Abstract
Research on gold catalysis has flourished over the last 20 years, and gold catalysts are now acknowledged as the “best choice” for a range of organic transformations. Gold complexes have emerged as promising candidates for this use in recent years because of their high reactivity, which enables them to induce a broad range of transformations under mild conditions. Extensive demonstrations have showcased the extraordinary efficiency of synthesizing complex organic compounds from the basic starting components. In addition to its traditional applications in catalysis, gold catalysis has expanded to include the total synthesis of natural compounds, which is a complex and demanding undertaking. The class of molecules known as carbo- and heterocycles, which is arguably the most important, has a significant impact on the synthesis of agrochemicals and pharmaceuticals among the numerous additional products made possible by the novel procedures pioneered. The main topic of this review is how to use Au salts in homogeneous catalysis to create cyclization processes for small heterocyclic and carbocyclic systems. This study gives an overview of most of the books and articles written after 2013 that discuss making three- and four-membered carbo- and heterocyclic rings with gold as a catalyst. We have made every effort to include all outstanding reports on this subject; nonetheless, we apologize for any omissions.
1 Introduction
Gold possesses unique characteristics that set it apart from other elements. The inherent inertness and exceptional resistance to oxidation and chemical attack of bulk metals have historically been considered undesirable qualities for catalytic purposes. Gold, being the metallic element with the highest electronegativity, exhibits a nearly equivalent value to carbon on the Pauling scale, measuring 2.54. The formation of hydrolytically stable and strongly covalent Au–C bonds is seen. In contrast to other noble metals, the delay in conducting investigations into the organometallic chemistry of this particular metal can be attributed to this factor [1]. Significant transformations have occurred at present.
Gold has a unique place in the Periodic Table because it tends to behave like main group elements when it has an oxidation state of +1 and a fully occupied d-shell. It is more likely for the compound to form linear complexes with two-coordinate ligands and much less likely to interact with donor ligands that are positioned in a way that is against the molecular axis [2]. In contrast to compounds of gold in the oxidation state of +I, those in the +III oxidation state possess all the characteristic properties of a transition metal. Furthermore, they primarily adopt the square-planar coordination geometry, which is commonly observed in other heavy metal ions with a d8 electron configuration. Furthermore, there are significant variations in both the chemical and structural features.
Biologically active natural and synthetic substances, medications, and agrochemicals often contain carbo- and heterocycles as structural architectures. Furthermore, these structural components may play a significant role in organic synthesis as synthons [3,4,5,6,7]. There are a lot of very good ways to make different carbo- and heterocyclic systems, but we are always looking for new ways that use easy-to-find starting materials, require fewer steps for transformation and purification, and are very selective in terms of chemo-, regio-, and stereoselectivity [8]. Gold catalysis has led to the creation of amazing, highly inventive, and ahead of the competition methods that can be used to make a wide range of chemical changes that are needed to make carbo- and heterocyclic compounds from basic starting materials. Several books and reviews on this field of study have been released in recent years [9,10,11].
2 Small-sized ring systems
The ubiquitous themes of three- and four-membered rings throughout medicinal chemistry and nature have captivated chemists ever since their discovery. Nevertheless, the construction of small rings often poses significant challenges due to energy considerations. Chemists have since demonstrated a significant level of interest in the characteristics and structural organization of these extensively stretched carbocycles and their derivatives [12,13,14,15]. As a result, significant endeavors have been undertaken over the course of time to obtain entry to diverse minor compounds [16] and to examine the distinctive patterns of reactivity exhibited by these molecules.
Due to the inherent strain caused by their 60° C–C bond angles, which deviate from the typical 109.5° Csp3–Csp3 bond angles found in conventional alkanes, three-membered rings often exhibit reactivity that is comparable to alkenes rather than alkanes [17,18]. The techniques used to construct medium- or large-sized rings are very different from those used to assemble severely strained little rings due to uphill thermodynamics.
3 Synthesis of three-membered ring systems
3.1 Gold-catalyzed diazo compound-mediated cyclopropanation
The strategy involving metal-catalyzed carbene transfer from diazo compounds is widely recognized as the most thoroughly investigated approach for the synthesis of acceptor or donor–acceptor cyclopropanes. Metal complexes, such as Au(i) catalysts, can facilitate the transition [19]. Over the past 15 years, several Au(i) complexes have been employed to facilitate carbene transfer processes towards diverse nucleophiles [20].
As of 2019, Pérez et al. revealed that ethylene 1, which is thought to be the basic alkene, can go through cyclopropanation when it meets ethyl diazoacetate (EDA) 2 and an NHC-Au(i) catalyst [21]. The reaction between 1 and 2 at room temperature is catalyzed by IPrAuCl in the presence of one equivalent of NaBArF4, producing 3 with yields of around 70% (based on EDA) (Scheme 1). This is the first instance of ethylene being directly cyclopropanated using this technology, with notable conversions.
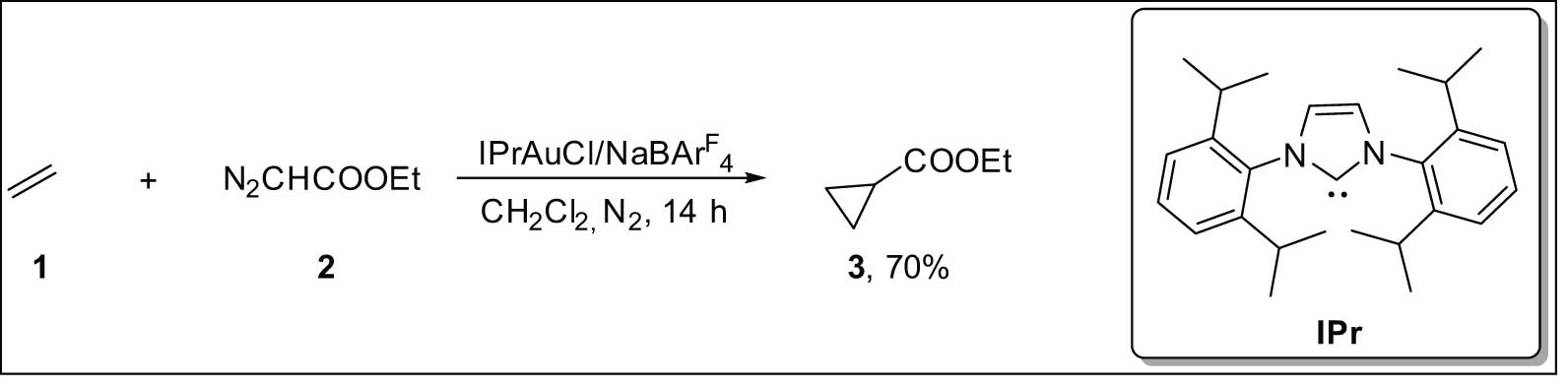
Gold-catalyzed cyclopropanation of ethylene with EDA.
Using diazooxindoles 4 and α-CH2F and CF2H styrenes 5, Cao et al. created a very selective cyclopropanation [22] to obtain 3-spirocyclopropyl oxindoles 6 (Scheme 2) with vicinal all-carbon stereocenters. These are interesting compounds for medical research or could be used to make different kinds of fluorinated spirocyclopropanes [23]. They demonstrate that the formation of C-F⋯H-X interactions between fluorinated solvents and reactive intermediates may significantly aid the reaction.
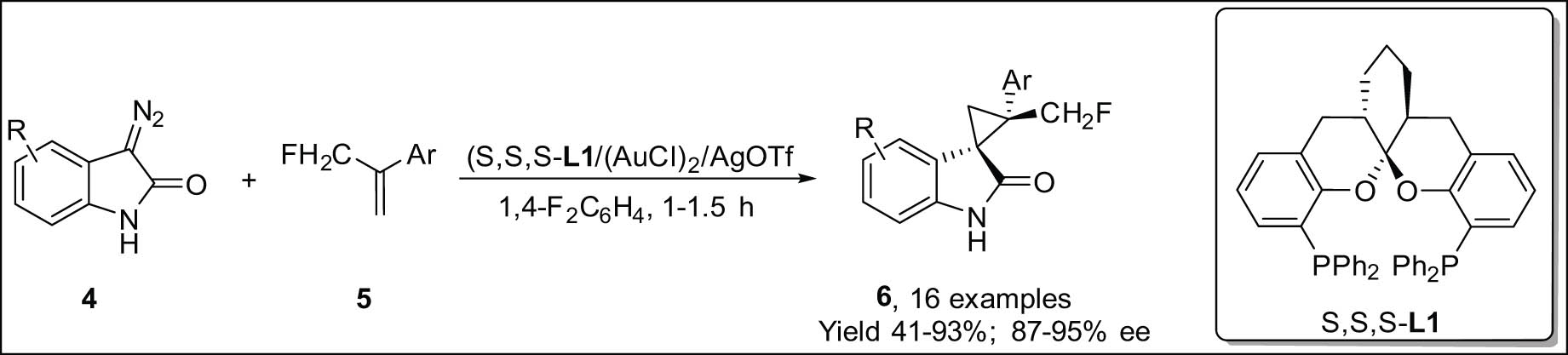
Gold(i)-mediated enantioselective cyclopropanation using diazooxindoles.
This study offers an unparalleled understanding of the role of fluorinated organic solvents in augmenting the reactivity of organic reactions while also presenting supplementary proof for the presence of C-F⋯H-X interactions.
Liu et al. explained the process of producing spirocyclopropyl oxindoles 9 using a gold(i) catalyst by reacting 1,2,4-substituted dienes 7 with diazooxindoles 8 (Scheme 3) [24]. The authors investigated a unique rearrangement of these spirocyclopropyl oxindoles 9, resulting in the formation of 3-(cyclopenta-1,3-dien-1-ylmethyl)oxindoles 11 as the product with the same gold catalyst. The reported products 11 of this rearrangement are generated through a thorough cleavage of the cyclopropane ring of spirocyclopropyl oxindoles 9 via a formal methyl C–H insertion at a C(3)–oxindole ring. Based on experimental results, the authors ruled out the possibility of a reversible gold-cyclopropanation pathway. In contrast, they proposed the formation of intricate pairs involving gold enolates and 1-methylen-2,3,4-cyclopentadienyl cations, leading to a 1,5-enolate shift.

Gold(i)-mediated diastereoselective cyclopropanation using diazooxindoles and dienes.
The majority of the time, throughout the cyclopropanation process, small quantities of minor products such as 3-(4-phenylcyclopenta-2,4-dien-1-yl)indolin-2-one derivatives (<10%) were produced, posing challenges in purification using column chromatography.
Zhang et al. [25] reported on the synthesis of densely substituted cyclopropanes 14 (Scheme 4). The authors obtained good yields and great enantioselectivity and diastereoselectivity by using α-diazoarylacetate compounds 13 to help with the enantioselective cyclopropanation of enamides 12. The reaction exhibited high efficiency when applied to internal or terminal enamides, yielding the desired products in satisfactory quantities with remarkable levels of enantioselectivity (up to 98% ee) and diastereoselectivity, particularly when employing a chiral binol-derived phosphoramidite-gold(i) complex. The present methodology offers a clear and feasible approach for the synthesis of chiral cyclopropylamines possessing consecutive stereogenic centers, a structural feature of utmost importance in the fields of biological investigation and pharmaceutical advancement. The utilization of Carreira ligand 15 [26] in the process led to the synthesis of a broad spectrum of densely substituted donor–acceptor cyclopropanes, exhibiting significant degrees of diastereo- and enantioselectivity.
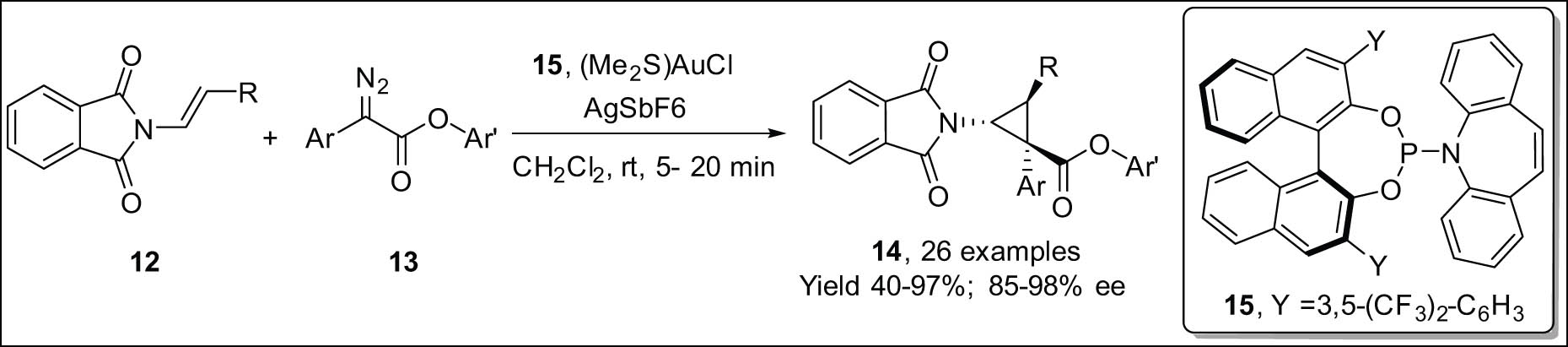
Au-catalyzed enantioselective cyclopropanation of enamides using α-diazoarylacetate.
3.2 Gold-catalyzed cyclopropanation by cycloisomerization of unsaturated systems
3.2.1 Cyclopropanation through cycloisomerization of enynes
Gold complexes are a kind of carbophilic π acid that have been intentionally designed to have the capability to activate various C–C bonds [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. This includes bonds present in alkynes, alkenes, and allenes. Extensive research has been conducted on the gold-catalyzed cycloisomerizations of enynes within the timeframe of the twenty-first century [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. Au(i) can undergo interaction with an enyne, resulting in the formation of η 2-complexes involving either the double bond or the triple bond [67,68]. The η 2-alkyne gold complex has a smaller lowest unoccupied molecular orbital, which makes it more vulnerable to nucleophilic attack. Consequently, this results in a heightened affinity for alkynes over alkenes, a phenomenon commonly referred to as alkynophilicity [69].
3.2.1.1 Cyclopropanation via cycloisomerization of 1,6-enynes
Liu and co-workers reported the cyclization of aryl diazo ketones 17 and 1,6-enynes 16 by gold catalysis, which offers easy access to 3-cyclopropyl-2-en-1-one derivatives 18. The reaction was initiated through the creation of 5-exo-dig cyclopropyl gold carbenes from 1,6-enynes. As shown in Scheme 5 [70], aryl diazo ketones subsequently trapped these carbenes.
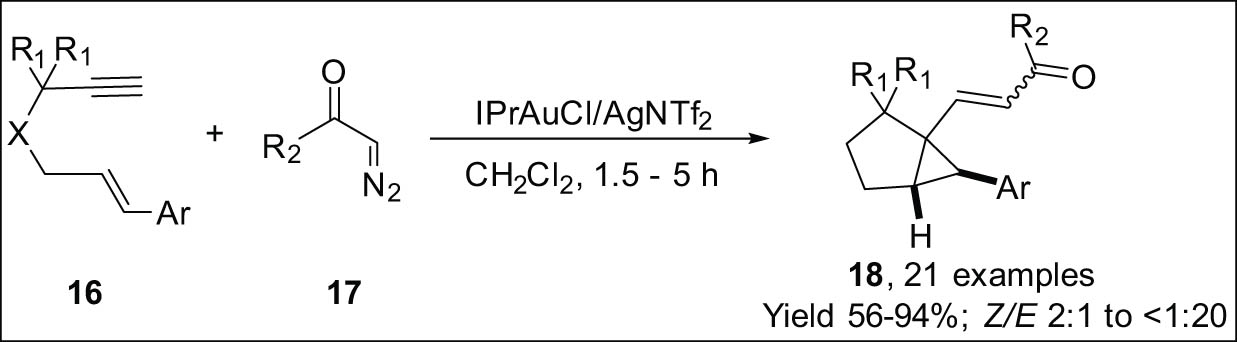
Gold-catalyzed cycloisomerization of 1,6-enynes using diazo ketone.
Fensterbank (year) documented the process of cycloisomerization of O-tethered 1,6-enynes 19 using gold catalysis. This reaction resulted in the formation of a four-membered ring [71] inside the molecular structure. An important stage is to expand the Wagner–Meerwein rearrangement onto intermediate carbene center 20. Good yields of the corresponding cyclopropane-fused tricyclic systems with a dihydropyran moiety 21 were achieved (Scheme 6).

Cycloisomerization of O-tethered 1,6-enynes via gold catalysis.
In 2020, Michelet et al. presented a robust method for the rapid synthesis of 3/6-membered moderately volatile enol ethers 23 with distinct olfactive characteristics. In this method, oxygen-tethered enynes 22 were cycloisomerized with the help of gold, as shown in Scheme 7 [72]. The fact that the reaction is feasible and can use a 0.04 mol% catalyst loading on a 25 g scale in mild conditions is proof of its viability. The olfactory characteristics of a library of synthetic bicyclic compounds were investigated. Depending on the substituents, these compounds’ odor characteristics can vary greatly. Some of them have aromas that are like some well-known synthetic perfumes, such as rhubafuran, hexalon, and hyacinth body. Various research groups have utilized diverse ligands in their investigations, exploring the gold-catalyzed cycloisomerization of enynes and producing analogous cyclopropane-fused six-membered heterocyclic products [73 74 75 76].

Gold-catalyzed bicyclic scent synthesis via cycloisomerization of oxygen-tethered enynes.
According to Calleja et al. (Scheme 8) [77], 1,6-enyne 24 undergoes a cyclization/1,5-OR migration/cyclopropanation reaction under gold catalysis to produce a tricyclic molecule 25. Given that racemization does not occur in the cascade reaction, it is not possible for propargyl carbocations to form. To synthesize (+)-schisanwilsonene A with better enantiomeric purity, this has been used to prepare important intermediates. Based on density functional theory (DFT) calculations, the 1,5-OR migration seems to move stepwise through a cyclic intermediate 26 after the first cyclization, even though the cleavage occurs through a low barrier.

Sequential cyclization, migration, and cyclopropanation in dienynes.
Karunakar et al. have recently devised a method for accessing the tetrahydrobenzo[h]cyclopropa[c]-chromenes 28 through the cycloisomerization of 1,6-ene-diynes 27 catalyzed by gold (Scheme 9) [78]. Regioselective yields of ≤92% tetrahydrobenzochromenes fused with cyclopropane were achieved. Three new C–C bonds were successively produced in one pot during this atom-economic biological transition. The reaction progresses through cycloisomerization of enyne with gold, producing the gold carbene intermediate 27′/1,2-hydride shift/deauration/6-endo-dig cyclization.
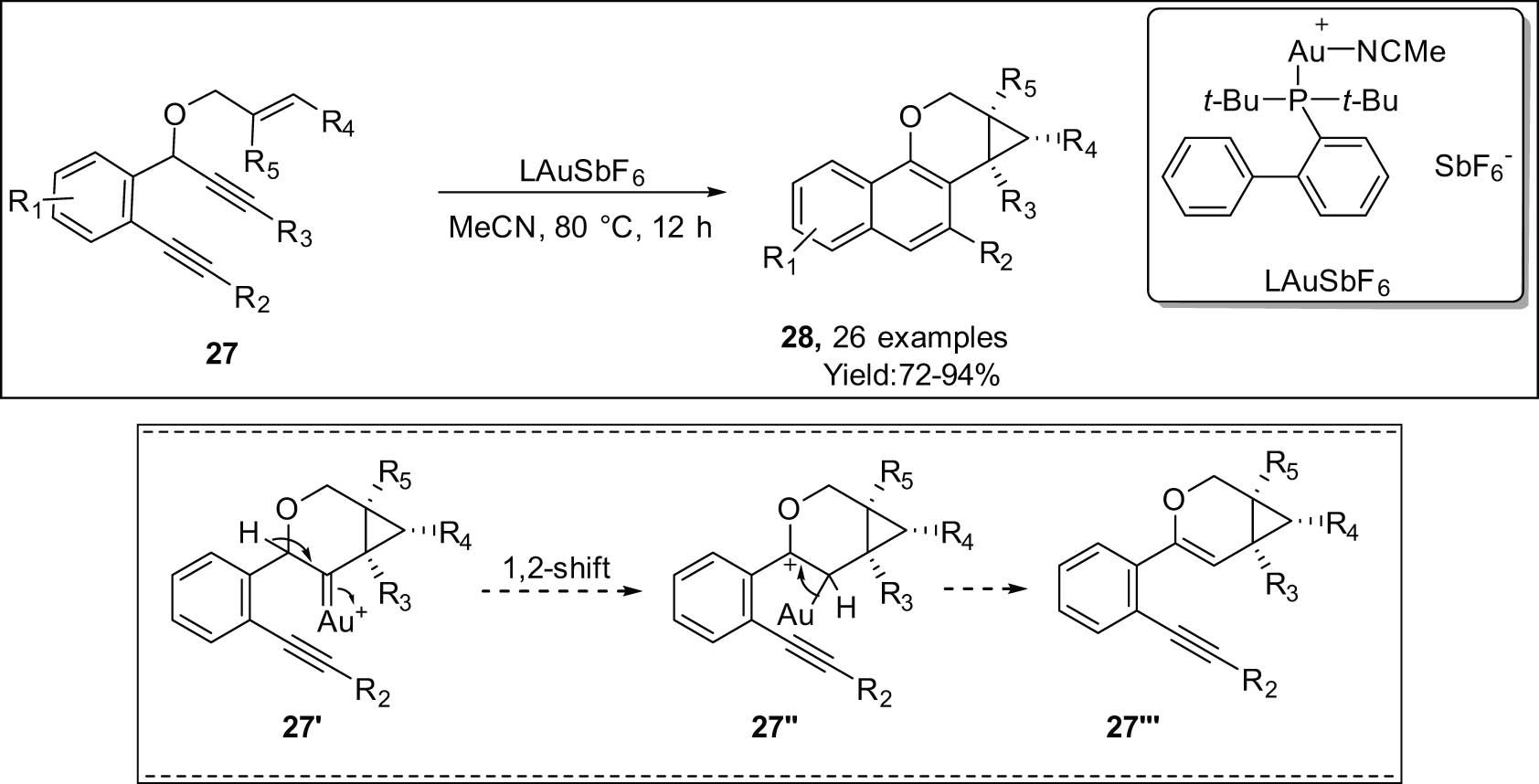
Au-catalyzed tandem cycloisomerization and 6-endo-dig cyclization of ene-diynes.
3.2.1.2 Cyclopropanation via cycloisomerization of 1,5-enynes
The enantioconvergent direct 1,5-enyne 29 cycloisomerization and kinetic resolution [79] are part of a new Au(iii)-catalyzed process shown in Scheme 10. The reaction also creates 1,5-enynes 31 that are optically more abundant and highly enantioenriched bicyclo[3.1.0]-hexenes 30 at all conversion levels without causing any racemization or symmetrization. Primarily, this finding provides proof for the exceptional capability of Au(iii) complexes in enantioselective catalysis. It is also the first time that a very selective enantioselective conversion has been made possible by a carefully studied cationic Au(iii) catalyst.
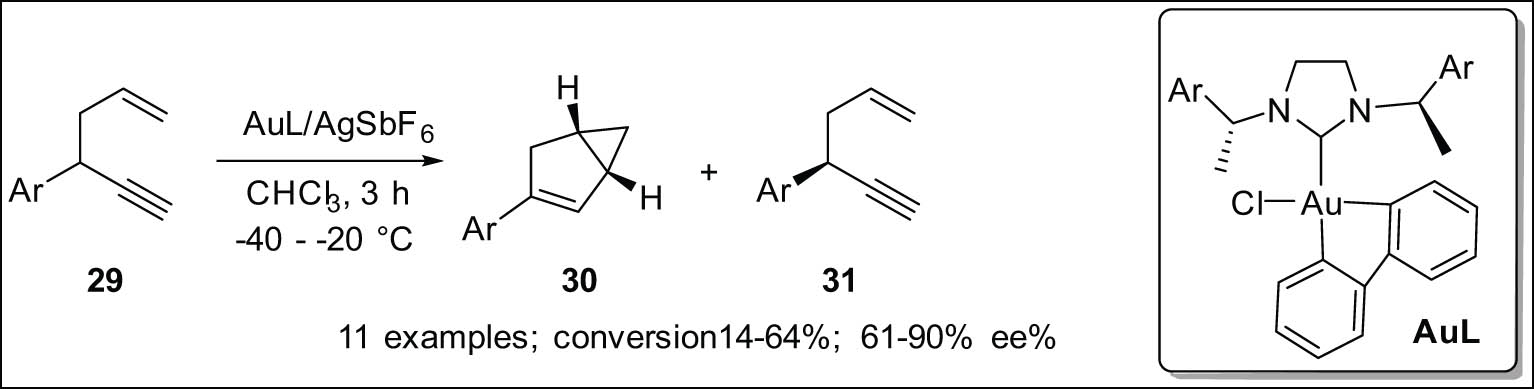
Enantioconvergent cycloisomerization and kinetic resolution of 1,5-enynes catalyzed by Au(iii).
Davies synthesized tetracyclic compounds 33 by a C–H insertion or cyclization cascade of N-homoallyl ynamides 32, catalyzed by Au(picolinate)Cl2. The generation of N-homoallyl ynamides 32 resulted in considerable diastereoselectivity in most cases (Scheme 11) [80].
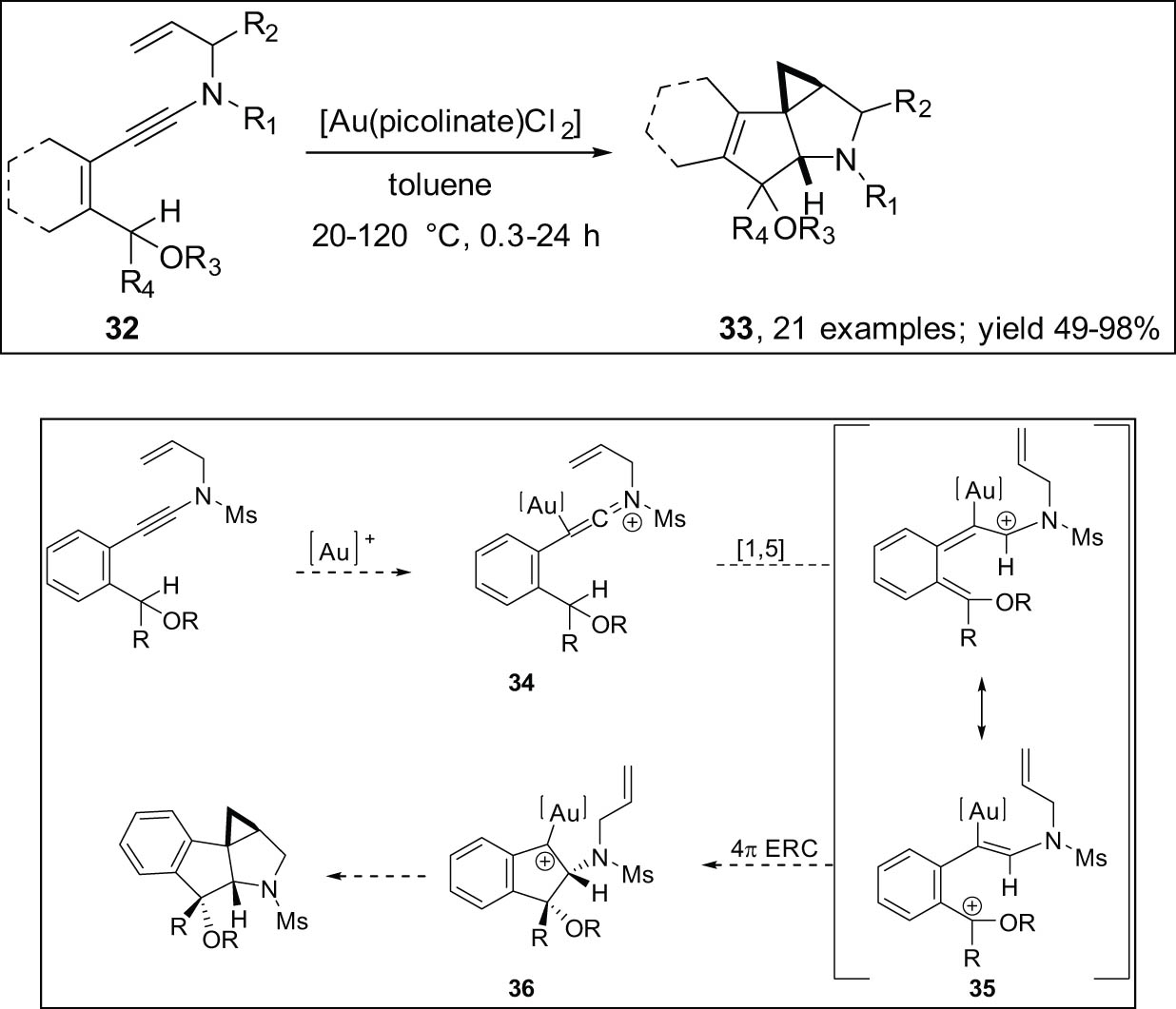
The C–H insertion/cyclization cascade of N-allyl ynamides catalyzed by gold.
The reaction is likely to occur along the gold keteniminium 34 pathways, which involves [1,5] hydride transfer to create benzylic cation 35. A 4π electrocyclic ring closure (4π ERC) process can result in the formation of the gold complex 36, and cyclopropanation can complete the cascade reaction.
Wei et al. have developed a cycloisomerization reaction using Au(i) catalysts to convert easily accessible 1,5-enynes 37 with a cyclopropane ring into biscyclopropane 38. This reaction has demonstrated moderate to good yields, as reported in Scheme 12 [81]. Through the manipulation of temperature and the choice of gold(i) catalyst, it is possible to selectively synthesize three different products that possess an ortho substituent. The required products are made by changing the key intermediate, tricyclic cyclobutene. This is done by first applying a standard enyne cycloisomerization reaction to the 1,5-enyne substrate.

Au(i)-mediated cycloisomerization of cyclopropyl 1,5-enynes.
3.2.2 Cyclopropanation through cycloisomerization of allenynes
The production of 1-naphthylcyclopropane-fused benzoheterocycle derivatives 41 was observed by Ohno’s group via the reaction of benzoheterocycles 40 with benzene-tethered 5-allenynes 39, as depicted in Scheme 13a and b [82]. In this transition, benzofuran 40 engages in an attack on the vinyl cation 42 that was formed earlier. The attack takes place at either the 2-position or 3-position of benzofuran 40. When the ring closes, aromatization and protodeauration occur, and the heterocycle is in the axial position that is least affected by steric hindrance. This yields either (E)-43 or (Z)-43. This process ultimately leads to the production of the regioselective product 41a.

(a) Gold(i)-catalyzed 5-allenynes cycloisomerization to 1-naphthylcyclopropane systems and (b) possible reaction mechanism.
3.2.3 Cycloisomerization of diynes via migration of 1,2- and 1,3-acyloxy groups
The tandem cycloisomerization, or 1,2-OAc shift, of trienynes 44, which incorporates a propargylic ester, was reported by the Gagosz group. This reaction was catalyzed by Au(i). Because of this, divinyl cyclopropanes 45 were made, and these then went through a thermal Cope rearrangement that created a complex polycyclic structure 46 (Scheme 14) [83].

Tandem Au(i) catalyst cycloisomerization/cope rearrangement.
By reacting styrene 47 with propargyl ester 48 as a model reaction, Reiersølmoen et al. reported employing Bis(pyridine)Au(iii) complexes as catalysts for the synthesis of vinyl cyclopropanes 49 (Scheme 15) [84]. They confirmed that the catalytic activity of Au(iii) is critically dependent on the electron density of the pyridine ligands. It appears that one pyridine must dissolve to produce the active species because ligands that have electron-withdrawing groups are noticeably more active. The use of 15N NMR (nuclear magnetic resonance) data confirmed the discovery. Furthermore, the application of DFT modeling elucidated that the energy associated with the pivotal transition state was contingent upon the electron density present on the nitrogen atom of the pyridine ligand. The same research group investigated the impact of various nitrogen ligands coordinated to gold salts in the cyclopropanation reaction involving styrene and propargyl ester [85,86].
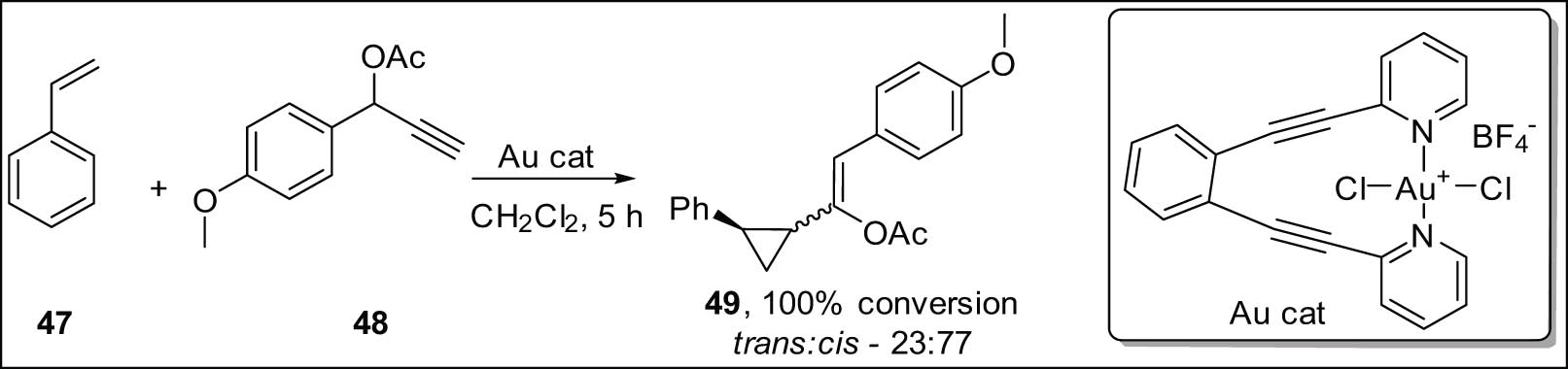
Au(i)-catalyzed enantioselective styrene cyclopropanation with propargylic esters.
Beginning with simple starting materials, ester-tethered terminal 1,6-diyne 50 and an alkene 51, the Hashmi group disclosed a unique synthesis method for producing cyclopropylnaphthalene derivatives 52 (Scheme 16a) [87]. A gold-catalyzed process involving the reaction of propargyl esters with a tethered alkynylphenyl group produced key intermediate naphthylcarbene complexes 53. Following the formation of the unusual carbene complex by a 1,2-carboxylate shift, an alkyne insertion produces a new naphthylcarbene complex. The next step is cyclopropanation with an alkene additive to capture this new complex. A smooth reaction inside the molecule of 1,6-diyne 54 attached to an alkene part also made the required cyclopropylnaphthalene derivatives 55 with a moderate yield (Scheme 16b). Notably, no competing enyne cyclization was seen, suggesting that the naphthyl carbenoid forms quickly. The research teams led by Oh [88] and Hashmi [89] delved into the significant role of gold carbenoids (formed by 1,2-carboxylate shift) in the cycloisomerization reaction of propargylic esters connected to alkyne motifs, aiming to produce naphthylene derivatives.

(a) Intermolecular cyclopropanation of 1,6-diynes using gold catalysis and alkene. (b) Intramolecular cyclopropanation of 1,6-diynes pendant with alkenes.
In a study conducted by Rao, Chan, and colleagues, the authors detail the synthesis of tetracyclodecene and tetracycloundecene compounds 57. We made these chemicals by a series of steps that included an Au(i)-catalyzed tandem 1,3-acyloxy migration and double cyclopropanation of 1-ene-4,9-diyne and 1-ene-4,10-diyne esters 56 (Scheme 17) [90].

Sequential tandem 1,3-migration and dual cyclopropanation in 1-ene-4,n-diyne esters.
3.3 Cyclopropanation by an oxygen-transfer agent via the oxidation of alkynes
Yeom and Shin showed that oxidative cyclopropanation of 1,6-enynes 58 with a propiolamide moiety attached could occur inside the molecule, creating cyclopropane carboxaldehydes 59 (shown in Scheme 18) [91]. In oxidative cyclopropanation, substrates containing terminal alkynes were initially utilized to produce cyclopropane carboxaldehydes, which possessed unique applications because of their aldehyde functionality. Diphenyl sulfoxide worked well as an oxidant for this kind of substrate, as opposed to pyridine-N-oxides, and this has been shown to have a broad universality about alkenes.

Intramolecular cyclization for cyclopropane formation in 1,6-enynes.
The Zhang group reported a catalytic asymmetric oxidative cyclopropanation of 1,6-enynes 60 in 2014. Because of this reaction, densely functionalized bicyclo[3.1.0]hexanes 63 were made, which had a lot of enantioselectivity (Scheme 19 [92]). A highly effective gold(i) complex with cationic properties was successfully synthesized, utilizing ligand 62. To facilitate the reaction, 8-methylquinoline N-oxide 61 was selected as the external oxidizing agent.

Asymmetric gold-catalyzed oxidative cyclopropanation of 1,6-enyne.
Ji et al. [93] developed a method for the enantioselective oxidative cyclization of 1,5-enynes 64, resulting in the formation of cyclopropane fused bicyclic derivatives 65 (Scheme 20). In order to achieve effective enantiocontrol, a hemilabile P,N-ligand [94,95] 66 containing a C2-symmetric piperidine ring was strategically developed. According to the existing literature, it has been suggested that piperidine offers a favorable chiral environment as a result of its capacity to coordinate with the metal center’s nitrogen atom and provide steric shielding. This helps mitigate the high reactivity of the highly electrophilic α-oxo gold carbene intermediate [96,97]. The approach performed by the Echavarren research team has been previously applied to produce enantioenriched 1,5-enyne 64, which was then utilized in the overall synthesis of (−)-nardoaristolone B 67 [98].
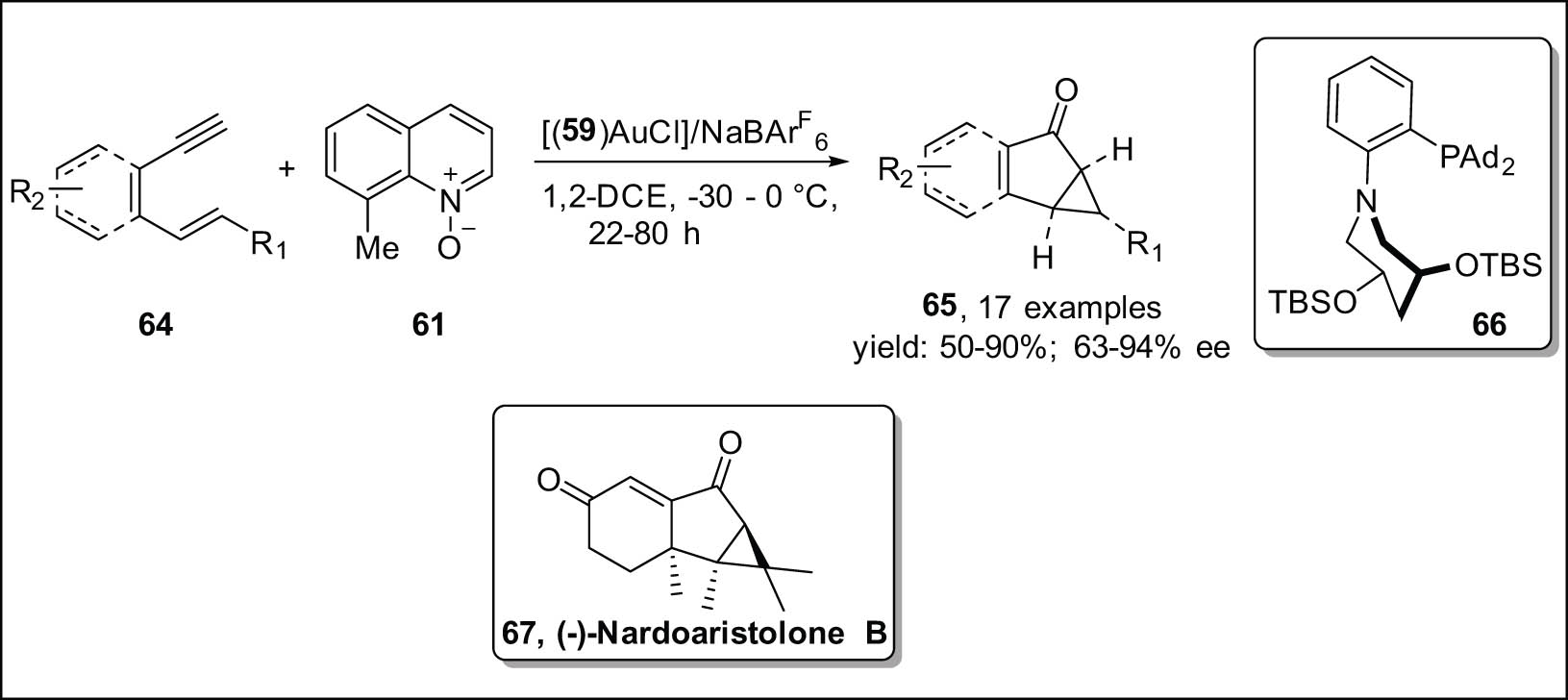
Asymmetric Au-catalyzed oxidative cyclopropanation in 1,5-enynes.
Employing a conformationally rigid P,N-bidentate ligand 70, Zhang and colleagues reported a highly efficient three-step intramolecular cyclopropanation of alkyne tethered enals 68 to bicyclic or tricyclic functionalized cyclopropyl ketones 69 (Scheme 21) [99]. The reaction was completed with largely good yields. When considering step economy and operational safety, this reaction exhibits favorable comparisons to similar approaches that rely on the diazo approach.

Gold-catalyzed synthesis of bi- and tricyclic cyclopropyl ketones through oxidative cyclizations.
The first-ever gold-catalyzed oxidative cyclopropanation of N-allyl ynamides 71 was reported by Li et al. The catalyst used in this process was [(IMes)AuCl]/AgBF4, and the oxidant employed was pyridine N-oxide 72. This reaction yielded diverse variants of 3-aza-bicyclo[3.1.0]hexan-2-one 73 (Scheme 22) [100]. Mechanistic studies were conducted to investigate the potential participation of the α-oxo gold carbene as an intermediary. The results of these tests indicated that the α-oxo gold carbene is not involved, hence suggesting that the alkene attacks the electrophilic β-oxypyridinium vinyl gold(i) species 74.

Gold-catalyzed oxidative cyclopropanation of N-allylynamides.
Because of overoxidation, the oxidative cyclization of ynamides with aromatic substituents rich in electrons at the alkynes terminal was not tolerable. By utilizing various reaction circumstances, Davis and his team were able to solve this concern (Scheme 23) [101]. Diketone 76 was made when N-allylynamides 75 with a tosyl substituent connected to nitrogen went through oxidative cyclization in 1,2-DCE. The cyclopropanation product 77 in nitromethane, on the other hand, was made by oxidative cyclization of N-allylynamides 75, which had a mesyl substituent connected to nitrogen. An instance of Au(i)-catalyzed yne-ynamide with pendant alkene undergoing cascade reactions involving cyclization, oxidation, and intramolecular cyclopropanation was recently reported by the Hashmi group utilizing diphenyl sulfoxide [102].

Oxidation of electron-rich ynamides by gold catalyst.
This study by Davies and his team showed how N-benzyl ynamides 78 react with oxygen in a Buchner-type way. This reaction creates [5.3.0] azabicycles 79′ (Scheme 24) [101]. The NMR technique effectively demonstrated the presence of a dynamic equilibrium between cyloheptatriene and norcaradiene. The synthesis of Polycycle 80 involved the utilization of a Diels–Alder reaction to capture the norcaradiene tautomer 79b, resulting in its formation. Conversely, the norcaradiene product 79a was obtained exclusively. It is easy to obtain benzyl propargyl ethers, which were used successfully by Ji and Zhang in their study to make a gold-catalyzed oxidative cyclization report of a similar nature [103].
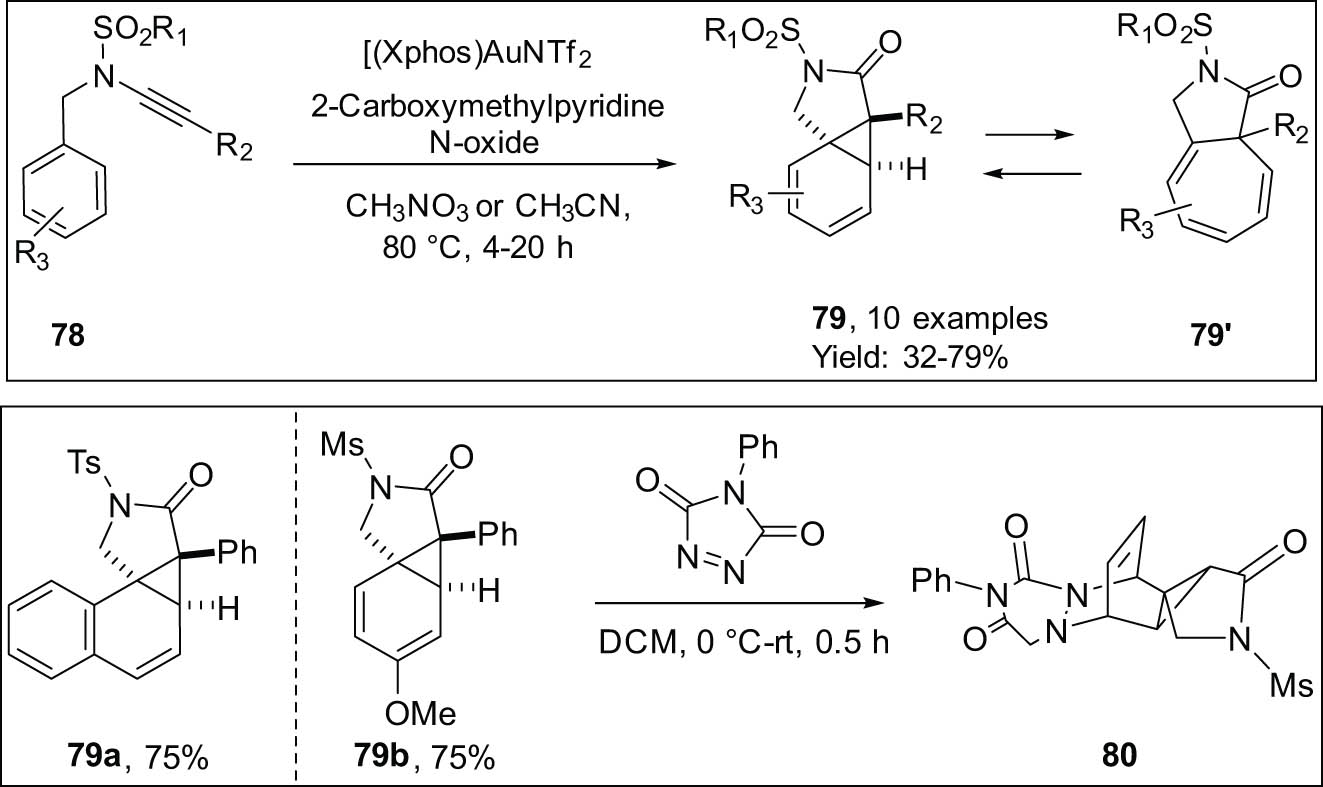
Intramolecular oxidative cyclopropanation of N-benzyl ynamides.
Zhang and others described a method to mix molecules called intermolecular cyclopropanation. They used 2-(tert-butyl)-6-chloro pyridine N-oxide as an outside oxidant to make α-oxo gold carbene from sulfonyl alkyne moiety 81 (Scheme 25) [104]. At first, carbene 84 is made when the carbon atom next to the alkyne group undergoes oxidation at the β position. A plausible chemical mechanism and this synthesis support the expected regioselectivity. Episulfone 85 would result from a Friedel–Crafts-style attack on the highly electrophilic carbene center, and it would fragment into α-oxo gold carbene 86 through SO2 extrusion. It is important to highlight that the oxidation of an internal aryl alkyne is the exclusive approach to generating the resulting carbene as the lesser regioisomer.

Gold-catalyzed intermolecular oxidative cyclopropanation.
The study focused on styrenes that were electronically unbiased, and a surplus of these styrenes, ten times greater than the required amount, was employed. Reactants with a significant electron density, such as 4-methoxystyrene or ethyl vinyl ether, were considered unfavorable. Instead of utilizing cyclopropane, the solvent α-methylstyrene resulted in the formation of a (3 + 2) cycloaddition product.
3.4 Oxidative cyclopropanation of alkynes with nitrogen-transfer agents
The first cyclopropanation that made cyclopropane-fused indanimines 90 (Scheme 26) was reported by Liu and colleagues [105]. They used α-imino gold carbene intermediates from earlier research on the oxidative cyclopropanation of 1,5-enynes 87 [106]. The application of iminopyridinium ylides, specifically compound 89, as nucleophilic nitrenoids is observed. The nitrenoids discussed here are nitrogen-based compounds that share similarities with pyridine N-oxides. Pyridine N-oxides are frequently used as oxidizing agents in the field of α-oxo gold carbenes.

Gold-catalyzed intramolecular cyclopropanation of 1,5-enynes with iminopyridinium ylides.
The delivery of cyclopropane-fused indoloquinolines 92 was reported by Ohno et al. through the reaction of (azido)-ynamides 91 with tethered alkenes, as depicted in Scheme 27 [107]. The α-imino gold carbene 93 played a critical role in the transformation of the ynamide. This was done by the azide attack inside the molecules, which caused the loss of nitrogen and the cyclopropanation of the alkene that was present around.

Intramolecular cyclopropanation of azidoynamides.
As part of their study, Hashmi et al. showed that adding external nitrene transfer reagents made α-imino gold carbenes more useful for intramolecular cyclopropanations of ynamides 94. By cutting N–S and attacking gold-activated ynamides across molecules, they made α-imino gold carbenes 97 using N-arylsulfilimines 95 (Scheme 28) [108]. The compound 3-azabicyclo[3.1.0] is of hexan-2-imines 96 and can be synthesized with high yields by employing various methods [109], including the utilization of a tethered alkene as one of the approaches. For the effective progression of the reaction, an electron-withdrawing group is attached to the arene of the sulfilimines, and a temperature of 80°C is necessary. Furthermore, it has been discovered that Au(iii) catalysts with Lewis acidity demonstrate superior efficiency in comparison to Au(i) catalysts.

Intermolecular cyclopropanation of ynamides using sulfilimines.
After that, the Hashmi group showed that ynamides 94 could turn into 99 under milder conditions by using different nucleophilic nitrenoids, namely anthranils 98 (Scheme 29) [110].

Anthranil-mediated intramolecular cyclopropanation of ynamides.
In this instance, the reaction might continue at room temperature with a catalytic quantity of NaAuCl4 dihydrate. A related work with α-imino gold carbenes is worth mentioning, as it allowed the creation of bicyclic compounds fused with cyclopropane in certain situations [111].
3.5 Gold-mediated synthesis of cyclopropanes containing heteroatom
Additionally, heterocyclic 3-membered rings such as aziridines and epoxides were produced under gold(i) catalysis in several mechanistic contexts.
The group demonstrated that reacting 3-hydroxybenzofurans 101 with 2H-azirines 102 under gold catalysis generates aziridines 103 (Scheme 30) [112].
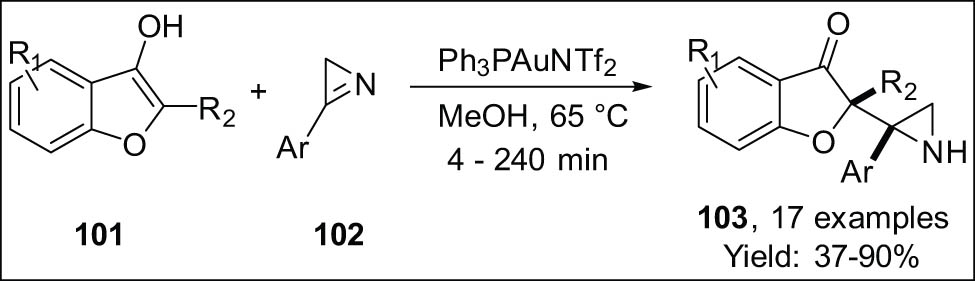
Synthesis of aziridines from 2H-azirines via gold catalysis.
In their important work, Sahani and Liu provided a full explanation of the intermolecular (4 + 2) cycloaddition reaction involving benzisoxazoles 105 and electron-poor alkynes 104, specifically propiolates. The use of gold catalysis in this process led to the formation of tetrahydroquinolines 107, which possess an epoxide ring. This reaction is illustrated in Scheme 31 [113].

Oxirane synthesis from benzisoxazoles under Au-catalysis.
Hashmi’s group used electron-rich alkynyl amines in a similar manner [114]. This led to the construction of a novel switchable approach that allowed for the synthesis of quinoline epoxides (containing an Au(iii) complex) or 6-acylindoles (containing an Au(i) complex) from the same two substrates by selecting the appropriate gold catalyst.
4 Gold-catalyzed formation of four-membered rings
Zhang [115] stated that Au(i) catalysis was used to make the first indoline-fused cyclobutanes. Subsequently, the region has experienced substantial development because of the recognition and implementation of a remarkable array of gold(i)-catalyzed methodologies, the majority of which rely on sequential [2 + 2] cycloadditions and ring expansions [27,28,29,30,31,32,33,34,116,117,118,119,120,121,122,123,124,125,126]. The utilization of these novel methodologies enables the atom-efficient synthesis of a wide range of molecules, encompassing both simple ring structures and complex frameworks seen in natural substances.
4.1 Formation of four-membered rings via cyclopropyl ring expansion
The Echavarren group has reported a diastereoselective Au(i)-catalyzed cascade transformation. In this process, a cyclopropyl group is changed into a cyclobutane through a series of chemical reactions that include ring expansion, enyne cyclization, and Prins cyclization. In particular, the change is made to cyclopropyl 1,6-enynes 109, which creates tricyclic compounds with a decahydrocyclobuta[a]pentalene skeleton 110. These compounds exhibit a syn/anti/syn relationship. Following this, the tricyclic compounds are changed into the natural product Repraesentin F 111 in six steps (Scheme 32) [127].
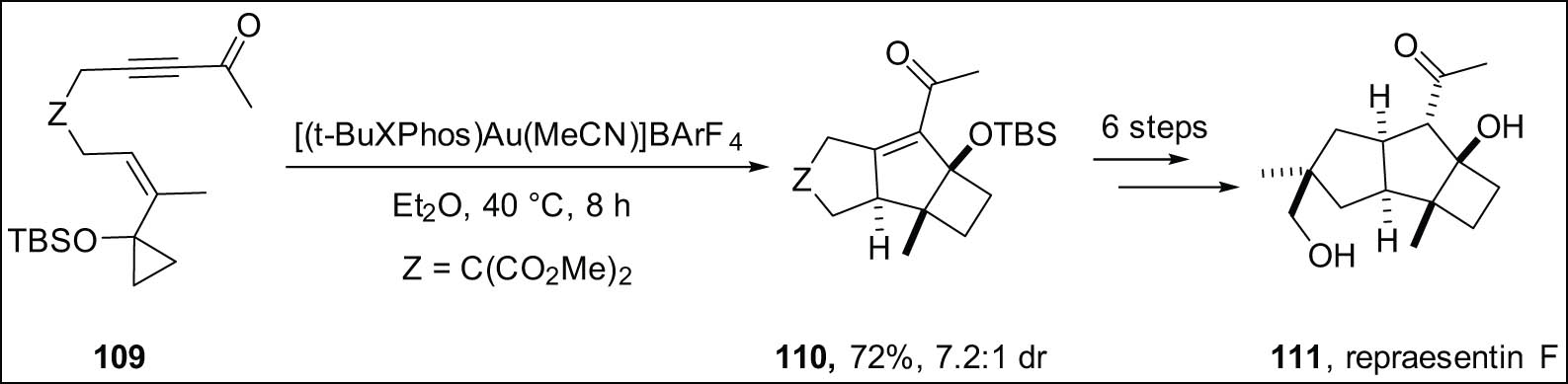
Synthesis of cyclobutanes via Au-catalyzed expansion of cyclopropanes access to repraesentin-F.
The tricyclic core [128] belonging to the protoilludanes family was successfully obtained by a one-step process utilizing a closely comparable Au(i)-catalyzed allene-vinylcyclopropane cycloisomerization.
Shi et al. [81] documented the cycloisomerization of 1,5-enynes 112, which were tethered with a cyclopropyl group, using divergent gold(i) catalysis (Scheme 33). Cyclobutane-fused 1,4-cyclohexadienes 114 were synthesized in cases where the aryl group did not possess ortho-substitution, as depicted in Scheme 33a. The ortho-substitution of the aryl group facilitated the generation of a switchable protocol. Under controlled temperature conditions, three unique polycyclic scaffolds were synthesized using a suitable gold catalyst. The scaffolds encompass spiro compound 116, fused-cyclobutane-conjugated cyclohexadiene 115, and tricyclic cyclobutene 117. The synthesis was carried out according to Scheme 33b–d, respectively. Both labeling investigations and computational analysis were employed to demonstrate that the ortho-substituent effect played a crucial role in determining the transformation of the common biscyclopropane Au(i) carbene intermediate 113 into different products.
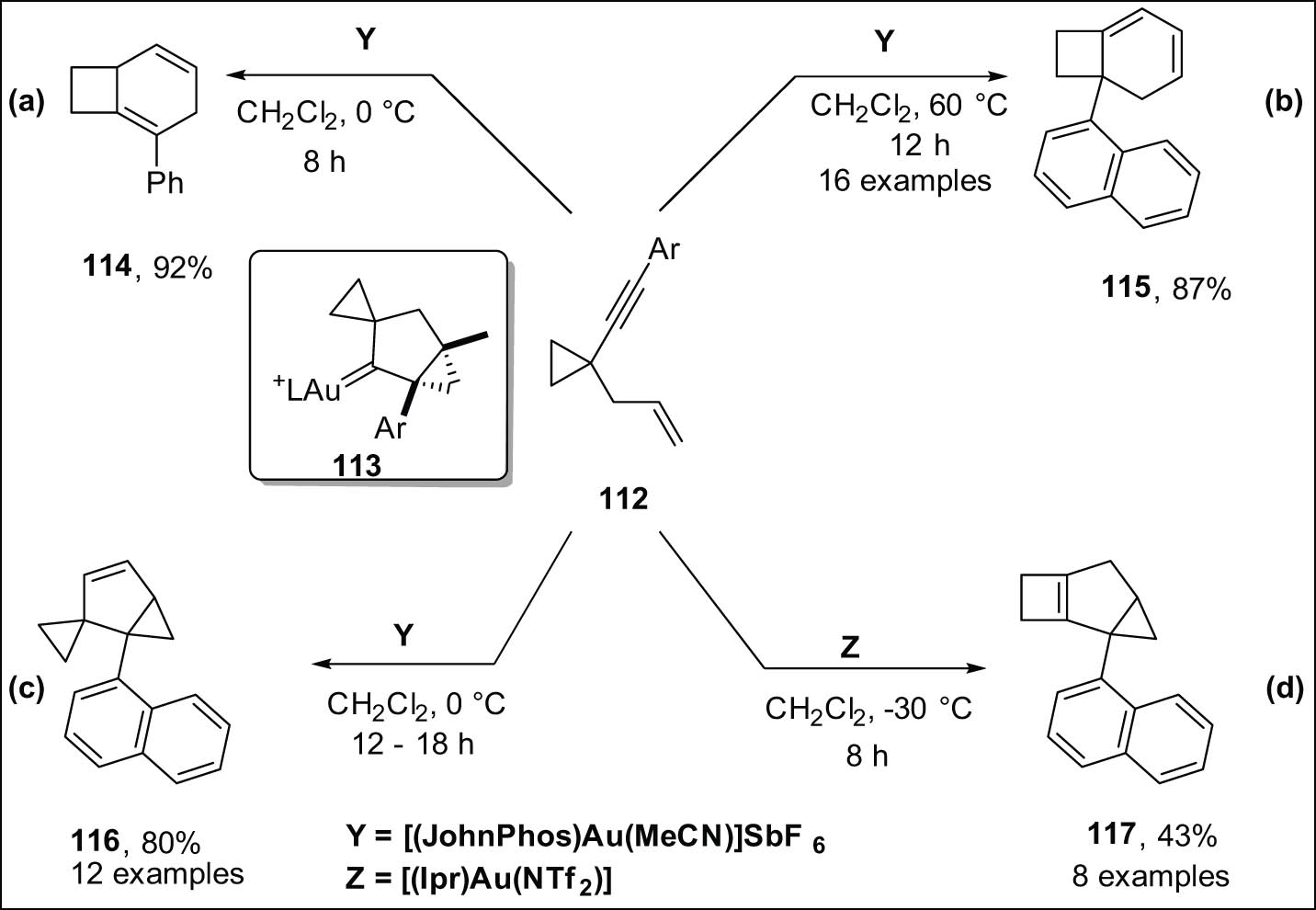
Gold-catalyzed transformation of 1,5-enynes with cyclopropyl group into (a) cyclobutane-fused 1,4-cyclohexadiene; (b) 1,3-cyclohexadiene; (c) biscyclopropane; (d) tricyclic cyclobutene derivatives.
The Gagne group reported cyclic 1,5-dienes 118 undergoing Cope rearrangement and cyclopropane ring expansion under gold(i) catalysis to synthesize tricyclic compounds 119 (Scheme 34) [129].
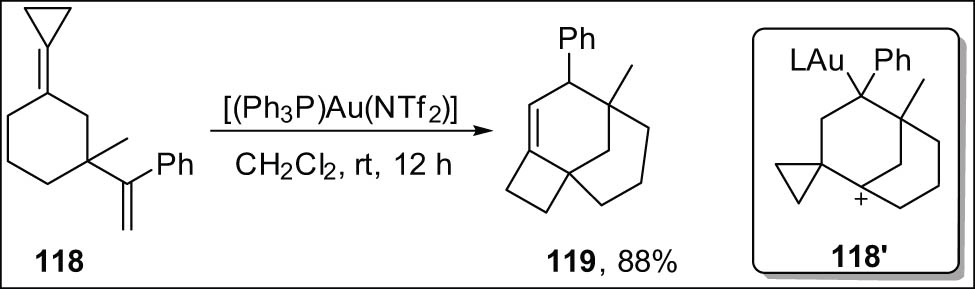
Cycloisomerization of cyclopropylidene-tethered frameworks.
The enantioselective cycloisomerization/ring expansion procedure for cyclopropylidene-containing 1,5-enynes 120 was found by the Gagne research team. This process led to the formation of enantioenriched bicycle [4.2.0] octanes 122 with an enantiomeric excess of up to 70% (Scheme 35, right) [130]. The same group of researchers later found that aldehydes could effectively capture Au(i)-stabilized allyl cation intermediates 121, which led to the formation of oxo-carbenium cations. The cations we talked about earlier went through Friedel–Crafts annulation, which created polycyclic structures 123 through a cascade reaction with high diastereoselectivity (shown in Scheme 35, left) [131].

Au(i)-Catalyzed synthesis of cyclobutane fused systems.
Carreira has successfully devised a process characterized by a high degree of diastereocontrol that enables the synthesis of cyclobutane derivatives 125 by the employment of Au(i)-catalyzed cyclo-isomerization of cyclic cyclopropylidene 1,5-enynes 124 in conjunction with a terminal alkyne. Harziane diterpenoid 126 (Scheme 36) was obtained through a series of 16 consecutive transformations, starting with the initial cyclobutane precursor [132].
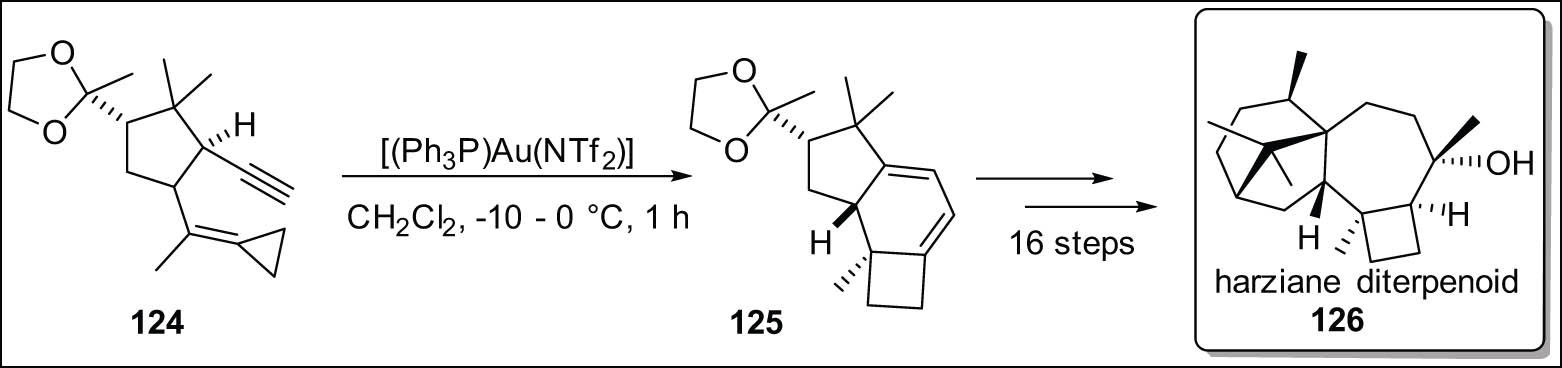
Cycloisomerization of cyclopropylidene1,5-enynes.
The Shi group made a big discovery about how ortho-substituents affect the phenyl group (R1) in the reaction between (propargyloxy)arenemethylenecyclopropanes 127 and an Au(i) catalyst that attracts electrons, specifically [(p-CF3C6H4)3PAuCl. This reaction exhibited chemodivergent results, as seen in Scheme 37 [133]. There have been observations in chemical reactions that when bulky substituents like t-Bu and t-Am are used, the methylene cyclopropane group moves instead of expanding. This leads to the creation of bicyclic products 129, as depicted in Scheme 37 on the left. Methoxy (MeO), halogens, or methyl (Me) groups are thought to be non-bulky and can help the ring become bigger and the propargyl group moves around. Au(i) facilitates the catalytic process, which results in the synthesis of 2,3-dihydrobenzofuran cyclobutane fused allene derivatives 131. The reaction is visually represented in Scheme 37, situated on the right-hand side.

Regiodivergent cycloisomerization of cyclopropylidene 1,7-enynes catalyzed by Au(i).
In addition, Shi effectively developed a regiodivergent catalytic cyclization/ring opening sequence (Scheme 38) utilizing alkynylamide-tethered alkylidenecyclopropanes 132 [134]. In this case, the gold(i) catalysts utilized resulted in the formation of two unique types of fused 4-membered rings, namely cyclobutenes 134 and cyclobutanes 135. The cationic Au(i) catalyst’s ligand and counteranion were discovered to be significant factors in the distribution of the product. Cyclobutenes 134 were the only compounds produced when [(Ph3P)AuCl] was employed in conjunction with AgOTf (Scheme 38, left). Cyclobutanes 135 was the lone product, however, because of using JohnPhos as a ligand with the BArF4 counteranion (Scheme 38, right). It is possible for a Friedel–Crafts annulation to create spiropolycyclic cyclobutanes 135 from the Au(i)-stabilized carbocation 133 after the ring has grown. When intermediate 133 is deprotonated, cyclobutene 134 is obtained as an alternative.
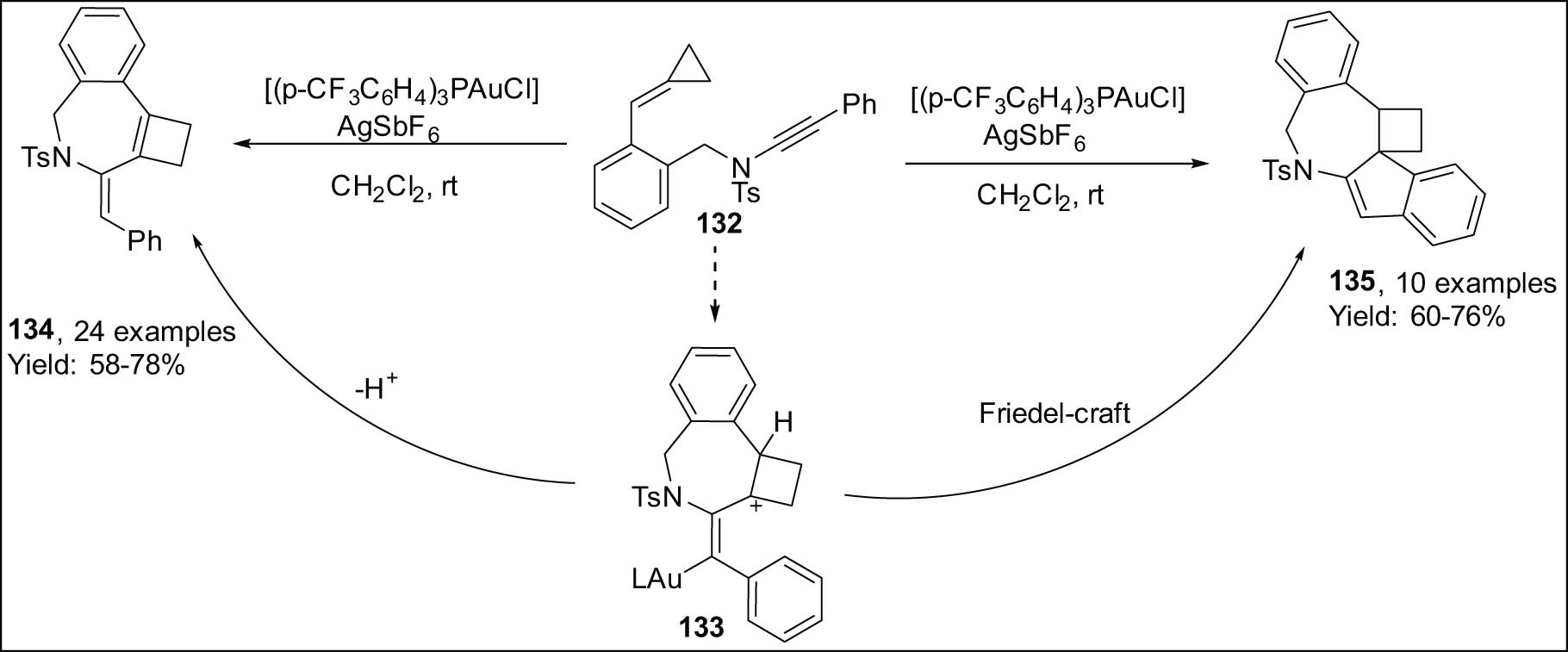
Cycloisomerizations of ynamide-cyclopropylidene 1,7-enyne.
Li and Yu used cyclopropylidene 1,6-enynes 136 as a starting point for the synthesis of azepine-fused cyclobutanes 137. As shown in Scheme 39 [135], a series of steps involving Au(i)-catalyzed cyclization, C–C cleavage, and Wagner–Meerwein rearrangement were necessary to achieve the result. The authors found that in this instance, the influence of both the counterion and the solvent enhanced enantioinduction. It was shown that AgSbF6, toluene, and [(R)-4-MeO-3,5-(t-Bu)2-MeOBIPHEP)(AuCl)2] can improve the enantioselectivity of cyclobutane products 137 by as much as 99% ee. Noting the creation of spirocyclic molecule 137′ during the reaction is very important because it shows great chemoselectivity, especially when aryl groups lacking electrons are connected to the cyclopropylidene moiety.

Enantioselective gold(i)-catalyzed cycloisomerization of cyclopropylidene 1,6-enynes.
Shi et al. reported a novel method of generating gold(i) carbenes based on vinylidenecyclopropanes’ ambiphilic properties. A vinylidene cyclopropane, like 138, is activated by gold(i) to produce cyclopropyl gold(i) intermediate 139, which then expands the ring to form Au(i) carbene 140 (Scheme 40) [136].
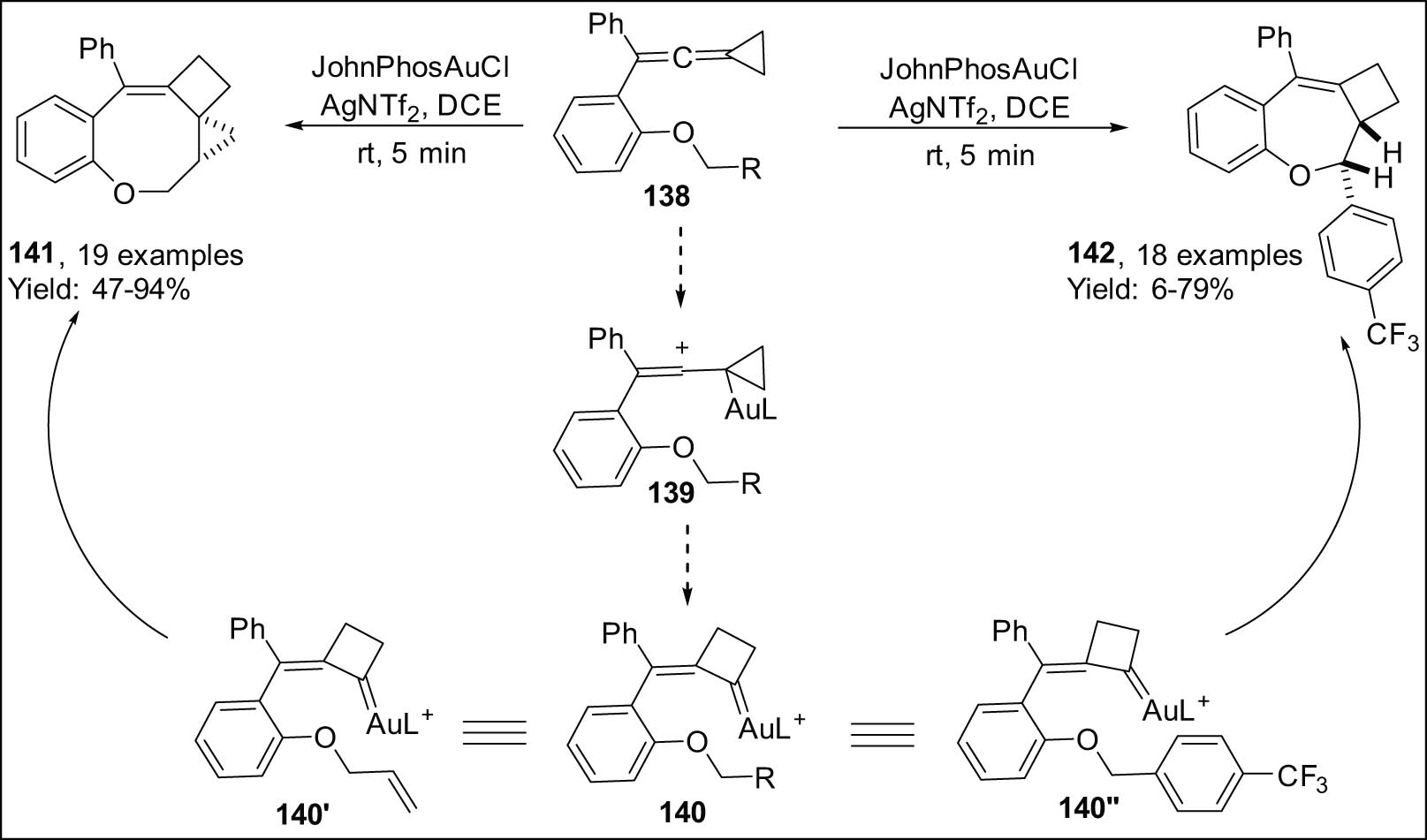
Cycloisomerization of vinylidenecyclopropanes coupled with O-allyl and benzyl moieties.
When this method was used, the pendant alkene part of vinylidenecyclopropane-enes of type 138′ underwent cyclopropanation by temporary Au(i) carbenes 140′. Therefore, 3-, 4-, and 8-membered fused rings 141 (Scheme 40, left) were constructed in a single step [136]. Vinylidenecyclopropane-enes were the only ones that worked as substrates because they only bound to aromatic rings without ortho substitution. The researchers came up with new methods to achieve this. The Au(i) carbene that was made in the reaction mixture was very important in a process called intramolecular C(sp3)-H insertion. This resulted in the successful synthesis of the required fused cyclobutane products, denoted as 142. This was achieved by strategically modifying the pendant chain of vinylidene cyclopropanes, as depicted in Scheme 40 (right) [137].
4.2 Gold(i)-catalyzed 1,3-acyloxy migration in propargylic carboxylates
The challenge associated with the 1,3-acyloxy migration of 1,3-diarylpropargyl carboxylates under the influence of a gold(i) catalyst has been ascribed to the formation of uncharacterized amalgamations. In the context of the intermolecular synthesis of cyclobutanes 144b catalyzed by Au(i), as described by Shi et al., they developed a silver-free process that demonstrates both chemo- and regioselectivity. This methodology was employed to investigate the characteristics of the various products, as depicted in Scheme 41 [138]. Here, the intermolecular [2 + 2] cycloaddition between the in situ-produced allenes 143′ is catalyzed by gold(i). Only 1,4-enyne 144a was produced when silver was present (Scheme 41). On the other hand, depending on the catalyst selected and the carboxylate moiety’s substitution pattern, cyclobutane isomer 144b may be produced as the main product in a silver-free environment.
![Scheme 41
Au(i)-mediated intermolecular 1,3-acyloxy shift followed by [2 + 2] cycloaddition.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_041.jpg)
Au(i)-mediated intermolecular 1,3-acyloxy shift followed by [2 + 2] cycloaddition.
Fiksdahl recently reported an isolated case of this reactivity in the context of [2 + 2 + 2] cyclotrimerizations catalyzed by Au(i) [139].
In the context of a computational modeling investigation on the structural properties of acyclic diaminocarbene ligands, an accompanying enantioselective reaction was performed. The goal of this reaction was to change indolyl substrates 145, which have a propargyl ester part, into complex tetracyclic scaffolds 146 (shown in Scheme 42) [140].

Enantioselective Au(i)-catalyzed tandem rearrangement and cyclization of indolyl propargylic esters with acyclic diaminocarbene complexes.
It is easier to tell the difference between enantiomers when gold catalyst 147 is used with bigger alkyl groups on the alkyne moiety. In contrast, complex 148 demonstrates an inverse impact. In general, it can be observed that complex 147 exhibits superior enantioselectivity and yields compared to those of complex 148.
4.3 Gold(i)-catalyzed [2 + 2]-cycloaddition between allenes and alkenes
The efficacy of 3-(Propa-1,2-dien-1-yl)oxazolidin-2-one 149 as a suitable two-carbon partner in a full regio- and stereocontrolled intramolecular [2 + 2] cycloaddition with alkenes 150 has been established by López et al. As shown in Scheme 43, the presence of gold acts as a catalyst to facilitate this reaction. The formation of trans isomer 152 is independent of the alkene 150′s structure. The proposed mechanism entails a sequential cationic pathway characterized by the formation of cationic intermediates. The regioselectivity of the reaction is determined by the nucleophilic attack of the alkene on a second cationic intermediate 151. This attack favors the creation of a more stable benzylic or iminium cation [141]. An important discovery was made by Chen et al. about the catalytic properties of the chiral bis-1,2,3-triazol-5-ylidene diAu(i) complex 153 in a recent study. This complex has amazing catalytic properties that the researchers used to show how it can help make cyclobutanes selectively using a [2 + 2] cycloaddition process [142]. This ligand can be used with a wider range of substrates, such as different allenamide and alkene chemical counterparts.
![Scheme 43
Gold-catalyzed allenamide-alkene [2 + 2] cycloaddition.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_043.jpg)
Gold-catalyzed allenamide-alkene [2 + 2] cycloaddition.
The asymmetric Au(i)-catalyzed cycloadditions of N-allenylsulfonamides 155 and 3-styrylindoles 154 were reported by Zhang and colleagues (Scheme 44) [143]. Here, it was discovered that the N-substituents’ electrical characteristics were responsible for switching the cycloaddition mode. The [4 + 2] cycloaddition reaction leading to the formation of tetrahydrocarbazole scaffolds 157 (Scheme 44, left) was facilitated by substituents with electron deficiency. Conversely, the [2 + 2] cycloaddition reaction resulting in the synthesis of 3-cyclobutylindole 156 (Scheme 44, right) was favored by substituents with electron-donating properties.
![Scheme 44
Enantioselective [2 + 2] and [4 + 2] cycloaddition of 3-styrylindoles with allenamides.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_044.jpg)
Enantioselective [2 + 2] and [4 + 2] cycloaddition of 3-styrylindoles with allenamides.
Interestingly, it was discovered that employing the identical chiral phosphoramidite gold(i) catalyst allowed for diastereo- and highlyenantioselectivity in both routes. After that, a new method was created using a chiral Au(i) monophosphine catalyst to make the [2 + 2] cycloaddition process possible with N-allenyl oxazolidinone as the electrophilic reactant [144].
The Bandini group devised Scheme 45, an asymmetric technique for the dearomative [2 + 2] cycloaddition of 1,2-substituted indoles 160 and allenamides 161 [145,146]. To obtain very good chemo-, diastereo-, and enantioselectivity in the production of indolincyclobutanes 162, an electron-rich phosphine-based gold catalyst had to be used. In a previous paper, the same research group showed that indoles could selectively become C3-functionalized when they were exposed to an extremely electrophilic Au(i) catalyst, which is like the scaffolds used in this study [147].
![Scheme 45
Enantioselective gold(i)-catalyzed [2 + 2] cycloaddition of indoles with allenes.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_045.jpg)
Enantioselective gold(i)-catalyzed [2 + 2] cycloaddition of indoles with allenes.
4.4 Gold(i)-catalyzed [2 + 2]-cycloadditions between alkynes and alkenes
Numerous studies have been conducted on the alkene–alkyne system to produce cyclobutanes, cyclobutenes, and cyclobutanones by intra- and intermolecular [2 + 2] cycloaddition [61]. With 1,6- [148–157], 1,7- [158–161], 1,8- [155,162,163], and 1,9-enynes [163] as substrates, many bicycles with four carbon rings have been formed. It was necessary to employ bulky phosphines, primarily biarylphosphines, as ligands for the majority of these unsaturated systems. Additionally, there have been documented instances of multiple situations involving the inclusion of macrocycles with ring sizes ranging from 9 to 15 members [164,165].
The access to cyclobutanones 166 is facilitated by the gold-catalyzed cycloisomerization of substituted 1,6-ene-ynamides 163, as described by Cossy [150,153] and Yeh (Scheme 46) [157]. This process involves the hydrolysis of the first formed cyclobutenes to obtain the desired cyclobutanones. The proposed mechanism for the formation of trimethylsilylanol involves the protonation of the double bond in the intermediate compound 164, which is a cyclobutene replaced with trimethylsilyl groups. This protonation event then triggers the removal of the trimethylsilyl group. The lack of rapid protosilylation in the presence of AuCl can be attributed to the behavior of trimethylsilyl-ynamides. The presence of H2O leads to the transformation of component 165 into cyclobutanone 166.
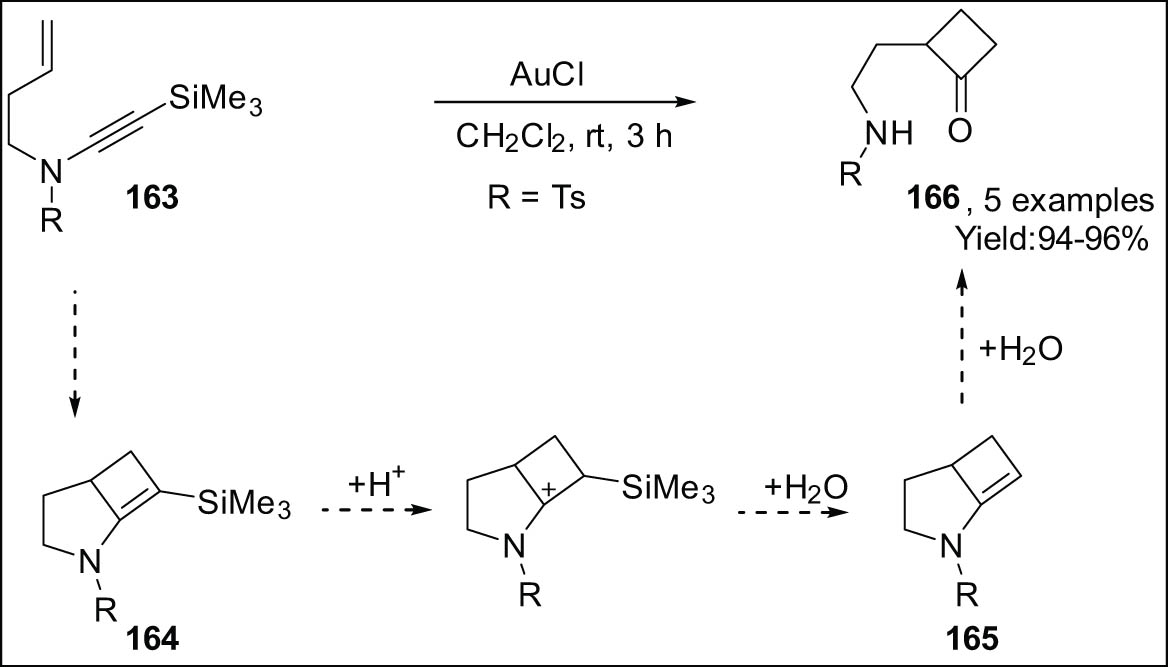
Cycloisomerization of trimethylsilyl-modified 1,6-ene-ynamide.
Furthermore, the formation of 6,6-diarylbicyclo[3.2.0]heptanes 169, which possess a quaternary core, is successfully accomplished by the utilization of a sequential gold-catalyzed cycloaddition/hydroarylation procedure involving 7-aryl-1,6-enynes 167. By using an arene 168 with a high electron density as the nucleophile, Scheme 47 [156] can perform the reaction. Many mono-, di-, and trisubstituted arenes have gone through this change, which creates diastereoisomer combinations in an endo/exo configuration. The ratios of these combinations vary from 1:7 to 25:1.
![Scheme 47
Sequential gold-catalyzed [2 + 2] enynes’ cycloaddition and hydroarylation.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_047.jpg)
Sequential gold-catalyzed [2 + 2] enynes’ cycloaddition and hydroarylation.
It is possible to selectively make substituted cyclobutenes by using Au(i) as an intermolecular catalyst in a [2 + 2] cycloaddition reaction with aromatic or aliphatic alkenes and terminal electron-rich alkynes. Kinetic measurements and DFT calculations support the mechanistic proposal. These results back up the idea that the rate-determining phase involves an associative ligand exchange between the gold-bound alkyne and the alkene. Additionally, the early intermediates are believed to be cyclopropyl Au(i) carbenes. The scope of the reaction includes reactions involving alkenes 171 and 1,3-butadiynes 172. When the highly substituted carbon of the alkene and the secondary carbon of the alkyne come into contact with each other, a chemoselective [2 + 2] cycloaddition reaction takes place. The only way this reaction can occur is through the terminal alkyne, which makes alkynyl cyclobutenes 172 (shown in Scheme 48) [166].
![Scheme 48
Gold-catalyzed regioselective [2 + 2] 1,3-butadiyne cycloaddition with alkenes.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_048.jpg)
Gold-catalyzed regioselective [2 + 2] 1,3-butadiyne cycloaddition with alkenes.
Similarly, alkenes 173 can combine with the equivalent 1,3-enynes 174 to produce 1-vinyl-3-substituted cyclobutenes 175 (Scheme 49) [167].
![Scheme 49
Gold-catalyzed chemoselective alkene-diyne [2 + 2] cycloaddition.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_049.jpg)
Gold-catalyzed chemoselective alkene-diyne [2 + 2] cycloaddition.
Recently, it was reported that the [2 + 2] cycloaddition of unactivated monosubstituted alkenes 177 with chloroethynyls 176 has very good selectivity in certain regions. With 1,2-disubstituted unactivated alkenes, the reaction is essentially stereo-specific (Scheme 50) [168]. The fact that the 1-chlorocyclobutene derivatives 178 worked well as substrates in cross-coupling reactions shows that they are useful for making new things.
![Scheme 50
Gold-catalyzed [2 + 2] cycloaddition of chloroalkynes with unactivated alkenes.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_050.jpg)
Gold-catalyzed [2 + 2] cycloaddition of chloroalkynes with unactivated alkenes.
They used a gold catalyst with a Josiphos ligand and a [BAr4F]− counterion to show enantioselectivity for the first time. This catalyst was applied to di- and trisubstituted alkenes ranging from 180 to limit the formation of digold species, as seen in Scheme 51 [169]. The utilization of this technology in the enantioselective total synthesis of Rumphellaone A, a terpenoid known for its cytotoxicity against human tumor cells, is of particular significance as it involves a sequence of nine steps.
![Scheme 51
Enantioselective [2 + 2] cycloaddition between trisubstituted alkenes and terminal alkynes.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_051.jpg)
Enantioselective [2 + 2] cycloaddition between trisubstituted alkenes and terminal alkynes.
Sparr et al. conducted an experimental investigation on the novel atropisomeric teraryl monophosphine ligand, Joyaphos, employing the same methodology as described in the study of Castrogiovanni et al. [170]. The study’s results show that using (Sa)-Ph2JoyaphosAuCl and Cy2JoyaphosAuCl with AgSbF6 can help the reaction occur, even though the yields aren’t very high and the enantioselectivity isn’t very good.
In a recent study, Echavarren et al. conducted research on the enantioselective synthesis of hushinone, a norsesquiterpenoid present in the essential oils obtained from Betula pubescens buds. The synthesis involved a series of 16 steps, resulting in a yield of around 1.1%. Additionally, they also successfully synthesized Rumphellaone A in 12 steps, achieving an approximate yield of 8% [165]. To synthesize the cyclobutene part 183, the 1,10-enyne 182 needs to be diastereoselectively [2 + 2] macrocyclized with the help of Au(i) catalysis (Scheme 52).
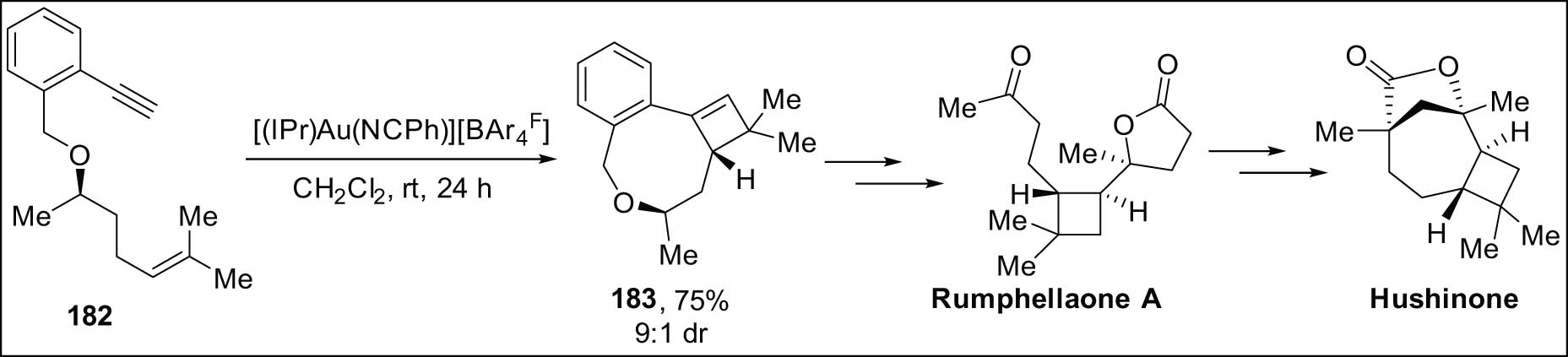
Crucial step strategy for the total syntheses of hushinone and rumellaone A.
Xu et al. have developed a convergent approach utilizing pinacol rearrangement and Au(i)-catalyzed [2 + 2] enyne 184 cycloaddition. This method enables the formation of the central bridging tricyclic BCD ring system found in norditerpenoid alkaloids, specifically Racemulsonine (Scheme 53) [160].

Synthesis of the bridged tricyclic BCD ring system core.
4.5 Gold(i)-mediated alkyne [2 + 2]-cycloadditions
With gold catalysis, 1,n-diynes have not been utilized nearly as frequently as 1,n-enynes. Every scenario given involves the formal [2 + 2] cycloaddition-mediated evolution of an allene molecule from its initial production.
Gold can be used as a catalyst to convert 1,7-diyn-3,6-bis(propargyl) carbonates 187 into functionalized naphtho[b]cyclobutenes 188 with great stereoselectivity. The double 3,3-rearrangement that forms bis(allenyl)carbonate 189 in the cascade sequence. The naphthyl derivative 190, which is produced via a 6π-electrocyclic reaction and can be thought of as a highly stabilized biradical 191, is then followed by cyclobutenyl dicarbonate 192 when it spontaneously cyclizes (Scheme 54) [171]. At last, 188 is obtained through a decarbonylative cyclization (pathway A). On the other hand, approach B suggests that the allenic group attacks the gold-activated allene with a nucleophilic attack inside the molecule, which creates oxocarbenium.
![Scheme 54
Gold-catalyzed diyne [2 + 2] cycloaddition for naphtho[b]cyclobutene formation.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_054.jpg)
Gold-catalyzed diyne [2 + 2] cycloaddition for naphtho[b]cyclobutene formation.
Alternatively, the intramolecular gold-catalyzed cycloisomerization of stable alkylidene tethered diynes 194 can be utilized to facilitate the production of cyclobutene-fused azepines 195. A viable mechanism, shown in Scheme 55, has been developed based on the findings obtained from the 1H NMR investigation. A single pair of electrons on the nitrogen atom facilitates a 6-endo-dig attack, which is how the reaction starts. This attack results in the nucleophilic addition of an alkene to an activated alkyne.
![Scheme 55
[2 + 2] Gold-catalyzed Diyne cycloaddition to cyclobutene-fused azepines.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_055.jpg)
[2 + 2] Gold-catalyzed Diyne cycloaddition to cyclobutene-fused azepines.
An allylic cation, 197, is produced by cleavage of the C–N bond, 196, and then demetallation to form the allene, 198. Finally, the activation of the vicinal alkyne [172] causes a [2 + 2] cycloaddition with the former allene.
Chan et al. investigated the cycloisomerization of 1,6-diyne esters 199 through 1,3-migration[2 + 2]-cycloaddition. This reaction resulted in the selective formation of bicyclo[3.2.0]hepta-1,5-dienes 200 (Scheme 56) [173].
![Scheme 56
Synthesis of alkylidencyclobutenes by [2 + 2] Diynes cycloaddition.](/document/doi/10.1515/hc-2022-0172/asset/graphic/j_hc-2022-0172_fig_056.jpg)
Synthesis of alkylidencyclobutenes by [2 + 2] Diynes cycloaddition.
4.6 Heteroatom-incorporating 4-membered ring synthesis
The approach to creating 4-membered rings, including O and N atoms, was devised by Zhang. This was achieved by utilizing the intramolecular insertion capacity of α-oxo gold carbenes into N–H and O–H bonds. The carbenes discussed in this study are generated from alkynes via a gold(i)-catalyzed intermolecular oxidation process [174].
A viable approach for the synthesis of oxetan-3-ones [175], 202, and 204 was devised by utilizing propargylic alcohols 201 [176] that are easily available. The synthesis was conducted under ambient air conditions, employing pyridine N-oxides as oxidizing agents and catalytic quantities of Au(i) complexes, as illustrated in Scheme 57 [177]. To mitigate potential side reactions arising from the generation of propargylic cations in an acidic environment, it was necessary to include an electron-withdrawing group at the alkyne terminus of tertiary propargylic alcohol substrates 203.

Gold(i)-catalyzed formation of oxetan-3-ones via oxidative synthesis.
The disclosed technique (Scheme 58) was employed to successfully synthesize a drug discovery library of 419 spirocyclic oxetane-piperidine scaffolds. These scaffolds are considered significant motifs in the field of medicinal chemistry [178–180].

Efficient gold(i)-catalyzed production of oxetane–piperidine system at scale.
Zhang et al. utilized a similar methodology in order to synthesize 208 from N-tert-butylsulfonyl propargylamines [181]. The requirement for acidic additives when working with secondary propargyl amine substrates was eliminated by utilizing a large Buchwald ligand and an electron-deficient, obstructed pyridine N-oxide, hence improving compatibility with functional groups.
Based on the previous research conducted by Ye et al. [181], a tripartite approach was developed to efficiently synthesize azetidin-3-ones 208 using readily available propargylic alcohols 207 (as shown in Scheme 59) [182]. A convenient and practical method for the synthesis of diverse azetidin-3-ones involves the oxidative cyclization of N-tosyl propargylamines with gold as the catalyst. Several other sulfonyl-protecting groups were found to be suitable for use in this process.
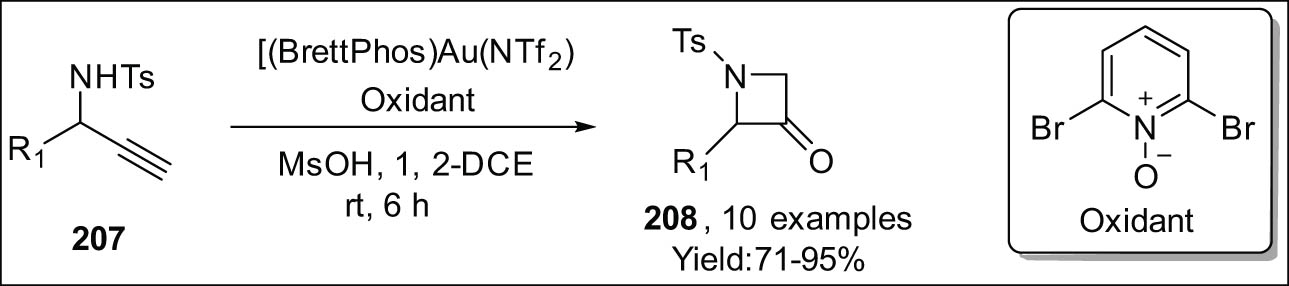
Au(i)-catalyzed formation of azetidin-3-ones via oxidative synthesis.
5 Conclusion
Over the past 20 years, Au(i) catalysis has emerged as a highly effective strategy for achieving rapid and efficient synthesis of complex molecules through single-step reactions. The first step in a series of changes that occur when Au(i) π-bonds are activated is the formation of Au(i) carbene intermediates. These intermediate compounds often undergo reactions either within the molecule or between molecules to form carbocycles consisting of three or four carbon atoms. This approach has been extensively utilized to formulate a variety of techniques that effectively enable the precise and streamlined fabrication of diminutive rings. One of the primary obstacles in this domain pertains to the establishment of adaptable procedures that are not dependent on overly complex or specialist beginning materials.
It is imperative to prevent the inclusion of undesirable functional groups in the resultant reaction products. Because these reactions involve a lot of complicated molecules, small changes to the substrate substitution pattern or reaction conditions can often lead to very different results. There are also still problems with making enantioselective versions of some transformations, even though there have been big steps forward in the field of asymmetric Au(i) catalysis in the last 10 years. Utilizing Au(i)-catalyzed intermolecular interactions can result in the synthesis of enantioenriched three- or four-membered rings. However, it is worth noting that these reactions remain infrequent and often exhibit a significant dependence on the specific substrates employed.
Acknowledgement
The authors would like to express their gratitude towards the management of Shri Vishnu Engineering College for Women (A), Bhimavaram, for their logistic support, without which it would not have been possible to carry out this work. The other co-authors would also like to acknowledge the help rendered by their respective managements.
-
Funding information: Authors state no funding involved.
-
Author contributions: Kasi Ganesh Kadiyala and Naresh Kumari Kattari are involved in the design and data collection part of the entire manuscript, and Goutham and Yamini are the authors involved in the analysis and writing part of the provided data in the form of the article.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
[1] Raubenheimer HG, Schmidbaur H. The late start and amazing upswing in gold chemistry. J Chem Educ. 2014;91(12):2024–36. 10.1021/ed400782p.Search in Google Scholar
[2] Rocchigiani L, Bochmann M. Recent advances in gold(III) chemistry: Structure, bonding, reactivity, and role in homogeneous catalysis. Chem Rev. 2021;121(14):8364–451. 10.1021/acs.chemrev.0c00552.Search in Google Scholar PubMed
[3] Fraga BM. Natural sesquiterpenoids. Nat Prod Rep. 2005;22(4):465–86. 10.1039/b501837b.Search in Google Scholar PubMed
[4] Kirsch SF. Syntheses of polysubstituted furans: recent developments. Org Biomol Chem. 2006;4(11):2076–80. 10.1039/b602596j.Search in Google Scholar PubMed
[5] Hou X-L, Yang Z, Yeung K-S, Wong HNC. Five-membered ring systems: furans and benzofurans. Prog Heterocycl Chem. 2008;19:176–207.10.1016/S0959-6380(08)80009-8Search in Google Scholar
[6] Comprehensive heterocyclic chemistry. In: Katritzky AR, Ramsden CA, Scriven EFV, Taylor RJK, editors. Oxford: Elsevier; 2008.Search in Google Scholar
[7] Heterocycles in natural product synthesis. In: Majumdar KC, Chattopadhyay SK, editors. Weinheim: Wiley-VCH Press; 2011.Search in Google Scholar
[8] Gulevich AV, Dudnik AS, Chernyak N, Gevorgyan V. Transition metal-mediated synthesis of monocyclic aromatic heterocycles. Chem Rev. 2013;113(5):3084–213. 10.1021/cr300333u.Search in Google Scholar PubMed PubMed Central
[9] Arcadi A. In: Toste FD, Michelet V, editors. Gold catalysis: A homogeneous approach. London: Imperial College Press; 2014. p. 175–224.10.1142/9781848168534_0005Search in Google Scholar
[10] Ahlsten N, Cambeiro XC, Perry GJP, Larrosa IC-H. Functionalisation of heteroaromatic compounds via gold catalysis. In: Bandini M, editor. Au-Catalyzed Synthesis and Functionalization of Heterocycles. Cham, Switzerland: Springer International Publishing; 2016. p. 175–226.10.1007/7081_2015_5005Search in Google Scholar
[11] Hashmi ASK. Special issue gold chemistry. Chem Rev. 2021;121(14):8309–10. 10.1021/acs.chemrev.1c00393.Search in Google Scholar PubMed
[12] Greenberg A, Liebman JF, editors. Strained organic molecules. New York: Academic Press; 1978.Search in Google Scholar
[13] de Meijere A, Kozhushkov SI. The chemistry of highly strained ligospirocyclopropane systems. Chem Rev. 2000;100(1):93–142. 10.1021/cr960153y.Search in Google Scholar PubMed
[14] Faust R. Fascinating natural and artificial cyclopropane architectures. Angew Chem Int Ed Engl. 2001;40(12):2251–3. 10.1002/1521-3773(20010618)40:12<2251: aid-anie2251>3.0.co;2-r.Search in Google Scholar
[15] Chen Z, Mercer JAM, Zhu X, Romaniuk JAH, Pfattner R, Cegelski L, et al. Mechanochemical unzipping of insulating Polyladderene to semiconducting polyacetylene. Science. 2017;357(6350):475–9. 10.1126/science.aan2797.Search in Google Scholar
[16] Mato M, Franchino A, Garcı A-Morales CG, Echavarren AM. Gold-catalyzed synthesis of small rings. Chem Rev. 2021;121(14):8613–84. 10.1021/acs.chemrev.0c00697.Search in Google Scholar
[17] Pauling L. The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J Am Chem Soc. 1931;53(4):1367–400. 10.1021/ja01355a027.Search in Google Scholar
[18] de Meijere A. Bonding properties of cyclopropane and their chemical consequences. Angew Chem Int Ed Engl. 1979;18:809–26.10.1002/anie.197908093Search in Google Scholar
[19] Coelho PS, Brustad EM, Kannan A, Arnold FH. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science. 2013;339(6117):307–10. 10.1126/science.1231434.Search in Google Scholar
[20] Fructos MR, Díaz-Requejo MM, Pérez PJ. Gold and diazo reagents: a fruitful tool for developing molecular complexity. Chem Commun (Camb). 2016;52(46):7326–35. 10.1039/c6cc01958g.Search in Google Scholar
[21] Rull SG, Olmos A, Pérez PJ. Gold-catalyzed ethylene cyclopropanation. Beilstein J Org Chem. 2019;15:67–71. 10.3762/bjoc.15.7.Search in Google Scholar
[22] Cao Z-Y, Wang W, Liao K, Wang X, Zhou J, Ma J. Catalytic enantioselective Synthesis of cyclopropanes Featuring Vicinal All-Carbon Quaternary Stereocenters with a CH2F Group; Study of the Influence of C−F···H−N interactions on reactivity. Org Chem Front. 2018;5(20):2960–8. 10.1039/C8QO00842F.Search in Google Scholar
[23] Xu PW, Liu JK, Shen L, Cao ZY, Zhao XL, Yan J, et al. Diastereo- and enantioselective [3 + 3] cycloaddition of spirocyclopropyl oxindoles using both aldonitrones and ketonitrones. Nat Commun. 2017;8(1):1619. 10.1038/s41467-017-01451-1.Search in Google Scholar
[24] Sasane AM, Kuo TC, Cheng MJ, Liu RS. Gold-catalyzed rearrangement of α carbonyl cyclopropanes to Form 3 (Cyclopenta-1,3-dien-1-ylmethyl)oxindoles via a postulated 1,5-enolate shift. Org Lett. 2022;24:5220–5. 10.1021/acs.orglett.2c02117.Search in Google Scholar PubMed
[25] Chu Z, Tang Z, Zhang K, Wang L, Li W, Wu H-H, et al. Gold(I)-catalyzed enantioselective cyclopropanation of α-aryl diazoacetates with enamides. Organometallics. 2019;38(20):4036–42. 10.1021/acs.organomet.9b00415.Search in Google Scholar
[26] Rössler SL, Petrone DA, Carreira EM. Iridium-catalyzed asymmetric synthesis of functionally rich molecules enabled by (Phopshoramidite, olefin) ligands. Acc Chem Res. 2019;52(9):2657–72. 10.1021/acs.accounts.9b00209.Search in Google Scholar PubMed
[27] Fürstner A, Davies PW. Cationic carbophilic activation: catalysis by platinum and Gold π acids. Angew Chem Int Ed. 2007;46:3410–49.10.1002/anie.200604335Search in Google Scholar PubMed
[28] Jiménez-Núñez E, Echavarren AM. Molecular diversity through gold catalysis with alkynes. Chem Commun (Camb). 2007;4:333–46. 10.1039/b612008c.Search in Google Scholar PubMed
[29] Kirsch SF. Construction of heterocycles by the strategic use of alkyne π-activation in catalyzed cascade reactions. Synthesis. 2008;2008(20):3183–204. 10.1055/s-0028-1083164.Search in Google Scholar
[30] Fürstner A. Gold and platinum catalysis – A convenient tool for generating molecular complexity. Chem Soc Rev. 2009;38(11):3208–21. 10.1039/b816696j.Search in Google Scholar PubMed
[31] Pérez AG, Álvarez R, de Lera ÁR. Characterization of pericyclic steps in the mechanisms of gold(I) catalyzed rearrangement of alkynes. WIREs Comput Mol Sci. 2013;3(3):211–25. 10.1002/wcms.1126.Search in Google Scholar
[32] Ohno H. Gold-catalyzed cascade reactions of alkynes for construction of polycyclic compounds. Isr J Chem. 2013;53(11–12):869–82. 10.1002/ijch.201300054.Search in Google Scholar
[33] Dorel R, Echavarren AM. Gold (I)-catalyzed activation of alkynes for the construction of molecular complexity. Chem Rev. 2015;115:9028–72.10.1021/cr500691kSearch in Google Scholar PubMed PubMed Central
[34] Fürstner A. Gold catalysis for heterocyclic chemistry: A representative case study on pyrone natural products. Angew Chem Int Ed Engl. 2018;57(16):4215–33. 10.1002/anie.201707260.Search in Google Scholar PubMed
[35] Braun I, Asiri AM, Hashmi ASK. Gold catalysis 2.0. ACS Catal. 2013;3(8):1902–7. 10.1021/cs400437s.Search in Google Scholar
[36] Asiri AM, Hashmi ASK. Gold-catalysed reactions of diynes. Chem Soc Rev. 2016;45(16):4471–503. 10.1039/c6cs00023a.Search in Google Scholar PubMed
[37] Day DP, Chan PWH. Gold-catalyzed cycloisomerizations of 1,n-Diyne carbonates and esters. Adv Synth Catal. 2016;358(9):1368–84. 10.1002/adsc.201600005.Search in Google Scholar
[38] Krause N, Winter C. Gold-catalyzed nucleophilic cyclization of functionalized allenes: A powerful access to carbo- and heterocycles. Chem Rev. 2011;111(3):1994–2009. 10.1021/cr1004088.Search in Google Scholar PubMed
[39] Yang W, Hashmi ASK. Mechanistic insights into the gold chemistry of allenes. Chem Soc Rev. 2014;43(9):2941–55. 10.1039/c3cs60441a.Search in Google Scholar PubMed
[40] Soriano E, Fernández I. Allenes and computational chemistry: from bonding situation to reaction mechanisms. Chem Soc Rev. 2014;43(9):3041–105. 10.1039/c3cs60457h.Search in Google Scholar PubMed
[41] Aubert C, Fensterbank L, Garcia P, Malacria M, Simonneau A. Transition metal catalyzed cycloisomerizations of 1,n-Allenynes and -Allenenes. Chem Rev. 2011;111(3):1954–93. 10.1021/cr100376w.Search in Google Scholar PubMed
[42] Cañeque T, Truscott FM, Rodriguez R, Maestri G, Malacria M. Electrophilic activation of Allenenes and Allenynes: analogies and differences between Brønsted and lewis acid activation. Chem Soc Rev. 2014;43(9):2916–26. 10.1039/c4cs00023d.Search in Google Scholar PubMed
[43] Mascareñas JL, Varela I, López F. Allenes and derivatives in gold(I)- and platinum(II)-catalyzed formal cycloadditions. Acc Chem Res. 2019;52(2):465–79. 10.1021/acs.accounts.8b00567.Search in Google Scholar PubMed PubMed Central
[44] Aubert C, Buisine O, Malacria M. The behavior of 1,n-Enynes in the presence of transition metals. Chem Rev. 2002;102(3):813–34. 10.1021/cr980054f.Search in Google Scholar PubMed
[45] Lloyd-Jones GC. Mechanistic aspects of transition metal catalysed 1,6-diene and 1,6-enyne cycloisomerisation reactions. Org Biomol Chem. 2003;1(2):215–36. 10.1039/b209175p.Search in Google Scholar PubMed
[46] Diver ST, Giessert AJ. Enyne metathesis (enyne bond reorganization). Chem Rev. 2004;104(3):1317–82. 10.1021/cr020009e.Search in Google Scholar PubMed
[47] Echavarren AM, Nevado C. Non-stabilized transition metal carbenes as intermediates in intramolecular reactions of alkynes with alkenes. Chem Soc Rev. 2004;33(7):431–6. 10.1039/b308768a.Search in Google Scholar PubMed
[48] Bruneau C. Electrophilic activation and cycloisomerization of enynes: A new route to functional cyclopropanes. Angew Chem Int Ed Engl. 2005;44(16):2328–34. 10.1002/anie.200462568.Search in Google Scholar PubMed
[49] Zhang L, Sun J, Kozmin SA. Gold and platinum catalysis of enyne cycloisomerization. Adv Synth Catal. 2006;348(16–17):2271–96. 10.1002/adsc.200600368.Search in Google Scholar
[50] Ma S, Yu S, Gu Z. Gold-catalyzed cyclization of enynes. Angew Chem Int Ed Engl. 2005;45(2):200–3. 10.1002/anie.200502999.Search in Google Scholar PubMed
[51] Nieto-Oberhuber C, López S, Jiménez-Núñez E, Echavarren AM. The mechanistic puzzle of transition-metal-catalyzed skeletal rearrangements of enynes. Chemistry. 2006;12(23):5916–23. 10.1002/chem.200600174.Search in Google Scholar PubMed
[52] Zhang Z, Zhu G, Tong X, Wang F, Xie X, Wang J, et al. Transition metal-catalyzed intramolecular enyne cyclization reaction. Curr Org Chem. 2006;10(12):1457–78. 10.2174/138527206778018203.Search in Google Scholar
[53] Michelet V, Toullec PY, Genêt JP. Cycloisomerization of 1,n-Enynes: challenging metal-catalyzed rearrangements and mechanistic insights. Angew Chem Int Ed Engl. 2008;47(23):4268–315. 10.1002/anie.200701589.Search in Google Scholar PubMed
[54] Jiménez-Núñez E, Echavarren AM. Gold-catalyzed cycloisomerizations of enynes: A mechanistic perspective. Chem Rev. 2008;108(8):3326–50. 10.1021/cr0684319.Search in Google Scholar PubMed
[55] Belmont P, Parker E. Silver and gold catalysis for cycloisomerization reactions. Eur J Org Chem. 2009;2009(35):6075–89. 10.1002/ejoc.200900790.Search in Google Scholar
[56] Abu Sohel SM, Liu RS. Carbocyclisation of alkynes with external nucleophiles catalysed by gold, platinum and other electrophilic metals. Chem Soc Rev. 2009;38(8):2269–81. 10.1039/b807499m.Search in Google Scholar PubMed
[57] Echavarren AM, Jiménez-Núñez E. Complexity via gold-catalyzed molecular gymnastics. Top Catal. 2010;53(13–14):924–30. 10.1007/s11244-010-9524-6.Search in Google Scholar
[58] Garayalde D, Nevado C. Gold-containing and gold-generated 1,n-dipoles as useful platforms toward cycloadditions and cyclizations. ACS Catal. 2012;2(7):1462–79. 10.1021/cs300043w.Search in Google Scholar
[59] Nunes dos Santos Comprido L, Hashmi ASK. Catalytic oxidative cyclisation reactions of 1,6-enynes: A critical comparison between gold and palladium. Isr J Chem. 2013;53:883–91.10.1002/ijch.201300072Search in Google Scholar
[60] Michelet V. Noble metal-catalyzed enyne cycloisomerizations and related reactions. Comprehensive organic synthesis. Vol. 5. 2nd edn. Amsterdam: Elsevier; 2014. p. 1483–536.10.1016/B978-0-08-097742-3.00531-0Search in Google Scholar
[61] Obradors C, Echavarren AM. Gold-catalyzed rearrangements and beyond. Acc Chem Res. 2014;47(3):902–12. 10.1021/ar400174p.Search in Google Scholar PubMed PubMed Central
[62] Obradors C, Echavarren AM. Intriguing mechanistic labyrinths in gold(I) catalysis. Chem Commun (Camb). 2014;50(1):16–28. 10.1039/c3cc45518a.Search in Google Scholar PubMed PubMed Central
[63] Dorel R, Echavarren AM. Gold-catalyzed reactions via cyclopropyl gold carbene-like intermediates. J Org Chem. 2015;80(15):7321–32. 10.1021/acs.joc.5b01106.Search in Google Scholar PubMed PubMed Central
[64] Lee Y-C, Kumar K. Catalyzed enyne cycloisomerization – A road map to privileged heterocyclic scaffolds. Isr J Chem. 2018;58(5):531–56. 10.1002/ijch.201700067.Search in Google Scholar
[65] Marín-Luna M, Nieto Faza O, Silva López C. Gold-catalyzed homogeneous (cyclo)isomerization reactions. Front Chem. 2019;7:296. 10.3389/fchem.2019.00296.Search in Google Scholar PubMed PubMed Central
[66] Echavarren AM, Muratore ME, López-Carrillo V, Escribano-Cuesta A, Huguet N, Obradors C. Gold-catalyzed cyclizations of alkynes with alkenes and arenes. Org React. 2017;92:1–411.10.1002/0471264180.or092.01Search in Google Scholar
[67] Schmidbaur H, Schier A. Gold η2-coordination to unsaturated and aromatic hydrocarbons: the key step in gold-catalyzed organic transformations. Organometallics. 2010;29(1):2–23. 10.1021/om900900u.Search in Google Scholar
[68] Zhdanko A, Ströbele M, Maier ME. Coordination chemistry of gold catalysts in solution: A detailed NMR study. Chemistry. 2012;18(46):14732–44. 10.1002/chem.201201215.Search in Google Scholar PubMed
[69] García-Mota M, Cabello N, Maseras F, Echavarren AM, Pérez-Ramírez J, Lopez N. Selective homogeneous and heterogeneous gold catalysis with alkynes and alkenes: similar behavior, different origin. ChemPhysChem. 2008;9(11):1624–9. 10.1002/cphc.200800246.Search in Google Scholar PubMed
[70] Kale BS, Lee H-F, Liu R-S. A sequential route to cyclopentenes from 1,6-Enynes and Diazo Ketones through Gold and Rhodium Catalysis. Adv Synth Catal. 2017;359(3):402–9. 10.1002/adsc.201600980.Search in Google Scholar
[71] Simonneau A, Harrak Y, Jeanne-Julien L, Lemière G, Mouriès-Mansuy V, Goddard J-P, et al. Ring expansions within the gold-catalyzed cycloisomerization of O -tethered 1,6-enynes. Application to the synthesis of natural-product-like macrocycles. ChemCatChem. 2013;5(5):1096–9. 10.1002/cctc.201200484.Search in Google Scholar
[72] Laher R, Marin C, Michelet V. When gold meets perfumes: synthesis of olfactive compounds via gold-catalyzed Cycloiosmerization reactions. Org Lett. 2020;22(11):4058–62. 10.1021/acs.orglett.0c00843.Search in Google Scholar PubMed
[73] Dubarle-Offner J, Barbazanges M, Augé M, Desmarets C, Moussa J, Axet MR, et al. Gold compounds anchored to a metalated Arene scaffold: synthesis, X-ray molecular structures, and cycloisomerization of enyne. Organometallics. 2013;32:1665–73.10.1021/om301101zSearch in Google Scholar
[74] Fourmy K, Mallet-Ladeira S, Dechy-Cabaret O, Gouygou M. Gold phosphole complexes as efficient catalysts for alkyne activation. Organometallics. 2013;32(6):1571–4. 10.1021/om301244m.Search in Google Scholar
[75] Medena C, Calogero F, Lemoine Q, Aubert C, Derat E, Fensterbank L, et al. Bisphosphinite ligand: synthesis and application in gold-catalyzed enynes cycloisomerization. Eur J Org Chem. 2019;2019:2129–37.10.1002/ejoc.201801722Search in Google Scholar
[76] Zhang PC, Wang Y, Zhang ZM, Zhang J. Gold(I)/xiang-phos-catalyzed asymmetric intramolecular cyclopropanation of indenes and trisubstituted alkenes. Org Lett. 2018;20(22):7049–52. 10.1021/acs.orglett.8b02999.Search in Google Scholar PubMed
[77] (a) Calleja P, Pablo Ó, Ranieri B, Gaydou M, Pitaval A, et al. α, β‐unsaturated gold(I) carbenes by tandem cyclization and 1, 5‐alkoxy migration of 1, 6‐enynes: Mechanisms and applications. Chem Eur J. 2016;22:13613–8; (b) Carreras J, Livendahl M, McGonigal PR, Echavarren AM. Gold(I) as an artificial cyclase: short stereodivergent syntheses of (-)-epiglobulol and (-)-4β,7α- and (-)-4α,7α-aromadendranediols. Angew Chem Int Ed Engl. 2014;53(19):4896–9. 10.1002/anie.201402044.Search in Google Scholar PubMed PubMed Central
[78] Bharath Kumar P, Raju CE, Chandubhai PH, Sridhar B, Karunakar GV. Gold(I)-catalyzed regioselective cyclization to access cyclopropane-fused tetrahydrobenzochromenes. Org Lett. 2022;24(37):6761–6. 10.1021/acs.orglett.2c02564.Search in Google Scholar PubMed
[79] Bohan PT, Toste FD. Well-defined chiral gold(III) complex catalyzed direct enantioconvergent kinetic resolution of 1,5-enynes. J Am Chem Soc. 2017;139(32):11016–9. 10.1021/jacs.7b06025.Search in Google Scholar PubMed PubMed Central
[80] Adcock HV, Chatzopoulou E, Davies PW. Divergent C–H insertion−cyclization cascades of N-allyl ynamides. Angew Chem Int Ed Engl. 2015;54(51):15525–9. 10.1002/anie.201507167.Search in Google Scholar PubMed PubMed Central
[81] Chen GQ, Fang W, Wei Y, Tang XY, Shi M. Divergent reaction pathways in gold-catalyzed cycloisomerization of 1,5-enynes containing a cyclopropane ring: dramatic Ortho substituent and temperature effects. Chem Sci. 2016;7(7):4318–28. 10.1039/c6sc00058d.Search in Google Scholar PubMed PubMed Central
[82] Ikeuchi T, Inuki S, Oishi S, Ohno H. Gold (I)-catalyzed cascade cyclization reactions of allenynes for the synthesis of fused cyclopropanes and acenaphthenes. Angew Chem Int Ed. 2019;58:7792–6.10.1002/anie.201903384Search in Google Scholar PubMed
[83] Cao Z, Gagosz F. Gold-catalyzed tandem cycloisomerization/Cope rearrangement: an efficient access to the hydroazulenic motif. Angew Chem Int Ed Engl. 2013;52(34):9014–8. 10.1002/anie.201304497.Search in Google Scholar PubMed
[84] Reiersølmoen AC, Csókás D, Øien-Ødegaard S, Vanderkooy A, Gupta AK, Carlsson A-CC, et al. Catalytic activity of trans-bis(pyridine)gold complexes. J Am Chem Soc. 2020;142(13):6439–46. 10.1021/jacs.0c01941.Search in Google Scholar PubMed PubMed Central
[85] Reiersølmoen AC, Østrem E, Fiksdahl A. Gold(III)-catalysed cis-to-trans cyclopropyl isomerization. Eur J Org Chem. 2018;2018(25):3317–25. 10.1002/ejoc.201800419.Search in Google Scholar
[86] Reiersølmoen AC, Fiksdahl A. Pyridine- and quinoline-based gold(III) complexes: synthesis, characterization, and application. Eur J Org Chem. 2020;2020:2867–77.10.1002/ejoc.202000139Search in Google Scholar
[87] Lauterbach T, Ganschow M, Hussong MW, Rudolph M, Rominger F, Hashmi ASK. Gold-catalyzed synthesis of 1-Naphthylcarbenoids and their synthetic utilization in cyclopropanation reactions. Adv Synth Catal. 2014;356(4):680–6. 10.1002/adsc.201400104.Search in Google Scholar
[88] Oh CK, Kim JK, Piao L, Yu J, Kim SY. Gold carbenes from substituted propargyl acetates: intramolecular ene-type reactions to 2-Acetoxynaphthalene derivatives. Chem Eur J. 2013;19:10501–5.10.1002/chem.201301384Search in Google Scholar PubMed
[89] Lauterbach T, Gatzweiler S, Nösel P, Rudolph M, Rominger F, Hashmi ASK. Carbene transfer − A new pathway for propargylic esters in gold catalysis. Adv Synth Catal. 2013;355(13):2481–87. 10.1002/adsc.201300572.Search in Google Scholar
[90] Chen C, Zou Y, Chen X, Zhang X, Rao W, Chan PWH. Gold-catalyzed tandem 1,3-Migration/Double cyclopropanation of 1-Ene-4,n-diyne esters to tetracyclodecene and tetracycloundecene derivatives. Org Lett. 2016;18:4730–33.10.1021/acs.orglett.6b02404Search in Google Scholar PubMed
[91] Yeom HS, Shin S. Au(I)-catalyzed intramolecular oxidative cyclopropanation of 1,6-enynes derived from propiolamides with diphenyl sulfoxide. Org Biomol Chem. 2013;11:1089–92.10.1039/c2ob27394bSearch in Google Scholar PubMed
[92] Qian D, Hu H, Liu F, Tang B, Ye W, Wang Y, et al. Gold(I)-catalyzed highly diastereo- and enantioselective alkyne oxidation/cyclopropanation of 1,6-enynes. Angew Chem Int Ed. 2014;53(50):13751–5. 10.1002/anie.201407717.Search in Google Scholar PubMed
[93] Ji K, Zheng Z, Wang Z, Zhang L. Enantioselective oxidative gold catalysis enabled by a designed chiral P,N-bidentate ligand. Angew Chem Int Ed Engl. 2015;54(4):1245–49. 10.1002/anie.201409300.Search in Google Scholar PubMed PubMed Central
[94] Lundgren RJ, Sappong-Kumankumah A, Stradiotto M, Highly A. Versatile catalyst system for the cross-coupling of aryl chlorides and amines. Chem Eur J. 2010;16:1983–91.10.1002/chem.200902316Search in Google Scholar PubMed
[95] Lundgren RJ, Peters BD, Alsabeh PG, Stradiotto MAP. N-ligand for palladium-catalyzed ammonia arylation: coupling of deactivated aryl chloride, chemoselective arylations, and room temperature reactions. Angew Chem Int Ed Engl. 2010;49(24):4071–74. 10.1002/anie.201000526.Search in Google Scholar PubMed
[96] Luo Y, Ji K, Li Y, Zhang L. Tempering the reactivities of postulated α-oxo gold carbenes using bidentate ligands: implication of tricoordinated gold intermediates and the development of an expedient bimolecular assembly of 2,4-disubstituted oxazoles. J Am Chem Soc. 2012;134(42):17412–5. 10.1021/ja307948m.Search in Google Scholar PubMed PubMed Central
[97] Zeineddine A, Rekhroukh F, Sosa Carrizo ED, Mallet-Ladeira S, Miqueu K, Amgoune A, et al. Isolation of a reactive tricoordinate α-oxo gold carbene complex. Angew Chem Int Ed Engl. 2018;57(5):1306–10. 10.1002/anie.201711647.Search in Google Scholar PubMed
[98] Homs A, Muratore ME, Echavarren AM. Enantioselective total synthesis of (−)-Nardoaristolone B via a gold(I)-catalyzed oxidative cyclization. Org Lett. 2015;17(3):461–63. 10.1021/ol503531n.Search in Google Scholar PubMed PubMed Central
[99] Ji K, Zhang L. A non-diazo strategy to cyclopropanation via oxidatively generated gold carbene: the benefit of a conformationally rigid P,N-bidentate ligand. Org Chem Front. 2014;1(1):34–8. 10.1039/C3QO00080J.Search in Google Scholar PubMed PubMed Central
[100] Wang KB, Ran RQ, Xiu SD, Li CY. Synthesis of 3-aza-bicyclo[3.1.0]hexan-2-one derivatives via gold-catalyzed oxidative cyclopropanation of N-Allylynamides. Org Lett. 2013 May 17;15(10):2374–77. 10.1021/ol4007629.Search in Google Scholar PubMed
[101] Sánchez-Cantalejo F, Priest JD, Davies PW, Gold A. Carbene manifold to prepare fused γ-lactams by oxidative cyclisation of ynamides. Chem Eur J. 2018;24:17215–19.10.1002/chem.201804378Search in Google Scholar PubMed PubMed Central
[102] Claus V, Molinari L, Büllmann S, Thusek J, Rudolph M, Rominger F, et al. Gold-catalyzed cyclisation by 1,4-Dioxidation. Chem A Eur J. 2019;25(40):9385–9. 10.1002/chem.201900996.Search in Google Scholar PubMed
[103] Ji K, Zhang L. Cyclopropanation of benzene rings by oxidatively generated α-oxo gold carbene: one-pot access to Tetrahydropyranone-fused cycloheptatrienes from propargyl benzyl ethers. Adv Synth Catal. 2018;360(4):647–51. 10.1002/adsc.201701322.Search in Google Scholar PubMed PubMed Central
[104] Chen H, Zhang L. A Desulfonylative approach in oxidative gold catalysis: regiospecific access to donor-substituted acyl gold carbenes. Angew Chem Int Ed. 2015;54(40):11775–9. 10.1002/anie.201504511.Search in Google Scholar PubMed PubMed Central
[105] Hung H-H, Liao Y-C, Liu R-S. Silver-catalyzed stereoselective [3 + 2] cycloadditions of cyclopropyl-Indanimines with carbonyl compounds. Adv Synth Catal. 2013;355(8):1545–52. 10.1002/adsc.201300090.Search in Google Scholar
[106] Vasu D, Hung HH, Bhunia S, Gawade SA, Das A, Liu RS. Gold-catalyzed oxidative cyclization of 1,5-enynes using external oxidants. Angew Chem Int Ed Engl. 2011;50(30):6911–4. 10.1002/anie.201102581.Search in Google Scholar PubMed
[107] Tokimizu Y, Oishi S, Fujii N, Ohno H. Gold-catalyzed Cascade cyclization of (azido)ynamides: an efficient strategy for the construction of indoloquinolines. Org Lett. 2014;16(11):3138–41. 10.1021/ol5012604.Search in Google Scholar PubMed
[108] Tian X, Song L, Rudolph M, Rominger F, Oeser T, Hashmi ASK. Sulfilimines as versatile nitrene transfer reagents: facile access to diverse aza-heterocycles. Angew Chem Int Ed Engl. 2019;58(11):3589–93. 10.1002/anie.201812002.Search in Google Scholar PubMed
[109] Tian X, Song L, Hashmi ASK. α-imino gold carbene intermediates from readily accessible sulfilimines: intermolecular access to structural diversity. Chemistry. 2020;26(15):3197–204. 10.1002/chem.201904869.Search in Google Scholar PubMed PubMed Central
[110] Song L, Tian X, Rudolph M, Rominger F, Hashmi ASK. Gold(III)-catalyzed chemoselective annulations of Anthranils with N-Allylynamides for the synthesis of 3-azabicyclo[3.1.0]hexan-2-imines. Chem Commun (Camb). 2019;55(61):9007–10. 10.1039/c9cc04027g.Search in Google Scholar PubMed
[111] Giri SS, Liu RS. Gold-catalyzed [4 + 3]- and [4 + 2]-annulations of 3-En-1-ynamides with isoxazoles via novel 6π-Electrocyclizations of 3-Azahepta Trienyl cations. Chem Sci. 2018;9(11):2991–5. 10.1039/c8sc00232k.Search in Google Scholar PubMed PubMed Central
[112] Sakharov PA, Rostovskii NV, Khlebnikov AF, Khoroshilova OV, Novikov MS. Transition metal-catalyzed synthesis of 3-coumaranone-containing NH-aziridines from 2H-Azirines: Nickel (II) versus Gold (I). Adv Synth Catal. 2019;361(14):3359–72. 10.1002/adsc.201900366.Search in Google Scholar
[113] Sahani RL, Liu RS. Gold-catalyzed [4 + 2] annulation/cyclization cascades of benzisoxazoles with propiolate derivatives to access highly oxygenated tetrahydroquinolines. Angew Chem Int Ed Engl. 2017;56(41):12736–40. 10.1002/anie.201707423.Search in Google Scholar PubMed
[114] Tian X, Song L, Farshadfar K, Rudolph M, Rominger F, Oeser T, et al. Acyl migration versus epoxidation in gold catalysis: facile, switchable, and atom-economic synthesis of acylindoles and quinoline derivatives. Angew Chem Int Ed Engl. 2020;59(1):471–8. 10.1002/anie.201912334.Search in Google Scholar PubMed PubMed Central
[115] Zhang L. Tandem Au-catalyzed 3,3-Rearrangement-[2 + 2] cycloadditions of propargylic Esters: expeditious access to Highly functionalized 2,3-indoline-fused cyclobutanes. J Am Chem Soc. 2005;127(48):16804–5. 10.1021/ja056419c.Search in Google Scholar PubMed
[116] Lu BL, Dai L, Shi M. Strained small rings in gold-catalyzed rapid chemical transformations. Chem Soc Rev. 2012;41(8):3318–39. 10.1039/c2cs15295a.Search in Google Scholar PubMed
[117] Brooner REM, Widenhoefer RA. Cationic, two-coordinate Gold π complexes. Angew Chem Int Ed Engl. 2013;52(45):11714–24. 10.1002/anie.201303468.Search in Google Scholar PubMed
[118] Ranieri B, Escofet I, Echavarren AM. Anatomy of gold catalysts: facts and myths. Org Biomol Chem. 2015;13(26):7103–18. 10.1039/c5ob00736d.Search in Google Scholar PubMed PubMed Central
[119] Gimeno MC, Laguna A. Three- and four-coordinate gold(I) complexes. Chem Rev. 1997;97(3):511–22. 10.1021/cr960361q.Search in Google Scholar PubMed
[120] Kumar R, Nevado C. Cyclometalated gold(III) complexes: synthesis, reactivity, and physicochemical properties. Angew Chem Int Ed Engl. 2017;56(8):1994–2015. 10.1002/anie.201607225.Search in Google Scholar PubMed
[121] Muratore ME, Homs A, Obradors C, Echavarren AM. Meeting the challenge of intermolecular gold(I)-catalyzed cycloadditions of alkynes and allenes. Chem – An Asian J. 2014;9(11):3066–82. 10.1002/asia.201402395.Search in Google Scholar PubMed PubMed Central
[122] García-Morales C, Echavarren AM. From straightforward gold(I)-catalyzed enyne cyclizations to more demanding intermolecular reactions of alkynes with alkenes. Synlett. 2018;29(17):2225–37. 10.1055/s-0037-1610203.Search in Google Scholar
[123] Wang Q, Shi M. Synthesis of cyclic and heterocyclic compounds via gold-catalyzed reactions. Synlett. 2017;28:2230–40.10.1055/s-0036-1590827Search in Google Scholar
[124] Fang W, Shi M. Recent advances in the cycloisomerizations of methylene cyclopropanes using gold catalysis. Chemistry. 2018;24(40):9998–10005. 10.1002/chem.201705788.Search in Google Scholar PubMed
[125] Garayalde D, Nevado C. Synthetic applications of gold-catalyzed ring expansions. Beilstein J Org Chem. 2011;7:767–80. 10.3762/bjoc.7.87.Search in Google Scholar PubMed PubMed Central
[126] Merino E, Fernández L, Nevado C. Gold-catalyzed migrations and ring expansions. Organogold compounds; Patai’s chemistry of functional groups. John Wiley & Sons, Ltd; 2014.10.1002/9780470682531.pat0811Search in Google Scholar
[127] Ferrer S, Echavarren AM. Total synthesis of Repraesentin F and configuration reassignment by a gold(I)-catalyzed cyclization Cascade. Org Lett. 2018;20(18):5784–8. 10.1021/acs.orglett.8b02478.Search in Google Scholar PubMed
[128] Pitaval A, Leboeuf D, Ceccon J, Echavarren AM. Access to the protoilludane core by gold-catalyzed Allene-vinylcyclopropane cycloisomerization. Org Lett. 2013;15(17):4580–3. 10.1021/ol402188b.Search in Google Scholar PubMed PubMed Central
[129] Felix RJ, Gutierrez O, Tantillo DJ, Gagné MR. Gold (I)-catalyzed formation of bicyclo. J Org Chem. 2013;78[4.2.0]oct-1-enes:5685–90.10.1021/jo400139gSearch in Google Scholar PubMed PubMed Central
[130] Zheng H, Felix RJ, Gagné MR. Gold-catalyzed enantioselective ring-expanding cycloisomerization of cyclopropylidene bearing 1,5-enynes. Org Lett. 2014;16(8):2272–5. 10.1021/ol5007955.Search in Google Scholar PubMed PubMed Central
[131] Roselli CA, Gagné MR. Gold (I)-catalyzed addition of aldehydes to cyclopropylidene bearing. Org Biomol Chem. 2016;14 6-Aryl-1,5-Enynes:11261–5.10.1039/C6OB02128JSearch in Google Scholar PubMed PubMed Central
[132] Hönig M, Carreira EM. Total synthesis and structural revision of a harziane diterpenoid. Angew Chem Int Ed Engl. 2020;59(3):1192–6. 10.1002/anie.201912982.Search in Google Scholar PubMed
[133] Fang W, Wei Y, Tang X-Y, Shi M. Gold (I)-catalyzed cycloisomerization of ortho-(Propargyloxy) arenemethylenecyclopropanes controlled by adjacent substituents at the aromatic rings. Chem Eur J. 2017;23:6845–52.10.1002/chem.201700600Search in Google Scholar PubMed
[134] Zhang J-H, Wei Y, Shi M, Ring G-C. Enlargement and cycloisomerization of alkynylamidetethered alkylidenecyclopropanes. Org Chem Front. 2018;5:2980–5.10.1039/C8QO00907DSearch in Google Scholar
[135] Li CL, Yu ZX. Asymmetric Synthesis of azepine-Fused cyclobutanes from Yne- methylene cyclopropanes involving cyclopropanation/C−C cleavage/Wagner−Meerwein rearrangement and reaction mechanism. J Org Chem. 2019;84(16):9913–28. 10.1021/acs.joc.9b01071.Search in Google Scholar PubMed
[136] Li DY, Wei Y, Marek I, Tang XY, Shi M. Gold(i)-catalyzed cycloisomerization of vinylidenecyclopropane-enes via carbene or non-carbene processes. Chem Sci. 2015;6(10):5519–25. 10.1039/c5sc01806d.Search in Google Scholar PubMed PubMed Central
[137] Li DY, Fang W, Wei Y, Shi MC. C(sp3)-H functionalizations promoted by the gold carbene generated from Vinylidenecyclopropanes. Chemistry. 2016;22(50):18080–4. 10.1002/chem.201604200.Search in Google Scholar PubMed
[138] Su Y, Zhang Y, Akhmedov NG, Petersen JL, Shi X. Ambient intermolecular [2 + 2] cycloaddition: an example of Carbophilicity and oxophilicity competition in Au/ag catalysis. Org Lett. 2014;16(9):2478–81. 10.1021/ol500854m.Search in Google Scholar PubMed
[139] Jońsson HF, Evjen S, Fiksdahl A. Gold (I). Catalyzed [2 + 2 + 2] cyclotrimerization of 1,3-diarylpropargyl acetals. Org Lett. 2017;19:2202–5.10.1021/acs.orglett.7b00411Search in Google Scholar PubMed
[140] Niemeyer ZL, Pindi S, Khrakovsky DA, Kuzniewski CN, Hong CM, Joyce LA, et al. Parameterization of acyclic diaminocarbene ligands applied to a gold(I)-catalyzed enantioselective tandem rearrangement/cyclization. J Am Chem Soc. 2017;139(37):12943–6. 10.1021/jacs.7b08791.Search in Google Scholar PubMed PubMed Central
[141] Faustino H, Alonso I, Mascareñas JL, López F. Gold(I)-catalyzed cascade cycloadditions between allenamides and carbonyl-tethered alkenes: an enantioselective approach to oxa-bridged medium-sized carbocycles. Angew Chem Int Ed Engl. 2013;52(25):6526–30. 10.1002/anie.201302713.Search in Google Scholar PubMed
[142] Huang W, Zhang Y-C, Jin R, Chen B-L, Chen Z. Synthesis of axially chiral 1,2,3-Triazol-5-ylidene-Au(I) complex and its application in enantioselective [2 + 2] cycloaddition of Alleneamides with alkenes. Organometallics. 2018;37(18):3196–209. 10.1021/acs.organomet.8b00524.Search in Google Scholar
[143] Wang Y, Zhang P, Liu Y, Xia F, Zhang J. Enantioselective gold-catalyzed intermolecular [2 + 2] versus [4 + 2]-cycloadditions of 3-styrylindoles with N-allenamides: observation of interesting substituent effects. Chem Sci. 2015;6(10):5564–70. 10.1039/c5sc01827g.Search in Google Scholar PubMed PubMed Central
[144] Hu H, Wang Y, Qian D, Zhang Z-M, Liu L, Zhang J. Enantioselective gold-catalyzed intermolecular [2 + 2]-cycloadditions of 3-Styrylindoles with N-allenyl oxazolidinone. Org Chem Front. 2016;3(6):759–63. 10.1039/C6QO00087H.Search in Google Scholar
[145] Ocello R, De Nisi A, Jia M, Yang Q-Q, Monari M, Giacinto P, et al. Gold (I)-catalyzed dearomative [2 + 2]-cycloaddition of indoles with activated allenes: A combined experimental-computational study. Chem Eur J. 2015;21:18445–53.10.1002/chem.201503598Search in Google Scholar PubMed
[146] Jia M, Monari M, Yang QQ, Bandini M. Enantioselective gold catalyzed dearomative [2 + 2]-cycloaddition between indoles and Allenamides. Chem Commun (Camb). 2015;51(12):2320–3. 10.1039/c4cc08736d.Search in Google Scholar PubMed
[147] Jia M, Cera G, Perrotta D, Monari M, Bandini M. Taming gold(I)-counterion interplay in the de-aromatization of indoles with Allenamides. Chemistry. 2014;20(32):9875–8. 10.1002/chem.201403155.Search in Google Scholar PubMed
[148] Mézailles N, Ricard L, Gagosz F. Phosphine gold(I) Bis- (trifluoromethanesulfonyl) imidate complexes as new highly efficient and air-stable catalysts for the cycloisomerization of enynes. Org Lett. 2005;7(19):4133–6. 10.1021/ol0515917.Search in Google Scholar PubMed
[149] Nieto-Oberhuber C, López S, Echavarren AM. Intramolecular [4 + 2] cycloadditions of 1, 3-enynes or arylalkynes with alkenes with highly reactive cationic phosphine Au(I) complexes. J Am Chem Soc. 2005;127(17):6178–9. 10.1021/ja042257t.Search in Google Scholar PubMed
[150] Couty S, Meyer C, Cossy J. Diastereoselective gold-catalyzed cycloisomerizations of ene-ynamides. Angew Chem Int Ed Engl. 2006;45(40):6726–30. 10.1002/anie.200602270.Search in Google Scholar PubMed
[151] Lee SI, Kim SM, Choi MR, Kim SY, Chung YK, Han WS, et al. Catalyzed Cyclizationof enynes bearing an olefinic cycle. J Org Chem. 2006;71(25):9366–72. 10.1021/jo061254r.Search in Google Scholar PubMed
[152] Nieto-Oberhuber C, Pérez-Galán P, Herrero-Gómez E, Lauterbach T, Rodríguez C, López S, et al. Gold (I)-catalyzed intramolecular [4 + 2] cycloadditions of arylalkynes or 1, 3-enynes with alkenes: Scope and mechanism. J Am Chem Soc. 2008;130:269–79.10.1021/ja075794xSearch in Google Scholar PubMed
[153] Couty S, Meyer C, Cossy J. Gold-catalyzed cycloisomerizations of ene-ynamides. Tetrahedron. 2009;65:1809–32.10.1016/j.tet.2008.10.108Search in Google Scholar
[154] Escribano-Cuesta A, Pérez-Galán P, Herrero-Gómez E, Sekine M, Braga AAC, Maseras F, et al. The role of cyclobutenes in gold(I)-catalysed skeletal rearrangement of 1, 6-enynes. Org Biomol Chem. 2012;10(30):6105–11. 10.1039/c2ob25419k.Search in Google Scholar PubMed
[155] Inagaki F, Matsumoto C, Okada Y, Maruyama N, Mukai C. Air-stable cationic gold(I) catalyst featuring a Z-type ligand: promoting enyne cyclizations. Angew Chem Int Ed. 2015;54(3):818–22. 10.1002/anie.201408037.Search in Google Scholar PubMed
[156] Robertson BD, Brooner REM, Widenhoefer RA. Gold T/Silver-catalyzed cycloaddition/hydroarylation of 7-Aryl-1, 6-enynes to Form 6,6-Diarylbicyclo [3.2.0] heptanes. Chem Eur J. 2015;21:5714–7.10.1002/chem.201500371Search in Google Scholar PubMed
[157] Zhong CZ, Tung PT, Chao TH, Yeh M-CP. Gold-catalyzed stereoselective synthesis of bicyclic lactams and ketones from N-Tosylynamidomethyl-Tethered cyclohexenes. J Org Chem. 2017;82(1):481–501. 10.1021/acs.joc.6b02479.Search in Google Scholar PubMed
[158] Nieto-Oberhuber C, López S, Muñoz MP, Cárdenas DJ, Buñuel E, Nevado C, et al. Divergent mechanisms for the skeletal rearrangement and [2 + 2] cycloaddition of enynes catalyzed by Gold. Angew Chem Int Ed Engl. 2005;44(38):6146–8. 10.1002/anie.200501937.Search in Google Scholar PubMed
[159] Huple DB, Liu RS. Gold-catalyzed diastereoselective [2 + 2 + 2] -Cycloaddition of 1, 7-enynes with carbonyl compounds. Chem Commun (Camb). 2012;48(89):10975–7. 10.1039/c2cc36219h.Search in Google Scholar PubMed
[160] Cao S-Y, Yue H-J, Zhu M-Q, Xu L. Synthesis of tricyclo[7.2.1.0 9,10]dodecan-11-one core ring systems of norditerpenoid alkaloids and racemulosine. Org Chem Front. 2020;7(7):933–7. 10.1039/D0QO00088D.Search in Google Scholar
[161] Zang W, Wei YShi,M. Gold (I)-catalyzed cascade cyclization of O-tethered 1, 7-enynes bearing a cyclopropane moiety: Construction of multi-substituted furans. Chem Commun. 2019;55:8126–9.10.1039/C9CC03735GSearch in Google Scholar PubMed
[162] Odabachian Y, Gagosz F. Cyclobutenes as isolable intermediates in the gold(I)-catalysed cycloisomerisation of 1, 8-enynes. Adv Synth Catal. 2009;351(3):379–86. 10.1002/adsc.200900056.Search in Google Scholar
[163] Iwai T, Ueno M, Okochi H, Sawamura M. Synthesis of cyclobutene-fused eight-membered carbocycles through gold-catalyzed intramolecular enyne [2 + 2] cycloaddition. Adv Synth Catal. 2018;360(4):670–5. 10.1002/adsc.201701193.Search in Google Scholar
[164] Obradors C, Leboeuf D, Aydin J, Echavarren AM. Gold (I)-catalyzed macrocyclization of 1,n-Enynes. Org Lett. 2013;15:1576–9.10.1021/ol400358fSearch in Google Scholar PubMed
[165] Ranieri B, Obradors C, Mato M, Echavarren AM. Synthesis of Rumphellaone A and Hushinone by a gold-catalyzed [2 + 2] cycloaddition. Org Lett. 2016;18(7):1614–7. 10.1021/acs.orglett.6b00473.Search in Google Scholar PubMed PubMed Central
[166] De Orbe ME, Amenós L, Kirillova MS, Wang Y, López-Carrillo V, Maseras F, et al. Cyclobutene vs 1,3-diene formation in the gold-catalyzed reaction of alkynes with alkenes: the complete mechanistic picture. J Am Chem Soc. 2017;139(30):10302–11. 10.1021/jacs.7b03005.Search in Google Scholar PubMed PubMed Central
[167] De Orbe ME, Echavarren AM. Broadening the scope of the gold-catalyzed [2 + 2] cycloaddition reaction: synthesis of Vinylcyclobutenes and further transformations. Eur J Org Chem. 2018;2740–52.10.1002/ejoc.201800170Search in Google Scholar
[168] Bai YB, Luo Z, Wang Y, Gao JM, Zhang L. Au-Catalyzed intermolecular [2 + 2] cycloadditions between Chloroalkynes and unactivated alkenes. J Am Chem Soc. 2018;140(17):5860–5. 10.1021/jacs.8b01813.Search in Google Scholar PubMed PubMed Central
[169] García-Morales C, Ranieri B, Escofet I, López-Suarez L, Obradors C, Konovalov AI, et al. Enantioselective synthesis of cyclobutenes by intermolecular [2 + 2] cycloaddition with non-C2 symmetric digold catalysts. J Am Chem Soc. 2017;139(39):13628–31. 10.1021/jacs.7b07651.Search in Google Scholar PubMed PubMed Central
[170] Castrogiovanni A, Lotter D, Bissegger FR, Sparr C. JoyaPhos: an atropisomeric Teraryl monophosphine ligand. Chemistry. 2020;26(44):9864–8. 10.1002/chem.202001269.Search in Google Scholar PubMed
[171] Chen M, Liu J, Wang L, Zhou X, Liu Y. Gold-Catalyzed Cascade Cycloisomerization of 1, 7-diyn-3,6-bis (propargyl carbonate) s: stereoselective Synthesis of naphtho [b] cyclobutenes. Chem Commun (Camb). 2013;49(77):8650–2. 10.1039/c3cc44757j.Search in Google Scholar PubMed
[172] Nayak S, Ghosh N, Sahoo AK. Access to cyclobutene-fused azepines through Au-catalyzed cycloisomerization of stable alkyne tethered ketene N,N-acetals. Org Lett. 2014;16(11):2996–9. 10.1021/ol501125r.Search in Google Scholar PubMed
[173] Li D, Rao W, Tay GL, Ayers BJ, Chan PWH. Gold-Catalyzed Cycloisomerization of 1, 6-diyne Esters to 1H-Cyclopenta [b] naphthalenes, cis-cyclopenten-2-yl δ-diketones, and bicyclo [3.2.0] hepta-1, 5-dienes. J Org Chem. 2014;79(23):11301–15. 10.1021/jo5020195.Search in Google Scholar PubMed
[174] Zhang L. A non-diazo approach to α-oxo gold carbenes via gold-catalyzed alkyne oxidation. Acc Chem Res. 2014;47(3):877–88. 10.1021/ar400181x.Search in Google Scholar PubMed PubMed Central
[175] Bull JA, Croft RA, Davis OA, Doran R, Morgan KF. Oxetanes: recent advances in synthesis, reactivity, and medicinal chemistry. Chem Rev. 2016;116(19):12150–233. 10.1021/acs.chemrev.6b00274.Search in Google Scholar PubMed
[176] Zhang B, Wang T. Gold-catalyzed transformations of propargyl alcohols and propargyl amines. Asian J Org Chem. 2018;7(9):1758–83. 10.1002/ajoc.201800324.Search in Google Scholar
[177] Ye L, He W, Zhang L. Gold-catalyzed one-step practical synthesis of Oxetan-3-ones from readily available propargylic alcohols. J Am Chem Soc. 2010;132(25):8550–1. 10.1021/ja1033952.Search in Google Scholar PubMed PubMed Central
[178] Geary GC, Nortcliffe A, Pearce CA, Hamza D, Jones G, Moody CJ. Densely functionalised spirocyclic oxetane-piperidine scaffolds for drug discovery. Bioorg Med Chem. 2018;26(4):791–7. 10.1016/j.bmc.2017.12.012.Search in Google Scholar PubMed
[179] Zheng Y, Tice CM, Singh SB. The use of spirocyclic scaffolds in drug discovery. Bioorg Med Chem Lett. 2014;24(16):3673–82. 10.1016/j.bmcl.2014.06.081.Search in Google Scholar PubMed
[180] Carreira EM, Fessard TC, Spyrocycles F-MR-C. Four-membered ring-containing spirocycles: Synthetic strategies and opportunities. Chem Rev. 2014;114(16):8257–322. 10.1021/cr500127b.Search in Google Scholar PubMed
[181] Ye L, He W, Zhang L. A flexible and stereoselective synthesis of Azetidin-3-ones through gold-catalyzed intermolecular oxidation of alkynes. Angew Chem Int Ed Engl. 2011;50(14):3236–9. 10.1002/anie.201007624.Search in Google Scholar PubMed PubMed Central
[182] Tan P, Xiang J-N. Excellent preparation of Azetidin-3-ones from propargylic alcohols by gold-catalysis. Adv Mater Res. 2013;634:970–4.10.4028/www.scientific.net/AMR.634-638.970Search in Google Scholar
© 2024 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids
Articles in the same Issue
- Research Articles
- Structural simplification of the 3‐nitroimidazo[1,2‐a]pyridine antileishmanial pharmacophore: Design, synthesis, and antileishmanial activity of novel 2,4-disubstituted 5-nitroimidazoles
- Synthesis of a novel water-soluble pyridine dicarboxylate and its application in fluorescence cell imaging
- Synthesis of novel meta-diamide compounds containing pyrazole moiety and their insecticidal evaluation
- Review Articles
- Inorganic nanoparticles promoted synthesis of oxygen-containing heterocycles
- Gold-catalyzed synthesis of small-sized carbo- and heterocyclic compounds: A review
- Synthesis of imidazole derivatives in the last 5 years: An update
- Current progress in the synthesis of imidazoles and their derivatives via the use of green tools
- Synthetic and therapeutic review of triazoles and hybrids

