Abstract
Composite molecular sieve Y/SBA-15(C-Y) was prepared by microwave method, while Ce was loaded by ion exchange method to the composite molecular sieves (Ce-Y/SBA-15 (C-X)). Productive-type middle distillate hydrocracking catalyst was prepared from C-X and C-Y loaded. FI-IR, XPS, Pyridine IR, and TG-DTG had been used to characterize the C-X’s and C-Y’s structure and acidity. The results showed that Ce loaded not only had not broken the original structure of C-Y, but also improved silica alumina ratio of C-X, furthermore improved its total acid content. Through polarization and entrainment, Ce increased the skeleton and hydroxyl silicon aluminum hydroxy on electronic probability of migration to the cage, thus enhance the C-X’s B acid strength, make it more suitable for heavy oil processing. As compared with C-Y, the selectivity and yield of middle distillates over C-X was 0.7 % and 1.8 % higher, respectively. C-X have the greatest relief wax oil viscosity index, best once cracking selectivity, and lowest levels of diesel oil solidifying point in the three catalysts.
Introduction
Because of the heavy oil and the pressure of environment protection, the oil industry must face the problem of how to better use of heavy oil. As is known to all, heavy oil is a mixture of macromolecular compounds, which contains more impurity atoms [1]. As for heavy oil using gradually thorough, the traditional catalyst was found to have not well adapt to the change of heavy oil. The emergence of mesoporous molecular sieves has solved the problem of macromolecular material into small hole [2, 3], but as a result of the mesoporous channel when the amorphous state [4, 5, 6, 7], the application of mesoporous molecular sieves in industry is not satisfying. Micro–mesoporous composite molecular sieves materials could solve these problems.
Hydrocracking developing rapidly in recent years is one of the important processing methods in the oil industry, which can achieve heavy oil, low-quality product modification and refine the complete target. The diameter of heavy oil is greater than 2.5 nm, which is difficult to enter the Y-type microporous molecular sieve (pore size is 2 nm) in the channel of reaction. In this case, the reactant is difficult to close to the catalyst activity center, so it reduced the catalytic efficiency and the service life of catalyst [8, 9]. At present, most of the hydrocracking catalyst are Y zeolite with modified to improved catalytic performance, which could adapt to the requirement of different raw materials and generate more light components [10].
However, Y zeolite with modified is still of low total acid and Brǿnsted acid, the method of modified did not solve the problem of the conversion, selectivity, and stability of the catalyst fundamentally. In the last decade, a high number of studies about the hydrogenation cracking of heavy oil with catalysts including zeolites with a number of metals modification, like Co [11], Ni [12], and Mo [13] were published. Molecular sieves with metals modified are environmentally friendly catalysts which have the advantages of high activity and selectivity. Dai et al. [14] have previously used Ni–Mo/Al2O3 as fluid catalytic cracking (FCC) for catalytic cracking the heavy oil. They obtained the conversion rate of dienes in the FCC gasoline reduce by more than 98 % under the following conditions: temperature is 80 ℃, liquid hourly space velocity (LHSV) is 10 h–1, volume ratio of hydrogen and oil is 10, and pressure is 1.5 MPa.
Rare earth elements have many applications in extraction due to its unique properties [15, 16]. The composite molecular sieves Y/SBA-15 synthesized by microwave, while by ion exchange method to load Ce [17, 18] to the sample [19, 20]. This method improves the Y/SBA–15 silica alumina ratio, furthermore, increases the active center of a composite molecular sieves. In the end, we examine the effect of the different molecular sieve catalysts hydrogenation cracking of daqing-mixed raw material oil.
Experimental
Reagents and apparatus
The heavy oil is daqing-mixing oil, while the main properties of it are shown in Table 1.
The main properties of Daqing heavy oil (conditions: density, paraffin content, condensation point, sulfur content, characterization factor, and nickel content).
| Density (g/mL) | Paraffin content | Condensation point (K) | Sulfur content | Characterization factor | Nickel content (g) |
|---|---|---|---|---|---|
| 0.9222 | 26–30 % | 303 | 0.10 % | 12.55 | 2×10−5 |
Sodium aluminate, sodium hydroxide, sodium silicate, cerium nitrate, heptane, toluene, and tetraethyl orthosilicate (TEOS) were all analytical pure reagents and purchased from Sinopharm Group Co. Ltd. Triblock copolymer poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) (Pluronic P123) was purchased from Sigma Aldrich, America. Y-type microporous materials are provided by Fushun Petrochemical Research Institute.
The catalyst was synthesized with XH-MC-1 laboratory microwave reactor, and was characterized by GC7890II gas chromatograph, WYS-2S digital thermogravimetric analyzer, and WAY-IS abbe refractometer. Strengthen the catalyst cracking performance is completed by strengthening cracking transpose (made by ourselves).
Synthetic experiments
A 4.0-g triblock copolymer poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol) P123 was dissolved in 30.0 g of deionized water and stirred until fully dissolved. To the above solution was added 9.5 g of TEOS and stirred magnetically and powerfully, followed by the addition of 2.0 g prepared Y-type microporous materials and 120 ml certain concentration of hydrochloric acid, resulting a mixture solution with pH of 2 by a powerful magnetic stirring. After reacting in microwave reaction apparatus at 100 °C for 2 h, the crystallized product was washed with fresh deionized water and transferred again in an autoclave dried at 110 °C. Finally, the solid was calcined at 550 °C to afford a powder micro–mesoporous Y/SBA-15 composite material.
Certain amount of cerium nitrate was dissolved in concentrated sulfuric acid to afford a saturated Ce(NO3)2–H2SO4 solution, followed by the addition of the activated Y/SBA-15. One gram of the calcined Y/SBA-15 composite material was impregnated with 15 ml saturated Ce(NO3)2–H2SO4 solution and stirred at room temperature for 24 h. The resulting mixture was filtrated several times and the resultant solid was transferred again in an autoclave dried at 100 °C for 24 h. Finally, the solid was calcined at 550 °C for 3 h to afford the desired catalyst.
First, we take a certain quality of molecular sieve (conditions: Ce-Y/SBA-15, Y/SBA-15, Y), Al2O3, field of well-known powder and deionized water, then mix them, at last, turn them into the dough by rolling method. We put the dough in a squeeze of the extruding machine, next get mitsubishi article carrier, and then cut them into small pieces. The pieces were dried at 100 ℃ for 6 h and calcinated at 823 K for 5 h. Catalyst samples are named respectively as: C-X, C-Y, and C-Z.
Catalyst characterization
FT-IR was conducted to analyze the components change during the catalyst preparation; KBr (IR grade) was used to prepare the pellet (1:200). Thus, the raw powder of the molecular sieve was grinded with KBr (IR) with a ratio of 1:200 to afford the KBr pellets, with a testing range of wace number of 4,000–400 cm−1.
X-ray fluorescence spectrometry (XRF) images were recorded on a JEOL X’Pert ProI-type X-ray fluorescence spectrum instrument.
Pyridine adsorption in spectrometry (Py-FTIR) was taken on a Fourier spectrometer Bio-Rad FTS15/C-type instrument.
Thermal analysis (TG-DTG) was conducted by NETZSCH STA449F3C 951-type instrument.
Hydrocracking reaction
Design parameters of hydro cracking unit are in Table 2.
Design technical parameters (conditions: reaction pressure, reaction temperature, gas feed rate, liquid feed rate, and catalyst loading).
| Parameter | Numerical range | Control precision |
|---|---|---|
| Reaction pressure | 1–8 MPa | ±1 % |
| Reaction temperature | ≤600 ℃ | ±1.0℃ |
| Gas feed rate | 20 mL/h | ±1 % |
| Liquid feed rate | 5 mL/min | 1 % |
| Catalyst loading | 10 mL | – |
The evaluation conditions of hydrocracking reaction
Heavy oil is Daqing-mixed VGO. Heavy oil is plunger into the vaporizing chamber by double-trace pump, which occurs gasification and mixed with hydrogen at high temperature, and then mixed gas went into the preheating chamber and the reactor. The material of the reactor is stainless steel, the length of 540 mm, inner diameter of 10 mm. Filling length is 10 cm of catalyst in the reactor, the rest of the ceramic is filled by bead with 30 purposes.
Evaluation criteria of the hydrocracking reaction
Miniature hydrocracking reactor increased its temperature to 180 °C by the rate of about 2 °C/min, when the temperature reaches 330 °C temperature for 6 h, and then we empty the separator. Continue reflecting for 4 h, the record into the fluid volume and the amount of produced liquid, and gas chromatography analysis. In the hole reaction process, the total reaction pressure is 3 mPa, space velocity is 3 h−1, and hydrogen oil volume is 700:1.
Results and discussion
IR specrum
Figure 1 illustrates the IR-spectra patterns of C-X, C-Y, and C-Z. C-X’s characteristic peak is 792 cm−1 and SBA-15’s characteristic peak 1,096 cm−1. OH-group (1,632 cm−1) and Si–OH–group (3,500 cm−1) of the C-Y is not the same as the single C-Z or SBA-15, respectively. The absorption peaks (488 cm−1, 1,096 cm−1) of C-X is more obvious than C-Y [21]. There are still C-Y’s skeleton characteristic absorption peaks in C-X, which shows that there is no distinction between C-X and C-Y at the endemic skeletal structures.

IR-spectra patterns of C-X, C-Y, and C-Z.
XPS specrum
Table 3 listed out silica alumina ratio of the three kinds of molecular sieve catalyst. Silica alumina ratio of C-X is the highest of the three kinds of molecular sieve catalyst. It indicated that the acid center density of molecular sieve loaded on Ce decreased and the acid strength increased.
The higher the silica alumina ratio is, the better thermal stability and hydrothermal stability of molecular sieve have. Because C-X’s silica alumina ratio is higher than C-Y’s, C-X has better thermal stability and hydrothermal stability than C-Y. Therefore, based on the XRF data, the order of acid strength, thermal stability, and hydrothermal stability from big to small is: C-X, C-Y, and C-Z. The type of acid and acid strength is needed to prove by virtue of further characterization methods such as Py-FTIR spectra.
Silica alumina ratio of the three kinds of molecular sieve catalyst (conditions: aluminum and silicon atoms and the molar ratio Si/Al of C-X, C-Y, and C-Z).
| Item | Aluminum and silicon atoms | Si/Al molar ratio |
|---|---|---|
| C-X | 93.6 | 46.8 |
| C-Y | 89 | 44.5 |
| C-Z | 7.6 | 3.8 |
Py-FTIR spectra
Figure 2 shows Py-FTIR spectra of pyridine desorbed on C-X, C-Y, and C-Z. The IR spectra of pyridine desorption at 473 and 673 K are shown in Figure 2. A characteristic absorption peak (1,442 cm−1) belongs to weak acid L of C-Z shows that the Na+ and non-framework aluminum species of C-Z are in weak acid L. The concentration of pyridinium ions (1,545 cm−1, the signature of Brǿnsted acid sites) is higher with Ce loaded at 473 and 673 K than without Ce [22, 23], whereas the coordinated pyridine (1,455 cm−1, the signature of Lewis acid sites) is of the basic quite with C-X and C-Y [24]. The value of L acid center strength from big to small is C-Y, C-X, and C-Z.
The addition of rare earth can improve the activity of the catalyst. In molecular sieve cage, rare earth ions increased the probability of electronic migration to the cage on the skeleton and hydroxyl silicon aluminum hydroxy through function and induced polarization effect. Due to the increased density of electron cloud of molecular sieve’s cage, hydroxy has the stronger B acid strength than without Ce, while accordingly improve the activity of C-X. The value of B acid center strength from big to small is C-X, C-Y, and C-Z.

Py-FTIR spectra of pyridine desorbed on C-X, C-Y, and C-Z.
TG-DTG specrum
The thermal decomposition curves of two kinds of catalysts were shown in Figure 3. Two kinds of catalyst’s decomposition temperature are both at 700 K, whereas they have good stability. It suggested that the two kinds of catalysts have no volatile under the condition of catalytic reaction’s temperature.
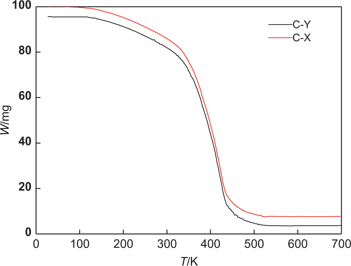
The thermal decomposition curves of two kinds of catalysts.
Catalytic of the catalyst
Table 4 shows the evaluation results of catalyst with homemade on the hydrocracking device. Hydrocracking catalysts for enhancing middle distillates yield were prepared by such composite supports [25, 26]. Composite molecular sieves’ selective of middle oil is higher and the product performance is better than microporous molecular sieves’, respectively. They have fantastic smoke point of aviation kerosene and tail oil of BMCI value. Under the same reaction temperature, as compared with C-Z, the selectivity and yield of middle distillates over C-X was 4.0 % and 4.7 % higher, respectively; besides, it is 0.7 % higher in contrast C-Y. It adequately embodies the advantages of Ce doped on composite molecular sieves which applied to the hydrocracking of heavy oil.
The evaluation results of catalyst with homemade on the hydrocracking device(conditions: reaction temperature, yield, smoke point, freezing point, viscosity index, BMCI value, yield of middle oil, and selective of middle oil of C-X, C-Y, and C-Z).
| Item | C-X | C-Y | C-Z |
|---|---|---|---|
| Reaction temperature (K) | 673 | 673 | 673 |
| The yield of distillate less than 373 K | 1.8 % | 2.0 % | 2.2 % |
| The fraction of straight-run gasoline between 373 and 473 K | |||
| Yield | 6.0 % | 5.8 % | 7.9 % |
| w(The potential aromatic hydrocarbon) | 21.5 % | 20.4 % | 19.0 % |
| The fraction of aviation kerosene between 473 and 573 K | |||
| Yield | 22.3 % | 21.8 % | 20.5 % |
| Smoke point (mm) | 25 | 25 | 27 |
| Freezing point (K) | <213 | <213 | <213 |
| The fraction of vacuum gas oil between 573 and 643 K | |||
| Yield | 23.2 % | 21.3 % | 21.2 % |
| Viscosity index | 111 | 108 | 99 |
| The tail oil (>643 k) | |||
| Yield | 40.0 % | 38.1 % | 35.3 % |
| BMCI value | 9.0 | 9.3 | 8.9 |
| Yield of middle oil | 55.7 % | 55.0 % | 51.7 % |
| Selective of middle oil | 85.9 % | 84.1 % | 81.2 % |
Reusability of the catalyst
The ability to be recycled and reused is an important criteria for catalysts, so the reusability of the three catalysts, C-X, C-Y, and C-Z, was investigated under the optimized esterification reaction conditions. The results are shown in Table 5.
The repeated use of the properties of the three kinds of molecular sieve catalyst (conditions: yield of middle oil of C-X, C-Y, and C-Z for three times of repeated use).
| The number of repeated use | Yield of middle oil (%) | ||
|---|---|---|---|
| C-X | C-Y | C-Z | |
| 1 | 55.7 | 55.0 | 51.7 |
| 2 | 42.5 | 40.2 | 37.6 |
| 3 | 30.7 | 28.1 | 25.3 |
From Table 5, it can be seen that there was an increase in the recycling times, and an obvious decrease in the yield of middle oil after reusing the catalyst for three cycles. This can be attributed to the loss of amount and surface activity of the catalyst caused by washing and filtration steps during the recycling process. In order to maintain the yield of middle oil, the quality of the catalyst should be increased and the catalysts should be activated.
Overall, the yield of middle oil by using C-X is higher than that by using C-Y or C-Z. This result is consistent with our expectations, as the value of B acid center strength from big to small is C-X, C-Y, and C-Z.
Conclusions
Composite molecular sieve C-Y was prepared by microwave method, while Ce was loaded by ion exchange method to the composite molecular sieves (C-X). It is found that
There is no distinction between C-X and C-Y at the endemic skeletal structures. The order of acid strength, thermal stability, and hydrothermal stability from big to small is C-X, C-Y, and C-Z.
Due to the increased density of electron cloud of molecular sieve's cage, hydroxy has the stronger B acid strength than without Ce, while accordingly improve the activity of C-X.
As compared with C-Z, the selectivity and yield of middle distillates over C-X was 4.0 % and 4.7 % higher, respectively; besides, it is 0.7 % higher in contrast C-Y. It adequately embodies the advantages of Ce doped on composite molecular sieves which applied to the hydrocracking of heavy oil.
Funding statement: This project was supported by Project supported by National Natural Science Foundation of China (51274060), the Scientific Research Special Foundation of Doctor Subject of Chinese Universities (20100042110008), Liaoning Shihua University college students’ innovative entrepreneurial training program Fund (2015041), and Fushun City Science and Technology Development Project Fund (FSKJHT201255).
References
[1] K.R. Kloetstra, H.W. Zandbergen and J.C. Jansen, Microporous Mater., 526 (1996) 287–293.10.1016/0927-6513(96)00036-3Search in Google Scholar
[2] F.X. Chen, H.K. Luo and Y.F. Han, Catal. Today., 131 (2008) 76–81.10.1016/j.cattod.2007.10.035Search in Google Scholar
[3] C.F. Ana, G.C. Cristiano and J.F. Maria, Microporous Mesoporous Mater., 172 (2013) 206–212.10.1016/j.micromeso.2013.01.022Search in Google Scholar
[4] X.H. Zhao, H. Wang and B.F. Dong, Microporous Mesoporous Mater., 151 (2012) 56–63.10.1016/j.micromeso.2011.11.016Search in Google Scholar
[5] J. Zhou, Z.L. Hua and X.Z. Cui, Chem. Commun., 46 (2010) 4994–4996.10.1039/c0cc00499eSearch in Google Scholar
[6] Z.Q. Cai, J. Liu and M.X.J. Shao, Chem. Soc. Pak., 36 (2014) 717–721.Search in Google Scholar
[7] Z.Q. Cai, W.Y. Ma and J. Liu, Chinese J. Struct. Chem., 36 (2014) 1383–1387.Search in Google Scholar
[8] H.S. Cho and R. Ryoo, Microporous Mesoporous Mater., 151 (2012) 107–112.10.1016/j.micromeso.2011.11.007Search in Google Scholar
[9] X.X. Wang, G. Li and W.H. Wang, Microporous Mesoporous Mater., 142 (2011) 494–502.10.1016/j.micromeso.2010.12.035Search in Google Scholar
[10] V. Stanislav, I. Irina and A.P. Olga, Microporous Mesoporous Mater., 164 (2012) 222–231.10.1016/j.micromeso.2012.08.017Search in Google Scholar
[11] Z. Jiao, W.C. Zhan, Y. Guo, Y.L. Guo, Z.Q. Gong and G.Z. Lu, Chinese J. Catal., 37 (2016) 273–280.10.1016/S1872-2067(16)62540-8Search in Google Scholar
[12] D. Dai, H.Y. Wang, M. Wei, J. Ma and X.D. Miao, J. Liaoning Shihua Univ., 25 (2005) 38–40.Search in Google Scholar
[13] X.Z. Zhou, Y. Liu, X.J. Meng, B.J. Shen and F.S. Xiao, Chinese J. Catal., 34 (2013) 1504–1512.10.1016/S1872-2067(12)60638-XSearch in Google Scholar
[14] Y.G. Dai, W.L. Cui, J.H. Yang and Y.J. Liu, J. Petrochem. Univ., 27 (2014) 19–23.Search in Google Scholar
[15] S.H. Yin, S.W. Li, W.Y. Wu, X. Bian, J.H. Peng and L.B. Zhang, RSC Adv., 104 (2014) 59997–60001.10.1039/C4RA10143JSearch in Google Scholar
[16] L.B. Zhang, F. Xie, S.W. Li, S.H. Yin, J.H. Peng and S.H. Ju, Green Process. Synth., 1 (2014) 3–10.Search in Google Scholar
[17] S.H. Yin, L.B. Zhang, J.H. Peng, S.W. Li, S.H. Ju and L.H. Zhang, Chem. Eng. Process. Process Intensif., 91C (2015) 1–6.10.1016/j.cep.2015.03.003Search in Google Scholar
[18] S.H. Yin, S.W. Li, J.H. Peng and L.B. Zhang, RSC Adv., 5 (2015) 48659–48664.10.1039/C5RA07113ESearch in Google Scholar
[19] R. Zhou and Y. Cao, Appl. Catal. A General, 236 (2012) 103–108.10.1016/S0926-860X(02)00281-8Search in Google Scholar
[20] M.A. Centeno, M. Paulis and M.P. Galis, Appl. Catal. A General, 234 (2002) 65–67.10.1016/S0926-860X(02)00214-4Search in Google Scholar
[21] T. Noda, K. Suzuki and N. Katada, J. Catal., 259 (2008) 203–208.10.1016/j.jcat.2008.08.004Search in Google Scholar
[22] X.W. Li, L.Q. She, X.Y. Liu and J. Chin, Catal. Lett., 4 (1983) 43–50.10.1007/BF00764869Search in Google Scholar
[23] P. Garcia, E. Lima, J. Aguilar and V. Lara, Catal. Lett., 128 (2009) 385–389.10.1007/s10562-008-9761-5Search in Google Scholar
[24] T. Noda, K. Suzuki and N. Katada, J. Catal., 259 (2008) 203–210.10.1016/j.jcat.2008.08.004Search in Google Scholar
[25] H.L. Chen, B.J. Shen and H.F. Pan, Chinese J. Catal., 25 (2004) 715–720.Search in Google Scholar
[26] S.Q. Yu, H.P. Tian and Y.X. Zhu, J. Acta Phys. Chim. Sin., 27 (2011) 2528–2534.10.3866/PKU.WHXB20111101Search in Google Scholar
© 2017 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Estimation for Iron Redox Equilibria in Multicomponent Slags
- The Effect of Multi-inclined Holes on the Creep Properties of Nickel-Based Superalloy
- Estimation of Various Properties of CaO–“FeO”–SiO2 System at 1,673 K by Mass Triangle Model
- The Enhancing Effect of Microwave Irradiation and Ultrasonic Wave on the Recovery of Zinc Sulfide Ores
- The Self-assembled Deposition on the Surface of Mono-crystalline Silicon Induced by High-Current Pulsed Electron Beam
- Numerical Model of Dephosphorization Reaction Kinetics in Top Blown Converter Coupled with Flow Field
- Morphological Evolution of Low-Grade Silica Fume at Elevated Temperature
- Discussion of Carbon Emissions for Charging Hot Metal in EAF Steelmaking Process
- Predictive Models for Modulus of Rupture and Modulus of Elasticity of Particleboard Manufactured in Different Pressing Conditions
- Photoluminescence Properties of Eu3+-activated Silicate Phosphors
- Synthesis, Acidity and Catalytic of the Rare Earth Ce Loaded on the Composite Pore Zeolite Catalyst for Hydrogenation Cracking
Articles in the same Issue
- Frontmatter
- Research Articles
- Estimation for Iron Redox Equilibria in Multicomponent Slags
- The Effect of Multi-inclined Holes on the Creep Properties of Nickel-Based Superalloy
- Estimation of Various Properties of CaO–“FeO”–SiO2 System at 1,673 K by Mass Triangle Model
- The Enhancing Effect of Microwave Irradiation and Ultrasonic Wave on the Recovery of Zinc Sulfide Ores
- The Self-assembled Deposition on the Surface of Mono-crystalline Silicon Induced by High-Current Pulsed Electron Beam
- Numerical Model of Dephosphorization Reaction Kinetics in Top Blown Converter Coupled with Flow Field
- Morphological Evolution of Low-Grade Silica Fume at Elevated Temperature
- Discussion of Carbon Emissions for Charging Hot Metal in EAF Steelmaking Process
- Predictive Models for Modulus of Rupture and Modulus of Elasticity of Particleboard Manufactured in Different Pressing Conditions
- Photoluminescence Properties of Eu3+-activated Silicate Phosphors
- Synthesis, Acidity and Catalytic of the Rare Earth Ce Loaded on the Composite Pore Zeolite Catalyst for Hydrogenation Cracking

