Abstract
Objectives
The patient’s family history of type 2 diabetes (FH-DM2) has been negatively associated with the functionality of endothelial cells (ECs). Our objectives in this work were to use human umbilical vein endothelial cells (HUVECs) as a model, to substantiate whether FH-DM2 influences endothelial phenotype and impairs NO and ROS synthesis, cell metabolism, and mitochondrial activity of ECs from individuals with FH-DM2.
Methods
In this study were evaluated the synthesis of reactive oxygen species (ROS) and nitric oxide (NO), mitochondrial membrane potential (MMP), mRNA of eNOS, glucose consumption, and lactate synthesis in HUVECs from newborns with FH-DM2. Furthermore, we also evaluated EC complexity and cell size through flow cytometry.
Results
Our results showed significant differences in HUVECs with FH-DM2, regarding their complexity and cell size, in the synthesis of ROS (p<0.01), and NO (p<0.05); they also reflected diminished glucose consumption and slight changes in the lactate levels.
Conclusion
In conclusion, our results showed that HUVECs from children with FH-DM2 have a reduced capability of synthesizing ROS and NO, which might be linked to the metabolism of endothelial cells. These results are relevant since early endothelial dysfunction has been reported in individuals with FH-DM2, and could be used to establish preventive measures to reduce the risk of developing atherosclerosis or cardiovascular diseases in healthy individuals, but with this family background.
Introduction
Endothelial cells (ECs) regulate the vasomotor tone, the glucose and lipids transport and metabolism, as well as the innate and adaptive immune response [1], [2], [3]. Interestingly, ECs can change their phenotypic characteristics depending on the actions they need to perform [4], 5]. In patients with diabetes and obesity, as well as in healthy subjects but with a type 2 diabetes family history (FH-DM2), the capability of these cells to change is associated with impaired functionality [6], [7], [8]. Interestingly, variations in glucose levels in healthy individuals with a strong FH-DM2 background induce a diminished nitric oxide (NO) bioavailability [9], 10]. Recently, a report mentioned that a family history of diabetes was associated with increased thickness of the carotid intima-media, including individuals without diagnosed diabetes [11]. Additionally, in normoglycaemic patients with FH-DM, a diminished postprandial skeletal muscle macro- and microvascular response was reported, therefore, the authors suggest that deficient vascular responses can be a risk factor for type 2 diabetes development [12]. On the other hand, a poor response to both NO synthesis inducers, as well as an impaired vascular function, was reported in young healthy adults with FH-DM2 [13]. Another relevant aspect is that endothelial dysfunction in different pathologies is characterized by a reduction in NO and reactive oxygen species (ROS) synthesis, and with alterations in the cell membrane and glucose metabolism [14], 15]. Previous results of our group showed an impaired synthesis of ROS, and NO in Human umbilical vein endothelial cells (HUVECs) from newborns with an FH-DM2 background when cultivated in the presence of high glucose [16], 17]. However, cell complexity was not evaluated in these studies, neither was glucose consumption nor lactate synthesis, factors recently related to endothelial dysfunction [18]. HUVECs are an experimental model extensively and are used in vascular dynamics or angiogenesis studies to evaluate ECs response. Consequently, our objectives were to use HUVECs as a model, to substantiate whether FH-DM2 influences endothelial phenotype, impairs the synthesis of NO and ROS, glucose metabolism, and the mitochondrial activity of the endothelial cells from individuals with a family diabetes background.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs), isolated as previously described [16], were obtained from 20 healthy newborn babies and healthy non-diabetic mothers (21–27 ± 2 years old) but with a family history of type 2 diabetes (three or more first-degree relatives with the disease), and 20 newborns with healthy mothers (20–26 years old) without FH-DM2. Endothelial cells (5 × 105) were incubated in M−199 medium (without phenol red), in a 5 % CO2 atmosphere at 37 °C until use. HUVECs were used within passages 4–6 and were identified as endothelial by their morphology and their uniform positive endoglin (CD105; BD Pharmigen, USA) staining. Cells were co-cultured for 24 and 48 h with 5, 15, or 30 mM of glucose. The Institutional Scientific and Bioethics Committees of the National Institute of Respiratory Diseases “Ismael Cosío Villegas” approved this protocol. All participants signed a written informed consent (Protocol number C09-14).
Determination of ROS and NO synthesis, and mitochondrial membrane potential by flow cytometry
The granularity, size, as well as intracellular synthesis of ROS, NO, and mitochondrial membrane potential (MMP) from HUVECs, were evaluated by flow cytometry (Becton Dickinson Facscalibur instrument, San Jose, CA, USA). The ROS synthesis was determined in 1 × 104 cells incubated for 15 min with 10 μM of dichlorofluorescein diacetate bis(acetoxymethyl) (DCFH-DA, Molecular Probes, Eugene, OR, USA) [16], whereas NO formation was evaluated in cells incubated with 8 μM 4-amino-5-methylamino-2′,7′-dichlorofluorescein diacetate (DAF-FM, Molecular Probes) for 30 min [17]; in both cases, cells incubated with 5, 15, or 30 mM glucose. Positive controls were 1 × 104 endothelial cells incubated with 200 μM H2O2 or with 2 mmol of S-nitroso-acetyl penicillamine (SNAP, a donor of NO, Molecular Probes). MMP was evaluated in 1 × 104 cells incubated for 15 min with 50 nM of 3,3′-dihexyloxacarbocyanine iodide (DiOC6 (3)), Molecular Probes) [16].
RT-PCR for endothelial nitric oxide synthase (eNOS)
Total RNA was obtained using 1 mL of Trizol (Molecular Research Center Inc.) from 1 × 106 cells cultured for 48 h in the experimental glucose concentrations. The RNA pellet was resuspended, and its concentration was determined. RNA (1 μg) was mixed with 1 μL of oligo-d[T], 4 μL of 5 × reverse transcriptase first strand buffer, and 10 μL of diethyl pyrocarbonate-treated water. Reverse transcription was performed using Moloney murine leukemia virus reverse transcriptase, this RT mixture was incubated at 42 °C for 50 min and then at 70 °C for 15 min and the resulting cDNA was amplified by PCR. The primers for eNOS were 5_-CCA GCT AGC CAA AGT CAC CAT-3 (upstream) and 5_- GTC TCG GAG CCA TAC AGG ATT-3 (downstream) [18]. The conditions for PCR were 94 °C for 1 min (denaturation), 55 °C for 1 min (annealing), and 72 °C for 2 min (extension) in a p × 2 Thermal Cycler (Thermo Electron Corporation, USA). The primers for β-actin, were 5_-CGT TCA CCT TGA TGA GCC CAT T-3_ (upstream) and 5-TCC AAG GGT CCG CTG CAG GTC-3_ (downstream) [17]. After 35 cycles, PCR products were evaluated by electrophoresis on 2 % agarose gel and stained with ethidium bromide, each band was analyzed, and its optical density was determined in a UVP image analyzer (Ultraviolet Products, CA, USA) at 255 nm using the Labworks 4.0 image acquisition and analysis software. The results of mRNA expression of eNOS were analyzed and are shown as the ratio calculated from their correspondent optical density divided by the optical density obtained of the β-actin band, ×100.
Evaluation of glucose consumption and lactate levels
Culture supernatants of HUVECs (1 × 104 cells) co-cultured in M−199 medium with 5, 15, or 30 mM glucose, for 24 and 48 h were used to determine glucose consumption with the Amplex® red glucose/glucose oxidase assay kit (Molecular Probes). The levels of lactate were obtained using a lactate reagent (Sigma, St. Louis, MO, USA) after growing HUVECs in our experimental conditions. Differences in glucose and lactate levels between control and glucose-exposed supernatants were determined. Results were normalized and expressed as microgram of glucose per microgram of protein (μg of Glc/μg of protein) or as microgram of lactate per microgram of protein (μg lactate/μg of protein), determined by the micro-Lowry method.
Statistical analysis
Variance analysis followed by Dunnett’s test was performed with SPSSv11. Results are expressed as mean ± SEM. Statistical significance was set at p<0.05.
Results
Determination of granularity, size, ROS, and NO synthesis, and expression of eNOS mRNA
Twenty-four-hour incubation with diverse glucose concentrations did not yield important differences either between groups or between treated and control cells. However, 48 h incubation showed significant differences. In Figure 1, one (R1) or two (R1 and R2) HUVEC populations from the newborns with (A) or without (B) FH-DM2 respectively, were observed. In addition, cells obtained from newborns with FH-DM2 (Figure 1A, forward scatter, FSC-H) were significantly smaller as opposed to cells from newborns without FH-DM2 (Figure 1B, FSC-H). Assessment of the synthesis of ROS, a vital parameter of endothelial cell function, revealed that the HUVECs from both groups secreted ROS. However, again, one (R1) or two populations (R1 and R2) associated with ROS synthesis were observed in cells from newborns with (Figure 1C) or without (Figure 1D) FH-DM2. Interestingly, when HUVECs with FH-DM2 were exposed to diverse glucose concentrations, the percentage of cell-producing ROS did not show important changes (Figure 1E, R1); whereas the R2 population was practically inexistent (Figure 1E). In comparison, the HUVECs without FH-DM2 showed a diminution in the percentage of cells in the population R1 (Figure 1F), whereas the percentage of the population of R2 (Figure 1F) showed an increase associated with the rise in glucose concentration (*p<0.05, and **p<0.01).
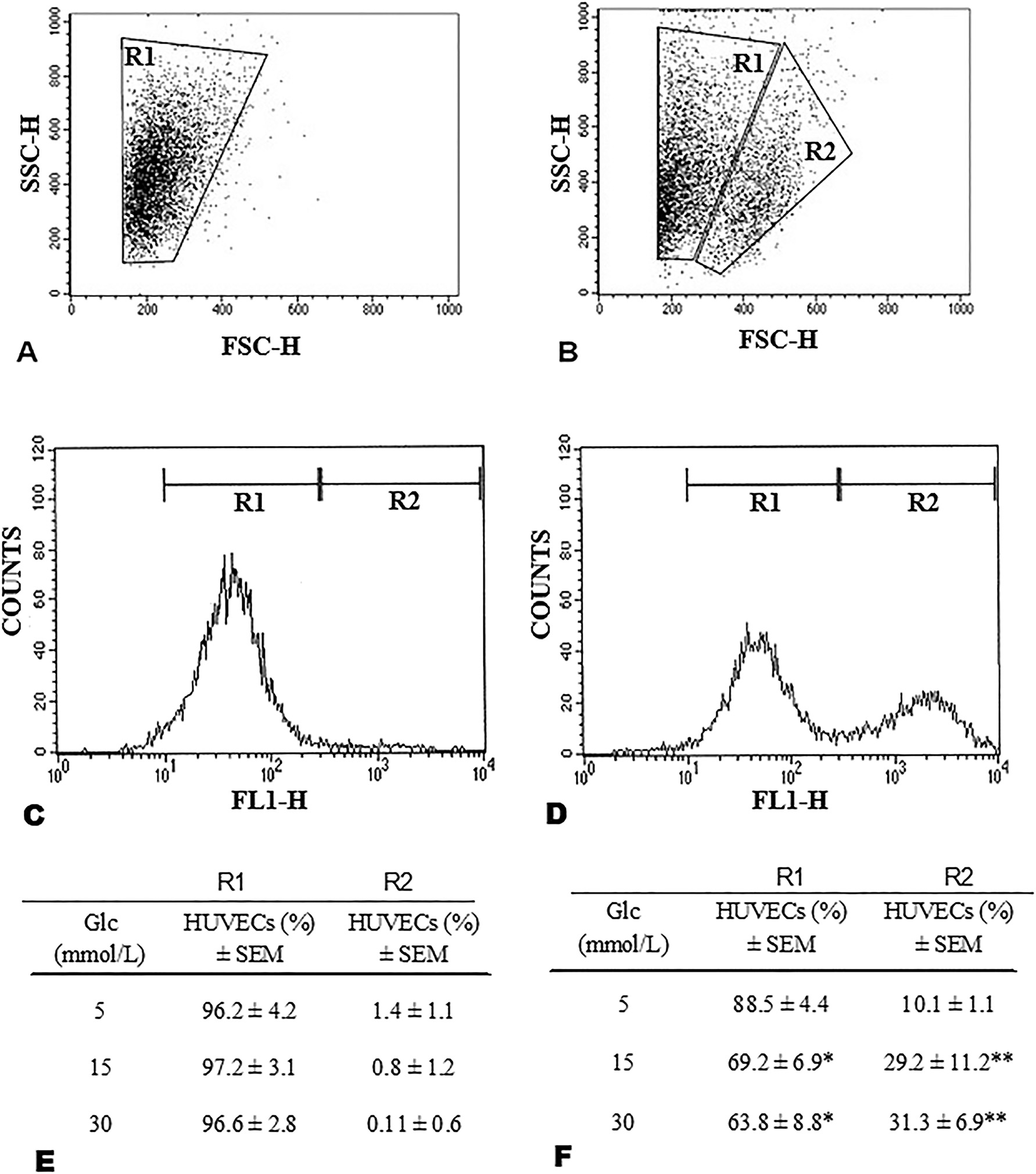
The population of endothelial cells from newborns with FH-DM2 (A) and without FH-DM2 (B), was differentiated by side scatter (SSC-H) and forward scatter (FSC-H). Histograms show HUVECs positive for the synthesis of ROS with (C) and without FH-DM2 (D). Percentage of endothelial cells that produce reactive oxygen species (ROS) from newborns with (E, n=20) or without FH-DM2 (D, n=20), after incubation with 5, 15, or 30 mM of glucose for 48 h. Results are shown as the mean percentage of positive cells to ROS synthesis ± SEM. *p<0.05 and **p<0.01 vs. 5 mM of glucose.
When intracellular NO synthesis was evaluated, the percentage of ECs with FH-DM2 (Figure 2A) that synthesized NO showed significant changes according to the glucose concentration (*p<0.05). In comparison, the percentage of ECs without FH-DM2 (Figure 2B) and positive to NO synthesis, showed a more pronounced increase in the percentage of these cells, an increase associated again with the glucose concentration. Expression of eNOS mRNA shows changes associated with the increase in glucose concentration. However, after comparison between groups, the HUVECs without FH-DM2 showed a slightly higher expression of eNOS mRNA (Figure 2D), in contrast with the HUVECs with FH-DM2 (Figure 2C). This last was confirmed after obtaining the eNOS/β-actin ratio, where although was observed an increase in this proportion in the cells with FH-DM2 (Figure 2E), this increase was major in the cells without diabetes background from a concentration of 15 mmol of glucose (Figure 2F).
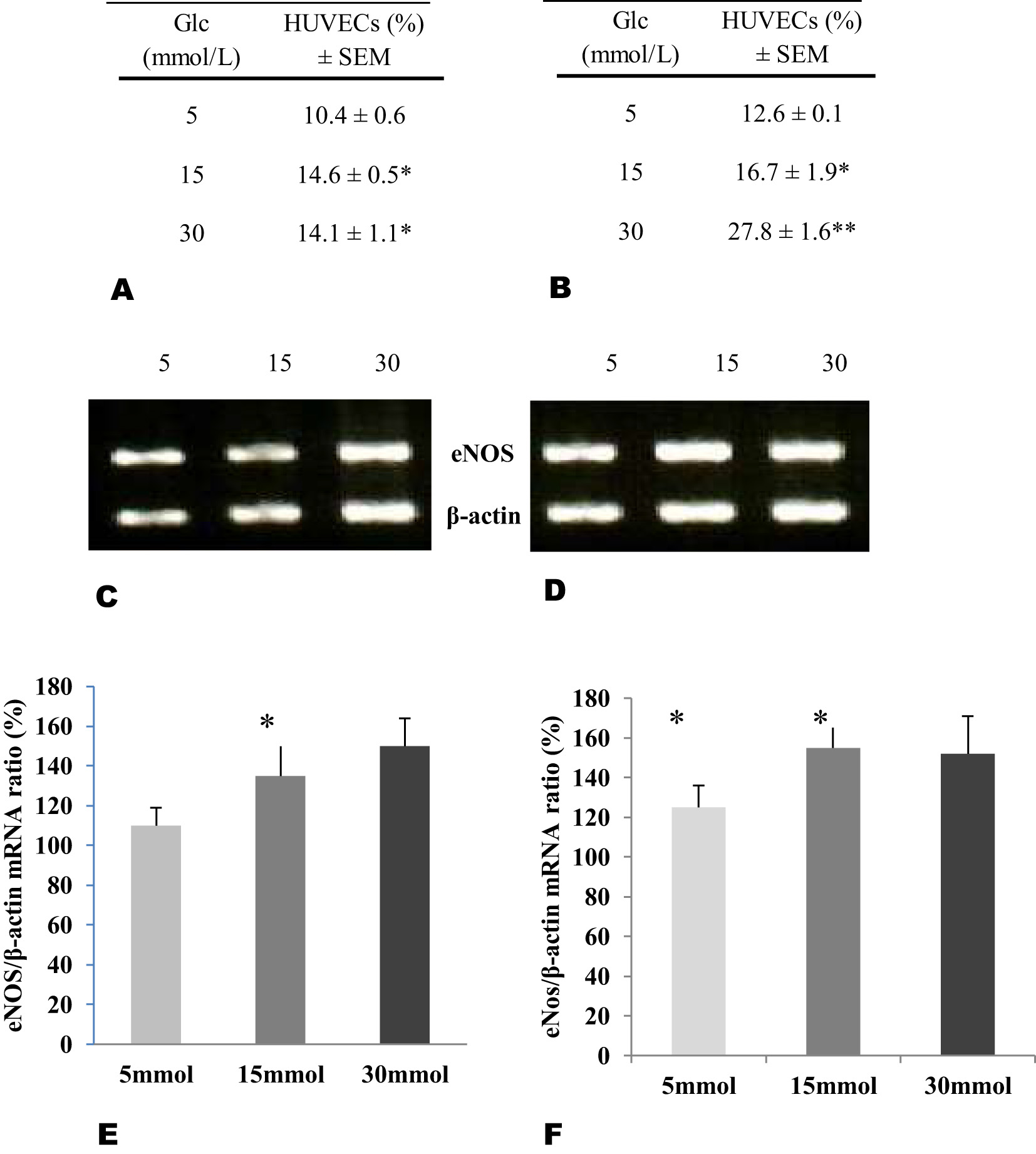
Percentage of endothelial cells producers of nitric oxide (NO) from newborns with (A, n=20) or without FH-DM2 (B, n=20), incubated with different glucose concentrations for 48 h. Expression of mRNA and ratio calculated from endothelial nitric oxide synthase (eNOS) in relation from β-actin in endothelial cells with (C–E) or without (D–F) HF-DM2, after incubation by 48 h in the glucose concentrations showed (*p<0.05 vs. 5 mmol of glucose).
Glucose consumption and lactate levels
As glucose consumption and high glucose concentration have shown both negative and positive effects on NO synthesis [9], 19], 20], we considered that it was important to determine what glucose uptake was in both ECs populations. The results showed a significant rise in glucose uptake in HUVECs with FH-DM2 (Figure 3A; *p<0.05); however, this increase in glucose consumption was lower than that observed in the ECs without FH-DM2 (Figure 3B), especially when 30 mM glucose was used (**p<0.01). In comparison, although lactate levels showed an increase in the cells with FH-DM2 specially after incubating them with 15 mM glucose (*p<0.05), they were not significantly different for any of the glucose concentrations, or between cells with or without an FH-DM2 (Figure 3C–and 3D, respectively).
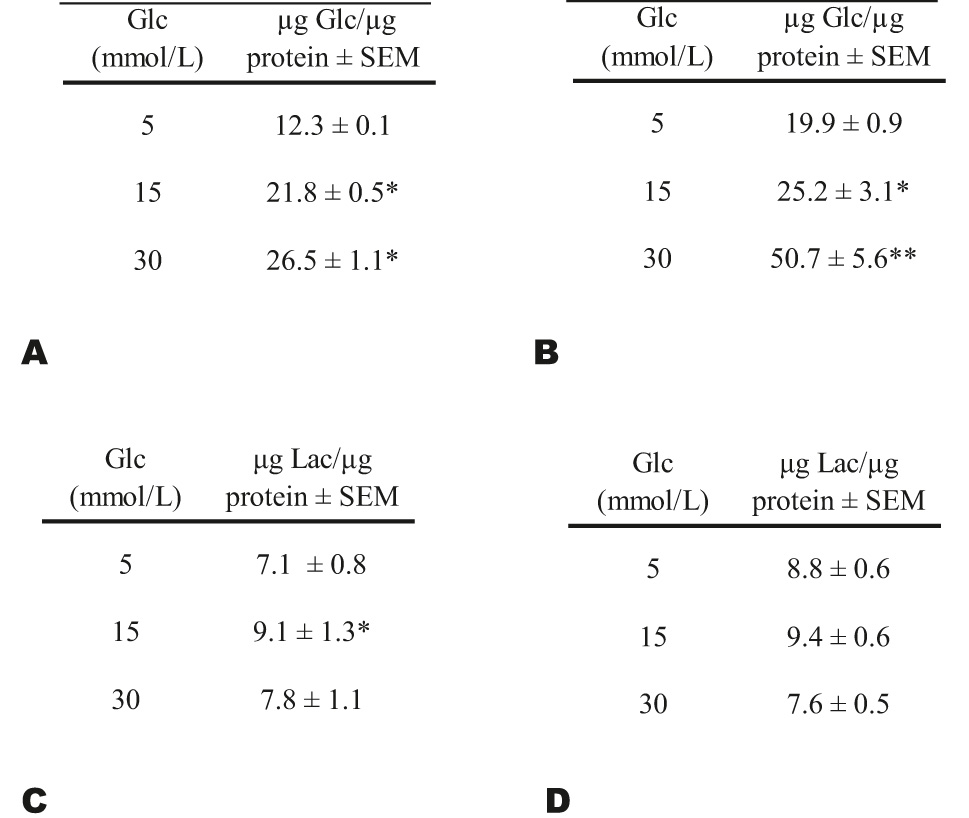
Glucose (Glc) consumption was evaluated using the culture medium of HUVECs with (A, n=20) or without FH-DM2 (B, n=20). Lactate levels (Lac) in culture medium of HUVECs with (C) or without (D) FH-DM2 after 48 h of culture. Results were normalized and expressed as micrograms of glucose or lactate per microgram of protein: µg Glc/µg protein ± SEM; or μg Lac/μg protein ± SEM. *p<0.05 and **p<0.01 vs. 5 mM of glucose.
Mitochondrial membrane potential
The mitochondrial membrane potential of the HUVECs, independently of their background, was not significantly modified by any of the glucose concentrations tested (Figure 4).
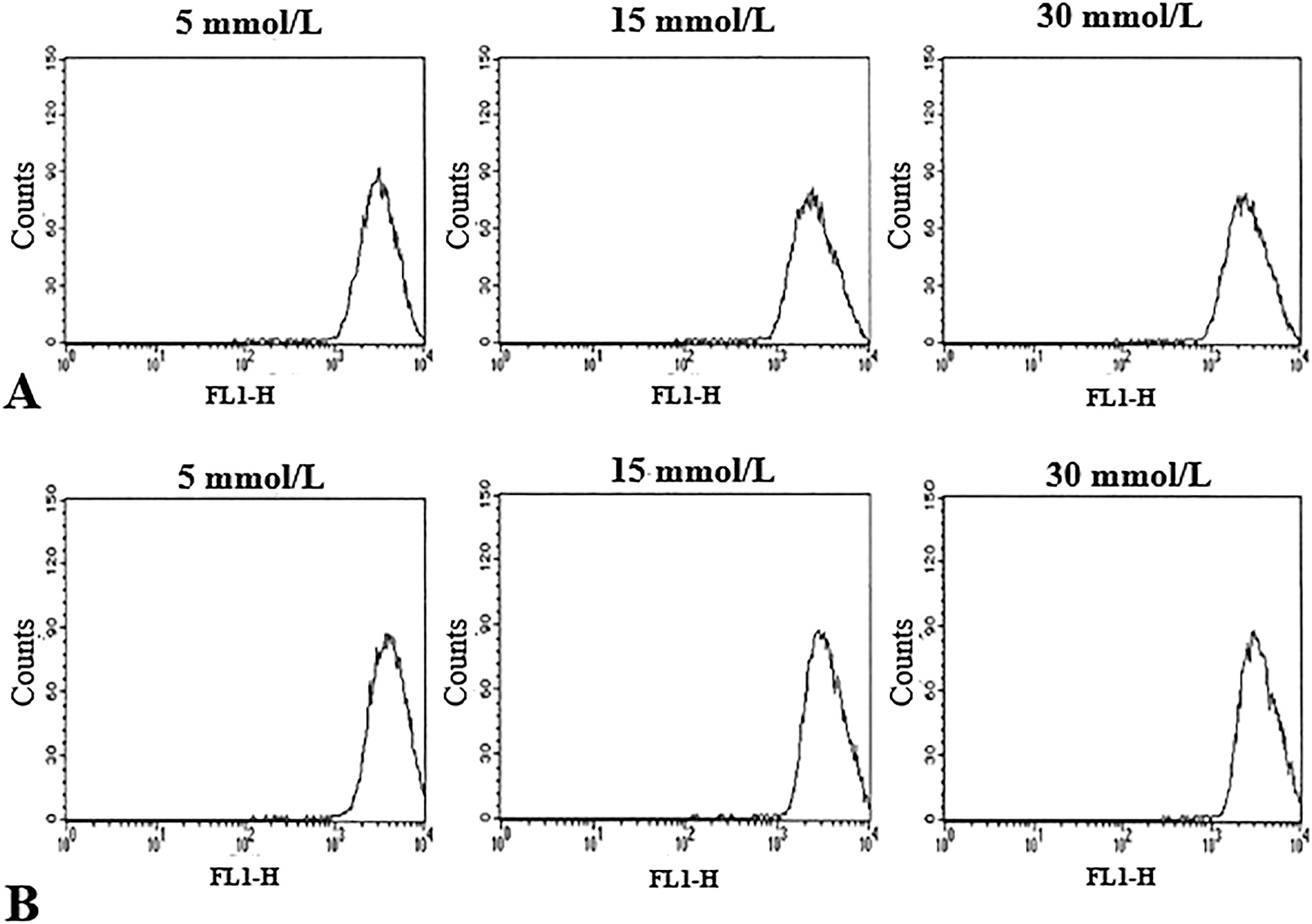
Histograms showing mitochondrial membrane potential (MMP) determined in HUVECs with (A) and without FH-DM2 incubated for 48 h with glucose plus 50 nM DiOC6 (3). The figure shows the results of a 48 h representative experiment. Our results did not show significant changes in the endothelial cells with/without FH-DM2 after 48 h of culture with the different glucose concentrations used; p>0.05.
Discussion
Although endothelial dysfunction has been reported in healthy subjects with an FH-DM2 [6], [7], [8, 10], the possible role of endothelial heterogeneity associated with endothelial dysfunction in patients with an FH-DM2 has not been evaluated. Our results showed a great variation in the complexity side scatter (SSC-H) and size (FSC-H) in the HUVECs isolated from newborns without an FH-DM2, which could be associated with different functionality or aging of these ECs [1], [2], [3], [4], [5, 21]. The latter could be additionally supported by evaluating ROS and NO synthesis [12], 22], both molecules highly relevant for the proliferation and survival of endothelial cells. Our results showed that high glucose concentrations do not affect the synthesis of ROS by HUVECs with FH-DM2. However, the response of ECs without FH-DM2 was different and related to the glucose concentration, and with the percentage of cells that synthesized ROS. This difference could be associated with the early endothelial dysfunction reported in subjects with an FH-DM2 [6], 7], 10], including normoglycemic people but with a diabetes family background [7], 12]. However, when NO synthesis was determined, it was not possible to find different populations from those observed during the ROS synthesis evaluation. In the case of NO, its synthesis was carried out by the total populations of endothelial cells. Nevertheless, there was an astonishing lack of response to high glucose concentrations in the HUVECs with an FH-DM2 in contrast to those without FH-DM2 which showed a significant increase in the percentage of NO-producing endothelial cells. This difference is probably related to a deficient capacity to properly metabolize glucose in ECs with a diabetes background [9], 13], 14], 23], 24], compared to those of a non-diabetic background, which might negatively influence NO synthesis [25]. The possible explanation for this difference remains unclear, even though some authors showed that high glucose inhibits NO synthesis [26], while others reported that high glucose induces NO synthesis in diabetic patients [20]. The aforementioned reports highlight the importance of this topic nowadays [27].
In this context, EC metabolism influences their capability to change. For instance, their ATP synthesis can be through oxidative phosphorylation, but a low number of mitochondria favors the use of the glycolytic pathway, which is upgraded in these cells [28]. Additionally, some authors suggest that high glycolytic activity could benefit endothelial cells by inducing a minor synthesis of ROS, indirectly favoring higher oxygen availability for other cells, and a major lactate synthesis (a factor proangiogenic) [29]. However, recent data show the importance of ATP generated by endothelial mitochondria for NO synthesis, involved in vascular tone regulation [30]. Based on the aforementioned facts, the metabolism of ECs could be a relevant point that enables endothelial cells to perform an ample diversity of functions by changing their phenotypic characteristics.
This discrepancy led us to determine glucose consumption by ECs. Our results showed that HUVECs with FH-DM2 had increased glucose consumption, but this intake was higher in the ECs without FH-DM2, which reinforces the importance of glucose as an inducer of NO synthesis [20]. However, this finding might point out that intermittent or chronic exposure to high glucose levels could impair long-run NO synthesis [27], 31]. Additionally, it is well established that the mitochondrion intervenes in apoptosis, ROS synthesis, glucose metabolism, and NO synthesis [32], [33], [34]. However, our results did not show significant changes in MMP, which suggests that in our study, high glucose did not play a relevant role in mitochondrial activity or cell death as reported by other authors [33], which suggests the importance of time high glucose exposition to observe negative results on endothelial cell functionality [27], 34].
Our work had some limitations, like the sample cell number, a consequence of the difficulty in finding pregnant women with a strong DM2 family history. Nonetheless, this circumstance gives our results particular relevance and, also noteworthy, our results derive from individualized non-pooled HUVECs therefore taking into consideration individual cell sample´s characteristics, and reducing the risk of a nonspecific response, as observed when using pooled HUVECs [34], 35]. Since it is impossible to obtain endothelial cells from normal and diabetic adults, we cannot extrapolate our results to grown-ups. However, we consider that our data can be compared with reports of individuals with FH-DM [6], [7], [8], [9], [10]. Additionally, it is important to mention that some reports showed a deficient glucose uptake and an increase in the mitochondrial area of HUVECs isolated from newborns with type 1 diabetic mothers [36], or in ECs incubated with high glucose concentrations for long periods as 6–10 days [37]. Likewise, it is important to mention that the HUVECs with an FH-DM2 background were more sensitive to changes in culture conditions, which unfortunately limits the number of passages as well as the type of questions that might be answered using this type of cell. Yet, HUVECs are considered an excellent model for studying different vascular and metabolic diseases [35], 38], 39].
Conclusions
In conclusion, our results suggest that FH-DM2 is an important modifier of the endothelial cell response in the presence of a common stressor such as high glucose concentration. This could be an important consideration for “healthy” subjects with FH-DM2, but with impaired glucose metabolism, which affects the synthesis of ROS and NO, and would favor early endothelial dysfunction in these individuals, a fact that is associated with the onset of vascular disease later in life [36]. Moreover, evidence supports the above. It shows an impaired ability to properly metabolize glucose in ECs with a diabetes background [9], 23], 25], negatively influencing NO synthesis [25], 40]. Moreover, our results suggest that this EC´s sensibility glucose levels associated with the FH-DM2 might have been an additional factor to consider in other pathologies, for example with the endothelial dysfunction reported in COVID-19 patients [37], 40], where a patient without diagnosed comorbidities usually has high glucose levels, which is linked with a bad prognosis [37], 38], 41].
-
Research ethics: The Institutional Scientific and Bioethics Committees of the National Institute of Respiratory Diseases “Ismael Cosío Villegas” approved this protocol.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: NAV: Conceptualization, methodology, data analysis, writing. BS: Conceptualization, methodology, writing. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This research was financed by Instituto Nacional de Enfermedades Respiratorias “Ismael Cosío Villegas”.
-
Data availability: Not applicable.
References
1. Aird, WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 2007;100:158–73. https://doi.org/10.1161/01.RES.0000255691.76142.4a.Search in Google Scholar PubMed
2. Aird, WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2012;2:a006429. https://doi.org/10.1101/cshperspect.a006429.Search in Google Scholar PubMed PubMed Central
3. Iiyama, K, Hajra, L, Iiyama, M, Li, H, DiChiara, M, Medoff, BD, et al.. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res 1999;85:199–207. https://doi.org/10.1161/01.res.85.2.199.Search in Google Scholar PubMed
4. Aird, WC. Endothelium in health and disease. Pharmacol Rep 2008;60:139–43.Search in Google Scholar
5. Hajra, L, Evans, AI, Chen, M, Hyduk, SJ, Collins, T, Cybulsky, MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA 2000;97:9052–7. https://doi.org/10.1073/pnas.97.16.9052.Search in Google Scholar PubMed PubMed Central
6. Goldfine, AB, Beckman, JA, Betensky, RA, Devlin, H, Hurley, S, Varo, N, et al.. Family history of diabetes is a major determinant of endothelial function. J Am Coll Cardiol 2006;47:2456–61. https://doi.org/10.1016/j.jacc.2006.02.045.Search in Google Scholar PubMed
7. Scuteri, A, Tesauro, M, Rizza, S, Iantorno, M, Federici, M, Lauro, D, et al.. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metabol Cardiovasc Dis 2008;18:349–56. https://doi.org/10.1016/j.numecd.2007.03.008.Search in Google Scholar PubMed
8. Rasooly, D, Moonesinghe, R, Littrell, K, Hull, L, Khoury, MJ. Association between a first-degree family history and self-reported personal history of obesity, diabetes, and heart and blood conditions: results from the all of us research program. J Am Heart Assoc 2023;12:e030779. https://doi.org/10.1161/JAHA.123.030779.Search in Google Scholar PubMed PubMed Central
9. Reynolds, SS, Yanek, LR, Vaidya, D, Mora, S, Moy, TF, Saudek, CD, et al.. Glucose levels in the normal range predict incident diabetes in families with premature coronary heart disease. Diabetes Res Clin Pract 2006;74:267–73. https://doi.org/10.1016/j.diabres.2006.03.021.Search in Google Scholar PubMed
10. Lopez, X, Bouché, C, Tatro, E, Goldfine, AB. Family history of diabetes impacts on interactions between minimal model estimates of insulin sensitivity and glucose effectiveness. Diabetes Obes Metabol 2009;11:123–30. https://doi.org/10.1111/j.1463-1326.2008.00913.x.Search in Google Scholar PubMed
11. Shim, SY, Lee, GB, Shim, JS, Jung, SJ, Kim, HC. Association between a family history of diabetes and carotid artery atherosclerosis in Korean adults. Epidemiol Health 2021;43:e2021049. https://doi.org/10.4178/epih.e2021049.Search in Google Scholar PubMed PubMed Central
12. Russell, RD, Roberts-Thomson, KM, Hu, D, Greenaway, T, Betik, AC, Parker, L, et al.. Impaired postprandial skeletal muscle vascular responses to a mixed meal challenge in normoglycaemic people with a parent with type 2 diabetes. Diabetologia 2022;65:216–25. https://doi.org/10.1007/s00125-021-05572-7.Search in Google Scholar PubMed
13. McSorley, PT, Bell, PM, Young, IS, Atkinson, AB, Sheridan, B, Fee, JP, et al.. Endothelial function, insulin action and cardiovascular risk factors in young healthy adult offspring of parents with type 2 diabetes: effect of vitamin E in a randomized double-blind, controlled clinical trial. Diabet Med 2005;22:703–10. https://doi.org/10.1111/j.1464-5491.2005.01506.x.Search in Google Scholar PubMed
14. Adarsh, R, Krushna, C, Maharana, SM, Sanjiv, S. Endothelial dysfunction and its relation in different disorders: recent update. Health Sciences Review 2023;7:100084. https://doi.org/10.1016/j.hsr.2023.100084.Search in Google Scholar
15. Jamwal, S, Sharma, S. Vascular endothelium dysfunction: a conservative target in metabolic disorders. Inflamm Res 2018;67:391–405. https://doi.org/10.1007/s00011-018-1129-8.Search in Google Scholar PubMed
16. Alvarado-Vásquez, N, Páez, A, Zapata, E, Alcázar-Leyva, S, Zenteno, E, Massó, F, et al.. HUVECs from newborns with a strong family history of diabetes show diminished ROS synthesis in the presence of high glucose concentrations. Diabetes Metab Res Rev 2007;23:71–80. https://doi.org/10.1002/dmrr.665.Search in Google Scholar PubMed
17. Alvarado-Vásquez, N, Zapata, E, Alcázar-Leyva, S, Massó, F, Montaño, LF. Reduced NO synthesis and eNOS mRNA expression in endothelial cells from newborns with a strong family history of type 2 diabetes. Diabetes Metab Res Rev 2007;23:559–66. https://doi.org/10.1002/dmrr.743.Search in Google Scholar PubMed
18. Ferreiro, CR, Chagas, ACP, Carvalho, MHC, Dantas, AP, Scavone, C, Souza, LC, et al.. Expression of inducible nitric oxide synthase is increased in patients with heart failure due to ischemic disease. Braz J Med Biol Res 2004;37:1313–20. https://doi.org/10.1590/s0100-879x2004000900005.Search in Google Scholar PubMed
19. Sakamuri, SSVP, Sure, VN, Kolli, L, Liu, N, Evans, WR, Sperling, JA, et al.. Glycolytic and oxidative phosphorylation defects precede the development of senescence in primary human brain microvascular endothelial cells. Geroscience 2022;44:1975–94. https://doi.org/10.1007/s11357-022-00550-2.Search in Google Scholar PubMed PubMed Central
20. Adela, R, Nethi, SK, Bagul, PK, Barui, AK, Mattapally, S, Kuncha, M, et al.. Hyperglycaemia enhances nitric oxide production in diabetes: a study from South Indian patients. PLoS One 2015;10:e0125270. https://doi.org/10.1371/journal.pone.0125270.Search in Google Scholar PubMed PubMed Central
21. Yoon, HJ, Cho, SW, Ahn, BW, Yang, SY. Alterations in the activity and expression of endothelial NO synthase in aged human endothelial cells. Mech Ageing Dev 2010;131:119–23. https://doi.org/10.1016/j.mad.2009.12.010.Search in Google Scholar PubMed
22. Cai, H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res 2005;68:26–36. https://doi.org/10.1016/j.cardiores.2005.06.021.Search in Google Scholar PubMed
23. Park, JL, Heilig, CW, Brosius, FC3rd. GLUT1-deficient mice exhibit impaired endothelium-dependent vascular relaxation. Eur J Pharmacol 2004;496:213–4. https://doi.org/10.1016/j.ejphar.2004.06.022.Search in Google Scholar PubMed
24. Madonna, R, Pieragostino, D, Rossi, C, Confalone, P, Cicalini, I, Minnucci, I, et al.. Simulated hyperglycemia impairs insulin signaling in endothelial cells through a hyperosmolar mechanism. Vasc Pharmacol 2020;130:106678. https://doi.org/10.1016/j.vph.2020.106678.Search in Google Scholar PubMed
25. Cui, J, Zhang, B, Gao, M, Liu, B, Dai, C, Dong, Y, et al.. The protective effect of tetrahydroxystilbene glucoside on high glucose-induced injury in human umbilical vein endothelial cells through the PI3K/Akt/eNOS pathway and regulation of bcl-2/bax. J Vasc Res 2021;58:301–10. https://doi.org/10.1159/000511035.Search in Google Scholar PubMed
26. Suresh, V, Reddy, A. Dysregulation of nitric oxide synthases during early and late pathophysiological conditions of diabetes mellitus leads to amassing of microvascular impediment. J Diabetes Metab Disord 2021;20:989–1002. https://doi.org/10.1007/s40200-021-00799-y.Search in Google Scholar PubMed PubMed Central
27. Liao, J, Lei, M, Chen, X, Liu, F. Effect of intermittent high glucose on synthesis of nitric oxide in human umbilical vein endothelial cells and its mechanism. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2010;35:295–300. https://doi.org/10.3969/j.issn.1672-7347.2010.04.003.Search in Google Scholar PubMed
28. Groschner, LN, Waldeck-Weiermair, M, Malli, R, Graier, WF. Endothelial mitochondria — less respiration, more integration. Pflügers Archiv 2012;464:63–76. https://doi.org/10.1007/s00424-012-1085-z.Search in Google Scholar PubMed PubMed Central
29. Mertens, S, Noll, T, Spahr, R, Krützfeldt, A, Piper, HM. Energetic response of coronary endothelial cells to hypoxia. Am J Physiol 1990;258:H689–694. https://doi.org/10.1152/ajpheart.1990.258.3.H689.Search in Google Scholar PubMed
30. Wilson, C, Lee, MD, Buckley, C, Zhang, X, McCarron, JG. Mitochondrial ATP production is required for endothelial cell control of vascular tone. Function 2023;4:zqac063. https://doi.org/10.1093/function/zqac063.Search in Google Scholar PubMed PubMed Central
31. Cappelli-Bigazzi, M, Battaglia, C, Pannain, S, Chiariello, M, Ambrosio, G. Role of oxidative metabolism on endothelium-dependent vascular relaxation of isolated vessels. J Mol Cell Cardiol 1997;29:871–9. https://doi.org/10.1006/jmcc.1996.0286.Search in Google Scholar PubMed
32. Quagliaro, L, Piconi, L, Assaloni, R, Da Ros, R, Szabó, C, Ceriello, A. Primary role of superoxide anion generation in the cascade of events leading to endothelial dysfunction and damage in high glucose treated HUVEC. Nutr Metabol Cardiovasc Dis 2007;17:257–67. https://doi.org/10.1016/j.numecd.2006.01.007.Search in Google Scholar PubMed
33. Zhang, J, Guo, Y, Ge, W, Zhou, X, Pan, M. High glucose induces apoptosis of HUVECs in a mitochondria-dependent manner by suppressing hexokinase 2 expression. Exp Ther Med 2019;18:621–9. https://doi.org/10.3892/etm.2019.7609.Search in Google Scholar PubMed PubMed Central
34. Schiffer, TA, Lundberg, JO, Weitzberg, E, Carlström, M. Modulation of mitochondria and NADPH oxidase function by the nitrate-nitrite-NO pathway in metabolic disease with focus on type 2 diabetes. Biochim Biophys Acta, Mol Basis Dis 2020;1866:165811. https://doi.org/10.1016/j.bbadis.2020.Search in Google Scholar
35. Cristina de Assis, M, Cristina Plotkowski, M, Fierro, IM, Barja-Fidalgo, C, de Freitas, MS. Expression of inducible nitric oxide synthase in human umbilical vein endothelial cells during primary culture. Nitric Oxide 2002;7:254–61. https://doi.org/10.1016/s1089-8603(02)00123-4.Search in Google Scholar PubMed
36. Medina-Leyte, DJ, Domínguez-Pérez, M, Mercado, I, Villarreal-Molina, MT, Jacobo-Albavera, L. Use of human umbilical vein endothelial cells (HUVEC) as a model to study cardiovascular disease: a review. Applied Sciences 2020;10:938. doi.org: https://doi.org/10.3390/app10030938.Search in Google Scholar
37. Cester, N, Rabini, RA, Salvolini, E, Staffolani, R, Curatola, A, Pugnaloni, A, et al.. Activation of endothelial cells during insulin-dependent diabetes mellitus: a biochemical and morphological study. Eur J Clin Invest 1996;26:569–73. https://doi.org/10.1046/j.1365-2362.1996.1750526.x.Search in Google Scholar PubMed
38. Mandal, AK, Ping, T, Caldwell, SJ, Bagnell, R, Hiebert, LM. Electron microscopic analysis of glucose-induced endothelial damage in primary culture: possible mechanism and prevention. Histol Histopathol 2006;21:941–50. https://doi.org/10.14670/HH-21.941.Search in Google Scholar PubMed
39. Regina, C, Panatta, E, Candi, E, Melino, G, Amelio, I, Balistreri, CR, et al.. Vascular ageing and endothelial cell senescence: Molecular mechanisms of physiology and diseases. Mech Ageing Dev 2016;159:14–21. https://doi.org/10.1016/j.mad.2016.05.003.Search in Google Scholar PubMed
40. Alvarado-Vasquez, N. Could a family history of type 2 diabetes be a risk factor to the endothelial damage in the patient with COVID-19? Med Hypotheses 2021;146:110378. https://doi.org/10.1016/j.mehy.2020.Search in Google Scholar
41. Makker, J, Sun, H, Patel, H, Mantri, N, Zahid, M, Gongati, S, et al.. Impact of prediabetes and type-2 diabetes on outcomes in patients with COVID-19. Internet J Endocrinol 2021;2021:5516192. https://doi.org/10.1155/2021/5516192.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Crosstalk between miRNAs and signaling pathways in the development of drug resistance in breast cancer
- Hormonal disorders in autism spectrum disorders
- An overview of the relationship between melatonin and drug resistance in cancers
- Original Articles
- Gestational diabetes mellitus (GDM): diagnosis using biochemical parameters and anthropometric measurements during the first trimester in the Indian population
- Endothelial cell phenotype is linked to endothelial dysfunction in individuals with a family history of type 2 diabetes
- Associations of serum levels of cGAMP in the context of COVID-19 infection, atherosclerosis, sterile inflammation, and functional endothelial biomarkers in patients with coronary heart disease and healthy volunteers
Articles in the same Issue
- Frontmatter
- Review Articles
- Crosstalk between miRNAs and signaling pathways in the development of drug resistance in breast cancer
- Hormonal disorders in autism spectrum disorders
- An overview of the relationship between melatonin and drug resistance in cancers
- Original Articles
- Gestational diabetes mellitus (GDM): diagnosis using biochemical parameters and anthropometric measurements during the first trimester in the Indian population
- Endothelial cell phenotype is linked to endothelial dysfunction in individuals with a family history of type 2 diabetes
- Associations of serum levels of cGAMP in the context of COVID-19 infection, atherosclerosis, sterile inflammation, and functional endothelial biomarkers in patients with coronary heart disease and healthy volunteers

