Abstract
This paper presents the results of investigations carried out on processing and characterization of CrB2-based novel composites. Niobium metal powder was used as additive to form new composites. Hardness and fracture toughness of chromium boride increased by addition of niobium. CrB2 composites were prepared by addition of 2.5, 10 and 20 wt.% Nb. Density of higher than 95% ρth was achieved in all the samples. Hot pressed samples were analyzed to contain reaction products of NbB2, Cr2B3 and Cr3B4 phases along with CrB2. Hardness of CrB2 composite was increased from 18.46 GPa to 21.89 GPa by increasing the Nb content from 2.5 to 10 wt.%. Fracture toughness of composites prepared by addition of 2.5 and 10% was measured as 3.11 and 3.38 MPa.m1/2 respectively. Addition of 20% Nb resulted in increased fracture toughness of 4.32 MPa.m1/2.
Introduction
CrB2 is a potential candidate for structural applications, which requires high temperature strength and stability under severe conditions [1]. It has high melting point (2,200°C), high hardness (~20 GPa) and good wear resistance [2, 3]. Chromium diboride has considerable potential for hard coatings on cutting tools and as a protective coating for material exposed to wear and corrosion [4, 5]. It fulfills the function of a protective layer in chromium alloys and stainless steels [5, 6]. CrB2 is also used as additive for improvement in properties of high performance ceramic materials like borides and carbides [7–9].
Most of the literature on CrB2 is related to thin film formations [10–12], whereas literature on bulk processing of CrB2 is very limited. In bulk form borides can be processed by powder metallurgy route. Chromium borides can be synthesized by (a) reaction between elemental boron and chromium powder [1, 13] (b) borothermic reduction of Cr2O3 [14] (c) boron carbide reduction of Cr2O3 [15].
Usually, densification of boride powders is extremely difficult due to presence of covalent bonding and low self-diffusivity. High temperature and external pressure is required to get dense shapes of borides. Pressureless sintering and hot pressing of CrB2 have been studied by Iizumi et al. [16] Hiroki et al. [17] have studied sintering of CrB powder by hot isostatic pressing. In this study, investigations were carried out on processing and characterization of CrB2-based composite. Niobium metal powder was used as additive to form new composites. Nb had been used as sinter additive to ZrB2 and was found to increase the mechanical properties of boride [18]. As per author’s knowledge, there is no report on effect of Nb addition on microstructure and properties of CrB2.
Experimental
Starting material
In-house synthesized CrB2 (D50: 6.7 µm, “C”: 0.9 wt.%, “O”: 0.6 wt.%) and commercial Nb (99.7% pure) powder were used as starting materials. CrB2 powder was prepared by boron carbide reduction of Cr2O3 in presence of carbon. Preparation details of CrB2 powder are presented elsewhere [15]. Figure 1 presents the XRD pattern of the starting powders.
Densification and characterization
For densification, weighed quantities of fine chromium diboride and Nb powder were mixed thoroughly using a motorized mortar and pestle in dry condition for 1 h to obtain samples of different compositions. Figure 2(a) and (b) presents the particle size distribution of CrB2 and Nb powders respectively. CrB2 powder has tri-modal distribution and particle size in the range of 0.1 μm to 20 μm. Median particle diameter was measured as 6.79 μm. Distribution of Niobium powder was monomodal and the particle size ranges from 10 μm to 70 μm. Median particle diameter was measured as 28.16 μm.
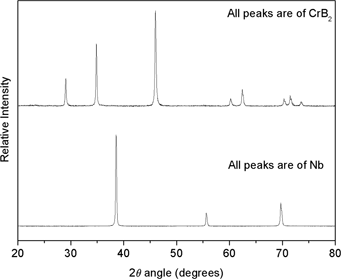
XRD pattern of starting CrB2 and Nb powder
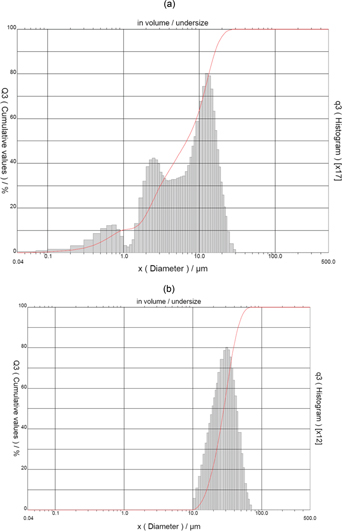
Particle size distribution of (a) CrB2 and (b) Nb powder
The powder mixtures were then loaded in a high density graphite die (12 mm dia) and hot pressed at a temperature of 1,600°C under a pressure of 35 MPa for 2 h in a high vacuum (1×10−5 mbar) chamber. The pellets were ejected from the die after cooling and the density measured by liquid displacement method. Densified samples were polished to mirror finish using diamond powder of various grades from 15 to 0.25 μm in an auto polisher (Laboforce-3, Struers). Microhardness was measured on the polished surface at a load of 100 g and dwell time of 10 s. The indentation fracture toughness (KIC) data were evaluated by crack length measurement of the crack pattern formed around Vickers indents (using 10 Kg load), adopting the model formulation proposed by Anstis et al. [19],
where E=Young’s modulus, H=Vickers’s hardness, P=Applied indentation load, c=Half crack length.
Elastic modulus of the samples was measured by ultrasonic method. The reported value of hardness and fracture toughness are the average of five measured values. Polished and fractured surfaces of dense pellets were analyzed by scanning electron microscope and energy dispersive spectroscopy (EDS). X-ray diffraction was also used for phase analysis.
Results and discussion
Densification
Table 1 summarizes the results of densification experiments carried out by hot pressing. CrB2 powder containing 0, 2.5, 10 and 20 wt.% Nb was hot pressed. A density of higher than 95% ρth was achieved in all the composites consolidated by hot pressing at 1,600°C and 34 MPa pressure. For monolithic CrB2, only 89.42% density was obtained under similar hot pressing condition. The enhanced densification by addition of Nb could be due to reaction sintering caused by reaction between CrB2 and Nb which resulted in formation of NbB2. More details of the reaction are discussed in Section “Phase Analysis and Microstructure”. Iizumi et al. [16] have reported that chromium borides (Cr2B, CrB and CrB2) obtained by solid state reaction of Cr and B in the temperature range of 1,400 to 1,500°C. These powders [16] could not be consolidated by pressureless sintering process and were densified by hot pressing at about 1,700°C.
Effect of Nb addition on densification and properties of CrB2
| Sample | Temperature (°C) | Pressure | Density | Hardness (GPa) | KIC (MPa.m1/2) | Elastic modulus (GPa) |
| CrB2 | 1,600 | 34 MPa | 89.42 | 11.45 | 3.10 | 481 |
| CrB2 + 2.5% Nb | 1,600 | 34 MPa | 98.66 | 18.46 | 3.11 | 510 |
| CrB2 + 10% Nb | 1,600 | 34 MPa | 98.32 | 21.89 | 3.38 | 559 |
| CrB2 + 20% Nb | 1,600 | 34 MPa | 96.9 | 17.34 | 4.32 | 570 |
Phase analysis and microstructure
XRD patterns of sintered CrB2 pellet processed with different content are shown in Figure 3. It indicates the presence of crystalline CrB2 and the reaction products such as Cr2B3, Cr3B4 and NbB2. The presence of NbB2 in XRD pattern indicates that niobium is converted into niobium boride. Niobium boride formation takes place due to reaction of niobium with chromium boride. Required boron to form NbB2 will be obtained from the matrix CrB2. As a result, boron-deficient chromium boride such as Cr2B3 and Cr3B4 is formed. The formation of NbB2, Cr2B3 and Cr3B4 can be explained by following possible reactions:
Reaction (1) is thermodynamically feasible at hot pressing temperature of 1,400°C in vacuum (1×10−5 mbar). Gibbs free energy change obtained using FactSage database for reaction 1 is presented in Figure 4. It shows that free energy change is negative for all the temperature. Elemental chromium, which formed as per reaction (1), reacts with CrB2 and forms Cr3B4 (reaction 2) and Cr2B3 (reaction 3). Simultaneously, CrB2 and Nb can react and result in formation of NbB2, Cr2B3 and Cr3B4 as per reaction (4). Thermodynamic calculations for reactions (2 to 4) could not be computed as data for Cr3B4 and Cr2B3 are not available.
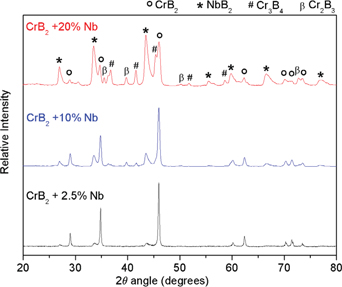
XRD pattern of hot pressed CrB2 composites prepared by addition of Nb
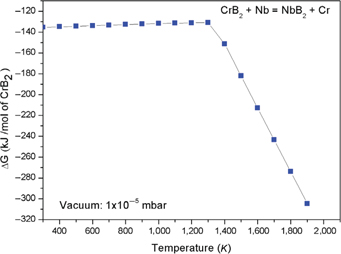
Free energy change for reaction between CrB2 and Niobium (calculated using FactSage software)
From XRD pattern, it is observed that as the percentage of niobium increases in chromium boride, the intensity of niobium boride is higher in composite material.
Figure 5 presents the BSE image of CrB2-based composite prepared by addition of 20% Nb, which shows the presence of gray matrix and in which, second phase (lighter shade) is dispersed. In EDS spectra, the gray matrix was analyzed to contain only Cr and B indicating that, it is CrB2. The lighter shade was analyzed to contain mainly Nb in which very little amount of Cr (~4 atom %) is present. Chromium, which formed as per the reaction (1), could be diffused into Nb. Other phases such as Cr3B4 and Cr2B3 could not be seen in microstructure, due to difference in atomic number is not sufficient to distinguish the contrast of different chromium boride phases.
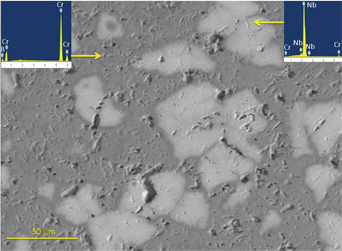
SEM image and elemental analysis of different phases in CrB2 composite prepared by addition of 20% Nb
Mechanical properties and fractography
Measured Vickers hardness, fracture toughness and elastic modulus of CrB2 composites are presented in Table 1. Hardness of CrB2 prepared with 2.5% Nb was measured as 18.46 GPa which increases to 21.89 GPa with increasing Niobium content to 10 mass%.This could be due to the higher hardness (25.5 GPa) of NbB2 phase as compared to monolithic CrB2(22 GPa) [2]. Further increase of Nb content decreased the hardness to 17.34 GPa. This could be attributed to the formation of Cr2B3 and Cr3B4 phases in significant amounts. Hardness of monolithic CrB2 was measured as 11.45 GPa. The low hardness obtained is due to the lower density (89.42%) of the sample. Microhardness of single crystal CrB2 has been reported as 22.6 GPa (1N load) by Okada et al. [20]. Sonber et al. [15] have reported hardness of hot pressed CrB2 as 22 GPa.
Fracture toughness of monolithic CrB2 was measured as 3.10 MPa.m1/2. Fracture toughness of composites prepared by addition of 2.5 and 10 wt.% Nb was measured as 3.11 and 3.38 MPa.m1/2 respectively. Addition of 20% Nb resulted in increased fracture toughness of 4.32 MPa.m1/2. The increase in the fracture toughness is due to presence of second phase. Figure 6 presents the fracture surfaces of CrB2 composites. Dense surfaces were observed in all the composites. The mode of fracture is observed to be transgranular.

Fracture surface of CrB2 composites prepared by addition of (a) 2.5% Nb (b) 10% Nb (c) 20% Nb
Reinforcement of metallic particles in ceramic material enhances fracture toughness of ceramics. Addition of Nb and Mo into ZrB2 has been reported to result in increased fracture toughness [18, 21]. This enhancement is due to plastic deformation of metals which consumes available energy. In the present study, Niobium metal was added to CrB2 matrix to form new composite. During hot pressing, Nb has reacted with CrB2 and formed NbB2 and thus in composite, ductile Nb metal is not present. However, presence of NbB2 resulted in enhancement of hardness and fracture toughness but effect is not as high as achieved by Nb addition in ZrB2 by Sun et al. [18].
Conclusion
CrB2-based novel composite has been developed by addition of Niobium powder. Hot pressing of CrB2 + Nb mixture at 1,600°C and 34 MPa pressure resulted in a density higher than 95% ρth. Monolithic CrB2 was densified to 89.42% under similar hot pressing condition. The enhanced densification of CrB2 by addition of Nb is attributed to reaction sintering. During hot pressing, Nb reacts with CrB2 and results in the formation of NbB2, Cr2B3 and Cr3B4. Hardness of CrB2 composite prepared by addition of 2.5% Nb was measured as 18.46 GPa which increases to 21.89 GPa by increasing Nb content to 10%. Fracture toughness of composites containing 2.5 and 10% Nb was measured as 3.11 and 3.38 MPa.m1/2 respectively. Addition of 20% Nb resulted in increased fracture toughness of 4.32 MPa.m1/2.
References
1. IizumiK, KudakaK, MaezawaD, SasakiT. Mechanochemical synthesis of chromium borides.J Ceram Soc Jpn1999;107:491–3.10.2109/jcersj.107.491Search in Google Scholar
2. BauccioMLASM engineered materials reference book. Materials Park, OH: ASM International, 1994.Search in Google Scholar
3. AudronisM, RosliZM, LeylandA, KellyPJ, MatthewsA. Tribological behaviour of pulsed magnetron sputtered CrB2 coatings examined by reciprocating sliding wear testing against aluminium alloy and steel.Surf Coat Technol2008;202:1470–8.10.1016/j.surfcoat.2007.06.057Search in Google Scholar
4. DahmKL, JordanLR, HaaseJ, DearnleyPA. Magnetron sputter deposition of chromium diboride coatings.Surf Coat Technol1998;108–109:413–18.10.1016/S0257-8972(98)00568-4Search in Google Scholar
5. JordanLR, BettsAJ, DahmKL, DearnleyPA, WrightGA. Corrosion and passivation mechanism of chromium diboride coatings on stainless steel.Corros Sci2005;47:1085–96.10.1016/j.corsci.2003.10.018Search in Google Scholar
6. GordienkoSP, EvtushokTM, RookitskayaEA, ZhunkovskiiGL. Thermodynamic analysis of chemical reaction between components of the Cr-CrB2-CrSi2 system with air at elevated temperatures.Powder Metall Met Ceram2003;42:408.10.1023/B:PMMC.0000004161.23791.d5Search in Google Scholar
7. MurthyTSRCh, SonberJK, SubramanianC, HubliRC, KrishnamurthyN, SuriAK. Densification and Oxidation behavior of a novel TiB2-MoSi2-CrB2 composite.Int J Refract Met Hard Mater2013;36:243–53.10.1016/j.ijrmhm.2012.09.006Search in Google Scholar
8. YamadaS, HiraoK, YamauchiY, KanzakiS. B4C-CrB2 composites with improved mechanical properties.J Eur Ceram Soc2003;23:561–5.10.1016/S0955-2219(02)00094-8Search in Google Scholar
9. MurthyTSRCh, SonberJK, SubramanianC, FotedarRK, GonalMR, SuriAK. Effect of CrB2 addition on densification, properties and oxidation resistance of TiB2.Int J Refract Met Hard Mater2009;27:976–84.10.1016/j.ijrmhm.2009.06.004Search in Google Scholar
10. JhaM, MarkaS, Ghanashyam KrishnaM, GanguliAK. Multifunctional nanocrystalline chromium boride thin films.Mater Lett2012;73:220–2.10.1016/j.matlet.2011.12.124Search in Google Scholar
11. ChoiHS, ParkB, LeeJJ. CrB2 coatings deposited by inductively coupled plasma assisted DC magnetron sputtering.Surf Coat Technol2007;202:982–6.10.1016/j.surfcoat.2007.06.020Search in Google Scholar
12. JayaramanS, KleinEJ, YangY, KimDY, GirolamiGS, AbelsonJR. Chromium diboride thin films by low temperature chemical vapor deposition.J Vac Sci Technol A2005;23:631–3.10.1116/1.1927534Search in Google Scholar
13. IizumiK, KudakaK. Synthesis and sintering of chromium borides via solid state reactions and mechanochemical processes of chromium-amorphous boron mixed powder.Chem Soc Jpn Chem Ind Chem J2000;6:379–80.10.1246/nikkashi.2000.369Search in Google Scholar
14. PeshevP, BliznakovG, LeyarovskaL. On the preparation of some chromium, molybdenum and tungsten borides.J Less Common Met1967;13:241–7.10.1016/0022-5088(67)90188-9Search in Google Scholar
15. SonberJK, MurthyTSRCh, SubramanianC, KumarS, FotedarRK, SuriAK. Investigation on synthesis, pressureless sintering and hot pressing of chromium diboride.Int J Refract Met Hard Mater2009;27:912–18.10.1016/j.ijrmhm.2009.05.008Search in Google Scholar
16. IizumiK, MaezavaD, SasakiT, KudakaK. Reactive sintering of CrB2 via mechanochemical processing of chromium and boron powders.J Jpn Soc Powder Powder Metall2000;47:3–8.10.2497/jjspm.47.3Search in Google Scholar
17. HirokiY, YoshinakaM, HirotaK, YamaguchiO. Hot isostatic pressing of CrB prepared by self propagating high temperature synthesis.J Jpn Soc Powder Powder Metall2003;50:367–71.10.2497/jjspm.50.367Search in Google Scholar
18. SunX, HanW, HuP, WangZ, ZhangX. Microstructure and mechanical properties of ZrB2–Nb composite.Int J Refract Met Hard Mater2010;28:472–4.10.1016/j.ijrmhm.2009.12.005Search in Google Scholar
19. AnstisGR, ChantikulP, LawnBR, MarshallDB. A criticalevaluation of indentation techniques for measuring fracture toughness. I: Direct crackmeasurements.J Am Ceram Soc1981;64:533–8.10.1111/j.1151-2916.1981.tb10320.xSearch in Google Scholar
20. OkadaS, KudouK, IizumiK, KudakaK, HigashiI, LundstromT. Single-crystal growth and properties of CrB, Cr3B4, Cr2B3 and CrB2 from high-temperature aluminum solutions.J Cryst Growth1996;166:429–35.10.1016/0022-0248(95)00890-XSearch in Google Scholar
21. WangH, ChenD, WangCA, ZhangR, FangD. Preparation and characterization of high-toughness ZrB2/Mo composites by hot-pressing process.Int J Refract Met Hard Mater2009;27:1024–6.10.1016/j.ijrmhm.2009.06.003Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- The Influence of the Induced Ferrite and Precipitates of Ti-bearing Steel on the Ductility of Continuous Casting Slab
- Study on Evolution of Ti-containing Intermetallic Compounds in Alloy 2618-Ti during Homogenization
- The Effects of Different P2O5 Content and Basicity on Phosphorus Enrichment in the CaO-SiO2-FetO-P2O5 Slag System
- Dry Sliding Wear Behaviours of Valve Seat Inserts Produced from High Chromium White Iron
- A Characterization for the Hot Flow Behaviors of As-extruded 7050 Aluminum Alloy
- A Characterization of Hot Flow Behaviors Involving Different Softening Mechanisms by ANN for As-Forged Ti-10V-2Fe-3Al Alloy
- Effect of Microwave Heating Conditions on the Preparation of High Surface Area Activated Carbon from Waste Bamboo
- Laboratory Study on Oxide Inclusions in High-Strength Low-Alloyed Steel Refined by Slag with Basicity 2–5
- Processing and Characterization of CrB2-Based Novel Composites
- Dispersion of Particles in the Emulsion by the Electric Current
- Description of Grain Refinement by Dynamic Recrystallization Under Hot Compressions for As-Extruded 3Cr20Ni10W2 Heat-Resistant Alloy
- Identification of Stable Processing Parameters in Ti–6Al–4V Alloy from a Wide Temperature Range Across β Transus and a Large Strain Rate Range
- Life Estimation and Creep Damage Quantification of Service Exposed Reformer Tube
Articles in the same Issue
- Frontmatter
- The Influence of the Induced Ferrite and Precipitates of Ti-bearing Steel on the Ductility of Continuous Casting Slab
- Study on Evolution of Ti-containing Intermetallic Compounds in Alloy 2618-Ti during Homogenization
- The Effects of Different P2O5 Content and Basicity on Phosphorus Enrichment in the CaO-SiO2-FetO-P2O5 Slag System
- Dry Sliding Wear Behaviours of Valve Seat Inserts Produced from High Chromium White Iron
- A Characterization for the Hot Flow Behaviors of As-extruded 7050 Aluminum Alloy
- A Characterization of Hot Flow Behaviors Involving Different Softening Mechanisms by ANN for As-Forged Ti-10V-2Fe-3Al Alloy
- Effect of Microwave Heating Conditions on the Preparation of High Surface Area Activated Carbon from Waste Bamboo
- Laboratory Study on Oxide Inclusions in High-Strength Low-Alloyed Steel Refined by Slag with Basicity 2–5
- Processing and Characterization of CrB2-Based Novel Composites
- Dispersion of Particles in the Emulsion by the Electric Current
- Description of Grain Refinement by Dynamic Recrystallization Under Hot Compressions for As-Extruded 3Cr20Ni10W2 Heat-Resistant Alloy
- Identification of Stable Processing Parameters in Ti–6Al–4V Alloy from a Wide Temperature Range Across β Transus and a Large Strain Rate Range
- Life Estimation and Creep Damage Quantification of Service Exposed Reformer Tube

