Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
-
Valentina Zagardo
Abstract
Renal cell carcinoma (RCC) often presents with tumor thrombus (TT) in the inferior vena cava (IVC), posing significant therapeutic challenges, particularly in cases of metastatic or inoperable disease. While surgical excision remains the standard treatment approach, recent advancements in radiotherapy techniques may offer alternative strategies. We present the clinical picture of a 74-year-old male with metastatic RCC, who presented with recurrent IVC-TT, detected by surveillance computed tomography imaging, and complained of mild lower leg edema. This was successfully managed with stereotactic body radiotherapy (SBRT), resulting in a slow but continuous shrinkage of the IVC-TT with almost complete regression of most lung, liver, and lymph node metastases, obtaining a full resolution of the mild bilateral leg edema. The case described here highlights the possibility of using radiotherapy as a safe and tolerable treatment for inoperable or metastatic patients with IVC-TT. Additionally, we conducted a literature review looking for evidence of the effectiveness of radiotherapy in RCC patients with IVC-TT across different treatment settings. This case-based review ultimately aims to shed light on the emerging evidence supporting the usefulness of radiotherapy in such complex clinical challenges, hopefully paving the way for well-organized trials.
Introduction
Renal cell carcinoma (RCC) accounts for about 3 % of all malignancy cases and is the most common histology of kidney cancer [1]. It is classified based on cell type characteristics into multiple histological subtypes, including clear cell carcinoma, papillary, and chromophobe, which represent the most common variants [2]. Its propensity to spread via hematogenous and lymphatic routes, combined with direct invasion into the venous system in approximately 25 % of cases, often leads to the formation of tumor thrombus (TT) [3]. Since TT is often asymptomatic, its detection typically occurs incidentally during routine follow-up computed tomography (CT) scans, notwithstanding its potential to provoke a varied spectrum of symptoms [4].
In patients with inferior vena cava tumor thrombus (IVC-TT) presenting with locally advanced disease, the current standard treatment approach involves complete surgical excision of the TT along with radical nephrectomy. However, this procedure carries high mortality rates (which increase depending on the degree of caval invasion), perioperative complications (e.g. transfusion, cardiac thromboembolism), and a high incidence of treatment failures [5]. Considering the complexity of such surgery, patients with IVC-TT, especially in cases of metastatic or inoperable disease, pose a significant therapeutic challenge. In such cases, alternative therapeutic strategies may include medical therapies such as tyrosine kinase inhibitors and/or immunotherapy. These treatments have shown potential in reducing thrombus levels, thereby enabling surgery in patients with inoperable disease, although the data remain limited [6]. While surgery remains the cornerstone of treatment, another therapeutic option is radiotherapy, whose role in addressing IVC-TT is still unknown, with limited cases reported in the literature.
Here we present the clinical case of a 74-year-old male diagnosed with metastatic RCC and a recurrent, inoperable large IVC-TT, who underwent stereotactic body radiotherapy (SBRT). Furthermore, we provided a narrative literature review, summarizing the limited available evidence on the effectiveness of radiotherapy in managing RCC patients with IVC-TT, with a focus on its different applications across different treatment settings. From these review objectives, useful suggestions for designing specific trials may stem.
Case description
In October 2018, a 69-year-old male with a medical history of arterial hypertension presented to our clinic (REM Radioterapia) with a left renal neoplasm. At that time, as no evidence of metastases was detected, he was referred for surgery, which consisted of radical nephrectomy. The post-operative pathological finding was consistent with the Fuhrman grade 3 clear cell RCC, pathologic stage pT3N0M0. However, one year later, surveillance CT imaging revealed an IVC-TT with few micronodular liver metastases and retroperitoneal lymph node involvement. Then, thrombectomy and lymphadenectomy with reconstruction of the IVC were performed. Although the lymph nodes had appeared pathological on CT imaging, all 13 resected lymph nodes were negative. The liver metastases were not removed during the surgery. Subsequent postoperative CT of the chest and abdomen showed no residual tumoral thrombus. Postoperative pathological analysis confirmed disease recurrence. The liver micronodular metastases were neither removed nor biopsied. Indeed, these were micronodular lesions, and in any case, given the recurrence of the disease, confirmed by post-operative pathological findings, the patient was a candidate for systemic therapy.
Therefore, medical oncology recommended starting first-line therapy with Nivolumab plus Ipilimumab, which transitioned to maintenance with Nivolumab once stable disease was documented six months later. In April 2023, despite ongoing immunotherapy, a contrast-enhanced total body CT scan revealed progressive disease in the form of micronodular pulmonary metastases, mediastinal and abdominal lymphadenopaties, and a large TT recurrence extending from the intrahepatic course of the IVC to the right atrium (41 × 28 × 45 mm). The patient had only mild bilateral leg edema. Since the extent of TT filling the IVC lumen and the elevated levels of direct bilirubin (0.58 mg/dL) and fibrinogen (527 mg/dL) discouraged any further surgical procedure, the patient was offered palliative radiotherapy after discontinuation of immunotherapy as he was considered developing immune resistance. His good performance status (ECOG 0) encouraged us to also consider the SBRT option.
After weighing the pros and cons, we decided to perform stereotactic radiotherapy via a Novalis Truebeam STx (Varian, Palo Alto, CA, USA). CT simulation was performed using a “slow” four-dimensional (4D) computed tomography (4D-CT) capturing any target motion during the respiratory cycle with a slice thickness of 1.25 mm. Once the most stable individual respiratory phases were selected, the IVC-TT was delineated as gross tumor volume (GTV) on the CT simulation, which was also merged with the diagnostic contrast-enhanced CT scan aiming for a more precise delineation of the target. To compensate for target motion and ensure target coverage, considering the non-negligible intra-fraction target displacement observed during CT simulation due to heartbeats, the planning target volume (PTV) was generated by adding 5 mm of isometric margins to the GTV. The tumor location concerned us about possible radiation-related cardiac toxicity. To reduce the dose received by the heart, two prescription levels were chosen: a dose of 32.5 Gy in five daily fractions had to include the entire TT, including its tract abutting and invading the right atrium, while a simultaneous integrated boost (SIB) up to 40 Gy was planned to target the intrahepatic tract exclusively (Figure 1). The treatment was delivered with a Varian Truebeam linear accelerator (Varian Medical Systems, Palo Alto, CA, USA) using 10 MV Flattening Filter Free (FFF) photon beams for faster delivery.
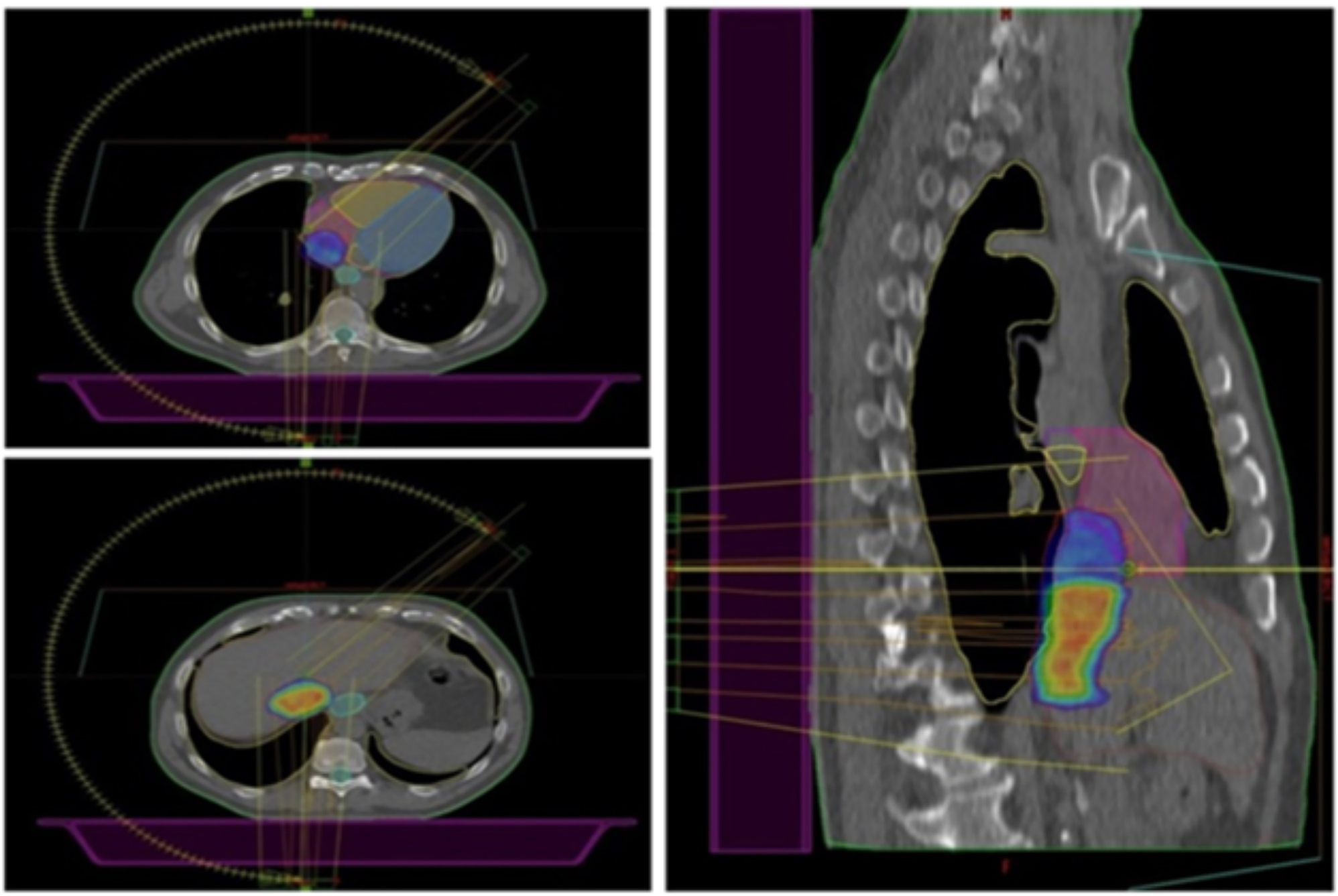
Axial (on the left) and sagittal (on the right) planes showing the 98 % dose distribution. Two prescription dose levels are depicted: the hotter yellowish/reddish dose color-wash refers to the highest dose prescription (40 Gy to the SIB volume), which gradually fades into the colder bluish lower dose (32.5 Gy) to the longitudinal section of the TT closer to the heart to reduce the risk of cardiac toxicity.
All dose-volume constraints for nearby organs at risk (OARs, e.g. heart, liver, spinal cord, lungs, esophagus) were met (Figure 2) as per Timmerman tables [7].

Dose-volume histogram (DVH) of the treatment plan: It shows the relation between the cumulative radiation dose (x-axis) and volume (y-axis). The red lines are for the PTV 32.5 Gy (the leftmost) and the SIB volume at 40 Gy (the rightmost). Purple, magenta, green, light blue and yellow lines are for the heart, right atrium, left atrium, esophagus and lungs, respectively.
The PTV received at least 98 % of the prescribed dose by two coplanar volumetric arcs (181.0–45.0 CW – 45.0–181.0 CCW) and the treatment was delivered under respiratory gating and daily cone beam CT guidance to ensure proper positioning.
The patient tolerated radiation treatment well without any acute or late toxicity: no pulmonary embolism occurred. Total body CT scans performed after 3, 6, and 12 months showed a slow but continuous shrinkage of the IVC-TT, reaching 11 mm at the latest follow-up (April 2024, Figure 3). At the time of writing, we have documented an abscopal response with almost complete regression of most lung, liver and lymph node metastases, a few being still stable. Furthermore, the mild bilateral leg edema is completely resolved.

Pre- and post-radiotherapy images: starting condition (A, B) and 12 months after radiotherapy treatment (C, D). The images show a partial response to the treatment (28 × 45 vs. 11 × 21 mm).
Thanks to the favorable response to radiotherapy, immunotherapy with Nivolumab, resumed after completion of SBRT, is still ongoing.
The patient’s disease course is summarized in Figure 4.

Timeline of patient’s treatment. CT, computed tomography; IVC-TT, inferior vena cava tumor thrombus; SBRT, stereotactic body radiation therapy.
Literature review
A search of the literature published in PubMed and Scopus from the inception of electronic databases to February 2024 was performed, looking for articles on the use of radiotherapy to treat RCC patients with IVC-TT. We used the following English search terms: “renal cell” or “kidney” “cancer” or “carcinoma” and “inferior vena cava” and “radiotherapy” or “stereotactic body radiotherapy” or “hypofractionated radiotherapy” and “tumour thrombus”. Given the rarity of the use of radiotherapy in this clinical scenario, we also included case reports and case series. The only exclusion criterion was non-English language. Table 1 lists the seven papers we reviewed; the last row shows our case.
Summary of studies available in literature about IVC-TT in patients with RCC treated with radiotherapy.
| Authors, year | Type of study | No of pts | Sex | Age, years | Symptoms | RT dose and fractionation | Acute/late toxicity | Clinical outcomes | Overall survival and local control |
|---|---|---|---|---|---|---|---|---|---|
| 1) Malkin et al. 1975 [8] | Case report | 1 | M | 59 | Fatigue | Conventional RT – 5,000 rads over six weeks | None | Complete symptom resolution |
|
| 2) Hannan et al. 2015 [9] | Case report | 2 | M | 74 | Shortness of breath with mild exertion | SBRT – 50 Gy in 5 Fx | None | Complete symptom resolution |
|
| M | 83 | Lower extremity edema | SBRT – 36 Gy in 4 Fx | None | Good symptom control |
|
|||
| 3) Beldzinska et al. 2022 [10] | Case report | 1 | F | 43 | Fatigue, swelling, massive edema, ascites, severe pain, disturbed sleep and anxiety | HFRT – 60 Gy in 12 Fx | Nausea, vomiting and abdominal pain | Complete symptom resolution |
|
| 4) Freifeld et al. 2022 [11] | R | 15 | NR | NR | Budd and Chiari, edema, pain, hematuria | SBRT – 40 Gy (25–50 in 5) in Fx (1–5) | Fatigue, nausea and dermatitis G1–G2 | NR |
|
| 5) Margulis et al. 2022 [12] | P II | 6 | 3M 3F |
Median 75 | NR | SBRT – 40 Gy in 5 Fx | Nausea G2, abdominal pain, ALT increased | NR |
|
| 6) Castelnau-Marchand et al. 2023 [13] | Case report | 1 | M | 62 | Lower limb edema | SBRT – 35 Gy in 5 Fx | Nausea G2 | Good general condition |
|
| 7) Chen et al. 2024 [14] | P | 8 | 6M 2F | Median 60 | NR | SBRT – 30 Gy in 5 Fx | Only G1–G2 AEs were reported | NR |
|
| 8) Zagardo et al. 2024 | Case report | 1 | M | 74 | Mild bilateral leg edema | SBRT with SIB – 32.5/40 Gy in 5 Fx | None | Asymptomatic |
|
-
R, retrospective; P, prospective; P II, phase II prospective; NR, not reported; SBRT, stereotactic body radiation therapy; OS, overall survival; LC, local control; PR, partial response; CR, complete response; PD, progressive disease; RT, radiation therapy; PTS, patients.
Neoadjuvant SBRT
The current body of literature on radiotherapy for RCC with IVC-TT is notably limited. In our opinion, the rationale behind combining preoperative radiotherapy with surgical intervention could primarily revolve around enhancing treatment outcomes, such as overall survival (OS) and disease-free survival (DFS), while concurrently mitigating surgical complexities associated with disease extent. While the reduction of the TT size might mitigate the complexity of the surgical procedure, surgery following radiotherapy, especially if delayed, might be more challenging due to potential fibrosis caused by the radiotherapy itself. Results from a recent phase II prospective trial involving six RCC patients presenting with IVC-TT indicated that neo-adjuvant SBRT delivered shortly prior to surgery represents an efficacious and well-tolerated therapeutic approach. Indeed, at a median follow-up of 24 months, all six patients were alive, indicating favorable treatment outcomes. No grade ≥3 treatment-related toxicities were observed, with nausea and abdominal pain being the most common adverse events. Immunohistochemistry analysis revealed lower Ki-67 expression and increased PD-L1 expression compared to non-irradiated lesions, suggesting the potential immunomodulatory effects of SBRT [12]. This finding supports the efficacy and safety of neo-adjuvant SBRT followed by surgery in patients with RCC IVC-TT. Furthermore, SBRT demonstrated potential immunomodulatory effects, highlighting its role in enhancing off-target effects such as abscopal and bystander effects [15].
While SBRT-related complications in the study by Margulis et al. were minimal, vigilance regarding the risk of pulmonary embolism is warranted [12]. Indeed, patients receiving SBRT for IVC-TT face a significant risk of pulmonary embolism, which can represent a life-threatening complication. This risk arises from the potential dislodgement of the thrombus into the right atrium during radiotherapy [16]. Despite this concern, no cases of pulmonary embolism after SBRT in RCC patients with IVC-TT have been documented in the existing literature. However, due to the scarcity of published studies addressing this specific aspect, it is not possible to establish definitive conclusions regarding the occurrence of pulmonary embolism after SBRT in this patient population.
Another prospective trial confirms the safety profile of neoadjuvant SBRT [14]. Within this study, eight patients received SBRT (total dose of 30 Gy administered over five fractions), followed by surgery, without significant adverse events. Nevertheless, SBRT did not elicit complete tumor regression in any patient; however, at least, partial responses were observed. The authors postulated that this partial response may be attributed to the relatively low radiation dose administered to a tumor recognized for its inherent radioresistant nature and encourages further research into dose escalation.
Finally, another phase I trial is currently investigating the role of SBRT in the neo-adjuvant setting [17].
Radiotherapy as definitive therapy for RCC IVC-TT
Historically, because the inherent radioresistance of RCC argues the need for dose escalation against the risk of excessive radioinduced toxicities, conventional radiotherapy has been administered primarily for palliative or salvage purposes [18]. However, recent technological advances over the past decade have facilitated the integration of radiotherapy into the comprehensive management of unresectable RCC with IVC-TT with curative intent. In particular, SBRT, which delivers a higher dose per fraction, has emerged as a promising modality capable of overcoming traditional radioresistance of RCC, thus achieving excellent local control while simultaneously minimizing damage to adjacent OARs [19].
Reviewing the limited available literature, definitive radiotherapy in the treatment of IVC-TT has shown early promise. In 2015, Hannan et al. reported a case series involving two RCC patients with IVC-TT who experienced disease progression while on immunotherapy. After radiotherapy treatment, both patients achieved good symptom control, with an OS of 18 and 24 months, which was comparable to that of surgically managed patients [9]. Similarly, a 43-year-old female with recurrent disease received hypofractionated radiotherapy (HFRT) at a dose of 60 Gy in 12 fractions, resulting in complete resolution of symptoms and three-year survival [10]. Interestingly, none of the above three patients were administered systemic treatments, underscoring the ability of radiotherapy to produce a sustained response while avoiding the potential toxicities of drug therapy [20]. A multicentre retrospective case series of 15 patients treated with SBRT further demonstrated a median OS of 34 months and no associated side effects. SBRT was suggested as a viable option for symptom palliation and in case of refractory or inoperable disease to effectively control local progression [11]. Additionally, effective use of SBRT at a dose of 35 Gy in five fractions was reported in an RCR patient with recurrent level IV IVC-TT refractory to sunitinib. Subsequent administration of nivolumab post-SBRT resulted in sustained survival [13]. In this study, radiotherapy seemed to restore sensitivity to immunotherapy, mirroring the findings in our patient in whom radiotherapy facilitated the continuation of systemic treatment despite acquired resistance. An intriguing observation, in our case report, is an abscopal response observed in almost all non-target lesions resulting in a nearly complete response, which obviated the need for additional radiation treatments unlike the patient in Marchand’s report, who was treated with 45 Gy in three fractions to the remaining liver metastases. This observation would suggest that varying the radiation dose/fraction could induce different immunotherapeutic effects [21].
Notably, we found only one report on the use of conventional dose fractionation, dating back to the era of two-dimensional radiotherapy [8]. The current availability of increasingly precise radiotherapy techniques makes radiation delivery safe enough to allow the use of ultra-hypofractionated regimens for dose escalation, which, in turn, translates into radiobiological advantages and improved patient compliance thanks to the shortened treatment schedule.
Within the context of this paragraph, it is worth highlighting that our patient is alive and well one year after radiotherapy, against a median survival of five months among untreated patients [22].
Taken together, these literature findings highlight the potential gain from the use of SBRT in achieving survival benefits, and symptom relief. Furthermore, it seems to restore the immune competence in RCC patients with IVC-TT.
However, the awareness of unpredictable adverse effects possibly resulting from the combination of high radiation doses per fraction and anti-PD1 immunotherapy for targets close to the spine such as the one in our case mandates careful monitoring for early diagnosis and timely management [23].
The main limitation of this study is represented by the paucity of data supporting the evidence reviewed here, which is therefore of low quality. Therefore, further studies are warranted to confirm the utility and efficacy of radiotherapy in treating patients with IVC-TT.
Conclusions
Based on this evidence, radiotherapy (especially SBRT) represents a valid therapeutic alternative for inoperable or metastatic patients with IVC-TT, offering a balance between efficacy and tolerability. Moreover, when combined with immunotherapy, stereotactic radiotherapy seems to hold promise in reversing immune resistance, thus allowing for the continuation of ongoing systemic therapy and postponing the therapeutic switch. Therefore, SBRT might play a pivotal role in the future management of these patients.
-
Research ethics: Not applicable.
-
Informed consent: Written informed consent was obtained from the patient.
-
Author contributions: All authors contributed to the study’s conception and design. Material preparation and analysis were performed by Valentina Zagardo and Gianluca Ferini. Validation: Gianluca Ferini, Silvana Parisi, Giacomo Ferrantelli, Miriam Sciacca. Data collection was performed by Francesco Cuccia, Antonio Piras, Silvana Parisi, Giacomo Ferrantelli, and Miriam Sciacca. The first draft of the manuscript was written by Valentina Zagardo and Gianluca Ferini. Writing review and editing: Valentina Zagardo, Francesco Cuccia, and Antonio Piras. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
-
Competing interests: The authors have no relevant financial or non-financial interests to disclose.
-
Research funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
-
Data availability: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
-
Consent to publish: The authors affirm that the human research participant provided informed consent for publication of the images included in this paper.
References
1. Bahadoram, S, Davoodi, M, Hassanzadeh, S, Bahadoram, M, Barahman, M, Mafakher, L. Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G Ital Nefrol 2022;39:2022-vol3.Suche in Google Scholar
2. Muglia, VF, Prando, A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras 2015;48:166–74. https://doi.org/10.1590/0100-3984.2013.1927.Suche in Google Scholar PubMed PubMed Central
3. Slaton, JW, Balbay, MD, Levy, DA, Pisters, LL, Nesbitt, JC, Swanson, DA, et al.. Nephrectomy and vena caval thrombectomy in patients with metastatic renal cell carcinoma. Urology 1997;50:673–7. https://doi.org/10.1016/s0090-4295(97)00329-4.Suche in Google Scholar PubMed
4. Klein-Weigel, PF, Elitok, S, Ruttloff, A, Reinhold, S, Nielitz, J, Steindl, J, et al.. Inferior vena cava-syndrome. Vasa 2021;50:250–64. https://doi.org/10.1024/0301-1526/a000919.Suche in Google Scholar PubMed
5. Ljungberg, B, Albiges, L, Abu-Ghanem, Y, Bedke, J, Capitanio, U, Dabestani, S, et al.. European Association of Urology guidelines on renal cell carcinoma: the 2022 update. Eur Urol 2022;82:399–410. https://doi.org/10.1016/j.eururo.2022.03.006.Suche in Google Scholar PubMed
6. Gu, L, Peng, C, Li, H, Jia, T, Chen, X, Wang, H, et al.. Neoadjuvant therapy in renal cell carcinoma with tumor thrombus: a systematic review and meta-analysis. Crit Rev Oncol Hematol 2024;196:104316. https://doi.org/10.1016/j.critrevonc.2024.104316.Suche in Google Scholar PubMed
7. Timmerman, R. A story of hypofractionation and the table on the wall. Int J Radiat Oncol Biol Phys 2022;112:4–21. https://doi.org/10.1016/j.ijrobp.2021.09.027.Suche in Google Scholar PubMed
8. Malkin, RB. Regression of renal carcinoma following radiation therapy. J Urol 1975;114:782–3. https://doi.org/10.1016/s0022-5347(17)67144-1.Suche in Google Scholar PubMed
9. Hannan, R, Margulis, V, Chun, SG, Cannon, N, Kim, DW, Abdulrahman, RE, et al.. Stereotactic radiation therapy of renal cancer inferior vena cava tumor thrombus. Cancer Biol Ther 2015;16:657–61. https://doi.org/10.1080/15384047.2015.1026506.Suche in Google Scholar PubMed PubMed Central
10. Bełdzińska, K, Gądek, K, Rutkowski, J. Hypofractionated radiotherapy for renal cell carcinoma with inferior vena cava tumour thrombus. Contemp Oncol 2022;26:310–3. https://doi.org/10.5114/wo.2023.124792.Suche in Google Scholar PubMed PubMed Central
11. Freifeld, Y, Pedrosa, I, Mclaughlin, M, Correa, RM, Louie, AV, Maldonado, JA, et al.. Stereotactic ablative radiation therapy for renal cell carcinoma with inferior vena cava tumor thrombus. Urol Oncol 2022;40:166.e9–13. https://doi.org/10.1016/j.urolonc.2021.12.018.Suche in Google Scholar PubMed PubMed Central
12. Margulis, V, Freifeld, Y, Pop, LM, Manna, S, Kapur, P, Pedrosa, I, et al.. Neoadjuvant SABR for renal cell carcinoma inferior vena cava tumor thrombus-safety lead-in results of a phase 2 trial. Int J Radiat Oncol Biol Phys 2021;110:1135–42. https://doi.org/10.1016/j.ijrobp.2021.01.054.Suche in Google Scholar PubMed PubMed Central
13. Castelnau-Marchand, P, Scher, N, Bollet, M, Chargari, C, Toledano, A. Stereotactic ablative radiotherapy for unresectable inferior vena cava tumor thrombus in a patient with renal cell carcinoma: a case report. Strahlenther Onkol 2023;199:420–4. https://doi.org/10.1007/s00066-023-02054-0.Suche in Google Scholar PubMed
14. Chen, J, Liu, Z, Peng, R, Liu, Y, Zhang, H, Wang, G, et al.. Neoadjuvant stereotactic ablative body radiotherapy combined with surgical treatment for renal cell carcinoma and inferior vena cava tumor thrombus: a prospective pilot study. BMC Urol 2024;24:31. https://doi.org/10.1186/s12894-024-01405-y.Suche in Google Scholar PubMed PubMed Central
15. Chen, Y, Gao, M, Huang, Z, Yu, J, Meng, X. SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: a focus on the mechanisms, advances, and future challenges. J Hematol Oncol 2020;13:105. https://doi.org/10.1186/s13045-020-00940-z.Suche in Google Scholar PubMed PubMed Central
16. Lee, SJ, Jang, HS, Choi, YK. Clinical outcome and toxicity of radiotherapy for inferior vena cava tumor thrombus in HCC patients: a retrospective study. Medicine 2021;100:e26390. https://doi.org/10.1097/md.0000000000026390.Suche in Google Scholar PubMed PubMed Central
17. Liu, Y, Liu, Z, Peng, R, Xiao, R, Wang, J, Wang, H, et al.. Preoperative stereotactic body radiotherapy combined with surgical treatment for renal cell carcinoma and inferior vena cava tumour thrombus: study protocol for a single-arm cohort trial. BMJ Open 2022;12:e055364. https://doi.org/10.1136/bmjopen-2021-055364.Suche in Google Scholar PubMed PubMed Central
18. Wei, Q, He, H, Lv, L, Xu, X, Sun, W. The promising role of radiotherapy in the treatment of advanced or metastatic renal cell carcinoma: a narrative review. Transl Androl Urol 2020;9:2821–30. https://doi.org/10.21037/tau-20-1466.Suche in Google Scholar PubMed PubMed Central
19. Cheung, P, Thibault, I, Bjarnason, GA. The emerging roles of stereotactic ablative radiotherapy for metastatic renal cell carcinoma. Curr Opin Support Palliat Care 2014;8:258–64. https://doi.org/10.1097/spc.0000000000000074.Suche in Google Scholar PubMed
20. Cao, G, Wu, X, Wang, Z, Tian, X, Zhang, C, Wu, X, et al.. What is the optimum systemic treatment for advanced/metastatic renal cell carcinoma of favourable, intermediate and poor risk, respectively? A systematic review and network meta-analysis. BMJ Open 2020;10:e034626. https://doi.org/10.1136/bmjopen-2019-034626.Suche in Google Scholar PubMed PubMed Central
21. Zagardo, V, Harikar, M, Ferini, G. Is an immune-oriented use of radiation therapy possible? An increasingly open question under the spotlight of immunotherapy. Oncologie 2024;26:487–91. https://doi.org/10.1515/oncologie-2024-0040.Suche in Google Scholar
22. Reese, AC, Whitson, JM, Meng, MV. Natural history of untreated renal cell carcinoma with venous tumor thrombus. Urol Oncol 2013;31:1305–9. https://doi.org/10.1016/j.urolonc.2011.12.006.Suche in Google Scholar PubMed
23. Parisi, S, Napoli, I, Lillo, S, Cacciola, A, Ferini, G, Iatì, G, et al.. Spine eburnation in a metastatic lung cancer patient treated with immunotherapy and radiotherapy. The first case report of bystander effect on bone. J Oncol Pharm Pract 2022;28:237–41. https://doi.org/10.1177/10781552211027348.Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Autophagy and radiotherapy in esophageal cancer: modulating treatment sensitivity and overcoming challenges
- Functions of CAFs in microenvironment of non-small cell lung cancer: based on updated hallmarks of cancer
- Cisplatin-induced pyroptosis: a double-edged sword in cancer treatment
- Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
- Research Articles
- A lipid metabolism-related gene model reveals the prognosis and immune microenvironment of cutaneous melanoma
- Boanmycin induces apoptosis and overcomes venetoclax resistance in acute myeloid leukemia
- Identification of GNB1 as a downstream effector of the circRNA-0133711/miR-145-5p axis involved in breast cancer proliferation and metastasis
- Clinical characteristics and prognosis of lung metastases from unknown primary cancer sites
- Integrating bulk-RNA and single-cell analysis reveals heterogeneous expression of cuproptosis-related sorafenib-resistant genes in hepatocellular carcinoma
- Exploring the mechanism of genistein in treating hepatocellular carcinoma through network pharmacology and molecular docking
- Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
- Exploring the anti-lung cancer mechanism of Ganoderma lucidum and its relationship with the level of immune cell infiltration based on network pharmacology and molecular docking
- Constructing a prognostic model for colon cancer patients on basis of coagulation genes enriched in cancer-associated fibroblasts to guide personalized immunotherapy
- Rapid Communication
- Evaluation of HER2 immunohistochemistry-positive and immunohistochemistry-negative FISH amplification breast cancers using next-generation sequencing
- Short Commentary
- Unveiling the unexplored: shedding light on a novel aspect of colorectal carcinoma
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Autophagy and radiotherapy in esophageal cancer: modulating treatment sensitivity and overcoming challenges
- Functions of CAFs in microenvironment of non-small cell lung cancer: based on updated hallmarks of cancer
- Cisplatin-induced pyroptosis: a double-edged sword in cancer treatment
- Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
- Research Articles
- A lipid metabolism-related gene model reveals the prognosis and immune microenvironment of cutaneous melanoma
- Boanmycin induces apoptosis and overcomes venetoclax resistance in acute myeloid leukemia
- Identification of GNB1 as a downstream effector of the circRNA-0133711/miR-145-5p axis involved in breast cancer proliferation and metastasis
- Clinical characteristics and prognosis of lung metastases from unknown primary cancer sites
- Integrating bulk-RNA and single-cell analysis reveals heterogeneous expression of cuproptosis-related sorafenib-resistant genes in hepatocellular carcinoma
- Exploring the mechanism of genistein in treating hepatocellular carcinoma through network pharmacology and molecular docking
- Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
- Exploring the anti-lung cancer mechanism of Ganoderma lucidum and its relationship with the level of immune cell infiltration based on network pharmacology and molecular docking
- Constructing a prognostic model for colon cancer patients on basis of coagulation genes enriched in cancer-associated fibroblasts to guide personalized immunotherapy
- Rapid Communication
- Evaluation of HER2 immunohistochemistry-positive and immunohistochemistry-negative FISH amplification breast cancers using next-generation sequencing
- Short Commentary
- Unveiling the unexplored: shedding light on a novel aspect of colorectal carcinoma

