Field evaluation of α-copaene enriched natural oil lure for detection of male Ceratitis capitata (Diptera: Tephritidae) in area-wide monitoring programs: results from Tunisia, Costa Rica and Hawaii
-
Saida Kharrat
Abstract
In an 18-week trial in Tunisia, a 20-week trial in Costa Rica and two 24-week trials in Hawaii a formulation of an α-copaene enriched natural oil bulb captured more male Mediterranean fruit flies (medflies) Ceratitis capitata (Wiedemann) than traps baited with trimedlure (TML). In the Tunisian and Costa Rican trials 2 g of TML were placed on a dental wick and renewed weekly. In the Hawaiian trials fresh and field aged 4 g TML plugs were used. For one Hawaiian trial 4 g TML plugs were field weathered under Hawaiian conditions while for the ancillary trial 4 g TML plugs were aged in Florida. During periods of the Tunisian and Costa Rican trials where traps baited with the TML wick captured less than an average of one male medfly per trap per week, traps baited with the natural oil bulb formulation captured significantly more male medflies. During the same low population periods a higher percentage of traps baited with the natural oil bulb formulation that captured at least a single medfly was greater than the percentage of traps baited with TML capturing at least one male medfly. Based on the present data, the replacement interval for NO bulbs is 3–4 times longer than for 4 g TML plugs.
Resumen
En un ensayo de 18 semanas en Túnez, un ensayo de 20 semanas en Costa Rica y dos ensayos de 24 semanas en Hawaii, se utilizó una formulación de un bulbo de aceite natural enriquecido con α-copaeno que encaja perfectamente en la canasta que se usa para el monitoreo con trimedlure (TML) en áreaslos programas de amplios. En los ensayos de Túnez y Costa Rica se colocaron 2 g de TML en una mecha dental y se renovaron semanalmente. En las pruebas hawaianas se utilizaron tapones de TML de 4 g, frescos y envejecidos en el campo, que se utilizan actualmente para el monitoreo en áreas amplias. Para una prueba hawaiana, se envejecieron tapones TML de 4 g en condiciones hawaianas, mientras que para la prueba auxiliar se envejecieron tapones TML de 4 g en Florida. Durante los períodos de las pruebas de Túnez y Costa Rica en los que las trampas cebadas con la mecha TML capturaron menos de un promedio de un macho de moscamed por trampa por semana, las trampas cebadas con la formulación de bulbo de aceite natural capturaron significativamente más moscamed machos. Durante los mismos períodos de baja población, un mayor porcentaje de trampas cebadas con la formulación de bulbo de aceite natural que capturan al menos una moscamed es mayor que el porcentaje de trampas cebadas con TML que capturan al menos un macho de moscamed. Según los datos actuales, el intervalo de sustitución de las bulbo NO es de 3 a 4 veces más largo que el de las tampones TML de 4 g.
1 Introduction
Fruit flies (Diptera: Tephritidae) are major economic pests in most fruit growing areas of the world (Papadopoulos et al. 2024). Due to their high intrinsic rate of population growth (Carey et al. 1998, 2005) and high dispersal ability (Iwahashi 1972; Meats and Smallridge 2007), early detection of infestations is important, as control and eradication efforts are more likely to be successful and less costly when enacted against small populations (Brockerhoff et al. 2010; Epanchin-Niell et al. 2014; Simberloff 2009). Fruit fly monitoring relies on male-specific attractants as well as food-based lures that emit odors associated with protein decomposition that attract both males and females (Broughton and Rahman 2017; Kean et al. 2024). The male-specific lures used most often are trimedlure (TML; Ceratitis spp.), cue-lure (CL; Bactrocera spp., Zeugodacus spp. and a few Dacus spp.) and methyl eugenol (ME; Bactrocera spp.) (Tan et al. 2014; White and Elson-Harris 1992). TML and CL, both synthetic compounds, were discovered during an intense chemical screening process undertaken by the United States Department of Agriculture (USDA) to identify potential insect attractants (Beroza and Green 1963). The attraction of Bactrocera spp. males to ME was discovered in the early 20th century by Howlett (Verghese et al. 2013), and this attractant was used in area-wide management of the oriental fruit fly, Bactrocera dorsalis (Hendel) as early as 1955 (Steiner and Lee 1955).
In its recent projections, the USDA Animal and Plant Health Inspection Service (APHIS) proposes to deploy approximately 153,000 traps per year to monitor exotic fruit flies (USDA APHIS 2024). One of the main targets of this trapping effort is the Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann), and monitoring for the medfly is currently projected to require between 100,000 and 400,000 TML dispensers (polymeric plugs) per year (SAM 2022). The maximum annual TML plug requirement will provide bait for approximately 61,500 traps, with plugs being replaced every 8 weeks (SAM 2022). Thus, the resources required for monitoring C. capitata represent a substantial portion of the total allocation for fruit fly surveillance within APHIS.
During its over half century of use (Shelly and Cloonan 2024), effort has been made to extend the useful field life of TML baits. Initially, TML was placed on cotton dental wicks from which it rapidly evaporated, and the useful lifetime of these TML baits was 2–4 weeks (King and Landolt 1984; Rice et al. 1984). Studies of entrainment of TML in cylindrical polymer plugs or laminated sheets yielded, as a preferred formulation, plugs that were easy to place in traps and emitted effective amounts of TML for 6 weeks (Dean et al. 2018; Leonhardt et al. 1984). In addition to the use of a polymeric dispenser, evaporation from wicks and plugs can be decreased by diluting TML with compounds with similar or lower boiling points (Leonhardt et al. 1984). TML diluted with such evaporation retardants (e.g., Capilure®, International Pheromone Systems, Neston, Chesire, United Kingdom) and placed on wicks was initially considered to be less expensive than TML on wicks, because traps baited with it were determined to attract more male medflies for longer periods of time (Hill 1987). However, in the decades following the introduction of Capilure®, mixed results were obtained when traps baited with Capilure® were compared with those baited with TML (Shelly 2013). For example, several studies reported that TML and Capilure® dispensers had similar attractiveness during the initial 8–11 weeks of field deployment, but that Capilure® had greater attractiveness thereafter (Hill 1987; Nakagawa et al. 1981; Wijesuriya and De Lima 1995). In contrast, Baker et al. (1988) and Shelly (2013) found that traps baited with TML captured more male medflies than traps baited with Capilure® during the initial 8–10 weeks of deployment (see also Manrakhan et al. 2021). Finally, the possibility that increasing the loading of TML might extend the lure’s effective longevity has been examined recently. Shelly and Kurashima (2020) and Francis et al. (2023) investigated whether doubling the TML loading to 4 g might correspondingly double the longevity of TML plugs to 12 weeks. While some increases were noted, boosting the loading to 4 g generally extended the effective life of the plugs by only 2–4 weeks (Francis et al. 2023; Shelly and Kurashima 2020).
An additional strategy for extending the longevity of C. capitata baits is to identify an attractant with a longer field life than TML. Work focusing on this strategy has centered on two approaches. First, the longevity of TML might be extended via its structural modification. For example, ceralure B1, an iodo analog of TML, is more attractive than TML and remains attractive for longer periods than TML (Jang et al. 2001; Warthen et al. 1994). Unfortunately, the high cost of synthesizing ceralure B1 renders this approach economically infeasible (Jang et al. 2005; Khrimian et al. 2003). Second, identification of a natural attractant with greater longevity could lead to the replacement of TML with an alternative lure. In fact, such replacement was recognized as a viable approach during the screening of attractants in the 1950’s (Beroza and Green 1963). Seed and root extract from the plant Angelica officinalis L. (syn archangelica, Apiales: Apiaceae) were both as attractive to male medflies as any early stage TML analog (Beroza and Green 1963). When Angelica seed oil was deployed in monitoring traps for medfly in Florida, it proved to be an excellent attractant (Steiner et al. 1957). While the monitoring program was successful, the high demand for Angelica seed oil exhausted the available supply (Steiner et al. 1957), which was an important incentive to develop TML as a male lure.
The active chemical in Angelica seed oil was determined to be α-copaene (Guitto et al. 1972), with the dextrorotary isomer being the predominant enantiomer (Jacobson et al. 1987; Takeoka et al. 1990). A 7-day trial comparing (+)-α-copaene and (−)-α-copaene revealed that the former was more attractive to male medflies than the latter and that (−)-α-copaene was less attractive than several other sesquiterpenes tested during this study (Flath et al. 1994). Ginger root oil (Zingiber officinale Roscoe, Zingiberales: Zingiberaceae) was later identified as an alternative source of (+)-α-copaene but repeated fractional distillation was required to increase (+)-α-copaene concentration from 0.3–0.4 % to the 3 % or more required for significant male C. capitata attraction (Shelly and Pahio 2002). Trials in multiple locations verified that (+)-α-copaene enriched ginger root oil (EGRO) baited traps can be more attractive than those baited with TML. In Tunisia, Hafsi et al. (2019) found traps baited with EGRO captured more male medflies than traps baited with TML. Likewise, in South Africa Mwatawala et al. (2013, 2015) and Manrakhan et al. (2017) reported that EGRO baited traps were equally or even more attractive to male medfly than those baited with TML. However, field tests in Hawaii showed that TML baited traps were significantly more attractive than those baited with EGRO (Shelly 2013; Shelly and Pahio 2002). The release rates of EGRO and TML almost certainly varied among these studies making interpretation difficult. In further investigating the role of α-copaene in medfly attraction to essential oils, Niogret et al. (2017) reported no correlation between the relative attractiveness of an oil and its α-copaene content, suggesting when α-copaene is a minor component in a natural oil other components may mask the attraction. Notably, in this study, traps baited with Angelica seed oil were less attractive than those baited with TML. The most recent search for alternative sources of α-copaene revealed that the terpene fraction of de-eugenolized clove oil yields a mixture of α-copaene enantiomers attractive to male C. capitata, although the chemical procedure had low yield and involved a purification method using high performance liquid chromatography (HPLC) (Lull et al. 2023).
In 2020, Chemtica Internacional in Costa Rica began a search for new sources of attractants for male C. capitata. Screening of natural oils from several sources led to identification of an α-copaene containing oil, which when enriched in α-copaene through efficient fractional distillation, was highly attractive to male C. capitata (Kharrat et al. 2024; Shelly et al. 2023). In a year-long study conducted in Tunisia and Costa Rica, we recently determined that Jackson traps, which are commonly used in fruit fly detection programs, baited with a sachet loaded with 4.2 g of a natural oil (NO) were more attractive than traps baited with 2 g TML plugs when the former were replaced at 12-weeks and the latter at 6-week intervals (Kharrat et al. 2024). A subsequent study in Hawaii showed that traps baited with the same sachet (and not replaced over the entire study) were more attractive than traps baited with 2 g of liquid TML applied to a dental wick renewed weekly for 20 weeks (Shelly et al. 2023). While the NO appeared highly attractive, installing the sachet dispenser inside traps is cumbersome and thus problematic for large-scale surveillance networks that include thousands or tens of thousands of traps for Ceratitis detection.
The current study was undertaken to evaluate a new, user-friendly NO formulation (a plastic bulb) designed to fit snugly in the basket positioned inside the Jackson trap and normally used to hold a TML plug (Figure 1). Field trials were conducted in three locations: Tunisia, Costa Rica, and Hawaii. In Tunisia and Costa Rica, we compared captures of male medflies in traps baited with weathered (not replaced) NO-containing bulbs against traps baited with TML wick dispensers replaced weekly. In Hawaii we compared captures of male medflies in traps baited with weathered NO bulbs against traps baited with fresh 4 g TML plugs and 4 g TML plugs weathered either in Hawaii or Sarasota, Florida.

α-Copaene enriched natural oil bulb in basket normally used to hold trimedlure plugs in Jackson traps.
2 Materials and methods
2.1 Tunisia
In Tunisia, field work was conducted in an orange (Citrus sinensis Osbeck, Sapindales: Rutaceae) orchard (8 ha; 36.577720°N, 10.639656°E) at 90 m.a.s.l. Trapping was conducted between March and July 2024, during which average weekly minimum and maximum temperatures were 12.4 °C and 37.0 °C, respectively. The average weekly daytime relative humidity varied between 26–87 % in the dry season (June to July) and 26–92 % in the wet season (March to May). There was no rainfall in the dry season, and rainfall averaged 36 mm/month in the wet season (Bouchrik Agricultural Weather Station, approximately 12.9 km from the field site).
Males of C. capitata were captured using Jackson traps, which are used worldwide in fruit fly surveillance programs (FAO/IAEA 2018). These traps were white ‘delta’ traps made of polymer-coated paper. A removable insert, coated with the polyisobutylene adhesive Stickem (Seabright Laboratories, California, USA) was placed on the floor of the trap to retain insects. Traps were suspended from branches using a metal hanger, with a straight rod positioned under the roof along the apex of the trap. Medfly captures were compared among three treatments: traps were baited with 2 mL of liquid TML (TML control; specific gravity ≈ 1.0; Farma Tech International Corporation, North Bend, Washington, USA) applied to dental wicks or bulbs containing 3 or 4 g of NO. The NO lures contained α-copaene enriched (>80 %) natural oil in a semi-rigid matrix held inside a flexible plastic bulb (NO bulb, P388PB, Chemtica Internacional, Sto Domingo, Heredia, Costa Rica, Figure 1). Both the TML-containing wicks and the NO-containing bulbs fit in the perforated, plastic baskets that were suspended from the metal hanger inside the traps.
Ten transects were established in the orchard, with each containing one trap per treatment (30 total traps). Traps were placed 30 m from each other, 1.5 m above ground, and in the canopy on the eastern side of trees. All traps were > 20 m from the border of the orchard. The TML-bearing wicks were replaced weekly, while the NO-bearing bulbs were not replaced over the 18-week long trial. Traps were checked weekly, and sticky inserts (covering the bottom of the trap) with captured insects were replaced. Within each transect, traps were advanced one position weekly to minimize location effects. The inserts were taken to the laboratory, and captured insects were identified and counted. If more than 100 C. capitata males were captured in any trap in a given week, at the next week, inserts were renewed twice per week trying to ensure that captures in all traps dropped below 100 males per week. This procedure was adopted to reduce the possibility that high catch reduced the capture efficiency of the sticky inserts in the traps and was used only for the Tunisian trial.
2.2 Costa Rica
In Costa Rica, trapping was performed in a farm located near Atenas, Alajuela, Costa Rica (4 ha; 10.0077778°N, 84.4086111°E) at 840 m.a.s.l. This farm contained orange (Citrus sinensis Osbeck, Sapindales: Rutaceae) and tangerine (Citrus reticulata Blanco, Sapindales: Rutaceae) trees planted to shade coffee (Coffea arabica L., Getianales: Rubiaceae). Trapping was conducted between November 2023 and April 2024. During November 2023, which was the last month of the wet season, the average daily minimum and maximum temperatures were 21.1 °C and 27.3 °C, respectively. Daytime minimum relative humidity averaged 59.8 %. Rainfall averaged 246 mm/month (data weather station of the Instituto Meteorológico Nacional located 5–10 km from the field site). During the dry season between December 2023 and the termination of the trial in April 2024 the average daily minimum and maximum temperatures were 16 °C and 28.8 °C, respectively. Daytime minimum relative humidity averaged 47.1 % and rainfall averaged 15 mm/month.
Medfly captures were compared among the same three treatments used in Tunisia: 2 mL of TML on wicks or 3 or 4 g of NO in bulbs. The trapping protocol was similar to that used in Tunisia. Initially, 10 transects were established in the orchard, with each containing one Jackson trap per treatment (30 total traps). Traps were placed 15–20 m from each other, 1.5–2.0 m above ground, and in the canopy on the eastern side of trees. All traps were > 10 m from the border of the orchard. The TML-bearing wicks were replaced weekly, while the NO-bearing bulbs were not replaced over the 18-week trial. Traps were checked weekly at which point the inserts replaced and used inserts were taken to the laboratory, and captured insects were identified and counted. Traps were advanced one position weekly to minimize location effects.
2.3 Hawaii
Field work in Hawaii was conducted in a commercial coffee field (65 ha, 100 m.a.s.l.) in central Oahu approximately 10 km southeast of Haleiwa, Hawaii, USA. Plant rows were separated by 3 m, and plants were maintained at a height of 2–3 m. Within a row, adjacent plants were 1–2 m apart, but foliage was contiguous between neighboring plants. Trapping was performed between October 2023 and April 2024 during which average daily minimum and maximum temperatures were 17.7 °C (range: 15.5–21.1 °C) and 25.5 °C (range: 23.8–30.0 °C), respectively, with daytime relative humidity generally between 60 and 80 %. Conditions were relatively dry, and rainfall averaged 18 mm/month over the study period (weather data from Wheeler Army Airfield, Wahiawa, Hawaii, about 10 km from the study site).
Jackson traps were baited either with 4 g TML plugs or 3 g NO bulbs. Two sets of TML plugs were deployed. In one set, the TML plugs were newly placed (i.e., were fresh) at the start of each sampling period and thus served as a standard or control lure. TML plugs in the second set were not replaced but weathered over the course of the experiment. The NO bulbs were likewise weathered over the experiment. Trapping was conducted when the TML plugs and NO plug/bulbs were weathered for 0 (i.e., when fresh), 6, 9, 12, 15, 18, 21, and 24 weeks. Trapping was conducted for 4–5 days at each of these weathering intervals after which the plugs and bulbs were placed in a shaded outdoor area at the USDA Hawaii Fruit Fly Laboratory, Aiea, Hawaii, under environmental conditions similar to the study site.
For a given trapping period, six Jackson traps, two for each of the three treatments, were placed in each of six rows of coffee plants (i.e., 36 total traps). Traps were placed in shaded locations 1.5–2.0 m above ground. Within a row, traps were separated by 20–25 m and were placed in repeating sequences (e.g., ABCABC), with the particular sequence selected randomly. The six rows used were 25–30 m apart. Over the trial, different sections of the coffee field were used for trapping, as harvesting was ongoing, and the mechanical harvester would have destroyed any traps encountered. Thus, harvesting necessitated shifting trapping locations within the field.
An additional trapping study was conducted in Hawaii in September 2024 using 3 g NO bulbs that had been weathered in Sarasota, Florida, and shipped to Hawaii for testing. Summer temperatures in central and south Florida are higher than those in Hawaii, and there was interest in evaluating the impact of these hotter conditions on the performance of NO bulbs. In Florida, NO bulbs were in placed in Jackson traps (without sticky inserts) and suspended in citrus trees (1.5–2.0 m above ground) in a grove in Sarasota. A total of 45 such traps were deployed, with 15 traps each being deployed on May 13, June 24, and August 5 (all 2024). All traps were collected on September 16, yielding NO bulbs weathered for 6, 12, or 18 weeks, which were then express mailed to Hawaii. Daily average minimum and maximum temperatures for the months when the bulbs were weathered were: May: 24.1–32.4 °C; June: 24.9–32.7 °C; July: 25.7–32.9 °C; August: 26.6–32.2 °C; September: 25.2–32.5 °C (weather data from Sarasota-Bradenton International Airport approximately 15 km from the weathering site).
In Hawaii, the weathered bulbs were placed in Jackson traps and then deployed in the same coffee field described above on September 19, 2024. As a control, 15 Jackson traps containing a fresh 4 g TML plug also were deployed. The traps (60 total) were placed in seven rows each with eight traps (two traps per treatment, with the treatments deployed following the same protocol described above for Hawaii) and one row of four traps (one trap per treatment). Traps were 25–30 m apart both within and between rows. Traps were collected 1 week later and returned to the laboratory for insect identification and counting.
2.4 Statistical analysis
Capture data were not normally distributed for any of the three study locations (Shapiro–Wilk test). Consequently, we compared the captures of the different treatments for each week using the Kruskal–Wallis test, a non-parametric one-way ANOVA. If significant variation was detected, the Tukey multiple comparisons test was used to identify significant differences (P < 0.05) among specific treatments. The Wilcoxon Mann Whitney U test was used to compare captures between fresh TML plugs and NO bulbs in week 0 in Hawaii (i.e., when the third treatment, weathered TML plugs, was not available). In both Tunisia and Costa Rica, we explored possible differences in medfly detection at low population sizes. For these locations, we defined low population periods as intervals during which captures in TML baited traps averaged less than 1 fly/week. In both countries, we tabulated the numbers of traps that captured 0 or ≥1 medflies for all collection dates within the low population period and compared the frequencies of these absent/present scores among the three treatments with chi-squared test.
3 Results
3.1 Tunisia
For the trial conducted in Tunisia, both the 3 and 4 g bulb formulations attracted more medflies than TML-baited traps at all evaluations, being significantly different for the first 11 weeks and week 13, Figure 2. No significant differences in capture rates for the NO- versus TML-baited traps were found for weeks 15–18. During the entire Tunisian trial, traps baited with 3 or 4 g NO bulbs captured 1.5× and 2.8× as many medflies as TML-baited traps, respectively.

Average male medfly captures in traps baited with trimedlure control wicks (TML; replaced weekly) or 3 or 4 g of natural oil bulbs (NO 3 g B and NO 4 g B, respectively; not replaced) in a citrus farm in Tunisia. Different letters in the same week indicate significant differences (Tukey, P < 0.05). If no letters are presented near data points for a given week, then all treatments at that collection date gave statistically equivalent captures.
Low capture periods were defined as weeks during which there was, on average, less than one medfly per trap in the TML-baited traps. In Tunisia, this corresponded to weeks 1–3 of the trial. During this interval, there were 89 trap evaluations (collections) for all treatments. At least one medfly was recorded for all 30 collections of traps baited with 4 g NO bulbs (100 %) and 26/29 (98 %) of traps baited with 3 g NO bulbs. In contrast, only 19/30 (63 %) of traps baited with liquid TML contained at least one medfly. The frequency of at least one capture varied significantly among the treatments (χ 2 = 37.11, df = 2, P < 0.0001). The total number of medfly captures during weeks 1–3 were 61, 62, and 16 for traps baited with 4 g NO bulbs, 3 g NO bulbs or TML control wicks, respectively.
3.2 Costa Rica
The trial in Costa Rica began in late November 2023, near the beginning of the dry season when medfly populations typically increase due to the ripening of coffee fruits. Traps baited with 3 g NO bulbs captured 2× and traps baited with 4 g NO bulbs captured 2.5× as many male medflies as those baited with TML control wicks over the 20-week trial (Figure 3). Traps baited with 3 or 4 g NO bulbs captured more C. capitata males every week, except week 6 when captures in traps baited with TML control wicks exceeded those in traps baited with 3 g NO bulbs and week 14 when TML-baited traps had higher capture numbers than traps baited with 4 g NO bulbs. Statistical differences in capture rates between NO bulb-baited traps and those baited with TML wicks were found only in the 1st and 9th weeks of the trial.
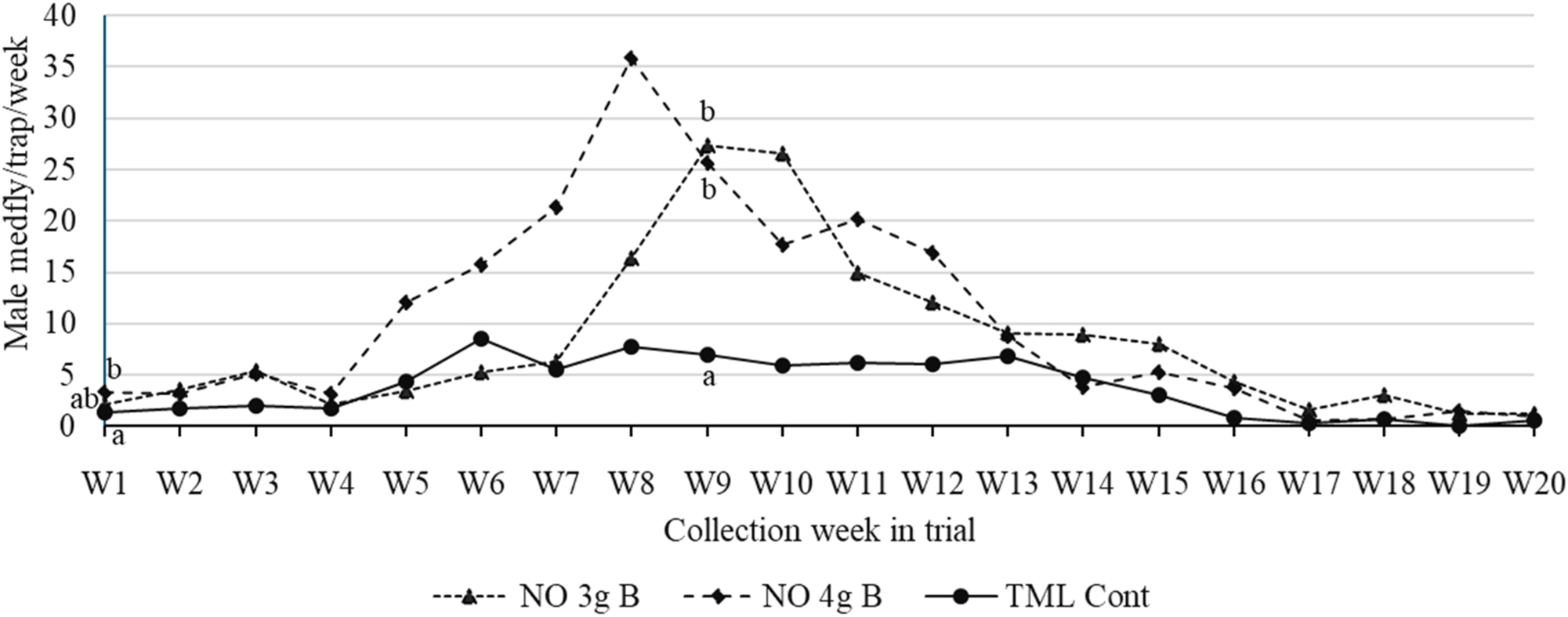
Average male medfly captures in traps baited with trimedlure (TML) control wicks replaced weekly versus traps baited with 3 or 4 g natural oil bulbs (NO 3 g B and NO 4 g B, respectively, not replaced) in a coffee farm in Costa Rica. Different letters in the same week indicate significant differences (Tukey, P < 0.05). If no letters are presented near data points for a given week, then all treatments at that collection date gave statistically equivalent captures.
For the low capture period, i.e., those weeks in which the average capture in the TML control wick baited traps was below one fly per trap (meaning weeks 16–20 in Figure 3), 68 % (34/50) of traps baited with TML captured at least one fly. In the case of the NO treatments, 54 % (25/46) and 70 % (30/43) of 3 and 4 g baited traps, respectively, captured at least one fly during the low capture periods. These percentages did not differ significantly (χ 2 = 3.43, df = 2, P = 0.18). During the same low capture period TML baited traps captured 25 male medflies while those baited with 3 g NO bulbs captured 103 and those baited with 4 g NO bulbs captured 66.
3.3 Hawaii
In Hawaii, captures of C. capitata males in 3 g NO bulb-baited traps were equal to or significantly greater than captures in traps baited with fresh 4 g TML plugs in all trapping periods (Figure 4). Except for weeks 6 and 24, traps with NO bulbs attracted significantly more medflies than traps baited with 4 g TML plugs weathered for the same duration. Traps baited with fresh or weathered TML plugs generally captured similar numbers of male medflies, except weeks 12 and 15 when captures with fresh plugs were significantly greater.

Average male medfly captures in traps baited with fresh or aged 4 g trimedlure (TML) plugs versus traps baited with 3 g NO bulbs in a coffee farm in Hawaii. Different letters in the same week indicate significant differences (Tukey, P < 0.05). If no letters are presented near data points for a given week, then all treatments at that collection date gave statistically equivalent captures.
In the ancillary test in Hawaii, the average (SE) numbers of medfly males captured per trap per day for the different treatments were: fresh 4 g TML plug = 1.03 (0.28); 6-week weathered NO bulbs = 2.27 (0.40); 12-week weathered NO bulbs = 1.68 (0.45); and 18-week weathered NO bulbs = 1.41 (0.30). The Kruskal–Wallis test revealed significant variation among the treatments (H = 8.79, df = 3, P = 0.03), with captures for the 6-week weathered NO bulbs being significantly greater than those for the fresh 4 g TML plugs. No significant differences were detected among the remaining pair wise comparisons. There was no collection date at which average captures in NO bulb baited traps were at or below 50 % of the average captures in TML plug baited traps.
4 Discussion
Since its discovery in the 1950’s, TML has been the gold standard for attraction of male medfly and is universally used as an attractant in area-wide monitoring programs (FAO/IAEA 2018). Of the three principal male fruit fly attractants (TML, CL, and ME), TML is considered the weakest (Shelly and Cloonan 2024). Although highly attractive synthetic analogs have been identified, there are none currently within economic reach (Shelly and Cloonan 2024). The most attractive natural product is (+)-α-copaene, which is present in Angelica seed oil (Steiner et al. 1957), and can be enriched in ginger root oil to levels that generate a level of attractancy equivalent to TML (Manrakhan et al. 2017; Mwatawala et al. 2013, 2015). Unfortunately, extensive fractional distillation is required to increase α-copaene content in ginger root oil to levels that produce attraction that rivals TML. This distillation process increases the cost of this attractant to several times that of TML while providing an attractant that is not more attractive than TML (Manrakhan et al. 2017; Mwatawala et al. 2013, 2015; Shelly 2013).
Earlier, we reported the discovery of an oil whose α-copaene content can be easily increased (Kharrat et al. 2024; Shelly et al. 2023). These studies also showed that traps baited with this α-copaene enriched natural oil (or NO), when presented in a sachet-type dispenser and weathered over long intervals, out-performed traps baited with the TML lures that were frequently renewed. Despite this result, installing the sachets in Jackson traps is time-consuming, rendering them impractical for large programs where thousands of traps require servicing. Here, we evaluated the effectiveness of a bulb dispenser of NO that is more easily handled given its snug fit in the basket used to hold male lure plugs in Jackson traps.
Trapping studies conducted in three different countries generated a consistent result: traps baited with NO-bearing bulbs, whether containing 3 or 4 g of the attractant, captured statistically equal or greater numbers of male medflies than traps baited with frequently refreshed TML over intervals of 18–24 weeks. Importantly, from a programmatic perspective, this finding indicates that NO bulbs have a longer replacement interval than the widely used 2 g TML plugs. Current guidelines (FAO/IAEA 2018) recommend replacing 2 g TML plugs every 6–8 weeks given their reduced attractancy after this duration of field use (Dean et al. 2018; Leonhardt et al. 1989). Thus, based on the present data, the replacement interval for NO bulbs is 3–4 times longer than for TML plugs, which would presumably reduce expenditures for medfly lures as well as labor costs associated with replacing lures.
In addition to greater longevity, there is evidence that the NO lure is more attractive to male medflies than TML. In testing the sachet dispenser in Hawaii, Shelly et al. (2023) found that traps baited with NO, which was not replaced, captured significantly more male medflies than traps baited with refreshed TML on wicks over the first 12 weeks of the study. In the present study, traps in Tunisia baited with NO bulbs, which were not replaced, had significantly higher catch than traps containing refreshed TML over the first 9 weeks of the study. Data from the low capture periods in Tunisia further indicated that NO lures might provide a higher probability of detecting small, incipient medfly populations, which would be particularly valuable for areas designated as pest free or having low pest prevalence. In Costa Rica and Hawaii, the numbers of male medflies captured in traps baited with NO bulbs were generally greater than those baited with TML plugs, although these differences were often not statistically significant.
In conclusion, the present findings indicate that the bulb formulation of α-copaene enriched natural oil effectively attracts male medflies for at least 12 weeks without replacement. The new formulation is easily handled and, importantly for worker safety, does not involve any contact with the oil. The bulb fits snugly in the basket normally used for TML in Jackson traps, and results from Tunisia and Hawaii (involving the bulbs weathered in Florida) show that the bulbs maintain higher potency even when daytime temperatures reach 32–37 °C.
Acknowledgments
In Hawaii we thank the Dole Corporation for allowing access to the Waialua coffee field and to Rick Kurashima for field assistance. Thanks are also extended to the farmers in Tunisia and Costa Rica for allowing access to their citrus fields in Tunisia and coffee fields in Costa Rica.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: KS site location and experimental execution in Tunisia; FG statistical analysis Tunisia & Costa Rica, CR & EGP experimental execution in Costa Rica, CC lure preparation, statistical analysis Costa Rica, TS experimental execution in Hawaii, statistical analysis, manuscript preparation, CO manuscript preparation, project coordination.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared: no LLM, AI assisted, or Machine Learning Tools were used in the reported work.
-
Conflict of interest: FG, CR, CC CO are employees of Chemtica International.
-
Research funding: None declared: no external funding was obtained.
-
Data availability: The raw data can be obtained from the corresponding author upon reasonable request.
References
Baker, P.S., Hendrichs, J., and Liedo, P. (1988). Improvement of attractant dispensing systems for the Mediterranean fruit fly (Diptera: Tephritidae) sterile release program in Chiapas, Mexico. J. Econ. Entomol. 81: 1068–1072, https://doi.org/10.1093/jee/81.4.1068.Suche in Google Scholar
Beroza, M. and Green, N. (1963). Materials tested as insect attractants. United States Department of Agriculture – Agricultural Research Service, Agriculture Handbook No. 239, Washington, D.C., U.S.A.Suche in Google Scholar
Brockerhoff, E.G., Liebhold, A.M., Richardson, B., and Suckling, D.M. (2010). Eradication of invasive forest insects: concepts, methods, costs and benefits. N.Z. J. For. Sci. 40: S117–S135.Suche in Google Scholar
Broughton, S. and Rahman, T. (2017). Evaluation of lures and traps for male and female monitoring of Mediterranean fruit fly in pome and stone fruit. J. Appl. Entomol. 141: 441–449, https://doi.org/10.1111/jen.12360.Suche in Google Scholar
Carey, J.R., Liedo, P., Müller, H.-G., Wang, J.-L., and Vaupel, J.W. (1998). A simple graphical technique for displaying individual fertility data and cohort survival: case study of 1000 Mediterranean fruit fly females. Funct. Ecol. 12: 359–363, https://doi.org/10.1046/j.1365-2435.1998.00197.x.Suche in Google Scholar
Carey, J.R., Liedo, P., Müller, H.-G., Wang, J.-L., Senturke, D., and Harshman, L. (2005). Biodemography of a long-lived tephritid: reproduction and longevity in a large cohort of female Mexican fruit flies, Anastrepha ludens. Exp. Gerontol. 40: 793–800, https://doi.org/10.1016/j.exger.2005.07.013.Suche in Google Scholar PubMed PubMed Central
Dean, D., Pierre, H., Mosser, L., Kurashima, R., and Shelly, T. (2018). Field longevity and attractiveness of trimedlure plugs to male Ceratitis capitata in Florida and Hawaii. Fla. Entomol. 101: 441–446, https://doi.org/10.1653/024.101.0322.Suche in Google Scholar
Epanchin-Niell, R.S., Brockerhoff, E.G., Kean, J.M., and Turner, J.A. (2014). Designing cost-efficient surveillance for early detection and control of multiple biological invaders. Ecol. Appl. 24: 1258–1274, https://doi.org/10.1890/13-1331.1.Suche in Google Scholar PubMed
FAO/IAEA (Food and Agriculture Organization of the United Nations/International Atomic Energy Agency) (2018). Trapping guidelines for area-wide fruit fly programmes, 2nd ed. Food and Agriculture Organization of the United Nations/International Atomic Energy Agency, Vienna, Austria.Suche in Google Scholar
Flath, R.A., Cunningham, R.T., Mon, T.R., and John, J.O. (1994). Additional male Mediterranean fruit fly (Ceratitis capitata Wied.) attractants from angelica seed oil (Angelica archangelica L.). J. Chem. Ecol. 20: 1969–1984, https://doi.org/10.1007/BF02066237.Suche in Google Scholar PubMed
Francis, A., Mesa Martin, R., Mosser, L., Bazelet, C., and Shelly, T. (2023). Relationship between field captures of Mediterranean fruit flies (Diptera: Tephritidae) and the residual amount and release rate of trimedlure lure from polymeric plugs. Fla. Entomol. 106: 97–103, https://doi.org/10.1653/024.106.0205.Suche in Google Scholar
Guitto, A.A., Fornasiero, U.U., and Baccichetti, F.F. (1972). Investigations on attractants for males of Ceratitis capitata. Farmaco; Edizione Scientifica 27: 663–669.Suche in Google Scholar
Hafsi, A., Rahmouni, R., and Chermiti, B. (2019). Detection of Ceratitis capitata Wiedemann (Diptera: Tephritidae) using trimedlure versus enriched ginger oil in citrus orchards. Int. J. Pest Manag. 66: 378–384, https://doi.org/10.1080/09670874.2019.1654632.Suche in Google Scholar
Hill, A.R. (1987). Comparison between trimedlure and Capilure® – attractants for male Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Aust. J. Entomol. 26: 35–36, https://doi.org/10.1111/j.1440-6055.1987.tb00254.x.Suche in Google Scholar
Iwahashi, O. (1972). Movement of the oriental fruit fly adults among islets of the Ogasawara Islands. Environ. Entomol. 1: 176–179, https://doi.org/10.1093/ee/1.2.176.Suche in Google Scholar
Jacobson, M., Uebel, E.C., Lusby, W.R., and Waters, R.M. (1987). Optical isomers of α-copaene derived from several plant sources. J. Agric. Food Chem. 35: 798–800, https://doi.org/10.1021/jf00077a038.Suche in Google Scholar
Jang, E.B., Raw, A.S., and Carvalho, L.A. (2001). Field attraction of Mediterranean fruit fly, Ceratitis capitata (Wiedemann), to synthetic stereoselective enantiomers of the ceralure B1 isomer. J. Chem. Ecol. 27: 235–242, https://doi.org/10.1023/A:1005620203504.Suche in Google Scholar
Jang, E.B., Khrimian, A., Holler, T.C., Casana-Giner, V., Lux, S., and Carvalho, L.A. (2005). Field response of Mediterranean fruit fly (Diptera: Tephritidae) to ceralure B1: evaluations of enantiomeric B1 ratios of fly captures. J. Econ. Entomol. 98: 1139–1143, https://doi.org/10.1603/0022-0493-98.4.1139.Suche in Google Scholar PubMed
Kean, J.M., Manoukis, N.C., and Dominiak, B.C. (2024). Review of surveillance systems for tephritid fruit fly threats in Australia, New Zealand, and the United States. J. Econ. Entomol. 117: 8–23, https://doi.org/10.1093/jee/toad228.Suche in Google Scholar PubMed PubMed Central
Kharrat, S., Gonzalez, F., Rodriguez, C., Calvo, C., and Oehlschlager, C. (2024). Relative captures and detection of male Ceratitis capitata using a natural oil lure or trimedlure plugs. Fla. Entomol. 107: 20240022, https://doi.org/10.1515/flaent-2024-0022.Suche in Google Scholar
Khrimian, A., Margaryan, A.K., and Schmidt, W.F. (2003). An improved synthesis of ethyl cis-5-iodo-trans-2-methylcyclohexanecarboxylate, a potent attractant for the Mediterranean fruit fly. Tetrahedron 59: 5475–5480, https://doi.org/10.1016/S0040-4020(03)00853-6.Suche in Google Scholar
King, J.R. and Landolt, P.J. (1984). Rate of loss of trimedlure from cotton wicks under south Florida field conditions. J. Econ. Entomol. 77: 221–224, https://doi.org/10.1093/jee/77.1.221.Suche in Google Scholar
Leonhardt, B.A., Rice, R.E., Harte, E.M., and Cunningham, R.T. (1984). Evaluation of dispensers containing trimedlure, the attractant for the Mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 77: 744–749, https://doi.org/10.1093/jee/77.3.744.Suche in Google Scholar
Leonhardt, B.A., Cunningham, R.T., Rice, R.E., Harte, E.M., and Hendrichs, J. (1989). Design, effectiveness, and performance criteria of dispenser formulations of trimedlure, an attractant of the Mediterranean fruit fly (Diptera: Tephritidae). J. Econ. Entomol. 82: 860–867, https://doi.org/10.1093/jee/82.3.860.Suche in Google Scholar
Lull, C., Gil-Ortiz, R., and Cantín, A. (2023). A chemical approach to obtaining α-copaene from clove oil and its application in the control of the medfly. Appl. Sci. 13: 5622, https://doi.org/10.3390/app13095622.Suche in Google Scholar
Manrakhan, A., Daneel, J.H., Beck, R., Virgilio, M., Meganck, K., and De Meyer, M. (2017). Efficacy of trapping systems for monitoring of Afro-tropical fruit flies. J. Appl. Entomol. 141: 825–840, https://doi.org/10.1111/jen.12373.Suche in Google Scholar
Manrakhan, A., Daneel, J.-H., Beck, R., Love, C.N., Gilbert, M.J., Virgilio, M., and De Meyer, M. (2021). Effects of male lure dispensers and trap types for monitoring of Ceratitis capitata and Bactrocera dorsalis (Diptera: Tephritidae). Pest Manag. Sci. 77: 2219–2230, https://doi.org/10.1002/ps.6246.Suche in Google Scholar PubMed
Meats, A. and Smallridge, C.J. (2007). Short- and long-range dispersal of medfly, Ceratitis capitata (Dipt., Tephritidae), and its invasive potential. J. Appl. Entomol. 131: 518–523, https://doi.org/10.1111/j.1439-0418.2007.01168.x.Suche in Google Scholar
Mwatawala, M., Virgilio, M., Quilici, S., Dominic, M., and De Meyer, M. (2013). Field evaluation of the relative attractiveness of enriched ginger root oil (EGO) lure and trimedlure for African Ceratitis species (Diptera: Tephritidae). J. Appl. Entomol. 137: 392–397, https://doi.org/10.1111/j.1439-0418.2012.01744.x.Suche in Google Scholar
Mwatawala, M., Virgilio, M., Joseph, J., and De Meyer, M. (2015). Niche partitioning among two Ceratitis rosa morphotypes and other Ceratitis pest species (Diptera: Tephritidae) along an altitudinal transect in Central Tanzania. ZooKeys 540: 429–442, https://doi.org/10.3897/zookeys.540.6016.Suche in Google Scholar PubMed PubMed Central
Nakagawa, S., Harris, E.J., and Keiser, I. (1981). Performance of capilure® in capturing Mediterranean fruit flies in Steiner plastic or cardboard sticky traps. J. Econ. Entomol. 74: 244–245, https://doi.org/10.1093/jee/74.2.244.Suche in Google Scholar
Niogret, J., Gill, M.A., Espinoza, H.R., Kendra, P.E., and Epsky, N.D. (2017). Attraction and electroantennogram responses of male Mediterranean fruit fly (Diptera: Tephritidae) to six essential oils. J. Entomol. Zool. Stud. 5: 958–964.Suche in Google Scholar
Papadopoulos, N.T., De Meyer, M., Terblanche, J.S., and Kriticos, D.J. (2024). Fruit flies: challenges and opportunities to stem the tide of global invasions. Annu. Rev. Entomol. 69: 355–373, https://doi.org/10.1146/annurev-ento-022723-103200.Suche in Google Scholar PubMed
Rice, R.E., Cunningham, R.T., and Leonhardt, B.A. (1984). Weathering and efficacy of trimedlure dispensers for attraction of Mediterranean fruit flies (Diptera: Tephritidae). J. Econ. Entomol. 77: 750–756, https://doi.org/10.1093/jee/77.3.750.Suche in Google Scholar
SAM. (2022). System for Award Management Site of US Government. Available at: https://sam.gov/opp/d5d62ac374a344eaa0545961f812dc87/view (Accessed 16 October 2024).Suche in Google Scholar
Shelly, T.E. (2013). Detection of male Mediterranean fruit flies (Diptera: Tephritidae): performance of trimedlure relative to capilure and enriched ginger root oil. Proc. Hawaii. Entomol. Soc. 45: 1–7.Suche in Google Scholar
Shelly, T.E. and Cloonan, K.R. (2024). Male lures and the detection of tephritid fruit flies: assessing the relationships between lure amount and release rate and trap captures of invasive pest species. Crop Prot. 176: 106504, https://doi.org/10.1016/j.cropro.2023.106504.Suche in Google Scholar
Shelly, T. and Kurashima, R. (2020). Field capture of male Mediterranean fruit flies (Diptera: Tephritidae) in traps baited with varying amounts of trimedlure. Fla. Entomol. 103: 16–22.https://doi.org/10.1653/024/1.3.0403.Suche in Google Scholar
Shelly, T.E. and Pahio, E. (2002). Relative attractiveness of enriched ginger root oil and trimedlure to male Mediterranean fruit flies (Diptera: Tephritidae). Fla. Entomol. 85: 545–551, https://doi.org/10.1653/0015-4040(2002)085[0545:RAOEGR]2.0.CO:2.10.1653/0015-4040(2002)085[0545:RAOEGR]2.0.CO;2Suche in Google Scholar
Shelly, T., Oehlschlager, C., and Kurashima, R. (2023). Natural oil lure outperforms trimedlure in capturing males of the Mediterranean fruit fly, Ceratitis capitata (Diptera: Tephritidae). Neotrop. Entomol. 52: 1138–1143, https://doi.org/10.1007/s13744-023-01079-5.Suche in Google Scholar
Simberloff, D. (2009). We can eliminate invasions or live with them. Successful management projects. Biol. Invasions 11: 149–157, https://doi.org/10.1007/s10530-008-9317-z.Suche in Google Scholar
Steiner, L.F. and Lee, R.K.S. (1955). Large area tests of a male-annihilation method for oriental fruit fly control. J. Econ. Entomol. 48: 311–317, https://doi.org/10.1093/jee/48.3.311.Suche in Google Scholar
Steiner, J.F., Miyashita, D.H., and Christenson, L.D. (1957). Angelic oils as Mediterranean fruit fly lures. J. Econ. Entomol. 50: 505, https://doi.org/10.1093/jee/50.4.505.Suche in Google Scholar
Takeoka, G., Flath, R.A., Mon, T.R., Buttery, R.G., Teranishi, R., Güntert, R., Lautamo, R., and Szejtli, J. (1990). Further applications of permethylated β-cyclodextrin capillary gas chromatographic columns. J. High Resolut. Chromatogr. 13: 202–206, https://doi.org/10.1002/jhrc.1240130317.Suche in Google Scholar
Tan, K.H., Nishida, R., Jang, E.B., and Shelly, T.E. (2014). Pheromones, male lures, and trapping of tephritid fruit flies. In: Shelly, T.E., Epsky, N.D., Jang, E.B., Reyes-Flores, J., and Vargas, R. (Eds.). Trapping and the detection, control, and regulation of tephritid fruit flies. Springer, Dordrecht, The Netherlands, pp. 15–74.10.1007/978-94-017-9193-9_2Suche in Google Scholar
USDA APHIS (United States Department of Agriculture Animal and Plant Health Inspection Service) (2024). Fruit fly exclusion and detection program strategy fiscal years 2024–2028, https://www.Aphis.Usda.Gov/Plant_health/Plant_pest_info/Fruit_flies/Downloads/Feed-Strategy-Plan.Pdf (Accessed 16 October 2024).Suche in Google Scholar
Verghese, A., Shivananda, T.N., Kamala Jayanthi, P.D., and Sreedevi, K. (2013). Frank Milburn Howlett (1877-1920): discoverer of the Pied Piper’s lure for the fruit flies (Tephritidae: Diptera). Curr. Sci. 105: 260–262.Suche in Google Scholar
Warthen, J.D., Cunningham, R.T., Demilo, A.B., and Spencer, S. (1994). Trans-ceralure isomers: differences in attraction for Mediterranean fruit fly, Ceratitis capitata (Wied.) (Diptera: Tephritidae). J. Chem. Ecol. 20: 569–578, https://doi.org/10.1007/BF02059598.Suche in Google Scholar
White, I.M. and Elson-Harris, M.M. (1992). Fruit flies of economic significance: their identification and bionomics. CAB International, Wallingford, UK.10.1079/9780851987903.0000Suche in Google Scholar
Wijesuriya, S. and De Lima, C. (1995). Comparison of two types of traps and lure dispensers for. Ceratitis capitata (Wiedemann) (Diptera: Tephritidae). Aust. J. Entomol. 34: 273–275, https://doi.org/10.1111/j.1440-6055.1995.tb01337.x.Suche in Google Scholar
© 2025 the author(s), published by De Gruyter on behalf of the Florida Entomological Society
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Research Articles
- First report of Phidotricha erigens (Lepidoptera: Pyralidae: Epipaschiinae) injuring mango inflorescences in Puerto Rico
- Seed predation of Sabal palmetto, Sabal mexicana and Sabal uresana (Arecaceae) by the bruchid Caryobruchus gleditsiae (Coleoptera: Bruchidae), with new host and distribution records
- Genetic variation of rice stink bugs, Oebalus spp. (Hemiptera: Pentatomidae) from Southeastern United States and Cuba
- Selecting Coriandrum sativum (Apiaceae) varieties to promote conservation biological control of crop pests in south Florida
- First record of Mymarommatidae (Hymenoptera) from the Galapagos Islands, Ecuador
- First field validation of Ontsira mellipes (Hymenoptera: Braconidae) as a potential biological control agent for Anoplophora glabripennis (Coleoptera: Cerambycidae) in South Carolina
- Field evaluation of α-copaene enriched natural oil lure for detection of male Ceratitis capitata (Diptera: Tephritidae) in area-wide monitoring programs: results from Tunisia, Costa Rica and Hawaii
- Abundance of Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and other thrips in commercial snap bean fields in the Homestead Agricultural Area (HAA)
- Performance of Salvinia molesta (Salviniae: Salviniaceae) and its biological control agent Cyrtobagous salviniae (Coleoptera: Curculionidae) in freshwater and saline environments
- Natural arsenal of Magnolia sarcotesta: insecticidal activity against the leaf-cutting ant Atta mexicana (Hymenoptera: Formicidae)
- Ethanol concentration can influence the outcomes of insecticide evaluation of ambrosia beetle attacks using wood bolts
- Post-release support of host range predictions for two Lygodium microphyllum biological control agents
- Missing jewels: the decline of a wood-nesting forest bee, Augochlora pura (Hymenoptera: Halictidae), in northern Georgia
- Biological response of Rhopalosiphum padi and Sipha flava (Hemiptera: Aphididae) changes over generations
- Argopistes tsekooni (Coleoptera: Chrysomelidae), a new natural enemy of Chinese privet in North America: identification, establishment, and host range
- A non-overwintering urban population of the African fig fly (Diptera: Drosophilidae) impacts the reproductive output of locally adapted fruit flies
- Fitness of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) on four economically important host fruits from Fujian Province, China
- Carambola fruit fly in Brazil: new host and first record of associated parasitoids
- Establishment and range expansion of invasive Cactoblastis cactorum (Lepidoptera: Pyralidae: Phycitinae) in Texas
- A micro-anatomical investigation of dark and light-adapted eyes of Chilades pandava (Lepidoptera: Lycaenidae)
- Scientific Notes
- Early stragglers of periodical cicadas (Hemiptera: Cicadidae) found in Louisiana
- Attraction of released male Mediterranean fruit flies to trimedlure and an α-copaene-containing natural oil: effects of lure age and distance
- Co-infestation with Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae): a threat for berry crops in Morelos, Mexico
- Observation of brood size and altricial development in Centruroides hentzi (Arachnida: Buthidae) in Florida, USA
- New quarantine cold treatment for medfly Ceratitis capitata (Diptera: Tephritidae) in pomegranates
- A new invasive pest in Mexico: the presence of Thrips parvispinus (Thysanoptera: Thripidae) in chili pepper fields
- Acceptance of fire ant baits by nontarget ants in Florida and California
- Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)
- Note on the nesting biology of Epimelissodes aegis LaBerge (Hymenoptera: Apidae)
- Mass rearing protocol and density trials of Lilioceris egena (Coleoptera: Chrysomelidae), a biological control agent of air potato
- Cardinal predation of the invasive Jorō spider Trichophila clavata (Araneae: Nephilidae) in Georgia
- Retraction
- Retraction of: Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)
Artikel in diesem Heft
- Frontmatter
- Research Articles
- First report of Phidotricha erigens (Lepidoptera: Pyralidae: Epipaschiinae) injuring mango inflorescences in Puerto Rico
- Seed predation of Sabal palmetto, Sabal mexicana and Sabal uresana (Arecaceae) by the bruchid Caryobruchus gleditsiae (Coleoptera: Bruchidae), with new host and distribution records
- Genetic variation of rice stink bugs, Oebalus spp. (Hemiptera: Pentatomidae) from Southeastern United States and Cuba
- Selecting Coriandrum sativum (Apiaceae) varieties to promote conservation biological control of crop pests in south Florida
- First record of Mymarommatidae (Hymenoptera) from the Galapagos Islands, Ecuador
- First field validation of Ontsira mellipes (Hymenoptera: Braconidae) as a potential biological control agent for Anoplophora glabripennis (Coleoptera: Cerambycidae) in South Carolina
- Field evaluation of α-copaene enriched natural oil lure for detection of male Ceratitis capitata (Diptera: Tephritidae) in area-wide monitoring programs: results from Tunisia, Costa Rica and Hawaii
- Abundance of Megalurothrips usitatus (Bagnall) (Thysanoptera: Thripidae) and other thrips in commercial snap bean fields in the Homestead Agricultural Area (HAA)
- Performance of Salvinia molesta (Salviniae: Salviniaceae) and its biological control agent Cyrtobagous salviniae (Coleoptera: Curculionidae) in freshwater and saline environments
- Natural arsenal of Magnolia sarcotesta: insecticidal activity against the leaf-cutting ant Atta mexicana (Hymenoptera: Formicidae)
- Ethanol concentration can influence the outcomes of insecticide evaluation of ambrosia beetle attacks using wood bolts
- Post-release support of host range predictions for two Lygodium microphyllum biological control agents
- Missing jewels: the decline of a wood-nesting forest bee, Augochlora pura (Hymenoptera: Halictidae), in northern Georgia
- Biological response of Rhopalosiphum padi and Sipha flava (Hemiptera: Aphididae) changes over generations
- Argopistes tsekooni (Coleoptera: Chrysomelidae), a new natural enemy of Chinese privet in North America: identification, establishment, and host range
- A non-overwintering urban population of the African fig fly (Diptera: Drosophilidae) impacts the reproductive output of locally adapted fruit flies
- Fitness of Bactrocera dorsalis (Hendel) (Diptera: Tephritidae) on four economically important host fruits from Fujian Province, China
- Carambola fruit fly in Brazil: new host and first record of associated parasitoids
- Establishment and range expansion of invasive Cactoblastis cactorum (Lepidoptera: Pyralidae: Phycitinae) in Texas
- A micro-anatomical investigation of dark and light-adapted eyes of Chilades pandava (Lepidoptera: Lycaenidae)
- Scientific Notes
- Early stragglers of periodical cicadas (Hemiptera: Cicadidae) found in Louisiana
- Attraction of released male Mediterranean fruit flies to trimedlure and an α-copaene-containing natural oil: effects of lure age and distance
- Co-infestation with Drosophila suzukii and Zaprionus indianus (Diptera: Drosophilidae): a threat for berry crops in Morelos, Mexico
- Observation of brood size and altricial development in Centruroides hentzi (Arachnida: Buthidae) in Florida, USA
- New quarantine cold treatment for medfly Ceratitis capitata (Diptera: Tephritidae) in pomegranates
- A new invasive pest in Mexico: the presence of Thrips parvispinus (Thysanoptera: Thripidae) in chili pepper fields
- Acceptance of fire ant baits by nontarget ants in Florida and California
- Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)
- Note on the nesting biology of Epimelissodes aegis LaBerge (Hymenoptera: Apidae)
- Mass rearing protocol and density trials of Lilioceris egena (Coleoptera: Chrysomelidae), a biological control agent of air potato
- Cardinal predation of the invasive Jorō spider Trichophila clavata (Araneae: Nephilidae) in Georgia
- Retraction
- Retraction of: Examining phenotypic variations in an introduced population of the invasive dung beetle Digitonthophagus gazella (Coleoptera: Scarabaeidae)

