Abstract
Cultivated blueberries (Vaccinium spp.) (Ericaceae) are among the top three exported agricultural commodities of Mexico. Since their commercial debut in 1995, the area of cultivation has increased annually, reflecting the economic importance of this crop. Because species of the moth family Tortricidae associated with blueberries elsewhere are of considerable economic importance, we investigated the tortricid fauna of commercial blueberry plantations in the states of Guanajuato, Jalisco, Michoacan, and Puebla, Mexico. From 2019 to 2021 we collected and reared tortricid larvae and noted the damage they inflicted. We identified six species feeding on leaves, flowers, and/or fruit: Amorbia cuneana (Walsingham), Argyrotaenia montezumae (Walsingham), Platynota stultana (Walsingham), Templemania sarothrura (Felder & Rogenhofer), Bonagota mexicana (Razowski & Becker), and Zomaria interruptolineana (Fernald). The first three are well-known agricultural pests of numerous crops, and are widely distributed throughout southwestern U.S. and northern Mexico; T. sarothrura and B. mexicana are polyphagous native species that are encountered uncommonly; and Z. interruptolineana is a documented Ericaceae-feeder that is widespread in the eastern U.S. For all but Zomaria, these are the first records of Vaccinium as hosts for these tortricids. We suggest that is important to monitor population levels of these species owing to their potential economic importance, and to expand sampling to other producer states to evaluate their levels of infestation.
Resumen
En México, el arándano (Vaccinium spp.) se encuentra entre los tres principales productos agrícolas de exportación. Desde su apertura comercial en 1995, la superficie de cultivo se ha incrementado anualmente, reflejando indiscutiblemente su importancia económica para el país. Por esta razón, es importante identificar las especies de Tortricidae asociadas a este cultivo que son, o pueden llegar a ser plagas. De 2019 a 2021, se recolectó material vegetal infestado con larvas de Tortricidae en los estados de Guanajuato, Jalisco, Michoacán y Puebla, México, y se criaron hasta su madurez. Durante la recolección, también se observaron los daños que causan en el cultivo. Se identificaron seis especies asociadas con hojas, flores y frutos: Amorbia cuneana (Walsingham), Argyrotaenia montezumae (Walsingham), Platynota stultana (Walsingham), Templemania sarothrura (Felder & Rogenhofer), Bonagota mexicana (Razowski & Becker) and Zomaria interruptolineana (Fernald). Las primeras tres especies son plagas de numerosos cultivos agrícolas y están ampliamente distribuidas por el suroeste de Estados Unidos y el norte de México. T. sarothrura y B. mexicana son especies nativas polífagas que se encuentran con poca frecuencia. Se ha documentado que Z. interruptolineana se alimenta de Ericaceae y está ampliamente distribuida en el este de los Estados Unidos. Para Para todas las especies, excepto Zomaria, este es el primer registro de Vaccinium como hospedante de estos tortrícidos. Sugerimos que es importante monitorear la población de estas especies debido a su potencial de importancia económica y ampliar el muestreo a otros estados productores para evaluar sus niveles de infestación.
1 Introduction
Cultivated species of blueberries (Vaccinium spp.; Ericaceae) originated in the Northern Hemisphere (Rodriguez-Saona et al. 2019). Currently, highbush blueberries (Vaccinium corymbosum L.) and rabbit eye blueberries (Vaccinium ashei Reade) (Ericaceae) are the primary species of Vaccinium cultivated in North, Central, and South America (Pescie and López 2007; Rocca and Brown 2013). In Mexico, the first commercial plantations of rabbit eye blueberries were reported in Puebla in 1995 (SIAP 2021). By 2007, production had accelerated, and today blueberries are grown in 10 Mexican states (SIAP 2021). Worldwide, Mexico is the fifth largest producer of blueberries, with an annual production of 50,293 tons (FAOSTAT 2022). This crop has the highest economic value of any berry crop in the country, with the highest benefit-cost ratio; most of the crop is exported (González-Ramírez et al. 2020).
Commonly known as “leaf rollers,” members of the family Tortricidae are economically important pests of fruit worldwide. Over 100 species have been reported to feed on Vaccinium species (Brown et al. 2008; Wagner et al. 1995), including several that are considered pests of blueberries (Anonymous 2016; Easterbrooke 1986; Mallampalli and Issacs 2002; Marucci 1966; McGregor et al. 1998; McQuillin 1992; Neunzig and Falter 1966; Ponder and Seabrook 1988, 1991; Rocca and Brown 2013; Tomlinson 1951; Vergeer 1954).
Tortricid larvae feed on foliage, buds, and fruit of their host plants, typically constructing a silken shelter of tied leaves. Their damage can result in diminished fruit production, reduced fruit quality, quarantine restrictions, and economic loss owing to the excessive costs for control (Kumar et al. 2015) and prevention of invasion into pest-free areas (Gilligan et al. 2020). Although tortricid pests have been reported for many crops in Mexico (e.g., Bautista 2006; Bautista et al. 2011, 2014; Martínez et al. 2014; Ruiz-Galván et al. 2023; Tejeda-Reyes et al. 2021; Varela-Fuentes et al. 2009), our knowledge of those that feed on blueberries is virtually non-existent. Many native species of Tortricidae, some that are polyphagous and some that feed on species related to crops, are known to move onto, and often thrive in, crop monocultures. To provide a glimpse into potential tortricid pests of blueberry in the highlands of central Mexico, we conducted a survey of the species of this family on cultivated blueberry in four states.
2 Materials and methods
2.1 Collection sites and rearing methods
Specimens of larval tortricids were collected in 17 commercial plantations of highbush blueberry (Vaccinium spp.) in the Mexican states of Guanajuato (n = 2), Jalisco (n = 8), Michoacán (n = 2), and Puebla (n = 5), at elevations of 1,502–2,408 m a.s.l., from October 2019 to December 2021. Blueberry varieties were primarily Biloxi, Stella, and Rocío. Studies were conducted during plant vegetative development, flowering, and fruiting. In each plantation, 1 ha was sampled. Within each sampling area we collected shoots, flowers, or fruits that harbored leaf roller larvae. Each vegetative part was labeled and placed in an individual plastic cup with floral foam and water, and transported to the Laboratory of Entomology at the Colegio de Postgraduados, Campus Montecillo, Texcoco, State of Mexico. Larvae were reared under laboratory conditions at 25 ± 2 °C, 60 ± 20 % humidity, and photoperiod of 12:12 h (light:darkness). When adults emerged, they were pinned, labeled, and photographed.
2.2 Species identification
Specimens were identified by comparison with photographs of adults and male genitalia in the literature or on various websites cited in the results section for each species. Male genitalia were dissected following standard methods (Padwal et al. 2018; Robinson 1976). Photographs were taken with a Photomicroscope III Carl Zeiss® camera (Carl Zeiss, Germany). Species identifications were corroborated by tortricid specialists, who compared our specimens and their genitalia with identified material in the collections of the Cornell University Insect Collection (CUIC), Ithaca, New York; private collection of Vitor Becker, Camacan, Brazil; and the National Museum of Natural History (USNM), Washington DC. Larva and adult specimens of the species found are located in the Entomological Collection of the Institute of Plant Health, Colegio de Postgraduados, Campus Montecillo, Texcoco, State of Mexico, Mexico.
3 Results
We collected a total of 825 plant samples infested with Tortricidae larvae, from which 174 adults emerged. We identified six tortricid species: Amorbia cuneana (Walsingham), Argyrotaenia montezumae (Walsingham), Platynota stultana (Walsingham), Bonagota mexicana (Razowski & Becker), Zomaria interruptolineana (Fernald) and Templemania sarothrura (Felder & Rogenhofer) (Table 1). Below we provide details for each species reared to the adult stage in this study.
Tortricidae reared to the adult stage from blueberries collected as larvae at the study sites in four Mexican states; number of adults emerged in parentheses.
| State | Municipality | Altitude m a.s.l. | Coordinates | Species |
|---|---|---|---|---|
| Jalisco | Zapotlán el grande | 1,632 | 19.7016389 °N, 103.5368056 °W | Amorbia cuneana (4♂, 5♀) |
| Zapotiltic | 1,502 | 19.6268056 °N, 103.4728889 °W |
Am. cuneana (8♂, 3♀) Platynota stultana (3♂) Argyrotaenia montezumae (1♀) |
|
| Tangancícuaro | 1,725 | 19.8706944 °N, 102.2159444 °W |
Am. cuneana (4♂, 3♀) Ar. montezumae (1♀) |
|
| Peribán | 1,900 | 19.4405833 °N, 102.4316667 °W |
Am. cuneana (2♂, 1♀) Ar. montezumae (14♂, 12♀) |
|
| 1,800 | 19.4509167 °N, 102.4403056 °W |
Am. cuneana (1♂) Ar. montezumae (11♂, 12♀) |
||
| Acuitzio | 2,270 | 19.5000000 °N, 101.2728611 °W |
Am. cuneana (1♂, 1♀) Ar. montezumae (1♂, 1♀) |
|
| 2,300 | 19.4813056 °N, 101.2768889 °W |
Am. cuneana (5♂ 2♀) Ar. montezumae (6♂, 7♀) Templemania sarothrura (1♂) |
||
| Villa Madero | 2,146 | 19.4240833 °N, 101.2927222 °W |
Am. cuneana (4♂, 3♀) Ar. montezumae (7♂, 9♀) |
|
| 2,408 | 19.3721667 °N, 101.3440278 °W |
Am. cuneana (2♀) Ar. montezumae (2♂, 8♀) |
||
| 2,337 | 19.38358333 °N, 101.3236389 °W | Ar. montezumae (1♂) | ||
| Guanajuato | Celaya | 1,770 | 20.6174722 °N, 100.7850000 °W |
Am. cuneana (2♂) Ar. montezumae (1♂) |
| Cortazar | 1,740 | 20.5082222 °N, 100.9285278 °W |
Am. cuneana (3♂ 2♀) Pl. stultana (1♂) |
|
| Puebla | Zacatlán | 2,174 | 19.9885278 °N, 97.9671944 °W | Te. sarothrura (1♂) |
| 1,847 | 20.0171944 °N, 97.9283056 °W |
Ar. montezumae (1♂ 2♀) Bonagota mexicana (2) |
||
| 2,020 | 19.9960000 °N, 97.9566111 °W |
Ar. montezumae (1♂, 1♀) Zomaria interruptolineana (2♂) Bo. mexicana (1) |
||
| 2,020 | 19.9965000 °N, 97.9400556 °W |
Ar. montezumae (1♂, 1♀) Te. sarothrura (1♂) Zo. interruptolineana (2♂) Bo. mexicana (1) |
||
| 2,015 | 20.0042778 °N, 97.9368889 °W |
Te. sarothrura (1♂) Bo. mexicana (1) |
3.1 Subfamily Tortricinae
3.1.1 Amorbia cuneana (Walsingham 1879) (Figure 1a and b)
Fifty-six (35♂ 21♀) Am. cuneana were reared to the adult stage (Table 1). Identification was based on forewing pattern, morphology of the male genitalia, and a feature of the abdomen (i.e., mid-dorsal pit). In males, the forewing is brown to cream-colored with dark spots in the costal area and well-defined transversal spots or dots. On the head, ocelli are absent, and on the abdomen, there is a mid-dorsal depression on segment II (Gilligan and Epstein 2014; Phillips-Rodriguez and Powell 2007; Powell and Brown 2012). In the male genitalia, the distal half of the valvae are slightly wider than the basal half, and there is a distinct articulation of the base of the uncus with the dorsum of the tegumen. Identifications of adults were corroborated by larval characteristics (MacKay 1962).
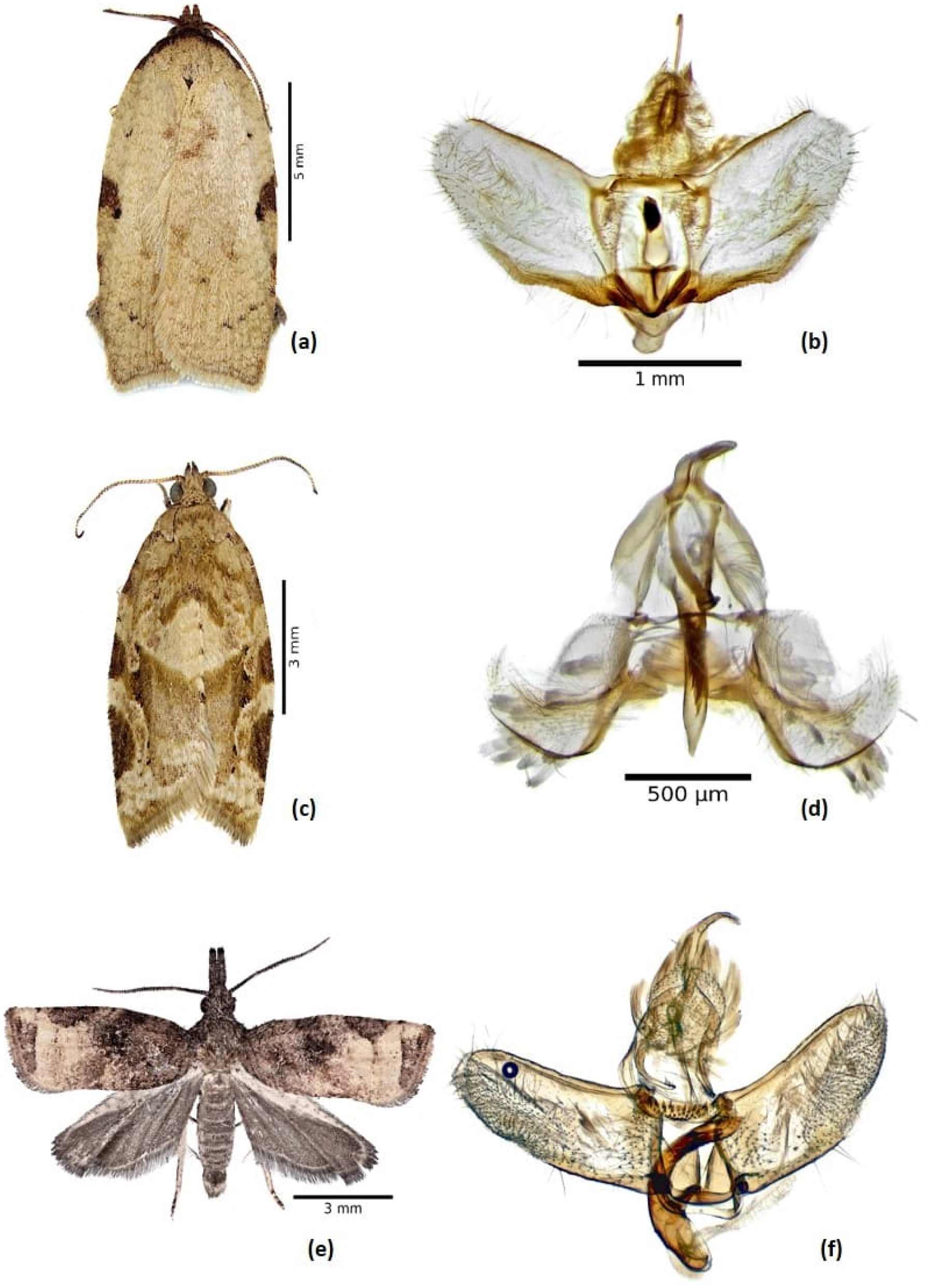
Tortricid adult male and genitalia. (a, b) Amorbia cuneana; (c, d) Argyrotaenia montezumae; and (e, f) Platynota stultana.
Hosts: Amorbia cuneana is a polyphagous tortricid, recorded on approximately 30 different plant species in 13 plant families, including Ericaceae (Gilligan and Epstein 2014; Powell and Brown 2012). Its use of Vaccinium as a larval host was not previously documented; hence, this is the first report of Am. cuneana on blueberry.
Distribution: Amorbia cuneana occurs in the western and southwestern United States and northwestern Mexico, from sea level to about 800 m a.s.l. (CAB International 2019; Juárez-Gutiérrez et al. 2015; Powell and Brown 2012). We collected specimens at nearly every site, over an elevational range of 1,502–2,300 m a.s.l.
Observations: This species was observed feeding on young leaves (Figure 2a) and to a lesser degree on inflorescences. The larvae may be cannibalistic and can survive on dry foliage. Moreover, Am. cuneana and Pl. stultana share the same microhabitat and may be found on the same shoot, but on different leaves.

Tortricid damage in blueberry. (a) Amorbia cuneana rolling apical leaves, (b) floral raceme consumed by Argyrotaenia montezumae, (c) larva feeding on fruits, and (d) apical death caused by Platynota stultana.
3.1.2 Argyrotaenia montezumae (Walsingham 1914) (Figure 1c and d)
We reared 101 (46♂, 55♀) Ar. montezumae to the adult stage (Table 1). Identification was based on comparison of images of adults and their male genitalia. The male genitalia are distinguished by short, rounded-triangular valvae; and a phallus that is slightly capitate with the coecum penis dilated and curved slightly downward (Brown 1999; Obraztsov 1961; Trematerra and Brown 2004).
Hosts: This species is recorded from Asteraceae, Lauraceae, Liliaceae, Malvaceae, and Rosaceae (Brown et al. 2008; López et al. 2014; McClay et al. 1995; Ruiz-Galván et al. 2023; Tejeda-Reyes et al. 2021). Its association with Ericaceae was previously unreported; this is the first report of Ar. montezumae feeding on cultivated blueberry.
Distribution: This species is known from southwestern United States, Central America (Mexico, Guatemala, and Honduras) and South America (Brazil) (Obraztsov 1961). This species was absent from only four of our 17 sites, from 1,502 to 2,408 m a.s.l.
Observations: The larvae feed on leaves, shoots, flowers, and fruits. On inflorescences they may completely consume the raceme (Figure 2b). The fruits are affected during the period from fruit-set to ripening. Larvae may completely consume the pulp, resulting in a fruit that is raisin-like (Figure 2c).
3.1.3 Platynota stultana (Walsingham 1884) (Figure 1e and f)
Four Pl. stultana individuals were reared to the adult stage (Table 1). Identification was confirmed based on illustrations in Miller and Hodges (1995), Powell and Brown (2012), Brown (2013), and Groenen and Baixeras (2013), all of whom indicate that this species can be distinguished by the transtilla, which is densely covered by large, evenly distributed spines; the V-shaped excavation in the middle of the juxta; and a strongly curved phallus. Superficially males have a darker area at the base of the wings, with a paler area in the distal half, and both sexes have long, porrect labial palpi.
Hosts: In the economic literature this species is referred to as the omnivorous leaf roller. Host plants include 27 different plant families, including vegetables, fruit crops, forest trees, and ornamental plants. Hosts of economic importance include pepper (Capsicum annuum L.; Solanaceae), citrus (Citrus spp.; Rutaceae), cotton (Gossypium hirsutum L.; Malvaceae), avocado (Persea americana Lauraceae), maize (Zea mays L.; Poaceae), peach (Prunus persica (L.) Batsch; Rosaceae), and grapes (Vitis vinifera L.; Vitaceae), among others (Gilligan and Epstein 2014; Powell 1983). Despite its extensive host range, these are the first reports of Pl. stultana on blueberry.
Distribution: Platynota stultana occurs in the southern United States and northern Mexico, and has been inadvertently introduced to the Hawaiian Islands (Miller and Hodges 1995) and Spain (CAB International 2022; Groenen and Baixeras 2013). This species was reared from larvae collected at two of our lowest elevation sites at 1,502 and 1,740 m a.s.l.
Observations: Platynota stultana larvae were found infesting apical shoots, which died from as a result of larval feeding damage (Figure 2d).
3.1.4 Templemania sarothrura (Felder & Rogenhofer, 1875) (Figure 3a and b)
Four Te. sarothrura were reared to the adult stage. Our specimens were compared with the adult image on Gilligan et al. (2018), and their identity confirmed by comparison with identified specimens in the USNM collection. Nonetheless, the male genitalia do not agree entirely with specimens in the USNM, so the identification of this species is provisional.
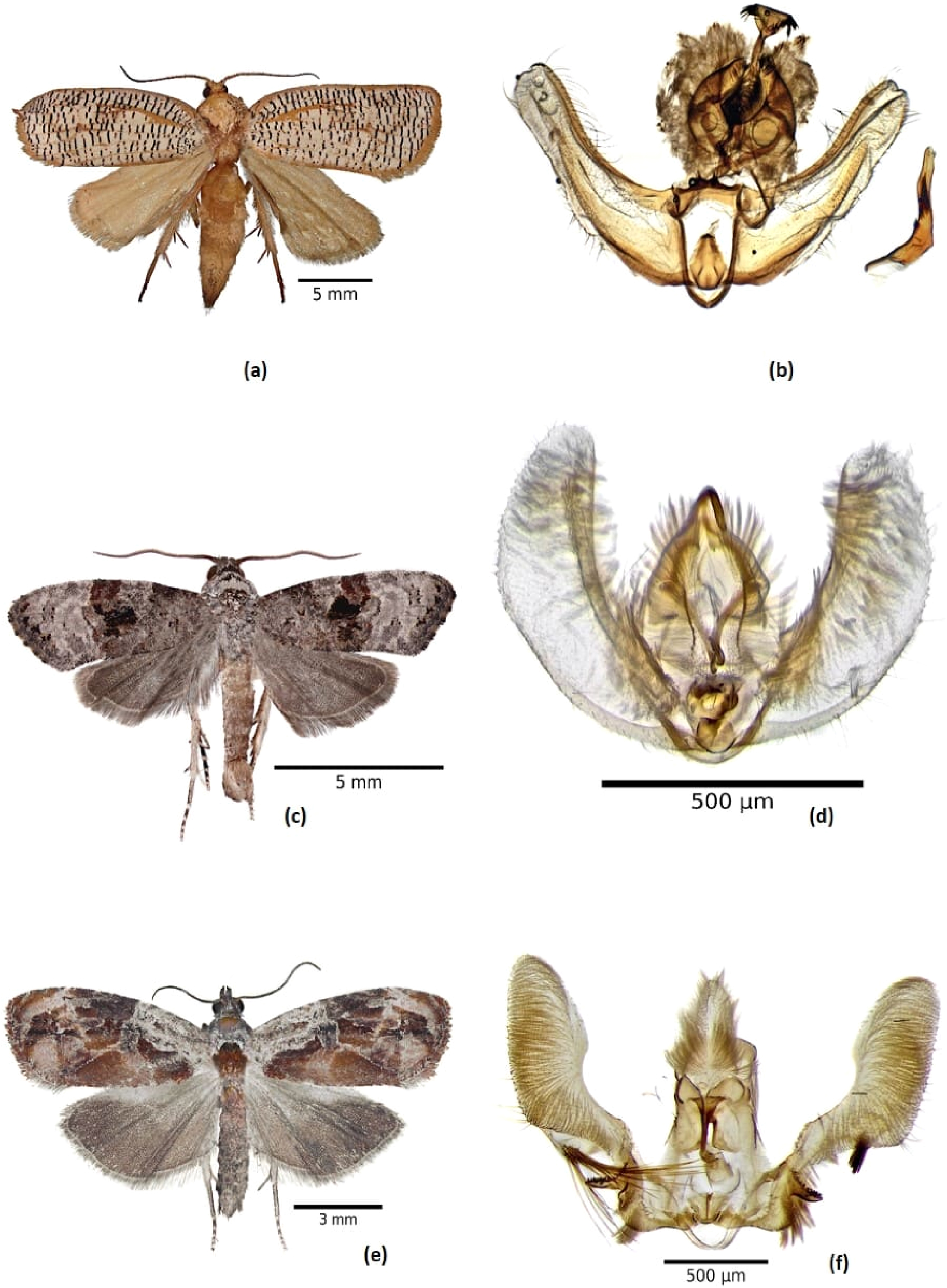
Male adults and genitalia. (a, b) Templemania sarothrura; (c, d) Bonagota mexicana; and (e, f) Zomaria interruptolineana.
Hosts: Although this is the first reported host for this species, the specimen illustrated by Tejeda-Reyes et al. (2021), reared from Crataegus mexicana Moc & Sessé ex DC (Rosaceae) and identified as Templemania millistriata (Walsingham), may also represent this or a closely related species.
Distribution: Templemania sarothrura is recorded from Mexico (Razowski and Becker 2011) and Honduras (specimens in USNM). We collected this species at three of our 17 sites, ranging from 2,015 to 2,174 m a.s.l.
Observations: Templemania sarothrura larvae feed on blueberry flowers and tender shoots. Their presence is evidenced by silk webbing on foliage and flowers where the larva takes refuge. Larvae were found in October and July.
3.1.5 Bonagota mexicana (Razowski & Becker 2000) (Figure 3c and d)
Five specimens of Bo. mexicana were reared to the adult stage (Table 1). Superficially adults of this species are characterized by a blackish gray head with black spots; white and gray forewings with dark hues, and a gray termen with some black spots on the apical half; and brown hindwings (Brown and Razowski 2003; Razowski and Becker 2000). The male genitalia have elongated, upturned, nearly parallel-sided valvae, with a broadly rounded apex.
Hosts: Species of Bonagota appear to be polyphagous, with 12 plant families reported as larval hosts for the genus. Among those of agricultural interest are Fabaceae, Rosaceae, Rutaceae and Vitaceae (Brown and Passoa 1998). Our rearings on blueberry represent the first report of Bonagota on Ericaceae.
Distribution: Although Bonagota is distributed throughout the New World tropics, from Uruguay to the United States, Bo. mexicana is reported only from Mexico (Brown and Razowski 2003; Razowski and Becker 2000). We collected this species at three sites, with elevations from 1,847 to 2,020 m a.s.l.
Observations: Larvae were recovered from apical buds where the leaves were folded and webbed together in October and June.
4 Subfamily Olethreutinae
4.1 Zomaria interruptolineana (Fernald 1882) (Figure 3e and f)
Four specimens of Zo. interruptolineana were reared to the adult stage on blueberries (Table 1). Identification was based on figures in Heinrich (1926), Miller (1987), Razowski and Becker (2016), and PBase (1999–2022). The male genitalia are characterized by the absence of spiny setae on the valvae; and a narrow, densely spined process on the venter of the middle part of the valvae.
Hosts: Larvae of Z. interruptolineana have been reported from four species of Ericaceae, including Vaccinium (Brown et al. 2008), and a single species of Sapotaceae. This is the first report on cultivated blueberry.
Distribution: This species ranges from southern Canada to Florida, east to Minnesota and Texas in eastern North America (BAMONA 2022). We reared this species from only two sites, both at 2,020 m a.s.l. Our records from Puebla represent a consider southward extension of the known range of the species.
Observations: Webs of these species were noticed on vegetative buds and inflorescence in October, April, and May.
5 Discussion
Within the past decade, a number of economically important pests have been reported in commercial blueberry plantations in Mexico, including Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) (Moreno et al. 2015; Ruiz-Galván et al. 2022), Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) (Ortiz et al. 2020), Dasineura oxycoccana Johnson (Diptera: Cecidomyiidae) (Toledo-Hernández et al. 2021), and Duponchelia fovealis Zeller (Lepidoptera: Crambidae) (Rosales-Escareño et al. 2021). However, there is limited knowledge of the broader entomofauna of this crop in Mexico. In an effort to begin to remedy this shortcoming, we investigated the family Tortricidae on this host in four Mexican states where commercial blueberries are cultivated. Our study documented six species of tortricids: Am. cuneana, Ar. montezumae, Pl. stultana, Te. sarothrura, Bo. mexicana and Zo. interruptolineana.
For the polyphagous tortricids, which include all but Zomaria, colonization of cultivated blueberry is not particularly surprising, and over time, the same may be expected from other polyphagous native and introduced species that are resident near blueberry plantations. For Zomaria, it is likely that it feeds on wild blueberries or related hosts, and has simply adapted to commercial blueberry, as documented in other species (Gassmann et al. 2006; Messina et al. 2020).
In our study, Am. cuneana (n = 56 individuals) and Ar. montezumae (n = 101 individuals) were the most common species that we reared to the adult stage. Both are polyphagous pests that feed on leaves, flowers, and fruits. In addition, they are multivoltine, present during the entire crop cycle. In addition, both appear to occur over a broad ecological and/or climatic range. Hence, they have the greatest potential to become economically important pests of blueberry in Central Mexico.
Although we reared few individuals of Pl. stultana (n = 4) to the adult stage, this polyphagous pest appears to be relatively invasive, with documented introductions from the southwestern U.S. into the Hawaiian Islands and Europe. While we observed larvae only on apical shoots, it is reported to damage foliage and fruit of a wide variety of crop plants, resulting in significant economic losses (e.g., AliNiazee and Stafford 1972; Atkins et al. 1957). However, based on its current geographical distribution, it may be more-or-less confined to warmer and/or Mediterranean climates (i.e., California, Florida, Hawaiian Islands, Spain) than can be found in the middle elevation sites in Mexico where blueberries are gown (1,502–2,408 m a.s.l.).
Bonagota mexicana also was documented in low density (n = 4), and hence, may have low potential to be become a pest. However, a congener in Brazil, Bonagota salubricola (Meyrick), has become a major pest of apple in that country (Brown and Razowski 2003). Templemania sarothrura also was documented in low density (n = 4), and is an unlikely candidate to achieve pest status. Although there are only two documented host records for the genus, Powell (specimen label data) has reared at least two species of Templemania to the adult stage on synthetic diet, suggesting that it may be polyphagous, as are other members of the tribe Atteriini. Finally, although Zo. interruptolineana appears to be a specialist on Ericaceae, it is not recorded as a pest of cultivated blueberries, even though its broad range in the U.S. includes areas of cultivated Vaccinium.
We suggest that future studies target a broader geographical coverage that includes all the blueberry-producing states of the Mexico. This may reveal additional species, as well as document wider distributions of the species reported herein. Undoubtedly, considerable research is required to fill the large gaps in our knowledge regarding these tortricid species, their host breadth, habits, biology, and the extent of damage caused. This knowledge is critical for establishing programs and control strategies to maintain populations below thresholds of economic damage.
Acknowledgments
We thank Jason J. Dombroskie of Cornell University, USA, and Vitor O. Becker of Serra Bonita, Camacan, Brazil, for help in corroborating the identity of the species. We also thank the growers for permission to conduct research in their blueberry plantations. The first author thanks Mexico’s National Council of Humanities Science and Technology (CONAHCYT) for a scholarship in support of her doctoral studies, and the National Service of Agri-Food Health Safety, and Quality (SENASICA) for funding part of this study. Finally, we thank the reviewers for many helpful comments and suggestions.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
AliNiazee, M.T. and Stafford, E.M. (1972). Notes on the biology, ecology, and damage of Platynota stultana on grapes. J. Econ. Entomol. 65: 1042–1044, https://doi.org/10.1093/jee/65.4.1042.Search in Google Scholar
Anonymous (2016). Cherry furitworm (Grapholita packardi) in blueberry. British Columbia Ministry of Agriculture, p. 4. Available at: https://www2.gov.bc.ca/assets/gov/farming-natural-resources-and-industry/agriculture-and-seafood/animal-and-crops/plant-health/phu-cherryfruitworm-blueberry.pdf (Accessed 28 Agust 2025).Search in Google Scholar
Atkins, E.L., Frost, M.H., Anderson, L.D., and Deal, A.S. (1957). The “Omnivorous leaf roller,” Platynota stultana Wlshm., on cotton in California: nomenclature, life history, and bionomics (Lepidoptera, Tortricidae). Ann. Entomol. Soc. Am. 50: 251–259, https://doi.org/10.1093/aesa/50.3.251.Search in Google Scholar
BAMONA (2022). Butterflies and moths of North America, Available at: https://www.butterfliesandmoths.org/ (Accessed 25 September 2022).Search in Google Scholar
Bautista, M.N. (2006). Insectos plaga, una guía ilustrada para su identificación, 1st ed. Colegio de Postgraduados, Estado de México, Texcoco, México, p. 113.Search in Google Scholar
Bautista, M.N., Chavarin, P.C., and López, B.E. (2011). Primer reporte del enrollador de bandas oblicuas, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae), en manzano en el Ejido Vista Hermosa, Ciudad Cuauhtémoc, Chihuahua, México. Acta Zool. Mex. (n.s.) 27: 819–824.10.21829/azm.2011.273784Search in Google Scholar
Bautista, M.N., Vargas, M.H., Ramirez, A.S., and Pérez, P.R. (2014). First report of the Platynota n. sp. (Lepidoptera: Tortricidae) genus in prickly pear (Opuntia spp.) in the municipality of Villa Milpa Alta, Mexico DF, Mexico. Southwest. Entomol. 39: 379–381, https://doi.org/10.3958/059.039.0215.Search in Google Scholar
Brown, J.W. (1999). Five new species of Argyrotaenia (Tortricidae: Archipini) from Mexico and the Southwestern United States. J. Lepidopterists’ Soc. 53: 114–125.Search in Google Scholar
Brown, J.W. (2013). Two new neotropical species of Platynota with comments on Platynota stultana Walsingham and Platynota xylophaea (Meyrick) (Lepidoptera: Tortricidae). Proc. Entomol. Soc. Wash. 115: 128–139, https://doi.org/10.4289/0013-8797.115.2.128.Search in Google Scholar
Brown, J.W. and Passoa, S. (1998). Larval foodplants of Euliini (Lepidoptera: Tortricidae): from Abies to Vitis. Pan-Pac. Entomol. 74: 1–11.Search in Google Scholar
Brown, J.W. and Razowski, J. (2003). Description of Ptychocroca, a new genus from Chile and Argentina, with comments on the Bonagota Razowski group of genera (Lepidoptera: Tortricidae: Euliini). Zootaxa 303: 1–31, https://doi.org/10.11646/zootaxa.303.1.1.Search in Google Scholar
Brown, J.W., Robinson, G., and Powell, J.A. (2008). Food plant database of the leafrollers of the world (Lepidoptera: Tortricidae) (Version 1.0), Available at: http://www.tortricid.net/foodplants.asp (Accessed 20 June 2022).Search in Google Scholar
CAB International (2019). Amorbia cuneana (avocado leafroller), Available at: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.4997 (Accessed 24 September 2022).Search in Google Scholar
CAB International (2022). Platynota stultana (omnivorous leaf roller), Available at: https://www.cabi.org/isc/datasheet/41858 (Accessed 24 September 2022).Search in Google Scholar
Easterbrooke, M.A. (1986). Damage to blueberry (Vaccinium corymbosum) by Cacoecimorpha pronubana (Hübner). Entomol. Rec. J. Var. 98: 218.Search in Google Scholar
FAOSTAT (Organización de las Naciones Unidas para la Alimentación y la Agricultura) (2022). Cultivos y productos de ganadería, Available at: http://www.fao.org/faostat/es/#data/QC (Accessed 14 July 2025).Search in Google Scholar
Gassmann, A.J., Levy, A., Tran, T., and Futuyma, D.J. (2006). Adaptations of an insect to a novel host plant: a phylogenetic approach. Funct. Ecol. 20: 478–485, https://doi.org/10.1111/j.1365-2435.2006.01118.x.Search in Google Scholar
Gilligan, T.M. and Epstein, E.M. (2014). Tortricids of agricultural importance. Interactive keys developed in Lucid 3.5. 2014, Available at: http://idtools.org/id/leps/tortai/tortricidae.html (Accessed 22 June 2022).Search in Google Scholar
Gilligan, T.M., Baixeras, J., and Brown, J.W. (2018). T@RTS: online world catalogue of the Tortricidae (Ver. 4.0), Available at: http://www.tortricid.net/catalogue.asp (Accessed 10 June 2022).Search in Google Scholar
Gilligan, T.M., Brown, J.W., and Baixeras, J. (2020). Immigrant Tortricidae: holarctic versus introduced species in North America. Insects 11: 594, https://doi.org/10.3390/insects11090594.Search in Google Scholar PubMed PubMed Central
González-Ramírez, M.G., Santoyo-Cortésa, V.H., Arana-Coronado, J.J., and Muñoz-Rodríguez, M. (2020). The insertion of Mexico into the global value chain of berries. World Dev. Perspect. 20: 100240, https://doi.org/10.1016/j.wdp.2020.100240.Search in Google Scholar
Groenen, F. and Baixeras, J. (2013). The omnivorous leafroller, Platynota stultana Walsingham, 1884 (Tortricidae: Sparganothini), a new moth for Europe. Nota Lepidopterol. 36: 53–55.Search in Google Scholar
Heinrich, C. (1926). Revision of the North American moths of the subfamilies Laspeyresiinae and Olethreutinae. Bull. U.S. Natl. Mus. 132: 1–216, https://doi.org/10.5479/si.03629236.132.1.Search in Google Scholar
Juárez-Gutiérrez, A.C., Martínez, A.M., Figueroa, J.I., Rebollar, A.A., Aguilera, P.M.M., and Pineda, S. (2015). Registro del enrollador de las hojas, Amorbia cuneana (Walsingham) (Lepidoptera: Tortricidae), en zarzamora en Rancho Huatarillo, Peribán, Michoacán. Acta Zool. Mex. (n.s.) 31: 341–343.10.21829/azm.2015.312998Search in Google Scholar
Kumar, S., Neven, L.G., Zhu, H., and Zhang, R. (2015). Assessing the global risk of establishment of Cydia pomonella (Lepidoptera: Tortricidae) using CLIMEX and MaxEnt niche models. J. Econ. Entomol. 108: 1708–1719, https://doi.org/10.1093/jee/tov166.Search in Google Scholar PubMed
López, I., Pineda, S., Figueroa, J.I., Sánchez, J.A., Martínez, A.M., Williams, R.N., and Rebollar-Alviter, A. (2014). Identification, parasitoids, and population dynamics of a blackberry leafroller (Lepidoptera: Tortricidae) from Michoacán, Mexico. Southwest. Entomol. 39: 503–510, https://doi.org/10.3958/059.039.0311.Search in Google Scholar
MacKay, M.R. (1962). Larvae of the North American Tortricinae (Lepidoptera: Tortricidae). Can. Entomol. 94: 5–182, https://doi.org/10.4039/entm9428fv.Search in Google Scholar
Mallampalli, N. and Issacs, R. (2002). Distribution of eggs and larval populations of cranberry fruitworm (Lepidoptera: Pyralidae) and cherry fruitworm (Lepidoptera: Tortricidae) in highbush blueberries. Environ. Entomol. 31: 852–858.10.1603/0046-225X-31.5.852Search in Google Scholar
Martínez, A., Barreto-Barriga, O., Pineda, S., Rebollar-Alviter, Á., Chavarrieta, J.M., and Figueroa, J.I. (2014). Parasitoides asociados a los enrolladores de hojas de zarzamora Argyrotaenia montezumae Walsingham y Amorbia sp. (Lepidoptera: Tortricidae), en Michoacán, México. Acta Zool. Mex. (n.s.) 30: 553–563.10.21829/azm.2014.30377Search in Google Scholar
Marucci, P.E. (1966). Insects and their control. In: Eck, P. and Childers, N.F. (Eds.). Blueberry culture. Rutgers University Press, New Brunswick, New Jersey, pp. 199–235.10.36019/9780813566412-012Search in Google Scholar
McClay, A.S., Palmer, W.A., Bennett, F.D., and Pullen, K.R. (1995). Phytophagous arthropods associated with Parthenium hysterophorus (Asteraceae) in North America. Environ. Entomol. 24: 796–809, https://doi.org/10.1093/ee/24.4.796.Search in Google Scholar
McGregor, R., Hueppelsheuser, T., Luczynski, A., and Henderson, D. (1998). Collection and evaluation of Trichogramma species (Hymenoptera: Trichogrammatidae) as biological controls of the oblique–banded leafroller, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae) in raspberries and blueberries. Biol. Control 11: 38–42, https://doi.org/10.1006/bcon.1997.0569.Search in Google Scholar
McQuillan, P.B. (1992). A checklist of the Tasmanian tortricid moths (Lepidoptera: Tortricidae) and their host-plant relationships. Pap. Proc. R. Soc. Tasman. 126: 77–89, https://doi.org/10.26749/rstpp.126.77.Search in Google Scholar
Messina, F.J., Lish, M.A., Springer, A., and Gompert, Z. (2020). Colonization of marginal host plants by seed beetles (Coleoptera: Chrysomelidae): effects of geographic source and genetic admixture. Environ. Entomol. 49: 938–946, https://doi.org/10.1093/ee/nvaa065.Search in Google Scholar PubMed
Miller, W.E. (1987). Guide to the Olethreutine moths of Midland North America (Tortricidae), 1st ed. United States Department of Agriculture, Washington, DC, pp. 20.Search in Google Scholar
Miller, S. and Hodes, R.W. (1995). Platynota stultana, the omnivorous leafroller, established in the Hawaiian Islands (Lepidoptera: Tortricidae). Bish. Mus. Occas. Pap. 42: 36–39.Search in Google Scholar
Moreno, C.G., Rodríguez, V.B., Sánchez, G.J.A., and Arredondo, B.H.C. (2015). Trampeo y registro del parasitoide Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae) sobre Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) en México. Southwest. Entomol. 40: 199–203, https://doi.org/10.3958/059.040.0118.Search in Google Scholar
Neunzig, H.H. and Falter, J.M. (1966). Insect and mite pests of blueberry in North Carolina. N.C. Agric. Exp. Stn. Bull. 427: 34.Search in Google Scholar
Obraztsov, N.S. (1961). Descriptions of and notes on North and Central American species of Argyrotaenia, with the description of a new genus (Lepidoptera, Tortricidae). Am. Mus. Novit. 2048: 1–49.Search in Google Scholar
Ortiz, J.A., Infante, F., Rodríguez, D., and Toledo-Hernández, R.A. (2020). Discovery of Scirtothrips dorsalis (Thysanoptera: Thripidae) in blueberry fields of Michoacan, Mexico. Fla. Entomol. 103: 408–410, https://doi.org/10.1653/024.103.0316.Search in Google Scholar
Padwal, K.G., Kumar, S.S., and Kumar, S.S. (2018). Dissection and slide mounting technique for male and female genitalia of Leucinodes orbonalis. J. Entomol. Res. Soc. 42: 259–262.10.5958/0974-4576.2018.00043.9Search in Google Scholar
PBase (1999–2022). Zomaria interruptolineana pictures, Available at: https://pbase.com/search?q=Zomaria+interruptolineana&b=Search+Photos&c=sp (Accessed 14 July 2025).Search in Google Scholar
Pescie, M.A. and López, C.G. (2007). Inducción floral en arándano alto del sur (Vaccinium corymbosum). RIA 36: 97–98.Search in Google Scholar
Phillips-Rodriguez, E. and Powell, J.A. (2007). Phylogenetic relationships, systematics, and biology of the species of Amorbia Clemens (Lepidoptera: Tortricidae: Sparganothini). Zootaxa 670: 1–109, https://doi.org/10.11646/zootaxa.1670.1.1.Search in Google Scholar
Ponder, B.M. and Seabrook, W.D. (1988). Biology of the blueberry leaftier Croesia curvalana (Kearfott) (Tortricidae): a field and laboratory study. J. Lepidopterists’ Soc. 42: 120–131.Search in Google Scholar
Ponder, B.M. and Seabrook, W.D. (1991). Sensitivity of blueberry leaftier moths (Lepidoptera: Tortricidae) (Kearfott) to their own sex pheromone: mating bioassay, electroantennogram, and trap attractancy studies. Can. Entomol. 123: 231–238, https://doi.org/10.4039/ent123231-1.Search in Google Scholar
Powell, J.A. (1983). Expanding geographical and ecological range of Platynota stultana in California (Lepidoptera: Tortricidae). Pan-Pac. Entomol. 59: 233–239.Search in Google Scholar
Powell, J.A. and Brown, J.W. (2012). The moths of North America. Fascicle 8.1, Tortricoidea, Tortricidae (Part), Sparganothini and Atteriini, Vol. 8. The Wedge Entomological Research Foundation, Washington, pp. 1–229.Search in Google Scholar
Razowski, J. and Becker, V.O. (2000). Revision of the New World Euliini – genus Bonagota Razowski, with notes on Apotomops Powell & Obraztsov (Lepidoptera: Tortricidae). Pol. J. Entomol. 69: 65–76.Search in Google Scholar
Razowski, J. and Becker, V.O. (2011). Systematic and faunistic data on Neotropical Tortricidae: Phricanthini, Tortricini, Atteriini, Polyorthini, Chlidanotini (Lepidoptera: Tortricidae). SHILAP 39: 161–181.Search in Google Scholar
Razowski, J. and Becker, V.O. (2016). Systematics and faunistics of Neotropical Olethreutini, 1: Lobesia Guenée, 1845, Ophiorrhabda Diakonoff, 1966, Megalota Diakonoff, 1966, Eumarozia Heinrich, 1926, Zomaria Heinrich, 1926 and Alexiloga Meyrick, 1922 (Lepidoptera: Tortricidae). Pol. J. Entomol. 85: 13–25, https://doi.org/10.1515/pjen-2016-0002.Search in Google Scholar
Robinson, G.S. (1976). The preparation of slides of Lepidoptera genitalia with special reference to the microlepidoptera. Entomol. Gaz. 27: 127–132.Search in Google Scholar
Rocca, M. and Brown, J.W. (2013). New host records for four species of tortricid moths (Lepidoptera: Tortricidae) on cultivated blueberries, Vaccinium corymbosum (Ericaceae), in Argentina. Proc. Entomol. Soc. Wash. 15: 167–172, https://doi.org/10.4289/0013-8797.115.2.167.Search in Google Scholar
Rodriguez-Saona, C., Vincent, C., and Isaacs, R. (2019). Blueberry IPM: past successes and future challenges. Annu. Rev. Entomol. 64: 95–114, https://doi.org/10.1146/annurev-ento-011118-112147.Search in Google Scholar PubMed
Rosales-Escareño, L., Lozano-Gutiérrez, J., España-Luna, M.P., Enriquez-Enriquez, D., and Balleza-Cadengo, J.J. (2021). Primer reporte de Duponchelia fovealis (Zeller, 1847) (Lepidoptera: Crambidae) en el estado de Aguascalientes, México. In: Resúmenes del LVI Congreso Nacional de Entomología, Cd. Victoria, Tamaulipas, México, 10 September 2021, p. 91.Search in Google Scholar
Ruiz-Galván, I., Quezada-Daniel, R.M.G., and Bautista-Martínez, N. (2022). First detection of the spotted wing vinegar fly, Drosophila suzukii Matsumura, 1931 (Diptera: Drosophilidae), in commercial blueberries in Puebla, Mexico. Pan-Pac. Entomol. 98: 163–165, https://doi.org/10.3956/2022-98.2.163.Search in Google Scholar
Ruiz-Galván, I., Bautista-Martínez, N., Soto-Rojas, L., Pineda-Guillermo, S., and Romero-Nápoles, J. (2023). Identification and distribution of leafrollers (Lepidoptera, Tortricidae) associated with berries (Rosaceae) cultivated in Mexico. ZooKeys 1146: 185–196, https://doi.org/10.3897/zookeys.1146.81734.Search in Google Scholar PubMed PubMed Central
SIAP (Servicio de Información Agroalimentaria y Pesquera (2021). Anuario Estadístico de la Producción Agrícola, Available at: https://nube.siap.gob.mx/cierreagricola/ (Accessed 10 May 2022).Search in Google Scholar
Tejeda-Reyes, M.A., Valdez-Carrasco, J.M., and González-Hernández, H. (2021). First report of Argyrotaenia montezumae and Templemania millistriata in hawthorn (Crataegus mexicana) in Chiautzingo, Puebla, Mexico. Southwest. Entomol. 46: 291–294, https://doi.org/10.3958/059.046.0133.Search in Google Scholar
Toledo-Hernández, R.A., Mikery, O., Ibañez, S., Aguilar, I., Sánchez, D., and Rodriguez, D. (2021). First record of invasive pest blueberry gall midge, Dasineura oxycoccana (Johnson) in Mexico: molecular and morphological confirmation. Southwest. Entomol. 46: 963–970.10.3958/059.046.0417Search in Google Scholar
Tomlinson, W.E. (1951). Control of insect larvae infesting immature blueberry fruit. J. Econ. Entomol. 44: 247–250, https://doi.org/10.1093/jee/44.2.247.Search in Google Scholar
Trematerra, P. and Brown, J.W. (2004). Argentine Argyrotaenia (Lepidoptera: Tortricidae): synopsis and descriptions of two new species. Zootaxa 574: 1–12, https://doi.org/10.11646/zootaxa.574.1.1.Search in Google Scholar
Varela-Fuentes, S., Brown, J.W., and Silva-Aguirre, G. (2009). Registro de Platynota rostrana (Walker, 1863) (Lepidoptera: Tortricidae) en cítricos de México. Acta Zool. Mex. (n.s.) 25: 651–654, https://doi.org/10.21829/azm.2009.253666.Search in Google Scholar
Vergeer, T. (1954). The cherry fruitworm (Grapholitha packardi) as a blueberry pest in Michigan. Mich. Agric. Exp. Stn. Q. Bull. 36: 370–373.Search in Google Scholar
Wagner, D.L., Peacock, J.W., Carter, J.L., and Talley, S.E. (1995). Spring caterpillar fauna of oak and blueberry in a Virginia deciduous forest. Ann. Entomol. Soc. Am. 88: 416–426, https://doi.org/10.1093/aesa/88.4.416.Search in Google Scholar
© 2025 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Research Articles
- Dynamics of citrus pest populations following a major freeze in northern Florida
- Control of Drosophila melanogaster (Diptera: Drosophilidae) by trapping with banana vinegar
- Establishment, distribution, and preliminary phenological trends of a new planthopper in the genus Patara (Hemiptera: Derbidae) in South Florida, United States of America
- Comparative evaluation of the infestation of five varieties of citrus by the larvae of Anastrepha ludens (Diptera: Tephritidae)
- Impact of land use on the density of Bulimulus bonariensis (Stylommatophora: Bulimulidae) and its parasitic mite, Austreynetes sp. (Trombidiformes: Ereynetidae)
- First record of native seed beetle Stator limbatus (Coleoptera: Chrysomelidae) on invasive earleaf acacia in Florida
- Establishment and monitoring of a sentinel garden of Asian tree species in Florida to assess potential insect pest risks
- Parasitism of Halyomorpha halys and Nezara viridula (Hemiptera: Pentatomidae) sentinel eggs in Central Florida
- Genetic differentiation of three populations of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in Mexico
- Tortricidae (Lepidoptera) associated with blueberry cultivation in Central Mexico
- First report of Phidotricha erigens (Lepidoptera: Pyralidae: Epipaschiinae) injuring mango inflorescences in Puerto Rico
- Seed predation of Sabal palmetto, Sabal mexicana and Sabal uresana (Arecaceae) by the bruchid Caryobruchus gleditsiae (Coleoptera: Bruchidae), with new host and distribution records
- Genetic variation of rice stink bugs, Oebalus spp. (Hemiptera: Pentatomidae) from Southeastern United States and Cuba
- Selecting Coriandrum sativum (Apiaceae) varieties to promote conservation biological control of crop pests in south Florida
- First record of Mymarommatidae (Hymenoptera) from the Galapagos Islands, Ecuador
- First field validation of Ontsira mellipes (Hymenoptera: Braconidae) as a potential biological control agent for Anoplophora glabripennis (Coleoptera: Cerambycidae) in South Carolina
- Field evaluation of α-copaene enriched natural oil lure for detection of male Ceratitis capitata (Diptera: Tephritidae) in area-wide monitoring programs: results from Tunisia, Costa Rica and Hawaii
- Abundance of Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and other thrips in commercial snap bean fields in the Homestead Agricultural Area (HAA)
- Performance of Salvinia molesta (Salviniae: Salviniaceae) and its biological control agent Cyrtobagous salviniae (Coleoptera: Curculionidae) in freshwater and saline environments
- Natural arsenal of Magnolia sarcotesta: insecticidal activity against the leaf-cutting ant Atta mexicana (Hymenoptera: Formicidae)
- Ethanol concentration can influence the outcomes of insecticide evaluation of ambrosia beetle attacks using wood bolts
- Post-release support of host range predictions for two Lygodium microphyllum biological control agents
- Missing jewels: the decline of a wood-nesting forest bee, Augochlora pura (Hymenoptera: Halictidae), in northern Georgia
- Biological response of Rhopalosiphum padi and Sipha flava (Hemiptera: Aphididae) changes over generations
- Argopistes tsekooni (Coleoptera: Chrysomelidae), a new natural enemy of Chinese privet in North America: identification, establishment, and host range
- A non-overwintering urban population of the African fig fly (Diptera: Drosophilidae) impacts the reproductive output of locally adapted fruit flies
- Fitness of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) on four economically important host fruits from Fujian Province, China
- Carambola fruit fly in Brazil: new host and first record of associated parasitoids
- Establishment and range expansion of invasive Cactoblastis cactorum (Lepidoptera: Pyralidae: Phycitinae) in Texas
- A micro-anatomical investigation of dark and light-adapted eyes of Chilades pandava (Lepidoptera: Lycaenidae)
- Scientific Notes
- Evaluation of food attractants based on fig fruit for field capture of the black fig fly, Silba adipata (Diptera: Lonchaeidae)
- Exploring the potential of Amblyseius largoensis (Acari: Phytoseiidae) as a biological control agent against Aceria litchii (Acari: Eriophyidae) on lychee plants
- Early stragglers of periodical cicadas (Hemiptera: Cicadidae) found in Louisiana
- Attraction of released male Mediterranean fruit flies to trimedlure and an α-copaene-containing natural oil: effects of lure age and distance
- Co-infestation with Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae): a threat for berry crops in Morelos, Mexico
- Observation of brood size and altricial development in Centruroides hentzi (Arachnida: Buthidae) in Florida, USA
- New quarantine cold treatment for medfly Ceratitis capitata (Diptera: Tephritidae) in pomegranates
- A new invasive pest in Mexico: the presence of Thrips parvispinus (Thysanoptera: Thripidae) in chili pepper fields
- Acceptance of fire ant baits by nontarget ants in Florida and California
- Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)
- Note on the nesting biology of Epimelissodes aegis LaBerge (Hymenoptera: Apidae)
- Mass rearing protocol and density trials of Lilioceris egena (Coleoptera: Chrysomelidae), a biological control agent of air potato
- Cardinal predation of the invasive Jorō spider Trichophila clavata (Araneae: Nephilidae) in Georgia
- Book Reviews
- Review: Harbach, R.E. 2024. The Composition and Nature of the Culicidae (Mosquitoes). Centre for Agriculture and Bioscience International and the Royal Entomological Society, United Kingdom. ISBN 9781800627994
- Retraction
- Retraction of: Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)
Articles in the same Issue
- Frontmatter
- Research Articles
- Dynamics of citrus pest populations following a major freeze in northern Florida
- Control of Drosophila melanogaster (Diptera: Drosophilidae) by trapping with banana vinegar
- Establishment, distribution, and preliminary phenological trends of a new planthopper in the genus Patara (Hemiptera: Derbidae) in South Florida, United States of America
- Comparative evaluation of the infestation of five varieties of citrus by the larvae of Anastrepha ludens (Diptera: Tephritidae)
- Impact of land use on the density of Bulimulus bonariensis (Stylommatophora: Bulimulidae) and its parasitic mite, Austreynetes sp. (Trombidiformes: Ereynetidae)
- First record of native seed beetle Stator limbatus (Coleoptera: Chrysomelidae) on invasive earleaf acacia in Florida
- Establishment and monitoring of a sentinel garden of Asian tree species in Florida to assess potential insect pest risks
- Parasitism of Halyomorpha halys and Nezara viridula (Hemiptera: Pentatomidae) sentinel eggs in Central Florida
- Genetic differentiation of three populations of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in Mexico
- Tortricidae (Lepidoptera) associated with blueberry cultivation in Central Mexico
- First report of Phidotricha erigens (Lepidoptera: Pyralidae: Epipaschiinae) injuring mango inflorescences in Puerto Rico
- Seed predation of Sabal palmetto, Sabal mexicana and Sabal uresana (Arecaceae) by the bruchid Caryobruchus gleditsiae (Coleoptera: Bruchidae), with new host and distribution records
- Genetic variation of rice stink bugs, Oebalus spp. (Hemiptera: Pentatomidae) from Southeastern United States and Cuba
- Selecting Coriandrum sativum (Apiaceae) varieties to promote conservation biological control of crop pests in south Florida
- First record of Mymarommatidae (Hymenoptera) from the Galapagos Islands, Ecuador
- First field validation of Ontsira mellipes (Hymenoptera: Braconidae) as a potential biological control agent for Anoplophora glabripennis (Coleoptera: Cerambycidae) in South Carolina
- Field evaluation of α-copaene enriched natural oil lure for detection of male Ceratitis capitata (Diptera: Tephritidae) in area-wide monitoring programs: results from Tunisia, Costa Rica and Hawaii
- Abundance of Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and other thrips in commercial snap bean fields in the Homestead Agricultural Area (HAA)
- Performance of Salvinia molesta (Salviniae: Salviniaceae) and its biological control agent Cyrtobagous salviniae (Coleoptera: Curculionidae) in freshwater and saline environments
- Natural arsenal of Magnolia sarcotesta: insecticidal activity against the leaf-cutting ant Atta mexicana (Hymenoptera: Formicidae)
- Ethanol concentration can influence the outcomes of insecticide evaluation of ambrosia beetle attacks using wood bolts
- Post-release support of host range predictions for two Lygodium microphyllum biological control agents
- Missing jewels: the decline of a wood-nesting forest bee, Augochlora pura (Hymenoptera: Halictidae), in northern Georgia
- Biological response of Rhopalosiphum padi and Sipha flava (Hemiptera: Aphididae) changes over generations
- Argopistes tsekooni (Coleoptera: Chrysomelidae), a new natural enemy of Chinese privet in North America: identification, establishment, and host range
- A non-overwintering urban population of the African fig fly (Diptera: Drosophilidae) impacts the reproductive output of locally adapted fruit flies
- Fitness of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) on four economically important host fruits from Fujian Province, China
- Carambola fruit fly in Brazil: new host and first record of associated parasitoids
- Establishment and range expansion of invasive Cactoblastis cactorum (Lepidoptera: Pyralidae: Phycitinae) in Texas
- A micro-anatomical investigation of dark and light-adapted eyes of Chilades pandava (Lepidoptera: Lycaenidae)
- Scientific Notes
- Evaluation of food attractants based on fig fruit for field capture of the black fig fly, Silba adipata (Diptera: Lonchaeidae)
- Exploring the potential of Amblyseius largoensis (Acari: Phytoseiidae) as a biological control agent against Aceria litchii (Acari: Eriophyidae) on lychee plants
- Early stragglers of periodical cicadas (Hemiptera: Cicadidae) found in Louisiana
- Attraction of released male Mediterranean fruit flies to trimedlure and an α-copaene-containing natural oil: effects of lure age and distance
- Co-infestation with Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae): a threat for berry crops in Morelos, Mexico
- Observation of brood size and altricial development in Centruroides hentzi (Arachnida: Buthidae) in Florida, USA
- New quarantine cold treatment for medfly Ceratitis capitata (Diptera: Tephritidae) in pomegranates
- A new invasive pest in Mexico: the presence of Thrips parvispinus (Thysanoptera: Thripidae) in chili pepper fields
- Acceptance of fire ant baits by nontarget ants in Florida and California
- Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)
- Note on the nesting biology of Epimelissodes aegis LaBerge (Hymenoptera: Apidae)
- Mass rearing protocol and density trials of Lilioceris egena (Coleoptera: Chrysomelidae), a biological control agent of air potato
- Cardinal predation of the invasive Jorō spider Trichophila clavata (Araneae: Nephilidae) in Georgia
- Book Reviews
- Review: Harbach, R.E. 2024. The Composition and Nature of the Culicidae (Mosquitoes). Centre for Agriculture and Bioscience International and the Royal Entomological Society, United Kingdom. ISBN 9781800627994
- Retraction
- Retraction of: Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)

