Abstract
Objectives
Paraganglioma (PGL) is a rare extra-adrenal neuroendocrine tumor, and intrapericardial PGL is extremely rare. We report a rare case of intrapericardial nonfunctional PGL, which may be used as a reference for further analysis.
Case presentation
This article presents the case of a 65-year-old woman with a 2-year history of lower extremity pain. Ultrasound revealed a hypoechoic mass adjacent to the right atrium, compressing and narrowing the right atrium. Computed tomography (CT) showed a low-density mass with marked enhancement suggestive of a solitary fibrous tumor or a vasogenic tumor of pericardial origin. Cardiac magnetic resonance imaging (MRI) confirmed the location and provided a primary diagnosis of solitary fibrous tumor, hemangioma, or hemangiosarcoma. The patient eventually underwent pericardial tumor resection, and the diagnosis of PGL was confirmed by postoperative histopathology.
Conclusions
Pathology is considered the gold standard for the diagnosis of PGL. Imaging examinations can provide valuable information for the diagnosis and management of intrapericardial PGL, and surgery remains the treatment of choice.
Introduction
Paraganglioma (PGL) is a rare neuroendocrine tumor that arises from the extra-adrenal sympathetic nervous system and may have hormonal secreting functions, leading to clinical syndromes such as increased blood pressure and metabolic changes in patients [1]. PGL is mainly found in the paravertebral sympathetic chain in the thorax, abdomen, and pelvis, and its occurrence in the pericardium is extremely rare [2, 3]. The localization and diagnosis of PGL is challenging and requires a combination of biochemical testing, imaging, and postoperative histopathologic evaluation to confirm the diagnosis [2]. This article presents a case of intrapericardial nonfunctional PGL and systematically analyzes the diagnosis and treatment of PGL in conjunction with a review of the literature, especially the imaging features of PGL. We expect that this case report will provide clinicians with valuable insights to consider in their future work.
Case presentation
A 65-year-old woman with unexplained bilateral lower extremity pain presented to another hospital two years ago. There were no abnormal findings associated with color Doppler ultrasonography of the lower extremity vasculature. However, no cardiac imaging examinations were performed. Recently, the patient returned to the same hospital, presenting with pain in both lower extremities. Computed tomography (CT) scan revealed an abnormal soft tissue mass in the pericardium. Magnetic resonance imaging (MRI) confirmed an occupying lesion in the right pericardium and a neoplastic lesion originating from the epicardial myocardium, possibly with a fibrous component, was diagnosed. The patient was then referred to the Cardiovascular Surgery Department of Daping Hospital for further treatment as a “cardiac tumor” and admitted for evaluation.
There was no history of hypertension, and no abnormalities were found on physical examination. Biochemical tests revealed an elevated B-type natriuretic peptide (BNP) level of 104.22 pg/mL. Bilateral carotid artery and bilateral lower extremity arteriovenous Doppler ultrasound results were normal (right femoral vein internal diameter of 9.7 mm and left femoral vein internal diameter of 9.2 mm). Echocardiography revealed a mass approximately 8.1×7.9 cm with a lower echo than the liver on the right lateral aspect of the heart that was regular and well defined, had an intact envelope, and no apparent colored blood flow within the mass. The mass caused a compression of the right atrium, which measured approximately 22 mm in transverse diameter. Furthermore, the internal diameter of the inferior vena cava at the entrance of the right atrium was approximately 10.6 mm, and the internal diameter of the inferior vena cava in the intrahepatic segment was approximately 15.8 mm. Based on ultrasound, the patient was diagnosed with an intrapericardial occupying lesion (Figure 1, Supplementary Video 1). A CT scan of the chest revealed a soft tissue mass adjacent to the right atrium causing significant compression and deformation of the right atrium and ventricle. After contrast administration, the mass showed apparent heterogeneous enhancement and multiple tortuous dilated vessels were seen within the mass. Coronary artery CTA showed a mass adjacent to the right atrium that was partially encircled by the right coronary artery (Figure 2, Supplementary Video 2). Cardiac MRI revealed a mass in the pericardium with hyperintensity on T2-weighted fat-suppressed (T2WI-FS) suppression imaging. The tumor was indistinguishable from the right heart, causing compression and deformation of the right heart. Cine MRI of the heart showed no significant myocardial thinning or thickening in the systolic or diastolic phases of the ventricle (Supplementary Video 3). The MRI suggested an intrapericardial occupying lesion between which an isolated fibrous tumor of epicardial or myocardial origin, hemangioma, or hemangiosarcoma was primarily considered (Figure 3). Since MRI suggests that the tumor is highly vascularized, the risk of bleeding after tumor biopsy is high. To assess the risk of bleeding after surgical removal of the tumor, the patient underwent coronary angiography. Coronary angiography revealed that the tumor received most of its blood supply from the right coronary artery.
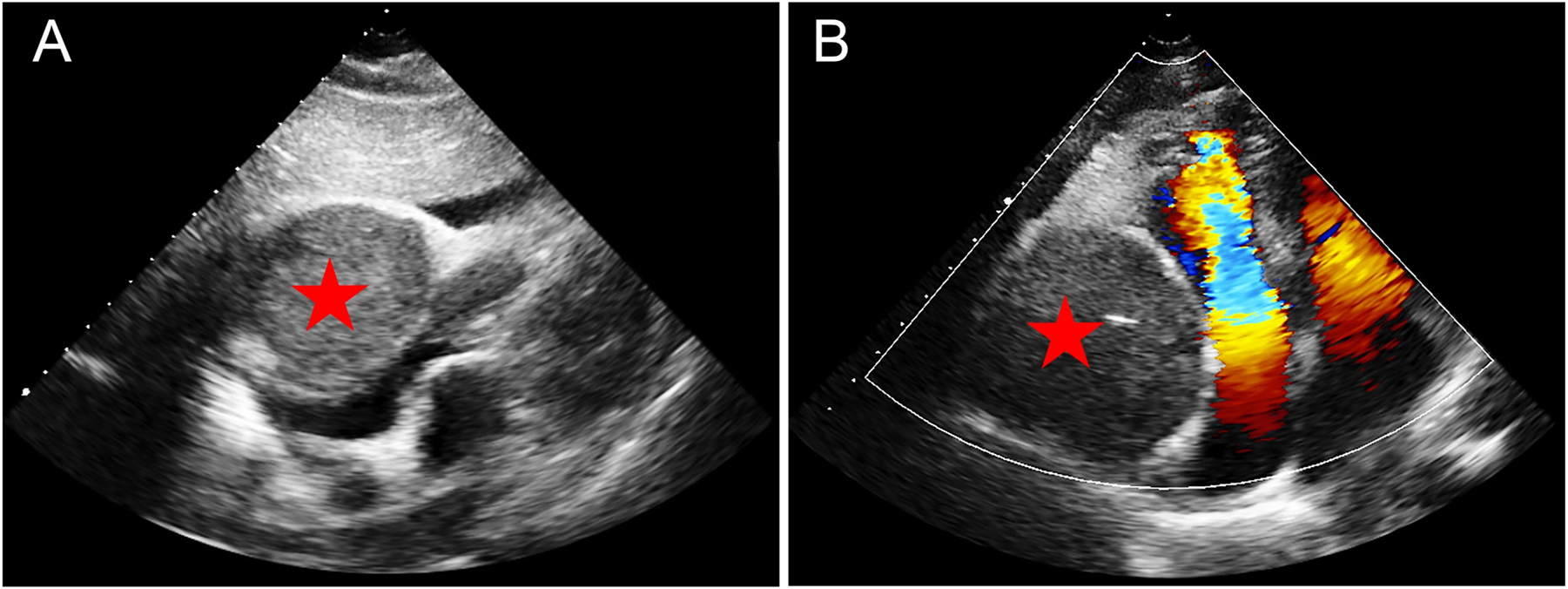
Echocardiography findings of PGL. (A) The four-chamber cardiac section below the xiphoid: a mass approximately 8.1×7.9 cm in size and less echogenic than the liver (red pentagram) was seen adjacent to the right side of the heart, causing compression and deformation of the right atrium and ventricle. (B) The apical four-chambered heart. This mass (red pentagram) compresses the right atrium and ventricle, resulting in increased blood flow velocity in the right heart and colored confusion artifacts.
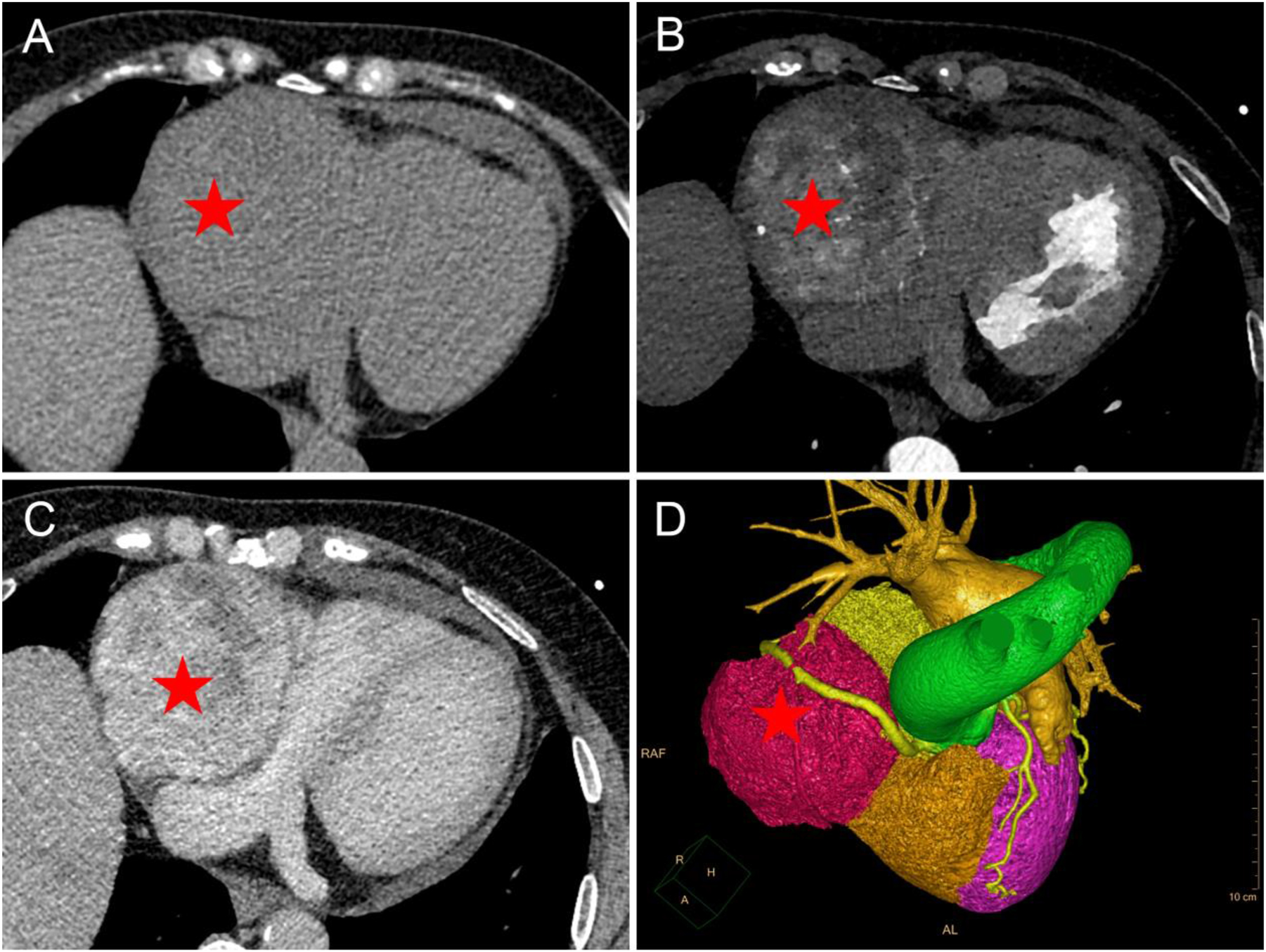
Chest CT and coronary CTA findings of PGL. (A–C) Chest CT. A large mass similar in density to the liver (red pentagram) was seen adjacent to the right side of the heart, which was not clearly delineated from the right atrium and ventricle. CT attenuation was 34 Hounsfield units (HU) on non-enhanced CT ((A) non-enhanced CT). After contrast administration, the mass (red pentagram) was continuously and heterogeneously enhanced (B) arterial phase; (C) venous phase. (D) Volume rendering showed that the mass was partially surrounded by the right coronary artery, which was moderately displaced by the mass.
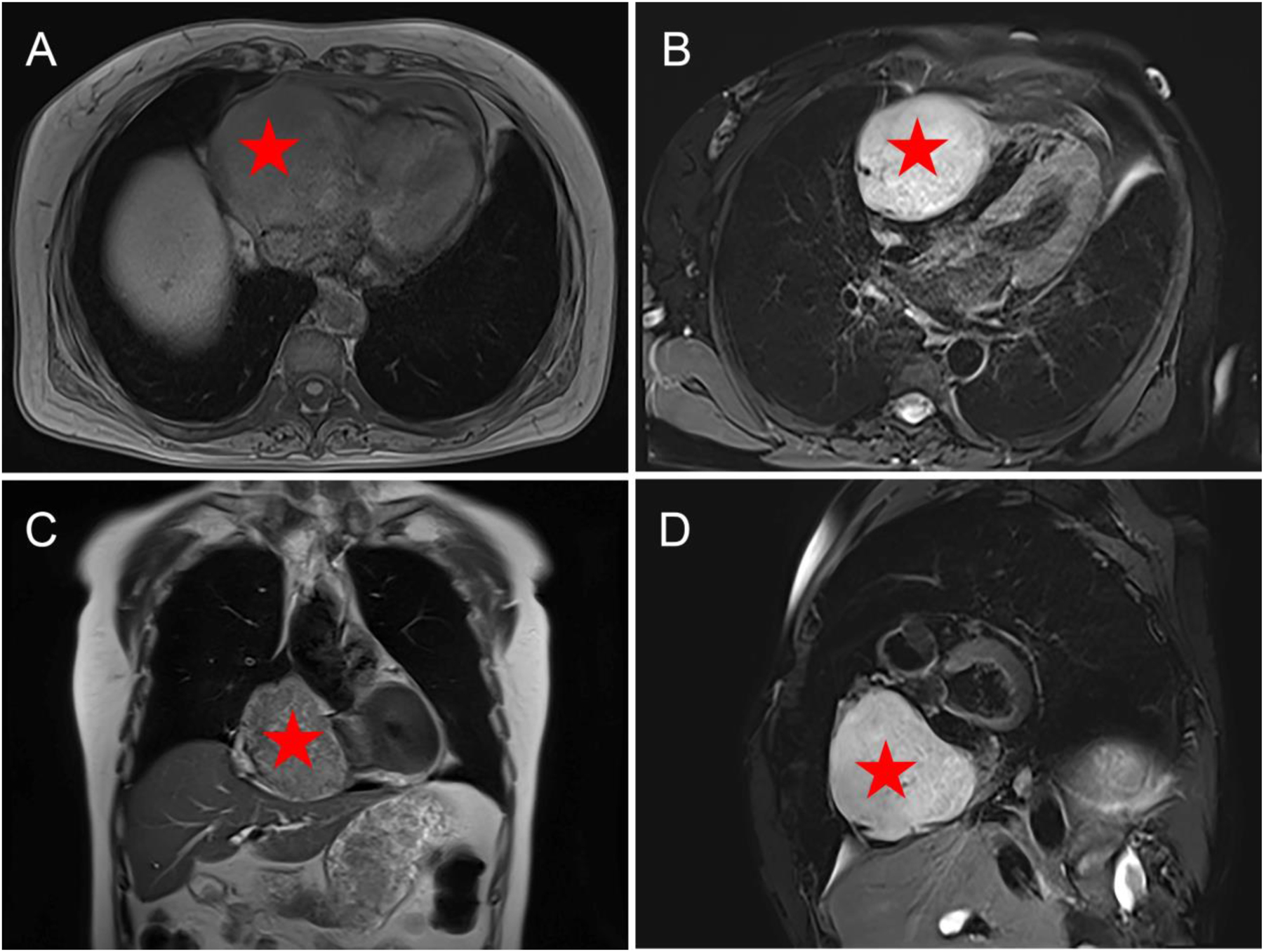
Cardiac MRI findings of PGL. A mass adjacent to the right side of the heart with long T1 and T2 signals completely surrounded by the outer edge of the pericardium. (A) Axial T1WI; (B) axial T2WI-FS; (C) coronary T2WI; (D) sagittal T2WI-FS.
A median sternotomy was performed, and the pericardium was incised and suspended. A solid tumor measuring approximately 7 cm×6 cm×6 cm extending from the right atrium to the surface of the right ventricle was visualized under the epicardium of the right heart, and the surface of the tumor was richly vascularized. Ultrasonography showed that the right coronary artery surrounded the tumor. Under extracorporeal circulation, the gap between the epicardium and the surface of the tumor was gradually separated, and the tumor was completely removed. There was no rupture or perforation of the ventricular wall. The cavity formed after tumor resection was heavily oozing blood, which was difficult to stop. A bovine pericardial patch was used to shunt blood from the tumor cavity into the right atrium. After complete hemostasis, a pericardial mediastinal drain was placed. The postoperative tumor specimen was analyzed by a pathologist. The tumor cells were arranged in cords and glands. And there were abundant interstitial blood vessels. The cytoplasm was eosinophilic and partially transparent. Immunohistochemical stains were positive for CD56, Synaptophysin, S-100, Chromogranin A, SDHB, Sox-10, ATRX, CD34, Des, FH, GATA-3, H3K27me3, and Vimentin, but negative for SC, CA9, CK, Calretinin, SMA, Stat6, and WT-1. Finally, this tumor was diagnosed as PGL (Figure 4). The patient had a good postoperative prognosis with stable vital signs and was discharged 10 days after surgery. The patient will be followed closely in the future.
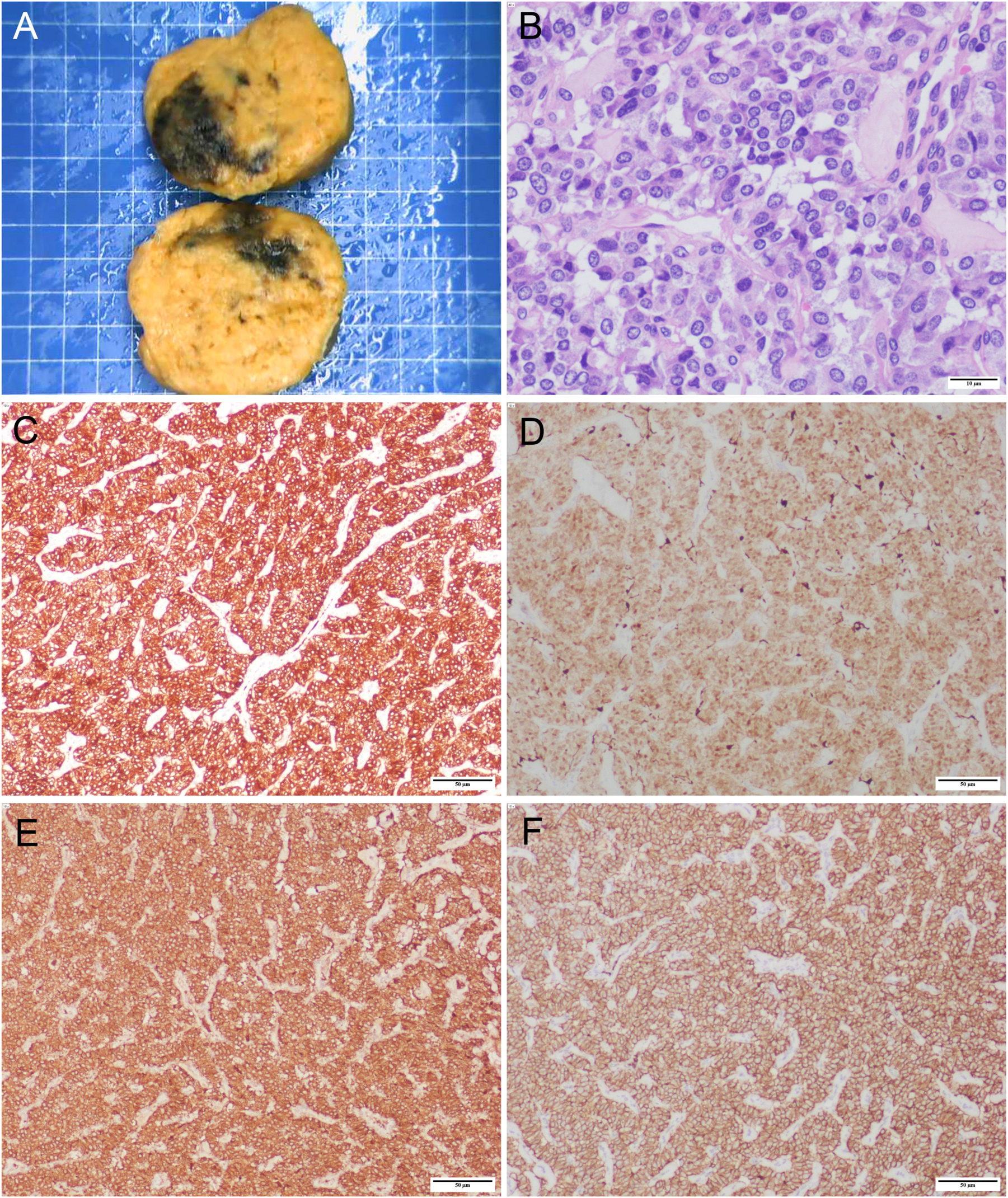
Final pathology slide of PGL specimen. (A) Surgical specimen (size: 7 cm×6 cm×6 cm). (B) Hematoxylin and eosin staining of paraganglioma (×400), scale bar: 10 μm. Immunohistochemistry results: (C) Synaptophysin (×100); (D) S-100 (×100); (E) Chromogranin A (×100); (F) CD56 (×100). Scale bar: 50 μm.
Discussion
PGL is a rare type of neuroendocrine neoplasm, with intrapericardial PGL accounting for less than 2 % of all paragangliomas [1]. Paragangliomas and pheochromocytomas are neuroendocrine tumors of the same pathologic type and cannot be distinguished by histologic type [4]. The only difference is the site of origin, which is called pheochromocytoma when it is located in the adrenal gland and PGL when it is located outside the adrenal gland [5]. Paragangliomas originate from neural crest tissue (paraganglia of visceral autonomic and branch nerves) and are strongly associated with mutations in the SDHx, FH, PHD1/2 and EPAS1/HIF2A genes, with the head and neck being the most common site [5, 6]. Clinical manifestations vary depending on whether the tumor tissue is functional or not. PGL is divided into non-functional PGL and functional PGL (non-functional PGL: the tumor does not secrete catecholamines and the patient has no clinical symptoms or only symptoms of tumor compression; functional PGL: the tumor secretes catecholamines and the patient has symptomatic hypertension) [1, 5, 7]. This patient in our study had no history of hypertension. And histologic analysis and immunohistochemical staining confirmed the diagnosis of PGL. Therefore, the present case can be classified as non-functional PGL.
Using “intrapericardial” and “paraganglioma” as search terms, we cumulatively searched 21 papers from 1987 to 2022 [3, 7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]. All the literature is a single case report with 12 females, eight males, and 1 case where gender was not explicitly reported. The minimum age of the patients was 25 years, and the maximum age was 78 years. In all cases, 11 cases of PGL were functional, 8 cases of PGL were nonfunctional, and the type of PGL was unknown in two patients. The smallest mass was 2.6 cm and the largest was 9.0 cm. 19 tumors were surgically resected, 1 tumor was treated with radiation, and 1 tumor had no clear reported treatment (see Supplementary Table 1 for more information).
We further analyzed the diagnostic program of these previously reported intrapericardial PGL and summarized some of their features. The gold standard for the diagnosis of PGL is a surgical biopsy of the lesion, but the benign or malignant nature of PGL is not determined by pathology, but by the presence of distant metastases and the biological behavior of recurrence (tumors larger than 5 cm have a higher risk of malignancy) [1, 27]. Imaging examinations also play an important role in its diagnosis [1]. Echocardiography is used as a routine preoperative test, and PGL presents as an intrapericardial hypoechoic mass on echocardiography, but its specificity is poor [27, 28]. Due to the low lipid content of PGL, non-enhanced CT is also valuable in the diagnosis of PGL (CT values greater than 10 HU are more likely to be PGL, at which point enhanced CT and MRI are required to further define PGL) [1, 29]. PGL shows isointense or hypointense relative to muscle on T1WI, but the signal intensity is increased if the lesion is accompanied by hemorrhage. PGL shows hyperintense on T2WI and there is no signal reduction in the fat-suppressed sequence [27]. Because PGL is a vascular-rich tumor, it shows significant enhancement on both contrast-enhanced CT scans and contrast-enhanced MRI scans. If necrosis is present within the PGL, the PGL will show heterogeneous enhancement [27, 29]. Functional imaging based on physiological metabolism (131I/123I-MIBG, 18F-FDOPA, 18F-FDG, 68GA-DOTA-SSA) also plays an important role in the diagnosis of parasympathetic neuroma when it is functional, especially for bone or distant soft tissue metastases that are difficult to detect on CT or MRI [5, 27].
Consistent with the previous references, the diagnosis of PGL in this case was made by pathological examination. The PGL lesion presented as a hypoechoic solid mass on echocardiography and had a CT of 34 HU. And the PGL in this case showed significant and heterogeneous enhancement on contrast-enhanced CT. The PGL in this case showed hypointense on T1WI and hyperintense on T2WI, and there was no significant signal reduction on compression fat T2WI. Nevertheless, the patient in our study did not have a PET-CT scan for economic reasons.
We also further analyzed the treatment of these previously reported intrapericardial PGL and summarized some of their features. Surgical resection is the treatment of choice for a well-defined diagnosis of PGL [5, 25, 27]. And echocardiography, CT, MRI, and functional imaging can help localize the PGL and clarify the degree of infiltration of the PGL with surrounding tissues, which in turn can help clinicians develop a surgical plan [14, 15, 18, 25, 27, 29]. The vascular nature of PGL not only determines the high risk of bleeding during biopsy but also increases the technical difficulty of surgical resection of PGL [18]. CT angiography can delineate the trophoblastic arteries of PGL, allowing the clinician to embolize the arteries and reduce the risk of intraoperative bleeding [25]. If this PGL is functional, the surgeon should give the patient α-adrenoceptor antagonist (phenoxybenzamine, doxazosin) and β-adrenoceptor antagonist (take-home pay, atenolol) two weeks before surgery to avoid intraoperative hypertensive crisis [3]. Because paragangliomas arising anywhere in the retroperitoneum or adjacent to the spine are usually adjacent to large blood vessels, most surgeons choose an open surgical approach, while a few may choose a minimally invasive approach depending on the circumstances [5]. Patients with multiple and metastatic lesions should receive pharmacologic treatment (including chemotherapy and targeted therapy) as appropriate [30]. Paragangliomas carry a risk of recurrence, so patients should be followed with biochemical tests and imaging throughout their lives after surgery [5].
Compared with previous studies, we report this case of PGL with rich imaging, which may provide some reference value for the study of PGL. However, there are some shortcomings in this case. First, the tumor was resected without a clear pathologic diagnosis of the tumor, which may pose a greater risk to the outcome. Second, after the tumor was diagnosed as PGL by postoperative pathology, no functional imaging was performed to assess for definitive metastases, which may be important during patient follow-up.
Conclusions
In conclusion, intrapericardial PGL is a rare occurrence. Our study reported a case of intrapericardial PGL diagnosed by pathology. We reviewed and analyzed the imaging features and treatment of PGL. This case report may serve as a reference for further analysis.
Funding source: the Central Government Guides Local Special Fund Projects for Science and Technology Development
Funding source: Innovation Ability Enhancement Project of Daping Hospital - Multimodal ultrasound in BI-RADS category 4 breast mass grading diagnosis and prognostic assessment of breast cancer
Award Identifier / Grant number: ZXZYTSLC07
Acknowledgments
We thank all the medical staff of the Department of Diagnostic Ultrasound and Cardiovascular Surgery for their support and assistance in our work.
-
Research ethics: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ Institutional Review Board or equivalent committee (Ratification NO: 2023(128)).
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: The authors confirm contribution to the paper as follows: study conception and design: Yuhong Fan, Jingqin Fang; data collection: Yuhong Fan, Jingqin Fang, Jiayin Hu; analysis and interpretation of results: Yuhong Fan, Jingqin Fang, Jiayin Hu, Tao Li; draft manuscript preparation: Yuhong Fan, Jiayin Hu. All authors reviewed the results and approved the final version of the manuscript.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: This work was supported by Innovation Ability Enhancement Project of Daping Hospital – Multimodal ultrasound in BI-RADS category 4 breast mass grading diagnosis and prognostic assessment of breast cancer (Grant Number: ZXZYTSLC07), the Central Government Guides Local Special Fund Projects for Science and Technology Development.
-
Data availability: All data generated and analyzed during this study are included in this article.
References
1. Carrasquillo, JA, Chen, CC, Jha, A, Ling, A, Lin, FI, Pryma, DA, et al.. Imaging of pheochromocytoma and paraganglioma. J Nucl Med 2021;62:1033–42. https://doi.org/10.2967/jnumed.120.259689.Search in Google Scholar PubMed PubMed Central
2. Neumann, HPH, Young, WFJr., Eng, C. Pheochromocytoma and paraganglioma. New Engl J Med 2019;381:552–65. https://doi.org/10.1056/nejmra1806651.Search in Google Scholar PubMed
3. Siejka, DA, Vittorio, AF, Thakur, S, Burgess, JR, Hardikar, A. Resection of a functioning intrapericardial paraganglioma associated with succinate dehydrogenase B mutation. SAGE Open Med Case Rep 2019;7:2050313x19839530. https://doi.org/10.1177/2050313x19839530.Search in Google Scholar
4. Ayala-Ramirez, M, Feng, L, Johnson, MM, Ejaz, S, Habra, MA, Rich, T, et al.. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab 2011;96:717–25. https://doi.org/10.1210/jc.2010-1946.Search in Google Scholar PubMed
5. Moon, JK, Mattei, P. Pheochromocytoma and paraganglioma. Semin Pediatr Surg 2020;29:150926. https://doi.org/10.1016/j.sempedsurg.2020.150926.Search in Google Scholar PubMed
6. Savvateeva, M, Kudryavtseva, A, Lukyanova, E, Kobelyatskaya, A, Pavlov, V, Fedorova, M, et al.. Somatic mutation profiling in head and neck paragangliomas. J Clin Endocrinol Metab 2022;107:1833–42. https://doi.org/10.1210/clinem/dgac250.Search in Google Scholar PubMed PubMed Central
7. Tsilimparis, N, Gregor, JI, Swierzy, M, Ismail, M, Rogalla, P, Weichert, W, et al.. Intrapericardial paraganglioma in a 78-year-old female patient. Am Surg 2010;76:450–1. https://doi.org/10.1177/000313481007600429.Search in Google Scholar
8. Shimoyama, Y, Kawada, K, Imamura, H. A functioning intrapericardial paraganglioma (pheochromocytoma). Br Heart J 1987;57:380–3. https://doi.org/10.1136/hrt.57.4.380.Search in Google Scholar PubMed PubMed Central
9. Banzo, J, Prats, E, Velilla, J, Monzón, FJ, Delgado, M. Functioning intrapericardial paraganglioma diagnosed by I-123 MIBG imaging. Clin Nucl Med 1991;16:860–1. https://doi.org/10.1097/00003072-199111000-00016.Search in Google Scholar PubMed
10. Casanova, J, Moura, CS, Torres, JP, Vouga, L, Graça, AS, Gomes, MM. Intrapericardial paraganglioma. Eur J Cardio Thorac Surg 1996;10:287–9. https://doi.org/10.1016/s1010-7940(96)80154-0.Search in Google Scholar PubMed
11. Boumzebra, DA, Charifchefchaouni, ZS, Belhadj, SA, Maazouzi, WA, Al-Halees, ZY. Intrapericardial paraganglioma. Saudi Med J 2002;23:1278–80.Search in Google Scholar
12. Hawari, M, Yousri, T, Hawari, R, Tsang, G. Intrapericardial paraganglioma directly irrigated by the right coronary artery. J Card Surg 2008;23:780–2. https://doi.org/10.1111/j.1540-8191.2008.00620.x.Search in Google Scholar PubMed
13. Lee, CC, Chua, S, Huang, SC, Lee, FY, Chung, SY. Intrapericardial paraganglioma with intratumoral coronary arterial aneurysm and an arteriovenous fistula. J Am Soc Echocardiogr 2009;22:211.e1–3. https://doi.org/10.1016/j.echo.2008.12.001.Search in Google Scholar PubMed
14. Rana, O, Gonda, P, Addis, B, Greaves, K. Image in cardiovascular medicine. Intrapericardial paraganglioma presenting as chest pain. Circulation 2009;119:e373–5. https://doi.org/10.1161/circulationaha.108.822197.Search in Google Scholar
15. Gökalp, G, Topal, U, Cavuşoğlu, G, Saraydaroğlu, O. Intrapericardial paraganglioma. Anadolu Kardiyol Derg 2010;10:E20–1. https://doi.org/10.5152/akd.2010.153.Search in Google Scholar PubMed
16. Pacheco Gómez, N, Marcos Gómez, G, Garcipérez de Vargas, FJ, Pérez Calvo, C. Intrapericardial paraganglioma. Rev Esp Cardiol 2010;63:116–7. https://doi.org/10.1016/s1885-5857(10)70020-7.Search in Google Scholar PubMed
17. Imperatori, A, De Monte, L, Rotolo, N, Dionigi, G, Uccella, S, Mariscalco, G, et al.. Hypertension and intrapericardial paraganglioma: an exceptional presentation of multiple endocrine neoplasia type IIA syndrome. Hypertension 2011;58:e189–90. https://doi.org/10.1161/hypertensionaha.111.180992.Search in Google Scholar
18. Matsumoto, J, Tanaka, N, Yoshida, Y, Yamamoto, T. Resection of an intrapericardial paraganglioma under cardiopulmonary bypass. Asian Cardiovasc Thorac Ann 2013;21:476–8. https://doi.org/10.1177/0218492312459641.Search in Google Scholar PubMed
19. Tracy, JC, Wein, RO. Intrapericardial paraganglioma associated with succinate dehydrogenase complex subunit C mutation syndrome. Head Neck 2013;35:E251–3. https://doi.org/10.1002/hed.23098.Search in Google Scholar PubMed
20. Brichon, PY, Chavanon, O, Thony, F, Wion, N. An intrapericardial paraganglioma with embolization of a large vessel from the left coronary artery. Eur J Cardio Thorac Surg 2014;45:e234. https://doi.org/10.1093/ejcts/ezu110.Search in Google Scholar PubMed
21. Ojiaku, M, Peña, E, Belanger, EC, Chan, KL, Dennie, C. Functioning intrapericardial paraganglioma: multimodality imaging findings and pathological correlation. Circulation 2014;130:e137–9. https://doi.org/10.1161/circulationaha.114.012458.Search in Google Scholar PubMed
22. Teodorescu, M, Coche, E, Ghaye, B. Intrapericardial paraganglioma. J Belg Radiol 2014;97:170–1. https://doi.org/10.5334/jbr-btr.67.Search in Google Scholar PubMed
23. Yamamoto, Y, Kodama, K, Yamato, H, Takeda, M. Successful removal of giant intrapericardial paraganglioma via posterolateral thoracotomy. Case Rep Surg 2014;2014:308462. https://doi.org/10.1155/2014/308462.Search in Google Scholar PubMed PubMed Central
24. Degrauwe, S, Monney, P, Qanadli, SD, Prior, J, Beigelmann-Aubry, C, Masci, PG, et al.. Intrapericardial paraganglioma: the role of integrated advanced multi-modality cardiac imaging for the assessment and management of rare primary cardiac tumors. Cardiol J 2017;24:447–9. https://doi.org/10.5603/cj.2017.0091.Search in Google Scholar PubMed
25. Chen, YY, Huang, WC, Huang, MH, Lu, TM, Hsu, CP. The intra-pericardial paraganglioma presenting as ascites and hemopericardium with impending tamponade. Int Heart J 2018;59:664–7. https://doi.org/10.1536/ihj.17-150.Search in Google Scholar PubMed
26. Gomes de Farias, LP, Teles, G, Baptista, LPS, de Albuquerque, AS. Intrapericardial paraganglioma. Radiol: Cardiothorac Imaging 2022;4:e220100. https://doi.org/10.1148/ryct.220100.Search in Google Scholar PubMed PubMed Central
27. Huang, WP, Gao, G, Chen, Z, Qiu, YK, Gao, JB, Kang, L. Multimodality imaging evaluation of primary right atrial paraganglioma: a case report and literature review. Front Med 2022;9:942558. https://doi.org/10.3389/fmed.2022.942558.Search in Google Scholar PubMed PubMed Central
28. Zhao, SH, Liang, S, Luo, J, Mo, HD, Jiang, Y, Zhang, MM, et al.. Heart combined with adrenal multiple pheochromocytomas. J Nucl Cardiol 2018;25:1040–3. https://doi.org/10.1007/s12350-017-0860-9.Search in Google Scholar PubMed
29. Gunawardane, PTK, Grossman, A. Phaeochromocytoma and paraganglioma. Adv Exp Med Biol 2017;956:239–59. https://doi.org/10.1007/5584_2016_76.Search in Google Scholar PubMed
30. Shindorf, ML, Chaudhuri, PK. Single-agent thalidomide for treatment of malignant paraganglioma of the organ of Zuckerkandl. Case Rep Med 2019;2019:7185973. https://doi.org/10.1155/2019/7185973.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/oncologie-2023-0483).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- Mesenchymal stem cell exosomes: a promising delivery system for glioma therapy
- Research Articles
- A multi-cancer analysis unveils ITGBL1 as a cancer prognostic molecule and a novel immunotherapy target
- MicroRNA-302a enhances 5-fluorouracil sensitivity in HepG2 cells by increasing AKT/ULK1-dependent autophagy-mediated apoptosis
- Integrated analysis of TCGA data identifies endoplasmic reticulum stress-related lncRNA signature in stomach adenocarcinoma
- Glioblastoma with PRMT5 gene upregulation is a key target for tumor cell regression
- BRCA mutation in Vietnamese prostate cancer patients: a mixed cross-sectional study and case series
- Microglia increase CEMIP expression and promote brain metastasis in breast cancer through the JAK2/STAT3 signaling pathway
- Integrative machine learning algorithms for developing a consensus RNA modification-based signature for guiding clinical decision-making in bladder cancer
- Transcriptome analysis of tertiary lymphoid structures (TLSs)-related genes reveals prognostic value and immunotherapeutic potential in cancer
- Prognostic value of a glycolysis and cholesterol synthesis related gene signature in osteosarcoma: implications for immune microenvironment and personalized treatment strategies
- Identification of potential biomarkers in follicular thyroid carcinoma: bioinformatics and immunohistochemical analyses
- Rapid Communication
- Comparison of the Molecular International Prognostic Scoring System (IPSS-M) and Revised International Prognostic Scoring System (IPSS-R) in predicting the prognosis of patients with myelodysplastic neoplasms treated with decitabine
- Case Reports
- Intrapericardial nonfunctional paraganglioma: a case report and literature review
- Treatment of central nervous system relapse in PLZF::RARA-positive acute promyelocytic leukemia by venetoclax combined with arubicin, cytarabine and intrathecal therapy: a case report
Articles in the same Issue
- Frontmatter
- Review Article
- Mesenchymal stem cell exosomes: a promising delivery system for glioma therapy
- Research Articles
- A multi-cancer analysis unveils ITGBL1 as a cancer prognostic molecule and a novel immunotherapy target
- MicroRNA-302a enhances 5-fluorouracil sensitivity in HepG2 cells by increasing AKT/ULK1-dependent autophagy-mediated apoptosis
- Integrated analysis of TCGA data identifies endoplasmic reticulum stress-related lncRNA signature in stomach adenocarcinoma
- Glioblastoma with PRMT5 gene upregulation is a key target for tumor cell regression
- BRCA mutation in Vietnamese prostate cancer patients: a mixed cross-sectional study and case series
- Microglia increase CEMIP expression and promote brain metastasis in breast cancer through the JAK2/STAT3 signaling pathway
- Integrative machine learning algorithms for developing a consensus RNA modification-based signature for guiding clinical decision-making in bladder cancer
- Transcriptome analysis of tertiary lymphoid structures (TLSs)-related genes reveals prognostic value and immunotherapeutic potential in cancer
- Prognostic value of a glycolysis and cholesterol synthesis related gene signature in osteosarcoma: implications for immune microenvironment and personalized treatment strategies
- Identification of potential biomarkers in follicular thyroid carcinoma: bioinformatics and immunohistochemical analyses
- Rapid Communication
- Comparison of the Molecular International Prognostic Scoring System (IPSS-M) and Revised International Prognostic Scoring System (IPSS-R) in predicting the prognosis of patients with myelodysplastic neoplasms treated with decitabine
- Case Reports
- Intrapericardial nonfunctional paraganglioma: a case report and literature review
- Treatment of central nervous system relapse in PLZF::RARA-positive acute promyelocytic leukemia by venetoclax combined with arubicin, cytarabine and intrathecal therapy: a case report

