A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes
-
Seung Mi Lee
, Joong Shin Park
Abstract
Aim: To describe a new device for the transcervical collection of amniotic fluid (AF) in patients with ruptured membranes, and to compare the concentration of proteins in fluid retrieved by transabdominal amniocentesis and the transcervical AF collector.
Study design: Paired AF samples were collected in patients with preterm prelabor rupture of membranes (PROM) (n=11) by transabdominal amniocentesis and with the transcervical AF collector (Yoon’s AF Collector™). Three proteins known to have high concentrations in AF [α-fetoprotein (AFP), β-human chorionic gonadotrophin (β-hCG), and prolactin] were measured.
Results: (1) There was a significant correlation between the concentrations of analytes in AF obtained by transabdominal amniocentesis and by the transcervical AF collector (r=0.94, P<0.001 for AFP; r=0.96, P<0.001 for β-hCG; r=0.72, P<0.05 for prolactin); (2) Bland-Altman plots showed no evidence of heteroscedasticity between transabdominal or transcervical AF concentrations of these markers.
Conclusions: There was a strong correlation between the concentrations of proteins in AF collected by amniocentesis or with the transcervical device.
Introduction
Rupture of membranes (ROM) occurs spontaneously during the course of labor [1]. Prelabor rupture of membranes (PROM) is defined as chorioamniorrhexis prior to the onset of labor and complicates 10% of all pregnancies [2–20]; in 2% of the cases, it occurs in preterm gestations [1, 21–37]. Preterm PROM accounts for 30–35% of all preterm births [21, 24, 38].
Amniotic fluid analysis is used to assess the likelihood of intra-amniotic infection, intra-amniotic inflammation [32, 39–79], and fetal lung maturity [73, 80–95] in preterm PROM. Amniotic fluid can be retrieved with amniocentesis; however, this procedure is technically demanding in the context of rupture of membranes due to the decreased volume of amniotic fluid frequently observed in these cases [37, 96, 97].
We report herein a novel device designed for transcervical collection of amniotic fluid. The purpose of this article is to describe the device and to report the concentration of proteins in amniotic fluid collected transabdominally, as well as that obtained transcervically with the use of the device. For this, we measured α-fetoprotein (AFP), beta-human chorionic gonadotropin (β-hCG), and prolactin, which are known to be present in high concentrations in amniotic fluid [98–102].
Materials and methods
Study design
Eleven women with singleton pregnancies admitted to the Seoul National University Hospital, Seoul National University Bundang Hospital, or Dongguk University Hospital, Korea, were included in the study. All patients had the diagnosis of preterm PROM (≤35 weeks of gestation). Amniotic fluid was collected using the new medical device described herein. Transabdominal amniocentesis was also performed to examine the presence or absence of intra-amniotic infection and/or inflammation and is part of the standard of practice in our institution.
The retrieval of amniotic fluid was performed after obtaining written informed consent from the patients, and the Institutional Review Boards of the Seoul National University Hospital, Seoul National University Bundang Hospital, and Dongguk University Hospital approved the collection and use of these samples and information for research purposes.
Collection of amniotic fluid
Transcervical amniotic fluid collection was performed with the transcervical amniotic fluid collector (Yoon’s AF Collector™), which was developed and patented by the authors (patent number: Korea 10-1170053-0000). The collector was placed against the cervix during a sterile speculum exam and fixed with a silicon balloon. Amniotic fluid that leaked through the cervix pooled into the collector (Figure 1). Transabdominal amniotic fluid was obtained after transcervical amniotic fluid collection by amniocentesis under ultrasonographic guidance as previously described [78, 103, 104]. Amniotic fluid was centrifuged for 10 min, and stored in polypropylene tubes at –70°C until assayed.
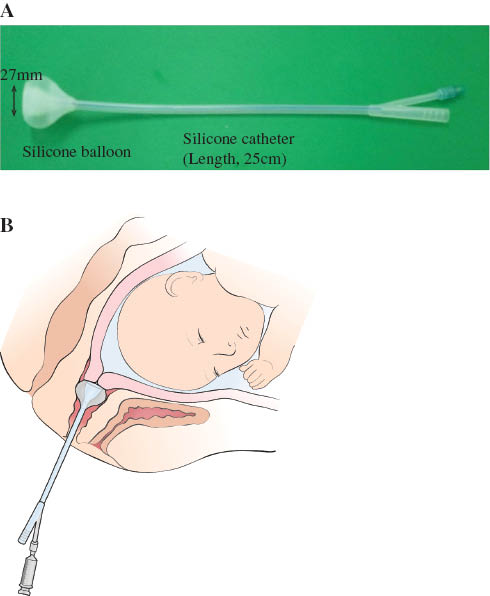
(A) The transcervical amniotic fluid collector (Yoon’s AF Collector™). (B) Schematic drawing of Yoon’s AF Collector™ placed in the vagina.
Amniotic fluid analysis
Amniotic fluid was assayed for AFP, β-hCG, and prolactin by immunoradiometric assays (IRMA) with commercially available kits (AFP: Immunotech, Prague, Czech Republic; β-hCG: Shinjin, Seoul, Korea; prolactin: Shinjin, Seoul, Korea).
Statistical analysis
The concentrations of amniotic fluid proteins in samples retrieved either by transabdominal amniocentesis or using the transcervical amniotic fluid collector were compared using Spearman’s correlation and Passing and Bablok regression analysis. Bland-Altman plots were used to evaluate the differences between protein concentrations obtained from transabdominal amniotic fluid and those retrieved transcervically. We used SPSS version 20.0 for Windows (SPSS Inc., Chicago, IL, USA) and MedCalc version 12.7.1.0 (MedCalc Software, Mariaherke, Belgium). A P-value<0.05 was considered significant.
Results
The clinical characteristics of patients enrolled in the study are summarized in Table 1. None of the patients developed clinical chorioamnionitis.
Clinical characteristics of the study population.
| n=11 | |
|---|---|
| Agea | 33 (29–36) |
| Nulliparity | 6 (55%) |
| Gestational age at amniocentesisa | 30.1 (27.3–34.1) |
| Positive AF culture | 2 (18%) |
| Intra-amniotic infection and/or inflammation | 3 (27%) |
| Gestational age at deliverya | 32.6 (27.4–34.4) |
| Cesarean delivery | 2 (18%) |
| Birthweighta | 1956 (1080–2800) |
| Histologic chorioamnionitis | 6/10 (60%) |
| Funisitis | 3/10 (30%) |
AF=amniotic fluid. aMedian (range).
Figure 2 shows the distribution of protein concentrations in fluid collected either by transabdominal amniocentesis or using the transcervical amniotic fluid collector. The transcervical collector was effective in collecting amniotic fluid from all patients. There was a significant correlation between the concentrations of analytes in transabdominal and transcervical amniotic fluid (Spearman correlation coefficients: r=0.936, P<0.001 for AFP; r=0.964, P<0.001 for β-hCG; r=0.718, P<0.05 for prolactin; Figure 3 and Table 2). Bland-Altman plots showed no evidence of heteroscedasticity between transabdominal and transcervical amniotic fluid concentrations of these markers (Figure 4). Passing-Bablok regression analysis revealed neither constant distance (with intercept including 0 for all of proteins) nor proportional difference (with slope including 1) for all three proteins (Table 2).
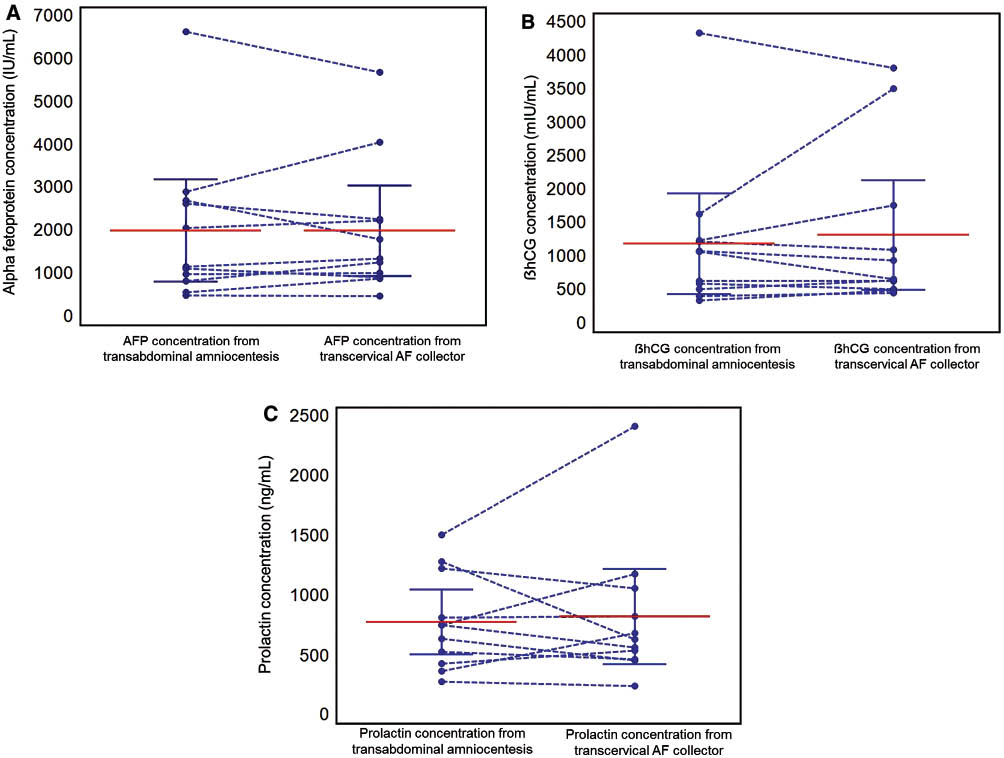
Protein concentrations in transabdominal amniotic fluid and those in transcervical amniotic fluid, with means and 95% confidence interval presented in solid line. (A) α-Fetoprotein (AFP) (IU/mL); (B) β-human chorionic gonadotrophin (β-hCG) (mIU/mL); (C) prolactin (ng/mL).
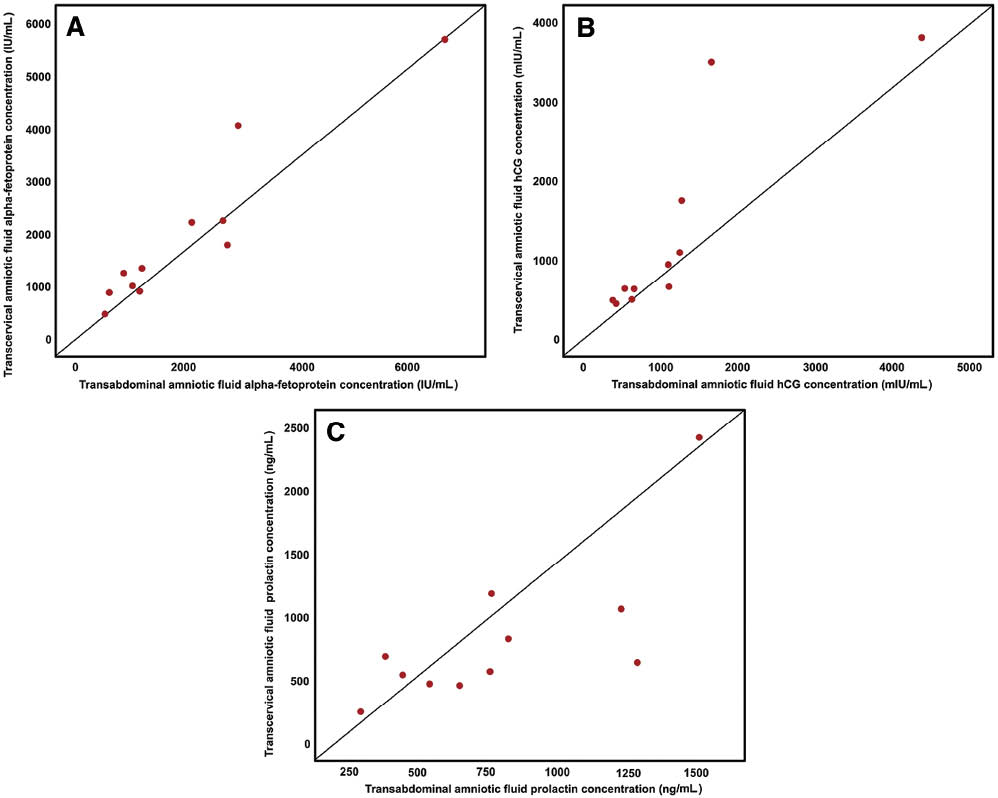
Scatter plot of correlation between protein concentrations in transabdominal amniotic fluid and those in transcervical amniotic fluid. (A) α-Fetoprotein (AFP) (IU/mL); (B) β-human chorionic gonadotrophin (β-hCG) (mIU/mL); (C) prolactin (ng/mL).
The protein concentrations in transabdominal amniotic fluid and those in transcervical amniotic fluid.
| Concentrations [mean (SD)] | α-Fetoprotein (IU/mL) | β-hCG (mIU/mL) | Prolactin (ng/mL) | |||
|---|---|---|---|---|---|---|
| Transabdominal AF | Transcervical AF | Transabdominal AF | Transcervical AF | Transabdominal AF | Transcervical AF | |
| 2006 (1771) | 1997 (1575) | 1190 (1122) | 1322 (1220) | 784 (400) | 829 (592) | |
| Wilcoxon signed rank test (P-value) | 0.93 | 0.82 | 1.00 | |||
| Spearman correlation coefficients (P-value) | 0.94 (P<0.001) | 0.96 (P<0.001) | 0.72 (P<0.05) | |||
| Passing-Bablok regression analysis | ||||||
| Intercept [95% CI] | 176.9 [–415.2 to 629.9] | 75.6 [–989.7 to 260.7] | –48.5 [–989.6 to 372.7] | |||
| Slope [95% CI] | 0.86 [0.62 to 1.46] | 0.89 [0.67 to 2.50] | 1.05 [0.39 to 2.26] | |||
SD=standard deviation, AF=amniotic fluid, AFP=α-fetoprotein, β-hCG=β-human chorionic gonadotropin.
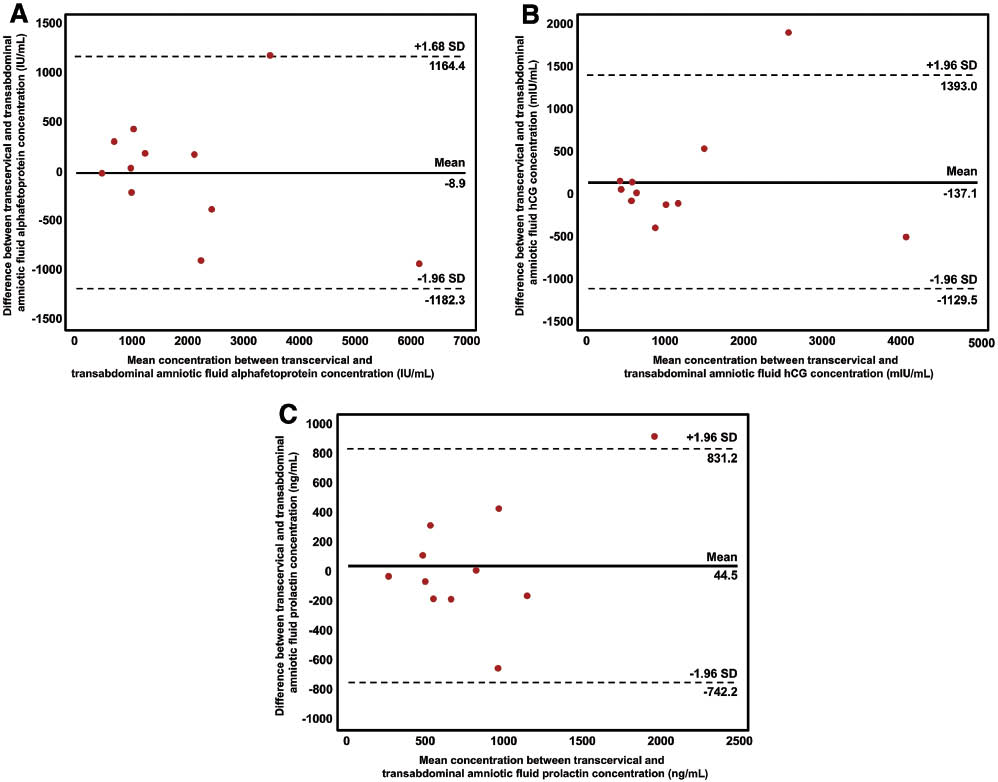
Bland-Altman plots of protein concentrations in transabdominal amniotic fluid and those in transcervical amniotic fluid. (A) α-Fetoprotein (AFP) (IU/mL); (B) β-human chorionic gonadotrophin (β-hCG) (mIU/mL); (C) prolactin (ng/mL).
Discussion
Principal findings of the study
(1) We describe a new device for the transcervical collection of amniotic fluid from patients with ruptured membranes; and (2) a strong and significant relationship was observed between the concentration of proteins in amniotic fluid obtained by the transabdominal and transcervical method.
Amniotic fluid analysis in the assessment of patients with ruptured membranes
Amniotic fluid analysis has been used to study fetal lung maturity [80–82, 84, 85, 89–92, 94, 105], karyotype, intra-amniotic inflammation, and microorganisms (bacteria and viruses) [32, 39–79] in patients with preterm PROM. Fluid obtained from the vaginal pool has been employed to assess fetal lung maturity [73, 80–95, 105]. However, this fluid cannot be used for the assessment of microbial invasion of the amniotic cavity or the assessment of intra-amniotic inflammation, because bacteria are normally present in the vagina, and vaginal fluid contains a high concentration of inflammatory mediators [44, 51, 54, 56, 59–61, 75, 76]. Therefore, we devised a method to obtain amniotic fluid noninvasively, by collecting this biological material directly through the cervix before it reached the vaginal canal. Ultrasound images demonstrate that this fluid is present in the cervix of patients who have ruptured membranes, and can be seen with color Doppler in the endocervical canal [106]. Obtaining amniotic fluid noninvasively can allow assessment of patients with severe oligohydramnios in which amniocentesis is not possible. Moreover, it allows serial evaluation of patients with preterm PROM without repeat amniocentesis.
Collection of amniotic fluid by a transcervical amniotic fluid collector
We obtained amniotic fluid using a transcervical amniotic fluid collector designed for this specific purpose. This device has the following features: (1) it allows collection at the level of the external cervical os, and thereby reduces the likelihood of dilution of amniotic fluid with vaginal discharge; (2) it prevents leakage of amniotic fluid into the vagina, and therefore, fluid can be obtained even in patients with scant vaginal pooling; and (3) placement of the device for a short period of time (60 min) allowed collection of enough fluid for analysis. Patients did not report discomfort during collection of fluid with this device (Figure 1). Importantly, there was an excellent correlation between the concentration of a set of analytes in fluid retrieved by transabdominal amniocentesis and the use of the transcervical amniotic fluid collector, suggesting that this method may be useful in assessing the composition of amniotic fluid in patients with preterm PROM. Future studies are required to determine if this material can be used to assess the likelihood of intra-amniotic inflammation and fetal lung maturity.
This was a feasibility study; therefore, the sample size was small. We measured AFP, prolactin, and hCG, all of which are known to be present in high concentrations in amniotic fluid. It would be important to assess other components of amniotic fluid, including “omics” characterization (proteomics, metabalomics, lipidomics, etc.).
Conclusion
We describe a new device for the collection of amniotic fluid from patients with ruptured membranes. The transcervical amniotic fluid collector might be useful in the evaluation of amniotic fluid in patients with ruptured membranes.
Acknowledgments
The participation of Roberto Romero in this research is funded by the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and, in part, by NICHD Contract No. HHSN275201300006C and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI12C0768). All patients were recruited in South Korea under IRB approved protocols, and assays were performed at the Seoul National University Hospital, Seoul National University Bundang Hospital or Dongguk University Hospital.
References
[1] Romero R, Yeo L, Gotsch F, Soto E, Hassan S, Kusanovic JP, et al. Prelabor rupture of the membranes. In: Winn HN, Chervanak FA, Romero R, editors. Clinical maternal-fetal medicine online. 2nd ed. London: Informa Healthcare; 2011, pp. 1–24.10.1201/9781003222590-2Search in Google Scholar
[2] Premature rupture of the membranes. Br Med J. 1979;1:1165–6.10.1136/bmj.1.6172.1165Search in Google Scholar
[3] Chan BC, Leung WC, Lao TT. Prelabor rupture of membranes at term requiring labor induction – a feature of occult fetal cephalopelvic disproportion? J Perinat Med. 2009;37:118–23.10.1515/JPM.2009.011Search in Google Scholar
[4] Christensen KK, Christensen P, Ingemarsson I, Mardh PA, Nordenfelt E, Ripa T, et al. A study of complications in preterm deliveries after prolonged premature rupture of the membranes. Obstet Gynecol. 1976;48:670–7.Search in Google Scholar
[5] Daikoku NH, Kaltreider DF, Khouzami VA, Spence M, Johnson JW. Premature rupture of membranes and spontaneous preterm labor: maternal endometritis risks. Obstet Gynecol. 1982;59: 13–20.Search in Google Scholar
[6] Eschenbach D. Reply to “Ismail AQT, Lahiri S. Management of prelabor rupture of membranes (PROM) at term”. J Perinat Med. 2013;41:653–5.10.1515/jpm-2013-0167Search in Google Scholar
[7] Fayez JA, Hasan AA, Jonas HS, Miller GL. Management of premature rupture of the membranes. Obstet Gynecol. 1978;52:17–21.Search in Google Scholar
[8] Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstet Gynecol. 1982;60:671–9.Search in Google Scholar
[9] Grunebaum A. Reply to “Management of prelabour rupture of membranes (PROM) at term”. J Perinat Med. 2013;41:651–2.10.1515/jpm-2013-0127Search in Google Scholar
[10] Gunn GC, Mishell DR Jr., Morton DG. Premature rupture of the fetal membranes. A review. Am J Obstet Gynecol. 1970;106:469–83.10.1016/0002-9378(70)90378-9Search in Google Scholar
[11] Ismail AQ, Lahiri S. Management of prelabour rupture of membranes (PROM) at term. J Perinat Med. 2013;41:647–9.10.1515/jpm-2013-0078Search in Google Scholar
[12] Ladfors L, Mattsson LA, Eriksson M, Milsom I. Prevalence and risk factors for prelabor rupture of the membranes (PROM) at or near-term in an urban Swedish population. J Perinat Med. 2000;28:491–6.10.1515/JPM.2000.066Search in Google Scholar
[13] Lebherz TB, Hellman LP, Madding R, Anctil A, Arje SL. Double-blind study of premature rupture of the membranes. A report of 1,896 cases. Am J Obstet Gynecol. 1963;87:218–25.10.1016/0002-9378(63)90502-7Search in Google Scholar
[14] Parry S, Strauss JF 3rd. Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–70.10.1056/NEJM199803053381006Search in Google Scholar PubMed
[15] Pintucci A, Meregalli V, Colombo P, Fiorilli A. Premature rupture of membranes at term in low risk women: how long should we wait in the “latent phase”? J Perinat Med. 2014;42:189–96.10.1515/jpm-2013-0017Search in Google Scholar PubMed
[16] Ramsauer B, Vidaeff AC, Hosli I, Park JS, Strauss A, Khodjaeva Z, et al. The diagnosis of rupture of fetal membranes (ROM): a meta-analysis. J Perinat Med. 2013;41:233–40.10.1515/jpm-2012-0247Search in Google Scholar PubMed
[17] Sacks M, Baker TH. Spontaneous premature rupture of the membranes. A prospective study. Am J Obstet Gynecol. 1967;97:888–93.10.1016/0002-9378(67)90512-1Search in Google Scholar
[18] Santolaya-Forgas J, Romero R, Espinoza J, Erez O, Friel L, Kusanovic JP, et al. Prelabour rupture of the membranes. In: Reece EA, Hobbins JC, editors. Clinical obstetrics: the fetus and mothers. 3rd ed. Malden, MA: Blackwell; 2008, pp. 1130–88.10.1002/9780470753293.ch63Search in Google Scholar
[19] Tagore S, Kwek K. Comparative analysis of insulin-like growth factor binding protein-1 (IGFBP-1), placental alpha-microglobulin-1 (PAMG-1) and nitrazine test to diagnose premature rupture of membranes in pregnancy. J Perinat Med. 2010;38:609–12.10.1515/jpm.2010.099Search in Google Scholar
[20] Wang T, Zhou R, Xiong W, Wang Y, Zhu C, Song C, et al. Clinical evaluation of soluble intercellular adhesion molecule-1 and insulin like growth factor-binding protein-1-based rapid immunoassays for the diagnosis of prelabor rupture of membranes. J Perinat Med. 2013;41:181–5.10.1515/jpm-2012-0094Search in Google Scholar
[21] August Fuhr N, Becker C, van Baalen A, Bauer K, Hopp H. Antibiotic therapy for preterm premature rupture of membranes – results of a multicenter study. J Perinat Med. 2006;34:203–6.10.1515/JPM.2006.035Search in Google Scholar
[22] Bujold E, Morency AM, Pasquier JC. Antibiotic therapy for preterm premature rupture of membranes. J Perinat Med. 2006;34:503; author reply 4.10.1515/JPM.2006.098Search in Google Scholar
[23] Dudenhausen JW. Primary prevention of preterm birth. J Perinat Med. 2014;42:431–3.10.1515/jpm-2014-0036Search in Google Scholar
[24] Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84.10.1016/S0140-6736(08)60074-4Search in Google Scholar
[25] Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infect Dis Clin North Am. 1997;11:135–76.10.1016/S0891-5520(05)70347-0Search in Google Scholar
[26] Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371:164–75.10.1016/S0140-6736(08)60108-7Search in Google Scholar
[27] Lee T, Silver H. Etiology and epidemiology of preterm premature rupture of the membranes. Clin Perinatol. 2001;28:721–34.10.1016/S0095-5108(03)00073-3Search in Google Scholar
[28] Maxwell GL. Preterm premature rupture of membranes. Obstet Gynecol Surv. 1993;48:576–83.10.1097/00006254-199308000-00026Search in Google Scholar
[29] Mercer B. Antibiotics in the management of PROM and preterm labor. Obstet Gynecol Clin North Am. 2012;39:65–76.10.1016/j.ogc.2011.12.007Search in Google Scholar
[30] Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am. 2005;32:411–28.10.1016/j.ogc.2005.03.003Search in Google Scholar
[31] Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5.10.1126/science.1251816Search in Google Scholar
[32] Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159:661–6.10.1016/S0002-9378(88)80030-9Search in Google Scholar
[33] Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. Br J Obstet Gynecol. 2006;113(Suppl 3):17–42.10.1111/j.1471-0528.2006.01120.xSearch in Google Scholar PubMed PubMed Central
[34] Singh K, Mercer B. Antibiotics after preterm premature rupture of the membranes. Clin Obstet Gynecol. 2011;54:344–50.10.1097/GRF.0b013e318217ec5dSearch in Google Scholar PubMed
[35] Waters TP, Mercer BM. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am J Obstet Gynecol. 2009;201:230–40.10.1016/j.ajog.2009.06.049Search in Google Scholar PubMed
[36] Waters TP, Mercer B. Preterm PROM: prediction, prevention, principles. Clin Obstet Gynecol. 2011;54:307–12.10.1097/GRF.0b013e318217d4d3Search in Google Scholar PubMed
[37] Weissmann-Brenner A, O’Reilly-Green C, Ferber A, Divon MY. Values of amniotic fluid index in cases of preterm premature rupture of membranes. J Perinat Med. 2009;37:232–5.10.1515/JPM.2009.078Search in Google Scholar
[38] Steer P. The epidemiology of preterm labor – a global perspective. J Perinat Med. 2005;33:273–6.10.1515/JPM.2005.053Search in Google Scholar
[39] Agrawal V, Hirsch E. Intrauterine infection and preterm labor. Semin Fetal Neonatal Med. 2012;17:12–9.10.1016/j.siny.2011.09.001Search in Google Scholar
[40] Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Curr Opin Obstet Gynecol. 1999;11:541–7.10.1097/00001703-199912000-00001Search in Google Scholar
[41] Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66:316–21.Search in Google Scholar
[42] Cobo T, Palacio M, Martinez-Terron M, Navarro-Sastre A, Bosch J, Filella X, et al. Clinical and inflammatory markers in amniotic fluid as predictors of adverse outcomes in preterm premature rupture of membranes. Am J Obstet Gynecol. 2011;205:126 e1–8.10.1016/j.ajog.2011.03.050Search in Google Scholar
[43] Cotton DB, Hill LM, Strassner HT, Platt LD, Ledger WJ. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol. 1984;63:38–43.Search in Google Scholar
[44] Di Quinzio MK, Oliva K, Holdsworth SJ, Ayhan M, Walker SP, Rice GE, et al. Proteomic analysis and characterisation of human cervico-vaginal fluid proteins. Aust N Z J Obstet Gynaecol. 2007;47:9–15.10.1111/j.1479-828X.2006.00671.xSearch in Google Scholar
[45] Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31:331–6.10.1111/j.1479-828X.1991.tb02814.xSearch in Google Scholar
[46] Font GE, Gauthier DW, Meyer WJ, Myles TD, Janda W, Bieniarz A. Catalase activity as a predictor of amniotic fluid culture results in preterm labor or premature rupture of membranes. Obstet Gynecol. 1995;85:656–8.10.1016/0029-7844(95)00026-NSearch in Google Scholar
[47] Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol. 1982;59:539–45.Search in Google Scholar
[48] Hatzidaki E, Gourgiotis D, Manoura A, Korakaki E, Bossios A, Galanakis E, et al. Interleukin-6 in preterm premature rupture of membranes as an indicator of neonatal outcome. Acta Obstet Gynecol Scand. 2005;84:632–8.10.1111/j.0001-6349.2005.00747.xSearch in Google Scholar PubMed
[49] Horvath B, Lakatos F, Toth C, Bodecs T, Bodis J. Silent chorioamnionitis and associated pregnancy outcomes: a review of clinical data gathered over a 16-year period. J Perinat Med. 2014;42:441–7.10.1515/jpm-2013-0186Search in Google Scholar PubMed
[50] Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325 e1–10.10.1016/j.ajog.2013.10.882Search in Google Scholar PubMed
[51] Kacerovsky M, Musilova I, Jacobsson B, Drahosova M, Hornychova H, Rezac A, et al. Cervical and vaginal fluid soluble Toll-like receptor 2 in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014; in press.10.3109/14767058.2014.944859Search in Google Scholar PubMed
[52] Kacerovsky M, Drahosova M, Hornychova H, Pliskova L, Bolehovska R, Forstl M, et al. Value of amniotic fluid interleukin-8 for the prediction of histological chorioamnionitis in preterm premature rupture of membranes. Neuro Endocrinol Lett. 2009;30:733–8.Search in Google Scholar
[53] Kacerovsky M, Musilova I, Khatibi A, Skogstrand K, Hougaard DM, Tambor V, et al. Intraamniotic inflammatory response to bacteria: analysis of multiple amniotic fluid proteins in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2012;25:2014–9.10.3109/14767058.2012.671873Search in Google Scholar PubMed
[54] Kacerovsky M, Musilova I, Jacobsson B, Drahosova M, Hornychova H, Janku P, et al. Vaginal fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor membrane ruptures. J Matern Fetal Neonatal Med. 2014; in press.10.3109/14767058.2014.917625Search in Google Scholar PubMed
[55] Kim KW, Romero R, Park HS, Park CW, Shim SS, Jun JK, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol. 2007;197:292 e1–5.10.1016/j.ajog.2007.06.040Search in Google Scholar PubMed
[56] Klein LL, Jonscher KR, Heerwagen MJ, Gibbs RS, McManaman JL. Shotgun proteomic analysis of vaginal fluid from women in late pregnancy. Reprod Sci. 2008;15:263–73.10.1177/1933719107311189Search in Google Scholar PubMed
[57] Korzeniewski SJ, Romero R, Cortez J, Pappas A, Schwartz AG, Kim CJ, et al. A ‘multi-hit’ model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J Perinat Med. 2014;42:731–43.10.1515/jpm-2014-0250Search in Google Scholar PubMed PubMed Central
[58] Lee SE, Romero R, Lee SM, Yoon BH. Amniotic fluid volume in intra-amniotic inflammation with and without culture-proven amniotic fluid infection in preterm premature rupture of membranes. J Perinat Med. 2010;38:39–44.10.1515/jpm.2009.123Search in Google Scholar PubMed PubMed Central
[59] Liong S, Di Quinzio MK, Heng YJ, Fleming G, Permezel M, Rice GE, et al. Proteomic analysis of human cervicovaginal fluid collected before preterm premature rupture of the fetal membranes. Reproduction. 2013;145:137–47.10.1530/REP-12-0264Search in Google Scholar PubMed
[60] Lo JO, Reddy AP, Wilmarth PA, Roberts VH, Kinhnarath A, Snyder J, et al. Proteomic analysis of cervical vaginal fluid proteins among women in recurrent preterm labor. J Matern Fetal Neonatal Med. 2014;27:1183–8.10.3109/14767058.2013.852172Search in Google Scholar PubMed
[61] Lucovnik M, Kornhauser-Cerar L, Premru-Srsen T, Gmeiner-Stopar T, Derganc M. Neutrophil defensins but not interleukin-6 in vaginal fluid after preterm premature rupture of membranes predict fetal/neonatal inflammation and infant neurological impairment. Acta Obstet Gynecol Scand. 2011;90:908–16.10.1111/j.1600-0412.2011.01177.xSearch in Google Scholar PubMed
[62] Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–16.10.1515/JPM.2001.044Search in Google Scholar PubMed
[63] Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–96.10.1515/JPM.2008.084Search in Google Scholar PubMed PubMed Central
[64] Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med. 2004;32:391–9.10.1515/JPM.2004.134Search in Google Scholar PubMed
[65] Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203:211e1–8.10.1016/j.ajog.2010.03.035Search in Google Scholar PubMed PubMed Central
[66] Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008;36:497–502.10.1515/JPM.2008.079Search in Google Scholar PubMed PubMed Central
[67] Park KH, Lee SY, Kim SN, Jeong EH, Oh KJ, Ryu A. Prediction of imminent preterm delivery in women with preterm premature rupture of membranes. J Perinat Med. 2012;40:151–7.10.1515/jpm.2011.124Search in Google Scholar
[68] Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, et al. Matrix metalloproteinase 3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31:12–22.10.1515/JPM.2003.002Search in Google Scholar PubMed
[69] Romero R, Espinoza J, Hassan S, Gotsch F, Kusanovic JP, Avila C, et al. Soluble receptor for advanced glycation end products (sRAGE) and endogenous secretory RAGE (esRAGE) in amniotic fluid: modulation by infection and inflammation. J Perinat Med. 2008;36:388–98.10.1515/JPM.2008.076Search in Google Scholar PubMed PubMed Central
[70] Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med. 2011;24:1444–55.10.3109/14767058.2011.591460Search in Google Scholar PubMed PubMed Central
[71] Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51.10.1016/0002-9378(93)90014-ASearch in Google Scholar
[72] Romero R, Miranda J, Chaemsaithong P, Kusanovic JP, Chaiwaropongsa T, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2014; in press.10.3109/14767058.2014.958463Search in Google Scholar
[73] Salim R, Zafran N, Nachum Z, Garmi G, Shalev E. Predicting lung maturity in preterm rupture of membranes via lamellar bodies count from a vaginal pool: a cohort study. Reprod Biol Endocrinol. 2009;7:112.10.1186/1477-7827-7-112Search in Google Scholar
[74] Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199:375e1–5.10.1016/j.ajog.2008.06.040Search in Google Scholar
[75] Soydinc HE, Sak ME, Evliyaoglu O, Evsen MS, Turgut A, Ozler A, et al. Prolidase, matrix metalloproteinases 1 and 13 activity, oxidative-antioxidative status as a marker of preterm premature rupture of membranes and chorioamnionitis in maternal vaginal washing fluids. Int J Med Sci. 2013;10: 1344–51.10.7150/ijms.4802Search in Google Scholar
[76] Taylor BD, Holzman CB, Fichorova RN, Tian Y, Jones NM, Fu W, et al. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum Reprod. 2013;28:942–52.10.1093/humrep/det019Search in Google Scholar
[77] Veleminsky M Jr., Stransky P, Veleminsky M Sr., Tosner J. Relationship of IL-6, IL-8, TNF and sICAM-1 levels to PROM, pPROM, and the risk of early-onset neonatal sepsis. Neuro Endocrinol Lett. 2008;29:303–11.Search in Google Scholar
[78] Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstet Gynecol. 1996;88:1034–40.10.1016/S0029-7844(96)00339-0Search in Google Scholar
[79] Zlatnik FJ, Cruikshank DP, Petzold CR, Galask RP. Amniocentesis in the identification of inapparent infection in preterm patients with premature rupture of the membranes. J Reprod Med. 1984;29:656–60.Search in Google Scholar
[80] Brame RG, MacKenna J. Vaginal pool phospholipids in the management of premature rupture of membranes. Am J Obstet Gynecol. 1983;145:992–1000.10.1016/0002-9378(83)90854-2Search in Google Scholar
[81] Dombroski RA, MacKenna J, Brame RG. Comparison of amniotic fluid lung maturity profiles in paired vaginal and amniocentesis specimens. Am J Obstet Gynecol. 1981;140:461–4.10.1016/0002-9378(81)90046-6Search in Google Scholar
[82] Estol PC, Poseiro JJ, Schwarcz R. Phosphatidylglycerol determination in the amniotic fluid from a PAD placed over the vulva: a method for diagnosis of fetal lung maturity in cases of premature ruptured membranes. J Perinat Med. 1992;20:65–71.10.1515/jpme.1992.20.1.65Search in Google Scholar PubMed
[83] Gleaton KD, White JC, Koklanaris N. A novel method for collecting vaginal pool for fetal lung maturity studies. Am J Obstet Gynecol. 2009;201:408 e1–4.10.1016/j.ajog.2009.06.070Search in Google Scholar
[84] Gluck L, Kulovich MV, Borer RC Jr., Keidel WN. The interpretation and significance of the lecithin-sphingomyelin ratio in amniotic fluid. Am J Obstet Gynecol. 1974;120:142–55.10.1016/0002-9378(74)90194-XSearch in Google Scholar
[85] Goldstein AS, Mangurten HH, Libretti JV, Berman AM. Lecithin/sphingomyelin ratio in amniotic fluid obtained vaginally. Am J Obstet Gynecol. 1980;138:232–3.10.1016/0002-9378(80)90044-7Search in Google Scholar
[86] How HY, Cook CR, Cook VD, Ralston KK, Greenwell ER, Goldsmith LJ, et al. The pattern of change in the lecithin/sphingomyelin ratio in patients with preterm premature rupture of membranes between 24 and 34 weeks’ gestation. J Perinatol. 2002;22:21–5.10.1038/sj.jp.7210579Search in Google Scholar
[87] Kacerovsky M, Pliskova L, Bolehovska R, Skogstrand K, Hougaard DM, Tsiartas P, et al. The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am J Obstet Gynecol. 2012;206:342e1–8.10.1016/j.ajog.2012.01.004Search in Google Scholar
[88] Kacerovsky M, Musilova I, Hornychova H, Kutova R, Pliskova L, Kostal M, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2014;211:385e1–9.10.1016/j.ajog.2014.03.069Search in Google Scholar
[89] Lewis DF, Towers CV, Major CA, Asrat T, Nageotte MP, Freeman RK, et al. Use of amniostat-FLM in detecting the presence of phosphatidylglycerol in vaginal pool samples in preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:573–6.10.1016/0002-9378(93)90624-RSearch in Google Scholar
[90] Schumacher RE, Parisi VM, Steady HM, Tsao FH. Bacteria causing false positive test for phosphatidylglycerol in amniotic fluid. Am J Obstet Gynecol. 1985;151:1067–8.10.1016/0002-9378(85)90382-5Search in Google Scholar
[91] Shaver DC, Spinnato JA, Whybrew D, Williams WK, Anderson GD. Comparison of phospholipids in vaginal and amniocentesis specimens of patients with premature rupture of membranes. Am J Obstet Gynecol. 1987;156:454–7.10.1016/0002-9378(87)90307-3Search in Google Scholar
[92] Spinnato JA, Shaver DC, Bray EM, Lipshitz J. Preterm premature rupture of the membranes with fetal pulmonary maturity present: a prospective study. Obstet Gynecol. 1987;69:196–201.10.1016/0020-7292(87)90096-8Search in Google Scholar
[93] Spinnato JA 2nd. Maturity testing with preterm premature rupture of the membranes. Clin Perinatol. 2001;28:819–36, vii.10.1016/S0095-5108(03)00080-0Search in Google Scholar
[94] Stedman CM, Crawford S, Staten E, Cherny WB. Management of preterm premature rupture of membranes: assessing amniotic fluid in the vagina for phosphatidylglycerol. Am J Obstet Gynecol. 1981;140:34–8.10.1016/0002-9378(81)90254-4Search in Google Scholar
[95] Tran SH, Cheng YW, Kaimal AJ, Caughey AB. Length of rupture of membranes in the setting of premature rupture of membranes at term and infectious maternal morbidity. Am J Obstet Gynecol. 2008;198:700e1–5.10.1016/j.ajog.2008.03.031Search in Google Scholar
[96] Beazley D, Lewis R. The evaluation of infection and pulmonary maturity in women with premature rupture of the membranes. Semin Perinatol. 1996;20:409–17.10.1016/S0146-0005(96)80008-5Search in Google Scholar
[97] Piazze J, Anceschi MM, Cerekja A, Brunelli R, Meloni P, Marzano S, et al. Validity of amniotic fluid index in preterm rupture of membranes. J Perinat Med. 2007;35:394–8.10.1515/JPM.2007.077Search in Google Scholar
[98] Esim E, Turan C, Unal O, Dansuk R, Cengizglu B. Diagnosis of premature rupture of membranes by identification of beta-HCG in vaginal washing fluid. Eur J Obstet Gynecol Reprod Biol. 2003;107:37–40.10.1016/S0301-2115(02)00277-4Search in Google Scholar
[99] Kishida T, Yamada H, Negishi H, Sagawa T, Makinoda S, Fujimoto S. Diagnosis of premature rupture of the membranes in preterm patients, using an improved AFP kit: comparison with ROM-check and/or nitrazine test. Eur J Obstet Gynecol Reprod Biol. 1996;69:77–82.10.1016/0301-2115(95)02519-7Search in Google Scholar
[100] Koninckx PR, Trappeniers H, Van Assche FA. Prolactin concentration in vaginal fluid: a new method for diagnosing ruptured membranes. Br J Obstet Gynaecol. 1981;88:607–10.10.1111/j.1471-0528.1981.tb01216.xSearch in Google Scholar PubMed
[101] Ni CY, Jia WX, Yi WM, Feng LH, Yu LZ. Practicability of using vaginal fluid markers in detecting premature rupture of membranes. Ann Clin Biochem. 2003;40:542–5.10.1258/000456303322326461Search in Google Scholar PubMed
[102] Shahin M, Raslan H. Comparative study of three amniotic fluid markers in premature rupture of membranes: prolactin, beta subunit of human chorionic gonadotropin, and alpha-fetoprotein. Gynecol Obstet Invest. 2007;63:195–9.10.1159/000097844Search in Google Scholar PubMed
[103] Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26.10.1016/S0002-9378(97)70432-0Search in Google Scholar
[104] Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6.10.1067/mob.2001.117680Search in Google Scholar
[105] Golde SH. Use of obstetric perineal pads in collection of amniotic fluid in patients with rupture of the membranes. Am J Obstet Gynecol. 1983;146:710–2.10.1016/0002-9378(83)91017-7Search in Google Scholar
[106] Cover image from Am J Obstet Gynecol. 2014;210(4).Search in Google Scholar
The authors stated that there are no conflicts of interest regarding the publication of this article.
Article note
The device described in this article was patented by the Seoul National University in Seoul, Korea. Dr. BH Yoon, Dr. JS Park, and Dr. SM Lee are inventors.
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- Editorial
- Erich Saling – The Father of Prenatal and Perinatal Medicine—Dedication to his 90th birthday
- Original articles - Obstetrics
- A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes
- Advanced cervical dilatation and spontaneous preterm labor: a comparison between twin and singleton gestations
- Comparison of a novel test for placental alpha microglobulin-1 with fetal fibronectin and cervical length measurement for the prediction of imminent spontaneous preterm delivery in patients with threatened preterm labor
- Does recent sexual intercourse during pregnancy affect the results of the fetal fibronectin rapid test? A comparative prospective study
- Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes
- Effect of blood on ROM diagnosis accuracy of PAMG-1 and IGFBP-1 detecting rapid tests
- Single versus combination tocolytic regimen in the prevention of preterm births in women: a prospective cohort study
- Recommendations of activity restriction in high-risk pregnancy scenarios: a Danish national survey
- Is pharmacologic research on pregnant women with psychoses ethically permissible?
- Women’s knowledge and attitude towards pregnancy in a high-income developing country
- Impact of maternal body mass index on the cesarean delivery rate in Germany from 1990 to 2012
- Justified skepticism about Apgar scoring in out-of-hospital birth settings
- The effect of the use of oxytocin on blood loss during different postpartum periods
- Original articles - Fetus
- The T/QRS ratio values in pregnancies complicated by threatened preterm labour treated with intravenous infusions of fenoterol
- Cardiotocography patterns and risk of intrapartum fetal acidemia
- Combined spinal epidural analgesia for labor using sufentanil epidurally versus intrathecally: a retrospective study on the influence on fetal heart trace
- Original articles - Newborn
- Predicting fetal growth deviation in parous women: combining the birth weight of the previous pregnancy and third trimester ultrasound scan
- Letter to the Editor
- A cerclage is not a modified total cervical occlusion!
- Letter Reply
- Reply to: a cerclage is not a modified Total Cervical Occlusion!
- Congress Calendar
- Congress Calendar
Articles in the same Issue
- Frontmatter
- Editorial
- Erich Saling – The Father of Prenatal and Perinatal Medicine—Dedication to his 90th birthday
- Original articles - Obstetrics
- A transcervical amniotic fluid collector: a new medical device for the assessment of amniotic fluid in patients with ruptured membranes
- Advanced cervical dilatation and spontaneous preterm labor: a comparison between twin and singleton gestations
- Comparison of a novel test for placental alpha microglobulin-1 with fetal fibronectin and cervical length measurement for the prediction of imminent spontaneous preterm delivery in patients with threatened preterm labor
- Does recent sexual intercourse during pregnancy affect the results of the fetal fibronectin rapid test? A comparative prospective study
- Usefulness of maternal serum C-reactive protein with vaginal Ureaplasma urealyticum as a marker for prediction of imminent preterm delivery and chorioamnionitis in patients with preterm labor or preterm premature rupture of membranes
- Effect of blood on ROM diagnosis accuracy of PAMG-1 and IGFBP-1 detecting rapid tests
- Single versus combination tocolytic regimen in the prevention of preterm births in women: a prospective cohort study
- Recommendations of activity restriction in high-risk pregnancy scenarios: a Danish national survey
- Is pharmacologic research on pregnant women with psychoses ethically permissible?
- Women’s knowledge and attitude towards pregnancy in a high-income developing country
- Impact of maternal body mass index on the cesarean delivery rate in Germany from 1990 to 2012
- Justified skepticism about Apgar scoring in out-of-hospital birth settings
- The effect of the use of oxytocin on blood loss during different postpartum periods
- Original articles - Fetus
- The T/QRS ratio values in pregnancies complicated by threatened preterm labour treated with intravenous infusions of fenoterol
- Cardiotocography patterns and risk of intrapartum fetal acidemia
- Combined spinal epidural analgesia for labor using sufentanil epidurally versus intrathecally: a retrospective study on the influence on fetal heart trace
- Original articles - Newborn
- Predicting fetal growth deviation in parous women: combining the birth weight of the previous pregnancy and third trimester ultrasound scan
- Letter to the Editor
- A cerclage is not a modified total cervical occlusion!
- Letter Reply
- Reply to: a cerclage is not a modified Total Cervical Occlusion!
- Congress Calendar
- Congress Calendar

