Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
-
Xiao-Qin Yang
, Meng-Zhe Liu
Abstract
Objectives
Melanoma remains a challenge due to the lack of effective and low-toxicity treatments. Litsea cubeba essential oil (LEO), known for its tyrosinase inhibitory activity, has shown promise as an anti-melanoma compound, although robust scientific evidence is lacking.
Methods
We conducted GC-MS analysis to identify the major components of LEO and screened for effective components were further evaluated on A375 and HaCaT cells using the CCK-8 assay. Network pharmacology was employed to predict potential targets using PharmMapper and SwissTarget Prediction databases, with melanoma-related targets sourced from the GeneCards database. Protein–protein interaction (PPI) network was created using STRING and Cytoscape. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were performed through the DAVI database. Additionally, we constructed a natural product-main components-core targets-pathways-disease (NMCPD) network in Cytoscape and conducted molecular docking using PyMOL and Autodock Vina.
Results
GC-MS analysis revealed neral (cis-citral) and geranial (trans-citral) as the primary active components of LEO. Cell assays demonstrated that a citral mixture, in combination with LEO, effectively inhibited A375 cell growth with IC50 values of 35.94 ± 1.23 μM and 12.00 ± 0.96 μg/mL, while exhibiting minimal toxicity to HaCaT cells with IC50 values of 67.72 ± 2.96 μM and 22.32 ± 2.53 μg/mL. Screening identified10 hub targets among 190 common targets between drug and disease-related targets. KEGG pathway enrichment analysis suggested therapeutic effects of citral on melanoma by modulating signaling pathways. Molecular docking revealed strong binding affinity of neral and geranial with RXRA and ESR1, suggesting that citral, the principal LEO component, regulates multiple pathways for potential melanoma therapy.
Conclusions
These findings support the potential utility of LEO as a treatment for melanoma and highlight the importance of exploring tyrosinase inhibitors for the development of novel anti-melanoma drugs.
Introduction
Litsea cubeba fruit, known as “Bì Chéng Qié” in China, has been utilized as a traditional herbal medicine for an extended period. This fruit is derived from a unique woody oil plant, L. cubeba (Lour.) Pers., belonging to the Lauraceae family [1]. Due to its valuable medicinal properties and economic benefits, the plant has seen increased cultivation in China in recent years, becoming an essential industrial crop [2]. The volatile oil extracted from L. cubeba fruit, known as L. cubeba essential oil (LEO), has been traded for several decades due to its diverse biological activities and extensive applications in the fields of food, medicine, cosmetics, and agriculture [3], [4], [5], [6]. The yield and composition of LEOs are influenced by various factors such as varieties, environmental conditions, and extraction methods [7], [8], [9]. Nevertheless, citral has consistently been identified as a predominant component in LEOs and is believed to be responsible for the strong bioactivity of LEO due to its multiple biological activities, including but not limited to antioxidant, anti-cancer, anti-inflammatory, antimicrobial, and insecticidal properties [10], [11], [12], [13].
Melanoma primarily arises from melanocytes in the skin and eyes, representing a highly aggressive form of skin cancer. It commonly affects adults and can result from various factors, including excessive exposure to ultraviolet radiation, genetics, and trauma to existing moles [14]. Conventional treatments for melanoma, including surgery, chemotherapy, radiation therapy, and immunotherapy, have unfortunately resulted in low survival rates for patients. In fact, less than 5 % of patients have survived beyond five years despite undergoing these treatments [15], [16], [17], [18]. Most patients with advanced-stage melanoma have a poor response to treatment and are at high risk of metastasis and recurrence, making treatment challenging and a serious public health concern [19]. Therefore, researchers are exploring alternative therapies for melanoma that are effective and have low toxicity [20]. In recent years, natural products have emerged as a promising alternative to conventional cancer treatments, with many demonstrating anti-cancer activity and being widely used in the treatment of melanoma [21], [22], [23], [24], [25].
In melanoma cells, melanin is a crucial pigment, and its biosynthesis is catalyzed by an enzyme called tyrosinase [26]. Tyrosinase, highly expressed in melanoma cells, has been identified as an ideal target for both diagnosis and treatment of the disease [27]. Some earlier studies have suggested that compounds with tyrosinase inhibitory activity can induce apoptosis in B16 melanoma cells in mice, indicating their potential therapeutic use [28]. Therefore, developing anti-melanoma drugs from compounds with tyrosinase inhibitory activity is a promising strategy. Relevant research has demonstrated that LEO and its main component, citral, exhibit significant inhibitory effects on both monophenolase and diphenolase activities of tyrosinase [29]. Additionally, citral has been found to exert antiproliferative and cytotoxic effects on B16F10, a melanoma cell line [30]. These findings suggest that LEO and its active ingredients could potentially offer therapeutic benefits in melanoma treatment. However, research on the impact and mechanisms of LEO and its active constituents on melanoma remains limited, and more research is needed to fully understand how they can benefit melanoma treatment.
In this study, we conducted an investigation into the primary components of LEO, their tyrosinase inhibitory activity, and performed a cytotoxicity analysis on A375 and HaCaT cells in this study. Subsequently, we utilized bioinformatics analysis techniques, including network pharmacology and molecular docking, to speculate on the potential therapeutic effects and underlying mechanisms of LEOs and citral in the treatment of melanoma. This approach can offer insights into the potential therapeutic targets and mechanisms of action of LEOs, ultimately paving the way for more effective treatments for melanoma.
Materials and methods
Materials
LEO was commercially purchased from Runsuo Natural Essential Oil Store (Jiangxi, China) and the components were further analyzed using Gas Chromatography-Mass Spectrometer (GC-MS). Citral (Adamas-beta®, purity≥94 % by GC) was a mixture of cis and trans isomers with a specific mixing ratio of 47.70 % neral (cis-trial) to 46.57 % geranial (trans-citral), and purchased from Shanghai Titan Scientific Co., Ltd. (Shanghai, China). Taxol (purity≥98 % by HPLC) was provided by Nanjing Pusheng Biomedical Technology Co., Ltd (Jiangsu, China). All other chemical agents used in the study were of analytical grade and were used without further purification.
Components analysis of LEO
An analysis of the components in LEO was performed using a Thermo Scientific™ TRACE 1310 gas chromatograph (GC, Thermo Fisher Scientific Inc., USA) coupled with a Thermo Scientific™ ISQ™ LT mass spectrometer (MS, Thermo Fisher Scientific Inc., USA) that operated in selected ion monitoring (SIM) mode with electron ionization (EI). The equipment was equipped with a Thermo Scientific™ Instant Connect split/splitless (SSL) injector (Thermo Fisher Scientific Inc., USA), and helium carrier gas was used as the carrier gas. The sample was diluted using hexane from 20 to 100 μM, and a split ratio of 10:1 was employed. Subsequently, 1 μL of the diluted sample was injected into the GC column at 250 °C. The sample was then passed through a Thermo Scientific™ TraceGOLD™ TG-5MS column (Thermo Fisher Scientific Inc., USA) with dimensions of 30 m (length) × 0.25 mm (Diameter) × 0.25 μm (film thickness) while maintaining a constant flow rate of 1 mL/min and using temperature programming. The oven temperature was initially set to 40 °C and maintained at that temperature for 5 min, then the temperature was raised at a rate of 4 °C/min until it reached 100 °C and held for an additional 1 min. Finally, the temperature was raised at a rate of 30 °C/min until it reached a final temperature of 250 °C, and held at this temperature for 10 min for analysis.
Component identification was conducted by comparing the recorded mass spectra with the NIST 2017 Mass Spectra Library (mainlib, replib) in an automated mode. The proportion of each component was determined using the peak area normalization method, and the experiment was conducted in triplicate.
Determination of tyrosinase inhibitory activities in vitro
The tyrosinase inhibitory activities of LEO and citral were measured by using SpectraMax® 190 (Molecular Devices, USA) following a modified method of the literature method [31]. To do this, 150 μL of substrate (1 mM L-tyrosine for monophenolase and 0.5 mM L-DOPA for diphenolase) in a phosphate-buffered saline (PBS) solution (0.05 M, pH 6.8) and 30 μL of inhibitors solvated in a PBS solution with 2 % dimethyl sulfoxide, at concentrations ranging from 0.05–0.2500 mg/mL, were mixed in a 96-well plate. After pre-incubation of 20 μL of 31.25 U/mL tyrosinase at 30 °C ± 2 °C for 10 min, it was added to the mixture. Each sample was measured using the SpectraMax® 190 (Molecular Devices, USA) and subjected to absorbance measurements three times at 475 nm, with an interval of 5 s between readings. For each experiment, kojic acid served as the positive control, and three replicates were conducted. The tyrosinase inhibition rate was calculated using Equation (1).
Where A 1 represents the absorbance of a mixture containing substrates and tyrosinase, A 2 represents the absorbance of a mixture containing only substrates, A 3 represents the absorbance of a mixture containing substrates, tyrosinase, and inhibitors, and A 4 represents the absorbance of a mixture containing both substrates and inhibitors.
Experimental verification in vitro
Cell lines and cell culture
Human melanoma A375 tumor cells and human immortalized keratinocyte (HaCaT) non-tumor cells were obtained from the Nanjing Pusheng Biomedical Technology Co., Ltd (Jiangsu, China). The cells (A375/HaCaT) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10 % (v/v) fetal bovine serum (FBS, Gibco, Grand Island, NY, USA), and incubated at 37 °C with 5 % CO2 in a humidified incubator.
Cell viability assay
Cell proliferation and cytotoxicity assays were conducted using the Cell counting kit-8 (CCK-8) on A375 and HaCaT cells. Briefly, the cells were initially seeded in 96-well plates at a density of 4 × 104 cells/well and incubated for 24 h at 37 °C with 5 % CO2 in a humidified incubator. Each treatment was performed in six duplicate wells. The tested compounds were added at various concentrations, and the cells were then incubated for 72 h. Following the incubation period, the CCK-8 solution (Dojindo Laboratories, Kumamoto, Tokyo, Japan) was added to each well, and the plates were further incubated for 2 h. The absorbance of each well at 450 nm was measured using an absorbance microplate reader (BioTek ELx800, USA). The assay included negative controls in which cells were treated with distilled water and positive controls in which cells were treated with 10 μg/mL taxol. The percentage of cell growth inhibition was calculated using the following Equation (2):
Where B 1 represents the absorbance of a mixture containing cells, medium, and CCK8, B 2 represents the absorbance of absorbance of a mixture containing cells, medium, CCK8, and the test compound; B 3 represents a mixture containing medium and CCK8.
Cell growth and morphology were observed using an inverted microscope (Olympus IX51, Japan). The experiments were conducted in triplicate to ensure accuracy and consistency of results.
Network pharmacology
Target collection
The chemical structures of neral and geranial were obtained from the National Institutes of Health (PubChem) database (https://pubchem.ncbi.nlm.nih.gov). Potential targets of “Homo sapiens” species for neral and geranial were retrieved from the PharmMapper (http://www.lilab-ecust.cn/pharmmapper/) and SwissTarget Prediction (https://www.swisstargetprediction.ch/) databases, and duplicates were removed. To standardize the retrieved targets into official gene symbols, the Universal Protein Resource (UniProt, https://www.uniprot.org/) and Biological DataBase network (bioDBnet, https://biodbnet-abcc.ncifcrf.gov/) databases were used. Targets related to melanoma in the “H. sapiens” species were retrieved from the Human Gene (GeneCards, https://www.genecards.org/) database by searching for the keywords “melanoma”. Finally, the online tool BioLadder (https://www.bioladder.cn/web/#/chart/17) was used to obtain the shared targets of neral, geranial, and melanoma.
Protein–protein-interaction (PPI) network construction
To perform a more detailed analysis of the shared target proteins resulting from the mapping of neral, geranial, and melanoma, the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (https://string-db.org/) was used for protein–protein interaction (PPI) network analysis. The “H. sapiens” option was selected, and only high-confidence interactions with a confidence score of 0.900 were included. The resulting PPI network was imported into Cytoscape 3.9.1 for topological analysis. To identify key regulatory proteins in the network, the node color and size were adjusted using Cytoscape. Complete PPI networks of overlapping targets were then constructed. The median degree centrality (DC), betweenness centrality (BC), and closeness centrality (CC) were used as screening criteria to draw the core network and identify the hub targets of citral in anti-melanoma.
GO and KEGG pathway enrichment analysis
The core targets resulting from the PPI network analysis of neral, geranial, and melanoma were further analyzed by performing Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG, https://www.kegg.jp/) pathway enrichment analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID, https://david.ncifcrf.gov/). The GO terms were classified into three groups, including Biological Process (BP), Cellular Component (CC), and Molecular Function (MF). A statistical significance level of p-value<0.01 was used to determine the significance of the results. Potential biological functions of citral against melanoma were considered based on functions with a high level of significance and a large proportion of frequency. Advanced bar graphs and bubble charts were generated using the Bioinformatics website (http://www.bioinformatics.com.cn).
Natural product-main components-core targets-pathways-disease (NMCPD) network construction
The associations between the compounds-targets-pathways of LEO and melanoma were illustrated through the NMCPD network using Cytoscape 3.9.1 software. This network consists of a compound-predicted target for LEO network, as well as the main component compounds-core targets-pathways network for LEO in anti-melanoma treatment.
Molecular docking
The top 10 hub target proteins identified from the PPI analysis were subjected to molecular docking, with crystal structures of the target proteins obtained from the UniProt database (https://www.uniprot.org/). Prior to docking, OpenBabel 2.4.1 (https://openbabel.org/) was used to convert the chemical format of neral and geranial. The proteins ligands were preprocessed using Autodock Tools 1.5.7, and AutoDock Vina 1.1.2 was used to perform the molecular docking calculations. The compounds with the lowest binding free energy were further analyzed for their interactions with the target proteins using Discovery Studio Visualizer 2022. To visualize the potential binding modes and interactions between the hub proteins and ligands, PyMol 2.6.0a0 software was used. Additionally, to evaluate the structural dissimilarities between the neural and geranial structures docked with the same target, the preferred conformations of both were subjected to Root mean squared deviation (RMSD) analysis using Discovery Studio Visualizer 2022.
Statistical data analysis
A one-way ANOVA was utilized to analyze the statistical data, with significant differences between groups being determined at a p<0.05. The results were presented as mean ± standard deviation (SD). The IC50 value was determined through non-linear regression curve analysis using Prism GraphPad Prism version 9.0 for Windows (GraphPad Software, San Diego, CA).
Results and Discussion
Component analysis of LEO
The chemical composition of LEO was determined using Gas Chromatography-Mass Spectrometer (GC-MS), as shown in Table 1, the result revealed that the presence of seven major terpenoids, including limonene (19.19 %), citronellal (2.34 %), isoneral (1.64 %), isogeranial (2.69 %), neral (34.73 %), geranial (37.48 %), and β-caryophyllene (1.91 %). The primary component of LEO was found to be citral, which is a racemic mixture of geometric isomers known as neral (cis-citral) and geranial (trans-citral). Recent studies have investigated the potential anti-cancer properties of LEO, particularly its main component citral, as a natural anti-cancer agent. While these findings suggest potential therapeutic applications for LEOs and citral in cancer treatment, further research is necessary to fully understand the mechanisms underlying these effects. Additional studies are needed to determine the efficacy and safety of LEOs and citral in treating cancer.
Component analysis of LEO.
| Retention time, min | Compound | Molecular formula | Molecular structure | Relative content (%) |
|---|---|---|---|---|
| 15.58 | D-Limonene | C10H16 |

|
19.19 ± 0.76 |
| 20.36 | Citronellal | C10H18O |

|
2.34 ± 0.33 |
| 20.67 | Isoneral | C10H16O |

|
1.64 ± 0.39 |
| 21.04 | Isogeranial | C10H16O |

|
2.69 ± 0.40 |
| 21.88 | Neral (cis-citral) | C10H16O |

|
34.73 ± 1.20 |
| 22.22 | Geranial (trans-citral) | C10H16O |
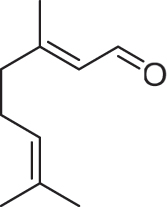
|
37.48 ± 0.77 |
| 23.50 | β-Caryophyllene | C15H24 |

|
1.91 ± 0.09 |
-
Data represents the mean values ± SD of triplicate independent experiments.
Tyrosinase inhibitory activity of LEO and citral
Tyrosinase is an enzyme that plays a crucial role in the biosynthesis of melanin, and its dysregulation has been associated with the development of melanoma, a malignant skin tumor originating from melanocytes. Previous studies have demonstrated the tyrosinase inhibitory activity of LEO, and further investigations have examined the inhibitory activity of major components of LEO, such as limonene, citral, and citronellal, on tyrosinase. However, the findings showed that only citral exhibited promising activity, while limonene and citronellal did not exhibit significant inhibitory activity on tyrosinase [29].
To further investigate the tyrosinase inhibitory activity of LEO and citral, the study conducted experiments on both monophenolase and diphenolase inhibition. As shown in Figure 1, the results indicated that LEO and citral had significant inhibitory effects on both monophenolase and diphenolase inhibition, with their inhibition rates reaching up to 56 and 43 %, and 39 and 37 %, respectively. The inhibition of tyrosinase activity by LEO and citral is due to the formation of Schiff bases between the aldehydes and nucleophilic groups surrounding the active site of tyrosinase. These aldehydes can react with crucial nucleophilic groups, such as sulfhydryl, amino, or hydroxyl groups in proteins, creating steric hindrance that hinders enzyme-substrate binding and ultimately leads to a reduction in enzyme activity.

Dose-dependent response of LEO (A) and citral (B) on the activity of monophenolase and diphenolase contained in tyrosinase. Data represent the mean values ± SD of triplicate independent experiments.
Given the significant association between tyrosinase and melanoma, further research is necessary to predict the potential anti-melanoma activity of LEO and citral.
Cytotoxicity of citral and LEO on A375 cells and HaCaT cells
In this study, we used the CCK-8 assay to investigate the effects of citral and LEO on cell proliferation and cytotoxicity in A375 and HaCaT cells. The results showed that citral inhibited cell proliferation in a dose-dependent manner in both cell types. As shown in Figure 2A, the results demonstrated that treating A375 and HaCaT cells with citral at concentrations of 100, 200, and 400 μM for 72 h significantly inhibited cell proliferation.

Dose-dependent response of citral (A) and LEO (B) on A375 cells and HaCaT cells after 72 h of treatment. Results are expressed as cell inhibition (%) related to positive control (cells stimulated with 10 μg/mL taxol). Data represented the mean values ± SD of triplicate independent experiments. Comparison among the groups was performed using the One-way ANOVA test followed by Dunnett’s post-test. (∗p<0.05; ∗∗p<0.01).
Morphological analysis of cells treated with citral revealed significant changes in cell morphology, including loss of cell-cell contacts, development of membrane blebs, and formation of vacuoles, in comparison to control cells, compared to control cells, as shown in Figure 3A and B. However, at lower concentrations of 3.125, 6.25, and 12.5 μM, the inhibitory effect on cell proliferation was minimal, with less than 20 % inhibition and no noticeable morphological changes. At higher concentrations of 25 and 50 μM, citral demonstrated a more potent inhibitory effect on A375 cells, with inhibition rates of 24.99 and 66.13 %, respectively, along with significant changes in cell morphology.

Morphological changes of A375 cells (A) and HaCaT cells (B) with citral and A375 cells (C) and HaCaT cells (D) with LEO treatment, shown by an Olympus inverted biological microscope. Scale bar: 100 μm.
In contrast, the inhibitory effect on HaCaT cells was relatively weaker, with only 8.19 and 14.07 % inhibition and no observable morphological changes. Furthermore, the IC50 values after 72 h of treatment indicated that citral was more toxic to A375 cells than HaCaT cells, with a lower IC50 value of 22.30 μM and a higher IC50 value of 67.7 μM, respectively. These findings suggest that citral has the potential to inhibit cell proliferation and induce cytotoxic effects, particularly in A375 cells, indicating its possible use in cancer treatment.
To further investigate the effect of LEO on A375 and HaCaT cells, we evaluated its impact on cell proliferation. As depicted in Figure 2B, LEO was found to have a similar potency in inhibiting cell proliferation as citral. Treatment with LEO at concentrations ranging from 25 to 400 μg/mL for 72 h resulted in significant inhibition of cell proliferation in A375 and HaCaT cells.
Further analysis, as shown in Figure 3C, revealed that cells treated with LEO showed marked changes in marked morphology compared to control cells. However, at concentrations of 3.125 and 6.25 μg/mL, the inhibitory effect on cell proliferation was minimal, with less than 20 % inhibition, and no noticeable morphological changes were observed. At a concentration of 12.5 μg/mL, LEO showed a stronger inhibitory effect on A375 cells, with an inhibition rate of 60.96 % and significant changes in cell morphology.
Conversely, as depicted in Figure 3D, the inhibitory effect on HaCaT cells was relatively weaker, with only 7.5 % inhibition and no observed morphological changes. Additionally, the IC50 values after 72 h of treatment revealed that LEO exhibited greater toxicity towards A375 cells, with a lower IC50 value of 12.00 ± 0.96 μg/mL, compared to HaCaT cells, which had a higher IC50 value of 22.32 ± 2.53 μg/mL, as shown in Table 2.
Inhibitory effect of LEO and citral on A375 cells and HaCaT cells.
| IC50 | ||
|---|---|---|
| A375 | HaCaT | |
| LEO | 12.00 ± 0.96 μg/mL | 22.32 ± 2.53 μg/mL |
| Citral | 35.94 ± 1.23 μM | 67.72 ± 2.96 μM |
Cultured A375 cells and HaCaT cells in exponential growth were treated with increasing concentrations of LEO and citral for 72 h. The IC50 values were calculated from dose-response curves obtained by nonlinear regression. Data represented the mean values ± SD of six independent experiments.
Experimental results have shown that citral and LEO can effectively suppress the growth of A375 cells, even at low concentrations, while causing minimal toxicity to HaCaT cells. This suggests that LEO and citral have promising therapeutic potential for the treatment of melanomas. Further verification of the anti-tumor mechanism and safety of LEO can be achieved through animal experiments.
Network pharmacology analysis
Targets of citral in melanoma-related targets
Citral, the primary bioactive component of LEO, is a promising candidate for potential targets for anti-melanoma treatment due to its significant inhibitory effect on tyrosinase and melanoma cell line B16F10 [32]. The isomers of citral, neral and geranial, possess unique 3D structures, which can result in different biological activities. Therefore, it is possible that neral and geranial may have isomeric effects on their interactions with biological targets, such as enzymes and receptors.
To identify potential melanoma therapeutic targets, separate network pharmacology analyses were conducted for neral and geranial. The PharmMapper and SwissTargetPrediction databases were used to search for potential targets associated with neral and geranial, resulting in a total of 294 and 288 potential targets, respectively, after removing duplicates (Table S1A and Table S1B). Additionally, 8,242 targets associated with melanoma were retrieved from the GeneCards database (Table S2). Venn diagram analysis revealed 190 overlapping targets between the drug-related targets and disease-related targets (Figure 2A, Table S3). Moreover, 26 and 32 unique overlapping targets were identified between disease-related targets and neral-related or geranial-related targets, respectively (Figure 4A, Table S3). These overlapping targets hold promise for the development of anti-melanoma therapies using citral and warrant further investigation to assess their efficacy in treating the disease.

Illustrate (A) a Venn diagram of potential melanoma targets in citral treatment, (B) a PPI network of citral and melanoma, and (C) hub gene identification from the PPI network using the Centiscape plugin, highlighting key nodes and interactions.
Protein–protein-interaction (PPI) network construction
The data on the 190 shared targets among neral, geranial, and melanoma, along with the 26 and 32 unique shared targets between neral-melanoma and geranial-melanoma, respectively, were utilized to construct a PPI network using the STRING database for investigating their interactions. As shown in Figure 4B, the PPI network resulting from the 190 shared targets exhibited 467 edges and had an average node degree of 4.920, indicating a highly interconnected network with a clustering coefficient of 0.424 among the 190 target genes. Conversely, the smaller networks generated from the 26 and 32 unique shared targets between neral-melanoma and geranial-melanoma, respectively, displayed fewer edges, less connectivity, lower average node degrees, and clustering coefficients. It is evident that the PPI network of the 190 shared targets has significantly more interactions than the smaller networks generated from the 26 and 32 unique shared targets between neral-melanoma and geranial-melanoma, respectively. This indicated that the screened proteins are biologically connected, and the network holds greater research value for exploring interactive relationships between targets and identifying potential therapeutic targets for melanoma.
The data obtained were imported into Cytoscape 3.9.1 for network mapping and topology analysis to gain a better understanding of the relationships among the targets. The Centiscape plugin was used to further analyze the 190 shared targets, revealing that the median values of BC, CC, and DC were 447, 0.0018, and 12, respectively. As presented in Figure 4C and Table 3, 24 core targets with values above the median were identified and further screened on their connectivity degree greater than 36, resulting in the identification of 10 hub targets that play significant roles in the pathogenesis of melanoma. These hub targets, MAPK1, SRC, AKT1, HSP90AA1, GRB2, PTPN11, MAPK14, RXRA, LCK, and ESR1, are often dysregulated, contributing to the growth, survival, metastasis, and drug resistance of melanoma cells.
Core targets of citral in melanoma treatment.
| No. | Core targets | Degree | Betweenness | Closeness | Description |
|---|---|---|---|---|---|
| 1 | MAPK1 | 70 | 3,809.07 | 0.0026 | Mitogen-activated protein kinase 1 |
| 2 | SRC | 66 | 2,955.43 | 0.0026 | Proto-oncogene tyrosine-protein kinase Src |
| 3 | AKT1 | 60 | 4,129.84 | 0.0025 | RAC-alpha serine/threonine-protein kinase |
| 4 | HSP90AA1 | 54 | 2011.18 | 0.0024 | Heat shock protein HSP 90-alpha |
| 5 | GRB2 | 50 | 749.51 | 0.0023 | Growth factor receptor-bound protein 2 |
| 6 | PTPN11 | 48 | 539.14 | 0.0023 | Tyrosine-protein phosphatase non-receptor type 11 |
| 7 | MAPK14 | 42 | 1792.56 | 0.0023 | Mitogen-activated protein kinase 14 |
| 8 | RXRA | 38 | 2,120.33 | 0.0023 | Retinoic acid receptor RXR-alpha |
| 9 | LCK | 38 | 793.81 | 0.0024 | Tyrosine-protein kinase Lck |
| 10 | ESR1 | 38 | 593.42 | 0.0023 | Estrogen receptor 1 |
| 11 | MAPK8 | 36 | 6,114.89 | 0.0025 | Mitogen-activated protein kinase 8 |
| 12 | ERBB4 | 28 | 1,063.16 | 0.0022 | Receptor tyrosine-protein kinase ErbB-4 |
| 13 | ABL1 | 26 | 906.26 | 0.0023 | Tyrosine-protein kinase ABL1 |
| 14 | RXRB | 26 | 558.42 | 0.0021 | Retinoic acid receptor RXR-beta |
| 15 | CASP3 | 24 | 1,310.36 | 0.0022 | Caspase 3 |
| 16 | AR | 24 | 999.59 | 0.0024 | Androgen receptor |
| 17 | CCND1 | 24 | 899.77 | 0.0021 | G1/S-specific cyclin-D1 |
| 18 | CTNNA1 | 22 | 622.34 | 0.0020 | Catenin alpha 1 |
| 19 | NR3C1 | 22 | 460.95 | 0.0023 | Glucocorticoid receptor |
| 20 | MDM2 | 22 | 459.41 | 0.0022 | E3 ubiquitin-protein ligase Mdm2 |
| 21 | F2 | 18 | 2082.37 | 0.0022 | Prothrombin |
| 22 | GSK3B | 18 | 519.34 | 0.0021 | Glycogen synthase kinase-3 beta |
| 23 | CCNA2 | 16 | 505.65 | 0.0019 | Cyclin A2 |
| 24 | PPARA | 16 | 494.41 | 0.0022 | Peroxisome proliferator-activated receptor alpha |
-
The core targets were sorted based on their betweenness values (>447), closeness values (>0.0018), and degree values (>12), with higher degree values indicating greater importance. 10 hub targets with degree values>36 are highlighted in orange.
Of these hub genes, the MAPK (mitogen-activated protein kinase) signaling pathway plays a crucial role in the development and progression of melanoma, with key components such as MAPK1 (mitogen-activated protein kinase 1) and MAPK14 (mitogen-activated protein kinase 14) playing important roles. Studies have shown that MAPK1 can reverse the promoting effect of LINC01296 in vitro in cutaneous malignant melanoma cells [33]. Moreover, the p38α-MAPK14 pathway can effectively inhibit the growth of melanoma both in vitro and in vivo, making it a promising therapeutic target for treating melanoma, particularly in cases involving oncogenic NRAS [34]. The tyrosine kinase signaling pathway, which is known to promote melanoma progression, involves the participation of SRC (proto-oncogene tyrosine-protein kinase Src) and LCK (tyrosine-protein kinase). Studies have indicated that overexpression and activation of SRC can significantly promote tumor progression, while citral has been found to inhibit the phosphorylation of Src (Y416). This suggests that targeting SRC could be an effective therapeutic approach for treating melanoma, particularly when using citral as a treatment agent [35]. LCK is involved in multiple cellular processes and has been highlighted as a potential biomarker for melanoma immunotherapy [36, 37]. Therapeutic strategies targeting LCK in tumor cells could offer a promising avenue for treating melanoma [37]. AKT1 (RAC-alpha serine/threonine-protein kinase) and HSP90AA1 (heat shock protein HSP 90-alpha) play essential roles in various cellular processes, such as cell growth, survival, and metabolism, and both are frequently overexpressed in melanoma. High expression of AKT is known to contribute to resistance against cell apoptosis in melanoma, and several AKT inhibitors are currently undergoing evaluation in preclinical and clinical studies for the treatment of this disease [38], [39], [40].
HSP90AA1 activates several oncogenic client proteins, which promote cell survival, growth, and invasiveness in cancer cells. However, there is currently no evidence of the effectiveness of HSP90AA1 in treating melanoma [39]. GRB2 (growth factor receptor-bound protein 2) and PTPN11 (tyrosine-protein phosphatase non-receptor type 11) are both involved in cell signaling and are potential prognostic markers for melanoma [40, 41]. GRB2 plays a crucial role in the progression of different types of human cancer and has been identified as a reliable prognostic marker for uveal melanoma [42]. PTPN11 is a multifunctional tyrosine phosphatase that has been implicated in the development of various types of tumors, and PTPN11 mosaicism can cause a range of pigmentary and vascular neurocutaneous disorders and increase the risk of melanoma [41, 43]. RXRA (retinoic acid receptor RXR-alpha) is a transcription factor that regulates cell differentiation, proliferation, and survival, and may potentially be involved in melanoma pathogenesis. However, there is limited evidence supporting its role in melanoma [44]. ESR1 (estrogen receptor 1), the primary estrogen receptor expressed in the human epidermis, has been implicated in melanoma development and progression, but is unlikely to be the sole factor involved. More research is needed to investigate the interplay between various estrogen receptors, miRNA expression, and their effects on the tumor microenvironment specific to melanoma [45]. Despite this complexity, targeting these genes remains a promising strategy for treating melanoma, and they should be considered as key targets for citral therapy.
The aforementioned analysis suggests that citral treatment for melanoma targets multiple proteins, and there exists a strong correlation among these target proteins. Gaining a comprehensive understanding of how these genes function and interact with each other could enhance our knowledge of the underlying mechanisms of citral treatment for melanoma. This understanding could also lead to the discovery of new targets and the development of more effective strategies for both prevention and treatment. However, additional research is required to fully comprehend the roles and interactions of these genes and their potential as therapeutic targets for citral in melanoma treatment.
GO and KEGG pathway enrichment analyses
In order to gain more insights into the mechanisms underlying the therapeutic effects of citral in melanoma, a total of 24 core target genes were subjected to GO annotation and KEGG pathway analysis using the DAVID database. Screening with a p-value≤0.01 enriched 125 GO terms, including 79 for BP, 11 for CC, and 35 for MF, as shown in Table S4A. The top 10 terms of each biological process were visualized in a bar graph as shown in Figure 5A. The results revealed that citral has the potential to regulate biological processes that are involved in the development and progression of melanoma. Specifically, the analysis indicated that citral may modulate BP such as the EGFR (epidermal growth factor receptor) signaling pathway (GO:0007173) and positive regulation of transcription and DNA-templated (GO:0045893), which are closely associated with the regulation of gene expression and play a crucial role in the progression of melanoma. In terms of CC, the analysis identified nucleoplasm (GO:0005654), cytosol (GO:0005829), mitochondrion (GO:0005739), and nucleus (GO:0005634) as closely related to the proliferation and survival of melanoma cells. Regarding MF, the analysis indicated that the regulation of melanoma development and progression is associated with RNA polymerase II transcription factor activity, ligand-activated sequence-specific DNA binding (GO:0004879), enzyme binding (GO:0019899), protein kinase binding (GO:0019901), and phosphotyrosine binding (GO:0001784). The aberrant activity of these cellular components and molecular functions has been associated with numerous diseases, including cancer. By comprehending the roles of these factors in the citral treatment of melanoma, we hope to acquire a better understanding of the mechanisms underlying melanoma progression and advance the development of novel therapeutic strategies.
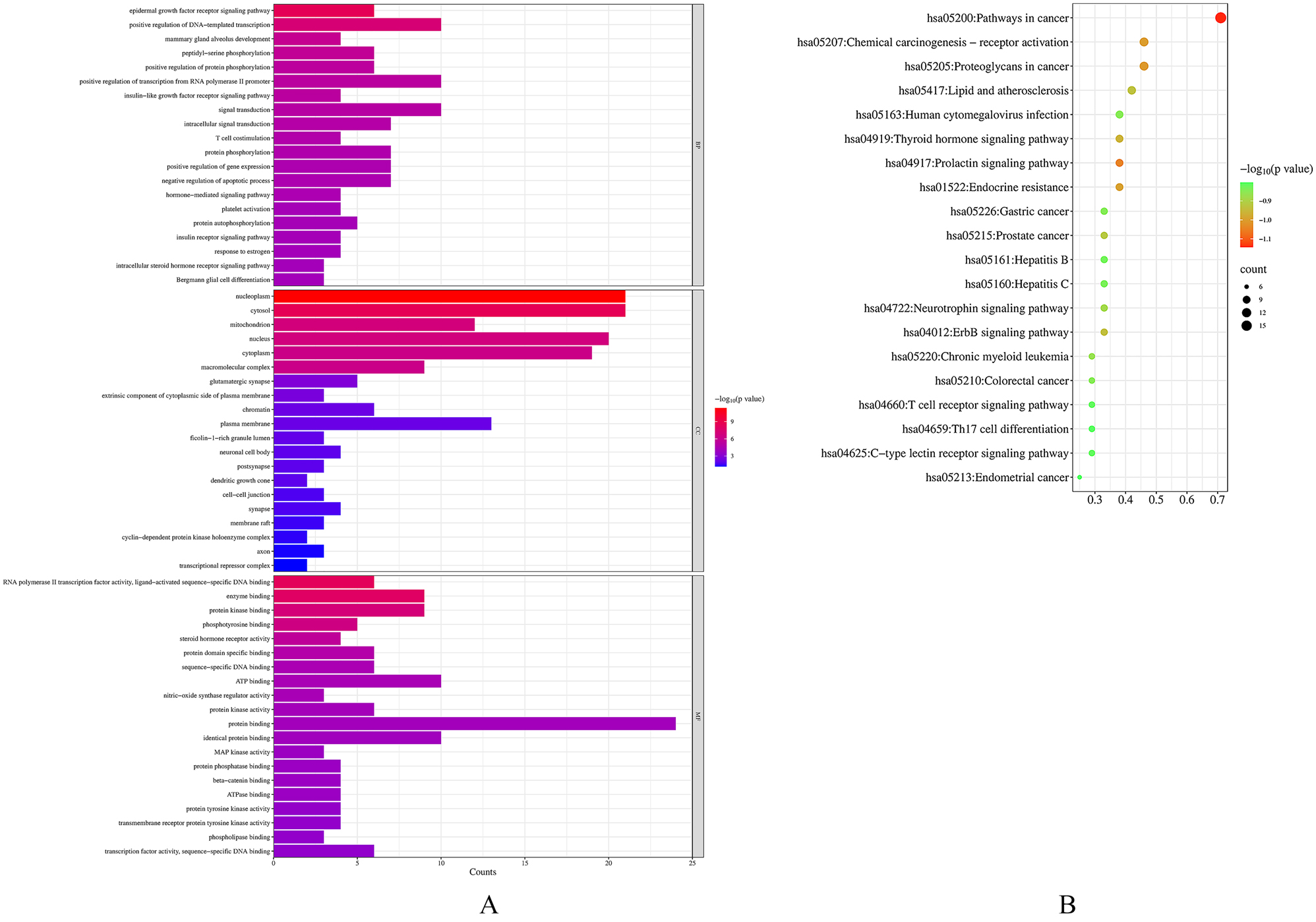
Illustrate (A) GO enrichment analysis showing the top 10 biological process terms with p-values <0.01, with bar length representing gene count and color indicating p-value significance, and (B) KEGG pathway enrichment analysis identifying the top 20 significant pathways with p-values <0.01, with dot size representing gene count and color indicating p-value significance.
Figure 5B and Table S4B presented the top 20 pathways ranked by gene ratio and their p-values, out of a total of 92 pathways identified through KEGG pathway enrichment analysis with a p-value of≤0.01. The results showed that core genes were significantly enriched in pathways in cancer (hsa05200), proteoglycans in cancer (hsa05205), chemical carcinogenesis - receptor activation (hsa05207), lipid and atherosclerosis (hsa05417), prolactin signaling pathway (hsa04917), endocrine resistance (hsa01522), thyroid hormone signaling pathway (hsa04919), ErbB signaling pathway (hsa040125), prostate cancer (hsa05215), and neurotrophin signaling pathway (hsa04722).
Among the pathways identified, pathways in cancer were found to be directly associated with melanoma. This signaling pathway involves many tumor-related pathways, such as Wnt, PI3K/Akt, MAPK, and p53 pathways. In melanoma, pathways in cancer may contribute to cancer cell proliferation, metastasis, invasion, and apoptosis, and targeting it may hold promise as a therapeutic strategy [46]. The proteoglycans in cancer and prolactin signaling pathways are closely related to melanoma metastasis and progression [47]. The endocrine resistance pathway is also of interest in melanoma, as it has been associated with therapy resistance, including resistance to targeted therapies [48]. Studies have shown that the thyroid hormone signaling pathway plays a critical role in the development of melanoma [49]. It has been observed that individuals with aggressive forms of melanoma are at a higher risk of developing thyroid malignancies [50]. This association may be due to elevated levels of thyroid-stimulating hormone found in patients with thyroid failure. Moreover, melanomas have been found to express high levels of thyroid-stimulating hormone receptors, which can be activated by thyroid-stimulating hormone and contribute to the conversion of melanocytes to melanoma [51]. These pathological associations between melanoma and thyroid cancer suggest that targeting the thyroid hormone signaling pathway may be an effective therapeutic strategy for melanoma. Several approved therapies target the ErbB signaling pathway, which has been found to play a crucial role in melanoma cell growth and survival [52]. Additionally, prostate cancer has been found to be linked to melanoma via mutual androgen-dependence. The inhibitory effects of citral on melanoma are likely achieved through targeting these signaling pathways.
Citral has shown potential as an anti-melanoma agent in various studies. Theoretical evidence suggests that it can regulate crucial signaling pathways associated with melanoma cell proliferation, metastasis, invasion, apoptosis, and therapeutic resistance. These findings provide promising prospects for the development of novel therapeutic approaches to treat melanoma. Additionally, the results suggest that LEO and citral may also be potential therapeutic agents for other types of cancer, including prostate cancer and thyroid cancer.
NMCPD network analysis
In order to gain a deeper understanding of the mechanism of LEO and citral action on melanoma, a systematic and comprehensive network was constructed using the two main active components of LEO, 24 shared core targets, and 20 KEGG signaling pathways. Figure 6 illustrates this network, which consists of 48 nodes and 244 edges. One notable finding is that a single compound in LEO can interact with multiple targets, and a single target can be regulated by both neral and geranial. The results of this analysis reveal that LEO and citral have a positive, multicomponent, and multi-target intervention in the treatment of melanoma. In the bioinformatics network, LEO is represented by the color brown, main active components, namely isomers of citral neral and geranial, are represented by green, target genes are represented by orange, signal pathways are represented by blue, and the disease is represented by red. It is noteworthy that one target can correspond to multiple components.
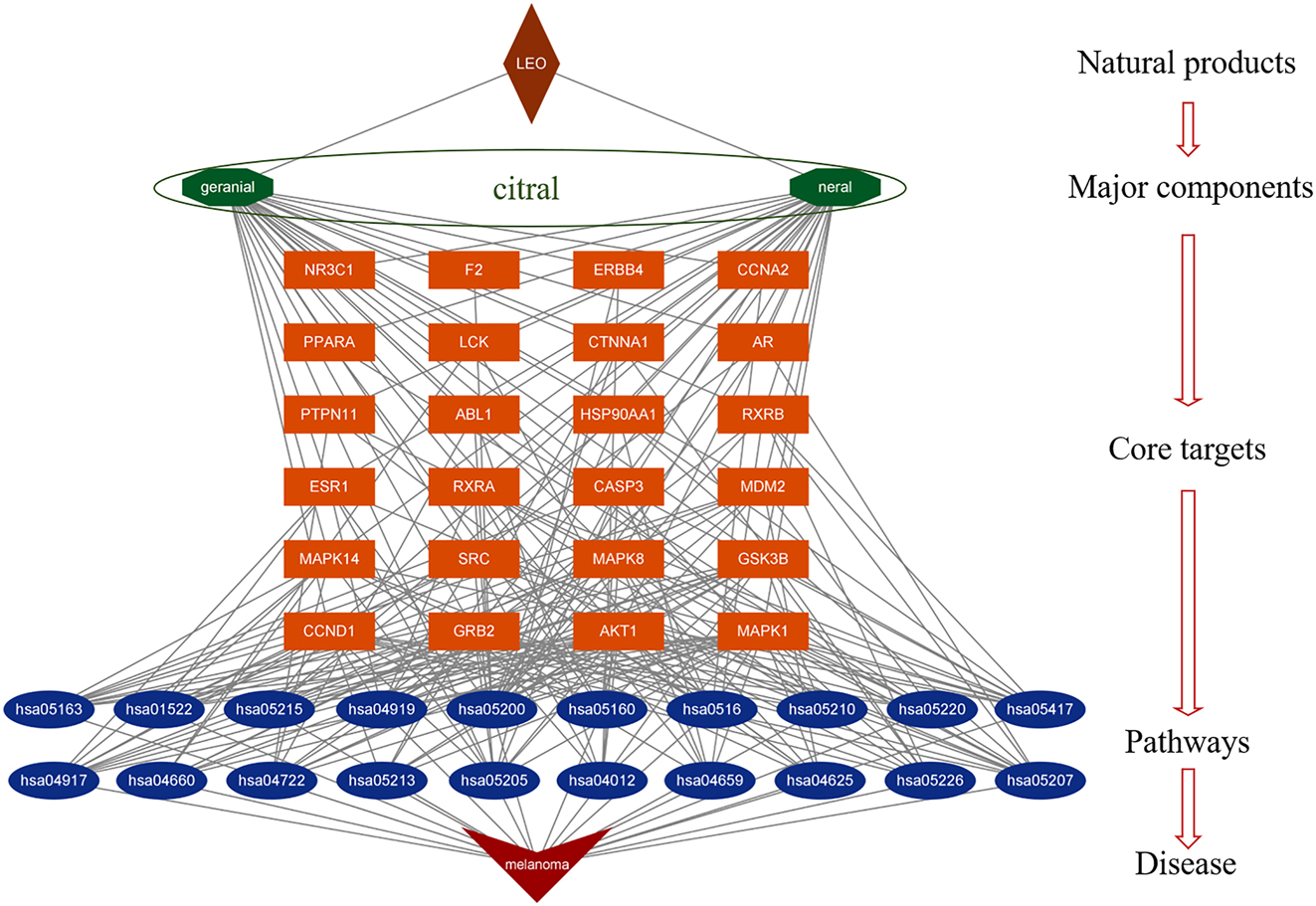
Natural products-major components-core targets-pathways-disease (NMCPD) network of LEO anti-melanoma. The NMCPD network of LEO anti-melanoma was composed of 2 components, 24 common targets, and 20 pathways, covering 48 nodes and 244 edges. The brown color represents LEO; green represents main bioactive compounds from LEO; orange represents target genes; blue represents signal pathways; red represents disease.
Specifically, the NMCPD system network suggests that several targets regulated by neral and geranial are closely associated with melanoma signaling pathways, such as pathways in cancer (hsa05200), proteoglycans in cancer (hsa05205), chemical carcinogenesis - receptor activation (hsa05207). Pathways in cancer (hsa05200) signal pathways were chosen for further investigation using the KEGG Mapper. The effects of neral and geranial on melanoma were visualized as a signaling pathway diagram in hsa05200 as shown in Figure 7. The results showed that citral could regulate 17 effective targets, which accounted for 71 % of the 20 shared targets between the active compounds and melanoma. These targets were distributed throughout the pathway and played significant roles in proliferation, survival, differentiation, apoptosis and malignancy of melanogenesis. The findings highlight the potential of LEO and citral as promising therapeutic agents for the treatment of melanoma.

The pathways in the cancer signaling pathway. The red boxes indicate the interactive targets.
To fully leverage the therapeutic potential of citral, further research is necessary to gain a more comprehensive understanding of the mechanisms by which it interacts with key targets, and to develop effective therapeutic strategies based on these insights. The development of such approaches could lead to the identification of novel therapies for combating melanoma, and improve outcomes for patients affected by this disease.
Overall, the anti-melanoma mechanism of natural products such as LEO and citral is characterized by multiple ingredients, multiple targets, and multiple mechanisms. This information will be valuable in developing new therapeutic strategies for melanoma treatment.
Molecular docking
We employed molecular docking analysis to investigate the binding interactions between the active components of LEO and their corresponding target genes. The binding energies were classified based on the conventional ligand binding activity scoring system, where binding energy >−4 kcal/mol was regarded as weak or no binding, −7 kcal/mol<binding energy ≤−4 kcal/mol was considered moderate binding, and binding energy ≤−7 kcal/mol was considered strong binding [53]. The 10 hub target proteins, including MAPK1, SRC, AKT1, HSP90AA1, GRB2, PTPN11, MAPK14, RXRA, LCK, and ESR1, were docked with neral and geranial to validate the predicted potential targets proteins. The structural dissimilarities between the neural and geranial structures docked with the same target were analyzed using RMSD analysis, where an RMSD value of 0 Å indicated identical structures. However, as depicted in Figure 6A1–J1, the RMSD analysis revealed that the neural and geranial structures docked with the same target were not identical, indicating notable structural differences between them.
Furthermore, as shown in Table 4, the molecular docking results showed that neral had a strong binding affinity with all the targets, with binding energies below −4 kcal/mol. Geranial, on the other hand, exhibited weak binding affinities with SRC and AKT1, as indicated by binding energies above −4 kcal/mol. However, geranial exhibited moderate binding activity with the remaining eight targets, with binding energies below −4 kcal/mol. Both neral and geranial displayed a relatively strong binding affinity with RXRA and ESR1, with binding energies close to −7 kcal/mol. The molecular docking results of neral and geranial with the 10 hub target proteins were visually analyzed using PyMOL software. As shown in Figure 8, the aldehyde group of neral and geranial formed conventional hydrogen bonds with amino acid residues in different proteins, including Thr183 of SRC (PDB ID: 1A07), Thr 347 of ESR1 (PDB ID: 1A52), Met 109 of MAPK14 (PDB ID: 1A9U), Lys 109 of GRB2 (PDB ID: 1BM2), Thr 184 of HSP90AA1 (PDB ID: 1BYQ), and Lys 54 of MAPK1 (PDB ID: 1PME). Neral formed a conventional hydrogen bond with Thr 183 of RXRA (PDB ID: 1FBY), whereas geranial formed a conventional hydrogen bond with Agr 316 of RXRA and a carbon hydrogen bond with Ser 312 of RXRA. Additionally, neral formed a conventional hydrogen bond with Met 109 of MAPK14, whereas geranial formed a conventional hydrogen bond with Leu 108 of MAPK14 and a carbon hydrogen bond with Met 109 of MAPK14. Furthermore, neral and geranial interacted with amino acid residues in LCK (PDB ID: 1BHF) and PTPN11 (PDB ID: 3B7O) through two conventional hydrogen bonds with Ser 158 and Glu 157, and Arg 465 and Gln 510, respectively. Finally, neral and geranial formed three and four conventional hydrogen bonds, respectively, with Glu 17, Ile 19, Agr 23, and Tyr 18 of AKT1 (PDB ID: 1H10).
Binding energy values of citral and hub targets.
| No. | Core targets | PDB ID | Binding energy, kcal/mol | |
|---|---|---|---|---|
| Neral (cis-citral) | Geranial (trans-citral) | |||
| 1 | MAPK1 | 1PME | −5.5 | −5.6 |
| 2 | SRC | 1A07 | −4.3 | −3.9 |
| 3 | AKT1 | 1H10 | −4.2 | −3.8 |
| 4 | HSP90AA1 | 1BYQ | −5.0 | −5.1 |
| 5 | GRB2 | 1BM2 | −4.5 | −4.6 |
| 6 | PTPN11 | 3B7O | −4.4 | −4.7 |
| 7 | MAPK14 | 1A9U | −5.0 | −5.0 |
| 8 | RXRA | 1FBY | −6.5 | −6.8 |
| 9 | LCK | 1BHF | −4.0 | −4.4 |
| 10 | ESR1 | 1A52 | −5.9 | −6.0 |
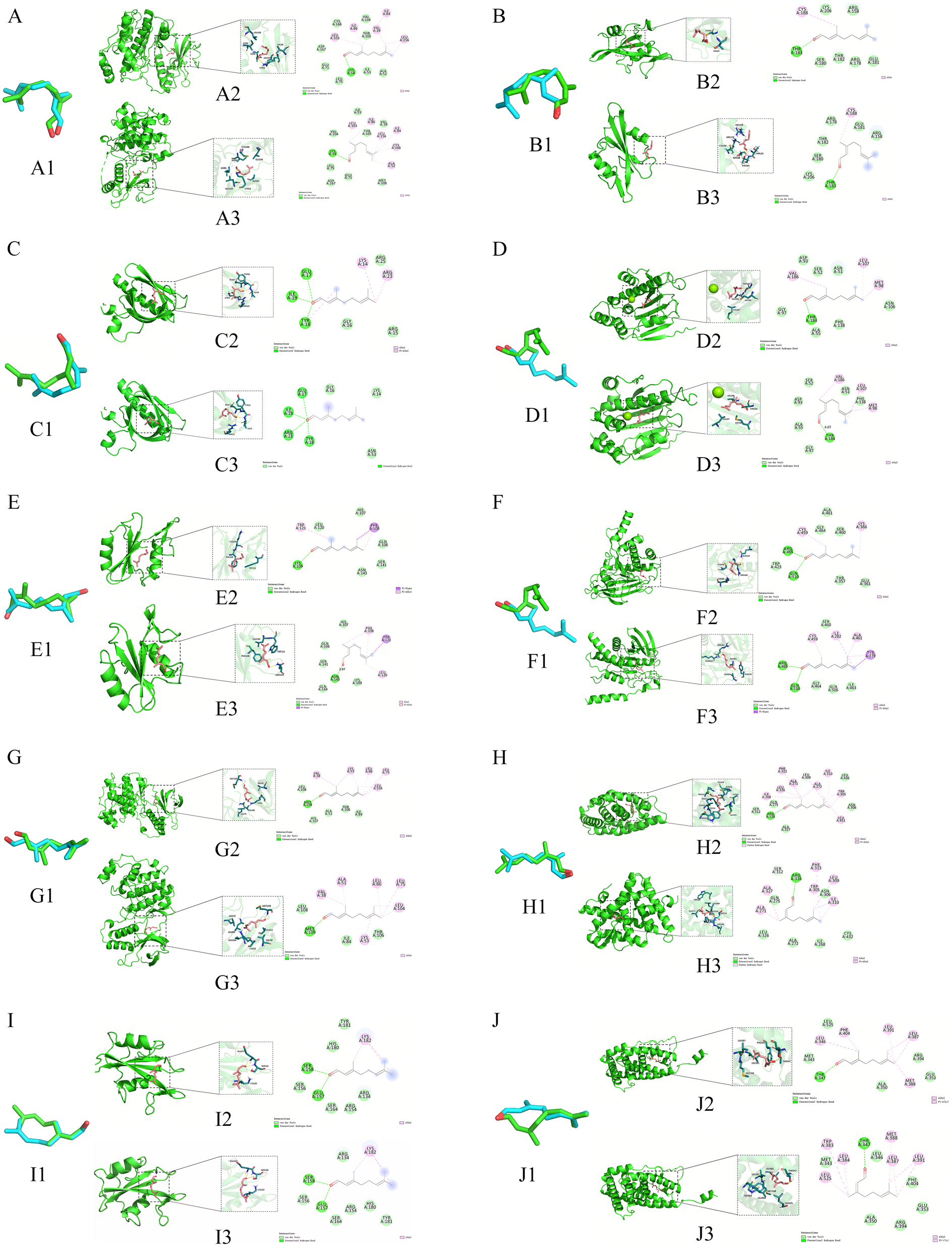
Virtual docking of neral and citral with A-MAPK1 (neral-A2, geranial-A3), B-SRC (neral-B2, geranial-B3), C-AKT1 (neral-C2, geranial-C3), D-HSP90AA1 (neral-D2, geranial-D3), E-GRB2 (neral-E2, geranial-E3), F-PTPN11 (neral-F2, geranial-F3), G-MAPK14 (neral-G2, geranial-G3),H- RXRA (neral-H2, geranial-H3), I-LCK (neral-I2, geranial-I3), and J-ESR1 (neral-J2, geranial-J3).
Based on the docking results, it can be inferred that both neral and geranial have the ability to effectively bind to the active pocket of the receptor protein, thereby forming a stable binding conformation [47]. This suggests that both neral and geranial hold promise as therapeutic agent and could potentially be developed further for medicinal purposes. However, it appears that geranial may require additional structural optimization in order to enhance its binding affinity with specific targets. Overall, these findings are encouraging and highlight the potential of these compounds for medicinal use.
Conclusions
LEO, rich in citral, has a long history of use in traditional Chinese medicine. Citral has been shown to have antiproliferative effects on melanoma cells. However, there is a lack of data on the efficacy of LEO and citral in treating melanoma and their mechanisms of action. Based on components analysis, network pharmacology predictions, and preliminary cell experiments, this study suggests that citral is the main active component of LEO with anti-melanoma activity. Citral may act on MAPK1, AKT1 and other proteins as core targets, mainly through signaling pathways such as pathways in cancer, proteoglycans in cancer, prolactin and thyroid hormone to exert its therapeutic effect on melanoma. These findings not only provide initial evidence for the validity and scientific rigor of network pharmacology analysis, but also offer insights and directions for further research into the anti-melanoma mechanism of LEO.
Funding source: Young and Middle-aged Academic and Technological Leaders of Yunnan Province
Award Identifier / Grant number: 202205AC160049
Funding source: Foundation of State Key Laboratory of Utilization of Woody Oil Resource
Award Identifier / Grant number: GZKF202109
Acknowledgment
We extend our sincere appreciation to State Key Laboratory of Utilization of Woody Oil Resource and Science and Technology Department of Hunnan Province and for their invaluable contributions to this research. Their Financial support has played a pivotal role in the successful completion of this study. Our heartfelt thanks go to Forestry Natural Products Development & Utilization Innovation Team for their experimental platform, without which the accomplishment of this research would not have been possible.
-
Research ethics: Not applicable.
-
Author contributions: The authors confirm their contribution to the paper as follows: study conception and design: X. Q. Yang, R. K. Liu and P. Zhao; data collection: M. Z. Liu and K. J. Han; analysis and interpretation of results: X. Q. Yang, M. Z. Liu, K. J. Han, L. Li, S. S. Liu, and B. Yang; draft manuscript preparation: M. Z. Liu and K. J. Han. All authors reviewed the results and approved the final version of the manuscript.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Conflicts of interest: The authors declare that we have no conflicts of interest to report regarding the present study.
-
Research funding: Financial support from the Foundation of State Key Laboratory of Utilization of Woody Oil Resource (Grant No. GZKF202109) and Young and Middle-aged Academic and Technological Leaders of Yunnan Province (No. 202205AC160049) is gratefully acknowledged.
-
Data availability: The data used and analyzed during the current study are available from the corresponding author on reasonable request.
References
1. Qiu, Y, Yu, Y, Lan, P, Wang, Y, Li, Y. An overview on total valorization of Litsea cubeba as a new woody oil plant resource toward a zero-waste biorefinery. Molecules 2021;26:3948. https://doi.org/10.3390/molecules26133948.Search in Google Scholar PubMed PubMed Central
2. Sivamaruthi, BS, Kesika, P, Chaiyasut, C. The composition, pharmacological and economic importance of essential oil of Litsea cubeba (Lour.) Pers. Food Sci Tech 2022;42:e35720. https://doi.org/10.1590/fst.35720.Search in Google Scholar
3. Hao, K, Xu, B, Zhang, G, Si, H. Antibacterial activity and mechanism of Litsea cubeba L. essential oil against Acinetobacter baumannii. Nat Prod Commun 2021;16:3. https://doi.org/10.1177/1934578x21999146.Search in Google Scholar
4. Borotová, P, Galovičová, L, Vukovic, NL, Vukic, M, Kunová, S, Hanus, P, et al.. Role of Litsea cubeba essential oil in agricultural products safety: antioxidant and antimicrobial applications. Plants 2022;11:1504. https://doi.org/10.3390/plants11111504.Search in Google Scholar PubMed PubMed Central
5. Wang, X, Gao, M, Wu, L, Zhao, Y, Wang, Y, Chen, Y. Antimicrobial activity of essential oils extracted from Litsea cubeba. For Res 2022;2:2. https://doi.org/10.48130/fr-2022-0002.Search in Google Scholar
6. Chen, CJ, Tseng, YH, Chu, FH. Neuropharmacological activities of fruit essential oil from Litsea cubeba Persoon. J Wood Sci 2012;58:538–43. https://doi.org/10.1007/s10086-012-1277-3.Search in Google Scholar
7. Barua, KN, Dutta, NB, Hazarika, P, Borah, P, Hazarika, P, Saikia, NJ, et al.. Variation in yield of essential oil from different population of Litsea cubeba in North East India with emphasis on identification of industrially adoptable elite genotypes. J Non-Timber For Prod 2022;29:65–9. https://doi.org/10.54207/bsmps2000-2022-4gb250.Search in Google Scholar
8. Gao, M, Chen, Y, Wang, Y. Evaluation of the yields and chemical compositions of the essential oils of different Litsea cubeba varieties. J Essent Oil Bear Plants 2016;19:1888–902. https://doi.org/10.1080/0972060x.2016.1252695.Search in Google Scholar
9. Fan, GR, Ning, XD, Chen, SX, Zhong, L, Guo, CC, Yang, YL, et al.. Differences in fruit yields and essential oil contents and composition among natural provenances of Litsea cubeba in China and their relationships with main habitat factors. Ind Crop Prod 2023;194:116285. https://doi.org/10.1016/j.indcrop.2023.116285.Search in Google Scholar
10. Guo, Y, Li, Y, Li, Z, Jiang, L, Cao, X, Gao, W, et al.. Deep eutectic solvent-homogenate based microwave-assisted hydrodistillation of essential oil from Litsea cubeba (Lour.) Pers. fruits and its chemical composition and biological activity. J Chromatogr A 2021;1646:462089. https://doi.org/10.1016/j.chroma.2021.462089.Search in Google Scholar PubMed
11. Sharma, S, Habib, S, Sahu, D, Gupta, J. Chemical properties and therapeutic potential of citral, a monoterpene isolated from lemongrass. Med Chem 2021;17:2–12. https://doi.org/10.2174/15734064mtazmmjyc2.Search in Google Scholar
12. She, QH, Li, WS, Jiang, YY, Wu, YC, Zhou, YH, Zhang, L. Chemical composition, antimicrobial activity and antioxidant activity of Litsea cubeba essential oils in different months. Nat Prod Commun 2020;34:3285–8. https://doi.org/10.1080/14786419.2018.1557177.Search in Google Scholar PubMed
13. Najar, B, Shortrede, JE, Pistelli, L, Buhagiar, J. Chemical composition and in vitro cytotoxic screening of sixteen commercial essential oils on five cancer cell lines. Chem Biodiversity 2019;17:e1900478. https://doi.org/10.1002/cbdv.201900478.Search in Google Scholar PubMed
14. Ahmed, B, Qadir, MI, Ghafoor, S. Malignant melanoma: skin cancer - diagnosis, prevention and treatment. Crit Rev Eukaryotic Gene Expression 2020;30:291–7. https://doi.org/10.1615/critreveukaryotgeneexpr.2020028454.Search in Google Scholar PubMed
15. Teixido, C, Castillo, P, Martinez-Vila, C, Arance, A, Alos, L. Molecular markers and targets in melanoma. Cells 2021;10:2320. https://doi.org/10.3390/cells10092320.Search in Google Scholar PubMed PubMed Central
16. Namikawa, K, Yamazaki, N. Targeted therapy and immunotherapy for melanoma in Japan. Curr Treat Options Oncol 2019;20:7. https://doi.org/10.1007/s11864-019-0607-8.Search in Google Scholar PubMed PubMed Central
17. Leonardi, GC, Falzone, L, Salemi, R, Zanghì, A, Spandidos, DA, Mccubrey, JA, et al.. Cutaneous melanoma: from pathogenesis to therapy (review). Int J Oncol 2018;52:1071–80. https://doi.org/10.3892/ijo.2018.4287.Search in Google Scholar PubMed PubMed Central
18. Guo, W, Wang, H, Li, C. Signal pathways of melanoma and targeted therapy. Signal Transduct Targeted Ther 2021;6:424. https://doi.org/10.1038/s41392-021-00827-6.Search in Google Scholar PubMed PubMed Central
19. Mishra, H, Mishra, PK, Ekielski, A, Jaggi, M, Iqbal, Z, Talegaonkar, S. Melanoma treatment: from conventional to nanotechnology. J Cancer Res Clin Oncol 2018;144:2283–302. https://doi.org/10.1007/s00432-018-2726-1.Search in Google Scholar PubMed
20. El-Harakeh, M, Al-Ghadban, S, Safi, R. Medicinal plants towards modeling skin cancer. Curr Drug Targets 2021;22:148–61. https://doi.org/10.2174/1389450121666201005103521.Search in Google Scholar PubMed
21. Biswas, R, Chowdhury, N, Mukherjee, R, Bagchi, A. Identification and analyses of natural compounds as potential inhibitors of TRAF6-basigin interactions in melanoma using structure-based virtual screening and molecular dynamics simulations. J Mol Graph Model 2018;85:281–93. https://doi.org/10.1016/j.jmgm.2018.09.008.Search in Google Scholar PubMed
22. Cho, H, Shen, Q, Zhang, L, Okumura, M, Kawakami, A, Ambrose, J. Cyp27a1-dependent anti-melanoma activity of limonoid natural products targets mitochondrial metabolism. Cell Chem Biol 2021;28:1407–19. https://doi.org/10.1016/j.chembiol.2021.03.004.Search in Google Scholar PubMed PubMed Central
23. Montuori, E, Capalbo, A, Lauritano, C. Marine compounds for melanoma treatment and prevention. Int J Mol Sci 2022;23:10284. https://doi.org/10.3390/ijms231810284.Search in Google Scholar PubMed PubMed Central
24. Danciu, C, Soica, C, Antal, D, Alexa, E, Pavel, IZ, Ghiulai, R, et al.. Natural compounds in the chemoprevention of malignant melanoma. Anti Cancer Agents Med Chem 2018;8:631–44. https://doi.org/10.2174/1871520617666171121142522.Search in Google Scholar PubMed
25. Seo, SY, Sharma, VK, Sharma, N. Mushroom tyrosinase: recent prospects. J Agric Food Chem 2003;51:2837–53. https://doi.org/10.1021/jf020826f.Search in Google Scholar PubMed
26. Qu, Y, Zhan, Q, Du, S, Ding, Y, Fang, B, Du, W. Catalysis-based specific detection and inhibition of tyrosinase and their application. J Pharm Anal 2020;10:414–25. https://doi.org/10.1016/j.jpha.2020.07.004.Search in Google Scholar PubMed PubMed Central
27. Liu, SC, Sheu, ML, Tsai, YC, Lin, YC, Chang, CW, Lai, DW. Attenuation of in vitro and in vivo melanin synthesis using a Chinese herbal medicine through the inhibition of tyrosinase activity. Phytomedicine 2022;95:153876. https://doi.org/10.1016/j.phymed.2021.153876.Search in Google Scholar PubMed
28. Huang, XW. Chemical composition and tyrosinase inhibition activity and antioxidant activities of three essential oils [Ph.D. Thesis]. Wuhai, Hubei: Huazhong University of Science and Technology; 2013.Search in Google Scholar
29. Sanches, LJ, Marinello, PC, Panis, C, Fagundes, TR, Morgado-Díaz, JA, de-Freitas-Junior, JC, et al.. Cytotoxicity of citral against melanoma cells: the involvement of oxidative stress generation and cell growth protein reduction. Tumor Biol 2017;39:1–15. https://doi.org/10.1177/1010428317695914.Search in Google Scholar PubMed
30. Mahdavi, A, Mohammadsadeghi, N, Mohammadi, F, Saadati, F, Nikfard, S. Evaluation of inhibitory effects of some novel phenolic derivatives on the mushroom tyrosinase activity: insights from spectroscopic analyses, molecular docking and in vitro assays. Food Chem 2010;387:132938. https://doi.org/10.1016/j.foodchem.2022.132938.Search in Google Scholar PubMed
31. Trott, O, Olson, AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 2010;31:455–61. https://doi.org/10.1002/jcc.21334.Search in Google Scholar PubMed PubMed Central
32. Wang, K, Luo, Q, Zhang, Y, Xie, X, Cheng, W, Yao, Q, et al.. LINC01296 promotes proliferation of cutaneous malignant melanoma by regulating miR-324-3p/MAPK1 axis. Aging 2022;15:2877–90. https://doi.org/10.18632/aging.204413.Search in Google Scholar PubMed PubMed Central
33. Banik, I, Cheng, PF, Dooley, CM, Travnickova, J, Merteroglu, M, Dummer, R, et al.. NRASQ61K melanoma tumor formation is reduced by p38-MAPK14 activation in zebrafish models and NRAS-mutated human melanoma cells. Pigm Cell Melanoma Res 2021;34:150–62. https://doi.org/10.1111/pcmr.12925.Search in Google Scholar PubMed
34. Maruoka, T, Kitanaka, A, Kubota, Y, Yamaoka, G, Kameda, T, Imataki, O. Lemongrass essential oil and citral inhibit Src/Stat3 activity and suppress the proliferation/survival of small-cell lung cancer cells, alone or in combination with chemotherapeutic agents. Int J Clin Oncol 2018;52:1738–48. https://doi.org/10.3892/ijo.2018.4314.Search in Google Scholar PubMed
35. Ouyang, FZ, Wu, RQ, Wei, Y, Liu, RX, Yang, D, Xiao, X, et al.. Dendritic cell-elicited B-cell activation fosters immune privilege via IL-10 signals in hepatocellular carcinoma. Nat Commun 2016;7:13453. https://doi.org/10.1038/ncomms13453.Search in Google Scholar PubMed PubMed Central
36. Wang, F, Zheng, A, Zhang, D, Zou, T, Xiao, M, Chen, J, et al.. Molecular profiling of core immune-escape genes highlights LCK as an immune-related prognostic biomarker in melanoma. Front Immunol 2022;13:1024931. https://doi.org/10.3389/fimmu.2022.1024931.Search in Google Scholar PubMed PubMed Central
37. Zhao, JJ, Zeng, X, Song, P, Wu, XH, Shi, HB. Akt1 as the pagerank hub gene is associated with melanoma and its functional annotation is highly related to the estrogen signaling pathway that may regulate the growth of melanoma. Oncol Rep 2016;36:2087–93. https://doi.org/10.3892/or.2016.5048.Search in Google Scholar PubMed
38. Liu, H, Zhang, Z, Huang, Y, Wei, W, Ning, S, Li, J. Plasma HSP90AA1 predicts the risk of breast cancer onset and distant metastasis. Front Cell Dev Biol 2021;9:639596. https://doi.org/10.3389/fcell.2021.639596.Search in Google Scholar PubMed PubMed Central
39. Hao, S, Li, S, Wang, J, Zhao, L, Zhang, C, Huang, W. Phycocyanin reduces proliferation of melanoma cells through downregulating GRB2/ERK signaling. J Agric Food Chem 2018;66:10921–9. https://doi.org/10.1021/acs.jafc.8b03495.Search in Google Scholar PubMed
40. Polubothu, S, Bender, N, Muthiah, S, Zecchin, D, Demetriou, C, Martin, SB, et al.. PTPN11 mosaicism causes a spectrum of pigmentary and vascular neurocutaneous disorders and predisposes to melanoma. J Invest Dermatol 2023;143:1042–51.e3. https://doi.org/10.1016/j.jid.2022.09.661.Search in Google Scholar PubMed PubMed Central
41. Chen, M, Li, Y, Sun, X, Zhang, B, Li, W, Wang, S, et al.. Grb2-associated binder 2 expression and its roles in uveal melanoma invasion. Mol Med Rep 2017;16:4577–82. https://doi.org/10.3892/mmr.2017.7151.Search in Google Scholar PubMed PubMed Central
42. Cao, Y, Duan, H, Su, A, Xu, L, Lai, B. A pan-cancer analysis confirms PTPN11’s potential as a prognostic and immunological biomarker. Aging 2022;14:5590–610. https://doi.org/10.18632/aging.204171.Search in Google Scholar PubMed PubMed Central
43. Yin, J, Liu, H, Yi, X, Wu, W, Amos, CI, Fang, S, et al.. Genetic variants in the Vitamin D pathway genes VDBP and RXRA modulate cutaneous melanoma disease-specific survival. Pigm Cell Melanoma Res 2016;29:176–85. https://doi.org/10.1111/pcmr.12437.Search in Google Scholar PubMed PubMed Central
44. Dika, E, Patrizi, A, Lambertini, M, Manuelpillai, N, Fiorentino, M, Altimari, A, et al.. Estrogen receptors and melanoma: a review. Cells 2019;8:1463. https://doi.org/10.3390/cells8111463.Search in Google Scholar PubMed PubMed Central
45. Hsin, KY, Ghosh, S, Kitano, H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One 2013;8:e83922. https://doi.org/10.1371/journal.pone.0083922.Search in Google Scholar PubMed PubMed Central
46. Berthenet, K, Ferrer, CC, Fanfone, D, Popgeorgiev, N, Neves, D, Bertolino, P, et al.. Failed apoptosis enhances melanoma cancer cell aggressiveness. Cell Rep 2020;31:107731. https://doi.org/10.1016/j.celrep.2020.107731.Search in Google Scholar PubMed
47. Tzanakakis, G, Giatagana, EM, Kuskov, A, Berdiaki, A, Tsatsakis, A, Neagu, M, et al.. Proteoglycans in the pathogenesis of hormone-dependent cancers: mediators and effectors. Cancers 2020;12:2401. https://doi.org/10.3390/cancers12092401.Search in Google Scholar PubMed PubMed Central
48. Slominski, AT, Zmijewski, MA, Semak, I, Zbytek, B, Pisarchik, A, Li, W, et al.. Cytochromes p450 and skin cancer: role of local endocrine pathways. Anti-Cancer Agent Me 2014;14:77–96. https://doi.org/10.2174/18715206113139990308.Search in Google Scholar PubMed PubMed Central
49. Handler, MZ, Ross, AL, Shiman, MI, Elgart, GW, Grichnik, JM. Potential role of human growth hormone in melanoma growth promotion. Arch Dermatol 2012;148:1179–82. https://doi.org/10.1001/archdermatol.2012.2149.Search in Google Scholar PubMed
50. Liu, S, Zhao, P, Chen, Y. ERBB1/2/3 expression, prognosis, and immune infiltration in cutaneous melanoma. Front Genet 2021;12:602160. https://doi.org/10.3389/fgene.2021.602160.Search in Google Scholar PubMed PubMed Central
51. Lazzara, DR, Zarkhin, SG, Rubenstein, SN, Glick, BP. Melanoma and thyroid carcinoma: our current understanding. J Clin Aesthet Dermatol 2019;12:39–41.Search in Google Scholar
52. Oakley, GM, Curtin, K, Layfield, L, Jarboe, E, Buchmann, LO, Hunt, JP. Increased melanoma risk in individuals with papillary thyroid carcinoma. JAMA Otolaryngol Head Neck Surg 2014;140:423–7. https://doi.org/10.1001/jamaoto.2014.78.Search in Google Scholar PubMed
53. Yang, G, Liu, Y, Liu, Y, Ma, Y, Li, Y, Chen, J. Integrating network pharmacology and an experimental validation strategy elucidates the protective effect and mechanism of callicarpa nudiflora against neuroinflammation. RSC Adv 2022;12:31124–41. https://doi.org/10.1039/d2ra05143e.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/oncologie-2023-0579).
© 2024 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Autophagy and radiotherapy in esophageal cancer: modulating treatment sensitivity and overcoming challenges
- Functions of CAFs in microenvironment of non-small cell lung cancer: based on updated hallmarks of cancer
- Cisplatin-induced pyroptosis: a double-edged sword in cancer treatment
- Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
- Research Articles
- A lipid metabolism-related gene model reveals the prognosis and immune microenvironment of cutaneous melanoma
- Boanmycin induces apoptosis and overcomes venetoclax resistance in acute myeloid leukemia
- Identification of GNB1 as a downstream effector of the circRNA-0133711/miR-145-5p axis involved in breast cancer proliferation and metastasis
- Clinical characteristics and prognosis of lung metastases from unknown primary cancer sites
- Integrating bulk-RNA and single-cell analysis reveals heterogeneous expression of cuproptosis-related sorafenib-resistant genes in hepatocellular carcinoma
- Exploring the mechanism of genistein in treating hepatocellular carcinoma through network pharmacology and molecular docking
- Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
- Exploring the anti-lung cancer mechanism of Ganoderma lucidum and its relationship with the level of immune cell infiltration based on network pharmacology and molecular docking
- Constructing a prognostic model for colon cancer patients on basis of coagulation genes enriched in cancer-associated fibroblasts to guide personalized immunotherapy
- Rapid Communication
- Evaluation of HER2 immunohistochemistry-positive and immunohistochemistry-negative FISH amplification breast cancers using next-generation sequencing
- Short Commentary
- Unveiling the unexplored: shedding light on a novel aspect of colorectal carcinoma
Articles in the same Issue
- Frontmatter
- Review Articles
- Autophagy and radiotherapy in esophageal cancer: modulating treatment sensitivity and overcoming challenges
- Functions of CAFs in microenvironment of non-small cell lung cancer: based on updated hallmarks of cancer
- Cisplatin-induced pyroptosis: a double-edged sword in cancer treatment
- Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
- Research Articles
- A lipid metabolism-related gene model reveals the prognosis and immune microenvironment of cutaneous melanoma
- Boanmycin induces apoptosis and overcomes venetoclax resistance in acute myeloid leukemia
- Identification of GNB1 as a downstream effector of the circRNA-0133711/miR-145-5p axis involved in breast cancer proliferation and metastasis
- Clinical characteristics and prognosis of lung metastases from unknown primary cancer sites
- Integrating bulk-RNA and single-cell analysis reveals heterogeneous expression of cuproptosis-related sorafenib-resistant genes in hepatocellular carcinoma
- Exploring the mechanism of genistein in treating hepatocellular carcinoma through network pharmacology and molecular docking
- Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
- Exploring the anti-lung cancer mechanism of Ganoderma lucidum and its relationship with the level of immune cell infiltration based on network pharmacology and molecular docking
- Constructing a prognostic model for colon cancer patients on basis of coagulation genes enriched in cancer-associated fibroblasts to guide personalized immunotherapy
- Rapid Communication
- Evaluation of HER2 immunohistochemistry-positive and immunohistochemistry-negative FISH amplification breast cancers using next-generation sequencing
- Short Commentary
- Unveiling the unexplored: shedding light on a novel aspect of colorectal carcinoma

