Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
-
Mariola Krawiecka
, Bożena Kuran
Abstract
In the search for new antimicrobial and anticancer agents, a series of (aryl/heteroaryl-piperazino-alkyl)-substituted derivatives of benzo[b]furans were prepared. All compounds were characterized by 1H NMR, 13C NMR, ESI-MS spectra and elemental analyses. Most of the investigated compounds had no antimicrobial activity (MIC > 512 mg/L) except for 2l, 2m and 2o, which showed activity against Candida albicans. None of the tested compounds showed significant anticancer activity in K562 and HeLa cells.
Introduction
The benzofuran system, as an important pharmacophore, is present in numerous compounds isolated from natural sources as well as in synthetic products. These heterocyclic compounds show a variety biological activity, including antiarrhythmic, spazmolitic, antiviral, anticancer, antifungal and anti-inflammatory properties [1–10]. The most recognized derivatives of the benzofurans are Khellinone and Visnaginone isolated from Ammi visnaga (Apiaceae) [11].
Our research group obtained a large group of compounds which show antimicrobial, antiviral and cytotoxic activities [12–15]. This article describes our latest research results on the design and synthesis of new compounds with potential biological activity (Scheme 1).
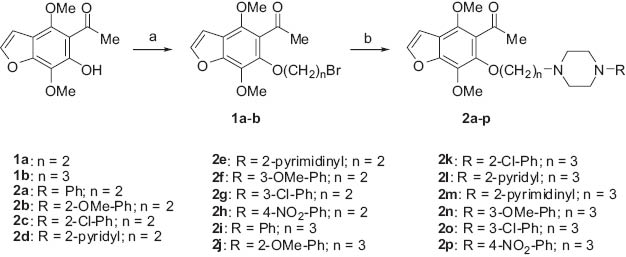
Synthesis of compounds 2a–p; (A) Br(CH2)2Br or Br(CH2)3Br, K2CO3, acetonitrile; (B) 1-aryl/heteroarylpiperazine, K2CO3, KI.
Results and discussion
Synthesis
The synthesis of compounds 1a,b and 2a–p was accomplished as presented in Scheme 1. The starting compound was 5-acetyl-6-hydroxy-4,7-dimethoxybenzofuran which was alkylated with 1,2-dibromoethane or 1,3-dibromopropane to give the respective products 1a or 1b. The final products 2a–p were prepared with good yields by condensation of the intermediate product 1a or 1b with the appropriate 1-aryl/heteroarylpiperazine in the presence of K2CO3 and KI in refluxing acetonitrile. The amino derivatives were converted into their hydrochlorides. All reported products showed 1H NMR, 13C NMR, ESI-MS spectra and elemental analyses in agreement with the assigned structures.
Antimicrobial activity
The obtained compounds 1a,b and 2a–p were tested for their antimicrobial activity against aerobic bacteria: Staphylococcus aureus, Escherichia coli, Stenotrophomonas maltophilia and yeast strain Candida albicans and some (1a, 2a–e) against selected anaerobes Propionibacterium acnes, Bacteroides thetaiotaomicron and Bacteroides fragilis.
Most of the investigated compounds showed no antimicrobial activity and did not inhibit growth even at the concentration of 512 mg/L. Some activity was observed for compounds 2l, 2m and 2o. They were active against C. albicans. The compounds inhibited the growth of yeast at a concentration of 512 mg/L. The highest activity was observed for compound 2o. For compounds 2l and 2m, the growth of fungi was observed on the second day.
Cytotoxic properties
The selected derivatives 1b, 2i, 2j, 2m, 2o and 2p were tested for their cytotoxic properties in K562 and HeLa cells. On the basis of dose-response curves it was not possible to calculate IC50 values, which indicates that none of the tested compounds show significant anticancer activity.
Conclusion
In the present study, we obtained and tested for antimicrobial and anticancer activity new aryl/heteroaryl-piperazino-alkyl derivatives of benzo[b]furans. The results show that this class of benzofurans possess low antimicrobial activity and do not show significant anticancer activity.
Experimental
Melting points were determined by the capillary method using the Electrothermal 9100 apparatus and were uncorrected. Unless stated otherwise, nuclear magnetic resonance spectra were recorded in DMSO (dimethyl sulfoxide)-d6 on a Bruker VMNRS300 operating at 300 MHz (1H NMR) and 75 MHz (13C NMR). Mass spectral (electrospray ionization, ESI) measurements were carried out on a Mariner Perspective – Biosystem instrument with a TOF detector. The spectra were obtained in the positive ion mode with a declustering potential of 140–300 V. Elemental analyses were recorded using a CHN model 2400 Perkin-Elmer analyzer. Chromatographic columns were filled with Merck Kieselgel 0.05–0.2 mm (70–325 mesh ASTM) silica gel. Reactions were monitored by thin layer chromatography (TLC) on silica gel (plates with 254 nm fluorescent indicator, layer thickness 0.2 mm, Kieselgel G., Merck), eluting with 9.8:0.2 or 9.5:0.5 chloroform/methanol.
Synthesis of compounds 1a and 1b
A solution of 5-acetyl-1-(6-hydroxy)-4,7-dimethoxybenzofuran (0.01 mol) in acetonitrile (30 mL) was treated with anhydrous K2CO3 (0.01 mol) and 1,2-dibromoethane (0.03 mol) or 1,3-dibromopropane (0.03 mol). The mixture was heated under reflux for 7–15 h, then filtered and concentrated. The residue was purified by column chromatography eluting with chloroform.
5-Acetyl-6-(2-bromoethoxy)-4,7-dimethoxybenzofuran (1a)
This compound was obtained in 84% yield as a colorless oil; 1H NMR (DCl3: δ 7.59 (d, 1H, C2-H, J = 2.1 Hz), 6.88 (d, 1H, C3-H, J = 2.1 Hz), 4.36 (t, 2H, C1′-H, J = 6.3 Hz), 4.09 (s, 3H, -OCH3), 3.99 (s, 3H, -OCH3), 3.58 (t, 2H, -C2′-H, J = 6.3 Hz), 2.55(s, 3H, -COCH3); 13C NMR: δ 31.5, 32.6, 60.6, 61.0, 73.8, 105.4, 115.8, 123.3, 133.3, 143.0, 143.6, 145.8, 147.9, 200.4; ESI-MS: m/z 343.17 [M+]. Anal. Calcd for C14H15BrO5: C, 49.00; H, 4.41. Found: C, 48.98; H, 4.39.
5-Acetyl-6-(3-bromopropoxy)-4,7-dimethoxybenzofuran (1b)
This compound was obtained in 80% yield as a colorless oil; 1H NMR: δ 7.57 (d, 1H, C2-H, J = 2.1 Hz), 6.87 (d, 1H, C3-H, J = 2.1 Hz), 4.22–4.14 (m, 2H, C3′-H), 4.09 (s, 3H, -OCH3), 3.99 (s, 3H, -OCH3), 3.62–3.58 (m, 2H, C1′-H), 2.55 (s, 3H, -COCH3), 2.29–2.21 (m, 2H, C2′-H); 13C NMR: δ 30.1, 30.5, 33.3, 33.8, 61.6, 73.0, 105.5, 117.0, 124.7, 134.8, 144.5, 144.6, 145.1, 149.1, 202.4; ESI-MS: m/z 380.9 [M+]. Anal. Calcd for C15H17BrO5: C, 50.44; H, 4.80. Found: C, 50.30; H, 4.59.
Synthesis of 4-arylpiperazino derivatives 2a–p
A mixture of N-bromoalkyl derivative 1a or 1b (0.01 mol), a powdered anhydrous K2CO3 (0.01 mol), a catalytic amount of KI in acetone (30 mL) and a substituted piperazine (0.01 mol) was heated under reflux for 10–20 h, then filtered and concentrated. The residue was purified by column chromatography eluting with chloroform or chloroform/methanol, 50:0.2. All products were converted into their hydrochlorides and the salts were crystallized from methanol/ether.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-(4-phenylpiperazino)ethoxy]benzofuran (2a)
The hydrochloride salt was obtained in 60% yield as a colorless oil; 1H NMR: δ 10.92 (s, 1H, NH+), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.30–7.24 (m, 3H, CH, C3-H), 7.04–7.01 (m, 2H, CH), 6.87 (t, 1H, CH, J = 7.2 Hz), 4.40–4.37 (m, 2H, -CH2-piperazine), 4.06 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.90–3.86 (m, 2H, C1′-H), 3.68–3.65 (m, 2H, -CH2-piperazine), 3.58–3.50 (m, 5H, -COCH3, -CH2-piperazine), 3.30–3.13 (m, 4H, -CH2-piperazine); 13C NMR: δ 32.7, 45.3, 51.0, 54.9, 60.6, 61.1, 68.8, 105.5, 115.9, 116.2, 119.9, 123.0, 129.1, 133.4, 142.6, 143.8, 146.2, 147.7, 149.5, 201.0; ESI-MS: m/z 425.2 [M+H+]. Anal. Calcd for C24H28N2O5‧HCl: C, 62.54; H, 6.34; N, 6.08. Found: C, 62.34; H, 6.14; N, 6.01.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-[4-(2-methoxyphenyl)piperazino]ethoxy]benzofuran (2b)
The hydrochloride salt was obtained in 70% yield as a white powder; mp 174–176°C; 1H NMR: δ 10.89 (s, 1H, NH+), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.26 (d, 1H, C-H, C3-H, J = 2.4 Hz), 7.06–6.88 (m, 4H, C-H), 4.39 (t, 2H, -CH2-piperazine, J= 4.8 Hz), 4.07 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.80 (s, 3H, -OCH3), 3.65–3.62 (m, 2H, C1′-H), 3.56–3.53 (m, 4H, -CH2-piperazine, C2′-H), 3.39–3.28 (m, 2H, -CH2-piperazine), 3.14–3.07 (m, 2H, -CH2-piperazine), 2.50 (s, 3H, -COCH3); 13C NMR: δ 32.7, 46.8, 51.4, 54.9, 55.3, 60.6, 61.1, 68.6, 105.5, 111.9, 116.1, 118.3, 120.8, 123.0, 123.5, 133.3, 139.2, 142.5, 143.8, 146.2, 147.7, 151.8, 201.0; ESI-MS: m/z 455.2 [M+H+]. Anal. Calcd for C25H30N2O6‧HCl: C, 61.16; H, 6.36; N, 5.71. Found: C, 61.11; H, 6.12; N, 5.80.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-[4-(2-chlorophenyl)piperazino]ethoxy]benzofuran (2c)
The hydrochloride salt was obtained in 72% yield as a white powder; mp 154–155°C; 1H NMR: δ 11.04 (s, 1H, NH+), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.48–7.45 (m, 1H, C-H), 7.37–7.32 (m, 1H, C-H), 7.26–7.22 (m, 2H, C-H, C3-H), 7.15–7.09 (m, 1H, C-H), 4.40–4.38 (m, 2H, -CH2-piperazine), 4.07 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.70–3.46 (m, 6H, -CH2-piperazine, C1′-H), 3.30–3.19 (m, 4H, -CH2-piperazine, C2′-H), 2.50 (s, 3H, -COCH3); 13C NMR: δ 32.6, 47.6, 51.5, 54.9, 60.6, 61.1, 68.6, 105.5, 116.2, 121.0, 123.0, 124.8, 127.5, 128.2, 130.4, 133.3, 142.5, 143.8, 146.2, 147.4, 147.7, 200.9; ESI-MS: m/z 459.1 [M+H+]. Anal. Calcd for C24H27ClN2O5‧HCl: C, 58.19; H, 5.70; N, 5.65. Found: C, 58.02; H, 5.80; N, 5.78.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-(4-(pyridin-2-yl)piperazino)ethoxy]benzofuran (2d)
The hydrochloride salt was obtained in 86% yield as a white powder, mp 156–157°C; 1H NMR: δ 10.88 (s, 1H, NH+), 8.18–8.15 (m, 1H, C-H), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.74–7.70 (m, 1H, C-H), 7.26 (d, 1H, C-H, C3-H, J = 2.4 Hz), 7.09–7.06 (m, 1H, C-H), 6.84–6.80 (m, 1H, C-H), 4.49–4.44 (m, 2H, -CH2-piperazine), 4.38–4.35 (m, 2H, -CH2-piperazine), 4.06 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.70–3.64 (m, 2H, C1′-H), 3.58–3.50 (m, 2H, -CH2-piperazine,) 3.41–3.34 (m, 2H, -CH2-piperazine), 3.23–3.16 (m, 2H, C2′-H), 2.50 (s, 3H, -COCH3); 13C NMR: δ 32.6, 42.3, 47.6, 50.5, 54.9, 60.6, 61.1, 68.6, 105.5, 109.6, 114.0, 116.2, 122.9, 130.4, 133.3, 140.3, 142.4, 143.8, 146.2, 147.7, 200.9; ESI-MS: m/z 426.2 [M+H+]. Anal. Calcd for C23H27N3O4‧HCl: C, 59.80; H, 6.11; N, 9.10. Found: C, 59.67; H, 6.01; N, 9.12.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-(4-(pyrimidin-2-yl)piperazino)ethoxy]benzofuran (2e)
The hydrochloride salt was obtained in 76% yield as a white powder; mp 189–190°C; 1H NMR: δ 10.49 (s, 1H, NH+), 8.46 (d, 2H, C-H, J = 4.8 Hz), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.26 (d, 1H, C-H, C3-H, J = 2.4 Hz), 6.79–6.76 (m, 1H, C-H), 4.77–4.73 (m, 2H, -CH2-piperazine), 4.38–4.30 (m, 2H, -CH2-piperazine), 4.05 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.68–3.64 (m, 2H, C1′-H), 3.56–3.48 (m, 2H, -CH2-piperazine,) 3.40–3.36 (m, 2H, -CH2-piperazine), 3.31 (s, 3H, -COCH3), 3.20–3.16 (m, 2H, C2′-H); 13C NMR: δ 32.6, 47.6, 50.7, 55.0, 60.6, 61.0, 68.4, 105.5, 111.3, 116.1, 122.9, 133.3, 142.4, 143.8, 146.2, 147.7, 158.1, 160.7, 200.9; ESI-MS: m/z 427.2 [M+H+]. Anal. Calcd for C22H26N4O4‧HCl: C, 57.08; H, 5.88; N, 12.10. Found: C, 57.17; H, 6.00; N, 12.01.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-[4-(3-methoxyphenyl)piperazino]ethoxy]benzofuran (2f)
The hydrochloride salt was obtained in 76% yield as an oil; 1H NMR: δ 10.94 (s, 1H, NH+), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.25 (d, 1H, C3-H, J = 2.4 Hz), 7.16 (t, 1H, C-H, J = 8.2 Hz), 6.62–6.54 (m, 2H, C-H), 6.47–6.43 (m, 1H, C-H), 4.80–4.70 (m, 2H, -CH2-piperazine), 4.38 (t, 2H, -CH2-piperazine, J = 4.8 Hz), 4.06 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.90–3.86 (m, 2H, -CH2-piperazine), 3.73 (s, 3H, -OCH3), 3.66–3.63 (m, 2H, C1′-H), 3.54–3.53 (m, 2H, -CH2-piperazine), 3.28–3.21 (m, 2H, C2′-H), 2.50(s, 3H, -COCH3); 13C NMR: δ 32.6, 45.2, 50.9, 54.9, 54.9, 60.6, 61.1, 68.6, 102.1, 105.2, 105.5, 108.3, 116.2, 119.1, 123.0, 129.8, 133.4, 142.4, 143.9, 146.2, 147.7, 150.8, 160.2, 200.9; ESI-MS: m/z 455.3 [M+H+]. Anal. Calcd for C25H30N2O6‧HCl: C, 61.16; H, 6.36; N, 5.71. Found: C, 61.20; H, 6.30; N, 5.68.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-[4-(3-chlorophenyl)piperazino]ethoxy]benzofuran (2g)
The hydrochloride salt was obtained in 76% yield as a white powder; mp 140–142°C; 1H NMR: δ 10.35 (s, 1H, NH+), 8.08 (d, 1H, C2-H, J = 2.1 Hz), 7.30–7.24 (m, 2H, C-H), 7.11–7.08 (m, 1H, C3-H), 7.01–6.98 (m, 1H, C-H), 6.89–6.87 (m, 1H, C-H), 4.36–4.30 (m, 2H, -CH2-piperazine), 4.06 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.69–3.65 (m, 2H, -CH2-piperazine) 3.58–3.54 (m, 2H, C1′-H), 3.43–3.12 (m, 5H, -COCH3, -CH2-piperazine), 2.50–2.48 (m, 4H, -CH2-piperazine, C2′-H); 13C NMR: δ 32.6, 44.7, 50.7, 54.8, 60.6, 61.1, 68.6, 105.5, 114.1, 115.2, 116.2, 119.1, 123.0, 130.6, 133.4, 133.9, 142.4, 143.8, 146.2, 147.7, 150.7, 200.9; ESI-MS: m/z 459.2 [M+H+]. Anal. Calcd for C24H27ClN2O5‧HCl: C, 58.19; H, 5.70; N, 5.65. Found: C, 58.01; H, 5.71; N, 5.69.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[2-[4-(4-nitrophenyl)piperazino]ethoxy]benzofuran (2h)
The hydrochloride salt was obtained in 54% yield as a white powder; mp 206–208°C; 1H NMR: δ 11.14 (s, 1H, NH+), 8.14–8.10 (m, 2H, C-H), 8.08 (d, 1H, C2-H, J = 2.4 Hz), 7.26 (d, 1H, C3-H, J = 2.4 Hz), 7.17–7.14 (m, 2H, C-H), 4.39–4.36 (m, 2H, -CH2-piperazine), 4.28–4.24 (m, 2H, -CH2-piperazine), 4.06 (s, 3H, -OCH3), 4.00 (s, 3H, -OCH3), 3.70–3.66 (m, 2H, -CH2-piperazine) 3.62–3.50 (m, 2H, C1′-H), 3.47–3.40 (m, 4H, -CH2-piperazine, C2′-H), 3.28 (s, 3H, -COCH3); 13C NMR: δ 32.6, 43.5, 50.5, 54.8, 60.6, 61.1, 68.6, 105.5, 113.5, 116.2, 123.0, 125.6, 133.4, 138.0, 142.4, 143.9, 146.2, 147.7, 153.7, 200.9; ESI-MS: m/z 492.2 [M+Na+]. Anal. Calcd for C24H27N3O7‧HCl: C, 56.97; H, 5.58; N, 8.31. Found: C, 56.91; H, 5.70; N, 8.21.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-(4-phenylpiperazino)propoxy]benzofuran (2i)
The hydrochloride salt was obtained in 60% yield as a white powder; mp 180–182°C (lit. mp 175°C [16]); 1H NMR: δ 10.84 (s, 1H, NH+), 8.04 (d, 1H, C2-H, J = 2.1 Hz), 7.29–7.22 (m, 1H, C3-H), 7.02–7.00 (m, 2H, C-H), 6.89–6.84 (m, 1H, C-H), 4.06 (t, 2H, C3′-H, J = 8.1 Hz), 4.01 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.84–3.81 (m, 2H, -CH2-piperazine), 3.61–3.58 (m, 2H, C1′-H), 3.30–3.25 (m, 2H, -CH2-piperazine), 3.19–3.09 (m, 4H, -CH2-piperazine), 2.46 (s, 3H, -COCH3), 2.19–2.14 (m, 2H, C2′-H); 13C NMR: δ 24.0, 32.6, 45.4, 50.6, 52.9, 60.5, 60.9, 71.8, 105.4, 115.7, 115.9, 120.0, 123.2, 129.1, 133.4, 143.3, 143.6, 145.9, 147.9, 149.5, 200.8; ESI-MS: m/z 439.1 [M+H+]. Anal. Calcd for C25H30N2O5‧HCl: C, 63.22; H, 6.58; N, 5.90. Found: C, 63.11; H, 6.56; N, 5.89.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-[4-(2-methoxyphenyl)piperazino]propoxy]benzofuran (2j)
The hydrochloride salt was obtained in 80% yield as a white powder; mp 181–184°C (lit.: an oil for the free base [17]); 1H NMR: δ 10.81 (s, 1H, NH+), 8.04 (d, 1H, C2-H, J = 2.4 Hz), 7.22 (d, 1H, C3-H, J = 2.4 Hz), 7.06–6.89 (m, 4H, C-H), 4.06 (t, 2H, C3′-H, J = 6 Hz), 4.01 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.80 (s, 3H, -OCH3), 3.60–3.49 (m, 4H, -CH2-piperazine), 3.30–3.23 (m, 2H, C1′-H), 3.19–3.03 (m, 4H, -CH2-piperazine), 2.47 (s, 3H, -COCH3), 2.21–2.11 (m, 2H, C2′-H); 13C NMR: δ 24.1, 32.6, 46.8, 51.1, 53.0, 55.3, 60.5, 60.9, 71.8, 105.5, 111.9, 115.7, 118.2, 120.8, 123.2, 123.5, 133.4, 139.3, 143.3, 143.6, 145.9, 147.9, 151.8, 200.9; ESI-MS: m/z 469.2 [M+H+]. Anal. Calcd for C26H32N2O6‧HCl: C, 61.84; H, 6.59; N, 5.55. Found: C, 61.80; H, 6.57; N, 5.61.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-[4-(2-chlorophenyl)piperazino]propoxy]-1-benzofuran (2k)
The hydrochloride salt was obtained in 70% yield as a white powder; mp 206–207°C; 1H NMR: δ 10.44 (s, 1H, NH+), 8.04 (d, 1H, C2-H, J = 2.1 Hz), 7.48–7.44 (m, 1H, C-H), 7.38–7.32 (m, 1H, C-H), 7.23–7.21 (m, 2H, C3-H), 7.14–7.09 (m, 1H, C-H), 4.07 (t, 2H, C3′-H, J = 6 Hz), 4.01 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.65–3.61 (m, 2H, -CH2-piperazine), 3.47–3.43 (m, 2H, -CH2-piperazine), 3.34–3.30 (m, 2H, C1′-H), 3.24–3.09 (m, 4H, -CH2-piperazine), 2.47 (s, 3H, -COCH3), 2.17–2.12 (m, 2H, C2′-H); 13C NMR: δ 24.1, 32.6, 47.6, 51.2, 53.0, 60.5, 60.9, 71.8, 105.4, 115.7, 120.9, 123.2, 124.7, 127.5, 128.2, 130.4, 133.4, 143.3, 143.6, 145.9, 147.3, 147.9, 200.8; ESI-MS: m/z 473.2 [M+H+]. Anal. Calcd for C25H29ClN2O5‧HCl: C, 58.94; H, 5.94; N, 5.50. Found: C, 58.80; H, 5.91; N, 5.59.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-(4-(pyridin-2-yl)piperazino)propoxy]benzofuran (2l)
The hydrochloride salt was obtained in 75% yield as a white powder; mp 188–189°C; 1H NMR: δ 10.67 (s, 1H, NH+), 8.17–8.15 (m, 1H, C-H), 8.03 (d, 1H, C2-H, J = 2.4 Hz), 7.76–7.71 (m, 1H, C-H), 7.22 (d, 1H, C3-H, J = 2.4 Hz), 7.10–7.07 (m, 1H, C-H), 6.85–6.81 (m, 1H, C-H), 4.50–4.40 (m, 2H, -CH2-piperazine), 4.08–4.06 (m, 2H, C3′-H), 4.00 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.64–3.60 (m, 2H, -CH2-piperazine), 3.34–3.24 (m, 4H, -CH2-piperazine, C1′-H), 3.16–3.11 (m, 2H, -CH2-piperazine), 2.46 (s, 3H, -COCH3), 2.17–2.12 (m, 2H, C2′-H); 13C NMR: δ 24.0, 32.6, 42.7, 50.0, 53.0, 54.9, 60.5, 60.9, 71.8, 105.4, 110.6, 113.9, 115.7, 123.2, 129.8, 133.4, 141.4, 143.3, 143.6, 145.9, 147.9, 200.8; ESI-MS: m/z 440.2 [M+H+]. Anal. Calcd for C24H29N3O5‧HCl: C, 60.56; H, 6.35; N, 8.83. Found: C, 60.51; H, 6.50; N, 8.81.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-(4-(pyrimidin-2-yl)piperazino)propoxy]benzofuran (2m)
The hydrochloride salt was obtained in 70% yield as a white powder; mp 172–173°C; 1H NMR: δ 11.09 (s, 1H, NH+), 8.45 (d, 2H, Harom., J = 4.8 Hz), 8.04 (d, 1H, C2-H, J = 2.4 Hz), 7.23 (d, 1H, C3-H, J = 2.4 Hz), 6.78–6.75 (m, 1H, C-H), 4.73–4.69 (m, 2H, -CH2-piperazine), 4.07–4.04 (m, 2H, C3′-H), 4.00 (s, 3H, -OCH3), 3.97 (s, 3H, -OCH3), 3.60–3.57 (m, 2H, -CH2-piperazine), 3.42–3.38 (m, 2H, C1′-H), 3.25–3.21 (m, 2H, -CH2-piperazine), 3.09–3.05 (m, 2H, -CH2-piperazine), 2.45 (s, 3H, -COCH3), 2.19–2.15 (m, 2H, C2′-H); 13C NMR: δ 24.1, 32.6, 47.6, 50.4, 53.1, 60.5, 60.9, 71.7, 105.4, 111.3, 115.7, 123.2, 133.4, 143.3, 143.6, 145.9, 147.9, 158.1, 160.6, 200.8; ESI-MS: m/z 441.3 [M+H+]. Anal. Calcd for C23H28N4O5‧HCl: C, 57.92; H, 6.13; N, 11.75. Found: C, 57.89; H, 6.16; N, 11.78.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-[4-(3-methoxyphenyl)piperazino]propoxy]benzofuran (2n)
The hydrochloride salt was obtained in 70% yield as a white powder; mp 172–174°C; 1H NMR: δ 10.42 (s, 1H, NH+), 8.04 (d, 1H, C2-H, J = 2.1 Hz), 7.23 (d, 1H, C3-H, J = 2.1 Hz), 7.16 (t, 1H, Harom., J = 8.1 Hz), 6.60–6.57 (m, 2H, C-H), 6.47–6.43 (m, 1H, C-H), 4.06–4.08 (m, 2H, C3′-H), 4.00 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.86–3.82 (m, 2H, -CH2-piperazine), 3.73 (s, 3H, -OCH3), 3.60–3.57 (m, 2H, -CH2-piperazine), 3.18–3.04 (m, 6H, C1′-H, -CH2-piperazine), 2.46 (s, 3H, -COCH3), 2.20–2.10 (m, 2H, C2′-H); 13C NMR: δ 24.0, 32.6, 45.3, 50.6, 52.9, 54.9, 60.5, 60.9, 71.8, 102.1, 105.2, 105.4, 108.3, 115.7, 123.2, 129.8, 133.4, 143.3, 143.6, 145.9, 147.9, 150.9, 160.2, 200.8; ESI-MS: m/z 469.2 [M+H+]. Anal. Calcd for C26H32N2O6‧HCl: C, 61.84; H, 6.59; N, 5.55. Found: C, 61.80; H, 6.57; N, 5.59.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-[4-(3-chlorophenyl)piperazino]propoxy]-1-benzofuran (2o)
The hydrochloride salt was obtained in 70% yield as a white powder; mp 189–192°C; 1H NMR: δ 10.52 (s, 1H, NH+), 8.04 (d, 1H, C2-H, J = 2.1 Hz), 7.28–7.26 (m, 1H, C-H), 7.24–7.22 (m, 1H, C3-H), 7.08–7.05 (m, 1H, C-H), 6.99–6.95 (m, 1H, C-H), 6.89–6.86 (m, 1H, C-H), 4.07–4.05 (m, 2H, C3′-H), 4.00 (s, 3H, -OCH3), 3.98 (s, 3H, -OCH3), 3.92–3.89 (m, 2H, -CH2-piperazine), 3.62–3.56 (m, 2H, C1′-H), 3.30–3.25 (m, 2H, -CH2-piperazine), 3.16–3.13 (m, 4H, -CH2-piperazine), 2.46 (s, 3H, -COCH3), 2.20–2.10 (m, 2H, C2′-H); 13C NMR: δ 24.0, 32.6, 44.8, 50.4, 52.9, 60.5, 60.9, 71.8, 105.4, 114.1, 115.2, 115.7, 119.1, 123.2, 130.6, 133.4, 133.9, 143.3, 143.6, 145.9, 147.9, 150.8, 200.8; ESI-MS: m/z 473.2 [M+H+]. Anal. Calcd for C25H29ClN2O5‧HCl: C, 58.94; H, 5.94; N, 5.50. Found: C, 58.96; H, 5.95; N, 5.49.
Hydrochloride of 5-acetyl-4,7-dimethoxy-6-[3-[4-(4-nitrophenyl)piperazino]propan-1-oxy]benzofuran (2p)
The hydrochloride salt was obtained in 80% yield as a white powder; mp 177–179°C; 1H NMR: δ 10.86 (s, 1H, NH+), 8.11–8.09 (m, 2H, C-H), 8.03 (d, 1H, C2-H, J = 2.1 Hz), 7.22 (d, 1H, C3-H, J = 2.1 Hz), 7.16–7.10 (m, 2H, C-H), 4.08–4.04 (m, 2H, C3′-H), 4.00 (s, 3H, -OCH3), 3.97 (s, 3H, -OCH3), 3.70–3.60 (m, 2H, -CH2-piperazine), 3.50–3.40 (m, 2H, C1′-H), 3.34–3.28 (m, 4H, -CH2-piperazine), 3.18–3.16 (m, 2H, -CH2-piperazine), 2.45 (s, 3H, -COCH3), 2.22–2.00 (m, 2H, C2′-H); 13C NMR: δ 24.1, 32.6, 43.6, 50.3, 53.0, 60.5, 60.9, 71.8, 105.4, 113.4, 115.7, 123.2, 125.6, 133.4, 137.9, 143.4, 143.6, 145.9, 147.9, 153.8, 200.8; ESI-MS: m/z 484.3 [M+H+]. Anal. Calcd for C25H29N3O7‧HCl: C, 57.75; H, 5.82; N, 8.08. Found: C, 57.81; H, 5.83; N, 8.06.
Microbiology
Organisms
Standard strains of S. aureus ATCC 25923, E. coli ATCC 25922, C. albicans ATCC 14053, P. acnes ATCC 6919, B. thetaiotaomicron ATCC 29741, B. fragilis ATCC 25285 and one clinical isolate S. maltophilia CO 2275 were used.
Screening for antimicrobial activity
Compounds were tested for bacteriostatic activity at high concentrations (512 mg/L) and if the bacteriostatic effect was observed, the concentration was reduced. A method according to CLSI (Clinical and Laboratory Standards Institute) [18] directives was applied. The tested substances were dissolved in DMSO and then the solutions were added to brain heart infusion broth (BHI-B) medium to a final concentration of 512 mg/L. The aerobic bacteria were cultured on plates with BHI agar (BHI-A) medium supplemented with 7% horse blood, at temperature 35–37°C, in an aerobic atmosphere, for 18–24 h. Anaerobes were cultured in Schaedler agar with 5% of sheep blood at 35–37°C for 48 h, in anaerobic atmosphere. The fungal strain was cultured in the Sabouraud agar (SA), at the same temperature and atmosphere, but for at least 24 h. The cultures which were in mid-logarithmic phase of growth were suspended in 0.9% NaCl to obtain 0.5 MacFarland’s optical density and in the case of anaerobes 1.0 in the same scale. Cells (1.0–9.0 × 105, 0.1 mL of the prepared suspension) were added to sample tubes with 2 mL of BHI-B medium containing the tested substances. For anaerobes, all media were pre-reduced. Samples were incubated at temperature 35–37°C for 24–48 h and in the case of anaerobes for 48–60 h. If after 48 h (60 h for anaerobes), growth was absent, the substance was noted as potentially possessing antimicrobial activity. In all experiments, strain vitality controls and a DMSO antimicrobial activity control were performed at the used concentrations.
Anticancer activity
The HeLa (human cervix carcinoma) and K562 (leukemia) cells were cultured in RPMI 1640 medium supplemented with antibiotics and 10% fetal calf serum, in a 5% CO2–95% air atmosphere. The cells (7 × 103) were seeded on each well on a 96-well plate (Nunc). After 48 h cells were exposed to the test compounds. Stock solutions (100 mm) of test compounds were freshly prepared in DMSO. The final concentrations of the compounds tested in the cell cultures were: 1 mm, 1 × 10–2 mm, 1 × 10–4 mm and 1 × 10–6 mm. The concentration of DMSO in the cell culture medium was 1%.
The values of IC50 (the concentrations of test compound required to reduce the cell survival fraction to 50% of the control) were calculated from dose-response curves and used as a measure of cellular sensitivity to a given treatment.
The cytotoxicity of all compounds was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; Sigma, St. Louis, MO, USA] assay as previously described [19]. Briefly, after 24 h or 48 h of incubation with drugs, the cells were treated with the MTT reagent and incubation was continued for 2 h. The MTT-formazan crystals were dissolved in 20% SDS and 50% DMF at pH 4.7 and absorbance was read at 570 and 650 nm on an ELISA-PLATE READER (FLUOstar Omega). Cells grown in the presence of vehicle (1% DMSO) only were used as a control (100% viability).
The cytotoxicity studies were performed in the Screening Laboratory, Department of Bioorganic Chemistry, Centre of Molecular and Macromolecular Studies of the Polish Academy of Sciences and financially supported by the Ministry of Science and Higher Education, project PBZ-MNiSW-07/I/2007 (2008–2010).
References
[1] Cui, B.; Chai, H.; Santisuk, T.; Reutrakul, V.; Farnsworth, N.; Cordell, G. A.; Pezzuto, J. A.; Kinghorn, A. D. Novel cytotoxic 1H-cyclopenta[b]-benzofuran lingnans from Aglaia elliptica. Tetrahedron 1997, 53, 17625–17632.Suche in Google Scholar
[2] Dumontet, V.; Thoison, O.; Omobuwajo, O. R.; Martin, M.-T.; Perromat, G.; Chiaroni, A.; Riche, C.; Pais, M.; Sevenet, T. New nitrogenous and aromatic derivatives from Aglaia argentea and A. forbesii. Tetrahedron 1996, 52, 6931–6942.Suche in Google Scholar
[3] Erber, S.; Ringshandl, R.; Vonangerer, E. 2-Phenylbenzo[b]furans: relationship between structure, estrogen receptor affinity and cytostatic activity against mammary tumor cells. Anticancer Drug Des. 1991, 5, 417–426.Suche in Google Scholar
[4] Gerard, B.; Jones, G., II; Porco, J. A. A biomimetic approach to the rocaglamides employing photogeneration of oxidopyryliums derived from 3-hydroxyflavones. J. Am. Chem. Soc. 2004, 126, 13620–13621.Suche in Google Scholar
[5] Hattori, M. T.; Hada, S.; Watahiki, A. Studies on dental caries prevention by traditional medicines. X. Antibacterial action of phenolic components from mace against Streptococcus mutans. Chem. Pharm. Bull. 1986, 34, 3885–3893.Suche in Google Scholar
[6] Hayakawa, I.; Shiota, R.; Agatsuma, T.; Furukawa, H.; Naruto, S.; Sugano, Y. 4-Hydroxy-3-methyl-6-phenylbenzofuran-2-carboxylic acid ethyl ester derivatives as potent anti-tumor agents. Bioorg. Med. Chem. Lett. 2004, 14, 455.Suche in Google Scholar
[7] Cencic, R.; Carrier, M.; Galicia-Vazquez, G.; Bordeleau, M. E.; Sukarieh, R.; Bourdeau, A.; Brem, B.; Teodoro, J. G.; Greger, H.; Tremblay, M. L.; et al. Antitumor activity and mechanism of action of the cyclopenta[b]benzofuran, Silvestrol. PLoS One 2009, 4, e5223.10.1371/journal.pone.0005223Suche in Google Scholar PubMed PubMed Central
[8] Ragab, F. A. F.; El-Sayed, N. A. M.; Eissa, A. A. H. M.; El Kedawry, A. M. Synthesis and anticonvulsant activity of certain substituted furochromone, benzofuran and flavone derivatives. Chem. Pharm. Bull. 2010, 58, 1148–1156.Suche in Google Scholar
[9] Harish Kumar, D. R.; Karvekar, M. D. Synthesis of benzofuran derivatives and their evaluation of antimicrobial activity. E J. Chem. 2010, 7, 636–640.Suche in Google Scholar
[10] Bohnenstengel, F. I.; Steube, K. G.; Meyer, C.; Quentmeier, H.; Nugroho, B. W.; Proksch, P. 1H-Cyclopenta[b]benzofuran lignans from Aglaia species inhibit cell proliferation and alter cell distribution in human monocytic leukemia cell lines. Z. Naturforsch. C 1999, 54, 1075–1085.Suche in Google Scholar
[11] Ismail, E.; Tawfik, A. A.; Elebrashi, N. M. A. Antibacterial and anthelmintic properties of visnaginone and khellinone derivatives. Arzneimittelforschung 1977, 27, 1393–1394.Suche in Google Scholar
[12] Kossakowski, J.; Ostrowska, K.; Hejchman, E.; Wolska, I. Synthesis and structural characterization of derivatives of 2- and 3-benzo[b]furan carboxylic acids with potential cytotoxic activity. Il Farmaco 2005, 60, 519–527.Suche in Google Scholar
[13] Kossakowski, J.; Ostrowska, K. Synthesis of new derivatives of 2,3-dihydro-7-benzo[b]furanol with potential pharmacological activity. Acta Pol. Pharm. 2006, 63, 271–275.Suche in Google Scholar
[14] Kossakowski, J.; Ostrowska, K.; Struga M.; Stefańska, J. Synthesis of new derivatives of 2,2-dimethyl-2,3-dihydro-7-benzo[b]furanol with potential antimicrobial activity. Med. Chem. Res. 2009, 18, 555–565.Suche in Google Scholar
[15] Kossakowski, J.; Krawiecka, M.; Kuran, B.; Stefańska, J.; Wolska, I. Synthesis and preliminary evaluation of the antimicrobial activity of selected 3-benzofurancarboxylic acid derivatives. Molecules 2010, 15, 4737–4749.Suche in Google Scholar
[16] Bourgery, G. P.; Lacour, A. 6-N-Arylpiperazinoalkoxy-4,7-dimetoxybenzofuran derivatives and their therapeutic use. Demande 1981, FR 2447378, A1 19800822.Suche in Google Scholar
[17] Schlecker, R.; Raschack, M.; Gries, J. Preparation of benzofurans as antihypertensives and cardiovascular agents. Eur. Pat. Appl. 1989, EP 303920 A1 19890222.Suche in Google Scholar
[18] Clinical and Laboratory Standards Institute. Antimicrobial susceptibility testing (M100-S16). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard, seventh edition (M7-A7). Performance standards for antimicrobial disc susceptibility test approved standard, ninth edition (M2-A9). Clinical and Laboratory Standards Institute, Wayne, PA, 2006.Suche in Google Scholar
[19] Maszewska, M.; Leclaire, J.; Cieslak, M.; Nawrot, B.; Okruszek, A.; Caminade, A.-M.; Majoral, J.-P. Water-soluble polycationic dendrimers with a phosphoramidothioate backbone: preliminary studies of cytotoxicity and oligonucleotide/plasmid delivery in human cell culture. Oligonucleotides 2003, 13, 193–205.Suche in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Artikel in diesem Heft
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety
Artikel in diesem Heft
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety

