Abstract
Complexes of Co(II), Ni(II), Cu(II), and Zn(II) containing N2S2 ligand have been synthesized from the template condensation reaction between 1,2-ethanedithiol, o-bromoaniline and dimethyl tin dichloride in 1:2:1 molar ratio. It resulted in the formation of new series of 11-membered N2S2-Tin macrocyclic complexes [MLX2Sn(CH3)2] (M=Co(II) or Zn(II); X=Cl or NO3); and [MLSn(CH3)2]X2 (M=Ni(II) or Cu(II); X=Cl or NO3). The complexes were characterized using techniques including elemental analyses, IR, 1H NMR, 119Sn NMR, EPR, electronic spectral studies, conductivity, and magnetic susceptibility measurements. The reducing power of the Co(II) and Cu(II) complexes have been checked and compared. The complexes derived from nickel and copper have been found to show square-planar geometry, while octahedral geometry is projected for the cobalt and zinc complexes.
Introduction
The coordination chemistry of macrocyclic precursors is a fascinating area which has attracted the attention of inorganic chemists. Macrocyclic complexes of transition metals have been of great interest due to their importance in view of their various applications (Song et al., 2008; Mathew et al., 2014), particularly in the field of catalysis (Bush and Alcock, 1994; Javanbakht et al., 1999). Multi-donor ligands and particularly mixed donors are important due to the presence of several potential donor centers and their flexibility to bind with biomolecules or to coordinate with various metal ions. Mixtures of two or more donor sites have also been employed to tune selectivity and stability (Singh et al., 2008). A number of mixed donor macrocycles have been reported (Richard et al., 2004; Ilhan et al., 2007; Lindoy et al., 2013) incorporating N2S2, O3N2, O2N3, or N4S2 donor sets, and investigation of the effects of incorporating a soft sulfur donor into a ligand framework containing harder nitrogen atoms is of great interest (Adhikari et al., 1997; Danks et al., 1998; Tavacoli et al., 2003; Quintana and Roglans, 2005). Although sulfides are generally treated as poor ligands to transition metal centers (Anderson et al., 1997), recent studies (Bottino et al., 1976; Bruce et al., 1996; White et al., 2005) have proved that macrocyclic sulfides readily bind to transition metal ions to form stable complexes. Template synthesis is the organization of an assembly of atoms with respect to one or more geometric loci to achieve a particular linking of atoms. The template macrocyclic synthesis utilizes metal ions as the “anchor” of the template complex. The advantages of this synthesis are the ease and high yield of the reaction, as well as work best for bridging adjacent nitrogens in polycyclic azamacrocycles (Mohamed et al., 2005). The considerable developments over recent decades in the use of organotin compounds as reagents or intermediates in organic synthesis prompted the preparation of many new organotin compounds (Chaudhary et al., 2006). A considerable number of organotin macrocyclic complexes with nitrogen donors have been reported over the past decades (Garnovskii et al., 1993; Chaudhary et al., 2002a,b; Khan et al., 2005). Here, we wish to report a new series of dithiadiaza macrocyclic complexes [MLX2] (M=Co(II) or Zn(II);X=Cl or NO3; and [ML]X2 (M=Ni(II) or Cu(II); X=Cl or NO3) obtained by the template condensation of 1,2-ethanedithiol, o-bromoaniline, and dimethyl tin (IV) dichloride in 1:2:1 molar ratio.
Results and discussion
All of the complexes are colored; stable at room temperature; non-hygroscopic; and were soluble in DMSO, DMF, and acetonitrile. Attempts to prepare metal-free ligands in methanol led to an oily product, which could not be isolated. However, when the condensation reaction was carried out in the presence of metal ions, solid products were obtained in good yields (65–85%). The complexes prepared by the template method have a 1:1 metal to ligand stoichiometry as well as the analytical results (Table 1) agree well with the proposed structures of the complexes, as shown in Figure 1. The purity of all the complexes was checked by thin-layer chromatography which results in a single spot corresponding to the final product. The molar conductance values of the cobalt and zinc complexes indicate that they are non-electrolytes while all other complexes are ionic (Geary, 1971; Siddiqi and Khan, 2004). The overall geometries were inferred from the various spectroscopic studies discussed below.
Yield, color, m.p., μeff, elemental analysis, molar conductance, and electronic spectral data of the compounds.
| Complexes | Yield (%) | Color | Melting point (°C) | μeff (BM) | Found (calc) % | Λm (cm-1 Ω-1mol-1) | Band position (cm-1) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Cl | C | H | N | |||||||
| [CoL(NO3)2] | 75 | Dark brown | 323 | 4.04 | 10.0 | – | 31.5 | 3.1 | 9.5 | 23 | 16 400 |
| (9.7) | (31.7) | (3.3) | (9.2) | 21 500 | |||||||
| [CoLCl2] | 72 | Dark brown | 314 | 4.08 | 10.8 | 12.5 | 34.3 | 3.8 | 5.3 | 21 | 16 600 |
| (10.6) | (12.8) | (34.7) | (3.6) | (5.1) | 21 450 | ||||||
| [NiL](NO3)2 | 70 | Blackish brown | 310 | 3.10 | 10.5 | – | 35.5 | 3.6 | 10.5 | 112 | 15 850 |
| (10.7) | (35.1) | (3.2) | (10.2) | 19 550 | |||||||
| [NiL]Cl2 | 69 | Dark brown | 316 | 3.14 | 12.3 | 14.5 | 38.7 | 3.8 | 6.0 | 103 | 15 900 |
| (11.9) | (14.3) | (38.9) | (4.1) | (5.7) | 19 300 | ||||||
| [CuL](NO3)2 | 62 | Dim green | 330 | 1.80 | 11.2 | – | 35.4 | 3.6 | 10.5 | 100 | 16 210 |
| (11.6) | (35.1) | (3.7) | (10.2) | 21 600 | |||||||
| 11 400 | |||||||||||
| [CuL]Cl2 | 68 | Dark green | 310 | 1.77 | 12.7 | 14.5 | 39.2 | 3.9 | 5.9 | 105 | 16 100 |
| (12.9) | (14.3) | (38.9) | (4.1) | (5.7) | 21 650 | ||||||
| 11 350 | |||||||||||
| [ZnL(NO3)2] | 69 | Grayish blue | 306 | – | 11.6 | – | 35.3 | 3.4 | 10.5 | 20 | – |
| (11.9) | (35.1) | (3.7) | (10.2) | ||||||||
| [ZnLCl2] | 70 | Grayish blue | 309 | – | 13.4 | 14.2 | 38.6 | 3.8 | 5.9 | 19 | – |
| (13.2) | (14.3) | (38.9) | (4.1) | (5.7) | |||||||
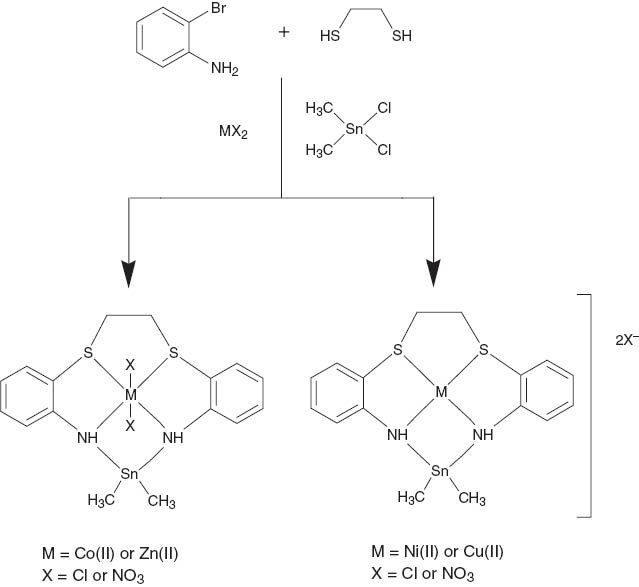
Proposed structure of the synthesized complexes.
IR spectra
The prominent IR spectral bands of the complexes are presented in Table 2. The absence of band characteristic of thiol groups and the appearance of bands corresponding to a secondary amino group strongly suggests that the ligand framework is formed. All complexes exhibit a single sharp band in the region 3230–3275 cm-1, ascribed to the coordinated secondary amino group suggesting that nitrogen is involved in the coordination to the metal ions, respectively (Khan et al., 2010). The presence of sharp band in the region 372–410 cm-1 in all the complexes is due to ν(M-N) vibrations (Khan and Ghani, 2005). Bands in the region 255–280 cm-1 are assigned to the ν(M-Cl) stretching vibrations. A new medium intensity band in the region 320–380 cm-1 may reasonably be assigned to ν(M-S). The appearance of a new band in the region 440–480 cm-1 assignable to the ν (Sn-N) vibrations suggested that the nitrogen is coordinated to the tin atom (Chaudhary et al., 2002a,b). The Sn-Cl stretching vibrations of compounds have been assigned at 485–500 cm-1 as reported earlier also (Dey et al., 2000). The phenyl ring and other fundamental vibrations (Khan et al., 2010) appeared at their expected positions.
IR vibrational frequencies (cm-1) and EPR spectral data of the complexes.
| Compound | ν(N-H) | ν(C-H) | ν(Sn-N) | ν(M-N) | ν(Sn-Cl) | ν(M-S) | ν(M-Cl) | Ring vibrations | EPR data | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| g∣∣ | g⊥ | G | |||||||||
| [CoL(NO3)2] | 3240 | 2960 | 445 | 372 | 490 | 330 | 260 | 1415, 1050, 740 | – | – | – |
| [CoLCl2] | 3275 | 2950 | 475 | 390 | 495 | 355 | 280 | 1410, 1020, 730 | – | – | – |
| [NiL](NO3)2 | 3260 | 2885 | 460 | 385 | 485 | 375 | – | 1405, 1015, 720 | – | – | – |
| [NiL]Cl2 | 3230 | 2920 | 440 | 410 | 500 | 370 | – | 1420, 1060, 750 | – | – | – |
| [CuL](NO3)2 | 3250 | 2925 | 480 | 380 | 495 | 380 | – | 1430, 1055, 720 | 2.36 | 2.11 | 3.4 |
| [CuL]Cl2 | 3260 | 2930 | 460 | 380 | 500 | 370 | – | 1430, 1020, 715 | 2.40 | 2.07 | 3.1 |
| [ZnL(NO3)2] | 3270 | 2925 | 450 | 390 | 485 | 320 | 255 | 1435, 1050, 725 | – | – | – |
| [ZnLCl2] | 3255 | 2910 | 470 | 410 | 495 | 350 | 270 | 1410,1030, 720 | – | – | – |
EPR spectra
The EPR spectra (Table 2) of copper(II) macrocyclic complexes have been recorded at room temperature and all the complexes gave the same type of spectrum. The g∣∣ and g⊥ were found to be 2.36–2.40 and 2.07–2.11, respectively. The magnitude of the ratio G=(g∣∣–2)/ (g⊥–2) is a measure of exchange interaction in the copper(II) complexes (Khan and Ghani, 2005). The calculated G values for the complexes appeared in the range 3.1–3.4 which suggests (G<4) a considerable exchange interaction in these solid complexes (Khan et al., 2010).
1H NMR spectra
The 1H NMR spectra of Zn(II) dithiadiaza-tin macrocyclic complexes recorded in DMSO-d6 show a multiplet in the 6.63–6.78 ppm region which can be assigned (Burgess et al., 1994) to the secondary amino protons of the o-bromoaniline indicating the replacement of primary amino proton by the diorganotin moiety. The 1H-NMR spectra of the complexes do not show any signal corresponding to primary amino protons of the condensed o-bromoaniline moiety, suggesting that the proposed macrocyclic frameworks have been formed. The spectrum showed a singlet peak at around 2.09–2.15 ppm assigned to methyl proton (CH3, 6H) of the dimethyltin moiety (Raman and Thangaraja, 2005). The 1H NMR spectra of all the zinc complexes gave a multiplet in the region δ 3.48–3.65 corresponding (Duckworth et al., 1993) to methylene protons adjacent to sulfur (S-CH2; 4H). Furthermore, the absence of thiol protons again confirms the proposed framework. The multiplets of aromatic protons were observed at δ 7.3–8.09 in the spectra of all the complexes.
The 119Sn NMR spectrum of all the complexes shows the signal at around -170 ppm which gives good agreement with a tetracoordinated tin (Holecek et al., 1986; Baul et al., 2006).
Magnetic moments and electronic spectra
The electronic spectral and magnetic moment data (Table 1) recorded at room temperature for all of the metal complexes are consistent with the proposed structure. The observed magnetic moment values of the cobalt complexes appeared in the range which is expected for three unpaired electrons. The electronic spectra of the complexes derived from the Co(II) ion showed two bands around 16 500 and 21 000 cm-1 which may reasonably be assigned to the 4T1g(F)→4A2g(F) and 4T1g(F)→4T1g(P) transitions, respectively, suggesting an octahedral geometry for the complexes (Lever, 1984).
The macrocyclic complexes derived from Ni(II) exhibit two bands in their electronic spectra (Table 1) at around 15 600 and 19 500 cm-1 and may be assigned to 1A1g→1B1g and 1A1g→1A2g transitions, respectively, suggesting (Siddiqi and Khan, 2004) a square planar geometry around the Ni(II) ion. The magnetic moment values also support the electronic spectral data. The electronic spectra of the copper(II) complexes showed a broadband centered at ca. 16 250 cm-1 assignable (Khan et al., 2005) to the 2B1g→2A1g transition. However, two weak shoulders appearing around at 21 600 and 11 500 cm-1 may be ascribed to the 2B1g→2Eg and 2B1g→2B2g transitions corresponding to a square-planar geometry around the Cu(II) ion. The magnetic moment values further confirm the above proposed geometry.
In order to determine if the metal complexes undergoes solvatochromic shifts in different solvents, the complexes were dissolved in a variety of polar solvents like DMSO, DMF, THF, CHCl3, and their UV visible spectra were recorded. Since the spectra do not exhibit any variation in the absorption pattern as a function of solvent, it is suggested that binding of solvent to the metal ions does not occur. This means that geometry of the metal ions does not change in solution.
Reducing power
The reducing capacity of the complexes acts as an indicator of its potential antioxidant activity (Meir et al., 1995). The Co(II) and Cu(II) complexes were tested for their relative reducing capacities (Figures 2 and 3). The antioxidant activities of recognized antioxidants are ascribed to various mechanisms, such as prevention of chain initiation, decomposition of peroxides, prevention of continued hydrogen abstraction and radical scavenging. Therefore, it is suggested that there is no correlation between total antioxidant activity and reducing power activity (Diplock, 1997). So, Co(II) complexes may have low reducing power and can have high total antioxidant activity than Cu(II) complexes.
![Figure 2 Comparison of immuno sorbent electron microscopy (ISEM) values of the three experiments showing reducing power of [CuLCl2] and [CoLCl2] as series 1 and series 2.](/document/doi/10.1515/mgmc-2014-0032/asset/graphic/mgmc-2014-0032_fig2.jpg)
Comparison of immuno sorbent electron microscopy (ISEM) values of the three experiments showing reducing power of [CuLCl2] and [CoLCl2] as series 1 and series 2.
![Figure 3 Comparison of immuno sorbent electron microscopy (ISEM) values of the three experiments showing reducing power of [CuL(NO3)2] and [CoL(NO3)2] as series 1 and series 2.](/document/doi/10.1515/mgmc-2014-0032/asset/graphic/mgmc-2014-0032_fig3.jpg)
Comparison of immuno sorbent electron microscopy (ISEM) values of the three experiments showing reducing power of [CuL(NO3)2] and [CoL(NO3)2] as series 1 and series 2.
Experimental
The chemicals o-bromoaniline, 1,2-ethanedithiol and dimethyl tin (IV) dichloride (all Fluka Chemicals, Gillingham, UK) were of analytical grade and were used as purchased without further purification. The metal salts MX2.6H2O (M=Co(II) or Ni(II); X=Cl or NO3), CuX2.2H2O, (X=Cl or NO3), ZnCl2 and Zn(NO3)2.6H2O (all BDH, Poole, UK) were commercially available pure samples.
Synthesis of 1:2, 7:8-dibenzo-3,6-dithia-9,11-diazacycloundecane metal(II)- dimethyl tin (IV) dichloride and dinitrate, [MLX2] (M=Co(II) or Zn(II); X=Cl or NO3); and 1:2, 7:8-dibenzo-3,6-dithia-9,11-diazacycloundecane metal(II)-dimethyl tin (IV) chloride and nitrate, [ML]X2 (M=Ni(II), or Cu(II); X=Cl or NO3).
A mixture of o-bromoaniline (20 mmol, 3.44 g) and 1,2-ethanedithiol (10 mmol, 0.83 mL) in methanol (∼75 mL) in a round bottomed flask was stirred with slight heating (38°C) for about 35 min. A warm (32°C) methanolic solution (∼50 mL) of the metal salt (10 mmol) was added drop wise followed by the addition of dimethyl tin (IV) dichloride (10 mmol, 0.22 g). The resulting mixture was stirred for 13 h. A brisk color change from bright to dim was observed after the addition of the metal salt solution. The solid product thus separated was filtered off, washed several times with methanol and then with diethylether and dried in vacuo.
Methodology
Analyses of C, H, and N were carried out on a Perkin-Elmer 240 elemental analyzer, Waltham, USA. IH NMR spectra in DMSO-d6 (Sigma-Aldrich, St. Louis, USA) were recorded on JEOL-FX-100-FT-NMR spectrometer, Cranford, USA with MeSi4 (Sigma-Aldrich, St. Louis, USA) as an internal standard, and 119Sn spectra were recorded on Bruker ACF300, MA, USA (at 300 MHz) with SnMe4 as an external standard. Metals and chlorides were determined volumetrically (Reilley et al., 1959) or gravimetrically (Vogel, 1961). The IR spectra (4000–400 cm-1) were recorded as KBr discs on an IR 408 Shimadzu spectrophotometer (Shandong, China). The electronic spectra in DMSO solution were recorded on a Pye-Unicam 8800 spectrophotometer (Cambrigde, UK). The EPR spectra were recorded on JEOL JES RE 2X EPR spectrometer (MA, USA). For reducing power, the complexes (25–800 mm) in DMSO were mixed with 2.5 mL of phosphate buffer (0.2 m, pH 6.6) and 2.5 mL potassium ferricyanide [K3Fe(CN)6] (1%), and then the mixture was incubated at 50°C for 30 min afterwards, 2.5 mL of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. Finally, 2.5 mL distilled water and 0.5 mL FeCl3 (0.1%), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. The electrical conductivities of 10-3 m solutions in DMSO were obtained on a Systronic type 302 Conductivity Bridge (Gujarat, India) equilibrated at 25±0.01°C. Magnetic susceptibility measurements were carried out using a Faraday balance (TN, USA).
Acknowledgments
The authors thank the Fiji Higher Education Commission for partially funding the research work with grant No. CCCEESD RPF 01. We are also thankful to the University of Fiji, for providing the necessary research facilities.
References
Adhikari, B.; Anderson, O. P.; Hazell, R.; Miller, S. M.; Oslen, C. E.; Toftlund, H. Nickel(II) N2X2 Schiff-base complexes incorporating pyrazole (X=NH, O or S): syntheses and characterization. J. Chem. Soc. Dalton Trans. 1997, 23, 4539–4548.Search in Google Scholar
Anderson, O. P.; Findeisen, M.; Hennig, L.; Simonsen, O.; Toftlund, H. Zinc(II) N2S2 Schiff-base complexes incorporating pyrazole: syntheses, characterization, tautomeric equilibria and racemization kinetics. J. Chem. Soc. Dalton Trans. 1997, 1, 111–120.Search in Google Scholar
Baul, T. S. B.; Singh, K. S.; Linden, A.; Song, X.; Eng. G. Synthesis, spectroscopic characterization of tribenzyltin(IV) complexes of polyaromatic carboxylic acid ligands: crystal and molecular structures of Bz3Sn[O2CC6H4{Ndouble bond; length as m-dashN(C6H3-4-OH(C(H)double bond; length as m-dashNC6H4X-4))}-o](OH2) (X=–Cl, –OCH3). Polyhedron 2006, 25, 3441–3448.Search in Google Scholar
Bottino, F.; Foti, S; Pappalardo, S. Synthesis and characterization of oxygen and sulfur bridged aromatic macrocycles. Tetrahedron. 1976, 32, 2567–2570.Search in Google Scholar
Bruce, J. I.; Donlevy, T. M.; Gahan, L. R.; Kennard, C. H. L.; Byriel, K. A. Zinc(II) complexes of the encapsulating ligands 13-dithia-6,10,16,19-tetraazabicyclo[6.6.6]icosane (amn4s2sar) and 3-thia-6,10,13,16,19-pentaazabicyclo[6.6.6]icosane (amn(5)ssar) - x-ray structural characterization. Polyhedron. 1996,15, 49–55.Search in Google Scholar
Burgess, K.; Lim, D.; Kantto, K.; Ke, C. Y. Asymmetric syntheses of protected derivatives of carnosadine and its stereoisomers as conformationally constrained surrogates for arginine. J. Org. Chem. 1994, 59, 2179–2185.Search in Google Scholar
Bush, D. H.; Alcock, N. W. Iron and cobalt “lacunar” complexes as dioxygen carriers. Chem. Rev., 1994, 94, 585–623.Search in Google Scholar
Chaudhary, A.; Singh, R. V.; Phor, A. Synthetic, spectroscopic and biocidal aspects of sixteen to twenty two-membered tetraazamacrocyclic complexes of tin(II). Indian J. Chem. 2002a,41A, 2536–2539.Search in Google Scholar
Chaudhary, A.; Swaroop, R.; Singh, R. Tetraazamacrocyclic complexes of tin(II): synthesis spectroscopy and biological screening. Bol. Soc. Chil. Quim. 2002b, 47, 203–211.Search in Google Scholar
Chaudhary, A.; Phor, A.; Singh, R. V. Studies on potentially biodynamic heterocyclic organotin(II) macrocyclic complexes. Heterocycl. Commun. 2006, 12, 53–60.Search in Google Scholar
Danks, J. P.; Champness, N. R.; Schröder, M. Chemistry of mixed nitrogen- and sulfur-donor tridentate macrocycles. Coord. Chem. Rev. 1998, 174, 417–468.Search in Google Scholar
Dey, D. K.; Saha, M. K.; Dahlenburg, L. Synthesis and characterization of diorganotin(IV) dichloride adducts of Schiff bases. Indian J. Chem. 2000, 39A, 1177–1181.Search in Google Scholar
Diplock, A. T. Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease? Free Radical Res. 1997, 27, 511–532.Search in Google Scholar
Duckworth, P. A.; Lindoy, L. F.; Tasker, P. A. New macrocyclic ligands. IV. Dibenzo-substituted rings incorporating five donor atoms. x-ray structures of an N4O-donor macrocycle, its protonated form, and its complex with barium perchlorate. Aust. J. Chem. 1993,46, 1787–1797.Search in Google Scholar
Garnovskii, A. D.; Novorozhkin, A. L.; Minkin, V. I. Ligand environment and the structure of Schiff bases adducts and tetracoordinated-chelates. Coord. Chem. Rev. 1993, 126, 1–69.Search in Google Scholar
Geary, W. J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122.Search in Google Scholar
Holecek, J.; Nadvornik, M.; Handlir, K.; Lycka, A. 13C and 119Sn NMR spectra of di-n-butyltin (IV) compounds. J. Organomet. Chem. 1986, 315, 299–308.Search in Google Scholar
Ilhan, S.; Temel, H.; Ziyadanoğullar, R.; Şekerci, M. Synthesis and spectral characterization of macrocyclic Schiff base by reaction of 2,6-diaminopyridine and 1,4-bis(2-carboxyaldehyde phenoxy)butane and its Cu(II), Ni(II), Pb(II), Co(III) and La(III) complexes. Transition Met. Chem. 2007, 32, 584–590.Search in Google Scholar
Javanbakht, M.; Ganjali, M. R.; Eshghi, H.; Sharghi, H.; Shamsipur, M. Mercury (II) ion-selective electrode based on dibenzo-diazathia-18-crown-6-dione Electroanalysis 1999, 11, 81–84.Search in Google Scholar
Khan, T. A.; Ghani, S. S. Synthesis and physico-chemical studies of 14-membered tetraazamacrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II) derived from 3,4-diaminobenzophenone. Pol. J. Chem. 2005,79, 817–823.Search in Google Scholar
Khan, T. A.; Ghani, S. S.; Siddiqui, M. Y. Synthesis and characterization of a new series of transition metal - tin macrocyclic complexes. Main Group Met. Chem. 2005, 28, 265–272.Search in Google Scholar
Khan, T. A.; Ghani, S. S.; Naseem, S. Interaction of Co(II), Ni(II), Cu(II), and Zn(II) with 12- and 14-membered macrocycles containing O2N2 donors. J. Coord. Chem. 2010, 63, 4411–4420.Search in Google Scholar
Lever A. B. P. Inorganic Electronic Spectroscopy; Amsterdam: Elsevier. 1984.Search in Google Scholar
Lindoy, L. F.; Park, K. M.; Lee, S. S. Metals, macrocycles and molecular assemblies – macrocyclic complexes in metallo-supramolecular chemistry. Chem. Soc. Rev. 2013, 42, 1713–1727.Search in Google Scholar
Mathew, S.; Rajith, L.; Lonappan, L. A.; Jos, T.; Kumar, K. G. A lead (II) selective PVC membrane potentiometric sensor based on a tetraazamacrocyclic ligand. J. Inclusion Phenom. Macrocyclic Chem. 2014, 78, 171–177.Search in Google Scholar
Meir, S.; Kanner, J.; Akiri, B.; Hadas S. P. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agric. Food Chem. 1995, 43, 1813–1819.Search in Google Scholar
Mohamed, G. G.; Omar, M. M.; Hindy, A. M. M. Synthesis, characterization and biological activity of some transition metals with schiff base derived from 2-thiophene carboxaldehyde and aminobenzoic acid. Spectrochim. Acta A. 2005, 62, 1140–1150.Search in Google Scholar
Quintana, A. P.; Roglans, A. ESI-mass spectrometry as a tool for investigating the mechanistic role of a 15-membered triolefinic macrocyclic palladium(0) complex in the Heck reaction. Arkivoc. 2005, 9, 51–62.Search in Google Scholar
Raman, N.; Thangaraja, C. Synthesis and structural characterization of a fully conjugated macrocyclic tetraaza(14)-membered Schiff base and its bivalent metal complexes. TransitionMet. Chem. 2005,30, 317–322.Search in Google Scholar
Reilley, C. N., Schmid, R. W.; Sadek, F. A. Chelon approach to analysis (II). Illustrative experiments. J. Chem. Educ. 1959, 36, 619–625.Search in Google Scholar
Richard, J. P.; Morrow, J. R.; O’Donoghue, A. C.; Pyun, S. Y. Kinetic studies of RNA cleavage by lanthanide(III) macrocyclic complexes. Bull. Kor. Chem. Soc. 2004, 25,403–406.Search in Google Scholar
Siddiqi, Z. A.; Khan, M. M. Synthesis and characterization of a novel 32-membered unsymmetrical dinucleating [N12] macrocycle: preparation of bimetallic complexes M2LX2(ClO4)2 (M=Zn, Cd or Hg; X=Cl, NCS or NO3). Synth. React. Inorg. Met.-Org. Chem. 2004, 34, 5, 897–917.Search in Google Scholar
Singh, R. V.; Fahmi, N.; Swami, M.; Chauhan, S. Synthesis, structural investigation and biological studies of new macrocyclic complexes of tin(II). J. Macromol. Sci. 2008, 45, 159–163.Search in Google Scholar
Song, W.; Wu, C.; Yin, H.; Liu, X.; Sa, P.; Hu, J. Preparation of PbS nanoparticles by phase-transfer method and application to Pb-selective electrode based on PVC membrane. Anal. Lett. 2008, 41, 2844–2859.Search in Google Scholar
Tavacoli, S.; Miller, T. A.; Paul, R. L.; Jeffery, J. C.; Ward, M. D. Synthesis and coordination chemistry of tetradentate ligands containing two bidentate thioquinoline units: mononuclear complexes with Cu(I) and Cu(II), and a coordination polymer with Cu(I). Polyhedron. 2003,22, 507–514.Search in Google Scholar
Vogel, A. I. Text Book of Quantitative Inorganic Analysis, Longmans: London, 1961.Search in Google Scholar
White, V. A.; Long N. J.; Robertson, N. Synthesis of nitrogen and sulfur macrocycles with cis exogenous oxygen and sulfur donor atoms. Org. Biomol. Chem. 2005, 3, 4268–4273.Search in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis and structure of an acyclic dialkylstannylene
- Two in one: the bismuth bromido clusters in [Bi6Cp*6Br9][Bi7Br24]
- 2D Hydrogen-bonded polymer assembled by zinc(II) tetraaza macrocyclic complex and 1,2-cyclopentanedicarboxylic acid
- An efficient synthesis of N-substituted pyrroles catalyzed by MgI2 etherate
- Synthesis and characterization of N2S2-tin macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II)
- Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates
- Short Communications
- Reactivity of a spirobis(pentagerma[1.1.1]propellane)
- A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3
- Synthesis and structure of bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn
- Synthesis and structure of diarylhalotelluronium hexahalotellurates [(8-Me2 NC10 H6)2 TeX]2 TeX6 (X=Cl, Br)
- Synthesis and structure of three molecular arylindium phosphinates
- Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis and structure of an acyclic dialkylstannylene
- Two in one: the bismuth bromido clusters in [Bi6Cp*6Br9][Bi7Br24]
- 2D Hydrogen-bonded polymer assembled by zinc(II) tetraaza macrocyclic complex and 1,2-cyclopentanedicarboxylic acid
- An efficient synthesis of N-substituted pyrroles catalyzed by MgI2 etherate
- Synthesis and characterization of N2S2-tin macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II)
- Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates
- Short Communications
- Reactivity of a spirobis(pentagerma[1.1.1]propellane)
- A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3
- Synthesis and structure of bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn
- Synthesis and structure of diarylhalotelluronium hexahalotellurates [(8-Me2 NC10 H6)2 TeX]2 TeX6 (X=Cl, Br)
- Synthesis and structure of three molecular arylindium phosphinates
- Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2

