Abstract
The title compound bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn was obtained by the metathesis reaction of the lithium organyl 2,6-Mes2 C6 H3 Li with zinc difluoride ZnF2 and fully characterised by X-ray crystallography.
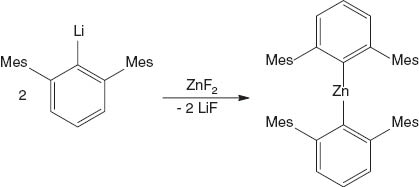
The beginning of organometallic chemistry is intimately related to the discovery of organozinc compounds such as Me2 Zn and Et2 Zn by Frankland in the mid-19th century (Seyferth, 2001). The handling of these pyrophoric compounds posed a substantial challenge at the time and required the development of hitherto unknown inert gas techniques. Despite being known for a long time, the structure of dialkylzinc compounds (Bacsa et al., 2011) and their hydrolysis/oxygenation products are still of current interest (Jana et al., 2007). This also holds true for diarylzinc compounds. Diphenylzinc is a dimer in the solid state (Markies et al., 1990), readily forms adducts with Lewis bases (e.g., pyridine or ethers) (Markies et al., 1992; Lennartson and Håkansson, 2009) and reacts with molecular oxygen to give phenylzinc phenolate (Lennartson and Håkansson, 2008). Diarylzinc compounds with bulkier substituents are monomers, show little tendency to form adducts and are substantially more stable towards oxidation. We prepared another member of this compound class, namely, (2,6-Mes2 C6 H3)2 Zn, by the reaction of 2,6-Mes2 C6 H3 Li with ZnF2, which was accidently present in a reaction mixture that was prepared to give a m-terphenylfluorosilane. After adjusting the reaction conditions, (2,6-Mes2 C6 H3)2 Zn was obtained as air-stable colourless crystals at a yield of 90% (Equation 1).
The molecular structure of (2,6-Mes2 C6 H3)2 Zn is shown in Figure 1. Selected geometrical parameters are collected in Table 1, along with known monomeric diarylzinc compounds for comparison.
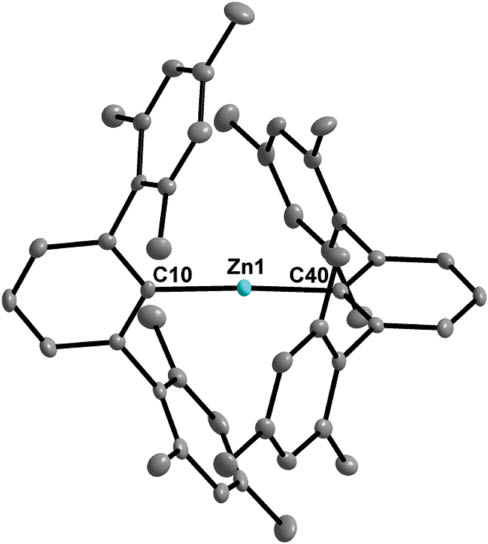
Molecular structure of monoclinic (2,6-Mes2 C6 H3)2 Zn showing 30% probability ellipsoids and the crystallographic numbering scheme.
Selected geometrical parameters of monomeric diarylzinc compounds.
| Compound | Zn-C (Å) | C-Zn-C (°) | Approx. point group symmetry | References |
|---|---|---|---|---|
| (C6 F5)2 Zn | 1.926(4), 1.930(5) | 172.5(2) | Cs | (Sun et al., 1998) |
| Mes2 Zn (monoclinic) | 1.942(2) | 180 | C2v | (Cole et al., 2003) |
| Mes2 Zn (tetragonal) | 1.951(4) | 180 | C2v | (Krieck et al., 2009) |
| [6-(CF3)C6 H4]2 Zn | 1.938(3), 1939(3) | 178.5(1) | Cs | (Chisholm et al., 2005) |
| [3,5-(CF3)2 C6 H3]2 Zn | 1.925(8), 1.938(8) | 177.0(2) | C2v | (Lai et al., 2012) |
| [2,4,6-(CF3)3 C6 H2]2 Zn | 1.949(3), 1.950(3) | 170.0(1) | C2 | (Brooker et al., 1992) |
| (2,4,6-t-Bu3 C6 H2)2 Zn | 1.945(6) | 165.9(3) | C2 | (Westerhausen et al., 2005) |
| (2,6-Naph2 C6 H3)2 Zn | 1.946(2), 1.955(2) | 168.27(9) | Cs | (Gridley et al., 2013) |
| [2,6-(2,6-Xyl)2 C6 H3]2 Zn | 1.944(4), 1.949(4) | 178.7(2) | Cs | (Blundell et al., 2014) |
| [2,6-(3,5-Xyl)2 C6 H3]2 Zn | 1.936(1), 1.940(1) | 171.18(5) | Cs | (Blundell et al., 2014) |
| [2,6-Pmp2 C6 H3]2 Zn | 1.939(3), 1.939(3) | 175.8(1) | Cs | (Blundell et al., 2014) |
| (2,6-Mes2 C6 H3)2 Zn | 1.945(3), 1.947(3) | 177.1(1) | Cs | This work |
Like those of other bulky diarylzinc compounds, the spatial arrangement of the Zn atom of (2,6-Mes2 C6 H3)2 Zn is nearly linear (C-Zn-C 177.1(1)°) and the Zn-C bond lengths [1.945(3), 1.947(3)] are consistent. Differences are observed in the relative orientation of the phenyl rings. Like (C6 F5)2 Zn (Sun et al., 1998), [6-(CF3)C6 H4]2 Zn (Chisholm et al., 2005), the closely related (2,6-Naph2 C6 H3)2 Zn (Gridley et al., 2013), [2,6-(2,6-Xyl)2 C6 H3]2 Zn, [2,6-(3,5-Xyl)2 C6 H3]2 Zn, [2,6-Pmp2 C6 H3]2 Zn (Blundell et al., 2014) and the isoelectronic (2,6-Mes2 C6 H3)2 Hg (Niemeyer and Power, 1997), (2,6-Mes2 C6 H3)2 Zn adopts a nearly Cs symmetry, while Mes2 Zn (Cole et al., 2003; Krieck et al., 2009) and [3,5-(CF3)2 C6 H3]2 Zn (Lai et al., 2012) possess a nearly C2v symmetry. Presumably due to the large steric congestion, (2,4,6-t-Bu3 C6 H2)2 Zn (Westerhausen et al., 2005) and the related [2,4,6-(CF3)3 C6 H2]2 Zn (Brooker et al., 1992) approach only C2 symmetry. We currently investigated the use of (2,6-Mes2 C6 H3)2 Zn as a transmetallation reagent.
Experimental
To a solution of 2,6-Mes2 C6 H3 Li (500 mg, 1.56 mmol) in diethyl ether (10 mL) at -78°C, a suspension of ZnF2 (81 mg, 0.78 mmol) in diethyl ether (10 mL) was added. After 2 h, the mixture was warmed up to room temperature and stirred for an additional 12 h. The mixture was filtered and the volatile materials were removed under reduced pressure. Recrystallisation of the residue from n-hexane/CH2 Cl2 afforded colourless crystals of (2,6-Mes2 C6 H3)2 Zn (0.488 g, 0.71 mmol, 90%; Mp. 122°C).
1H NMR (CDCl3): δ=7.48 (t, 3J(1H,1H)=7.6 Hz, 2H), 7.12 (d, 3J(1H,1H)=7.6 Hz, 4H), 6.97 (s, 8H), 2.35 (s, 12H), 2.07 (s, 24H). 13C{1H} NMR (CDCl3): δ=141.3, 139.2, 136.6, 136.0, 130.4, 128.6, 128.2, 127.7, 21.2, 20.9. Anal. Calcd. for C48 H50 Zn (692.31): C, 83.28; H, 7.28. Found: C, 83.31; H, 7.11.
Crystallography
Single crystals of (2,6-Mes2 C6 H3)2 Zn·CHCl3 were obtained by crystallisation from CHCl3 at room temperature. Intensity data were collected on a Bruker Venture D8 diffractometer at 173 K with graphite-monochromated Mo-Kα (0.7107 Å) radiation. The structure was solved by direct methods and difference Fourier synthesis using SHELXS-97 and SHELXL-97 implemented in the program WinGX 2002 (Farrugia, 1999). Full-matrix least-squares refinements on F2, using all data. The absolute configuration was established by refinement of the Flack parameter [0.418(9)] (Flack, 1983). All non-hydrogen atoms were refined using anisotropic displacement parameters. Hydrogen atoms attached to carbon atoms were included in geometrically calculated positions using a riding model. Crystal and refinement data are collected in Table 2. Figures were created using the DIAMOND software (Brandenburg and Putz, 2006). Crystallographic data (excluding structure factors) for the structural analyses have been deposited with the Cambridge Crystallographic Data Centre (CCDC no. 1007854). Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk).
Crystal data and structure refinement of (2,6-Mes2 C6 H3)2 Zn·CHCl3.
| Formula | C49 H51 Cl3 Zn |
|---|---|
| Formula weight, g/mol | 811.62 |
| Crystal system | Monoclinic |
| Crystal size, mm | 0.1×0.1×0.1 |
| Space group | P21 |
| a, Å | 10.008(5) |
| b, Å | 17.134(5) |
| c, Å | 12.567(5) |
| α, ° | 90.00 |
| β, ° | 101.379(5) |
| γ, ° | 90.00 |
| V, Å3 | 2112(2) |
| Z | 2 |
| ρcalcd, mg/m3 | 1.276 |
| T, K | 173 |
| μ (Mo Kα), mm-1 | 0.144 |
| F(000) | 852 |
| θ range, ° | 2.38–26.59 |
| Index ranges | -12≤h≤10 |
| -21≤k≤21 | |
| -15≤l≤15 | |
| No. of reflections collected | 24,651 |
| Completeness to θmax | 99.8% |
| No. of independent reflections | 8747 |
| No. of observed reflections with [I>2σ(I)] | 7872 |
| No. of refined parameters | 490 |
| GooF (F2) | 1.037 |
| R1 (F) [I>2σ(I)] | 0.0413 |
| wR2 (F2) (all data) | 0.0943 |
| (Δ/σ)max | <0.001 |
| Largest difference peak/hole, e/Å-3 | 0.701/-0.360 |
GooF, goodness of fit.
References
Bacsa, J.; Hanke, F.; Hindley, S.; Odedra, R.; Darling, G. R.; Jones, A. C.; Steiner, A. The solid-state structures of dimethylzinc and diethylzinc. Angew. Chem. Int. Ed. 2011, 50, 11685–11687.Search in Google Scholar
Blundell, T.J.; Hastings, F. R.; Gridley, B. M.; Moxey, G. J.; Lewis, W.; Blake, A. J.; Kays, D. L. Ligand influences on homoleptic Group 12 m-terphenyl complexes. Dalton Trans. 2014, DOI: 10.1039/c4dt00647j.10.1039/C4DT00647JSearch in Google Scholar
Brandenburg, K.; Putz, H. DIAMOND V3.1d: Crystal Impact GbR: Bonn, Germany, 2006.Search in Google Scholar
Brooker, S.; Bertel, N.; Stalke, D.; Noltemeyer, M.; Roesky, H. W.; Sheldrick, G. M.; Edelmann, F. T. Main-group chemistry of the 2,4,6-tris(trifluoromethyl)phenyl substituent: X-ray crystal structures of [2,4,6-(CF3)3 C6 H2]2 Zn, [2,4,6-(CF3)3 C6 H2]2 Cd(MeCN), and [2,4,6-(CF3)3 C6 H2]2 Hg. Organometallics1992, 11, 192–195.Search in Google Scholar
Chisholm, M. H.; Gallucci, J. C.; Yin, H.; Zhen, H. Arylzinc alkoxides: [ArZnOCH Pri2]2 and Ar2 Zn3(OCHPri2)4 when Ar = C6 H5, p-CF3 C6 H4, 2,4,6-Me3 C6 H2, and C6 F5. Inorg. Chem. 2005, 44, 4777–4785.Search in Google Scholar
Cole, S. C.; Coles, M. P.; Hitchcock, P. B. Dimesitylzinc: a strictly 2-coordinate, homoleptic diarylzinc compound. Dalton Trans. 2003, 3663–3664.10.1039/b309709aSearch in Google Scholar
Farrugia, L. J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838.Search in Google Scholar
Flack, H. D. On enantiomorph-polarity estimation. Acta Crystallogr. 1983, A39, 876–881.Search in Google Scholar
Gridley, B. M.; Moxey, G. J.; Lewis, W.; Blake, A. J.; Kays, D. L. Conformational isomerism in monomeric, low-coordinate group 12 complexes stabilized by a naphthyl-substituted m-terphenyl ligand. Chem. Eur. J. 2013, 19, 11446–11453.Search in Google Scholar
Jana, S.; Berger, R. J. F.; Fröhlich, R; Pape, T.; Mitzel, N. W. Oxygenation of simple zinc alkyls: surprising dependence of product distributions on the alkyl substituents and the presence of water. Inorg. Chem. 2007, 46, 4293–4297.Search in Google Scholar
Krieck, S.; Görls, H.; Westerhausen, M. Bis(2,4,6-trimethylphenyl)zinc(II). Acta Crystallogr. 2009, 65E, m809.Search in Google Scholar
Lai, Y. Y.; Bornand, M.; Chen, P. Homogeneous model complexes for supported rhenia metathesis catalysts. Organometallics2012, 31, 7558–7565.Search in Google Scholar
Lennartson, A.; Håkansson, M. Slow atmospheric oxidation of diphenylzinc: di-mu-phenoxido-bis[(diethyl ether)phenylzinc(II)]. Acta Crystallogr. 2008, C64, m10–m12.Search in Google Scholar
Lennartson, A.; Håkansson, M. Diphenyldipyridinezinc(II): partial spontaneous resolution of an organometallic reagent. Acta Crystallogr. 2009, C65, m205–m207.Search in Google Scholar
Markies, P. R.; Schat, G.; Akkerman, O. S.; Bickelhaupt, F.; Smeets, W. J. J.; Spek, A. L. Coordinational behavior of solvent-free diorganylzinc compounds: the remarkable X-ray structure of dimeric diphenylzinc. Organometallics1990, 9, 2243–2247.Search in Google Scholar
Markies, P. R.; Schat, G.; Akkerman, O. S.; Bickelhaupt, F.; Spek, A. L. Complexation of diphenylzinc with simple ethers. Crystal structures of the complexes Ph2 Zn · glyme and Ph2 Zn · diglyme. J. Organomet. Chem. 1992, 430, 1–13.Search in Google Scholar
Niemeyer, M.; Power, P. P. Synthesis and structure of the solvent-free sodium aryl (NaC6 H3-2,6-Mes2)2. Organometallics1997, 16, 3258–3260.10.1021/om970247dSearch in Google Scholar
Seyferth, D. Zinc alkyls, Edward Frankland, and the beginnings of main-group organometallic chemistry. Organometallics2001, 20, 2940–2955.10.1021/om010439fSearch in Google Scholar
Sun, Y.; Piers, W. E.; Parvez, M. The solid-state structure of bis(pentafluorophenyl)zinc Can. J. Chem. 1998, 76, 513–517.Search in Google Scholar
Westerhausen, M.; Oßberger, M. W.; Alexander, J. S.; Ruhlandt-Senge, K. Influence of the steric demand of the 2,4,6-trialkylphenyl substituents on the structures and reactivity of diarylzinc. Z. Anorg. Allg. Chem.2005, 631, 2836–2841.Search in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis and structure of an acyclic dialkylstannylene
- Two in one: the bismuth bromido clusters in [Bi6Cp*6Br9][Bi7Br24]
- 2D Hydrogen-bonded polymer assembled by zinc(II) tetraaza macrocyclic complex and 1,2-cyclopentanedicarboxylic acid
- An efficient synthesis of N-substituted pyrroles catalyzed by MgI2 etherate
- Synthesis and characterization of N2S2-tin macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II)
- Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates
- Short Communications
- Reactivity of a spirobis(pentagerma[1.1.1]propellane)
- A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3
- Synthesis and structure of bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn
- Synthesis and structure of diarylhalotelluronium hexahalotellurates [(8-Me2 NC10 H6)2 TeX]2 TeX6 (X=Cl, Br)
- Synthesis and structure of three molecular arylindium phosphinates
- Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis and structure of an acyclic dialkylstannylene
- Two in one: the bismuth bromido clusters in [Bi6Cp*6Br9][Bi7Br24]
- 2D Hydrogen-bonded polymer assembled by zinc(II) tetraaza macrocyclic complex and 1,2-cyclopentanedicarboxylic acid
- An efficient synthesis of N-substituted pyrroles catalyzed by MgI2 etherate
- Synthesis and characterization of N2S2-tin macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II)
- Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates
- Short Communications
- Reactivity of a spirobis(pentagerma[1.1.1]propellane)
- A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3
- Synthesis and structure of bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn
- Synthesis and structure of diarylhalotelluronium hexahalotellurates [(8-Me2 NC10 H6)2 TeX]2 TeX6 (X=Cl, Br)
- Synthesis and structure of three molecular arylindium phosphinates
- Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2

