in cell viability, which was identical to the trend seen with the positive control, doxorubicine. Notably, amino acid compound11 was more toxic to MCF-7 cells than telluro compound 12 at various doses. The IC50 value for complex 11 and 12 was 7.29 ± 0.27 μg/mL and 25.36 ± 0.12 μg/mL, respectively. 68
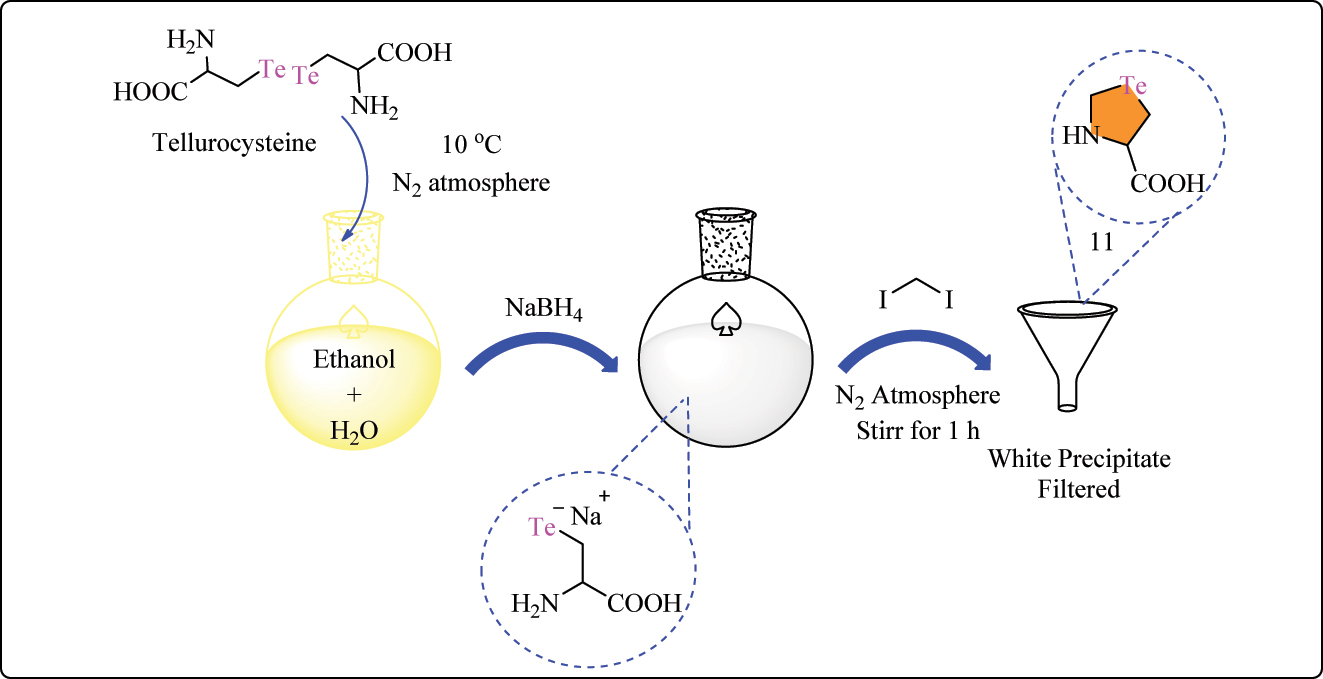
Methodology for the synthesis of telluro amino acid complex 11.
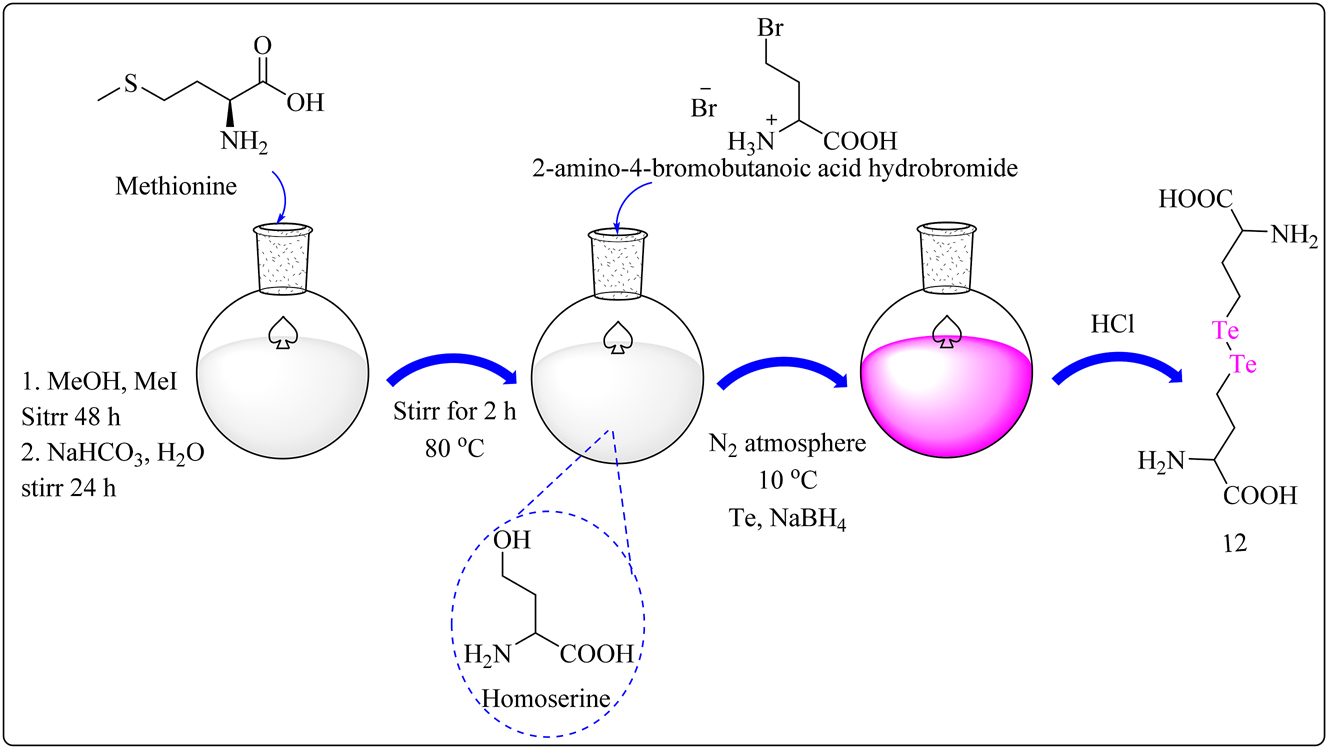
Methodology for the synthesis of ditelluride amino acid complex 12.
2.4 β-hydroxy alkylated Te complexes (13–20)
Li2Te was produced by reacting LiEt3BH with elemental tellurium powder in THF solution, as described in the literature. 69 The resultant Li2Te suspension in THF was treated with an epoxide and stirred at room temperature for 12 h (Scheme 5A). The mixture was then diluted with Et2O, filtered, and washed with saturated NH4Cl and H2O. The organic phase was dried over Na2SO4, filtered, and evaporated at low pressure. The crude product was purified by flash column chromatography, resulting in β-hydroxy tellurides 13–20. Additionally, ditellurides 13 and 14 were produced utilizing a similar technique that took advantage of Li2Te2’s reactivity. 70 , 71 , 72
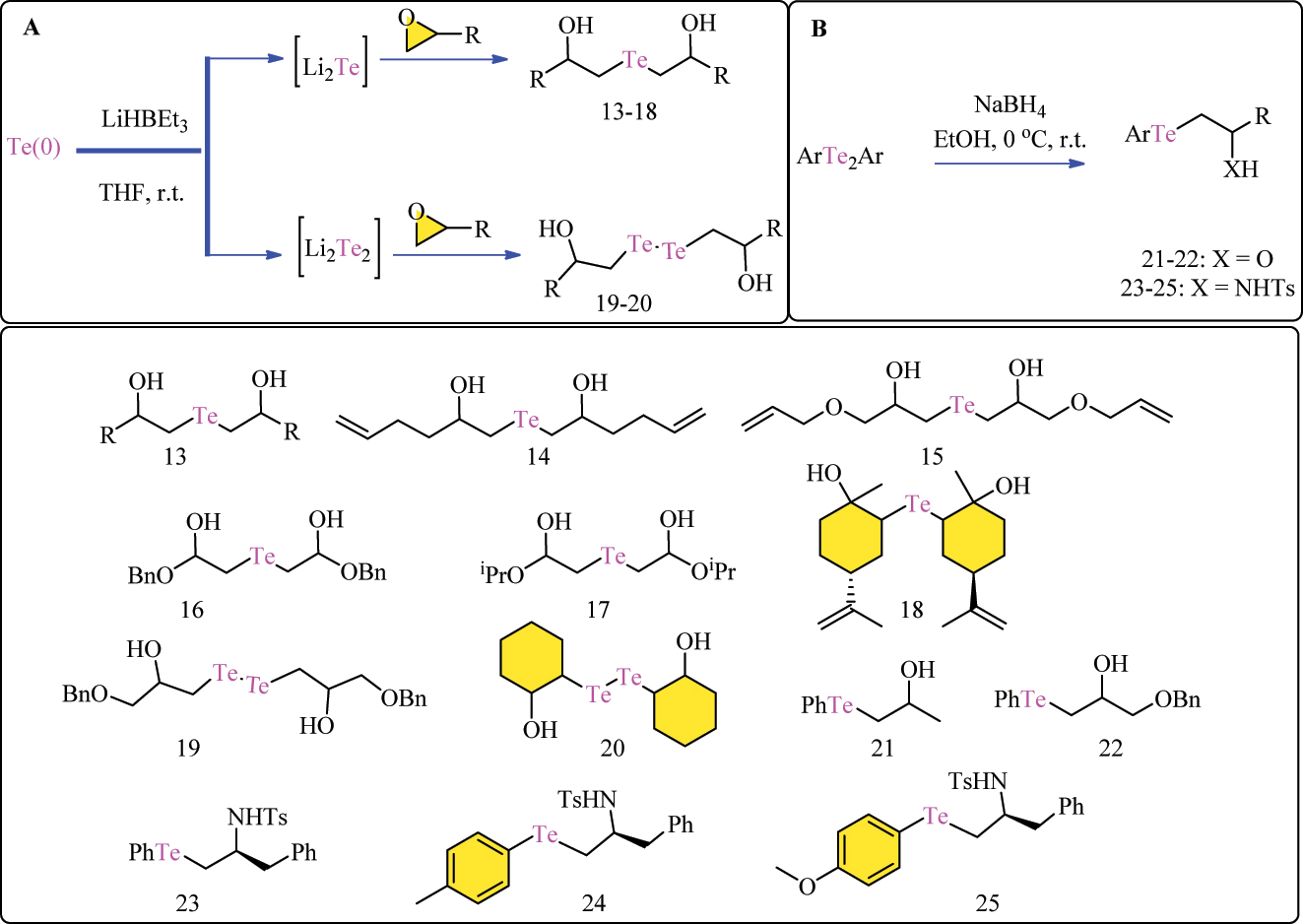
Methodology for the synthesis of (A) β-hydroxy alkylated Te complexes (13–20) and (B) telluride based β-phenyltelluro alcohols 21–22 and β-aryltelluro amines 23–25 complexes.
2.5 β-phenyltelluro alcohols (21–22) and β-aryltelluro amines (23–25) Te complexes
Unsymmetrical aryl-alkyl tellurides 23–25 with hydroxy and amino functionalities were produced by ring-opening reactions between epoxides or aziridines and aryl tellurolates. 73 , 74 These tellurolates were produced in situ by reducing diaryl ditellurides with NaBH4 in EtOH at 0 °C in an inert environment (Scheme 5B). The following addition of epoxides or aziridines started the reaction, which continued until completion, as measured by TLC. The intended β-phenyltelluro-alcohols 21–22 or β-aryltelluro-amines 23–25 were obtained after quenching with saturated aqueous NH4Cl, extraction, and purification with flash chromatography. 75
Following the DTT oxidation test results, researchers investigated the cell-protective properties of their manufactured compounds. Prior to undertaking these tests, they assessed the cytotoxicity of the organochalcogen derivatives, taking into account the shortage and inconsistent nature of the available data. The majority of compounds showed negligible cytotoxicity, with certain tellurated derivatives exhibiting no deleterious effects on cell viability at dosages up to 100 μM. However, complexes 23–25 were insoluble during testing. Notably, among the examined series, GPx-like tellurated complexes 13 and 15 had the lowest toxicity. As Table 3 shows the IC50 values of synthesized complexes (13–25), complex 21 and 22 proved to be the most efficient of all the telluride complexes. 71
Illustrate the IC50 values of complexes 13–25.
| Compound | IC50 (μM) |
|---|---|
| 13 | >100 |
| 14 | 100 * |
| 15 | > 100 |
| 16 | 20.7 |
| 17 | 80.7 |
| 18 | 22.9 |
| 19 | 31.9 |
| 20 | 49.3 |
| 21 | 8.5 |
| 22 | 19.8 |
| 23 | ND |
| 24 | ND |
| 25 | ND |
2.6 β-aryl-chalcogenium azides based Te complexes (26–27)
Complex 26 and 27 β-aryl-chalcogenium azides with tellurium (Figure 2), were synthesized using recognized techniques, as previously published. 76 Phenylalaninol, valinol, and leucinol, which are commercially available α-amino alcohols, were the starting materials for the chiral β-chalcogenium amine compounds 26–27. Using di-tert-butyl dicarbonate, they were transformed into N-Boc-protected amino alcohols. Alcohol mesylates were produced through further mesylation in THF and Et3N using mesyl chloride. Then, utilizing aryl dichalcogenides and NaBH4 in THF-ethanol, the β-aminomesylates were transformed with organochalcogenium moieties via organochalcogenolate synthesis. 77 In the initial study, researchers evaluated the potential anticancer effects of complexes 26 and 27 on bladder cancerous cells in vitro. The MTT assay demonstrated a dose-dependent reduction in the survival of the human bladder cancer cell line (5,637) after 48 h of incubation with both drugs. This suggests a dose-dependent link between drug concentration and cellular response, since higher doses lead to a greater loss in cell survival. It also showed that the action of the drugs on the cancer cells is directly correlated with the concentration of given dosage. Surprisingly, at concentrations as low as 1 μM, these organochalcogen compounds displayed an average suppression of more than 50 % of cancer cell viability. After 48 h of exposure, the IC50 values for 26 and 27 were 1.57 ± 0.70 μM and 0.48 ± 0.13 μM, respectively. Compound 27 showed slightly higher cytotoxicity, inhibiting 50 % of cellular viability at lower doses. The IC50 concentration values of 1.6 μM for complex 26 and 0.5 μM for complex 27 were chosen for further investigations. 78
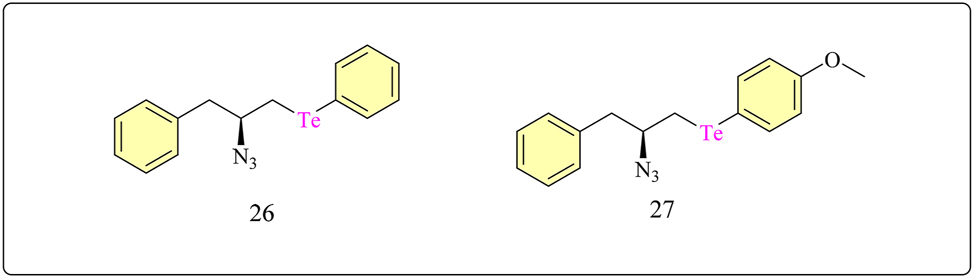
Structural representation of Te complex 26 and 27.
2.7 Chalcogenobiotin Te complexes (28–29)
Ligands L3 and L4 were synthesized, by mixing 1,4-dibromobutane and 1,5-dibromopentane separately with biotin in a two-necked rounded bottom flask under an argon environment. In order to preserve the integrity of reactive chemicals and ensure the success of complex reactions, an argon atmosphere is necessary during the organic synthesis process. Researchers may more effectively control the reaction conditions and produce higher yields of the intended products with fewer side reactions or contaminants by keeping oxygen and moisture out of the reaction 79 Se. The flask had a reflux condenser and magnetic stirring. 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) was dissolved in acetonitrile, and the reaction mixture was allowed to reflux for 3 h with stirring (Scheme 6). The solvent was then evaporated and obtained solid material was purified using silica gel column chromatography, resulting in the desired product. A rotary evaporator was used to evaporate the solvent, yielding a vacuum-dried product. The synthesized ligands L3 and L4 were used in the development of biotin-based Te(IV) complexes, 28 and 29. 80 Chalcogenolate generation was started by combining diorganoyl ditelluride, NaBH4, and EtOH in an argon environment. Biotinyl bromoalkyl ester L3 and L4 were added to THF and stirred for 6 h. The mixture was then washed with NH4Cl and ethyl acetate, before being dried, filtered, and evaporated to remove the organic phase. The complexes 28 and 29 were purified using silica gel chromatography. Tumor cells, due to their increased metabolic activity, have a higher demand for important vitamins like biotin, which is required for cellular function. Overexpression of biotin receptors in malignancies promotes vitamin intake, which contributes to cell growth and function. 81 , 82 Cytotoxicity of biotin, 28 and 29 complexes were evaluated on 5,637 cell lines at various concentrations. Biotin and its Selenium complexes exhibited no cytotoxicity against tumor cells. 80 , 83 However, tellurium complexes 28 and 29 displayed potent antiproliferative activity against 5,637 cells at lower concentrations. Like biotin, heptyl(phenyl)tellane had minimal cytotoxicity, which decreased over time, emphasizing the necessity of phenyl tellurium-biotin conjugation for improved antitumoral activity. Tellurium biotin derivatives 28 and 29 significantly reduced 5,637 cell viability in a time-dose dependent manner, but selenium derivatives did not cause cytotoxicity. The IC50 values for complex 28 and 29 shown in Table 4, that were measured using the MTT reduction assay, showed that they had cytotoxic effects on 5,637 cells at low concentrations, indicating that chalcogenobiotin derivatives are attractive anticancer possibilities for bladder cancer. 80
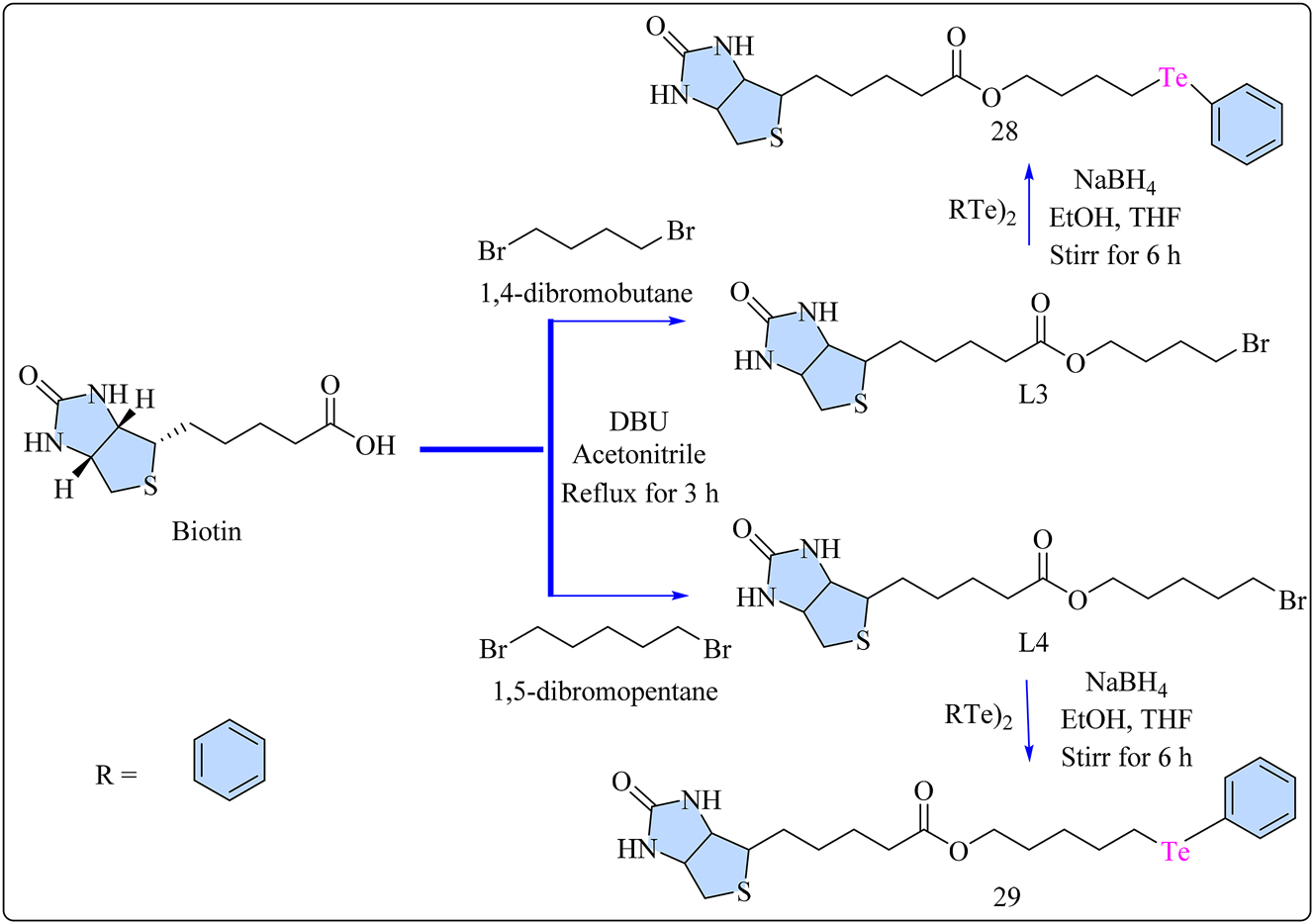
Methodology for the synthesis of biotin ligand L3 and L4 and their Te(IV) complexes 28 as well as 29.
The obtained IC50 values for the Te complex 28 and 29.
| Compound | IC50 24 h | IC50 48 h |
|---|---|---|
| L3-L4 | – | – |
| 28 | 5.8 ± 3.11 | 4.73 ± 2.13 |
| 29 | 7.63 ± 3.17 | 6.71 ± 1.87 |
| Heptyl(phenyl)tellane | – | – |
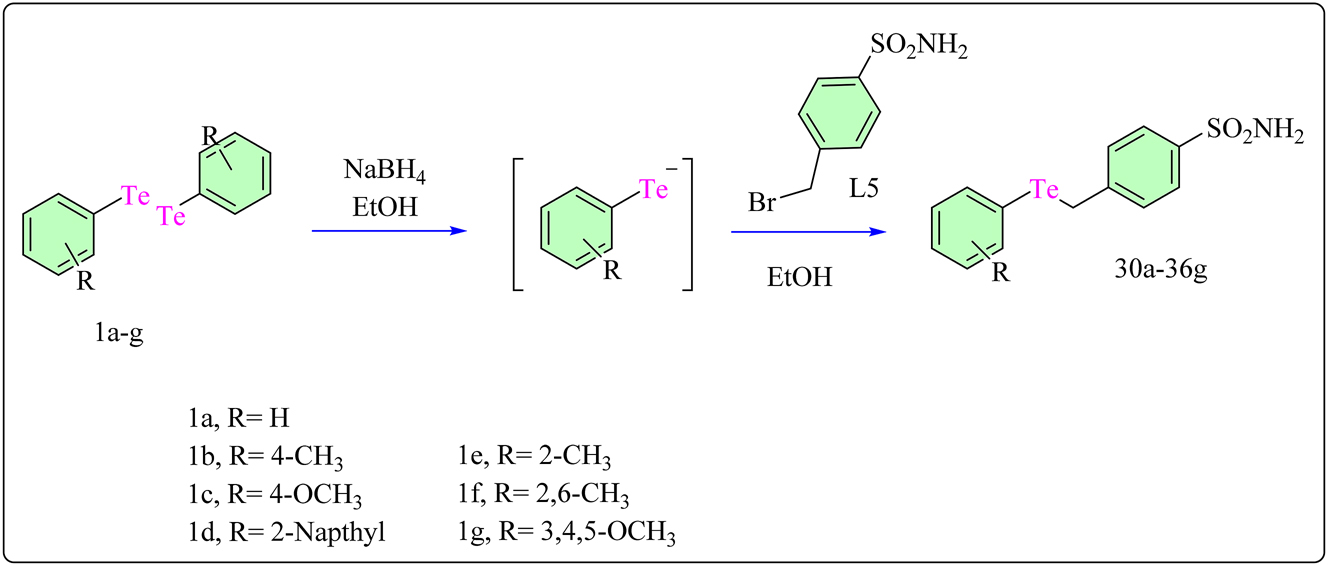
2.8 Benzenesulfonamide based Te complexes (30a–36g)
Diphenylditellurides 1a–g were reduced with NaBH4 before being treated with 4-(bromomethyl)benzenesulfonamide (L5) to get complexes 30a–36g. This two-step procedure produces telluride complexes 30a–36g in high yield. The reduction step with NaBH4 promotes the synthesis of telluride intermediates, which combine with 4-(bromomethyl)benzenesulfonamide to yield the desired molecules (Scheme 7). This synthetic approach has been previously reported and shows efficiency in synthesizing the target chemicals. 84 , 85
Methodology for the synthesis of benzenesulfonamide based Te complexes.
Several tellurides containing sulfonamide (30a–36g) and acetazolamide (AAZ) were tested for inhibitory activity against several human carbonic anhydrase (CA) isoforms (I, II, IV, VII, and IX) using a stopped-flow carbon dioxide hydration assay. 86 Telluride complexes 30a–36g with benzensulfonamide had variable inhibitory efficacy among CA isoforms, which was modulated by aromatic ring alterations. Compounds containing para and ortho substituents (31b, 32c, 34e, and 35f) showed higher inhibition than 30a. CA IX, one of the 15 human isoforms, is significantly overexpressed in several hypoxic malignancies, making it a recognized pharmacological target. 87 hCA IX is typically found in tumor cells and has restricted diffusion in cells that are normal. Its presence makes a major contribution to exogenous acidification along with cytosolic alkalization. 88 Complex 30a and 36g, which contain sulfonamide, were tested against MDA-MB-231 cells because they increased hCA IX expression in hypoxic circumstances. 89 At 1 μM, 30a demonstrated significant activity, killing over 90 % of cells in normoxia, while 36g had slightly lower efficacy with 18 % viability. At 10 μM, both drugs reduced viability to less than 5 %. In hypoxia, 30a had a viability of 16 % at 1 μM and 13 % at 10 μM, while 36g had high activity, killing 65 % and over 85 % of cells. Despite a decrease in efficacy in hypoxia, both drugs maintained significant potency. Their redox characteristics are thought to contribute to this action by changing the amounts of oxidative agents. Notably, their success in normoxia shows a larger therapeutic potential than hCA IX inhibition, warranting further investigation against various tumor types. 85
2.9 Carbonic anhydrase based Te complexes (37–44)
To target the tumor-associated hCA IX and hCA XII isoforms, a main sulfonamide moiety was combined with an organotellurium tail to control selectivity against these carbonic anhydrase (CAs). 90 , 91 , 92 Recent study on chalcogen CA inhibitors has led to the development of organotellurides for this particular purpose. 75 A simple, modular, and fast two-step synthesis produced a variety of functionalized tellurides with a benzensulfonamide moiety. Ligands L6-L8 were synthesized, by dissolving amino sulfonamide 2a–c and K2CO3 in acetone at 0 °C, which was then added to 4-(chloromethyl)benzoyl chloride (Scheme 8). After 1 h of stirring, the solvent was withdrawn and water was added to precipitate the result, which was then filtered and washed with diethyl ether. Complexes 37–44 were synthesized by treating ditelluride 2d–j in ethanol/THF with NaBH4 until reduced. The mixture was then agitated for 2 h with a THF solution containing the appropriate amide derivative L6-L8. The reaction was then saturated with NH4Cl, extracted with ethyl acetate, and rinsed with water and brine. The organic phase was dried with Na2SO4, while the solvent was evaporated under vacuum. The crude product was purified using flash column chromatography. 93
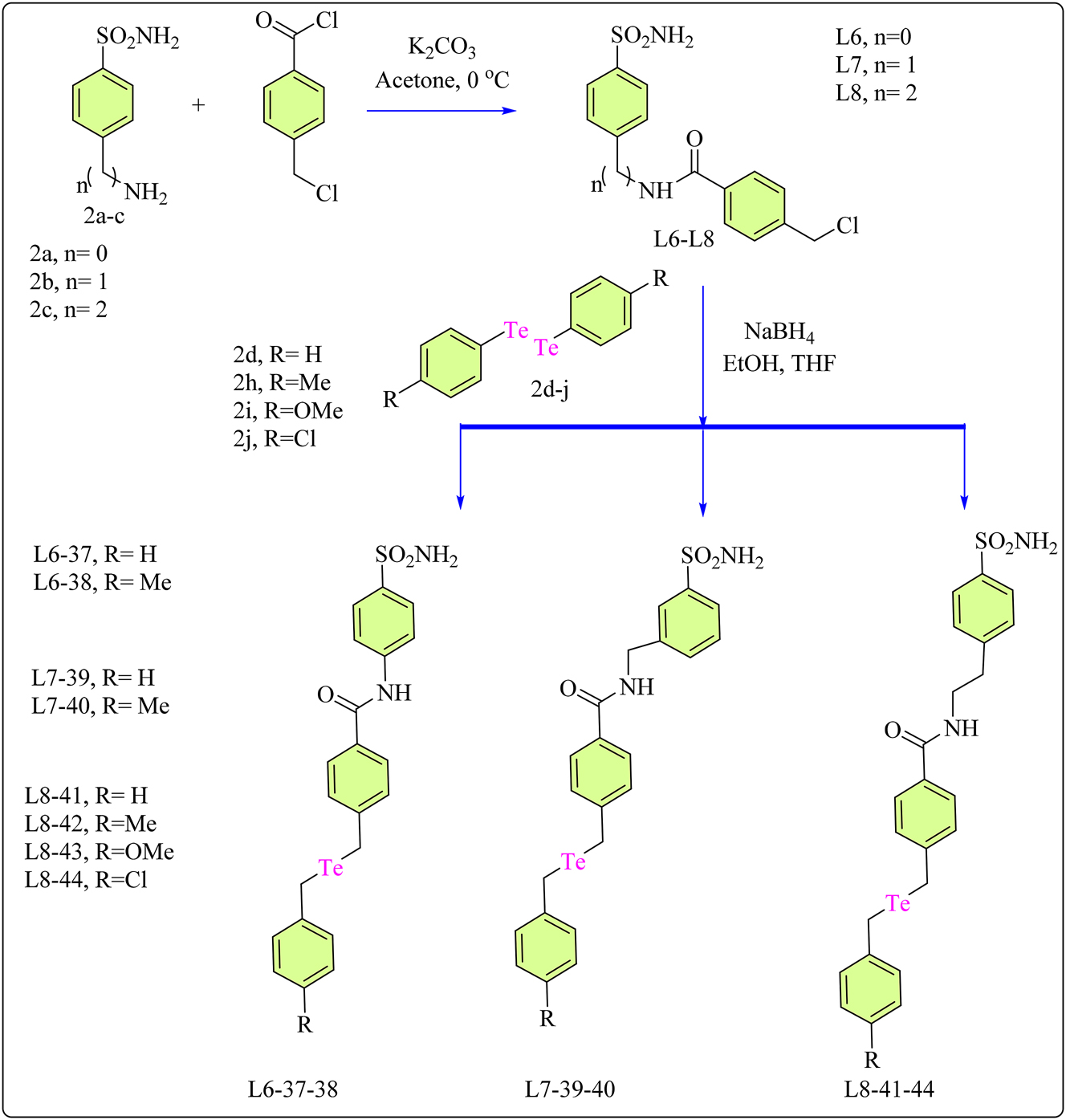
Methodology for the synthesis of CA based ligands (L6-L8) and their Telluride complexes.
The synthesized complexes were tested for cytotoxicity, compound 38 proved cytotoxic to NHDF and cancer cell lines LN-229, SaOS-2, and HepG2 at a concentration of roughly 100 μM (Table 5). It was likewise cytotoxic to MeWo cells at lesser dosages. Compound 42 caused cytotoxicity in NHDF, LN-229, and HepG2 cells at 100 μM, whereas MeWo and SaOS-2 cells were cytotoxic at 70 μM and 30 μM, respectively. Compound 43 exhibited cytotoxicity against NHDF cells at 30 μM and had significant effects on cancer cells at lower doses. These findings were verified by the IC50 values for each cancer cell line. The selective index (SI) is an important statistic for determining chemical activity against cancer, with larger values suggesting stronger selectivity. Complexes 38 and 42 did not meet the SI threshold (≥10) for the cancer cell lines examined. 94 Similarly, compound 43 does not meet this threshold in LN-229 and SaOS-2 cells. Nonetheless, compound 43 has promisingly high SIs for HepG2 (SI = 26.5) and MeWo (SI = 87.8) cells, indicating prospective applicability for targeted therapy in hepatocellular carcinoma and melanoma, respectively. 93
IC50 values obtained for CA based complexes against different tumor cell lines.
| Compound | NHDF | MeWo | LN-229 | SaOS-2 | HepG2 | |
|---|---|---|---|---|---|---|
| 38 | IC50 (μM) | 300.9 | 81.76 | 357.7 | 305.4 | 544.9 |
| SI | – | 3.7 | 0.8 | 1.0 | 0.6 | |
| 42 | IC50 (μM) | 409.3 | 97.75 | 184.7 | 85.49 | 321.2 |
| SI | – | 4.2 | 2.2 | 4.8 | 1.3 | |
| 43 | IC50 (μM) | 62.5 | 0.7121 | 12.11 | 7.834 | 2.356 |
| SI | – | 87.8 | 5.2 | 8.0 | 26.5 |
2.10 Sulpha based Te complexes (45–48)
Complex 3a was prepared by heating mercuric acetate and 4-aminobenzenesulfonic acid in ethanol for 14 h, then adding NaCl to boiling methanol and filtering, washing, and drying (Scheme 9). This complex was subsequently utilized to create Te complex 45 by refluxing tellurium tetrabromide with complex 3a in 1,4-dioxane under an argon environment, followed by recrystallization from dichloromethane and methanol to obtain brown crystals. 95 Complex 3a was also used to create Hg complex 3b, which serves as a precursor for Te complex 46. Complex 3b was synthesized by refluxing a mixture of 3,4-dihydroxybenzaldehyde and complex 3a in ethanol with the addition of sulfuric acid, providing a yellowish solid after filtration, washing, and recrystallization. Similarly, complex 3c was created by combining 4,4′-sulfonyldianiline and mercuric acetate with NaCl, using the same steps as complex 3a. Complex 3c was then used to synthesize Te complex 47 and Hg complex 3d, following procedures similar to those employed for complexes 45 and 3b. Finally, complex 3d was used to generate Te complex 48. In vitro, several complexes were tested for their anticarcinogenic properties against T24 bladder cells and PC-3 human prostate cells. Complexes 45, 46, 47, and 48 showed significant anticarcinogenic efficacy. Complex 46 and 47 performed particularly well towards both types of tumor cells, outperforming complex 46 and 48, as seen in Table 6. This observed activity is most likely attributable to the presence of chemically active elements such as sulfur, oxygen, nitrogen, and tellurium. 96 , 97 , 98 Tellurium, in specifically, has shown potential in blocking enzymes involved in tumor proliferation. 46 , 99 Notably, complex 46 had a lower IC50 value than complex 47 against cancer cells, indicating greater efficacy. Table 6 shows that complex 46 has a higher activity than complex 47. This difference could be ascribed to the presence of both phenolic and azomethine groups, as well as intermolecular chalcogen bonding aided by tellurium atoms. These interactions allow the chemicals to effectively engage cancer cell proteins, resulting in powerful inhibitory effects. 95
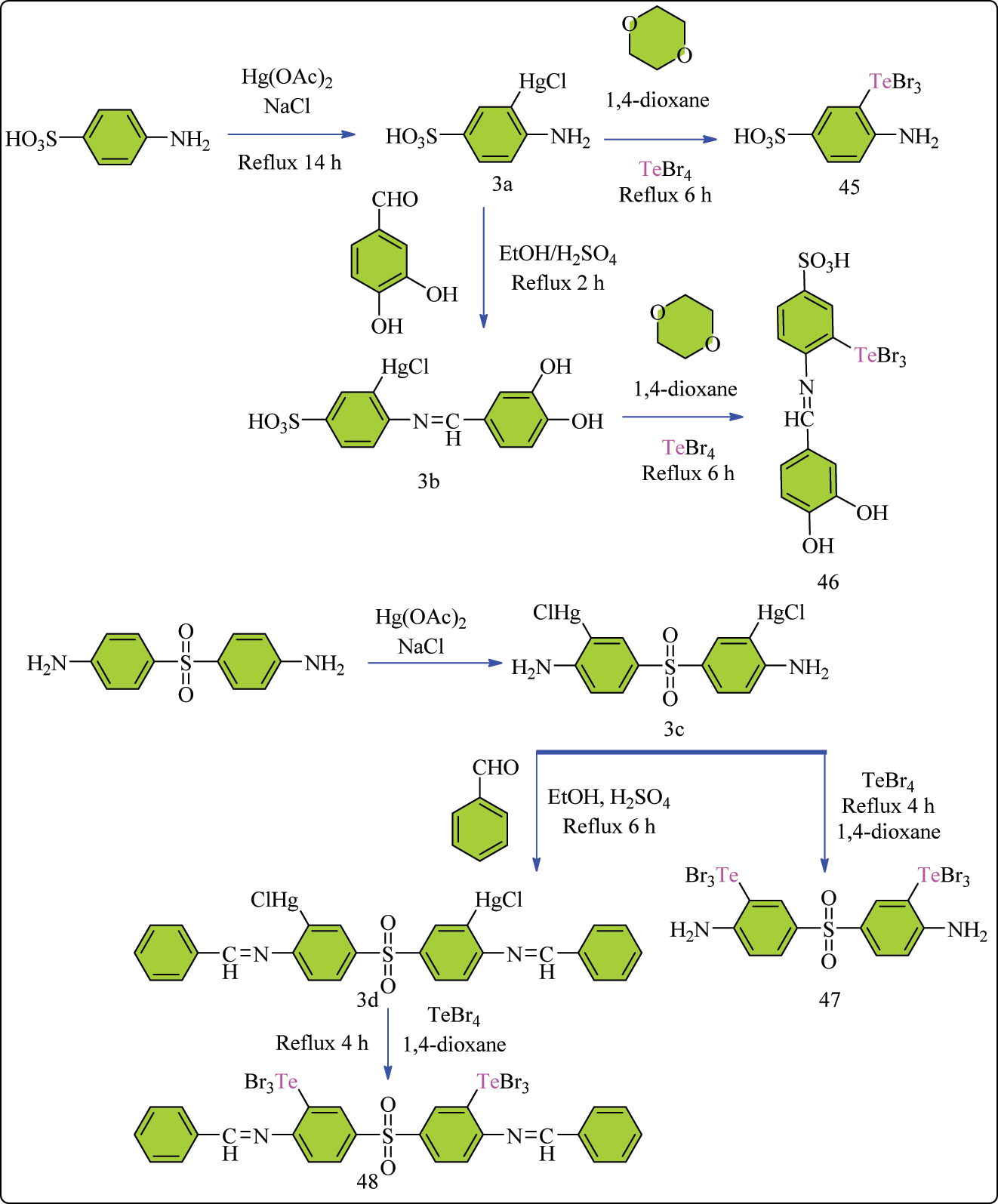
Representation of reaction pathways for the synthesis of Sulpha based Te complexes.
Obtained IC50 values for Sulpha based Te complex 46 and 47, showed their anticancer potential.
| Compound | IC50 (μM) | |
|---|---|---|
| PC-3 | T24 | |
| 46 | 22.26 ± 3.21 | 24.12 ± 4.44 |
| 47 | 27.53 ± 4.72 | 48.82 ± 4.84 |
3 Synthesis of Te complexes and antioxidant activity
3.1 Schiff base Te(IV) complexes
The synthesis of the HNED (L9) Schiff base ligand involved slowly mixing 2-Hydroxy-1-naphthaldehyde with ethylenediamine in warm ethanol under reflux for 5 h (Scheme 10). The resulting crude precipitate was purified through petroleum ether and CH3CH2OH, then dried. Complexes were prepared by mixing equivalent amounts of L9 (1 mmol) with various aryl Te(IV) chlorides (RTeCl3/R2TeCl2) dissolved in warm CH3OH, followed by continuous stirring for 4 h. The obtained complexes, acquired through solvent evaporation, underwent further purification, recrystallization using a mixture of petroleum ether and CH3OH, and drying. Both the ligand and complexes were then stored in a desiccator over CaCl2. L9 Schiff base coordinates to tellurium in octahedral geometry (Figure 3). The synthesized compounds were evaluated for antioxidant capabilities using the DPPH scavenging method, with ascorbic acid as standard. The results, reported in Table 7, show the IC50 values. The L9 ligand exhibited low scavenging ability (IC50 = 227.05 ± 0.63 μg/mL). However, coordination with tellurium ion significantly enhanced scavenging ability in complexes 49–54 (IC50 = 81.90 ± 0.31 to 191.84 ± 0.50 μg/mL). Complex 51 and 54 showed the highest scavenging ability (IC50 = 91.48 ± 0.38 and 81.90 ± 0.31 μg/mL), in comparison to ascorbic acid. 100
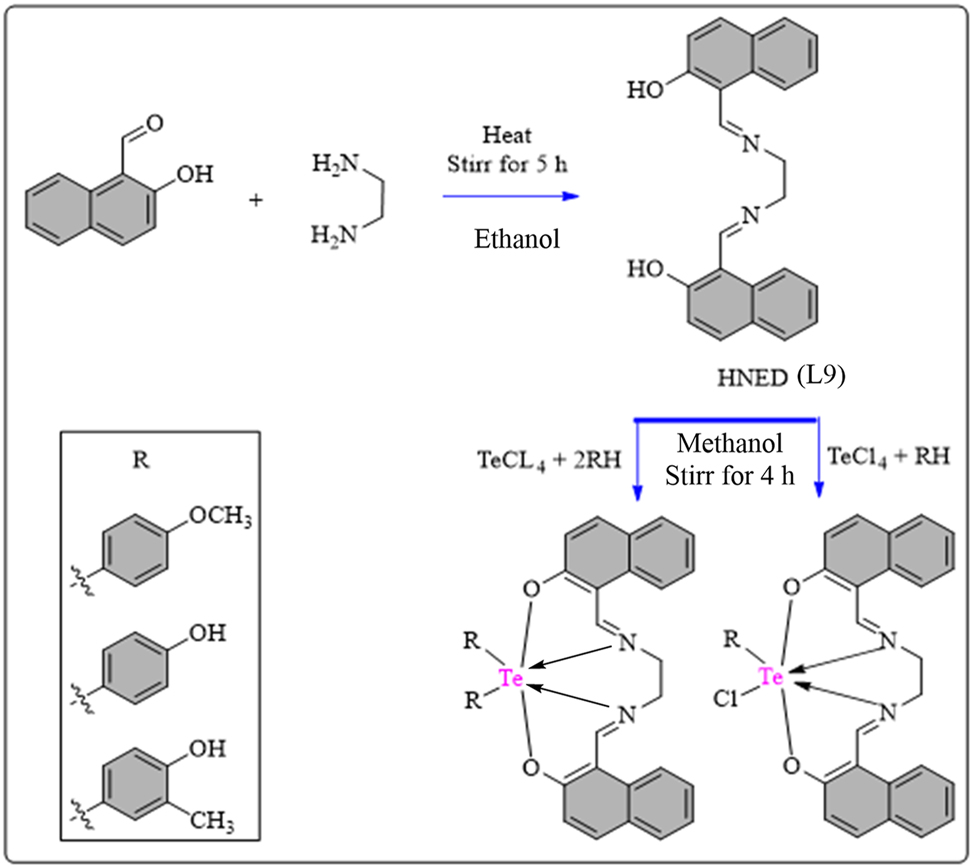
Methodology for the synthesis of HNED Schiff base ligand and Te complexes.

Structural representation of L9 based hexa-coordinated Te(IV) complexes.
Analysis of minimal inhibitory concentrations of ligand L9, L10 and corresponding Te complexes for their antioxidant potential.
| Compound | % Scavenging activity at various concentration (in µg/mL) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | ||
| L9 | 7.23 | 13.54 | 15.56 | 19.00 | 24.81 | 227.05 ± 0.63 |
| 49 | 22.56 | 31.04 | 35.56 | 40.02 | 51.00 | 102.38 ± 0.40 |
| 50 | 5.01 | 14.03 | 18.99 | 24.98 | 28.01 | 171.55 ± 0.30 |
| 51 | 30.00 | 38.41 | 42.98 | 47.00 | 51.30 | 91.48 ± 0.38 |
| 52 | 26.20 | 34.22 | 39.45 | 43.32 | 48.13 | 104.38 ± 0.38 |
| 53 | 9.00 | 14.05 | 19.31 | 25.07 | 27.09 | 191.84 ± 0.50 |
| 54 | 29.99 | 39.10 | 44.50 | 50.10 | 54.00 | 81.90 ± 0.31 |
| Ascorbic acid | 37.01 | 42.06 | 48.03 | 51.33 | 55.66 | 73.71 ± 0.37 |
| L10 | 10.49 | 12.01 | 14.04 | 17.20 | 19.07 | 379.46 ± 0.91 |
| 55 | 22.35 | 31.40 | 36.08 | 40.89 | 51.02 | 100.96 ± 0.32 |
| 56 | 5.42 | 12.88 | 17.29 | 25.12 | 37.06 | 141.02 ± 0.35 |
| 57 | 5.03 | 10.75 | 17.72 | 22.03 | 34.14 | 152.21 ± 0.38 |
| 58 | 23.14 | 31.53 | 38.03 | 44.04 | 51.14 | 96.32 ± 0.40 |
| 59 | 12.74 | 15.76 | 17.48 | 22.46 | 26.64 | 239.66 ± 0.91 |
| 60 | 9.48 | 10.38 | 15.11 | 18.22 | 21.24 | 283.90 ± 0.69 |
| Ascorbic acid | 37.01 | 42.06 | 48.03 | 51.33 | 55.66 | 418.52 ± 0.02 |
The HNTD (L10) Schiff base ligand was developed through a condensation reaction beyween 2-Hydroxy-1-naphthaldehyde and 2,4,6-trimethyl-1,3-phenylenediamine dissolved in ethanol (Scheme 11). After refluxing the colored mixture at 60 °C for a duration of 5 h, yellow solid precipitates were produced and recrystallized using an ethanol and petroleum ether solution. The resultant L10 ligand was dried under CaCl2 and then used to synthesize Te(IV) complexes via reactions with different organotellurium chlorides (Figure 4). The synthesized complexes 55 , 56 , 57 , 58 , 59 , 60 showed higher reactivity against free radicals, with IC50 values ranging from 96.32 ± 0.40 to 283.90 ± 0.69 μg/mL, compared to L10’s IC50 of 379.46 ± 0.91 μg/mL (Table 7). This improvement shows a significant increase in radical quenching capabilities by chelation with tellurium ion. Complexes 55 and 58 showed IC50 values (100.96 ± 0.32 and 96.32 ± 0.40 μg/mL) similar to ascorbic acid, indicating high antioxidant activity. 101 These findings emphasize the potential utility of the produced complexes as efficient antioxidants, underlining the relevance of chelation with tellurium ions in boosting their radical quenching.
Methodology for the synthesis of L10 ligand based Te hexa-coordinated complexes.
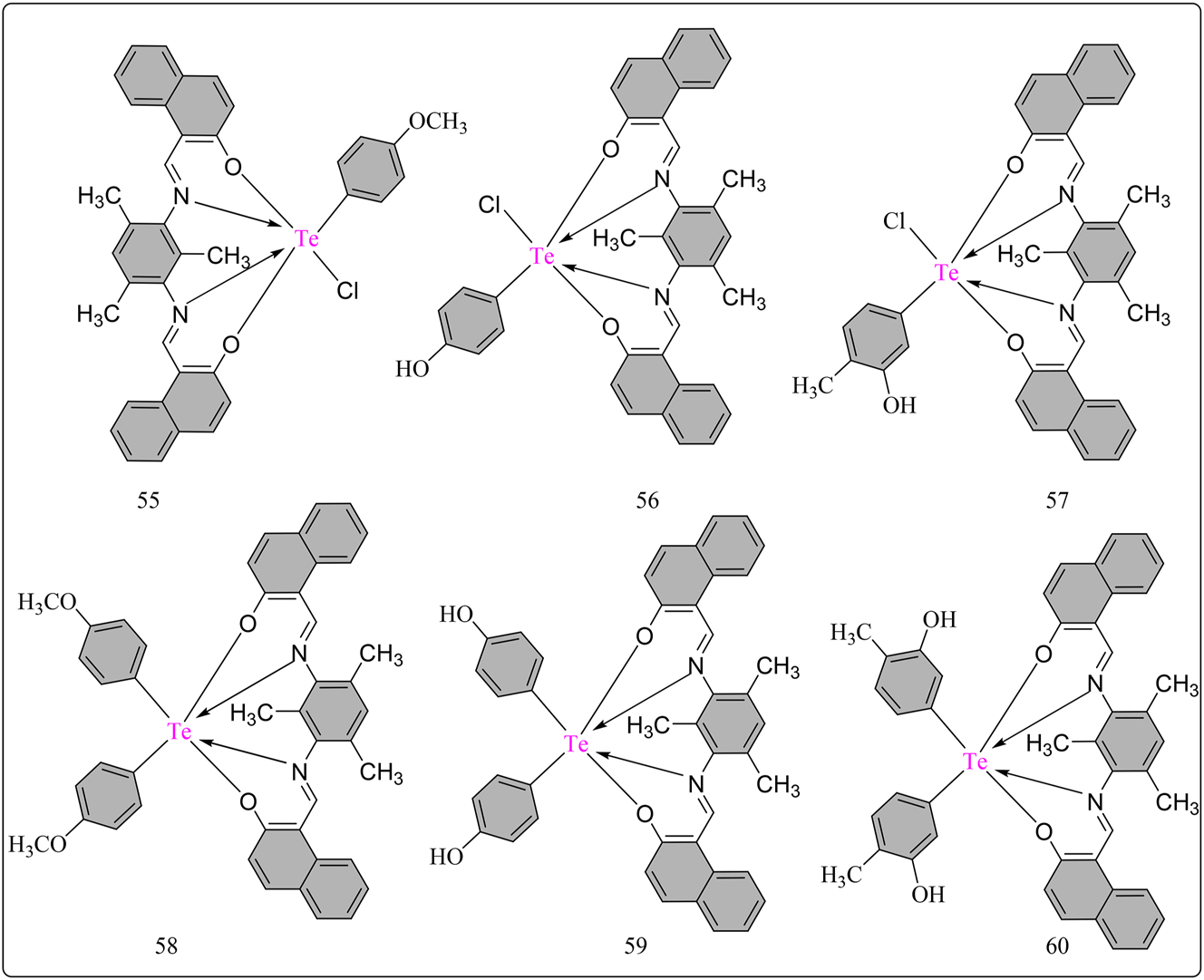
Structural representation of L10 ligand based hexa-coordinated Te(IV) complexes.
H2NA (L11) Schiff base was synthesized using a known method. 102 2-Hydroxy-1-naphthaldehyde was dissolved in 20 mL of CH3CH2OH and combined separately with ethanolic solutions of 2-nitroaniline and 4-nitroaniline to synthesize the L11 and L12 ligands, respectively (Scheme 12). The resulting mixtures were refluxed over a water bath for 4 h, yielding precipitates. These precipitates were subsequently filtered, purified using absolute ethanol, and dried under vacuum. The synthesis of L11 and L12 schiff base Te(IV) complexes followed a method analogous to that described for the L9 Te(IV) complexes, resulting in complexes exhibiting a distorted square bipyramidal geometry. The antioxidant potential of ligand L11 and its Te(IV) complexes (61–66) shown in Figure 5 was evaluated using the DPPH assay, 103 with results compiled in Table 8. The percentage radical scavenging power increased with increasing sample concentration. L11 exhibited low radical scavenging activity (IC50 = 2,487.77 ± 0.05 μM) compared to the standard ascorbic acid (IC50 = 418.52 ± 0.02 μM). Chelation with tellurium atom enhanced scavenging activity. Complexes 61, 62, and 64 showed higher scavenging activity (IC50 = 109.22 ± 0.08 μM, 106.23 ± 0.03 μM, and 106.34 ± 0.09 μM, respectively) compared to the standard antioxidant. Complexes 61, 62, and 64 demonstrate potential as effective free radical scavengers, while complex 65 displayed moderate scavenging activity (IC50 = 123.90 ± 0.07 μM) comparable to the standard antioxidant. 104 The complexes 67, 68, and 69, originating from the L12 schiff base ligand, displayed notable scavenging efficacy against free radicals, as evidenced by their low IC50 values of 80.32 ± 0.45, 83.60 ± 0.43, and 79.80 ± 0.29 μg/mL, respectively. These values are comparable to the IC50 value of the well-established antioxidant, ascorbic acid (IC50 = 73.71 ± 0.37 μg/mL), highlighting the substantial antioxidant potential of these complexes. 105 This enhancement in activity can be attributed to the presence of either a p-hydroxy or p-methoxy substituent on the aryl group of the complexes. Previous research has indicated that such substituents tend to augment the antioxidant activity of compounds derived from pyrazolones. The inclusion of these specific substituents is likely altering the electronic and steric properties of the complexes, thereby enhancing their ability to effectively scavenge free radicals. 106
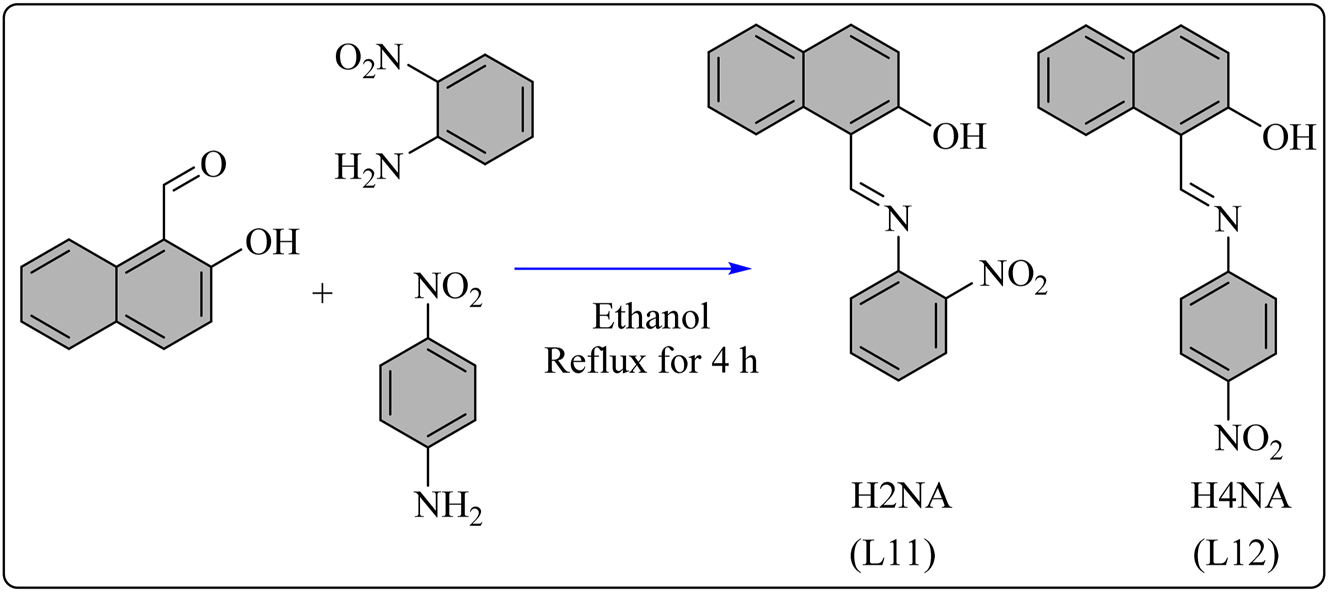
Methodology for the synthesis of L11 and L12 ligand and their Te complexes.

Structural representation of H2NA and H4NA based penta-coordinated Te(IV) complexes.
Comparison of IC50 values of ligands L11, L12, L13, and L14 with their Te complexes.
| Compound | % Scavenging activity at various concentration (in µg/mL) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | ||
| L11 | 3.70 | 4.60 | 5.76 | 7.01 | 9.18 | 2,487.77 ± 0.05 |
| 61 | 26.05 | 38.21 | 49.14 | 59.03 | 64.98 | 109.22 ± 0.08 |
| 62 | 29.00 | 40.02 | 51.50 | 58.54 | 65.64 | 106.23 ± 0.03 |
| 63 | 26.04 | 27.79 | 28.95 | 30.70 | 32.18 | 560.44 ± 0.04 |
| 64 | 31.55 | 40.33 | 48.55 | 53.25 | 58.17 | 106.34 ± 0.09 |
| 65 | 28.64 | 35.62 | 43.52 | 52.14 | 55.90 | 123.90 ± 0.07 |
| 66 | 25.09 | 27.71 | 29.01 | 31.96 | 33.18 | 391.32 ± 0.05 |
| Ascorbic acid | 37.01 | 42.06 | 48.03 | 51.33 | 55.66 | 418.52 ± 0.02 |
| L12 | – | – | – | – | – | 313.25 ± 0.94 |
| 67 | – | – | – | – | – | 80.32 ± 0.45 |
| 68 | – | – | – | – | – | 83.60 ± 0.43 |
| 69 | – | – | – | – | – | 79.80 ± 0.29 |
| Ascorbic acid | – | – | – | – | – | 73.71 ± 0.37 |
| L13 | 14.98 | 20.79 | 28.61 | 34.14 | 42.32 | 124.16 |
| 70 | 16.41 | 23.98 | 33.51 | 39.12 | 45.21 | 110.46 |
| 71 | 29.14 | 39.54 | 48.40 | 58.91 | 65.31 | 63.78 |
| 72 | 30.57 | 38.14 | 44.17 | 52.36 | 59.24 | 74.26 |
| 73 | 15.63 | 24.39 | 31.14 | 39.35 | 44.40 | 112.46 |
| 74 | 18.92 | 25.41 | 33.54 | 42.70 | 47.22 | 104.48 |
| 75 | 30.14 | 36.75 | 47.61 | 52.23 | 58.11 | 74.09 |
| Ascorbic acid | 26.60 | 39.34 | 47.72 | 56.18 | 63.90 | 67.13 |
| L14 | – | – | – | – | – | 124.01 |
| 76 | – | – | – | – | – | 110.55 |
| 77 | – | – | – | – | – | 59.20 |
| 78 | – | – | – | – | – | 141.09 |
| 79 | – | – | – | – | – | 90.01 |
| 80 | – | – | – | – | – | 155.91 |
| 81 | – | – | – | – | – | 86.37 |
5MTCPAP (L13) schiff base ligand was synthesized by refluxing 5-methyl-2-thiophene carboxaldehyde and p-aminophenol in ethanol for 4 h, yielding brown filtrates, which were subsequently recrystallized using absolute ethanol. Complexes were synthesized by combining 2 mmol of Te(IV) chlorides with 2 mmol of L13 in methanol and refluxing for 4 h at 80 °C (Scheme 13). The reaction mixture was then concentrated and filtered, followed by purification with petroleum ether and methanol. Both the ligand and complexes were dried using anhydrous CaCl2. The radical scavenging activity of Schiff base L13 ligand and its Te(IV) complexes (70–75) shown in Figure 6 was investigated using the standard DPPH assay, 107 with Table 8 presenting antioxidant behavior data for the complexes. UV–Vis spectroscopy was employed to obtain results, correlated with Ascorbic acid as the standard. The antioxidant activity increased with the % radical scavenging ability and sample complex concentration. At 100 μg/mL concentration, L13 exhibited 14.98 % radical scavenging activity, which increased upon chelation with Te(IV) complexes. The scavenging activity of ligand L13 was enhanced upon chelation with Te atom, with complex 71 exhibiting higher radical scavenging ability 65.3 % than ascorbic acid. However, the L13 displayed lower antioxidant ability (IC50 = 124.16 ± 0.03 µM) compared to the standard ascorbic acid (IC50 = 67.12 ± 0.05 µM). Complexes 71 (IC50 = 63.78 µM) and 72 (IC50 = 74.26 µM) displayed moderate antioxidant activity compared to ascorbic acid, indicating their potential as antioxidant agents. 108 NMeIATP (L14) schiff base ligand was synthesized by refluxing N-methylisatin with 2-aminothiophenol in 50 mL EtOH for 4 h, yielding an orange solid upon cooling and recrystallization with methanol (Scheme 14). Schiff base ligand (15 mmol) was then combined with solutions of RTeCl3/R2TeCl2 in 50 mL methanol and refluxed for 5 h. The resulting products were filtered, adjusted to a pH of around seven with CH3ONa, recrystallized from methanol, and dried over anhydrous CaCl2. L14 ligand and all Te(IV) complexes shown in Figure 7 were assessed for antioxidant activity using DPPH free radical assay in DMSO/methanol with UV–Visible spectroscopy, compared to Ascorbic acid. All complexes (76–81) exhibited good antioxidant activity (IC50). Complexes 77, 79, and 81 displayed stronger activity (Table 8), with complex 77 showing the highest activity (IC50 = 59.20 μg/mL), surpassing Ascorbic acid (IC50 = 71.03 μg/mL). Except for complexes 78 and 80, other complexes demonstrated higher activity than L14. 109
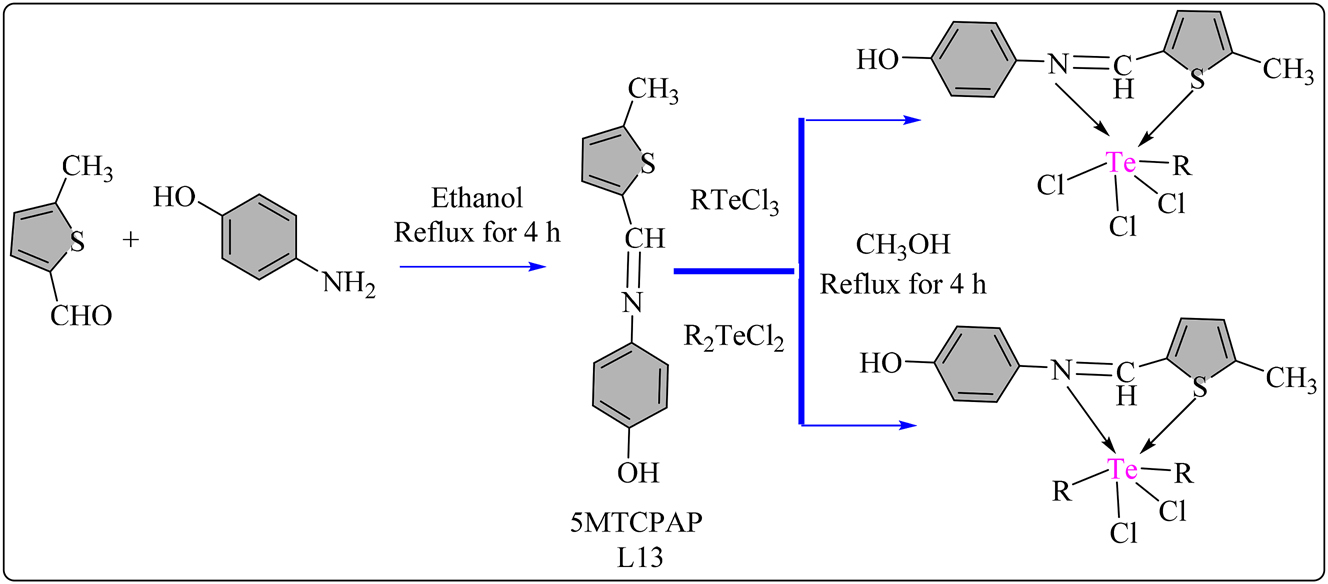
Methodology for the synthesis of L13 ligand and its Te complexes.

Structural representation of L13 ligand based hexa-coordinated Te complexes.
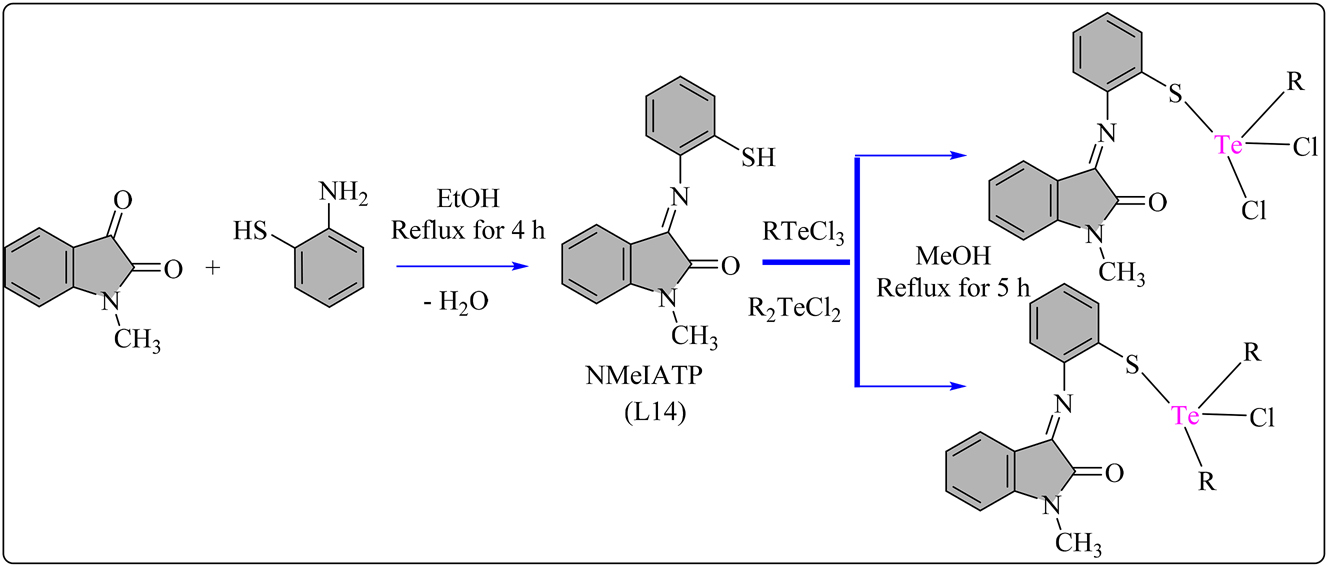
Methodology for the synthesis of L14 ligand and its Te complexes.
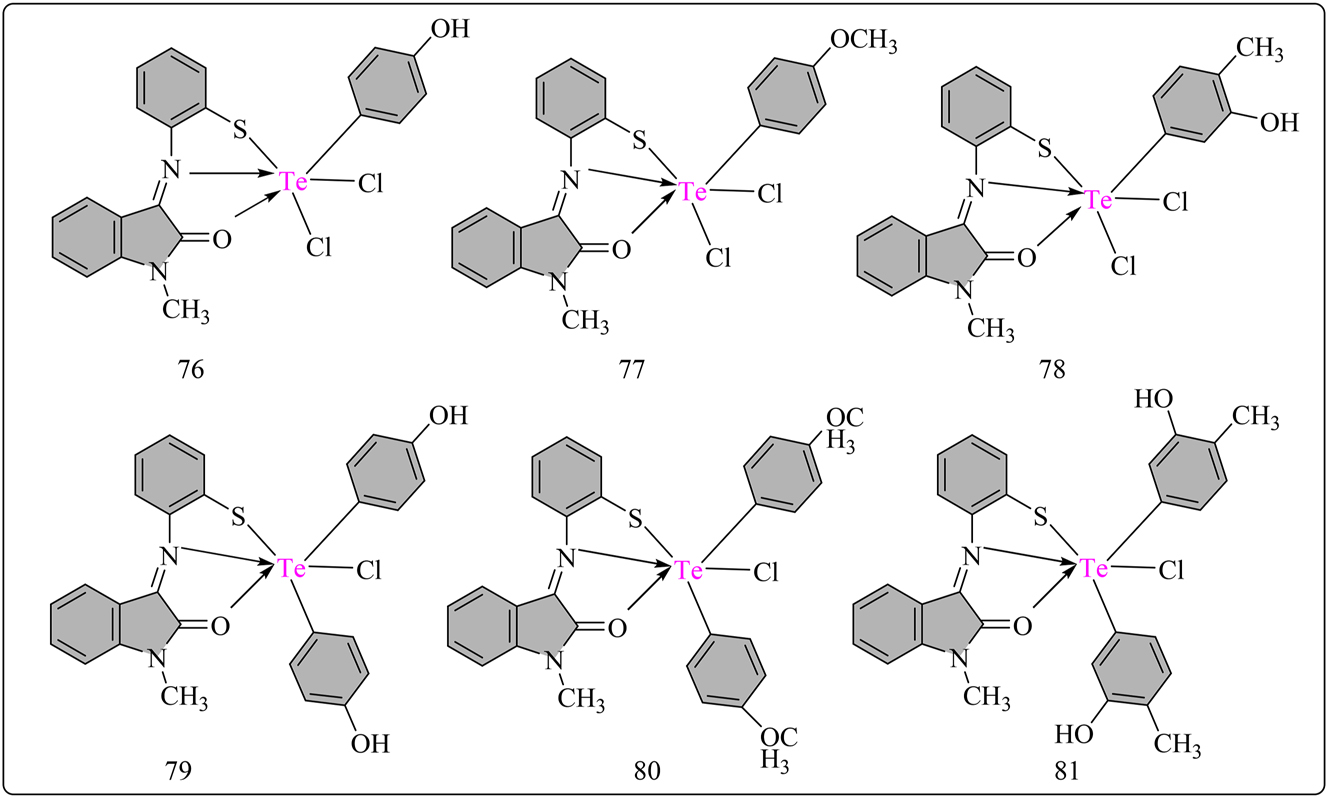
Structural representation of L14 ligand based hexa-coordinated Te complexes.
5-methyl-2-thiophene carboxaldehyde and 2-nitroaniline were refluxed in ethanol to produce the 5MTCONA (L15) schiff base ligand, which was subsequently used to create L15-containing Te complexes. The process required combining L15 with arylated Te chlorides in the presence of methyl alcohol and refluxing for 4 h (Scheme 15). To obtain the final products, the chemicals were filtered and purified using petroleum ether and methanol, and then dried over an anhydrous solution of calcium chloride. The antioxidant activity results show that as the percentage radical scavenging ability increases, correspondingly increases the concentration of experimental complexes (82–85). The Schiff base L15 had the lowest radical scavenging activity among the investigated complexes (IC50 = 187.36 ± 0.45 μg/mL) compared to ascorbic acid as a reference (IC50 = 70.49 ± 0.28 μg/mL) (Table 9). The complexes 82, 83, and 85, with IC50 values of 73.91 ± 0.50 μg/mL, 68.870 ± 0.43 μg/mL, and 80.39 ± 0.37 μg/mL, respectively, exhibit the strongest radical scavenging activity equivalent to ascorbic acid. The complex 84 (IC50 = 91.94 ± 0.50 μg/mL) has modest antioxidant activity. 110
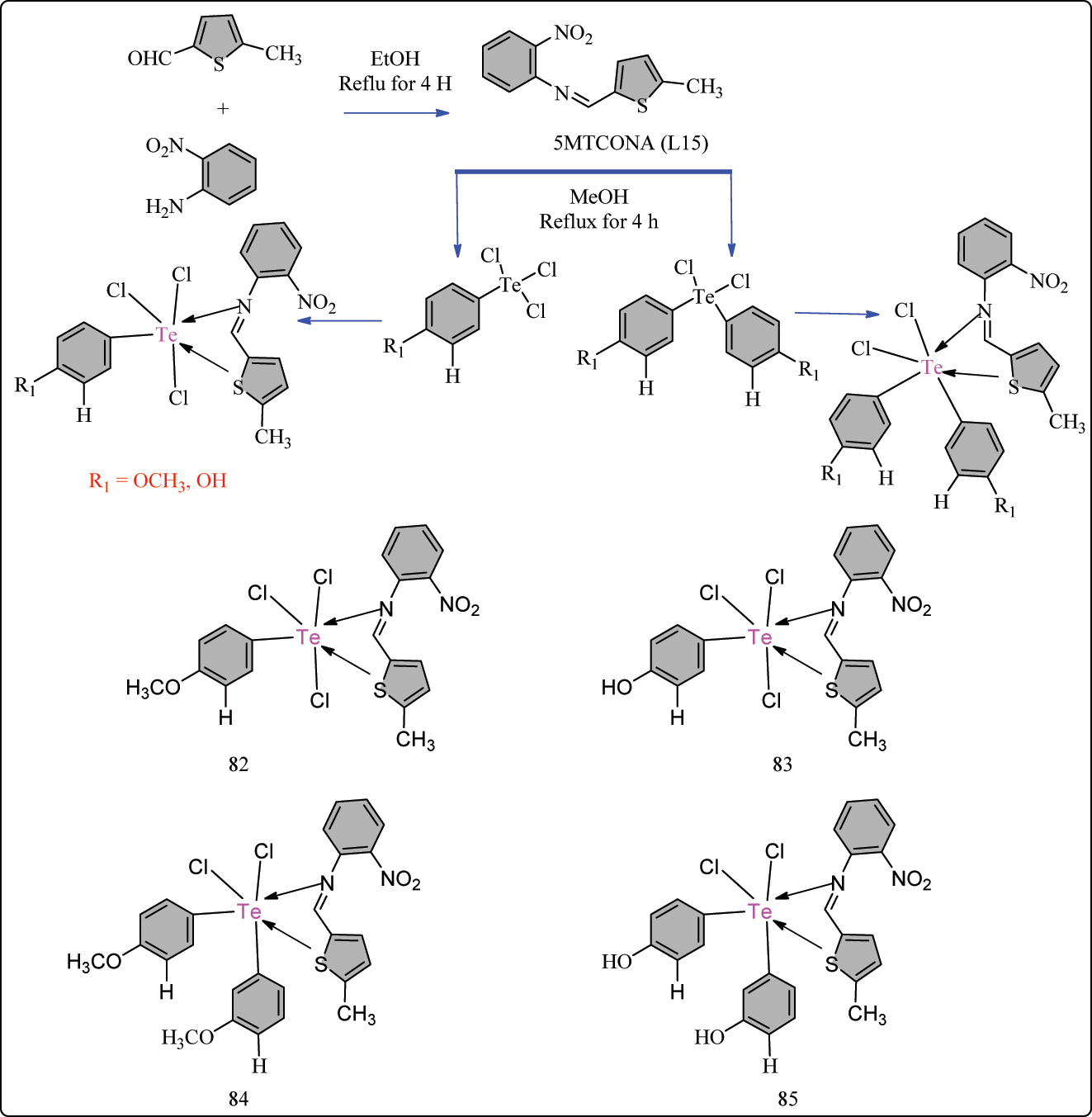
Methodology for the synthesis of L15 ligand and its Te hexa-coordinated complexes.
Illustration of IC50 values of ligand L15 and its Te complexes at various concentrations.
| Compound |
% Scavenging activity at various concentration (in µg/mL) | IC50 (μM) | ||||
|---|---|---|---|---|---|---|
| 20 | 40 | 60 | 80 | 100 | ||
| L15 | 10.98 | 13.65 | 20.79 | 24.56 | 29.20 | 187.36 |
| 82 | 30.62 | 37.89 | 45.12 | 52.41 | 59.11 | 73.90 |
| 83 | 27.70 | 37.25 | 46.52 | 53.21 | 64.40 | 68.87 |
| 84 | 20.89 | 28.14 | 36.21 | 45.65 | 53.32 | 91.94 |
| 85 | 26.12 | 33.54 | 40.61 | 49.55 | 58.74 | 80.39 |
| Ascorbic acid | 29.81 | 36.15 | 48.21 | 54.64 | 60.31 | 70.49 |
4 Summary and outlook
This in-depth review covers important synthetic techniques and navigates the rapidly expanding field of organotellurium compounds, illuminating its numerous applications in the biological and material science fields. It emphasizes how crucial it is to synthesis these compounds with a variety of ligands, such as Schiff bases, amino acids, naphthoquinones, and β-hydroxy alkylated compounds all of which are well-known for their stability and adaptability in assisting the synthesis of organotellurium complexes. This study demonstrates the proven efficacy of organotellurium complexes as antioxidants and anticancer agents, emphasizing their future outlook. By means of comparisons to standard drugs, the research highlights their capacity for addressing significant health issues. Furthermore, the study shows that although organic ligands by themselves have some anticancer and antioxidant potential, when they are complexed with tellurium, they become much more effective, highlighting the amazing improvement in their therapeutic potency.
Although organotellurium compounds have a lot of potential, there are also a lot of challenges and disadvantages. One major drawback compared to other compounds derived from metals is the very limited knowledge of their mechanisms of action. Further study is required to fully understand their potential in solving complex biological and material science problems. Moreover, the complex andresource-intensive nature of the synthesis of organotellurium compounds may pose challenges to scalability and cost-effectiveness for large-scale applications. Additionally, concerns about their potential toxicity and environmental impacts necessitate the design of mitigation measures and the use of extreme caution in their development and implementation. In conclusion, all of the potential that organotellurium compounds hold for solving significant problems in biology and materials science remains untapped. This review offers valuable insights on synthetic methods, potential applications, and future directions for advancing our understanding and exploration of organotellurium compounds in several scientific domains.
Funding source: Pakistan Science Foundation
Award Identifier / Grant number: PSF/CRP/Consr-676
-
Research ethics: Not applicable.
-
Author contributions: Fadhil Farhood M.Al-joborae: writing-original draft, software. Sawsan.S Al-Rawi: resources and validation. Ahmad H. Ibrahim: data curation, visualization. Abbas Washeel Salman: review and editing. Muhammad Adnan Iqbal: conceptualization, resources, supervision, overall guidance.
-
Competing interests: The authors declare no conflict of interest.
-
Research funding: The authors are thankful to the Pakistan Science Foundation (PSF) for awarding the research grant PSF/Cons/676.
-
Data availability: Data will be provided on demand.
References
1. Jamier, V.; Ba, L. A.; Jacob, C. Selenium‐and Tellurium‐containing Multifunctional Redox Agents as Biochemical Redox Modulators with Selective Cytotoxicity. Chem. Eur. J. 2010, 16 (36), 10920–10928. https://doi.org/10.1002/chem.201000884.Suche in Google Scholar PubMed
2. Koop, C. E. Health and Health Care for the 21st Century: For All the People. Am. J. Public Health. 2006, 96, 2090–2092; https://doi.org/10.2105/AJPH.2006.098962.Suche in Google Scholar PubMed PubMed Central
3. Weitzel, J. N.; Blazer, K. R.; MacDonald, D. J.; Culver, J. O.; Offit, K. Genetics, Genomics, and Cancer Risk Assessment: State of the Art and Future Directions in the Era of Personalized Medicine. Ca - Cancer J. Clin. 2011, 61 (5), 327–359. https://doi.org/10.3322/caac.20128.Suche in Google Scholar PubMed PubMed Central
4. Hirales Casillas, C. E.; Flores Fernandez, J. M.; Camberos, E. P.; Herrera Lopez, E. J.; Pacheco, G. L.; Velázquez, M. M. Current Status of Circulating Protein Biomarkers to Aid the Early Detection of Lung Cancer. Future Oncol. 2014, 10 (8), 1501–1513. https://doi.org/10.2217/fon.14.21.Suche in Google Scholar PubMed
5. Lucas, R.; Yazar, S.; Young, A.; Norval, M.; De Gruijl, F.; Takizawa, Y.; Rhodes, L. E.; Sinclair, C. A.; Neale, R. E. Human Health in Relation to Exposure to Solar Ultraviolet Radiation under Changing Stratospheric Ozone and Climate. Photochem. Photobiol. Sci. 2019, 18 (3), 641–680. https://doi.org/10.1039/c8pp90060d.Suche in Google Scholar PubMed
6. Pervaiz, M.; Sadiq, S.; Sadiq, A.; Younas, U.; Ashraf, A.; Saeed, Z.; Zuber, M.; Adnan, A. Azo-Schiff Base Derivatives of Transition Metal Complexes as Antimicrobial Agents. Coord. Chem. Rev. 2021, 447, 214128. https://doi.org/10.1016/j.ccr.2021.214128.Suche in Google Scholar
7. Malik, M. A.; Dar, O. A.; Gull, P.; Wani, M. Y.; Hashmi, A. A. Heterocyclic Schiff Base Transition Metal Complexes in Antimicrobial and Anticancer Chemotherapy. Med. Chem. Comm. 2018, 9 (3), 409–436. https://doi.org/10.1039/c7md00526a.Suche in Google Scholar PubMed PubMed Central
8. Zafar, A.; Iqbal, M. A.; Iram, G.; Shoukat, U. S.; Jamil, F.; Saleem, M.; Yousif, M.; Abidin, Z. U.; Asad, M. Advances in Organocatalyzed Synthesis of Organic Compounds. RSC Adv. 2024, 14 (28), 20365–20389. https://doi.org/10.1039/d4ra03046j.Suche in Google Scholar PubMed PubMed Central
9. Haas, K. L.; Franz, K. J. Application of Metal Coordination Chemistry to Explore and Manipulate Cell Biology. Chem. Rev. 2009, 109 (10), 4921–4960. https://doi.org/10.1021/cr900134a.Suche in Google Scholar PubMed PubMed Central
10. Guarra, F.; Pratesi, A.; Gabbiani, C.; Biver, T. A Focus on the Biological Targets for Coinage Metal-NHCs as Potential Anticancer Complexes. J. Inorg. Biochem. 2021, 217, 111355. https://doi.org/10.1016/j.jinorgbio.2021.111355.Suche in Google Scholar PubMed
11. Schattschneider, C.; Kettenmann, S. D.; Hinojosa, S.; Heinrich, J.; Kulak, N. Biological Activity of Amphiphilic Metal Complexes. Coord. Chem. Rev. 2019, 385, 191–207. https://doi.org/10.1016/j.ccr.2018.12.007.Suche in Google Scholar
12. Cervo, R.; Lopes, T. R.; de Vasconcelos, A. R.; Cargnelutti, J. F.; Schumacher, R. F.; Tirloni, B.; dos Santos, S. S.; Abram, U.; Lang, E. S. Coordination Compounds Containing 2-pyridylselenium Ligands: Synthesis, Structural Characterization, and Antibacterial Evaluation. New J. Chem. 2021, 45 (29), 12863–12870. https://doi.org/10.1039/d1nj02374h.Suche in Google Scholar
13. Boros, E.; Dyson, P. J.; Gasser, G. Classification of Metal-Based Drugs According to Their Mechanisms of Action. Chemisty 2020, 6 (1), 41–60. https://doi.org/10.1016/j.chempr.2019.10.013.Suche in Google Scholar PubMed PubMed Central
14. Miranda, V. M. Medicinal Inorganic Chemistry: An Updated Review on the Status of Metallodrugs and Prominent Metallodrug Candidates. Rev. Inorg. Chem. 2022, 42 (1), 29–52. https://doi.org/10.1515/revic-2020-0030.Suche in Google Scholar
15. Gelasco, A.; Lippard, S. J. Anticancer Activity of Cisplatin and Related Complexes. Metallopharmaceuticals I. DNA Interact. 1999, 1–43.10.1007/978-3-662-03815-4_1Suche in Google Scholar
16. Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. https://doi.org/10.1016/j.bioorg.2019.102925.Suche in Google Scholar PubMed
17. Ndagi, U.; Mhlongo, N.; Soliman, M. E. Metal Complexes in Cancer Therapy–An Update from Drug Design Perspective. Drug Des. Dev. Ther. 2017, 599–616. https://doi.org/10.2147/dddt.s119488.Suche in Google Scholar PubMed PubMed Central
18. Yousuf, I.; Bashir, M.; Arjmand, F.; Tabassum, S. Advancement of Metal Compounds as Therapeutic and Diagnostic Metallodrugs: Current Frontiers and Future Perspectives. Coord. Chem. Rev. 2021, 445, 214104. https://doi.org/10.1016/j.ccr.2021.214104.Suche in Google Scholar
19. Dasari, S.; Tchounwou, P. B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. https://doi.org/10.1016/j.ejphar.2014.07.025.Suche in Google Scholar PubMed PubMed Central
20. Markert, B. The Biological System of the Elements (BSE) for Terrestrial Plants (Glycophytes). Sci. Total Environ. 1994, 155 (3), 221–228. https://doi.org/10.1016/0048-9697(94)90501-0.Suche in Google Scholar
21. Markert, B. Presence and Significance of Naturally Occurring Chemical Elements of the Periodic System in the Plant Organism and Consequences for Future Investigations on Inorganic Environmental Chemistry in Ecosystems. Vegetatio 1992, 103, 1–30. https://doi.org/10.1007/bf00033413.Suche in Google Scholar
22. Taylor, A. Biochemistry of Tellurium. Biol. Trace Elem. Res. 1996, 55, 231–239. https://doi.org/10.1007/bf02785282.Suche in Google Scholar PubMed
23. Wilber, C. G. Toxicology of Selenium: a Review. Clin. Toxicol. 1980, 17 (2), 171–230. https://doi.org/10.3109/15563658008985076.Suche in Google Scholar PubMed
24. Fränzle, S.; Markert, B. The Biological System of the Elements (BSE). Part II: a Theoretical Model for Establishing the Essentiality of Chemical Elements. The Application of Stoichiometric Network Analysis to the Biological System of the Elements. Sci. Total Environ. 2000, 249 (1-3), 223–241. https://doi.org/10.1016/s0048-9697(99)00520-3.Suche in Google Scholar PubMed
25. Chasteen, T. G.; Fuentes, D. E.; Tantaleán, J. C.; Vásquez, C. C. Tellurite: History, Oxidative Stress, and Molecular Mechanisms of Resistance. FEMS Microbiol. Rev. 2009, 33 (4), 820–832. https://doi.org/10.1111/j.1574-6976.2009.00177.x.Suche in Google Scholar PubMed
26. Ashraf, M. W.; Haider, S. I.; Solangi, A. R.; Memon, A. F. Toxicity of Tellurium and its Compounds. Phys. Sci. Rev. 2023, 8 (11), 4375–4390. https://doi.org/10.1515/psr-2021-0112.Suche in Google Scholar
27. Rayman, M. P. The Importance of Selenium to Human Health. Lancet 2000, 356 (9225), 233–241. https://doi.org/10.1016/s0140-6736(00)02490-9.Suche in Google Scholar
28. Chasteen, T. G.; Bentley, R. Biomethylation of Selenium and Tellurium: Microorganisms and Plants. Chem. Rev. 2003, 103 (1), 1–26. https://doi.org/10.1002/chin.200315286.Suche in Google Scholar
29. Behne, D.; Kyriakopoulos, A. Mammalian Selenium-Containing Proteins. Annu. Rev. Nutr. 2001, 21 (1), 453–473. https://doi.org/10.1146/annurev.nutr.21.1.453.Suche in Google Scholar PubMed
30. Sredni, B. Immunomodulating Tellurium Compounds as Anti-Cancer Agents. Semin. Cancer Biol. 2012, 22 (1), 60–69.10.1016/j.semcancer.2011.12.003Suche in Google Scholar PubMed
31. Widy-Tyszkiewicz, E.; Piechal, A.; Gajkowska, B.; Śmiałek, M. Tellurium-induced Cognitive Deficits in Rats Are Related to Neuropathological Changes in the Central Nervous System. Toxicol. Lett. 2002, 131 (3), 203–214. https://doi.org/10.1016/s0378-4274(02)00050-4.Suche in Google Scholar PubMed
32. Morell, P.; Toews, A. D.; Wagner, M.; Goodrum, J. F. Gene Expression during Tellurium-Induced Primary Demyelination. Neurotoxicology 1994, 15 (1), 171–180.Suche in Google Scholar
33. Morell, P.; Toews, A. D. Schwann Cells as Targets for Neurotoxicants. Neurotoxicology 1996, 17 (3-4), 685–695.Suche in Google Scholar
34. Santos, D.; Schiar, V.; Paixão, M.; Meinerz, D.; Nogueira, C.; Aschner, M.; Rocha, J.; Barbosa, N. Hemolytic and Genotoxic Evaluation of Organochalcogens in Human Blood Cells In Vitro. Toxicol. Vitro 2009, 23 (6), 1195–1204. https://doi.org/10.1016/j.tiv.2009.05.010.Suche in Google Scholar PubMed
35. Roy, S.; Hardej, D. Tellurium Tetrachloride and Diphenyl Ditelluride Cause Cytotoxicity in Rat Hippocampal Astrocytes. Food Chem. Toxicol. 2011, 49 (10), 2564–2574. https://doi.org/10.1016/j.fct.2011.06.072.Suche in Google Scholar PubMed
36. Yang, F.; Wong, K.-H.; Yang, Y.; Li, X.; Jiang, J.; Zheng, W.; Wu, H. Purification and In Vitro Antioxidant Activities of Tellurium-Containing Phycobiliproteins from Tellurium-Enriched Spirulina Platensis. Drug Des. Dev. Ther. 2014, 1789–1800. https://doi.org/10.2147/dddt.s62530.Suche in Google Scholar
37. Krivovichev, V. G.; Krivovichev, S. V.; Charykova, M. V. Tellurium Minerals: Structural and Chemical Diversity and Complexity. Minerals 2020, 10 (7), 623. https://doi.org/10.3390/min10070623.Suche in Google Scholar
38. González-Ibarra, A.; Nava-Alonso, F.; Dávila-Pulido, G.; Carrillo-Pedroza, F.; Rodríguez-Flores, A. Dissolution Behavior of Elemental Tellurium and Tellurium Dioxide in Alkaline Cyanide Solutions. Hydrometallurgy 2021, 203, 105702. https://doi.org/10.1016/j.hydromet.2021.105702.Suche in Google Scholar
39. Sun, Y.; Zhao, Y.; Lei, Q.; Du, W.; Yao, Z.; Zhang, W.; Si, J.; Ren, Z.; Chen, J.; Gao, Y.; Wen, W.; Tai, R.; Li, X.; Zhu, D. Initiating Reversible Aqueous Copper–Tellurium Conversion Reaction with High Volumetric Capacity through Electrolyte Engineering. Adv. Mater. 2023, 35 (9), 2209322. https://doi.org/10.1002/adma.202209322.Suche in Google Scholar PubMed
40. Jain, V. K.; Chauhan, R. S. New Vistas in the Chemistry of Platinum Group Metals with Tellurium Ligands. Coord. Chem. Rev. 2016, 306, 270–301. https://doi.org/10.1016/j.ccr.2015.07.009.Suche in Google Scholar
41. Chauhan, R. S.; Shivran, N. Emerging Trends in Organotellurolate Chemistry Derived from Platinoids. RSC Adv. 2017, 7 (87), 55175–55198. https://doi.org/10.1039/c7ra09480a.Suche in Google Scholar
42. Orian, L.; Toppo, S. Organochalcogen Peroxidase Mimetics as Potential Drugs: A Long Story of a Promise Still Unfulfilled. Free Radic. Biol. Med. 2014, 66, 65–74. https://doi.org/10.1016/j.freeradbiomed.2013.03.006.Suche in Google Scholar PubMed
43. Hassan, W.; Narayanaperumal, S.; Santos, M.; Gul, K.; Mohammadzai, I.; Braga, A.; Rodrigues, O.; Teixeira da Rocha, J. Understanding the Mechanism of Antioxidant Potential of Organochalcogens in Rat’s Brain Preparation. Pharm. Anal. Acta S 2011, 3, 2.10.4172/2153-2435.S3-002Suche in Google Scholar
44. Tiekink, E. R. Therapeutic Potential of Selenium and Tellurium Compounds: Opportunities yet Unrealised. Dalton Trans. 2012, 41 (21), 6390–6395. https://doi.org/10.1039/c2dt12225a.Suche in Google Scholar PubMed
45. Persike, D. S.; Cunha, R. L. O. R.; Juliano, L.; Silva, I. R.; Rosim, F. E.; Vignoli, T.; Dona, F.; Cavalheiro, E. A.; Fernandes, M. J. d. S. Protective Effect of the Organotelluroxetane RF-07 in Pilocarpine-Induced Status Epilepticus. Neurobiol. Dis. 2008, 31 (1), 120–126. https://doi.org/10.1016/j.nbd.2008.04.001.Suche in Google Scholar PubMed
46. Cunha, R. L.; Urano, M. E.; Chagas, J. R.; Almeida, P. C.; Bincoletto, C.; Tersariol, I. L.; Comasseto, J. V. Tellurium-based Cysteine Protease Inhibitors: Evaluation of Novel Organotellurium (IV) Compounds as Inhibitors of Human Cathepsin B. Bioorg. Med. Chem. Lett 2005, 15 (3), 755–760. https://doi.org/10.1016/j.bmcl.2004.11.012.Suche in Google Scholar PubMed
47. Abondanza, T.; Oliveira, C.; Barbosa, C.; Pereira, F.; Cunha, R. L. O. R.; Caires, A.; Comasseto, J.; Queiroz, M.; Valadares, M.; Bincoletto, C. Bcl-2 Expression and Apoptosis Induction in Human HL60 Leukaemic Cells Treated with a Novel Organotellurium (IV) Compound RT-04. Food Chem. Toxicol. 2008, 46 (7), 2540–2545. https://doi.org/10.1016/j.fct.2008.04.010.Suche in Google Scholar PubMed
48. Cunha, R. L.; Gouvea, I. E.; Juliano, L. A Glimpse on Biological Activities of Tellurium Compounds. An Acad. Bras Ciências 2009, 81, 393–407. https://doi.org/10.1590/s0001-37652009000300006.Suche in Google Scholar PubMed
49. Dalal, M.; Devi, J.; Antil, N.; Kumar, B.; Verma, Y. K.; Kumar, S.; Wati, M.; Garg, S. Exploring the Antimicrobial, Antioxidant and Cytotoxic Activities of Organyltellurium (IV) Complexes Incorporating 2‐hydroxy‐1‐naphthaldehyde Schiff Base Ligand: Synthesis, Spectroscopic Investigations and Theoretical Studies. Appl. Organomet. Chem. 2024, 38 (2), e7338. https://doi.org/10.1002/aoc.7338.Suche in Google Scholar
50. Kınalı, M.; Çol, S.; Çoban, C. Ç.; Türk, M.; Aydın, G.; Emirik, M.; Baran, A. Chalcone-based Dipolar Cycloaddition of Novel Heteroaromatic Compounds: Their Anticancer Examination. J. Mol. Struct. 2023, 1293, 136244. https://doi.org/10.1016/j.molstruc.2023.136244.Suche in Google Scholar
51. Reddy, P. N.; Sharon, N.; Padmaja, P.; Ugale, V. G.; Lokwani, D.; Pragati, P.; P, P.; K, A. Molecular Hybridization-Based Design, PASE Three-Component Synthesis, Antiproliferative Activity and Molecular Modelling Studies of N, N-Dimethylaminophenyl Substituted 5H-Chromeno [2, 3-b] Pyridine Analogs. J. Mol. Struct. 2023, 1286, 135589. https://doi.org/10.1016/j.molstruc.2023.135589.Suche in Google Scholar
52. Graat, H.; Witlox, M.; Schagen, F.; Kaspers, G.; Helder, M.; Bras, J.; Schaap, G. R.; Gerritsen, W. R.; Wuisman, P. I. J. M.; van Beusechem, V. W. Different Susceptibility of Osteosarcoma Cell Lines and Primary Cells to Treatment with Oncolytic Adenovirus and Doxorubicin or Cisplatin. Br. J. Cancer 2006, 94 (12), 1837–1844. https://doi.org/10.1038/sj.bjc.6603189.Suche in Google Scholar PubMed PubMed Central
53. da Rocha, D. R.; Mota, K.; da Silva, I. M.; Ferreira, V. F.; Ferreira, S. B.; da Silva, F. C. Synthesis of Fused Chromene-1, 4-naphthoquinones via Ring-Closing Metathesis and Knoevenagel-Electrocyclization under Acid Catalysis and Microwave Irradiation. Tetrahedron 2014, 70 (20), 3266–3270. https://doi.org/10.1016/j.tet.2013.11.068.Suche in Google Scholar
54. Reich, H. J.; Ml, C.; Ps, C. Reagents for synthesis of organoselenium compounds: diphenyl diselenide and benzeneselenenyl chloride, 1980.Suche in Google Scholar
55. Goulart, T. A.; Back, D. F.; Zeni, G. Copper‐Catalyzed Carbon‐Nitrogen/Carbon‐Selenium Bonds Formation: Synthesis of 2‐(Organochalcogenyl)‐indolizines. Adv. Synth. Catal. 2017, 359 (11), 1901–1911. https://doi.org/10.1002/adsc.201700166.Suche in Google Scholar
56. Gomes, L. S.; Neto, J. S.; di Leo, I.; Barbosa, C. G.; Moraes, C. B.; Freitas-Junior, L. H.; Rizzuti, B.; Santi, C.; Nascimento, V. Ecofriendly Aminochalcogenation of Alkenes: a Green Alternative to Obtain Compounds with Potential Anti-SARS-CoV-2 Activity. New J. Chem. 2023, 47 (14), 6591–6601. https://doi.org/10.1039/d2nj06218f.Suche in Google Scholar
57. Gomes, L. S.; Costa, E. O.; Duarte, T. G.; Charret, T. S.; Castiglione, R. C.; Simões, R. L.; Pascoal, V. D. B.; Döring, T. H.; da Silva, F. D. C.; Ferreira, V. F.; de Oliveira, A.; Pascoal, A. C. R. F.; Cruz, A. L. S.; Nascimento, V. New Chalcogen-Functionalized Naphthoquinones: Design, Synthesis, and Evaluation, In Vitro and In Silico, against Squamous Cell Carcinoma. ACS Omega 2024, 9 (20), 21948–21963. https://doi.org/10.1021/acsomega.3c10134.Suche in Google Scholar PubMed PubMed Central
58. Zorzanelli, B. C.; de Queiroz, L. N.; Santos, R. M.; Menezes, L. M.; Gomes, F. C.; Ferreira, V. F.; da Silva, F. D.; Robbs, B. K. Potential Cytotoxic and Selective Effect of New Benzo [b] Xanthenes against Oral Squamous Cell Carcinoma. Future Med. Chem. 2018, 10 (10), 1141–1157. https://doi.org/10.4155/fmc-2017-0205.Suche in Google Scholar PubMed
59. Macedo, A. L.; da Silva, D. P.; Moreira, D. L.; de Queiroz, L. N.; Vasconcelos, T. R.; Araujo, G. F.; Kaplan, M. A. C.; Pereira, S. S.; de Almeida, E. C.; Valverde, A. L.; Robbs, B. K. Cytotoxicity and Selectiveness of Brazilian Piper Species towards Oral Carcinoma Cells. Biomed. Pharmacother. 2019, 110, 342–352. https://doi.org/10.1016/j.biopha.2018.11.129.Suche in Google Scholar PubMed
60. Hartner, L. Chemotherapy for Oral Cancer. Dental Clinics 2018, 62 (1), 87–97. https://doi.org/10.1016/j.cden.2017.08.006.Suche in Google Scholar PubMed
61. Khasraw, M.; Bell, R.; Dang, C. Epirubicin: Is it like Doxorubicin in Breast Cancer? A Clinical Review. Breast 2012, 21 (2), 142–149. https://doi.org/10.1016/j.breast.2011.12.012.Suche in Google Scholar PubMed
62. Shafei, A.; El-Bakly, W.; Sobhy, A.; Wagdy, O.; Reda, A.; Aboelenin, O.; Marzouk, A.; El Habak, K.; Mostafa, R.; Ali, M. A.; Ellithy, M. A Review on the Efficacy and Toxicity of Different Doxorubicin Nanoparticles for Targeted Therapy in Metastatic Breast Cancer. Biomed. Pharmacother. 2017, 95, 1209–1218. https://doi.org/10.1016/j.biopha.2017.09.059.Suche in Google Scholar PubMed
63. Radha Abbas Hasoon, M.; Jawad Kadhim, N. Improvement of the Selectivity Index (SI) and Cytotoxicity Activity of Doxorubicin Drug by Panax Ginseng Plant Extract. Arch. Razi Inst. 2021, 76 (3), 659–666. https://doi.org/10.22092/ari.2021.355413.1681.Suche in Google Scholar PubMed PubMed Central
64. Gomes, L. S.; Costa, E. O.; Duarte, T. G.; Charret, T. S.; Castiglione, R. C.; Simões, R. L.; Pascoal, V. D. B.; Döring, T. H.; da Silva, F. D. C.; Ferreira, V. F.; de Oliveira, A.; Pascoal, A. C. R. F.; Cruz, A. L. S.; Nascimento, V. New Chalcogen-Functionalized Naphthoquinones: Design, Synthesis, and Evaluation, In Vitro and In Silico, against Squamous Cell Carcinoma. ACS Omega 2024, 9, 21948–21963; https://doi.org/10.1021/acsomega.3c10134.Suche in Google Scholar PubMed PubMed Central
65. Tripathi, A.; Khan, A.; Kiran, P.; Shetty, H.; Srivastava, R. Screening of AS101 Analog, Organotellurolate (IV) Compound 2 for its In Vitro Biocompatibility, Anticancer, and Antibacterial Activities. Amino Acids 2023, 55 (7), 891–902. https://doi.org/10.1007/s00726-023-03280-7.Suche in Google Scholar PubMed
66. Alberto, E. E.; Nascimento, V. D.; Braga, A. L. Catalytic Application of Selenium and Tellurium Compounds as Glutathione Peroxidase Enzyme Mimetics. J. Braz. Chem. Soc. 2010, 21, 2032–2041. https://doi.org/10.1590/s0103-50532010001100004.Suche in Google Scholar
67. Bothwell, I. R.; Luo, M. Large-scale, Protection-free Synthesis of Se-Adenosyl-L-Selenomethionine Analogues and Their Application as Cofactor Surrogates of Methyltransferases. Org. Lett. 2014, 16 (11), 3056–3059. https://doi.org/10.1021/ol501169y.Suche in Google Scholar PubMed PubMed Central
68. Tripathi, A.; Khan, A.; Srivastava, R. Synthesis and Screening for Anticancer Activity of Two Novel Telluro-Amino Acids: 1, 3-Tellurazolidine-4-Carboxylic Acid and Tellurohomocystine. Amino Acids 2023, 55 (10), 1361–1370. https://doi.org/10.1007/s00726-023-03314-0.Suche in Google Scholar PubMed
69. Detty, M. R.; Seidler, M. D. Bis (Trialkylsilyl) Chalcogenides. 1. Preparation and Reduction of Group VIA Oxides. J. Org. Chem. 1982, 47 (7), 1354–1356. https://doi.org/10.1021/jo00346a041.Suche in Google Scholar
70. Tanini, D.; Grechi, A.; Dei, S.; Teodori, E.; Capperucci, A. An Easy One-step Procedure for the Synthesis of Novel β-functionalised Tellurides. Tetrahedron 2017, 73 (38), 5646–5653. https://doi.org/10.1016/j.tet.2017.07.061.Suche in Google Scholar
71. Capperucci, A.; Coronnello, M.; Salvini, F.; Tanini, D.; Dei, S.; Teodori, E.; Giovannelli, L. Synthesis of Functionalised Organochalcogenides and In Vitro Evaluation of Their Antioxidant Activity. Bioorg. Chem. 2021, 110, 104812. https://doi.org/10.1016/j.bioorg.2021.104812.Suche in Google Scholar PubMed
72. Tanini, D.; Capperucci, A. Unexpected Ethyltellurenylation of Epoxides with Elemental Tellurium under Lithium Triethylborohydride Conditions. Chemistry 2020, 2 (3), 652–661. https://doi.org/10.3390/chemistry2030041.Suche in Google Scholar
73. Marques, N. B.; Jacob, R. G.; Perin, G.; Lenardão, E. J.; Alves, D.; Silva, M. S. NMR Chiral Discrimination of Chalcogen Containing Secondary Alcohols. Chirality 2019, 31 (1), 41–51. https://doi.org/10.1002/chir.23030.Suche in Google Scholar PubMed
74. Berlin, S.; Ericsson, C.; Engman, L. Radical Carbonylation/reductive Cyclization for the Construction of Tetrahydrofuran-3-Ones and Pyrrolidin-3-Ones. J. Org. Chem. 2003, 68 (22), 8386–8396. https://doi.org/10.1021/jo030153f.Suche in Google Scholar PubMed
75. Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C. T. First Evaluation of Organotellurium Derivatives as Carbonic Anhydrase I, II, IV, VII and IX Inhibitors. Bioorg. Chem. 2018, 76, 268–272. https://doi.org/10.1016/j.bioorg.2017.12.010.Suche in Google Scholar PubMed
76. Tabarelli, G.; Dornelles, L.; Iglesias, B. A.; Gonçalves, D. F.; Terra Stefanello, S.; Soares, F. A.; Piccoli, B. C.; D’Avila da Silva, F.; da Rocha, J. B. T.; Schultze, E.; Bonemann Bender, C.; Collares, T.; Kömmling Seixas, F.; Peterle, M. M.; Braga, A. L.; Rodrigues, O. E. D. Synthesis and Antitumoral Lung Carcinoma A549 and Antioxidant Activity Assays of New Chiral β‐Aryl‐Chalcogenium Azide Compounds. ChemistrySelect 2017, 2 (27), 8423–8430. https://doi.org/10.1002/slct.201701107.Suche in Google Scholar
77. Miyashita, M.; Hoshino, M.; Yoshikoshi, A. Sodium Phenylseleno (Triethoxy) Borate, Na+ (PhSeB (OEt) 3]−: The Reactive Species Generated from (PhSe) 2 with NaBH4 in Ethanol. Tetrahedron Lett. 1988, 29 (3), 347–350. https://doi.org/10.1016/s0040-4039(00)80092-1.Suche in Google Scholar
78. Lucia Ruiz Benitez, M.; Severo Sabedra Sousa, F.; Peter, F. I.; Carlos, R. J. J.; Victoria, M. B. M.; Vieira, S. N.; Tabarelli, G.; Klein Couto, G.; Júlia Damé Fonseca Paschoal, M.; Silveira Pacheco, B.; Rodrigues, O.; Collares, T.; Kömmling Seixas, F. Chiral β‐arylchalcogenium Azide Induce Apoptosis and Regulate Oxidative Damage on Human Bladder Cancer Cells. ChemistrySelect 2022, 7 (42), e202203207. https://doi.org/10.1002/slct.202203207.Suche in Google Scholar
79. Gunnarsson, R.; Brenning, N.; Boyd, R. D.; Helmersson, U. Nucleation of Titanium Nanoparticles in an Oxygen-Starved Environment. I: Experiments. J. Phys. Appl. Phys. 2018, 51 (45), 455201. https://doi.org/10.1088/1361-6463/aae117.Suche in Google Scholar
80. Leitemberger, A.; Sonego, M. S.; Garcia, F. D.; Dos Santos, A. C.; Piccoli, B. C.; da Silva, F. D.; Oliveira, C. S.; Seixas, F. K.; Dornelles, L.; Rocha, J. B.; Nogara, P. A.; Schachtschneider, K. M.; Collares, T.; Rodrigues, O. E. Synthesis and Biological Evaluation of New Antioxidant and Antiproliferative Chalcogenobiotin Derivatives for Bladder Carcinoma Treatment. Bioorg. Med. Chem. 2020, 28 (9), 115423. https://doi.org/10.1016/j.bmc.2020.115423.Suche in Google Scholar PubMed
81. Tripodo, G.; Mandracchia, D.; Collina, S.; Rui, M.; Rossi, D. New Perspectives in Cancer Therapy: the Biotin-Antitumor Molecule Conjugates. Med. Chem. 2014, 8, 1–4.Suche in Google Scholar
82. Plazuk, D.; Zakrzewski, J.; Salmain, M.; Błauż, A.; Rychlik, B.; Strzelczyk, P.; Bujacz, A.; Bujacz, G. Ferrocene–biotin Conjugates Targeting Cancer Cells: Synthesis, Interaction with Avidin, Cytotoxic Properties and the Crystal Structure of the Complex of Avidin with a Biotin–Linker–Ferrocene Conjugate. Organometallics 2013, 32 (20), 5774–5783. https://doi.org/10.1021/om4003126.Suche in Google Scholar
83. Xu, H.; Hou, W. Selenium-Containing Heterocycles. Privileged Scaffolds in Drug Discovery; Elsevier: Cambridge, MA, 2023; pp 915–930.10.1016/B978-0-443-18611-0.00006-1Suche in Google Scholar
84. Angeli, A.; Etxebeste-Mitxeltorena, M.; Sanmartín, C.; Espuelas, S.; Moreno, E.; Azqueta, A.; Parkkila, S.; Carta, F.; Supuran, C. T. Tellurides Bearing Sulfonamides as Novel Inhibitors of Leishmanial Carbonic Anhydrase with Potent Antileishmanial Activity. J. Med. Chem. 2020, 63 (8), 4306–4314. https://doi.org/10.1021/acs.jmedchem.0c00211.Suche in Google Scholar PubMed
85. Angeli, A.; Pinteala, M.; Maier, S. S.; Toti, A.; Mannelli, L. D. C.; Ghelardini, C.; Selleri, S.; Carta, F.; Supuran, C. T. Tellurides Bearing Benzensulfonamide as Carbonic Anhydrase Inhibitors with Potent Antitumor Activity. Bioorg. Med. Chem. Lett 2021, 45, 128147. https://doi.org/10.1016/j.bmcl.2021.128147.Suche in Google Scholar PubMed
86. Khalifah, R. G. The Carbon Dioxide Hydration Activity of Carbonic Anhydrase: I. Stop-Flow Kinetic Studies on the Native Human Isoenzymes B and C. J. Biol. Chem. 1971, 246 (8), 2561–2573. https://doi.org/10.1016/s0021-9258(18)62326-9.Suche in Google Scholar
87. Neri, D.; Supuran, C. T. Interfering with pH Regulation in Tumours as a Therapeutic Strategy. Nat. Rev. Drug Discov. 2011, 10 (10), 767–777. https://doi.org/10.1038/nrd3554.Suche in Google Scholar PubMed
88. Supuran, C. T. Experimental Carbonic Anhydrase Inhibitors for the Treatment of Hypoxic Tumors. J. Exp. Pharmacol. 2020, 603–617. https://doi.org/10.2147/jep.s265620.Suche in Google Scholar PubMed PubMed Central
89. Li, Y.; Wang, H.; Oosterwijk, E.; Tu, C.; Shiverick, K. T.; Silverman, D. N.; Frost, S. C. Expression and Activity of Carbonic Anhydrase IX Is Associated with Metabolic Dysfunction in MDA-MB-231 Breast Cancer Cells. Cancer Invest. 2009, 27 (6), 613–623. https://doi.org/10.1080/07357900802653464.Suche in Google Scholar PubMed PubMed Central
90. Tanini, D.; Ricci, L.; Capperucci, A.; Mannelli, L. D. C.; Ghelardini, C.; Peat, T. S.; Carta, F.; Angeli, A.; Supuran, C. T. Synthesis of Novel Tellurides Bearing Benzensulfonamide Moiety as Carbonic Anhydrase Inhibitors with Antitumor Activity. Eur. J. Med. Chem. 2019, 181, 111586. https://doi.org/10.1016/j.ejmech.2019.111586.Suche in Google Scholar PubMed
91. Scozzafava, A.; Menabuoni, L.; Mincione, F.; Briganti, F.; Mincione, G.; Supuran, C. T. Carbonic Anhydrase Inhibitors. Synthesis of Water-Soluble, Topically Effective, Intraocular Pressure-Lowering Aromatic/heterocyclic Sulfonamides Containing Cationic or Anionic Moieties: Is the Tail More Important Than the Ring? J. Med. Chem. 1999, 42 (14), 2641–2650. https://doi.org/10.1021/jm9900523.Suche in Google Scholar PubMed
92. Kumar, A.; Siwach, K.; Supuran, C. T.; Sharma, P. K. A Decade of Tail-Approach Based Design of Selective as Well as Potent Tumor Associated Carbonic Anhydrase Inhibitors. Bioorg. Chem. 2022, 126, 105920. https://doi.org/10.1016/j.bioorg.2022.105920.Suche in Google Scholar PubMed
93. Petreni, A.; Iacobescu, A.; Simionescu, N.; Petrovici, A.-R.; Angeli, A.; Fifere, A.; Pinteala, M.; Supuran, C. T. Carbonic Anhydrase Inhibitors Bearing Organotelluride Moieties as Novel Agents for Antitumor Therapy. Eur. J. Med. Chem. 2022, 244, 114811. https://doi.org/10.1016/j.ejmech.2022.114811.Suche in Google Scholar PubMed
94. Peña-Morán, O. A.; Villarreal, M. L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera Fagaroides Var. Fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21 (8), 1013. https://doi.org/10.3390/molecules21081013.Suche in Google Scholar PubMed PubMed Central
95. Alharis, R.; Al-Asadi, R. H.; Hassan, D. A. New Mercurated and Tellurated Sulpha Compounds: Synthesis, Invitro Anticancer Study and DFT Calculation. Egypt. J. Chem. 2021, 64 (10), 5755–5764.Suche in Google Scholar
96. Ray, P.; Ferraro, M.; Haag, R.; Quadir, M. Dendritic Polyglycerol‐derived Nano‐architectures as Delivery Platforms of Gemcitabine for Pancreatic Cancer. Macromol. Biosci. 2019, 19 (7), 1900073. https://doi.org/10.1002/mabi.201900073.Suche in Google Scholar PubMed
97. Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. Natural Compounds in Prostate Cancer Prevention and Treatment: Mechanisms of Action and Molecular Targets. Cells 2020, 9 (2), 460. https://doi.org/10.3390/cells9020460.Suche in Google Scholar PubMed PubMed Central
98. Ray, P.; Nair, G.; Ghosh, A.; Banerjee, S.; Golovko, M. Y.; Banerjee, S. K.; Reindl, K. M.; Mallik, S.; Quadir, M. Microenvironment-sensing, Nanocarrier-Mediated Delivery of Combination Chemotherapy for Pancreatic Cancer. J. Cell Commun. Signal. 2019, 13, 407–420. https://doi.org/10.1007/s12079-019-00514-w.Suche in Google Scholar PubMed PubMed Central
99. Roos, F.; Binder, K.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F. K. H.; Juengel, E.; Blaheta, R. A. The Antitumor Effect of Curcumin in Urothelial Cancer Cells Is Enhanced by Light Exposure In Vitro. Evid. base Compl. Alternative Med. 2019, 2019. https://doi.org/10.1155/2019/6374940.Suche in Google Scholar PubMed PubMed Central
100. Dalal, M.; Antil, N.; Kumar, B.; Devi, J.; Garg, S. Exploring the Novel Aryltellurium (IV) Complexes: Synthesis, Characterization, Antioxidant, Antimicrobial, Antimalarial, Theoretical and ADMET Studies. Inorg. Chem. Commun. 2024, 159, 111743. https://doi.org/10.1016/j.inoche.2023.111743.Suche in Google Scholar
101. Dalal, M.; Antil, N.; Kumar, B.; Garg, S. Unveiling the Therapeutic Potential of Organotellurium (IV) Schiff Base Complexes through Structural Elucidation, Antimalarial, Antioxidant and Antimicrobial Studies. J. Mol. Struct. 2024, 138558. https://doi.org/10.1016/j.molstruc.2024.138558.Suche in Google Scholar
102. Yahaya, N. P.; Ndahi, N. P.; Bako, L.; Madugu, M. L.; Zulqiflu, A.; Mamman, Y. Synthesis and Partial Characterization of Two Schiff Base Ligands with (2 and 4-nitroaniline) and Their Transition Metal (II)(Co and Cu) Complexes. Dutse J. Peace Appl. Sci. 2018, 4 (2), 584–591.Suche in Google Scholar
103. Pachwania, S.; Devi, J.; Taxak, B.; Boora, A. Synthesis, Characterization, and Biological Evaluation of Organotin (IV) Complexes Derived from Schiff Bases of 3-methoxybenzohydrazide. Phosphorus, Sulfur, Silicon Relat. Elem. 2023, 198 (2), 102–113. https://doi.org/10.1080/10426507.2022.2116637.Suche in Google Scholar
104. Dalal, M.; Dubey, A.; Tufail, A.; Antil, N.; Sehrawat, N.; Garg, S. Organyltellurium (IV) Complexes Incorporating Schiff Base Ligand Derived from 2-Hydroxy-1-Naphthaldehyde: Preparation, Spectroscopic Investigations, Antimicrobial, Antioxidant Activities, DFT, MESP, NBO, Molecular Docking and ADMET Evaluation. J. Mol. Struct. 2023, 1287, 135590. https://doi.org/10.1016/j.molstruc.2023.135590.Suche in Google Scholar
105. Dalal, M.; Dubey, A.; Antil, N.; Tufail, A.; Garg, S. Synthesis, Structural Investigation of Schiff Base Endowed Organyltellurium (IV) Complexes: Biological Activities, Molecular Docking, Quantum Chemical Computations and ADMET Prediction. Res. Chem. Intermed. 2023, 49 (7), 2889–2917. https://doi.org/10.1007/s11164-023-05015-5.Suche in Google Scholar
106. Mustafa, G.; Zia-ur-Rehman, M.; Sumrra, S. H.; Ashfaq, M.; Zafar, W.; Ashfaq, M. A Critical Review on Recent Trends on Pharmacological Applications of Pyrazolone Endowed Derivatives. J. Mol. Struct. 2022, 1262, 133044. https://doi.org/10.1016/j.molstruc.2022.133044.Suche in Google Scholar
107. Dawar, N.; Devi, J.; Kumar, B.; Dubey, A. Synthesis, Characterization, Pharmacological Screening, Molecular Docking, DFT, MESP, ADMET Studies of Transition Metal (II) Chelates of Bidentate Schiff Base Ligand. Inorg. Chem. Commun. 2023, 151, 110567. https://doi.org/10.1016/j.inoche.2023.110567.Suche in Google Scholar
108. Bhardwaj, A.; Kumar, M.; Garg, S.; Kumar, D. Organotellurium (IV) Complexes Derived from Thiophene Based Schiff Base 5-Methyl-2-Thiophene Carboxaldehyde: Synthesis, Spectral Characterization, Thermal Analysis Potent Antimicrobial and Antioxidant Activities Supported by Molecular Docking, DFT Studies and ADMET Prediction. Inorg. Chem. Commun. 2023, 157, 111332. https://doi.org/10.1016/j.inoche.2023.111332.Suche in Google Scholar
109. Kumar, M.; Chauhan, S.; Sindhu, M.; Darolia, P. J.; Bhardwaj, A.; Garg, S. Organotellurium (IV) Complexes of N-Methylisatin-O-Aminothiophenol Schiff Base: Preparation, Characterization, DFT, Molecular Docking Studies, Antimicrobial and Antioxidant Activity. J. Indian Chem. Soc. 2023, 100 (2), 100797. https://doi.org/10.1016/j.jics.2022.100797.Suche in Google Scholar
110. Bhardwaj, A.; Kumar, M.; Bendi, A.; Garg, S. Theoretical and Experimental In‐vitro Studies of Novel Thiophene Based Organotellurium (IV) Complexes. Chem. Biodivers. 2024, 21 (2), e202301544. https://doi.org/10.1002/cbdv.202301544.Suche in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Comprehensive reviews on the potential applications of inorganic metal sulfide nanostructures in biological, environmental, healthcare, and energy generation and storage
- Comparative analysis of dye degradation methods: unveiling the most effective and environmentally sustainable approaches, a critical review
- A review: evaluating methods for analyzing kidney stones and investigating the influence of major and trace elements on their formation
- Revolutionizing Metal-organic Frameworks (MOFs) in Wastewater Treatment Applications
- Advances in synthesis and anticancer applications of organo-tellurium compounds
- Effect of doping of metal salts on polymers and their applications in various fields
- Recent trends in medicinal applications of mercury based organometallic and coordination compounds
- A review of organometallic compounds as versatile sensors in environmental, medical, and industrial applications
- A comprehensive overview of fabrication of biogenic multifunctional metal/metal oxide nanoparticles and applications
- Semiconductor-attapulgite composites in environmental and energy applications: a review
Artikel in diesem Heft
- Frontmatter
- Comprehensive reviews on the potential applications of inorganic metal sulfide nanostructures in biological, environmental, healthcare, and energy generation and storage
- Comparative analysis of dye degradation methods: unveiling the most effective and environmentally sustainable approaches, a critical review
- A review: evaluating methods for analyzing kidney stones and investigating the influence of major and trace elements on their formation
- Revolutionizing Metal-organic Frameworks (MOFs) in Wastewater Treatment Applications
- Advances in synthesis and anticancer applications of organo-tellurium compounds
- Effect of doping of metal salts on polymers and their applications in various fields
- Recent trends in medicinal applications of mercury based organometallic and coordination compounds
- A review of organometallic compounds as versatile sensors in environmental, medical, and industrial applications
- A comprehensive overview of fabrication of biogenic multifunctional metal/metal oxide nanoparticles and applications
- Semiconductor-attapulgite composites in environmental and energy applications: a review

