Abstract
Objectives
To compare short term variation (STV) and phase rectified signal averaging (PRSA) and their association with fetal outcome in early onset fetal growth restriction (FGR).
Methods
Data were used from a retrospective cohort study of women who were admitted for FGR and/or pre-eclampsia and who were delivered by pre-labor Cesarean section or had a fetal death before 32 weeks’ gestation. Computerized cardiotocography (cCTG) registrations of the 5 days before delivery or fetal death were used for calculation of STV and PRSA. PRSA was expressed as the average acceleration capacity (AAC) and average deceleration capacity (ADC). FHR decelerations were classified visually as absent, 1–2 per hour or recurrent. Abnormality of STV and of PRSA was either analyzed as a single parameter or in combination with recurrent decelerations. Endpoints were defined as composite adverse condition at birth consisting of fetal death, low Apgar score, low umbilical pH, the need for resuscitation after birth and as major neonatal morbidity or neonatal death.
Results
Included were 367 pregnancies of which 20 resulted in fetal death. An abnormal cCTG with either recurrent decelerations and/or low STV or recurrent decelerations and/or low PRSA were similarly associated with composite adverse condition at birth (n=99), but neither with major neonatal morbidity.
Conclusions
PRSA and STV have similar efficacy for measuring fetal heart rate variation in early onset FGR. An increased risk of a composite adverse condition at birth is indicated by a low value of either parameter and/or the presence of recurrent decelerations.
Introduction
Early onset fetal growth restriction (FGR) is a rare condition, but with high neonatal morbidity and mortality [1]. This patient group requires intensive monitoring and has a high likelihood of iatrogenic premature delivery, both for fetal and for maternal indications [2]. Fetal monitoring consists of ultrasound assessment (fetal Doppler profile and biometry) and cardiotocography (CTG), mainly focused at optimal timing of delivery, a topic in which currently significant practice variation exists.
The CTG is a standard monitoring tool that enables visual assessment of the pattern of the fetal heart rate (FHR). This method has been widely used since the introduction after 1965, but it has the shortcoming of high inter- and intra-observer variability [3, 4]. To overcome this, computerized techniques have been developed to calculate the FHR variability and reduce observer bias [5, 6]. FHR variability as expressed in the short-term variation (STV) has been established as an identifier of fetal acid-base status [7], and low STV has strong associations with adverse fetal outcomes [8, 9].
Recently, phase-rectified signal averaging (PRSA) has been suggested as an additional or alternative method for fetal CTG analysis [10]. PRSA analysis studies quasi-periodicities in non-stationary data. It is recognized as a predictor of mortality in patients who survived acute myocardial infarction [11]. PRSA can extract periodicities and periodic components from biodata such as electrocardiograms (ECG) but also from CTG’s. This method can calculate the variation of FHR, but can also describe the speed of changes in the FHR, expressed as the average acceleration capacity (AAC) and average deceleration capacity (ADC).
From data of the Trial of Umbilical and Fetal Flow in Europe (TRUFFLE) study cut-off values for PRSA (AAC and ADC) were calculated to predict adverse perinatal outcome in early onset fetal growth restriction (FGR) [12]. In a secondary study of TRUFFLE data cut-off levels for STV were evaluated [13]. In this study we aim to compare pre-specified cut-off values for PRSA and STV in computerized CTG’s (cCTG) in a cohort of women who received routine CTG as part of the monitoring strategy in early-onset FGR/preeclampsia (PE).
Materials and methods
Study population
All women who had been admitted to the Amsterdam University Medical Center – location AMC Amsterdam, the Netherlands, between the years 2003 and 2015 because of early onset FGR and/or preeclampsia, and who had a Cesarean delivery before onset of labor or had a fetal demise before 32°weeks of gestation were included in this analysis.
The data from this cohort were used earlier in a study that assessed the association of STV, recurrent decelerations and fetal arterial Doppler abnormalities and short-term and long-term two-year infant outcome, using predefined cCTG cut-off values that had been defined for the TRUFFLE study (Trial of Randomized Umbilical and Fetal Flow in Europe) [14]. Sixty-one women in the current study were participants in the TRUFFLE study. In an earlier post-hoc study of the TRUFFLE cohort, these data were used in a comparison of PRSA and STV among the data of 279 other women who participated in the TRUFFLE study [12, 15].
FGR was defined as an estimated fetal weight below the 10th centile and an umbilical artery Doppler pulsatility index (PI) higher than the 95th centile. Percentiles and Z-scores for fetal weight, birth weight, and Doppler parameters were calculated according reference values published by Verburg and Arduini [16, 17]. Preeclampsia was defined according to the International Society for the Study of Hypertension in Pregnancy (ISSHP) consensus [18].
The management of preeclampsia was temporizing as long as fetal and maternal conditions were deemed acceptable [19]. Preferred antihypertensive drugs were methyldopa, nifedipine and labetalol. Magnesium sulphate was used in clinical preeclampsia for the prevention of seizures; administration around delivery for neuro-prophylaxis for the neonate was not standard practice during the study period. Corticosteroids for fetal maturity were prescribed when it was presumed that early delivery due to change of fetal condition would be necessary. Only one course of corticosteroids was given.
Fetal monitoring consisted of arterial Doppler assessment one or two times a week and CTG’s at least once a day, depending on the fetal condition. An abnormal CTG was continued or repeated after a short time to check consistency and obstetric management decisions were made by perinatologists, using visual assessment of CTG. Computerized CTG (and ductus venosus Dopplers) were solely used in a subset of women who participated in the TRUFFLE study [15].
Software
CTG’s were registered using Philips series 50A or M1350A machines (Philips Healthcare, Amsterdam, the Netherlands). The files were stored digitally on a server, using Mosos CTG monitoring and archiving software (BMA Health Care Solutions, Houten, the Netherlands). For this study all cCTG’s recorded during the last five days before delivery or fetal death, with a duration of at least 20 min, were analyzed post-hoc using STVcalc and PRSA method [10, 20].
STVcalc is locally developed software for fetal heart rate analysis, based on literature description of FetalCare, a commercially available tool for STV calculation (Huntleigh Healthcare, Cardiff, UK) [21], [22], [23], [24] Comparison of FetalCare version 2 with STVcalc showed similar test performance [6]. The software code of STVcalc is available on GitHub (https://github.com/hwolf46), a repository for freeware and host for collaborating not-for-profit software developers. STV <2.6 ms (ms) is considered abnormal at <29°weeks of gestation, and STV <3.0 ms at an age ≥29°weeks of gestation. These cut-off points have been assessed extensively in early onset FGR [5, 13, 15, 20].
Identical traces of cCTG were analyzed using MatLab software version R2017a (MathWorks, USA) for PRSA analysis. The method is described in detail by Lobmaier et al. [10]. The ascending anchor points were calculated over windows of 10 s before and after the potential anchor point. Anchor points with differences of >0.05% between the average before and after the anchor point were not considered. Anchor points were synchronized and PRSA AAC and PRSA ADC were calculated from the difference between the average FHR after and before the anchor point, using a window of 100 s. AAC<1.41 beats per minute (bpm) and/or ADC>−1.43 bpm were used as cut-off values for abnormality. AAC/ADC cut-off thresholds had been derived from a secondary analysis of the TRUFFLE data for later use, although they were not published earlier [12]. Values were chosen resulting in a sensitivity of 70% with a maximum specificity for prediction of the composite adverse outcome: 5 min Apgar score <7 (sensitivity 70% and specificity 42%), umbilical artery pH <7.1 (sensitivity 70% and specificity 62%) or fetal death (sensitivity 70% and specificity 67%) [12].
Because neither STVcalc nor the PRSA calculation application are reliable for exact recognition of decelerations, we classified decelerations visually as either absent, or 1–2 decelerations per hour, or recurrent decelerations defined as more than two decelerations per hour (HW). Using prelabour CTG’s the uterine pressure is often registered inadequate. Therefore we did not differentiate between variable decelerations vs. late decelerations.
Outcome endpoints
Antenatal death could be unexpected or anticipated in cases where it was decided not to deliver the baby by Cesarean delivery when fetal condition was assessed as abnormal. This could be decided, after counselling and discussion with the parents, if the chance of healthy survival was estimated low, based on poor fetal condition, gestational age and estimated fetal weight, or if the parents refused intervention.
Composite adverse condition at birth was defined by an Apgar score <5 after 5 min before 29°weeks and <7 thereafter, an arterial umbilical pH <7.1 or a venous pH <7.2, the need for resuscitation after birth by intubation or cardiac compressions, or fetal death. The pH cut-off values are approximately 2 standard deviations below the mean in an average population [25, 26]. We assumed that, in this selected population, fetal death and neonatal asphyxia at birth were similarly caused by placental insufficiency and supply shortage, but only differed in severity of clinical expression.
Major neonatal morbidity was defined by intraventricular hemorrhage grade 3 or 4 (IVH) [27], periventricular leukomalacia grade 2 or 3 (PVL) [28], moderate or severe bronchopulmonary dysplasia (BPD) [29], sepsis or meningitis with microbiological confirmation, or necrotizing enterocolitis grade 2 or 3 [30].
Statistical analysis
We used the last computerized CTG (cCTG) before delivery or fetal death to assess the predictive value for the outcome endpoints using different thresholds for abnormal cCTG: recurrent decelerations only, low STV only, low AAC/ADC only, and the combination of low STV or low AAC/ADC and/or recurrent decelerations. Low STV was defined at a threshold of 2.6 ms before 29°weeks and 3.0 ms thereafter, and low PRSA at AAC<1.41 bpm and/or ADC>−1.43 bpm [12].
For each threshold and endpoint 2×2 tables were made and unadjusted relative risk (RR) with 95% confidence interval (CI) was calculated. For composite adverse condition at birth odds ratios were calculated of an abnormal cCTG according to PRSA criteria and according to STV criteria, with adjustment for preeclampsia, umbilical artery PI Z-score, umbilical artery absent or reversed diastolic (ARED) flow, gestational age and birth weight Z-score, using logistic regression analysis.
AAC, ADC and STV were assessed longitudinally during the last five days before delivery or fetal death. For all women only the last cCTG recorded on each day was used. Values were compared pair-wise non-parametrically with Kruskal–Wallis test. Outcome groups were compared by Fisher exact test.
Calculations were performed using IBM SPSS version 25 (IBM, New York, USA).
Medical ethical approval
Medical ethical approval was not required under contemporary Dutch law, as this was an anonymous retrospective quality evaluation of data from women who had been treated in our center. All data had been anonymized before analysis.
Results
A total of 367 women were included in this study with a total number of 3295 CTG’s that were recorded in the 5 days prior to either delivery or fetal demise. The median gestational age at delivery was 29.6 (IQR 28.3 to 30.7) weeks, median birth weight 910 (IQR 750 to 1,090) gram. Demographic and obstetric details are presented in Table 1. Except for the cases of fetal death, all women had a cesarean delivery before start of labor.
Demographic and perinatal data of the study population.
| n | 367 |
| Nulliparous | 232 (63%) |
| Gestational age admission hospital, weeks | 27.9 (26.6–29.3) |
| Duration of antenatal hospitalization, days | 8 (4–16) |
| Preeclampsia | 289 (79%) |
| Antihypertensive medication | 250 (68%) |
| Corticosteroids for fetal maturation | 353 (96%) |
| Last umbilical Doppler ARED flow | 153 (42%) |
| Last UCR (n=322) | 1.4 (1.1–2.1) |
| Last UCR Z-score (n=322) | 3.2 (1.7–5.8) |
| Last STV | 3.1 (2.4–3.9) |
| Last cCTG<STV cut-off (2.6–3.0 ms) or deceleration >2/h | 241 (66%) |
| Last AAC | 1.43 (1.18–1.75) |
| Last ADC | −1.43 (−1.74 to −1.18) |
| Last cCTG<PRSA cut-off (AAC 1.41 bpm, ADC>−1.43 bpm, or decelerations >2/h) | 277 (76%) |
| Cesarean delivery fetal distress | 325 (94%) |
| Maternal condition | 22 (6%) |
| Gestational age at delivery, weeks | 29.6 (28.3–30.7) |
| Birth weight, grams | 910 (750–1,090) |
| Birth weight <10th percentile | 358 (98%) |
| Birth weight Z-score | −3.5 (−4.2 to −2.8) |
| Male | 169 (46%) |
| Composite adverse condition at birth | 99 (27%) |
| Fetal death | 20 (5%) |
| Apgar 5’ >0 and <5 at <29°weeks or <7 at ≥29°weeks | 19 (6%) |
| Low pH (arterial <7.1 or venous <7.2) (n=213) | 55 (26%) |
| Resuscitation | 18 (5%) |
| Major neonatal morbidity | 149 (43%) |
| Cerebral abnormality | 17 (5%) |
| Bronchopulmonary dysplasia | 47 (14%) |
| Necrotizing enterocolitis or sepsis/meningitis | 127 (37%) |
| Neonatal death <=4°weeks | 29 (8%) |
-
Data presented as number (percentage) or median (interquartile range); ARED, absent and reversed end-diastolic; UCR, umbilical-cerebral ratio; STV, short-term variation; cCTG, computerized cardiotocography; AAC, average acceleration capacity; ADC, average deceleration capacity; PRSA, phase-rectified signal averaging.
There were 20 cases of fetal death (5%). In 14 women this was expected, with a prior made decision to abstain from intervention due to the anticipated low chances of intact infant survival. In 6 women fetal death occurred unexpectedly despite regular monitoring. Details of perinatal outcomes are described in Table 1. The median interval between the last cCTG and delivery or fetal death was 3 h (IQR 2–5 h).
The relative risks (RR) values with 95% confidence intervals (CI) for composite adverse condition at birth and its components are displayed in Table 2, using the different thresholds for an abnormal last cCTG before delivery or fetal death, based on combinations of low AAC/ADC, low STV and/or recurrent decelerations. For fetal death and low 5 min Apgar score only the threshold using STV and/or recurrent decelerations reached statistical significance. For the composite asphyxia at birth all RR calculations that included recurrent decelerations reached statistical significance, but STV only and PRSA only did not. No association with low pH was observed. The statistically significant indices had a sensitivity of 80–90%, and a specificity of 30–40%.
Unadjusted relative risk with 95% confidence limits of fetal death, low Apgar score, low pH, composite asphyxia at birth, perinatal mortality, major neonatal morbidity and impairment at two-year follow-up for low AAC/ADC only, low STV only and abnormal cCTG using low AAC/ADC or low STV in combination with recurrent decelerations.
| Fetal death (n=20) | Low apgar 5’ (n=19) | Low pHa (n=55) | Composite adverse condition at birth (n=99) | Perinatal mortality (n=60) | Major neonatal morbidity (n=149) | |
|---|---|---|---|---|---|---|
| AAC/ADC low only | 2.0 (0.8–5.1) | 1.5 (0.6–3.8) | 0.9 (0.6–1.4) | 1.3 (0.9–1.9) | 1.2 (0.8–1.9) | 1.3 (1.0–1.7) |
| STV low only | 1.4 (0.6–3.2) | 3.9 (1.4 to 10.5) | 0.8 (0.5–1.2) | 1.2 (0.9–1.7) | 1.3 (0.8–2.0) | 1.2 (1.0–1.6) |
| PRSA cCTG abnormal | 2.9 (0.7–12.4) | 6.1 (0.8–45) | 0.9 (0.5–1.6) | 1.8 (1.1 to 3.0) | 2.1 (1.0 to 4.3) | 1.1 (0.8–1.5) |
| STV cCTG abnormal | 4.7 (1.1 to 20.0) | 10.0 (1.4 to 74) | 1.1 (0.6–1.7) | 2.1 (1.3 to 3.2) | 2.1 (1.2 to 3.8) | 1.0 (0.8–1.3) |
| Recurrent decelerations only | 1.6 (0.7–3.7) | 2.2 (0.9–5.4) | 1.4 (0.9–2.2) | 1.8 (1.3 to 2.6) | 1.4 (0.9–2.2) | 0.9 (0.7–1.2) |
-
a154 cases umbilical pH missing. Text bold: p<0.05 Fisher exact test 2-sided. AAC, average acceleration capacity; ADC, average deceleration capacity; STV, short-term variation; PRSA, phase-rectified signal averaging; cCTG, computerized cardiotocography.
The different cCTG thresholds were not associated with neonatal morbidity or infant 2-year neurodevelopmental outcome (data not shown).
Comparison of AAC, ADC and STV in cCTG’s with or without recurrent decelerations showed that AAC and ADC were significantly higher in the presence of recurrent decelerations, while STV was not affected (Table 3).
STV, AAC and ADC of the last cCTG before fetal death or delivery, compared for the presence of recurrent decelerations or not.
| Decelerations | Median (IQR) | n=367 | |
|---|---|---|---|
| STV | Recurrent decelerations | 3.1 (2.4–3.8) | 143 (39%) |
| ≤2 decelerations. | 3.0 (2.4–3.9) | 224 (61%) | |
| AACa | Recurrent decelerations | 1.56 (1.29–1.83 | 143 (39%) |
| ≤2 decelerations. | 1.36 (1.09–1.67) | 224 (61%) | |
| ADCa | Recurrent decelerations | −1.54 (−1.84 to −1.30) | 143 (39%) |
| ≤2 decelerations. | −1.37 (−1.67 to −1.06) | 224 (61%) |
-
ap<0.05 Mann–Whitney. STV, short-term variation; AAC, average acceleration capacity; ADC, average deceleration capacity.
The odds ratio of an abnormal cCTG according to AAC/ADC criteria (=abnormal AAC/ADC and/or recurrent decelerations) for composite asphyxia at birth was 1.9 (95% CI 1.0 to 3.6) after adjustment for umbilical artery PI, umbilical artery ARED flow and gestational age. The AUC for the prediction model was 0.64 (95% CI 0.58 to 0.70). For an abnormal cCTG based on criteria with STV in a similar analysis these values were OR 2.5 (95% CI 1.4 to 4.3) and AUC 0.67 (95% CI 0.61 to 0.73) (Figure 1). Birth weight Z-score and preeclampsia were ejected from the model (p>0.1).

Odds ratios for composite adverse condition at birth with 95% CI of a last cCTG, abnormal according to PRSA criteria or to STV criteria (low value and/or recurrent deceleration), adjusted by umbilical artery PI Z-score, umbilical artery ARED flow, and gestational age at delivery. ROC AUC for the model including PRSA 0.64 (0.58–0.70); sensitivity 70% at specificity 40%, for the model including STV 0.67 (95% CI 0.61 to 0.73); sensitivity 70% at specificity 50%. Birth weight Z-score and preeclampsia were ejected from the model (p>0.1). PRSA, phase-rectified signal averaging; cCTG, computerized cardiotocography; STV, short-term variation; ARED, absent and reversed end-diastolic.
In 14 women it was decided not to intervene. The last cCTG in this group had a STV of 2.34 (IQR 1.67–3.84) ms, AAC 1.10 (IQR 0.84–1.60) bpm, ADC −1.06 (IQR −1.50 to −0.85) bpm and 8 (57%) had recurrent decelerations. All were classified as abnormal based on the criteria specified earlier for STV or PRSA.
Longitudinal assessment of AAC, ADC and STV during the last five days before delivery or fetal death showed that these indices only changed within two days of delivery or fetal death (Figure 2). For all three indices values at day 0 and day 1 before delivery or fetal death were significantly lower (STV or AAC), or higher (ADC) than values on earlier days (Kruskal–Wallis pairwise comparison).
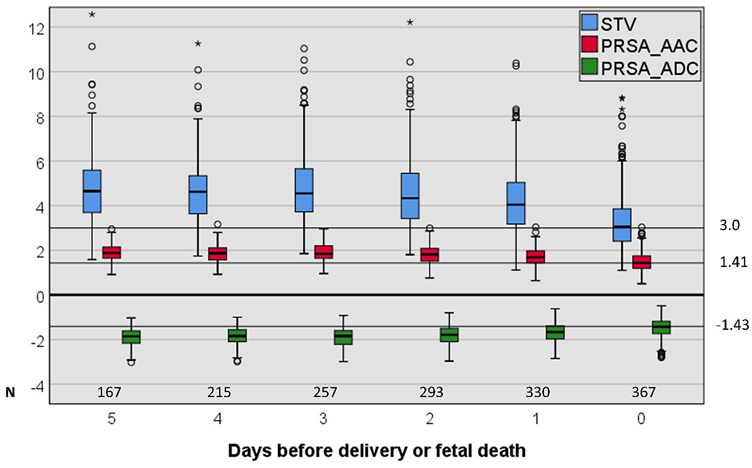
Box plot of AAC, ADC, and STV at the last five days before delivery or fetal death (only the last cCTG for each day was selected). STV, short-term variation; PRSA, phase-rectified signal averaging; AAC, average acceleration capacity; ADC, average deceleration capacity. Values at day 1 and 0 are significantly different from earlier values for all three indices.
Discussion
Test characteristics of a low STV, low AAC or ADC and/or recurrent decelerations for the prediction of fetal death or asphyxia were similar and showed reasonable sensitivity of approximately 80–90%, but very low specificity of 30–40%. Following standard test evaluation this would make both measurements useless for clinical application. However, all outcome endpoints have been influenced largely by obstetric management, potentially causing significant intervention bias. All women in this study were admitted for fetal monitoring with the goal to try to optimize the timing of delivery, weighing the risk of iatrogenic prematurity against the risk of intrauterine fetal asphyxia. Clinical management, using CTG, is obviously set on improving outcome parameters, which consequently strongly influences the results of this study. Considering this, poor test characteristics of cCTG might be considered reassuring, as many cases of adverse neonatal outcome are probably prevented. The fetal deaths in the 14 cases in which it was decided not to intervene by parents and caregivers (because of poor neonatal prognosis) after an abnormal CTG by low STV or low PRSA and/or recurrent decelerations, demonstrate that intervention is urgently needed when cCTG becomes abnormal as defined in this study, if fetal death is not acceptable.
Our data show that the combination of abnormal STV or AAC/ADC and/or recurrent decelerations improves the prediction of adverse condition at birth compared to using FHR variability only. This differs from a paper by Dawes, which concluded in an analysis of 89 women with FGR and a STV <3.0 ms that the presence or absence of decelerations was an unreliable guide to outcome [23]. Possibly this is influenced by the specific selection in this study of women with a very low STV. We observed that decelerations could increase FHR variability, as shown by higher values for AAC/ADC in the presence of decelerations. Although STV was not significantly different in the presence or absence of recurrent decelerations, it should be noted that the localization of decelerations is not exact, neither by FetalCare nor by STVcalc [6]. In the current study decelerations were verified visually. Furthermore, the Oxford system, which uses FetalCare and is based on research by Dawes, includes decelerations in its criteria for fetal normality [31].
There is a limited number of studies to compare our findings to. Only one study, a secondary analysis of the TRUFFLE study, is comparable to our study [12]. This study concluded that the predictive value of AAC and ADC compared to STV in severe FGR fetuses had a similar predictive performance for adverse outcomes (Apgar score <7, pH <7.1 and antenatal death) [12]. This study compared averages of STV, AAC and ADC, while the current study assesses cut off levels for STV, AAC and ADC, which are essential for clinical application.
Two studies concluded that FHR variability, as measured by STV, AAC or ADC was lower in pregnancies complicated by FGR than with normal growth, and that low AAC/ADC had a stronger association with FGR than STV [10, 32]. However, the difference in birth weight between the FGR and the normally grown neonates in these studies was such, that more commonly used ultrasound biometry and Doppler would probably be more effective for diagnosis of FGR.
One retrospective study compared AAC, ADC and STV during second stage of labor for the association with fetal acidosis and concluded that ADC predicted acidemia better than STV [33]. However, the predictive efficacy of both measurements was too low for clinical application. Furthermore, the CTG signal during the second stage of labour differs largely from pre-labour CTG.
Both STV and AAC or ADC have a comparable and rather poor association with adverse condition at birth with an AUC of a ROC graph between 0.6 and 0.7. A meta-analysis of STV presents similar results [34]. The advantage of STV over AAC or ADC is that there are many papers that assess the clinical application of STV [34], while for AAC or ADC several papers show associations, but none present clinically applicable thresholds. Furthermore, there are several commercial systems for computerized CTG and STV calculation and a number of non-commercial applications [6], while these are absent for AAC or ADC.
The advantage of computerized assessment of CTG over commonly used visual assessment is supported by several observational studies [9, 14] but can only be determined by a randomized trial. The combination of different fetal surveillance techniques might be tested using machine learning and artificial intelligence methods.
Novelty and clinical implications
In pregnancies complicated by fetal growth restriction, increased risk of an adverse condition at birth is indicated by a low value of either STV or PRSA and/or the presence of recurrent decelerations in the 5 days before birth or fetal demise.
The data of this study do not show superiority of either PRSA and STV in the prediction of adverse outcomes in growth restricted fetuses. The combination of these parameters with recurrent decelerations best predict adverse neonatal outcome.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: The local Institutional Review Board deemed the study exempt from review.
References
1. Spencer, R, Rossi, C, Lees, M, Peebles, D, Brocklehurst, P, Martin, J, et al.. Achieving orphan designation for placental insufficiency: annual incidence estimations in Europe. BJOG 2019;126:1157–67. https://doi.org/10.1111/1471-0528.15590.Search in Google Scholar PubMed
2. Ganzevoort, W, Mensing Van Charante, N, Thilaganathan, B, Prefumo, F, Arabin, B, Bilardo, CM, et al.. How to monitor pregnancies complicated by fetal growth restriction and delivery before 32 weeks: post-hoc analysis of TRUFFLE study. Ultrasound Obstet Gynecol 2017;49:769–77. https://doi.org/10.1002/uog.17433.Search in Google Scholar PubMed
3. Figueras, F, Albela, S, Bonino, S, Palacio, M, Barrau, E, Hernandez, S, et al.. Visual analysis of antepartum fetal heart rate tracings: inter- and intra-observer agreement and impact of knowledge of neonatal outcome. J Perinat Med 2005;33:241–5. https://doi.org/10.1515/jpm.2005.044.Search in Google Scholar
4. Alfirevic, Z, Devane, D, Gyte, GM, Cuthbert, A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev 2017;2:CD006066.10.1002/14651858.CD006066.pub3Search in Google Scholar PubMed PubMed Central
5. Dawes, GS, Redman, CW, Smith, JH. Improvements in the registration and analysis of fetal heart rate records at the bedside. Br J Obstet Gynaecol 1985;92:317–25. https://doi.org/10.1111/j.1471-0528.1985.tb01103.x.Search in Google Scholar PubMed
6. Wolf, H, Bruin, C, Dobbe, JGG, Gordijn, SJ, Ganzevoort, W. Computerized fetal cardiotocography analysis in early preterm fetal growth restriction – a quantitative comparison of two applications. J Perinat Med 2019;47:439–47. https://doi.org/10.1515/jpm-2018-0412.Search in Google Scholar PubMed
7. Henson, GL, Dawes, GS, Redman, CW. Antenatal fetal heart-rate variability in relation to fetal acid-base status at caesarean section. Br J Obstet Gynaecol 1983;90:516–21. https://doi.org/10.1111/j.1471-0528.1983.tb08958.x.Search in Google Scholar PubMed
8. Lees, C, Marlow, N, Arabin, B, Bilardo, CM, Brezinka, C, Derks, JB, et al.. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013;42:400–8. https://doi.org/10.1002/uog.13190.Search in Google Scholar PubMed
9. Ganzevoort, W, Thornton, JG, Marlow, N, Thilaganathan, B, Arabin, B, Prefumo, F, et al.. Comparative analysis of the 2-year outcomes in the GRIT and TRUFFLE trials. Ultrasound Obstet Gynecol 2019;24:24.Search in Google Scholar
10. Lobmaier, SM, Huhn, EA, Pildner von Steinburg, S, Muller, A, Schuster, T, Ortiz, JU, et al.. Phase-rectified signal averaging as a new method for surveillance of growth restricted fetuses. J Matern Fetal Neonatal Med 2012;25:2523–8. https://doi.org/10.3109/14767058.2012.696163.Search in Google Scholar PubMed
11. Bauer, A, Kantelhardt, JW, Barthel, P, Schneider, R, Makikallio, T, Ulm, K, et al.. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet 2006;367:1674–81. https://doi.org/10.1016/s0140-6736(06)68735-7.Search in Google Scholar PubMed
12. Lobmaier, SM, Mensing van Charante, N, Ferrazzi, E, Giussani, DA, Shaw, CJ, Muller, A, et al.. Phase-rectified signal averaging method to predict perinatal outcome in infants with very preterm fetal growth restriction- a secondary analysis of TRUFFLE-trial. Am J Obstet Gynecol 2016;215:630e1–7. https://doi.org/10.1016/j.ajog.2016.06.024.Search in Google Scholar PubMed
13. Wolf, H, Arabin, B, Lees, CC, Oepkes, D, Prefumo, F, Thilaganathan, B, et al.. Longitudinal study of computerized cardiotocography in early fetal growth restriction. Ultrasound Obstet Gynecol 2017;50:71–8. https://doi.org/10.1002/uog.17215.Search in Google Scholar PubMed
14. Wolf, H, Gordijn, SJ, Onland, W, Vliegenthart, RJS, Ganzevoort, W. Computerized fetal heart rate analysis in early preterm fetal growth restriction. Ultrasound Obstet Gynecol 2019;12:12. https://doi.org/10.1002/uog.21887.Search in Google Scholar PubMed
15. Lees, CC, Marlow, N, van Wassenaer-Leemhuis, A, Arabin, B, Bilardo, CM, Brezinka, C, et al.. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet 2015;385:2162–72. https://doi.org/10.1016/s0140-6736(14)62049-3.Search in Google Scholar PubMed
16. Verburg, BO, Steegers, EA, De Ridder, M, Snijders, RJ, Smith, E, Hofman, A, et al.. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol 2008;31:388–96. https://doi.org/10.1002/uog.5225.Search in Google Scholar PubMed
17. Arduini, D, Rizzo, G. Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat Med 1990;18:165–72. https://doi.org/10.1515/jpme.1990.18.3.165.Search in Google Scholar PubMed
18. Tranquilli, AL, Dekker, G, Magee, L, Roberts, J, Sibai, BM, Steyn, W, et al.. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens 2014;4:97–104. https://doi.org/10.1016/j.preghy.2014.02.001.Search in Google Scholar PubMed
19. Ganzevoort, W, Sibai, BM. Temporising versus interventionist management (preterm and at term). Best Pract Res Clin Obstet Gynaecol 2011;25:463–76. https://doi.org/10.1016/j.bpobgyn.2011.01.004.Search in Google Scholar PubMed
20. Wolf, H, Gordijn, SJ, Onland, W, Vliegenthart, RJS, Ganzevoort, W. Computerized fetal heart rate analysis in early preterm fetal growth restriction. Ultrasound Obstet Gynecol 2020;56:51–60. https://doi.org/10.1002/uog.21887.Search in Google Scholar
21. Dawes, GS, Visser, GH, Goodman, JD, Redman, CW. Numerical analysis of the human fetal heart rate: the quality of ultrasound records. Am J Obstet Gynecol 1981;141:43–52. https://doi.org/10.1016/0002-9378(81)90673-6.Search in Google Scholar PubMed
22. Dawes, GS, Houghton, CR, Redman, CW. Baseline in human fetal heart-rate records. Br J Obstet Gynaecol 1982;89:270–5. https://doi.org/10.1111/j.1471-0528.1982.tb04695.x.Search in Google Scholar PubMed
23. Dawes, GS, Moulden, M, Redman, CW. Short-term fetal heart rate variation, decelerations, and umbilical flow velocity waveforms before labor. Obstet Gynecol 1992;80:673–8.Search in Google Scholar
24. Dawes, GS, Moulden, M, Redman, CW. Computerized analysis of antepartum fetal heart rate. Am J Obstet Gynecol 1995;173:1353–4. https://doi.org/10.1016/0002-9378(95)91391-2.Search in Google Scholar PubMed
25. Dildy, GA, Thorp, JA, Yeast, JD, Clark, SL. The relationship between oxygen saturation and pH in umbilical blood: implications for intrapartum fetal oxygen saturation monitoring. Am J Obstet Gynecol 1996;175:682–7. https://doi.org/10.1053/ob.1996.v175.a74922.Search in Google Scholar PubMed
26. Victory, R, Penava, D, Da Silva, O, Natale, R, Richardson, B. Umbilical cord pH and base excess values in relation to adverse outcome events for infants delivering at term. Am J Obstet Gynecol 2004;191:2021–8. https://doi.org/10.1016/j.ajog.2004.04.026.Search in Google Scholar PubMed
27. Volpe, JJ. Intraventricular hemorrhage and brain injury in the premature infant. Neuropathology and pathogenesis. Clin Perinatol 1989;16:361–86. https://doi.org/10.1016/s0095-5108(18)30637-7.Search in Google Scholar
28. de Vries, LS, Eken, P, Dubowitz, LM. The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 1992;49:1–6. https://doi.org/10.1016/s0166-4328(05)80189-5.Search in Google Scholar PubMed
29. Jobe, AH, Bancalari, E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. https://doi.org/10.1164/ajrccm.163.7.2011060.Search in Google Scholar PubMed
30. Walsh, MC, Kliegman, RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 1986;33:179–201. https://doi.org/10.1016/s0031-3955(16)34975-6.Search in Google Scholar PubMed PubMed Central
31. Pardey, J, Moulden, M, Redman, CW. A computer system for the numerical analysis of nonstress tests. Am J Obstet Gynecol 2002;186:1095–103. https://doi.org/10.1067/mob.2002.122447.Search in Google Scholar PubMed
32. Graatsma, EM, Mulder, EJ, Vasak, B, Lobmaier, SM, Pildner von Steinburg, S, Schneider, KT, et al.. Average acceleration and deceleration capacity of fetal heart rate in normal pregnancy and in pregnancies complicated by fetal growth restriction. J Matern Fetal Neonatal Med 2012;25:2517–22. https://doi.org/10.3109/14767058.2012.704446.Search in Google Scholar PubMed
33. Georgieva, A, Papageorghiou, AT, Payne, SJ, Moulden, M, Redman, CW. Phase-rectified signal averaging for intrapartum electronic fetal heart rate monitoring is related to acidaemia at birth. BJOG 2014;121:889–94. https://doi.org/10.1111/1471-0528.12568.Search in Google Scholar PubMed
34. Pels, A, Mensing van Charante, NA, Vollgraff Heidweiller-Schreurs, CA, Limpens, J, Wolf, H, de Boer, MA, et al.. The prognostic accuracy of short term variation of fetal heart rate in early-onset fetal growth restriction: a systematic review. Eur J Obstet Gynecol Reprod Biol 2019;234:179–84. https://doi.org/10.1016/j.ejogrb.2019.01.005.Search in Google Scholar PubMed
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Disquiet concerning cesarean birth
- Prenatal care and pregnancy outcome among incarcerated pregnant individuals in the United States: a systematic review and meta-analysis
- Corner of Academy
- Does COVID-19 infection acquired in different pregnancy trimester influence placental pathology?
- Original Articles – Obstetrics
- Did the first wave of the COVID-19 pandemic impact the cesarean delivery rate? A retrospective cohort study at a primary care center in Switzerland
- Hypertensive disorders of pregnancy and severe acute respiratory syndrome coronavirus-2 infection
- Hidden in plain sight in the delivery room – the Apgar score is biased
- Comparison of phase rectified signal averaging and short term variation in predicting perinatal outcome in early onset fetal growth restriction
- Serum levels of kynurenine in pregnancies with fetal growth restriction and oligohydramnios
- Effect of third trimester maternal vitamin D levels on placental weight to birth weight ratio in uncomplicated pregnancies
- One-third of patients with eclampsia at term do not have an abnormal angiogenic profile
- Chorioangioma: a single tertiary care center retrospective study
- A prospective cohort study: can advanced ultrasonography replace magnetic resonance imaging in the diagnosis of placental adhesion disorders?
- Original Articles – Fetus
- Fetal left brachiocephalic vein diameters in normal and growth restricted fetuses
- Functional assessment of atrial wall excursion and foramen ovale flap tracings in 3rd trimester as predictor of short-term hemodynamic stability in congenital heart defects fetuses
- Original Articles – Neonates
- Developing a new pediatric extracorporeal membrane oxygenation (ECMO) program
- Patterns of placental injury in various types of fetal congenital heart disease
- Short Communication
- Prenatal care in the era of economic collapse
- Letters to the Editor
- Does fetus feel stress or pain on uterine contraction?
- Pregnancy associated plasma protein-A for the prediction of small for gestational age
- Reply to: Pregnancy associated plasma protein-A for the prediction of small for gestational age
Articles in the same Issue
- Frontmatter
- Reviews
- Disquiet concerning cesarean birth
- Prenatal care and pregnancy outcome among incarcerated pregnant individuals in the United States: a systematic review and meta-analysis
- Corner of Academy
- Does COVID-19 infection acquired in different pregnancy trimester influence placental pathology?
- Original Articles – Obstetrics
- Did the first wave of the COVID-19 pandemic impact the cesarean delivery rate? A retrospective cohort study at a primary care center in Switzerland
- Hypertensive disorders of pregnancy and severe acute respiratory syndrome coronavirus-2 infection
- Hidden in plain sight in the delivery room – the Apgar score is biased
- Comparison of phase rectified signal averaging and short term variation in predicting perinatal outcome in early onset fetal growth restriction
- Serum levels of kynurenine in pregnancies with fetal growth restriction and oligohydramnios
- Effect of third trimester maternal vitamin D levels on placental weight to birth weight ratio in uncomplicated pregnancies
- One-third of patients with eclampsia at term do not have an abnormal angiogenic profile
- Chorioangioma: a single tertiary care center retrospective study
- A prospective cohort study: can advanced ultrasonography replace magnetic resonance imaging in the diagnosis of placental adhesion disorders?
- Original Articles – Fetus
- Fetal left brachiocephalic vein diameters in normal and growth restricted fetuses
- Functional assessment of atrial wall excursion and foramen ovale flap tracings in 3rd trimester as predictor of short-term hemodynamic stability in congenital heart defects fetuses
- Original Articles – Neonates
- Developing a new pediatric extracorporeal membrane oxygenation (ECMO) program
- Patterns of placental injury in various types of fetal congenital heart disease
- Short Communication
- Prenatal care in the era of economic collapse
- Letters to the Editor
- Does fetus feel stress or pain on uterine contraction?
- Pregnancy associated plasma protein-A for the prediction of small for gestational age
- Reply to: Pregnancy associated plasma protein-A for the prediction of small for gestational age

