Abstract
In this review, we discuss the synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes, containing pyrane ring of coumarin as part of the photosensitive hexatriene system or bearing a coumarin unit as a substituent outside of hexatriene. Coumarinylethenes with noncyclic double bond as well as coumarinyl(thienyl)thiazoles, coumarinyl(thienyl)maleic anhydride (or maleimide) derivatives, and 3,4-bis(thiazolyl)coumarins possess both photochromism and fluorescence switching properties upon UV/vis irradiation.
Introduction
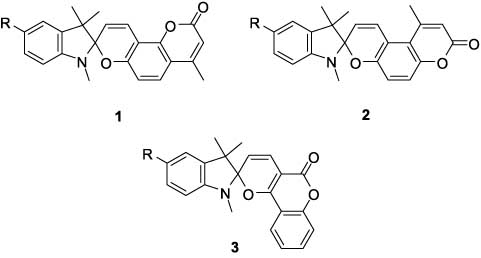
We have previously reviewed the photochromism of indoline spiropyrans – coumarin derivatives of general structures 1–3 [1].
Photochromism of these compounds is based on reversible transformations of cyclic (spiro form A) and open (merocyanine form B) structures, as shown below [2–9]. The photoinduced pyran ring opening of spiro form A is followed by the thermal cis-trans isomerization of the open structure with formation of a colored merocyanine form and the subsequent thermal or photochemical recyclization of the open form B to the starting colorless spiro form A.
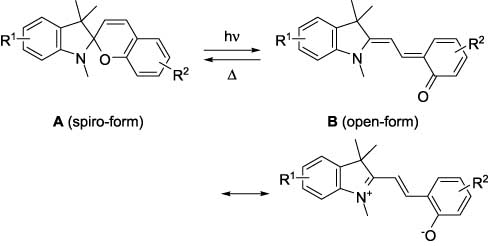
It has been found that inclusion of a coumarin fragment into the spiropyran moiety provides a dramatic effect on the stability of the open form and, therefore, on the photochromic transformations of the related spiro forms as a whole. Moreover, coumarin-condensed spiropyranes (e.g., 1) significantly change their fluorescence when transforming from the spiro form to the open form [10, 11].
In this review, we discuss the photochromism of aryl(heteroaryl)- and diheteroarylethenes containing a coumarin unit, shown schematically as C and D.

In contrast to spiropyranes (which show thermally reversible photochromism), diheteroarylethenes behave as thermally irreversible photochromes. That is why diheteroarylethenes are considered as perspective compounds for their use in optoelectronics, in particular, for the development of light-sensitive recording media with the ultra-high informational capacity for three-dimensional optical memory [12–14]. The introduction of a bridged double bond into a cycle fixes its cis-configuration, which increases the quantum yield of the photocyclization reaction. Therefore, diheteroarylethenes containing fragments of cyclopentene and perfluorocyclopentene as the ethene bridge are studied the most [12]. In addition, diheteroarylethenes in which the heteroaryl fragments are bonded by a heteroarene ‘bridge’ [14], in particular, the thiazole one [15–18], are also known.
Among diheteroarylethenes, derivatives containing at least one thiophene fragment conjugated with the C=C double bond possess particularly valuable photochromic characteristics. In recent years, photochromic thienylethenes able to function as molecular photoswitches, which upon the action of light reversibly change not only color but also other properties, for example, fluorescence, are under intensive study [19]. Such compounds may be used, in particular, for the development of photochromic data recording media with non-destructive readout of recorded information [20–26].
1-Coumarinyl-2-(aryl/heteroaryl)ethenes with noncyclic double bond
1-(4-Coumarinyl)-2-arylethenes
There are several approaches to synthesis of 1-(4-coumarinyl)-2-arylethenes 5. One approach involves the reaction of 4-coumarinylacetic acids 4 with aromatic aldehydes in the presence of bases (Scheme 1) [27–29]. Syntheses have been carried out in pyridine with piperidine addition, similar to classical conditions of the Knoevenagel reaction. Mechanism of this reaction is likely to be similar to the Knoevenagel synthesis, because 4-coumarinylacetic acids may be considered as vinylogs of malonic acid. Other 4-styrylcoumarin derivatives [30–32], including related furan, thiophene, and pyridine derivatives [33–36], have been synthesized in a similar way with 30–65% yields.

In an analogous manner, 7-diethylamino-4-methylcoumarin 6 upon reaction with aromatic aldehydes affords 7-diethylamino-4-styrylcoumarins 7 with 9–51% yields (Scheme 2). However, stronger base (potassium tert-butoxide in dimethyl sulfoxide) should be used in this case [37].

Electron-withdrawing substituents (e.g., acetyl- [38] or cyano- [39–46]) at position 3 of coumarin increase the reactivity of the methyl group at position 4 and, as a result, a weaker base piperidine is sufficient for the condensation reaction to proceed. It is noteworthy that 3-acetyl-4-methylcoumarin 8 undergoes reaction at the 4-methyl group instead of acetyl function [38]. With use of excess aldehyde (2 eq.) both 4-methyl- and 3-acetyl- functions are involved in the reaction (Scheme 3), but yield of bis-condensation product 10 does not exceed 5%.

4-Styrylcoumarins may be prepared starting from 2-hydroxychalcones. 3-Phеnyl-4-styrylcoumarins have been obtained in low yields (20–40%) after prolonged (24–48 h) heating of such chalcones with sodium phenylacetate in acetic anhydride [29]. Later, the Wittig reaction involving 2-hydroxychalcones 11 and ethoxycarbonylmethylidene(triphenyl)phosphorane 12 has been reported to give 4-styrylcoumarins 13 in high yields (60–92%) upon reflux in toluene for 24 h (Scheme 4) [47–53]. No data have been published about photoinduced cyclizations of 1-(4-coumarinyl)-2-(aryl)ethenes.

1-(3-Coumarinyl)-2-(aryl/heteroaryl)ethenes
1-(3-Coumarinyl)-2-arylethenes have been prepared more recently. The synthesis of 16 (Scheme 5) starts with the preparation of 3-(2′-hydroxybenzylidene)-5-aryl-2-furanones 14, the rearrangement of 14 to 3-(benzoylmethyl)coumarins 15 followed by reduction and dehydration of the corresponding alcohols [54]. The Knoevenagel reaction of 3-coumarinylacetic acid 17 with aromatic aldehydes, in a way similar to the reaction of 4-coumarinylacetic acids, also gives 3-styrylcoumarins [54]. Alternatively, the Knoevenagel reaction of 7-(diethylamino)-3-formylcoumarin 18 (Scheme 5) with arylacetic acids affords 7-(diethylamino)-3-styrylcoumarins (Table 1, entries 10, 12) [55]. Syntheses are best conducted in tributylamine at 220–230°C or in pyridine with addition of piperidine (Table 1, entry 13). Synthesis of 1-(3-coumarinyl)-2-(2/4-pyridyl)ethenes has been performed by condensation of 7-(diethylamino)-3-formylcoumarin 18 with salts prepared in situ from benzoyl chloride and 2- or 4-methylpyridine (Table 1, entries 17, 18) [55]. Condensation of 18 with 2-methylquinoline in acetic anhydride has been reported (Scheme 6).
Reported 1-(3-coumarinyl)-2-arylethenes.
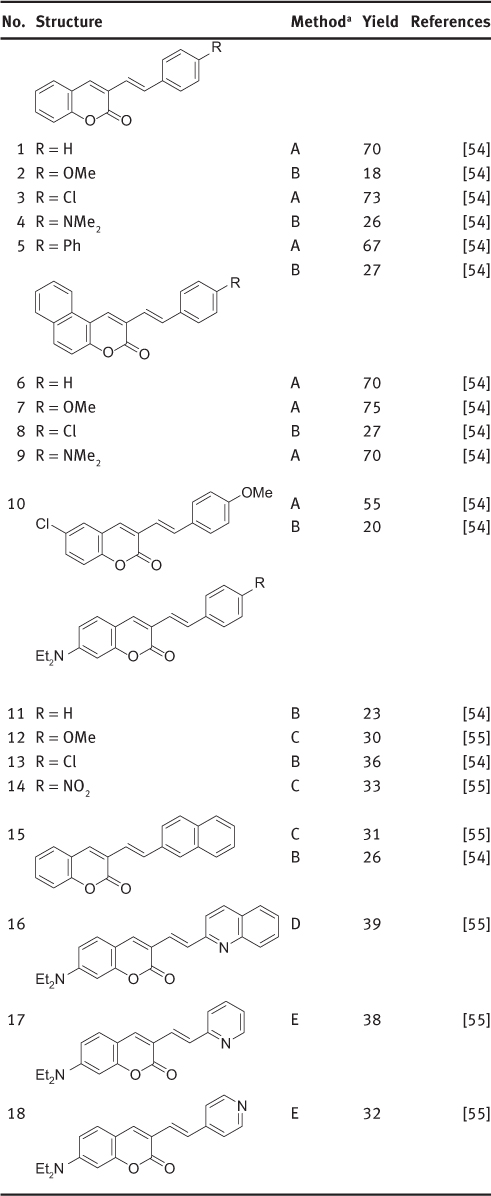
aМеthod А: condensation of 3-coumarinylacetic acid with aromatic aldehydes; method B: condensation of salicylaldehyde and 4-аryl-4-oxobutanoic acids; method C: condensation of 3-formylcoumarin with arylacetic acids; method D: condensation of 3-formylcoumarin with 2-methylquinoline; method E: condensation of 3-formylcoumarin with picolinium salts.


1-(3-Coumarinyl)-2-phenylethene has also been prepared by the Horner-Wadsworth-Emmons reaction from (3-coumarinylmethyl)phosphonate 19 and benzaldehyde (Scheme 7) [56].

Reaction of 3-coumarinylmethyl(triphenyl)phosphonium chloride 20 with aldehyde 21 has been used later for double bond formation at the first step of synthesis of coumarin analog 22 of retinal (Scheme 8) [57].

The Heck reaction was used for efficient synthesis of 3-styrylcoumarins (Scheme 9) [58, 59]. Pd-catalyzed coupling of styrene derivatives with 3-bromocoumarins 23 has been used in parallel synthesis of library of fluorescent 3-styrylcoumarins [58]. More recently, a similar reaction of 3-vinylcoumarins 24 with haloarenes has been reported [59].

1,2-Bis(3-coumarinyl)ethenes have been prepared from 1,4-dicyano-2-butene and salicylic aldehydes (Scheme 10) [60].

New 1-(3-coumarinyl)-2-heteroarylethenes have been synthesized starting from ketones 25 (Scheme 11) [61]. Butenolide 14 has been prepared by condensation of salicylic aldehyde with 4-phenyl-4-оxobutanoic acid (45% yield) followed by treatment with a mixture of acetic and hydrochloric acids under reflux. The formation of 15 has been suggested [54]; however, on the basis of fragmentation in the mass spectrum the structure 25а was established [61]. Compound 25a can easily be transformed to 3-styrylcoumarin 27а.

Then the synthetic route to ketone 25а and its heteroanalogs has been optimized using Friedel-Crafts acylation of arenes (and heteroarenes) with 3-coumarinylacetyl chloride. 3-Coumarinylacetic acid 28 has been prepared by reaction of salicylic aldehyde with succinic anhydride (55% yield) in the presence of triethylamine (instead of sodium succinate [62] Scheme 12).

Treatment of acid 28 with excess of thionyl chloride in CH2Cl2 at room temperature provides quantitative yield of acid chloride 29. Acylation of benzene with 29 in the presence of AlCl3 gives ketone 25а with 75% yield. It is important that more than 2 eq. of AlCl3 is used in acylation with 3-coumarinylacetyl chloride due to complex formation of Lewis acid with lactone function of 29 (Scheme 13).
This acylation procedure also works smoothly with thiophenes. Ketones 25b and 25c have been prepared under similar conditions in 71% and 63% yields, respectively. Reduction of 25 and dehydration of the corresponding alcohols has furnished 1-(3-coumarinyl)-2-heteroarylethenes 27 (Scheme 13).

Using a previously reported method for 3-styrylcoumarins [54], 1-(3-coumarinyl)-2-heteroarylethenes 27 have been prepared in one step by condensation of 3-coumarinylacetic acid 28 with heteroarene aldehydes (Scheme 14) [61].

This reaction is catalyzed by piperidine and activated by microwave irradiation. The use of microwave irradiation increases yield of the ethene product almost twofold and makes its isolation easier. All the 1-(3-coumarinyl)-2-heteroarylethenes 27a–g were isolated as (E)-isomers, showing two doublets in 1H NMR spectra at 6.84–7.14 and 7.54–7.88 ppm with trans-spin-spin coupling constants of 16–16.5 Hz.
Photochromism of 1-(3-coumarinyl)-2-heteroarylethenes
Spectral properties and photochromism of 1-(3-coumarinyl)-2-heteroarylethenes series in toluene have been studied (Table 2) [61]. All 1-(3-coumarinyl)-2-heteroarylethenes 27 show an absorption band in the UV region and a fluorescence band in the visible region. Their E-forms undergo photochemical isomerization under irradiation at 365 nm (Scheme 15). Changes in absorption and emission spectra of compound 27c due to its photoinduced transformations are shown in Figures 1 and 2. Appearance of photoinduced absorption, which is shifted 70–72 nm into the visible region, is specific for cyclic forms of dithienylethenes [63].
Spectral properties of 3-coumarinyl(hetaryl)ethenes.
 | ε, L/(mol×cm) | 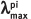 | 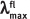 | |
|---|---|---|---|---|
| 27a | 362 | 28 000 | 433 | 440 |
| 27b | 378 | 22 200 | 450 | 452 |
| 27c | 371 | 11 500 | 442 | 458 |
| 27d | 378 | 13 000 | <300 | 442 |
| 27e | 385 | 23 700 | <300 | 450 |
| 27f | 380 | 23 400 | <300 | 453 |
| 27g | 390 | 22 200 | 323 | 465 |
Note: 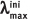 : the absorption maxima of initial form;
: the absorption maxima of initial form; 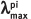 : the absorption maxima of photoinduced form;
: the absorption maxima of photoinduced form; 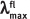 : maxima of fluorescence emission; ε: coefficient of molecular extinction.
: maxima of fluorescence emission; ε: coefficient of molecular extinction.
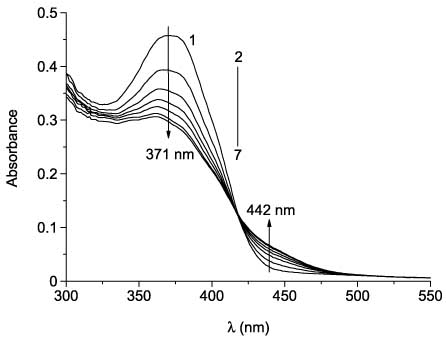
Absorption spectra of 27c in toluene before (1) and after UV irradiation (365 nm) for 5 min (2), 10 min (3), 15 min (4), 20 min (5), 30 min (6), and 40 min (7).

Fluorescence emission spectra of solution of 27c in toluene before (1) and after UV irradiation (365 nm) for 0.25 min (2) and 1.75 min (3).
Compounds 27a and 27b undergo similar transformations. These transformations are reversible. Changes of absorption spectra of compound 27a during its irradiation at 436 nm are shown in Figure 3.

Some of the compounds 27 do not show changes in the visible region of absorption spectra; however, they also undergo reversible photochromic transitions (Figure 3). Compounds 27b,c also show high thermal stability of photoinduced forms. Certain changes in emission spectra can also be seen.
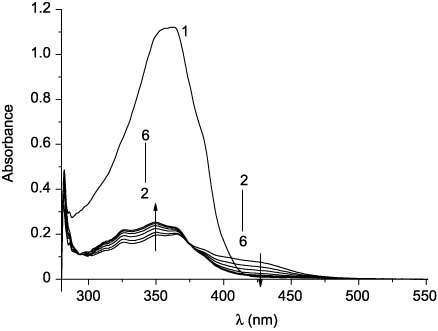
Absorption spectra of 27a in toluene before (1) and after UV irradiation (365 nm) for 210 s (2) and subsequent irradiation with visible light (436 nm) for 5 s (3), 15 s (4), 30 s (5), and 45 s (6).
By contrast, compounds 27d,e,g do not undergo photocyclization reactions. Nevertheless, they show reversible E-Z photoisomeric transformations. Appearance of photoinduced short-wave absorption bands (Figure 4) is specific for E-Z isomerization of stilbenes and their analogs under irradiation [63]. Compound 27f does not undergo E-Z isomerization upon irradiation.
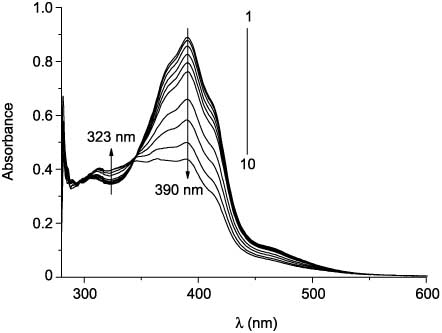
Absorption spectra of 27g in toluene before (1) and after UV irradiation (385 nm) for 5 min (2), 15 min (3), 30 min (4), 45 min (5), 60 min (6), 2 h (7), 3 h (8), 4 (9), and 5 h (10).
Dithienylethenes containing coumarin outside of the hexatriene system
In continuation of the study of photochromic diheteroarylethenes, which incorporate the coumarin unit [61] and show photomodulated fluorescence, a dithienylethene, bearing a coumarin substituent in the thiophene ring, has been synthesized [64]. It was proposed that coumarin-substituted 3,4-bis(thienyl)furan-2,5-dione would combine high photochromic performance (thermal stability of cyclic form and high fatigue resistance) and intense fluorescence of open form, because 3-arylcoumarins exhibit high fluorescence quantum yields [65]. Therefore, the synthetic route to dithienylethene 30, including a coumarin unit, has been developed. Retrosynthetic analysis of 30 has led to a key intermediate – earlier unreported 3-(5-methyl-2-thienyl)coumarin 31. This compound is now available via two synthetic approaches, widely used for synthesis of analogous 3-substituted coumarins (Scheme 16).

Suzuki-Miyaura coupling of 5-methyl-2-thienylboron derivatives and 3-halocoumarins is an important route to compound 31, and several examples of Suzuki cross-coupling of 3-halocoumarins and arylboron derivatives have been reported [66–69]. Another method of synthesis of 3-arylcoumarins involves Knoevenagel condensation of arylacetic acid derivatives and o-hydroxycarbonyl compounds. Condensation of arylacetyl chlorides with salicylaldehydes was reported in acetone/K2CO3 [70] and under phase transfer conditions (K2CO3 aq./benzene [71] or K2CO3 aq./DCM [72, 73]). Arylacetic acids may be condensed with salicylaldehydes or 2-hydroxyacetophenones in acetic anhydride in the presence of bases (acetates or triethylamine) to give 3-arylcoumarins [74, 75]. Carboxy-activating reagents have also been successfully used for synthesis of various 3-arylcoumarins from arylacetic acids: dicyclohexylcarbodiimide [76], carbonyldiimidazole [77], phenyldichlorophosphate [78], and Mukaiyama reagent (2-chloro-1-methylpyridinium iodide) [79]. The use of arylacetates [80–82] and arylacetonitriles [83] affords 3-arylcoumarins upon condensation with o-hydroxycarbonyl compounds under basic conditions.
Optimization of the Suzuki-Miyaura coupling reaction for preparation of 31.
| No. | Catalyst | Solvent | Base | Additive | Time (h) | Yield (%) |
|---|---|---|---|---|---|---|
| 1 | Pd(PPh3)4 | Dioxane | CsF (3 eq.) | – | 10 | 34 |
| 2 | Pd(PPh3)4 | Dioxane | Na2CO3 (2.5 eq.) | H2O | 6 | Traces |
| 3 | Pd(PPh3)4 | CH3CN | Na2CO3 (2.5 eq.) | H2O | 6 | 36 |
| 4 | Pd(PPh3)4 | CH3CN | CsF (5 eq.) | – | 48 | 21 |
| 5 | Pd(PPh3)4 | CH3CN | K3PO4 (3 eq.) | Bu4NBr | 9 | 15 |
| 6 | Pd(dppf)Cl2 | CH3CN | Na2CO3 (2.5 eq.) | H2O | 6 | 55 |
| 7 | Pd(dppf)Cl2 | CH3CN | CsF (5 eq.) | – | 6 | 80 |
| 8 | Pd(dppf)Cl2 | DMF | Na2CO3 (5 eq.) | H2O | 3 | 75 |
| 9 | Pd(dppf)Cl2 | DMF | CsF (5 eq.) | – | 3 | 84 |
3-Bromocoumarin 32 was synthesized via a two-step procedure, including bromination of coumarin to give 3,4-dibromocoumarin [84] and subsequent HBr elimination [85]. 5-Methyl-2-thienylboronic acid 33 was prepared by a standard procedure through directed metallation of 2-methylthiophene with n-butyllithium followed by addition of tri(isopropyl)borate (Scheme 17). Suzuki coupling of 3-bromocoumarin 32 and 5-methyl-2-thienylboronic acid 33 gave poor yields of 31, although various solvents (dioxane, acetonitrile), bases (Na2CO3, K3PO4, CsF), and catalysts [Pd(PPh3)4, Pd(dppf)Cl2] have been tested. Thin layer chromatography (TLC) control of the reactions showed a rapid consumption of 33 (0.5–1 h), whereas 32 was still present in the mixture.
Synthesis of 31 was successfully optimized using pinacol ester 34 (Scheme 18 and Table 3). The highest yields of 3-(5-methyl-2-thienyl)coumarin 31 were obtained using Pd(dppf)Cl2 as a catalyst and CsF as a base in polar aprotic solvent [CH3CN or dimethylformamide (DMF)].


7-Diethylaminocoumarins exhibit a strong bathochromic shift and higher quantum yields in comparison with unsubstituted analogs [65]; therefore, synthesis of 7-diethylamino-substituted analog of 31 would be of interest. However, the above optimized procedure turned out to be unsuccessful for the synthesis of 7-(diethylamino)-3-(5-methyl-2-thienyl)coumarin from 7-(diethylamino)-3-bromocoumarin and borate 34. The strongly electron-donating diethylamino substituent is likely to complicate the oxidative addition step and thus the slowing cross-coupling reaction. Knoevenagel condensation was considered to be more convenient and versatile for variation of substituents in the benzene ring of coumarin of 3-arylcoumarins, as many substituted salicylaldehydes are commercially available.
To date, no convenient procedure for the preparation of 5-methyl-2-thienylacetic acid 35 or its esters has been reported. The most common method for synthesis of arylacetic acids is the Willgerodt-Kindler reaction starting from corresponding methylketones via intermediate thiomorfolides [86]. Under standard reaction conditions, reported to be successful for the synthesis of some thienylacetic acids [87], unsatisfactory yield of thiomorfolide 36 (21%) was obtained. Use of microwave irradiation [88] and addition of catalytic amounts of TsOH [89] gave only slight yield increase to 29% (Scheme 19, route 1).

Reduction of corresponding 2-oxoacetate with triethylsilane in trifluoroacetic acid [90–93] turned out to be a more convenient approach to the desired ester 37. Ethyl 2-oxo-2-(5-methyl-2-thienyl)acetate 38 was obtained via Friedel-Crafts acylation of 2-methylthiophene with ethyl oxalyl chloride, similar to the reported procedure [94]. Further reduction with Et3SiH/TFA was completed in 2 h at 0°C affording ethyl 5-methyl-2-thienylacetate 37 in 85% yield (Scheme 19, route 2).
Synthesis of 31 was attempted using standard reaction conditions for the preparation of 3-arylcoumarins: salicylaldehyde and 37 were heated under reflux in ethanol for 16 h [80]. However, these conditions did not provide full conversion of 37. To improve yield of 31 and shorten the reaction time, this condensation was performed under microwave irradiation under solvent-free conditions. This procedure gave high yields of desired 3-(5-methyl-2-thienyl)coumarins: 73% for 31 and 84% for 39 (Scheme 20).

3-(5-Methyl-2-thienyl)coumarin 31 was transformed to the goal compound 30 via a five-step pathway (Scheme 21). Friedel-Crafts acylation of 3-(5-methyl-2-thienyl)coumarin 31 with ethyl oxalyl chloride required addition of at least 3 eq. of aluminum chloride. Acylation of the intermediate product 40 was not detected if a lower amount of aluminum chloride was added. This may be due to complexation of AlCl3 (1 eq.) with coumarin ester functionality. Ketoester 40 was isolated in excellent yield (95%). The 1H NMR NOE correlation of the thiophene proton and 4-coumarin proton confirmed the regioselectivity of acylation. Further reduction of 40 using Et3SiH/TFA gave thienylacetate 41 in 86% yield. Alkaline hydrolysis of 41 provided arylacetic acid 42 in 75% yield, which was then transformed into corresponding chloride 43 by treatment with SOCl2. Triethylamine promoted condensation of arylacetic acid chloride 43 and (2,5-dimethylthien-3-yl)glyoxylic acid according to the reported procedure [95] gave desired furan-2,5-dione 30.

Acylation of 7-(diethylamino)-3-(5-methyl-2-thienyl)coumarin 39 under similar reaction conditions as for the synthesis of 31 gave a complex mixture of unidentified products; the desired 2-oxoacetate could not be isolated.
Dithienylethene 30 shows photochromism in solution (Scheme 22). UV irradiation of 30 leads to photocyclization, as determined by the appearance of a strong absorption band at 610 nm. It is noteworthy that the spectrum does not show any significant changes in the UV region during irradiation (Figure 5). Visible light irradiation of cyclic form leads to recovery of open form absorption.
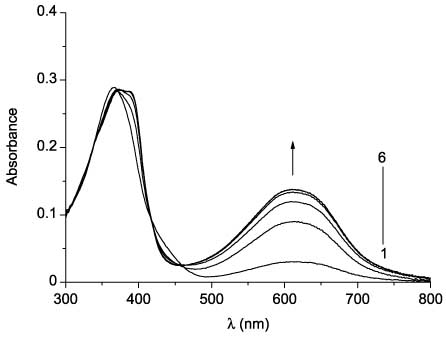
Absorption spectra of 30 in toluene before (line 1) and after UV irradiation (365 nm) for 1s (2), 2 s (3), 4 s (4), 8 s (5), and 16 s (6).

Dithienylethene 30 shows relatively high photostability. The photochromic cycles can be performed many times without a significant decrease of optical density in the cyclic form absorption maximum. After 50 cycles of photochromic transformation (no exclusion of air), its photostationary optical density at 612 nm makes up 95% from the initial value (Figure 6). Both oxidation and photochemical byproduct formation may be responsible for the observed photodegradation. Dithienylethenes non-substituted at 4 and 4′ positions of thiophene rings can be transformed into a tetracyclic non-photochromic byproduct, isomeric to closed form, thus loosing photochromic performance [96]. No significant decrease of optical density in cyclic form absorption maxima was observed during an 8-day-long storage (darkness, 20°C) of solution irradiated to reach the photostationary state.
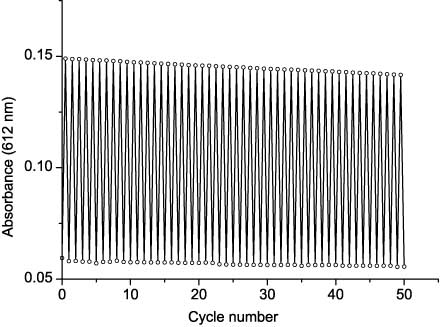
Reversibility of photochromic transformations of 30 in toluene solution.
No changes in fluorescence spectrum are observed upon photoisomerization of 30. This may be due to fluorescence maximum of open form (441 nm) lies nearby to isosbestic point (457 nm) (Figure 7) and intersection with absorption band of cyclic form is not significant.
Non-conjugated coumarin-substituted dithienylethene 44 has been reported as a multiphoto-responsive photochromic compound (Scheme 23) [97]. Besides reversible photochromism due to cyclization of the dithienylethene unit, compound 44 undergoes photodimerization involving a three to four double bond of the coumarin. These two photoinduced transformations may produce four corresponding states which may be switched by light. It was found that photodimerization occurs only in the presence of BF3 and both dimeric forms may be switched by irradiation with UV or visible light. Dimerization is not completely reversible due to photolysis of the compound when exposed to short wavelength UV light (<290 nm).

Coumarinyl(thienyl)thiazoles as new fluorescent molecular photoswitches
A number of new unsymmetrical diheteroarylethenes which possess properties of fluorescent photoswitches have been prepared [98, 99] during the study of coumarin derivatives capable of the photoinduced changes of fluorescence [10, 11]. These diheteroarylethenes contain a coumarin fragment at position 5 of the thiazole ‘bridge’ and the thiophene or benzothiophene fragment at position 4. Such compounds show an intensive fluorescence that reversibly changes under UV and visible light irradiation. Earlier [15–18], the synthesis and photochromism of dithienylthiazoles 45 (Scheme 24) have been described. Coumarin-containing analogs were prepared using ethanones 25 as key intermediates (Scheme 25). The reaction of ethanones 25 with bromine in dichloromethane at room temperature afforded α-bromoketones 46 in 83–92% yields. Thiazoles 48 were obtained in good yields (54–94%) by reflux of methanol solutions of α-bromoketones 46 with thioamide-containing compounds 47.
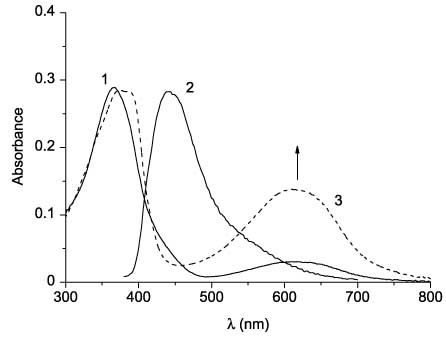
Absorption (line 1) and fluorescence spectra (line 2) of the open form and absorption spectrum of the cyclic form (line 3) of 1.


The results of spectral and photokinetic studies of thiazoles 48 are summarized in Table 4. In the absorption spectra of 4-phenylthiazoles 48aa–48ae, two partially overlapping bands are found in the region 300–410 nm (Figure 8, curve 1). In the sequence of compounds 48aa (X = Me), 48ab (X = Ph), 48ac (X = 4-MeOC6H4), 48ad (X = NH2), 48ae (X = NHPh), a bathochromic shift of the long-wave absorption band maximum is observed, which is caused, apparently, by the enhancement of donor properties of substituents in this sequence. 3-(4-Phenylthiazol-5-yl)coumarins 48aa–48ae exhibit fluorescence in the visible region of the spectrum, their emission maxima lying in the region at 420–550 nm (Figure 8, curve 2) and they are also bathochromically shifted in the sequence from 48aa to 48ae (Table 4).
Spectral properties of open and closed forms of 3-(4-arylthiazol-5-yl)coumarins 48.
| Compound | 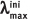 | ε, L/(mol × cm) | 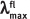 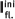 | 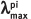 | 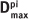 |
|---|---|---|---|---|---|
| 48aa | 338 | 7400 | 426 (230) | – | – |
| 48ab | 362 | 9360 | 445 (80) | – | – |
| 48ac | 390 | 7440 | 458 (410) | – | – |
| 48ad | 392 | 6880 | 474 (165) | – | – |
| 48ae | 402 | 8780 | 552 (360) | – | – |
| 48ba | 350 | 7300 | 504 (125) | 500 | 0.22 |
| 48bb | 374 | 14 100 | 501 (295) | 517 | 0.22 |
| 48bc | 382 | 16 300 | 510 (280) | 515 | 0.30 |
| 48bd | 398 | 7400 | 543 (7) | 496 | 0.28 |
| 48be | 410 | 13 400 | 551 (50) | 500 | 0.25 |
| 48ca | 365 | 3200 | 466 (125) | 481 | 0.06 |
| 48cb | 376 | 5450 | 468 (75) | 502 | 0.04 |
| 48cc | 378 | 5400 | 473 (260) | 502 | 0.04 |
| 48cd | 406 | 6450 | 505 (220) | 480 | 0.21 |
| 48ce | 390 | 8530 | 490 (215) | 495 | 0.10 |
Note: 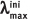 : the absorption maxima of initial form;
: the absorption maxima of initial form; 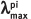 : the absorption maxima of photoinduced form;
: the absorption maxima of photoinduced form; 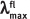 : maxima of fluorescence emission;
: maxima of fluorescence emission; 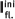 : relative intensity of fluorescence of initial form; ε: coefficient of molecular extinction;
: relative intensity of fluorescence of initial form; ε: coefficient of molecular extinction; 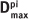 : absorbance value in maxima of photoinduced form in photostationary state.
: absorbance value in maxima of photoinduced form in photostationary state.
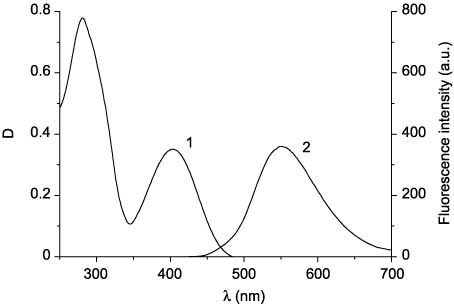
Absorption (1) and emission (2) spectra of 48ae.
Absorption and fluorescence spectra of compounds 48ba–48be before irradiation are analogous to the spectra of compounds 48aa–48ae. In the series of compounds 48ba–48be, a bathochromic shift of the absorption and fluorescence band maxima is also observed. However, in contrast to compounds 48aa–48ae, the presence of the amino group at position 2 of the thiazole ring (compounds 48bd, 48be) leads to a significant decrease in the intensity of fluorescence in comparison with 2-aryl- and 2-alkylthiazoles 48ba–48bc. In the absorption spectra of compounds 48ca–48ce, two overlapping absorption bands in the region 280–400 nm (Figure 9, curve 1) can also be highlighted. The absorption and emission bands in the spectra of compounds 48ca–48ce are additionally bathochromically shifted. Photochemical transformations of compounds 48 were studied by irradiation of their acetonitrile solutions with the filtered light of the mercury-xenon lamp. Irradiation of solutions of 3-(4-phenylthiazol-5-yl)coumarins 48aa–48ae with the UV light did not cause any changes in the spectra, which suggests the absence of the electrocyclization reaction. Conversely, compounds 48ba–48be and 48ca–48ce turned out to be capable of photoinduced changes in both the absorption and the fluorescence spectra. The irradiation of solutions of the open forms of compounds 48 with filtered UV light leads to a decrease in intensity of the absorption band in the region 350–400 nm and appearance of the absorption band in the visible region of the spectrum (496–517 nm), the intensity of which increases during irradiation (Figure 10, curves 2–6). The intensity of fluorescence on irradiation of compounds 48 decreases by three to six times (Figure 11) without changing emission maxima wavelength (Table 4). Retention of the fluorescence maxima positions suggests that only the initial form of these compounds causes their fluorescence.
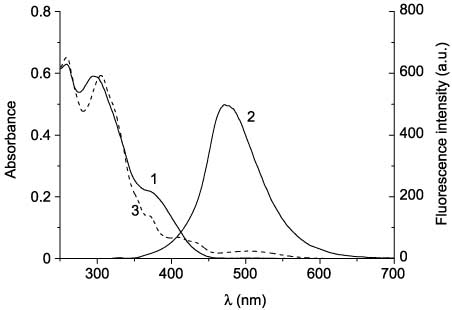
Absorption (1) and emission (2) spectra of the open form and absorption spectra of the cyclic form (3) of 48bc.
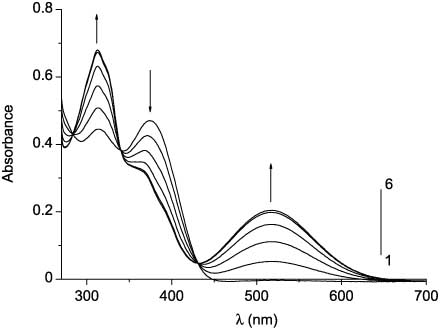
Absorption spectra of 48bb before (curve 1) and after UV irradiation (365 nm) for 1 s (2), 2 s (3), 4 s (4), 8 s (5), and 12 s (6).
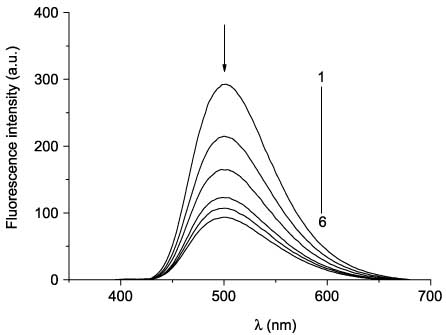
Fluorescence spectra of 48bb before (curve 1) and after UV irradiation (365 nm) for 1 s (2), 2 s (3), 4 s (4), 8 s (5), and 12 s (6).
The irradiation of the photoinduced cyclic forms of compounds 48 with light of the wavelength corresponding to their absorption maximum in the visible region leads to the reverse transformations of the closed form into the open one with virtually complete recovery of the absorption and fluorescence spectra of the compound initial form. The photochromic transformations of compounds 48 observed are explained by the photoinduced electrocyclic reaction, rather than, for example, by the [2+2]-cyclodimerization of compounds 48 at the three to four double bond of the coumarin fragment. The growth of the absorption intensity in the region of 500 nm on irradiation of the starting forms follows first order kinetics rather than second order kinetics, which would have been the case for the photoinduced reaction of a [2+2]-cycloaddition (Figure 12).
Compounds 48 are able to undergo repeated mutually reversible phototransformations. However, after 10–12 cycles of phototransformations, absorbance of the colored form is lower by 50% of the initial value (Figure 13). The photocyclization reactions of compounds 48 is thermally reversible. After irradiation of solutions with the UV light until the photostationary state is reached, a slow decrease in the value of optical density in the absorption band maximum of the photoinduced cyclic form is observed upon keeping the solutions in the dark.
The rate of spontaneous bleaching in the dark depends on the nature of substituent in the thiazole ring. Compound 48bb having the phenyl substituent undergoes a complete decolorization after 8 days, whereas other compounds decolorize only partially during this period. Probably, the donor substituents (methyl, p-methoxyphenyl, and phenylamino groups) stabilize the closed form of compounds 48. The effect mentioned is the strongest in the case of phenylamino derivative 48be: the optical density in the absorption band maximum of the closed form of compound 48be decreases by only 27% for 8 days. This is in agreement with the fact that the closed forms of dithienylethenes connected by the azole bridges are thermally unstable [15–18]. Half-life of the closed form of 45 in the dark is only several hours. From the data obtained, it follows that the introduction of the coumarin fragment into the conjugated system of thienylthiazole significantly increases the thermal stability of the closed form.
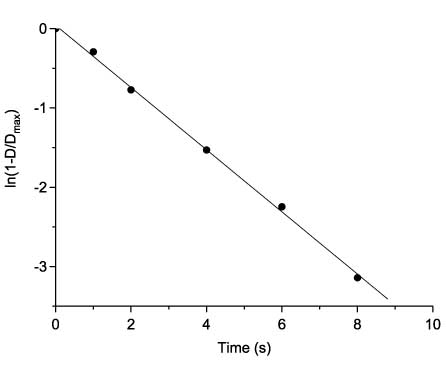
Changes of 48bb absorbance upon its coloration in first order kinetics coordinates.
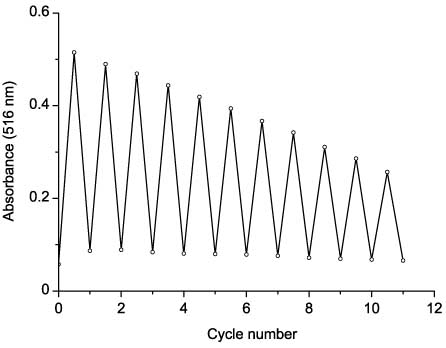
Reversibility of photochromic transformations of 48bb in acetonitrile.
On the prolonged irradiation of solutions of compounds 48 with the non-filtered light of a mercury-xenon lamp, photodecomposition is observed. The photodecomposition rate depends on the nature of substituent at position 2 of the thiazole ring. Compounds containing methyl and p-methoxyphenyl substituents are the least light-resistant. Complete decomposition was observed after irradiation for 80–120 s.
It had been shown earlier that introduction of coumarin-fused spiropyrans into the poly(methylmethacrylate) significantly increases stability of the photoinduced form in comparison with the solution [11]. Photochromism of compounds 48 was further studied in poly(methylmethacrylate) film. The spectral changes observed on irradiation of the film with UV and visible light are similar to the changes observed in solution. The positions of the absorption and fluorescence bands are virtually unchanged relatively to the solution in acetonitrile.
However, the introduction of 3-(4-heteroarylthiazol-5-yl)coumarins into the polymer matrix provided an increase in fatigue resistance of phototransformations: a 50% decrease of optical density in the absorption maximum of the cyclic form was observed after 18 cycles of phototransformations (Figure 14). In addition, dark stability of the cyclic forms in the polymer is significantly higher than in solution: the optical density in the absorption maximum of the closed form in the film, irradiated with the UV light until the photostationary state, decreases by 50% for 30 days (compound 48bb).
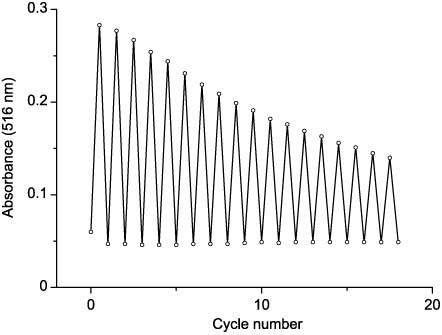
Reversibility of photochromic transformations of 48bb in polymethylmethacrylate (PMMA) film.
In conclusion, a number of differences between 3-(4-arylthiazol-5-yl)coumarins [98, 99] and dithienylthiazoles described earlier [15–18] should be highlighted. The introduction of the coumarin fragment leads to the appearance of intensive fluorescence in the open form, which significantly decreases upon photocyclization. In addition, it turned out to be possible to increase the stability of the closed form in the dark up to several days instead of several hours, which was reported earlier, by the variation of the substituent at position 2 of the thiazole ring in coumarin derivatives. An additional increase in the thermal stability of the closed forms of 3-(4-arylthiazol-5-yl)coumarins is achieved by their incorporation into the poly(methylmethacrylate) film.
Coumarinyl(thienyl)ethenes – maleic acid cyclic derivatives
In the previous sections, syntheses and photochromism of diheteroarylethenes, bearing the coumarin unit as a part of the photosensitive hexatriene system, including non-annelated [61] and thiazole-annelated [98, 99] diheteroarylethenes have been discussed. Those compounds along with photochromic transformations show fluorescence switching upon UV/vis irradiation, open forms being fluorescent and closed forms being non-fluorescent. In search for coumarinyl(thienyl)ethenes, which would have better fatigue-resistant properties, the thiazole ring in the photochromic system has been substituted with a maleic anhydride (or maleimide) bridge.
In this section, the synthesis, the study of photochromic properties, and the estimation of photoreversibility of coumarinyl(thienyl)maleic anhydride and maleimide are presented.
The synthesis of coumarinyl(thienyl)maleic anhydride 53 has been performed as shown in Scheme 26 [100]. Methyl ester of 7-hydroxy-4-methylcoumarin-3-yl acetic acid 49 was prepared via Pechmann condensation of dimethyl acetyl succinate and resorcinol in concentrated H2SO4. Further methylation with dimethyl sulfate and alkaline hydrolysis gave acid 51, which was converted to a corresponding acid chloride 52 by a standard procedure using thionyl chloride. The furandione 53 was obtained by slow addition of triethylamine to a cooled solution of (2,5-dimethylthien-3-yl)glyoxylic acid and acid chloride 52 in dichloromethane. Furandione 53 was transformed into functionalized maleimide 54 upon heating under reflux with ethanolamine in methanol. The free hydroxyl group of maleimide 54 may be used for covalent binding of photochrome to polymers.

The photochromic properties of 53 and 54 were studied in toluene solution. The UV/Vis absorption spectra of 53 and 54 and their changes upon UV irradiation are shown in Figures 15 and 16. Upon irradiation with UV light (365 nm), the absorption band intensity at 350–400 nm decreases and a simultaneous increase of optical density at the visible range (516 nm maximum for 53 and 485 nm maximum for 54) is observed due to photocyclization. It is noteworthy that maleimide derivative 54 shows a definite hypsochromic shift relative to 53.
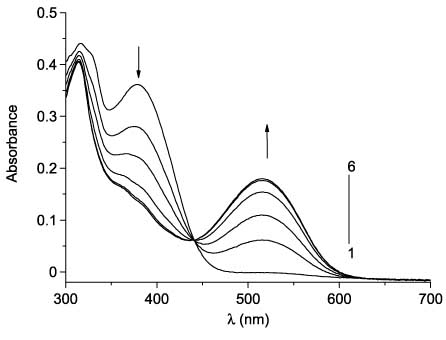
UV/VIS absorption spectra of 53 (4×10-5 m, toluene, 25°C) before (line 1) and after irradiation with UV light (365 nm) for 2 s (2), 4 s (3), 8 s (4), 16 s (5), and 32 s (6).
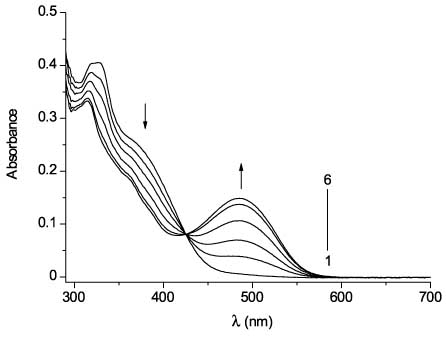
UV/VIS absorption spectra of 54 (4 × 10-5 m, toluene, 25°C) before (line 1) and after irradiation with UV light (365 nm) for 2 s (2), 4 s (3), 8 s (4), 16 s (5), and 32 s (6).
The cyclic forms of 53 and 54 return to the initial state upon irradiation with visible light (>450 nm). The photochromic cycle may be performed many times without a significant decrease in optical density. In contrast to photochromes 48, bearing the thiazole bridge, maleic acid cyclic derivatives seem to be much more fatigue-resistant. Moreover, maleimide 54 is more fatigue-resistant than maleic anhydride 53: after 50 cycles of photochromic transformation (in the presence of air) its photostationary optical density makes up 94% of the initial value (Figure 17). Maleic anhydride 53 shows 83% of initial optical density after 50 cycles and previously studied coumarinyl(thienyl)thiazoles 48 did not reach even 50% of initial optical density after 5–10 cycles.
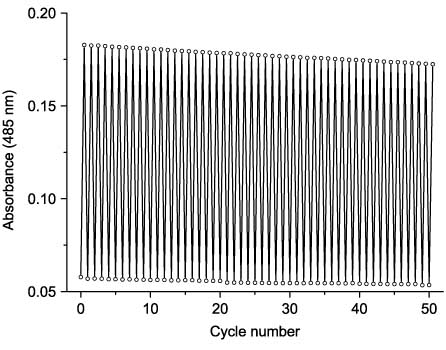
Reversibility of photochromic transformations of 54 in toluene solution.
No direct comparison of fatigue-resistance properties of maleic anhydride- and maleimide-bridged diheteroarylethenes was performed earlier. The conversion of maleic anhydride function to N-alkylmaleimide was assumed to improve fatigue resistance of diheteroarylethenes.
The coumarinyl(thienyl)maleic anhydride 53 and maleimide 54 show a fluorescence in toluene solution in an open form (at 565 and 540 nm, respectively) (Figure 18), which slightly decreases upon photocyclization. The cyclic forms of 53 and 54 are stable in the dark: after 8-day storage of irradiated (to the photostationary state) solutions in the absence of light, only a 7% decrease of optical density in cyclic form absorption maxima was observed. Thiazole-bridged coumarinyl(thienyl)ethenes 48 showed 27–100% decoloration in the dark depending on two substituents in the thiazole ring during the same period of time [98, 99].
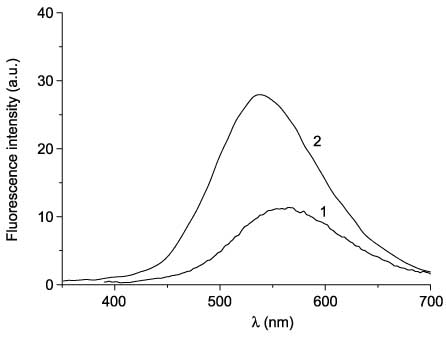
Fluorescence spectra of open forms of 53 (line 1) and 54 (line 2).
Synthesis and photochromism of 3,4-bis(thiazolyl)coumarins
Novel diheteroarylethenes 55, containing coumarin as a bridge connecting two thiazole units in the hexatriene system, have been reported recently [101]. Synthesis of 55 was accomplished in one step from the known building block for the preparation of 3,4-diarylcoumarins, 3-bromo-4-(trifluoromethylsulfonyloxy)coumarin [102], using microwave-assisted Suzuki coupling (Scheme 27).

Compounds 55 show photochromism in solution with remarkably high conversion ratios in photostationary states both for cyclization- and ring-opening reactions (Scheme 28). Ring closure of 3,4-bis(thiazolyl)coumarins leads to a significant decrease of fluorescence intensity due to disruption of coumarin chromophore.

By addition of 1,8-diazabicycloundec-7-ene (DBU) to dichloromethane solution of the hydroxy derivative 55c, it is converted to corresponding phenolate anion, which, as expected, shows bathochromically shifted absorption and emission maxima. The fluorescence intensity of 55c in water/methanol media is dramatically sensitive to pH changes in a biologically significant range: fluorescence intensity of the open form increases 14-fold by changing pH of medium from 6.05 to 7.58. Remarkably, upon photocyclization of anionic form of 55c in mixed water-containing solvent fluorescence intensity decreases by 1.5% from the initial value before irradiation. Thus, 7-hydroxy-3,4-bis(thiazolyl)coumarin 55c, including two switchable functionalities (pH-sensitive phenolic and photosensitive hexatriene) and fluorescent coumarin core can be introduced as a dual-mode fluorescent switch.
Conclusions
Photoinduced transformations of 1-(3-coumarinyl)-2-heteroarylethenes with a noncyclic double bond depend on their structure. These derivatives undergo an E-Z photoisomerization reaction. However, these structural changes are followed by cyclization only in the case of phenyl- or thienyl-substituted ethenes (compounds 27a–c). It is important to note that photocyclization reactions are also accompanied by fluorescence changes of the substrate.
New photochromic dithienylethene 30, containing a fluorescent coumarin unit conjugated with the photochromic system, shows long wavelength absorption of cyclic form, good fatigue resistance, and thermal irreversibility. However, photoisomerization of 30 is not accompanied by fluorescence changes because the fluorescence maximum of the open form lies near the isosbestic point, and cross-section with absorption band of cyclic form is not significant.
Photochromic coumarinyl(thienyl)thiazoles possess prominent fluorescence in the open form, which is quenched upon photocyclization. Thermal stability of their cyclic forms is higher compared with that of bis(thienyl)thiazoles.
Coumarinyl(thienyl)maleic acid cyclic derivatives, anhydride and maleimide, show better photochromic performance (fatigue resistance and dark stability of cyclic forms) in comparison with that of coumarinyl(thienyl)thiazoles.
Novel diheteroarylethenes, containing coumarin as a bridge connecting two thiazole units in the hexatriene system, show significant changes in fluorescence upon photocyclization and exhibit remarkably high conversion ratios in photostationary states. A hydroxy-substituted bis(thiazolyl)coumarin includes two switchable functionalities (pH-sensitive phenolic and photosensitive hexatriene) and a fluorescent coumarin core comprising together a dual-mode fluorescent switch.
References
[1] Traven, V. F. Coumarin polymethines, their boron complexes and analogs. In Topics in Heterocyclic Chemistry-14. Heterocyclic Polymethine Dyes; Strekowski, L., Ed., Springer: Berlin, 2008; pp. 107–131.10.1007/7081_2008_114Search in Google Scholar
[2] Bertelson, R. C. The Photochromism. Brown, G. H., Ed. Wiley-Interscience: New York, 1971.Search in Google Scholar
[3] Krongauz, V. A.; Goldburt, E. I. Quasi-crystals from irradiated photochromic dyes in an applied electric field. Nature1978, 271, 43–45.Search in Google Scholar
[4] Chibisov, A. K.; Gorner, H. Complexes of spiropyran-derived merocyanines with metal ions: relaxation kinetics, photochemistry and solvent effects. Chem. Phys. 1998, 237, 425–442.Search in Google Scholar
[5] Bertelson, R. C. Spiropyrans. In Organic Photochromic and Thermochromic Compounds; Vol. 1. Crano, J. C., Guglielmetti, R., Eds. Plenum Press: New York, 1999; pp. 11–83.10.1007/0-306-46911-1_2Search in Google Scholar
[6] Maeda, S. Spirooxazines. In Organic Photochromic and Thermochromic Compounds; Vol. 1. Crano, J. C., Guglielmetti, R., Eds. Plenum Press: New York, 1999; pp. 85–109.10.1007/0-306-46911-1_3Search in Google Scholar
[7] Barachevsky, V. A. Photofluorochromic spirocompounds and their application. J. Fluorescence2000, 10, 185–191.Search in Google Scholar
[8] Antipin, S. A.; Petrukhin, A. N.; Gostev, F. E.; Marevtsev, V. S.; Titov, A. A.; Barachevsky, V. A.; Strokach, Y. P.; Sarkisov, O. M. Femtosecond transient absorption spectroscopy of non-substituted photochromic spirocompounds. Chem. Phys. Lett. 2000, 331, 378–386.Search in Google Scholar
[9] Lokshin, V. A.; Samat, A.; Metelitsa, A. V. Spirooxazines: synthesis, structure, spectral and photochromic properties. Russ. Chem. Rev. 2002, 71, 893–916.Search in Google Scholar
[10] Traven, V. F.; Miroshnikov, V. S.; Chibisova, T. A.; Barachevsky, V. A.; Venidiktova, O. V.; Strokach, Y. P. Synthesis and structure of indoline spiropyrans of the coumarin series. Russ. Chem. Bull. Int. Ed. 2005, 54, 2417–2424.Search in Google Scholar
[11] Barachevsky, V. A.; Karpov, R. E.; Venidiktova, O. V.; Valova, T. M.; Strokach, Y. P.; Miroshnikov, V. S.; Chibisova, T. A.; Traven, V. F. Photochromic properties of indoline spiropyrans of the coumarin series. Russ. Chem. Bull. Int. Ed. 2005, 54, 2425–2431.Search in Google Scholar
[12] Irie, M. Diarylethenes for memories and switches. Chem. Rev. 2000, 100, 1685–1716.Search in Google Scholar
[13] Barachevsky, V. A.; Strokach, Y. P.; Puankov, Y. A.; Krayushkin, M. M. Thermally irreversible organic photochromic compounds for optical memory. J. Phys. Org. Chem. 2007, 20, 1007–1020.Search in Google Scholar
[14] Krayushkin, M. M. Synthesis of photochromic dihetarylethenes. Chem. Heterocycl. Compd. 2001, 37, 15–36.Search in Google Scholar
[15] Krayushkin, M. M.; Ivanov, S. N.; Martynkin, A. Y.; Lichitskii, B. V.; Dudinov, A. A.; Uzhinov, B. M. Photochromic dihetarylethenes. 7. Synthesis of bis(thienylazoles), photochromic analogs of diarylethenes. Russ. Chem. Bull. Int. Ed. 2001, 50, 116–121.Search in Google Scholar
[16] Krayushkin, M. M.; Lichitskii, B. V.; Mikhalev, A. P.; Nabatov, B. V.; Dudinov, A. A.; Ivanov, S. N. Behavior of benzoins and hydroxy ketones in acid medium: II. Reactions of 1,2-bis(2,5-dimethyl-3-thienyl)-2-hydroxy-ethan-1-one with N,S-binucleophiles in trifluoroacetic acid. Russ. J. Org. Chem. 2006, 42, 860–864.Search in Google Scholar
[17] Nakashima, T.; Atsumi, K.; Kawai, S.; Nakagawa, T.; Hasegawa, Y.; Kawai, T. Photochromism of thiazole-containing triangle terarylenes. Eur. J. Org. Chem.2007, 3212–3218.10.1002/ejoc.200700074Search in Google Scholar
[18] Kawai, S.; Nakashima, T.; Atsumi, K.; Sakai, T.; Harigai, M.; Imamoto, Y.; Kamikubo, H.; Kataoka, M.; Kawai, T. Novel photochromic molecules based on 4,5-dithienyl thiazole with fast thermal bleaching rate. Chem. Mater.2007, 19, 3479–3483.Search in Google Scholar
[19] Tian, H.; Yang, S. Recent progresses on diarylethene based photochromic switches. Chem. Soc. Rev. 2004, 33, 85–97.Search in Google Scholar
[20] Raymo, M. F.; Tomasulo, M. Electron and energy transfer modulation with photochromic switches. Chem. Soc. Rev. 2005, 34, 327–336.Search in Google Scholar
[21] Kawai, T.; Sasaki, T.; Irie, M. A photoresponsive laser dye containing photochromic dithienylethene units. Chem. Commun. 2001, 711–712.10.1039/b100330pSearch in Google Scholar
[22] Fukaminato, T.; Sasaki, T.; Kawai, T.; Tamai, N.; Irie, M. Digital photoswitching of fluorescence based on the photochromism of diarylethene derivatives at a single-molecule level. J. Am. Chem. Soc. 2004, 126, 14843–14849.Search in Google Scholar
[23] Odo, Y.; Fukaminato, T.; Irie, M. Photoswitching of fluorescence based on intramolecular electron transfer. Chem. Lett. 2007, 36, 240–241.Search in Google Scholar
[24] Golovkova, T. A.; Kozlov, D. V.; Neckers, D. C. Synthesis and properties of novel fluorescent switches. J. Org. Chem. 2005, 70, 5545–5549.Search in Google Scholar
[25] Norsten, T. B.; Branda, N. R. Photoregulation of fluorescence in a porphyrinic dithienylethene photochrome. J. Am. Chem. Soc. 2001, 123, 1784–1785.Search in Google Scholar
[26] Dvornikov, A. S.; Liang, Y.; Cruse, C. S.; Rentzepis, P. M. Spectroscopy and kinetics of a molecular memory with nondestructive readout for use in 2D and 3D storage systems. J. Phys. Chem. B2004, 108, 8652–8658.Search in Google Scholar
[27] Dey; Row. J. Indian Chem. Soc. 1923/1924, 1, 287.Search in Google Scholar
[28] Dey; Seshadri. J. Indian Chem. Soc. 1931, 8, 247.Search in Google Scholar
[29] Mahal, H. S.; Venkataraman, K. Some 4-styrylcoumarins. J. Chem. Soc. 1933, 616–617.10.1039/jr9330000616Search in Google Scholar
[30] Mustafa, A.; Kamel, M. 4-Styrylcoumarins and 2,3-dimethylquinoxaline in diene syntheses. J. Am. Chem. Soc. 1955, 77, 1828–1830.Search in Google Scholar
[31] Mustafa, A.; Kamel, M. 4-Styrylcoumarins in diene syntheses. II. J. Am. Chem. Soc. 1956, 78, 4692–4694.Search in Google Scholar
[32] Gharde, A. D.; Ghiya, B. J. Synthesis of novel 4-styrylcoumarins from coumarin-4-acetic acids. J. Ind. Chem. Soc. 1992, 69, 397–398.Search in Google Scholar
[33] Moorthy, J. N.; Samant, S. D.; Venkatesan, K. Studies in crystal engineering: structure-reactivity correlations of substituted styrylcoumarins and related systems. J. Chem. Soc. Perkin Trans.1994, 2, 1223–1228.Search in Google Scholar
[34] Shridhar, C. V.; Reddy Sastry, C. V.; Vaidya, N. K.; Moorty, S. R.; Reddi, G. S.; Thapar, G. S. Antimicrobal agents: synthesis and antibacterial activity of some new 4-[2-(heteroaryl)vinyl]coumarins. Ind. J. Chem. Sect. B1978, 16, 704–708.Search in Google Scholar
[35] Shridhar, D. R.; Reddy Sastry, C. V.; Lal, B. Synthesis and biological activity of 9-substituted 4-[2-(5′-nitro-2′-furyl-5′-nitro-2′-thienyl)vinyl]-8,10-dihydro-2H-pyrano[2,3-f][1,3]-benzoxazin-2-ones. Ind. J. Chem. Sect. B1980, 18, 1065–1067.Search in Google Scholar
[36] Gharde, A. D.; Ghiya, B. J. Synthesis of novel 4-styrylcoumarins from coumarin-4-acetic acids. J. Ind. Chem. Soc. 1992, 69, 397–398.Search in Google Scholar
[37] Tkach, I. I.; Andronova, N. A.; Savvina, L. P.; Lukyanets, E. A. Synthesis and spectral-luminescence properties of 7-diethylamino-4-(2-arylethenyl)coumarins. Chem. Heterocycl. Compd. 1991, 27, 259–262.Search in Google Scholar
[38] Dimitrova, E.; Angelova, Y. Condensation of 3-acetyl- and 3-acetyl-4-methyl-2H-1-benzopyran-2-one with aromatic aldehydes. Synth. Commun. 1986, 16, 1195–1205.Search in Google Scholar
[39] Hafez, E.; Elnagdi, M. H.; Elagamey, A. G.; El-Taweel, F. M. Nitriles in heterocyclic synthesis: novel synthesis of benzo[c]coumarin and of benzo[c]pyrano[3,2-c]quinoline derivatives. Heterocycles1987, 26, 903–907.Search in Google Scholar
[40] Brufola, G.; Fringuelli, F.; Piermatti, O.; Pizzo, F. Simple and efficient one-pot preparation of 3-substituted coumarins in water. Heterocycles1996, 43, 1257–1266.Search in Google Scholar
[41] Boutome, H.; Hartmann, H. On the condensation of 2,2-difluoro-4-methyl-benzo[d]-l,3,2-dioxaborines with cyano acetic acid derivatives. Formation and transformation of 3-cyano-4-methyl-benzo[b]pyran-2-ones and their 2-imine precursors. Monatsh. Chem. 1997, 128, 71–78.Search in Google Scholar
[42] Nabil, M.; Khodeir, M. Nitriles in heterocyclic synthesis: synthesis of pyridine, pyrone, benzocoumarin and pyrido[3,4-c]coumarin derivatives. Ind. J. Chem. Sect. B1993, 32, 783.Search in Google Scholar
[43] Madkour, H. M. F. Synthesis and reactions of some 3-cyano-4-methylcoumarins. Heterocycles1993, 36, 947–959.10.3987/COM-92-6079Search in Google Scholar
[44] Jun, H.; Hong, S. Y.; Yoon, S. S.; Kang, C.; Suh, M. Fluorescent hydrophobic probes based on intramolecular charge transfer state for sensitive protein detection in solution. Chem. Lett. 2004, 33, 690–691.Search in Google Scholar
[45] Kawase, M.; Varu, B.; Shah, A.; Motohashi, N.; Tani, S.; Saito, S.; Debnath, S.; Mahapatra, S.; Dastidar, S. G.; Chakrabarty, A. N. Antimicrobial activity of new coumarin derivatives. Arzn. Forsch. 2001, 51, 67–71.Search in Google Scholar
[46] Upadhyay, K.; Bavishi, A.; Thakrar, S.; Radadiya, A.; Vala, H.; Parekh, S.; Bhavsar, D.; Savant, M.; Parmar, M.; Adlakha, P.; et al. Synthesis and biological evaluation of 4-styrylcoumarin derivatives as inhibitors of TNF-α and IL-6 with anti-tubercular activity. Bioorg. Med. Chem. Lett. 2011, 21, 2547–2549.Search in Google Scholar
[47] Joshi, U. K.; Keltaar, R. M.; Paradker, M. V. A convenient synthesis of 4-styrylcoumarins. Ind. J. Chem. Sect. B1983, 22, 1151–1152.Search in Google Scholar
[48] Vishnumurthy, K.; Guru Row, T. N.; Venkatesan, K. Studies in crystal engineering: crystal packing, topological photodimerization and structure-reactivity correlations in fluoro-substituted styrylcoumarins. J. Chem. Soc. Perkin Trans.1997, 2, 615–619.Search in Google Scholar
[49] Vishnumurthy, K.; Guru Row, T. N.; Venkatesan, K. Studies in crystal engineering: steering ability of fluorine in 4-styrylcoumarins. Tetrahedron1999, 55, 4095–4108.Search in Google Scholar
[50] Ahluwalia, V. K., Kaila, N., Bala, S. Synthesis and antifungal and antibacterial activities of some 2-amino-4,6-substituted-pyrimidines and 4-styryl-6,7-pyranocoumarins. Ind. J. Chem. Sect. B1987, 26, 700–702.10.1002/chin.198805203Search in Google Scholar
[51] Ahluwalia, V. K.; Adhikari, R.; Singh, R. P. A facile synthesis of 4-cinnamoyl-dibenzofurans. Ind. J. Chem. Sect. B1988, 27, 274–276.Search in Google Scholar
[52] Ahluwalia, V. K.; Mann, R. R.; Bala, S. Synthesis and antifungal and antibacterial activities of some 4-arylethenyl- and 4-arylethyl-6,7-dihydropyranocoumarins. Ind. J. Chem. Sect. B1989, 28, 608–610.Search in Google Scholar
[53] Henkel, B. Resin-bound (succinimid-1-yloxycarbonylmethyl)triphenylphosphonium ylide – a synthon for rapid access to diverse heterocycles under microwave heating. Synlett2008, 355–358.10.1055/s-2008-1032046Search in Google Scholar
[54] Chodankar, N. K.; Joshi, S. D.; Sequeria, S.; Sehadri, S. Synthesis of 3-styrylcoumarins from coumarin-3-acetic acids and 3-phenacylcoumarins. Indian J. Chem. 1987, 26, 427–430.Search in Google Scholar
[55] Tkach, I. I.; Mikhailova, T. A.; Savvina, L. P.; Lukyanets, E. A. Synthesis, luminescence, and spectral characteristics of 7-diethylamino-3-(2-arylethenyl)coumarins. Chem. Heterocycl. Compd. 1992, 28, 985–989.Search in Google Scholar
[56] Janecki, T.; Bodalski, R. A convenient Horner-Emmons approach to the synthesis of substituted ethyl 1,3-butadiene-2-carboxylates, and related compounds. Synthesis1989, 506–510.10.1055/s-1989-27301Search in Google Scholar
[57] Ivanova, D. I.; Eremin, S. V.; Shvets, V. I. The synthesis of a coumarin analogue of retinal. Tetrahedron1996, 52, 9581–9588.Search in Google Scholar
[58] Schiedel, M.; Briehn, C. A.; Baeuerle, P. Single-compound libraries of organic materials: parallel synthesis and screening of fluorescent dyes. Angew. Chem. Int. Ed. 2001, 40, 4677–4680.Search in Google Scholar
[59] Gordo, J.; João Avó, A.; Parola, J.; Lima, J. C.; Pereira, A.; Branco, P. S. Convenient synthesis of 3-vinyl and 3-styryl coumarins. Org. Lett. 2011, 13, 5112–5115.Search in Google Scholar
[60] Colonge, F. Bull. Soc. Chim. Fr. 1964, 3090–3093.Search in Google Scholar
[61] Bochkov, A. Y.; Yarovenko, V. N.; Krayushkin, M. M.; Chibisova, T. A.; Valova, T. M.; Barachevsky, V. A.; Traven, V. F.; Beletskaya, I. P. Synthesis and photoinduced fluorescence of 3-(2-hetarylethenyl)chromen-2-ones. Russ. J. Org. Chem. 2008, 44, 595–601.Search in Google Scholar
[62] Chodankar, N. K.; Sehadri, S. Studies in the Vilsmeier-Haack reaction: part XXV – synthesis of 3-hetarylcoumarins by the application of the Vilsmeier-Haack reaction. Dyes Pigm.1985, 6, 313–319.Search in Google Scholar
[63] Waldeck, D. H. Photoisomerization dynamics of stilbenes. Chem. Rev. 1991, 91, 415–436.Search in Google Scholar
[64] Bochkov, A. Y.; Krayushkin, M. M.; Yarovenko, V. N.; Barachevsky, V. A.; Beletskaya, I. P.; Traven, V. F. Synthesis of 3-(5-methylthiophen-2-yl)coumarins and their photochromic dihetarylethene derivatives. J. Heterocycl. Chem. 2013, in press. DOI: 10.1002/jhet.931.10.1002/jhet.931Search in Google Scholar
[65] Kuznetsova, N. A.; Kaliya, O. L. Photochemistry of coumarins. Russ. Chem. Rev. 1992, 61, 683–696.10.1070/RC1992v061n07ABEH000992Search in Google Scholar
[66] Schiedel, M.; Briehn, C. A.; Baeuerle, P. Single-compound libraries of organic materials: parallel synthesis and screening of fluorescent dyes. Angew. Chem. Int. Ed. 2001, 40, 4677–4680.Search in Google Scholar
[67] Hara, K.; Kurashige, M.; Dan-oh, Y.; Kasada, C.; Shinpo, A.; Suga, S.; Sayama, K.; Arakawa, H. Design of new coumarin dyes having thiophene moieties for highly efficient organic-dye-sensitized solar cells. New J. Chem. 2003, 27, 783–785.Search in Google Scholar
[68] Yee, D. J.; Balsanek, V.; Sames, D. New tools for molecular imaging of redox metabolism: development of a fluorogenic probe for 3a-hydroxysteroid dehydrogenases. J. Am. Chem. Soc. 2004, 126, 2282–2283.Search in Google Scholar
[69] Zhang, L.; Meng, T.; Fan, R.; Wu, J. General and efficient route for the synthesis of 3,4-disubstituted coumarins via Pd-catalyzed site-selective cross-coupling reactions. J. Org. Chem. 2007, 72, 7279–7286.Search in Google Scholar
[70] Rao, P.; Srimannaryana, G. A novel and convenient synthesis of 3-phenylcoumarins. Synthesis1981, 887–888.10.1055/s-1981-29633Search in Google Scholar
[71] Sabitha, G.; Subba Rao, A. V. Synthesis of 3-arylcoumarins, 2-aroylbenzofurans and 3-aryl-2H-1,4-benzoazines under phase-transfer catalysis conditions. Synth. Commun. 1987, 17, 341–354.Search in Google Scholar
[72] Rao, P.; Reddy, K.; Ashok, D. A facile synthesis of 2-benzoyl-3-methyl-6-phenyl-5-(substituted Styryl)-7H-furo [3,2-g] [1] benzopyran-7-ones and their antifeedant activity. Synth. Commun. 1997, 27, 3181–3189.Search in Google Scholar
[73] Reddy, K.; Sabitha, G.; Subba Rao, A. V. Synthesis of 6H-bis-[1]-benzopyrano[2,3-b:3′,4′-e]pyridin-8(8H)ones and 3-(2′-hydroxybenzoyl)-5H-[1]benzopyrano[4,3-b]pyridines and their derivatives. Org. Prep. Proc. Int. 1996, 28, 325–332.Search in Google Scholar
[74] Walker, G. N. Reduction of phenols. New synthesis of oxyhexahydro-3-ketophenanthrenes by cyclodehydration of 4-(ß-arylethyl)-1,3-cyclohexandiones. J. Am. Chem. Soc. 1958, 80, 645–652.Search in Google Scholar
[75] Salman, M.; Ray, S.; Agarwal, A. K.; Durani, S.; Setty, B. S.; Kamboj, V. P.; Anand, N. Antifertility agents. 38. Effect of the side chain and its position on the activity of 3,4-diarylchromans. J. Med. Chem. 1983, 26, 592–595.Search in Google Scholar
[76] Zhang, X.; Sui, Z. A convenient synthesis of 3-pyridinyl- and 3-pyrimidinylcoumarins. Synthesis2006, 2568–2572.10.1055/s-2006-942461Search in Google Scholar
[77] Waldmann, H.; Kuehn, M.; Liu, W.; Kumar, K. Reagent-controlled domino synthesis of skeletally-diverse compound collections. Chem. Commun. 2008, 1211–1213.10.1039/b717635jSearch in Google Scholar PubMed
[78] Oliveira, A. M.; Raposo, M.; Oliveira-Campos, A. M. F.; Machado, A. E. H.; Puapairoj, P.; Pedro, M.; Nascimento, M. S. J.; Portela, C.; Afonso, C.; Pinto, M. Psoralen analogues: synthesis, inhibitory activity of growth of human tumor cell lines and computational studies. Eur. J. Med. Chem. 2006, 41, 367–372.Search in Google Scholar
[79] Mashraqui, S. H.; Vashi, D.; Mistry, H. D. Efficient synthesis of 3-substituted coumarins. Synth. Commun. 2004, 34, 3129–3134.Search in Google Scholar
[80] Wolfbeis, O. S.; Marhold, H. Syntheses, absorption and fluorescence spectra of 7-hydroxy-3-pyridylcoumarins, their esters, ethers, and quaternized derivatives. Chem. Ber. 1985, 118, 3664–3672Search in Google Scholar
[81] Takechi, H.; Kamada, S.; Machida, M. 3-[4-(Bromomethyl)phenyl]-7-(diethylamino)-2H-1-benzopyran-2-one (MPAC-Br): a highly sensitive fluorescent derivatization reagent for carboxylic acids in high-performance liquid chromatography. Chem. Pharm. Bull. 1996, 44, 793–799.Search in Google Scholar
[82] Lee, S.; Sivakumar, K.; Shin, W.-S.; Xie, F.; Wang, Q. Synthesis and anti-angiogenesis activity of coumarin derivatives. Bioorg. Med. Chem. Lett. 2006, 16, 4596–4599.Search in Google Scholar
[83] Brufola, G.; Fringuelli, F.; Piermatti, O.; Pizzo, F. Simple and efficient one-pot preparation of 3-substituted coumarins in water. Heterocycles1996, 43, 1257–1266.Search in Google Scholar
[84] Fuson, R. C.; Kneisley, J. W.; Kaiser, E. W. Coumarilic acid. Org. Synth. 1944, 24, 33.Search in Google Scholar
[85] Kim, K.-M.; Park, I.-H. A convenient halogenation of α,β-unsaturated carbonyl compounds with OXONE® and hydrohalic acid (HBr, HCl). Synthesis2004, 2641–2644.10.1055/s-2004-831232Search in Google Scholar
[86] Brown, E. V. The Willgerodt reaction. Synthesis1975, 358–375.10.1055/s-1975-23756Search in Google Scholar
[87] Press, J. B.; McNally, J. J. Thiophene systems. 10. The synthesis and chemistry of some thienopyridinols. J. Heterocycl. Chem. 1988, 25, 1571–1581.Search in Google Scholar
[88] Darabi, H. R.; Aghapoora, K.; Tajbakhsh, M. Extension of the Willgerodt-Kindler reaction: protected carbonyl compounds as efficient substrates for this reaction. Tetrahedron Lett. 2004, 45, 4167–4169.Search in Google Scholar
[89] Rolfs, A.; Liebscher, J. 3-Morpholino-2-phenylthioacrylic acid morpholide and 5-4-bromobenzoyl-2-(4-morpholino)-3-phenylthiophene (morpholine, 4-[3-(4-morpholinyl)-2-phenyl-1-thioxo-2-propenyl]-,) and (thiophene, 5-(4-bromobenzoyl)-2-(4-morpholino)-3-phenyl-). Org. Synth. 1997, 74, 257–262.Search in Google Scholar
[90] Thiault, G. A.; Le Guen, Y.; Boucherle, A.; Walrant, P. N-Aryl pyrrole derivatives with analgesic and antiinflammatory activity. Part II. Variations on the 1-arylpyrrole model. Farm. Ed. Sci. 1984, 39, 765–780.Search in Google Scholar
[91] Roue, N.; Levy, J.; Barret, R. Efficient synthesis of 1,3,4,5-tetrahydropyrrolo-[4,3,2-de]quinolone. Synth. Commun. 1995, 25, 681–690.Search in Google Scholar
[92] Hu, S.; Huang, Y.; Poss, M. A.; Gentles, R. G. A novel route to the 5,6-dihydro-4-H-thieno[3,2-b]pyrrol-5-one ring system involving an intermediate substituted-thiophene synthesis. J. Heterocycl. Chem. 2005, 42, 661–667.Search in Google Scholar
[93] Biava, M.; Porretta, G. C.; Cappelli, A.; Vomero, S.; Manetti, F.; Botta, M.; Sautebin, L.; Rossi, A.; Makovec, F.; Anzini, M. 1,5-Diarylpyrrole-3-acetic acids and esters as novel classes of potent and highly selective cyclooxygenase-2 inhibitors. J. Med. Chem. 2005, 48, 3428–3432.Search in Google Scholar
[94] Fabrichnyi, B. P.; Shalavina, I. F.; Gol′dfarb, Y. L. J. Org. Chem. USSR1979, 15, 1371.Search in Google Scholar
[95] Yamaguchi, T.; Irie, M. One pot synthesis of photochromic maleic anhydride derivatives. Chem. Lett. 2005, 34, 64–65.Search in Google Scholar
[96] Irie, M.; Lifka, T.; Uchida, K.; Kobatake, S.; Shindo, Y. Fatigue resistant properties of photochromic dithienylethenes: by-product formation. Chem. Commun. 1999, 747–748.10.1039/a809410aSearch in Google Scholar
[97] Xiao, S.; Yi, T.; Li, F.; Huang, C. A multi-photo responsive photochromic dithienylethene containing coumarin derivative. Tetrahedron Lett. 2005, 46, 9009–9012.Search in Google Scholar
[98] Traven, V. F.; Bochkov, A. Y.; Krayushkin, M. M.; Yarovenko, V. N.; Nabatov, B. V.; Dolotov, S. M.; Barachevsky, V. A.; Beletskaya, I. P. Coumarinyl(thienyl thiazoles – novel photochromes with modulated fluorescence. Org. Lett. 2008, 10, 1319–1322.Search in Google Scholar
[99] Bochkov, A. Y.; Yarovenko, V. N.; Barachevskii, V. A.; Nabatov, B. V.; Krayushkin, M. M.; Dolotov, S. M.; Traven, V. F.; Beletskaya, I. P. Coumarinyl(thienyl)thiazoles as new fluorescent molecular photoswitches. Russ. Chem. Bull. 2009, 58, 162–169.Search in Google Scholar
[100] Traven, V. F.; Bochkov, A. Y.; Krayushkin, M. M.; Yarovenko, V. N.; Barachevsky, V. A.; Beletskaya, I. P. Novel photochromic 3-(3-coumarinyl)-4-(3-thienyl)maleic acid cyclic derivatives. Mend. Commun. 2010, 20, 22–24.Search in Google Scholar
[101] Suzuki, K.; Ubukata, T.; Yokoyama, Y. Dual-mode fluorescence switching of photochromic bisthiazolylcoumarin. Chem. Commun. 2012, 48, 765–767.Search in Google Scholar
[102] Zhang, L.; Meng, T.; Fan, R.; Wu, J. General and efficient route for the synthesis of 3,4-disubstituted coumarins via Pd-catalyzed site-selective cross-coupling reactions. J. Org. Chem. 2007, 72, 7279–7286.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety
Articles in the same Issue
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety

