Abstract
The oxidation behavior of Ti-5Al-2.5Sn and Ti-6Al-4V produced by hot isostatic pressing (HIP) has been studied at 650–850°C in air for 24 h. The oxidation kinetics of both alloys followed the parabolic law with good approximation, except for Ti-5Al-2.5Sn oxidized at 850°C. Multi-layered scales formed on both alloys at 750°C and 850°C. Ternary additions of Sn and V accounted for the different morphology of the scales formed on these two alloys. In addition, the oxidation behavior of HIP alloys is compared with that of the corresponding cast alloys and the scaling mechanism is discussed.
Introduction
Pure Ti and Ti-base alloys are widely used in aerospace, automotive and biomedical industry due to their high strength-to-weight ratio, good mechanical properties and corrosion resistance [1]. However, poor oxidation resistance and high oxygen solubility restrict their application at high temperatures. Many researchers have investigated the oxidation behavior of pure Ti [2–6] and the relative Ti-base alloys [5, 7–16]. The oxidation behavior of pure Ti below 1,000°C was controlled by the inward diffusion of oxygen in TiO2 which is an n-type semiconductor and contains oxygen ion vacancies [2, 17]. The addition of Al improves the oxidation resistance of Ti by modifying the oxidation mechanism due to the formation of a protective layer of alumina [9, 10, 18].
The hot isostatic pressing (HIP) technology, commonly applied for aerospace, watercraft and automobile industries, has been developed particularly to produce the components with complicated shapes for its potential economical convenience with respect to the conventional processing methods [19]. The Ti-based alloy components synthesized by this process have various advantages such as fine grain size and homogeneous microstructure and no internal defects, showing good mechanical properties close to those of the wrought alloys [20].
The present work was designed to investigate the oxidation behavior of the two alloys Ti-5Al-2.5Sn and Ti-6Al-4V made by HIP technique and to determine the upper limit temperature of application in air. The air oxidation behavior of the two HIP alloys is finally compared with that of the corresponding cast alloys.
The corrosion behavior of cast alloys with composition similar to Ti-6Al-4V has been widely studied in various atmospheres, such air [11, 14, 17], H2/H2O [5], H2/H2O/H2S [5], water vapor [3] at various temperature from 650 to 950°C, while only a single study has concerned the oxidation of a conventional Ti-5Al-2.5Sn alloys [9]. Conversely, the oxidation behavior of Ti-base alloys produced by HIP has never been reported so far.
Experimental
The alloys used for this study are denoted as Ti-5Al-2.5Sn and Ti-6Al-4V, respectively (all in mass%, if not otherwise specified). Ti-5Al-2.5Sn is a single α phase, while Ti-6Al-4V is composed of a mixture of the two phases α and β. The microstructures of the two alloys are shown in Figure 1: the dark strip-like β particles of Ti-6Al-4V are evenly distributed in α substrate.

Optical microstructure of Ti-5Al-2.5Sn (a) and Ti-6Al-4V (b) alloys. (a) single α phase; (b) dark = β phase, light matrix = α phase.
The two alloys Ti-5Al-2.5Sn and Ti-6Al-4V [19–21] were prepared by powder metallurgy (PM) HIP process. Pre-alloyed powders with particle size less than 250 μm were prepared by electrode induction melting gas atomization process. The powders were put into cylindrical metal capsules under pressures varying from 120 to 150 Mpa for 2–4 h. The temperature was controlled between 920°C and 940°C. The average grain size of the two HIP alloys was around 30 μm.
The Ti-6Al-4V specimens with a size of 10 × 8 × 1.5 mm and the cylindrical specimens of Ti-5Al-2.5Sn with a diameter of around 10 mm and 2 mm thick were cut from ingots using a line saw and a 1 mm hole was drilled near one edge. All the specimens were mechanically abraded on successively finer abrasive papers down to 2,000 grit and finally cleaned with water, acetone and ethanol and dried immediately before each test. The oxidation experiments were carried out in a microbalance Setaram B-92.
All the HIP alloy samples used in this work were oxidized in air for 24 h at 650, 750 and 850°C, respectively. The corroded samples were examined by means of scanning electron microscopy (SEM), generally using the back-scattered electron image (BEI) mode, energy dispersive X-ray microanalysis (EDX), and XRD to study the structure of the scales, to identify the nature of the phases and their distribution.
Results
Corrosion kinetics
The kinetic curves for the oxidation of Ti-5Al-2.5Sn and Ti-6Al-4V for 24 h in air at 650–850°C are shown separately in Figures 2(a) and 3(a) as linear plot and in Figures 2(b) and 3(b) as parabolic plots, respectively.
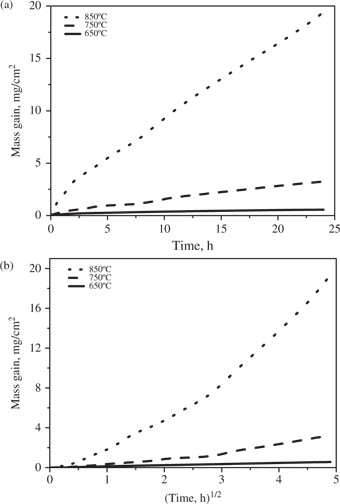
Kinetic curves for the oxidation of Ti-5Al-2.5Sn in air at 650, 750 and 850°C for 24 h: (a) linear plots; (b) parabolic plots.
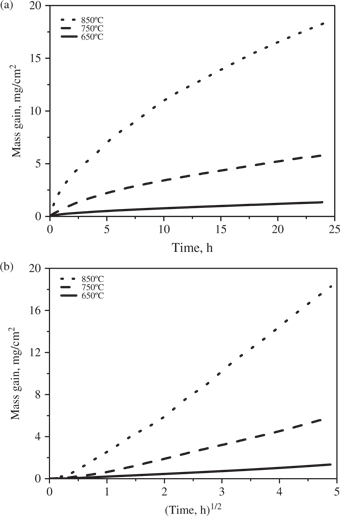
Kinetic curves for the oxidation of Ti-6Al-4V in air at 650, 750 and 850°C for 24 h: (a) linear plots; (b) parabolic plots.
After an initial period of slow rate of mass gain for the first 0.1 h, denoted as an incubation period, the corrosion kinetics of Ti-5Al-2.5Sn at 650°C followed the parabolic rate law with a parabolic rate constant (kp) equal to 3.52 × 10−12 g2 cm−4 s−1. Conversely, the kinetic curves at 750°C were more complex because, after an initial incubation period, they followed two parabolic stages connected by a period lasting from 2.5 h to 10 h during which the mass gain first decreased and then increased, indicating the spallation and cracking of the scales. The kp for the first parabolic stage, lasting from 0.1 h to 2.5 h, was equal to 4.83 × 10−11 g2 cm−4 s−1, while the kp for the second parabolic stage, lasting from 10 h to 24 h, was equal to 2.50 × 10−10 g2 cm−4 s−1. At 850°C, the oxidation of Ti-5Al-2.5Sn initially (from the start to 1.5 h) obeyed approximately the parabolic rate law with a kp equal to 1.595 × 10−8 g2 cm−4 s−1, and then followed the linear law with a rate constant kc equal to 5.94 × 10−7 g cm−2 s−1 up to the end of the test.
The oxidation of Ti-6Al-4V, at all temperatures followed the parabolic rate law after an initial incubation period of variable durations and of small rates of mass gain. At 650°C, the parabolic period lasted from 0.5 h to 24 h with a kp equal to 2.82 × 10−11 g2 cm−4 s−1. The oxidation kinetics at 750°C followed the parabolic law from 2.3 h up to 24 h with a kp equal to 4.86 × 10−10 g2 cm−4 s−1 after a transition period presenting an increase of the instantaneous slope of the parabolic plot. At 850°C, the parabolic period lasted from 2 h to 24 h with a kp value equal to 4.47 × 10−9 g2 cm−4 s−1.
For both alloys, the mass gains during the oxidation after a fixed time increased with temperature, especially in going from 750°C to 850°C.
Morphology and structure of the scales
The scale formed on Ti-5Al-2.5Sn oxidized at 650°C for 24 h (Figure 4) was compact and contained mainly TiO2 with some Al in solution, as shown by the EDX results. At 750°C (Figure 5), the scale was mainly composed of a porous TiO2 layer with a thickness of around 20 μm which was overgrown by a thin layer of about 3 μm composed of a mixture of Al and Ti oxides. In addition, a small part of the inner layer close to the interface with the outer layer was rich in Sn. According to the EDX results the scale formed at 850°C (Figure 6) was divided into two layers. The outer zone was composed of a mixture of oxides of Al and Ti. In addition, the Al2O3 particles were agglomerated into large regions with a ribbon-like shape and were perpendicular to the alloy surface and surrounded with voids. The inner layer was made of pure TiO2 and was more compact than the outer region. In addition, Sn particles were distributed within the inner sub-layer of TiO2 and were aggregated into a thin layer of the base alloy close to the interface with the scale.
The scale formed on Ti-6Al-4V after 24 h at 650°C (Figure 7) was mainly composed of TiO2 and Al2O3 according to the XRD result. The darker regions of the scale close to the scale/gas interface were rich in Al2O3. Moreover, close to the alloy the scale was porous and peeled off from the matrix. At 750°C (Figure 8), the scale morphology was similar to that formed at 650°C but thicker and more porous. The sandwiched sub-layers of Al2O3 were parallel to the scale/alloy interface and spalled off from the TiO2 sub-layers. This phenomenon was more evident at 850°C (Figure 9).

Micrograph (SEM/BEI) of a cross section of Ti-5Al-2.5Sn oxidized in air at 650°C for 24 h.
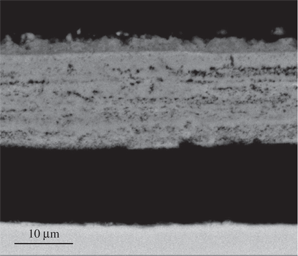
Micrograph (SEM/BEI) of a cross section of Ti-5Al-2.5Sn oxidized in air at 750°C for 24 h.
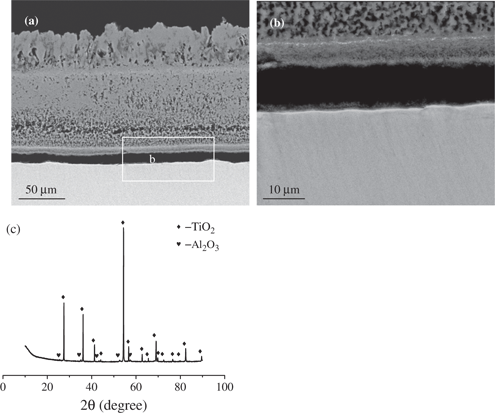
Micrograph (SEM/BEI) of a cross section and XRD spectrum of Ti-5Al-2.5Sn oxidized in air at 850°C for 24 h: (a) general view, (b) enlarged view of the selected area of (a), (c) XRD spectrum.
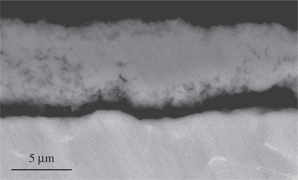
Micrograph (SEM/BEI) of a cross section of Ti-6Al-4V oxidized in air at 650°C for 24 h.
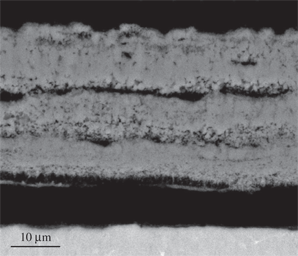
Micrograph (SEM/BEI) of a cross section of Ti-6Al-4V oxidized in air at 750°C for 24 h.
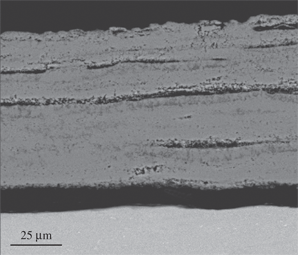
Micrograph (SEM/BEI) of a cross section of Ti-6Al-4V oxidized in air at 850°C for 24 h.
Discussion
Mechanism of formation of the scales
Rutile is a n-type semiconductor caused by an oxygen deficit and the activation energy for the diffusion of oxygen within this oxide is much lower than that of titanium. Thus, at relatively low temperatures the growth of TiO2 in the oxidation of pure Ti is mainly due to the inward diffusion of oxygen. However, above 1,000°C both Ti and O contribute to the growth of the oxide by diffusing in opposite directions.
The growth mechanism of the scales formed on the Ti-Al alloys has been rather extensively investigated [9, 10, 18, 22, 23]. Du et al. [5] proposed that the oxides of Ti and Al nucleate at active sites on the alloy surface and grow simultaneously. However, later Al2O3 is wrapped up by TiO2. Conversely, Maximovitch et al. [9] claimed that the oxidation of Al occurs only when the Al content exceeds 12.5 wt%. TiO2 forms at both the scale/alloy and the scale/gas interface due to the inward diffusion of oxygen and the outward diffusion of Ti through the TiO2 layer with a large density of defects. Al also can diffuse outward to the outer region of the scale to form Al2O3 due to a displacement reaction with TiO2.
Typical scales formed on dilute Ti-Al alloys contain two layers [7, 24]. The external layer with large columnar crystals and huge voids is formed by an outward diffusion of cations and is composed of a mixture of TiO2 and Al2O3 where Al2O3 is mainly distributed in the inner region. This structure is partly attributed to the fact that the solubility of Al2O3 in TiO2 decreases with an increase of the oxygen pressure [22]. The inner thick layer with fine grains and numerous small voids is mainly composed of TiO2. Beyond that, other structures involving the presence of alternate layers of rutile and alumina have also been observed [5, 11, 14].
The reactivity of the various metals involved in the composition of the present alloys with oxygen increases in the order of Sn, V, Ti and Al. Therefore, when Ti-Al alloys with additions of V or Sn are oxidized in air, Al and Ti react with O to form the corresponding oxides. Conversely, Sn is stable as such in the scale and agglomerates into large metal particles. V is oxidized into VO2 and then into V2O5 [25]. However, V2O5 in the scale close to the scale/gas interface is exposed to volatilization due to its low melting point (670–690°C).
The protective scale formed on Ti-5Al-2.5Sn at 650°C is mainly promoted by the outward diffusion of Al and Ti. As the oxidation temperature raises to 750°C, the rate of the inward diffusion of oxygen increases more rapidly than that of the outward diffusion of Ti and Al and becomes the dominating factor for the growth of the scale, resulting in the exclusive growth of the TiO2 at the scale/alloy interface. However, by oxidation at 850°C all the elements are able to diffuse quickly: the outward diffusion of Ti and Al results in the growth of the outer layer of Ti and Al oxides, while the inward diffusion of oxygen results in the growth of the inner TiO2 layer. In addition, this TiO2 layer is divided into two sub-layers, the outer region being more porous than the inner zone. It is proposed that the porous structure results from a transformation of an initially compact layer due to the recrystallization of TiO2. During the recrystallization of the new formed TiO2 layer, most of Sn is segregated back into the base alloy, while the remaining fraction is kept in the scale.
However, by oxidation at 650 and 750°C there is no enrichment of Sn in the substrate, probably because at lower temperatures the scales are thinner and the content of Sn segregated from the scale into the alloy is not sufficient to form a thin layer of Sn.
The growth mechanism of the scales formed on Ti-6Al-4V has already been investigated [11]. A thin TiO2 scale formed on the alloy due to its high growth rate at the gas/alloy interface during an initial stage. Later on, Al diffuses outward to form Al2O3 at the gas/TiO2 interface, while oxygen diffuses inward and reacts with Ti at the scale/alloy interface. This double-layered oxide scale grows up to a critical thickness and then cracks partly from the base alloy due to the accumulation of growth stresses at the alloy/scale interface. This detachment prevents the outward diffusion of Al and Ti, while oxygen is still able to diffuse inward. As the oxygen pressure increases, the above oxidation process is repeated and forms the double-layered scales. This mechanism is demonstrated by the enrichment of the Al oxide in the upper side of the sub-layers.
The structure of the scales formed on Ti-5Al-2.5Sn differs from that of the scales formed on Ti-6Al-4V, especially at 850°C, due to the different nature of the alloying elements of the two alloys. Some researchers believe that the addition of V is detrimental for the oxidation resistance [26] by reducing the activity of Al in Ti-6Al-4V and suppressing the formation of Al2O3. In addition, the oxidation states of V in the inner layer of the scale are +5 and +4 [25]. It is assumed that V4+ ions dissolved into TiO2 scale take the interstitial positions and consequently decrease the concentration of interstitial Ti4+ ions. According to Wagner–Hauffe theory [27], the presence of V5+ is expected to reduce the concentration of interstitial Ti4+ ions. Therefore, the outward diffusion rate of Ti4+ decreases and the thickness ratio between the outward- and inward- growing sections of the scale decreases. Al2O3 forms at the outer region of the layer. When the initial double-layered scale reaches a critical thickness, it spalls off from the base alloy. However, the addition of Sn has no clear effect on the mass gains in the oxidation of Ti-based alloys [26]: for Ti-5Al-2.5Sn, according to the scale morphology, Sn dissolves slightly in TiO2. Most of the Sn precipitates at the interface between the scale and the alloy. Therefore, Sn has only small effects on the diffusing processes in the scale. The morphology of the scale formed on Ti-5Al-2.5Sn is similar to that of typical scales formed on dilute Ti-Al alloys (with Al content varying from 3.65–10 wt%) [10].
Differences between conventional and HIP alloys
The parabolic rate constants for the air oxidation of a conventional Ti-6Al-4V [11] at 650°C, 750°C and 850°C are of the same order of magnitude as those of the HIP alloy. The scales formed on the conventional alloy are similar to those grown on the HIP alloys. An Arrhenius plot of the parabolic rate constant for the oxidation of HIP Ti-6Al-4V, shown in Figure 10, yields an activation energy, Ea, equal to 217 kJ/mol: this value is lower than that of the conventional alloy (267 kJ/mol) [11], but close to that found for the air oxidation of pure titanium (214 kJ/mol) [10]. Thus, the oxidation resistance of Ti-6Al-4V made by HIP in air is weaker than that of the cast alloy. This difference may be caused by the larger density of grain boundaries in the HIP alloy than that in the cast alloys. Grain boundaries provide rapid diffusion channels and may play an important role in the diffusion of all species which controls the rate of growth of the scale and generates lower values of Ea.
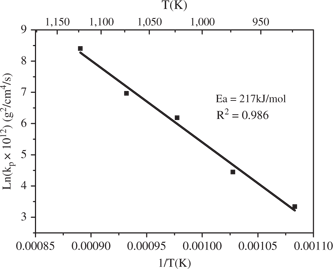
Arrhenius plot for the air oxidation of Ti-6Al-4V made by HIP process.
The activation energy for the air oxidation of Ti-5Al-2.5Sn calculated from the present data, about 255 kJ/mol, cannot be compared with that for the corresponding conventional alloy, never reported so far. It may only be observed that this Ea value is rather close to that of the conventional Ti-6Al-4V alloy and significantly larger than that of HIP Ti-6Al-4V.
Conclusions
The air oxidation of the alloys Ti-5Al-2.5Sn and Ti-6Al-4V produced by HIP followed the parabolic rate law at 650–850°C, except for the oxidation of Ti-5Al-2.5Sn at 850°C which obeyed the linear rate law. Lamellar scales were formed on both alloys at 750°C and 850°C. The scale formed on Ti-5Al-2.5Sn at 650°C was protective, while the scales grown at the other two temperatures were composed of a mixed outer layer of Al2O3 and TiO2 and an inner layer of pure TiO2. The addition of Sn to Ti-based alloy had negligible effects on the oxidation behavior, while Sn was mostly segregated back into the base alloy. Conversely, the scales grown on Ti-6Al-4V consisted of alternating sub-layers of Al2O3 and TiO2 with Al2O3 preferentially located in the upper side of the sub-layers. Ternary addition of V decreased the Al activity in Ti-6Al-4V, which resulted initially in the exclusive formation of TiO2, leading later to the formation of sandwiched Al2O3 sub-layers. Due to the larger density of grain boundaries, the oxidation resistance in air of the Ti-6Al-4V alloy made by HIP is weaker than that of the conventional alloy.
Funding statement: Funding: A financial support by the NSFC (China) under the research project (No. 51371183) is gratefully acknowledged.
References
[1] R.R.Boyer, Mater. Sci. Eng. A, 213 (1996) 103–114.Search in Google Scholar
[2] P.Kofstad, K.Hauffe and H.Kjollesdal, Acta Chem. Scand., 12 (1958) 239–266.Search in Google Scholar
[3] F.Motte, C.Coddet, P.Sarrazin, et al., Oxid. Met., 10 (1976) 113–126.Search in Google Scholar
[4] J.E.L.Gomes and A.M.Huntz, Oxid. Met., 14 (1980) 471–498.Search in Google Scholar
[5] H.L.Du, P.K.Datta, D.B.Lewis, et al., Oxid. Met., 45 (1996) 507–527.Search in Google Scholar
[6] Y.Wouters, A.Galerie and J.P.Petit, Solid State Ionics, 104 (1997) 89–96.Search in Google Scholar
[7] R.N.Shenoy, J.Unnam and R.K.Clark, Oxid. Met., 26 (1986) 105–124.Search in Google Scholar
[8] A.M.Chaze and C.Coddet, J. Mater. Sci., 22 (1987) 1206–1214.Search in Google Scholar
[9] G.G.Maksimovich, V.N.Fedirko, A.T.Sobolevskii, et al., Soviet Mater. Sci., 25 (1989) 276–279.Search in Google Scholar
[10] A.M.Chaze and C.Coddet, J. Less-Common Metals, 157 (1990) 55–70.Search in Google Scholar
[11] H.L.Du, P.K.Datta, D.B.Lewis, et al., Corros. Sci., 36 (1994) 631–642.Search in Google Scholar
[12] S.Thongtem, T.Thongtem and M.McNallan, Surf. Interface Anal., 32 (2001) 306–309.Search in Google Scholar
[13] Y.Xiong, S.Zhu and F.Wang, Surf. Coat. Technol., 190 (2005) 195–199.Search in Google Scholar
[14] H.Guleryuz and H.Cimenoglu, J. Alloy. Compd., 472 (2009) 241–246.Search in Google Scholar
[15] D.Poquillon, C.Armand and J.Huez, Oxid. Met., 79 (2013) 249–259.Search in Google Scholar
[16] W.L.Xiao, H.Murakami, D.H.Ping, et al., J. Mater. Sci., 48 (2013) 3363–3369.Search in Google Scholar
[17] Z.X.Zhang, H.Dong, T.Bell, et al., Oxid. Met., 66 (2006) 91–106.Search in Google Scholar
[18] R.A.Perkins, K.T.Chiang and G.H.Meier, Scripta. Metal., 21 (1987) 1505–1510.Search in Google Scholar
[19] L.Xu, J.Wu, Y.Y.Liu, et al., Titanium, 28 (2011) 19–23.Search in Google Scholar
[20] W.X.Cheng, L.Xu, J.F.Lei, et al., J. Nonferr. Met. Chin., 23 (2013) 362–369.Search in Google Scholar
[21] R.P.Guo, L.Xu, C.G.Ba, et al., J. Nonferr. Met. Chin., 8 (2014) 2050–2056.Search in Google Scholar
[22] S.Becker, A.Rahmel, M.Schorr, et al., Oxid. Met., 38 (1992) 425–464.10.1007/BF00665663Search in Google Scholar
[23] X.Yuming, Z.Shenglong, W.Fuhui, et al., J. Coat. Technol. Res., 5 (2008) 93–98.10.1007/s11998-007-9057-5Search in Google Scholar
[24] L.Zhang, J. Nonferr. Met. Chin., 16 (2006) 899–903.Search in Google Scholar
[25] M.Hou, An comparative investigation on thermal oxidation of Ti6Al7Nb and Ti6Al4V medical titanium alloys, Master thesis: Zhejiang University(2010).Search in Google Scholar
[26] Y.Shida, H.Anada, Oxid. Met., (1996) 197–219.10.1007/BF01046826Search in Google Scholar
[27] M.M.Li, Chapter 2 in High Temperature Corrosion of Metals, Metallurgical Industry Press, Beijing (2001) 67.Search in Google Scholar
©2016 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of Cations on Corrosion of Inconel 625 in Molten Chloride Salts
- Thermodynamic Analysis on the Minimum of Oxygen Content in the Deoxidation Equilibrium Curve in Liquid Iron
- Air Oxidation Behavior of Two Ti-Base Alloys Synthesized by HIP
- Generation of Constant Life Diagram under Elevated Temperature Ratcheting of 316LN Stainless Steel
- Effect of c-BN Size and Content on the Self-Propagating High-Temperature Synthesis of c-BN Composites Bonded with Ti-Al-C System Multiphase Products
- Effect of Welding Speeds on Mechanical Properties of Level Compensation Friction Stir Welded 6061-T6 Aluminum Alloy
- Simulation of Thermo-viscoplastic Behaviors for AISI 4140 Steel
- Transition Metal Nitrides: A First Principles Study
- The Constitutive Relationship and Processing Map of Hot Deformation in A100 steel
- Preparation of Granular Red Mud Adsorbent using Different Binders by Microwave Pore – Making and Activation Method
- On the Structure and Some Properties of LaCo Co-substituted NiZn Ferrites Prepared Using the Standard Ceramic Technique
- Effect of MgO and MnO on Phosphorus Utilization in P-Bearing Steelmaking Slag
- Dependence of Temperature and Slag Composition on Dephosphorization at the First Deslagging in BOF Steelmaking Process
Articles in the same Issue
- Frontmatter
- Research Articles
- Effects of Cations on Corrosion of Inconel 625 in Molten Chloride Salts
- Thermodynamic Analysis on the Minimum of Oxygen Content in the Deoxidation Equilibrium Curve in Liquid Iron
- Air Oxidation Behavior of Two Ti-Base Alloys Synthesized by HIP
- Generation of Constant Life Diagram under Elevated Temperature Ratcheting of 316LN Stainless Steel
- Effect of c-BN Size and Content on the Self-Propagating High-Temperature Synthesis of c-BN Composites Bonded with Ti-Al-C System Multiphase Products
- Effect of Welding Speeds on Mechanical Properties of Level Compensation Friction Stir Welded 6061-T6 Aluminum Alloy
- Simulation of Thermo-viscoplastic Behaviors for AISI 4140 Steel
- Transition Metal Nitrides: A First Principles Study
- The Constitutive Relationship and Processing Map of Hot Deformation in A100 steel
- Preparation of Granular Red Mud Adsorbent using Different Binders by Microwave Pore – Making and Activation Method
- On the Structure and Some Properties of LaCo Co-substituted NiZn Ferrites Prepared Using the Standard Ceramic Technique
- Effect of MgO and MnO on Phosphorus Utilization in P-Bearing Steelmaking Slag
- Dependence of Temperature and Slag Composition on Dephosphorization at the First Deslagging in BOF Steelmaking Process

