Abstract
In this work, a pleonastic compound, a compound with a composition between hercynite and spinel sensu stricto FeAl2O4–MgAl2O4, was synthesized by a non-conventional method of arc-plasma synthesis (APS). The structure of the obtained spinel compound was characterized by means of X-ray diffraction and Mössbauer effect measurements. The microstructure was observed by applying scanning electron microscope (SEM)/energy dispersive spectrometer (EDS) method. It was found that the arc-plasma synthesized material was characterized by a monophasic character, a low-inversion parameter and a compact microstructure.
Introduction
Compounds with a spinel structure, like hercynite or spinel sensu stricto, are today of great technological importance due to their beneficial physicochemical and mechanical properties, such as high melting points, corrosion resistance to Portland clinker and alkali environment, and thermal shock resistance, and because they can act as “substitutes” in the production of toxic chrome-containing refractories [1–4]. FeAl2O4 and MgAl2O4 are the examples of the spinel minerals rarely encountered in nature, and characterized by the high melting points of 1,780 °C and 2,135 °C, respectively [5], and low thermal expansion coefficients. They may form a complete solid solution, Fe2+/∑(Mg2++Fe2+), with ratio ranging from 0.25 to 0.75 called pleonaste [6].
Hercynite (FeAl2O4) and regular (or true) spinel (MgAl2O4) belong to AB2O4sensu lato oxide spinel group possessing the Fd3m symmetry (227 space group number). In the spinel crystal lattice all the divalent A2+ ions occupy 8a positions (1/8,1/8,1/8), whereas all of the trivalent Al3+ ions are placed in the 16d Wyckoff sites (1/2, 1/2,1/2), and oxygen ions O2– are in 32e positions (1/4,1/4,1/4). Endmembers FeAl2O4 and MgAl2O4 are characterized by the normal cation distribution. This means that all of the divalent ferrous (Fe2+) or magnesium Mg2+ ions occupy the tetrahedral (Td) sites (the so-called A ...sites), while all of the Al3+ ions fill up the octahedral (Oh) ones (called as B sites) in the spinel unit cell [7], which is illustrated in Figure 1.
![Figure 1:
Structure of spinel, where green tetrahedrons represent [FeO4] or [MgO4], while the blue octahedrons denote [AlO6] coordinates, and light blue balls refer to oxygen atoms.](/document/doi/10.1515/htmp-2015-0252/asset/graphic/j_htmp-2015-0252_fig_001.jpg)
Structure of spinel, where green tetrahedrons represent [FeO4] or [MgO4], while the blue octahedrons denote [AlO6] coordinates, and light blue balls refer to oxygen atoms.
Nevertheless, such a distribution of cations is true but only for a low-temperature origin, because above the absolute temperature ions begin to exchange with their positions, where Fe2+/Mg2+ cations begin to occupy Oh cavities, while Al3+ ions hop to Td sites. Therefore, in practice at ambient conditions mixed spinels prevail. The introduced disorder, with contrast to the ordered endmember species, is described by the so-called inversion parameter x, which is the fraction of trivalent aluminium ions that passed from Oh to Td sites [7]. This term was first introduced by Verwey and Heilmann [8]. Together with the lattice parameter a and the oxygen positional parameter u, they constitute parameters fully characterizing the spinel crystal structure. Worth mentioning here is the fact that inversion parameter x determines spinel type and takes values in range from 0 to 1 for the perfectly normal and inverse spinel, respectively. On the other hand, when the x parameter is between these values the spinel is partially inversed. And besides, random spinel is additionally distinguished by x equal to 2/3 [7].
Hercynite was reported to be found in the metallurgical slag as the product of reaction between refractories containing SiO2 and iron melts with oxygen [9–11]. It was also observed in the slag when metallic Al was applied as the deoxidizer additive [12]. Moreover, the formation of FeAl2O4 was also reported by Okamoto et al. [13] in the intermediate layer occurring as the bonding interface in the process of joining of metal with alumina ceramics.
Hercynite, although not as popular as spinel [14–17], because of the difficulties encountered during its synthesis, possesses a great potential of the usefulness as the elastifying component of basic refractories. It was previously reported that addition of hercynite to MgO–CaZrO3 material significantly improved its thermal shock resistance by a generation of microcracks that inhibited cracks propagation [18]. Moreover, Chenn et al. [19] investigated that hercynite facilitates formation of a protective coating on the refractory material in burning zone of cement rotary kiln during cement clinker production, which prevents the materials from premature damage.
Magnesia spinel materials were installed for the first time at 30 years of twentieth century and have been state of the art since the mid-1980s. Magnesia–hercynite materials have been developed only in the early 1990s of the same century and applied first in 1995 in cement rotary kiln [20, 21].
The ability of hercynite to interdiffuse with magnesia matrix, when it acts as a component of magnesia refractory material, makes the material more flexible by the local formation of secondary spinel phases, and therefore material is able to withstand thermal as well as mechanical strains, which constantly accompany the rotating kiln, and in result internal refractory lining [22]. The exchange of ions between hercynite and magnesia, or other material constituents, is of great interest, because by creating transitional spinel solid solutions or hybrid spinels [22] during the entire operational period they heal the cracks, which generate during the operation, and for this reason can be called as “intelligent materials”. The interdiffusion with magnesia matrix is realized by the mutual exchange of ferrous/ferric ions with magnesium ones, which is possible due to the similarity in effective ionic radii [23] (Fe2+=0.063 nm, Fe3+=0.049 nm, Mg2+=0.057 nm, all values given for tetrahedral coordination).
Currently, materials prepared by using arc-plasma synthesis (APS) possess a great potential of applicability due to their high density, good corrosion resistance resulting from larger crystals formed and higher thermal stability [24].
Therefore, this method was applied for the synthesis of pure hercynite-spinel sensu stricto transitional compound (pleonaste), which was investigated in this work by means of structure and microstructure analysis.
Experimental
The starting reagents, which were reagents of high-grade powders of Fe2O3 and Al2O3 and MgO, were composed in the proportion expressed by the formula Fe0.5Mg0.5Al2O4 (pleonastic composition). Then, the starting powder mixture was pressed into the form of pellet of 20 mm in diameter with the pressure of 100 MPa. Subsequently, material was subjected to APS. The process of synthesis was carried out in SpekoArc300 arc plasma furnace (Spaw-Projekt, Kraków, www.arc-furnace.pl) in a flow of the protective atmosphere of a noble gas, which was pure argon. In order to get a fully reacted and homogenized material, sample was melted twice. Owing to a non-contact spark ignition a risk of the sample pollution was avoided. The arc-plasma furnace was powered by an invertor source of power with the current regulation within the range of 10–300 A and equipped with a water-cooled copper crucible and a tungsten electrode.
The synthesized spinel material sample was subsequently powdered to the granulation below 0.063 mm and subjected to X-ray diffraction (XRD) analysis in order to identify its phase composition. The XRD measurement was conducted at ambient temperature, utilizing the PANalytical X’Pert Pro MPD X-ray diffractometer, in Bragg–Brentano geometry, with Cu-Kα radiation (λ=1.54056 Å), in the 2θ range 5°–90°. The XRD pattern was processed applying Rietveld refinement as implemented in the Maud code [25].
Mössbauer spectroscopy (MS) measurement of the spinel material was conducted in a transmission mode by means of the RENON MsAa-3 spectrometer, equipped with the LND Kr-filled proportional detector and the He–Ne laser-based interferometer, which is used to calibrate a velocity scale. A commercial 57Co(Rh) source kept at room temperature was applied for 14.41 keV resonant transition in 57Fe. The obtained spectrum was fitted using Lorentzian shape line. The values of isomer shift (IS) were given relatively to α-Fe. The Mössbauer absorbers were prepared in a powder form.
The obtained material was observed under the ultra-high-definition Nova NanoSEM 200 scanning electron microscope (SEM) equipped with an energy dispersive spectrometer (EDS). The sample for the microscopic observations was both in the form of surface fracture and polished cross section prepared by the traditional ceramographic technique.
Results and discussion
XRD study
Figure 2 shows the refined XRD pattern for the APS spinel material. The all registered reflexes were characteristic for cubic spinel phase (space group Fd3m). The spinel crystal lattice parameter was determined on a=8.1148(2)Å, while the oxygen positional parameter equalled u=0.2600. In comparison to pure hercynite spinel [26], which lattice and oxygen parameter were previously estimated as a=8.1320(9)Å and u=0.262, respectively, the values obtained in this study for mixed spinel are decreased. Simultaneously, they are increased with respect to parameters for spinel sensu stricto synthesized by flux growth method. The XRD pattern was refined by means of Rietveld method and using XRD data for Fe0.494Mg0.544Al1.962O4 obtained by Andreozzi and Lucchesi [27], and with the attained parameters Rw= 9.0321 % and sigma=1.0124, confirming a satisfactory fitting procedure.
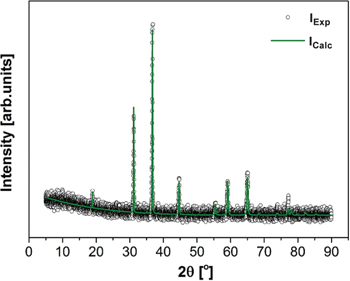
The refined XRD pattern of the arc-plasma synthesized spinel compound.
Mössbauer effect study
In order to fully characterize the spinel crystal structure by determining its inversion parameter x, the Mössbauer spectroscopy (MS) measurement was performed. The obtained ambient temperature Mössbauer spectrum is illustrated in Figure 3.
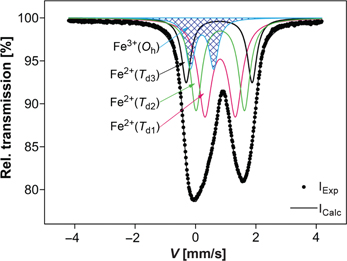
The Mössbauer spectrum at ambient temperature of the arc-plasma synthesized pleonaste spinel.
The fitted continuous spectrum (corresponding to the experimental points) was resolved into four subspectra that were described in the plot. As it can be observed, there are ferrous (Fe2+) and ferric (Fe3+) ions in the spinel structure [28], which was determined based on the obtained IS values. Moreover, they occupy different positions in the spinel crystal lattice. The average value of the IS for the Fe2+ ions was 0.910 mm/s, while for the Fe3+ ones it was estimated as 0.321 mm/s. Besides, it was found that divalent iron is distributed among the tetrahedral positions of different symmetry, with the mostly distorted Td3 one, which was characterized by the highest quadrupole splitting (QS) value of 2.181(10) mm/s, whereas for the rest ones it was equal to 1.601(7) mm/s (Td2) and 1.011(10) mm/s (Td1). The component of the lowest relative area and hyperfine parameters of IS=0.320(4) mm/s and QS=0.776(7) mm/s (marked by blue colour in Figure 3) were attributed as responsible for the octahedrally coordinated Fe3+ ions.
Additionally, it was found that the ratio of the percentage surface area for Fe2+/Fe3+ was in proportion of 85:15. Based on the obtained area ratio and assuming that all the magnesium ions occupy tetrahedral sites, due to their high site preference energy in Td positions [29], the chemical formula of the synthesized spinel material was established as follows (eq. (1)):
where indices Td and Oh denote tetrahedral and octahedral coordinations, respectively and square designates an ion vacancy.
Taking under consideration the surplus of the positive charge at the tetrahedral site, the presence of vacancies at Oh sites was reflected in the chemical formula to account for charge balance. The preference of vacancies occupation in the octahedral positions of spinel lattice was stated in Ref. [30]. The established chemical formula of the investigated spinel compound shows that its inversion parameter is equal to x=7.5 % and is much lower than x for pure hercynite which was reported to be x=23 % [26]. Such a decline in the inversion parameter value with the exchange of iron by magnesium ions is probably associated with the high-site preference energy of Mg2+ in Td cavities, which prevent aluminium ions against passing from Oh to Td sites.
SEM/EDS study
Figure 4(a) and (b) shows SEM images taken with the use of BSED mode for the cross-sectional sample (Figure 4(a)), and applying LVD (Low Vacuum Detector) mode for the fracture surface (Figure 4(b)), both in magnification of 1,000×.
It can be observed from the image of the cross section of the spinel sample that there are light- and dark-grey regions, especially able to distinguish on the right side of the image. The first ones prevail in the vicinity of the zig-zag-like cracks, which constitute boundaries between spinel crystals. On the other hand, inside of these crystals are visible by dark-grey colour. The observed microregions were examined by EDS in points 1 and 2, marked in Figure 3(a). The elemental composition measured in points 1 and 2 was as follows: point 1: Fe-12.2 %, Mg-4.2 %, Al-32.4 %, O-51.2 %; point 2: Fe-3.9 %, Mg-11.3 %, Al-33.8 %, O-51.0 %. So, light-grey regions are enriched with iron atoms, while dark-grey areas are enriched with aluminium atoms, with respect to approximate stoichiometry of spinel compound. The ratio of Al/(Fe+Mg) in point 1 equals 2, which is in compliance with the composition of stoichiometric spinel, while in point 2 it slightly increased to the value of 2.2.
The observed difference in the content of Fe atoms in points 1 and 2 (Figure 4(a)) was probably the effect of the diffusion of iron atoms from the higher temperature zone (point 2) to the lower temperature zone (point 1: boundaries between the crystals), as a result of temperature gradient across the crystal.
The morphology of the APS material, exhibited in Figure 4(b), is revealed by the small crystals that grew from the larger massive ones. The microstructure is rather dense and well compacted with the sparsely occurring voids. The image of the fracture surface does not allow the microregions with the chemical gradient to be distinguished, as it was observed for the cross-sectional sample.
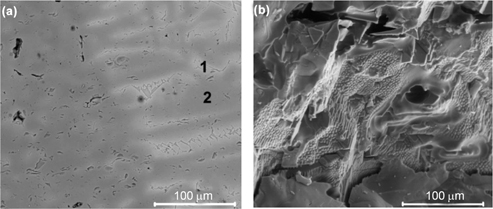
SEM images of the investigated spinel material: (a) polished cross section and (b) fracture surface.
Conclusions
The pleonastic Fe(50 %)Mg(50 %) aluminate spinel was synthesized in this work by the non-conventional method for the synthesis of ceramic compounds (APS) and investigated in view of its structure and microstructure.
The APS method permitted to obtain the monophasic spinel compound, confirmed by the XRD analysis, with the low inversion parameter of x=7.5 % determined by means of the Mössbauer effect measurement. The microstructure of the obtained material was compact, with sparsely occurring voids.
It was found that iron atoms tend to diffuse in the direction of outside crystal surfaces that constitute lower temperature zones.
The obtained results showed that it is possible to produce, in a fast route (by APS method), the material of pleonastic composition, which can be applied as the effective and good quality addition to refractory materials, for example in cement rotary kiln.
Acknowledgements
This work was supported by the statutory funds of the Faculty of Materials Science and Ceramics AGH in Kraków.
References
[1] [1] F. Baruthio, Biol. Trace Elem. Res., 32 (1992) 145–153.10.1007/BF02784599Search in Google Scholar PubMed
[2] [2] L. Guangpink, L. Nan, Y. Wen, G. Changhe, Z. Wei and L. Yuanyuan, Ceram. Int., 40(6) (2014) 8149–8155.10.1016/j.ceramint.2014.01.010Search in Google Scholar
[3] [3] E. Rodíguez, G.A. Castillo, J. Contreras, R. Puente-Ornelas, J.A. Aguilar-Martínez, L. García and C. Gómez, Ceram. Int., 38 (2012) 6769–6775.10.1016/j.ceramint.2012.05.071Search in Google Scholar
[4] [4] G. Gelbmann, R. Krischanitz and S. Jörg, RHI Bull., 2 (2013) 10–12.Search in Google Scholar
[5] [5] C.A. Schach, Refractories Handbook, Marcel Dekker, New York (2004).Search in Google Scholar
[6] [6] W.A. Deer, R.A. Howie and J. Zussman, An Introduction to the Rock-Forming Minerals, Longman Scientific & Technical, Harlow, England (1992), p. 695.Search in Google Scholar
[7] [7] K.E. Sickafus, J.M. Wills and N.W. Grimes, J. Am. Ceram. Soc., 82(12) (1999) 3279–3292.10.1111/j.1151-2916.1999.tb02241.xSearch in Google Scholar
[8] [8] E.J.W. Verwey and E.L. Heilmann, J. Chem. Phys., 15(174) (1947) 174–180.10.1063/1.1746464Search in Google Scholar
[9] [9] T. Zienert and O. Fabrichnaya, J. Europ. Ceram. Soc., 35 (2015) 1317–1326.10.1016/j.jeurceramsoc.2014.10.033Search in Google Scholar
[10] [10] E. Kapilashrami, V. Sahajwalla and S. Seetharaman, Proceedings of the VII International Conference on Molten Slags Fluxes and Salts, Johannesburg (2004), pp. 417–422.Search in Google Scholar
[11] [11] E. Kapilashrami, V. Sahajwalla and S. Seetharaman, J. Mat. Sci., 40 (2005) 2371–2375.10.1007/s10853-005-1961-4Search in Google Scholar
[12] [12] K. Wasai, K. Mukai, H. Fuchiwaki and A. Yoshida, ISIJ Int., 39(8) (1999) 760–766.10.2355/isijinternational.39.760Search in Google Scholar
[13] [13] I. Okamoto, M. Naka and K. Asami, Trans. JWRI, 11 (1982) 131–133.Search in Google Scholar
[14] [14] P. Biswas, K. Rajeswari, V. Mahendar and R. Johnson, Ceram. Int., 39 (2013) 9819–9821.10.1016/j.ceramint.2013.05.091Search in Google Scholar
[15] [15] I. Reimanis and H.J. Kleebe, J. Am. Ceram. Soc., 92(7) (2009) 1472–1480.10.1111/j.1551-2916.2009.03108.xSearch in Google Scholar
[16] [16] R. Salomao, M.O.C. Villas Bôas and V.C. Pandolfelli, Ceram. Int., 37 (2011) 1393–1399.10.1016/j.ceramint.2011.01.012Search in Google Scholar
[17] [17] J. Szczerba, I. Jastrzębska, Z. Pędzich and M.M. Bućko, J. Mat. Sci. Chem. Eng., 2 (2014) 16–25.10.4236/msce.2014.210003Search in Google Scholar
[18] [18] E.A. Rodríguez, A.K. Limones, J.E. Contreras, J.J. Ruiz-Valdés, R. Puente-Ornelas, A.M. Arato and J.A. Aguilar-Martínez, J. Eur. Ceram. Soc., 35 (2015) 2631–2639.10.1016/j.jeurceramsoc.2015.03.018Search in Google Scholar
[19] [19] J. Chenn, M. Yan, J. Su, B. Li and J. Sun, Ceram. Int., 42(1) (2016) 569–575.10.1016/j.ceramint.2015.08.148Search in Google Scholar
[20] [20] M. Geith, C. Majcenovic and A. Wiry, RHI Bull., 1 (2003) 25.Search in Google Scholar
[21] [21] R. Krischanitz. 20 Years Hercynite Technology. Website access in 27.08.2015: http://www.rhi-ag.com/linkableblob/internet_en/74150/data/Magnesia_Hercynit_Art-data.pdfSearch in Google Scholar
[22] [22] G. Liu, N. Li, W. Yan, G. Tao and Y. Li, J. Ceram. Proc. Res., 13(4) (2012) 480–485.Search in Google Scholar
[23] [23] R.D. Shannon, Acta Cryst. A, 32 (1976) 751–767.10.1107/S0567739476001551Search in Google Scholar
[24] [24] I. Jastrzębska and J. Szczerba, Proceedings of the X Krakow Conference of Young Scientists, Krakow (2015), pp. 9–10.Search in Google Scholar
[25] [25] L. Lutterotti, S. Matthies and H.R. Wenk, Proceedings of the 17th International Conference on Textures of Materials ICOTOM 12, Montreal (1999).Search in Google Scholar
[26] [26] I. Jastrzębska, J. Szczerba, P. Stoch, A. Błachowski, K. Ruebenbauer, R. Prorok and E. Śnieżek, Nukleonika (J. Nucl. Res.), 60(1) (2015) 45–47.10.1515/nuka-2015-0012Search in Google Scholar
[27] [27] G.B. Andreozzi and S. Lucchesi, Am. Miner., 74 (1989) 339–351.Search in Google Scholar
[28] [28] I. Dézsi, I. Szűcs and E. Sváb, J. Radioanal. Nucl. Chem., 246(1) (2000) 15–19.10.1023/A:1006796022996Search in Google Scholar
[29] [29] A. Navrotsky, C. Ma, K. Lilova and N. Birkner, Science, 330 (2010) 199–201.10.1126/science.1195875Search in Google Scholar PubMed
[30] [30] R. Dupree, M.H. Lewis and M.E. Smith, Phil. Mag. Lett., 53(2) (1986) L17–L20.10.1080/01418618608242816Search in Google Scholar
©2017 by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Research Articles
- Kinetics and Tribological Characterization of Pack-Borided AISI 1025 Steel
- A Comparative Study of Hot Deformation Behaviors for Sand Casting and Centrifugal Casting Q235B Flange Blanks
- Effects of Annealing Temperature on the Microstructure and Mechanical Properties of Electrodeposited Ni-Fe Alloy Foils
- Thermochemical Approach for Screening of Alternative Metal Oxides as a Flame Retardant of Modacrylic Fiber
- Hot Corrosion Behavior of Stainless Steel with Al-Si/Al-Si-Cr Coating
- Calculation of the Combined Heat Transfer Coefficient of Hot-face on Cast Iron Cooling Stave Based on Thermal Test
- The Corrosion Behavior of Stainless Steel 316L in Novel Quaternary Eutectic Molten Salt System
- Corrosion of Nickel-Based Alloys in Ultra-High Temperature Heat Transfer Fluid
- Superplastic Behaviour of AZ61-F Magnesium Composite Materials
- Effects of Laser Shock Processing on Fatigue Performance of Ti-17 Titanium Alloy
- Effect of the Platinum Electroplated Layer Thickness on the Coatings’ Microstructure
- Structural and Microstructural Study on the Arc-Plasma Synthesized (APS) FeAl2O4–MgAl2O4 Transitional Refractory Compound
- Retraction
- Retraction of: Mechanical and Electrochemical Characterization of Super-Solidus Sintered Austenitic Stainless Steel (316L)
Articles in the same Issue
- Frontmatter
- Research Articles
- Kinetics and Tribological Characterization of Pack-Borided AISI 1025 Steel
- A Comparative Study of Hot Deformation Behaviors for Sand Casting and Centrifugal Casting Q235B Flange Blanks
- Effects of Annealing Temperature on the Microstructure and Mechanical Properties of Electrodeposited Ni-Fe Alloy Foils
- Thermochemical Approach for Screening of Alternative Metal Oxides as a Flame Retardant of Modacrylic Fiber
- Hot Corrosion Behavior of Stainless Steel with Al-Si/Al-Si-Cr Coating
- Calculation of the Combined Heat Transfer Coefficient of Hot-face on Cast Iron Cooling Stave Based on Thermal Test
- The Corrosion Behavior of Stainless Steel 316L in Novel Quaternary Eutectic Molten Salt System
- Corrosion of Nickel-Based Alloys in Ultra-High Temperature Heat Transfer Fluid
- Superplastic Behaviour of AZ61-F Magnesium Composite Materials
- Effects of Laser Shock Processing on Fatigue Performance of Ti-17 Titanium Alloy
- Effect of the Platinum Electroplated Layer Thickness on the Coatings’ Microstructure
- Structural and Microstructural Study on the Arc-Plasma Synthesized (APS) FeAl2O4–MgAl2O4 Transitional Refractory Compound
- Retraction
- Retraction of: Mechanical and Electrochemical Characterization of Super-Solidus Sintered Austenitic Stainless Steel (316L)

