Abstract
This review provides a detailed inventory analysis of the manufacturing process of a cosmetic cream using gold nanoparticles (AuNPs) and hydroxylated fullerene water complex (3HFWC) as novel nanocomponents for cream. The inventory analysis was focused on the evaluation of the two raw materials of the nanocomponents, the consumption of electricity and water, which enabled an insight into the process flows within the production process. The data obtained from this analysis of the inventory of nanocomponents provide an insight into the potential improvements that can be made in the manufacturing process of nanocomponents, in order to reduce the environmental impact of the production of new cosmetic creams. These results will serve as the basis for the second part of the analysis, where a life cycle analysis will be carried out to assess the environmental impacts of cream production from the acquisition of raw materials to the disposal of the final product.
1 Introduction
Sustainable and environmentally friendly practices are becoming more and more important in various industries, including cosmetics. 1 , 2 As the demand for environmentally friendly products continues to increase, it has become imperative for companies to conduct a Life Cycle Analysis (LCA) to assess the environmental impact of their products throughout their life cycle.
The global and profitable cosmetics industry offers a variety of products to improve the way consumers look and feel. 3 Cosmetic cream is one of the most popular and widely used products in this industry, with applications ranging from moisturising, anti-ageing and sun protection to skin whitening. The cosmetics industry enhances these effects with novel ingredients, such as gold nanoparticles (AuNPs). AuNPs are reported to have anti-ageing and skin-hydrating properties. 4 However, there is concern regarding their potential toxicity, especially in relation to their size. Smaller particles, up to about 20 nm, have more potential for skin penetration than larger ones from 40 nm upwards, as tested. 5 There are reports of 28 nm AuNPs having a weak toxicological response on hairless mouse skin, while larger sizes of 49 and 73 nm showed no toxicological response. 6 This response is also achieved with AuNPs embedded in cosmetic creams, as the interaction between the AuNPs and cosmetics’ ingredients seems to play a critical role in toxicity enhancement or reduction. Studies showed the toxic and non-toxic nature of AuNPs, depending on their characteristics, preparations and physicochemical properties. As such, their overall effects are not considered conclusive, with more studies required to focus on their changes in physical properties before and after treatment with biological media. 7
The production and consumption of cosmetic creams also have significant environmental impacts that must be assessed and mitigated. 4 The purpose of all studies is to use a life cycle analysis to assess the environmental impacts that occur throughout the entire life cycle of cosmetic cream production, i.e. from the acquisition of raw materials, the supply and transport of materials and packaging, as well as the production itself, to the handling of the products after use. 5 The primary task of the LCA method is to provide information on the basis of which we can make environmentally oriented business decisions, for example, comparing the environmental impacts of different products, choosing different materials, developing a new product, choosing technological processes that are less harmful to the environment, introducing new recycling processes, or choosing suitable packaging. The purpose of modern research is to assess the environmental impacts of cosmetic creams in their life cycle, and to stimulate marketing activities that will increase the use of more environmentally friendly products. The inventory analysis involves the following steps:
Identification of the system boundaries: Definition of the assessment’s functional unit and system boundaries. This includes determining which processes and activities are included in the inventory analysis, and which inputs and outputs are considered.
Collection of data: The inputs and outputs of each process and activity within the system boundaries. These data may include information on the raw materials used, energy consumed, water consumed, emissions released, and waste generated at each life cycle stage.
Conversion of the data to a standard unit: Conversion of the data collected to a standard unit of measurement allows for data comparison and aggregation. For example, energy inputs may be converted to kilowatt-hours, while emissions may be converted to kilogrammes of carbon dioxide equivalents.
Quality check: Review the data for accuracy, completeness and consistency. This involves validating the data with industry experts, or performing sensitivity analyses to test the impact of different assumptions and data sources.
Calculation of the inventory results: We used software tools and databases to calculate the total inputs and outputs for each life cycle stage and the total inventory results for the entire life cycle. This involved using the specialised LCA software OpenLCA, which contains pre-populated environmental data databases for various products and processes.
Documentation of the inventory: The inventory data and methods used in the analysis will be documented transparently and consistently, to facilitate the reproducibility and comparability of the results.
In this review, we have analysed the raw materials of the cosmetic cream, which will serve as the basis for the assessment of the product’s life cycle. More precisely, we have studied all the procedures of making a cosmetic cream at different stages of the production process. We also assessed the environmental impacts of the production process, such as energy consumption, greenhouse gas emissions and water consumption. The purpose of the inventory analysis was to provide a comprehensive understanding of the environmental impact of the cosmetic cream, and to identify opportunities to improve the production process. These results will help the entire cosmetics industry to improve the sustainability of their products and reduce their environmental footprint. The inventory analysis performed in this review will serve as the basis for a comprehensive life cycle assessment of the cosmetic cream. The LCA will assess the environmental impact of a product throughout its life cycle, including the procurement of raw materials, production, distribution, use and disposal. By performing the LCA we aim to identify areas of the product’s life cycle with the most significant impact on the environment, and evaluate the effectiveness of possible improvement strategies. In addition, the LCA will allow us to compare the environmental impact of the cosmetic cream with other similar products on the market and determine its overall sustainability. The findings of this inventory analysis and LCA will be valid for other decision-makers in the cosmetics industry, including product designers, manufacturers and marketers, so that they can make informed decisions on how to improve the sustainability of their products. Overall, the aim of this review was to provide a better understanding of the environmental impact of cosmetic creams, and to promote more sustainable practices in the cosmetic industry.
2 Materials and methods
Cosmetic creams are used widely by people worldwide for various purposes, such as moisturising, anti-ageing and skin-brightening effects. 6 , 7 The demand for cosmetic creams with innovative and effective ingredients is increasing constantly. In this context, the use of AuNPs 8 , 9 and hydroxylated fullerene water complex (3HFWC) as key ingredients in cosmetic creams has gained popularity, due to their unique properties and potential benefits for the skin. The production process of cosmetic cream that includes these ingredients involves several steps, including the synthesis, purification, and drying of the AuNPs, the synthesis of 3HFWC, and the mixing of the cream with other ingredients. 2 Figure 1 outlines the detailed process of producing cosmetic cream that includes AuNPs and 3HFWC as key ingredients. A detailed inventory analysis for each process is provided in the continuation of the manuscript.
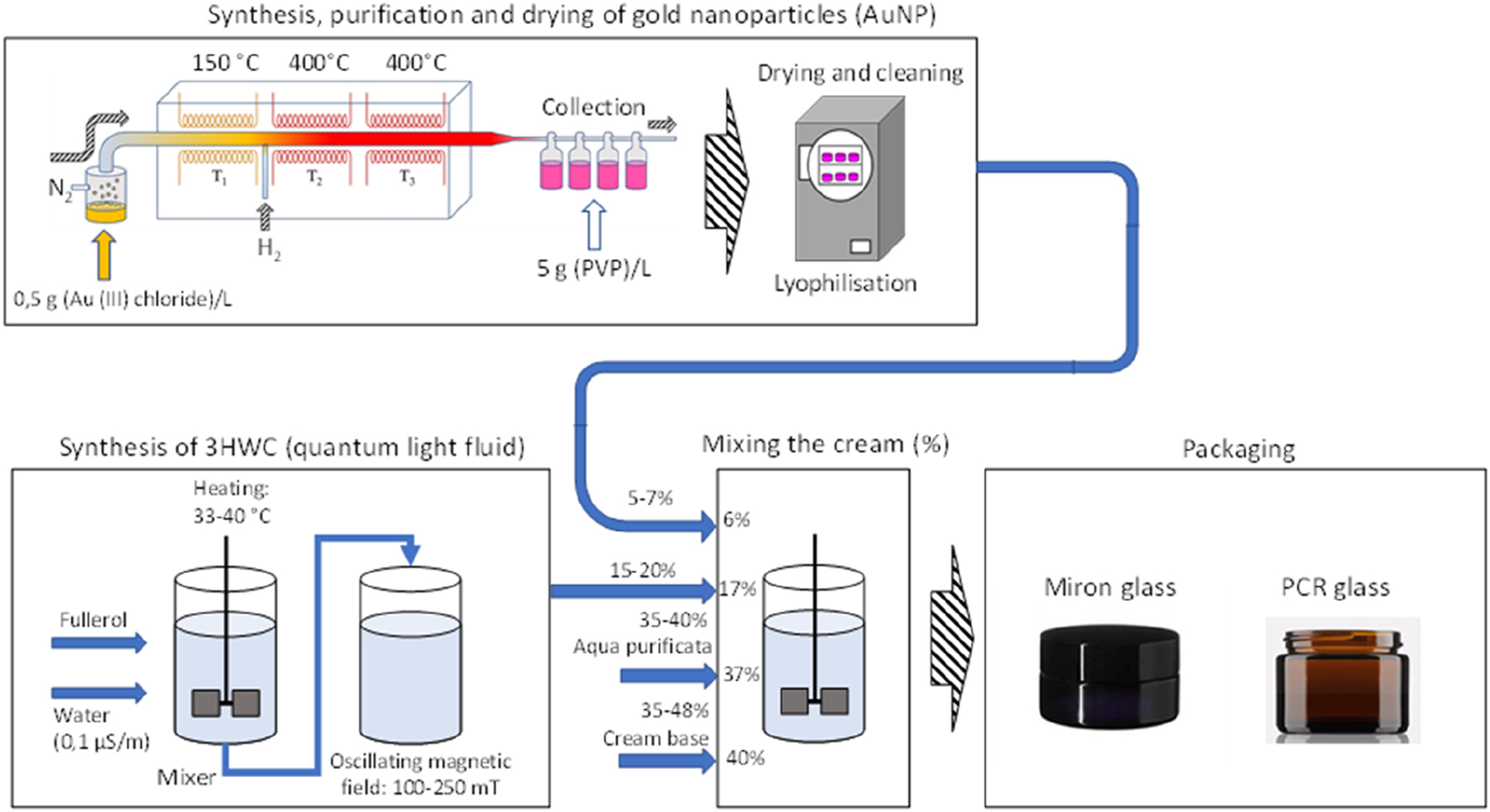
Detailed process involved in producing cosmetic cream.
3 Results and discussion
3.1 Inventory data for the synthesis, purification and drying of AuNPs
The data inventory for the synthesis, purification and drying of AuNPs was first carried out by reviewing and processing the input data received from the manufacturing process. The manufacturing processes used in the evaluated system were reviewed next.
3.1.1 Synthesis of HAuCl4·3H2O
A common chemical reaction for synthesising HAuCl₄·3H₂O involves the dissolution of gold metal in aqua regia, a mixture of nitric acid (HNO₃) and hydrochloric acid (HCl). Aqua regia is one of the few reagents capable of dissolving gold. This unique capability arises not from hydrochloric acid alone, but from the combined action of both acids, where the nitric acid oxidises the gold, and the hydrochloric acid stabilises the gold ions in the solution.
The reaction proceeds as follows:
Upon cooling and partial evaporation of the aqueous solution obtained from the reaction above, gold(III) chloride trihydrate crystallises out of the solution:
The process involves controlling the evaporation rate and the temperature, to ensure that the product retains its trihydrate form.
To synthesise HAuCl4·3H2O, we need to determine the amounts of each required starting reagent. The molar mass of HAuCl4·3H2O is 393.83 g/mol. Therefore, 1 g of HAuCl4·3H2O is equivalent to:
Now we can calculate the mass inputs of each starting chemical:
We need 1 mol of gold (Au) to synthesise 1 mol of HAuCl4·3H2O. Therefore, we need the following:
We also need 4 mol of hydrochloric acid (HCl) and 1 mol of nitric acid (HNO3) for each mole of HAuCl4·3H2O. The molar masses of HCl and HNO3 are 36.46 g/mol and 63.01 g/\mol, respectively. Therefore, we need the following:
Therefore, to synthesise 1 g of HAuCl4·3H2O, we need:
The data used for individual input process streams for the production of HAuCl4·3H2O are as follow:
3.1.1.1 Gold (Au)
The input data for pure gold were the EcoInvent dataset »market for gold | gold | Cutoff, U«. This dataset describes the production of 1 kg of gold. Data were taken from the 2005 Environmental Report of Newmont 10 for the Kalgoorlie, Jundee, Tanami and Pajingo Mines – all of which produce only gold. The data for the reference flows, by-products and most exchanges were calculated by piling up the corresponding inputs and outputs of each mine, and then scaling them to the production of 1 kg of gold. Tanami and Pajingo are underground mines. Kalgoorlie is an open pit, and Jundee is both an open pit and underground. The system includes the transport of raw materials to the mine. The activity ends with the Wohlwill electrolysis of gold. 11 The dataset includes the mining and beneficiation of the metals, the smelting, the energy, materials and land consumption, and the air and water emissions. The dataset does not include the emissions from the refining step.
3.1.1.2 Hydrogen chloride (HCl)
This dataset represents the production of 1 kg of hydrochloric acid via the combustion of chlorine with hydrogen. Hydrogen chloride (HCl) is a colourless to yellowish-fuming liquid with a sharp, pungent odour. It is a strong, highly corrosive acid. This dataset estimates the amounts of raw materials and emissions based on stoichiometric calculations. 12 The electricity consumption is approximated based on data from a large chemical factory. 13 The activity starts when the raw materials enter the process. This activity ends with the production of hydrochloric acid. The dataset includes the input materials, energy uses, infrastructure and emissions. As an input for hydrochloric acid we considered the dataset from the EcoInvent database 14 »market for hydrochloric acid, without water, in a 30 % solution state | hydrochloric acid, without water, in a 30 % solution state | Cutoff, U«.
3.1.1.3 Nitric acid (HNO3)
This nitric acid process represents a market activity. This dataset is a consumption mix, representing the supply of “nitric acid, without water, in a 50 % solution state” from activities that produce it, to activities that consume it within the geography of this dataset, Europe without Russia. The dataset considers transportation from the producer to the consumer of this product. The transportation distances are based on the default transport distances for markets. 14
»Nitric acid, without water, in a 50 % solution state« is an inorganic substance whose molecular formula is HNO3. It is liquid in normal conditions of temperature and pressure. This dataset represents a pure substance (100 % active substance). The reference to a “50 % solution state” pertains to the common industrial usage of nitric acid, which is typically utilised in a diluted form for safety and practicality. However, in the Ecoinvent database used, this substance is described as 100 % active, implying a pure form. On a consumer level, »nitric acid, without water, in a 50 % solution state« is used in the following products: washing & cleaning products, fertilisers, polishes and waxes and air care products. On industrial sites, the substance is used for the manufacture of products in the following sectors: pH regulators and water treatment products, washing and cleaning products, non-metal-surface treatment products, metal surface treatment products, water treatment chemicals, semiconductors and laboratory chemicals. The input data for nitric acid were from the EcoInvent dataset 14 »market for nitric acid, without water, in a 50 % solution state | nitric acid, without water, in a 50 % solution state | Cutoff, U«.
3.1.2 Nitrogen
This dataset for nitrogen represents the production of liquefied nitrogen, oxygen and argon by cryogenic air separation. The liquefaction process of air represents an average cryogenic air separation process. Nitrogen is often used as an »inert« gas, due to its non-reactive nature with many materials. Commercial nitrogen is produced by different air separation processes, such as cryogenic liquefaction and distillation, pressure swing adsorption (PSA) and membrane separation. Gaseous nitrogen is used in the chemical and petroleum industries for storage tank blanketing and vessel inerting applications, in the food industries to pack oxidisable foods, and in the electronics and metals industries for inert properties. This is the market for “nitrogen liquid” in the geography of Europe and is a constrained market for consequential system models. For attributional system models, this is a stock market. In the case of the consequential system model, details about the marginal consumer can be found in the comment on the conditional exchange (by-product).
Nitrogen, a liquid, is usually produced as a by-product of different activities. This means its production volume always depends on the number of reference products produced in those activities. The consequence is that the market for nitrogen liquid is not fully flexible, but constrained. In the case when the demand increases, the supply will not increase.
Special transport modelling for liquid gases: No ship transport and normal transport modelling for chemicals reduced by 90 %. This activity starts at the gate of the activities that produce »nitrogen, liquid« within the geography of Europe. This activity ends with the supply of »nitrogen, liquid« to the consumers of this product. Transport is included. Product losses during transportation are assumed negligible, and are therefore not included.
The input data for nitrogen were from the EcoInvent dataset 14 »market for nitrogen, liquid | nitrogen, liquid | Cutoff, U«. For a typical synthesis, 1.21 N2 cylinders are used (data from a practical example). The volume of the cylinder is 50 L, filled to 200 bar. The ideal gas law can be used to calculate the mass of nitrogen gas at a pressure of 200 bar and a volume of 60.5 L:
where p is the pressure, V is the volume, n is the number of moles, R is the gas constant and T the temperature. We assumed a standard temperature of 0 °C (273.15 K) for the calculation.
First, we can calculate the number of moles of nitrogen gas:
Then, using the molar mass value, we can calculate the mass of nitrogen gas:
Therefore, the mass of nitrogen gas at a pressure of 200 bar and a volume of 50 L is approximately 14.934 kg.
3.1.3 Hydrogen
The hydrogen production process represents a market activity for hydrogen production. When relevant, they also account for transport to the consumer and the losses during that process. This is the »hydrogen gaseous« market in the Global geography. This product is generally considered an intermediate product, and is expected to be used at or near the production site. Therefore, the market does not contain any transport.
For processes demanding gaseous hydrogen, it is advised to set direct activity links to the producing activities. »hydrogen, gaseous« is an inorganic substance called »molecular hydrogen« under IUPAC naming, and its molecular formula is H2. It is gas under normal temperature and pressure conditions and has no odour. The substance is modelled as a pure substance. On industrial sites the substance is used to manufacture products in the following sectors: Fuels and fuel additives, functional fluids (closed systems), intermediates, laboratory chemicals, odour agents, oxidising/reducing agents, and processing aids specific to petroleum production. The activity begins with the product leaving the production site. The activity ends with the product arriving at the user. As this product is generally considered to be used on or near the production site, no transport requirements are included.
The hydrogen input data were from the Ecoinvent database 14 »market for hydrogen, gaseous | hydrogen, gaseous | Cutoff, U«.
For a normal synthesis, one cylinder of H2 is used (data from a practical example). The volume of the cylinder is 50 L, filled to 200 bar.
The ideal gas law can be used to calculate the mass of hydrogen gas at a pressure of 200 bar and a volume of 50 L:
where p is the pressure, V is the volume, n is the number of moles, R is the gas constant and T the temperature. We assumed a standard temperature of 0 °C (273.15 K) for the calculation.
First, we can calculate the number of moles of hydrogen gas:
Then, using the molar mass value, we can calculate the mass of hydrogen gas:
Therefore, the mass of hydrogen gas at a pressure of 200 bar and a volume of 50 L at standard temperature is approximately 0.88 kg.
3.1.4 Polyvinylpyrrolidone (PVP)
PVP stands for Polyvinylpyrrolidone, a water-soluble polymer used commonly in various industrial and medical applications. PVP is a linear polymer comprised of repeating units of the monomer N-vinylpyrrolidone. In the medical field, PVP is often used as a binder, thickener and emulsifier in various pharmaceutical and cosmetic products. It is also used as a coating for tablets and capsules, and a dispersant for pigments and other insoluble substances. PVP is used as a binder and dispersant in industrial applications, to produce ceramics, inks and adhesives. It is also used in the manufacture of textiles, paper and detergents.
PVP (Polyvinylpyrrolidone) is purified through a series of steps that typically involve dissolution in a suitable solvent, followed by filtration to remove insoluble impurities. After filtration, the solution might undergo further purification steps, such as dialysis against a solvent to remove low molecular weight substances, or repeated precipitation and redissolution in solvents of varying polarity to separate the PVP from other soluble impurities. The final step usually involves drying the purified PVP under specific conditions, to obtain it in a solid form suitable for use in subsequent applications or research studies.
In our application PVP is used as a stabilising agent: the amount of stabilising agent required in AuNPs’ synthesis depends on the specific stabilising agent used and the desired size and morphology of the AuNPs. Common stabilising agents used for the synthesis of AuNPs include cetyltrimethylammonium bromide (CTAB), polyvinylpyrrolidone (PVP) and bovine serum albumin (BSA). PVP was used in our case. The input for PVP was the Ecoinvent database 14 dataset »market for N-methyl-2-pyrrolidone | N-methyl-2-pyrrolidone | Cutoff, U«.
3.1.5 Water (0.1 μS/m)
This is a market activity representing the consumption mix of a product in a given geography, connecting suppliers with consumers of the same product in the same geographical area. Ultra-pure water is a standard requirement in many industrial applications. As such, these industries can only invest in high-quality water production equipment for their plants and facilities. 15 Thus, ultrapure water production is usually carried out at the premises of each industry. No transport and losses are assumed. This activity starts at the gate of the activities that produce »water, ultrapure« within the geography of Europe. This activity ends with the supply of »water, ultrapure« to the consumers of this product. Transport or losses are considered irrelevant for this product. This market represents the local market for 1 kg of ultrapure water in Europe. As water input, we considered the Ecoinvent database 14 dataset »market for water, ultrapure | water, ultrapure | Cutoff, U«.
3.1.6 Miron glass jar 1 L with lid
Miron glass is a biophotonic glass that filters different light frequencies to protect and revitalise natural products, extending their shelf life drastically (https://www.mironglass.com). It is also known as violet glass, because of its dark purple colour. It allows only beneficial rays, such as violet, ultraviolet and infrared, to pass through, while blocking the harmful rays that can degrade the quality of natural products. Miron glass is used for packaging various natural products, such as cosmetics, skincare, foods, drinks, natural healing and nutrition.
Miron glass is made by adding a blend of minerals to the molten glass during manufacturing, giving it its distinctive violet colour. The minerals used in Miron glass are believed to block out harmful light frequencies selectively, while allowing in beneficial light, such as violet, ultraviolet and infrared. This selective filtering is thought to help protect the contents from the damaging effects of light, which can cause degradation and loss of potency over time.
Miron glass’s specific properties and benefits are still being studied, and the scientific evidence is limited. However, some studies have suggested that Miron glass may help to maintain the quality and shelf life of certain products, such as essential oils, herbs and supplements, by protecting them from the damaging effects of light and oxidation. As no Miron glass production reports were available in the database, we used as an alternative a dataset that represents the production of 1 kg of brown packaging glass from a mix of primary and secondary raw materials. The material inputs were estimated based on literature sources and a Swiss production site. The energy and water consumption, emissions, and waste production were estimated based on literature sources. Infrastructure is an estimation based on the average European packaging glass factories. The CO2 emissions have been recalculated from the carbon content in raw materials and fuels. The dataset includes the reception of the precursors silica sand, soda, unsorted glass cullets, limestone, dolomite, feldspar, and chemicals inorganics at the factory gate. The activity ends with the production of 1 kg of brown packaging glass. The dataset includes material and energy inputs, water consumption, emissions to air and water, waste production and infrastructure.
The input for packaging glass was the dataset from the Ecoinvent database 14 »packaging glass production, brown | packaging glass, brown | Cutoff, U«.
The data for all the input components for synthesising, purifying and drying AuNPs s are shown in Table 1.
Inventory for synthesising, purifying and drying AuNPs. 14
| Data | Quantity | Unit | Dataset |
|---|---|---|---|
| HAuCl4 × 3H2O | |||
| Gold (Au) | 0.501 | g | »Market for gold | gold | Cutoff, U« |
| Hydrogen chloride (HCl) | 0.370 | g | »Market for hydrochloric acid, without water, in 30 % solution state | hydrochloric acid, without water, in 30 % solution state | Cutoff, U«. |
| Nitric acid (HNO3) | 0.160 | g | »Market for nitric acid, without water, in 50 % solution state | nitric acid, without water, in 50 % solution state | Cutoff, U« |
| Water | 0.163 | g | »Market for water, ultrapure | water, ultrapure | Cutoff, U« |
| Nitrogen | 14.934 | kg | »Market for nitrogen, liquid | nitrogen, liquid | Cutoff, U« |
| Hydrogen | 0.888 | kg | »Market for hydrogen, gaseous | hydrogen, gaseous | Cutoff, U« |
| Polyvinylpyrrolidone (PVP) | 32.400 | g | »Market for N-methyl-2-pyrrolidone | N-methyl-2-pyrrolidone | Cutoff, U« |
| Water (0.1 μS/m) | 15.200 | L | »Market for water, ultrapure | water, ultrapure | Cutoff, U« |
| Miron glass jar 1 L with lid | 1 | Item | »Packaging glass production, brown | packaging glass, brown | Cutoff, U« |
3.1.7 Electricity for synthesis, purification and drying of AuNPs
For an inventory of the electricity data for the base cream, we used the »market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« from the Ecoinvent database. 14 This dataset does not include losses and relevant exchanges (transport, transport infrastructure, etc.). They are present in the contained markets. This is the market group for »electricity, medium voltage, in Europe without Switzerland«. This activity starts at the gate of markets that deliver »electricity, medium voltage« in geographies contained within the geography of this dataset. This activity ends with the supply of »electricity, medium voltage« to the consumers of this product within the geography of Europe without Switzerland.
The inventory of electricity used for synthesising, purifying, and drying AuNPs is presented in Table 2.
Inventory of electricity use for synthesising, purifying, and drying AuNPs. 14
| Data | Quantity | Unit | Dataset |
|---|---|---|---|
| Electricity for USP synthesis cycle (tube furnace, US generators, chiller) | 28.7 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
| Electricity for supporting equipment for the USP synthesis cycle (fume scrubber, ventilation) | 32.0 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
| Electricity for rotary evaporation of the prepared Au nanoparticle suspension | 12.0 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
| Electricity for the Lyophilisation cycle (nanoparticle suspension drying) | 105.0 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
| Electricity for Lyophilisation air conditioning | 172.8 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
3.1.8 Water consumption for the synthesis, purification and drying of AuNPs
This dataset represents the production of 1 kg of deionised water delivered to the user by ion exchange. The process includes an anionic resin or combined cation/anion exchange resin. The unit is operated with counterflow regeneration. Obtained water quality: about 1–0.1 μS/cm for the conductivity and silica content (as SiO2) of 5–25 μg/L. For the water source, tap water from a public supply with a total hardness assumed of 1.71 mol/m3 (range 0.7–3.2). 12
The data for all the inputs and outputs for water use for the synthesising, purifying and drying AuNPs are shown in Table 3.
Inventory of inputs and outputs for water use for the synthesising, purifying and drying of AuNPs. 14
| Data | Quantity | Unit | Dataset |
|---|---|---|---|
| Inputs | |||
| Cleaning of the USP system | 50 | L | »Water production, deionised | water, deionised | Cutoff, U« |
| Chiller water for rotary evaporation | 2400 | L | »Water production, deionised | water, deionised | Cutoff, U« |
| Outputs | |||
| Discharge from cleaning of the USP system | 50 | L | Water, Europe without Switzerland (emissions to water, unspecified) |
| Discharge of chiller water for rotary evaporation | 2400 | L | Water, Europe without Switzerland (emissions to water, unspecified) |
3.2 Inventory data for the synthesis of 3HFWC
3.2.1 Synthesis of 3HFWC
3.2.1.1 Fullerol
Fullerol is a term that refers to a class of water-soluble fullerene derivatives that have hydroxyl groups attached to the carbon atoms of the fullerene cage. Fullerols can have various structures, depending on the number and position of the hydroxyl groups and other possible functional groups. 16 Fullerols have been studied for their physicochemical properties and applications in various fields, such as Nanotechnology, Nanomaterials, and Nanomedicine. Some potential applications of fullerols include polymers, paints, concrete, drug delivery, antioxidant therapy and tissue engineering. 16 Since fullerol is a relatively new and emerging material, it is not yet included in the Ecoinvent database. Therefore, we used Phenol as a reference for fullerol in terms of the life cycle assessment. Fullerol and Phenol share similarities in their chemical structure, with both compounds containing hydroxyl functional groups (–OH). Both are also used as antioxidants, and have been studied for their potential use in various biomedical applications. It is important to note that these compounds are not identical or very similar to fullerol in all aspects, and each compound’s potential uses and properties may differ significantly. In the analysis, we used Phenol as the best proxy as an alternative process for producing fullerol, as it would be too time-consuming to simulate the process. We used the Ecoinvent 3.8 database 14 process for »market for phenol | phenol | Cutoff, U« and data from the literature. 17 , 18 This dataset represents the supply of 1 kg of Phenol from activities that produce it within the geography RER. The transport amounts are based on the Eurostat transport statistics for 2016. 19 This activity starts at the gate of the activities that produce Phenol within the geography RER, with the product ready for transportation. This activity ends with the supply of 1 kg of Phenol to the consumers of this product. Transport is included. Product losses during transportation are assumed negligible, and are therefore not included.
3.2.1.2 Water
For the water the dataset from the Ecoinvent 3.8 database 14 was used: »market for water, deionised | water, deionised | Cutoff, U« for the modelling. The process includes an anionic resin or combined cation/anion exchange resin. The unit is operated with counterflow regeneration. Obtained water quality: about 1–0.1 μS/cm for the conductivity and silica content (as SiO2) of 5–25 μg/L. For the water source, tap water from a public supply where a total hardness of 1.71 mol/m3 (range 0.7–3.2) was assumed. 12 Table 4 presents the inventory data of the 3HFWC synthesis.
Inventory of the 3HFWC synthesis. 14
| Data | Quantity | Unit | Dataset |
|---|---|---|---|
| Fullerol | 3 | g | »Market for phenol | phenol | Cutoff, U« |
| Water (0.1 μS/m) | 20 | L |
3.2.2 Electricity use for 3HFWC synthesis
To inventory the electricity data for the base cream, we used the »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« from the Ecoinvent database 14 shown in Table 5.
Inventory of electricity data for 3HFWC synthesis. 14
| Data | Quantity | Unit | Dataset |
|---|---|---|---|
| Electricity for heating | 1 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
| Electricity for production | 3 | kWh | »Market group for electricity, medium voltage | electricity, medium voltage | Cutoff, U« |
3.2.3 Water use for 3HFWC synthesis
This dataset represents the production of 1 kg of deionised water delivered to the user by ion exchange. The process includes an anionic resin or combined cation/anion exchange resin. The unit is operated with counterflow regeneration. Obtained water quality: about 1–0.1 μS/cm for the conductivity and silica content (as SiO2) of 5–25 μg/L. For the water source, tap water from a public supply where a total hardness of 1.71 mol/m3 (range 0.7–3.2) was assumed. 12
The data for all the input and output components for water use for 3HFWC synthesis are shown in Table 6.
Inventory of data for water use for 3HFWC synthesis. 14
| Data | Quantity | Unit | Dataset |
|---|---|---|---|
| Inputs | |||
| Cleaning of the system | 20 | L | »Water production, deionised | water, deionised | Cutoff, U« |
| Cleaning of the equipment | 10 | L | »Water production, deionised | water, deionised | Cutoff, U« |
| Output water missions | |||
| Cleaning discharge | 30 | L | Water, Europe without Switzerland (emissions to water, unspecified) |
3.3 Final remarks
This study undertook a Life Cycle Inventory (LCI) analysis to pave the way for a comprehensive Life Cycle Assessment (LCA) of a cosmetic cream manufacturing process, emphasising the integration of innovative ingredients such as gold nanoparticles (AuNPs) and three-dimensional hydrating water cubes (3HWC). The LCI analysis, a foundational aspect of LCA, plays a critical role in evaluating the environmental footprint of products, including those within the cosmetics industry. Our objective was to generate precise inventory data for a detailed assessment of the environmental impacts at each stage of the cosmetic cream production process, ranging from raw material extraction to the synthesis of the final product. This in-depth environmental profiling is vital for identifying both direct and indirect environmental impacts associated with cosmetic products accurately, thereby facilitating a comprehensive understanding of their environmental footprint.
The inventory analysis, a cornerstone of this review, quantified the consumption of critical raw materials, electricity, and water meticulously. By focussing specifically on synthesising AuNPs and 3HWC, the study shed light on the substantial environmental burdens of producing cosmetic creams containing advanced nanocomponents. While including AuNPs and 3HWC enhances product quality, it poses significant environmental challenges, particularly concerning resource consumption and energy use. The LCI analysis effectively identifies environmental hotspots in the production of cosmetic creams, such as stages characterised by high energy consumption, intensive water use, or the release of hazardous substances. Recognising these hotspots is crucial for directing efforts towards environmental improvements, be it through changes in formulation, process optimisations, or packaging innovations. The outcomes of this analysis are instrumental for subsequent environmental impact assessments, and for exploring potential enhancements in the production processes of nanocomponents.
A significant aspect of the research focused on quantifying the environmental burdens associated with using electricity and water during the synthesis, purification and drying of the AuNPs. Utilising the Ecoinvent database for inventorying electricity and water consumption provided robust and reliable data essential for an accurate environmental impact assessment. Our detailed analysis of resource consumption underscored the energy-intensive nature of the production process, and highlighted the critical need for optimising energy and water use to bolster overall sustainability.
This review offers pivotal insights into the environmental impacts associated with the production of cosmetic creams incorporating advanced nanocomponents. It underscores the importance of adopting sustainable manufacturing practices within the cosmetics industry, and is a foundational reference for further scholarly inquiry. By delineating environmental challenges and pinpointing opportunities for advancement, this study aids producers and stakeholders in making well-informed decisions towards more sustainable practices.
LCI analysis enables cosmetics manufacturers to benchmark the environmental performance of their products, thereby aiding in monitoring improvements over time. Such benchmarking is indispensable for establishing and meeting sustainability targets, communicating environmental credentials to consumers, and adhering to Regulatory and Certification Standards focused on environmental sustainability. The comprehensive data yielded by LCI analyses of cosmetic creams lays a solid groundwork for informed decision-making by industry stakeholders and policy-makers. It facilitates the development of sustainability Standards and certifications tailored to the Cosmetics sector, guides regulatory policies on environmental protection, and assists consumers in making more sustainable choices based on reliable environmental information. Furthermore, LCI analysis allows for the comparative analysis of different cosmetic cream formulations or packaging options, highlighting the relative environmental advantages of each. This comparative capability is vital for fostering life cycle thinking within the cosmetics industry, enabling brands to select ingredients, processes and packaging solutions that minimise environmental impacts and appeal to eco-conscious consumers.
The application of LCI in the cosmetics industry contributes significantly to broader global sustainability efforts, by promoting resource efficiency, reducing waste, and mitigating the environmental impacts associated with cosmetic products. By standardising the methodology for environmental impact assessment, LCI fosters cross-industry and international collaboration, propelling the Cosmetics sector towards more sustainable practices on a global scale. Although incorporating nanocomponents in cosmetics introduces sustainability challenges, notably in resource consumption and energy use, there are ample opportunities for improvement. The cosmetics industry can mitigate the environmental impacts of Nanotechnology by optimising production processes, minimising energy consumption, exploring sustainable raw material sources, and implementing recycling and recovery strategies. This holistic approach, underpinned by regulatory frameworks and fuelled by informed consumer demand, paves the way for developing more sustainable cosmetic products that leverage the benefits of Nanotechnology without compromising environmental integrity.
4 Conclusions
The conclusions of this review underscore the critical role of comprehensive inventory analysis in enhancing the sustainability of the cosmetic cream production process, particularly when incorporating advanced nano-components such as gold nanoparticles (AuNPs) and hydroxylated fullerenol water complexes (3HWC). This study illuminates the multifaceted environmental impacts engendered by integrating these innovative ingredients, by evaluating the consumption of key resources, including raw materials, electricity, and water, meticulously. Despite the enhancement in product quality attributed to AuNPs and 3HWC, their inclusion introduces considerable environmental challenges, notably in resource and energy utilisation. The findings from this analysis serve as an indispensable foundation for subsequent environmental impact assessments, and underscore the imperative for targeted improvements within the nano-component production process.
This investigation has further quantified the specific environmental burdens associated with the electricity and water used in the synthesis, purification and drying phases of AuNPs’ production. Utilising the Ecoinvent database for a robust inventory of these consumptions has provided essential, reliable data for an accurate and comprehensive environmental impact assessment. The detailed scrutiny of resource use highlights the energy-intensive nature of this production process, and stresses the urgency for optimising resource efficiency to bolster overall sustainability.
Moreover, this study contributes significantly to the ongoing discourse on sustainable production methodologies within the cosmetics industry. It accentuates the necessity for continuous evaluation and mitigation of the environmental repercussions associated with emerging production technologies. The insights garnered from this review advocate for implementing more eco-friendly practices across the industry, thereby fostering a paradigm shift towards sustainability in cosmetic product manufacturing. The elucidation of environmental challenges and opportunities for improvement aids stakeholders in formulating informed, sustainable choices.
Despite the challenges, the review on monitoring environmental pollution with AuNPs is progressing. By refining existing techniques and exploring novel approaches, we can understand these nanomaterials’ potential risks better, and develop effective strategies to mitigate their environmental impact. Continued research and collaboration between scientists, engineers and regulatory bodies are crucial, to ensure the responsible development and application of AuNPs in a sustainable manner.
In essence, this review examines the current environmental ramifications of incorporating advanced nano-components into cosmetic cream production critically, and establishes a solid groundwork for future endeavours to achieve sustainable production in the Cosmetics sector. By delineating a path for adopting greener practices and technologies, this study makes a pivotal contribution to the advancement of environmental stewardship in cosmetic manufacturing, ultimately paving the way for developing more sustainable cosmetic products and production techniques.
Funding source: SPIRIT Slovenia–Public Agency for Entrepreneurship, Internationalization, Foreign Investments and Technology
Award Identifier / Grant number: Agreement P-RRIO-NOO/00042
Acknowledgements
We would like to thank Djuro Koruga from Nano Lab, Faculty of Mechanical Engineering, University of Belgrade, Serbia for the technical support in obtaining the necessary technical data.
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interests.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Majerič, P., Jović, Z., Švarc, T., Jelen, Ž., Horvat, A., Koruga, D., Rudolf, R. Physicochemical Properties of Gold Nanoparticles for Skin Care Creams. Materials 2023, 16(8), 1–14; https://doi.org/10.3390/ma16083011.Search in Google Scholar PubMed PubMed Central
2. Rudolf, R.; Jelen, M. Z.; Majerič, P.; Jović, Z.; Vuksanović, M.; Stankovic, I.; Matija, L.; Dragicevic, A.; Miso Thompson, N.; Horvat, A.; Koruga, D. A Gold Nanoparticles and Hydroxylated Fullerene Water Complex as a New Product for Cosmetics. Adv. Prod. Eng. Manage. 2022, 17 (1), 89–107. https://doi.org/10.14743/apem2022.1.423.Search in Google Scholar
3. Liu, J. K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12(1), 1–43; https://doi.org/10.1007/s13659-022-00363-y.Search in Google Scholar PubMed PubMed Central
4. Secchi, M.; Castellani, V.; Collina, E.; Mirabella, N.; Sala, S. Assessing Eco-Innovations in Green Chemistry: Life Cycle Assessment (LCA) of a Cosmetic Product with a Bio-Based Ingredient. J. Clean. Prod. 2016, 129 (June 2007), 269–281. https://doi.org/10.1016/j.jclepro.2016.04.073.Search in Google Scholar
5. Pacheco, R.; Huston, K. Life Cycle Assessment (LCA) of Naturally-Sourced and Petroleum-Based Glycols Commonly Used in Personal Care Products Life Cycle Assessment (LCA) of Naturally-Sourced and Petroleum-Based Glycols Commonly Used in Personal Care Products. SOFW J. 2018, 144, 11–15.Search in Google Scholar
6. Cao, M.; Li, J.; Tang, J.; Chen, C.; Zhao, Y. Gold Nanomaterials in Consumer Cosmetics Nanoproducts: Analyses, Characterisation, and Dermal Safety Assessment. Small 2016, 12 (39), 5488–5496. https://doi.org/10.1002/smll.201601574.Search in Google Scholar PubMed
7. Liu, C., Wang, Y., Zhang, G., Pang, X., Yan, J., Wu, X., Qiu, Y., Wang, P., Huang, H., Wang, X., Zhang, H. Dermal Toxicity Influence of Gold Nanomaterials after Embedment in Cosmetics. Toxics 2022, 10(6), 1–16; https://doi.org/10.3390/toxics10060276.Search in Google Scholar PubMed PubMed Central
8. Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The Emerging Role of Nanotechnology in Skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. https://doi.org/10.1016/j.cis.2021.102437.Search in Google Scholar PubMed
9. Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals – A Review. Gels 2022, 8 (153), 1–31.10.3390/gels8030173Search in Google Scholar PubMed PubMed Central
10. Renner, H., Schlamp, G., Hollmann, D., Martin Lüschow, H., Tews, P., Rothaut, J., Dermann, K., Knödler, A., Hecht, C., Schlott, M., Drieselmann, R., Peter, C., Schiele, R. Gold, Gold Alloys, and Gold Compounds. Ullmann’s Encycl. Ind. Chem. 2000, 17, 93–143; https://doi.org/10.1002/14356007.a12_499.Search in Google Scholar
11. Newmont Mining. Newmount: Now & beyond 2005, 2005.Search in Google Scholar
12. Althaus, H., Chudacoff, M., Hischier, R., Jungbluth, N., Osses, M., Primas, A., Hellweg, S. Life Cycle Inventories of Chemicals. Ecoinvent Report No.8, v2.0. Final Report Ecoinvent Data 2007, 2(8), 1–957.Search in Google Scholar
13. Gendorf. Umwelterklärung 2015, 2016. www.gendorf.de.Search in Google Scholar
14. Ecoinvent. Version 3.8, 2021. https://www.ecoinvent.org/.Search in Google Scholar
15. WaterWorld, I., n.d. https://www.watertechonline.com/magazine/61278.Search in Google Scholar
16. Semenov, K. N.; Charykov, N. A.; Postnov, V. N.; Sharoyko, V. V.; Vorotyntsev, I. V.; Galagudza, M. M.; Murin, I. V. Fullerenols: Physicochemical Properties and Applications. Prog. Solid State Chem. 2016, 44 (2), 59–74. https://doi.org/10.1016/J.PROGSOLIDSTCHEM.2016.04.002.Search in Google Scholar
17. Kushnir, D.; Sandén, B. A. Energy Requirements of Carbon Nanoparticle Production. J. Ind. Ecol. 2008, 12 (3), 360–375. https://doi.org/10.1111/j.1530-9290.2008.00057.x.Search in Google Scholar
18. Anctil, A.; Babbitt, C. W.; Raffaelle, R. P.; Landi, B. J. Material and Energy Intensity of Fullerene Production. Environ. Sci. Technol. 2011, 45 (6), 2353–2359. https://doi.org/10.1021/es103860a.Search in Google Scholar PubMed
19. Eurostat. Transport, 2016. https://ec.europa.eu/eurostat/data/database.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/revic-2024-0012).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Inorganic hydrogels: synthetic strategies, properties and applications
- A review on biogenic synthesized zinc oxide nanoparticles: synthesis, characterization, and its applications
- Photochemical synthesis in inorganic chemistry
- Variable heterotridentate ligands in Pt(ƞ3-X1C1X2)(PL) (X1,2 = N or S), Pt(ƞ3-X1N1Y1)(PL) (X, Y = O, C; C, S; or O, S) and Pt(ƞ3-S1B1S2)(PL), derivatives – structural aspects
- Inorganic-polymer composite electrolytes: basics, fabrications, challenges and future perspectives
- Applications of samarium complexes as cytotoxic, bioimaging and DNA interacting agents: a comprehensive review
- Graphene-based nanocomposites for gas sensors: challenges and opportunities
- The environmental impact of using gold nanoparticles and 3HFWC in cosmetics, as determined with LCA methodology
Articles in the same Issue
- Frontmatter
- Inorganic hydrogels: synthetic strategies, properties and applications
- A review on biogenic synthesized zinc oxide nanoparticles: synthesis, characterization, and its applications
- Photochemical synthesis in inorganic chemistry
- Variable heterotridentate ligands in Pt(ƞ3-X1C1X2)(PL) (X1,2 = N or S), Pt(ƞ3-X1N1Y1)(PL) (X, Y = O, C; C, S; or O, S) and Pt(ƞ3-S1B1S2)(PL), derivatives – structural aspects
- Inorganic-polymer composite electrolytes: basics, fabrications, challenges and future perspectives
- Applications of samarium complexes as cytotoxic, bioimaging and DNA interacting agents: a comprehensive review
- Graphene-based nanocomposites for gas sensors: challenges and opportunities
- The environmental impact of using gold nanoparticles and 3HFWC in cosmetics, as determined with LCA methodology

