Crosstalk between miRNAs and signaling pathways in the development of drug resistance in breast cancer
-
Reza Amiri
und Mohammad Valilo
Abstract
One of the biggest challenges of today’s society is cancer, which imposes a significant financial, emotional and spiritual burden on human life. Breast cancer (BC) is one of the most common cancers that affects people in society, especially women, and due to advanced treatment strategies and primary prevention, it is still the second cause of cancer-related deaths in society. Various genetic and environmental factors are involved in the development of BC. MicroRNAs (miRNA)s are non-coding RNAs, that the degradation or inhibition of them plays an important role in the prevention or development of cancer by modulating many cellular pathways including apoptosis, drug resistance, and tumorigenesis. Drug resistance is one of the important defense mechanisms of cancer cells against anticancer drugs and is considered one of the main causes of cancer treatment failure. Different miRNAs, including mir-7, mir-21, mir-31, and mir-124 control different cell activities, including drug resistance, through different pathways, including PI3K/AKT/mTOR, TGF-β, STAT3, and NF-kB. Therefore, cell signaling pathways are one of the important factors that miRNAs control cellular activities. Hence, in this study, we decided to highlight an overview of the relationship between miRNAs and signaling pathways in the development of drug resistance in BC.
Introduction
Contrary to the increasing prevalence of breast cancer (BC) in recent decades, the death rate from this cancer has decreased [1]. In the past few years, the prevalence of BC has statistically increased more than lung cancer. BC, which accounted for 11.7 % of all cancers in 2020, affected about 2.3 million individuals. Since the use of mammography, the rate of BC detection has increased, a trend that continues with the aging of the population. The death rate from BC is lower in developed countries than in developing countries. In developed countries, due to the improvements in technology, modern treatments, and diagnostic methods, the death rate from BC has declined [2], 3]. The mortality rate of BC in China is the highest among women aged 13–44 years. The average age at which BC is diagnosed in China is about 49 years, which is significantly different from that in the United States, which is about 60 years [4], 5]. The pathophysiology of BC is complex and not fully understood. Female gender and aging are the most important risk factors [6]. Among the key genetic mutations associated with BC are BRCA1-associated RING domain 1 [BARD1], BC1/2 [BRCA1/2], RAD51 homolog C [RAD51C], ATM, BRCA2 [PALB2], and checkpoint kinase 2 [CHEK2]. Premature menstruation, late menopause, a high body mass index (BMI), dense breasts, and hormone therapy after menopause are BC risk factors [7], [8], [9]. One of the most basic methods of BC treatment is surgery along with auxiliary treatments, such as chemotherapy, hormone therapy, radiotherapy, or a combination of these approaches. Cancer cells become resistant to drugs and treatment via various mechanisms that have been identified during treatment. Multiple drug resistance (MDR), ATP-binding cassette (ABC) transporter, adaptive signaling pathways, unprogrammed apoptosis, and epigenetic changes are among the mechanisms that contribute to drug resistance in cancer cells [10]. miRNAs are a group of non-coding RNAs with approximately 20–25 nucleotides that bind to the 3′ untranslated region (UTR) of mRNA through their 5′ UTR and suppress gene expression after transcription. Gene expression is involved in metastasis, carcinogenesis, and cancer response. Growing studies have shown that the dysfunction of miRNAs is associated with drug resistance in cancer cells. miRNAs can affect the expression of hundreds of genes, or a target gene can be regulated by several different miRNAs. One of the most important factors that regulate the function of miRNAs are exosomes, which transfer miRNAs from donor cells to recipient cells in the tumor microenvironment [11], [12], [13], [14]. On the other hand, various studies have revealed that miRNAs play a role in different cellular processes, including angiogenesis, apoptosis, cell growth, cell proliferation, and inflammation. miRNAs contribute to these cellular processes mostly through interaction with cellular signaling pathways [15]. Moreover, signaling pathways play a vital role in controlling cellular processes, including cell division, transcription, translation, cell cycle, cell growth and proliferation, and apoptosis [16], 17]. According to the above-mentioned findings, miRNAs are one of the main regulators of cellular processes, exerting their control mostly through interaction with signaling pathways. Therefore, in this review, we decided to clarify the relationship between miRNAs and signaling pathways in order to examine their role in the development and progression of BC, or conversely, in preventing the development and progression of this disease.
Methods
In the present study, we conducted literature search using the PubMed database, Semantic Scholar, and Google Scholar search engines. It is noteworthy that in this review, we identified articles that were related to our research topic and were written in English. In addition, the search for articles was conducted between November 2022 and December 2023. To find articles related to the topic, keywords including drug resistance, miRNAs, breast cancer, and signaling pathways were used. Moreover, in the review article, we were willing to examine the articles related to this topic from 2001 to 2023. Therefore, articles that were outside the specific time frame of our study or were in languages other than English were excluded.
BC
The second leading cause of cancer death among women is BC. One of the most effective methods to prevent BC is early detection. In developed countries, due to early detection, the survival rate of patients with BC is about 80 %. In recent years, significant progress has been made in understanding the pathogenesis of the disease, diagnostic methods, and drug resistance mechanisms through the study of BC stem cells (BCSCs) [18]. There are two main theories about the origin of BC: The random theory and the cancer stem cell theory. The random theory states that cancer originates from a single cell and various mutations can accumulate in this cell, leading to its transformation into a cancerous cell. Cancer stem cell theory suggests that stem cells are the origin of all tumor subtypes. Different genetic and epigenetic mutations in progenitor and stem cells lead to different cancer phenotypes [19]. BC is divided into different groups based on the presence of biomarkers, metastasis, tumor size, estrogen and progesterone receptors, and ERBB2 receptor. Stage zero BC, referred to as ductal carcinoma in situ (DCIS), is a non-invasive form of BC. Stages I, IIa, and IIb are the initial invasive stages, and stages IIIa and IIIb involve advanced local invasiveness. The fourth stage is the metastatic stage [20], 21]. DCIS involves the microcalcification of the ducts of the breast. Women with DCIS are treated with radiation therapy, lumpectomy, and mastectomy. Systemic treatments include immunotherapy with monoclonal antibodies, chemotherapy, radiation therapy, and endocrine treatments before and after surgery for non-metastatic BC. In the metastatic stage, BC metastasizes to the lungs, liver, and brain. Patients with metastasis can rarely be cured, and their survival rate is 2–3 years [22], 23].
However, treatments such as radiation therapy, chemotherapy, immunotherapy, and surgery are performed for purposes such as reducing the symptoms and improving patients’ quality of life [24]. BC treatment faces many challenges. The emergence of miRNAs as biological biomarkers has provided a new perspective on the treatment and diagnosis of BC. In BC, miRNAs play a significant role in the growth and proliferation of cancer cells and tumorigenesis [25]. There are many similarities between the growth of cancerous cells and normal cells. In healthy humans, cell growth is controlled by complex signaling pathways that allow cells to communicate with their surrounding environment. However, this regulatory system is disrupted in cancer cells, causing them to escape from the mechanisms that control the growth, proliferation, and migration of cells. Therefore, the activating mutations of proto-oncogenes lead to the activation of more signaling pathways and the inactivation of tumor suppressors, resulting in the elimination of negative regulators of signals [26], 27].
Drug resistance
One of the main problems in cancer treatment is the drug resistance that develops in cancer cells when exposed to drugs and chemicals. This complex mechanism operates via correlation with complicated signaling pathways. Cells usually develop drug resistance via increasing uptake and decreasing efflux through ABC drug transporters, the glutathione S-transferase π (GSTπ) gene, tumor microenvironment regulation, evasion from apoptosis, and the BC resistance protein (BCRP) gene. One of the most important members of the ABC transporter group is P-glycoprotein (P-gp), which is encoded by the MDR1 gene. Overexpression of P-gp causes resistance to anticancer drugs including tamoxifen, anthracyclines, platinum-based drugs, plant alkaloids, and taxanes. According to previous studies, changes in the structure and function of the estrogen receptor (ER), including ER gene mutations, reduction or loss of ER expression, abnormality of ER activators, post-translational modification of ER, and interference between ER and phosphoinositide 3-kinase (PI3K)/AKT/mTOR, HER2, mitogen-activated protein kinase (MAPK) pathways/ERK and HER2 are among the most important endocrine resistance mechanisms [28]. One of the factors controlling P-gp is miRNAs, which modulate the drug resistance of cancer cells [29], 30]. As an example, in drug-resistant MCF-7, the expressions of miR-345 and miR-326 are suppressed. Increasing the levels of these miRNAs directly by decreasing MRP enhances the sensitivity of MCF-7 cells to chemotherapy drugs [31]. In one study, miR-19 expression was reduced in the sensitive MCF-7 cell line contrary to three MDR BC cell lines (BCRP, MRP-1, and over-expressing MDR-1). miR-19 decreased the sensitivity of cells to chemotherapeutic drugs in MDR cells through modulating PTEN [32]. This paraclinical evidence indicates that miRNAs modulate the drug resistance of BC cells through different mechanisms, particularly signaling pathways.
miRNAs
miRNAs, which are non-coding RNAs about 22 nucleotides in length, are transcribed by RNA polymerases II and III. The pre-miRNAs produced by Drosha and DGCR8 are processed and generate 70-nucleotide RNAs. Pre-miRNAs are transported to the cytoplasm by exportin 5 and separated by Dicer, producing 22-nucleotide RNAs. Then these mature miRNAs interact with Argonautes and complexes with other proteins to form the RNA-induced silencing complex (miRISC), which combines with target mRNAs and causes the degradation or inhibition of mRNA functions [33], 34]. Transcription factors involved in mRNA transcription also control the transcription of miRNAs. In addition to the transcription factor that plays a crucial role in the biogenesis of miRNAs, the expression of miRNAs can also be regulated by other factors, such as DNA methylation. The miRNA binds to the 3′ UTR of the target mRNA through its 5′ UTR. If the miRNA is fully complementary to the target mRNA, the target mRNA undergoes degradation; however, if the miRNA is complementary to the mRNA in only a limited number of nucleotides, the target mRNA is inhibited [35], 36] (Figure 1). Growing studies have revealed that miRNAs significantly contribute to tumor progression. miRNAs are differentially expressed based on the tumor subtype, and tumors can be classified based on the expression profile of miRNAs. Tumor suppressor miRNAs are mostly located in fragile chromosomal regions; therefore, mutation or damage to these regions leads to the disruption of tumor suppressor genes and causes the upregulation of oncogenes. Moreover, the proliferation of chromosomal regions encoding oncogenic miRNAs that suppress tumor suppressor genes plays a key role in cancer development [37], [38], [39]. According to the literature, miRNAs can affect self-renewal and drug resistance by regulating the characteristics of cancer stem cells, such as tumorigenesis. Therefore, many miRNAs are known as oncogenes or tumor suppressors in BC and regulate tumor initiation, drug resistance, and metastasis [40], 41]. By targeting ABC, miRNAs increase the chemosensitivity of cells to chemical drugs. Additionally, miRNAs are associated with drug resistance via other mechanisms. For example, miRNAs cause drug resistance by regulating proteins and signaling pathways that control important cellular processes, such as cell cycle and drug resistance [42]. Mir-452 causes the resistance of MCF-7 BC cells to adriamycin (ADR) by expressing insulin-like growth factor 1 receptor (IGF-1R) [43], 44]. The study by Zhang et al. revealed that miR-3646 leads to the resistance of MCF-7 cells to docetaxel chemotherapy by inhibiting the expression of glycogen synthase kinase 3β (GSK-3β) and the subsequent induction of the GSK-3β/β-catenin pathway [45] (Table 1).
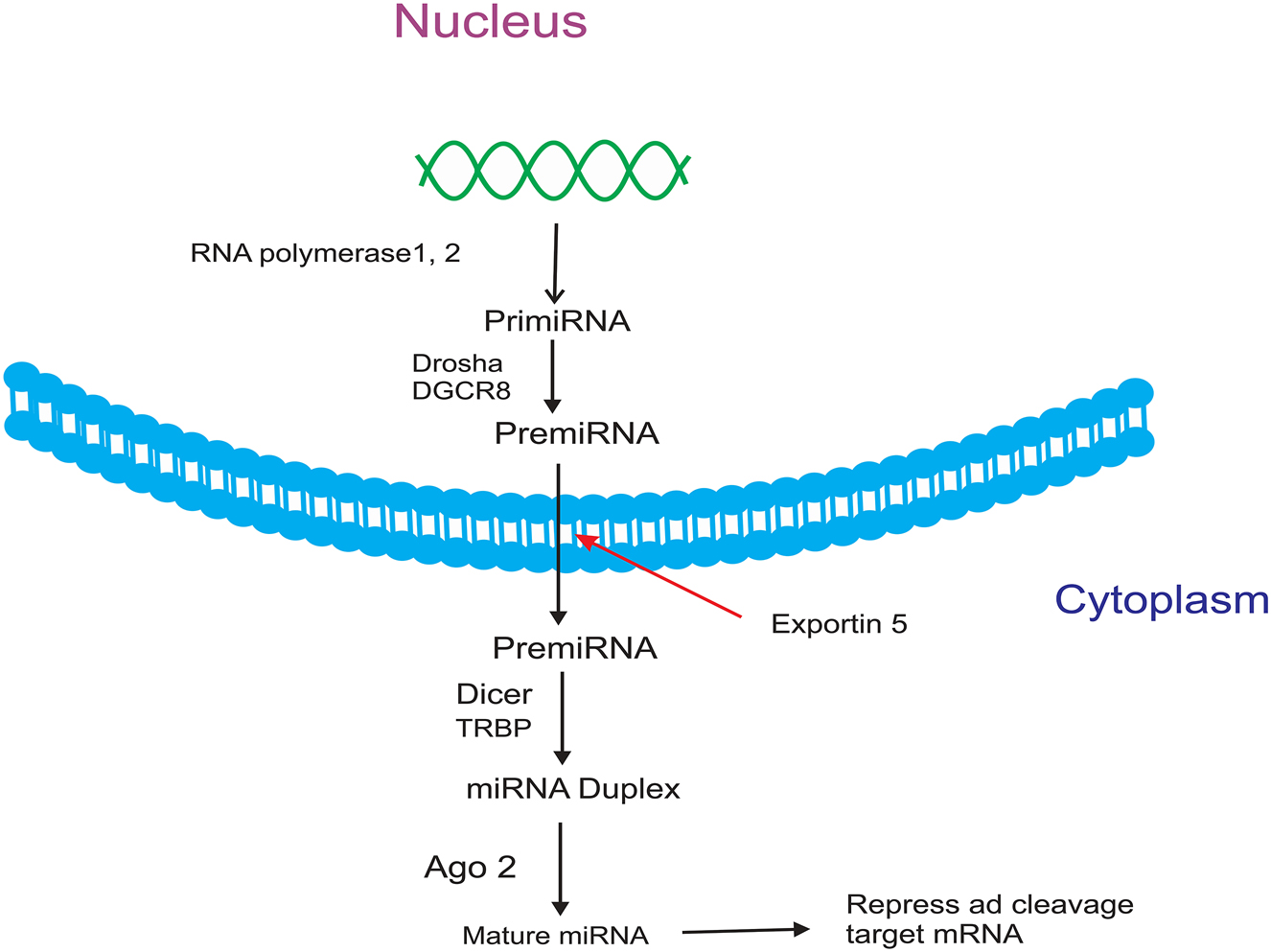
miRNA biosynthesis.
miRNAs that regulate drug resistance in breast cancer.
| Drug | miRNA | Ref |
|---|---|---|
| Anastrozole | miR-125b or miR-205, miR-424 | [75] |
| Capivasertib | miR-4649-5p | [76] |
| Fulvestrant | microRNA-221/222, miR-3188, miR-21, miR-149 | [77], [78], [79] |
| Lapatinib ditosylate | miR-630 | [80] |
| Letrozole | miR-128a, miRNA181a, miRNA22 | [81], 82] |
| Olaparib | miRNAs-181a/b | [83] |
| Palbociclib | miRNA-3613-3p, miR-29b-3p, miR-223, miR-432-5p, miR-106b, miR-432-5p | [84], [85], [86], [87], [88] |
| Pertuzumab | miRNA-150, miR-146a-5p | [89], 90] |
| Trastuzumab | miRNA-542-3p, miR-21, miR-23b-3p, miR-195-5p, miR-656-5p, miR-340-5p, miR-1246, miR-155 | [91], [92], [93], [94], [95] |
| Tamoxifen | miR-221, miR-222, miR-15a, miR-16, miR-200b, miR-200c, miR-375, miR-301, miRNA-27b-3p, miR-342 | [96], [97], [98], [99], [100], [101] |
| Doxorubicin | miR-200c, miR-298, miR-221-3p, miR-451, miR-145, miR-124-3p, miR-134, miR-140-5p, miR-128, miR-519a | [102], [103], [104], [105], [106] |
| Adriamycin | mir-452, mir-29a, mir‐221, mir-222, miR-7 | [107], [108], [109], [110], [111] |
| Cisplatin | Let-7i, miR-569, miR-214, miR-146a, miR-10a, mir-221/222, mir-345, mir-200b, mir-200c | [31], 112] |
Tumor microenvironment in drug resistance of BC
The tumor microenvironment (TME), which is made of connective tissue, consists of different cells and a network of glycoprotein, proteoglycan and collagen called the extracellular matrix. Cellular components include endothelial cells, immune cells, fat cells, and fibroblasts. Each of the cells plays its role in the microenvironment according to its nature. Mutations in the stroma or epithelium lead to mutagenesis. Healthy stromal cells are affected by factors secreted from malignant cells, including transforming growth factor-beta TGF-β, fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), epidermal (EGF), and vascular endothelial growth factor (VEGF) or microvesicles such as exosomes are placed to get the characteristics similar to them [46], [47], [48]. In BC, TME plays a fundamental role in tumor treatment response, tumor behavior, and pathological examination for treatment management [49]. Cancer cells with metastatic property had a tendency to migrate to the desired places and grow. The seed and soil theory was first presented by Stephen Paget. In this theory, metastatic cells are considered as seeds and the place of growth is considered as soil, and it points to the role of TME in cancer metastasis [50], 51]. TME leads to chemoresistance to chemotherapy drugs through an acquired mechanism or de novo pathway. Expression of ABCs, expression of tumor suppressor genes, and oncogenes are involved in acquired MDR through cell-to-TME matrix communication and cell-cell interaction. In the path of drug resistance that is created e novo, cancer cells after being exposed to treatment, the stromal tissue inside the TME shelters the cancer cells and induces stem formation in them, causing chemical resistance. On the other hand, cancer cells that have previously been exposed to drugs change their phenotype leading to resistance to subsequent treatments [52], [53], [54].
miRNAs and breast CSCs
Growing evidence shows that many cancers are curable after they grow and metastasize to other organs, although early-stage cancers are easier and more cost-effective to treat. However, by treating cancers in the early stages, a number of cancer source cells remain that can grow later and cause cancer recurrence and metastasis. These stem cells are said to be found in every stage of cancer and are similar to stem cells and are known as CSCs. It is believed that the activity of CSCs is mostly seen in malignant and solid cancers including breast, lung, liver, and brain cancer [28], 55]. CSCs show resistance to chemotherapy and cause cancer resistance [56]. It is said that the normal homeostasis of cancer stem cells is controlled by signaling pathways. Nevertheless, it is not far-fetched to expect that these signaling pathways are activated or suppressed in breast CSCs. Among these pathways, β-catenin, JAK/STAT, Notch, phosphoinositide 3-kinase (PI3K)/Akt, TGF-β, and NF-κB pathways are involved in CSC homeostasis [57], 58]. Since CSCs cause drug and chemical resistance, therefore, targeting CSCs is one of the ways to treat cancers, including breast cancer. One of the treatment methods is to target the signaling pathways of CSCs. Targeting the surface markers of CSCs by monoclonal antibodies is one of the new treatment methods. But this method of treatment is because: 1). Similarity of markers of CSCs with normal SCs and other CSCs, 2). Lack of some markers to classify CSCs, 3). The very small presence of CSCs in cancer faces challenges. On the other hand, miRNAs are another treatment option that can be used alone or together with other treatment methods for CSCs. One of the basic problems in the use of miRNAs is the methods of providing miRNAs. The most basic delivery system is non-viral systems, which due to their low efficiency compared to viral systems, recently many efforts have been made to increase their efficiency by enlarging the size of the particles or increasing their surface area [59], [60], [61], [62], [63].
Interaction between signaling pathways and miRNAs in BC
PTEN induces tumor suppression by inhibiting the PI3K signaling pathway. PTEN, which is a phosphatase upstream of Akt, converts phosphatidyl-inositol-3,4,5-triphosphate (PIP3) to phosphatidyl-inositol-4,5-bisphosphate (PIP2), thus inhibiting the PI3K pathway. Various studies have been conducted on the relationship between the PTEN pathway and miRNAs in the development of drug resistance in BC cells. The study by Yuan et al. indicated that miR-130b in MCF-7 and MCF-7/ADR cells causes resistance to ADR and increases proliferation by targeting the PTEN-PI3K/Akt pathway. In addition, in BC cells, miR-130b increases the expression of p-Akt308 and p-Akt473. Interestingly, the effect of miR-130b on p-Akt308 and p-Akt473 is reduced by increasing the expression of PTEN. Overall, this finding revealed that miR-130b, by acting on PTEN through the PI3K/Akt pathway, increases the drug resistance and growth of BC cells [64], [65], [66]. Manxin et al. found that the expression of miR-132/212 through the PTEN pathway enhanced the resistance of BC cells [67]. A study on the relationship between miRNA-200c and drug resistance in MCF-7 cancer cells showed that miRNA-200c increases the sensitivity of MCF-7 cells to ADR by promoting the expression of PTEN and E-cadherin while decreasing the expression of Akt and ZEB1 [68]. In the study by Shen et al., it was revealed that miR-155 suppresses SOCS6 mRNA and protein. By reducing miR-155, SOCS6 levels are elevated, which can inhibit STAT3 through miR-155 and cause cell growth [69]. The results of a study in 2021 indicated that exosomal miR-205 causes resistance to tamoxifen, proliferation, and migration by targeting E2F transcription factor 1 (E2F1) in BC cells and suppresses apoptosis by phosphorylating Akt and activating the caspase pathway. On the other hand, the overexpression of E2F1 or the knockdown of miRNA-205 reverses this result [70]. Experiments on LINC00968 in BC showed that its expression in BC is low, while the expression of WNT2 is higher. In vitro and animal studies have shown that the overexpression of LINC00968 reduces drug resistance by silencing WNT2 through the recruitment of HEY1 and inhibiting the Wnt2/β-catenin signaling pathway [71]. A study on the relationship between LncRNA-HOX transcript antisense RNA (HOTAIR) and resistance to doxorubicin (DOX) in BC cells showed that lncRNA-HOTAIR reduces DOX resistance in these cells through the PI3K/AKT/mTOR signaling pathway. It also promotes apoptosis in MCF-7 cells and the DOX-resistant BC cell line (DOXR-MCF-7) and can be a therapeutic target in BC [72]. miR-124 causes BCSC sensitivity to DOX by interfering with HIF-1 and STAT3 signaling pathways in DOX-resistant BCSCs [73]. The research conducted by Bergamaschi et al. showed that miR-451 increases the sensitivity of BC cells to chemotherapy drugs by suppressing the epidermal growth factor receptor (EGFR) and increases apoptosis by targeting 14-3-3ζ [74] (Figure 2). Hence, considering the emerging data, there is substantial evidence elucidating that some miRNAs contribute to drug resistance in BC through signaling pathways. However, more studies are required in this field.
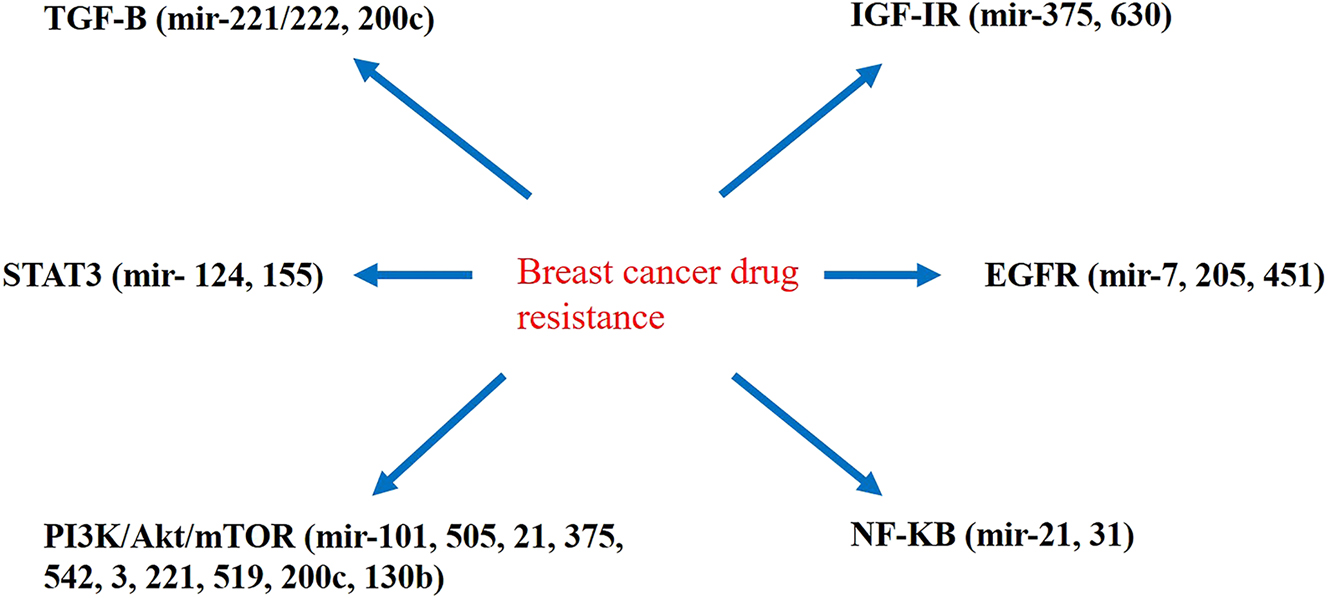
The interference of miRNAs with signaling pathways associated with drug resistance in breast cancer cells.
Conclusion
Initially, it was believed that miRNAs had no role in controlling the functions of the body and cells. However, subsequent research revealed that miRNAs play a pivotal role in controlling various cellular activities. Some miRNAs prevent cancer development and the growth of cancer cells by controlling cell pathways, and on the contrary, other miRNAs cause cell growth, invasion, and progression. Our findings demonstrated that miRNAs increase or eliminate drug resistance in BC cells through different mechanisms, particularly signaling pathways. According to the findings in this field, drug resistance, which is one of the strategies cancer cells use to escape from treatment, can be mediated through miRNA interference in cancer cells, which either increases or decreases their sensitivity to drugs. Therefore, more studies in this field are required to shed light on the function of miRNAs in the development of drug resistance in BC cells via interference with signaling pathways.
Acknowledgments
We thank the Urmia University of Medical Sciences for all support of this research.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: A. F, F. R. and A. N. were involved in writing the article. M. H. was involved in drawing the figures and data collecting. M. V. participated in the study design. R. A, and P. N. were involved in revising.
-
Use of Large Language Models, AI and Machine Learning Tools: No.
-
Conflict of interest: No.
-
Research funding: No.
-
Data availability: No.
References
1. Giaquinto, AN, Sung, H, Miller, KD, Kramer, JL, Newman, LA, Minihan, A, et al.. Breast cancer statistics, 2022. CA Cancer J Clin 2022;72:524–41. https://doi.org/10.3322/caac.21754.Suche in Google Scholar PubMed
2. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al.. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. https://doi.org/10.3322/caac.21660.Suche in Google Scholar PubMed
3. Hong, R, Xu, B. Breast cancer: an up‐to‐date review and future perspectives. Cancer Commun 2022;42:913–36. https://doi.org/10.1002/cac2.12358.Suche in Google Scholar PubMed PubMed Central
4. Zheng, R, Zhang, S, Zeng, H, Wang, S, Sun, K, Chen, R, et al.. Cancer incidence and mortality in China, 2016. J Nat Cancer Center 2022;2:1–9. https://doi.org/10.1016/j.jncc.2022.02.002.Suche in Google Scholar PubMed PubMed Central
5. Li, T, Mello-Thoms, C, Brennan, PC. Descriptive epidemiology of breast cancer in China: incidence, mortality, survival and prevalence. Breast Cancer Res Treat 2016;159:395–406. https://doi.org/10.1007/s10549-016-3947-0.Suche in Google Scholar PubMed
6. Watkins, EJ. Overview of breast cancer. J Am Acad PAs 2019;32:13–7. https://doi.org/10.1097/01.jaa.0000580524.95733.3d.Suche in Google Scholar PubMed
7. Obstetricians ACo, Gynecologists. Hereditary cancer syndromes and risk assessment. Obstet Gynecol 2019;134:e143–9.10.1097/AOG.0000000000003562Suche in Google Scholar PubMed
8. Sargazi, Z, Yazdani, Y, Tahavvori, A, Youshanlouei, HR, Alivirdiloo, V, Beilankouhi, EAV, et al.. NFR2/ABC transporter axis in drug resistance of breast cancer cells. Mol Biol Rep 2023;50:1–8. https://doi.org/10.1007/s11033-023-08384-7.Suche in Google Scholar PubMed
9. Xia, L, Ma, W, Afrashteh, A, Sajadi, MA, Fakheri, H, Valilo, M. The nuclear factor erythroid 2-related factor 2/p53 axis in breast cancer. Biochem Med 2023;33:266–78. https://doi.org/10.11613/bm.2023.030504.Suche in Google Scholar PubMed PubMed Central
10. Hu, W, Tan, C, He, Y, Zhang, G, Xu, Y, Tang, J. Functional miRNAs in breast cancer drug resistance. OncoTargets Ther 2018;11:1529–41. https://doi.org/10.2147/ott.s152462.Suche in Google Scholar PubMed PubMed Central
11. Milane, L, Singh, A, Mattheolabakis, G, Suresh, M, Amiji, MM. Exosome mediated communication within the tumor microenvironment. J Contr Release 2015;219:278–94. https://doi.org/10.1016/j.jconrel.2015.06.029.Suche in Google Scholar PubMed
12. Si, W, Shen, J, Zheng, H, Fan, W. The role and mechanisms of action of microRNAs in cancer drug resistance. Clin Epigenetics 2019;11:1–24. https://doi.org/10.1186/s13148-018-0587-8.Suche in Google Scholar PubMed PubMed Central
13. Medarova, Z, Pantazopoulos, P, Yoo, B. Screening of potential miRNA therapeutics for the prevention of multi-drug resistance in cancer cells. Sci Rep 2020;10:1970. https://doi.org/10.1038/s41598-020-58919-2.Suche in Google Scholar PubMed PubMed Central
14. Mamalo, AS, Alivirdiloo, V, Sadeghnejad, A, Hajiabbasi, M, Gargari, MK, Valilo, M. Potential roles of the exosome/microRNA axis in breast cancer. Pathol Res Pract 2023;251:154845. https://doi.org/10.1016/j.prp.2023.154845.Suche in Google Scholar PubMed
15. Wang, Z, Li, Y, Kong, D, Ahmad, A, Banerjee, S, Sarkar, FH. Cross-talk between miRNA and notch signaling pathways in tumor development and progression. Cancer Lett 2010;292:141–8. https://doi.org/10.1016/j.canlet.2009.11.012.Suche in Google Scholar PubMed PubMed Central
16. Karamboulas, C, Ailles, L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochimica et Biophysica Acta 2013;1830:2481–95. https://doi.org/10.1016/j.bbagen.2012.11.008.Suche in Google Scholar PubMed
17. Mohammadi, M, Fazilat, A, Mamalo, AS, Ojarudi, M, Hemmati-Dinarvand, M, Beilankouhi, EAV, et al.. Correlation of PTEN signaling pathway and miRNA in breast cancer. Mol Biol Rep 2024;51:221. https://doi.org/10.1007/s11033-023-09191-w.Suche in Google Scholar PubMed
18. Sun, Y-S, Zhao, Z, Yang, Z-N, Xu, F, Lu, H-J, Zhu, Z-Y, et al.. Risk factors and preventions of breast cancer. Int J Biol Sci 2017;13:1387. https://doi.org/10.7150/ijbs.21635.Suche in Google Scholar PubMed PubMed Central
19. Sun, YS, Zhao, Z, Yang, ZN, Xu, F, Lu, HJ, Zhu, ZY, et al.. Risk factors and preventions of breast cancer. Int J Biol Sci 2017;13:1387–97. https://doi.org/10.7150/ijbs.21635.Suche in Google Scholar
20. Waks, AG, Winer, EP. Breast cancer treatment: a review. JAMA 2019;321:288–300. https://doi.org/10.1001/jama.2018.19323.Suche in Google Scholar PubMed
21. Hortobagyi, GN, Connolly, JL, D’Orsi, CJ, Edge, SB, Mittendorf, EA, Rugo, HS, et al.. Breast. AJCC Cancer Staging Manual 2017;8:589–636.Suche in Google Scholar
22. Peart, O. Metastatic breast cancer. Radiol Technol 2017;88:519M–39M.Suche in Google Scholar
23. Network NCC. NCCN clinical practice guidelines in oncology; 2008. Available from: http://wwwnccnorg/professionals/physician_gls/PDF/occultpdf.Suche in Google Scholar
24. Yeo, B, Turner, NC, Jones, A. An update on the medical management of breast cancer. BMJ 2014;348:g3608. https://doi.org/10.1136/bmj.g3608.Suche in Google Scholar PubMed
25. Goh, JN, Loo, SY, Datta, A, Siveen, KS, Yap, WN, Cai, W, et al.. microRNAs in breast cancer: regulatory roles governing the hallmarks of cancer. Biol Rev 2016;91:409–28. https://doi.org/10.1111/brv.12176.Suche in Google Scholar PubMed
26. Huebner, RJ, Ewald, AJ. Cellular foundations of mammary tubulogenesis. Semin Cell Dev Biol 2014;31:124–31.10.1016/j.semcdb.2014.04.019Suche in Google Scholar PubMed PubMed Central
27. Feng, Y, Spezia, M, Huang, S, Yuan, C, Zeng, Z, Zhang, L, et al.. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 2018;5:77–106. https://doi.org/10.1016/j.gendis.2018.05.001.Suche in Google Scholar PubMed PubMed Central
28. Fakhrioliaei, A, Tanhaei, S, Pakmehr, S, Noori Shakir, M, Qasim, MT, Hariri, M, et al.. Potential role of Nrf2, HER2, and ALDH in cancer stem cells: a narrative review. J Membr Biol 2024;257:3–16. https://doi.org/10.1007/s00232-024-00307-2.Suche in Google Scholar PubMed
29. Gottesman, MM, Ling, V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett 2006;580:998–1009. https://doi.org/10.1016/j.febslet.2005.12.060.Suche in Google Scholar PubMed
30. Kutanzi, KR, Yurchenko, OV, Beland, FA, Checkhun, VF, Pogribny, IP. MicroRNA-mediated drug resistance in breast cancer. Clin Epigenetics 2011;2:171–85. https://doi.org/10.1007/s13148-011-0040-8.Suche in Google Scholar PubMed PubMed Central
31. Pogribny, IP, Filkowski, JN, Tryndyak, VP, Golubov, A, Shpyleva, SI, Kovalchuk, O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int J Cancer 2010;127:1785–94. https://doi.org/10.1002/ijc.25191.Suche in Google Scholar PubMed
32. Liang, Z, Li, Y, Huang, K, Wagar, N, Shim, H. Regulation of miR-19 to breast cancer chemoresistance through targeting PTEN. Pharm Res 2011;28:3091–100. https://doi.org/10.1007/s11095-011-0570-y.Suche in Google Scholar PubMed
33. O’Day, E, Lal, A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res 2010;12:1–10.10.1186/bcr2484Suche in Google Scholar PubMed PubMed Central
34. Jang, JY, Kim, YS, Kang, KN, Kim, KH, Park, YJ, Kim, CW. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol Clin Oncol 2021;14:1. https://doi.org/10.3892/mco.2020.2193.Suche in Google Scholar PubMed PubMed Central
35. Abolghasemi, M, Tehrani, SS, Yousefi, T, Karimian, A, Mahmoodpoor, A, Ghamari, A, et al.. MicroRNAs in breast cancer: roles, functions, and mechanism of actions. J Cell Physiol 2020;235:5008–29. https://doi.org/10.1002/jcp.29396.Suche in Google Scholar PubMed
36. Rehman, O, Zhuang, H, Muhamed Ali, A, Ibrahim, A, Li, Z. Validation of miRNAs as breast cancer biomarkers with a machine learning approach. Cancers (Basel) 2019;11:431. https://doi.org/10.3390/cancers11030431.Suche in Google Scholar PubMed PubMed Central
37. Yamamoto, Y, Yoshioka, Y, Minoura, K, Takahashi, RU, Takeshita, F, Taya, T, et al.. An integrative genomic analysis revealed the relevance of microRNA and gene expression for drug-resistance in human breast cancer cells. Mol Cancer 2011;10:1–16. https://doi.org/10.1186/1476-4598-10-135.Suche in Google Scholar PubMed PubMed Central
38. Blenkiron, C, Goldstein, LD, Thorne, NP, Spiteri, I, Chin, S-F, Dunning, MJ, et al.. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol 2007;8:1–16. https://doi.org/10.1186/gb-2007-8-10-r214.Suche in Google Scholar PubMed PubMed Central
39. Takahashi, R-U, Miyazaki, H, Ochiya, T. The roles of microRNAs in breast cancer. Cancers (Basel) 2015;7:598–616. https://doi.org/10.3390/cancers7020598.Suche in Google Scholar PubMed PubMed Central
40. Shimono, Y, Zabala, M, Cho, RW, Lobo, N, Dalerba, P, Qian, D, et al.. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009;138:592–603. https://doi.org/10.1016/s9999-9994(09)20394-1.Suche in Google Scholar
41. Ruan, Q, Wang, P, Wang, T, Qi, J, Wei, M, Wang, S, et al.. MicroRNA-21 regulates T-cell apoptosis by directly targeting the tumor suppressor gene Tipe2. Cell Death Dis 2014;5:e1095. https://doi.org/10.1038/cddis.2014.47.Suche in Google Scholar PubMed PubMed Central
42. Ma, M-T, He, M, Wang, Y, Jiao, X-Y, Zhao, L, Bai, X-F, et al.. MiR-487a resensitizes mitoxantrone (MX)-resistant breast cancer cells (MCF-7/MX) to MX by targeting breast cancer resistance protein (BCRP/ABCG2). Cancer Lett 2013;339:107–15. https://doi.org/10.1016/j.canlet.2013.07.016.Suche in Google Scholar PubMed
43. Majumder, S, Jacob, ST. Emerging role of microRNAs in drug-resistant breast cancer. Gene Exp 2011;15:141–51. https://doi.org/10.3727/105221611x13176664479287.Suche in Google Scholar PubMed PubMed Central
44. Xie, XH, Zhao, H, Hu, YY, Gu, XD. Germacrone reverses Adriamycin resistance through cell apoptosis in multidrug-resistant breast cancer cells. Exp Ther Med 2014;8:1611–5. https://doi.org/10.3892/etm.2014.1932.Suche in Google Scholar PubMed PubMed Central
45. Duda, P, Akula, SM, Abrams, SL, Steelman, LS, Gizak, A, Rakus, D, et al.. GSK-3 and miRs: master regulators of therapeutic sensitivity of cancer cells. Biochim Biophys Acta Mol Cell Res 2020;1867:118770. https://doi.org/10.1016/j.bbamcr.2020.118770.Suche in Google Scholar PubMed
46. Kim, IS, Zhang, XH-F. One microenvironment does not fit all: heterogeneity beyond cancer cells. Cancer Metastasis Rev 2016;35:601–29. https://doi.org/10.1007/s10555-016-9643-z.Suche in Google Scholar PubMed PubMed Central
47. Costa, A, Kieffer, Y, Scholer-Dahirel, A, Pelon, F, Bourachot, B, Cardon, M, et al.. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell 2018;33:463–79.e10. https://doi.org/10.1016/j.ccell.2018.01.011.Suche in Google Scholar PubMed
48. Bartoschek, M, Oskolkov, N, Bocci, M, Lövrot, J, Larsson, C, Sommarin, M, et al.. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun 2018;9:5150. https://doi.org/10.1038/s41467-018-07582-3.Suche in Google Scholar PubMed PubMed Central
49. Li, JJ, Tsang, JY, Tse, GM. Tumor microenvironment in breast cancer—updates on therapeutic implications and pathologic assessment. Cancers (Basel) 2021;13:4233. https://doi.org/10.3390/cancers13164233.Suche in Google Scholar PubMed PubMed Central
50. Seyfried, TN, Huysentruyt, LC. On the origin of cancer metastasis. Crit Rev Oncog 2013;18:43–73. https://doi.org/10.1615/critrevoncog.v18.i1-2.40.Suche in Google Scholar PubMed PubMed Central
51. Hanahan, D, Coussens, LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012;21:309–22. https://doi.org/10.1016/j.ccr.2012.02.022.Suche in Google Scholar PubMed
52. Castells, M, Thibault, B, Delord, J-P, Couderc, B. Implication of tumor microenvironment in chemoresistance: tumor-associated stromal cells protect tumor cells from cell death. Int J Mol Sci 2012;13:9545–71. https://doi.org/10.3390/ijms13089545.Suche in Google Scholar PubMed PubMed Central
53. Dauer, P, Nomura, A, Saluja, A, Banerjee, S. Microenvironment in determining chemo-resistance in pancreatic cancer: neighborhood matters. Pancreatology 2017;17:7–12. https://doi.org/10.1016/j.pan.2016.12.010.Suche in Google Scholar PubMed PubMed Central
54. Khalaf, K, Hana, D, Chou, JT-T, Singh, C, Mackiewicz, A, Kaczmarek, M. Aspects of the tumor microenvironment involved in immune resistance and drug resistance. Front Immunol 2021;12:656364. https://doi.org/10.3389/fimmu.2021.656364.Suche in Google Scholar PubMed PubMed Central
55. Zhang, L, Chen, W, Liu, S, Chen, C. Targeting breast cancer stem cells. Int J Biol Sci 2023;19:552. https://doi.org/10.7150/ijbs.76187.Suche in Google Scholar PubMed PubMed Central
56. Ayob, AZ, Ramasamy, TS. Cancer stem cells as key drivers of tumour progression. J Biomed Sci 2018;25:1–18. https://doi.org/10.1186/s12929-018-0426-4.Suche in Google Scholar PubMed PubMed Central
57. Humphries, B, Wang, Z, Yang, C. MicroRNA regulation of breast cancer stemness. Int J Mol Sci 2021;22:3756. https://doi.org/10.3390/ijms22073756.Suche in Google Scholar PubMed PubMed Central
58. Yang, L, Shi, P, Zhao, G, Xu, J, Peng, W, Zhang, J, et al.. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Targeted Ther 2020;5:8. https://doi.org/10.1038/s41392-020-0110-5.Suche in Google Scholar PubMed PubMed Central
59. Jabbour, E, O’Brien, S, Ravandi, F, Kantarjian, H. Monoclonal antibodies in acute lymphoblastic leukemia. Blood 2015;125:4010–6. https://doi.org/10.1182/blood-2014-08-596403.Suche in Google Scholar PubMed PubMed Central
60. Ohno, S-i, Takanashi, M, Sudo, K, Ueda, S, Ishikawa, A, Matsuyama, N, et al.. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther 2013;21:185–91. https://doi.org/10.1038/mt.2012.180.Suche in Google Scholar PubMed PubMed Central
61. O’brien, K, Khan, S, Gilligan, K, Zafar, H, Lalor, P, Glynn, C, et al.. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene 2018;37:2137–49. https://doi.org/10.1038/s41388-017-0116-9.Suche in Google Scholar PubMed
62. Roma-Rodrigues, C, Pereira, F, de Matos, APA, Fernandes, M, Baptista, PV, Fernandes, AR. Smuggling gold nanoparticles across cell types – a new role for exosomes in gene silencing. Nanomedicine 2017;13:1389–98.10.1016/j.nano.2017.01.013Suche in Google Scholar PubMed
63. Naseri, Z, Oskuee, RK, Jaafari, MR, Forouzandeh Moghadam, M. Exosome-mediated delivery of functionally active miRNA-142-3p inhibitor reduces tumorigenicity of breast cancer in vitro and in vivo. Int J Nanomed 2018;13:7727–47. https://doi.org/10.2147/ijn.s182384.Suche in Google Scholar
64. Miao, Y, Zheng, W, Li, N, Su, Z, Zhao, L, Zhou, H, et al.. MicroRNA-130b targets PTEN to mediate drug resistance and proliferation of breast cancer cells via the PI3K/Akt signaling pathway. Sci Rep 2017;7:41942. https://doi.org/10.1038/srep41942.Suche in Google Scholar PubMed PubMed Central
65. Xu, M, Mo, Y-Y. The AKT-associated microRNAs. Cell Mol Life Sci 2012;69:3601–12. https://doi.org/10.1007/s00018-012-1129-8.Suche in Google Scholar PubMed PubMed Central
66. Yu, T, Cao, R, Li, S, Fu, M, Ren, L, Chen, W, et al.. MiR-130b plays an oncogenic role by repressing PTEN expression in esophageal squamous cell carcinoma cells. BMC Cancer 2015;15:1–9. https://doi.org/10.1186/s12885-015-1031-5.Suche in Google Scholar PubMed PubMed Central
67. Xie, M, Fu, Z, Cao, J, Liu, Y, Wu, J, Li, Q, et al.. MicroRNA-132 and microRNA-212 mediate doxorubicin resistance by down-regulating the PTEN-AKT/NF-κB signaling pathway in breast cancer. Biomed Pharmacother 2018;102:286–94. https://doi.org/10.1016/j.biopha.2018.03.088.Suche in Google Scholar PubMed
68. Chen, Y, Sun, Y, Chen, L, Xu, X, Zhang, X, Wang, B, et al.. miRNA-200c increases the sensitivity of breast cancer cells to doxorubicin through the suppression of E-cadherin-mediated PTEN/Akt signaling. Mol Med Report 2013;7:1579–84. https://doi.org/10.3892/mmr.2013.1403.Suche in Google Scholar PubMed
69. Shen, R, Wang, Y, Wang, CX, Yin, M, Liu, HL, Chen, JP, et al.. MiRNA-155 mediates TAM resistance by modulating SOCS6-STAT3 signalling pathway in breast cancer. Am J Transl Res 2015;7:2115–26.Suche in Google Scholar
70. Beilankouhi, EAV, Valilo, M, Dastmalchi, N, Teimourian, S, Safaralizadeh, R. The function of autophagy in the initiation, and development of breast cancer. Curr Med Chem 2024;31:2974–90. https://doi.org/10.2174/0929867330666230503145319.Suche in Google Scholar PubMed
71. Xiu, D-H, Liu, G-F, Yu, S-N, Li, L-Y, Zhao, G-Q, Liu, L, et al.. Retracted article: long non-coding RNA LINC00968 attenuates drug resistance of breast cancer cells through inhibiting the Wnt2/β-catenin signaling pathway by regulating WNT2. J Exp Clin Cancer Res 2019;38:94. https://doi.org/10.1186/s13046-019-1100-8.Suche in Google Scholar PubMed PubMed Central
72. Li, Z, Qian, J, Li, J, Zhu, C. Knockdown of lncRNA-HOTAIR downregulates the drug-resistance of breast cancer cells to doxorubicin via the PI3K/AKT/mTOR signaling pathway. Exp Ther Med 2019;18:435–42. https://doi.org/10.3892/etm.2019.7629.Suche in Google Scholar PubMed PubMed Central
73. Liu, C, Xing, H, Guo, C, Yang, Z, Wang, Y, Wang, Y. MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle 2019;18:2215–27. https://doi.org/10.1080/15384101.2019.1638182.Suche in Google Scholar PubMed PubMed Central
74. Bergamaschi, A, Katzenellenbogen, BS. Tamoxifen downregulation of miR-451 increases 14-3-3ζ and promotes breast cancer cell survival and endocrine resistance. Oncogene 2012;31:39–47. https://doi.org/10.1038/onc.2011.223.Suche in Google Scholar PubMed PubMed Central
75. Vilquin, P, Donini, CF, Villedieu, M, Grisard, E, Corbo, L, Bachelot, T, et al.. MicroRNA-125b upregulation confers aromatase inhibitor resistance and is a novel marker of poor prognosis in breast cancer. Breast Cancer Res 2015;17:1–20. https://doi.org/10.1186/s13058-015-0515-1.Suche in Google Scholar PubMed PubMed Central
76. Jonas, K, Prinz, F, Ferracin, M, Krajina, K, Pasculli, B, Deutsch, A, et al.. MiR-4649-5p acts as a tumor-suppressive microRNA in triple negative breast cancer by direct interaction with PIP5K1C, thereby potentiating growth-inhibitory effects of the AKT inhibitor capivasertib. Breast Cancer Res 2023;25:119. https://doi.org/10.1186/s13058-023-01716-2.Suche in Google Scholar PubMed PubMed Central
77. Rao, X, Di Leva, G, Li, M, Fang, F, Devlin, C, Hartman-Frey, C, et al.. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene 2011;30:1082–97. https://doi.org/10.1038/onc.2010.487.Suche in Google Scholar PubMed PubMed Central
78. Liu, P, Sun, M, Jiang, W, Zhao, J, Liang, C, Zhang, H. Identification of targets of miRNA-221 and miRNA-222 in fulvestrant-resistant breast cancer. Oncol Lett 2016;12:3882–8. https://doi.org/10.3892/ol.2016.5180.Suche in Google Scholar PubMed PubMed Central
79. Zhou, Q, Zeng, H, Ye, P, Shi, Y, Guo, J, Long, X. Differential microRNA profiles between fulvestrant-resistant and tamoxifen-resistant human breast cancer cells. Anti Cancer Drugs 2018;29:539–48. https://doi.org/10.1097/cad.0000000000000623.Suche in Google Scholar PubMed
80. Corcoran, C, Rani, S, Breslin, S, Gogarty, M, Ghobrial, IM, Crown, J, et al.. miR-630 targets IGF1R to regulate response to HER-targeting drugs and overall cancer cell progression in HER2 over-expressing breast cancer. Mol Cancer 2014;13:1–12. https://doi.org/10.1186/1476-4598-13-71.Suche in Google Scholar PubMed PubMed Central
81. Masri, S, Liu, Z, Phung, S, Wang, E, Yuan, Y-C, Chen, S. The role of microRNA-128a in regulating TGFbeta signaling in letrozole-resistant breast cancer cells. Breast Cancer Res Treat 2010;124:89–99. https://doi.org/10.1007/s10549-009-0716-3.Suche in Google Scholar PubMed PubMed Central
82. Kazi, AA, Sabnis, G, Zhou, Q, Chumsri, S, Schech, A, Shah, P, et al.. HER2 regulated miRNA expression in letrozole resistant breast cancer. Cancer Res 2014;74:1471. https://doi.org/10.1158/1538-7445.am2014-1471.Suche in Google Scholar
83. Ashmawy, AM, Sheta, MA, Zahran, F, Wahab, AHAA. MiRNAs-181a/b as Predictive biomarkers for olaparib sensitivity in triple-negative breast cancer cells. Biochem Lett 2017;13:221–9. https://doi.org/10.21608/blj.2017.47612.Suche in Google Scholar
84. Yu, Y, Liao, H, Xie, R, Zhang, Y, Zheng, R, Chen, J, et al.. Overexpression of miRNA-3613-3p enhances the sensitivity of triple negative breast cancer to CDK4/6 inhibitor palbociclib. Front Oncol 2020;10:590813. https://doi.org/10.3389/fonc.2020.590813.Suche in Google Scholar PubMed PubMed Central
85. Torrisi, R, Vaira, V, Giordano, L, Fernandes, B, Saltalamacchia, G, Palumbo, R, et al.. Identification of a panel of miRNAs associated with resistance to palbociclib and endocrine therapy. Int J Mol Sci 2024;25:1498. https://doi.org/10.3390/ijms25031498.Suche in Google Scholar PubMed PubMed Central
86. Andrikopoulou, A, Shalit, A, Zografos, E, Koutsoukos, K, Korakiti, A-M, Liontos, M, et al.. MicroRNAs as potential predictors of response to CDK4/6 inhibitor treatment. Cancers (Basel) 2021;13:4114. https://doi.org/10.3390/cancers13164114.Suche in Google Scholar PubMed PubMed Central
87. Ji, W, Zhang, W, Wang, X, Shi, Y, Yang, F, Xie, H, et al.. c-myc regulates the sensitivity of breast cancer cells to palbociclib via c-myc/miR-29b-3p/CDK6 axis. Cell Death Dis 2020;11:760. https://doi.org/10.1038/s41419-020-02980-2.Suche in Google Scholar PubMed PubMed Central
88. Krasniqi, E, Goeman, F, Pulito, C, Palcau, AC, Ciuffreda, L, Di Lisa, FS, et al.. Biomarkers of response and resistance to CDK4/6 inhibitors in breast cancer: hints from liquid biopsy and MicroRNA exploration. Int J Mol Sci 2022;23:14534. https://doi.org/10.3390/ijms232314534.Suche in Google Scholar PubMed PubMed Central
89. Wuerkenbieke, D, Wang, J, Li, Y, Ma, C. miRNA-150 downregulation promotes pertuzumab resistance in ovarian cancer cells via AKT activation. Arch Gynecol Obstet 2015;292:1109–16. https://doi.org/10.1007/s00404-015-3742-x.Suche in Google Scholar PubMed
90. Cabello, P, Torres-Ruiz, S, Adam-Artigues, A, Forés-Martos, J, Martínez, MT, Hernando, C, et al.. miR-146a-5p promotes angiogenesis and confers trastuzumab resistance in HER2+ breast cancer. Cancers (Basel). 2023;15:2138. https://doi.org/10.3390/cancers15072138.Suche in Google Scholar PubMed PubMed Central
91. Ma, T, Yang, L, Zhang, J. miRNA-542-3p downregulation promotes trastuzumab resistance in breast cancer cells via AKT activation. Oncol Rep 2015;33:1215–20. https://doi.org/10.3892/or.2015.3713.Suche in Google Scholar PubMed
92. Decker, JT, Hall, MS, Blaisdell, RB, Schwark, K, Jeruss, JS, Shea, LD. Dynamic microRNA activity identifies therapeutic targets in trastuzumab-resistant HER2+ breast cancer. Biotechnol Bioeng 2018;115:2613–23. https://doi.org/10.1002/bit.26791.Suche in Google Scholar PubMed PubMed Central
93. Gong, C, Yao, Y, Wang, Y, Liu, B, Wu, W, Chen, J, et al.. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem 2011;286:19127–37. https://doi.org/10.1074/jbc.m110.216887.Suche in Google Scholar
94. Rezaei, Z, Sebzari, A, Kordi-Tamandani, DM, Dastjerdi, K. Involvement of the dysregulation of miR-23b-3p, miR-195-5p, miR-656-5p, and miR-340-5p in trastuzumab resistance of HER2-positive breast cancer cells and system biology approach to predict their targets involved in resistance. DNA Cell Biol 2019;38:184–92. https://doi.org/10.1089/dna.2018.4427.Suche in Google Scholar PubMed
95. Zhang, Z, Zhang, L, Yu, G, Sun, Z, Wang, T, Tian, X, et al.. Exosomal miR-1246 and miR-155 as predictive and prognostic biomarkers for trastuzumab-based therapy resistance in HER2-positive breast cancer. Cancer Chemother Pharmacol 2020;86:761–72. https://doi.org/10.1007/s00280-020-04168-z.Suche in Google Scholar PubMed
96. Cittelly, DM, Das, PM, Spoelstra, NS, Edgerton, SM, Richer, JK, Thor, AD, et al.. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol Cancer 2010;9:1–12. https://doi.org/10.1186/1476-4598-9-317.Suche in Google Scholar PubMed PubMed Central
97. Zhang, W, Xu, J, Shi, Y, Sun, Q, Zhang, Q, Guan, X. The novel role of miRNAs for tamoxifen resistance in human breast cancer. Cell Mol Life Sci 2015;72:2575–84. https://doi.org/10.1007/s00018-015-1887-1.Suche in Google Scholar PubMed PubMed Central
98. Ward, A, Shukla, K, Balwierz, A, Soons, Z, König, R, Sahin, Ö, et al.. MicroRNA-519a is a novel oncomir conferring tamoxifen resistance by targeting a network of tumour-suppressor genes in ER+ breast cancer. J Pathol 2014;233:368–79. https://doi.org/10.1002/path.4363.Suche in Google Scholar PubMed PubMed Central
99. Ye, P, Fang, C, Zeng, H, Shi, Y, Pan, Z, An, N, et al.. Differential microRNA expression profiles in tamoxifen-resistant human breast cancer cell lines induced by two methods. Oncol Lett 2018;15:3532–9. https://doi.org/10.3892/ol.2018.7768.Suche in Google Scholar PubMed PubMed Central
100. Zhu, J, Zou, Z, Nie, P, Kou, X, Wu, B, Wang, S, et al.. Downregulation of microRNA-27b-3p enhances tamoxifen resistance in breast cancer by increasing NR5A2 and CREB1 expression. Cell Death Dis 2016;7:e2454–e. https://doi.org/10.1038/cddis.2016.361.Suche in Google Scholar PubMed PubMed Central
101. Zhao, J-J, Lin, J, Yang, H, Kong, W, He, L, Ma, X, et al.. MicroRNA-221/222 negatively regulates estrogen receptorα and is associated with tamoxifen resistance in breast cancer. J Biol Chem 2008;283:31079–87. https://doi.org/10.1074/jbc.m806041200.Suche in Google Scholar
102. Jamialahmadi, K, Zahedipour, F, Karimi, G. The role of microRNAs on doxorubicin drug resistance in breast cancer. J Pharm Pharmacol 2021;73:997–1006. https://doi.org/10.1093/jpp/rgaa031.Suche in Google Scholar PubMed
103. Hu, D, Li, M, Su, J, Miao, K, Qiu, X. Dual-targeting of miR-124-3p and ABCC4 promotes sensitivity to adriamycin in breast cancer cells. Genet Test Mol Biomarkers 2019;23:156–65. https://doi.org/10.1089/gtmb.2018.0259.Suche in Google Scholar PubMed
104. Zhu, Y, Yu, F, Jiao, Y, Feng, J, Tang, W, Yao, H, et al.. Reduced miR-128 in breast tumor–initiating cells induces chemotherapeutic resistance via Bmi-1 and ABCC5. Clin Cancer Res 2011;17:7105–15. https://doi.org/10.1158/1078-0432.ccr-11-0071.Suche in Google Scholar PubMed
105. Gao, M, Miao, L, Liu, M, Li, C, Yu, C, Yan, H, et al.. miR-145 sensitizes breast cancer to doxorubicin by targeting multidrug resistance-associated protein-1. Oncotarget 2016;7:59714–26. https://doi.org/10.18632/oncotarget.10845.Suche in Google Scholar PubMed PubMed Central
106. Lu, L, Ju, F, Zhao, H, Ma, X. MicroRNA-134 modulates resistance to doxorubicin in human breast cancer cells by downregulating ABCC1. Biotechnol Lett 2015;37:2387–94. https://doi.org/10.1007/s10529-015-1941-y.Suche in Google Scholar PubMed
107. Hu, Q, Gong, J-P, Li, J, Zhong, S-L, Chen, W-X, Zhang, J-Y, et al.. Down-regulation of miRNA-452 is associated with adriamycin-resistance in breast cancer cells. Asian Pac J Cancer Prev 2014;15:5137–42. https://doi.org/10.7314/apjcp.2014.15.13.5137.Suche in Google Scholar PubMed
108. Shen, H, Li, L, Yang, S, Wang, D, Zhong, S, Zhao, J, et al.. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene 2016;593:84–90. https://doi.org/10.1016/j.gene.2016.08.016.Suche in Google Scholar PubMed
109. Yin, Y, Wang, X, Li, T, Ren, Q, Li, L, Sun, X, et al.. MicroRNA-221 promotes breast cancer resistance to adriamycin via modulation of PTEN/Akt/mTOR signaling. Cancer Med 2020;9:1544–52. https://doi.org/10.1002/cam4.2817.Suche in Google Scholar PubMed PubMed Central
110. Shen, H, Wang, D, Li, L, Yang, S, Chen, X, Zhou, S, et al.. MiR-222 promotes drug-resistance of breast cancer cells to adriamycin via modulation of PTEN/Akt/FOXO1 pathway. Gene 2017;596:110–8. https://doi.org/10.1016/j.gene.2016.10.016.Suche in Google Scholar PubMed
111. Huang, Q, Wu, Y-Y, Xing, S-J, Yu, Z-W. Effect of miR-7 on resistance of breast cancer cells to adriamycin via regulating EGFR/PI3K signaling pathway. Eur Rev Med Pharmacol Sci 2019;23:5285–92. https://doi.org/10.26355/eurrev_201906_18195.Suche in Google Scholar PubMed
112. Chen, X, Lu, P, Wu, Y, Wang, DD, Zhou, S, Yang, SJ, et al.. MiRNAs-mediated cisplatin resistance in breast cancer. Tumor Biol 2016;37:12905–13. https://doi.org/10.1007/s13277-016-5216-6.Suche in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Crosstalk between miRNAs and signaling pathways in the development of drug resistance in breast cancer
- Hormonal disorders in autism spectrum disorders
- An overview of the relationship between melatonin and drug resistance in cancers
- Original Articles
- Gestational diabetes mellitus (GDM): diagnosis using biochemical parameters and anthropometric measurements during the first trimester in the Indian population
- Endothelial cell phenotype is linked to endothelial dysfunction in individuals with a family history of type 2 diabetes
- Associations of serum levels of cGAMP in the context of COVID-19 infection, atherosclerosis, sterile inflammation, and functional endothelial biomarkers in patients with coronary heart disease and healthy volunteers
Artikel in diesem Heft
- Frontmatter
- Review Articles
- Crosstalk between miRNAs and signaling pathways in the development of drug resistance in breast cancer
- Hormonal disorders in autism spectrum disorders
- An overview of the relationship between melatonin and drug resistance in cancers
- Original Articles
- Gestational diabetes mellitus (GDM): diagnosis using biochemical parameters and anthropometric measurements during the first trimester in the Indian population
- Endothelial cell phenotype is linked to endothelial dysfunction in individuals with a family history of type 2 diabetes
- Associations of serum levels of cGAMP in the context of COVID-19 infection, atherosclerosis, sterile inflammation, and functional endothelial biomarkers in patients with coronary heart disease and healthy volunteers

