Placenta Accreta Spectrum Part I: anesthesia considerations based on an extended review of the literature
-
Rick Enste
, Patrick Cricchio
, Thorsten Braun
, Christopher O. Leonards
, Claudia Spies
, Wolfgang Henrich
Abstract
“Placenta Accreta Spectrum” (PAS) describes abnormal placental adherence to the uterine wall without spontaneous separation at delivery. Though relatively rare, PAS presents a particular challenge to anesthesiologists, as it is associated with massive peripartum hemorrhage and high maternal morbidity and mortality. Standardized evidence-based PAS management strategies are currently evolving and emphasize: “PAS centers of excellence”, multidisciplinary teams, novel diagnostics/pharmaceuticals (especially regarding hemostasis, hemostatic agents, point-of-care diagnostics), and novel operative/interventional approaches (expectant management, balloon occlusion, embolization). Though available data are heterogeneous, these developments affect anesthetic management and must be considered in planed anesthetic approaches. This two-part review provides a critical overview of the current evidence and offers structured evidence-based recommendations to help anesthesiologists improve outcomes for women with PAS. This first part discusses PAS management in centers of excellence, multidisciplinary care team, anesthetic approach and monitoring, surgical approaches, patient safety checklists, temperature management, interventional radiology, postoperative care and pain therapy. The diagnosis and treatment of hemostatic disturbances and preoperative prepartum anemia, blood loss, transfusion management and postpartum venous thromboembolism will be addressed in the second part of this series.
Introduction
“Placenta Accreta Spectrum” (PAS), or “Abnormally Invasive Placenta” (AIP), are umbrella terms that describe the abnormal adherence of the placenta to the uterine wall without spontaneous separation at delivery [1]. PAS encompasses a range of invasively growing placental villi—including, placenta accreta, increta, and percreta. In placenta percreta, the most severe form of PAS, the placental villi penetrate through the decidua, myometrium, and uterine serosa (often into the bladder and pelvis), which makes it difficult to surgically remove the placenta without causing a massive peripartum hemorrhage (PPH) [1], [2], [3]. Because pregnancy following caesarean section (C-section) is one of the leading causes of PAS, it is anticipated that PAS rates, estimated between 0.8 and 3.1 per 1,000 births after previous cesarean section, will continue to increase in the coming years as rates of C-sections continue to rise worldwide [1, 4, 5].
Most PAS diagnoses occur well before childbirth (and C-section), so an individually tailored PAS treatment regime in a center of excellence using a multidisciplinary care team approach is feasible and recommended [6], [7], [8], [9], [10], [11], [12], [13], [14]. Although those trials propose the superiority of a treatment in centers of excellence, a universally accepted definition of exactly what a “center of excellence” is mostly missing. The International Society for Placenta Accreta Spectrum (IS-PAS; former International Society for Abnormally Invasive Placenta; IS-AIP) defines a center of excellence by the “any-time-availability” of a multidisciplinary care team with the corresponding personal and technical infrastructure [1]. These multidisciplinary care teams include an expert obstetrician, obstetric anesthesiology specialist, a surgeon experienced with complex pelvic surgery—often a gynecological oncologist, an urologist (e.g., for ureteric re-implantation), a neonatologist, and a radiologist experienced with PAS diagnostics and interventional management. There should be rapid onsite access to colorectal and vascular surgeons, hematologists, adult intensive care facilities, (gestational age appropriate) neonatal intensive care facilities, massive transfusion facilities, and intraoperative autotransfusion (cell salvage) services. After initial medical assessment, an interdisciplinary presentation and case review should be performed (involving every attending discipline) to work up the planned approach as well as necessary technical and staff preparations. The planned delivery date and interdisciplinary approach should be written in the patient’s chart. A multidisciplinary PAS team should be available 24 h a day, 7 days a week to ensure that expertise is available for emergency situations. This systematic multidisciplinary approach reduces the need for massive transfusion, the incidence of coagulopathy, the duration of surgery, the incidence of hypotension, the length of intensive care unit (ICU) stay, the incidence of ureteral injuries, and the need for early reoperation [15], [16], [17], [18], [19].
There are different surgical procedures and therapeutic options to manage PAS — namely, (1) cesarean hysterectomy, (2) local uterine resection/uterus conserving surgery, and (3) conservative therapy — i.e., leaving the placenta in situ, depending on placental localization, invasion (e.g., into the cervix or parametrium), and the extent of adherence [1]. To a lesser extent, patients are additionally stratified based on factors like overall pregnancy risk, number of prior preterm deliveries, individual patient wishes, family planning considerations, comorbidities, and history of bleeding. Women with PAS generally undergo planned C-section at between 34 and 36 weeks gestation [1]. Caesarean hysterectomy is the most commonly accepted approach [20]. Here, the placenta is left in situ after delivery of the fetus, since forced placental removal attempts are associated with significant risk of hemorrhage [20]. In the conservative approach, the entire placenta remains untouched (in situ) until it is either completely resorbed or the adherent/invasive interface is significantly reduced. This area can then be locally resected in the postpartum period [1]. Though initial blood loss and transfusion requirements during delivery seem to be reduced, obstetricians and anesthesiologists should be aware that there is still an increased risk of (1) secondary bleeding, (2) hemostatic consumption and/or disseminated intravascular coagulation (DIC), and (3) postpartum infection in the following weeks [1]. Surgical removal of part of the myometrium where the placenta is abnormally adherent (local surgical resection) has been proposed as a technique for managing PAS while conserving the uterus [20]. It is reasonably successful and may have both slightly less blood loss and maternal morbidity than hysterectomy [1]. Nevertheless, hysterectomy can be indicated for primary, secondary, or emergency procedures [1].
In regard to tailored PAS treatment regimes, interventional radiology techniques are being increasingly used in PAS management (Supplementary Table S2).
Anesthesiologists should be aware of the implications associated with PAS: including prepartum lab workup, patient blood and anemia management, cell salvage, prewarming, special patient safety checklists and team time out, anesthesiology approach, monitoring, hemostatic management, access to interventional radiology, point-of-care testing, PPH algorithms, and postoperative care, e.g., ICU, pain therapy, and venous thromboembolism (VTE) prophylaxis.
In this two-part literature review, we survey the current literature and recommend a multimodal step-by-step approach to perioperative PAS management in an effort to optimize patient safety. This first part of the two-part review focuses on general therapeutic and anesthesiology management approaches to PAS. The second part of this series addresses hemostatic changes, blood loss, and transfusion management in PAS patients.
Literature findings: placenta accreta spectrum part I and II: anesthesia and hemostatic considerations based on an extended review of the literature
We conducted a computerized literature review of the Medline database using the following search queries “((abnorm invasive placenta OR placenta percreta OR placenta accreta OR placenta accreta spectrum)) AND (anaesth OR anesth)” to identify studies, case reports, series and reviews that investigated the impact of anesthesia management on PAS outcome. We then screened publications for inclusion based on the following criteria: (1) anesthetic approach, (2) monitoring, (3) anesthetic medication and uterotonics, (4) post-operative care, (5) cell salvage, (6) hemostatic products — e.g., tranexamic acid (TXA), fibrinogen or prothrombin complex concentrate (PCC), (7) estimated blood loss (EBL) and transfusion and (8) laboratory work-up. We included publications that reported information concerning anesthesiology approach, anesthetic drugs, or data from at least two of the six other criteria in the primary analysis. We additionally noted the (1) type of placental abnormally and the (2) surgical approach used.
Additionally, we extracted data from publications that presented results from PAS patients who underwent interventional radiology procedures for a separate radiological analysis and analyzed blood loss, transfusion/hemodynamics, hysterectomy rate, ICU admission rates, operative approach, and procedure duration. We also performed hand searching of references for all included publications.
We identified 7,723 publications published between 1973 and February 28 2021. Of those, 7,639 publications were excluded based on initial screening criteria (abstract, title duplicates). Of the remaining 78 publications, 28 were excluded following full-text screening. We identified 17 additional articles following hand searching of references from the retrieved full-texts, making 67 publications eligible for inclusion (Figure 1). We included 55 case reports/trials for anesthetic management analysis, which contained data of 3,459 women (Supplementary Table S1) [6], [7], [8], [9, 12, 14, 21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69]. For the analysis of interventional radiology in PAS 37 trials/reports met the criteria for inclusion, containing the data of 4,507 women (Supplementary Table S2) [12, 23], [24], [25], [26, 29, 36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51, 64, 66, 68, 70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]. 25 studies were included in both analyses.
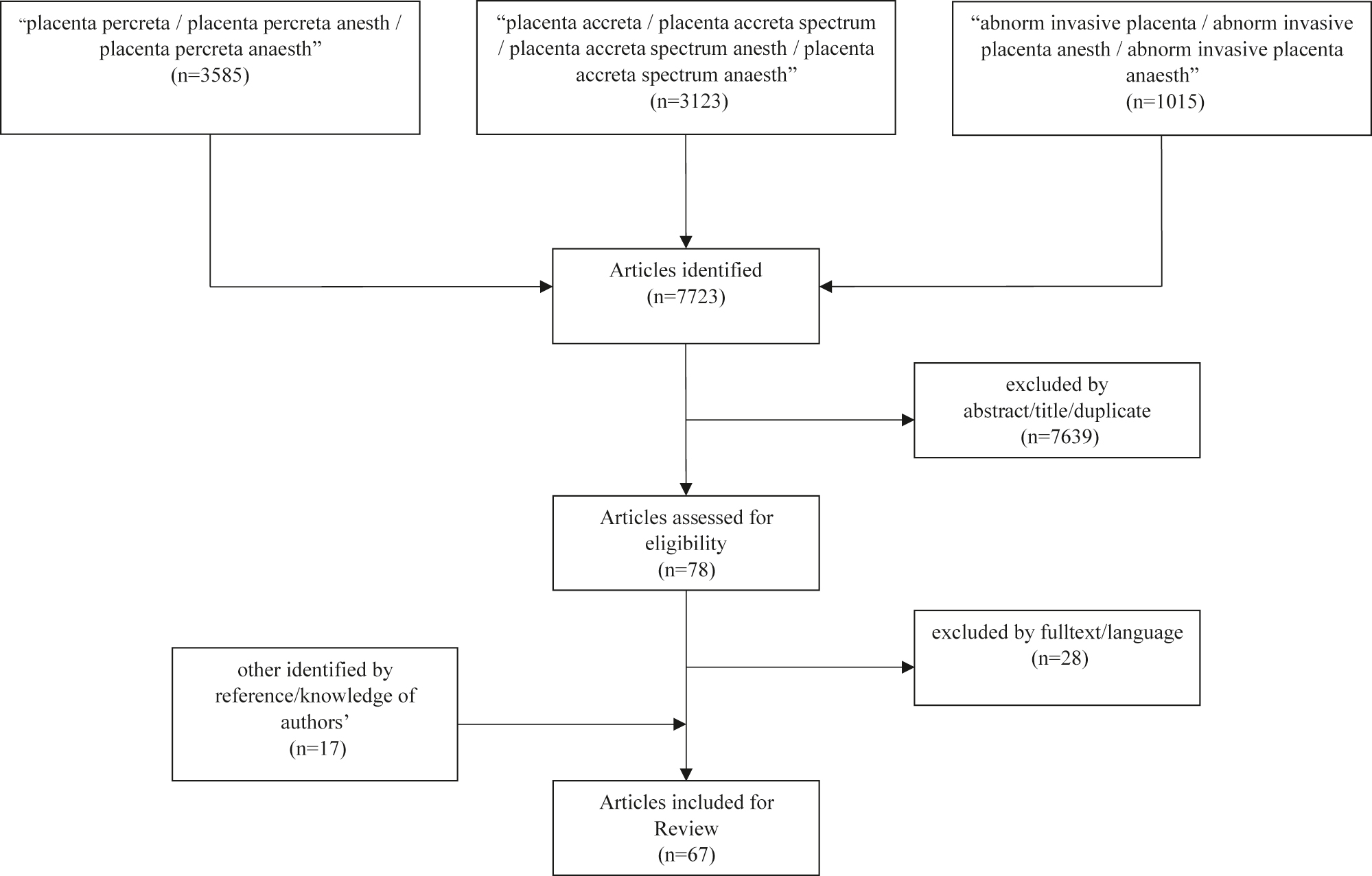
Flowchart: trial inclusion in anesthesia and hemostatic considerations analysis.
Of the 55 publications included in the primary analysis, we identified two randomized-controlled trials (RCTs) and no meta-analysis or systematic reviews (Figure 2, Supplementary Table S1).

Type of included trials in anesthesia and hemostatic considerations analysis and interventional radiology analysis.
This first part of the two-part review only the presents the results of this literature review with regard to anesthetic approach, surgical approach, monitoring, temperature management, interventional radiology, post-operative care and pain therapy. The results regarding hemostatic considerations, coagulopathy, anemia, laboratory work-up, blood loss, transfusion, use of hemostatic agents and postpartum venous thromboembolism prophylaxis will be addressed in the second part of this review.
Patient (surgical) safety checklist and team time-out
World Health Organization (WHO) initiatives have shown that surgical safety checklists and team time-outs prior to surgical incision help improve patient safety [82, 83]. None of the trials identified in this review reported the routine use of either checklists or team time-outs in PAS patients. Both checklists and team time-outs can be performed quickly and easily adapted to various clinical settings, to improve the treatment team’s understanding of the clinical situation prior to surgery and synchronize the team members involved [82, 84]. We recommend that checklists and team time-outs for PAS-surgery are used both preoperatively and intraoperatively. We advise that immediately prior to skin incision a pause be made for the first team time-out, where the entire team introduces themselves and their functions. In this team time-out the scheduled interdisciplinary approach, as discussed in the initial interdisciplinary case review, should be present briefly, including the intended operating procedure, expected surgical difficulties, and special operating room and anesthesia considerations (e.g., expected blood loss estimate, cell salvage system preparation, blood product availability, etc.). A second short team time-out during PAS-surgery should be performed following delivery of the placenta immediately after intraoperative PAS evaluation by the obstetrician/surgeon. The following information is of particular relevance to the healthcare team and should be explicitly stated in the second team time-out:
PAS confirmed, intraoperative staging, spontaneous or manual removal of the placenta [85]?
Planed operating procedure: Placenta in situ, local surgical resection or hysterectomy?
Uterotonics: Yes/No (which?)?
Bleeding?
Cardio-pulmonary state?
Conversion from neuraxial to general anesthesia?
Recent screening, diagnosis, and management guidelines of PAS disorders from the Society of Obstetricians and Gynaecologists of Canada (SOGC) highly recommend surgical safety checklists when all members of the health care team and their resources are assembled in the operating room (OR) [86].
Anesthetic considerations
|
Anesthesia (general vs. neuraxial) in PAS operative procedures
None of the trials that we identified in this review specifically assessed the difference between neuraxial vs. general anesthesia in PAS patients (Supplementary Table S1).
General anesthesia
Several studies reported findings from PAS patients who underwent general anesthesia (Supplementary Table S1). In one prospective observational trial, for instance, all women at high risk for placenta accreta received general anesthesia [32]. A wide variety of anesthetics were used to induce and maintain general anesthesia, which may simply reflect local standard operating procedures (Supplementary Table S1). Because incision-to-cord-clamping times can be much longer in PAS patients than in standard C-sections, anesthesia-related new-born distress and/or anesthesia of the new-born can occur if PAS patients undergoing general anesthesia for the entire operative procedure. Therefore, in our opinion, it is important to alert neonatology prior to surgery and discuss this as part of the initial team time-out.
Neuraxial anesthesia
Case reports, small retrospective, and prospective series have demonstrated the safety of neuraxial anesthesia for PAS management [33, 87]. However, the likelihood of conversion from neuraxial to general anesthesia increases (more than 50%) as bleeding increases [33, 88].
Each anesthetic approach has several benefits and detriments (Table 1). Therefore, a combination of neuraxial and planned secondary general anesthesia seems reasonable to maximize both patient comfort and safety. Initial neuraxial anesthesia enables women to take part in childbirth. It remains unclear which neuraxial anesthetic approach is superior. Proponents of spinal anesthesia, either as a single-shot spinal anesthesia (SPA) or as a combined spinal and epidural anesthesia (CSE), argue that this approach offers improved analgesia and patient comfort. However, neuraxial anesthesia may induce hypotension and therefore secondary fetal bradycardia or fetal distress. There are two systematic Cochrane Database reviews—one comparing SPA and epidural anesthesia (EDA) for C-section, another comparing CSE to EDA for analgesia for women in labor. Both found this effect, also known as spinal anesthesia induced hypotension (SAIH), to be more distinctive in SPA than in EDA [89, 90]. Furthermore, both failed to show any superiority of SPA or CSE in relation to analgesia or patient comfort. Whether this SAIH leads to otherwise preventable emergency situations related to C-sections or PAS in particular or has relevant effect on neonatal outcome is currently unclear. Nevertheless, SPA cannot be generally recommended for these reasons. Given the minor hemodynamic changes an EDA with e.g., ropivacaine 0.75% at a slow rate and 5–10 µg sufentanil appears to be favorable. This grants the opportunity to slowly “titrate” the EDA to an optimal level. With this slow rate hemodynamic changes can be easily countered with norepinephrine. The epidural catheter can also be used for postoperative (see below) and intraoperative analgesia in cases of prolonged surgery.
Anesthetic approach – considerations of (dis-advantages).
| Approach | GA | SPA | EDA | CSE | EDA + sec.planned GA |
|---|---|---|---|---|---|
| Advantage |
|
|
|
|
|
| Disadvantage |
|
|
|
|
|
-
AL, arterial line; CSE, combined spinal and epidural anesthesia; CVL, central venous line; EDA, epidural anesthesia; GA, general anesthesia; PCEA, patient controlled epidural analgesia; SAIH, spinal anesthesia induced hypotension; sec., secondary; SPA, spinal anesthesia.
If PAS is confirmed following childbirth, a planned conversion to general anesthesia before resection of the placenta minimizes stress and possible complications intraoperatively. Once PAS has been confirmed intraoperatively or if massive bleeding after birth is anticipated (second team time-out) switching from neuraxial to general anesthesia should be strongly considered in order to avoid emergency conversion under difficult conditions [33, 88].
Anesthetic considerations
|
Monitoring
We did not identify any studies that reported advanced monitoring during anesthesia in PAS patients (Supplementary Table S1). Standard monitoring included electrocardiogram (ECG), pulse oximetry, and non-invasive blood pressure (NIBP) and, in most cases, an arterial line (AL) was placed (invasive blood pressure; IBP) (Supplementary Table S1) [32, 69]. In addition to continuous IBP, advanced hemodynamic monitoring might be useful to detect hypovolemic changes, though none was reported in trials/case reports included in this review. Because massive bleeding with high volume resuscitation and transfusion rates frequently occurs in PAS, pulse pressure variation (PPV), stroke volume variation (SVV), stroke volume (SV) and cardiac output (CO) for targeted volume management should be considered, especially in women with placental invasion of the surrounding organs who are at a high risk of bleeding. A central venous line (CVL) was placed in most cases where general anesthesia was induced prior to surgery (Supplementary Table S1) and might be beneficial when high doses of vasopressors are required or in patients where peripheral intravenous cannulation is difficult (requiring small bore needles). Repetitive central venous or arterial blood gas analysis to determine lactate, hemoglobin (Hb), electrolyte levels including potassium and calcium and gas exchange parameters (e.g., pH value, carbon dioxide, bicarbonate) should be taken before birth and during the PAS management.
Additionally, body temperature, neuromuscular monitoring, and brain function monitoring (electroencephalogram) might be indicated in general anesthesia.
Anesthetic considerations
|
Temperature management and prewarming
We did not identify any studies in this review that reported data regarding preoperative or intraoperative warming or temperature management. Inadvertent perioperative hypothermia, defined as a body core temperature below 36 °C, is mainly caused by a shift in vasoconstriction and shivering thresholds, and sympathicolysis related vasodilatation (during neuraxial anesthesia) [91, 92]. Induction of general anesthesia aggravates the redistribution of warm blood from the body core to the periphery [93] with a decrease of the body core temperature from 0.8 to 1.5 °C [94]. In PAS patients with PPH, massive bleeding, and volume shifts, perioperative hypothermia may affect coagulation by altering platelet function [95, 96] and thereby perpetuating intraoperative blood loss [97]. A brief forced-air warming at 43 °C (prewarming) during epidural catheter placement and/or 10–20 min before induction of general anesthesia is effective for maintaining intra- and postoperative normothermia [94]. National and international guidelines recommend active warming before and during anesthesia induction in general [92, 98]. In addition to maintenance of normothermia, using a forced-air warming gown during neuraxial anesthesia placement or before general anesthesia induction may reduce patient agitation and improve preoperative well-being. This forced-air warming should be continued during the entire surgical procedure. Due to expected large in- and transfusion-volumes a fluid warmer should be used.
Anesthetic considerations
|
Interventional radiology: balloon occlusion and/or embolization, hybrid-OR
Our literature search yielded 37 relevant publications where interventional radiology performed either (1) balloon occlusion of the infrarenal abdominal aorta (IAABO), the internal iliac arteries (IIABO), common iliac arteries (CIABO) or (2) embolization of the uterine arteries (UAE) (Supplementary Table S2). The type of trials included here is shown in Figure 2.
Studies reported balloon occlusion interventions used in both emergency procedures and routine elective procedures. Usually, the epidural catheter was placed before the patients were transferred to the radiology suite where the balloon occlusion catheter was positioned under X-ray imaging [26, 36, 37]. Patients were then transferred to the OR for C-section where balloon catheter position was either re-confirmed or in some cases the catheter used without confirmation [38]. Meller et al. and Yamada et al. demonstrated the possibility of performing the entire procedure in a hybrid-OR [39, 74]. In general, the balloons were inflated routinely during C-section (1) after the umbilical cord had been clamped and (2) before any attempt of a surgical separation of the placenta or begin of hysterectomy [40, 41]. In some studies, balloons were only inflated if deemed necessary intraoperatively [75].
The regime of inflation and removal of the catheters and sheaths was not always reported but if varied widely. The duration of inflation and deflation till next inflation varied according to position of catheter and approach between several minutes up to an hour [25, 37, 41, 42, 76]. Generally, estimated blood loss (EBL), transfusion rate, operation time, hysterectomy rates, and ICU admission were analyzed. The two RCTs as well as Peng et al. did not identify any advantage for the use of IIABO compared to standard care [23, 43, 68]. In contrast, Cali et al. showed a significant reduction of EBL and RBC transfusion for the IIABO group in a non-randomized prospective trial in PAS [26]. These findings dovetail with the retrospective data presented by Zhou et al. [38]. Mei et al. compared IIABO with IAABO and found less EBL and shorter operating times for the IAABO group [40].
In a meta-analysis of 1811 women with PAS, Shahin et al. found a significant reduction in EBL and red blood cell concentrates (RBCs) transfusion for all endovascular procedures except UAE [70]. Moreover, they found that IAABO was associated with lower hysterectomy rates and blood loss than other endovascular methods [70]. Due to anastomosis from the ovarian or renal arteries, Matsubara et al. concluded that occlusion of the aorta may be beneficial to other locations [99]. Liu et al. found that aorta occlusion at the level of the renal arteries was associated with less blood loss in 57 retrospective analyzed PAS patients [44]. The postoperative levels of creatinine and lactate dehydrogenase were not affected [44].
Balloon occlusion-related complications occur frequently and were reported in 28 studies/case reports (Supplementary Table S2). The prevalence of arterial thrombosis, reported in 10 trials, varies from 1 to 14% (Supplementary Table S2) [42, 81]. Arterial dissection, rupture, aneurysm, and AV-fistula were reported in five trials, and single trials observed neurological symptoms of the lower limb, fever, vasospasm, gastrointestinal symptoms (requiring a conversion from neuraxial to general anesthesia), arterial wall hematoma and a vascular injury with need of a ilio-femoral bypass [24, 29, 36, 37, 42, 43, 45, 70, 76, 78]. Further complications are associated with the procedure itself, like balloon rupture, misplacement, catheter migration, or failure to provide adequate intraoperative hemostasis for other reasons [36, 46, 70, 77]. On the other hand, six trials reported that no catheter-related (major) complications occurred [12, 26, 47, 48, 68, 75]. In a systematic review, catheter-associated complications were found in 7.5% of total cases (4.5% minor and 3% major complications) [71]. The fewest complications occurred with the use of IAABO [71].
Various approaches were used for uterine artery embolization. Either UAE was used routinely to prevent PPH, or as a therapy if PPH occurs [29, 49], [50], [51]. The two largest trials assessing the use of UAE found divergent results. While Shahin et al. reported no significant benefit for UAE, Labarta et al. found it effective in 89.4% (1739 patients) with PPH due to uterine atony [70, 72]. However, it remains unclear if these results are transferrable to PAS patients. The prevalence of UAE related complications differed as well. While Yuan et al. and Meller et al. had no serious vascular complications, Kim et al. pointed to a high incidence of severe complications, namely: uterine necrosis, intra-abdominal hematoma, paralytic ileus, and late complications (two patients had total uterine necrosis and subsequent hysterectomies) [49, 51, 74]. In a meta-analysis, 43 of 263 women with abnormal placental implantation had a self-limiting post-embolization syndrome after UAE, whereas two women suffered uterine necrosis with subsequent hysterectomy and 10 women developed groin hematoma [70].
Additionally, cross-clamping of the aorta or surgical ligation of arteries, especially the uterine artery, were reported as alternative surgical approaches [25, 36, 46, 50, 73, 77]. Further, an intraarterial infusion of pituitrin (a formerly in obstetrics used extract of bovine posterior hormones by now replaced by other drugs with oxytocic activity) in the internal iliac arteries in addition to an IIABO was proposed [25]. Though not mentioned in any of the reviewed studies, manual compression of the aorta is another possible approach [100]. Aortic compression may be situational conducted either open or closed. Given that mostly used as an emergency maneuver if massive bleeding occurs, no standardized approach can be proposed.
In conclusion, endovascular interventions seem beneficial for women with PAS undergoing cesarean section, especially regarding EBL, transfusion rate, and uterus preservation.
Abdominal aorta balloon occlusion appears to be more beneficial than more distal occlusions for three reasons. First, the procedure is easier to perform with a single catheter and does not require a cross-over approach. Blood loss may be reduced due to occlusion of possible anastomoses from ovarian (and renal) arteries. Moreover, this procedure is advantageous as it is becoming more routinely performed in the clinical setting among both obstetric and trauma patients. For these reasons we, as well as the new SCOG guideline, recommend the IAABO as a promising approach [86]. The localization of the occlusion, whether at the level of the renal arteries or below, is currently being discussed and warrants further investigation. Because results even across IAABO studies vary widely, there may be an underlying effect of the inflation regimes which have yet to be assessed.
Still, balloon occlusion may not be indicated in every woman with PAS. The circulatory disturbances caused by occlusion and re-opening may lead to a decompensation in women with cardiac diseases. Women with contusion or intracranial hemorrhage could be threatened by the presumable increased intracranial pressure due to elevated blood pressure following the occlusion [101]. Finally, the blood pressure decreases following balloon deflation and a reperfusion-syndrome with severe metabolic derangements may occur [102, 103]. These adverse effects have to be anticipated to avoid potentially lethal complications and may limit the use of vascular occlusions.
UAE can lead to serious complications, like post-embolization syndrome, uterine necrosis, and vascular complications. Given the risks, it seems that the prophylactic use of UAE to prevent PPH in PAS is unjustifiable. Conversely, in women with ongoing PPH, the current American College of Obstetricians and Gynecologists, the Royal College of Obstetricians and Gynaecologists, and the Society of Obstetricians and Gynaecologists of Canada recommend UAE only if patients are (1) actively bleeding, and (2) stable enough to transfer [13, 104, 105]. Using a hybrid-OR decreases risks associated with patient transport and UAE or balloon occlusion may help stabilize PAS patients with active bleeding during C-section [39, 74].
The frequency with which methodological failures associated with endovascular techniques have been reported (balloon rupture, catheter displacement, or other causes), indicates that interventional radiologist experience may be a deciding factor in success/failure rates [36, 46, 70, 74, 77]. On account of the risks of severe complications like thrombosis, dissection, or hemodynamic disturbances, anesthesiologists should familiarize with these procedures and be prepared to respond accordingly if a severe complication arises.
Finally, endovascular techniques expose the fetus to radiation, though the dosage is normally below the maximal recommended fetal radiation dose (100 mGy) [106].
Anesthetic considerations
|
Postoperative care
Roughly half of the trials/case reports included in this review reported information regarding postoperative care. Overall, there was a high rate of postoperative ICU admission. Because there is a high risk of massive bleeding and large volume transfusion, critical care specialists should be involved prior to surgery as part of the multidisciplinary team [7, 107]. The primary reasons cited for postoperative ICU admission were (1) the need for further fluid resuscitation, (2) further correction of coagulopathy, (3) management of perioperative hypofibrinogenemia and potential reduction of other coagulation factors (e.g., consumption of FXIII), (4) identification and management of arterial catheter complications, (5) timing of venous-thrombo-embolism-prophylaxis, and (6) pain therapy [107]. Patil et al. reported hypothermia (from large fluid infusions and prolonged surgery), hyperkalemia, hypocalcemia, acidosis, volume depletion or overload or transfusion reactions as most common reasons for ICU admission in their review [10]. Therefore, standard monitoring, respiratory rate, level of consciousness, body core temperature, blood glucose levels (every 4 h for the first 24 h), electrolytes as well as arterial blood gas analysis (hourly for the first 2 h, then every 4 h for the following 12 h), pain, bleeding, and urine output as a marker of volume depletion should be monitored for roughly 24 h postoperatively [10].
Postoperative ICU admission or maternity-based high dependency unit (HDU) admission (with the objective of allowing early mother-child-bonding) should be determined dependent on the course of the operation and subsequent homeostatic disturbances. Regardless, close observation is necessary to identify postoperative hemorrhage and potential adverse events following surgery and interventional radiology procedures.
Anesthetic considerations
|
Postoperative pain therapy
Adequate pain control after C-section is one of patients’ foremost concerns. Optimal analgesia is also a key point in the enhanced recovery after surgery (ERAS) concept. Correspondingly, maternal mobility improves the interaction with the newborn. Inadequate pain control leads to delayed consequences such as postpartum depression, chronic pain, and opioid consumption [108]. Because opiates are avoided before umbilical cord clamping in general anesthesia, ketamine and neuraxial techniques are the only options for providing analgesia starting from skin incision. Out of 179 different surgical procedures, C-section is ranked ninth most painful postoperatively [109]. Women who received general anesthesia for C-section generally require high levels of opiates (on average 27 mg morphine equivalent) to alleviate postoperative pain [109]. Even when neuraxial anesthesia was applicated, postoperative numeric rating scale (NRS) pain scores amount up to 6–7 under mobilization (e.g., caring for the newborn) and chronic postsurgical pain were detected in upwards of 11% of women after planned caesarean section without PAS [110, 111]. C-section in women with PAS is thought to be even more painful, due to the more invasive nature of the operation. For its possible side effects (e.g., addiction, obstipation, weariness) postoperative systemic opioids should be considered as a recue medication. Therefore, advanced planning of postoperative analgesia is warranted.
None of the 73 trials/case reports included in this review specifically assessed postoperative analgesia in women with PAS. According to the anesthetic technique different approaches for the postoperative analgesia can be offered. In 17 of the included trials/case reports an epidural catheter was placed (as an EDA or CSE), in 24 publications the women received a primary general anesthesia (Supplementary Table S1).
3 mg morphine epidural can alleviate postoperative pain and spare other analgesics for the first 24 h if route is available [110, 112]. A catheter left in situ may prolong the postoperative immobilization, but it might be reasonable to leave the catheter in place for patient controlled epidural analgesia (PCEA) in dependency on the extent of the operation, especially if early postoperative mobilization appears unattainable.
Other regional anesthetic techniques such as transversus abdominal plane (TAP) block, a quadratus lumborum (QL) block, or erector spinae block has been shown to reduce postoperative opioid requirements and pain scores if no neuraxial morphine has been administered [113], [114], [115]. These approaches seem beneficial if cesarean section was conducted under general anesthesia alone.
Though primary developed for cancer pain, the use of the WHO analgesics ladder seems advisable [116]. We recommend additional postoperative analgesia with paracetamol and non-steroidal anti-inflammatory drugs, like ibuprofen (WHO level 1).
This multimodal analgesia is the current gold standard for pain management after cesarean delivery and can effectively reduce systemic opioid administration [108, 117, 118].
If the pain relief measures described above are insufficient, rescue medication using opioids at WHO levels 2 and 3 is inevitable [116]. The oral route is as effective as the intravenous and should be preferred if available [119]. Oral opioids (morphine sulfate, hydrocodone, oxycodone and tramadol) are currently available and acceptable. Codeine is contraindicated during breastfeeding [108]. In special cases of extraordinarily severe postoperative pain, patient controlled intravenous analgesia (PCIA) can be utilized [120].
Dexamethasone, which is frequently used for prevention of postoperative nausea and vomiting, may have an additional analgesic effect [121]. Other co-analgesics like ketamine, clonidine, gabapentin, lidocaine, or magnesium have been cited as possible co-analgesic treatments, however there is currently no data regarding the efficacy of these additional approaches in PAS [107].
Anesthetic considerations
|
PAS-algorithm
None of the trials/case reports included in this review reported a multidisciplinary PAS(-PPH) algorithm including detailed anesthetic considerations. Although, anesthetic PAS management is challenging due to requirements, such as preoperative planning, pre- and postpartum anesthetic approach and postoperative monitoring, possible further interventions or structural considerations of the hospital and department (Figure 3). Scheduled and structured team time outs may help improving multidisciplinary communication and matching current anesthetic/hemodynamic/hemostatic state with EBL, ongoing surgical procedures and excepted complications or additional interventions (Figure 3). Therefore, we propose this PAS-algorithm as a possible approach regarding the most challenging aspects in PAS management. This algorithm includes considerations concerning hemostatic and anemia management, which will be discussed detailed in the second part of this review.
Anesthetic considerations
|
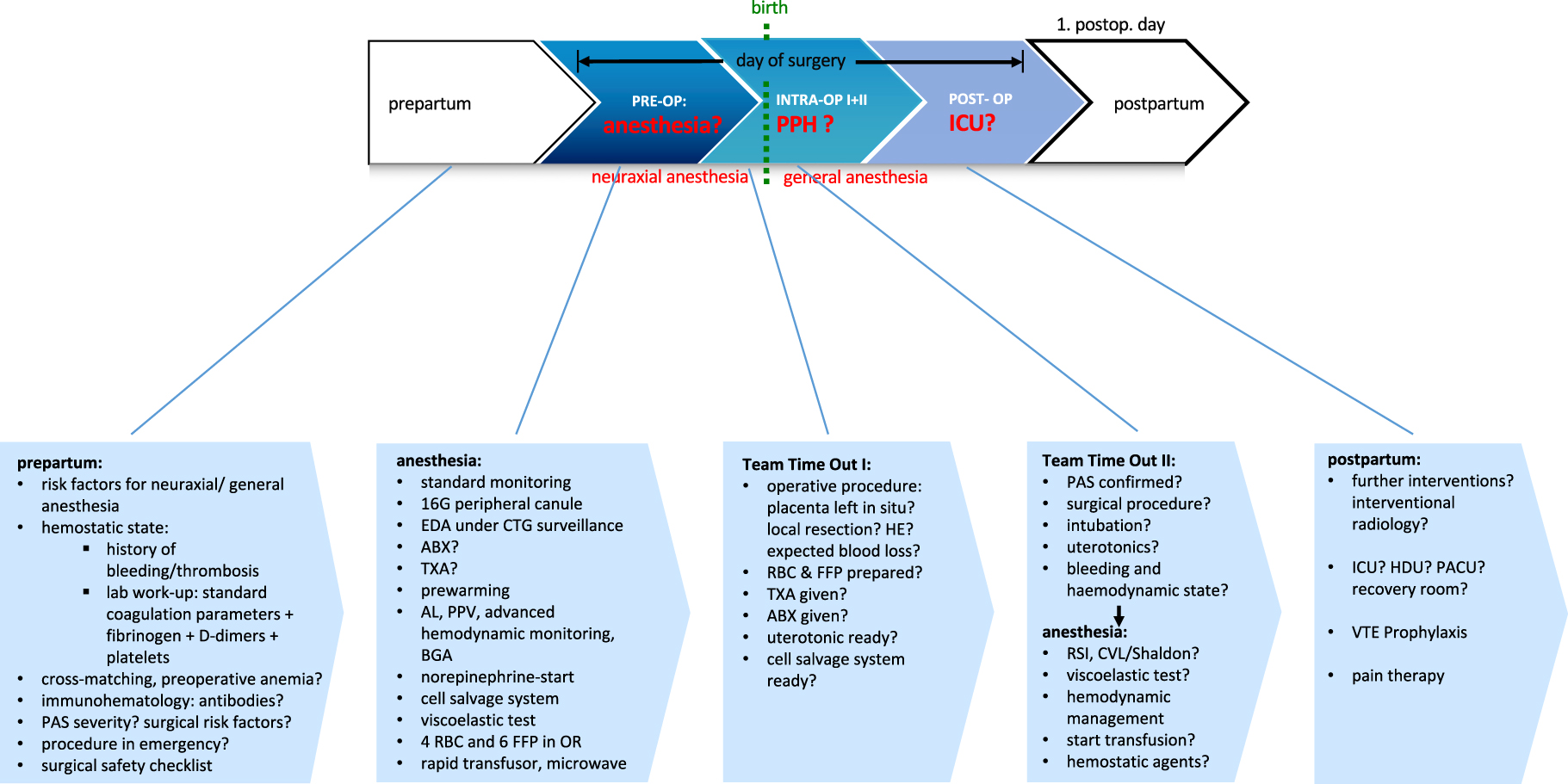
PAS-algorithm. AL, arterial line; ABX, antibiosis; BGA, blood gas analysis; CVL, central venous line; EDA, epidural anesthesia; FFP, fresh frozen plasma; HE, hysterectomy; ICU, intensive care unit; INTRA-OP, intraoperative; OR, operating room; PACU, post-anesthesia care unit; PAS, placenta accreta spectrum; PCEA, patient-controlled epidural anesthesia; POST-OP, postoperative; PPH, peripartum hemorrhage; PPV, pulse pressure variation; PRE-OP, preoperative; RBC, red blood cell concentrates; RSI, rapid sequence induction; TTO, team time out; TXA, tranexamic acid.
Limitations
PAS is a rare and heterogeneous condition. As such, PAS definitions and terms vary, it is therefore possible that the presented research was incomplete due to terminology variation (e.g., suspected “morbidly adherent placenta”). Moreover, though many publications were included in this review, few were specifically designed to primarily assess anesthesiology PAS management. Anesthesiology parameters were frequently reported as either secondary outcome measures or basic characteristics. In addition, the quality of the included studies is heterogenous. Currently, there are very few meta-analyses, systematic reviews, or randomized-controlled trials available. Most PAS data are retrospective and are frequently presented as case reports or case series. A few topics of interest for this review—for example temperature management and postoperative pain therapy in PAS patients—were not addressed at all in the available literature. Therefore, recommendations have to be generated from general therapeutic approaches and reasonable adaption according to PAS circumstances.
Furthermore, it has to be assumed, that especially precarious PAS cases are those to be published. Due to the large ratio of case reports and series in the available data, this may cause a serious bias.
Conclusions
Women presenting with PAS are at high risk of PPH, extensive surgical, anesthetic and/or interventional procedures and might be threatened by e.g., potential massive transfusion, hysterectomy, neonatal impairment, surgical complications and/or pain.
This part of the two-part review summarized current anesthesiology approaches, considering different PAS management strategies including interventional radiology. The importance of preoperative scheduling including e.g., modified surgical safety checklists and team time outs was highlighted. The anesthetic approach, monitoring, and preparation of potential massive transfusion as well as postoperative ICU-admission and pain therapy should be adjusted to structural conditions on site. A multidisciplinary algorithm of PAS management including anesthetic considerations and PPH management might be helpful to improve patient outcome. Due to the complexity of PAS and the personal and technical infrastructure needed to ensure the best possible outcome for women suffering from PAS, it seems reasonable to postulate a treatment of these women in centers of excellence providing the required recourses.
The level of evidence appears to be better for radiological interventions than for anesthesia considerations in PAS patients. Further high-quality trials are needed.
In the second part of this review, we will discuss possible hemostatic changes (e.g., a “DIC-like” hemostatic state) in PAS cases and their management. Moreover, we will address pre- and intrapartum anemia, transfusion management and the use of autologous blood transfusion systems.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: All authors state no conflict of interest in relation to this study. R.E. states no conflict of interest. P.C. states no conflict of interest. PY.D. received honoraria for lecture and consulting for CAF-DCF an LFB company. T.B. received research funds in relation to DFG-project BR2925/11-1. C.O.L. states no conflict of interest. P.N. states no conflict of interest. C.S. reports grants from Deutsche Forschungsgemeinschaft/German Research Society, grants from Deutsches Zentrum für Luft-und Raumfahrt e.V. (DLR)/German Aerospace Center, grants from Einstein Stiftung Berlin/Einstein Foundation Berlin, grants from Gemeinsamer Bundesausschuss/Federal Joint Committee (G-BA), grants from Inneruniversitäre Forschungsförderung/Inner University Grants, grants from Projektträger im DLR/Porject Management Agency, grants from Stifterverband/Non-Profit Society Promoting Science and Education, grants from European Society of Anaesthesiology and Intensive Care, grants from Baxter Deutschland GmbH, grants from Fresenius Medical Care, grants from Grünenthal GmbH, grants from Masimo Europe Ltd., grants from Pfizer Pharma PFE GmbH, personal fees from Georg Thieme Verlang, grants from Dr. F. Köhler Chemie GmbH, grants from Sintetica GmbH, grants from Stifterverband für die deutsche Wissenschaft e.V./Philips, grants from Stiftung Charité, grants from AGUETTANT Deutschland GmbH, grants from AbbVie Deutschland GmbH & Co. KG, grants from Amomed Pharma GmbH, grants from InTouch Health, grants from Copra System GmbH, grants from Correvio GmbH, grants from Gemeinsamer Bundesausschuss/Federal Joint Committee (G-BA) Innovationsfond, grants from Max-Planck-Gesellschaft zur Förderung der Wissenschaft e.V., grants from Deutsche Gesellschaft für Anesthesiologie & Intensivmedizin (DGAI), grants from Stifterverband für die deutsche Wissenschaft e.V. /Medtronic, grants from Philips Electronics Nederland BV, grants from BMBF/RKI, grants from BMBF, grants from Deutsche Forschungsgemeinschaft/German Research Societ, grants from Drägerwerk AG & Co. KGaA outside the submitted work. In addition C.S. has the patents 10 2014 215 211.9, 10 2018 114 364.8, 10 2018 110 275.5, 50 2015 010 534.8, 50 2015 010 347.7, 10 2014 215 212.7 licensed. W.H. states no conflict of interest. L.K. reports honoraria for lecture from Novo Nordisk, CSL Behring and HICC Deutschland GbR.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Collins, SL, Alemdar, B, van Beekhuizen, HJ, Bertholdt, C, Braun, T, Calda, P, et al.. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the international society for abnormally invasive placenta. Am J Obstet Gynecol 2019;220:511–26. https://doi.org/10.1016/j.ajog.2019.02.054.Search in Google Scholar PubMed
2. Parva, M, Chamchad, D, Keegan, J, Gerson, A, Horrow, J. Placenta percreta with invasion of the bladder wall: management with a multi-disciplinary approach. J Clin Anesth 2010;22:209–12. https://doi.org/10.1016/j.jclinane.2009.03.018.Search in Google Scholar PubMed
3. Wlodarz-Ulman, I, Nowosielski, K, Poreba, R, Poreba, A. P372 Placenta praevia increta with cesarean section scar invasion. Int J Gynecol Obstet 2009;107:S520. https://doi.org/10.1016/s0020-7292(09)61863-4.Search in Google Scholar
4. Jauniaux, E, Chantraine, F, Silver, RM, Langhoff-Roos, J. For the FIGO placenta accreta diagnosis and management expert consensus panel. FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynecol Obstet 2018;140:265–73. https://doi.org/10.1002/ijgo.12407.Search in Google Scholar PubMed
5. Beekhuizen, HJ, Stefanovic, V, Schwickert, A, Henrich, W, Fox, KA, Gziri, MM, et al.. A multicenter observational survey of management strategies in 442 pregnancies with suspected placenta accreta spectrum. Acta Obstet Gynecol Scand 2021;100:12–20. https://doi.org/10.1111/aogs.14096.Search in Google Scholar PubMed PubMed Central
6. Quist-Nelson, J, Crank, A, Oliver, EA, Kim, CH, Richard, S, George, B, et al.. The compliance with a patient-safety bundle for management of placenta accreta spectrum. J Matern Fetal Neonatal Med 2019;34:2880–6. https://doi.org/10.1080/14767058.2019.1671349.Search in Google Scholar PubMed
7. Nieto, AJ, Echavarría, MP, Carvajal, JA, Messa, A, Burgos, JM, Ordoñez, C, et al.. Placenta accreta: importance of a multidisciplinary approach in the Colombian hospital setting. J Matern Fetal Neonatal Med 2020;33:1321–9. https://doi.org/10.1080/14767058.2018.1517328.Search in Google Scholar PubMed
8. Khokhar, RS, Baaj, J, Khan, MU, Dammas, FA, Rashid, N. Placenta accreta and anesthesia: a multidisciplinary approach. Saudi J Anaesth 2016;10:332–4. https://doi.org/10.4103/1658-354x.174913.Search in Google Scholar PubMed PubMed Central
9. Sivasankar, C. Perioperative management of undiagnosed placenta percreta: case report and management strategies. Int J Womens Health 2012;4:451–4. https://doi.org/10.2147/ijwh.s35104.Search in Google Scholar PubMed PubMed Central
10. Patil, V, Ratnayake, G, Fastovets, G, Wijayatilake, DS. Clinical pearls part 3: anaesthetic management of abnormally invasive placentation. Curr Opin Anaesthesiol 2018;31:280–9. https://doi.org/10.1097/aco.0000000000000601.Search in Google Scholar PubMed
11. Mauritz, AA, Dominguez, JE, Guinn, NR, Gilner, J, Habib, AS. Blood-conservation strategies in a blood-refusal parturient with placenta previa and placenta percreta. A A Case Rep 2016;6:111–3. https://doi.org/10.1213/xaa.0000000000000258.Search in Google Scholar PubMed
12. Fratto, VM, Conturie, CL, Ballas, J, Pettit, KE, Stephenson, ML, Truong, YN, et al.. Assessing the multidisciplinary team approaches to placenta accreta spectrum across five institutions within the university of California fetal consortium (UCfC). J Matern Fetal Neonatal Med 2019;34:2971–6.10.1080/14767058.2019.1676411Search in Google Scholar PubMed
13. Leduc, D, Senikas, V, Lalonde, AB. Clinical Practice Obstetrics Committee. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can 2009;31:980–93. https://doi.org/10.1016/s1701-2163(16)34329-8.Search in Google Scholar PubMed
14. Nieto-Calvache, AJ, López-Girón, MC, Quintero-Santacruz, M, Bryon, AM, Burgos-Luna, JM, Echavarría-David, MP, et al.. A systematic multidisciplinary initiative may reduce the need for blood products in patients with abnormally invasive placenta. J Matern Fetal Neonatal Med 2020;35:738–44.10.1080/14767058.2020.1731460Search in Google Scholar PubMed
15. Eller, AG, Bennett, MA, Sharshiner, M, Masheter, C, Soisson, AP, Dodson, M, et al.. Maternal morbidity in cases of placenta accreta managed by a multidisciplinary care team compared with standard obstetric care. Obstet Gynecol 2011;117:331–7. https://doi.org/10.1097/aog.0b013e3182051db2.Search in Google Scholar PubMed
16. Walker, MG, Allen, L, Windrim, RC, Kachura, J, Pollard, L, Pantazi, S, et al.. Multidisciplinary management of invasive placenta previa. J Obstet Gynaecol Can 2013;35:417–25. https://doi.org/10.1016/s1701-2163(15)30932-4.Search in Google Scholar
17. Shamshirsaz, AA, Fox, KA, Salmanian, B, Diaz-Arrastia, CR, Lee, W, Baker, BW, et al.. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol 2015;212:218.e1–9. https://doi.org/10.1016/j.ajog.2014.08.019.Search in Google Scholar PubMed
18. Smulian, JC, Pascual, AL, Hesham, H, Qureshey, E, Thomas, MB, Depuy, AM, et al.. Invasive placental disease: the impact of a multi-disciplinary team approach to management. J Matern Fetal Neonatal Med 2017;30:1423–7. https://doi.org/10.1080/14767058.2016.1216099.Search in Google Scholar PubMed
19. Silver, RM, Fox, KA, Barton, JR, Abuhamad, AZ, Simhan, H, Huls, CK, et al.. Center of excellence for placenta accreta. Am J Obstet Gynecol 2015;212:561–8. https://doi.org/10.1016/j.ajog.2014.11.018.Search in Google Scholar PubMed
20. Cahill, A, Beigi, R, Phillips Heine, R, Silver, R, Wax, J, American College of Obstetricians and Gynecologists and the Society for Maternal–Fetal Medicine. Obstetric care consensus no. 7: placenta accreta spectrum. Obstet Gynecol 2018;132:e259–75.10.1097/AOG.0000000000002983Search in Google Scholar PubMed
21. Biele, C, Kaufner, L, Schwickert, A, Nonnenmacher, A, von Weizsäcker, K, Muallem, MZ, et al.. Conservative management of abnormally invasive placenta complicated by local hyperfibrinolysis and beginning disseminated intravascular coagulation. Arch Gynecol Obstet 2021;303:61–8. https://doi.org/10.1007/s00404-020-05721-0.Search in Google Scholar PubMed PubMed Central
22. Matsuzaki, S, Yoshino, K, Endo, M, Tomimatsu, T, Takiuchi, T, Mimura, K, et al.. Successful anticoagulant therapy for disseminated intravascular coagulation during conservative management of placenta percreta: a case report and literature review. BMC Pregnancy Childbirth 2017;17:443. https://doi.org/10.1186/s12884-017-1634-8.Search in Google Scholar PubMed PubMed Central
23. Salim, R, Chulski, A, Romano, S, Garmi, G, Rudin, M, Shalev, E. Precesarean prophylactic balloon catheters for suspected placenta accreta: a randomized controlled trial. Obstet Gynecol 2015;126:1022–8. https://doi.org/10.1097/aog.0000000000001113.Search in Google Scholar PubMed
24. Zhu, H, Wang, S, Shi, J, Yao, L, Wang, L, Chen, H, et al.. Prophylactic endovascular balloon occlusion of the aorta in cases of placenta accreta spectrum during caesarean section: points from the anaesthesiologist’s perspective. BMC Pregnancy Childbirth 2020;20:446. https://doi.org/10.1186/s12884-020-03136-y.Search in Google Scholar PubMed PubMed Central
25. Dai, M, Jin, G, Lin, J, Zhang, Y, Chen, Y, Zhou, Q, et al.. Control of postpartum hemorrhage in women with placenta accreta spectrum using prophylactic balloon occlusion combined with Pituitrin intra-arterial infusion. Eur Radiol 2020;30:4524–33. https://doi.org/10.1007/s00330-020-06813-w.Search in Google Scholar PubMed
26. Cali, G, Forlani, F, Giambanco, L, Amico, ML, Vallone, M, Puccio, G, et al.. Prophylactic use of intravascular balloon catheters in women with placenta accreta, increta and percreta. Eur J Obstet Gynecol Reprod Biol 2014;179:36–41. https://doi.org/10.1016/j.ejogrb.2014.05.007.Search in Google Scholar PubMed
27. Sentilhes, L, Ambroselli, C, Kayem, G, Provansal, M, Fernandez, H, Perrotin, F, et al.. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol 2010;115:526–34. https://doi.org/10.1097/aog.0b013e3181d066d4.Search in Google Scholar PubMed
28. Schwickert, A, van Beekhuizen, HJ, Bertholdt, C, Fox, KA, Kayem, G, Morel, O, et al.. Association of peripartum management and high maternal blood loss at cesarean delivery for placenta accreta spectrum (PAS): a multinational database study. Acta Obstet Gynecol Scand 2021;100(1 Suppl):29–40. https://doi.org/10.1111/aogs.14103.Search in Google Scholar PubMed
29. Wolf, MF, Maymon, S, Shnaider, O, Singer-Jordan, J, Maymon, R, Bornstein, J, et al.. Two approaches for placenta accreta spectrum: B-lynch suture versus pelvic artery endovascular balloon. J Matern Fetal Neonatal Med 2020;33:2711–7. https://doi.org/10.1080/14767058.2018.1558199.Search in Google Scholar PubMed
30. Bartal, MF, Papanna, R, Zacharias, NM, Soriano-Calderon, N, Limas, M, Blackwell, SC, et al.. Planned versus unplanned delivery for placenta accreta spectrum. Am J Perinatol 2020;39:252–8. https://doi.org/10.1055/s-0040-1714676.Search in Google Scholar PubMed
31. Schröder, L, Pötzsch, B, Rühl, H, Gembruch, U, Merz, WM. Tranexamic acid for hyperfibrinolytic hemorrhage during conservative management of placenta percreta. Obstet Gynecol 2015;126:1012–5. https://doi.org/10.1097/aog.0000000000000915.Search in Google Scholar PubMed
32. Weiniger, CF, Elram, T, Ginosar, Y, Mankuta, D, Weissman, C, Ezra, Y. Anaesthetic management of placenta accreta: use of a pre-operative high and low suspicion classification. Anaesthesia 2005;60:1079–84. https://doi.org/10.1111/j.1365-2044.2005.04369.x.Search in Google Scholar PubMed
33. Taylor, NJ, Russell, R. Anaesthesia for abnormally invasive placenta: a single-institution case series. Int J Obstet Anesth 2017;30:10–5. https://doi.org/10.1016/j.ijoa.2017.01.008.Search in Google Scholar PubMed
34. Bourrellier, L, Bensalem, R, Bersot, Y, Bertrand, A, Duminil, L, Malinovsky, JM, et al.. Disseminated intravascular coagulation syndrome two months after conservative management of placenta accreta. About two patients. Eur J Obstet Gynecol Reprod Biol 2017;215:266–7. https://doi.org/10.1016/j.ejogrb.2017.06.031.Search in Google Scholar PubMed
35. Shamshirsaz, AA, Fox, KA, Erfani, H, Clark, SL, Hui, SK, Shamshirsaz, AA, et al.. Coagulopathy in surgical management of placenta accreta spectrum. Eur J Obstet Gynecol Reprod Biol 2019;237:126–30. https://doi.org/10.1016/j.ejogrb.2019.04.026.Search in Google Scholar PubMed
36. Papillon-Smith, J, Hobson, S, Allen, L, Kingdom, J, Windrim, R, Murji, A. Prophylactic internal iliac artery ligation versus balloon occlusion for placenta accreta spectrum disorders: a retrospective cohort study. Int J Gynaecol Obstet 2020;151:91–6. https://doi.org/10.1002/ijgo.13256.Search in Google Scholar PubMed
37. Saito, K, Mariya, T, Fujibe, Y, Saito, M, Hirokawa, N, Ishioka, S, et al.. Common iliac artery dissection as a complication of common iliac artery balloon occlusion for placenta percreta: a case report. J Obstet Gynaecol Res 2021;47:1172–7. https://doi.org/10.1111/jog.14601.Search in Google Scholar PubMed
38. Zhou, X, Sun, X, Wang, M, Huang, L, Xiong, W. The effectiveness of prophylactic internal iliac artery balloon occlusion in the treatment of patients with pernicious placenta previa coexisting with placenta accreta. J Matern Fetal Neonatal Med 2021;34:93–8. https://doi.org/10.1080/14767058.2019.1599350.Search in Google Scholar PubMed
39. Yamada, T, Hirahata, E, Ihara, N, Nishimura, D, Inoue, K, Kato, J, et al.. Cesarean hysterectomy in a hybrid operating room for placenta percreta: a report of three cases. JA Clin Rep 2019;5:9. https://doi.org/10.1186/s40981-019-0230-5.Search in Google Scholar PubMed PubMed Central
40. Mei, Y, Luo, D, Wei, S, Wang, L, Liao, X, Jing, H, et al.. Comparison of emergency cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters in patients with placenta accreta spectrum. J Matern Fetal Neonatal Med 2020;35:3190–5.10.1080/14767058.2020.1815187Search in Google Scholar PubMed
41. Pinas-Carrillo, A, Bhide, A, Moore, J, Hartopp, R, Belli, AM, Arulkumaran, S, et al.. Outcomes of the first 50 patients with abnormally invasive placenta managed using the “Triple p procedure” conservative surgical approach. Int J Gynaecol Obstet 2020;148:65–71. https://doi.org/10.1002/ijgo.12990.Search in Google Scholar PubMed
42. Li, P, Liu, X, Li, X, Wei, X, Liao, J. Clinical outcomes and anesthetic management of pregnancies with placenta previa and suspicion for placenta accreta undergoing intraoperative abdominal aortic balloon occlusion during cesarean section. BMC Anesthesiol 2020;20:133. https://doi.org/10.1186/s12871-020-01040-8.Search in Google Scholar PubMed PubMed Central
43. Peng, W, Shen, L, Wang, S, Wang, H. Retrospective analysis of 586 cases of placenta previa and accreta. J Obstet Gynaecol 2020;40:609–13. https://doi.org/10.1080/01443615.2019.1634019.Search in Google Scholar PubMed
44. Liu, J, Xu, J, Jiao, D, Duan, X, Han, X. Comparison of the efficacy of prophylactic balloon occlusion of the abdominal aorta at or below the level of the renal artery in women with placenta accreta undergoing cesarean section. J Matern Fetal Neonatal Med 2019;34:1–211. https://doi.org/10.1080/14767058.2019.1667325.Search in Google Scholar PubMed
45. Whittington, JR, Pagan, ME, Nevil, BD, Kalkwarf, KJ, Sharawi, NE, Hughes, DS, et al.. Risk of vascular complications in prophylactic compared to emergent resuscitative endovascular balloon occlusion of the aorta (REBOA) in the management of placenta accreta spectrum. J Matern Fetal Neonatal Med 2022;35:3049–52. https://doi.org/10.1080/14767058.2020.1802717.Search in Google Scholar PubMed
46. Stubbs, MK, Wellbeloved, MA, Vally, JC. The management of patients with placenta percreta: a case series comparing the use of resuscitative endovascular balloon occlusion of the aorta with aortic cross clamp. Indian J Anaesth 2020;64:520–3. https://doi.org/10.4103/ija.ija_121_20.Search in Google Scholar PubMed PubMed Central
47. Tokue, H, Tokue, A, Tsushima, Y, Kameda, T. Safety and efficacy of aortic vs internal iliac balloon occlusion for cesarean delivery in coexisting placenta accreta and placenta previa. Cardiovasc Intervent Radiol 2020;43:1277–84. https://doi.org/10.1007/s00270-020-02548-9.Search in Google Scholar PubMed
48. Cho, SB, Hong, SJ, Lee, S, Won, JH, Choi, HC, Ha, JY, et al.. Preoperative prophylactic balloon-assisted occlusion of the internal iliac arteries in the management of placenta increta/percreta. Medicina 2020;56:368. https://doi.org/10.3390/medicina56080368.Search in Google Scholar PubMed PubMed Central
49. Yuan, Q, Jin, Y, Chen, L, Ling, L, Bai, XM. Prophylactic uterine artery embolization during cesarean delivery for placenta previa complicated by placenta accreta. Int J Gynaecol Obstet 2020;149:43–7. https://doi.org/10.1002/ijgo.13072.Search in Google Scholar PubMed
50. Titapant, V, Tongdee, T, Pooliam, J, Wataganara, T. Retrospective analysis of 113 consecutive cases of placenta accreta spectrum from a single tertiary care center. J Matern Fetal Neonatal Med 2020;33:3324–31. https://doi.org/10.1080/14767058.2018.1530757.Search in Google Scholar PubMed
51. Kim, MJ, Kim, IJ, Kim, S, Park, IY. Postpartum hemorrhage with uterine artery embolization: the risk of complications of uterine artery embolization. Minim Invasive Ther Allied Technol 2020;31:276–83.10.1080/13645706.2020.1789662Search in Google Scholar PubMed
52. Frasca, D. A Cesarean hysterectomy for invading placenta percreta: anesthetic safety considerations-a case report. AANA J 2012;80:373–8.Search in Google Scholar
53. Kamani, AA, Gambling, DR, Christilaw, J, Flanagan, ML. Anaesthetic management of patients with placenta accreta. Can J Anaesth 1987;34:613–7. https://doi.org/10.1007/bf03010522.Search in Google Scholar
54. Desbriere, R, Pascal, A, Katsogiannou, M, Mace, P, Laplane, C, Amar-Millet, A, et al.. Delayed disseminated intravascular coagulation revealed by spontaneous hematomas after conservative treatment of placenta percreta. Eur J Obstet Gynecol Reprod Biol 2018;226:77–8. https://doi.org/10.1016/j.ejogrb.2018.05.020.Search in Google Scholar PubMed
55. Kume, K, Tsutsumi, MY, Soga, T, Sakai, Y, Kambe, N, Kawanishi, R, et al.. A case of placenta percreta with massive hemorrhage during cesarean section. J Med Invest 2014;61:208–12. https://doi.org/10.2152/jmi.61.208.Search in Google Scholar PubMed
56. Binici, O, Büyükfırat, E. Anesthesia for cesarean section in parturients with abnormal placentation: a retrospective study. Cureus 2019;11:e5033. https://doi.org/10.7759/cureus.5033.Search in Google Scholar PubMed PubMed Central
57. Karacaer, F, Biricik, E, Ilgınel, M, Tunay, D, Sucu, M, Ünlügenç, H. Retrospective analysis of eighty-nine caesarean section cases with abnormal placental invasion. Turk J Anaesthesiol Reanim 2019;47:112–9. https://doi.org/10.5152/tjar.2018.31799.Search in Google Scholar PubMed PubMed Central
58. Atallah, D, Zeid, HA, Moubarak, M, Moussa, M, Nassif, N, Jebara, V. “You only live twice”: multidisciplinary management of catastrophic case in placenta accreta spectrum-a case report. BMC Pregnancy Childbirth 2020;20:135. https://doi.org/10.1186/s12884-020-2817-2.Search in Google Scholar PubMed PubMed Central
59. Ma, Y, You, Y, Jiang, X, Lin, X. Use of nitroglycerin for parallel transverse uterine cesarean section in patients with pernicious placenta previa and placenta accrete and predicted difficult airway: a case report and review of literature. Medicine 2020;99:e18943. https://doi.org/10.1097/md.0000000000018943.Search in Google Scholar PubMed PubMed Central
60. Ito, M, Oshita, K, Tanaka, K, Hara, M, Hiraki, T. Massive obstetric hemorrhage during cesarean section in a patient after conception by frozen-thawed embryo transfer: a case report. JA Clin Rep 2020;6:2. https://doi.org/10.1186/s40981-019-0308-0.Search in Google Scholar PubMed PubMed Central
61. Cojocaru, L, Lankford, A, Galey, J, Bharadwaj, S, Kodali, BS, Kennedy, K, et al.. Surgical advances in the management of placenta accreta spectrum: establishing new expectations for operative blood loss. J Matern Fetal Neonatal Med 2020;35:4496–505.10.1080/14767058.2020.1852213Search in Google Scholar PubMed
62. Urfalıoglu, A, Öksüz, G, Bilal, B, Teksen, S, Calışır, F, Boran, ÖF, et al.. Retrospective evaluation of anesthetic management in cesarean sections of pregnant women with placental anomaly. Anesthesiol Res Pract 2020;1358258. https://doi.org/10.1155/2020/1358258.Search in Google Scholar PubMed PubMed Central
63. Bartels, HC, Mulligan, KM, Craven, S, Rogers, AC, Higgins, S, O’Brien, DJ, et al.. Maternal morbidity in placenta accreta spectrum following introduction of a multi-disciplinary service compared to standard care: an Irish perspective. Ir J Med Sci 2021;190:1451–7. https://doi.org/10.1007/s11845-020-02473-3.Search in Google Scholar PubMed
64. Khoiwal, K, Gaurav, A, Kapur, D, Kumari, O, Sharma, P, Bhandari, R, et al.. Placenta percreta – a management dilemma: an institutional experience and review of the literature. J Turk Ger Gynecol Assoc 2020;21:228–35. https://doi.org/10.4274/jtgga.galenos.2020.2020.0106.Search in Google Scholar PubMed PubMed Central
65. Imtiaz, R, Masood, Z, Husain, S, Husain, S, Izhar, R, Hussain, S. A comparison of antenatally and intraoperatively diagnosed cases of placenta accreta spectrum. J Turk Ger Gynecol Assoc 2020;21:84–9. https://doi.org/10.4274/jtgga.galenos.2019.2019.0063.Search in Google Scholar PubMed PubMed Central
66. Herbert, K, Buchbinder, L, Seshachellam, V, Lee, L. Resuscitative endovascular balloon occlusion of the aorta and concomitant tranexamic acid for cesarean hysterectomy complicated by common femoral artery thrombosis: a case report. Cureus 2020;12:e11197. https://doi.org/10.7759/cureus.11197.Search in Google Scholar PubMed PubMed Central
67. Bluth, A, Schindelhauer, A, Nitzsche, K, Wimberger, P, Birdir, C. Placenta accreta spectrum disorders-experience of management in a German tertiary perinatal centre. Arch Gynecol Obstet 2021;303:1451–60. https://doi.org/10.1007/s00404-020-05875-x.Search in Google Scholar PubMed PubMed Central
68. Chen, M, Liu, X, You, Y, Wang, X, Li, T, Luo, H, et al.. Internal iliac artery balloon occlusion for placenta previa and suspected placenta accreta: a randomized controlled trial. Obstet Gynecol 2020;135:1112–9. https://doi.org/10.1097/aog.0000000000003792.Search in Google Scholar PubMed
69. Bergakker, SA. Case report: management of elective cesarean delivery in the presence of placenta previa and placenta accreta. AANA J 2010;78:380–4.Search in Google Scholar
70. Shahin, Y, Pang, CL. Endovascular interventional modalities for haemorrhage control in abnormal placental implantation deliveries: a systematic review and meta-analysis. Eur Radiol 2018;28:2713–26. https://doi.org/10.1007/s00330-017-5222-0.Search in Google Scholar PubMed
71. Makary, M, Chowdary, P, Westgate, JA. Vascular balloon occlusion and planned caesarean hysterectomy for morbidly adherent placenta: a systematic review. Aust N Z J Obstet Gynaecol 2019;59:608–15. https://doi.org/10.1111/ajo.13027.Search in Google Scholar PubMed
72. Labarta, FJR, Recarte, MPP, Luque, AA, Prieto, LJ, Martín, LP, Leyte, MG, et al.. Outcomes of pelvic arterial embolization in the management of postpartum haemorrhage: a case series study and systematic review. Eur J Obstet Gynecol Reprod Biol 2016;206:12–21. https://doi.org/10.1016/j.ejogrb.2016.07.510.Search in Google Scholar PubMed
73. Lee, AY, Ballah, D, Moreno, I, Dong, PR, Cochran, R, Picel, A, et al.. Outcomes of balloon occlusion in the university of California morbidly adherent placenta registry. Am J Obstet Gynecol MFM 2020;2:100065. https://doi.org/10.1016/j.ajogmf.2019.100065.Search in Google Scholar PubMed
74. Meller, CH, Garcia-Monaco, RD, Izbizky, G, Lamm, M, Jaunarena, J, Peralta, O, et al.. Non-conservative management of placenta accreta spectrum in the hybrid operating room: a retrospective cohort study. Cardiovasc Intervent Radiol 2019;42:365–70. https://doi.org/10.1007/s00270-018-2113-y.Search in Google Scholar PubMed
75. Nicholson, PJ, O’Connor, O, Buckley, J, Spence, LD, Greene, RA, Tuite, DJ. Prophylactic placement of internal iliac balloons in patients with abnormal placental implantation: maternal and foetal outcomes. Cardiovasc Intervent Radiol 2018;41:1488–93. https://doi.org/10.1007/s00270-018-1983-3.Search in Google Scholar PubMed
76. Duan, X, Chen, P, Han, X, Wang, Y, Chen, Z, Zhang, X, et al.. Intermittent aortic balloon occlusion combined with cesarean section for the treatment of patients with placenta previa complicated by placenta accreta: a retrospective study. J Obstet Gynaecol Res 2018;44:1752–60. https://doi.org/10.1111/jog.13700.Search in Google Scholar PubMed
77. Ono, Y, Murayama, Y, Era, S, Matsunaga, S, Nagai, T, Osada, H, et al.. Study of the utility and problems of common iliac artery balloon occlusion for placenta previa with accreta. J Obstet Gynaecol Res 2018;44:456–62. https://doi.org/10.1111/jog.13550.Search in Google Scholar PubMed PubMed Central
78. Rosner-Tenerowicz, A, Fuchs, T, Pomorski, M, Sliwa, J, Zimmer-Stelmach, A, Zimmer, M. The clinical evaluation of internal iliac arteries balloon occlusion for placenta accreta spectrum. Ginekol Pol 2021;92:210–5. https://doi.org/10.5603/gp.a2020.0180.Search in Google Scholar
79. Nieto-Calvache, AJ, Hidalgo-Cardona, A, Lopez-Girón, MC, Rodriguez, F, Ordoñez, C, Garcia, AF, et al.. Arterial thrombosis after REBOA use in placenta accreta spectrum: a case series. J Matern Fetal Neonatal Med 2020;35:4031–4.10.1080/14767058.2020.1846178Search in Google Scholar PubMed
80. Nieto-Calvache, AJ, Vergara-Galliadi, LM, Rodríguez, F, Ordoñez, CA, García, AF, López, MC, et al.. A multidisciplinary approach and implementation of a specialized hemorrhage control team improves outcomes for placenta accreta spectrum. J Trauma Acute Care Surg 2021;90:807–16. https://doi.org/10.1097/ta.0000000000003090.Search in Google Scholar PubMed
81. Peng, Y, Jiang, L, Peng, C, Wu, D, Chen, L. The application of prophylactic balloon occlusion of the internal iliac artery for the treatment of placenta accreta spectrum with placenta previa: a retrospective case-control study. BMC Pregnancy Childbirth 2020;20:349. https://doi.org/10.1186/s12884-020-03041-4.Search in Google Scholar PubMed PubMed Central
82. Anwer, M. WHO surgical safety checklist, compliance and its effectiveness: a JPMC audit. Pakistan J Med Sci 1969;32:831–5. https://doi.org/10.12669/pjms.324.9884.Search in Google Scholar PubMed PubMed Central
83. WHO. WHO surgical safety checklist and implementation manual [Online]. Available from: https://www.who.int/patientsafety/safesurgery/ss_checklist/en/.Search in Google Scholar
84. Oszvald, Á, Vatter, H, Byhahn, C, Seifert, V, Güresir, E. “Team time-out” and surgical safety—experiences in 12, 390 neurosurgical patients. Foc 2012;33:E6. https://doi.org/10.3171/2012.8.focus12261.Search in Google Scholar
85. Jauniaux, E, Alfirevic, Z, Bhide, A, Belfort, M, Burton, G, Collins, S, et al.. Placenta praevia and placenta accreta: diagnosis and management: green-top guideline no. 27a. Int J Obstet Gynecol 2019;126:e1–48. https://doi.org/10.1111/1471-0528.15306.Search in Google Scholar PubMed
86. Hobson, SR, Kingdom, JC, Murji, A, Windrim, RC, Carvalho, JCA, Singh, SS, et al.. No. 383-screening, diagnosis, and management of placenta accreta spectrum disorders. J Obstet Gynaecol Can 2019;41:1035–49. https://doi.org/10.1016/j.jogc.2018.12.004.Search in Google Scholar PubMed
87. Markley, JC, Farber, MK, Perlman, NC, Carusi, DA. Neuraxial anesthesia during cesarean delivery for placenta previa with suspected morbidly adherent placenta: a retrospective analysis. Anesth Analg 2018;127:930–8. https://doi.org/10.1213/ane.0000000000003314.Search in Google Scholar
88. Lilker, SJ, Meyer, RA, Downey, KN, Macarthur, AJ. Anesthetic considerations for placenta accreta. Int J Obstet Anesth 2011;20:288–92. https://doi.org/10.1016/j.ijoa.2011.06.001.Search in Google Scholar PubMed
89. Simmons, SW, Taghizadeh, N, Dennis, AT, Hughes, D, Cyna, AM. Combined spinal-epidural versus epidural analgesia in labour. Cochrane Database Syst Rev 2012;10:CD003401. https://doi.wiley.com/10.1002/14651858.CD003401.pub3.10.1002/14651858.CD003401.pub3Search in Google Scholar PubMed PubMed Central
90. Ng, KW, Parsons, J, Cyna, AM, Middleton, P. Spinal versus epidural anaesthesia for caesarean section. Cochrane Database Syst Rev 2004;CD008100. http://doi.wiley.com/10.1002/14651858.CD003765.pub2.10.1002/14651858.CD003765.pub2Search in Google Scholar PubMed PubMed Central
91. Forstot, RM. The etiology and management of inadvertent perioperative hypothermia. J Clin Anesth 1995;7:657–74. https://doi.org/10.1016/0952-8180(95)00099-2.Search in Google Scholar PubMed
92. Grote, R, Wetz, AJ, Bräuer, A, Menzel, M. [Prewarming according to the AWMF S3 guidelines on preventing inadvertant perioperative hypothermia 2014 : retrospective analysis of 7786 patients]. Anaesthesist 2018;67:27–33. https://doi.org/10.1007/s00101-017-0384-3.Search in Google Scholar PubMed
93. Sessler, DI. Perioperative thermoregulation and heat balance. Lancet 2016;387:2655–64. https://doi.org/10.1016/s0140-6736(15)00981-2.Search in Google Scholar
94. Kaufner, L, Niggemann, P, Baum, T, Casu, S, Sehouli, J, Bietenbeck, A, et al.. Impact of brief prewarming on anesthesia-related core-temperature drop, hemodynamics, microperfusion and postoperative ventilation in cytoreductive surgery of ovarian cancer: a randomized trial. BMC Anesthesiol 2019;19:161. https://doi.org/10.1186/s12871-019-0828-1.Search in Google Scholar PubMed PubMed Central
95. Kurz, A, Go, JC, Sessler, DI, Kaer, K, Larson, MD, Bjorksten, AR. Alfentanil slightly increases the sweating threshold and markedly reduces the vasoconstriction and shivering thresholds. Anesthesiology 1995;83:293–9. https://doi.org/10.1097/00000542-199508000-00009.Search in Google Scholar PubMed
96. Michelson, AD, MacGregor, H, Barnard, MR, Kestin, AS, Rohrer, MJ, Valeri, CR. Reversible inhibition of human platelet activation by hypothermia in vivo and in vitro. Thromb Haemostasis 1994;71:633–40. https://doi.org/10.1055/s-0038-1642495.Search in Google Scholar
97. Schmied, H, Kurz, A, Sessler, DI, Kozek, S, Reiter, A. Mild hypothermia increases blood loss and transfusion requirements during total hip arthroplasty. Lancet 1996;347:289–92. https://doi.org/10.1016/s0140-6736(96)90466-3.Search in Google Scholar PubMed
98. National Institute for Health and Care Excellence (UK). Addendum to clinical guideline CG65, inadvertent perioperative hypothermia. London, (UK): National Institute for Health and Care Excellence; 2016. [Online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK550689/.Search in Google Scholar
99. Matsubara, S, Takahashi, H, Takei, Y, Nakamura, H, Yagisawa, T. Prophylactic aortic balloon occlusion for placenta accreta spectrum disorders: occlusion where? Arch Gynecol Obstet 2020;302:1553–4. https://doi.org/10.1007/s00404-020-05434-4.Search in Google Scholar PubMed
100. Muñoz, M, Stensballe, J, Ducloy-Bouthors, AS, Bonnet, MP, De Robertis, E, Fornet, I, et al.. Patient blood management in obstetrics: prevention and treatment of postpartum haemorrhage. A NATA consensus statement. Blood Transfusion 2019;17:112–36. https://doi.org/10.2450/2019.0245-18.Search in Google Scholar PubMed PubMed Central
101. Uchino, H, Tamura, N, Echigoya, R, Ikegami, T, Fukuoka, T. “REBOA” – is it really safe? A case with massive intracranial hemorrhage possibly due to endovascular balloon occlusion of the aorta (REBOA). Am J Case Rep 2016;17:810–3. https://doi.org/10.12659/ajcr.900267.Search in Google Scholar PubMed PubMed Central
102. Abid, M, Neff, LP, Russo, RM, Hoareau, G, Williams, TK, Grayson, JK, et al.. Reperfusion repercussions: a review of the metabolic derangements following resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg 2020;89:S39–44. https://doi.org/10.1097/ta.0000000000002761.Search in Google Scholar PubMed
103. Davidson, AJ, Russo, RM, Ferencz, SAE, Cannon, JW, Rasmussen, TE, Neff, LP, et al.. Incremental balloon deflation following complete resuscitative endovascular balloon occlusion of the aorta results in steep inflection of flow and rapid reperfusion in a large animal model of hemorrhagic shock. J Trauma Acute Care Surg 2017;83:139–43. https://doi.org/10.1097/ta.0000000000001502.Search in Google Scholar
104. American College of Obstetricians and Gynecologists. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists number 76, October 2006: postpartum hemorrhage. Obstet Gynecol 2006;108:1039–47.Search in Google Scholar
105. Mavrides, E, Allard, S, Chandraharan, E, Collins, P, Green, L, Hunt, BJ, et al., Royal College of Obstetricians & Gynaecologists. Prevention and management of postpartum haemorrhage: green-top guideline no. 52. BJOG 2017;124:e106–49.10.1111/1471-0528.14178Search in Google Scholar PubMed
106. Valentin, J, Brent, R, Mettler, F, Wagner, L, Streffer, C, Berry, M, et al., International Commission on Radiological Protection. ICRP Publication 84: Pregnancy and medical radiation. Annals of the ICRP 2000;30:9–19.10.1016/S0146-6453(00)00027-0Search in Google Scholar
107. Hawkins, R, Evans, M, Hammond, S, Hartopp, R, Evans, E. Placenta accreta spectrum disorders – peri-operative management: the role of the anaesthetist. Best Pract Res Clin Obstet Gynaecol 2021;72:38–51. https://doi.org/10.1016/j.bpobgyn.2020.08.003.Search in Google Scholar PubMed
108. Sutton, CD, Carvalho, B. Optimal pain management after cesarean delivery. Anesthesiol Clin 2017;35:107–24. https://doi.org/10.1016/j.anclin.2016.09.010.Search in Google Scholar PubMed
109. Gerbershagen, HJ, Aduckathil, S, van Wijck, AJM, Peelen, LM, Kalkman, CJ, Meissner, W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology 2013;118:934–44. https://doi.org/10.1097/aln.0b013e31828866b3.Search in Google Scholar PubMed
110. Kaufner, L, Heimann, S, Zander, D, Weizsäcker, K, Correns, I, Sander, M, et al.. Neuraxial anesthesia for pain control after cesarean section: a prospective randomized trial comparing three different neuraxial techniques in clinical practice. Minerva Anestesiol 2016;82:514–24.Search in Google Scholar
111. Weibel, S, Neubert, K, Jelting, Y, Meissner, W, Wöckel, A, Roewer, N, et al.. Incidence and severity of chronic pain after caesarean section: a systematic review with meta-analysis. Eur J Anaesthesiol 2016;33:853–65. https://doi.org/10.1097/eja.0000000000000535.Search in Google Scholar
112. Palmer, CM, Nogami, WM, Van Maren, G, Alves, DM. Postcesarean epidural morphine: a dose-response study. Anesth Analg 2000;9:887–91. https://doi.org/10.1213/00000539-200004000-00021.Search in Google Scholar
113. Mitchell, KD, Smith, CT, Mechling, C, Wessel, CB, Orebaugh, S, Lim, G. A review of peripheral nerve blocks for cesarean delivery analgesia. Reg Anesth Pain Med 2019;rapm-2019-100752. https://doi.org/10.1136/rapm-2019-100752.Search in Google Scholar PubMed PubMed Central
114. Champaneria, R, Shah, L, Wilson, MJ, Daniels, JP. Clinical effectiveness of transversus abdominis plane (TAP) blocks for pain relief after caesarean section: a meta-analysis. Int J Obstet Anesth 2016;28:45–60. https://doi.org/10.1016/j.ijoa.2016.07.009.Search in Google Scholar PubMed
115. Blanco, R, Ansari, T, Riad, W, Shetty, N. Quadratus lumborum block versus transversus abdominis plane block for postoperative pain after cesarean delivery: a randomized controlled trial. Reg Anesth Pain Med 2016;41:757–62. https://doi.org/10.1097/aap.0000000000000495.Search in Google Scholar PubMed
116. World Health Organization. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents; 2018. [Online]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK537492/.Search in Google Scholar
117. Zeng, AM, Nami, NF, Wu, CL, Murphy, JD. The analgesic efficacy of nonsteroidal anti-inflammatory agents (NSAIDs) in patients undergoing cesarean deliveries: a meta-analysis. Reg Anesth Pain Med 2016;41:763–72. https://doi.org/10.1097/aap.0000000000000460.Search in Google Scholar PubMed
118. Munishankar, B, Fettes, P, Moore, C, McLeod, GA. A double-blind randomised controlled trial of paracetamol, diclofenac or the combination for pain relief after caesarean section. Int J Obstet Anesth 2008;17:9–14. https://doi.org/10.1016/j.ijoa.2007.06.006.Search in Google Scholar PubMed
119. Bonnal, A, Dehon, A, Nagot, N, Macioce, V, Nogue, E, Morau, E. Patient-controlled oral analgesia versus nurse-controlled parenteral analgesia after caesarean section: a randomised controlled trial. Anaesthesia 2016;71:535–43. https://doi.org/10.1111/anae.13406.Search in Google Scholar PubMed
120. McNicol, ED, Ferguson, MC, Hudcova, J. Patient controlled opioid analgesia versus non-patient controlled opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2015;CD003348. http://doi.wiley.com/10.1002/14651858.CD003348.pub3.10.1002/14651858.CD003348.pub3Search in Google Scholar PubMed PubMed Central
121. Cardoso, MMS, Leite, AO, Santos, EA, Gozzani, JL, Mathias, LAST. Effect of dexamethasone on prevention of postoperative nausea, vomiting and pain after caesarean section: a randomised, placebo-controlled, double-blind trial. Eur J Anaesthesiol 2013;30:102–5. https://doi.org/10.1097/eja.0b013e328356676b.Search in Google Scholar
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/jpm-2022-0232).
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Placenta Accreta Spectrum Part I: anesthesia considerations based on an extended review of the literature
- Placenta Accreta Spectrum Part II: hemostatic considerations based on an extended review of the literature
- Corner of Academy
- The impact of lateral placenta on preeclampsia and small for gestational age neonates: a systematic review and meta-analysis
- Original Articles – Obstetrics
- Fetal intelligent navigation echocardiography (FINE) has superior performance compared to manual navigation of the fetal heart by non-expert sonologists
- Evaluation of fetal middle adrenal artery Doppler and fetal adrenal gland size in pregnancies with fetal growth restriction: a case-control study
- First trimester low maternal serum pregnancy associated plasma protein-A (PAPP-A) as a screening method for adverse pregnancy outcomes
- Time interval to delivery in asymptomatic twin pregnancies with a short cervix at 23–28 weeks’ gestation
- Hepatic arterial buffer response in monochorionic diamniotic pregnancies with twin-to-twin transfusion syndrome
- Healthcare of pregnant women with diabetes during the COVID-19 pandemic: a Southern Brazilian cross-sectional panel data
- Attitudes toward COVID-19 vaccination of pregnant and lactating women in Hungary
- Maternal vitamin D levels correlate with fetal weight and bone metabolism during pregnancy: a materno-neonatal analysis of bone metabolism parameters
- Removal of pregnancy categories and likelihood of prescribing: a randomized trial
- Prenatal prediction of Shone’s complex. The role of the degree of ventricular disproportion and speckle-tracking analysis
- Original Article – Fetus
- The effect of middle cerebral artery peak systolic velocity on prognosis in early and late-onset fetal growth restriction
- Original Articles – Neonates
- Assessment of salivary cortisol concentrations for procedural pain monitoring in newborns
- Impact on neonatal morbidities after a change in policy to administer antenatal corticosteroids to mothers at risk for late preterm delivery
- The Apgar score in clinical research: for what, how and by whom it is used
- Short Communication
- Visitor restriction during the COVID-19 pandemic did not impact rates of Staphylococcus aureus colonization in the NICU patients
- Letter to the Editor
- Knowledge and attitudes of pregnant women on maternal immunization against COVID-19: correspondence
Articles in the same Issue
- Frontmatter
- Reviews
- Placenta Accreta Spectrum Part I: anesthesia considerations based on an extended review of the literature
- Placenta Accreta Spectrum Part II: hemostatic considerations based on an extended review of the literature
- Corner of Academy
- The impact of lateral placenta on preeclampsia and small for gestational age neonates: a systematic review and meta-analysis
- Original Articles – Obstetrics
- Fetal intelligent navigation echocardiography (FINE) has superior performance compared to manual navigation of the fetal heart by non-expert sonologists
- Evaluation of fetal middle adrenal artery Doppler and fetal adrenal gland size in pregnancies with fetal growth restriction: a case-control study
- First trimester low maternal serum pregnancy associated plasma protein-A (PAPP-A) as a screening method for adverse pregnancy outcomes
- Time interval to delivery in asymptomatic twin pregnancies with a short cervix at 23–28 weeks’ gestation
- Hepatic arterial buffer response in monochorionic diamniotic pregnancies with twin-to-twin transfusion syndrome
- Healthcare of pregnant women with diabetes during the COVID-19 pandemic: a Southern Brazilian cross-sectional panel data
- Attitudes toward COVID-19 vaccination of pregnant and lactating women in Hungary
- Maternal vitamin D levels correlate with fetal weight and bone metabolism during pregnancy: a materno-neonatal analysis of bone metabolism parameters
- Removal of pregnancy categories and likelihood of prescribing: a randomized trial
- Prenatal prediction of Shone’s complex. The role of the degree of ventricular disproportion and speckle-tracking analysis
- Original Article – Fetus
- The effect of middle cerebral artery peak systolic velocity on prognosis in early and late-onset fetal growth restriction
- Original Articles – Neonates
- Assessment of salivary cortisol concentrations for procedural pain monitoring in newborns
- Impact on neonatal morbidities after a change in policy to administer antenatal corticosteroids to mothers at risk for late preterm delivery
- The Apgar score in clinical research: for what, how and by whom it is used
- Short Communication
- Visitor restriction during the COVID-19 pandemic did not impact rates of Staphylococcus aureus colonization in the NICU patients
- Letter to the Editor
- Knowledge and attitudes of pregnant women on maternal immunization against COVID-19: correspondence

