Abstract
Esophageal cancer (ESCA) is one of the most fatal gastrointestinal cancers worldwide. ESCA is often diagnosed in its middle or late stages since the first symptoms are not identifiable. The use of radiotherapy, either alone or in conjunction with surgical intervention and chemotherapy, is essential to achieve a positive prognosis. Radiotherapy is an essential component of treatment for ESCA. Autophagy, a prevalent biological phenomenon, has a twofold impact on the incidence, progression, and treatment response of malignant tumors. This review explores the intricate mechanisms by which autophagy modulates radiation sensitivity in ESCA, including its effects on DNA repair, oxidative stress responses, and apoptosis. We provide a comprehensive analysis of recent advancements in the modulation of autophagy, focusing on the use of autophagy inhibitors and inducers to enhance radiotherapy efficacy. We discuss how autophagy inhibitors such as chloroquine and 3-methyladenine can overcome radiation resistance by blocking autophagic processes, while autophagy inducers like rapamycin can sensitize cancer cells to radiotherapy-induced cell death. Additionally, we examine the potential therapeutic benefits of combining autophagy regulation with existing treatment modalities, offering new strategies to improve patient outcomes. This review highlights the critical role of autophagy in ESCA and underscores the promise of autophagy-targeted therapies in enhancing the effectiveness of radiotherapy, thereby providing a novel avenue for overcoming treatment resistance and improving prognosis in ESCA patients.
Introduction
Esophageal cancer (ESCA) is one of the deadliest gastrointestinal tract cancers in the world. It is also the eighth most common cancer globally. It ranks sixth in mortality and seventh in worldwide incidence, which is especially concerning in terms of death. Crucially, the yearly incidence rate of ESCA continues to rise [1]. By 2023, the estimated number of new cases in the United States will be 21,560, with 16,120 fatalities [2]. Esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) are the principal forms of ESCA. Squamous cell carcinoma constitutes 85 % of all ESCA globally and is the most prevalent subtype [3]. The five-year relative survival rate of the ESCA patient population is approximately 21 % [2]. China has a high incidence of ESCA, accounting for approximately half of all cases worldwide in terms of both new and fatal cases [4].
Owing to the nonspecific early symptoms of ESCA, 80–90 % of patients are diagnosed at an advanced stage, which greatly limits the effectiveness of treatment and increases its complexity. Radiation therapy is one of the key strategies for treating ESCA, but treatment outcomes are often affected by tumor resistance to radiotherapy, especially in locally advanced patients [5].
Radiation therapy is a major method for treating ESCA, but its challenge lies in the varied responses of tumor cells to treatment, resulting in significant radiation resistance in some patients [6]. This resistance is primarily driven by multiple biological mechanisms, including enhanced DNA repair capabilities, regulated oxidative stress responses, abnormal autophagic activity, inhibition of cell apoptosis, and epithelial-mesenchymal transition [7]. These complex resistance mechanisms significantly reduce the efficacy of radiation therapy, highlighting the need for new treatment strategies to overcome these challenges [8]. Studies have shown that chemotherapy resistance and radiation resistance during treatment often coincide with abnormal autophagic activity, which is a major factor in reducing the efficacy of ESCA treatment [9]. Modulating autophagy can improve this issue to some extent.
Autophagy is a protective cellular response that not only maintains homeostasis in normal cells but also plays a protective role in malignant cells [10, 11]. Although the activation of autophagy can protect tumor cells from radiation damage, its specific role in different types of cancer remains unpredictable [12]. Furthermore, the normal function of autophagy is crucial for immune surveillance, aiding in antitumor responses and preventing tumor immune evasion [13]. Recent advances in research on autophagy and ESCA have revealed a close connection between autophagy and the onset and progression of ESCA. A more in-depth understanding of autophagy could provide new perspectives for the diagnosis and treatment of ESCA. This review explores the function of autophagy in the radiotherapeutic context of ESCA and summarizes the prospects of forthcoming treatment strategies.
Autophagy mechanisms and esophageal cancer
Introduction to the context of autophagy
Using electron microscopy in 1957, Clark identified many dense bodies with membrane-like structures in the epithelial cells of the proximal tubules in newborn mouse kidneys, which included cytoplasmic structures similar to mitochondria [14]. The Belgian scientist Christian de Duve first proposed the idea of cellular autophagy in 1963 and suggested that lysosomes are involved in the autophagic process. Therefore, he is acknowledged as the forefather of autophagy research [15]. The Japanese scientists Miki Tsukada and Yoshinori Ohsumi were the first to fully articulate and clarify the mechanism of autophagy. In 1993, Tsukada and Ohsumi reported on autophagy in yeast and used it as a model to identify many genes related to autophagy [16]. This marked the commencement of research on autophagy in humans. The discovery that almost all yeast autophagy-related genes have functional equivalents in higher eukaryotes, including humans, has greatly advanced the field of autophagy research. Klionsky et al. unified the names of these genes and called them autophagy-related genes (Atg) in 2003 so that scientists could better describe how their proteins interact with each other and how they work in the autophagic process [17].
The biological processes of autophagy
Autophagy initiates with the appearance of an isolation membrane (IM) in the cytoplasm, which then grows and expands. The IM encloses parts of the cytoplasm and organelles, proteins, and other elements to create an autophagosome. This then fuses with lysosomes to form an autophagolysosome. Proteolytic enzymes break down contents to renew some organelles and meet the metabolic needs of the cell (Figure 1). In summary, the autophagy process includes four stages: autophagy induction, vesicle nucleation, autophagosome formation, autophagolysosome formation, and degradation [18].
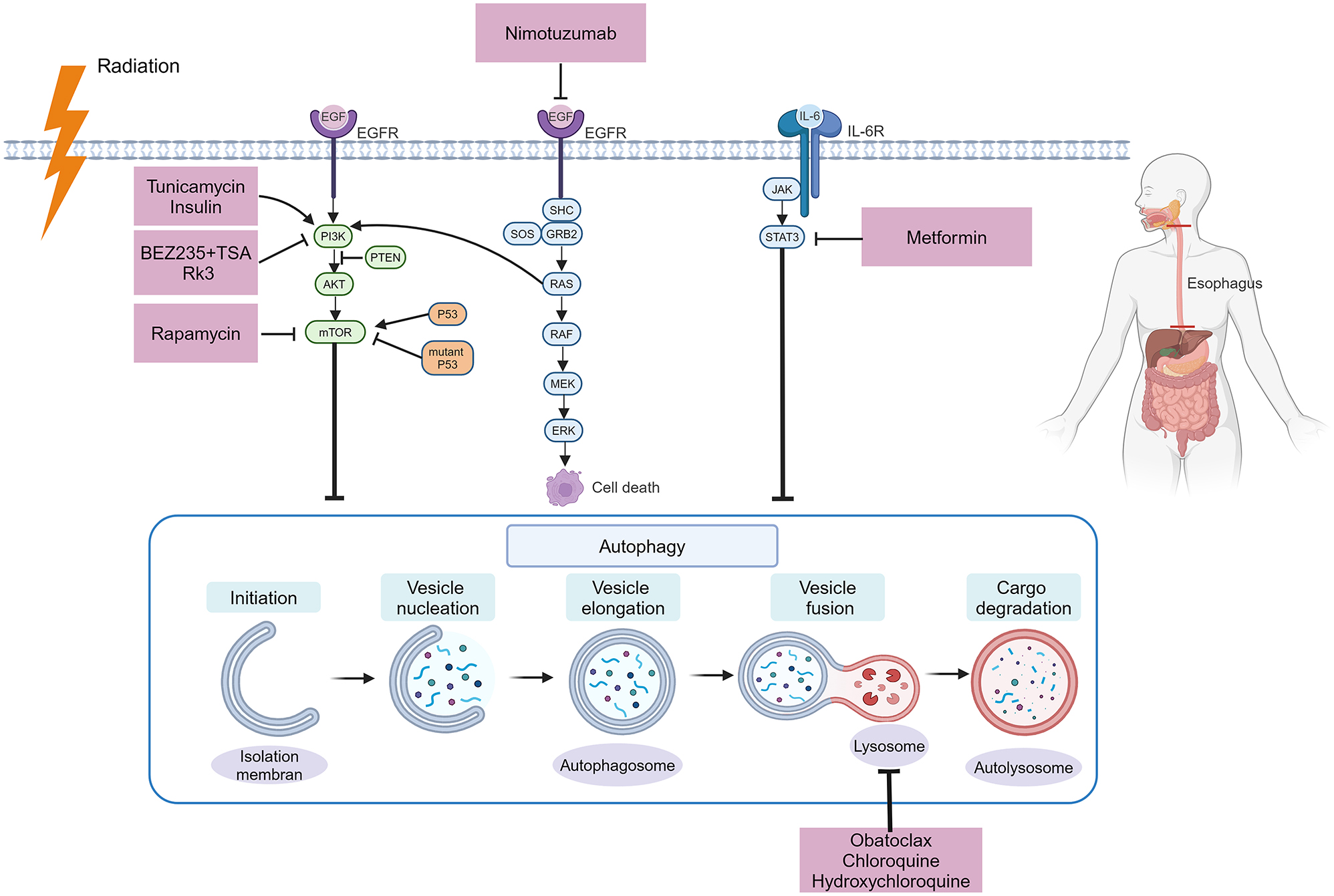
The process of autophagy and the application of autophagy modulators in the treatment of ESCA. Autophagy is a process consisting of several stages: Initiation, vesicle nucleation, vesicle elongation, vesicle fusion, and cargo degradation. Molecular pathways related to autophagy, such as the PI3K/Akt/mTOR, STAT3, and EGFR pathways, can be modulated by drugs. EGFR, epidermal growth factor receptor; GRB2, growth factor receptor-bound protein 2; IL-6, interleukin-6; JAK, Janus kinase; mTOR, mechanistic target of the rapamycin kinase; PI3K, phosphatidylinositol-3 kinase; STAT, signal transducer and activator of transcription (created with BioRender.com).
Based on the different pathways through which substrates enter lysosomes, three categories of autophagy have been identified: chaperone-mediated autophagy (CMA), microautophagy, and macroautophagy [19]. The most sophisticated form of cellular autophagy, known as macroautophagy, is commonly characterized. This is the variant with the most published research and is the primary subject of discussion in this article.
Autophagy in tumor occurrence and esophageal cancer development
Autophagy, an intracellular degradation and recycling mechanism, is crucial for maintaining the homeostasis of proteins and the functionality of organelles. In the process of tumorigenesis, autophagy plays a complex role [20]. On the one hand, by clearing damaged organelles and misfolded proteins, autophagy helps suppress tumor initiation. On the other hand, in established tumors, autophagy is essential for supporting cellular growth and increasing metabolic activity; thus, tumors rely on autophagy [21]. Autophagy plays a crucial role in tumorigenesis by maintaining cellular homeostasis and supporting tumor growth. Nunn and Guo demonstrated that systemic autophagy inhibition, achieved through inducible Atg5 knockdown, significantly reduced lung tumor growth and impaired tumor metabolism by decreasing glucose and lactate uptake. This inhibition also increased T-cell infiltration, promoting tumor cell killing, thus underscoring the dual role of autophagy in both sustaining tumor metabolism and facilitating immune evasion [22]. Research has indeed demonstrated that several types of human malignancies, including breast and prostate cancer, frequently exhibit a scarcity of autophagy-related genes, including Atg4 and Beclin1 [23, 24].
Several investigations have demonstrated that autophagy continuously provides cellular protection throughout the whole tumor growth process. Its primary characteristic, which is mostly related to changes in its protective targets, is that it has contrasting functions in the early and late phases of cancer promotion and repression. At the onset of tumorigenesis, autophagy chiefly acts by curbing inflammation, maintaining cellular genomic stability, fostering the aging of damaged cells, and restraining p62 accumulation, thus safeguarding the equilibrium of normal cells and impeding their conversion to cancerous cells. During the advanced stages of tumor formation, primary cancerous cells are established. This phase largely entails the persistent growth and evolution of initial tumor cells in a relatively nutrient-scarce environment, culminating in tumor development. Autophagy principally contributes to this process by assisting tumor cells in resisting intrinsic and extrinsic pressures and bolstering their robust metabolic mode, thereby enhancing their survival and proliferation [18, 25], [26], [27], [28].
During the development of tumors, the role of autophagy is even more complex. Autophagy not only functions within tumor cells (intrinsic role) but also plays a role in stromal cells within the tumor microenvironment (extrinsic role), affecting tumor growth and drug resistance. For instance, autophagy supports growth and metabolism in tumor cells, whereas in the tumor microenvironment, autophagy promotes tumor development by influencing the functions of cancer-associated fibroblasts (CAFs) [29]. In the tumor microenvironment, autophagy plays a significant role in regulating the metabolic adaptation of tumor cells to nutrient deprivation and hypoxic conditions [30]. Under conditions of nutrient scarcity, tumor cells can support their growth and survival by degrading intracellular components through autophagy to provide energy and substrates for biosynthesis [31].
The precise role of autophagy in ESCA is multifaceted and involves various mechanisms contributing to both tumor suppression and promotion. Understanding these mechanisms requires a detailed look at the specific autophagy-related events and their implications in ESCA. Autophagy safeguards esophageal epithelial cells against oxidative stress injuries caused by ethanol and its primary metabolite, acetaldehyde, demonstrating the function of autophagy-mediated cellular protection in alcohol-associated ESCA genesis [32]. Transforming growth factor (TGF)-β is capable of inducing autophagy in esophageal cells, further facilitating epithelial–mesenchymal transition (EMT) and elevating cluster of differentiation 44 (CD44) expression, ultimately causing esophageal cell carcinogenesis. This discovery indicates that autophagy can mediate ESCA genesis by modulating oxidative stress reactions [33]. Beclin1, a key autophagy protein, has been found to have reduced expression in tumor tissues of patients with esophageal squamous cell carcinoma (ESCC) compared to non-tumorous adjacent tissues. This decrease is significantly associated with advanced stages of clinical disease and poor prognosis. The alteration of Beclin1 expression levels can modulate the proliferation rate of ESCA cells, indicating its pivotal role in tumor progression [34, 35]. Metastasis-associated colon cancer-1 (MACC1) primarily induces autophagy through the AMP-activated protein kinase (AMPK)-Unc51-like kinase 1 (ULK1) pathway, promoting lymph node metastasis in ESCC cells [36]. Similarly, autophagy maintains the stem-like properties of OV6 + cells by stabilizing β-catenin in an ATG7-dependent manner, thus supporting the progression of ESCA [37]. In ESCC tissues and cell lines, a marked increase in claudin 1 (CLDN1) expression is observed. Abnormal expression and distribution of CLDN1 activate autophagy through the AMPK/STAT1/ULK1 signaling pathway, resulting in the growth, migration, and invasion of ESCC cells, with these phenotypes being suppressed by 3-methyladenine [38]. Family with sequence similarity 134, member B (FAM134B), an autophagy regulator for the endoplasmic reticulum, is overexpressed in ESCC and contributes to tumor aggressiveness by promoting proliferation, migration, and invasion [39]. Furthermore, elevated levels of Piwi-like RNA-mediated gene silencing 2 (PIWIL2) correlate with an adverse prognosis in ESCC patients. PIWIL2 competitively blocks the interaction between IκB kinase (IKK) and tuberous sclerosis 1 (TSC1), thus inhibiting mechanistic target of rapamycin complex 1 (mTORC1) signal transduction and enhancing autophagy [40]. Collectively, these findings underscore the intricate relationship between autophagy and the development and progression of ESCA. Targeting autophagy pathways could offer potential therapeutic strategies to enhance treatment sensitivity and overcome the challenges posed by ESCA.
Autophagy and radiotherapy
Autophagy induced by radiotherapy
Exposure to radiation in cancer cells can trigger protective autophagy, which helps maintain cellular homeostasis and allows cells to survive under stress. Specifically, in breast cancer MCF-7 cells, radiation therapy can induce autophagy as a defensive mechanism. However, inhibiting radiation-induced autophagy in these cells leads to cell death [41]. The p53 gene plays a crucial role in this process. Functional p53 promotes cytoprotective autophagy, while its inactivation converts autophagy into a harmful process. Thus, the status of the p53 gene determines whether autophagy induced by radiation is protective or not. Furthermore, the ability of p53 to regulate autophagy is essential for increasing cell radiosensitivity and improving the effectiveness of radiotherapy [42]. The liver kinase B1 (LKB1) pathway can help ESCA cells (Eca-109) survive by initiating autophagy when they are exposed to radiation. Conversely, the suppression of autophagy greatly hampers the survival of these cells [43].
Nevertheless, a different study revealed that radiation could promote autophagy in mouse breast tumor cells (4T1), but inhibiting this autophagy did not modify the radiation sensitivity of these cells. Additionally, chloroquine did not affect the radiotherapy sensitivity of mouse breast tumor cells (4T1) in animal models with normal immune function [44, 45].
The discrepancies in these studies indicate that additional investigations are needed before cytoprotective autophagy may be used in a therapeutic context.
Autophagy modifies the radiosensitivity of tumors
Autophagy plays a dual role in modifying the radiosensitivity of tumor cells. Due to variations in tumor types and the tumor environment, autophagy can either enhance or reduce the radiosensitivity of tumor cells.
Various studies have verified that autophagy can increase radiosensitivity. For example, in breast and lung cancer cells, exposure to radiation increases the levels of pro-autophagic proteins such as the ATG5-ATG12 complex and Beclin-1. However, when autophagy inhibitors are added, the cells’ sensitivity to radiation significantly decreases. Therefore, enhancing autophagy in radioresistant tumor cells during radiotherapy can improve their sensitivity to treatment, inducing autophagic cell death in cells with strong survival capabilities. Under continuous and stable stress conditions, autophagy disrupts cellular structures, ultimately leading to apoptosis of tumor cells to some extent [46]. The enhanced radiosensitivity of A549 lung cancer cells is attributed to autophagy triggered by rapamycin, which is linked to the increased production of phosphorylated γ-H2AX and the delayed repair of radiation-induced DNA double strand breaks (DSBs) [47]. This suggests that autophagy induced by rapamycin impairs DNA damage repair mechanisms, thereby enhancing the effectiveness of radiotherapy. Furthermore, the combination of rapamycin and suberoylanilide hydroxamic acid (SAHA) stimulates both autophagy and acetylation, leading to increased radiosensitivity of non-small cell lung cancer (NSCLC) cells by creating a more hostile intracellular environment for cancer survival [48]. Additionally, inhibition of the Aurora kinase a (AURKA)-CXCL5 axis has been shown to induce autophagy and enhance radiation sensitivity in NSCLC, indicating a multifaceted approach to leveraging autophagy for therapeutic gain in radiotherapy [49]. Tenacissoside H, a plant extract, can induce excessive cellular autophagy by blocking the phosphatidylinositol-3 kinase (PI3K)/AKT/mechanistic target of the rapamycin kinase (mTOR) pathway, thereby increasing the radiosensitivity of liver cancer cells [50]. The induction of autophagy by saikosaponin-d (SSd) also has a notable impact on increasing the radiation sensitivity of liver cancer cells [51]. Radiation-induced liver cancer cells undergo enhanced apoptosis as a result of the enhancement of autophagy by SSd via a reduction in mTOR phosphorylation. This effect may be somewhat counteracted by the addition of an mTOR activator (MHY1485) or the autophagy inhibitor chloroquine (CQ) [52]. The medications listed above offer a possible method for liver cancer radiosensitization treatment. The use of borneol to suppress the mTORC1/eukaryotic initiation factor 4E (eIF4E) signaling axis, which induces autophagy and reduces hypoxia-inducible factor-1α (HIF-1α) expression, enhances the radiosensitivity of malignant glioma cells [53]. In glioma cells, ionizing radiation (IR) suppresses autophagic cell death through decreased ubiquitination of PRDX1, leading to increased interleukin-1 receptor-associated kinase 1 (IRAK1) expression and decreased radiosensitivity. Gliomas become more radiosensitive when IRAK1 is downregulated, both in vitro and in vivo [54].Prostate cancer cells become more radiosensitive when fructose-1,6-bisphosphatase 1 (FBP1) is knocked down because this increases autophagy via the AMPK-mTOR signaling pathway [55].Through the downregulation of silencing information regulator 2 related enzyme 1 (Sirt1), autophagy increases the radiosensitivity of prostate cancer cells [56]. At its maximum dosage, tetrandrine inhibits the phosphorylation of ERK and MEK induced by radiation, induces autophagy in nasopharyngeal cancer cells, and enhances their radiosensitivity [57]. ATP6V1C1 likely suppresses autophagy by activating mTOR signaling, thereby decreasing the radiosensitivity of ESCC cells [58]. Increased autophagy results in increased radiosensitivity of cervical cancer cells [59]. The regulation of autophagy may have significant implications for enhancing the radiosensitivity of tumors.
Conversely, autophagy can also reduce radiosensitivity in some cancers. Many studies have demonstrated that autophagy reduces the radiosensitivity of malignancies, a finding that is well acknowledged among academics. In unfavorable environmental circumstances such as a lack of nutrients, a reduction in growth hormones, or hypoxia, autophagy might be triggered. Changes in autophagy can be observed in any cell line following ionizing radiation. Radiation treatment induces autophagy in cancerous and normal cells. Radiation therapy leads to cancer cell death predominantly due to DNA damage, and damage to deoxyribonucleic acid is deemed the most detrimental type of radiation-induced DNA damage. There are two distinct biochemical processes by which deoxyribonucleic acid can be repaired: homologous recombination and nonhomologous end joining. These repair pathways are abnormally activated in cancer cells, and such aberrant activation of DNA repair genes is one of the reasons for resistance to chemotherapy and radiotherapy [60]. According to the theory of autophagy-mediated radioresistance, radiation-induced cellular stress interferes with the ability of tumor cells to digest proteins, which results in the accumulation of misfolded proteins in the endoplasmic reticulum. To endure this strain, tumor cells must promptly clear these misfolded proteins through autophagy, which is an important mechanism of radioresistance in tumors [61].
By selectively inhibiting PLK1, GSK461364, a pharmacological inhibitor, decreases autophagy and increases the radiosensitivity of breast cancer cells [62]. Glioblastoma cells are more radiosensitive when CTSD is inhibited because it has an impact on the fusion of autophagosomes and lysosomes. Reduced autophagy levels are also observed [63]. Lys05 (Lys01 trihydrochloride) is a novel lysosomal enhancer with autophagy-inhibiting effects. In glioma cells treated with Lys05, autophagic flux is inhibited, lysosomal function is impaired, and radiosensitivity is increased [64]. Forkhead box G1 (FOXG1) increases autophagy, which reduces glioma cell radiosensitivity [65]. Upregulation of nuclear receptor binding factor 2 (NRBF2) can activate autophagy, reducing the radiosensitivity of glioblastoma [66]. In part, by blocking PGRMC1-mediated autophagy, ultrasound-triggered microbubble destruction (UTMD) increases the radiosensitivity of glioblastoma [67]. The autophagy inducer rapamycin and the autophagy inhibitor chloroquine diphosphate were applied separately to CNE-2R nasopharyngeal carcinoma cells. Autophagy and radioresistance are linked in nasopharyngeal cancer CNE-2R cells, as evidenced by enhanced radiosensitization following treatment with chloroquine diphosphate and decreased radiosensitivity following treatment with rapamycin [68]. Eliminating the VEGF gene activates the mTOR pathway and suppresses autophagy, leading to enhanced radiosensitivity in nasopharyngeal cancer cells [69]. Clioquinol and zinc together increase the radiosensitivity of nasopharyngeal cancer stem-like cells by blocking autophagy [70]. Within nasopharyngeal cancer cells, protective autophagy is induced by latent membrane protein 1 (LMP1), which is expressed by Epstein‒Barr virus (EBV) and promotes radiation resistance [71]. In vitro and in vivo research has shown that esophageal squamous cell carcinoma is more radiosensitive when autophagy is suppressed [72]. LKB1, via the AMPK pathway, suppresses cell apoptosis and activates autophagy, increasing the radioresistance of cancer cells in the esophagus [73]. High mobility group box 1 (HMGB1) stimulates autophagy, thereby promoting radiation resistance; knockout of the HMGB1 gene in esophageal squamous cancer cells leads to increased radiosensitivity [74]. IR-125 triggers autophagy in ESCC cells, and inhibiting this autophagy with 3-methyladenine improves radiosensitivity [75]. Downregulating Claudin5 facilitates the malignant progression and radioresistance of ESCA cells via Beclin1-mediated autophagy [76]. 3-MA inhibits the formation of autophagosomes by acting as an autophagy inhibitor. In human colorectal cancer cells, the use of 3-MA to restrict autophagy increases apoptosis and increases radiosensitivity [77]. In in vivo and in vitro studies, sodium orthovanadate (SOV) blocks autophagy triggered by radiation, increasing the radiosensitivity of stomach cancer cells and making SOV a potential radiosensitizing agent for gastric cancer [78]. A study in human pancreatic cancer cells revealed that autophagy is triggered by radiation through activation of the G2 checkpoint, both of which lead to decreased radiosensitivity in human pancreatic ductal adenocarcinoma (PDAC) cells [79]. A disintegrin and a metalloprotease (ADAM9) trigger the autophagic process in liver cancer cells by reducing the expression of Nrf2, reducing the radiosensitivity of these cells [80]. Through gamma-aminobutyric acid receptor-associated protein (GABARAP), nuclear-enriched abundant transcript 1 (NEAT1) causes autophagy, reducing the radiosensitivity of hepatocellular carcinoma cells [81]. Chloroquine, a widely used autophagy inhibitor, functions by preventing the union of lysosomes and autophagosomes, thereby halting the progression of autophagy. The use of chloroquine increases the apoptosis rate of bladder cancer cells in vitro and decreases tumor proliferation in vivo, confirming the radiosensitizing effect of autophagy inhibition on tumors both in vitro and in vivo [82].
The reduction in the radiosensitivity of tumor cells due to autophagy is related to DNA repair and ROS clearance. Protective autophagy induced by hypoxia accelerates the removal of ROS, thereby increasing cellular resistance to radiotherapy. In MG-63 human osteosarcoma cells, hypoxic treatment activates autophagy and accelerates the clearance of ROS, increasing cell survival after radiotherapy [83]. By inhibiting autophagy, the Chk1 inhibitor MK8776 weakens the ability to repair DNA in triple-negative breast cancer cells, consequently enhancing radiosensitivity [84]. Radiation-resistant human non-small cell lung cancer cells can reduce the content of ROS in cells by inducing autophagy [85]. In hepatocellular carcinoma, unconventional prefoldin RPB5 interactor 1 (URI1) activates autophagy via the AMPK/Forkhead box O3 (FOXO3) signaling pathway to inhibit radiation-induced ROS [86].
In summary, autophagy plays a complex role in modulating tumor radiosensitivity. Understanding and manipulating this process could lead to improved therapeutic strategies in radiotherapy. Targeting autophagy may enhance radiosensitivity in certain cancers while inhibiting it could overcome radioresistance in others.
Radiotherapy and autophagy in esophageal cancer
In ESCA cells, the activation of autophagy can promote resistance to radiotherapy. This resistance mechanism may involve several pathways, including the repair of DNA damage caused by radiation, inhibition of apoptosis, and alterations in cellular metabolism [87]. Numerous studies have indicated that the expression levels of autophagy-related genes are closely associated with the radiosensitivity of ESCA. For instance, the expression levels of key autophagic regulators, such as ATG5, ATG7, and BECN1, in ESCA are negatively correlated with the effectiveness of radiotherapy [72]. Additionally, high mobility group box 1(HMGB1) activates autophagy, thereby promoting radiation resistance. In esophageal squamous carcinoma cells, knockout of the HMGB1 gene results in increased radiosensitivity [74]. LKB1 has also been proven to regulate autophagy through the AMPK signaling pathway, thereby influencing the radiosensitivity of ESCA [73].
Researchers have proposed multiple potential therapeutic strategies targeting autophagy for the treatment of ESCA with radiotherapy. One strategy is to enhance the effectiveness of radiotherapy by inhibiting autophagy. For instance, the use of autophagy inhibitors such as 3-methyladenine (3-MA) or chloroquine can block the autophagy pathway, thereby increasing the sensitivity of ESCA cells to radiotherapy [88]. Another strategy involves the use of autophagy inducers, such as rapamycin, to promote cell death caused by radiotherapy [89]. Additionally, the combined use of radiotherapy and autophagy-regulating factors is a viable treatment strategy. For example, by targeting autophagy-related proteins such as ATG4B, ATG5, or ATG7, the autophagic activity of ESCA cells can be effectively inhibited, thereby enhancing the cytotoxic effects of radiotherapy [90]. Moreover, interventions targeting signaling pathways such as HMGB1 and LKB1 may also become new approaches for enhancing the radiosensitivity of ESCA [73, 74].
In summary, autophagy plays a crucial role in ESCA radiotherapy. By thoroughly understanding the regulatory mechanisms of autophagy and its role in the radiotherapy of ESCA, it is hoped that new treatment strategies can be developed to overcome radiation tolerance and improve therapeutic outcomes and quality of life for patients with ESCA. Future research should further explore the complex relationship between autophagy and radiosensitivity in ESCA and validate the efficacy and safety of these treatment strategies in clinical trials.
Strategic implications of autophagy modulation in esophageal cancer therapy
In the field of ESCA treatment, as the understanding of disease mechanisms has deepened, and therapeutic strategies have shifted from solely surgical resection to a diversified approach that includes radiotherapy, chemotherapy, and combined chemoradiotherapy. During treatment, chemotherapy resistance and radiotherapy resistance are often accompanied by abnormal autophagic activity, which is a significant factor in reducing the efficacy of ESCA treatment. Regulating autophagy can improve this issue to some extent. Recent clinical studies have begun to explore the role of autophagy modulation in enhancing the efficacy of radiotherapy for ESCA. These studies underscore the potential of targeting autophagy as a therapeutic strategy to overcome radioresistance and improve the efficacy of radiotherapy in ESCA patients. Therefore, the development and application of autophagy modulators hold significant strategic importance in the treatment of ESCA. In recent years, research on autophagy modulators has made significant progress. These modulators affect the autophagic process through various mechanisms, including activating or inhibiting autophagy and enhancing the effectiveness of treatments for ESCA through interactions with other signaling pathways. The following section summarizes the significant progress made in the study of autophagy modulators in recent years (Table 1).
Autophagy modulators in the treatment of ESCA.
| Compounds | Effect on autophagy | Study model | References |
|---|---|---|---|
| Tunicamycin, TM | Activation | in vitro: EC109 | [92] |
| Insulin | Inhibition | in vitro: EC9706 | [93] |
| Rapamycin | Activation | in vitro: TE-1 | [94] |
| 3-MA | Inhibition |
in vitro: EC9706 in vivo: mice |
[88] |
| MG-132 | Activation | in vitro: EC9706 | [95] |
| BEZ235 and TSA | Activation | in vitro: ECA109, TE-1 | [96] |
| Ginsenoside Rk3 | Activation |
in vitro: ECA109, KYSE150 in vivo: mice |
[97] |
| Metformin | Activation |
in vitro: EC109, EC9706 in vivo: mice |
[100] |
| Obatoclax | Inhibition | in vitro: EC109, HKESC-1 | [103] |
| Chloroquine | Inhibition | in vitro: ECA109, TE-1 | [105] |
| Chloroquine | Inhibition | in vitro: OE19 | [106] |
| Chloroquine | Inhibition | in vitro: KYSE30, KYSE450, EC1, EC109 | [107] |
| Nimotuzumab | Activation | in vitro: ECA109 | [94] |
| Resveratrol | Activation | in vitro:EC109, EC9706 | [112] |
| EDHB | Activation | in vitro: KYSE170 | [113] |
| Gyp-L | Inhibition | in vitro: EC109, TE-1 | [114] |
| NH125 | Inhibition |
in vitro: ECA109, TE-13 in vivo: mice |
[116] |
| Fasudil | Activation | in vitro: EC109, KYSE150 | [115] |
Autophagy regulators of the PI3K pathway
Inhibiting PI3K/Akt/mTOR can cause autophagy, and this pathway is essential for controlling autophagy. PI3K/Akt acts as a precursor signal to mTOR, modulating the activation of mTOR. mTOR-associated signaling pathways are upregulated in tumor cells, thereby inhibiting the initiation of autophagy [91].
The PI3K/Akt/mTOR signaling pathway is involved in the mechanism by which tunicamycin (TM) induces autophagy in esophageal squamous carcinoma cells. In vivo and in vitro, TM and ionizing radiation (IR) work together to stop tumor growth by increasing the sensitivity of human ESCA cells to radiation through apoptosis and autophagy [92]. Consequently, tunicamycin significantly aids in reducing the resistance of ESCA cells to radiotherapy. Insulin activates the PI3K/AKT/mTOR pathway, which reduces autophagy, augmenting the anticancer action of cisplatin in human ESCA cells [93]. The mTOR inhibitor rapamycin promotes autophagy in TE-1 cells, which increases the susceptibility of resistant ESCA cells to radiation and chemotherapy [94]. By activating the class III PI3K pathway, the proteasome inhibitor MG-132 significantly upregulates the expression of the Beclin-1 protein, thereby activating autophagy. In EC9706 cells treated with MG-132, a noticeable increase in the number of autophagic vacuoles and cell death were observed [95]. The use of the dual PI3K/mTOR inhibitor BEZ235 and the histone deacetylase inhibitor trichostatin A (TSA), either alone or in combination, for the ex vivo treatment of the human ESCC cell lines Eca-109 and TE-1 revealed that combined treatment with BEZ235 and TSA can inhibit the PI3K/mTOR pathway, significantly induce autophagy, and enhance antitumor activity [96]. The ginsenoside Rk3 inhibits the PI3K/Akt/mTOR pathway, which in turn triggers autophagy and apoptosis, hence suppressing the growth of Eca109 and KYSE150 cells [97].
The aforementioned autophagy regulators of the PI3K pathway have achieved breakthrough progress in the study of ESCA cells, but they have not yet been used in patients receiving treatment for ESCA, necessitating further exploration of the potential adverse reactions and application scope of these drugs. A detailed investigation of the involvement of autophagy in the cytotoxic consequences of these substances will be advantageous for developing new combination therapies aimed at antitumor treatment through the specific modulation of autophagy.
Autophagy regulators of the signal transducer and activator of transcription 3 (STAT3)/Bcl-2 pathway
By serving as a junction for several carcinogenic signaling pathways, STAT3 enhances tumor cell growth by modulating downstream target genes, such as survivin, Bcl-2, Bcl-xL, cyclin D1, and c-Myc, among others, and is significant in cancer progression [98].
Bcl-2 acts as an inhibitor of both autophagy and apoptosis, while STAT3 stimulates the expression of Bcl-2, leading to the suppression of autophagy-mediated cellular apoptosis [99].
In human esophageal squamous carcinoma cells, metformin inhibits the STAT3/Bcl-2 pathway, activates autophagy, and upregulates Beclin-1/Atg5 expression, thereby inhibiting cell proliferation [100].
At present, it has only been discovered in cell experiments that metformin can inhibit tumor cell proliferation by modulating the autophagy of ESCA cells. Given the multifaceted effects of metformin, it has not yet been incorporated into adjunctive cancer treatments for patients with ESCA. There is an ongoing need to explore how to better harness and maximize its positive impact on ESCA therapy.
Lysosomal autophagy regulators
Obatoclax is a compound that blocks the activity of Bcl-2, a protein involved in cell survival. The substance has the capacity to be employed in cancer treatment, and its effectiveness is significantly improved when combined with radiation and chemotherapy [101]. Obatoclax accumulates in lysosomes, affecting their function and hindering the degradation process of autophagy [102]. By inhibiting autophagic flow, obatoclax strengthens the response of ESCA cells to cisplatin, leading to increased cell death [103].
The Food and Drug Administration (FDA) of the United States has approved only two autophagy inhibitors: chloroquine and its derivative hydroxychloroquine. They primarily inhibit autophagy by impairing the fusion between autophagosomes and lysosomes [104]. Research indicates that protective autophagy produced by cancer cells can lead to treatment resistance in ESCA patients. At this point, inhibiting autophagy helps to block the invasion and spread of cancer cells and restores the sensitivity of ESCC cells to therapeutic drugs. Endostar can trigger autophagy in esophageal squamous carcinoma cells, namely, Eca109 and TE-1 cells. Compared to monotherapy with Endostar, the use of chloroquine to inhibit autophagy enhances the antiproliferative effect of Endostar and increases the number of apoptotic cells [105]. Autophagic flux is increased in lapatinib-resistant esophageal adenocarcinoma cells, and inhibiting autophagy with chloroquine substantially diminishes the viability of these cells [106]. The blockade of autophagy with chloroquine amplifies the growth-inhibitory and pro-apoptotic effects of the deubiquitinase (DUB) inhibitor PR-619 on ESCC cells [107].
Thus, the concurrent inhibition of protective autophagy generated by cancer cells during the administration of anticancer drugs could represent a novel, prospective approach to improve therapeutic outcomes for ESCA patients.
Autophagy regulators in targeted therapy
Nimotuzumab, a monoclonal antibody targeting the epidermal growth factor receptor (EGFR), is a prominent pharmaceutical agent used to treat ESCA. Its mechanism includes competitively blocking the binding of EGF to its receptor, thereby inhibiting the activation of EGFR and downstream signaling [108]. In one study, 56 esophageal squamous carcinoma tissue samples were analyzed, revealing the overexpression of EGFR in 30 patients (53.6 %) [109]. Another study used two ESCC cell lines with different levels of EGFR expression, Eca109 (high expression) and TE-1 (low expression), to assess the combined effects of nimotuzumab administration with chemotherapeutic drugs (such as paclitaxel and cisplatin) and radiotherapy. In Eca109 cells treated with nimotuzumab, there was increased expression of the microtubule-associated protein light chain 3-II (LC3-II), and electron microscopy analysis also revealed increased autophagosome formation. Nimotuzumab enhances the sensitivity of cells to chemotherapy and radiotherapy by activating autophagy. However, in TE-1 cells with lower EGFR expression, nimotuzumab did not significantly improve the sensitivity to chemotherapy or radiotherapy. However, when nimotuzumab is used in conjunction with the autophagy inducer rapamycin, the sensitivity of cells resistant to chemotherapy and radiation therapy can be improved. These results confirm that nimotuzumab can enhance the sensitivity of ESCC cells to chemotherapy and radiotherapy by activating autophagy, especially in cells with high EGFR expression [94]. At present, targeted therapies have notably improved cancer patient survival. Understanding the interaction between autophagy and apoptosis induced by these drugs is beneficial for advancing personalized treatment of ESCA.
Other autophagy regulators
Natural substances and herbs have been used for thousands of years to prevent and fight various forms of cancer [110]. Resveratrol, a plant-based antitoxin present in a variety of foods, possesses anticancer effects [111]. Resveratrol leads to the upregulation of Beclin-1, ATG5 (autophagy-related gene-5), and LC3-Ⅱ in EC109 and EC9706 cells, suppressing cell proliferation and facilitating cell death [112]. Ethyl 3,4-dihydroxybenzoate (EDHB) is a polyphenolic chemical found in peanut hulls that is frequently employed as a food additive. It has been shown to induce cell cycle arrest in the S phase, trigger autophagy, and ultimately lead to the apoptosis of esophageal squamous carcinoma cells [113]. Gypenoside L (Gyp-L) induces lysosome-associated cell death through reactive oxygen species (ROS)-endoplasmic reticulum (ER)-Ca2+ signaling. Treating esophageal squamous carcinoma cells with Gyp-L leads to lysosomal swelling, the obstruction of autophagosome-lysosome fusion, the suppression of autophagic flux, the activation of intracellular reactive oxygen species, the induction of protein ubiquitination and the endoplasmic reticulum stress response, the enhancement of calcium release, and ultimately cell death. Thus, Gyp-L offers an alternative therapeutic approach for the treatment of ESCA in humans [114]. In vitro, fasudil inhibited RhoA/ROCK pathway activity in EC109 and KYSE150 cells and induced autophagy. When the process of autophagy, triggered by fasudil, is inhibited either by decreasing the expression of crucial autophagy genes (Beclin 1 or ATG7) or by administering a medication (chloroquine), both interventions greatly increase the susceptibility of ESCC to apoptosis produced by fasudil, resulting in a notable decrease in the survival rate of cells in vitro [115].
With in-depth research on autophagy regulators in ESCA treatment, more such regulators are being identified. These drugs regulate autophagy through different mechanisms and hold significant potential for improving ESCA treatment outcomes. Although these autophagy regulators have shown promise in basic research on ESCA cells, additional work remains before they can be extensively utilized in clinical therapy. It is vital to deepen the understanding of how autophagy influences ESCA progression and to discover more effective and less toxic autophagy regulators, with the goal of enhancing treatment efficacy for ESCA patients. As research progresses, autophagy modulators are anticipated to become an integral part of future ESCA treatments.
Conclusion and future perspectives
For ESCA patients at various tumor stages, radiation therapy, chemotherapy, targeted therapy, and immunotherapy constitute a crucial part of the treatment strategy. The development of radiation therapy, a significant supplementary treatment approach, entails identifying potential radiosensitizing drugs and researching their mechanisms to enhance the efficacy of radiation for cancer of the esophagus. Autophagy plays a complex role in the biological processes of ESCA, both by inducing tumor cell death and providing a protective mechanism for tumor cell survival. Therefore, considering autophagy as a potential therapeutic target, especially in cases of resistance to conventional treatments, is particularly important in exploring its role in radiotherapy. In clinical applications, modulating autophagy could pave the way for a new therapeutic approach, especially when combined with existing cytotoxic and targeted treatments. By enhancing radiosensitivity, this strategy could significantly improve treatment outcomes for patients with ESCA. A deeper investigation into the mechanisms of autophagy activation and its interaction with apoptosis will aid in developing more effective treatment strategies. In the future, we anticipate the development of additional autophagy modulators that will specifically alter the tumor cell response to treatment, offering new hope in the fight against ESCA.
Funding source: Beijing Natural Science Foundation Program
Award Identifier / Grant number: 7222032
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 82100539
Award Identifier / Grant number: 82173056
Acknowledgments
We would like to extend our sincere appreciation to Haolin Sun, Jingting Wang, Jiexuan Hu, and Pengfei Zhao for their invaluable assistance and support throughout this study. We are also grateful for the financial support provided by the Beijing Natural Science Foundation Program and the National Natural Science Foundation of China.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors confirm their contributions to the paper as follows: study conception and design: Yuan Gao and Bangwei Cao; data collection: Yuan Gao; analysis and interpretation: Yuan Gao, Wei Hao, and Haishan Lin; and draft manuscript preparation: Yuan Gao and Bangwei Cao. All authors reviewed and approved the final version of the manuscript.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: This work was supported by the Beijing Natural Science Foundation Program (grant number 7222032), the National Natural Science Foundation of China (grant numbers 82173056 and 82100539).
-
Data availability: Not applicable.
References
1. Uhlenhopp, DJ, Then, EO, Sunkara, T, Gaduputi, V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. J Clin Gastroenterol 2020;13:1010–21. https://doi.org/10.1007/s12328-020-01237-x.Search in Google Scholar PubMed
2. Siegel, RL, Miller, KD, Wagle, NS, Jemal, A. Cancer statistics, 2023. CA-Cancer J Clin 2023;73:17–48. https://doi.org/10.3322/caac.21763.Search in Google Scholar PubMed
3. Morgan, E, Soerjomataram, I, Rumgay, H, Coleman, HG, Thrift, AP, Vignat, J, et al.. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology 2022;163:649–58.e2. https://doi.org/10.1053/j.gastro.2022.05.054.Search in Google Scholar PubMed
4. Yang, S, Lin, S, Li, N, Deng, Y, Wang, M, Xiang, D, et al.. Burden, trends, and risk factors of esophageal cancer in China from 1990 to 2017: an up-to-date overview and comparison with those in Japan and South Korea. J Hematol Oncol 2020;13:146. https://doi.org/10.1186/s13045-020-00981-4.Search in Google Scholar PubMed PubMed Central
5. Ajani, JA, D’Amico, TA, Bentrem, DJ, Cooke, D, Corvera, C, Das, P, et al.. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2023;21:393–422. https://doi.org/10.6004/jnccn.2023.0019.Search in Google Scholar PubMed
6. Graf, D, Vallböhmer, D, Knoefel, WT, Budach, W, Häussinger, D. Multimodal treatment of esophageal carcinoma. Dtsch Med Wochenschr 2014;139:2141–7. https://doi.org/10.1055/s-0034-1370297.Search in Google Scholar PubMed
7. Tsao, T, Beretov, J, Ni, J, Bai, X, Bucci, J, Graham, P, et al.. Cancer stem cells in prostate cancer radioresistance. Cancer Lett 2019;465:94–104. https://doi.org/10.1016/j.canlet.2019.08.020.Search in Google Scholar PubMed
8. Kim, BM, Hong, Y, Lee, S, Liu, P, Lim, JH, Lee, YH, et al.. Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci 2015;16:26880–913. https://doi.org/10.3390/ijms161125991.Search in Google Scholar PubMed PubMed Central
9. Xin, Y, Jiang, F, Yang, C, Yan, Q, Guo, W, Huang, Q, et al.. Role of autophagy in regulating the radiosensitivity of tumor cells. J Cancer Res Clin Oncol 2017;143:2147–57. https://doi.org/10.1007/s00432-017-2487-2.Search in Google Scholar PubMed
10. Germic, N, Frangez, Z, Yousefi, S, Simon, H-U. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ 2019;26:715–27. https://doi.org/10.1038/s41418-019-0297-6.Search in Google Scholar PubMed PubMed Central
11. Galluzzi, L, Pietrocola, F, Bravo-San Pedro, JM, Amaravadi, RK, Baehrecke, EH, Cecconi, F, et al.. Autophagy in malignant transformation and cancer progression. EMBO J 2015;34:856–80. https://doi.org/10.15252/embj.201490784.Search in Google Scholar PubMed PubMed Central
12. Saadh, MJ, Almoyad, MAA, Arellano, MTC, Maaliw, RR3rd, Castillo-Acobo, RY, Jalal, SS, et al.. Long non-coding RNAs: controversial roles in drug resistance of solid tumors mediated by autophagy. Cancer Chemother Pharmacol 2023;92:439–53. https://doi.org/10.1007/s00280-023-04582-z.Search in Google Scholar PubMed
13. Amaravadi, RK, Kimmelman, AC, Debnath, J. Targeting autophagy in cancer: recent advances and future directions. Cancer Discov 2019;9:1167–81. https://doi.org/10.1158/2159-8290.cd-19-0292.Search in Google Scholar
14. Clark, SLJr. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol 1957;3:349–62. https://doi.org/10.1083/jcb.3.3.349.Search in Google Scholar PubMed PubMed Central
15. De Duve, C The lysosome. Sci Am 1963;208:64–72. https://doi.org/10.1038/scientificamerican0563-64.Search in Google Scholar PubMed
16. Tsukada, M, Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett 1993;333:169–74. https://doi.org/10.1016/0014-5793(93)80398-e.Search in Google Scholar PubMed
17. Klionsky, DJ, Cregg, JM, Dunn, WAJr, Emr, SD, Sakai, Y, Sandoval, IV, et al.. A unified nomenclature for yeast autophagy-related genes. Dev Cell 2003;5:539–45. https://doi.org/10.1016/s1534-5807(03)00296-x.Search in Google Scholar PubMed
18. Levy, JMM, Towers, CG, Thorburn, A. Targeting autophagy in cancer. Nat Rev Cancer 2017;17:528–42. https://doi.org/10.1038/nrc.2017.53.Search in Google Scholar PubMed PubMed Central
19. Kuchitsu, Y, Taguchi, T. Lysosomal microautophagy: an emerging dimension in mammalian autophagy. Trends Cell Biol 2023;34:606–16. https://doi.org/10.1016/j.tcb.2023.11.005.Search in Google Scholar PubMed
20. Lim, SM, Mohamad Hanif, EA, Chin, SF. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci 2021;11:56. https://doi.org/10.1186/s13578-021-00570-z.Search in Google Scholar PubMed PubMed Central
21. Debnath, J, Gammoh, N, Ryan, KM. Autophagy and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol 2023;24:560–75. https://doi.org/10.1038/s41580-023-00585-z.Search in Google Scholar PubMed PubMed Central
22. Nunn, K, Yanxiang Guo, J. Transient systemic autophagy ablation irreversibly inhibits lung tumor cell metabolism and promotes T-cell mediated tumor killing. Autophagy 2023;19:1879–81. https://doi.org/10.1080/15548627.2022.2141534.Search in Google Scholar PubMed PubMed Central
23. Hamurcu, Z, Delibaşı, N, Nalbantoglu, U, Sener, EF, Nurdinov, N, Tascı, B, et al.. FOXM1 plays a role in autophagy by transcriptionally regulating Beclin-1 and LC3 genes in human triple-negative breast cancer cells. J Mol Med 2019;97:491–508. https://doi.org/10.1007/s00109-019-01750-8.Search in Google Scholar PubMed
24. Chen, HE, Lin, JF, Tsai, TF, Lin, YC, Chou, KY, Hwang, TI. Allyl isothiocyanate induces autophagy through the up-regulation of beclin-1 in human prostate cancer cells. Am J Chin Med 2018;46:1–19. https://doi.org/10.1142/s0192415x18500830.Search in Google Scholar
25. White, E The role for autophagy in cancer. J Clin Investig 2015;125:42–6. https://doi.org/10.1172/jci73941.Search in Google Scholar
26. Camuzard, O, Santucci-Darmanin, S, Carle, GF, Pierrefite-Carle, V. Autophagy in the crosstalk between tumor and microenvironment. Cancer Lett 2020;490:143–53. https://doi.org/10.1016/j.canlet.2020.06.015.Search in Google Scholar PubMed
27. Chavez-Dominguez, R, Perez-Medina, M, Lopez-Gonzalez, JS, Galicia-Velasco, M, Aguilar-Cazares, D. The double-edge sword of autophagy in cancer: from tumor suppression to pro-tumor activity. Front Oncol 2020;10. https://doi.org/10.3389/fonc.2020.578418.Search in Google Scholar PubMed PubMed Central
28. Suares, A, Medina, MV, Coso, O. Autophagy in viral development and progression of cancer. Front Oncol 2021;11. https://doi.org/10.3389/fonc.2021.603224.Search in Google Scholar PubMed PubMed Central
29. Sahai, E, Astsaturov, I, Cukierman, E, DeNardo, DG, Egeblad, M, Evans, RM, et al.. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer 2020;20:174–86. https://doi.org/10.1038/s41568-019-0238-1.Search in Google Scholar PubMed PubMed Central
30. Ren, Y, Wang, R, Weng, S, Xu, H, Zhang, Y, Chen, S, et al.. Multifaceted role of redox pattern in the tumor immune microenvironment regarding autophagy and apoptosis. Mol Cancer 2023;22:130. https://doi.org/10.1186/s12943-023-01831-w.Search in Google Scholar PubMed PubMed Central
31. Yan, RL, Chen, RH. Autophagy and cancer metabolism-The two-way interplay. IUBMB life 2022;74:281–95. https://doi.org/10.1002/iub.2569.Search in Google Scholar PubMed
32. Tanaka, K, Whelan, KA, Chandramouleeswaran, PM, Kagawa, S, Rustgi, SL, Noguchi, C, et al.. ALDH2 modulates autophagy flux to regulate acetaldehyde-mediated toxicity thresholds. Am J Cancer Res 2016;6:781–96.Search in Google Scholar
33. Whelan, KA, Chandramouleeswaran, PM, Tanaka, K, Natsuizaka, M, Guha, M, Srinivasan, S, et al.. Autophagy supports generation of cells with high CD44 expression via modulation of oxidative stress and Parkin-mediated mitochondrial clearance. Oncogene 2017;36:4843–58. https://doi.org/10.1038/onc.2017.102.Search in Google Scholar PubMed PubMed Central
34. Du, H, Che, J, Shi, M, Zhu, L, Hang, JB, Chen, Z, et al.. Beclin 1 expression is associated with the occurrence and development of esophageal squamous cell carcinoma. Oncol Lett 2017;14:6823–8. https://doi.org/10.3892/ol.2017.7015.Search in Google Scholar PubMed PubMed Central
35. Weh, KM, Howell, AB, Kresty, LA. Expression, modulation, and clinical correlates of the autophagy protein beclin-1 in esophageal adenocarcinoma. Mol Carcinog 2016;55:1876–85. https://doi.org/10.1002/mc.22432.Search in Google Scholar PubMed PubMed Central
36. Wu, J, Zhang, D, Li, J, Deng, X, Liang, G, Long, Y, et al.. MACC1 induces autophagy to regulate proliferation, apoptosis, migration and invasion of squamous cell carcinoma. Oncol Rep 2017;38:2369–77. https://doi.org/10.3892/or.2017.5889.Search in Google Scholar PubMed
37. Wang, C, Yan, FH, Zhang, Jj, Huang, H, Cui, QS, Dong, W, et al.. OV6(+) cancer stem cells drive esophageal squamous cell carcinoma progression through ATG7-dependent beta-catenin stabilization. Cancer Lett 2017;391:100–13. https://doi.org/10.1016/j.canlet.2017.01.026.Search in Google Scholar PubMed
38. Wu, J, Gao, F, Xu, T, Li, J, Hu, Z, Wang, C, et al.. CLDN1 induces autophagy to promote proliferation and metastasis of esophageal squamous carcinoma through AMPK/STAT1/ULK1 signaling. J Cell Physiol 2020;235:2245–59. https://doi.org/10.1002/jcp.29133.Search in Google Scholar PubMed
39. Islam, F, Gopalan, V, Law, S, Tang, JC-o, Lam, AK-y. FAM134B promotes esophageal squamous cell carcinoma in vitro and its correlations with clinicopathologic features. Hum Pathol 2019;87:1–10. https://doi.org/10.1016/j.humpath.2018.11.033.Search in Google Scholar PubMed
40. Zhao, X, Huang, L, Lu, Y, Jiang, W, Song, Y, Qiu, B, et al.. PIWIL2 interacting with IKK to regulate autophagy and apoptosis in esophageal squamous cell carcinoma. Cell Death Differ 2021;28:1941–54. https://doi.org/10.1038/s41418-020-00725-4.Search in Google Scholar PubMed PubMed Central
41. Hashemi, M, Paskeh, MDA, Orouei, S, Abbasi, P, Khorrami, R, Dehghanpour, A, et al.. Towards dual function of autophagy in breast cancer: a potent regulator of tumor progression and therapy response. Biomed Pharmacother 2023;161. https://doi.org/10.1016/j.biopha.2023.114546.Search in Google Scholar PubMed
42. Chakradeo, S, Sharma, K, Alhaddad, A, Bakhshwin, D, Le, N, Harada, H, et al.. Yet another function of p53-the switch that determines whether radiation-induced autophagy will be cytoprotective or nonprotective: implications for autophagy inhibition as a therapeutic strategy. Mol Pharmacol 2015;87:803–14. https://doi.org/10.1124/mol.114.095273.Search in Google Scholar PubMed PubMed Central
43. Lu, C, Xie, C. Radiation-induced autophagy promotes esophageal squamous cell carcinoma cell survival via the LKB1 pathway. Oncol Rep 2016;35:3559–65. https://doi.org/10.3892/or.2016.4753.Search in Google Scholar PubMed
44. Zheng, D, Li, J, Yan, H, Zhang, G, Li, W, Chu, E, et al.. Emerging roles of aurora-a kinase in cancer therapy resistance. Acta Pharm Sin. B 2023;13:2826–43. https://doi.org/10.1016/j.apsb.2023.03.013.Search in Google Scholar PubMed PubMed Central
45. Lee, MJ, Park, JS, Jo, SB, Joe, YA. Enhancing anti-cancer therapy with selective autophagy inhibitors by targeting protective autophagy. Biomol Ther 2023;31:1–15. https://doi.org/10.4062/biomolther.2022.153.Search in Google Scholar PubMed PubMed Central
46. Kadhim, MM, Ramírez-Coronel, AA, Jalil, AT, Talib, HA, Gupta, J, Jawhar, ZH, et al.. Autophagy as a self-digestion signal in human cancers: regulation by microRNAs in affecting carcinogenesis and therapy response. Pharmacol Res 2023;189. https://doi.org/10.1016/j.phrs.2023.106695.Search in Google Scholar PubMed
47. Li, Y, Liu, F, Wang, Y, Li, D, Guo, F, Xu, L, et al.. Rapamycin-induced autophagy sensitizes A549 cells to radiation associated with DNA damage repair inhibition. Thorac cancer 2016;7:379–86. https://doi.org/10.1111/1759-7714.12332.Search in Google Scholar PubMed PubMed Central
48. Wang, Y, Liu, F, Fang, C, Xu, L, Chen, L, Xu, Z, et al.. Combination of rapamycin and SAHA enhanced radiosensitization by inducing autophagy and acetylation in NSCLC. Aging 2021;13:18223–37. https://doi.org/10.18632/aging.203226.Search in Google Scholar PubMed PubMed Central
49. Wang, J, Hu, T, Wang, Q, Chen, R, Xie, Y, Chang, H, et al.. Repression of the AURKA-CXCL5 axis induces autophagic cell death and promotes radiosensitivity in non-small-cell lung cancer. Cancer Lett 2021;509:89–104. https://doi.org/10.1016/j.canlet.2021.03.028.Search in Google Scholar PubMed
50. Lin, J, Ruan, J, Zhu, H, Chen, Z, Chen, J, Yu, H. Tenacissoside H induces autophagy and radiosensitivity of hepatocellular carcinoma cells by PI3K/Akt/mTOR signaling pathway. Dose Response 2021;19. https://doi.org/10.1177/15593258211011023.Search in Google Scholar PubMed PubMed Central
51. Tian, YD, Lin, S, Yang, PT, Bai, MH, Jin, YY, Min, WL, et al.. Saikosaponin-d increases the radiosensitivity of hepatoma cells by adjusting cell autophagy. J Cancer 2019;10:4947–53. https://doi.org/10.7150/jca.30286.Search in Google Scholar PubMed PubMed Central
52. Wang, B, Min, W, Lin, S, Song, L, Yang, P, Ma, Q, et al.. Saikosaponin-d increases radiation-induced apoptosis of hepatoma cells by promoting autophagy via inhibiting mTOR phosphorylation. Int J Med Sci 2021;18:1465–73. https://doi.org/10.7150/ijms.53024.Search in Google Scholar PubMed PubMed Central
53. Li, Q, Xia, L, Sun, C, Zhang, H, Zheng, M, Zhang, H, et al.. Role of borneol induced autophagy in enhancing radiosensitivity of malignant glioma. Front Oncol 2021;11. https://doi.org/10.3389/fonc.2021.749987.Search in Google Scholar PubMed PubMed Central
54. Li, J, Sun, Y, Zhao, X, Ma, Y, Xie, Y, Liu, S, et al.. Radiation induces IRAK1 expression to promote radioresistance by suppressing autophagic cell death via decreasing the ubiquitination of PRDX1 in glioma cells. Cell Death Dis 2023;14:259. https://doi.org/10.1038/s41419-023-05732-0.Search in Google Scholar PubMed PubMed Central
55. Li, XR, Zhou, KQ, Yin, Z, Gao, YL, Yang, X. Knockdown of FBP1 enhances radiosensitivity in prostate cancer cells by activating autophagy. Neoplasma 2020;67:982–91. https://doi.org/10.4149/neo_2020_190807n728.Search in Google Scholar
56. Wang, KX, Yan, C, Yang, X, Zhu, PY, Cui, WW, Ye, C, et al.. Enhanced autophagy promotes radiosensitivity by mediating Sirt1 downregulation in RM-1 prostate cancer cells. Biochem Bioph Res Co 2022;609:84–92. https://doi.org/10.1016/j.bbrc.2022.03.142.Search in Google Scholar PubMed
57. Wang, J, Yao, Z, Lai, X, Bao, H, Li, Y, Li, S, et al.. Tetrandrine sensitizes nasopharyngeal carcinoma cells to irradiation by inducing autophagy and inhibiting MEK/ERK pathway. Cancer Med 2020;9:7268–78. https://doi.org/10.1002/cam4.3356.Search in Google Scholar PubMed PubMed Central
58. Yao, X, Chen, H, Xu, B, Lu, J, Gu, J, Chen, F, et al.. The ATPase subunit of ATP6V1C1 inhibits autophagy and enhances radiotherapy resistance in esophageal squamous cell carcinoma. Gene 2021;768. https://doi.org/10.1016/j.gene.2020.145261.Search in Google Scholar PubMed
59. Yoo, JG, Lee, YK, Lee, KH. Enhancing autophagy leads to increased cell death in radiation-treated cervical cancer cells. J Obstet Gynaecol 2023;43. https://doi.org/10.1080/01443615.2023.2171281.Search in Google Scholar PubMed
60. Chaurasia, M, Bhatt, AN, Das, A, Dwarakanath, BS, Sharma, K. Radiation-induced autophagy: mechanisms and consequences. Free Radic Res 2016;50:273–90. https://doi.org/10.3109/10715762.2015.1129534.Search in Google Scholar PubMed
61. Wang, X, Xu, F, Kou, H, Zheng, Y, Yang, J, Xu, Z, et al.. Stromal cell-derived small extracellular vesicles enhance radioresistance of prostate cancer cells via interleukin-8-induced autophagy. J Extracell Vesicles 2023;12:e12342. https://doi.org/10.1002/jev2.12342.Search in Google Scholar PubMed PubMed Central
62. Wang, B, Huang, X, Liang, H, Yang, H, Guo, Z, Ai, M, et al.. PLK1 inhibition sensitizes breast cancer cells to radiation via suppressing autophagy. Int J Radiat Oncol Biol Phys 2021;110:1234–47. https://doi.org/10.1016/j.ijrobp.2021.02.025.Search in Google Scholar PubMed
63. Zheng, W, Chen, Q, Wang, C, Yao, D, Zhu, L, Pan, Y, et al.. Inhibition of Cathepsin D (CTSD) enhances radiosensitivity of glioblastoma cells by attenuating autophagy. Mol Carcinogen 2020;59:651–60. https://doi.org/10.1002/mc.23194.Search in Google Scholar PubMed
64. Zhou, W, Guo, Y, Zhang, X, Jiang, Z. Lys05 induces lysosomal membrane permeabilization and increases radiosensitivity in glioblastoma. J Cell Biochem 2020;121:2027–37. https://doi.org/10.1002/jcb.29437.Search in Google Scholar PubMed
65. Xiao, N, Li, C, Liao, W, Yin, J, Zhang, S, Zhang, P, et al.. FOXG1 mediates the radiosensitivity of glioma cells through regulation of autophagy. Int J Radiat Biol 2021;97:139–48. https://doi.org/10.1080/09553002.2021.1846816.Search in Google Scholar PubMed
66. Kim, J, Kang, H, Son, B, Kim, MJ, Kang, J, Park, KH, et al.. NRBF2-mediated autophagy contributes to metabolite replenishment and radioresistance in glioblastoma. Exp Mol Med 2022;54:1872–85. https://doi.org/10.1038/s12276-022-00873-2.Search in Google Scholar PubMed PubMed Central
67. He, Y, Dong, XH, Zhu, Q, Xu, YL, Chen, ML, Liu, Z. Ultrasound-triggered microbubble destruction enhances the radiosensitivity of glioblastoma by inhibiting PGRMC1-mediated autophagy in vitro and in vivo. Mil Med Res 2022;9:9. https://doi.org/10.1186/s40779-022-00369-0.Search in Google Scholar PubMed PubMed Central
68. Liang, ZG, Lin, GX, Yu, BB, Su, F, Li, L, Qu, S, et al.. The role of autophagy in the radiosensitivity of the radioresistant human nasopharyngeal carcinoma cell line CNE-2R. Cancer Manag Res 2018;10:4125–34. https://doi.org/10.2147/cmar.s176536.Search in Google Scholar
69. Chen, L, Lin, G, Chen, K, Wan, F, Liang, R, Sun, Y, et al.. VEGF knockdown enhances radiosensitivity of nasopharyngeal carcinoma by inhibiting autophagy through the activation of mTOR pathway. Sci Rep 2020;10:16328. https://doi.org/10.1038/s41598-020-73310-x.Search in Google Scholar PubMed PubMed Central
70. Ke, Y, Wu, C, Zeng, Y, Chen, M, Li, Y, Xie, C, et al.. Radiosensitization of clioquinol combined with zinc in the nasopharyngeal cancer stem-like cells by inhibiting autophagy in vitro and in vivo. Int J Biol Sci 2020;16:777–89. https://doi.org/10.7150/ijbs.40305.Search in Google Scholar PubMed PubMed Central
71. Xu, S, Zhou, Z, Peng, X, Tao, X, Zhou, P, Zhang, K, et al.. EBV-LMP1 promotes radioresistance by inducing protective autophagy through BNIP3 in nasopharyngeal carcinoma. Cell Death Dis 2021;12:344. https://doi.org/10.1038/s41419-021-03639-2.Search in Google Scholar PubMed PubMed Central
72. Tao, H, Qian, P, Lu, J, Guo, Y, Zhu, H, Wang, F. Autophagy inhibition enhances radiosensitivity of Eca-109 cells via the mitochondrial apoptosis pathway. Int J Oncol 2018;52:1853–62. https://doi.org/10.3892/ijo.2018.4349.Search in Google Scholar PubMed PubMed Central
73. He, Q, Li, J, Dong, F, Cai, C, Zou, X. LKB1 promotes radioresistance in esophageal cancer cells exposed to radiation, by suppression of apoptosis and activation of autophagy via the AMPK pathway. Mol Med Rep 2017;16:2205–10. https://doi.org/10.3892/mmr.2017.6852.Search in Google Scholar PubMed
74. Ma, H, Zheng, S, Zhang, X, Gong, T, Lv, X, Fu, S, et al.. High mobility group box 1 promotes radioresistance in esophageal squamous cell carcinoma cell lines by modulating autophagy. Cell Death Dis 2019;10:136. https://doi.org/10.1038/s41419-019-1355-1.Search in Google Scholar PubMed PubMed Central
75. Wang, C, Li, TK, Zeng, CH, Fan, R, Wang, Y, Zhu, GY, et al.. Iodine-125 seed radiation induces ROS-mediated apoptosis, autophagy and paraptosis in human esophageal squamous cell carcinoma cells. Oncol Rep 2020;43:2028–44. https://doi.org/10.3892/or.2020.7576.Search in Google Scholar PubMed PubMed Central
76. Huang, S, Zhang, J, Li, Y, Xu, Y, Jia, H, An, L, et al.. Downregulation of Claudin5 promotes malignant progression and radioresistance through Beclin1-mediated autophagy in esophageal squamous cell carcinoma. J Transl Med 2023;21:379. https://doi.org/10.1186/s12967-023-04248-7.Search in Google Scholar PubMed PubMed Central
77. Dong, Y, Wu, Y, Zhao, GL, Ye, ZY, Xing, CG, Yang, XD. Inhibition of autophagy by 3-MA promotes hypoxia-induced apoptosis in human colorectal cancer cells. Eur Rev Med Pharmacol Sci 2019;23:1047–54. https://doi.org/10.26355/eurrev_201902_16992.Search in Google Scholar PubMed
78. Wang, W, Jiang, XG, Bai, YP, Li, H, Gao, LX, Zhang, T, et al.. SOV sensitizes gastric cancer cells to radiation by suppressing radiation-induced autophagy in vitro and in vivo. Tissue Cell 2023;82. https://doi.org/10.1016/j.tice.2023.102109.Search in Google Scholar PubMed
79. Suzuki, M, Anko, M, Ohara, M, Matsumoto, KI, Hasegawa, S. Radiation-induced autophagy in human pancreatic cancer cells is critically dependent on G2 checkpoint activation: a mechanism of radioresistance in pancreatic cancer. Int J Radiat Oncol Biol Phys 2021;111:260–71. https://doi.org/10.1016/j.ijrobp.2021.04.001.Search in Google Scholar PubMed
80. Zhu, L, Zhao, Y, Yu, L, He, X, Wang, Y, Jiang, P, et al.. Overexpression of ADAM9 decreases radiosensitivity of hepatocellular carcinoma cell by activating autophagy. Bioengineered 2021;12:5516–28. https://doi.org/10.1080/21655979.2021.1965694.Search in Google Scholar PubMed PubMed Central
81. Sakaguchi, H, Tsuchiya, H, Kitagawa, Y, Tanino, T, Yoshida, K, Uchida, N, et al.. NEAT1 confers radioresistance to hepatocellular carcinoma cells by inducing autophagy through GABARAP. Int J Mol Sci 2022;23:711. https://doi.org/10.3390/ijms23020711.Search in Google Scholar PubMed PubMed Central
82. Wang, F, Tang, J, Li, P, Si, S, Yu, H, Yang, X, et al.. Chloroquine enhances the radiosensitivity of bladder cancer cells by inhibiting autophagy and activating apoptosis. Cell Physiol Biochem 2018;45:54–66. https://doi.org/10.1159/000486222.Search in Google Scholar PubMed
83. Feng, H, Wang, J, Chen, W, Shan, B, Guo, Y, Xu, J, et al.. Hypoxia-induced autophagy as an additional mechanism in human osteosarcoma radioresistance. J Bone Oncol 2016;5:67–73. https://doi.org/10.1016/j.jbo.2016.03.001.Search in Google Scholar PubMed PubMed Central
84. Zhou, ZR, Yang, ZZ, Wang, SJ, Zhang, L, Luo, JR, Feng, Y, et al.. The Chk1 inhibitor MK-8776 increases the radiosensitivity of human triple-negative breast cancer by inhibiting autophagy. Acta Pharmacol Sin 2017;38:513–23. https://doi.org/10.1038/aps.2016.136.Search in Google Scholar PubMed PubMed Central
85. Chen, X, Wang, P, Guo, F, Wang, X, Wang, J, Xu, J, et al.. Autophagy enhanced the radioresistance of non-small cell lung cancer by regulating ROS level under hypoxia condition. Int J Radiat Biol 2017;93:764–70. https://doi.org/10.1080/09553002.2017.1325025.Search in Google Scholar PubMed
86. Xu, Y, Ji, Y, Li, X, Ding, J, Chen, L, Huang, Y, et al.. URI1 suppresses irradiation-induced reactive oxygen species (ROS) by activating autophagy in hepatocellular carcinoma cells. Int J Biol Sci 2021;17:3091–103. https://doi.org/10.7150/ijbs.55689.Search in Google Scholar PubMed PubMed Central
87. Xia, D, Zhang, XR, Ma, YL, Zhao, ZJ, Zhao, R, Wang, YY. Nrf2 promotes esophageal squamous cell carcinoma (ESCC) resistance to radiotherapy through the CaMKIIα-associated activation of autophagy. Cell Biosci 2020;10:90. https://doi.org/10.1186/s13578-020-00456-6.Search in Google Scholar PubMed PubMed Central
88. Chen, Y, Li, X, Guo, L, Wu, X, He, C, Zhang, S, et al.. Combining radiation with autophagy inhibition enhances suppression of tumor growth and angiogenesis in esophageal cancer. Mol Med Rep 2015;12:1645–52. https://doi.org/10.3892/mmr.2015.3623.Search in Google Scholar PubMed PubMed Central
89. Xiang, H, Zhang, J, Lin, C, Zhang, L, Liu, B, Ouyang, L. Targeting autophagy-related protein kinases for potential therapeutic purpose. Acta Pharm Sin B 2020;10:569–81. https://doi.org/10.1016/j.apsb.2019.10.003.Search in Google Scholar PubMed PubMed Central
90. Zhou, S, Sun, X, Jin, Z, Yang, H, Ye, W. The role of autophagy in initiation, progression, TME modification, diagnosis, and treatment of esophageal cancers. Crit Rev Oncol Hemat 2022;175. https://doi.org/10.1016/j.critrevonc.2022.103702.Search in Google Scholar PubMed
91. Zheng, W, Wu, C, Wu, X, Cai, Y, Liu, B, Wang, C. Genetic variants of autophagy-related genes in the PI3K/Akt/mTOR pathway and risk of gastric cancer in the Chinese population. Gene 2021;769. https://doi.org/10.1016/j.gene.2020.145190.Search in Google Scholar PubMed
92. Pang, XL, He, G, Liu, YB, Wang, Y, Zhang, B. Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation. World J Gastroentero 2013;19:1736–48. https://doi.org/10.3748/wjg.v19.i11.1736.Search in Google Scholar PubMed PubMed Central
93. Yang, Y, Wen, F, Dang, L, Fan, Y, Liu, D, Wu, K, et al.. Insulin enhances apoptosis induced by cisplatin in human esophageal squamous cell carcinoma EC9706 cells related to inhibition of autophagy. Chin Med J 2014;127:353–8. https://doi.org/10.3760/cma.j.issn.0366-6999.20130996.Search in Google Scholar
94. Song, H, Pan, B, Yi, J, Chen, L. Featured article: autophagic activation with nimotuzumab enhanced chemosensitivity and radiosensitivity of esophageal squamous cell carcinoma. Exp Biol Med 2014;239:529–41. https://doi.org/10.1177/1535370214525315.Search in Google Scholar PubMed
95. Liu, D, Gao, M, Yang, Y, Qi, YU, Wu, K, Zhao, S. Inhibition of autophagy promotes cell apoptosis induced by the proteasome inhibitor MG-132 in human esophageal squamous cell carcinoma EC9706 cells. Oncol Lett 2015;9:2278–82. https://doi.org/10.3892/ol.2015.3047.Search in Google Scholar PubMed PubMed Central
96. Wu, N, Zhu, Y, Xu, X, Zhu, Y, Song, Y, Pang, L, et al.. The anti-tumor effects of dual PI3K/mTOR inhibitor BEZ235 and histone deacetylase inhibitor Trichostatin A on inducing autophagy in esophageal squamous cell carcinoma. J Cancer 2018;9:987–97. https://doi.org/10.7150/jca.22861.Search in Google Scholar PubMed PubMed Central
97. Liu, H, Zhao, J, Fu, R, Zhu, C, Fan, D. The ginsenoside Rk3 exerts anti-esophageal cancer activity in vitro and in vivo by mediating apoptosis and autophagy through regulation of the PI3K/Akt/mTOR pathway. PloS one 2019;14:e0216759. https://doi.org/10.1371/journal.pone.0216759.Search in Google Scholar PubMed PubMed Central
98. Aziz, MA, Sarwar, MS, Akter, T, Uddin, MS, Xun, S, Zhu, Y, et al.. Polyphenolic molecules targeting STAT3 pathway for the treatment of cancer. Life Sci 2021;268. https://doi.org/10.1016/j.lfs.2020.118999.Search in Google Scholar PubMed
99. Ranjan, A, Kaushik, I, Srivastava, SK. Pimozide suppresses the growth of brain tumors by targeting STAT3-mediated autophagy. Cells 2020;9:2141. https://doi.org/10.3390/cells9092141.Search in Google Scholar PubMed PubMed Central
100. Feng, Y, Ke, C, Tang, Q, Dong, H, Zheng, X, Lin, W, et al.. Metformin promotes autophagy and apoptosis in esophageal squamous cell carcinoma by downregulating Stat3 signaling. Cell Death Dis 2014;5:e1088. https://doi.org/10.1038/cddis.2014.59.Search in Google Scholar PubMed PubMed Central
101. Or, CR, Huang, CW, Chang, CC, Lai, YC, Chen, YJ, Chang, CC. Obatoclax, a pan-BCL-2 inhibitor, downregulates survivin to induce apoptosis in human colorectal carcinoma cells via suppressing WNT/β-catenin signaling. Int J Mol Sci 2020;21:1773. https://doi.org/10.3390/ijms21051773.Search in Google Scholar PubMed PubMed Central
102. Stamelos, VA, Fisher, N, Bamrah, H, Voisey, C, Price, JC, Farrell, WE, et al.. The BH3 mimetic obatoclax accumulates in lysosomes and causes their alkalinization. PloS one 2016;11:e0150696. https://doi.org/10.1371/journal.pone.0150696.Search in Google Scholar PubMed PubMed Central
103. Yu, L, Wu, WK, Gu, C, Zhong, D, Zhao, X, Kong, Y, et al.. Obatoclax impairs lysosomal function to block autophagy in cisplatin-sensitive and -resistant esophageal cancer cells. Oncotarget 2016;7:14693–707. https://doi.org/10.18632/oncotarget.7492.Search in Google Scholar PubMed PubMed Central
104. Mauthe, M, Orhon, I, Rocchi, C, Zhou, X, Luhr, M, Hijlkema, KJ, et al.. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018;14:1435–55. https://doi.org/10.1080/15548627.2018.1474314.Search in Google Scholar PubMed PubMed Central
105. Han, X, Wang, Z, Hu, B, Xu, J. Autophagy inhibition contributes to Endostar sensitization in esophageal squamous cell carcinoma. Oncol Lett 2017;14:6604–10. https://doi.org/10.3892/ol.2017.7017.Search in Google Scholar PubMed PubMed Central
106. Janser, FA, Adams, O, Bütler, V, Schläfli, AM, Dislich, B, Seiler, CA, et al.. Her2-Targeted therapy induces autophagy in esophageal adenocarcinoma cells. Int J Mol Sci 2018;19:3069. https://doi.org/10.3390/ijms19103069.Search in Google Scholar PubMed PubMed Central
107. Wang, L, Li, M, Sha, B, Hu, X, Sun, Y, Zhu, M, et al.. Inhibition of deubiquitination by PR-619 induces apoptosis and autophagy via ubi-protein aggregation-activated ER stress in oesophageal squamous cell carcinoma. Cell Prolif 2021;54:e12919. https://doi.org/10.1111/cpr.12919.Search in Google Scholar PubMed PubMed Central
108. Wang, L, Liu, L, Cao, Y, Chen, X, Liu, S, Li, X, et al.. Simultaneous integrated boost intensity-modulated radiotherapy (SIB-IMRT) combined with nimotuzumab for locally advanced esophageal squamous cell carcinoma (ESCC): a phase II clinical trial. BMC cancer 2024;24:679. https://doi.org/10.1186/s12885-024-12427-y.Search in Google Scholar PubMed PubMed Central
109. Lin, G, Sun, XJ, Han, QB, Wang, Z, Xu, YP, Gu, JL, et al.. Epidermal growth factor receptor protein overexpression and gene amplification are associated with aggressive biological behaviors of esophageal squamous cell carcinoma. Oncol Lett 2015;10:901–6. https://doi.org/10.3892/ol.2015.3277.Search in Google Scholar PubMed PubMed Central
110. Avtanski, D, Poretsky, L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol Med 2018;24:29. https://doi.org/10.1186/s10020-018-0032-7.Search in Google Scholar PubMed PubMed Central
111. Vervandier-Fasseur, D, Latruffe, N. The potential use of resveratrol for cancer prevention. Molecules 2019;24:4506. https://doi.org/10.3390/molecules24244506.Search in Google Scholar PubMed PubMed Central
112. Tang, Q, Li, G, Wei, X, Zhang, J, Chiu, JF, Hasenmayer, D, et al.. Resveratrol-induced apoptosis is enhanced by inhibition of autophagy in esophageal squamous cell carcinoma. Cancer Lett 2013;336:325–37. https://doi.org/10.1016/j.canlet.2013.03.023.Search in Google Scholar PubMed
113. Han, B, Li, W, Sun, Y, Zhou, L, Xu, Y, Zhao, X. A prolyl-hydroxylase inhibitor, ethyl-3,4-dihydroxybenzoate, induces cell autophagy and apoptosis in esophageal squamous cell carcinoma cells via up-regulation of BNIP3 and N-myc downstream-regulated gene-1. PloS one 2014;9:e107204. https://doi.org/10.1371/journal.pone.0107204.Search in Google Scholar PubMed PubMed Central
114. Liao, C, Zheng, K, Li, Y, Xu, H, Kang, Q, Fan, L, et al.. Gypenoside L inhibits autophagic flux and induces cell death in human esophageal cancer cells through endoplasm reticulum stress-mediated Ca2+ release. Oncotarget 2016;7:47387–402. https://doi.org/10.18632/oncotarget.10159.Search in Google Scholar PubMed PubMed Central
115. Xie, FJ, Zheng, QQ, Qin, J, Zhang, LL, Han, N, Mao, WM. Autophagy inhibition stimulates apoptosis in oesophageal squamous cell carcinoma treated with fasudil. J Cancer 2018;9:1050–6. https://doi.org/10.7150/jca.23388.Search in Google Scholar PubMed PubMed Central
116. Zhu, H, Song, H, Chen, G, Yang, X, Liu, J, Ge, Y, et al.. eEF2K promotes progression and radioresistance of esophageal squamous cell carcinoma. Radiother Oncol 2017;124:439–47. https://doi.org/10.1016/j.radonc.2017.04.001.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Autophagy and radiotherapy in esophageal cancer: modulating treatment sensitivity and overcoming challenges
- Functions of CAFs in microenvironment of non-small cell lung cancer: based on updated hallmarks of cancer
- Cisplatin-induced pyroptosis: a double-edged sword in cancer treatment
- Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
- Research Articles
- A lipid metabolism-related gene model reveals the prognosis and immune microenvironment of cutaneous melanoma
- Boanmycin induces apoptosis and overcomes venetoclax resistance in acute myeloid leukemia
- Identification of GNB1 as a downstream effector of the circRNA-0133711/miR-145-5p axis involved in breast cancer proliferation and metastasis
- Clinical characteristics and prognosis of lung metastases from unknown primary cancer sites
- Integrating bulk-RNA and single-cell analysis reveals heterogeneous expression of cuproptosis-related sorafenib-resistant genes in hepatocellular carcinoma
- Exploring the mechanism of genistein in treating hepatocellular carcinoma through network pharmacology and molecular docking
- Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
- Exploring the anti-lung cancer mechanism of Ganoderma lucidum and its relationship with the level of immune cell infiltration based on network pharmacology and molecular docking
- Constructing a prognostic model for colon cancer patients on basis of coagulation genes enriched in cancer-associated fibroblasts to guide personalized immunotherapy
- Rapid Communication
- Evaluation of HER2 immunohistochemistry-positive and immunohistochemistry-negative FISH amplification breast cancers using next-generation sequencing
- Short Commentary
- Unveiling the unexplored: shedding light on a novel aspect of colorectal carcinoma
Articles in the same Issue
- Frontmatter
- Review Articles
- Autophagy and radiotherapy in esophageal cancer: modulating treatment sensitivity and overcoming challenges
- Functions of CAFs in microenvironment of non-small cell lung cancer: based on updated hallmarks of cancer
- Cisplatin-induced pyroptosis: a double-edged sword in cancer treatment
- Radiotherapy directed to inferior vena cava tumor thrombus among patients with renal cell carcinoma: an illustrative case and review of the literature
- Research Articles
- A lipid metabolism-related gene model reveals the prognosis and immune microenvironment of cutaneous melanoma
- Boanmycin induces apoptosis and overcomes venetoclax resistance in acute myeloid leukemia
- Identification of GNB1 as a downstream effector of the circRNA-0133711/miR-145-5p axis involved in breast cancer proliferation and metastasis
- Clinical characteristics and prognosis of lung metastases from unknown primary cancer sites
- Integrating bulk-RNA and single-cell analysis reveals heterogeneous expression of cuproptosis-related sorafenib-resistant genes in hepatocellular carcinoma
- Exploring the mechanism of genistein in treating hepatocellular carcinoma through network pharmacology and molecular docking
- Investigating the potential mechanisms of Litsea cubeba essential oil for anti-melanoma through experimental validation, network pharmacology, and molecular docking analysis
- Exploring the anti-lung cancer mechanism of Ganoderma lucidum and its relationship with the level of immune cell infiltration based on network pharmacology and molecular docking
- Constructing a prognostic model for colon cancer patients on basis of coagulation genes enriched in cancer-associated fibroblasts to guide personalized immunotherapy
- Rapid Communication
- Evaluation of HER2 immunohistochemistry-positive and immunohistochemistry-negative FISH amplification breast cancers using next-generation sequencing
- Short Commentary
- Unveiling the unexplored: shedding light on a novel aspect of colorectal carcinoma

