Abstract
Objectives
Polycyclic Aromatic Hydrocarbons (PAHs) are ubiquitous, toxic environmental chemicals that can cause adverse reproductive health effects. The objectives of this mini-review are to highlight the adverse reproductive outcomes due to PAH exposure with the main focus on polycystic ovary syndrome (PCOS) and premature ovarian failure (POF) and to provide perspectives on future research needs.
Content
We reviewed studies that have reported the adverse reproductive outcomes associated with PAHs exposures in women through a comprehensive search of bibliographic databases and gray literature sources. In addition, potentially modifiable sources of exposure to PAHs and associated reproductive outcomes were also investigated.
Summary
A total of 232 papers were retrieved through a comprehensive search of bibliographic databases, out of which three studies met the eligibility criteria and were included in the review. Results showed that exposure to PAHs is associated with adverse reproductive outcomes defined as PCOS, POF, and reproductive hormone imbalance. Sources of PAH exposure associated with adverse reproductive outcomes include active and passive tobacco smoking, specific cooking methods, and pesticides.
Outlook
Future studies are warranted to examine the mechanisms by which PAHs result in adverse reproductive endpoints in women. Further, environmental exposures that are potentially modifiable such as exposure to tobacco smoke, may contribute to PAH exposure, and these exposures should be targeted in future policies and interventions.
Introduction
Polycyclic Aromatic Hydrocarbons (PAHs) are a ubiquitous class of toxic environmental chemicals [1] to which humans can be exposed via inhalation, ingestion, and dermal contact [2]. PAHs are predominantly emitted during incomplete combustion and pyrolysis of various anthropogenic and natural sources, such as coal, coke, petrol, wood, forest fires, vehicle exhausts, and tobacco smoke [3], [4], [5]. Once entering the body, PAHs are metabolized and excreted in the urine, mainly as hydroxylated metabolites, with the most common being 1-hydroxypyrene (1-OHP) [6, 7]. The Environmental Protection Agency (EPA) has listed multiple PAHs such as benzo(a)pyrene (BaP), benzo(k)fluoranthene (BkF), and benz(a)anthracene (BaA) as high-priority chemicals of concern based on their carcinogenicity, mutagenicity, and toxicity [8]. The relationship between exposure to PAHs and adverse human health consequences has been well-documented, as exposure results in cardiopulmonary, reproductive, and neurological toxicity, carcinogenic effects, and damage to body organs such as the kidney and liver [5, 7, 9].
Human biomonitoring has been widely used as a complementary approach to exposure science to assess humans’ exposure to various chemicals, including PAHs in the general population and occupational settings [10], [11], [12], [13]. Previous epidemiological studies indicate that exposure to PAHs is associated with adverse reproductive outcomes such as polycystic ovary syndrome (PCOS) among women [14, 15]. PCOS is a complex endocrine syndrome with multiple factors contributing to its development, such as imbalances of reproductive hormones, insulin resistance, low-grade chronic inflammation, and heredity. PCOS is the most common cause of ovulatory infertility in women, affecting 5–10% of reproductive-aged women globally [14, 16]. Because women’s fertility is sensitive to hormone imbalances, exposure to chemicals such as PAHs, which may irritate the ovaries during women’s peak reproductive years, can interrupt ovarian follicular development [14, 15]. Since it is important to understand the existing research on the endocrine-disrupting potential of PAHs to explore the gaps and highlight future research needs, the present mini-review provides insight into the adverse reproductive outcomes in women defined as PCOS, premature ovarian failure (POF), and abnormal levels of reproductive hormones due to PAH exposure as well as perspectives on future research needs.
Methods
We conducted a comprehensive search on bibliographic databases, including Scopus, Web of Science, Embase, Medline, and PubMed, in July 2022. In addition, we searched the gray literature on Google Scholar and ProQuest. The search strategy was formed based on the PECOS format (population, exposure, comparator, outcome, and studies) as follows: (P) pregnant women or expecting mothers or women of reproductive age, (E) exposure to PAHs, (C) pregnant women or women at reproductive age who were not exposed to PAHs, (O) PECOS or POF, and (S) epidemiological observational studies (Supplementary material).
The retrieved studies from the databases that met the study’s aim were then transferred to the Covidence platform, where two independent reviewers assessed them, considering the set inclusion and exclusion criteria. The inclusion criteria were as follows: Human-based studies, primary/original research, studies focused on pregnant women or women of reproductive age, field studies, and studies that investigated PCOS and POF in pregnant women or women of reproductive age. For the exclusion criteria, we excluded animal and lab-based studies, simulation and modeling studies, studies that did not include a comparator group (e.g., controls), studies that investigated other reproductive outcomes resulting from PAHs exposure among women, systematic reviews, mini-reviews, highlight papers, abstracts presented in conferences without full-text available, and studies that focused on exposure to non-PAH pollutants. Two independent reviewers conducted the screening and full-text review stages on the Covidence platform, and disagreements were resolved through discussions until consensus was reached.
Data extraction was performed using a data collection form that included information on each study, such as the number of participants, year of publication, details of PAHs exposures, outcomes of interest, methods used to characterize PAH exposures, and assessment of adverse reproductive outcomes.
Results and discussion
General characteristics of the studies
We retrieved 232 published papers through a comprehensive search on bibliographic databases and gray literature sources, out of which three studies [14, 17, 18] met the eligibility criteria and were included in the present study (Figure 1). Studies were conducted in China and Canada, and the number of participants in the case and control groups was 226 and 140, respectively. Outcomes of interest (PCOS and POF) were assessed by measuring anti-mullerian hormone (AMH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) among the studied populations using chemiluminescence immunoassay.
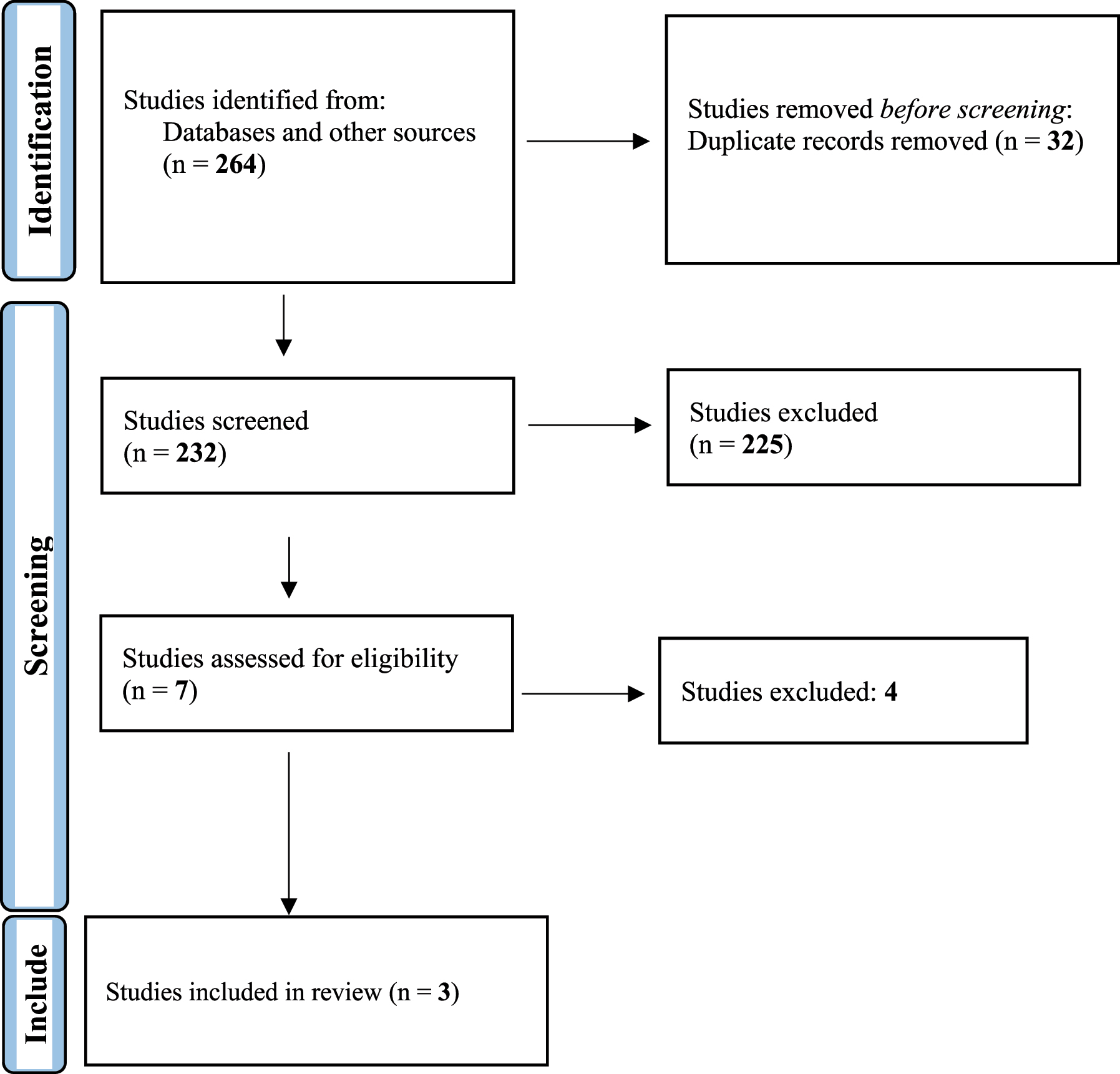
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram.
PAHs exposure and adverse reproductive outcomes in women
Two studies included in this study examined the associations between exposure to PAHs and adverse reproductive outcomes in women. In the study by Ye et al. [18]; a biomonitoring approach was performed among reproductive-aged women in China to assess the relationship between exposure to PAHs and the risk of premature ovarian failure (POF) [18]. The serum levels of low and high-molecular-weight PAHs (H-PAHs) were measured using a gas chromatography-triple quadrupole mass spectrometer system. In addition, serum levels of AMH, LH, and FSH were measured using an immunoassay. Ye et al. [18] observed significantly higher total serum PAH levels in the case group compared to controls, with pyrene (PYR) and naphthalene (NAP) being the most prevalent PAH congeners in both groups. Further, they found that serum concentrations of ƩPAHs, BaP, benzo(b)fluoranthene (BbF), BkF, phenanthrene (PHE), acenaphthene (ACE), NAP, acenaphthylene (ACY), chrysene (CHR), anthracene (ANT), and fluoranthene (FLT) were significantly associated with the risk of POF in the case group (Table 1).
Summary of the information and findings of the included studies.
| Study | No. | Age (mean ± SD) | Measured PAHs | Reproductive hormone levels (cases) (mean ± SD) | Reproductive hormone levels (controls) (mean ± SD) |
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | FSH, IU/L | LH, IU/L | AMH, ng/mL | FSH, IU/L | LH, IU/L | AMH, ng/mL | |||
| Ye et al. [18] | 157 | 120 | 34 ± 6 | 33 ± 6 | NAP, ACE, ACY, FLO, PHE, anthracene ANT, FLA, PYR, CHR, BbF, BkF, and BaP | 67.50 ± 30.49 | 39.76 ± 20.04 | 0.12 ± 0.67 | 6.80 ± 2.61 | 4.78 ± 2.61 | 3.08 ± 2.35 |
|
| Yang et al. [14] | 50 | 30 | 29.7 + 3.2 | 30.6 + 2.9 | Nap, Biphenyl (BP), acenaphthylene (ACN), ACE, Dibenzofuran (DBF), FLU, PHE, ANT, FLT, PYR, BaA, CHR, 1-Methylphenanthrene (1-MePhe), 2-Methylphenanthrene (2-MePhe), 1-Methylpyrene (1-MePyr), 1-hydroxy naphthalene (1-HO-Nap) | 6.37 + 1.62 | 7.14 + 3.38 | – | 6.96 + 1.73 | 3.73 + 1.86 | – |
|
| Neal et al. [17] | 19 | 10 | 33.24 ± 1.06 | 34.10 ± 1.13 | ACE, PHE, PYR, CHR, and BaP | 6.19 ± 0.60 | – | – | 5.42 ± 0.88 | – | – |
|
Ye et al. [18] also found that exposure to H-PAHs was significantly correlated with higher risks of POF and ovarian dysfunction than L-PAHs. Although research on the toxicological effects of exposure to PAHs and PCOS is limited, these findings are in parallel with other studies that reported exposure to PAHs could disrupt estrogen synthesis in follicular granulosa cells [14, 19]. Similarly, other studies revealed that exposure to some H-PAHs, such as BaP, may destroy the follicles and result in early menopause, suggesting that PAHs have endocrine-disrupting potential [20, 21]. One possible reason for these observed results could be due to the effect of exposure to PAHs on the aryl hydrocarbon receptor (AhR), a transcription factor expressed in ovarian tissues, which contributes to women’s ovarian follicular growth regulation. PAH congeners act as ligands for AhR, and exposure to some H-PAHs such as BaP may stimulate follicle apoptosis through the AhR pathway, which could increase POF risk [22], [23], [24].
Regarding the effect of PAHs exposures on reproductive hormones, the findings of this same study indicate that serum levels of all H-PAHs and L-PAHs, except PYR and FLO, were significantly correlated with FSH serum levels of the case group [18]. Moreover, serum levels of all H-PAHs and some L-PAHs, including ACE, NAP, and ANT, were significantly correlated with LH levels. The authors also found significant positive correlations between total serum PAHs with FSH and LH levels in the case group, and BaP was the most significantly correlated PAH congener with measured reproductive hormones [18]. Another study reported increased FSH and LH levels in women with POF, and significant correlations between exposure to PHE, PYR, and NAP with FSH and LH have also been observed [25]. Animal studies have reported a correlation between exposure to PHE and inhibition of LH-stimulated progesterone secretion and increased POF risk in female rats [26]. In addition, the estrogenic activity of PAHs, especially PHE, through interaction with the estrogen receptor alpha, has been observed in previous studies [27, 28].
Concerning reproductive hormones, it is also important to examine the effects of PAHs on AMH concentrations as AMH is correlated with ovarian cycle development, and monitoring AMH levels can be used to assess follicular depletion [29], [30], [31]. Since AMH levels are associated with the size of the ovarian follicular reserve, decreases in the level of this reproductive hormone could be a clinical marker of women’s ovarian disorders, including PCOS and POF [32, 33]. A recent study among Chinese reproductive-age women found negative correlations between AMH levels with all H-PAHs and L-PAHs except for FLO and PYR. The PAH serum levels were also inversely correlated with AMH concentration in women with PCOS [18].
The second study included in this review was conducted by Yang et al. [14]. Yang’s team conducted a case-control study among 50 women with PCOS and 30 normal non-pregnant female control participants from Northern China. Serum levels of 15 species of PAHs were measured using gas chromatography-mass spectrometry. Their results indicated that compared to control participants, participants with PCOS had significantly higher serum concentrations of 6 individuals and the sum of 15 compounds (ƩPAHs). Further, there were significant associations between the NAP and CAN and PCOS and marginally significant associations between PHE, FLU, ACE, ƩPAHs, and PCOS. The authors concluded that the findings of this preliminary study suggest that PAHs could be an etiologic exposure that results in PCOS.
The role of smoking in adverse reproductive consequences
Regarding smoking, one of the included studies [17] reported that BaP is one of the main PAHs present in mainstream and side-stream cigarette smoke. BaP could inhibit follicle development in active smokers and in individuals exposed to second-hand tobacco smoke [17, 24]. However, Ye et al. [18]; did not report significant correlations between cigarette smoking and the risk of reproductive endpoints in the studied population. However, this unexpected finding may be because only two active smokers were identified out of the 157 women who participated in that study, which precluded the examination of the effect of smoking on reproductive endpoints.
Previous studies have recognized that pollutants from active smoking and tobacco smoke exposure (i.e., second-hand smoke and/or third-hand smoke exposure) are important sources of exposure to PAHs [10, 34] that have potential ovo-toxic effects on reproductive hormones in rodents [35]. In addition, some studies have revealed a significant correlation between smoking and earlier menopause and the risk of POF [36, 37]. Cigarette smoking may interfere with AMH circulation in women of late-reproductive age [38], and significant correlations have been reported between exposure to high levels of BaP and a decrease in follicle AMH levels in mice exposed to cigarette smoke [24]. Although the mechanism underlying how smoking and PAH exposure affects follicular development is not well understood, some studies have reported that exposure to smoking could induce ovarian follicle destruction by caspase-3 activation [24, 39]. Another study found that there was a decrease in the circulating levels of estradiol (E2) and/or progesterone (P) due to exposure to cigarette smoke, suggesting that these decreases could affect women’s reproductive potential [24]. Given these prior findings, future toxicological studies are warranted to examine how exposure to PAHs can lead to adverse reproductive outcomes and explore the contribution of active and passive smoking in developing PCOS, POF, and reproductive hormone imbalance in women.
Other PAHs intake sources and concluding remarks
Dietary exposure is another important source and predictor of internal exposure to PAHs, as consumption of grilled and smoked meats is correlated with increases in urinary metabolites of PAHs [40]. Thus, future studies should explore the contribution of dietary PAH exposures to the risk of reproductive outcomes, such as ovulatory status and PCOS in adolescent girls and women of late-reproductive age, in order to investigate how different dietary habits can affect women’s reproductive health. Similarly, future studies are warranted to investigate the effects of other predictors of exposure to PAHs, such as cooking, on disrupting reproductive hormones in women. For example, high PAH levels could be emitted during high-temperature cooking methods such as stir and deep-frying, and PAH can subsequently enter the body through inhalation [41]. Moreover, the correlation between urinary H-PAHs with cooking was reported [42], and PAH levels have been found to be significantly correlated with the risk of POF in women [18].
In summary, the extant literature indicates that exposure to PAHs is associated with adverse reproductive outcomes, including PCOS, POF, and abnormal levels of reproductive hormones. Future studies are warranted to examine the mechanisms by which PAHs result in these outcomes. Further, environmental exposures that are potentially modifiable such as exposure to tobacco smoke, may contribute to PAH exposure, and reductions in these involuntary exposures should be targeted in future policies and interventions.
Funding source: National Institute of Environmental Health Sciences
Award Identifier / Grant number: R01 ES030743
Award Identifier / Grant number: R01 ES027815
-
Research funding: This work was supported, in part, by the National Institute of Environmental Health Sciences (NIH Grant Number R01 ES030743 and R01 ES027815, to Dr. Mahabee-Gittens).
-
Author contributions: Ata Rafiee: Conceptualization, Methodology, Project administration, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Mohammad Hoseini: Writing – review & editing. Sadaf Akbari: Screening of the studies. E. Melinda Mahabee-Gittens: Supervision, Writing – original draft, Writing – review & editing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Gearhart-Serna, LM, Tacam, MJr, Slotkin, TA, Devi, GR. Analysis of polycyclic aromatic hydrocarbon intake in the US adult population from NHANES 2005–2014 identifies vulnerable subpopulations, suggests interaction between tobacco smoke exposure and sociodemographic factors. Environ Res 2021;201:111614. https://doi.org/10.1016/j.envres.2021.111614.Suche in Google Scholar PubMed PubMed Central
2. Control CfD, Prevention. Fourth national report on human exposure to environmental chemicals, updated tables, February 2015. Atlanta, GA: US Department of Health and Human Services; 2015.Suche in Google Scholar
3. Famiyeh, L, Chen, K, Xu, J, Sun, Y, Guo, Q, Wang, C, et al.. A review on analysis methods, source identification, and cancer risk evaluation of atmospheric polycyclic aromatic hydrocarbons. Sci Total Environ 2021;789:147741. https://doi.org/10.1016/j.scitotenv.2021.147741.Suche in Google Scholar PubMed
4. Shen, G, Wang, W, Yang, Y, Ding, J, Xue, M, Min, Y, et al.. Emissions of PAHs from indoor crop residue burning in a typical rural stove: emission factors, size distributions, and gas−particle partitioning. Environ Sci Technol 2011;45:1206–12. https://doi.org/10.1021/es102151w.Suche in Google Scholar PubMed PubMed Central
5. Wang, S, Bai, Y, Deng, Q, Chen, Z, Dai, J, Li, X, et al.. Polycyclic aromatic hydrocarbons exposure and lung function decline among coke-oven workers: a four-year follow-up study. Environ Res 2016;150:14–22. https://doi.org/10.1016/j.envres.2016.05.025.Suche in Google Scholar PubMed
6. Li, Z, Trinidad, D, Pittman, EN, Riley, EA, Sjodin, A, Dills, RL, et al.. Urinary polycyclic aromatic hydrocarbon metabolites as biomarkers to woodsmoke exposure—results from a controlled exposure study. J Expo Sci Environ Epidemiol 2016;26:241–8. https://doi.org/10.1038/jes.2014.94.Suche in Google Scholar PubMed PubMed Central
7. Ramesh, A, Archibong, AE, Hood, DB, Guo, Z, Loganathan, BG. Global environmental distribution and human health effects of polycyclic aromatic hydrocarbons. In: Global contamination trends of persistent organic chemicals. Boca Raton, FL: CRC Press; 2011:95–124 pp.Suche in Google Scholar
8. ATSDR A. Toxicological profile for polycyclic aromatic hydrocarbons. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2005.Suche in Google Scholar
9. Niehoff, N, White, AJ, McCullough, LE, Steck, SE, Beyea, J, Mordukhovich, I, et al.. Polycyclic aromatic hydrocarbons and postmenopausal breast cancer: an evaluation of effect measure modification by body mass index and weight change. Environ Res 2017;152:17–25. https://doi.org/10.1016/j.envres.2016.09.022.Suche in Google Scholar PubMed PubMed Central
10. Hoseini, M, Nabizadeh, R, Delgado-Saborit, JM, Rafiee, A, Yaghmaeian, K, Parmy, S, et al.. Environmental and lifestyle factors affecting exposure to polycyclic aromatic hydrocarbons in the general population in a Middle Eastern area. Environ Pollut 2018;240:781–92. https://doi.org/10.1016/j.envpol.2018.04.077.Suche in Google Scholar PubMed
11. Rafiee, A, Delgado-Saborit, JM, Sly, PD, Amiri, H, Hoseini, M. Lifestyle and occupational factors affecting exposure to BTEX in municipal solid waste composting facility workers. Sci Total Environ 2019;656:540–6. https://doi.org/10.1016/j.scitotenv.2018.11.398.Suche in Google Scholar PubMed
12. Rafiee, A, Delgado-Saborit, JM, Sly, PD, Amiri, H, Mosalaei, S, Hoseini, M. Health consequences of disinfection against SARS-CoV-2: exploring oxidative stress damage using a biomonitoring approach. Sci Total Environ 2022;814:152832. https://doi.org/10.1016/j.scitotenv.2021.152832.Suche in Google Scholar PubMed PubMed Central
13. Rafiee, A, Delgado-Saborit, JM, Sly, PD, Quémerais, B, Hashemi, F, Akbari, S, et al.. Environmental chronic exposure to metals and effects on attention and executive function in the general population. Sci Total Environ 2020;705:135911. https://doi.org/10.1016/j.scitotenv.2019.135911.Suche in Google Scholar PubMed
14. Yang, Q, Zhao, Y, Qiu, X, Zhang, C, Li, R, Qiao, J. Association of serum levels of typical organic pollutants with polycystic ovary syndrome (PCOS): a case–control study. Hum Reprod 2015;30:1964–73. https://doi.org/10.1093/humrep/dev123.Suche in Google Scholar PubMed
15. Yin, S, Tang, M, Chen, F, Li, T, Liu, W. Environmental exposure to polycyclic aromatic hydrocarbons (PAHs): the correlation with and impact on reproductive hormones in umbilical cord serum. Environ Pollut 2017;220:1429–37. https://doi.org/10.1016/j.envpol.2016.10.090.Suche in Google Scholar PubMed
16. Teede, H, Deeks, A, Moran, L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 2010;8:41. https://doi.org/10.1186/1741-7015-8-41.Suche in Google Scholar PubMed PubMed Central
17. Neal, MS, Zhu, J, Foster, WG. Quantification of benzo [a] pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod Toxicol 2008;25:100–6. https://doi.org/10.1016/j.reprotox.2007.10.012.Suche in Google Scholar PubMed
18. Ye, X, Pan, W, Li, C, Ma, X, Yin, S, Zhou, J, et al.. Exposure to polycyclic aromatic hydrocarbons and risk for premature ovarian failure and reproductive hormones imbalance. J Environ Sci 2020;91:1–9. https://doi.org/10.1016/j.jes.2019.12.015.Suche in Google Scholar PubMed
19. Fowler, PA, Childs, AJ, Courant, F, MacKenzie, A, Rhind, SM, Antignac, J-P, et al.. In utero exposure to cigarette smoke dysregulates human fetal ovarian developmental signalling. Hum Reprod 2014;29:1471–89. https://doi.org/10.1093/humrep/deu117.Suche in Google Scholar PubMed
20. Archibong, AE, Ramesh, A, Inyang, F, Niaz, MS, Hood, DB, Kopsombut, P. Endocrine disruptive actions of inhaled benzo (a) pyrene on ovarian function and fetal survival in Fisher F-344 adult rats. Reprod Toxicol 2012;34:635–43. https://doi.org/10.1016/j.reprotox.2012.09.003.Suche in Google Scholar PubMed PubMed Central
21. Borman, S, Christian, P, Sipes, I, Hoyer, PB. Ovotoxicity in female Fischer rats and B6 mice induced by low-dose exposure to three polycyclic aromatic hydrocarbons: comparison through calculation of an ovotoxic index. Toxicol Appl Pharmacol 2000;167:191–8. https://doi.org/10.1006/taap.2000.9006.Suche in Google Scholar PubMed
22. Ji, C, Yan, L, Chen, Y, Yue, S, Dong, Q, Chen, J, et al.. Evaluation of the developmental toxicity of 2, 7-dibromocarbazole to zebrafish based on transcriptomics assay. J Hazard Mater 2019;368:514–22. https://doi.org/10.1016/j.jhazmat.2019.01.079.Suche in Google Scholar PubMed
23. Matikainen, T, Perez, GI, Jurisicova, A, Pru, JK, Schlezinger, JJ, Ryu, H-Y, et al.. Aromatic hydrocarbon receptor-driven Bax gene expression is required for premature ovarian failure caused by biohazardous environmental chemicals. Nat Genet 2001;28:355–60. https://doi.org/10.1038/ng575.Suche in Google Scholar PubMed
24. Sadeu, J, Foster, WG. Effect of in vitro exposure to benzo [a] pyrene, a component of cigarette smoke, on folliculogenesis, steroidogenesis and oocyte nuclear maturation. Reprod Toxicol 2011;31:402–8. https://doi.org/10.1016/j.reprotox.2010.12.006.Suche in Google Scholar PubMed
25. Luderer, U, Christensen, F, Johnson, WO, She, J, Ip, HSS, Zhou, J, et al.. Associations between urinary biomarkers of polycyclic aromatic hydrocarbon exposure and reproductive function during menstrual cycles in women. Environ Int 2017;100:110–20. https://doi.org/10.1016/j.envint.2016.12.021.Suche in Google Scholar PubMed PubMed Central
26. Nykamp, JA, Bols, NC, Carlson, JC. Phenanthrenequinone disrupts progesterone production in rat luteal cells. Reprod Toxicol 2001;15:393–8. https://doi.org/10.1016/s0890-6238(01)00140-x.Suche in Google Scholar PubMed
27. Cathey, AL, Watkins, DJ, Rosario, ZY, Vega, CMV, Loch-Caruso, R, Alshawabkeh, AN, et al.. Polycyclic aromatic hydrocarbon exposure results in altered CRH, reproductive, and thyroid hormone concentrations during human pregnancy. Sci Total Environ 2020;749:141581. https://doi.org/10.1016/j.scitotenv.2020.141581.Suche in Google Scholar PubMed PubMed Central
28. Kamelia, L, Louisse, J, De Haan, L, Maslowska-Gornicz, A, Ketelslegers, HB, Brouwer, A, et al.. The role of endocrine and dioxin-like activity of extracts of petroleum substances in developmental toxicity as detected in a panel of CALUX reporter gene assays. Toxicol Sci 2018;164:576–91. https://doi.org/10.1093/toxsci/kfy114.Suche in Google Scholar PubMed PubMed Central
29. Huang, X, Xu, X, Dai, Y, Cheng, Z, Zheng, X, Huo, X. Association of prenatal exposure to PAHs with anti-Müllerian hormone (AMH) levels and birth outcomes of newborns. Sci Total Environ 2020;723:138009. https://doi.org/10.1016/j.scitotenv.2020.138009.Suche in Google Scholar PubMed
30. Long, W-Q, Ranchin, V, Pautier, P, Belville, C, Denizot, P, Hln, C, et al.. Detection of minimal levels of serum anti-Mullerian hormone during follow-up of patients with ovarian granulosa cell tumor by means of a highly sensitive enzyme-linked immunosorbent assay. J Clin Endocrinol Metab 2000;85:540–4. https://doi.org/10.1210/jc.85.2.540.Suche in Google Scholar
31. Seifer, DB, MacLaughlin, DT, Christian, BP, Feng, B, Shelden, RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril 2002;77:468–71. https://doi.org/10.1016/s0015-0282(01)03201-0.Suche in Google Scholar PubMed
32. Hayes, E, Kushnir, V, Ma, X, Biswas, A, Prizant, H, Gleicher, N, et al.. Intra-cellular mechanism of Anti-Müllerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol 2016;433:56–65. https://doi.org/10.1016/j.mce.2016.05.019.Suche in Google Scholar PubMed
33. Visser, JA, Schipper, I, Laven, JS, Themmen, AP. Anti-Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol 2012;8:331–41. https://doi.org/10.1038/nrendo.2011.224.Suche in Google Scholar PubMed
34. Jacob, PIII, Benowitz, NL, Destaillats, H, Gundel, L, Hang, B, Martins-Green, M, et al.. Thirdhand smoke: new evidence, challenges, and future directions. Chem Res Toxicol 2017;30:270–94. https://doi.org/10.1021/acs.chemrestox.6b00343.Suche in Google Scholar PubMed PubMed Central
35. Camlin, NJ, Sobinoff, AP, Sutherland, JM, Beckett, EL, Jarnicki, AG, Vanders, RL, et al.. Maternal smoke exposure impairs the long-term fertility of female offspring in a murine model. Biol Reprod 2016;94:1–12.10.1095/biolreprod.115.135848Suche in Google Scholar PubMed
36. Chang, SH, Kim, C-S, Lee, K-S, Kim, H, Yim, SV, Lim, YJ, et al.. Premenopausal factors influencing premature ovarian failure and early menopause. Maturitas 2007;58:19–30. https://doi.org/10.1016/j.maturitas.2007.04.001.Suche in Google Scholar PubMed
37. Harlow, BL, Signorello, LB. Factors associated with early menopause. Maturitas 2000;35:3–9. https://doi.org/10.1016/s0378-5122(00)00092-x.Suche in Google Scholar PubMed
38. Plante, BJ, Cooper, GS, Baird, DD, Steiner, AZ. The impact of smoking on antimüllerian hormone levels in women aged 38 to 50 years. Menopause 2010;17:571. https://doi.org/10.1097/gme.0b013e3181c7deba.Suche in Google Scholar PubMed PubMed Central
39. Mattison, D, White, N, Nightingale, M. The effect of benzo (a) pyrene on fertility, primordial oocyte number, and ovarian response to pregnant mare’s serum gonadotropin. Pediatr Pharmacol 1980;1:143–51.Suche in Google Scholar
40. Motorykin, O, Santiago-Delgado, L, Rohlman, D, Schrlau, JE, Harper, B, Harris, S, et al.. Metabolism and excretion rates of parent and hydroxy-PAHs in urine collected after consumption of traditionally smoked salmon for Native American volunteers. Sci Total Environ 2015;514:170–7. https://doi.org/10.1016/j.scitotenv.2015.01.083.Suche in Google Scholar PubMed PubMed Central
41. Chen, J-W, Wang, S-L, Hsieh, DPH, Yang, H-H, Lee, H-L. Carcinogenic potencies of polycyclic aromatic hydrocarbons for back-door neighbors of restaurants with cooking emissions. Sci Total Environ 2012;417:68–75. https://doi.org/10.1016/j.scitotenv.2011.12.012.Suche in Google Scholar PubMed
42. Cao, L, Wang, D, Wen, Y, He, H, Chen, A, Hu, D, et al.. Effects of environmental and lifestyle exposures on urinary levels of polycyclic aromatic hydrocarbon metabolites: a cross-sectional study of urban adults in China. Chemosphere 2020;240:124898. https://doi.org/10.1016/j.chemosphere.2019.124898.Suche in Google Scholar PubMed
Supplementary Material
The article contains supplementary material (https://doi.org/10.1515/reveh-2022-0182).
© 2022 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Reviews
- The lack of international and national health policies to protect persons with self-declared electromagnetic hypersensitivity
- The use of micronucleus assay in oral mucosa cells as a suitable biomarker in children exposed to environmental mutagens: theoretical concepts, guidelines and future directions
- Improving the purification of aqueous solutions by controlling the production of reactive oxygen species in non-thermal plasma; a systematic review
- Ochratoxin A in coffee and coffee-based products: a global systematic review, meta-analysis, and probabilistic risk assessment
- Green space in health research: an overview of common indicators of greenness
- The effects of fine particulate matter on the blood-testis barrier and its potential mechanisms
- Evaluation of chemicals leached from PET and recycled PET containers into beverages
- The association between bisphenol a exposure and attention deficit hyperactivity disorder in children: a meta-analysis of observational studies
- A review on arsenic pollution, toxicity, health risks, and management strategies using nanoremediation approaches
- The impact of air pollution and climate change on eye health: a global review
- Exposure to Polycyclic Aromatic Hydrocarbons and adverse reproductive outcomes in women: current status and future perspectives
- Mechanisms of cholera transmission via environment in India and Bangladesh: state of the science review
- Effects of sulfur dioxide inhalation on human health: a review
- Health effects of alkaline, oxygenated, and demineralized water compared to mineral water among healthy population: a systematic review
- Toxic effects due to exposure heavy metals and increased health risk assessment (leukemia)
- A systematic review on environmental perspectives of monkeypox virus
- How does formal and informal industry contribute to lead exposure? A narrative review from Vietnam, Uruguay, and Malaysia
- Letter to the Editor
- Comments on “Personal protective equipment (PPE) and plastic pollution during COVID-19: strategies for a sustainable environment”, by Fatima Ali Mazahir and Ali Mazahir Al Qamari
Artikel in diesem Heft
- Frontmatter
- Reviews
- The lack of international and national health policies to protect persons with self-declared electromagnetic hypersensitivity
- The use of micronucleus assay in oral mucosa cells as a suitable biomarker in children exposed to environmental mutagens: theoretical concepts, guidelines and future directions
- Improving the purification of aqueous solutions by controlling the production of reactive oxygen species in non-thermal plasma; a systematic review
- Ochratoxin A in coffee and coffee-based products: a global systematic review, meta-analysis, and probabilistic risk assessment
- Green space in health research: an overview of common indicators of greenness
- The effects of fine particulate matter on the blood-testis barrier and its potential mechanisms
- Evaluation of chemicals leached from PET and recycled PET containers into beverages
- The association between bisphenol a exposure and attention deficit hyperactivity disorder in children: a meta-analysis of observational studies
- A review on arsenic pollution, toxicity, health risks, and management strategies using nanoremediation approaches
- The impact of air pollution and climate change on eye health: a global review
- Exposure to Polycyclic Aromatic Hydrocarbons and adverse reproductive outcomes in women: current status and future perspectives
- Mechanisms of cholera transmission via environment in India and Bangladesh: state of the science review
- Effects of sulfur dioxide inhalation on human health: a review
- Health effects of alkaline, oxygenated, and demineralized water compared to mineral water among healthy population: a systematic review
- Toxic effects due to exposure heavy metals and increased health risk assessment (leukemia)
- A systematic review on environmental perspectives of monkeypox virus
- How does formal and informal industry contribute to lead exposure? A narrative review from Vietnam, Uruguay, and Malaysia
- Letter to the Editor
- Comments on “Personal protective equipment (PPE) and plastic pollution during COVID-19: strategies for a sustainable environment”, by Fatima Ali Mazahir and Ali Mazahir Al Qamari

