Abstract
Selected 6- and 7-carboxyflavins activate H2O2, oxidizing thioanisole to its sulfoxide. The greater reactivity of the 6-carboxyflavin derivatives are ascribed to the intramolecular hydrogen bonding between N(5) and the carboxyl group of the flavin system.
Introduction
Flavoenzymes are a ubiquitous and diverse class of biological redox catalysts [1, 2]. The active site responsible for the activation of molecular oxygen has been established as the enzyme-bound 4a-hydroperoxyflavin [1–4]. One of the important roles of the proteins has been suggested to stabilize the intermediate 4a-hydroperoxyflavins by hydrogen bonds of the N(5) to the proteins. Otherwise, the 4a-hydroperoxyflavins undergo spontaneous elimination of H2O2 to yield the oxidized flavins [5]. Thus, the intramolecular hydrogen bonding of N(5) to the carboxylic acid group at C(6) in the flavin system has been anticipated to stabilize the 4a-hydroperoxide intermediate by suppression of the elimination of H2O2 (Scheme 1). The model reactions for flavoenzymes performed so far have used the flavinium derivatives for the purpose of the stabilization of the 4a-hydroperoxyflavins as well as flavin semiquinone radicals [6–9]. Also, the oxidation of thiols to disulfides, which involves nucleophilic attack to C-4a position of isoalloxazine, is stimulated by the activation of C-4a position through hydrogen bonding [10].
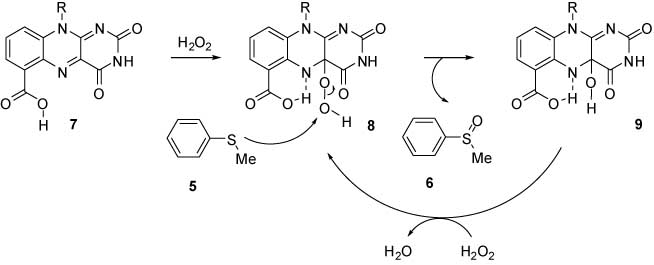
In this work, to obtain an insight into the role of hydrogen bonding to control the reactivities of flavin coenzymes, the oxidation of thioanisole (5) in the presence of carboxyflavins 1–4 has been studied.
Results and discussion
The reaction of thioanisole (5) with H2O2 in acetonitrile in the presence of flavin derivatives (1–4, Figure 1) afforded thioanisole S-oxide (6) in varying yields (Table 1). In the absence of the flavin derivatives, only 10% of the sulfide 5 was oxidized. In the presence of flavin derivatives without the carboxyl group (compounds 1 and 2), the conversion was only 9–12%. This shows that the oxidized flavin without the carboxy group does not form 4a-hydroperoxide intermediate and, as such, does not play a role in the oxygenation of sulfides. Further, the reaction of 5 with H2O2 in the presence of 10-butyl-6-carboxyflavin (4a) gave the S-oxide 6 in 65% yields, whereas the same reaction in the presence of 10-butyl-7-carboxyflavin (3a) afforded 6 in a much smaller yield of 35% (Table 1). Similar results have been obtained with decyl substituted flavins (Table 1). These results suggest that the intramolecular hydrogen bonding of N(5) to the carboxyl group at C-6 is essential for this type of activation of H2O2 by the flavin derivatives. The stabilization of 4a-hydroperoxide intermediate is found to be optimal with carboxylate group at C(6) in comparison with C(7). The C(7) derivative may be involved in a much weaker intermolecular hydrogen bond. Further, the esterification of the carboxyl groups of flavin 4b leads to the loss of reactivity (entry 10, Table 1), again suggesting the role of hydrogen bonding in the activation of H2O2. The generation of the intermediate C4a-hydroperoxyflavin is consistent with the appearance of absorption at λ = 384 nm in the UV-visible spectrum [11]. The role of hydrogen bonding in the activation of H2O2 was further confirmed in the experiments conducted in the presence of benzoic acid, which may form hydrogen bonding between N(5) and activate the 4a position. The addition of benzoic acid results in the enhancement of yields from 9–12% to 42–45%.

Flavins used in this study.
Oxidation of thioanisole (5) with flavin and H2O2 in acetonitrile at 25–30°C.

| Entry | System | Isolated yield of 6 (%) |
|---|---|---|
| 1 | 5/H2O2 | 10 |
| 2 | 5/H2O2/10-butylflavin (1) | 9 |
| 3 | 5/H2O2/10-decylflavin (2) | 12 |
| 4 | 5/H2O2/10-butylflavin (1)/benzoic acid | 42 |
| 5 | 5/H2O2/10-decylflavin (2)/benzoic acid | 45 |
| 6 | 5/H2O2/10-butyl-7-carboxylflavin (3a) | 35 |
| 7 | 5/H2O2/7-carboxy-10-decylflavin (3b) | 28 |
| 8 | 5/H2O2/10-butyl-6-carboxyflavin (4a) | 65 |
| 9 | 5/H2O2/6-carboxy-10-decylflavin (4b) | 68 |
| 10 | 5/H2O2/6-methoxycarbonyl-10-decylflavin (4c) | 10 |
| 11 | 5/H2O2/4c/benzoic acid | 40 |
| 12 | 5/H2O2/benzoic acid | 34 |
Flavin derivative, 0.15 mmol; thioanisole, 0.3 mmol; H2O2, 0.3 mmol; CH3CN, 15 mL; benzoic acid, 0.15 mmol.
Experimental
General
The flavins 1–3 have been synthesized by the acidic cyclocondensation of corresponding N-substituted-2-aminoanilines with alloxan monohydrate [12, 13]. The flavins 4 have been synthesized by following the literature procedure [14]. The purities of the compounds were determined on silica-coated Al plates (Merck).
Reaction of thioanisole (5) with H2O2 in the presence of flavins
A solution of a flavin 1–4 (0.15 mmol) and a thioanisole (0.3 mmol) in acetonitrile (15 mL) was treated with 30% H2O2 (70 μL, 0.3 mmol). Benzoic acid (0.15 mmol) was occasionally used (Table 1). The mixture was stirred for 24 h at room temperature under nitrogen, then poured into dichloromethane (100 mL) and washed with saturated solution of NaHCO3. The organic layer was dried over anhydrous sodium sulfate and the solvent was removed under reduced pressure. The residue was purified by preparative thin layer chromatography to yield the S-oxide 6.
The author Geetanjali is thankful to University Grant Commission (UGC), New Delhi, India for the financial support through minor project.
References
[1] Muller, F. Chemistry and Biochemistry of Flavoenzymes; Muller, F., Ed. CRC Press: Boca Raton, FL, 1991; Vol. 1, pp. 1–77.Search in Google Scholar
[2] Stankovich, M. T. Chemistry and Biochemistry of Flavoenzymes; Muller, F., Ed. CRC Press: Boca Raton, FL, 1991; Vol. 1, pp. 401–425.Search in Google Scholar
[3] Bruice, T. C. Mechanisms of flavin catalysis. Acc. Chem. Res. 1980, 13, 256–262.Search in Google Scholar
[4] Imada, Y.; Iida, H.; Ono, S.; Murahashi, S.-I. Flavin catalyzed oxidations of sulfides and amines with molecular oxygen. J. Am. Chem. Soc. 2003, 125, 2868–2869.Search in Google Scholar
[5] Entsch, B.; Ballou, D. P.; Massey, V. Flavin-oxygen derivatives involved in hydroxylation by p-hydroxybenzoate hydroxylase. J. Biol. Chem. 1976, 251, 2550–2563.Search in Google Scholar
[6] Jonsson, S. Y.; Farnegardh, K.; Backvall, J. E. Osmium-catalyzed asymmetric dihydroxylation of olefins by H2O2 using a biomimetic flavin-based coupled catalytic system. J. Am. Chem. Soc. 2001, 123, 1365–1371.Search in Google Scholar
[7] Chaudhary, S.; Awasthi, A.; Chauhan, S. M. S. Biomimetic oxidations of nicotine with hydrogen peroxide and 5-ethylflavin mononucleotide perchlorate. Ind. J. Chem. 1998, 37B, 294–297.Search in Google Scholar
[8] Kemal, C.; Chan, T. W.; Bruice, T. C. Reaction of 3O2 with dihydroflavins. 1. N3,5-Dimethyl-1,5-dihydrolumiflavin and 1,5-dihydroisoalloxazines. J. Am. Chem. Soc. 1977, 99, 7272–7286.Search in Google Scholar
[9] Kemal, C.; Bruice, T. C. Simple synthesis of a 4a-hydroperoxy adduct of a 1,5-dihydroflavine: preliminary studies of a model for bacterial luciferase. Proc. Natl. Acad. Sci. USA 1976, 73, 995–999.10.1073/pnas.73.4.995Search in Google Scholar PubMed PubMed Central
[10] Ball, S.; Bruice, T. C. Oxidation of amines by a 4a-hydroperoxyflavin. J. Am. Chem. Soc. 1980, 102, 6498–6503.Search in Google Scholar
[11] Ballou, D. P.; Entsch, B.; Cole, L. J. Dynamics involved in catalysis by single-component and two-component flavin-dependent aromatic hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 590–598.Search in Google Scholar
[12] Chauhan, S. M. S.; Geetanjali; Singh, R. A mild and efficient synthesis of 10-substituted isoalloxazines in the presence of solid acids. Ind. J. Heterocycl. Chem. 2000, 10, 157–158.Search in Google Scholar
[13] Chauhan, S. M. S.; Singh, R.; Geetanjali. Microwave assisted synthesis of 10-substituted isoalloxazines in the presence of solid acids. Synth. Commun. 2003, 33, 1179–1184.Search in Google Scholar
[14] Akiyama, T.; Simeno, F.; Murakami, M.; Yoneda, F. Flavin-6-carboxylic acids as novel and simple flavoenzyme models. Nonenzymatic stabilization of the flavin semiquinone radical and the 4a-hydroperoxyflavin by intramolecular hydrogen bonding. J. Am. Chem. Soc. 1992, 114, 6613–6620.Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety
Articles in the same Issue
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety

