Abstract
Available literature data on the aqueous chemistry of radium are compiled. There are limited available experimental data and a significant portion of the data has been estimated using electrostatic techniques, typically based on the corresponding data of barium. The available data are compared with the corresponding data of barium (and strontium) and a methodology for estimating additional radium thermochemical data is described.
1 Introduction
All the isotopes of radium are radioactive and emit high intensity radiation (often alpha particles and gamma rays) and, as such, very few laboratories are equipped to work with milligram amounts of radium that are required for the determination of good quality thermodynamic data. Consequently, the aqueous chemistry of radium is relatively unexplored and the vast majority of the chemical and thermodynamic properties of radium have been estimated, being generally based on the similarity of radium with the other alkaline earth metals, in particular, barium. Similar problems are found with other heavy elements that have no stable isotopes, especially the actinides.
Radium release from high level nuclear waste repositories is predicted to be one of the major contributors of radiation dose. Radium is also associated with uranium tailings and scale formation in the oil and gas industry. To understand the behavior of radium in these settings, it is essential to have thermodynamic data in relation to the aqueous chemistry of radium, both the stability of its aqueous species and solubility of its phases, so that its behavior in the environment can be understood.
Presently, radium finds applications in radiopharmaceutical chemistry [1, 2] where studies have shown that an aqueous solution of 223RaCl2 (trademark Xofigo®) is an efficient bone-seeking radiopharmaceutical which can reduce pain and delay disease progression in patients with bone metastases from advanced stage prostate cancer. These encouraging successes have sparked an interest in radium chemistry and much effort has been directed towards development of organic chelating ligands that can strongly and efficiently bind 223Ra2+ in mild conditions [3], [4], [5]. These radium-based molecules can be later conjugated to a biomolecule (e.g., amino acid or peptide) and can then be used to selectively target specific cancer cells. Moreover, various inorganic particles have been considered as alternative carriers for 223Ra and 224Ra, where the particles (e.g., surface functionalized BaSO4 or CaCO3 [6, 7]) labeled with radium can target various biological moieties.
Chemically, radium is similar to barium because they have similar ionic radii, however, their ionic radii are sufficiently different for measurable differences in stability/solubility to occur between Ba and Ra species/phases with the same ligand. The similar ionic radii of Ra and Ba have enabled the thermochemistry of radium to be estimated (when experimental data have not been determined) on the basis of the measured thermochemical behavior of barium and, in general, the stability of radium complexes is weaker than those of barium and radium phases less soluble. However, on occasion, it has been found that the opposite behavior occurs, where radium has a more stable complex than that of barium or its solid phase is more soluble than the corresponding phase of barium.
The present study compiles the available thermochemical data for radium and compares it to the associated data of barium. It describes how additional radium thermochemical data can be estimated depending on available measured data for both strontium and barium.
2 Aqueous chemistry of radium
2.1 Radium ion (Ra2+)
The standard oxidation potential of the Ra2+-Ra couple was calculated by Latimer [8] to be −2.916 V (equivalent to a log10 K = −98.6). Parker et al. [9] list a Gibbs energy of formation for Ra2+ of −561.5 kJ/mol that is equivalent to an oxidation potential of −2.910 V, in agreement with the value derived by Latimer [8]. All three values are very similar to the corresponding values for barium [10]. The Gibbs energy is more negative than the value recently estimated by Kitamura and Yoshida [11] (−555.6 ± 1.8 kJ/mol [−2.879 V]) from the trend in the Gibbs energy values for the other alkaline earth couples (M2+-M). This latter value is discussed further below.
2.2 Inorganic species and phases of radium
Experimental data for stability and solubility constants are only available for a relatively small number of radium species and solid phases, due to the experimental difficulties outlined above. Radium hydroxide is the most soluble of the alkaline earth hydroxide phases and, as such, is more basic than barium hydroxide [10]. This is supported by the data of Kitamura and Yoshida [11] who gave a solubility for Ra(OH)2(s) in acid of log10 K s ° = 31.2; this solubility constant was determined from Gibbs energy data listed in the JAEA thermochemical database [12]. The formation of RaOH+ has been experimentally measured [13, 14] and has also been estimated using electrostatic techniques several times [11, 15, 16]. Matyskin et al. [13] determined a stability constant for RaOH+ formation of log10 *K° = −13.3 ± 0.2 that was in good agreement with the earlier data of Zielińska and Bilewicz [14]. Brown and Ekberg [15] quoted a value of log10 *K° = −13.49 ± 0.20 from the earlier work of Langmuir and Riese [16], who estimated their value. Brown and Ekberg [15] also estimated a value of log10 *K° = −13.36 using the unified theory of metal ion complexation (UTMIC). Finally, the Gibbs energy data estimated by Kitamura and Yoshida [11] lead to log10 *K° = −13.56. The average of these values, which are all in reasonable agreement considering the weak stability, is log10 *K° = −13.42 ± 0.23 (equivalent to log10 K° = 0.57 ± 0.23 using the protonation constant of water from Brown and Ekberg [15]; where multiple data are available, the uncertainty is determined from the 95% confidence interval of the cited data).
The solubility of radium sulfate has been experimentally determined in several studies across the temperature range of 10–70 °C [17], [18], [19], [20], [21], [22], with all the data being in reasonable agreement. Experimental solubility data for 25 °C are only from Matyskin [22] (log10 K s ° = −10.23) whereas estimated values using electrostatic techniques have been derived by Brown et al. [23], Langmuir and Riese [16] (both log10 K s ° = −10.26) and Paige et al. [19] (log10 K s ° = −10.21). Aqueous complexes can form in solubility experiments, in this case RaSO4(aq), and data are only used in the present study where aqueous speciation has been considered (for all solubility constants). The average solubility constant is log10 K s ° = −10.24 ± 0.05. Use of the Gibbs energy data (where necessary, Gibbs energy data for inorganic anions are taken from the Nuclear Energy Agency series, e.g., ref. [24]) from Kitamura and Yoshida [11] leads to a solubility constant that is inconsistent with the other values listed and may question the Gibbs energy value they obtained for Ra2+. The crystal structures of Ra and Ba sulfate are both orthorhombic and they easily form a solid solution with a small Gibbs energy of mixing [22]. The stability of aqueous radium sulfate has not been measured experimentally, but has been estimated using electrostatic techniques several times [11, 16, 25]. The stability of the aqueous alkaline earth sulfate complexes exhibit an unusual behavior of increasing stability with increasing size of the alkaline earth ion, although a similar trend is also observed for cations in other valences (e.g., alkali and lanthanide metals). Consequently, it has been estimated that the stability of radium sulfate is greater than that of barium sulfate. Reported stability constants for aqueous radium sulfate are log10 K° = 2.63 [11], 2.76 [16], and 2.48 [25], with an average of log10 K° = 2.61 ± 0.33.
The solubility of most radium solid phases is less than that of the corresponding barium phase. However, radium carbonate is an exception with Nikitin [26] indicating that radium carbonate was 10 times more soluble than barium carbonate. Recently, Matyskin [22] conducted solubility studies from both undersaturation and oversaturation and confirmed that radium carbonate was significantly more soluble than barium carbonate, obtaining the first solubility constant for radium carbonate of log10 K s ° = −7.5 that is one log unit more than the solubility constant of barium carbonate. This latter study noted that witherite (BaCO3(s)) has an orthorhombic crystallinity whereas RaCO3(s) is cubic containing carbonate oxygen atoms that are highly disordered. This disordered nature possibly leads to the increase in solubility of RaCO3(s). Again, the stability constant of aqueous radium carbonate has not been measured experimentally, but data are available for the constant that have been determined using electrostatic techniques. The stability constants that have been derived are log10 K° = 2.5 [16], 2.61 [11], and 2.43 [25], leading to an average value of log10 K° = 2.53 ± 0.14.
A solubility constant has been estimated for RaHPO4(s) using electrostatic methods with confirmation of the solubility in a biogeochemical model of metal uptake (as a surrogate for calcium) into aquatic invertebrates [27, 28]. The average of the two solubility constants was log10 K s ° = −7.55.
Radium chloride is a relatively soluble salt. The solubility has been measured by Erbacher [29] and from the data a solubility constant of log10 K s ° = 0.35 can be determined. In addition, from the Gibbs energy data given by Kitamura and Yoshida [11] a solubility constant of log10 K s ° = 0.29 was determined. The latter value was estimated using electrostatic techniques, but is in very good agreement with the experimentally determined value. The average solubility constant is log10 K s ° = 0.32 ± 0.10 (where the uncertainty has been estimated in the present study). A stability constant has been estimated for the formation of RaCl+ in two studies [11, 16]. The estimated stability constants are log10 K° = −0.10 and −0.55 indicating that the stability of the complex is very weak, and potentially, actual formation of the complex may be questionable. The average stability constant is log10 K° = −0.32 ± 0.23 (where the uncertainty is selected to cover the range of the two values).
A stability constant for RaF+ can be estimated from Kitamura and Yoshida [11] using the provided Gibbs energy data. A stability constant of log10 K° = −0.27 was derived indicating that the stability of the species is weak. However, the stability of the corresponding barium and strontium phases might suggest that the stability of this phase is greater than indicated from the stability constant of Kitamura and Yoshida [11] and, as such, it is not considered reliable (see discussion with barium species below). In addition, recently Butkalyuk et al. [29] measured the solubility of RaF2(s). They measured a solubility of 0.763 g per 100 mL of water. Use of this value, and correction for the ionic strength of the solution (using the Davies equation; this equation has been used to correct stability or solubility constants to zero ionic strength throughout the present study) and complexation of radium with fluoride, leads to a solubility constant of log10 K s ° = −4.66 (considering the solution speciation of RaF+ with the stability constant listed below).
Erbacher [30] also measured the solubility of radium bromide and found that it was more soluble than radium chloride. From the data, a solubility constant of log10 K s ° = 1.39 has been determined.
Polesitsky and Tolmatsheff [31] measured the solubility of radium iodate. They found that it was less soluble than the corresponding barium salt. The solubility constant determined from the data was log10 K s ° = −8.85.
The solubility of radium nitrate was also measured by Erbacher [30]. Similar to the case for radium carbonate, the nitrate phase of radium is more soluble than the corresponding phase for barium. This has also been confirmed more recently by Butkalyuk et al. [32]. From the data of Erbacher [30], a solubility constant of log10 K s ° = −0.60 is determined, indicating that expectedly that the nitrate phase is quite soluble.
2.3 Organic species of radium
The majority of data on the complexation of radium by organic ligands has come from the work of Schubert and coworkers [33, 34] and Sekine et al. [35], although other data have been published on the stability of radium with ethylenediamine N,N,N′,N′-tetraacetic acid (EDTA) [36], [37], [38], [39]. In all of these studies, it has been found that the stability of the radium complex is less than that of the corresponding barium complex.
Matyskin et al. [39] have recently studied the complexation of both radium and barium with EDTA at two different pH values and as a function of ionic strength (0.2–2.5 mol/L) using NaCl as the medium. From the data, the stability constant of a single complex, RaEDTA2−, was determined with a value of log10 K° = 9.13. Previous data, when corrected to zero ionic strength [39], have been found to be in good agreement with this value, with stability constants of log10 K° = 9.22 [36], 9.2 [37], 8.9 [38], and 9.09 [35]; the average of all five measurements is log10 K° = 9.11 ± 0.25. Two of these studies utilized a temperature of 20 °C [36, 38], but the difference in stability that results from use of this temperature is believed to be within the uncertainty of the measurements.
Sekine et al. [35] also determined stability constants of the alkaline earth metals, including radium, with a range of other organic ligands. The ligands included cyclohexane 1,2-diamine N,N,N′,N′-tetraacetic acid (CyDTA), diethylenetriamine N,N,N′,N″,N″-pentaacetic acid (DTPA), nitrilotriacetic acid (NTA), 2,2′-ethylenedioxyl bis[ethyliminodi(acetic acid)] (EGTA) and N′-(2-hydroxyethyl)ethylenediamine N,N,N′-triacetic acid (HEDTA). Stability constants were determined in 0.1 mol/L NaClO4 and have been corrected to zero ionic strength [38] (although two [DTPA and HEDTA] have been redetermined in the present study using the correct charge for the ligand). The stability constants obtained were log10 K° = 10.09 (RaCyDTA2−), 9.49 (RaEGTA2−), 10.74 (RaDTPA3−), 6.95 (RaHEDTA−), and 6.45 (RaNTA−). The latter stability constant has been corrected to zero ionic strength in the present work from log10 K = 5.1 (0.1 mol/L NaClO4).
Schubert and coworkers [33, 34] studied the complexation of radium with several other organic acids. The acids studied included citric (Cit), tartaric (Tar), malic (Mal), succinic (Suc), aspartic (Asp), pyruvic (Pyr), oxaloacetic (OxAc), fumaric (Fum), and sulfosalicylic (SalSO4). The data were obtained using an ionic strength of 0.16 mol/L NaCl. They have also been corrected to zero ionic strength [39]. The stability constants obtained for zero ionic strength are log10 K° = 3.91 and 3.55 (for RaCit−; with an average of log10 K° = 3.73), 2.27 and 2.23 (for RaTar(aq); with an average of log10 K° = 2.25), 1.98 (RaMal(aq)), 2.03 (RaSuc(aq)), 1.38 (RaAsp+), 1.41 (RaPyr+), 2.63 (RaFum(aq)), 2.83 (RaOxAc(aq)), and 2.93 (RaSalSO4(aq)).
It is clear from the data for all organic ligands that the stability, as expected, increases with increasing charge of the complexing ligand. Moreover, the stability of the neutral species is also similar to the stability of the neutrally species of radium with inorganic ligands.
2.4 Comparison with the aqueous chemistry of barium
Stability and solubility data can also be compiled for barium. The data for radium with the inorganic and organic ligands listed above are compared with those of barium, where available, in Table 1. The data are split into two; those where the barium stability or solubility constant (log10 K° or log10 K s °) is less than the corresponding radium constant (there are only a few examples of this behavior) and those where it is larger. There are some examples where the barium constant is less than the radium constant, but it has been placed in the second of the two datasets. For these few cases, it is believed that either the radium or barium (or both) constant may be slightly in error and the barium constant should be the larger. In all three cases, this behavior is seen for organic acid ligands where the typical behavior for these ligands is for the barium constant to be larger than that of radium (see also discussion below).
Comparison of the solubility and stability constants of radium, barium, and strontium with various ligands at 25 °C and zero ionic strength.
| Species | log10 K° | References for Ba | References for Sr | |||
|---|---|---|---|---|---|---|
| Ra | Ba (Ba > Ra) | Ba (Ba < Ra) | Sr | |||
| MOH+ | 0.57 ± 0.23 | 0.67 ± 0.07 | 0.84 ± 0.05 | [15] | [15] | |
| MSO4(aq) | 2.61 ± 0.33 | 2.40 ± 0.10b | 2.21 ± 0.11 | [19, 40] | [40, 56] | |
| MCO3(aq) | 2.53 ± 0.14 | 2.56 ± 0.10b | 2.81 ± 0.10b | [16] | [57] | |
| MEDTA2- | 9.11 ± 0.25 | 9.86 ± 0.11 | 10.31 ± 0.37 | [39] | [58], [59], [60] | |
| MCyDTA2- | 10.09 ± 0.10b | 9.79 ± 0.10a,b | 10.71 ± 0.10b | [35] | [58] | |
| MEGTA2- | 9.49 ± 0.10b | 10.19 ± 0.10b | [35] | |||
| MDTPA3- | 10.74 ± 0.10b | 11.04 ± 0.10b | 11.94 ± 0.10b | [35] | [61] | |
| MHEDTA- | 6.95 ± 0.10b | 7.55 ± 0.10b | 8.15 ± 0.10b | [35] | [62] | |
| MNTA- | 6.45 ± 0.10b | 6.41 ± 0.10a,b | 6.73 ± 0.10b | [41] (20 °C) | [41] (20 °C) | |
| MCit- | 3.73 ± 0.18 | 4.09 ± 0.05 | 4.41 ± 0.03 | [32, 42, 43] | [33, 63] | |
| MTar(aq) | 2.25 ± 0.05 | 2.64 ± 0.12 | 2.85 ± 0.29 | [33, 44] | [63, 64] | |
| MSuc(aq) | 2.03 ± 0.10b | 2.25 ± 0.11 | 2.29 ± 0.10b | [33, 45] | [45] | |
| MAsp+ | 1.38 ± 0.10b | 1.59 ± 0.10b | 1.93 ± 0.10b | [46] | [46] | |
| MPyr+ | 1.41 ± 0.10b | |||||
| MFum(aq) | 2.63 ± 0.10b | 2.49 ± 0.10a,b | [47] | |||
| MOxAc(aq) | 2.83 ± 0.10b | |||||
| MMal(aq) | 1.98 ± 0.10b | 2.31 ± 0.11 | 2.45 ± 0.28 | [33, 44, 48] | [33, 48] | |
| MSalSO4(aq) | 2.93 ± 0.10b | |||||
| MCl+ | −0.32 ± 0.23 | −0.01 ± 0.05 | 0.21 ± 0.10b | [49], [50], [51] | [51] | |
| MSO4(s) | −10.24 ± 0.05 | −9.96 ± 0.05 | −6.62 ± 0.02 | [23] | [23] | |
| MHPO4(s) | −7.55 ± 0.05b | −7.46 ± 0.05b | −6.97 ± 0.11 | [27, 28] | [27, 28] | |
| M(OH)2(s) | 31.2 ± 0.5b,c | 30.2 ± 0.2b | 27.5 ± 0.2b | [11] | [11] | |
| MCO3(s) | −7.49 ± 0.10 | −8.56 ± 0.02 | −9.27 ± 0.02 | [23] | [23] | |
| MCl2(s) | 0.32 ± 0.10b | 1.11 ± 0.10b | [22] | |||
| M(NO3)2(s) | −0.60 ± 0.10b | −0.84 ± 0.10b | [22] | |||
| M(IO3)2(s) | −8.85 ± 0.10b | −8.75 ± 0.10b | −6.49 ± 0.08 | [22] | [52, 65, 66] | |
| MBr2(s) | 1.39 ± 0.20b | 2.23 ± 0.20b | [22] | |||
| MF2(s) | −4.66 ± 0.20b | −5.87 ± 0.05 | −8.54 ± 0.15 | [53], [54], [55] | [29, 59, 67] | |
-
aAlthough these values are less than the corresponding Ba constant, the stability behavior within the alkaline earth series (particularly that of strontium) would suggest that the constant should be larger than that of radium – see text. bUncertainty estimated in the present study. cEstimated stability or solubility constant.
Linear free energy relationships can be used to compare the magnitude of the solubility and stability constants of radium and barium complexes and phases. Figure 1 illustrates the relationship between the larger dataset, where the barium constant is larger than that of radium. As can be seen from the figure, there is very good correlation between the two sets of constants, with a coefficient of determination (r 2) of 0.999. As would be expected when the barium solubility and stability constants are larger than those of radium, the intercept is negative. The slope is marginally larger than unity. Given that the stability and solubility constants are displayed in logarithmic form, the relationship extends over more than 40 orders of magnitude in stability.
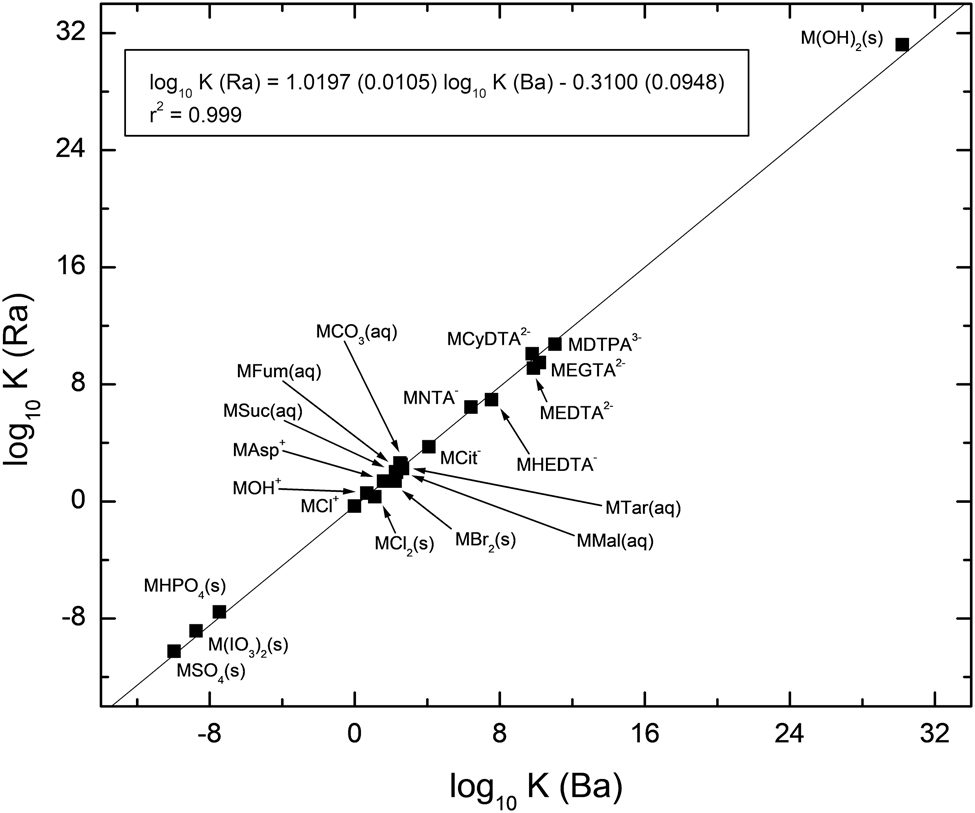
Correlation of the stability and solubility of Ba and Ra complexes and phases where the Ba constant is greater than that of Ra.
The relationship between the much smaller dataset, where the radium constant is larger than that of barium is shown in Figure 2. Again, there is an excellent correlation between the two sets of data, with a coefficient of determination of 0.9988. Even considering the small amount of data in the relationship, the correlation between the data indicates that the fit is significant. Converse to the behavior for the larger set of data, as might be expected for this dataset the intercept is greater than zero. The slope is significantly less than unity. For this set, the relationship spans about 10 orders of magnitude.
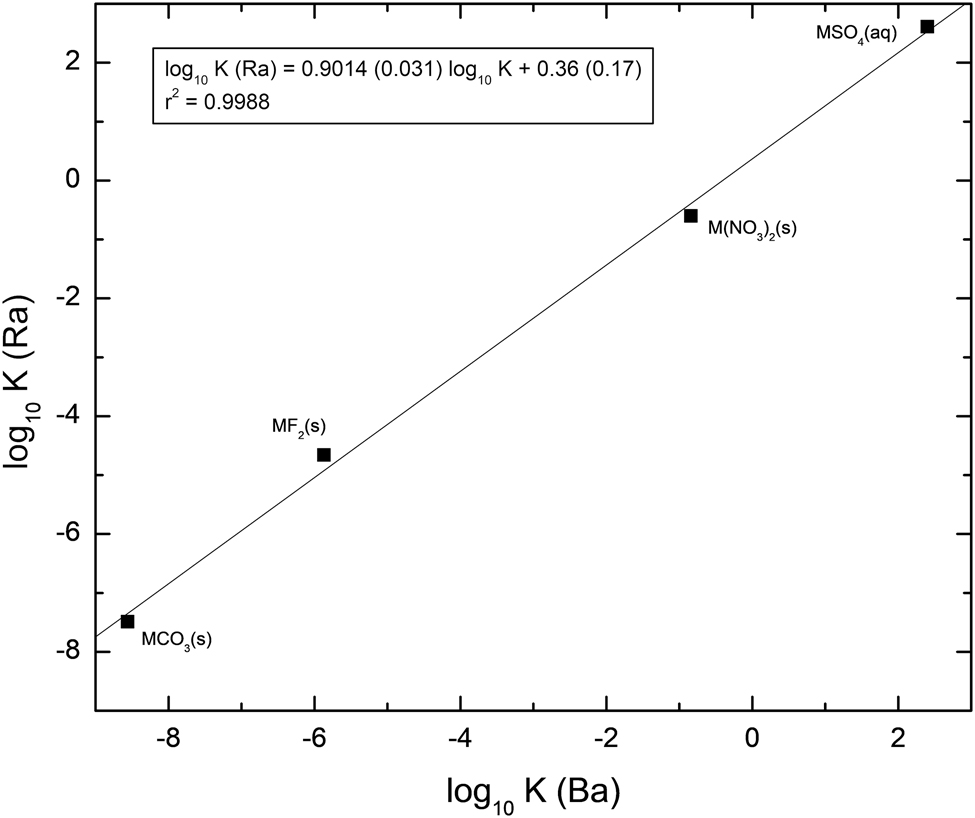
Correlation of the stability and solubility of Ba and Ra complexes and phases where the Ba constant is less than that of Ra.
It is important to note that a similar linear free energy relationship is not observed between the stability and solubility constants of radium with those of strontium. For example, the solubility constant of strontium with iodate has been determined to be log10 K s ° = −6.48 [52], whereas that for the solubility of strontium with sulfate is quite similar with log10 K s ° = −6.62 [23]. As can be seen in Table 1, the solubility constants for these two ligands both differ by more than an order of magnitude for both radium and barium.
What is always consistent from the data shown in Table 1, that further enables the selection of whether the solubility or stability constant of barium is greater than that of radium, is that when the Ba constant is greater than that of Ra then the corresponding constant of strontium is always greater than that of barium. Conversely, if the constant of barium is less than that of radium then the constant of strontium will always be less than that of barium. For example, at 25 °C, strontium is the least soluble of the alkaline earth carbonate phases. The stability or solubility constants of radium and strontium rarely overlap ensuring that the occurrence of the Ba constant being greater or less than the corresponding Ra constant can be easily ascertained.
Further, it is probable that when Ba and Ra phases have the same crystal structure that the solubility of the Ra phase will be less than that of the corresponding Ba phase. Conversely, when they are different and the radium phase may have a disordered structure, then the solubility of the Ra phase will be greater than that of the barium phase.
Stability constant data are available in the literature for the complexation of the alkaline earth metals, except radium, with fluoride. For magnesium the data span an ionic strength range of 0–1 mol/L (NaClO4), whereas there are less data for the heavier alkaline earth metals. However, the data that are available enable estimates to be made for all the metals at zero ionic strength. The data so derived indicate that the zero ionic strength stability constants decrease with increasing alkaline earth metal ion size, with BaF+ having a stability constant of log10 K° = 0.63. Based on this stability, the decreasing stability down the series and the regression equation listed in Figure 1, the calculated stability constant for RaF+ is log10 K° = 0.33. This stability constant is substantially more positive than that proposed by Kitamura and Yoshida [11] (log10 K° = −0.27). This may appear to again question the Gibbs energy derived for Ra2+ in this latter work, however, using the Gibbs energy listed in the present work actually leads to a more negative stability constant for RaF+. Use of the stability constants for SrF+ and BaF+ derived in the present study to determine Gibbs energy of reaction values, and combining them with the Gibbs energy values given by Kitamura and Yoshida [11] for Sr2+ and Ba2+ and that for F− from the NEA series [24], leads to Gibbs energy of formation values for both SrF+ and BaF+ that are 4.4 kJ/mol more negative than indicated by Kitamura and Yoshida [11]. Based on the prediction methodology used in the latter study [11], then the predicted Gibbs energy of formation value for RaF+ would also be 4.4 kJ/mol more negative, leading to a stability constant of log10 K° = 0.49, in closer agreement with the stability constant derived in the present study.
The regression equations given in Figures 1 and 2 can be used to estimate stability and solubility constants for either radium or barium complexes or phases where they have not been previously measured experimentally. However, before undertaking such calculations it is worthwhile to show the difference that occurs between the regression equations of the two figures. For this purpose, the solubility of radium carbonate is an excellent example. If the solubility of radium carbonate was consistent of a phase that was less soluble than the corresponding barium phase, then the regression equation shown in Figure 1 would indicate a solubility constant of log10 K s ° = −9.03. This is about 1.5 orders of magnitude less soluble than has been observed experimentally [22] and also estimated using electrostatic techniques [23]. There is a clear chemical behavior difference when radium solubility or stability constants are greater than the corresponding barium constants. Importantly, as indicated above, when the stability or solubility constant of radium is greater than that of barium, then also the constant of barium will be greater than that of strontium.
These important relationships between the stability and solubility constants in the strontium, barium and radium series can then be used to derive constants that have not, to date, been measured experimentally. As a first example, the stability of thiosulfate complexes with the alkaline earth metals exhibits the same behavior as that of the sulfate complexes. The average stability constant for the barium species, BaS2O3(aq), determined from literature data [68, 69] is log10 K° = 2.27. It is noted that the solubility constant for barium is greater than that of strontium which, in turn, is greater than that of calcium. Based on this information, and using the regression equation given in Figure 2, the calculated solubility constant for RaS2O3(aq) is log10 K° = 2.41, i.e., larger than that for BaS2O3(aq).
Similarly, data from Stary [70] indicate that the solubility of alkaline earth metals with oxalate increases with increasing ionic size of the alkaline earth metal ion. Stary [70] measured a solubility constant for BaOx(s) of log10 K s ° = −6.0 (using 0.1 mol/L KClO4), from which a zero ionic strength solubility constant of log10 K s ° = −6.43 can be derived. In the same study, the solubility of the strontium salt was found to be less than that of the barium salt and, in addition, that of the strontium salt less than that of the corresponding calcium salt. Regarding this behavior, the solubility of RaOx(s) is calculated, using the regression equation given in Figure 2, to be log10 K s ° = −5.43.
As shown in Table 1, there are a few ligands where stability constants have been determined for radium complexes, but no similar measurements have been made for barium complexes. Given that these complexes are all with organic acids, it is expected that the stability of the barium complexes will be greater than those of the radium complexes. Consequently, using the regression equation given in Figure 1, the following stability constants are calculated, log10 K° = 1.69 (BaPyr+), 3.08 (BaOxAc(aq)), and 3.18 (BaSalSO4(aq)).
There are also multiple examples of where the stability or solubility constant of barium is greater than that of radium. The following are some examples of prediction of the solubility or stability constant of radium complexes where not only is the stability of the barium complex larger than that of radium, but also the strontium complex stability is larger than that of barium. The data derived are for both inorganic and organic ligands. The stability constants derived for the various radium complexes are: RaAc+ (log10 K° = 0.44 [based on an average stability constant for BaAc+ of log10 K° = 0.74 [71], [72], [73]; where Ac is acetate]); RaOx(aq) (log10 K = 1.09 [based on a stability constant for BaOx(aq) of log10 K = 0.58 [74]; and corrected to zero ionic strength using other related data]); RaProp+ (log10 K° = −0.16 [based on a stability constant for BaProp+ of log10 K° = 0.15 [71]; where Prop is propionate]); RaGly+ (log10 K° = 0.47 [based on a stability constant for BaGly+ of log10 K° = 0.77 [75]; where Gly is glycine]); RaATP2− (log10 K° = 4.87 [based on a stability constant for BaATP2− of log10 K = 3.29 [76] and corrected to zero ionic strength; where ATP is adenosine triphosphoric acid]); RaHQSO4(aq) (log10 K° = 2.05 [based on a stability constant for BaHQSO4(aq) of log10 K° = 2.31 [77]; where HQSO4 is 8-hydroxyquinoline-5-sulfonic acid]); RaDiPic(aq) (log10 K° = 4.10 [based on a stability constant for BaDiPic(aq) of log10 K = 3.43 [78] and corrected to zero ionic strength; where DiPic is dipicolinic acid]); and RaP3O10 3− (log10 K° = 4.10 [based on a stability constant for BaP3O10 3− of log10 K° = 4.33 [79]; where P3O10 5− is the triphosphate ion]).
3 Conclusions
Available literature data for the aqueous chemistry of radium shows that in the majority of cases that the stability or solubility (with respect to log10 K° or log10 K s °) of a radium species/phase is less than the corresponding barium species/phase. This would indicate that the aqueous species are less stable, but also that the solid phases are less soluble. However, there are a limited number of cases where the opposite behavior is observed. Nevertheless, in all cases, the same trend that is observed between strontium and barium will also be observed between barium and radium. These trends enable the estimation of thermochemical data for radium if the corresponding data are available for strontium and barium.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: None declared.
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
1. Bruland, Ø. S., Jonasdottir, T. J., Fisher, D. R., Larsen, R. H. Radium-223: from radiochemical development to clinical applications in targeted cancer therapy. Curr. Rad. 2008, 1, 203; https://doi.org/10.2174/1874471010801030203.Suche in Google Scholar
2. Parker, C., Nilsson, S., Heinrich, D., Helle, S. I., O’Sullivan, J. M., Fosså, S. D., Chodacki, A., Wiechno, P., Logue, J., Seke, M., Widmark, A., Johannessen, D. C., Hoskin, P., Bottomley, D., James, N. D., Coleman, R., Franzén, L., O’Bryan-Tear, C. G., Staudacher, K., Bruland, Ø. S., Solberg, A., Kliment, J., Wedel, S., Boehmer, S., Dall’Oglio, M., Vogelzang, N. J., Garcia-Vargas, J., Shan, M., Sartor, O. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213; https://doi.org/10.1056/nejmoa1213755.Suche in Google Scholar
3. Bauer, D., Blumberg, M., Köckerling, M., Mamat, C. A comparative evaluation of calix[4]arene-1,3-crown-6 as a ligand for selected divalent cations or radiopharmaceutical interest. RSC Adv. 2019, 9, 32357; https://doi.org/10.1039/c9ra07293d.Suche in Google Scholar
4. Henriksen, G., Hoff, P., Larsen, R. H. Evaluation of potential chelating agents for radium. Appl. Radiat. Isot. 2002, 56, 667; https://doi.org/10.1016/s0969-8043(01)00282-2.Suche in Google Scholar
5. Abou, D. S., Thiele, N. A., Gutsche, N. T., Villmer, A., Zhang, H., Woods, J. J., Baidoo, K. E., Escorcia, F. E., Wilson, J. J., Thorek, D. L. J. Towards the stable chelation of radium for biomedical applications with an 18-membered macrocyclic ligand. R. Soc. Chem. Sci. 2021, 12, 3733; https://doi.org/10.1039/d0sc06867e.Suche in Google Scholar PubMed PubMed Central
6. Li, R. G., Napoli, E., Jorstad, I. S., Børnsdorff, T. B., Juzeniene, A., Bruland, Ø. S., Larsen, R. H., Westrøm, S. Calcium carbonate microparticles as carriers of 224Ra: impact of specific activity in mice with intraperitoneal ovarian cancer. Curr. Rad. 2021, 14, 145; https://doi.org/10.2174/1874471013666201201102056.Suche in Google Scholar PubMed
7. Reissig, F., Hübner, R., Steinbach, J., Pietzsch, H.-J., Mamat, C. Facile preparation of radium-doped, functionalized nanoparticles as carriers for targeted alpha therapy. Inorg. Chem. Front. 2019, 6, 1341; https://doi.org/10.1039/c9qi00208a.Suche in Google Scholar
8. Latimer, W. M. Oxidation Potentials, 2nd ed.; Prentice-Hall: New York, 1952.10.1097/00010694-195210000-00019Suche in Google Scholar
9. Parker, V. B., Wagman, D. D., Evans, W. H. Selected values of chemical thermodynamic properties. Tables for the alkaline earth elements (elements 92 through 97 in the standard order of arrangement); NBS Technical Note 270-6, 1971.Suche in Google Scholar
10. Kirby, H. W., Salutsky, M. L. The Radiochemistry of Radium. National Academy of Sciences, Nuclear Science Series, NAS-NS 3057; Springfield: U.S. Atomic Energy Commission, 1964.10.2172/4560824Suche in Google Scholar
11. Kitamura, A., Yoshida, Y. Prediction of thermodynamic data for radium suitable for thermodynamic database for radioactive waste management using an electrostatic model and correlation with ionic radii among alkaline earth metals. J. Radioanal. Nucl. Chem. 2021, 327, 839; https://doi.org/10.1007/s10967-020-07527-5.Suche in Google Scholar
12. Kitamura, A. JAEA-TDB-RN in 2020: Update of JAEA’s Thermodynamic Database for Solubility and Speciation of Radionuclides for Performance Assessment of Geological Disposal of High-Level and TRU Wastes. JAEA-Data/Code, 2020-020; Tokai-mura: Japan Atomic Energy Agency, 2021.Suche in Google Scholar
13. Matyskin, A. V., Brown, P. L., Ekberg, C. Weak barium and radium hydrolysis using an ion exchange method and its uncertainty assessment. J. Chem. Thermodyn. 2019, 128, 362; https://doi.org/10.1016/j.jct.2018.08.037.Suche in Google Scholar
14. Zielińska, B., Bilewicz, A. Influence of relativistic effects on hydrolysis of Ra2+. J. Radioanal. Nucl. Chem. 2005, 266, 339.10.1007/s10967-005-0913-4Suche in Google Scholar
15. Brown, P. L., Ekberg, C. Hydrolysis of Metal Ions; Wiley VCH: Weinheim, 2016; p. 917.10.1002/9783527656189Suche in Google Scholar
16. Langmuir, D., Riese, A. C. The thermodynamic properties of radium. Geochem. Cosmochim. Acta 1985, 49, 1593; https://doi.org/10.1016/0016-7037(85)90264-9.Suche in Google Scholar
17. Hedström, H. Radium Sulfate and its Co-Precipitation Behaviour with Barium and Strontium. Ph.D. dissertation, Chalmers University of Technology: Gothenburg, Sweden, 2013.Suche in Google Scholar
18. Nikitin, B., Tolmatscheff, P. Ein Beitrag zur Gültigkeit des Massenwirkungsgesetzes. II. Quantitative Bestimmung der Löslichkeit des Radiumsulfats in Natriumsulfatlösungen und in Wasser. Z. Phys. Chem. 1933, A167, 260; https://doi.org/10.1515/zpch-1933-16728.Suche in Google Scholar
19. Paige, C. R., Kornicker, W. A., Hileman, O. E., Snodgrass, W. J. Solution equilibria for uranium ore processing: the BaSO4-H2SO4-H2O system and the RaSO4-H2SO4-H2O system. Geochem. Cosmochim. Acta 1998, 62, 15; https://doi.org/10.1016/s0016-7037(97)00320-7.Suche in Google Scholar
20. Brandt, F., Curti, E., Klinkenberg, M., Rozov, K., Bosbach, D. Replacement of barite by a (Ba,Ra)SO4 solid solution at close-to-equilibrium conditions: a combined experimental and theoretical study. Geochem. Cosmochim. Acta 2015, 155, 1; https://doi.org/10.1016/j.gca.2015.01.016.Suche in Google Scholar
21. Langmuir, D., Melchior, D. The geochemistry of Ca, Sr, Ba and Ra sulfates in some deep brines from Palo Duro Basin, Texas. Geochem. Cosmochim. Acta 1985, 49, 2423; https://doi.org/10.1016/0016-7037(85)90242-x.Suche in Google Scholar
22. Matyskin, A. V. Solubility and Crystal Structure of Radium Sulfate and Carbonate. Ph.D. dissertation, Chalmers University of Technology: Gothenburg, Sweden, 2018.Suche in Google Scholar
23. Brown, P. L., Ekberg, C., Matyskin, A. V. On the solubility of radium and other alkaline earth sulfate and carbonate phases at elevated temperature. Geochem. Cosmochim. Acta 2019, 255, 88; https://doi.org/10.1016/j.gca.2019.04.009.Suche in Google Scholar
24. Brown, P. L., Curti, E., Grambow, B. Chemical Thermodynamics of Zirconium; Elsevier: Amsterdam, 2005; p. 512.Suche in Google Scholar
25. Beneš, P., Obdržálek, M., Čejchanová, M. The physicochemical forms of traces of radium in aqueous solutions containing chlorides, sulfates and carbonates. Radiochem. Radioanal. Lett. 1982, 50, 277.Suche in Google Scholar
26. Nikitin, B. A. Studies on the analytical chemistry of radium. II. Reactions of pure radium. Proc. Khlopin Radium Inst. 1937, 3, 228.Suche in Google Scholar
27. Jeffree, R. A., Markich, S. J., Brown, P. L. Comparative accumulation of alkaline-earth metals by two freshwater mussel species from the Nepean River, Australia: consistencies and a resolved paradox. Aust. J. Mar. Freshw. Res. 1993, 44, 609; https://doi.org/10.1071/mf9930609.Suche in Google Scholar
28. Markich, S. J., Brown, P. L., Jeffree, R. A. Divalent metal accumulation in freshwater bivalves: an inverse relationship with metal phosphate solubility. Sci. Total Environ. 2001, 275, 27; https://doi.org/10.1016/s0048-9697(00)00721-x.Suche in Google Scholar
29. Butkalyuk, P. S., Butkalyuk, I. L., Agapov, A. A., Kupriyanov, A. S., Kazakova, E. V. Determining the solubility of RaF2 in water. Radiochemistry 2021, 63, 21; https://doi.org/10.1134/s1066362221010045.Suche in Google Scholar
30. Erbacher, O. Löslichkeits-Bestimmungen einiger Radiumsalze. Ber. Dtsch. Chem. Ges. 1930, 63, 141; https://doi.org/10.1002/cber.19300630120.Suche in Google Scholar
31. Polesitsky, A., Tolmatscheff, P. Solubility of radium iodate. Dokl. USSR Akad. Sci. 1936, 3, 319.Suche in Google Scholar
32. Butkalyuk, P. S., Butkalyuk, I. L., Kuznetsov, R. A., Kupriyanov, A. S., Abdullov, R. G. Solubility of radium nitrate in nitric acid solutions. Radiochemistry 2019, 61, 13; https://doi.org/10.1134/s106636221901003x.Suche in Google Scholar
33. Schubert, J. Complexes of alkaline earth cations including radium with amino acids and related compounds. J. Am. Chem. Soc. 1954, 76, 3442; https://doi.org/10.1021/ja01642a021.Suche in Google Scholar
34. Schubert, J., Russell, E. R., Myers, L. S. Dissociation constants of radium-organic acid complexes measured by ionic exchange. J. Biol. Chem. 1950, 185, 387; https://doi.org/10.1016/s0021-9258(18)56429-2.Suche in Google Scholar
35. Sekine, T., Kawshima, Y., Unmai, T., Sakairi, M. Studies of the alkaline earth complexes in various solutions. IV. Solvent extraction study of radium(II) complexes with some aminocarboxylic acids in perchlorate media. Bull. Chem. Soc. Jpn. 1968, 41, 3013; https://doi.org/10.1246/bcsj.41.3013.Suche in Google Scholar
36. Baetsle, L., Bengsch, E. Ion-exchange characteristics of the radium-ethylene diaminetetraacetic acid complex. J. Chromatogr. 1962, 8A, 265; https://doi.org/10.1016/s0021-9673(01)99257-x.Suche in Google Scholar
37. Nelson, F., Day, R. A., Kraus, K. A. Anion exchange studies – XXX. A number of elements in ethylene diaminetetraacetic acid solutions. J. Inorg. Nucl. Chem. 1960, 15, 140; https://doi.org/10.1016/0022-1902(60)80022-x.Suche in Google Scholar
38. Nikolsky, B. P., Trofimov, A. M. Complex formation of barium and radium in Trilon B solutions. Radiochemistry 1959, 1, 147.Suche in Google Scholar
39. Matyskin, A. V., Hansson, N. L., Brown, P. L., Ekberg, C. Barium and radium complexation with ethylene diaminetetraacetic acid in aqueous alkaline sodium chloride media. J. Solut. Chem. 2017, 46, 1951; https://doi.org/10.1007/s10953-017-0679-7.Suche in Google Scholar PubMed PubMed Central
40. Lieser, K. H. Radiochemische Messung der Löslichkeit von Erdalkalisulfaten in Wasser und in Natriumsulfatlösungen. Z. Anorg. Allg. Chem. 1965, 335, 225; https://doi.org/10.1002/zaac.19653350502.Suche in Google Scholar
41. Schwarzenbach, G., Kampitsch, E., Steiner, R. Komplexone I. Über die Salzbildung der Nitrilotriessigsäure. Helv. Chim. Acta 1945, 28, 828; https://doi.org/10.1002/hlca.194502801121.Suche in Google Scholar
42. Campi, E., Ostacoli, G., Meirone, M., Saini, G. Stability of complexes of tricarballylic and citric acids with bivalent metal ions in aqueous solution. J. Inorg. Nucl. Chem. 1964, 26, 553; https://doi.org/10.1016/0022-1902(64)80288-8.Suche in Google Scholar
43. Hirokawa, T., Kiso, Y. Complex-forming equilibria in isotachphoresis: II. Evaluation of stability constants of tartrate and citrate complexes. J. Chromatogr. 1982, 248, 341; https://doi.org/10.1016/s0021-9673(00)85045-1.Suche in Google Scholar
44. Topp, N. E., Davies, C. W. The extent of dissociation of salts in water. Part IX. Calcium and barium salts of dicarboxylic acids. J. Chem. Soc. 1940, 87.10.1039/jr9400000087Suche in Google Scholar
45. de Robertis, A., de Stefano, C., Scarcella, R., Rigano, C. Thermodynamics of formation of magnesium(II), calcium(II), strontium(II) and barium(II) – succinate complexes in aqueous solution. Thermochim. Acta 1984, 80, 197; https://doi.org/10.1016/0040-6031(84)87199-3.Suche in Google Scholar
46. Lumb, R. F., Martell, A. E. Metal chelating tendencies of glutamic and aspartic acids. J. Phys. Chem. 1953, 57, 690; https://doi.org/10.1021/j150508a021.Suche in Google Scholar
47. Martell, A. E., Smith, R. M. Other Organic Ligands. Critical Stability Constants; Plenum Press: New York, 3, 1977.10.1007/978-1-4757-1568-2Suche in Google Scholar
48. Cannan, R. K., Kibrick, A. Complex formation between carboxylic acids and divalent metal cations. J. Am. Chem. Soc. 1938, 60, 2314; https://doi.org/10.1021/ja01277a012.Suche in Google Scholar
49. Macdougall, G., Davies, C. W. The solubility of barium iodate in salt solutions. J. Chem. Soc. 1935, 1416.10.1039/jr9350001416Suche in Google Scholar
50. Majer, V., Stulik, K. A study of the stability of alkaline-earth metal complexes with fluoride and chloride ions at various temperatures by potentiometry with ion-selective electrodes. Talanta 1982, 29, 145; https://doi.org/10.1016/0039-9140(82)80039-8.Suche in Google Scholar
51. Sucha, L., Cadek, J., Hrabek, K., Vesely, J. The stability of the chloro complexes of magnesium and of the alkaline earth metals at elevated temperatures. Collect. Czech Chem. Commun. 1975, 40, 2020.10.1135/cccc19752020Suche in Google Scholar
52. Colman-Porter, C. A., Monk, C. B. The electrolytic dissociation of strontium iodate and strontium hydroxide. J. Chem. Soc. 1952, 1312; https://doi.org/10.1039/jr9520001312.Suche in Google Scholar
53. Carter, R. H. Solubilities of some inorganic fluorides in water at 25 °C. Ind. Eng. Chem. 1928, 20, 1195; https://doi.org/10.1021/ie50227a024.Suche in Google Scholar
54. Talipov, S. T., Khadeev, V. A. Fiziko-khimicheskii analiz troinykh vodnykh sistem. Sostoyashchikh iz ftoridov shchelochno-zemelnykh metallov i ftoridov shchelochnykh metallov. I. Troinye sistemy BaF2-KF-H2O i BaF2-NaF-H2O pri 25-degrees. Zh. Obshch. Khim. 1950, 20, 774; https://doi.org/10.1021/ie50227a024.Suche in Google Scholar
55. Kohlrausch, F. Über gesättigte wässerige Lösungen schwerlöslicher Salze. Z. Phys. Chem. 1908, 64, 129; https://doi.org/10.1515/zpch-1908-6407.Suche in Google Scholar
56. Reardon, E. Determination of SrSO4° ion pair formation using conductimetric and ion exchange techniques. Geochem. Cosmochim. Acta 1983, 47, 1917; https://doi.org/10.1016/0016-7037(83)90208-9.Suche in Google Scholar
57. Busenberg, E., Plummer, L. N., Parker, V. B. The solubility of strontianite (SrCO3) in CO2-H2O solutions between 2 and 91 °C, the association constants of SrHCO3+(aq) and SrCO3°(aq) between 5 and 80 °C, and an evaluation of the thermodynamic properties of Sr2+(aq) and SrCO3(cr) at 25 °C and 1 atm total pressure. Geochem. Cosmochim. Acta 1984, 48, 2021; https://doi.org/10.1016/0016-7037(84)90383-1.Suche in Google Scholar
58. Bohigian, T., Martell, A. Progress report US Atomic Energy Commission Contract No. AT, (30-1)-1823, 1960.Suche in Google Scholar
59. Sillén, L. G., Martell, A. E. Stability Constants of Metal-Ion Complexes; London: The Chemical Society, Special Publication No. 17, 1964.Suche in Google Scholar
60. Charles, R. G. Heats and entropies of reaction of metal ions with ethylenediaminetetraacetate. J. Am. Chem. Soc. 1954, 76, 5854; https://doi.org/10.1021/ja01651a094.Suche in Google Scholar
61. Wanninen, E. Complexometric titrations with diethylenetriaminepentaacetic acid. Acta Acad. Aboensias 1960, 21, 17.Suche in Google Scholar
62. Holloway, J. H., Reilly, C. N. Metal chelate stability constants of aminopolycarboxylate ligands. Anal. Chem. 1960, 32, 249; https://doi.org/10.1021/ac60158a033.Suche in Google Scholar
63. Joseph, N. R. The dissociation constants of organic calcium complexes. J. Biol. Chem. 1946, 164, 529; https://doi.org/10.1016/s0021-9258(17)41256-7.Suche in Google Scholar
64. Schubert, J. Ion exchange studies of complex ions as a function of temperature, ionic strength, and presence of formaldehyde. J. Phys. Chem. 1952, 56, 113; https://doi.org/10.1021/j150493a022.Suche in Google Scholar
65. Bousquet, J., Mathurin, D., Vermande, P. Solubility of anhydrous and hydrated calcium, strontium and barium iodates in aqueous solutions. Bull. Soc. Chim. Fr. 1969, 1111.Suche in Google Scholar
66. Fedorov, V. A., Robov, A. M., Shmyd’ko, I. I., Vorontsova, N. A., Mironov, V. E. On interaction of alkaline earth metal ions with nitrate ions in aqueous solutions. Zh. Neorg. Khim. 1974, 19, 1746.Suche in Google Scholar
67. Talipov, S. T., Khadeev, V. A. Fiziko-khimicheskii analiz troinykh vodnykh sistem. Sostoyashchikh iz ftoridov shchelochno-zemelnykh metallov i ftoridov shchelochnykh metallov. II. Troinye sistemy SrF2-KF-H2O i SrF2-NaF-H2O pri 25-degrees. Zh. Obshch. Khim. 1950, 20, 783.Suche in Google Scholar
68. Davies, C. W., Wyatt, P. A. H. The extent of dissociation of salts in water. Part XI. Calcium and barium thiosulfates and barium bromoacetate. Trans. Faraday Soc. 1949, 45, 770; https://doi.org/10.1039/tf9494500770.Suche in Google Scholar
69. Denney, T. O., Monk, C. B. Ion pair formation in thiosulfate solutions. Trans. Faraday Soc. 1951, 47, 992; https://doi.org/10.1039/tf9514700992.Suche in Google Scholar
70. Stary, J. Systematic study of the solvent extraction of metal oxinates. Anal. Chim. Acta 1963, 28, 132; https://doi.org/10.1016/s0003-2670(00)87211-6.Suche in Google Scholar
71. Colman-Porter, C. A., Monk, C. B. Dissociation constants of the alkaline-earth salts of some monocarboxylic acids. J. Chem. Soc. 1952, 4363; https://doi.org/10.1039/jr9520004363.Suche in Google Scholar
72. Archer, D. W., Monk, C. B. Ion-association constants of some acetates by pH (glass electrode) measurements. J. Chem. Soc. 1964, 3117.10.1039/jr9640003117Suche in Google Scholar
73. Khokhlova, A., Fedorov, V. A. Barium carboxylate complexes. Zh. Neorg. Khim. 1977, 22, 3167.Suche in Google Scholar
74. Sekine, T., Sakairi, M., Hasegawa, Y. Studies of the alkaline earth complexes in various solutions. I. Barium(II) complexes with sulfate and oxalate ions in 1 M sodium perchlorate media. Bull. Chem. Soc. Jpn. 1966, 39, 2141; https://doi.org/10.1246/bcsj.39.2141.Suche in Google Scholar
75. Monk, C. B. Electrolytes in solutions of amino acids. Part V. The solubilities of calcium, barium and lanthanum iodates in glycine, alanine and glycyl-glycine. Trans. Faraday Soc. 1951, 47, 1233; https://doi.org/10.1039/tf9514701233.Suche in Google Scholar
76. Taqui Khan, M. M., Martell, A. E. Metal chelates of adenosine triphosphate. J. Phys. Chem. 1962, 66, 10; https://doi.org/10.1021/j100807a003.Suche in Google Scholar
77. Näsänen, R., Uusitalo, E. Potentiometric and spectrophotometric studies on 8-quinolinol and its derivatives. IX. Stability of some metal chelates of 8-quinolinol-5-sulfonic acid in aqueous solution. Acta Chem. Scand. 1954, 8, 112.10.3891/acta.chem.scand.08-0112Suche in Google Scholar
78. Bennett, W. E., Skovlin, D. O. Anion-exchange behaviour of the alkaline earth metals. J. Inorg. Nucl. Chem. 1966, 28, 591; https://doi.org/10.1016/0022-1902(66)80341-x.Suche in Google Scholar
79. Wolhoff, J. A., Overbeek, J. T. G. Determination of equilibrium constants for a number of metal-phosphate complexes. Recl. Trav. Chim. 1959, 78, 759; https://doi.org/10.1002/recl.19590781005.Suche in Google Scholar
© 2022 Paul L. Brown et al., published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Editorial: Diamond Jubilee Issue
- Sixty years of Radiochimica Acta: a brief overview with emphasis on the last 10 years
- A. Chemistry of Radioelements
- Five decades of GSI superheavy element discoveries and chemical investigation
- Chemistry of the elements at the end of the actinide series using their low-energy ion-beams
- Sonochemistry of actinides: from ions to nanoparticles and beyond
- Theoretical insights into the reduction mechanism of neptunyl nitrate by hydrazine derivatives
- The speciation of protactinium since its discovery: a nightmare or a path of resilience
- On the volatility of protactinium in chlorinating and brominating gas media
- The aqueous chemistry of radium
- B. Energy Related Radiochemistry
- Selective actinide(III) separation using 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol) in the innovative-SANEX process: laboratory scale counter current centrifugal contactor demonstration
- Fate of Neptunium in nuclear fuel cycle streams: state-of-the art on separation strategies
- Uranium adsorption – a review of progress from qualitative understanding to advanced model development
- Targeted synthesis of carbon-supported titanate nanofibers as host structure for nuclear waste immobilization
- Progress of energy-related radiochemistry and radionuclide production in the Republic of Korea
- C. Nuclear Data
- How accurate are half-life data of long-lived radionuclides?
- Status of the decay data for medical radionuclides: existing and potential diagnostic γ emitters, diagnostic β+ emitters and therapeutic radioisotopes
- An overview of nuclear data standardisation work for accelerator-based production of medical radionuclides in Pakistan
- An overview of activation cross-section measurements of some neutron and charged-particle induced reactions in Bangladesh
- Nuclear reaction data for medical and industrial applications: recent contributions by Egyptian cyclotron group
- Nuclear data for light charged particle induced production of emerging medical radionuclides
- D. Radionuclides and Radiopharmaceuticals
- The role of chemistry in accelerator-based production and separation of radionuclides as basis for radiolabelled compounds for medical applications
- Production of neutron deficient rare earth radionuclides by heavy ion activation
- Evaluation of 186WS2 target material for production of high specific activity 186Re via proton irradiation: separation, radiolabeling and recovery/recycling
- Special radionuclide production activities – recent developments at QST and throughout Japan
- China’s radiopharmaceuticals on expressway: 2014–2021
- E. Environmental Radioactivity
- A summary of environmental radioactivity research studies by members of the Japan Society of Nuclear and Radiochemical Sciences
Artikel in diesem Heft
- Frontmatter
- Editorial: Diamond Jubilee Issue
- Sixty years of Radiochimica Acta: a brief overview with emphasis on the last 10 years
- A. Chemistry of Radioelements
- Five decades of GSI superheavy element discoveries and chemical investigation
- Chemistry of the elements at the end of the actinide series using their low-energy ion-beams
- Sonochemistry of actinides: from ions to nanoparticles and beyond
- Theoretical insights into the reduction mechanism of neptunyl nitrate by hydrazine derivatives
- The speciation of protactinium since its discovery: a nightmare or a path of resilience
- On the volatility of protactinium in chlorinating and brominating gas media
- The aqueous chemistry of radium
- B. Energy Related Radiochemistry
- Selective actinide(III) separation using 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl]pyridine (PyTri-Diol) in the innovative-SANEX process: laboratory scale counter current centrifugal contactor demonstration
- Fate of Neptunium in nuclear fuel cycle streams: state-of-the art on separation strategies
- Uranium adsorption – a review of progress from qualitative understanding to advanced model development
- Targeted synthesis of carbon-supported titanate nanofibers as host structure for nuclear waste immobilization
- Progress of energy-related radiochemistry and radionuclide production in the Republic of Korea
- C. Nuclear Data
- How accurate are half-life data of long-lived radionuclides?
- Status of the decay data for medical radionuclides: existing and potential diagnostic γ emitters, diagnostic β+ emitters and therapeutic radioisotopes
- An overview of nuclear data standardisation work for accelerator-based production of medical radionuclides in Pakistan
- An overview of activation cross-section measurements of some neutron and charged-particle induced reactions in Bangladesh
- Nuclear reaction data for medical and industrial applications: recent contributions by Egyptian cyclotron group
- Nuclear data for light charged particle induced production of emerging medical radionuclides
- D. Radionuclides and Radiopharmaceuticals
- The role of chemistry in accelerator-based production and separation of radionuclides as basis for radiolabelled compounds for medical applications
- Production of neutron deficient rare earth radionuclides by heavy ion activation
- Evaluation of 186WS2 target material for production of high specific activity 186Re via proton irradiation: separation, radiolabeling and recovery/recycling
- Special radionuclide production activities – recent developments at QST and throughout Japan
- China’s radiopharmaceuticals on expressway: 2014–2021
- E. Environmental Radioactivity
- A summary of environmental radioactivity research studies by members of the Japan Society of Nuclear and Radiochemical Sciences


