Abstract
Placental abruption has classically been defined as the premature separation of a normally located placenta before delivery of the fetus. Traditionally, this diagnosis was based on clinical symptoms, including vaginal bleeding, pain, and fetal distress. This definition, however, preceded the advent of obstetric ultrasound. Ultrasound frequently identifies various hemorrhagic lesions, such as retroplacental, subchorionic, intraamniotic, intraplacental, and preplacental hematomas in both symptomatic and asymptomatic patients. These variable ultrasound findings lead to new challenges as to what to define as an abruption, particularly in the absence of symptoms. This ambiguity in defining placental abruption affects clinical decision-making and hinders our understanding of the pathophysiology of abruption, presenting challenges in studying abruption. It is likely that these varying sonographic findings may precede the classic presentation of vaginal bleeding and pain and therefore are often concealed abruptions. This commentary highlights the importance of developing clear diagnostic guidelines for placental abruption, given its association with severe outcomes including a high rate of perinatal mortality and maternal morbidity. We aim to elucidate the complexities of ultrasound diagnosis in placental abruption, advocating for precise criteria to better guide clinical practice. We propose that these ultrasound findings of hemorrhagic placental lesions after 20 weeks of gestation in asymptomatic patients should be considered part of the spectrum of abruption, while in symptomatic patients should be taken as confirmation of the diagnosis of abruption.
Introduction
Placental abruption is associated with substantial risk of death to the fetus by depriving it of blood, oxygen, and nutrients [1], 2]. It is a leading cause of preterm delivery and contributes to up to 25 % of perinatal deaths, and even those infants surviving the neonatal period have increased childhood mortality [1], [2], [3], [4], [5], [6]. Furthermore, abruption often results in severe maternal morbidity and even mortality [7]. It has long been defined as the premature separation of a normally located placenta prior to the delivery of the fetus, a clinical diagnosis typically made when patients present with symptoms such as vaginal bleeding, abdominal pain, contractions, uterine tenderness, and fetal distress [2], 5]. However, these criteria for diagnosing abruption preceded the widespread use of obstetric ultrasound, which has now become an integral part of prenatal care. Ultrasound frequently detects a variety of placental hemorrhagic lesions in both symptomatic and asymptomatic patients. These findings have introduced significant ambiguity regarding what constitutes placental abruption. Such haziness inevitably affects clinical decision-making, complicates research on the incidence and natural history of abruption, and challenges efforts to standardize management. This commentary explores, with ultrasound examples (Figures 1–9), the challenges posed by inconsistent definitions, proposes that sonographically-detected hemorrhagic placental lesions and clinical abruption are on a continuum, and emphasizes the need for standardized diagnostic criteria to improve clinical care and research outcomes.
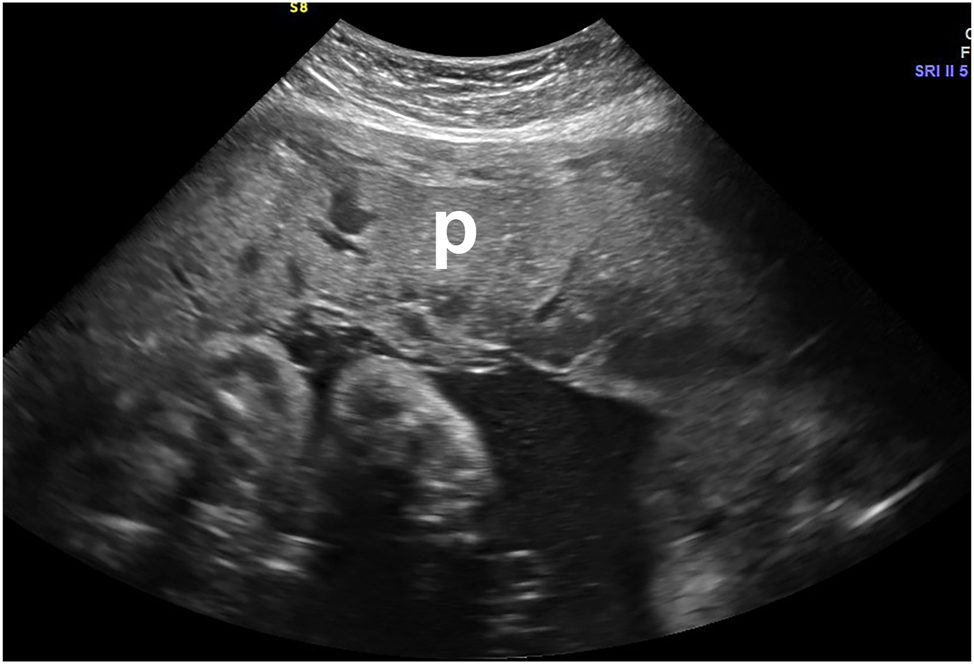
Thickened heterogeneous placenta (p) in a symptomatic patient who presented with bleeding and contractions, representing an intraplacental hemorrhage. This met the clinical criteria for abruption. Abruption was confirmed on placental examination and pathology after birth.

Thickened anterior heterogeneous placenta (p) in a symptomatic patient who presented with bleeding and contractions, with a posterior subchorionic hematoma (h). This met the clinical criteria for abruption. Abruption was confirmed on placental examination and pathology after birth.
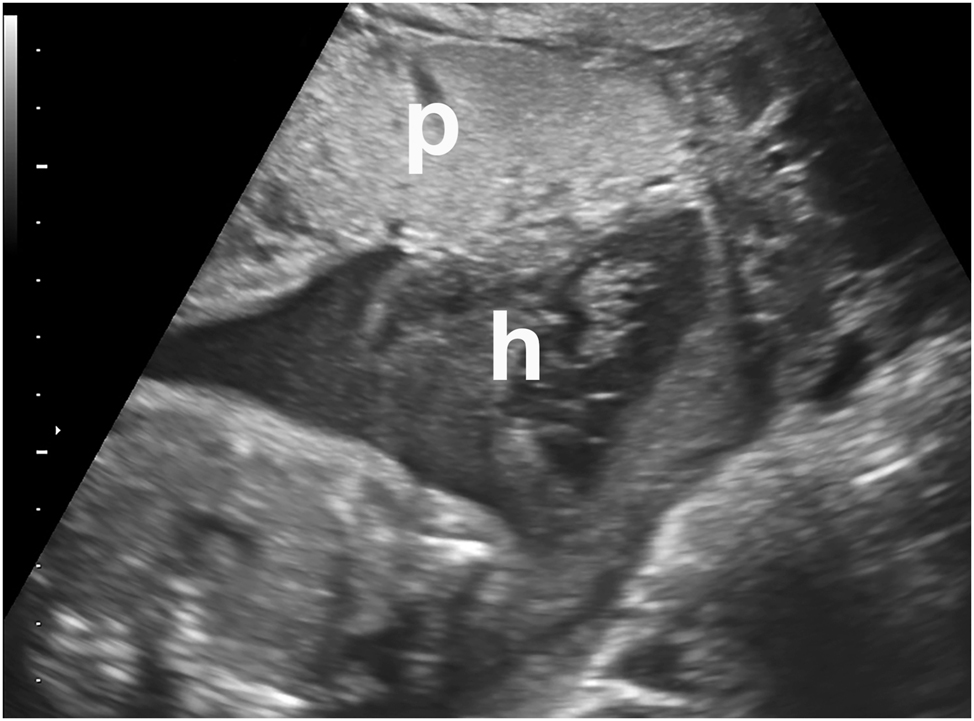
Intraamniotic hematoma (h) in a symptomatic patient who presented with bleeding and contractions. Placenta=“p” This met the clinical criteria for abruption. Abruption was confirmed on placental examination and pathology after birth.

Large subamniotic (preplacental) hematoma located below the amnion and above the chorionic tissue (Breus’ mole) in a patient without bleeding or pain but with fetal growth restriction. “p”=placenta. The hematoma was confirmed on gross and pathologic placental examination after birth.
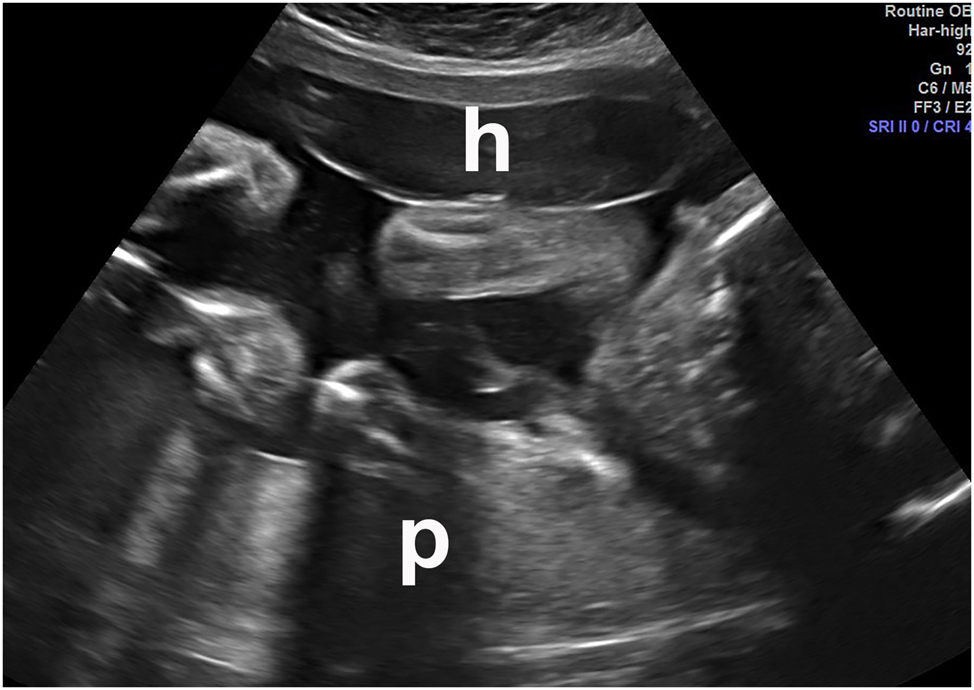
Anterior subchorionic hematoma (h) in a patient who presented with vaginal bleeding. The placenta (p) was posterior. This met the clinical criteria for abruption. “p”=placenta. Abruption was confirmed at delivery.
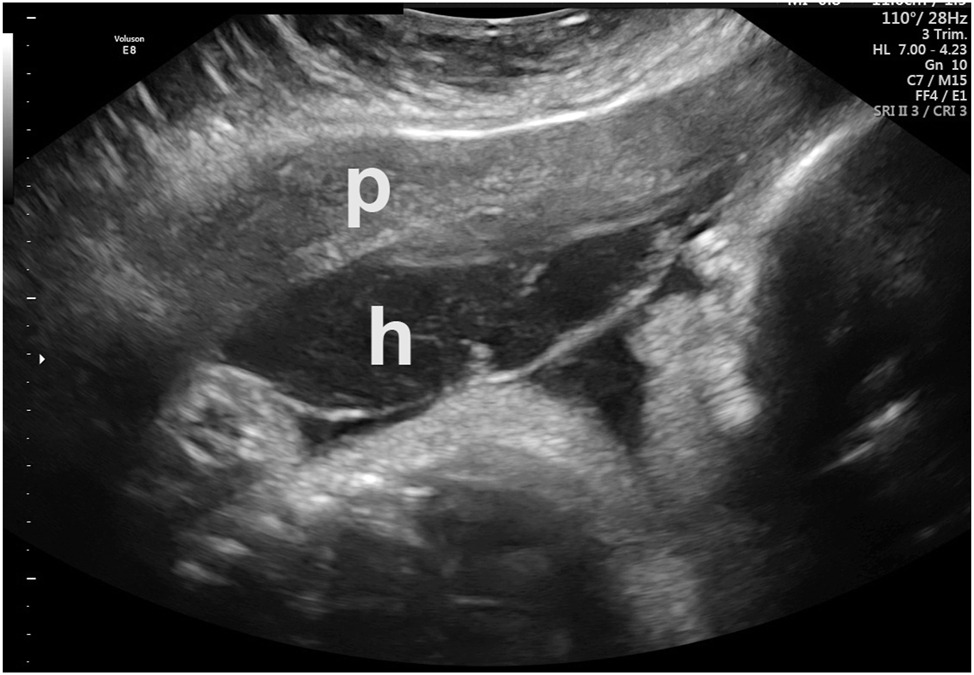
Preplacental hematoma in an asymptomatic patient. Placenta=“p”. Hematoma=“h”. Abruption was confirmed at delivery.

Retroplacental hematoma in an asymptomatic patient. This patient also had a subchorionic hematoma on ultrasound (Figure 8). Abruption was confirmed at delivery.
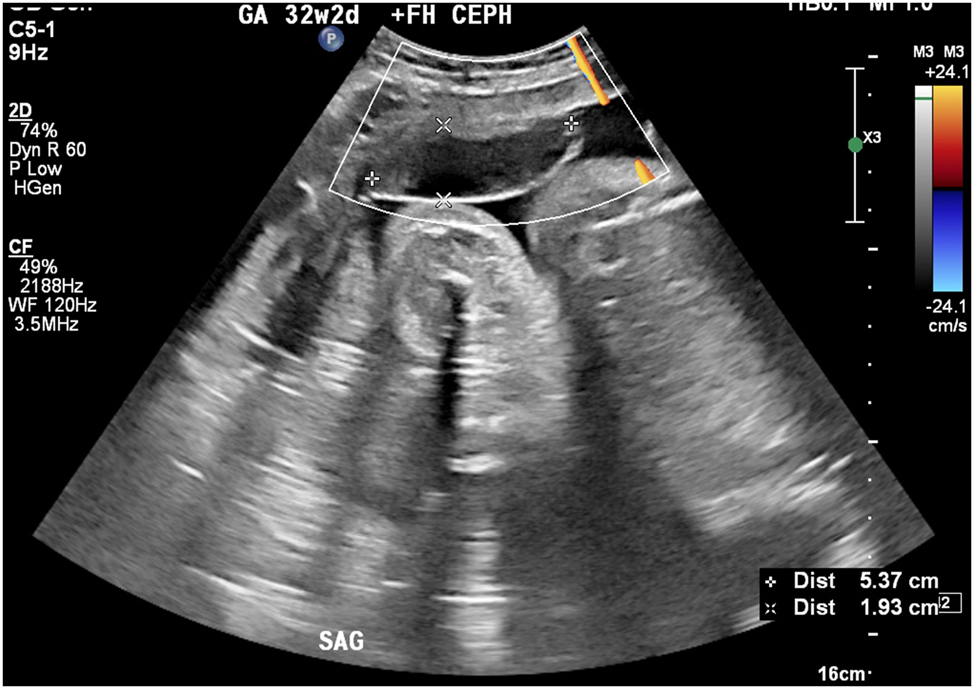
Anterior subchorionic hematoma in same patient as shown in Figure 7.
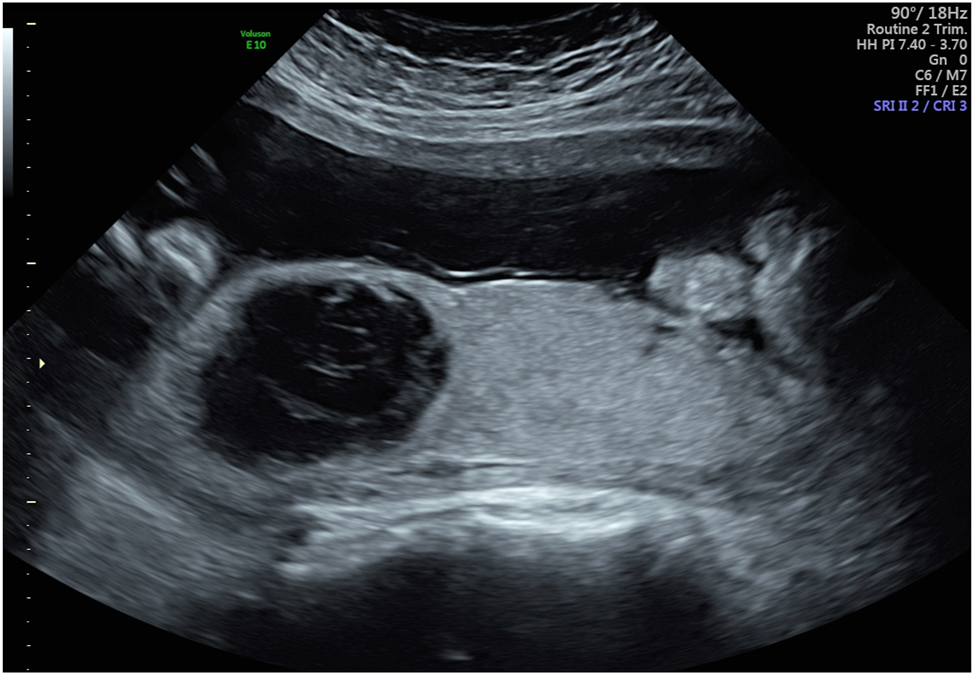
Large rounded intraplacental hematoma in a patient with preeclampsia at 32 weeks who was otherwise asymptomatic. A week later, the patient had a massive clinical abruption requiring urgent cesarean delivery. Placental pathology confirmed a 60 % placental abruption.
Evolving definitions and perspectives
First, we must correct the common erroneous belief that abruption must be symptomatic.
The traditional definition of placental abruption as the “premature separation of the normally located placenta” makes no mention of the presence or absence of bleeding. Historically, abruptions were classified as revealed (with visible vaginal bleeding) or concealed (without visible bleeding). These classifications were based solely on clinical presentation and did not account for imaging findings [8]. When catastrophic separation of the placenta occurs with uterine tenderness, contractions, fetal death, and an associated Couvelaire uterus, as well as coexistent massive obstetric hemorrhage and disseminated intravascular coagulopathy, a diagnosis of placental abruption is undisputed. With the advent of ultrasound however, it has become evident that the finding of hemorrhagic placental lesions that were previously unrecognized may precede clinical symptoms. Whether these findings represent early stages of abruption or distinct pathological processes remains a subject of debate.
The challenge of ultrasound detected hemorrhagic placental lesions
Obstetric ultrasound may sometimes reveal a spectrum of placental hemorrhagic lesions including retroplacental hematomas (hematomas behind the placenta), subchorionic hematomas (between the membranes and the uterine wall), preplacental hematomas (beneath the amnion, also known as Breus’ mole), intraplacental hematomas (within the placental parenchyma), and intraamniotic clots (Figures 1–9) [2], 5], 9], 10]. While some of these findings correlate strongly with clinical abruption, others may be incidental, occurring in asymptomatic patients (Figures 7–9) [11], 12]. This creates challenges for clinicians, who must decide whether to classify these findings as abruption and how to manage such pregnancies. Importantly, while several authoritative texts have considered these sonographic findings to represent abruption [2], 5], 13], 14], consensus is lacking. This may be the main reason that reported sensitivities of ultrasound for the diagnosis of placental abruption have varied widely, from Tikkanen et al. finding a sensitivity of 15 % [15] to Yeo and colleagues reporting that ultrasound detected 80 % of abruptions [13].
Ultrasound has given us an “eye” into the pregnant uterus. We are now able, using ultrasound, to observe several processes as they evolve, prior to symptomatic presentation. This has greatly enhanced our understanding of the pathophysiology and evolution of several pregnancy complications. We propose that several of the hemorrhagic placental lesions we observe on ultrasound precede the clinical phenotype we call placental abruption. This progression can be compared to the evolution of our understanding of another classic obstetrical syndrome: preeclampsia. Historically, eclampsia was diagnosed only when a pregnant individual in the second half of pregnancy experienced seizures. Over time, it was observed that these patients often exhibited swelling of the feet, hands, and face prior to the onset of eclamptic seizures.
It was only within the past two centuries, with the advent of blood pressure (BP) measurement and measurement of urinary protein, that elevated BP and proteinuria were identified as precursors to seizures. This discovery led to the recognition of a distinct condition, now known as preeclampsia. Today, when patients present with new-onset hypertension and proteinuria during pregnancy, they are diagnosed with preeclampsia, even though not all such cases progress to seizures. Similarly, not all these hemorrhagic lesions found on ultrasound end up with bleeding, pain, and fetal distress or demise, the clinical presentation of abruption.
Prior ultrasound studies of placental abruption
Lundberg in 1971 described three patients with retroplacental hematomas noted on ultrasound where abruption was confirmed after delivery [16]. Subsequently, Ylöstalo and colleagues reported on 16 patients with bleeding who were found to have a hematoma on ultrasound, and after delivery made a clinical diagnosis of abruption in five of them [17]. These authors concluded “a clinically important finding was that, in placental abruption, a haematoma may accumulate between the fetal membranes and the uterine wall instead of in the retroplacental space” [17]. Thus, these authors considered subchorionic hematomas to be abruptions. In 1981, Jaffe and colleagues reported on the sonographic appearances of symptomatic patients with clinical abruptions and found retroplacental and intraplacental hematomas, subchorionic hematomas, placental edge abnormalities, and thickened placentas [18]. Hill and colleagues in 1984 reported a case of confirmed placental abruption after delivery in a symptomatic patient in whom ultrasound had shown an intraamniotic clot [19]. They suggested that blood from a retroplacental hemorrhage may have gained access to the amniotic cavity [19]. In 1987, Nyberg et al. published a study of the sonographic spectrum of placental abruption in a cohort of 57 patients, of which 33 were at 20 weeks of gestation or greater [20]. All but two of the patients had vaginal bleeding. They reported that the locations of the hematomas were subchorionic in 81 %, retroplacental in 16 %, and preplacental in 4 % [20]. They classified a sonographically-detected subchorionic hematoma as an abruption if the subchorionic hematoma was noted to be contiguous to the placental edge [20]. These authors expanded on the sonographic findings that would be labelled “abruption”, not merely restricting this term to retroplacental hematomas, stating “while most sonographers recognize a retroplacental hematoma as evidence of placental abruption, other findings are less well known” [20]. They went on to state that “abruption may have a wide spectrum of sonographic findings that may be overlooked or misdiagnosed” [20]. Importantly, almost all these patients were symptomatic and therefore gave a unique opportunity to examine the relationship between ultrasound findings and patients with a clinical diagnosis of abruption.
Yeo and colleagues reported the following sonographic findings in 73 patients presenting with vaginal bleeding to be diagnostic of placental abruption: 1) preplacental collection of blood under the chorionic plate (between placenta and amniotic fluid) [2]; “jello-like” movement of the chorionic plate with fetal activity [3]; retroplacental collection (between placenta and myometrium) [4]; marginal collection (at the placental margin) [5]; subchorionic membranous collection (between the membranes and uterine wall) [6]; increased placental thickness or echogenicities; and [7] intra-amniotic hematomas (defined as collection within the amniotic fluid) [13]. Using these criteria, these authors found that ultrasound had sensitivity, specificity, positive predictive value, and negative predictive value the diagnosis of placental abruption of 80 %, 92 %, 95 %, and 69 %, respectively [13]. These diagnoses of abruptions were all confirmed macroscopically after delivery, which raises the question whether these exact findings in the absence of clinical bleeding should not have the same diagnosis.
Glantz and Purnell in a study of 147 women with vaginal bleeding or suspected abruption found that ultrasound had a sensitivity of only 28 % for abruption. However, these authors limited their definition of ultrasound detected abruption to only retroplacental and subchorionic hematomas [21]. These authors found a 96 % specificity for a clinical diagnosis of abruption for these sonographic findings. While most consider a retroplacental hematoma detected on ultrasound to represent an abruption, there is less consistency on how to term these other hemorrhagic lesions seen on sonography. Evidence that most of these lesions are placental abruptions comes from the studies of Nyberg et al., who demonstrated the presence of these ultrasound findings in patients who were symptomatic and had clinical diagnoses of abruption [20], 22]. Walker and colleagues described a finding of a retroplacental hypoechoic mass and a thick placenta in a patient who presented at 30 weeks of gestation with preeclampsia but with no traditional signs of abruption (bleeding, pain, contractions, abnormal fetal heart tracing) [12]. The mass increased in size over the next 2 days, prompting a cesarean delivery, at which a central organized retroplacental hematoma was found [12]. Pathology examination confirmed this retroplacental hematoma. Thus, this was a concealed abruption diagnosed solely based on ultrasound in an asymptomatic patient and was not based on the clinical diagnosis that is often used. The authors described this as a “chronic abruption.” The recognition that these sonographic findings represented a concealed abruption may have led to a favorable outcome in this case. In one of the cases we present, the patient was evaluated at 30 weeks of gestation for preeclampsia but had no bleeding or pain, with reassuring fetal testing. Sonography revealed a rounded heterogeneous avascular mass in the placenta (Figure 9). A week later, the patient had a massive clinical abruption with bleeding, pain, and fetal distress requiring an emergency delivery and developed disseminated intravascular coagulopathy. Pathology revealed a 60 % placental abruption. Thus, again, this case demonstrated a sonographically-detected abruption in an asymptomatic patient.
A recent study involving 420 patients diagnosed with placental abruption, all of whom underwent ultrasound evaluation, reported no sonographic findings consistent with abruption in 370 of these cases [23]. The authors noted that placental abruption accompanied by supporting sonographic features was associated with higher rates of adverse perinatal outcomes and pathological placental lesions compared to cases without sonographic evidence. However, despite dedicating substantial portions of the study to patient demographics, clinical presentation, neonatal outcomes, and pathological findings indicative of abruption, the specific sonographic findings consistent with abruption were not described [23]. The only reference to these findings was the vague term “ultrasonographic signs of placental abruption.”
Challenges in ultrasound diagnosis of abruption
Using the term “abruption” to collectively describe these diverse placental hemorrhagic lesions creates several problems. First, there is wide variability in the sonographic appearance of these hemorrhagic placental lesions as well as the language used to describe them. There is an absence of agreement on a standard ultrasound definition for placental hematoma, with terms such as “chronic abruption”, “Breus’ mole”, and “placental hemorrhage” often used in describing these placental hemorrhagic lesions detected on prenatal sonography [12], 14], [24], [25], [26]. Second, these lesions may have different etiologies and may have differing significance to the mother and baby. Third, the clinical significance of these lesions in symptomatic vs. asymptomatic patients remains inadequately studied and poorly understood. Finally, the discrepancies between clinical, ultrasound, and pathology findings presents a significant challenge. Clearly, our lack of clarity about the definition of placental abruption greatly inhibits our ability to appropriately study the condition and may lead to errors in ascertainment of the true incidence of abruption, its clinical significance and natural history as well as to the impact of different methods of management. However, all the aforementioned ultrasound findings (subchorionic hematomas, retroplacental hematomas, intraplacental hemorrhage, preplacental hemorrhage), as well as rupture of chorionic vessels may eventually result in vaginal bleeding and a clinical diagnosis of abruption.
Divergent vantage points
Clinicians, sonographers, and pathologists have different vantage points. The clinician has the perspective of history, a physical examination, vital signs, and laboratory tests. The sonographer or sonologist frequently has the history and then must decide how to interpret the sonographic findings. The pathologist relies on accurate transmission of clinical findings and has access to the placenta itself, and then must assess what likely occurred. Are we all like the proverbial six blind men, assessing an elephant by feel, with the men describing it as a tree trunk, spear, fan, rope, wall, or snake depending on whether they were touching the elephant’s leg, tusk, ear, tail, side, or trunk? [27].
Importantly, ultrasound may fail to detect an abruption when the blood loss occurs so rapidly that there is no accumulation of blood in the uterus, and in this situation, ultrasound may be of no utility [5]. Thus, the diagnosis of abruption must involve a combination of clinical findings and imaging, and neither clinical findings, ultrasound, nor pathology alone can exclude an abruption. The problem is compounded because vaginal bleeding in the absence of placenta previa or other clear etiologies are often arbitrarily labelled placental abruptions. The sensitivity and specificity of ultrasound for diagnosing placental abruption depends on what is termed an abruption in the first place. Bleeding is among the most common complications of third trimester pregnancy, and sonography is performed on most of these patients. We need clear criteria on which to base a diagnosis of abruption by ultrasound, since this has tremendous clinical implications. If, as has been argued by Nyberg et al., the variable findings of placental abruption are often not classified or recognized as such, it will have a profound impact on the role of ultrasound in the diagnosis and management of abruption [20].
A recent commentary highlights a critical gap in obstetric ultrasound training: while most education focuses on fetal examination, the placenta often receives minimal attention [28]. This oversight is particularly concerning, as a comprehensive PubMed search reveals fewer than 50 studies examining the role of ultrasound in diagnosing placental abruption. Among these, only a few specifically address the criteria for diagnosis and the outcomes associated with sonographically-detected hemorrhagic placental lesions [20], 22]. It is imperative to enhance training for practitioners of obstetric ultrasound to accurately identify the sonographic features of both normal and abnormal placental appearances. Sonographic misdiagnosis of abruption is common, as normal hypoechoic features within the placenta – such as placental lakes, lacunae, the retroplacental hypoechoic space, and even the decidua – are often mistaken for abruption by inexperienced examiners. Additionally, uterine fibroids may mimic placental abnormalities on ultrasound to the untrained eye. Improved education and a focused understanding of these nuances are essential to reduce diagnostic errors and optimize patient outcomes. Color flow Doppler, especially using SlowFlow HD may help in distinguishing these physiologic placental findings from abruption.
The situation becomes even more murky because pathology cannot offer a gold standard definition for abruption, both due to artifactual changes during delivery and the time interval needed to develop histologic changes after a bleed. The sensitivity of a pathology exam for confirmation of clinical acute abruption is estimated to be only 30 % [29]. Thus, pathology cannot and should not be considered a “gold standard” for a diagnosis of abruption. Pathology exam can identify risk factors for abruption (such as chorioamnionitis in the second trimester, marginal cord insertion, and hemosiderin-laden macrophages in the chorionic plate and membranes suggesting prior smaller hemorrhage) that may complement the clinical findings. In clinically diagnosed abruption, histology can also suggest the timing; hemosiderin-laden macrophages, villous necrosis or infarction and plasma cell deciduitis are compatible with subacute abruption, while villous stromal hemorrhage and acute inflammation suggest acute abruption [30]. Pathologists generally report abruption in 3 categories (acute central or complete abruption, acute marginal abruption, and chronic abruption) and then describe the features specific to the case to facilitate clinical correlation. These include gross findings: retroplacental hematoma with indentation of the disc (acute) or brown membrane staining or circumvallate membrane insertion (chronic); and microscopic features as described above. Development of a common terminology and reporting template would certainly improve communication between obstetricians and pathologists.
Suggested management of sonographically-detected hemorrhagic lesions
We propose that placental abruption should be viewed as a continuum rather than a binary condition, and that ultrasound findings that indicate hemorrhagic placental lesions be considered in the diagnosis and management of abruption. These findings may represent early stages of this continuum, even in asymptomatic patients. In these patients, sonographic evidence of hemorrhagic lesions should prompt closer monitoring, as these lesions may evolve into clinical abruption [11], 12]. Surveillance strategies could include serial ultrasounds and enhanced fetal testing to detect early signs of compromise, and delivery when these lesions are found at or near term. In symptomatic patients, ultrasound findings should be considered confirmatory of abruption and guide decisions regarding timing of delivery and maternal-fetal monitoring. This will help guide the surveillance and management of the patient, including moving toward delivery if the gestational age is near term.
Toward consensus: guidelines for sonographically-detected placental lesions
Clinicians, sonographers, and pathologists must work together to develop standardized terminology and diagnostic criteria for both abruption and sonographically-detected hemorrhagic lesions. Greater emphasis should be placed on placental imaging during obstetric ultrasound training [28]. In addition, more research needs to be done to study the prevalence and natural history of these sonographically-detected hemorrhagic lesions. Knowledge gaps exist regarding the diverse etiologies and outcomes of these lesions depending on their location, the most effective sonographic techniques for their assessment and monitoring, including the role of Doppler, and the utility of prospective pathological examinations in cases where such ultrasound findings are observed. Further prospective studies are essential to address these gaps and enhance our understanding. Pregnancies with these sonographic findings should undergo enhanced surveillance, including serial ultrasounds and fetal monitoring, and delivery should be considered when these lesions are found close to term. Finally, professional organizations should prioritize the development of evidence-based guidelines for managing pregnancies complicated by placental hemorrhagic lesions.
Conclusions
Placental abruption remains a complex and multifaceted condition. The integration of ultrasound into prenatal care has introduced new diagnostic challenges. Recognizing ultrasound-detected hemorrhagic placental lesions as part of the continuum of abruption may improve diagnostic accuracy, clinical management, and research outcomes. Standardized criteria and interdisciplinary collaboration will help in addressing these challenges and improving the care of pregnant individuals and their babies.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: Yinka Oyelese has received royalties as an author for chapters on abruption for UpToDate and BMJ Best Practice. Yinka Oyelese is on the Scientific Advisory Board for Sonio LLC, for which he has received honoraria. Wendy Kinzler has received royalties for a chapter in abruption for UpToDate. The remaining authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Ananth, CV, Berkowitz, GS, Savitz, DA, Lapinski, RH. Placental abruption and adverse perinatal outcomes. JAMA 1999;282:1646–51. https://doi.org/10.1001/jama.282.17.1646.Suche in Google Scholar PubMed
2. Brandt, JS, Ananth, CV. Placental abruption at near-term and term gestations: pathophysiology, epidemiology, diagnosis, and management. Am J Obstet Gynecol 2023;228:S1313–29. https://doi.org/10.1016/j.ajog.2022.06.059.Suche in Google Scholar PubMed PubMed Central
3. Waller, JA, Saade, G. Stillbirth and the placenta. Semin Perinatol 2023:151871. https://doi.org/10.1016/j.semperi.2023.151871.Suche in Google Scholar PubMed
4. Downes, KL, Shenassa, ED, Grantz, KL. Neonatal outcomes associated with placental abruption. Am J Epidemiol 2017;186:1319–28. https://doi.org/10.1093/aje/kwx202.Suche in Google Scholar PubMed PubMed Central
5. Oyelese, Y, Ananth, CV. Placental abruption. Obstet Gynecol 2006;108:1005–16. https://doi.org/10.1097/01.aog.0000239439.04364.9a.Suche in Google Scholar
6. Riihimaki, O, Metsaranta, M, Paavonen, J, Luukkaala, T, Gissler, M, Andersson, S, et al.. Placental abruption and child mortality. Pediatrics 2018;142. https://doi.org/10.1542/peds.2017-3915.Suche in Google Scholar PubMed
7. Downes, KL, Grantz, KL, Shenassa, ED. Maternal, labor, delivery, and perinatal outcomes associated with placental abruption: a systematic review. Am J Perinatol 2017;34:935–57. https://doi.org/10.1055/s-0037-1599149.Suche in Google Scholar PubMed PubMed Central
8. Cunningham, FLK, Dashe, JS, Hoffman, BL, Spong, CY, Casey, BM. William’s Obstetrics. New York, NY: McGraw Hill; 2022.Suche in Google Scholar
9. Ott, J, Pecnik, P, Promberger, R, Pils, S, Binder, J, Chalubinski, KM. Intra- versus retroplacental hematomas: a retrospective case-control study on pregnancy outcomes. BMC Pregnancy Childbirth 2017;17:366. https://doi.org/10.1186/s12884-017-1539-6.Suche in Google Scholar PubMed PubMed Central
10. Jouppila, P, Kirkinen, P. Problems associated with the ultrasonic diagnosis of abruptio placentae. Int J Gynaecol Obstet 1982;20:5–11. https://doi.org/10.1016/0020-7292(82)90038-8.Suche in Google Scholar PubMed
11. Sherer, DM, Kheyman, M, Benayoun, J, Dalloul, M. Incidental sonographic finding of a concealed placental abruption leading to delivery at 37 weeks’ gestation. J Clin Ultrasound 2021;49:630–1. https://doi.org/10.1002/jcu.22971.Suche in Google Scholar PubMed
12. Walker, M, Whittle, W, Keating, S, Kingdom, J. Sonographic diagnosis of chronic abruption. J Obstet Gynaecol Can 2010;32:1056–8. https://doi.org/10.1016/s1701-2163(16)34713-2.Suche in Google Scholar
13. Yeo, L, Vintzileos Anthony, M. Placental abruption. London: The Global Libary of Women’s Medicine; 2009. https://www.glowm.com/section-view/heading/Placental%20Abruption/item/122 [Accessed 29 Apr 2025].10.3843/GLOWM.10122Suche in Google Scholar
14. Melamud, K, Wahab, SA, Smereka, PN, Dighe, MK, Glanc, P, Kamath, A, et al.. Imaging of antepartum and postpartum hemorrhage. Radiographics 2024;44:e230164. https://doi.org/10.1148/rg.230164.Suche in Google Scholar PubMed
15. Tikkanen, M, Nuutila, M, Hiilesmaa, V, Paavonen, J, Ylikorkala, O. Clinical presentation and risk factors of placental abruption. Acta Obstet Gynecol Scand 2006;85:700–5. https://doi.org/10.1080/00016340500449915.Suche in Google Scholar PubMed
16. Lundberg, J. Diagnosis of abruptio placentae by ultrasound. Lancet 1971;1:806.10.1016/S0140-6736(71)91253-0Suche in Google Scholar PubMed
17. Ylostalo, P, Ammala, P, Seppala, M. Intrauterine haematoma and placental protein 5 in patients with uterine bleeding during pregnancy. Br J Obstet Gynaecol 1984;91:353–6.10.1111/j.1471-0528.1984.tb05922.xSuche in Google Scholar
18. Jaffe, MH, Schoen, WC, Silver, TM, Bowerman, RA, Stuck, KJ. Sonography of abruptio placentae. AJR Am J Roentgenol 1981;137:1049–54. https://doi.org/10.2214/ajr.137.5.1049.Suche in Google Scholar PubMed
19. Hill, LM, Breckle, R, Gehrking, W. Abruptio placentae: an unusual ultrasonic presentation. Am J Obstet Gynecol 1984;148:1144–5. https://doi.org/10.1016/0002-9378(84)90646-x.Suche in Google Scholar PubMed
20. Nyberg, DA, Cyr, DR, Mack, LA, Wilson, DA, Shuman, WP. Sonographic spectrum of placental abruption. AJR Am J Roentgenol 1987;148:161–4. https://doi.org/10.2214/ajr.148.1.161.Suche in Google Scholar PubMed
21. Glantz, C, Purnell, L. Clinical utility of sonography in the diagnosis and treatment of placental abruption. J Ultrasound Med 2002;21:837–40. https://doi.org/10.7863/jum.2002.21.8.837.Suche in Google Scholar PubMed
22. Nyberg, DA, Mack, LA, Benedetti, TJ, Cyr, DR, Schuman, WP. Placental abruption and placental hemorrhage: correlation of sonographic findings with fetal outcome. Radiology 1987;164:357–61. https://doi.org/10.1148/radiology.164.2.3299486.Suche in Google Scholar PubMed
23. Mor, L, Erteschik, N, Gandelsman, E, Vartkova, A, Kleiner, I, Barda, G, et al.. Obstetric and neonatal outcomes in pregnancies complicated by placental abruption with vs. without supporting sonographic findings- A retrospective cohort study. Placenta 2024;156:14–9. https://doi.org/10.1016/j.placenta.2024.08.017.Suche in Google Scholar PubMed
24. Hernandez-Andrade, E, Huntley, ES, Bartal, MF, Soto-Torres, EE, Tirosh, D, Jaiman, S, et al.. Doppler evaluation of normal and abnormal placenta. Ultrasound Obstet Gynecol 2022;60:28–41. https://doi.org/10.1002/uog.24816.Suche in Google Scholar PubMed
25. Alanjari, A, Wright, E, Keating, S, Ryan, G, Kingdom, J. Prenatal diagnosis, clinical outcomes, and associated pathology in pregnancies complicated by massive subchorionic thrombohematoma (Breus’ mole). Prenat Diagn 2013;33:973–8. https://doi.org/10.1002/pd.4176.Suche in Google Scholar PubMed
26. Kocak, M, Kandemir, O, Sen, S, Baskan, B, Demir, OF. A rare form of abruptio placenta and clinical presentation in a preterm labor case: Breus’ mole. Fetal Diagn Ther 2006;21:540–3. https://doi.org/10.1159/000095669.Suche in Google Scholar PubMed
27. Saxe, J. The poems of John Godfrey Saxe. Mifflin: Houghton; 1884.Suche in Google Scholar
28. Jauniaux, E, Lees, C, Bhide, A, Daly-Jones, E, Srinivasan, D, Oyelese, Y. The placenta and umbilical cord in prenatal care: Unseen, overlooked and misunderstood. BJOG 2025;132:12–4. https://doi.org/10.1111/1471-0528.17936.Suche in Google Scholar PubMed PubMed Central
29. Elsasser, DA, Ananth, CV, Prasad, V, Vintzileos, AM, New Jersey-Placental Abruption Study, I. Diagnosis of placental abruption: relationship between clinical and histopathological findings. Eur J Obstet Gynecol Reprod Biol 2010;148:125–30. https://doi.org/10.1016/j.ejogrb.2009.10.005.Suche in Google Scholar PubMed PubMed Central
30. Chen, AL, Goldfarb, IT, Scourtas, AO, Roberts, DJ. The histologic evolution of revealed, acute abruptions. Hum Pathol 2017;67:187–97. https://doi.org/10.1016/j.humpath.2017.08.007.Suche in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Editorial
- Perinatal responsibility in a fragmented world: reflections from the 2024 international academy of perinatal medicine New York meeting
- Corner of Academy
- Global education – impressive results of Ian Donald School
- Cicero’s universal law: a timeless guide to reproductive justice
- Enhancing patient understanding in obstetrics: the role of generative AI in simplifying informed consent for labor induction with oxytocin
- Faculty retention in academic OB/GYN: comprehensive strategies and future directions
- Hemolytic disease of the fetus and newborn: pregnant person’s and fetal immune systems interaction
- Viability of extremely premature neonates: clinical approaches and outcomes
- Reviews
- Standardizing cord clamping: bridging physiology and recommendations from leading societies
- Thrombotic thrombocytopenic purpura in pregnancy: a comprehensive review
- Mini Review
- Looking for a needle in a haystack: a case study of rare disease care in neonatology
- Opinion Paper
- Hemorrhagic placental lesions on ultrasound: a continuum of placental abruption
- Original Articles – Obstetrics
- Amnioreduction safety in singleton pregnancies; systematic review and meta-analysis
- Outpatient management of prelabour rupture of membranes (PROM) at term – a re-evaluation and contribution to the current debate
- Breastfeeding in HIV-positive mothers under optimized conditions: ‘real-life’ results from a well-resourced healthcare setting
- Intervention using the Robson classification as a tool to reduce cesarean section rates in six public hospitals in Brazil
- Short Communication
- Continuous positive airway pressure vs. high velocity nasal cannula for weaning respiratory support of preterm infants
Artikel in diesem Heft
- Frontmatter
- Editorial
- Perinatal responsibility in a fragmented world: reflections from the 2024 international academy of perinatal medicine New York meeting
- Corner of Academy
- Global education – impressive results of Ian Donald School
- Cicero’s universal law: a timeless guide to reproductive justice
- Enhancing patient understanding in obstetrics: the role of generative AI in simplifying informed consent for labor induction with oxytocin
- Faculty retention in academic OB/GYN: comprehensive strategies and future directions
- Hemolytic disease of the fetus and newborn: pregnant person’s and fetal immune systems interaction
- Viability of extremely premature neonates: clinical approaches and outcomes
- Reviews
- Standardizing cord clamping: bridging physiology and recommendations from leading societies
- Thrombotic thrombocytopenic purpura in pregnancy: a comprehensive review
- Mini Review
- Looking for a needle in a haystack: a case study of rare disease care in neonatology
- Opinion Paper
- Hemorrhagic placental lesions on ultrasound: a continuum of placental abruption
- Original Articles – Obstetrics
- Amnioreduction safety in singleton pregnancies; systematic review and meta-analysis
- Outpatient management of prelabour rupture of membranes (PROM) at term – a re-evaluation and contribution to the current debate
- Breastfeeding in HIV-positive mothers under optimized conditions: ‘real-life’ results from a well-resourced healthcare setting
- Intervention using the Robson classification as a tool to reduce cesarean section rates in six public hospitals in Brazil
- Short Communication
- Continuous positive airway pressure vs. high velocity nasal cannula for weaning respiratory support of preterm infants

