Abstract
The sphenopalatine (pterygopalatine) ganglion (SPG) is the most superficial ganglia to manipulate from the oral cavity. It has parasympathetic and sensory fibers directly affecting the paranasal sinuses as well as the palatine, nasal, pharyngeal, and lacrimal glands. The SPG can be manipulated intraorally by students and physicians utilizing osteopathic manipulative treatment (OMT) to relieve congestion associated with sinusitis, allergies, headaches, and upper respiratory infections. Within osteopathic medical education programs, students have anecdotally had difficulty identifying this ganglion due to its deep anatomic location and lack of direct visualization. In this article, we discuss that cadaveric dissection with a superficial to deep approach to the SPG has the ability to allow medical students and physicians to better understand the three-dimensional location and osteopathic clinical relevance of this ganglion.
The sphenopalatine (pterygopalatine) ganglion (SPG) is the most superficial ganglia to manipulate from the oral cavity utilizing osteopathic manipulative treatment (OMT). It has parasympathetic and sensory fibers directly affecting the paranasal sinuses as well as the palatine, nasal, pharyngeal, and lacrimal glands [1]. Parasympathetic stimulation produces watery secretions, whereas sympathetic stimulation leads to more viscous-mucoid secretions [2]. Therefore, due to the SPG’s parasympathetic innervation, it can be manipulated intraorally by physicians utilizing OMT to relieve congestion associated with sinusitis, allergies, headaches, and upper respiratory infections [3, 4]. Further studies have demonstrated that injection of this ganglion with lidocaine has also been shown to reduce postoperative nausea and vomiting after sinus surgery [5]. Additionally, reduction of nasal congestion may assist with the relief of obstructive sleep apnea symptoms [6]. In the documented SPG release technique, the physician inserts their fifth digit into the oral cavity along the maxilla, laterally to the posterior molars, until the finger contacts the pterygoid plate. Digital pressure is directed superiorly and in a rotatory motion to stimulate the ganglia and initiate serous drainage of the ipsilateral eye, nose, sinus, and throat [2] (Figure 1). This technique is usually done for 15–30 s and is known to be mildly uncomfortable when done correctly because the pterygoid plate is anecdotally sensitive for most people.
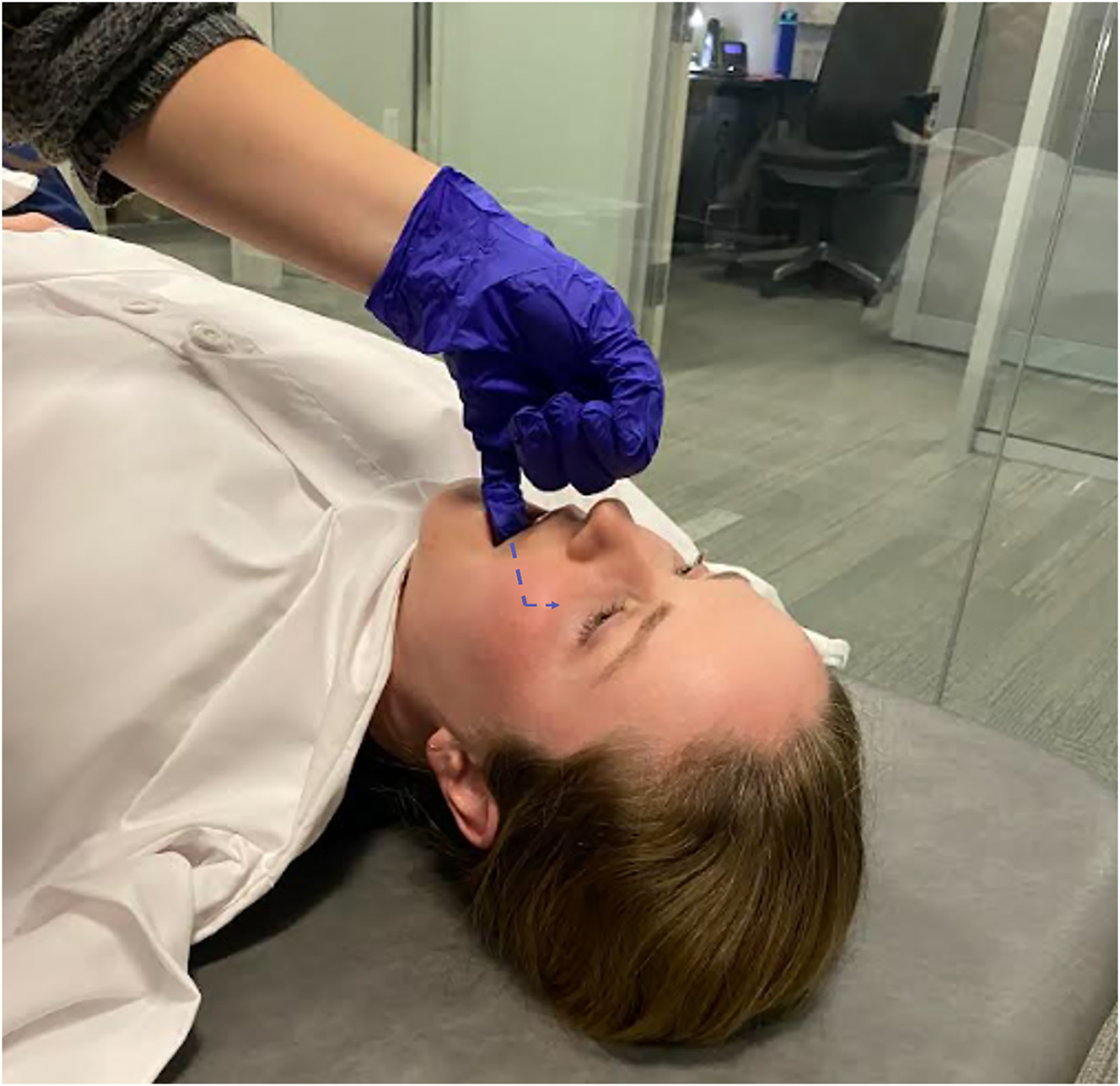
To perform the SPG release, insert a gloved finger into the oral cavity. Run your fifth finger along the maxilla within the oral cavity, lateral to the molars, until your finger contacts the pterygoid plate at the posterior aspect of the mouth. Then, direct your pressure superiorly and provide a rotatory motion to stimulate the SPG.
Although this technique is effective, it can be challenging to teach students and physicians how to perform it properly because the SPG’s exact anatomic location is difficult to visualize in the traditional curriculum of the medical school cadaver lab. When medical students learn to identify this ganglion in the cadaver lab, a deep to superficial approach is utilized by bisecting the head [7, 8]. Although this technique is quick and efficient to provide direct visualization, there is little osteopathic clinical relevance for this midline approach because the SPG is not palpated from a deep to superficial approach. Due to the discomfort associated with the intraoral nature of the SPG release and challenging anatomic visualization, the authors sought to find a way to teach students this technique with more accuracy and precision. Utilizing superficial to deep anatomic dissection, the learner better visualizes the orientation and depth of this ganglion in a manner that parallels palpation and has the potential to improve their SPG manipulation technique on future patients.
Clinical summary
Anatomy of the sphenopalatine (pterygopalatine) ganglion (SPG)
The SPG resides within the pterygopalatine fossa, which is a depression found within the superior pterygomaxillary fissure and is typically located near the lateral insertion of the posterior middle turbinate [6, 9]. This fissure is formed between the posterosuperior border of the maxilla and the anterosuperior border of the pterygoid plates. The lateral wall consists of the coronoid process of the mandibular ramus, whereas the medial wall consists of the process of the palatine bone that articulates with the maxilla and the lateral pterygoid plate [10].
The ganglion carries parasympathetic fibers from the facial nerve (cranial nerve [CN] VII) and sensory fibers from the maxillary nerve (CN V2) to the nasal, oral, and pharyngeal regions of the head. CN V has three main branches: ophthalmic (CN V1), maxillary (CN V2), and mandibular (CN V3), which exit the skull through three separate foramina: the superior orbital fissure, foramen rotundum, and foramen ovale, respectively [10]. The maxillary nerve crosses through the pterygopalatine fossa to enter the floor of the orbit through the inferior orbital fissure, where it becomes the infraorbital nerve. The infraorbital nerve distributes onto the face as it passes through the infraorbital foramen. Within this article, we are focusing on CN V2 due to its many connections with the SPG. The nerves that connect to the SPG are the nasopalatine nerve, greater palatine nerve, lesser palatine nerve, posterior/superior/inferior lateral nasal branches, and the pharyngeal branch, all of which originate from the maxillary (CN V2) nerve [6]. The preganglionic parasympathetic neurons (PrPNs) destined for the SPG are located within the superior salvatory nucleus in the pontine intermediate zone of the brainstem. The neuronal fibers from the brainstem emerge to form the facial nerve (CN VII) at the cerebellopontine angle and enter the internal auditory meatus with the vestibulocochlear nerve (CN VIII). These fibers travel through the facial canal to the geniculum of the facial nerve. The PrPNs will emerge to enter the floor of the middle cranial fossa as the greater petrosal branch of the facial nerve (CN VII). This branch eventually becomes a component of the nerve of the pterygoid canal with its sympathetic counterpart, the deep petrosal nerve, to finally arrive at the SPG, which is the site of the postganglionic parasympathetic neurons (PoPNs) within the pterygomaxillary fissure. The PoPNs are distributed with the branches of the maxillary (CN V2) nerve distal to the SPG. In totality, the SPG supplies parasympathetic innervation to the lacrimal gland and mucosal glands of the nose, nasopharynx, sinuses, and soft palate. The secretomotor fibers enter the pterygopalatine ganglion in the pterygopalatine fossa. The ganglion is suspended and joined by general sensory fibers from the maxillary branch of the trigeminal nerve (CN V2), which travel superior to the ganglion by way of the foramen rotundum [11]. The surrounding bony structures and the nerves that diverge onto the SPG influenced our development of the proposed dissection protocol.
Dissection protocol
Our superficial to deep dissection utilized one 72-year-old White male cadaver that was granted for use through the Des Moines University Osteopathic Medical School’s body donor program. The dissection began with removing skin from the face, followed by separating and identifying the muscles of facial expression. Next, we identified the three terminal branches of the trigeminal nerve (the supraorbital, infraorbital, and mental nerves) as they emerged from the supraorbital foramen, infraorbital foramen, and mental foramen, respectively (Figure 2). Next, we specifically targeted the infraorbital nerve (branch of CN V2) due to its anatomic proximity to the SPG. To properly follow the infraorbital nerve deep through the infraorbital foramen, along the infraorbital groove, and through the inferior orbital fissure as it turns into the maxillary nerve (CN V2), we enucleated the eyeball (Figure 3). Then, we utilized a Dremel® (Bosch, Mount Prospect, IL) to carefully remove portions of the inferior orbital wall and zygomatic arch while keeping the nerve intact. We followed CN V2 deep into the face, inferior to the orbit, until it converged into a spherical structure and identified the palatine nerves branching inferiorly to the SPG (Figure 4). By following the path and branching of the infraorbital and maxillary nerves, we could confirm that we had located the SPG from a superficial to deep approach.
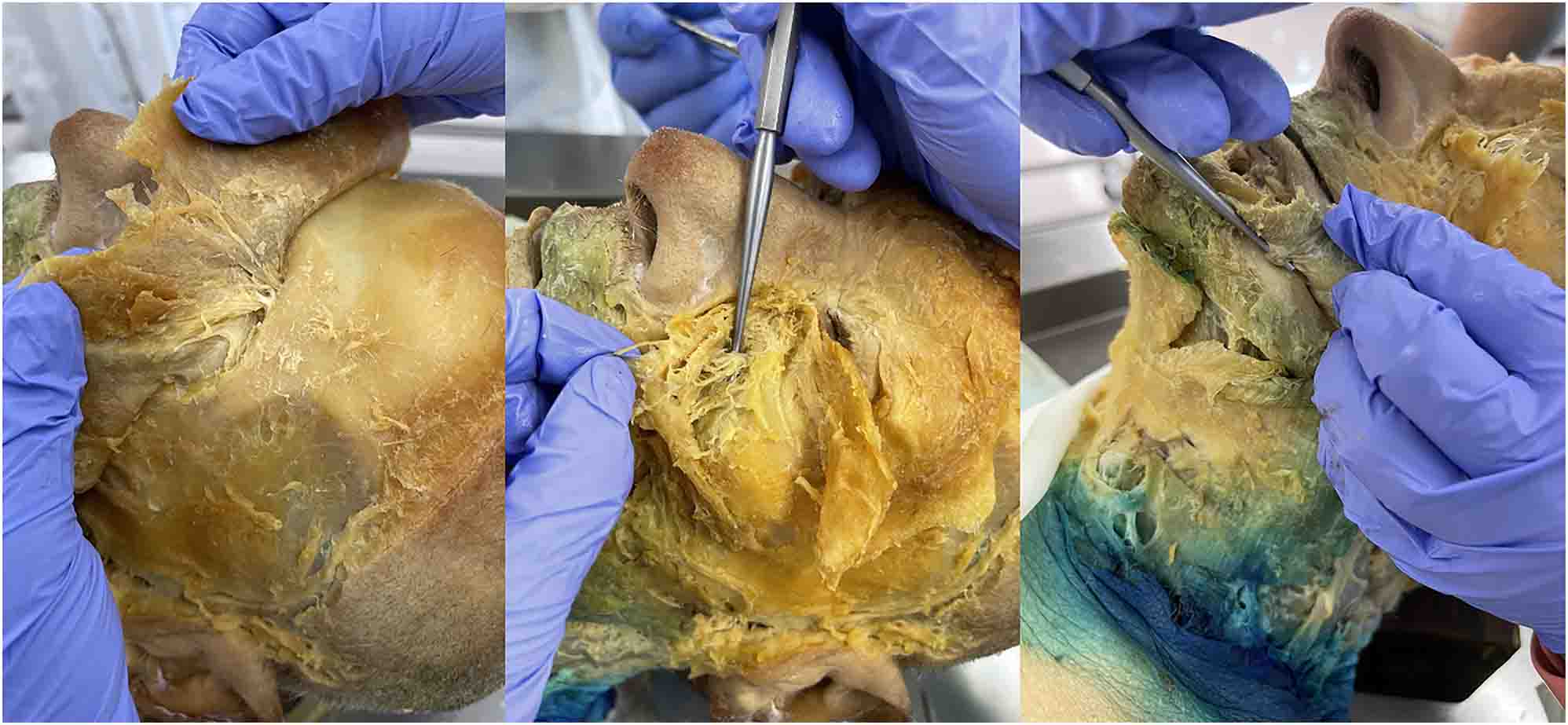
After removing the skin and dissecting the muscles of facial expression, the main branches of the trigeminal nerve were identified. From left to right: supraorbital (branch of CN V1) through the supraorbital foramen, infraorbital (branch of CN V2) through the infraorbital foramen, and mental (branch of CN V3) through the mental foramen.
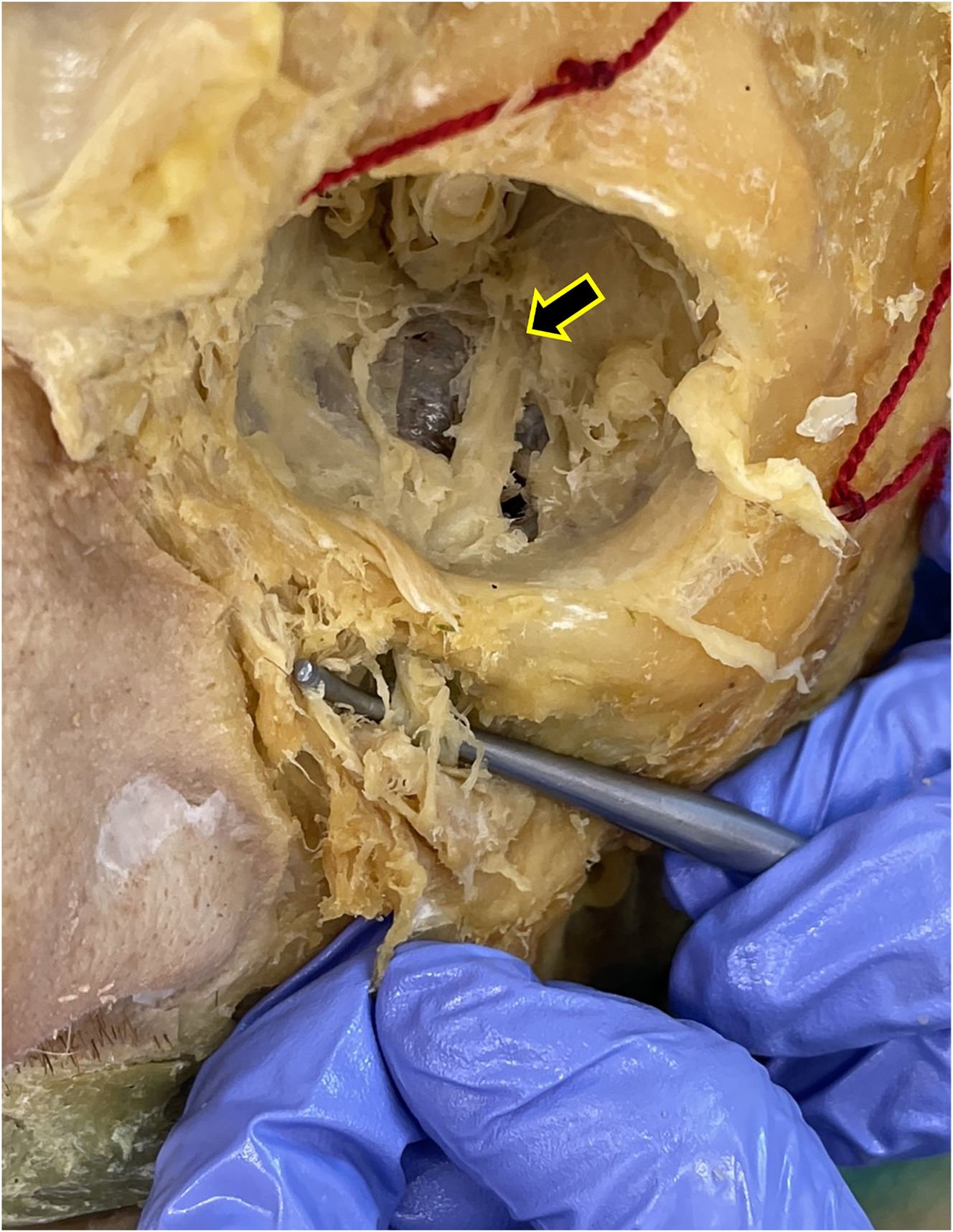
After enucleating the eyeball, we followed the infraorbital nerve as it travels along the infraorbital groove and emerges from the inferior orbital fissure (black arrow). To the left of the image is the nose, the ear is positioned to the right, and the superior edge of the orbit is positioned at the top of the image. The probe shows the infraorbital nerve (branch of CN V2) emerging from the infraorbital foramen.
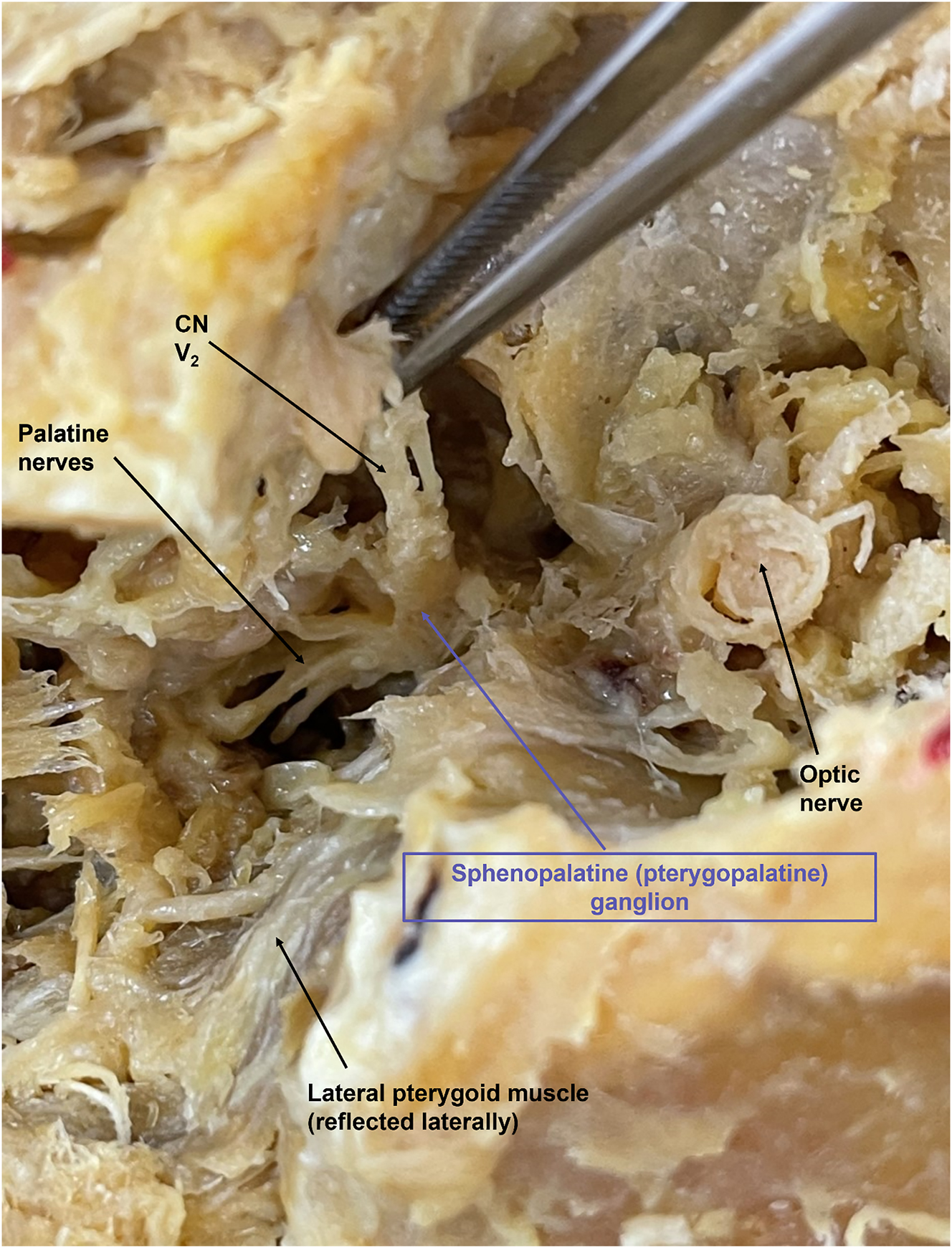
The SPG is demonstrated with the purple arrow. CN V2 is seen branching anteriorly, with the palatine nerves branching inferiorly. The cut optic nerve is seen superiorly. To the left of the image is the nose, the ear is positioned to the right, the superior edge of the orbit is positioned at the top, and the cut zygomatic arch is positioned at the bottom of the image.
Discussion
This dissection identifies the SPG from a perspective that parallels the approach from which it is palpated in the SPG release with OMT. Because intra-oral manipulation can be uncomfortable for patients, we wanted to streamline the procedure by enhancing the learner’s understanding of the SPG’s exact anatomic location in three-dimensional space. This has the ability to help osteopathic medical students and physicians deliver accurate palpation to the SPG to provide efficient and effective drainage and congestion relief to patients. While this article describes the technique of dissection, another study to demonstrate the benefits of observing a dissection of this magnitude should be conducted, either through student feedback or better demonstration of the SPG release in a practical examination setting. Future studies may also consider repeating this dissection on multiple donors of multiple sexes or ethnicities to identify the anatomic variation of the SPG. One could also specifically compare the two main dissection (superficial to deep, and deep to superficial) approaches to demonstrate which is most efficient or beneficial for educational purposes. Limitations include a lack of variability in human anatomy due to dissection of one cadaver at one medical school by medical students and by utilizing a single dissection technique. The SPG can be found following CN V or CN VII. Utilizing only one method of identification can result in a limitation as well. This dissection was completed by four medical students, two of whom hold master’s degrees in anatomy, with assistance by anatomy faculty holding a PhD. Due to the advanced anatomical education of this group, this dissection could be considered challenging for medical students to repeat without similar advanced anatomy training.
Conclusions
The SPG is the most superficial ganglia to palpate from the oral cavity and can be manipulated by students and physicians utilizing OMT to relieve congestion associated with sinusitis, allergies, headaches, and upper respiratory infections. Utilizing cadaveric dissection with a superficial to deep approach, learners have an opportunity to better understand the SPG’s anatomic location in a manner that parallels palpation, making this approach more clinically relevant. The proposed approach may augment learners’ manipulation technique and make the SPG release more comfortable and effective for future patients.
Acknowledgments
We would like to thank Ariel Gubatina Jr., MS (Anatomy Lab Specialist) and Edward Christopherson (Body Donation Coordinator) for their assistance with this project.
-
Research ethics: The Des Moines University Body Donation program and the individuals that voluntarily donate their body and tissues have permitted the utilization of their human remains for publications and presentations.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: None declared.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Shapira, IL. Neuromuscular dentistry and the role of the autonomic nervous system: sphenopalatine ganglion blocks and neuromodulation. An International College of Cranio Mandibular Orthopedics (ICCMO) position paper. Cranio 2019;37:201–6, https://doi.org/10.1080/08869634.2019.1592807.Suche in Google Scholar PubMed
2. Robbins, MS, Robertson, CE, Kaplan, E, Ailani, J, Charleston 4th, L, Kuruvilla, D, et al.. The sphenopalatine ganglion: anatomy, pathophysiology, and therapeutic targeting in headache. Headache 2016;56:240–58, https://doi.org/10.1111/head.12729.Suche in Google Scholar PubMed
3. Kuchera, ML, Kuchera, WA. The OCSD approach: techniques for homeostasis, healing, and symptom relief. In: Osteopathic considerations in EENT disorders, 1st ed. Dayton, OH: Greyden Press, LLC; 2011:180 p.Suche in Google Scholar
4. Crespi, J, Bratbak, D, Dodick, D, Matharu, M, Jamtøy, KA, Aschehoug, I, et al.. Measurement and implications of the distance between the sphenopalatine ganglion and nasal mucosa: a neuroimaging study. J Headache Pain 2018;19:14, https://doi.org/10.1186/s10194-018-0843-5.Suche in Google Scholar PubMed PubMed Central
5. Abubaker, AK, Al-Qudah, MA. The role of endoscopic sphenopalatine ganglion block on nausea and vomiting after sinus surgery. Am J Rhinol Allergy 2018;32:369–73, https://doi.org/10.1177/1945892418782235.Suche in Google Scholar PubMed
6. Attali, V, Jacq, O, Martin, K, Arnulf, I, Similowski, T. Osteopathic manipulation of the sphenopalatine ganglia versus sham manipulation, in obstructive sleep apnea syndrome: a randomised controlled trial. J Clin Med 2021;11:99. https://doi.org/10.3390/jcm11010099.Suche in Google Scholar PubMed PubMed Central
7. Agur, AMR, Dalley, AF. Grant’s atlas of anatomy. Philadelphia: Wolters Kluwer; 2021.Suche in Google Scholar
8. Seffinger, MA, King, HH, Ward, RC. Autonomic nervous system. In: Foundations of osteopathic medicine, 4th ed Philadelphia: Lippincott Williams & Wilkins; 2018:221–67 pp.Suche in Google Scholar
9. Moore, KL, Dalley, AF. Neck. In: Clinically oriented anatomy, 5th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2006:1078 p.Suche in Google Scholar
10. Crumbie, L. Pterygopalatine ganglion. Kenhub, Kenhub; 2022. Available from: https://www.kenhub.com/en/library/anatomy/pterygopalatine-ganglion.Suche in Google Scholar
11. Shen, SH. Pterygopalatine ganglion | Radiology Reference Article| Radiopaedia.org. Radiopaedia; 2022. https://radiopaedia.org/articles/pterygopalatineganglion?land= [Accessed 1 Nov].Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Medical Education
- Original Article
- The impact of emergency medicine residents on clinical productivity
- Clinical Practice
- A superficial dissection approach to the sphenopalatine (pterygopalatine) ganglion to emphasize osteopathic clinical relevance
- Musculoskeletal Medicine and Pain
- Original Article
- Effect of osteopathic manipulative treatment and Bio-Electro-Magnetic Energy Regulation (BEMER) therapy on generalized musculoskeletal neck pain in adults
- Neuromusculoskeletal Medicine (OMT)
- Brief Report
- Augmentation of immune response to vaccinations through osteopathic manipulative treatment: a study of procedure
- Pediatrics
- Original Article
- Effects of osteopathic manipulative treatment on children with plagiocephaly in the context of current pediatric practice: a retrospective chart review study
- Public Health and Primary Care
- Commentary
- Telehealth in opioid use disorder treatment: policy considerations for expanding access to care
- Clinical Image
- Traumatic tattooing: case description and a comprehensive review of the therapeutic management
Artikel in diesem Heft
- Frontmatter
- Medical Education
- Original Article
- The impact of emergency medicine residents on clinical productivity
- Clinical Practice
- A superficial dissection approach to the sphenopalatine (pterygopalatine) ganglion to emphasize osteopathic clinical relevance
- Musculoskeletal Medicine and Pain
- Original Article
- Effect of osteopathic manipulative treatment and Bio-Electro-Magnetic Energy Regulation (BEMER) therapy on generalized musculoskeletal neck pain in adults
- Neuromusculoskeletal Medicine (OMT)
- Brief Report
- Augmentation of immune response to vaccinations through osteopathic manipulative treatment: a study of procedure
- Pediatrics
- Original Article
- Effects of osteopathic manipulative treatment on children with plagiocephaly in the context of current pediatric practice: a retrospective chart review study
- Public Health and Primary Care
- Commentary
- Telehealth in opioid use disorder treatment: policy considerations for expanding access to care
- Clinical Image
- Traumatic tattooing: case description and a comprehensive review of the therapeutic management

