Abstract
A few crystals of the title compound Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2 (1·CH2 Cl2) were isolated accidentally from a reaction mixture that contained the starting materials TeCl4 and t-BuMgCl in THF and that was recrystallized from CH2 Cl2. The molecular structure of 1·CH2 Cl2 determined by X-ray crystallography features a rare TeCl3- moiety.
The VSEPR concept suggests tellurium tetrachloride, TeCl4, to adopt a trigonal bipyramidal structure when taking into account the stereochemically active lone pair. This spatial arrangement is indeed observed in the gas phase; however, in the condensed phase, the structure looks different (Shlykov et al., 2010). Tellurium tetrachloride contains isolated Te4(μ3-Cl4)Cl12 units (I) comprising a cube-like structure (Scheme 1) (Buss and Krebs, 1970, 1971). The spatial arrangement of the Te atoms is distorted octahedral and defined by three short (covalent) and three longer (ionic) Te-Cl bonds.
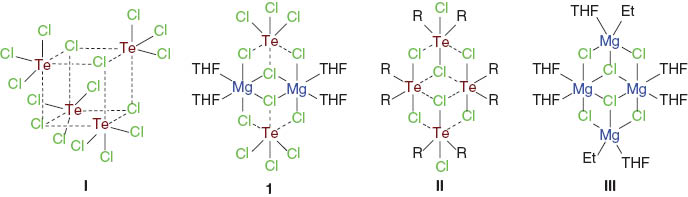
Molecular structures of Te4(μ3-Cl4)Cl12 (I), Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4 (1), (R2 Te)4(μ3-Cl2)(μ2-Cl4)Cl2 (II, R=4-MeC6 H4, 4-MeOC6 H4), and (EtMg)2 Mg2(μ3-Cl2)(μ2-Cl4)(THF)6 (III).
The three TeCl3+ ions situated at the apexes of the cube are also present in solutions of TeCl4 in polar solvents as well as in (TeCl3)AlCl4 (Krebs et al., 1971) and (TeCl3)Al[OC(CF3)3]4 (Engesser et al., 2012).
Magnesium dichloride, MgCl2, is readily soluble in THF and known to undergo complex formation with a number of Lewis acids including FeCl3, AlCl3, TiCl4, and MoOCl2. Isolated and well-defined complexes involve [Mg(THF)4(μ2-Cl)2 TiCl4] (Gordon and Wallbridge, 1984), [(THF)3 Mg(μ2-Cl)3 Mg(THF)3]TiCl5(THF) (Sobota et al., 1984a), [MgCl(THF)5]FeCl4·THF, [MgCl(THF)5]AlCl4·THF (Sobota et al., 1989), [Mg(THF)4(μ2-Cl)2 FeCl2] (Sobota et al., 1984b), [Mg(THF)6]MoOCl4(THF), and [(THF)3 Mg(μ2-Cl)3 Mg(THF)3]MoOCl4(THF) (Sobota et al., 1986).
We have now found that the reaction of TeCl4 with t-BuMgCl in THF, originally intended to give t-Bu2 Te (Jones and Sharma, 1983; Gedridge et al., 1991), proceeded with the formation of volatile isobutene (recognized by the characteristic smell) and produced after removal of almost all of the solvent a colorless solid, which was recrystallized from CH2 Cl2 to furnish a small crop of single crystals of the title compound Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2 (1·CH2 Cl2). The molecular structure of 1 is shown in Figure 1. Selected bond lengths are collected in the caption.
It consists of a centrosymmetric tetranuclear structure that strongly resembles those of the diaryltellurium dichlorides (R2 Te)4(μ3-Cl2)(μ2-Cl4)Cl2 (II, R=4-MeC6 H4; Beckmann et al., 2003), 4-MeOC6 H4 (Chadha and Drake, 1984), and the Grignard reagent (EtMg)2 Mg2(μ3-Cl2)(μ2-Cl4)(THF)6 (III) (Scheme 1) (Toney and Stucky, 1971). The spatial arrangement of the Te atom is distorted octrahedral and defined by a Cl6 donor set. Like in the parent Te4(μ3-Cl4)Cl12 (I) (Buss and Krebs, 1970, 1971), there are three short Te-Cl bonds and three long Te-Cl bonds, which are associated with the terminal chlorine atoms Cl1, Cl2, and Cl3 [2.340(1), 2.385(1), and 2.384(1) Å], the two μ2-chlorine atoms Cl5 and Cl6 [2.6921(9) and 2.6909(9) Å], and the μ3-chlorine atom Cl4 [2.8545(9) Å], respectively. Thus, the structure contains a rare TeCl3- moiety. The spatial arrangement of the Mg atom is also distorted octrahedral and defined by an O2 Cl4 donor set. The two Mg-O bond lengths [2.049(3) and 2.035(3) Å] and the four Mg-Cl bond lengths, which are associated with the two μ2-chlorine atoms Cl5 and Cl6a [2.505(1) and 2.514(1) Å] and the two μ3-chlorine atoms Cl4 and Cl4 [2.595(1) and 2.567(1) Å], respectively, are very similar to those observed in (EtMg)2 Mg2(μ3-Cl2)(μ2-Cl4)(THF)6 (III) (Toney and Stucky, 1971), [(THF)3 Mg(μ2-Cl)3 Mg(THF)3]TiCl5(THF) (Sobota et al., 1984a), [MgCl(THF)5]FeCl4·THF, [MgCl(THF)5]AlCl4·THF (Sobota et al., 1989), [Mg(THF)4(μ2-Cl)2 FeCl2] (Sobota et al., 1984b), [Mg(THF)6]MoOCl4(THF), and [(THF)3 Mg(μ2-Cl)3 Mg(THF)3]MoOCl4(THF) (Sobota et al., 1986) respectively.
![Figure 1 Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6·4 THF·CH2 Cl2 showing 30% probability ellipsoids and the crystallographic numbering scheme.Selected bond parameters [Å]: Te1-Cl1 2.340(1), Te1-Cl2 2.385(1), Te1-Cl3 2.384(1), Te1-Cl4 2.8545(9), Te1-Cl5 2.6921(9), Te1-Cl6 2.6909(9), Mg1-O1 2.049(3), Mg1-O2 2.035(3), Mg1-Cl4 2.595(1), Mg1-Cl4a 2.567(1), Mg1-Cl5 2.505(1), Mg1-Cl6a 2.514(1), Cl1-Te1-Cl2 93.32(5), Cl1-Te1-Cl3 92.78(5), Cl1-Te1-Cl4 167.53(4), Cl1-Te1-Cl5 90.93(4), Cl1-Te1-Cl6 91.16(4), Cl2-Te1-Cl3 90.05(4), Cl2-Te1-Cl4 95.29(4), Cl2-Te1-Cl5 175.68(4), Cl2-Te1-Cl6 90.07(4), Cl3-Te1-Cl4 96.21(4), Cl3-Te1-Cl5 88.97(4), Cl3-Te1-Cl6 176.04(4), Cl4-Te1-Cl5 80.64(3), Cl4-Te1-Cl6 79.83(2), Cl5-Te1-Cl6 90.63(3), O1-Mg1-O2 96.1(1), O1-Mg1-Cl4 174.74(9), O1-Mg1-Cl4a 92.61(8), O1-Mg1-Cl5 89.49(9), O1-Mg1-Cl6a 89.95(9), O2-Mg1-Cl5 93.48(8), O2-Mg1-Cl6a 89.14(8), O2-Mg1-Cl4 89.10(9), O2-Mg1-Cl4a 171.07(9), Cl4-Mg1-Cl5a 88.48(4), Cl4a-Mg1-Cl6a 88.97(4), Cl5-Mg1-Cl6a 177.36(6), Cl4-Mg1-Cl5 89.49(4), Cl4-Mg1-Cl6a 90.83(5), Cl4-Mg1-Cl4a 82.20(4).](/document/doi/10.1515/mgmc-2014-0024/asset/graphic/mgmc-2014-0024_fig1.jpg)
Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6·4 THF·CH2 Cl2 showing 30% probability ellipsoids and the crystallographic numbering scheme.
Selected bond parameters [Å]: Te1-Cl1 2.340(1), Te1-Cl2 2.385(1), Te1-Cl3 2.384(1), Te1-Cl4 2.8545(9), Te1-Cl5 2.6921(9), Te1-Cl6 2.6909(9), Mg1-O1 2.049(3), Mg1-O2 2.035(3), Mg1-Cl4 2.595(1), Mg1-Cl4a 2.567(1), Mg1-Cl5 2.505(1), Mg1-Cl6a 2.514(1), Cl1-Te1-Cl2 93.32(5), Cl1-Te1-Cl3 92.78(5), Cl1-Te1-Cl4 167.53(4), Cl1-Te1-Cl5 90.93(4), Cl1-Te1-Cl6 91.16(4), Cl2-Te1-Cl3 90.05(4), Cl2-Te1-Cl4 95.29(4), Cl2-Te1-Cl5 175.68(4), Cl2-Te1-Cl6 90.07(4), Cl3-Te1-Cl4 96.21(4), Cl3-Te1-Cl5 88.97(4), Cl3-Te1-Cl6 176.04(4), Cl4-Te1-Cl5 80.64(3), Cl4-Te1-Cl6 79.83(2), Cl5-Te1-Cl6 90.63(3), O1-Mg1-O2 96.1(1), O1-Mg1-Cl4 174.74(9), O1-Mg1-Cl4a 92.61(8), O1-Mg1-Cl5 89.49(9), O1-Mg1-Cl6a 89.95(9), O2-Mg1-Cl5 93.48(8), O2-Mg1-Cl6a 89.14(8), O2-Mg1-Cl4 89.10(9), O2-Mg1-Cl4a 171.07(9), Cl4-Mg1-Cl5a 88.48(4), Cl4a-Mg1-Cl6a 88.97(4), Cl5-Mg1-Cl6a 177.36(6), Cl4-Mg1-Cl5 89.49(4), Cl4-Mg1-Cl6a 90.83(5), Cl4-Mg1-Cl4a 82.20(4).
Experimental
To a suspension of Mg turnings (134 mg, 5.5 mmol) in THF (25 mL), tert-butyl chloride (463 mg, 5.0 mmol) was slowly added via a syringe. After stirring overnight all volatile materials were removed invacuo. The resulting residue was extracted with hot CH2 Cl2. The solution was filtered and left to stand in a closed Schlenk tube. After 3 weeks, a small crop of pale yellow single crystals formed.
X-ray crystallography
Intensity data were collected on a STOE IPDS 2T diffractometer at 150 K with graphite-monochromated Mo-Kα (0.7107 Å) radiation. The structure was solved by direct methods and difference Fourier synthesis using SHELXS-97 and SHELXL-97 implemented in the program WinGX 2002 (Farrugia, 1999). Full-matrix least-squares refinements on F2 were performed using all data. All nonhydrogen atoms were refined using anisotropic displacement parameters. Hydrogen atoms attached to carbon atoms were included in geometrically calculated positions using a riding model. Crystal and refinement data are collected in Table 1. Figures were created using DIAMOND (Brandenburg and Putz, 2006). Crystallographic data (excluding structure factors) for the structural analyses have been deposited with the Cambridge Crystallographic Data Centre, CCDC number 998857. Copies of this information may be obtained free of charge from The Director, CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or http://www.ccdc.cam.ac.uk).
Crystal data and structure refinement of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6·4 THF·CH2 Cl2.
| Formula | C17 H34 Cl14 Mg2 O4 Te2 |
| Formula weight, g mol-1 | 1102.56 |
| Crystal system | Monoclinic |
| Crystal size, mm | 0.45×0.35×0.30 |
| Space group | C2/c |
| a, Å | 16.8786(11) |
| b, Å | 12.1698(5) |
| c, Å | 20.2367(14) |
| α, ° | 90.00 |
| β, ° | 110.930(5) |
| γ, ° | 90.00 |
| V, Å3 | 3882.5(4) |
| Z | 4 |
| ρcalcd, mg m-3 | 1.886 |
| T, K | 150 |
| μ(Mo-Kα), mm-1 | 2.523 |
| F(000) | 2136 |
| θ range, deg | 2.71–25.02 |
| Index ranges | -19≤h≤20 |
| -13≤k≤14 | |
| -24≤l≤24 | |
| No. of reflns collected | 13401 |
| Completeness to θmax | 99.8% |
| No. indep. reflns | 3433 |
| No. obsd reflns with (I>2σ(I)) | 3064 |
| No. refined param | 177 |
| GooF (F2) | 1.034 |
| R1 (F) (I>2σ(I)) | 0.0287 |
| wR2 (F2) (all data) | 0.0705 |
| (Δ/σ)max | <0.001 |
| Largest diff peak/hole, e Å–3 | 0.922/-0.691 |
References
Beckmann, J.; Dakternieks, D.; Duthie, A.; Smith, N. A. Secondary bonding in para-substituted diphenyltellurium dichlorides (p-XC6 H4)2 TeCl2 (X=H, Me, MeO) probed by 125Te MAS NMR spectroscopy. Crystal and molecular structure of (p-MeC6 H4)2 TeCl2. J. Organomet. Chem. 2003, 669, 149–153.Search in Google Scholar
Brandenburg, K.; Putz, H. DIAMOND V3.1d. Crystal Impact GbR, Bonn, Germany, 2006.Search in Google Scholar
Buss, B.; Krebs, B. Crystal structure of TeCl4: presence of tetramers in solid chalcogen(IV) halides. Angew. Chem. Int. Ed. 1970, 9, 463.Search in Google Scholar
Buss, B.; Krebs, B. The crystal structure of tellurium tetrachloride. Inorg. Chem. 1971, 10, 2795–2800.Search in Google Scholar
Chadha, R. K.; Drake, J. E. Structure of dichlorobis(p-methoxyphenyl)tellurium(IV), [TeCl2(C7 H7 O)2]. Acta Crystallogr. 1984, C40, 1349–1352.Search in Google Scholar
Engesser, T. A.; Hrobárik, P.; Trapp, N.; Eiden, P.; Scherer, H.; Kaupp, M.; Krossing, I. [TeX3]+ cations stabilized by the weakly coordinating [Al(ORF)4]- anion: FIR spectra, Raman spectra, and evaluation of an abnormal halogen dependence of the 125Te NMR chemical shifts. ChemPlusChem2012, 77, 643–651.Search in Google Scholar
Farrugia, L. J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr.1999, 32, 837–838.Search in Google Scholar
Gedridge Jr., R. W.; Higa, K. T.; Nissan, R. A. Syntheses and mechanistic studies of symmetric tetraorganotellurium(IV) (R4 Te) and diorganyltellurium(II) (R′2 Te) compounds (R=R′=Me, n-Bu, Me3 SiCH2, and CH2=CH; R′=t-Bu and Allyl). Organometallics1991, 10, 286–291.10.1021/om00047a062Search in Google Scholar
Gordon, D.; Wallbridge, M. G. H. Characterisation and solution properties of complexes involving magnesium chloride, a Lewis Base, and the tetrachlorides of titanium, zirconium and tin. Inorg. Chim. Acta1984, 88, 15–20.10.1016/S0020-1693(00)81864-2Search in Google Scholar
Jones, C. H. W.; Sharma, R. D. The preparation of di-t-butyl ditelluride and di-t-butyltelluride and the 125Te NMR and Mössbauer spectra of some dialkyl tellurides and ditellurides. J. Organomet. Chem. 1983, 255, 61–70.Search in Google Scholar
Krebs, B.; Buss, B.; Altena, D. Die Kristallstruktur von Trichlorotellur(IV)-tetraaluminat TeCl3+AlCl4-. Z. Anorg. Allg. Chem. 1971, 386, 257–369.Search in Google Scholar
Shlykov, S. A.; Giricheva, N. I.; Titov, A. V.; Szwak, M.; Lentz, D.; Girichev, G. V. The structures of tellurium(IV) halides in the gas phase and as solvated molecules. Dalton Trans. 2010, 39, 3245–3255.Search in Google Scholar
Sobota, P.; Utko, J.; Lis, T. Preparation and crystal structure of tri-μ-chloro-hexakis(tetrahydrofuran)dimagnesium(II) pentachloro(tetrahydrofuran)titanate(IV). J. Chem. Soc. Dalton Trans. 1984a, 2077–2079.Search in Google Scholar
Sobota, P.; Pluzinski, T.; Lis, T. Reaction between magnesium chloride and iron(III) chloride in tetrahydrofuran. The crystal structure of di-μ-chlorodichloroiron(II) tetrakis(tetrahydrofuran)magnesium(II). Polyhedron1984b, 3, 45–47.10.1016/S0277-5387(00)84710-5Search in Google Scholar
Sobota, P.; Pluzinski, T.; Lis, T. Reaction between [MgCl2(THF)2] and [MoOCl3(THF)2]. The crystal structures of [Mg(THF)6][MoOCl4(THF)]2 and [Mg2(μ-Cl)3(THF)6][MoOCl4(THF)]. Z. Anorg. Allg. Chem. 1986, 533, 215–224.Search in Google Scholar
Sobota, P.; Pluzinski, T.; Utko, J.; Lis, T. Evidence for the existence of the [MgCl(THF)5]+ cation. Crystal structures of [MgCl(THF)5][FeCl4]·THF and [MgCl(THF)5][AlCl4]·THF. Inorg. Chem. 1989, 28, 2217–2219.Search in Google Scholar
Toney, J.; Stucky, G. D. The stereochemistry of polynulcear compounds of the main group elements. [C2 H5 Mg2 Cl3(C4 H8 O)3]2, a tetrameric grignard reagent. J. Organomet. Chem.1971, 28, 5–20.Search in Google Scholar
©2014 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis and structure of an acyclic dialkylstannylene
- Two in one: the bismuth bromido clusters in [Bi6Cp*6Br9][Bi7Br24]
- 2D Hydrogen-bonded polymer assembled by zinc(II) tetraaza macrocyclic complex and 1,2-cyclopentanedicarboxylic acid
- An efficient synthesis of N-substituted pyrroles catalyzed by MgI2 etherate
- Synthesis and characterization of N2S2-tin macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II)
- Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates
- Short Communications
- Reactivity of a spirobis(pentagerma[1.1.1]propellane)
- A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3
- Synthesis and structure of bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn
- Synthesis and structure of diarylhalotelluronium hexahalotellurates [(8-Me2 NC10 H6)2 TeX]2 TeX6 (X=Cl, Br)
- Synthesis and structure of three molecular arylindium phosphinates
- Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2
Articles in the same Issue
- Frontmatter
- Research Articles
- Synthesis and structure of an acyclic dialkylstannylene
- Two in one: the bismuth bromido clusters in [Bi6Cp*6Br9][Bi7Br24]
- 2D Hydrogen-bonded polymer assembled by zinc(II) tetraaza macrocyclic complex and 1,2-cyclopentanedicarboxylic acid
- An efficient synthesis of N-substituted pyrroles catalyzed by MgI2 etherate
- Synthesis and characterization of N2S2-tin macrocyclic complexes of Co(II), Ni(II), Cu(II) and Zn(II)
- Synthesis, characterization and in vitro cytotoxic activity of bis(triorganotin) 2,6-pyridinedicarboxylates
- Short Communications
- Reactivity of a spirobis(pentagerma[1.1.1]propellane)
- A monoclinic polymorph of 2,6-Mes2 C6 H3 SiF3
- Synthesis and structure of bis(m-terphenyl)zinc (2,6-Mes2 C6 H3)2 Zn
- Synthesis and structure of diarylhalotelluronium hexahalotellurates [(8-Me2 NC10 H6)2 TeX]2 TeX6 (X=Cl, Br)
- Synthesis and structure of three molecular arylindium phosphinates
- Molecular structure of Te2 Mg2(μ3-Cl2)(μ2-Cl4)Cl6(THF)4·CH2 Cl2

