Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
-
Karine A. Eliazyan
Abstract
2-Arylsulfonylimino-3,4-dimethyl-2,3-dihydrothiazole-5-carboxylic acid hydrazides 2 were obtained from corresponding 2-arylsulfonylimino-4-methyl-2,3-dihydrothiazole-5-carboxylic acid ethyl esters 1 in a two-step synthesis. Heterocyclization of the hydrazide moiety yielded 2-arylsulfonylimino-3,4-dimethyl-5-(5-thioxo-4,5-dihydro-[1,3,4]oxadiazol-2-yl)-2,3-dihydro-thiazoles 3. Treatment of compounds 3 with various alkyl halides gave corresponding S-substituted derivatives 4–6. The plant growth stimulant activities of synthesized compounds were compared with those of similar [1,2,4]triazol- and [1,3,4,]thiadiazol-2-yl-thiazoles.
Introduction
Derivatives of N,S,O-containing five-membered heterocycles, in particular thiazole and [1,3,4]oxadiazole, exhibit a broad spectrum of biological activity. In agriculture, the thiazole derivatives are widely used for chemical means of plant protection and growth regulation. They are known as herbicides (benazolin, benzthiazuron, fenthiaprop mefenacet, metabenzthiazuron, thiazopir), fungicides (ethaboxam, fluthianil, isotianil, metsulfovax, octhilinone, thiabendazole, thiadifluor, thifluzamide,), insecticides (clothianidin, imidaclothiz, tazimcarb, thiacloprid, thiamethoxam, thiapronil), acaricides (flubenzimine, hexythiazox), and bactericides (amicarthiazol). The arsenal of pesticides based on [1,3,4]oxadiazole is more limited and includes oxadiazolone herbicides (dimefuron, oxadiargyl, oxadiazon) and insecticide (metoxadiazone) (http://www.alanwood.net/pesticides/class_pesticides.html). However, the search for new biologically active compounds continues among the new, previously unexplored series of thiazole [1–9] and [1,3,4]oxadiazole derivatives [10–21].
The aim of the present investigation was to develop facile and efficient methods for the synthesis of non-condensed 2-arylsulfonylimino-substituted biheterocyclic systems with combination of [1,3,4]oxadiazole and thiazole rings and to study their physiological activity.
Results and discussion
Synthesis
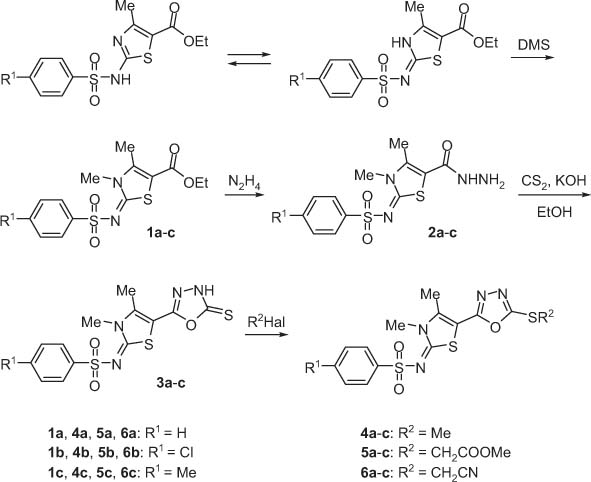
The treatment of 2-arylsulfonylimino-4-methyl-2,3-dihydrothiazole-5-carboxylic acid ethyl esters with dimethyl sulfate gave the corresponding N-methylated derivatives 1a–c (Scheme 1). In principle, alkylation can proceed on both exocyclic and endocyclic nitrogen atoms of the substrate. The structure determination could not be based on analysis of 13C NMR spectra due to lack of model compounds. Therefore, X-ray diffraction analysis of methyl derivative 1a was carried out, which showed that methylation occurs exclusively at the endocyclic nitrogen atom (Figure 1). The reaction of compounds 1a–c with hydrazine hydrate yielded corresponding hydrazides 2a–c. By their heterocyclization with carbon disulfide and potassium hydroxide in ethanol 2-arylsulfonylimino-3,4-dimethyl-5-(5-thioxo-4,5-dihydro-[1,3,4]oxadiazol-2-yl)-2,3-dihydro-thiazoles 3a–c were obtained. These compounds have thione structure because their 13C NMR spectra contain the signal for the C=S moiety at 176 ppm. Treatment of 3a–c with dimethyl sulfate, chloroacetic acid methyl ester or chloroacetonitrile yielded the S-substituted derivative 4a–c, 5a–c, or 6a–c, respectively. Alkylation of these compounds occurs at the sulfur atom because 13C NMR spectra display new signals for a C=N bond of [1,3,4]oxadiazole and the C=S signals are not observed.

Molecular structure of compound 1a.
Biological activity
The object of study was the seeds and seedlings of dicotyledonous common bean (Phaseolus vulgaris L.) collected in September 2012 from the Syunik region of Armenia. At preliminary screening, the possible herbicidal, fungicidal, and growth regulatory activities of novel compounds 3–6 were studied. All preparations did not show noticeable herbicidal or antifungal properties, but they showed a growth stimulant effect. Subsequently, more detailed studies of the activity of compounds 3–6 on the germination, growth, and survivability of seeds and seedlings of common bean were performed.
In the first set of experiments, the effect of aqueous solutions of compounds 3–6 and heteroauxin (indole-3-acetic acid, IAA) in concentrations of 25 and 50 mg/L was studied. The seeds were incubated for 24 h in an appropriate medium in the dark at 25°C. Then, the seeds were transplanted into soil and watered daily.
In the second experimental setup, the bean seeds were placed in the soil in small vessels. When the length of the stems reached 15–20 cm, the plants were dug out, the root parts were washed with water and cut off. A series of 8–10 cut plants were immersed in the prepared aqueous solutions of IAA or 3–6 at concentrations of 25 mg/L and 50 mg/L. After 24 h, they were washed and dipped into the vessels with water. Water in the vessels was changed every day. For every compound, the two experimental schemes were repeated three times. The experiments were conducted for 20–25 days. The number of plant roots of each series, and their length and weight in the moist and dry forms were calculated. The results were compared with similar data of plants placed in IAA solutions, and the activities of preparations in comparison with IAA (in %) were determined (Table 1). The data show that the growth stimulant properties increase in the following order 4 ≈ 6 < 3 < 5.
Growth stimulant activity of compounds 3–6.
| Compound | Concentration, mg/L | Activity |
| IAA | 50 | 100 |
| 25 | 100 | |
| 3a | 50 | 74.6 |
| 25 | 75.3 | |
| 3b | 50 | 73.8 |
| 25 | 63.4 | |
| 3c | 50 | 47.2 |
| 25 | 49.5 | |
| 4a | 50 | 60.5 |
| 25 | 55.5 | |
| 4b | 50 | 40.2 |
| 25 | 41.7 | |
| 4c | 50 | 41.1 |
| 25 | 42.2 | |
| 5a | 50 | 93.0 |
| 25 | 85.1 | |
| 5b | 50 | 73.8 |
| 25 | 62.1 | |
| 5c | 50 | 81.7 |
| 25 | 59.6 | |
| 6a | 50 | 62.3 |
| 25 | 79.9 | |
| 6b | 50 | 60.1 |
| 25 | 73.5 | |
| 6c | 50 | 42.6 |
| 25 | 60.5 |
Conclusions
This research is the continuation of our previous efforts on the synthesis of new non-condensed biheterocyclic systems with 1,2,3-triazole, 1,3,4-thiadiazole, or 1,3,4-oxadiazole moieties, additionally substituted with a thiazolyl, arylsulfonylimino [22], arylsulfonylethylamino [23], or thioxo [24–26] group. Practically, all derivatives of these systems manifest growth stimulant activity, in certain cases equal to the activity of heteroauxin. The triazolyl-thiazole derivatives also possess fungicidal properties [24]. The results of preliminary bioassays show that the obtained new compounds show promise for the development as new growth stimulators and fungicides.
Experimental
General
1H NMR (300 MHz) and 13C NMR (75 MHz, decoupled and coupled modes) spectra were recorded on a Varian Mercury 300 spectrometer in the mixture of DMSO-d6 and CCl4 (1:3) or in pure DMSO-d6. The reaction progress and purity of the obtained substances were checked by using TLC on ‘Silufol UV-254’ plates with an acetone/hexane mixture (2:1) as eluent. Melting points were determined in open capillaries and are uncorrected. An X-ray diffraction experiment was carried out at room temperature on an Enraf-Nonius automatic diffractometer CAD-4.
General procedure for 1а–с
2-Arylsulfonylimino-3-methylthiazol-5-carboxylic acid ethyl ester potassium salts were obtained by treatment of esters (0.01 mol) with KOH (0.01 mol) at 0°C. These products (0.01 mol) in water (10 mL) were allowed to react with dimethyl sulfate for 24 h at 20–25°C. The precipitate was filtered off, washed with water, and dried.
2-Benzenesulfonylimino-3,4-dimethyl-2,3-dihydrothiazol-5-carboxylic acid ethyl ester (1a)
Flesh-colored crystals; mp 125–127°C; yield 3.05 g (90%); 1H NMR: δ 1.36 (t, 3H, J = 7.0 Hz, OCH2CH3), 2.58 (s, 3H, 4-CH3), 3.47 (s, 3H, 3-NCH3), 4.32 (q, 2H, J = 7.0 Hz, OCH2CH3), 7.45–7.90 (m, 5H, C6H5). Anal. Calcd for C14H16N2O4S2: C, 49.40; H, 4.74; N, 8.23; S, 18.84. Found: C, 49.26; H, 4.63; N, 7.88; S, 18.48.
Crystal data for 1a
C14H14N4O3S3, monoclinic, space group P21/c, a = 10.914(2), b = 15.176(3), c = 10.285(2) Å, β = 92.45(3)°, V = 1702.0(6) Å3, Z = 4, T = 293(2)K, dx = 1.493 g cm-3, μ(MoKα) = 0.71073 mm-1, 4967 data were collected up to θmax = 30° (Rint= 0.0675, Rσ= 0.1575). Final R-indices for 1802 reflections with I > 2σ(I) and 220 refined parameters are: R1 = 0.0626, wR2 = 0.1150 (R1 = 0.2395, wR2 = 0.1532 for all 4967 data). Crystallographic data for compound 1a have been deposited with the Cambridge Crystallographic Data Centre, deposition no. CCDC 940247.
2-(4-Chlorobenzenesulfonylimino)-3,4-dimethyl-2,3-dihydrothiazole-5-carboxylic acid ethyl ester (1b)
Flesh-colored crystals; mp 156–158°C; yield 3.08 g (82%); 1H NMR: δ 1.35 (t, 3H, J = 7.0 Hz, OCH2CH3), 2.59 (s, 3H, 4-CH3), 3.48 (s, 3H, 3-NCH3), 4.31 (q, 2H, J = 7.0 Hz, OCH2CH3), 7.47–7.87 (m, 4H, C6H4). Anal. Calcd for C14H15СlN2O4S2: C, 44.86; H, 4.03; N, 7.47; S, 17.11. Found: C, 44.72; H, 3.91; N, 7.08; S, 16.77.
2-(4-Methylbenzenesulfonylimino)-3,4-dimethyl-2,3-dihydrothiazole-5-carboxylic acid ethyl ester (1c)
Flesh-colored crystals; mp 184–186°C; yield: 3.18 g (90%); 1H NMR: δ 1.35 (t, 3H, J = 7.0 Hz, OCH2CH3), 2.42 (s, 3H, CH3C6H4), 2.61 (s, 3H, 4-CH3), 3.48 (s, 3H, NCH3), 4.30 (q, 2H, J = 7.0 Hz, OCH2CH3), 7.26–7.75 (m, 4H, C6H4); 13C NMR (coupled): δ 12.9 (4-CH3); 14.0 (OCH2CH3), 20.8 (CH3C6H4), 32.7 (3-NCH3), 61.2 (OCH2CH3), 106.0 (C-5; 3J = 4.2 Hz), 125.9; 129.4, 138.7; 146.6, (Ar); 142.8 (C-4; 2J = 6.6 Hz), 160.4 (С=О, 3J = 3.2 Hz), 165.2 (С-2, 3J = 3.2 Hz). Anal. Calcd for C15H18N2O4S2: C, 50.83; H, 5.12; N, 7.90; S, 18.09. Found: C, 50.60; H, 5.06; N, 7.69; S, 17.88.
General procedure for 2а–c
The suspension of 2-arylsulfonylimino-3,4-dimethylthiazol-5-carboxylic acid ethyl ester 1 (0.01 mol) in 15 mL of hydrazine hydrate (48%) was stirred at 20°С for 2 days. The precipitate was filtered off, washed with water, and dried. Compounds 2 were purified by boiling in hexane.
2-Benzenesulfonylimino-3,4-dimethyl-2,3-dihydrothiazole-5-carboxylic acid hydrazide (2a)
White crystals; mp 174–176°C (with decomp.); yield 2.28 g (70%); 1H NMR: δ 2.45 (s, 3H, 4-CH3), 3.42 (s, 3H, 3-NCH3), 4.50 and 4.84 (brs, 2H, NH2), 7.50–7.87 (m, 5H, C6H5), 8.85 and 9.50 (brs, 1H, NH); 13C NMR: δ 13.0 (4-CH3), 32.7 (3-NCH3), 109.7 (C-5), 125.9, 128.9, 132.3, 140.0 (Ph), 141.7 (C-4), 160.2 (C=O), 164.8 (C-2). Anal. Calcd for C12H14N4O3S2: C, 44.16; H, 4.32; N, 17.17; S, 19.65. Found: C, 44.02; H, 4.20; N, 16.89; S, 19.44.
2-(4-Chlorobenzenesulfonylimino)-3,4-dimethyl-2,3-dihydrothiazole-5-carboxylic acid hydrazide (2b)
White crystals; mp 191–193°C (with decomp.); yield 3.25 g (90%); 1H NMR: δ 2.50 (s, 3H, 4-CH3), 3.43 (s, 3H, 3-NCH3), 4.50 and 4.80 (brs, 2H, NH2), 7.61–7.92 (m, 4H, C6H4), 8.80 and 9.40 (brs, 1H, NH); 13C NMR: δ 13.0 (4-CH3), 32.7 (3-NCH3), 109.7 (C-5), 127.8, 129.3, 137.3, 140.1 (Ar), 141.7 (C-4), 160.2 (C=O), 164.8 (C-2). Anal. Calcd for C12H13ClN4O3S2: C, 39.94; H, 3.63; N, 15.53; S, 17.77. Found: C, 39.85; H, 3.54; N, 15.30; S, 17.52.
2-(4-Methylbenzenesulfonylimino)-3,4-dimethyl-2,3-dihydrothiazole-5-carboxylic acid hydrazide (2c)
White crystals; mp 190–192°C (with decomp.); yield 2.70 g (79%); 1H NMR: δ 2.36 (s, 3H, PhCH3), 2.45 (s, 3H, 4-CH3), 3.41 (s, 3H, 3-NCH3), 4.51 and 4.83 (brs, 2H, NH2), 7.30–7.78 (m, 4H, C6H4), 8.83 and 9.49 (brs, 1H, NH); 13C NMR (coupled): δ 13.0 (4-CH3), 20.9 (PhCH3), 32.6 (2-NCH3), 109.6 (m, C-5), 125.9, 129.4, 138.9 (t, 2J =8.3 Hz), 140.0 (m) (Ar), 142.6 (q, 3J = 6.6 Hz, C-4), 160.3 (brs, C=O), 164.7 (q, 3J = 3.0 Hz, C-2). Anal. Calcd for C13H16N4O3S2: C, 45.87; H, 4.74; N, 16.46; S, 18.84. Found: C, 45.69; H, 4.62; N, 16.20; S, 18.61.
General procedure for 3а–с
To a mixture of hydrazide 2 and 1.2 mL (0.02 mol) of carbon disulfide in 20 mL of ethanol, at boiling, 1.12 g (0.02 mol) of KOH in 10 mL of ethanol was added dropwise with continuous stirring. After 7 h ethanol was evaporated, 10 mL of water was added, and the resultant suspension was acidified with HCl to pH 4. After 2–3 h the precipitate was filtered off, washed with water, and purified by boiling in ethanol (50%).
2-Benzenesulfonylimino-3,4-dimethyl-5-(5-thioxo-4,5-dihydro-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (3a)
Yellow crystals; mp 236–238°C (with decomp.); yield 2.87 g (78%); 1H NMR: δ 2.60 (s, 3H, 4-CH3), 3.55 (s, 3H, 3-NCH3), 7.50–7.90 (m, 5H, C6H5), 14.50 (brs, 1H, NH); 13C NMR (coupled): δ 13.2 (4-CH3), 32.8 (3-NCH3), 99.0 (q, 3J = 4.8 Hz, C-5), 125.8, 128.4, 131.8, 140.3 (Ph), 141.5 (q, 2J = 6.6 Hz, C-4), 154.5 (C-2′), 164.6 (q, 3J = 3.1 Hz, C-2), 176.4 (C=S). Anal. Calcd for C13H12N4O3S3: C, 42.38; H, 3.28; N, 15.21; S, 26.11. Found: C, 42.21; H, 3.16; N, 15.00; S, 25.92.
2-(4-Chlorobenzenesulfonylimino)-3,4-dimethyl-5-(5-thioxo-4,5-dihydro-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (3b)
Yellow crystals; mp 242–244°C (with decomp.); yield 3.30 g (82%); 1H NMR: δ 2.61 (s, 3H, 4-CH3), 3.53 (s, 3H, 3-NCH3), 7.60–7.92 (m, 4H, C6H4), 14.55 (brs, 1H, NH); 13C NMR (coupled): δ 13.12 (4-CH3), 32.8 (3-NCH3), 99.3 (q, 3J = 4.8 Hz, C-5), 127.6, 128.5, 137.5, 140.1 (Ar), 140.3 (q, 2J = 6.6 Hz, C-4), 154.3 (C-2′), 160.0 (q, 3J= 3.1 Hz, C-2), 176.35 (C=S). Anal. Calcd for C13H11ClN4O3S3: C, 38.75; H, 2.75; N, 13.91; S, 23.88. Found: C, 38.62; H, 2.69; N, 13.70; S, 23.61.
2-(4-Methylbenzenesulfonylimino)-3,4-dimethyl-5-(5-thioxo-4,5-dihydro-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (3c)
Yellow crystals; mp 242–244°C (with decomp.); yield 3.30 g (82%); 1H NMR: δ 2.43 (s, 3H, PhCH3), 2.60 (s, 3H, 4-CH3), 3.53 (s, 3H, 3-NCH3), 7.28–7.78 (m, 4H, C6H4), 14.50 (brs, 1H, NH); 13C NMR (coupled): δ 13.3 (4-CH3), 20.9 (PhCH3), 33.1 (2-NCH3), 98.6 (q, 3J = 4.8 Hz, C-5), 125.9, 129.4, 138.4, 141.4 (Ar), 142.7 (q, 2J = 6.6 Hz, C-4), 154.9 (C-2′), 164.6 (q, 3J = 3.2 Hz, C-2), 176.4 (C=S). Anal. Calcd for C14H14N4O3S3: C, 43.96; H, 3.69; N, 14.65; S, 25.15. Found: C, 43.82; H, 3.57; N, 14.31; S, 24.85.
General procedure for 4а–с
To a solution of compound 3 (0.01 mol) and KOH (0.01 mol) in 20 mL of water at 20°C, 1 mL (0.01 mol) of dimethyl sulfate was added portion-wise. The mixture was stirred at 20°С for 24 h, and the resultant precipitate of compound 3 was filtered off, washed with water, dried, and crystallized from benzene.
2-Benzenesulfonylimino-3,4-dimethyl-5-(5-methylsulfanyl-[1,3,4]oxadiazol-2-yl-)-2,3-dihydro-thiazole (4a)
White crystals; mp 180–182°C; yield 2.98 g (78%); 1H NMR: δ 2.62 (s, 3H, 4-CH3), 2.78 (s, 3H, SCH3), 3.57 (s, 3H, 3-NCH3), 7.48–7.90 (m, 5H, C6H5); 13C NMR: δ 13.1 (4-CH3), 14.1 (SCH3), 32.8 (3-NCH3), 99.3 (C-5), 125.7, 128.2, 131.6, 139.6 (Ph); 141.5 (C-4), 158.9 (C-2′), 163.3 (C-5′), 164.7 (C-2). Anal. Calcd for C14H14N4O3S3: C, 43.96; H, 3.69; N, 14.65; S, 25.15. Found: C, 43.82; H, 3.60; N, 14.32; S, 24.84.
2-(4-Chlorobenzenesulfonylimino)-3,4-dimethyl-5-(5-methylsulfanyl-[1,3,4]oxadiazol-2-yl-)-2,3-dihydrothiazole (4b)
White crystals; mp 158–160°C; yield 3.12 g (75%); 1H NMR: δ 2.57 (s, 3H, 4-CH3), 2.75 (s, 3H, SCH3), 3.50 (s, 3H, 3-NCH3), 7.60–7.92 (m, 4H, C6H4); 13C NMR (coupled): δ 13.3 (4-CH3), 14.3 (SCH3), 33.3 (3-NCH3), 99.3 (q, 3J = 4.8 Hz, C-5), 127.8, 129.2, 137.3, 140.2 (Ar), 141.0 (q, 2J = 6.6 Hz, C-4), 159.1 (C-2′), 164.0 (C-5′), 165.0 (q, 3J = 3.1 Hz, C-2). Anal. Calcd for C14H13ClN4O3S3: C, 40.33; H, 3.14; N, 13.44; S, 23.07. Found: C, 40.20; H, 3.05; N, 13.21; S, 22.79.
2-(4-Methylbenzenesulfonylimino)-3,4-dimethyl-5-(5-methylsulfanyl-[1,3,4]oxadiazol-2-yl-)-2,3-dihydrothiazole (4c)
White crystals; mp 230–232°C; yield 3.37 g (85%); 1H NMR: δ 2.42 (s, 3H, PhCH3), 2.62 (s, 3H, 4-CH3), 2.78 (s, 3H, SCH3), 3.54 (s, 3H, 3-NCH3), 7.27–7.78 (m, 4H, C6H4); 13C NMR: δ 13.1 (4-CH3), 14.2 (SCH3), 20.8 (PhCH3), 32.9 (3-NCH3), 99.1 (C-5); 125.8, 129.0; 138.6, 140.1 (Ar), 142.3 (C-4), 159.0 (C-2′), 163.6 (C-5′), 164.6 (C-2). Anal. Calcd for C15H16N4O3S3: C, 45.44; H, 4.07; N, 14.13; S, 24.26. Found: C, 45.22; H, 3.97; N, 13.88; S, 24.01.
General procedure for 5a–c and 6а–с
A potassium salt of 3a–c (0.01 mol) was obtained by treatment of compound 3a–c (0.01 mol) with KOH (0.01 mol) in 30 mL of acetone at 10–15°C. A suspension of this salt was treated with chloroacetic acid methyl ester or chloroacetonitrile (0.011 mol), and the mixture was heated at 50–60°C for 3 h. After filtration the solution was concentrated, and the residue was treated with water. The resultant precipitate was filtered off and dried. Compounds 5a and 6a were crystallized from benzene and others were purified by boiling in hexane.
2-Benzenesulfonylimino-3,4-dimethyl-5-(5-methoxycarbonyl-methylsulfanyl-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (5a)
White crystals; mp 168–169°C; yield 3.87 g (88%); 1H NMR: δ 2.62 (s, 3H, 4-CH3), 3.53 (s, 3H, 3-NCH3), 3.80 (s, 3H, OCH3), 4.20 (s, 2H, SCH2), 7.47–7.88 (m, 5H, C6H5); 13C NMR: δ 13.1 (4-CH3); 32.7 (3-NCH3); 33.5 (SCH2); 52.2 (OCH3); 99.2 (C-5); 125.7, 128.2, 131.6, 139.6 (Ph); 142.1 (C-4); 159.2 (C-2′); 162.3 (C-5′); 164.6 (C-2); 167.2 (C=O). Anal. Calcd for C16H16N4O5S3: C, 43.62; H, 3.66; N, 12.72; S, 21.84. Found: C, 43.50; H, 3.55; N, 12.46; S, 21.56.
2-(4-Chlorobenzenesulfonylimino)-3,4-dimethyl-5-(5-methoxycarbonyl-methylsulfanyl-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (5b)
White crystals; mp 190–192°C; yield 3.94 g (83%); 1H NMR: δ 2.60 (s, 3H, 4-CH3), 3.50 (s, 3H, 3-NCH3), 3.80 (s, 3H, OCH3), 4.20 (s, 2H, SCH2), 7.60–7.92 (m, 4H, C6H4); 13C NMR: δ 13.1 (4-CH3); 32.9 (3-NCH3); 33.4 (SCH2); 52.3 (OCH3); 99.2 (C-5); 127.8, 129.1, 137.3, 140.3 (Ar); 141.6 (C-4); 159.0 (C-2′); 162.5 (C-5′); 164.6 (C-2); 167.2 (C=O). Anal. Calcd for C16H15ClN4O5S3: C, 40.46; H, 3.18; N, 11.80; S, 20.25. Found: C, 40.29; H, 3.07; N, 11.52; S, 20.02.
2-(4-Methylbenzenesulfonylimino)-3,4-dimethyl-5-(5-methoxycarbonyl-methylsulfanyl-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (5c)
White crystals; mp 194–196°C; yield 4.10 g (90%); 1H NMR: δ 2.42 (s, 3H, PhCH3), 2.62 (s, 3H, 4-CH3), 3.55 (s, 3H, 3-NCH3), 3.78 (s, 3H, OCH3), 4.18 (s, 2H, SCH2), 7.25–7.78 (m, 4H, C6H4); 13C NMR: δ 13.1 (4-CH3), 20.8 (PhCH3), 32.8 (3-NCH3), 33.4 (SCH2), 52.2 (OCH3), 99.1 (C-5), 125.8, 128.8; 138.7, 140.0 (Ar), 142.0 (C-4), 159.2 (C-2′), 161.6 (C-5′), 164.6 (C-2), 167.1 (C=O). Anal. Calcd for C17H18N4O5S3: C, 44.92; H, 3.99; N, 12.33; S, 21.16. Found: C, 44.79; H, 3.88; N, 12.03; S, 20.90.
2-Benzenesulfonylimino-3,4-dimethyl-5-(5-cyanomethylsulfanyl-[1,3,4]oxadiazol-2-yl)-2,3-dihydro-thiazole (6a)
Flesh-colored crystals; mp 198–200°C; yield 3.38 g (83%); 1H NMR: δ 2.62 (s, 3H, 4-CH3), 3.55 (s, 3H, 3-NCH3), 4.42 (s, 2H, SCH2), 7.48–7.89 (m, 5H, C6H5); 13C NMR: δ 13.2 (4-CH3); 17.7 (SCH2); 32.7 (3-NCH3); 99.9 (C-5); 115.6 (C≡N); 125.7, 128.1, 131.6, 139.6 (Ph); 142.1 (C-4); 159.9 (C-2′); 160.3 (C-5′); 164.6 (C-2). Anal. Calcd for C15H13N5O3S3: C, 44.21; H, 3.22; N, 17.19; S, 23.61. Found: C, 44.09; H, 3.13; N, 16.88; S, 23.30.
2-(4-Chlorobenzenesulfonylimino)-3,4-dimethyl-5-(5-cyanomethylsulfanyl-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (6b)
Flesh-colored crystals; mp 158–160°C; yield 3.97 g (90%); 1H NMR: δ 2.62 (s, 3H, 4-CH3), 3.53 (s, 3H, 3-NCH3), 4.40 (s, 2H, SCH2), 7.58–7.90 (m, 4H, C6H4); 13C NMR: δ 13.21 (4-CH3); 17.6 (SCH2); 32.8 (3-NCH3); 99.9 (C-5); 115.5 (C≡N); 127.8, 129.2, 137.4, 140.3 (Ar); 141.9 (C-4); 159.9 (C-2′); 160.2 (C-5′); 164.6 (C-2). Anal. Calcd for C15H12ClN5O3S3: C, 40.77; H, 2.74; N, 15.85; S, 21.77. Found: C, 40.70; H, 2.69; N, 15.62; S, 21.49.
2-(4-Methylbenzenesulfonylimino)-3,4-dimethyl-5-(5-cyanomethylsulfanyl-[1,3,4]oxadiazol-2-yl)-2,3-dihydrothiazole (6c)
Flesh-colored crystals; mp 188–190°C; yield 4.0 g (95%); 1H NMR: δ 2.42 (s, 3H, PhCH3), 2.62 (s, 3H, 4-CH3), 3.53 (s, 3H, 3-NCH3), 4.40 (s, 2H, SCH2), 7.27–7.78 (m, 4H, C6H4); 13C NMR (coupled): δ 13.2 (4-CH3), 17.5 (SCH2), 20.9 (PhCH3), 32.8 (3-NCH3), 100.0 (q, 3J = 4.8 Hz, C-5), 115.6 (t, 2J = 8.1 Hz, C≡N), 125.8, 128.8; 138.6, 140.5 (Ar), 142.1 (q, 2J = 6.6 Hz, C-4), 159.9 (C-2′), 160.2 (t, 3J = 6.2 Hz, C-5′), 164.6 (q, 3J = 3.1 Hz, C-2). Anal. Calcd for C16H15N5O3S3: C, 45.59; H, 3.59; N, 16.61; S, 22.82. Found: C, 45.41; H, 3.48; N, 16.36; S, 22.58.
References
[1] Kumar, R.; Kotha, V.; Kumar, S. Simple and convenient synthesis of 2-(substituted-benzylsulfanyl)-4,5-dihydrothiazoles and their antimicrobial activity studies. J. Heterocycl. Chem. 2005, 42, 1191–1193.Search in Google Scholar
[2] Manju, S.; Devi, S.; Rajasekharan, K. Synthesis and antimicrobial studies of novel bis(diamino)thiazoles. J. Heterocycl. Chem. 2009, 46, 455–458.Search in Google Scholar
[3] El-Aasar, N.; Saied, K. Synthesis of new thiazolidine and imidazolidine derivatives of pharmacological interest. J. Heterocycl. Chem. 2008, 45, 645–652.Search in Google Scholar
[4] Dayan, F.; Vincent, A.; Romagni, J.; Allen, S.; Duke, S.; Duke, M.; Bowling, J.; Zjawiony, J. Amino- and urea-substituted thiazoles inhibit photosynthetic electron transfer. J. Agric. Food Chem. 2000, 48, 3689–3693.Search in Google Scholar
[5] Wang, Q.; Li, H.; Li, Y.; Huang, R. Synthesis and herbicidal activity of 2-cyano-3-(2-chlorothiazol-5-yl)methylaminoacrylates. J. Agric. Food Chem. 2004, 52, 1918–1922.Search in Google Scholar
[6] Sanemitsu, Y.; Kawamura, S.; Satoh, J.; Katayama, T.; Hashimoto, S. Synthesis and herbicidal activity of 2-acylimino-3-phenyl-1,3-thiazolines. A new family of bleaching herbicides. J. Pestic. Sci. 2006, 31, 305–310.Search in Google Scholar
[7] Mori, M.; Takagi, M.; Noritake, C.; Kagabu, S. 2,4-Dioxo-1,3-thiazolidine derivatives as a lead for new fungicides. J. Pestic. Sci. 2008, 33, 357–363.Search in Google Scholar
[8] Kayagil, I.; Demirayak, S. Synthesis and anticancer activities of some thiazole derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2009, 184, 2197–2207.Search in Google Scholar
[9] Wang, T.; Bing, G.; Zhang, X.; Qin, Z.; Yu, H.; Qin, X.; Dai, H.; Miao, W.; Wu, S.; Fang, J. Synthesis and herbicidal activities of 2-cyano-3-benzylaminoacrylates containing thiazole moiety. Bioorg. Med. Chem. Lett. 2010, 20, 3348–3351.Search in Google Scholar
[10] Chen, H.; Li, Z.; Han, Y. Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl (alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles). J. Agric. Food Chem. 2000, 48, 5312–5315.Search in Google Scholar
[11] Liu, F.; Luo, X.-Q.; Song, B.-A.; Bhadury, P. S.; Yang, S.; Jin, L.-H.; Xue, W.; Hu, D.-Y. Synthesis and antifungal activity of novel sulfoxide derivatives containing trimethoxyphenyl substituted 1,3,4-thiadiazole and 1,3,4-oxadiazole moiety. Bioorg. Med. Chem. 2008, 16, 3632–3640.Search in Google Scholar
[12] Husain, A.; Ajmal, M. Synthesis of novel 1,3,4-oxadiazole derivatives and their biological properties. Acta Pharm. 2009, 59, 223–233.Search in Google Scholar
[13] Jiang, L.-L.; Tan, Y.; Zhu, X.-L.; Wang, Z.-F.; Zuo, Y.; Chen, Q.; Xi, Z.; Yang, G.-F. Design, synthesis, and 3D-QSAR analysis of novel 1,3,4-oxadiazol-2(3H)-ones as protoporphyrinogen oxidase inhibitors. J. Agric. Food Chem. 2010, 58, 2643–2651.Search in Google Scholar
[14] Macaev, F.; Ribkovskaia, Z.; Pogrebnoi, S.; Boldescu, V.; Rusu, G.; Shvets, N.; Dimoglo, A.; Geronikaki, A.; Reynolds, R. The structure-antituberculosis activity relationships study in series of 5-aryl-2-thio-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. 2011, 19, 6792–6807.Search in Google Scholar
[15] Padmavathi, V.; Reddy, S.; Reddy, G.; Venkatesh, B.; Padmaja, A. Synthesis and antimicrobial studies of pyrazolyl oxadiazoles and thiadiazoles. J. Heterocycl. Chem. 2011, 48, 1197–1201.Search in Google Scholar
[16] Zou, X.-J.; Lai, L.-H.; Jin, G.-Y.; Zhang, Z.-X. Synthesis, fungicidal activity, and 3D-QSAR of pyridazinone-substituted 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem. 2002, 50, 3757–3760.Search in Google Scholar
[17] Zarghi, A.; Hamedi, S.; Tootooni, F.; Amini, B.; Sharifi, B.; Faizi, M.; Tabatabai, S; Shafiee, A. Synthesis and pharmacological evaluation of new 2-substituted-5-{2-[(2-halobenzyl)thio]phenyl}-1,3,4-oxadiazoles as anticonvulsant agents. Sci. Pharm. 2008, 76, 185–201.Search in Google Scholar
[18] Shashikant, R.; Rabara, P.; Jayashri, S.; Bukitagar, A.; Wakale, V.; Musmade, D. Synthesis and evaluation of some novel substituted 1,3,4-oxadiazole and pyrazole derivatives for antitubercular activity. Ind. J. Chem. 2009, 48B, 1453–1456.Search in Google Scholar
[19] Almasirad, A.; Shafiee, A.; Abdollahi, M.; Noeparast, A.; Shahrokhinejad, N.; Vousooghi, N.; Tabatabai, S.; Abbas, R. Synthesis and analgesic activity of new 1,3,4-oxadiazoles and 1,2,4-triazoles. Med. Chem. Res. 2011, 20, 435–442.Search in Google Scholar
[20] Dash, S.; Ashok, B.; Singh, J.; Maiti, B.; Maity, T. Synthesis of some novel 3,5-disubstituted 1,3,4-oxadiazole derivatives and anticancer activity on EAC animal model. Med. Chem. Res. 2010, 20, 1206–1213.Search in Google Scholar
[21] Tajik, H.; Dadras, A. Synthesis and herbicidal activity of novel 5-chloro-3-fluoro-2-phenoxypyridines with a 1,3,4-oxadiazole ring. J. Pest. Sci. 2011, 36, 27–32.Search in Google Scholar
[22] Eliazyan, K.; Hakobyan, R.; Pivazyan, V.; Ghazaryan, E.; Yengoyan, A. Synthesis of 2-(arylsulfonylmethylamino)-4-methyl-thiazole-5-carboxylic acids derivatives and their transformations. Chem. J. Armenia 2013, 66, 90–100.Search in Google Scholar
[23] Eliazyan, K.; Hakobyan, R.; Pivazyan, V.; Ghazaryan, E.; Yengoyan, A. Synthesis and transformations of 1-[2-(toluene-4-sulfonamido)ethyl]-thiourea. Heterocycl. Commun. 2013, 19, 121–124.Search in Google Scholar
[24] Knyazyan, A. Synthesis of 5-(2-thioxo-3H-thiazol-5-yl)-[1,2,4]triazole-3-thion derivatives with fungicidal and growth stimulant activities. Chem. J. Armenia 2012, 65, 94–104.Search in Google Scholar
[25] Knyazyan, A.; Eliazyan, K.; Pivazyan, V.; Ghazaryan, E.; Harutyunyan, S.; Yengoyan, A. Synthesis and growth regulatory activity of novel 5-(3-alkyl-4-methyl-2-thioxo-2,3-dihydro-thiazol-5-yl)-3H-[1,3,4]-thiadiazole(oxadiazole)-2-thiones and their derivatives. Heterocycl. Commun. 2012, 18, 103–108.Search in Google Scholar
[26] Knyazyan, A.; Eliazyan, K.; Pivazyan, V.; Ghazaryan, E.; Harutyunyan, S.; Yengoyan, A. Synthesis of novel 2-amino-(2-thioxo-3-alkyl-4-methyl-3H-thiazol-5-yl-[1,3,4]-thiadiazole derivatives and their growth stimulant activity. J. Heterocycl. Chem. 2013, in press.10.1002/jhet.1667Search in Google Scholar
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety
Articles in the same Issue
- Masthead
- Masthead
- Review
- Synthesis and photochromism of aryl(heteroaryl)- and diheteroarylethenes – coumarin derivatives
- Preliminary Communications
- Fluorescent labeling of oleanolic acid using ‘click’ chemistry
- Facile synthetic entry into rotationally restricted 9-arylacridines
- Research Articles
- Selective fluorescence sensing of ferric ion with novel triazolethione Schiff bases probes
- Synthesis and antimicrobial activities of some novel thiophene containing azo compounds
- Effect of intramolecular hydrogen bonding on biomimetic reactions by flavins
- Synthesis of some novel bis-1,2,4-triazole and bis-1,3,4-thiadiazole derivatives from terephthaloyl and isophthaloyl chlorides
- Synthesis of heterocyclic compounds by reaction of dialkyl acetylenedicarboxylates with thiourea derivatives
- Synthesis of novel 2-arylsulfonylimino-3,4- dimethyl-5-(5-S-substituted [1,3,4]oxadiazol- 2-yl)-2,3-dihydrothiazole derivatives and their plant growth stimulant activity
- Synthesis and biological activity of 6-substituted 5-acetyl-4,7-dimethoxybenzofuran derivatives
- Synthesis and biological activity of novel series of heterocyclic compounds containing succinimide moiety

