Abstract
The timing of umbilical cord clamping has stirred much greater debate and evolution in the field of obstetrics and neonatology, spurred by advances in medical science as well shifting clinical paradigms. This review seeks to address the history, physiology and clinical applications of different umbilical cord clamping practices around a common theme. The history of these practices and their effects on the mothers as well as new-borns have been addressed in this article along with how modern evidence has been shaping our guidelines. By examining the physiological mechanisms underlying umbilical cord clamping (UCC) and the evolving clinical standards, this article seeks to inform healthcare providers and policymakers on the best approaches for optimizing maternal and neonatal health.
Introduction
The timing of umbilical cord clamping has stirred much greater debate and evolution in the field of obstetrics and neonatology, spurred by advances in medical science as well shifting clinical paradigms [1]. Historically, it was common practice to immediately clamp the umbilical cord within 15–30 s of birth. In recent years, research and guidelines have changed the scope from early cord clamping to waiting at least 1–3 min after birth before clamping or even longer if needed, until pulsation of the cord has finished. This change in protocol is based on advancements in the understanding of neonatal physiology as it pertains to the value benefit from enhanced placental transfusion. This review seeks to address the history, physiology and clinical applications of different umbilical cord clamping (UCC) practices around a common theme. The present review addresses the history of these practices and their effects on the mothers as well as new born, along with how modern evidence has been shaping our guidelines. By examining the physiological mechanisms underlying UCC and the evolving clinical standards, this article seeks to inform healthcare providers and policymakers on the best approaches for optimizing maternal and neonatal health.
Historical context of cord clamping
While cutting the umbilical cord is essential, the rationale behind clamping it is more debated. In 1968, Botha reviewed early literature on cord tying or clamping dating back to 1668 [2]. Initially, the neonatal tie or clamp was used to prevent blood loss from the baby before the umbilical vessels closed naturally. Two additional reasons for clamping the placental side of the cord also emerged: to identify when the cord lengthened, signalling placental separation, and to keep bed linen clean by preventing placental blood from leaking from the cut end of the cord. Botha argued that these reasons were ‘not sufficient to justify… clamping’ [3]. In 1773, Charles White wrote that ‘the common method of tying and cutting the navel string in the instant the child is born… has nothing to plead in its favour but custom [4]. In 1801, Erasmus Darwin wrote, ‘Another thing very injurious to the child, is the tying and cutting of the navel string too soon; which should always be left till the child has not only repeatedly breathed but till all pulsation in the cord ceases. As otherwise the child is much weaker than it ought to be’ [5].
Nevertheless, early cord clamping gained popularity over time. In 1899, Magennis introduced a ‘midwifery surgical clamp’ to replace the traditional cloth tie, claiming that this instrumentation would reduce the risk of infection. He recommended clamping the cord ‘when it has ceased to pulsate.’ While the clamp became a standard tool in third stage management, the exact timing of its application was seldom recorded [6]. One reason clamping before placental delivery became common was the discovery in 1938 of placental and umbilical cord blood as a valuable source for transfusions. Due to its unique immunological and hematopoietic properties, cord blood has been used ever since for treating various conditions, from malaria to malignancies [7]. In the 1940s, research into erythroblastosis fetalis (haemolytic disease of the newborn) highlighted the role of maternal isoimmunization in the disease’s pathophysiology. It was believed that early clamping of the umbilical cord would prevent the transfer of ‘excessive amounts of maternal antibody-containing blood’ to the newborn. The subsequent development of Rh(D) Immune Globulin in the 1960s reduced the need for early clamping, but by that time, the practice had already become routine [8].
Immediate cord clamping, was initially incorporated in the 1960s as a component of active management during the third stage of labor, aimed to mitigate postpartum hemorrhage risk. This approach, encompassing a set of interventions, traditionally included the administration of a prophylactic uterotonic drug alongside immediate cord clamping and controlled cord traction. The rationale behind early cord clamping and controlled cord traction stemmed from concerns about potential complications following uterotonic drug administration, such as the placenta becoming entrapped in the uterus thereby leading to retained placenta and its related complications [9], 10]. A re-evaluation of the elements comprising active management has revealed that while uterotonic drugs effectively decrease the risk of postpartum hemorrhage, controlled cord traction has been found to lack significant additional benefits. Similarly, recent evidence claims that the timing of cord clamping seems to have minimal impact on the risk of postpartum hemorrhage or retained placenta. Moreover, there is accumulating evidence suggesting potential benefits for infants when cord clamping is delayed [11]. Figure 1 summarizes the timeline of events, from delayed to early and back to delayed cord clamping.
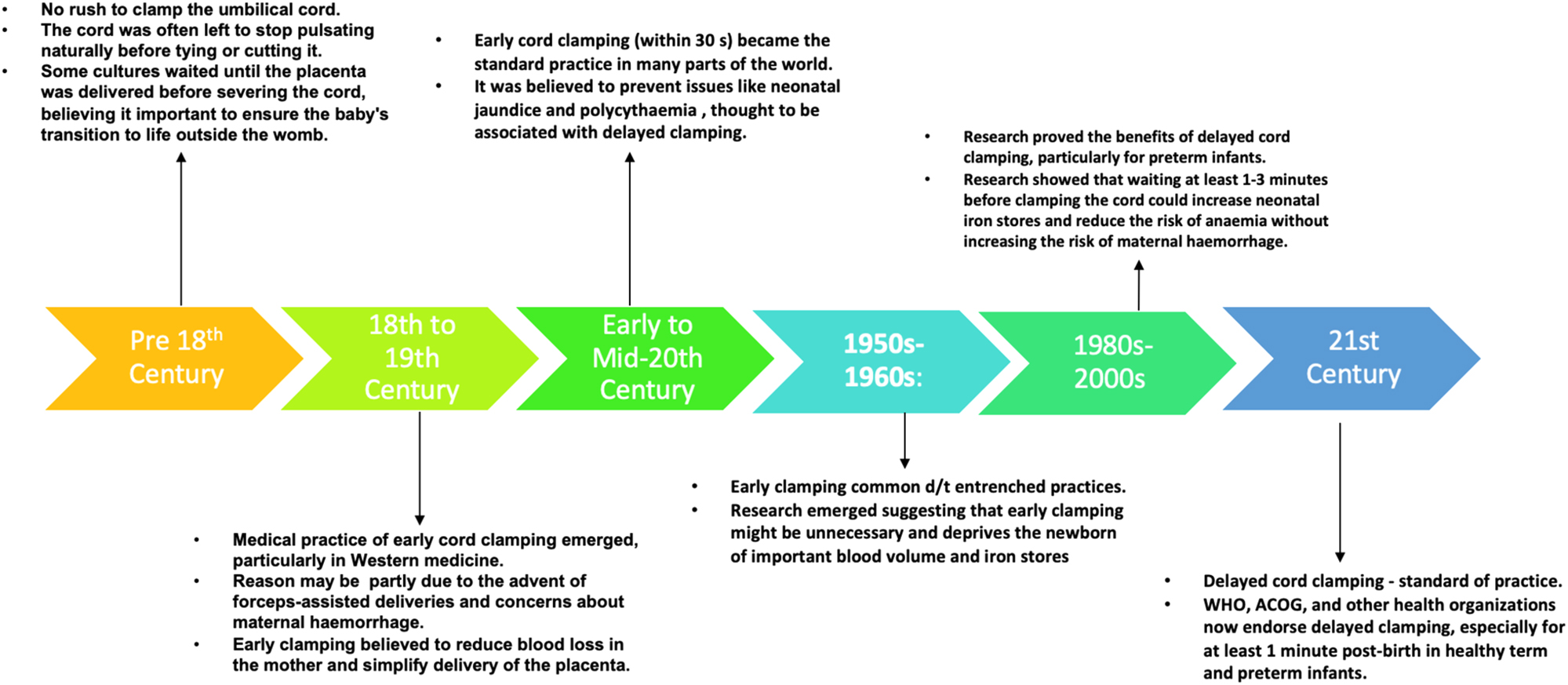
The timeline of events, from delayed to early and back to delayed cord clamping.
Why timing matters in umbilical cord clamping
The timing of UCC is important because it affects the newborn’s transition from fetal to neonatal life, impacting their immediate and long-term health outcomes. When the umbilical cord remains unclamped immediately after birth, there is a brief period during which blood continues to flow between the baby and the placenta, known as “placental transfusion.” In term births, this blood flow typically concludes within 2 min, though it may persist for up to 5 min [12], 13]. During this time, the infant receives an average of 80–100 mL of additional blood, which can constitute a significant portion, around a third to a quarter, of the newborn’s total blood volume at birth. The extra plasma gained from placental transfusion is rapidly integrated into the circulation, resulting in a higher red blood cell count. Consequently, this process contributes approximately 20–30 mg/kg of iron to the infant’s iron stores [14], 15]. DCC provides extra blood volume, which supports the cardiovascular system and helps the newborn transition to breathing outside the womb by providing adequate oxygen delivery during the first few minutes of life. It also increases the transfer of stem cells to the newborn, which can have regenerative properties and potentially improve organ development and repair.
Fetal to newborn physiology
Fetal circulation is characterized by three key shunts, the ductus venosus, foramen ovale, and ductus arteriosus, which direct oxygenated blood from the placenta to vital organs while bypassing the non-functioning lungs. The umbilical vein carries oxygen-rich blood from the placenta to the fetus, while deoxygenated blood returns via the umbilical arteries [16]. Pulmonary vascular resistance remains high due to fluid-filled alveoli, limiting blood flow to the lungs [17]. Cord clamping after birth initiates several physiological changes in a newborn, transitioning from fetal to neonatal circulation. Once the umbilical cord is clamped, the placental circulation is cut off, leading to an increase in systemic vascular resistance. This causes blood pressure in the newborn to rise, which helps maintain blood flow to vital organs. The increase in pressure on the left side of the heart, due to increased pulmonary blood flow, causes the foramen ovale to close. The increased oxygen levels in the blood and loss of placental prostaglandins cause the ductus arteriosus to constrict and eventually close. The ductus venosus, which shunts a portion of the blood flow from the umbilical vein directly to the inferior vena cava, also closes as the umbilical cord is clamped. Before birth, the lungs are fluid-filled, and only a small amount of blood passes through them [16], 17]. After birth, as the lungs expand and the newborn takes its first breaths, pulmonary resistance decreases significantly, allowing more blood to flow through the lungs for oxygenation. These physiological changes are essential for the newborn to adapt to life outside the womb (Figure 2).
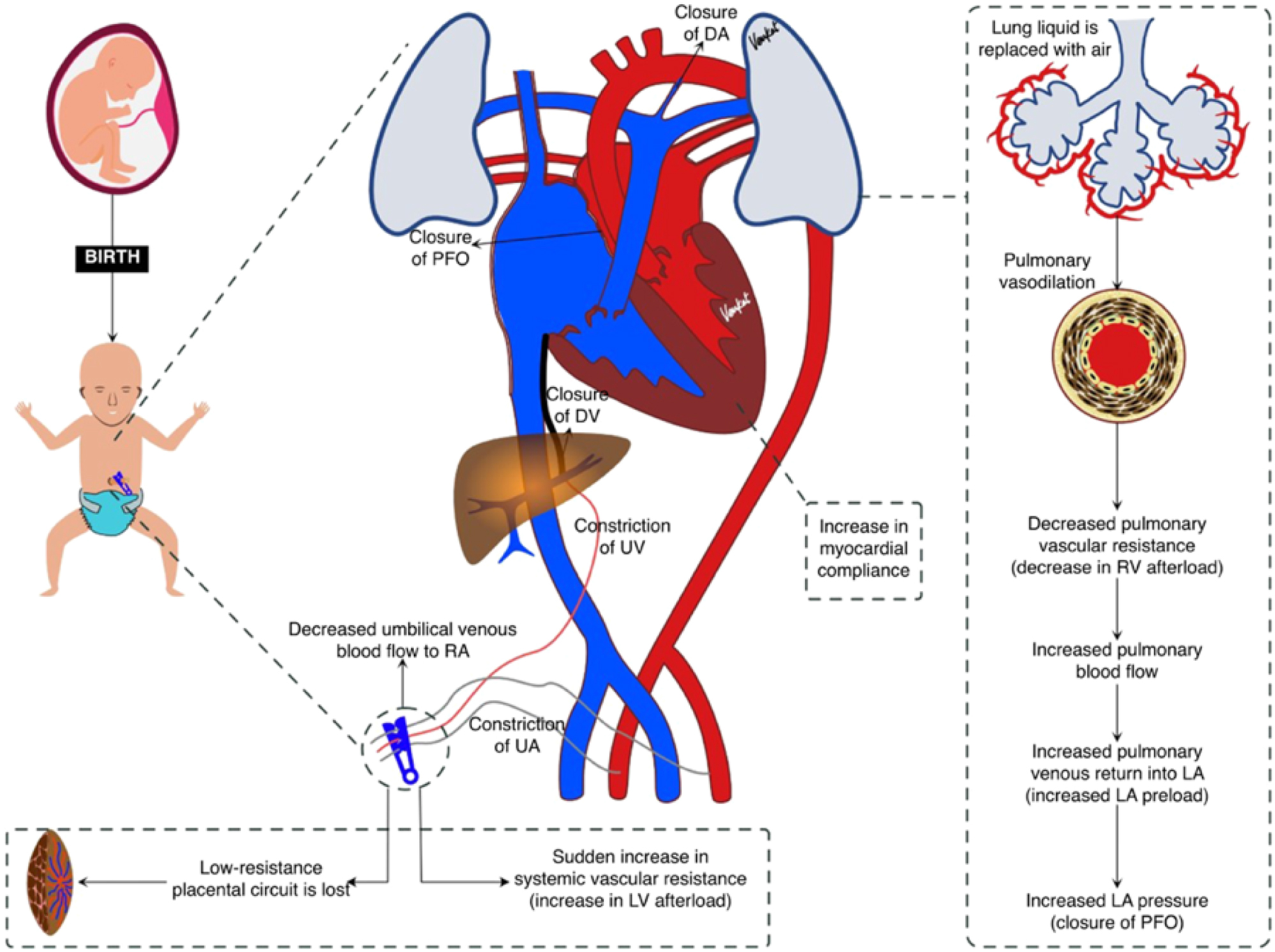
Fetal to neonatal transition after clamping of the umbilical cord (20) (Reproduced from Chakkarapani AA, Roehr CC, Hooper SB, Te Pas AB, Gupta S; ESPR neonatal resuscitation section writing group. Transitional circulation and hemodynamic monitoring in newborn infants. Pediatr res. 2024;96:595–603).
Physiological cord clamping or physiology based cord clamping (PBCC)
Physiological-based cord clamping (PBCC) is an individualized approach to umbilical cord management that prioritizes the newborn’s transition to independent breathing and circulation before clamping the cord. Unlike delayed cord clamping (DCC), which follows a set time frame, PBCC ensures the infant has established effective breathing and cardiovascular stability before separation from the placenta [18]. A heart rate above 100 beats per minute, improving skin colour, and increasing oxygen saturation indicate readiness for clamping. Additionally, the cord is typically clamped once pulsations slow or cease, signalling that placental transfusion is near completion. By waiting for these physiological signs rather than following a fixed time frame, PBCC supports a smoother neonatal transition, reducing risks such as hypoxia and hemodynamic instability. This approach allows continued placental transfusion, reducing the risks of hypoxia, hypotension, and intraventricular haemorrhage, especially in preterm infants. By maintaining umbilical circulation until the baby is physiologically ready, PBCC supports better oxygenation, hemodynamic stability, and overall neonatal adaptation [19], 20]. While PBCC offers significant benefits, its implementation requires bedside access to neonatal resuscitation and well-trained healthcare providers. It may not be feasible in emergencies where immediate intervention is needed. Compared to immediate and delayed cord clamping, PBCC provides a more individualized method, ensuring each newborn receives the optimal timing for cord clamping based on physiological readiness rather than a fixed duration.
Extrauterine placental perfusion
Extrauterine placental perfusion (EPP) is a technique used during neonatal resuscitation, particularly in very low birth weight (VLBW) infants, born via caesarean delivery. In EPP, after the baby is delivered, the umbilical cord remains attached to the placenta, which is then kept outside the mother’s body (hence “extrauterine”). This allows continued blood flow from the placenta to the baby, potentially aiding in the transition from prenatal to postnatal circulation. The method involves transferring the infant, with the placenta still connected, to a resuscitation unit where additional respiratory support, like mask CPAP (Continuous Positive Airway Pressure), can be provided. This approach is believed to offer the benefits of placental transfusion even after the placenta is detached from the uterus, supporting the infant’s circulation and oxygenation during the critical moments after birth [21], 22]. PBCC may stabilize the transition from prenatal to postnatal circulation by allowing blood flow from the placenta, when the cord is not clamped before lung aeration. However, it’s unclear if a detached placenta has the same effect. Dunn [23] explored delayed cord clamping (DCC) after placental removal in preterm caesarean births decades ago, finding it safe for mother and infant, even at term. A retrospective analysis showed no adverse outcomes associated with EPP. Delayed cord clamping, physiologic cord clamping and EPP are terms often used interchangeably, but they have some nuanced differences in their definitions and implications (Table 1; Figure 3) [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44].
A comparison of delayed cord clamping, physiological cord clamping and extra-uterine placental perfusion.
| Delayed cord clamping | Physiologic cord clamping | Extrauterine placental perfusion (EPP) [21] | |
|---|---|---|---|
| Definition | Delayed cord clamping (DCC) refers to the practice of waiting for a specific period (usually between 30 s to 3 min) after birth before clamping and cutting the umbilical cord. | Physiologic cord clamping (PCC) is the practice of clamping the cord based on physiological signs rather than a specific time period. This usually means waiting until the umbilical cord has stopped pulsating naturally, indicating that the placental transfusion is complete. | The infant and placenta, still connected by an intact umbilical cord, are removed from the uterus and transferred to a resuscitation unit, and thus initiating the respiratory support while allowing continued blood flow through the umbilical cord. Clamped is done, after onset of regular spontaneous breathing, stable HR>100, and adequate oxygen saturation. |
| Timing | The timing is usually standardized and may be influenced by clinical guidelines or protocols. | The timing is more flexible and individualized, often ranging from 1 to 5 min or longer, depending on the cessation of cord pulsation. | Variable and individualised, as the cord is clamped after achieving a stable HR>100, and adequate oxygen saturation levels. |
| Objective | The primary aim is to allow a significant amount of blood from the placenta to transfer to the newborn, improving blood volume and iron stores. | The goal is to align the clamping with the newborn’s natural physiological transition from fetal to neonatal circulation, ensuring maximal blood transfer and optimal neonatal outcomes. | To improve the newborn’s transition from intrauterine to extrauterine life, potentially reducing the risk of complications associated with immediate cord clamping. |
| Clinical practice | Easier to standardize and implement in clinical settings with specific protocols. | Requires more clinical judgment and observation, potentially varying more between practitioners and situations. | Still experimental, has been tried for a few cases of very low birth weight babies |
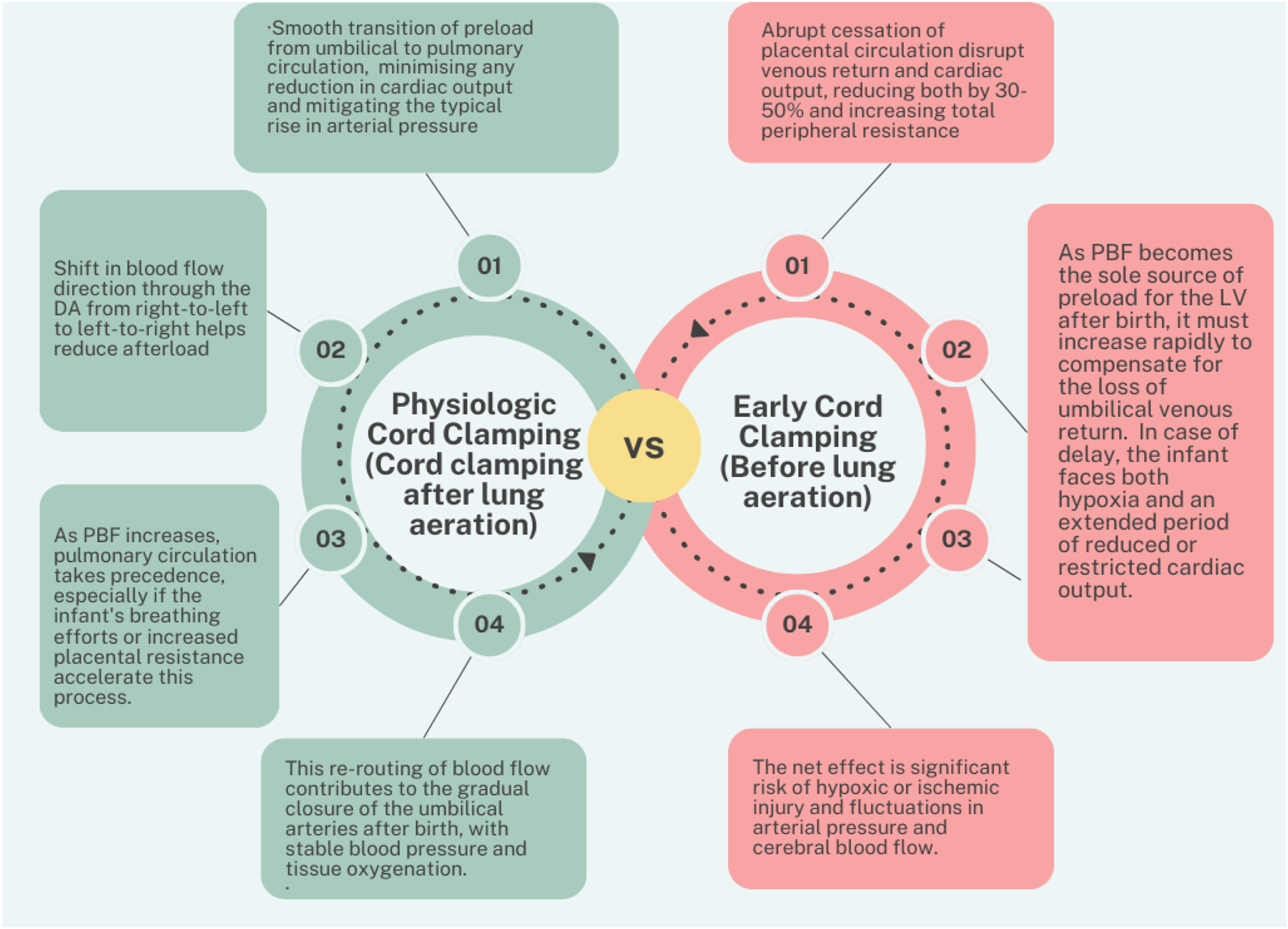
Comparison of physiologic vs. early cord clamping.
Clinical implications, controversies and debates in umbilical cord clamping
There is no universally accepted definition for the duration of “delayed” clamping, leading to varying practices worldwide. Some guidelines recommend waiting 30 s, while others suggest 1–3 min or until the umbilical cord stops pulsating. The lack of consensus complicates clinical decision-making and creates variability in care, particularly in diverse healthcare settings with different resources and protocols. A summary of the leading guidelines in the field are summarised in Table 2. The timing of umbilical cord clamping (UCC) has sparked significant debate among healthcare professionals, researchers, and policymakers due to its implications for both maternal and neonatal outcomes. While there is growing support for delayed cord clamping (DCC), controversies remain regarding its optimal timing, safety, and applicability across various clinical settings [45], 46].
A summary of the leading guidelines on umbilical cord clamping.
| Scientific body | Recommendation according to GA and type of delivery | At what time | Benefits/risks of DCC |
|---|---|---|---|
| Italian Recommendation for Placental Transfusion Strategies 2018 [24] |  - VD (T//LPT/PT) - VD (T//LPT/PT)CD (T/LPT/PT)  - CD - CD - Monochorionic twins, HIV + mothers with higher viral load, CD under GA, foetal hydrops, doubts about integrity of U. Cord, new born with perinatal asphyxia, rhesus disease - Monochorionic twins, HIV + mothers with higher viral load, CD under GA, foetal hydrops, doubts about integrity of U. Cord, new born with perinatal asphyxia, rhesus disease |
T/LPT - 1min PT- 30 s, reassess, if stable 60 s |
Benefits – Positive effect on neonate haematological parameters, and maintaining cardiac output and less systemic arterial pressure fluctuations Risks – NM |
| ACOG 2017 [25] |  - VD (T/PT) - VD (T/PT)CD(T/PT)  - Multiple gestation, neonates requiring immediate resuscitation, and fetuses in which placental circulation is compromised (abruption, previa, abnormal umbilical artery Dopplers) - Multiple gestation, neonates requiring immediate resuscitation, and fetuses in which placental circulation is compromised (abruption, previa, abnormal umbilical artery Dopplers) |
30–60 s | Benefits – Increases haemoglobin levels and iron stores Risks – Small increase in the risk of jaundice requiring phototherapy |
| WHO 2014 [26] |  VD/CD (T/PT) VD/CD (T/PT) |
1–3 min | Benefits – Improves iron reserves, and decreases the risk of IVH, NEC, late onset sepsis in PT. Risks – Increased risk of vertical transmission in HIV |
| RCOG [27] |  T/PT T/PT - Cases with cord prolapse, if newborn is not stable - Cases with cord prolapse, if newborn is not stable |
60 s | Benefits – Infants requiring resuscitation Risks – NM |
| European Association of Perinatal Medicine 2024 [28] |
 - PT - PT |
>30 s | Benefits – Increase hemoglobin and hematocrit & decrease risk of IVH & NEC Risks – NM |
| SOGC 2018 [29] |  - PT/T>37 weeks (weigh risk of jaundice with benefits of DCC) - PT/T>37 weeks (weigh risk of jaundice with benefits of DCC) |
60 s | Benefits – Less IVH Less need for transfusions Risks – NM |
| NICE 2015 [30] |  - PT if stable - PT if stable Unstable PT Unstable PT |
60 s | Benefits – Not mentioned Risks – NM |
| UK Resuscitation Council 2021 [31] |  T/PT (VD/CD) T/PT (VD/CD) >28 weeks if DCC is not possible. >28 weeks if DCC is not possible. |
60 s | Benefits – Not mentioned Risks – NM |
| Saudi Neonatology Society on Managing Very Low Birth Weight Infants 2016 [32] |
 - VLBW - VLBW |
30–60 s | Benefits – Not mentioned Risks – NM |
| Singapore Resuscitation Council 2016 [33] |  - T/PT - T/PT < 29 weeks < 29 weeks - Monochorionic twins, placental circulation compromised (Abruption, previa, cord avulsion), immediate requirement of resuscitation - Monochorionic twins, placental circulation compromised (Abruption, previa, cord avulsion), immediate requirement of resuscitation |
30–60 s | Benefits – Lower incidence of IVH, NEC, and lower requirement of blood transfusion Risks – NM |
| ANZCOR 2024 [34] |  - T/LPT/PT - T/LPT/PT - PT<28 weeks - PT<28 weeks - Individualized decisions based on maternal and neonatal risk in multiple gestation, placental abnormalities, foetal anemia/alloimmunization, maternal illness, congenital anomalies and in infants requiring resuscitation - Individualized decisions based on maternal and neonatal risk in multiple gestation, placental abnormalities, foetal anemia/alloimmunization, maternal illness, congenital anomalies and in infants requiring resuscitation |
At least 30 s, preferably 60 s | Benefits – Not mentioned Risks – NM |
| Swiss Society of Neonatology 2017 [35] |  (VD/CD) T/PT (VD/CD) T/PT (CD – T/PT) and in PT (CD – T/PT) and in PTConsidered if immediate cord clamping is required |
60 s | Benefits – Not mentioned Risks – NM |
| Queensland Clinical Guidelines 2022 [36] |  - T/LPT/PT - T/LPT/PT PT (28–33+6weeks: Alternative to DCC. Not recommended in PT<28 weeks PT (28–33+6weeks: Alternative to DCC. Not recommended in PT<28 weeks |
60 s | Benefits – Increase cardiac output and stable blood pressure, and higher Hb levels Risks – Increase incidence of jaundice requiring phototherapy |
| Association of Ontario Midwives 2024 [37] |  - T/PT - T/PT |
NM | Benefits – Improves iron stores and Hb levels Risks – NM |
| FIGO 2014 [38] |  - T/PT - T/PT - Placenta previa, vasa previa, Rh negative mother, newborn needing immediate resuscitation - Placenta previa, vasa previa, Rh negative mother, newborn needing immediate resuscitation |
30–40 s | Benefits – Reduces neonatal anemia Risks – NM |
| Canadian Paediatric Society 2015 [39] |  - T/PT (not requiring resuscitation) - T/PT (not requiring resuscitation) : No sufficient evidence to suggest as routine practice : No sufficient evidence to suggest as routine practice |
NM | Benefits – Not mentioned Risks – NM |
| European Consensus on Management of Respiratory Distress [40] |  - PT(Stable) - PT(Stable) (alternative) (alternative) |
30–60 s | Benefits – Not mentioned Risks – NM |
| ERAS – C Section 2018 [41] |
 - T/PT - T/PT |
T- 60 s PT- at least 30 s |
Benefits – T: improves iron stores, decreases anemia during infancy and improves neurological outcomes PT- decreases IVH, NEC, less need for transfusions Risks – NM |
| Turkish Neonatal Society Guidelines in Management of RDS 2018 [42] |
 - T/PT - T/PT : In emergency situations such as PT requiring resuscitation, maternal bleeding. : In emergency situations such as PT requiring resuscitation, maternal bleeding. - Infants requiring resuscitation, maternal haemorrhage - Infants requiring resuscitation, maternal haemorrhage |
30–120 s | Benefits – higher hematocrit level, less transfusion requirement, higher blood pressure, lesser NEC and IVH Risks – NM |
| ILCOR 2015 [43] |  - PT(Stable) - PT(Stable) - PT (unstable) - PT (unstable) - PT (28 weeks) - PT (28 weeks) |
30–60 s | Benefits – Increase HB levels, requirement of less transfusions, and decrease incidence of IVH, NEC Risks – NM |
| American Academy of Paediatrics (AAP) [44] |  - T/PT - T/PT - Infants requiring resuscitation - Infants requiring resuscitation |
30 s | Benefits – Improves anaemia Increases iron stores Risks – NM |
-
 - delayed cord clamping;
- delayed cord clamping;  - umbilical cord milking;
- umbilical cord milking;  - immediate cord clamping; T, term; LPT, late preterm; PT, preterm; VD, vaginal delivery; CD, caesarean delivery; IVH, intraventricular haemorrhage; NEC, necrotizing enterocolitis.
- immediate cord clamping; T, term; LPT, late preterm; PT, preterm; VD, vaginal delivery; CD, caesarean delivery; IVH, intraventricular haemorrhage; NEC, necrotizing enterocolitis.
One of the primary concerns associated with DCC is the potential for increased neonatal jaundice due to the additional red blood cell volume transferred to the newborn, which may require phototherapy. Some studies have reported a higher incidence of jaundice in infants who undergo DCC [47], while others find the risk to be manageable and outweighed by the benefits of improved iron status [48], 49]. This has led to disagreements on the safety and feasibility of DCC, particularly in low-resource settings where access to phototherapy may be limited [50], 51]. DCC can result in higher haematocrit levels, potentially causing polycythaemia and hyper viscosity syndrome, which can impair blood flow and oxygen delivery. Critics argue that these risks, though generally rare, necessitate careful monitoring and may not justify DCC in all cases, particularly for high-risk new-borns, such as those with maternal diabetes or growth restriction.
Cord milking has been proposed as an alternative to DCC, especially in situations where immediate care is required for the newborn. Some studies suggest cord milking may provide similar benefits to DCC [52], 53], such as improved blood volume and oxygenation, while minimizing delays in neonatal resuscitation. However, evidence is mixed, and concerns about potential risks like increased intraventricular haemorrhage in preterm infants have led to cautious adoption [54], 55]. There is debate over whether DCC is appropriate for all births, particularly for preterm infants and those delivered via caesarean section. While some studies indicate that DCC can improve outcomes in preterm infants (e.g., reduced need for blood transfusions, decreased intraventricular haemorrhage), others caution that it could delay urgent interventions or resuscitation. The current recommendation for preterm infants <34 weeks’ gestation, who do not require resuscitation, delaying cord clamping can be beneficial compared to early cord clamping. In infants between 28 and 34 weeks’ gestation where delayed cord clamping cannot be performed, intact cord milking may be reasonable and for infants <28 weeks’ gestation, intact cord milking is not recommended due to potential risks.
Controversy also surrounds the use of DCC in specific populations, such as babies born to mothers with certain conditions (e.g., infections, diabetes) or infants with congenital abnormalities. The variability in recommendations reflects the complex interplay of potential risks and benefits that need to be individualized for each patient. In low- and middle-income countries, the debate is often focused on the feasibility and safety of DCC in environments with limited resources. Challenges such as inadequate access to neonatal care, phototherapy, and the training required to implement new practices create hesitancy to adopt DCC universally [12], 50]. Advocates for DCC in these settings argue for tailored approaches that consider local resources and practices, while opponents stress the need for more robust evidence and infrastructure [12], 50].
The optimal approach to cord management in non-vigorous new-borns also remains uncertain, as few studies have validated effective strategies for this high-risk group. Current guidelines recommend delayed cord clamping for 30–60 s while initiating basic stabilization, such as drying and stimulation, unless immediate resuscitation is required. If the infant remains apnoeic or hypotonic, early clamping may be necessary to facilitate advanced resuscitation, including positive pressure ventilation (PPV) [56], 57]. EPP method may offer benefits similar to delayed cord clamping by improving blood volume and oxygenation, but logistical challenges and feasibility in emergency settings remain concerns. Umbilical cord milking has also been explored as an alternative, though its safety in non-vigorous infants, particularly preterm neonates, is still debated [58], 59]. It has been found to be associated with improved hemoglobin levels, reduced need for resuscitation, and lower incidence of hypoxic-ischemic encephalopathy (HIE) requiring therapeutic hypothermia [60]. The choice of cord management strategy should be individualized, balancing the potential benefits of enhanced placental transfusion against the urgency of resuscitation.
The controversies and debates around umbilical cord clamping underscore the complexity of finding a “one-size-fits-all” solution. As research continues to evolve, it remains critical for healthcare providers to consider individual clinical scenarios, weigh the benefits and risks, and apply evidence-based practices tailored to each case. Disagreements between major health organizations, such as the World Health Organization (WHO) and the American College of Obstetricians and Gynaecologists (ACOG), reflect the need for further research and consensus-building efforts. The authors have tried to summarise the best practices for cord management as of now in Figure 4.
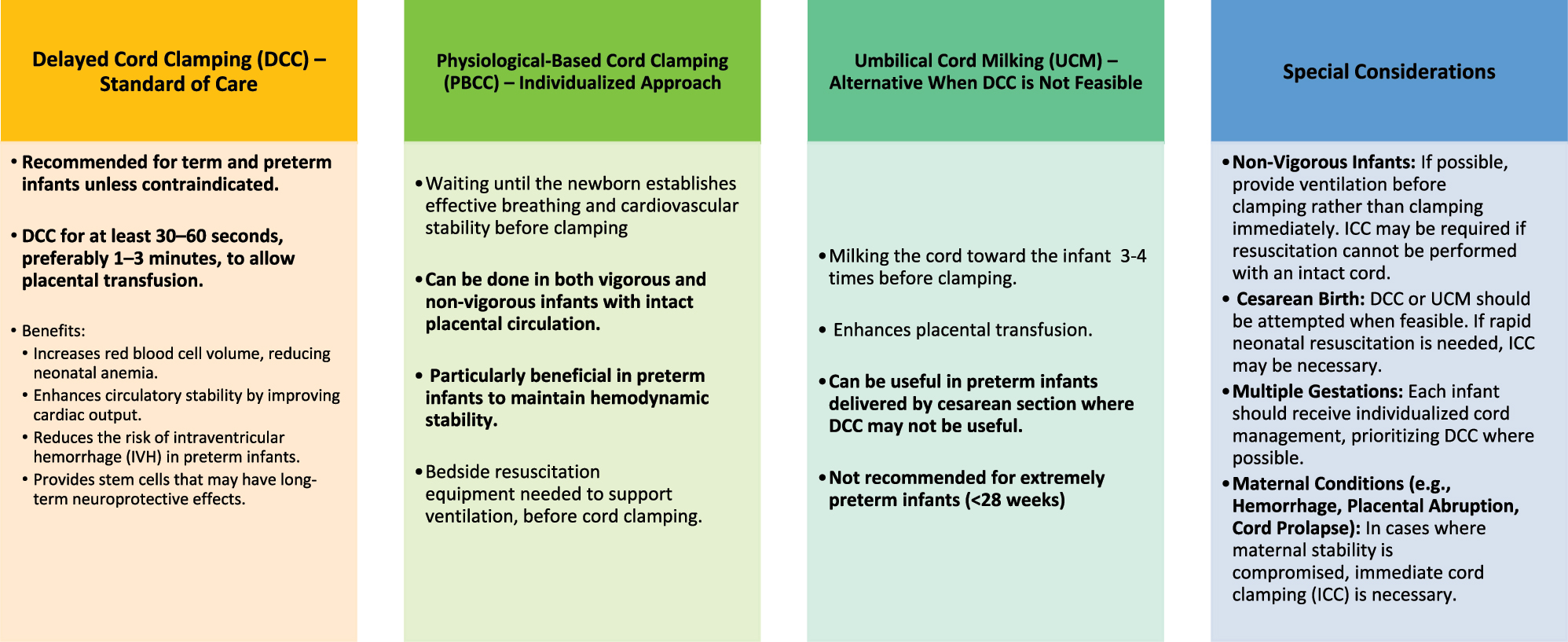
Summary of cord clamping practices: considerations and recommendations.
Future directions and research needs
As the field of neonatal care evolves, further research is essential to refine UCC practices and address existing uncertainties. While significant progress has been made, several areas require ongoing investigation to optimize outcomes and ensure evidence-based practices across diverse clinical settings. Research is needed to establish the most beneficial duration for delayed clamping, balancing the advantages of increased blood volume and iron stores against potential risks such as jaundice and polycythaemia. Studies should investigate varying time frames for clamping to identify the optimal period that maximizes benefits while minimizing adverse effects. Research should focus on developing guidelines that are adaptable to individual patient needs and conditions, ensuring that both the neonate and the mother receive the best possible care. Research should explore how delayed clamping can be safely implemented in low- and middle-income countries where resources are limited. Studies should assess practical approaches, including training, equipment needs, and cost-effectiveness, to ensure that the benefits of delayed clamping are accessible in all healthcare settings.
Conclusions
The timing of UCC is a significant aspect of neonatal care that has evolved from traditional practices to evidence-based guidelines. This comprehensive review has explored the historical context of UCC, highlighting how practices have shifted from immediate to delayed clamping based on advancements in medical understanding. The physiological insights presented underscore the benefits of delayed cord clamping, including enhanced blood volume, improved iron stores, and reduced risk of anaemia, while also addressing potential risks such as neonatal jaundice and polycythaemia. The review has also examined the clinical implications of different UCC practices, revealing how immediate and delayed clamping can impact both maternal and neonatal health. Current guidelines advocate for delayed clamping in most cases, given its demonstrated benefits for neonatal outcomes. However, the review also acknowledges ongoing debates and controversies, including concerns about the timing of clamping in specific scenarios, such as preterm births or caesarean deliveries, and in resource-limited settings.
As the evidence base continues to grow, it is crucial for healthcare providers to stay informed about the latest research and guidelines. The implementation of UCC practices should be individualized, taking into account the specific circumstances of each birth, including the health of the mother and the newborn, as well as available resources. Future research should focus on addressing existing gaps, such as the optimal duration of delayed clamping, the management of potential risks, and the application of UCC practices in diverse clinical settings. By integrating historical knowledge, physiological understanding, and current clinical evidence, this review aims to provide a comprehensive perspective on umbilical cord clamping, ultimately guiding practitioners towards informed decision-making and improved outcomes for mothers and their new-borns.
Funding source: Department of Health Research (DHR), Ministry of Health and family welfare, Government of India
Award Identifier / Grant number: HRD/DHR-ICMR/PG-2024/1192
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: A financial support has been received for the present article, from the Department of Health Research (DHR), Ministry of Health and family welfare, Government of India as a part of MD/MS thesis program (2024 Batch).
-
Data availability: Not applicable.
References
1. Mercer, J, Katheria, A, Backes, CH. Contemporary controversies in umbilical cord clamping practices. Semin Perinatol 2023;47:151782. https://doi.org/10.1016/j.semperi.2023.151749.Search in Google Scholar PubMed
2. Botha, MC. The management of the umbilical cord in labour. S Afr J Obstet Gynaecol 1968;6:30–3.Search in Google Scholar
3. Inch, S. Management of the third stage of labour – a cascade of intervention? Midwifery 1985;1:114–22. https://doi.org/10.1016/s0266-6138(85)80006-1.Search in Google Scholar
4. White, C. A treatise on the management of pregnant and lying-in women: and the means of curing, but more especially of preventing the principal disorders to which they are liable; together with some new directions concerning the delivery of the child and placenta in natural births; illustrated with cases, 1st Worcester ed. Worcester, MA: Isaiah Thomas; 1793. Available from: http://resource.nlm.nih.gov/2577003R.Search in Google Scholar
5. Darwin, E. Zoonomia; or The laws of Organic life. Dublin: B Dugdale, III:1731–802 pp. Available from: https://www.biodiversitylibrary.org/bibliography/25759.Search in Google Scholar
6. Magennis, E. A midwifery surgical clamp. Lancet 1899;153:1373. https://doi.org/10.1016/s0140-6736(01)50497-3.Search in Google Scholar
7. Goodall, A. A new source of blood for transfusion. JAMA 1938;110:1113–4.10.1001/jama.1938.02790140045013Search in Google Scholar
8. Smith, NJ. Management of erythroblastosis fetalis. Calif Med 1956;84:313–7.Search in Google Scholar
9. World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva: World Health Organization; 2012. [cited 2025 Feb 23]. Available from: https://apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng.pdf.Search in Google Scholar
10. McDonald, SJ, Middleton, P, Dowswell, T, Morris, PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database Syst Rev 2013;2013:CD004074.2384313410.1002/14651858.CD004074.pub3Search in Google Scholar PubMed PubMed Central
11. Rabe, H, Diaz-Rossello, JL, Duley, L, Dowswell, T. Effect of timing of umbilical cord clamping and other strategies to influence placental transfusion at preterm birth on maternal and infant outcomes. Cochrane Database Syst Rev 2019;9:CD003248.31529790 In this issue.10.1002/14651858.CD003248.pub4Search in Google Scholar PubMed PubMed Central
12. McAdams, RM, Lakshminrusimha, S. Management of placental transfusion to neonates after delivery. Obstet Gynecol 2022;139:121–37. https://doi.org/10.1097/AOG.0000000000004625.Search in Google Scholar PubMed PubMed Central
13. Katheria, AC, Lakshminrusimha, S, Rabe, H, McAdams, R, Mercer, JS. Placental transfusion: a review. J Perinatol 2017;37:105–11. https://doi.org/10.1038/jp.2016.151.Search in Google Scholar PubMed PubMed Central
14. Farrar, D, Airey, R, Law, G, Tuffnell, D, Cattle, B, Duley, L. Measuring placental transfusion for term births: weighing babies with cord intact. BJOG 2011;118:70–5. https://doi.org/10.1111/j.1471-0528.2010.02781.x.Search in Google Scholar PubMed
15. Saigal, S, O’Neill, A, Surainder, Y, Chua, LB, Usher, R. Placental transfusion and hyperbilirubinemia in the premature infant. Pediatrics 1972;49:406–19.10.1542/peds.49.3.406Search in Google Scholar
16. Hillman, NH, Kallapur, SG, Jobe, AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol 2012;39:769–83. https://doi.org/10.1016/j.clp.2012.09.009.Search in Google Scholar PubMed PubMed Central
17. Morton, SU, Brodsky, D. Fetal physiology and the transition to extrauterine life. Clin Perinatol 2016;43:395–407. https://doi.org/10.1016/j.clp.2016.04.001.Search in Google Scholar PubMed PubMed Central
18. Knol, R, Brouwer, E, van den Akker, T, DeKoninck, P, van Geloven, N, Polglase, GR, et al.. Physiological-based cord clamping in very preterm infants – randomised controlled trial on effectiveness of stabilisation. Resuscitation 2020;147:26–33. https://doi.org/10.1016/j.resuscitation.2019.12.007.Search in Google Scholar PubMed
19. Badurdeen, S, Davis, PG, Hooper, SB, Donath, S, Santomartino, GA, Heng, A, et al.. Physiologically based cord clamping for infants ≥32 weeks gestation: a randomised clinical trial. PLoS Med 2022;19:e1004029. https://doi.org/10.1371/journal.pmed.1004029.Search in Google Scholar PubMed PubMed Central
20. Chakkarapani, AA, Roehr, CC, Hooper, SB, Te Pas, AB, Gupta, S. ESPR Neonatal Resuscitation section writing group. Transitional circulation and hemodynamic monitoring in newborn infants. Pediatr Res 2024;96:595–603. https://doi.org/10.1038/s41390-022-02427-8.Search in Google Scholar PubMed PubMed Central
21. Kuehne, B, Grüttner, B, Hellmich, M, Hero, B, Kribs, A, Oberthuer, A. Extrauterine placental perfusion and oxygenation in infants with very low birth weight: a randomized clinical trial. JAMA Netw Open 2023;6:e2340597. https://doi.org/10.1001/jamanetworkopen.2023.40597.Search in Google Scholar PubMed PubMed Central
22. Roberts, CT. Placental perfusion rather than placental transfusion–key to umbilical cord management at birth? JAMA Netw Open 2023;6:e2340490. https://doi.org/10.1001/jamanetworkopen.2023.40490.Search in Google Scholar PubMed
23. Dunn, PM. The importance of the umbilical circulation in preventing respiratory distress syndrome following premature caesarean delivery, 1957–1973. West Engl Med J 2017;116:1–7.Search in Google Scholar
24. Ghirardello, S, Di Tommaso, M, Fiocchi, S, Locatelli, A, Perrone, B, Pratesi, S, et al.. Italian recommendations for placental transfusion strategies. Front Pediatr 2018;6:372. https://doi.org/10.3389/fped.2018.00372.Search in Google Scholar PubMed PubMed Central
25. American College of Obstetricians and Gynecologists. Committee Opinion No. 684: delayed umbilical cord clamping after birth. Obstet Gynecol 2017;129:1.10.1097/AOG.0000000000001860Search in Google Scholar PubMed
26. World Health Organization. Guideline: delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. Geneva: World Health Organization; 2014. Available from: https://www.ncbi.nlm.nih.gov/books/NBK310522/.Search in Google Scholar
27. Royal College of Obstetricians and Gynaecologists. Umbilical cord prolapse (Green-top Guideline No. 50). London: RCOG; 2014. Available from: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/umbilical-cord-prolapse-green-top-guideline-no-50/.Search in Google Scholar
28. Jauniaux, E, Ebbing, C, Oyelese, Y, Maymon, R, Prefumo, F, Bhide, A. European Association of Perinatal Medicine (EAPM) position statement: screening, diagnosis and management of congenital anomalies of the umbilical cord. Eur J Obstet Gynecol Reprod Biol 2024;298:61–5. https://doi.org/10.1016/j.ejogrb.2024.04.044.Search in Google Scholar PubMed
29. Leduc, D, Senikas, V, Lalonde, AB. No. 235–active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2018;40:e841–55. https://doi.org/10.1016/j.jogc.2018.09.024.Search in Google Scholar PubMed
30. National Collaborating Centre for Women’s and Children’s Health (UK). Preterm labour and birth. London: National Institute for Health and Care Excellence (UK); 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK327571/.Search in Google Scholar
31. Resuscitation Council UK. 2021 resuscitation guidelines [Internet]. [cited 2024 Sep 8]. Available from: https://www.resus.org.uk/library/2021-resuscitation-guidelines.Search in Google Scholar
32. Al-Salam, Z, Al-Alaiyan, S, Alallah, J, Al-Hazzani, F, Alfaleh, K, Alsaedi, S, et al.. The golden hour approach: practical guidelines of the Saudi Neonatology Society on managing very low birth weight infants in the first hour of life. J Clin Neonatol 2016;5:222–9. https://doi.org/10.4103/2249-4847.194178.Search in Google Scholar
33. Yeo, CL, Biswas, A, Ee, TT, Chinnadurai, A, Baral, VR, Chang, AS, et al.. Singapore neonatal resuscitation guidelines 2016. Singapore Med J 2017;58:391–403. https://doi.org/10.11622/smedj.2017066.Search in Google Scholar PubMed PubMed Central
34. Australian and New Zealand Council on Resuscitation (ANZCOR). Newborn resuscitation [Internet]; 2024. [cited 2024 Sep 8]. Available from: https://resus.org.au/anzcor-neonatal-guidelines/.Search in Google Scholar
35. Swiss Society of Neonatology. Support of adaptation and resuscitation of the newborn infant: revised recommendations of the Swiss Society of Neonatology; 2017. Available from: https://www.ssapm.ch/fileadmin/user_upload/ssapm/public/Ueber-uns/Interessengruppen_und_Partnergesellschaften/Interessengruppen/SGKA/Links/Support_of_Adaptation_and_Resuscitation_of_the_Newborn_Infant_engl-1.pdf.Search in Google Scholar
36. Queensland Clinical Guidelines. Neonatal resuscitation. Document No. MN22.5-V6-R27. Brisbane: Queensland Health; 2022. Available from: https://www.health.qld.gov.au/__data/assets/pdf_file/0019/140932/ed-resus.pdf.Search in Google Scholar
37. Association of Ontario Midwives. Prevention and management of postpartum hemorrhage. Clinical practice guideline No. 17. Toronto: association of ontario midwives; 2024. Available from: https://www.ontariomidwives.ca/sites/default/files/2024-06/CPG-Postpartum%20Hemorrhage-2024-PUB.pdf.Search in Google Scholar
38. Federation of Obstetric and Gynaecological Societies of India. Consensus statement for prevention of postpartum hemorrhage (PPH). Mumbai: FOGSI; 2014. Available from: https://www.fogsi.org/wp-content/uploads/2015/11/pph.pdf.Search in Google Scholar
39. Ryan, M, Lacaze-Masmonteil, T, Mohammad, K. Neuroprotection from acute brain injury in preterm infants. Paediatr Child Health 2019;24:276–90. https://doi.org/10.1093/pch/pxz056.Search in Google Scholar PubMed PubMed Central
40. Sweet, DG, Carnielli, VP, Greisen, G, Hallman, M, Klebermass-Schrehof, K, Ozek, E, et al.. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology 2023;120:3–23. https://doi.org/10.1159/000528914.Search in Google Scholar PubMed PubMed Central
41. Wilson, RD, Caughey, AB, Wood, SL, Macones, GA, Wrench, IJ, Huang, J, et al.. Guidelines for antenatal and preoperative care in cesarean delivery: enhanced Recovery after Surgery Society recommendations. Am J Obstet Gynecol 2018;219:523.e1–15. https://doi.org/10.1016/j.ajog.2018.09.015.Search in Google Scholar PubMed
42. Özkan, H, Erdeve, Ö, Kanmaz Kutman, HG. Turkish Neonatal Society guideline on the management of respiratory distress syndrome and surfactant treatment. Turk Pediatri Ars 2018;53:S45–54. https://doi.org/10.5152/TurkPediatriArs.2018.01806.Search in Google Scholar PubMed PubMed Central
43. Perlman, JM, Wyllie, J, Kattwinkel, J, Wyckoff, MH, Aziz, K, Guinsburg, R, et al.. Part 7: neonatal resuscitation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015;132:S204–41. https://doi.org/10.1161/cir.0000000000000276.Search in Google Scholar
44. Tarnow-Mordi, W, Morris, J, Kirby, A, Robledo, K, Askie, L, Brown, R, et al.. Delayed cord clamping in preterm infants. N Engl J Med 2017;377:2445–55. https://doi.org/10.1056/nejmoa1711281.Search in Google Scholar PubMed
45. Levy, T, Blickstein, I. Timing of cord clamping revisited. J Perinat Med 2006;34:293–7. https://doi.org/10.1515/jpm.2006.056.Search in Google Scholar
46. Mwakawanga, DL, Mselle, LT. Early or delayed umbilical cord clamping? Experiences and perceptions of nurse-midwives and obstetricians at a regional referral hospital in Tanzania. PLoS One 2020;15:e0234854. https://doi.org/10.1371/journal.pone.0234854.Search in Google Scholar PubMed PubMed Central
47. Bashir, BA, Othman, SA. Neonatal polycythaemia. Sudan J Paediatr 2019;19:81–3. https://doi.org/10.24911/sjp.106-1566075225.Search in Google Scholar
48. Rana, N, Ranneberg, LJ, Målqvist, M, Kc, A, Andersson, O. Delayed cord clamping was not associated with an increased risk of hyperbilirubinaemia on the day of birth or jaundice in the first 4 weeks. Acta Paediatr 2020;109:71–7. https://doi.org/10.1111/apa.14913.Search in Google Scholar PubMed
49. Pan, S, Lu, Q, Lan, Y, Peng, L, Yu, X, Hua, Y. Differential effects of delayed cord clamping on bilirubin levels in normal and diabetic pregnancies. Eur J Pediatr 2022;181:3111–7. https://doi.org/10.1007/s00431-022-04536-2.Search in Google Scholar PubMed
50. van Rheenen, PF, Gruschke, S, Brabin, BJ. Delayed umbilical cord clamping for reducing anaemia in low birthweight infants: implications for developing countries. Ann Trop Paediatr 2006;26:157–67. https://doi.org/10.1179/146532806x120246.Search in Google Scholar PubMed
51. Mercer, JS, Erickson-Owens, DA, Collins, J, Barcelos, MO, Parker, AB, Padbury, JF. Effects of delayed cord clamping on residual placental blood volume, hemoglobin and bilirubin levels in term infants: a randomized controlled trial. J Perinatol 2017;37:260–4. https://doi.org/10.1038/jp.2016.222.Search in Google Scholar PubMed PubMed Central
52. Koo, J, Kilicdag, H, Katheria, A. Umbilical cord milking–benefits and risks. Front Pediatr 2023;11:1146057. https://doi.org/10.3389/fped.2023.1146057.Search in Google Scholar PubMed PubMed Central
53. Jeevan, A, Ananthan, A, Bhuwan, M, Balasubramanian, H, Rao, S, Kabra, NS. Umbilical cord milking versus delayed cord clamping in term and late-preterm infants: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2022;35:5478–88. https://doi.org/10.1080/14767058.2021.1884676.Search in Google Scholar PubMed
54. Balasubramanian, H, Ananthan, A, Jain, V, Rao, SC, Kabra, N. Umbilical cord milking in preterm infants: a systematic review and meta-analysis. Arch Dis Child Fetal Neonatal Ed 2020;105:572–80. https://doi.org/10.1136/archdischild-2019-318627.Search in Google Scholar PubMed
55. Katheria, A, Reister, F, Essers, J, Mendler, M, Hummler, H, Subramaniam, A, et al.. Association of umbilical cord milking vs delayed umbilical cord clamping with death or severe intraventricular hemorrhage among preterm infants. JAMA 2019;322:1877–86. https://doi.org/10.1001/jama.2019.16004.Search in Google Scholar PubMed PubMed Central
56. Koo, J, Aghai, ZH, Katheria, A. Cord management in non-vigorous newborns. Semin Perinatol 2023;47:151742. https://doi.org/10.1016/j.semperi.2023.151742.Search in Google Scholar PubMed PubMed Central
57. Katheria, AC, Rich, WD, Bava, S, Lakshminrusimha, S. Placental transfusion for asphyxiated infants. Front Pediatr 2019;7:473. https://doi.org/10.3389/fped.2019.00473.Search in Google Scholar PubMed PubMed Central
58. Knol, R, Brouwer, E, van den Akker, T, DeKoninck, PLJ, Onland, W, Vermeulen, MJ, et al.. Physiological versus time-based cord clamping in very preterm infants (ABC3): a parallel-group, multicentre, randomised, controlled superiority trial. Lancet Reg Health Eur 2024;48:101146. https://doi.org/10.1016/j.lanepe.2024.101146.Search in Google Scholar PubMed PubMed Central
59. Winter, J, Kattwinkel, J, Chisholm, C, Blackman, A, Wilson, S, Fairchild, K. Ventilation of preterm infants during delayed cord clamping (VentFirst): a pilot study of feasibility and safety. Am J Perinatol 2017;34:111–6. https://doi.org/10.1055/s-0036-1584521.Search in Google Scholar PubMed
60. Katheria, AC, Clark, E, Yoder, B, Schmölzer, GM, Yan Law, BH, El-Naggar, W, et al.. Umbilical cord milking in nonvigorous infants: a cluster-randomized crossover trial. Am J Obstet Gynecol 2023;228:217.e1–e14. https://doi.org/10.1016/j.ajog.2022.08.015.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Perinatal responsibility in a fragmented world: reflections from the 2024 international academy of perinatal medicine New York meeting
- Corner of Academy
- Global education – impressive results of Ian Donald School
- Cicero’s universal law: a timeless guide to reproductive justice
- Enhancing patient understanding in obstetrics: the role of generative AI in simplifying informed consent for labor induction with oxytocin
- Faculty retention in academic OB/GYN: comprehensive strategies and future directions
- Hemolytic disease of the fetus and newborn: pregnant person’s and fetal immune systems interaction
- Viability of extremely premature neonates: clinical approaches and outcomes
- Reviews
- Standardizing cord clamping: bridging physiology and recommendations from leading societies
- Thrombotic thrombocytopenic purpura in pregnancy: a comprehensive review
- Mini Review
- Looking for a needle in a haystack: a case study of rare disease care in neonatology
- Opinion Paper
- Hemorrhagic placental lesions on ultrasound: a continuum of placental abruption
- Original Articles – Obstetrics
- Amnioreduction safety in singleton pregnancies; systematic review and meta-analysis
- Outpatient management of prelabour rupture of membranes (PROM) at term – a re-evaluation and contribution to the current debate
- Breastfeeding in HIV-positive mothers under optimized conditions: ‘real-life’ results from a well-resourced healthcare setting
- Intervention using the Robson classification as a tool to reduce cesarean section rates in six public hospitals in Brazil
- Short Communication
- Continuous positive airway pressure vs. high velocity nasal cannula for weaning respiratory support of preterm infants
Articles in the same Issue
- Frontmatter
- Editorial
- Perinatal responsibility in a fragmented world: reflections from the 2024 international academy of perinatal medicine New York meeting
- Corner of Academy
- Global education – impressive results of Ian Donald School
- Cicero’s universal law: a timeless guide to reproductive justice
- Enhancing patient understanding in obstetrics: the role of generative AI in simplifying informed consent for labor induction with oxytocin
- Faculty retention in academic OB/GYN: comprehensive strategies and future directions
- Hemolytic disease of the fetus and newborn: pregnant person’s and fetal immune systems interaction
- Viability of extremely premature neonates: clinical approaches and outcomes
- Reviews
- Standardizing cord clamping: bridging physiology and recommendations from leading societies
- Thrombotic thrombocytopenic purpura in pregnancy: a comprehensive review
- Mini Review
- Looking for a needle in a haystack: a case study of rare disease care in neonatology
- Opinion Paper
- Hemorrhagic placental lesions on ultrasound: a continuum of placental abruption
- Original Articles – Obstetrics
- Amnioreduction safety in singleton pregnancies; systematic review and meta-analysis
- Outpatient management of prelabour rupture of membranes (PROM) at term – a re-evaluation and contribution to the current debate
- Breastfeeding in HIV-positive mothers under optimized conditions: ‘real-life’ results from a well-resourced healthcare setting
- Intervention using the Robson classification as a tool to reduce cesarean section rates in six public hospitals in Brazil
- Short Communication
- Continuous positive airway pressure vs. high velocity nasal cannula for weaning respiratory support of preterm infants

