Copper corrosion mechanisms, influencing factors, and mitigation strategies for water circuits of heat exchangers: critical review and current advances
-
Zohra Benzarti
, Nadia Arrousse
Abstract
This review examines copper corrosion mechanisms and their key influencing factors, including microstructure effects, surface treatments, manufacturing conditions, temperature, water chemistry parameters, fluid velocity, and microbial effects in water-based systems, with a particular focus on heat exchangers. This addresses a critical gap in the existing literature, which often examines copper corrosion in a broader context. By critically analyzing the literature, the review provides an in-depth understanding of the factors that govern copper corrosion in heat exchanger applications. Copper corrosion in heat exchangers can have significant technical and social detrimental consequences, leading to substantial economic losses. By focusing on heat exchangers, the review offers valuable insights and best practices for engineers, researchers, and practitioners working with copper in this domain. Furthermore, the review evaluates the latest mitigation strategies, including advancements in material selection, surface treatments, water treatment techniques, and robust monitoring/maintenance methods. Finally, the review explores promising new concepts for corrosion prevention for long-term performance, paving the way for future research in developing innovative technologies and refining highly effective strategies under diverse operating conditions relevant to combat deleterious copper corrosion effects in heat exchanger applications.
1 Introduction
Copper, renowned for its exceptional thermal and electrical conductivity, has found extensive application across various industries, encompassing plumbing systems, heat exchangers, electrical wiring, and electronic components (Singer et al. 2017). Nevertheless, its vulnerability to corrosion presents substantial challenges in preserving the integrity and durability of copper-based systems, particularly when exposed to aqueous environments (Sarver and Edwards 2012). Corrosion, constituting a significant technological, economic, and societal concern, necessitates a comprehensive knowledge of metal behavior under real-world conditions, along with the judicious material selection and the design solutions (Zanotto et al. 2018). Notably, the corrosion of brass alloys, widely employed in drinking water distribution systems for components such as tube fittings, valves, and ancillaries, can raise health concerns due to potential lead (Pb) and copper (Cu) release for the drinking water (Latva et al. 2017).
In heat exchangers, copper corrosion carries substantial economic implications. The formation of oxide layers and deposits on heat exchanger surfaces diminishes their efficiency (Rushing and Edwards 2004), resulting in elevated energy consumption and increased operational expenses. Furthermore, the need for more frequent maintenance and cleaning to combat the corrosion-induced damage can lead to an increased downtime and labor costs. The consequent loss of productivity during downtime exacerbates the economic impact of copper corrosion in heat exchangers. Thus, the effective management and mitigation of copper corrosion become imperative for sustainable efficiency of industrial processes and to minimize economic losses, especially in systems reliant on these critical components for heating, cooling, and refrigeration purposes.
Copper corrosion in water is a complex electrochemical process influenced by several interrelated factors. Among these parameters, temperature (Rushing and Edwards 2004), dissolved oxygen content (Huang et al. 2022), pH (Schock et al. 1995), and the presence of contaminants play crucial roles in determining the corrosion rate and mechanisms. Water chemistry properties, including the levels of chloride, sulfate, alkalinity, and hardness, can significantly impact the corrosivity of the aqueous environment towards copper (Lytle and Nadagouda 2010; Zlatanović et al. 2017). For instance, elevated chloride concentrations are known to accelerate copper corrosion, while high alkalinity levels can offer protective properties by forming passive films on the metal surface.
Due to multitude factors that may affect copper performance in various electrolyte systems, predicting their effects is not an easy task. However, using laboratory test results as a support to select a specific copper alloy for a certain application in a simulated environment, can provide information on the corrosion resistance of the copper alloys before their use, during the design and the preselected material for drinking water distribution systems. Implementing effective protection methods against copper corrosion is crucial for minimizing the economic burden associated with corrosion-related damages (Hou et al. 2017). Given the importance of mitigating copper corrosion, various protection methods have been developed and applied in practice.
Several methods exist to combat copper’s susceptibility to corrosion in specific environments. These methods encompass both preventive measures and active corrosion control techniques. Preventive strategies play a crucial role, including proper design considerations to minimize stagnant water areas and material selection that prioritizes corrosion-resistant options like copper-nickel alloys for aggressive environments (Fabjan et al. 2011; Metikoš-Huković et al. 2011). Additionally, barrier protection through coating systems with organic paints or inorganic coatings acts as a shield, preventing direct contact between copper and corrosive agents (Savita et al. 2016). Inhibitors offer another layer of preventive defense. These chemicals work by adsorbing onto the copper surface, forming a protective film or altering the electrochemical reactions that drive corrosion (Arrousse et al. 2023). Both organic and inorganic inhibitors have been proven effective in reducing the corrosion rate and extending the lifespan of copper components (Arrousse et al. 2023; Edwards et al. 2002; Otmacic Curkovic et al. 2010). Finally, when preventive measures are insufficient, active corrosion control techniques come into play. These techniques, such as cathodic protection, essentially force the copper surface to become a cathode, thereby hindering the oxidation reactions responsible for corrosion (Ngaotrakanwiwat et al. 2020).
In order to offer profound insights into the significance of the copper corrosion topic and the prevailing research trends, Figure 1 provides a comprehensive graphical representation covering the period from 2000 to 2022 (data sourced from app. dimensions.ai). This figure elucidates the evolving research landscape and underscores the increasing importance of addressing copper corrosion within the engineering domain over the past two decades. The sustained upward trajectory of both publication and citation counts signifies the active and ongoing investigation of copper corrosion, with researchers building upon prior studies to develop effective strategies for corrosion prevention and control in engineering contexts. Consequently, it becomes imperative to ensure that researchers in this field remain adequately informed and up-to-date.
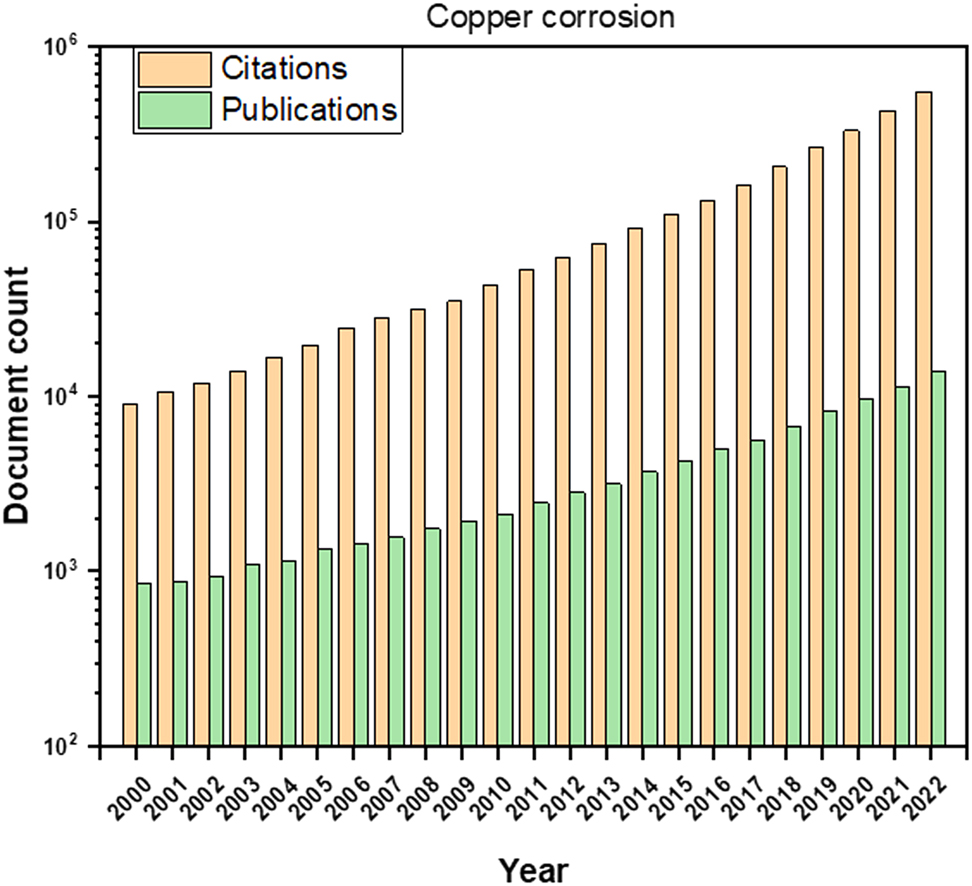
Evolution of the number of publications and citations for copper corrosion.
This review comprehensively examines copper corrosion mechanisms and their key influencing parameters in water-based systems, with a specific focus on heat exchangers. It explores various protection methods and critically analyzes the literature to provide an in-depth understanding of the chemical, physical, and biological factors that govern copper corrosion in this critical application. By focusing specifically on heat exchangers, an important application for copper where corrosion can have significant technical and social consequences, which cause together substantial economic losses. Additionally, this review presents the latest mitigation strategies, including advancements in material selection and design, water treatment techniques, and maintenance/monitoring methods. This review addresses a gap in the existing literature, which often examines copper corrosion in a broader context, neglecting the specific challenges faced in heat exchangers. This targeted approach offers valuable insights for engineers, researchers, and practitioners working with copper in this essential application. Future research in copper corrosion for heat exchangers should focus on the continuous development and refinement of effective prevention strategies. This includes quantifying the effectiveness of new technologies under diverse operating conditions and water chemistry parameters relevant to heat exchanger operation.
2 Copper corrosion
Copper, valued for its strength and durability, is a mainstay material in engineering equipment and structures, particularly within heat exchangers. However, a major drawback is its reactivity and chemical instability in various harsh environments. Despite its good resistance to corrosion, copper can be susceptible to degradation under certain water chemistry feature and operating conditions. This susceptibility can lead to significant technical, energy and economic consequences (Winston 2011; Winston and Uhlig 2008). In water-based systems, copper undergoes corrosion through an electrochemical process. This process involves two half-reactions: the oxidation of copper atoms at the metal surface (releasing electrons) and the reduction of another chemical species in the water that accepts these electrons. The specific mechanisms by which copper corrodes can be categorized based on their visual characteristics and the resulting surface morphology. Eight primary types of copper corrosion have been identified in the literature (Farooq et al. 2022; Fontana 1987). The following types are suggested: (i) pitting corrosion, (ii) erosion corrosion, (iii) stress corrosion and cracking, (iv) uniform corrosion, (v) galvanic corrosion, (iv) intergranular corrosion, (v) crevice corrosion and (vii) selective leaching. While other corrosion types like galvanic corrosion or intergranular corrosion can occur in copper under specific conditions, this review will focus on four mechanisms, which are the most commonly observed in copper water pipes and heat exchangers. These include, pitting corrosion, erosion corrosion, stress corrosion cracking, and uniform corrosion.
2.1 Copper corrosion mechanisms
Copper and its alloys are extensively utilized in various industries due to their exceptional mechanical and physical properties, making them the preferred materials for products and equipment such as water pipes and heat exchangers. However, it is crucial to carefully consider the susceptibility of copper to localized corrosion in aerated environments, despite its thermodynamic stability in less aerated conditions. In specific applications, the formation of a passive film under oxidic conditions on copper surface significantly affects the functionality of copper (King et al. 2017). For instance, in heat exchangers, the formation of scales and copper oxides can lead to a reduction in system efficiency. Nonetheless, these oxide films also serve as a protective barrier, preventing further dissolution of copper in corrosive environments. It is important to note that the passive film’s effectiveness in protecting copper from corrosion depends on both the metal itself (surface finish, microstructure) and its environment (aggressive ions, exposure time). These factors influence how well the film forms and how stable it is, ultimately affecting its ability to shield copper from corrosion. To effectively prevent corrosion and ensure the reliable performance of copper-based materials in diverse applications, it is essential to understand the formation and behavior of copper oxide films, as well as their interaction with the surrounding environment (Naseer and Khan 2009). Lu et al. (2021) studied the corrosion behavior of pure copper exposed to the harsh marine atmospheric environment. They revealed a high corrosion rate, with the formation of Cu2O, Cu2Cl(OH)3, and CuO as the main corrosion products. The corrosion product layer exhibits a double-layered structure, with differing trends in resistance values on the front and back sides.
Additionally, a comprehensive analysis of how the color of copper patina evolves and is influenced by different exposure conditions is required, which can be valuable for producers and end users, including architects, of copper sheet/pipe materials. In their study, Leygraf et al. (2019) focused on characterizing the color combined with determining the thickness of different patina constituents. Their research provided new insights into the origin and evolution of copper patina color, drawing from a rich collection of copper and copper-based materials exposed in marine, urban, and rural settings, along with selected historic copper artifacts.
2.1.1 Pitting corrosion
Extensive research on pitting corrosion in drinking water systems (Burstein et al. 2004; Duthil et al. 1996; Edwards et al. 1994a; Frankel 1998; Harrison et al. 2004; Lytle and Schock 2008; Marshall and Edwards 2005) has explored various factors influencing its initiation and growth. Theories propose microbial activity, material imperfections, and water chemistry (anions, pH) as potential contributors, but a single definitive explanation remains elusive due to the complexity of localized corrosion. Experimental variations (electrochemical versus full-scale) likely contribute to these discrepancies. While a comprehensive theory is lacking, the breakdown of the protective cuprous oxide film on copper surfaces is widely accepted as the primary failure mechanism. Chemical attack, inadequate film formation, or surface nonuniformities can cause this breakdown. The fine-grained cuprous oxide film, typically overlaid with basic cupric salts, offers excellent protection in potable water. However, specific water quality factors can either promote or mitigate pitting depending on their concentrations.
Pitting corrosion of copper requires a passive surface for anode/cathode separation. Passive films form through dissolution-precipitation or solid-state reactions. Custalow (2009) proposed a four-step mechanism for pitting in high-chlorine, high-pH waters: (1) anodic copper dissolution at the pit, (2) electron transport through the pipe wall, (3) cathodic consumption by disinfectants/oxygen, and (4) migration of anions into the pit. As shown in Figure 2, these steps are crucial for pitting regardless the initiation source.
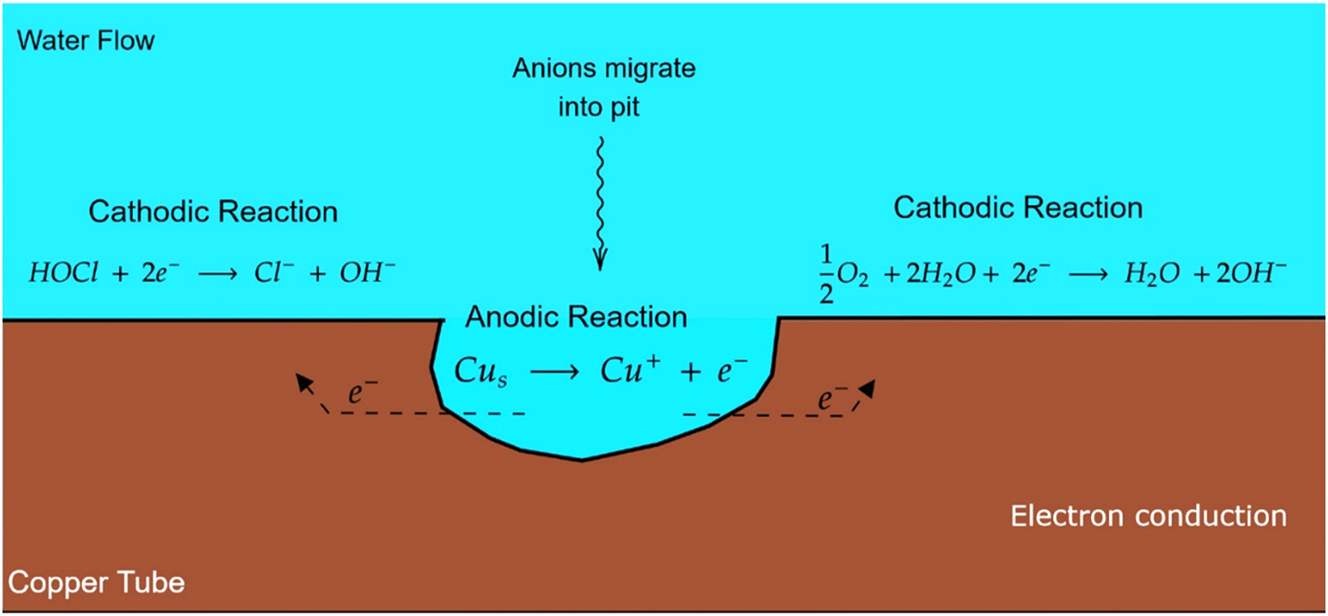
Pitting reaction in water with high chlorine and high pH.
Based on environmental conditions, three distinct types of pitting corrosion have been observed for copper and its alloys. Three main types have been identified based on their occurrence: cold water pitting (Type I), hot water pitting (Type II), and soft water pitting (Type III) (Campbell 1950). Each exhibits distinct chemical and physical characteristics.
2.1.1.1 Type I pitting
Type I pitting, a rapid form of corrosion observed in cold, hard waters (typically occurring within less than 3 months), is significantly influenced by water chemistry characteristics such as calcium carbonate saturation, organic content, and pH (Cornwell et al. 1973). Additionally, cleaning processes and temperature fluctuations within tanks and cylinders can contribute to its development (Cornwell et al. 1973). The mechanism behind Type I pitting involves the formation of cuprous chloride at a rate exceeding its conversion to copper oxide through oxidation or hydrolysis (Edwards et al. 1994a). The presence of phosphate in water can further exacerbate this issue by promoting the formation of a porous copper phosphate film that traps cuprous chloride, accelerating the corrosion process (Lytle and White 2014). Researchers have proposed models to predict the susceptibility of copper to Type I pitting based on water chemistry parameters (Lucey 1967). Especially, this type of corrosion is characterized as a direct chemical attack on the copper surface, as depicted in Figure 3.
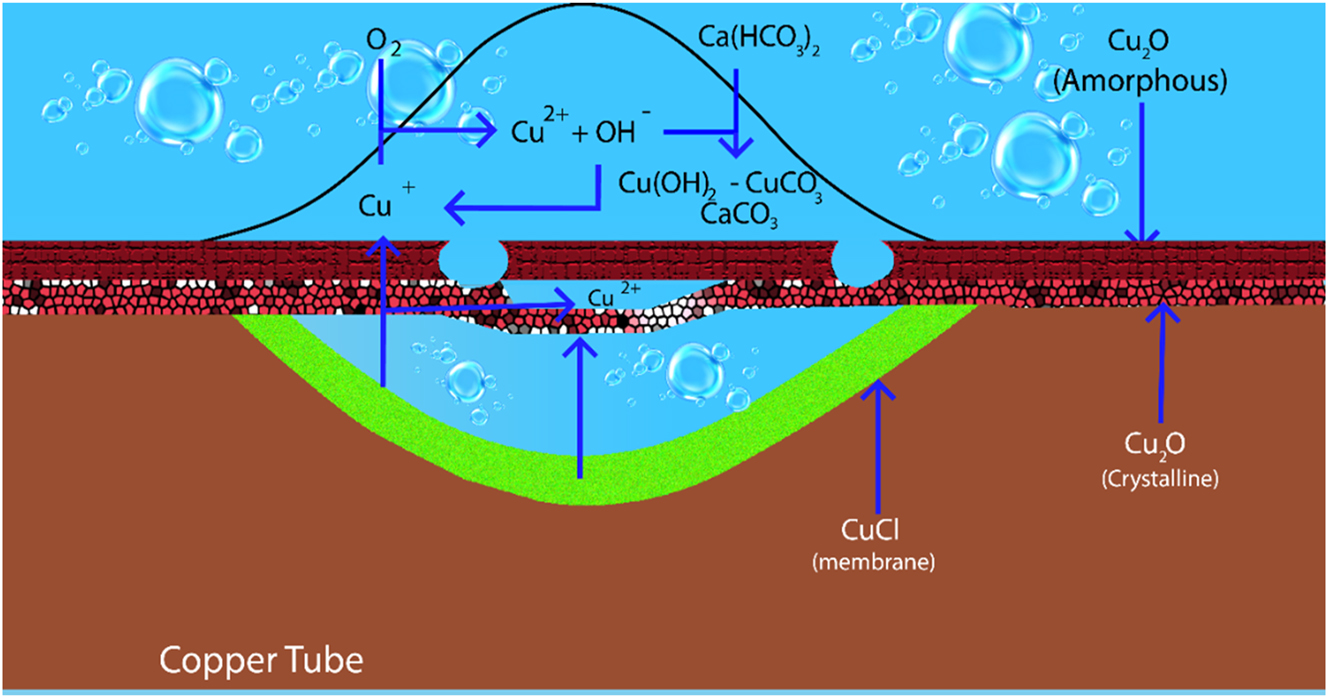
Corrosion products and reactions in type I pitting.
2.1.1.2 Type II pitting
Type II pitting, a slower form of corrosion compared to Type I, primarily observed in hot water systems with soft waters, low pH (below 7.4), and high temperatures (above 60 °C), manifests over a longer timeframe (typically 8–12 years) (Mattsson 1980). It manifests as deep pits containing crystalline cuprous oxide and basic copper sulfate (Figure 4). The presence of chlorine can significantly accelerate this process, leading to failures in less than 3 years (Sato et al. 1982). Baba et al. (1981) identified key factors influencing Type II pitting, including the bicarbonate-to-sulfate ratio ([HCO3 −]/[SO4 2−]) and critical pitting potential. Their observations revealed similar pit structures containing Cu2O, CuCl, and green Cu4(OH)6SO4 deposits (Figure 4).
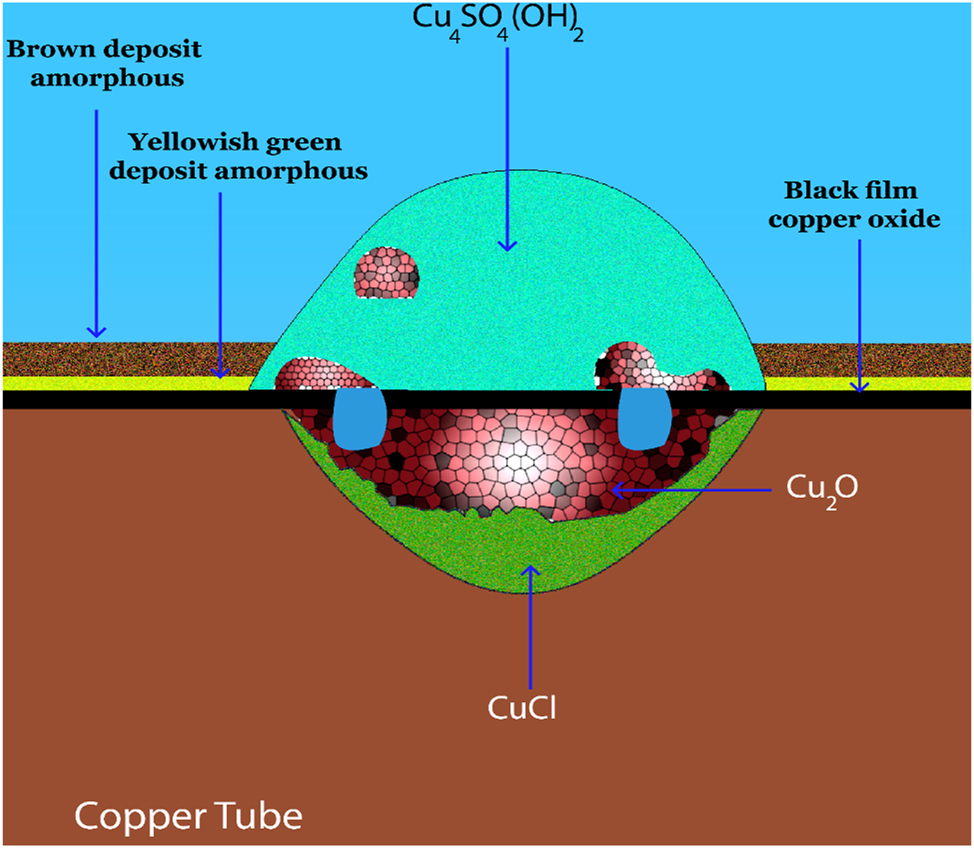
Corrosion products and reactions in type II pitting.
2.1.1.3 Type III pitting
Type III pitting corrosion, as reviewed by Campbell (Campbell 1950), specifically affects copper pipes in soft, acidic water environments, occurring in both cold and warm conditions. This phenomenon can manifest in cold water pipes exposed to warm surroundings or in hot water circuit systems at locations distant from the heat source, typically around 50 °C. The leading theory suggests a two-step process: firstly, a biofilm forms between the copper surface and its protective oxide-hydroxide layer. When this biofilm breaks down, it exposes a concentrated area of copper ions, potentially due to their accumulation within the biofilm itself. Secondly, the presence of peroxide and hydroperoxide ions within the biofilm accelerates the corrosion process compared to the typical reduction of dissolved oxygen.
2.1.1.4 Moundless pitting
In a recent study, Sakai (2022) investigated a newly discovered form of pitting corrosion in copper, known as “moundless” corrosion, which has been observed primarily in Japan. This research focused on elucidating the distinctive morphological characteristics of moundless corrosion, which differentiate it from conventional pitting corrosion as illustrated in Figure 5. Using surface analysis of the moundless pits, Sakai (2022) observed the presence of thin silica films covering their openings of pits. These findings suggest a strong influence of water quality, particularly the presence of silica, on the development of moundless pits.
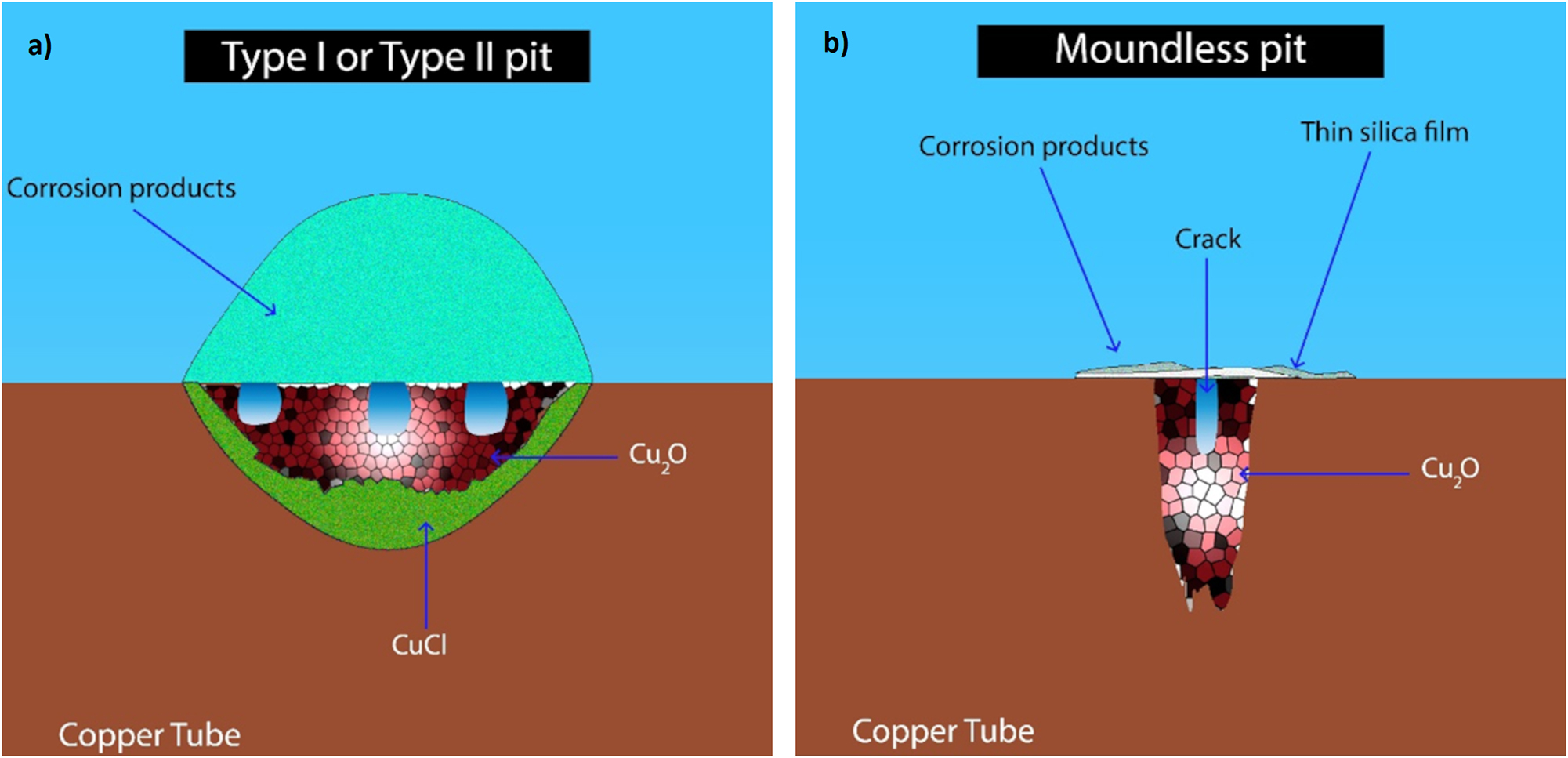
Comparison between (a) conventional pits (type I and type II) and (b) a moundless pit.
In conclusion, copper’s susceptibility to pitting corrosion is influenced by a complex interplay of factors. The types of oxides present on the copper surface can significantly impact its resistance, with chromium- or molybdenum-rich oxides offering better protection than defective or deficient ones (Akpanyung and Loto 2019). Water chemistry also plays a critical role. Stagnant water with high sulfate content and soft water with specific characteristics (low conductivity, high pH, low alkalinity, and high chloride/sulfate ratios) can increase the likelihood of pitting compared to flowing water, as reported by Lytle and Schock (2008), and Lytle et al. (2005). Additionally, recent research has identified novel forms of pitting corrosion, such as moundless corrosion, highlighting the need for continuous exploration of this phenomenon (Sakai 2022). Understanding these factors and their interactions is crucial for selecting appropriate copper alloys, implementing effective water treatment strategies, and designing systems that minimize the risk of all form of pitting corrosion in copper water pipes and heat exchangers.
2.1.2 Erosion-corrosion
Erosion corrosion, characterized by excessive turbulence and rapid flow-induced wear, resulting in removing protective films, significantly accelerates copper corrosion in water systems (Knutsson et al. 1972; Myers and Cohen 2005). In fact, the mechanism of erosion corrosion involves the synergistic effects of mechanical wear and electrochemical dissolution on copper pipes. This mechanism exposes copper to the corrosive agents in the water. This exposure leads to accelerated electrochemical reactions, particularly in the presence of aggressive ions such as chlorides and sulfates, which potentially disrupts protective surface layers, further degrade the copper as shown in Figure 6. High-velocity jets are particularly detrimental, preventing the formation of protective surface layers. Additionally, the jets induce separation of the anodic and cathodic regions of the copper, resulting in accelerated corrosion via the formation of a concentration cell due to uneven ion distribution in the electrolyte and the corrosive nature of water, leading to localized anodic dissolution (where metal corrodes) and adjacent cathodic regions (Murakami et al. 2003). However, copper pipe loops exposed to intermittent flow showed no erosion corrosion as opposed to severe damage in constant flow at the same velocity, suggesting that the removal of protective scale layers takes time. Coyne (2009) highlighted erosion corrosion as a significant problem in pipe water systems, particularly in hot water recirculation systems. Roy et al. (2018) achieved a comprehensive review on the rapid failures of copper-based plumbing materials in potable water systems due to erosion or velocity-induced corrosion. They enumerated several factors influencing erosion corrosion in copper pipes, including water chemistry (pH, hardness, alkalinity, and the presence of chlorides, sulfates, and other ions). Murakami et al. (2003) described the erosion corrosion mechanism as a removal protective surface films process, which fully exposes the copper to the corrosive nature of the water. The presence of abrasive particulate matter (Knutsson et al. 1972; Lyman and Cohen 1972; Schleich 2004), and microbiological activity such as biofilms and microbiologically influenced corrosion (MIC). Flow characteristics, such as turbulence, flow velocity, and flow patterns, significantly affect the erosion rate (Edwards et al. 1994a; Myers and Obrecht 1972; Obrecht and Quill 1960; Sakamoto et al. 1995). Particularly, Obrecht and Quill (1960) used water flow rate between 0.45 m/s and 4.0 m/s at temperature ranging from 10 °C to 93.3 °C in recirculating copper loop, where the pH of water is 7.0. Moreover, Mahmood and Suryanto and Al Hazza 2017 carried out copper corrosion tests in operating conditions of water flow rate range between 0.05 m/s and 3.5 m/s, the temperature ranges between 20 °C and 45 °C. Dissolved oxygen concentration was in the range between 6.1 mg/L and 9.2 mg/L. They found that the corrosion rate attains higher rates during the initial stage of the turbulent flow condition. Elevated temperatures increased chemical reaction rates and changes in water chemistry. Hydrodynamic conditions, including the overall flow regime and areas of stagnation and high shear stress, also impact erosion corrosion rate (Efird 1977). Additionally, the metallurgical properties of the copper, including its composition, microstructure, and surface condition, influence its susceptibility to erosion corrosion. However, copper alloyed with elements such as iron, aluminum, and chromium are more resistant to erosion corrosion (Syrett 1976). Based on their investigation, Roy et al. (2018) highlighted an urgent need for high-quality research to understand the specific processes driving copper failures in potable water systems. This includes the necessity to recreate erosion corrosion accurately in laboratory settings and to generate reliable and reproducible data.
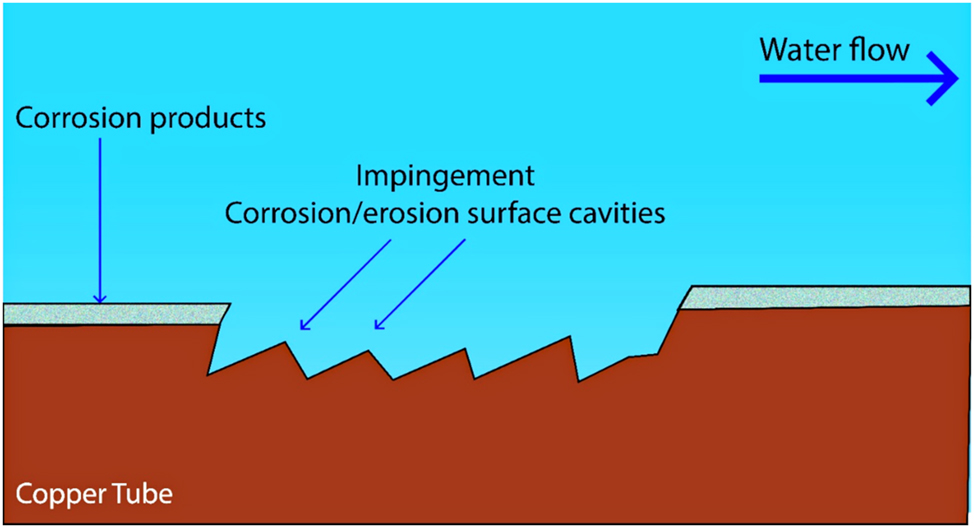
Erosion-corrosion mechanism.
In this context, Wu et al. (2022) investigated the erosion–corrosion behavior of pure copper tubes using a self-built loop apparatus. This study reveals the significant influence of flow velocity and intergranular oxidation on substrate grains, explaining the flow velocity-dependent corrosion rate in practical applications. Two critical velocities are identified: the first, associated with the stripping of outer cupric (II) corrosion products (0.5 m/s–1.5 m/s), and the second, linked to the stripping of the oxidized copper grain layer (4 m/s–4.7 m/s). The intergranular erosion–corrosion process forms detached and partially oxidized copper grains in the corrosion layer, proposing a nut structure to describe their composition evolution.
2.1.3 Stress corrosion cracking
In order to gain insights into the stress corrosion cracking behavior of copper pipes, Chae et al. (2020) conducted a comprehensive investigation involving metallurgical and mechanical analyses. They observed typical characteristics of stress corrosion cracking features, with the fracture surface revealing an intergranular mode on the outer surface (where corrosion pits initiate) and a mixed intergranular and transgranular mode closer to the inner surface (Figure 7). This aligns with the four stages of the stress corrosion cracking mechanism. First, there is a pit formation, where the corrosive environment triggers pit development on the outer pipe surface. Next, it happens a crack initiation under tensile hoop stress, where these pits can grow into small stress corrosion cracking. Then, the stress corrosion crackings propagate inwards, potentially along grain boundaries or through the grains themselves. Finally, the critical crack size is reached, leading to pipe rupture. The study carried out by Chae et al. (2020) reinforces the crucial role of both environmental factors (presence of a corrosive environment) and mechanical stresses (tensile hoop stress) in stress corrosion cracking, with the possible involvement of cupric oxide and cuprous oxide further influencing the process.
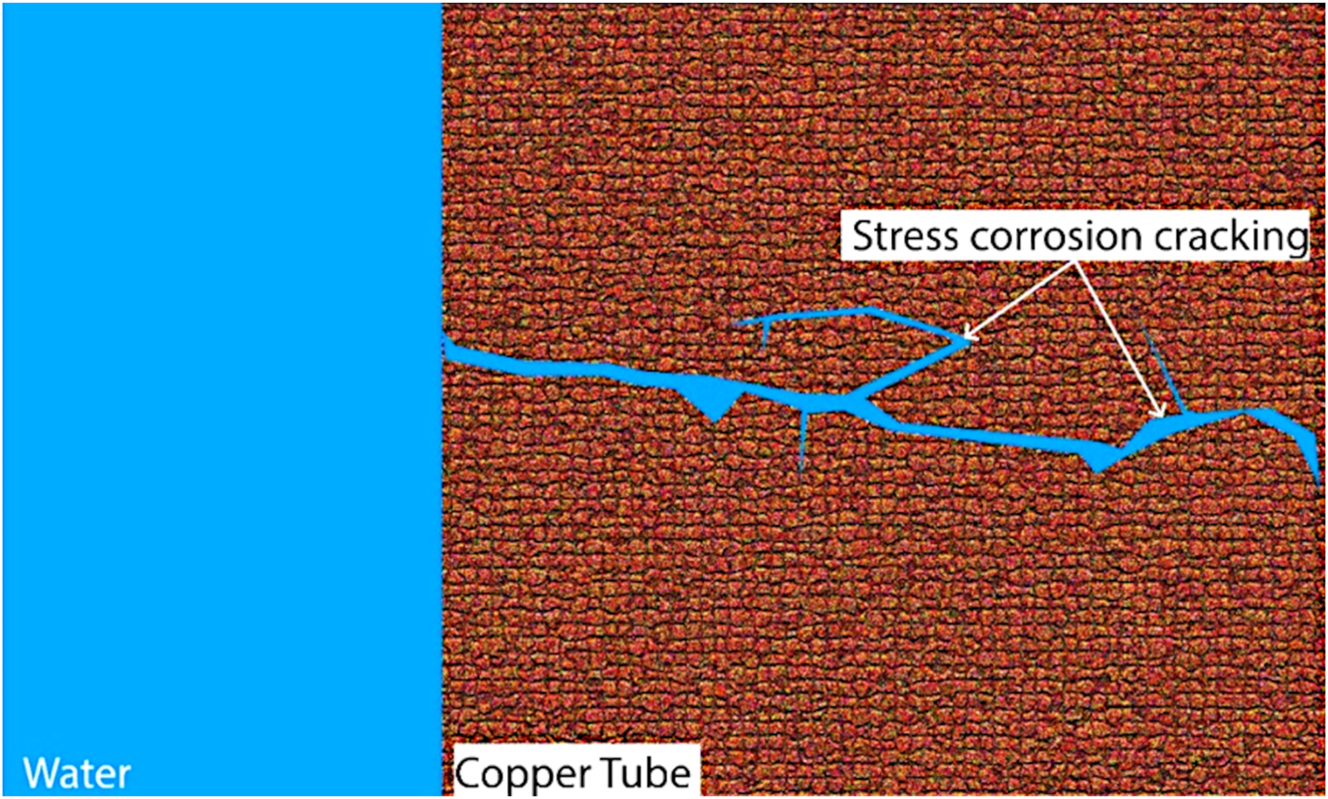
Stress corrosion cracking.
2.1.4 Uniform corrosion
Uniform corrosion is another type of corrosion that affects a metal surface evenly across its entire area, resulting in a relatively constant corrosion rate. This process initiates with the dissolution of copper ions due to their interaction with water (Pedeferri 2018). Dissolved oxygen acts as a catalyst, while chloride ions destabilize the protective oxide layer on the copper surface. As corrosion propagates, copper ions and chloride ions diffuse, and the flow rate and temperature can influence the corrosion rate. It is important to note that uniform corrosion is particularly prevalent in acidic environments (Pedeferri 2018). Unlike localized forms of corrosion, such as pitting corrosion and erosion corrosion, uniform corrosion spreads across the entire surface, potentially leading to a gradual reduction in the structural integrity of the metal over time.
In 2019, a study reported on uniform corrosion within copper piping of a closed-loop water heater system. This corrosion was attributed to microbiological attack by sulfur-reducing bacteria, which remove the protective oxide layer on the copper surface (Khan 2019b). Uniform corrosion is less prevalent in copper water circuits of heat exchangers, as the water flow and temperature are enough aggressive to generate the susceptible conditions for the pitting and erosion corrosion mechanisms (Roy et al. 2018).
2.2 Factors influencing copper corrosion
The main factors that impact the copper corrosion are the effect of microstructure and surface treatments, manufacturing conditions, temperature effect, water chemistry parameters, chemical composition, water flow, and microbial adhesion. These factors are reviewed and discussed.
2.2.1 Microstructure effects and surface treatments
While traditional corrosion studies have primarily focused on macroscopic characteristics, recent researches suggest that the behavior of corrosion is heavily influenced by local surface reactivity, which, in turn, can be strongly affected by the crystallographic orientation of the surface. Martinez-Lombardia et al. (2014) found that the corrosion properties of copper are not solely determined by the grain orientation of individual grains but also by the orientation of adjacent grains. To investigate this further, they examined the local electrochemical reactivity of high purity copper, considering the grain orientation within the microstructure. Their findings revealed that grain orientation does influence the electrochemical reactivity of the passive layer formed on copper surface. Moreover, grain size has been identified as a factor influencing the mechanical properties as well as corrosion behavior. The impact of grain size on the corrosion resistance of copper and its alloys has been the subject of numerous studies. The relationship between grain size and corrosion resistance is a topic of debate. While some researchers suggest that reducing grain size enhances corrosion resistance, others have observed the opposite effect. For instance, Ma et al. (2019) conducted experiments using copper samples with different grain sizes and assessed their pitting corrosion resistance in various solutions. They discovered that the effect of grain size on copper’s corrosion behavior varied depending on the solution. In NaOH solution, smaller grain sizes led to increased corrosion resistance as copper formed a protective passive film without pitting. Conversely, in a Na2SO4 solution, copper with smaller grain sizes exhibited higher susceptibility to pitting corrosion. Nonetheless, in a Na2SO4 + NaOH solution, smaller grain sizes demonstrated improved resistance to pitting corrosion due to the breakdown of the passive film. Additionally, Lapeire et al. (2017) conducted a study examining the impact of grain size on the electrochemical characteristics of pure copper in a 0.1 M HCl solution. The findings indicated that a smaller grain size was associated with a decreased corrosion potential and an increased corrosion current density. Moreover, it was observed that small-grained copper corroded more uniformly compared to samples with larger grain sizes, where a clear preferential attack of the grain boundaries was visualized. Furthermore, Jesus (2008) revealed that the corrosion mechanism varied depending on the concentration of the chloride solution. Localized attack was observed in solutions with concentrations between 0.06 mol L−1 and 0.12 mol L−1, while generalized attack predominated in the 0.6 mol L−1 solution. While the influence of grain size on copper corrosion has been explored, a more comprehensive understanding necessitates investigating a broader spectrum of corrosive environments. Employing advanced microscopy techniques could offer valuable insights into the interactions between corrosive species, passive films, and grain boundaries.
Furthermore, surface finishing treatments were also investigated, demonstrating that treatments resulting in a reduction in carbon content were beneficial for corrosion resistance. However, it is important to consider other factors as well. For example, while sandblasting reduced carbon content, it also increased surface roughness, which decreased corrosion resistance. Surface treatments such as degreasing and annealing, which resulted in lower carbon levels, showed improved corrosion resistance.
2.2.2 Manufacturing conditions
2.2.2.1 Soldering flux
Several studies have linked the presence of soldering flux to pitting corrosion in copper pipes. The literature indicates that the flux used in copper soldering can contain chloride (Cl−) elements such as in ammonium chloride (NH4Cl), zinc chloride (ZnCl2), tin chloride (SnCl2) or hydrochloric acid (HCl). These chloride elements have been found to promote pitting corrosion when the flux is introduced in excessive amounts into the pipes. Pits tend to develop primarily along narrow regions that run parallel to the longitudinal axes of the pipes and fittings. Myers and Cohen (2005) reported that to prevent soldering-flux-induced pitting, it is recommended that plumbing technicians adhere to industry-standard materials and practices during the installation of copper-tube systems. Research indicates that the aluminum content in domestic hot water can be kept within acceptable limits by refraining from using sacrificial aluminum-alloy anodes in hot-water heaters. Reiber (2013) reported that the copper tubing pitting was likely the result of poor fabrication and soldering practice during the original construction of the homes. The report recommends that the whole-household cold water tubing replacement is not warranted currently unless a clear pattern of poor soldering practice affecting the entire home is evident. It is crucial to acknowledge that the pitting and flux runs, which eventually resulted in perforation and leakage, occurred early in the life of the plumbing system.
2.2.2.2 Lubricant impact
In literature, several authors have mentioned carbon as a significant factor in triggering pitting corrosion. The presence of carbon films has also been related to pitting corrosion in copper. Campbell was one of the early researchers to discuss this topic (Campbell 1950). Campbell’s research demonstrated the correlation between pitting corrosion in copper pipes and the existence of carbon films. He suggested that these films, functioning as effective cathodes, while the remaining surface acted as an anode, facilitating pit formation. Other researchers highlighted that localized corrosion in copper pipes primarily arises from the breakdown of lubricants employed during the pipe drawing process. Later, Vincente (1996) noted that the residues of the lubricant persist within the pipes and undergo decomposition, leading to the formation of carbon during annealing. Yan et al. (2018) explored the corrosion mechanism of copper in an oil-in-water emulsion. It revealed that copper exhibits an incubation period before its corrosion rate increases linearly. The corrosion products identified include Cu2O, CuO, Cu(OH)2, CuCO3, and Cu2(OH)2CuCO3, leading to color changes in the emulsion. Emulsion droplets adsorb on copper surfaces, causing corrosion pitting and “emulsion spot” defects. Copper reacts with oxygen to form Cu+ and Cu2+ cations, which adsorb hydroxide and carboxylate anions, consuming surfactants and resulting in the emulsion separation. For this reason, it is suggested to add inhibitors to protect copper surfaces. In a more recent investigation, Iyasu et al. 2020, observed that only the copper pipe containing a residual carbon content of 6.1 mg/m2 exhibited a notable rise in corrosion potential and subsequent pitting corrosion after two weeks, under the existing chemical conditions of water treatment. The study highlighted that the extent of pitting corrosion was strongly influenced by the residual carbon content and the specific chemical water treatment employed. Currently, it is believed that the inhibitory effect on pitting corrosion by this chemical water treatment is attributed to the formation of a composite film, which comprises phosphonic acid, an azole-based anticorrosive agent presents in the chemical water treatment, and dissolved components in the water.
2.2.3 Temperature effect
Temperature exerts a significant influence on various aspects of copper tube corrosion, including corrosion rates, solubility of corrosive species on pipe walls, and susceptibility to erosion corrosion. Recent research has also highlighted the role of temperature variations in inducing stress corrosion within pipes. This phenomenon arises from the disparate thermal expansion coefficients between the protective scale on copper pipes and the underlying metal, potentially leading to scale detachment and subsequent exposure of copper to the water supply. Consequently, these temperature changes could account for the observed increase in copper release during winter months and the correlation between temperature fluctuations and copper pinhole failures reported by plumbers. To gain a comprehensive understanding of copper corrosion in domestic plumbing systems, it is crucial to grasp the influence of temperature gradients and pipe orientation. The orientation and differential heating of pipes can have a direct impact on the release of copper into water by influencing solubility changes, mixing dynamics, and stability. Furthermore, the presence of a temperature gradient along a pipe can give rise to thermogalvanic currents, which can accelerate the corrosion processes. Although the influence of these currents on copper solubility and mixing may not be as pronounced, they might contribute to increased copper release or diminished tube lifetimes (Rushing and Edwards 2004). Additionally, Touzé and Cougnon (2018) investigated the electrochemical behavior of copper surfaces that were pre-oxidized by heating in air at 90 °C, aiming to assess the influence of the resulting oxide layer on the corrosion in a chloride-rich environment. They found that shorter heating times led to the formation of Cu2O-rich films, while longer heating times resulted in CuO-rich films. They suggested that the composition and structure of the oxide layer play a crucial role in determining the corrosion behavior. Furthermore, Faes et al. (2019) reviewed the existing knowledge regarding the impact of temperature on corrosion in heat exchangers. They discussed how temperature influences the corrosion rates and mechanisms of various metallic alloys used in the construction of heat exchangers. Indeed, corrosion kinetics in heat exchangers are influenced by temperature and fluid flow characteristics. Elevated temperatures near the heat exchanger wall enhance corrosion rates due to increased chemical reaction rates and mass transport of corrosive species. Conversely, lower temperatures decelerate corrosion kinetics. Furthermore, temperature variations impact the solubility of corrosive species. This affects the formation and stability of protective corrosion product layers. At lower temperatures, higher oxygen solubility promotes the formation of stable oxide layers that shield the metal surface. However, at elevated temperatures, these oxide layers may become unstable and dissolve, exposing the metal to accelerated corrosion. It was observed that corrosion damage was worse at 80 °C compared to 60 °C, but the worst corrosion rate was found at 60 °C in conditions between 30 °C and 70 °C, indicating that hotter temperatures alone do not always lead to severe corrosion (Kristiansen 1977). In a separate investigation of 365 public water systems regulated under the U.S. EPA’s Lead and Copper Rule (LCR), no significant trend was found between temperature and copper concentration in water, indicating a consistent copper dissolution rate (Dodrill and Edwards 1995).
2.2.4 Water chemistry parameters
Water chemistry parameters play a significant role in the occurrence of water corrosion (Custalow 2009). Soft water, in drinking water distribution systems, is characterized by high pH levels and low alkalinity (i.e., low bicarbonate concentrations). Sulfate and chloride ions are commonly associated with all forms of pitting, although their relative importance in copper-pitting corrosion remains debatable. Previous studies have investigated the role of chloride in copper pitting corrosion and traditionally attributed it as the main cause (Akkaya and Ambrose 1985; Drogowska et al. 1987; Nishikata et al. 1990; Shalaby et al. 1989). However, more recent research suggests that sulfate and nitrate ions may play crucial roles in pit initiation and development, possibly even more significant than chloride (Duthil et al. 1996; Edwards et al. 1994b). The presence of either chloride or sulfate alone in water can lead to copper pitting, but when both anions are present, the effect of chloride depends on the relative concentrations of sulfate and chloride (Duthil et al. 1996).
Accelerated corrosion rate experiments have shown that chloride initially increases copper corrosion rates, but over time it can produce protective surfaces at pH 7.6. On the other hand, sulfate is not initially aggressive, but corrosion rates increase over time as scale forms, surpassing the rates of chloride-induced corrosion. The formation of brochantite, Cu4(OH)6(SO4), over soft water pits has been observed, and it has been postulated that its presence may be the key to hot water pitting. Thermodynamic calculations indicate that brochantite formation is favored in waters with high sulfate-to-chloride or sulfate-to-bicarbonate ratios (Edwards et al. 1994a). Further investigations have revealed that sulfate typically activates copper pitting corrosion, chloride has a passivating effect, and bicarbonate acts as a buffering agent. However, the combination of all three anions in a specific concentration range can lead to pitting corrosion (Schmitt et al. 2001). The presence of aluminum in drinking water, along with high chlorine residual and relatively high pH levels, has also been shown to cause pinhole leaks. Nonetheless, aluminum alone did not induce pitting, emphasizing its role in conjunction with other factors (Marshall and Edwards 2005).
Lytle and Schock (2008) reported that pitting corrosion of copper plumbing is more likely to occur in water with low chlorine concentration and dissolved inorganic carbon (DIC) levels of 5 and 10 mg C/L, possibly extending to 25 mg C/L. High-pH water with a pH of 9, in the presence of chloride (14–38 mg/L), also promotes pitting corrosion. Pitting corrosion was not observed at pH levels of 6.5 and 7, and at pH 8, it only occurred when higher chlorine levels were present. While sulfate was not essential for the development of pitting corrosion, it did impact the composition of the corrosion by-products associated with pitting corrosion. The presence of chloride ions was crucial for pit propagation, as it was the only ion in contact with the copper surface at the point of degradation (anode). Increasing the DIC to 50 mg C/L or introducing orthophosphate at a concentration of 3 mg/L, PO4 prevented the initiation of pitting corrosion at pH 9.
Moreover, Lytle and Nadagouda (2010) reported that the investigation of a community-wide copper pitting corrosion case yielded several key conclusions. Pinhole leaks in copper pipes were predominantly associated with chlorinated cold water having high pH (8.8), low alkalinity or total inorganic carbon (8.8 mg C/L), and significant chloride (64 mg/L) and sulfate levels (120 mg/L). Copper pits exhibited a structure comprising a pit cap, porous membrane, and the pit itself, with external corrosion observed near the pinholes. Analysis confirmed the presence of sulfur in the pit cap and localized copper corrosion at the pit floor. X-ray diffraction (XRD) analysis revealed basic copper sulfate minerals in the pit cap and cuprite crystals in the pit. Tenorite mineral formation was observed exclusively in hot water pipes. Notably, neighboring communities with similar water sources and treatment processes did not report copper pitting, possibly due to the addition of blended phosphate compounds to their water.
Additionally, during a laboratory test, Sakai (2022) successfully replicated the formation of moundless pits. This was achieved by subjecting artificial freshwater, containing specific concentrations of 40 ppm silica, 50 ppm sulfate ions, 10 ppm chloride ions, and 10 ppm bicarbonate ions, to a one-year test period. It was found that the concentration of chemical species in water affects growth rates and the formation of corrosion products.
Ranjbar (2010) investigated the causes of failure in a tubular heat exchanger constructed from copper-based alloys. The circulating water within the heat exchanger contained a high concentration of total dissolved solids (TDS) ranging from 2065 mg/L to 5,053 mg/L, primarily composed of calcium (Ca2⁺), magnesium (Mg2⁺), sodium (Na⁺), bicarbonates (HCO₃⁻), sulfates (SO₄2⁻), and chlorides (Cl⁻) ions. This high TDS, particularly the abundance of calcium compounds, led to extensive fouling deposition inside the tubes.
Ha et al. (2011) found that sulfate-containing waters have higher pit propagation rates and deeper pits, while chloride-containing waters have lower propagation rates and shallower pits. The growth rate of pits is influenced by the morphology and chemical nature of corrosion products, which impact the effective resistance and control pit growth. Indeed, copper chloride corrosion products with high resistance decrease pit growth, allowing for repassivation, while copper sulfate and copper carbonate corrosion products with lower resistance led to slower pit growth. Li et al. (2018) and Schindelholz et al. (2018) reported that copper is oxidized to CuCl2 − and CuCl3 2− in 4 M NaCl solution which further reacts with oxygen in solution to form clinoatacamite (Cu2(OH)3Cl). Furthermore, Zhang et al. (2013) reported that, on one hand, dissolved oxygen in water plays a crucial role in copper corrosion. It leads to the formation of a double-layered oxide membrane on the copper surface, consisting of Cu2O and Cu2O/CuO mixture. Corrosive products migrate through the membrane, triggering secondary reactions. On the other hand, increased dissolved carbon dioxide exacerbates copper corrosion by making the solution acidic and damaging the passivating membrane. This leads to the formation of cupric carbonate, which can be easily eroded, exposing the copper to further corrosion. Higher concentrations of dissolved carbon dioxide result in an increased corrosion rate. Hedin et al. (2018) showed no significant hydrogen evolution above the experimental background when copper was immersed in in pure, oxygen-free water, regardless of copper qualities and surface treatments. The absence of corrosion products in long-term studies further supports these findings. The theoretical background indicates that no stable Cu–O–H compound has been discovered to act as the driving force for sustained corrosion in oxygen-free water, contrary to previous claims (King 2010). Natural organic matter (NOM) also plays a crucial role in copper behavior within water systems. Its complexing action and interactions with copper ions can significantly impact copper corrosion. NOM acts as a chelating agent, forming stable complexes with copper, which affects the solubility and speciation of copper in water (Liu et al. 2007). These complexes can either enhance or hinder corrosion processes, depending on various factors such as NOM composition, concentration, and water chemistry (Broo et al. 1998). The presence of NOM has been found to prevent the formation of certain copper corrosion products and inhibit the growth of crystals on metal surfaces (Korshin et al. 2005), influencing the overall corrosion rate and release of copper into the water and potentially reducing pitting corrosion (Sarver and Edwards 2012).
Referring to the aforementioned, copper pitting corrosion is a complex phenomenon influenced by a multitude of factors beyond just chloride ions. Sulfate, bicarbonate, pH, DIC, dissolved oxygen, and even the presence of aluminum can all play crucial roles. Future research should prioritize a more holistic understanding of copper pitting corrosion. This includes investigating a wider range of anion combinations and their synergy with other water chemistry parameters, such as pH and DIC. Additionally, elucidating the mechanisms by which aluminum and dissolved gasses (oxygen and carbon dioxide) influence corrosion, along with defining their threshold concentrations for impact, would be valuable. Furthermore, a thorough understanding of how different corrosion products form and how their specific characteristics affect pit growth rates is crucial. By exploring these areas, we can bridge the knowledge gaps and develop comprehensive strategies to mitigate copper pitting corrosion and ensure the longevity of copper used in plumbing systems and heat exchangers.
On the other hand, the electrical conductivity of water has also a significant influence on the corrosion behavior of copper. Higher conductivity can accelerate electrochemical reactions, potentially leading to increased corrosion rates. Pehkonen et al. (2002) conducted a study to examine the effect of conductivity on dissolved copper levels at three different pH values. The results revealed a rapid-to-moderate increase in dissolved copper levels as conductivity increased, reaching slightly over 400 µS. At pH 8.5, the highest observed increase in dissolved copper levels was 87 % when conductivity rose from approximately 150 µS to 450 µS. However, beyond 500 µS, the dissolved copper levels became nearly independent of conductivity. The increased conductivity affected the mobility of free Cu+ and Cu2+ ions, reducing their tendency to accumulate on the surface of the coupon used in the study. This shift in the equilibrium of Cu ↔ Cu2+ + 2e− favored higher Cu2+ levels in the solution.
2.2.5 Impact of water flow
Pitting corrosion in home plumbing systems is influenced by various flow parameters, including pipe design characteristics and water velocity. Marshall (2004) conducted studies demonstrating that pitting corrosion becomes more severe with longer durations of flows when water with specific composition flows through new copper pipes under different flow cycles. Among these conditions, continuous flow was identified as the most aggressive for pitting corrosion. Building upon this research, Lattyak (2007) also found that water velocity exerts a significant influence on pitting corrosion in the presence of specific water composition under continuous flow conditions. Interestingly, their findings indicated that a temporary change in velocity had no long-term impact on electrification effects. This suggests that electrification involves both short-term effects resulting from fluid movement and long-term effects associated with the formation of scale on the pipes over time. Custalow (2009) observed that corrosion currents and the severity of pitting corrosion increase with water velocity, particularly at hot temperatures. While pipe diameter was found to have some influence on corrosion, its impact was not as significant as fluid velocity. Similarly, Coyne (2009) reported that water velocity, particularly heated saltwater, caused substantial pitting and damage to copper surfaces. Contributing factors such as cavitation, high-velocity impingement, and concentration cell corrosion were identified. Particularly, cavitation was found to remove protective scales on copper surfaces, leading to corrosion and electron flow that could result in failure. However, the effects were not observed with synthetic tap water. Roy et al. (2018) reported that high-velocity water flow can detach copper scale, disrupt protective films, and accelerate corrosion in copper pipes. Turbulent flow conditions and gas bubbles further contribute to pipe damage. Nevertheless, establishing reliable threshold velocities for copper pipes in plumbing standards remains challenging. More recently, Rahman and Ahmed (2020) examined the corrosion behavior of copper tubes in a heat exchanger. They investigated the corrosion rates of copper tubes in seawater and river water at different flow velocities (1.0 m/s, 1.5 m/s, and 2.0 m/s), while maintaining a constant tube side entry temperature of 20 °C. The findings demonstrated a substantial increase in corrosion rate with higher flow velocities, consequently reducing the lifespan of the copper tubes.
2.2.6 Microbial effects
To effectively manage distribution systems and control copper corrosion, it is crucial to understand the micro-organisms involved in copper plumbing corrosion. Bremer et al. (2001) highlighted the potential influence of microbial activity on unusual pitting corrosion and excessive copper by-product release. Factors such as plumbing design, water quality, and temperature contribute to this phenomenon. Additionally, sediment accumulation, poor soldering, and intermittent system usage increase the risk of corrosion, particularly in small-bore pipes and systems with moderate shear stresses. Further investigation conducted by Pavissich et al. (2010) focused on biofilm formation and copper resistance in heterotrophic bacteria isolated from copper pipes affected by ‘blue water’ corrosion. Their findings revealed higher copper concentrations in stagnant water from nonsterile treatments, suggesting the influence of microbially influenced corrosion in copper plumbing. Notably, gamma- and beta-proteobacteria were identified as dominant species in the bacterial community within corroded copper piping, highlighting their potential role in biofilm formation and promoting bacterial corrosion. In a study conducted by Burleigh et al. (2014) on copper tube pitting caused by microbial induced corrosion in Santa municipal water, the presence of actinobacteria within the pits was observed, matching the description of Type I pitting corrosion. The authors proposed that residual organic films, such as extrusion lubricants or degreasing compounds, facilitated the attachment of actinobacteria to the copper surface. Vargas et al. (2017) discussed the health impacts of copper ingestion, mechanisms of microbial involvement in copper release, and the effects of biofilms and hydrodynamics. They emphasized the challenges of repairing copper pinholes in plumbing and the importance of considering both acute and chronic effects of copper ingestion. The authors also highlighted various metal mobilization and immobilization processes facilitated by microbial activity. Additionally, temperature and water stagnation were identified as factors affecting the quality of domestic drinking water systems in the study conducted by Zlatanović et al. (2017). Stagnation caused leaching of copper and zinc from pipes and fixtures, while overnight stagnation in copper pipes influenced microbial parameters. The composition of biofilms varied, influenced by microclimates and consumption patterns. Shower pipes exhibited higher cell quantities, with Alphaproteobacteria dominant in shower biofilms and a mix of Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria in kitchen tap biofilms.
Seth and Edyvean (2006) investigated the role of sulfate-reducing bacteria (SRB) in water mains and found that SRB significantly increase corrosion rates of steel and cast iron. Jacobs and Edwards (2000) demonstrated that sulfate compounds transformed into sulfides by SRB, leading to a substantial increase in corrosion rates of copper even at pH 9.2. They showed that sulfide-containing scale accelerated corrosion rates by affecting both anodic and cathodic reactions. Moreover, Scardina et al. (2008) discovered SRB growth within copper pits, indicating their involvement in pit growth and pinhole leak failures. They also observed localized production of H2S by SRBs in and around the pits. Additionally, the corrosion of copper piping in a closed-loop water heater system was attributed to the synergistic effects of microbiologically influenced corrosion and erosion of the vulnerable oxide layer (Khan 2019b). The investigation provided evidence supporting microbiological attack, specifically by SRB. Lavanya (2021) reported that the presence of dissolved oxygen hindered the growth of SRB, resulting in sluggish growth and rapid decay. The growth process of SRB affected the conductivity, pH, and sulfide anion concentration under both aerobic and anaerobic conditions. Moreover, Amendola and Acharjee (2022) reported that microbiologically influenced corrosion, often attributed to SRB, can affect various metallic substrates and has economic implications. Despite the antimicrobial properties of copper and its alloys, they are susceptible to microbial corrosion in potable water systems and marine environments. Conversely, certain bacteria, such as Stenotrophomonas maltophilia, exhibited protective effects on copper, which the authors attributed to the binding of chloride ions to exopolymeric substances (Critchley et al. 2003).
Actually, there is a potential link between biological activity and pitting corrosion in water infrastructure. However, a more nuanced understanding is necessary before implementing widespread biocontrol strategies. Future research should focus on identifying the specific biological culprits, evaluating the cost-effectiveness of control measures, and addressing critical research gaps related to chemical transients, as well as the impact of disinfectant choice.
3 Effective strategies for copper corrosion mitigation
3.1 Strategies based on inhibitors and passivation techniques
Copper corrosion prevention and mitigation strategies play a critical role in maintaining the integrity and longevity of copper water infrastructure. With its widespread use in various industries, protecting copper surfaces from corrosion is essential to ensure optimal performance and sustainability. There are a number of methods and strategies that can control or reduce the corrosion damage and protect the metals and increase their durability. The most employed methods are cathodic protection, addition of corrosion inhibitors and application of protective coatings.
3.1.1 Cathodic protection
Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell (Gurrappa 2005). The principle behind CP is relatively simple: it involves connecting the metal to be protected (in this case, copper) to a more easily corroded ‘sacrificial’ metal to act as the anode (Tamhane et al. 2021). The sacrificial metal then corrodes instead of the copper, thus protecting the copper component. Recent years have seen significant developments in CP techniques (Sun et al. 2021), particularly in the design and application of sacrificial anodes. Traditional sacrificial anodes are typically made of zinc, magnesium, or aluminum, which have a more negative electrochemical potential than copper (Yang et al. 2021a,b). When these anodes are connected to the copper component and exposed to the same environment, they preferentially corrode, thereby protecting the copper. One of the recent advancements in this field is the development of alloy-based sacrificial anodes (Yang et al. 2021a,b). These anodes, made from alloys of zinc, aluminum, or magnesium, offer several advantages over traditional sacrificial anodes. For instance, they can provide a more uniform current distribution, leading to more effective corrosion protection. Additionally, they tend to have a longer lifespan, reducing the need for frequent replacement. Another significant development in CP is the integration of this technique with other corrosion protection strategies. For example, combining cathodic protection with protective coatings can offer enhanced corrosion resistance.
Lattyak (2007) conducted a study and revealed that the initial copper pipe in the system, prior to inhibitor addition, exhibited an anodic behavior in comparison to later sections of the pipe. The incorporation of an orthophosphate-polyphosphate blend resulted in a reduction of pitting currents, although not to the same extent as observed with zinc polyphosphate. Building upon previous research, Ngaotrakanwiwat et al. (2020) conducted a study that investigated the effect of the composition of a nanocomposite film containing Cu-doped TiO2 particles (Cu/TiO2) and TiO2 particles on the photoelectrochemical cathodic anti-corrosion properties of copper terminal lugs in a NaCl solution. The study demonstrated that the film exhibited enhanced corrosion resistance, characterized by higher corrosion potential (Ecorr) and lower corrosion current (Icorr), when compared to the bare lug. Remarkably, the film exhibited even better anti-corrosion performance when exposed to UV irradiation.
Remarkably, these advancements highlight the continuous development of CP techniques for copper pipe protection. Future research should focus on optimizing alloy design for sacrificial anodes, maximizing lifespan, current distribution, and cost-effectiveness. Additionally, exploring synergistic combinations of CP with advanced, long-lasting protective coatings specifically designed for copper pipes holds promise. Furthermore, investigating the long-term performance and potential limitations of emerging CP strategies like nanocomposite films under real-world plumbing conditions is crucial for ensuring their practical application in safeguarding water infrastructure.
3.1.2 Inhibitor additives for copper corrosion mitigation
The addition of organic and inorganic corrosion inhibitors is a widely studied method to prevent copper corrosion. These inhibitors have shown promising results in protecting copper surfaces and reducing degradation.
3.1.2.1 Organic inhibitors
A growing body of research investigates the potential of various organic derivatives, plant extracts, and even pharmaceutical compounds as corrosion inhibitor additives for copper in acidic environments. These findings hold promise for the development of more sustainable and effective corrosion prevention strategies applicable in diverse industrial settings. While heterocyclic azoles demonstrate promise, it’s important to recognize the vast landscape of organic corrosion inhibitors. These diverse compounds find application in mitigating corrosion of copper across a wide range of acidic environments (Chaubey et al. 2021; Fouda et al. 2013; Hawsawi 2022; Patel and Vashi 2016; Savita et al. 2016; Vashi et al. 2021; Vaštag et al. 2013) (see Table 1). However, a comprehensive exploration of all such environments and inhibitors falls outside the scope of this current review.
Inhibition efficiency of organic and inorganic corrosion inhibitors for copper in water.
| Inhibitor | Concentration | Medium | Efficiency (%) | Reference |
|---|---|---|---|---|
| Benzotriazole | 0.14 × 10−3M | Ground water | 57.83 (EIS) | Gopi et al. (2009) |
| 1-(2-Thienyl carbonyl)- benzotriazole (TCBT) | 0.12 × 10−3M | 70.81 (EIS) | ||
| 1-(2-Pyrrole carbonyl)- benzotriazole (PCBT) | 0.095 × 10−3M | 72.84 (EIS) | ||
| Triton X-100 | 0.16 × 10−3M | 61.64 (EIS) | ||
| Benzotriazole + Triton X-100 | (0.14 + 0.16) × 10−3M | 81.62 (EIS) | ||
| Triton X-100 + TCBT | (0.12 + 0.16) × 10−3M | 89.04 (EIS) | ||
| Triton X-100 + PCBT | (0.095 + 0.16) × 10−3M | 92.07(EIS) | ||
| Fernox® provided by Mitsubishi electric, U. | 25 % (mass percent) | Tap water | The efficiency follows the order using PDP method: Fernox® > BTA > (BTA + TEA) > TEA | Bi et al. (2016) |
| Benzotriazole + triethanolamine | 4.7 mM + 30 mM | |||
| Benzotriazole (BTA) | 4.7 mM | |||
| Triethanolamine (TEA) | 30 mM | |||
| 1,2,5-Thiadiazole | 0.5 × 10−3 M | Oil-in-water emulsion | 94.17 (EIS) | Xiong et al. (2020) |
| NaNO2 | 2,000 ppm | Simulated cooling water | 61.8 (EIS) | Rizvi et al. (2021) |
| 5-(4′-Dimetylamino-benzylidene)-2,4-dioxotetrahydro-1,3-thiazole (DABDT) | 100 × 10−5 mol L−1 | Acidic sulphate-containing solution | 90 (PDP) | Vaštag et al. (2013) |
| (1E,4Z,6E)-5-Hydroxy-1,7-bis(3-hydroxy-4-methoxyphenyl)hepta-1,4,6-trien-3-one | 15 × 10−6 mol L−1 | 1 M HNO3 solution | 69 (EIS) | Fouda et al. (2013) |
| (E)-5-(4-hydroxystyryl)benzene-1,3-diol | 15 × 10−6 mol L−1 | 66.6 (EIS) | ||
| Leaf extracts | 0.1 g L−1 | 3 M HNO3 | 96 (EIS) | Savita et al. (2016) |
| Citrus aurantium extract | 0.3 g L−1 | Nitric acid | 62 (WL) | Patel and Vashi (2016) |
| Moringa oleifera extract | 0.3 g L−1 | 71 (WL) | ||
| Capsicum annuum extract | 0.3 g L−1 | 85 (WL) | ||
| 2-Amino-5-ethyl-1,3,4-thiadiazole | 10−2 mol L−1 | Acidic medium | 84.44 (PDP) | Božinović (2017) |
| Solupred | 300 ppm | 1.0 M sulfamic acid solution | 95.6 (PDP) | Hawsawi (2022) |
| 90.1 (EIS) | ||||
| 1-Octanethiol, 1-dodecanethiol | 0.5 mmol L−1 | Simulated acid rain | 90.64 (PDP) | Martinović et al. (2019) |
| 1-Dodecanethiol | 0.10 mmol L−1 | 90.28 (PDP) | ||
| 1-Octadecanethiol | 0.05 mmol L−1 | 73.67 (PDP) | ||
| Iodate ions from potassium iodate | 20 ppm | 0.5 M sodium chloride medium | 72.72 (EIS) | Guibadj and Carlo (2017) |
| Phosphate | 1 mg L−1 | Low-alkalinity waters | Not mentioned | Gibson and Karney (2021) |
Gopi et al. (2009) conducted a study on the effectiveness of new benzotriazole derivatives and their synergistic effect as organic inhibitors on copper corrosion in groundwater, utilizing electrochemical methods. The results indicated a significant synergy effect between triton X-100 and 1-(2-Thienyl carbonyl)-benzotriazole at a concentration of (0.095 + 0.16) × 10−3 M, with an impressive efficiency value of 92.07 %, as measured using the electrochemical impedance spectroscopy method (EIS). Bi et al. (2016) investigated the anti-corrosion behavior of commercial corrosion inhibitors for copper plumbing (Fernox) and analyzed their chemical composition, including benzotriazole (BTA) and triethanolamine (TEA), to understand the mechanism of copper corrosion inhibition in tap water. The efficiency of corrosion inhibition, as determined using the PDP method, followed the order: Fernox® > BTA > (BTA + TEA) > TEA.
Xiong et al. (2020) investigated the inhibition mechanism of copper immersed in an oil-in-water (O/W) emulsion. They found that among the tested compounds, furan < pyrrole < thiophene < 1,2,5-oxadiazole < 2H-1,2,3-triazole < 1,2,5-thiadiazole exhibited excellent inhibitory properties, with 1,2,5-thiadiazole at a concentration of 0.5 mM demonstrating the most effective inhibition on copper immersed in the emulsion (94.17 %). The inhibitors were found to primarily undergo chemical adsorption on the copper matrix, following the Langmuir adsorption isotherm. Both quantum chemical and molecular dynamics simulations revealed that these compounds adhered to the copper matrix in a flat-adsorption mode, effectively protecting against emulsion-induced copper corrosion. The adsorbed inhibitors acted as a barrier on the copper matrix, impeding corrosion and enhancing hydrophobicity.
3.1.2.2 Inorganic inhibitors
Several studies have explored effective strategies for mitigating copper corrosion in various water conditions. These investigations have yielded valuable insights into the use of different inorganic inhibitors and their impact on corrosion prevention.
Lattyak (2007) conducted a study that highlighted the effectiveness of zinc and phosphate in mitigating non-uniform corrosion of copper in water conditions characterized by high pH, high chlorine levels, and aluminum solids. The combination of zinc and phosphate acted as a mixed inhibitor, resulting in notable reductions in potentials, currents, and chlorine decay. Building on this research, Sarver and Edwards (2012) investigated the causes of copper pitting corrosion, focusing on aggressive potable waters with high pH, free chlorine residual, and low alkalinity. Their study examined the effectiveness of inhibitors, including phosphate, silica, and natural organic matter, in preventing pitting under continuous flow conditions. The results demonstrated that a phosphate concentration of 1 mg/L effectively prevented pit initiation, while 5 mg/L of silica significantly slowed down the occurrence of pitting. However, lower inhibitor showed limited benefits and, in some cases, even accelerated corrosion rates. Additionally, the study explored the dose-response effects of free chlorine and alkalinity, revealing that higher free chlorine levels accelerated pit initiation, with even moderate levels (around 0.4 mg/L) leading to severe pitting. While high alkalinity reduced pit propagation rates, it did not prevent pit formation. Guibadj and Carlo (2017) investigated the inhibitive effect of iodate ions from potassium iodate as an inorganic inhibitor for copper corrosion in a sodium chloride medium. Their research demonstrated that iodate ions acted as a mixed-type inhibitor, exhibiting significant inhibition efficiency. Moreover, the inhibition efficiency of IO3 − remained stable over time, with a substantial inhibition efficiency of 72.72 % observed after 16 days of immersion as mentioned in Table 1. These studies collectively contribute to our understanding of corrosion mitigation strategies and the inhibitive effects of various substances on copper corrosion. Recently, Gibson and Karney (2021) used phosphate corrosion inhibitors that may be beneficial to prevent pitting corrosion, particularly in low-alkalinity waters (see Table 1). However, inadequate surface coverage can stimulate corrosion, and phosphates have the potential to increase biological growth in some cases.
Additionally, the synergistic effect of temperature and disinfectant concentration can impact the corrosion patterns. Montes et al. (2014) reported that the investigation into disinfection treatments’ impact on copper pipe corrosion revealed that temperature and disinfectant concentration play a significant role in the corrosion processes. They observed uniform corrosion at 70 °C with 25 ppm of sodium hypochlorite and localized corrosion at 50 °C with the same disinfectant concentration. Pitting corrosion occurred after an extended incubation period, likely linked to the transformation of the cuprite film on the copper surface. Higher sodium hypochlorite concentrations accelerated corrosion product formation. This study proposed a disinfectant concentration threshold beyond which localized corrosion may occur at 50 °C.
Rizvi et al. (2021) studied the effect of sodium nitrite against pure copper corrosion in simulated cooling water the efficiency of 61.8 % was obtained using EIS method at 2,000 ppm as revealed in Table 1.
By analyzing organic and inorganic copper corrosion inhibitors, it is revealed that both inhibitors show promise, and some of them offer potential eco-friendly solutions. Nevertheless, further research is needed on inhibitor mechanisms, tailoring to specific environments, and the long-term impact of organic inhibitors on the environment. Exploring combinations of these inhibitor types and computational design tools could lead to the development of more efficient and sustainable corrosion control strategies for copper.
Table 1 summarizes of the above-mentioned organic and inorganic corrosion inhibitors for copper in water.
3.1.3 Application of protective coatings
The application of protective coatings is a widely recognized and effective approach for copper corrosion prevention and mitigation. Protective coatings are applied to copper surfaces to create a barrier that shields the metal from corrosive agents and environmental factors. There are three types of coatings: organic, inorganic, and hybrid coatings.
3.1.3.1 Organic coatings
Ghelichkhah et al. (2015) introduced a novel approach to enhance corrosion resistance by applying a polydopamine (PDA) coating on a l-cysteine-modified copper surface. The incorporation of the l-cysteine interlayer improves the stability of the PDA coating, resulting in lower corrosion rates compared to bare copper, as demonstrated by electrochemical experiments. Based upon this work, Xu et al. (2017) proposed a one-step immersion method for creating stable superhydrophobic copper surfaces with exceptional corrosion resistance. This method employs fatty acids in a water-based solution, which offers a faster process compared to traditional methods that use ethanol. The research findings highlight the remarkable corrosion resistance exhibited by the superhydrophobic copper surfaces and underscore the enhanced safety in industrial manufacturing when producing superhydrophobic metal surfaces on a commercial scale. While superhydrophobic layers have garnered attention as corrosion inhibitors, particularly for copper in chloride media, their effectiveness in other corrosive solutions, such as sulfate or nitrate, requires further investigation. Tasić et al. (2019) delved into the development of superhydrophobic coatings as corrosion inhibitors for copper. The study emphasizes the significance of achieving high surface roughness and low surface energy to attain superhydrophobic properties. Various techniques, including chemical etching, chemical vapor deposition, and solution immersion, are discussed as efficient, simple, and cost-effective approaches for creating rough surfaces. Furthermore, experimental studies are needed to explore the impact of high temperature and pressure on the corrosion properties of superhydrophobic coatings and to gain a deeper understanding of their synergistic mechanisms.
3.1.3.2 Inorganic coatings
Research findings highlighted the potential of innovative techniques and materials in addressing challenges related to surface coating and corrosion protection.
Li et al. (2008) conducted a study on a copper plating system used to coat the inner surface of tube-shaped workpieces. The researchers explored various design scenarios aimed at achieving superior coating quality in terms of thickness and uniformity. Their findings revealed that the optimized design yielded significant improvements in coating quality compared to the original design. This optimized approach holds the potential to save considerable time, energy, and cost associated with quality control in manufacturing processes. In a separate study, Ngaotrakanwiwat et al. (2020) focused on Cu-doped TiO2 particles (Cu/TiO2) with an optimal Cu doping of 0.5 mol %. These particles exhibited a notably higher photocurrent and were utilized to create a nanocomposite film for effectively mitigating corrosion of copper terminal lugs in a NaCl solution. The application of the coated terminal lugs demonstrated enhanced corrosion resistance and photoelectrochemical cathodic protection under UV irradiation.
3.1.3.3 Hybrid coatings
Several researches highlighted the development and effectiveness of various coatings and treatments in enhancing the corrosion resistance of copper. In a study conducted by Dagdag et al. (2017), it was found that the use of a formulated matrix zinc inorganic filler provided the best corrosion protection for copper. The combination of the standard polymeric epoxy resin matrix with zinc resulted in the formation of a protective layer that effectively limited electrolyte penetration. The authors also utilized density functional theory to calculate the parameters of 4,4′-Ethylene bis(N,N-diglycidylaniline), which exhibited a strong correlation with the corrosion performance of the coatings. Wan et al. (2019) studied a conductive polypyrrole film codoped with silica and benzotriazole (BTA) to enhance the corrosion resistance of copper. The incorporation of silica and BTA contributed to a reduction in film thickness and improved compactness. The codoped film demonstrated significant improvements in corrosion resistance, durability, and electrical conductivity compared to films doped with silica or BTA alone. This enhancement was attributed to the synergistic effect between the silica nanofiller and BTA inhibitor. Similarly, Pasha et al. (2022) developed a high-performance organic coating for the corrosion protection of copper surfaces. This coating combined epoxy with para toluene sulphonic acid doped polypyrrole and manganese iron oxide nanoparticles. The resulting nanocomposite coating exhibited improved conductivity, dielectric performance, and adhesion strength compared to pure epoxy. It provided superior corrosion protection, with a corrosion inhibition efficiency of 99 % compared to only 22 % for pure epoxy. This innovative coating shows promise as an effective solution for safeguarding copper surfaces in corrosive environments. Additionally, Arshad et al. (2022) introduced a graphene oxide-triazole hybrid coating for the corrosion protection of copper. The hybrid coating demonstrated enhanced corrosion inhibition efficiency, primarily targeting the anodic reaction.
3.2 Strategies based on water treatment techniques
3.2.1 Advances in pH adjustment methods
The pH level of water plays a significant role in the corrosion process. Acidic water can accelerate the corrosion of copper, while alkaline water can slow it down. Therefore, maintaining the pH of water within an optimal range is crucial for controlling copper corrosion. Recent advances in pH adjustment methods have made it possible to control the pH of water more accurately and efficiently. Traditional methods of pH adjustment, such as the addition of acids or bases, are being complemented with more sophisticated techniques. One of the significant advancements in this area is the development of automated pH adjustment systems (Pomberger et al. 2023). These systems continuously monitor the pH of the water and adjust it as needed, providing more precise control over the pH and reducing the risk of over- or under-adjustment. Another breakthrough is the use of pH adjustment agents (Martić et al. 2015). These agents can provide more stable and long-lasting pH control compared to traditional acids and bases. For instance, some of these agents can buffer the pH of the water, preventing sudden changes in pH that could trigger rapid corrosion. Furthermore, advances in technology have also enabled the development of pH sensors that can provide real-time monitoring of water pH (Viciano-Tudela et al. 2023). These sensors can be integrated with automated pH adjustment systems to provide continuous, real-time control of water pH, further enhancing the effectiveness of pH adjustment methods. Despite these advancements, challenges remain in pH adjustment methods. These include the need for regular calibration and maintenance of pH sensors and the potential for chemical contamination from pH adjustment agents.
The study found that free carbon dioxide increases the corrosion rate of copper. The presence of carbon dioxide disrupts the formation of a protective layer on the copper surface, making it more susceptible to corrosion. Other factors like temperature and pH also affect the corrosion rate. The study suggests controlling the concentration of carbon dioxide in water to prevent or slow down copper corrosion. These findings can help develop effective corrosion control strategies in water distribution systems (Churchill et al. 2000) (Figure 8). The study involved raising the pH by adding lime (Ca(OH)2) and increasing alkalinity by adding sodium bicarbonate (NaHCO3). The zinc orthophosphate used was a liquid with 8.3 % zinc content and a zinc-to-phosphate ratio of 1:3. The pH-alkalinity treatment had positive effects, reducing metal levels in faucets and flowing water. This cost-effective method has proven successful in field studies and actual distribution systems. The study emphasizes the importance of maintaining a stable pH to reduce metal levels. It suggests that a lower, stable pH may be easier to maintain and could be more beneficial for other water treatment considerations. For instance, higher pH levels can reduce the effectiveness of free chlorine as a disinfectant and increase the formation of trihalomethanes and the precipitation of naturally occurring iron if chlorine is present.
Evaluation of target treatment values of four pH–alkalinity combinations and different concentrations of zinc orthophosphate within pipe loop system.
Xuejun et al. (2018) investigated thermodynamically the effect of pH value on copper corrosion in desalted water. The corrosion problem here caused by oxygen and the method using to prevent copper corrosion in this case is to control pH value. From the obtained results in cooling water pH value could be ranging between 7.86 and 8.86 and between, 7.86–9.26 in dual water inner cooling water.
3.2.2 Breakthroughs in oxygen removal techniques
Oxygen significantly accelerates copper corrosion in water by forming copper oxides that react further to create corrosive substances. Recent advancements have diversified oxygen removal techniques beyond traditional methods like boiling or chemical deoxygenation (Martić et al. 2015; Zhao et al. 2017). Membrane deoxygenation emerges as a particularly promising strategy. This technique employs semi-permeable membranes to selectively remove oxygen from water (Martić et al. 2015). This approach offers advantages like superior efficiency, lower energy consumption, and the elimination of chemical residues compared to traditional methods. Another significant strategy involves the development of novel oxygen scavengers. These substances effectively reduce oxygen concentration by absorbing it from the water. Recent research has yielded more efficient scavengers capable of faster and more complete oxygen removal than traditional options. Despite these breakthroughs, challenges remain. Precise control over the oxygen removal process is crucial for optimal performance, and potential impacts on other water quality parameters require further investigation.
4 Copper corrosion in water circuits of heat exchangers
Despite the widespread use of copper in heat exchangers due to its excellent thermal properties, a comprehensive understanding of copper corrosion within these systems remains limited. This knowledge gap necessitates further research to effectively mitigate corrosion and ensure the long-term performance and reliability of copper heat exchangers. Several researchers have addressed various aspects of heat exchanger performance, including material selection, operating conditions, maintenance schedules, and corrosion assessment methods (Faes et al. 2019). However, a critical review focusing specifically on copper corrosion in these systems is lacking. Corrosion assessment plays a crucial role in maintaining heat exchanger integrity. To analyse copper corrosion mechanisms, atomic absorption spectroscopy was employed to measure copper concentration in heat exchangers, revealing significant corrosion. Such analyses can contribute to developing expert systems for comprehensive corrosion assessment. Recent studies have investigated mechanisms and risk factors for copper corrosion in heat exchangers. Hoshi et al. (2016) analyzed cooling water from air-conditioning units in Japan to investigate copper pitting corrosion in heat exchanger tubes. A two-dimensional diagram (2DD) was established to correlate acid consumption with corrosion susceptibility. Then, Hoshi et al. developed a three-dimensional diagram (3DD) considering acid consumption, pH, and ion ratios to categorize corrosion risk more accurately than traditional two-dimensional methods. Simulated tests validated the effectiveness of the 3DD approach in screening cooling water for pitting corrosion risk. Lee et al. (2010) investigated the mechanisms of pitting corrosion in copper tubes used in heating systems. Their study identified the presence of a Cu(I) oxide layer and the presence of iron species as key factors influencing pitting corrosion, with stagnant water conditions further promoting this process. These studies demonstrate ongoing efforts to understand the copper corrosion mechanisms in heat exchangers. Another case study conducted by Khan (Khan 2019a) reported efficiency reduction and leakage issues in a heat exchanger used for cooling a coal pulverizer in Georgia (USA). The inner surfaces of the copper tubing were exposed to non-potable cooling water from a local lake, while the outer surfaces were in contact with hot oil. Corrosion products, primarily consisting of copper oxide, were detected, but a substantial amount of iron oxides was also present. Investigation revealed corrosion pits on the inner surfaces of the copper tubing caused by corrosive water, affecting both the copper tubing and upstream steel pipes.
Hong et al. (2019) conducted a study on corrosion failure in district heating systems. They identified fractures with an open window shape, which resulted from internal wall thinning caused by caustic corrosion and the formation of sodium and iron oxides. The protective magnetite layer was weakened by tensile residual stress, leading to accelerated caustic corrosion and the formation of cavities or micro-voids at grain boundaries due to hydrogen diffusion. Additionally, high temperature creep promoted cavity evolution in areas subjected to tensile residual stress. The cracks initiated at grain boundaries due to hydrogen embrittlement and thermal creep, exhibiting an intergranular feature on the inside and eventually transitioning to a transgranular fracture mode on the outside. Moreover, Chae et al. (2020) investigated the stress corrosion cracking behavior of a copper pipe in a heating water supply system, employing both metallurgical and mechanical analyses. They reported typical stress corrosion cracking characteristics in the copper pipe fracture, which resulted from corrosive conditions and high tensile stresses. Different corrosion products were observed on the outer and inner surfaces of the pipe. The primary cause of tensile hoop stresses on the outer surface was the U-bolt force, leading to plastic deformation.
Peltola and Lindgren (2015) identified the ant-nest corrosion to be the cause of heat exchanger leakage in copper tubes. While other potential failure mechanisms were ruled out, similarities to stress corrosion cracking were noted. Ant-nest corrosion is a well-known phenomenon, and methods for its control have been proposed. The examination of ant-nest corrosion using optical and scanning electron microscopy (SEM) revealed a correlation between the orientation of corrosion tunnels and copper grain boundaries. The preferential attack of grain boundaries, potentially influenced by surface morphology and the presence of non-metallic inclusions can act as initiation sites for localized corrosion. European standards specify the maximum acceptable value for lubricant residues, referring to the total carbon content on the inner surface of tubes (Standards France 2016). Additionally, Lachowicz (2020) conducted corrosion tests on the inner surface of copper tubes in a shell and tube heat exchanger. They reported that the corrosion was attributed to organic decomposition products, resulting in the formation of carboxylic acids (Bastidas et al. 2006; Chandra et al. 2014; Corbett and Elliott 2002).
The presence of carboxylic acid (e.g., RCOOH) form an unstable complex following the reaction (1):
The second step include the oxidization of Cu(I) to Cu(II) carboxylate complex following the reaction (2):
Then, the wedging effect of the Cu(I) to Cu(II) carboxylate complexes deposition following the reaction (3):
On this matter, reactions 2 and 3 repeat over and over until tunnels are formed leading to ultimate through-wall penetration. Ranjbar (2010) investigated the effect of flow induced corrosion and erosion on failure of a tubular copper heat exchanger. This study highlights the importance of maintaining optimal water flow velocity in heat exchangers. Low velocity of circulating water (0.4 m/s) leads to build up of sediments to accumulate, creating sites for under deposit corrosion that can reduce tube diameter. Conversely, excessively high locally velocity water (0.6 m/s) can destroy the surface protecting film on the tube surface and yield to severe erosion within the tubes operating at the temperature range (104 °C–110 °C), ultimately causing failure. To address this issue, the study recommends a minimum flow rate of 0.5 m/s. This velocity helps to minimize sediment build up and reduce the risk of corrosion while staying below the threshold for significant erosion. Schmitt and Bakalli (2010) mentioned that high-velocity fluids with entrained gas bubbles can damage protective scales, layers, or films on pipe walls, especially at locations like heat exchanger inlets where air bubbles can develop significant radial velocity. This damage starts with isolated bends that grow and considerably roughen the pipe surfaces.
Rahman and Ahmed (2020) investigated the corrosion behavior of copper tubes in heat exchangers to assess their lifespan for on-board applications. A series of experiments were performed to examine the corrosion rates of copper tubes in sea water and river water, considering various flow velocities and tube and shell side temperatures. The investigations revealed that corrosion in sea water was approximately three times higher than that in river water. Furthermore, the increase in temperature and flow velocity significantly accelerated the rate of corrosion, contributing to a shortened lifespan. Also, Huang et al. (2019) studied the fireside corrosion in gas instantaneous water heaters. The study suggests that in heat exchangers without a lead coating, the main corrosion product was Cu4(OH)6SO4, while PbSO4 was the primary corrosion product in heat exchangers with a lead coating. The experiments revealed that the corrosion rate decreased as the heat input increased. Additionally, the thermal efficiency of the gas water heaters decreased to different degrees in the range of 2.4 %–6 % caused by the accumulation of flue gas side corrosion products, which have hostile effects on the heat transfer performance of heat exchangers.
5 Copper corrosion mitigation in water circuits of heat exchangers
Corrosion represents a significant challenge in heat exchangers, hindering their performance and lifespan. Extensive research has addressed this challenge by investigating the potential mitigation strategies to minimize detrimental effects of corrosion, with particular emphasis placed on material selection, protective coatings, corrosion inhibitors, and innovative design approaches. Suzuki et al. (2005) conducted a study on copper heat exchanger tubes in water heaters and found that they experienced Type I pitting corrosion, like pass-through soft copper plumbing tubes, when exposed to well water. However, heat exchangers with copper tubes that were treated with tin coating after manufacturing exhibited the highest resistance against pitting corrosion. This resistance was attributed to the formation of a stable tin oxide layer on the coating surface. Additionally, heat exchangers made of copper tubes with residual free carbon below 4 mg/m2 also showed excellent resistance against pitting corrosion. Notably, heat exchangers with manufactured Cu-0.24 mass_%Sn alloy tubes corroded regardless of the presence of a carbon film. Recently, Zhang et al. (2022) proposed a coupling solution to optimize flue gas condensation heat exchangers. They suggested using materials with good thermal conductivity and anti-corrosive modified coatings, such as a Ni–Cu–P organic pure-coated layer and a Ni–Cu–P chemical organic-coated composite layer. Experimental studies were conducted on the condensation heat transfer performance of wet air on 3-D finned-tube heat exchanger surfaces with different coatings. The results indicated that the pure-coating layer achieved a higher heat transfer coefficient compared to the composite coating, while the copper-based heat exchanger outperformed the steel-based surface. The study also established and validated non-dimensional correlations for flue gas convection condensation heat transfer with different coating surfaces, demonstrating good agreement between theoretical and experimental data. These correlations provide reliable guidance for the design of similar anti-corrosive heat exchangers.
Moreover, Skrypnikova et al. (2014) emphasized the effectiveness of benzotriazole as a copper pitting corrosion inhibitor in heat exchange equipment and water supply systems. They demonstrated that the efficiency of benzotriazole increased with temperature, and complete protection against pitting corrosion was achieved at specific benzotriazole concentrations. For instance, at 60 °C, a benzotriazole concentration of 5 × 10−7 M resulted in the full suppression of pitting corrosion. Furthermore, Chaitanya Kumar and Appa Rao (2016) studied two methods for mitigating microbially influenced corrosion of copper alloy in heat exchangers. The first method involves using the inhibitor formulation 1,2,3-benzotriazole (BTAH) and tetrakis (hydroxymethyl)phosphonium sulfate (THPS). The second method uses 5-(3-aminophenyl)tetrazole and THPS. Both formulations have an inhibition efficiency of greater than 99 %. Faes et al. (2019) conducted a study and reported that heat exchangers are susceptible to corrosion due to their metallic construction. The researchers provided an overview of corrosion in heat exchangers made from various metallic alloys, including the different types of corrosion encountered, vulnerable areas within the devices. They addressed prevention and control measures for corrosion in heat exchangers, outlining several methods to mitigate corrosion issues. These methods include general considerations such as material selection and design phase modifications, focusing on maximizing the heat transfer coefficient on the side with the cooler fluid in heat exchangers. This reduces the effective temperature on that side, thereby suppressing corrosion kinetics and the solubility of corrosive species, leading to reduction corrosion. Moreover, coatings and linings, such as metallic and organic coatings, are recommended to provide a protective barrier against corrosion. These approaches help preserve the integrity of protective layers and prolong the heat exchanger’s lifespan.
Tan et al. (2018) conducted a study on gas water heaters in residential buildings, which often suffer from poor thermal efficiency and low-temperature corrosion. To address these issues, researchers developed a phase transition heat exchanger, resulting in improved heat transfer efficiency. Experimental results demonstrated that the new heat exchanger had 6 % higher heat transfer efficiency compared to the existing one. Furthermore, the phase transition heat exchanger prevented low-temperature corrosion by maintaining exhaust gas temperature above the dew point (see Table 2). The new heater exhibited low sensitivity to heat source fluctuations and provided a better user experience. In terms of economic efficiency, the study showed that the cost recovery could be achieved in approximately 2.5 years, with potential natural gas savings of 1.1 × 107 m3 in China.
Corrosion problem and recommendations in heat-exchanger.
| Problem | Type of water | Recommendations | Reference |
|---|---|---|---|
| The issue of corrosion in the heat exchanger used to warm up cold basin water in Sharm El Sheikh, Egypt, is due to the high concentration of chloride. | Desalinated water | 1. Use corrosion-resistant materials: employ materials like stainless steel or titanium which have high resistance to chloride-induced corrosion. 2. Water treatment: implement desalination or dichlorination processes to reduce chloride concentration in the basin water. 3. Protective coatings: apply anti-corrosive coatings to the heat exchanger to provide a barrier against chloride attack. |
Ghayad et al. (2015) |
| Copper pipes utilized in air conditioning systems are susceptible to formicary, or ant-nest, corrosion induced by organic acids. | Tap water | 1. Material selection: use corrosion-resistant materials for the tubes, such as stainless steel or specific copper alloys known for their resistance to organic acids. 2. Coating: apply protective coatings to the copper tubes to provide a barrier against the organic acids. 3. Regular maintenance and inspection: implement a routine of regular checks and maintenance to detect early signs of corrosion and take necessary action. 4. Control of acid source: identify and control the source of organic acids causing the corrosion. |
Cozzarini et al. (2020) |
| Failure of the heat exchanger caused by corrosive organic species. | Chilled water | 1. Material selection: use materials that are resistant to corrosion by organic species for the heat exchanger, such as stainless steel or specific alloys. 2. Protective coatings: apply protective coatings to the heat exchanger to provide a barrier against the corrosive organic species. 3. Regular maintenance and inspection: implement a routine of regular checks and maintenance to detect early signs of corrosion and take necessary action. 4. Control of organic species: Identify and control the source of corrosive organic species. |
Peltola and Lindgren (2015) |
| The deterioration of the copper tube was a result of erosion–corrosion triggered by two elements: the turbulent flow of water carrying suspended solid particles and the water’s chemical composition, which was high in chlorides, leading to the destabilization of the protective cuprous oxide layer. | Hard water | 1. Water treatment: implement water treatment processes to remove suspended solids and reduce the disturbed flow of water. 2. Protective coatings: apply protective coatings to the copper tubes to provide a barrier against erosion-corrosion. 3. Design modifications: modify the design of the system to minimize areas of disturbed flow. |
Kuźnicka (2009) |
| Microstructure problem of heat exchanger. | Water | 1. Prevention and control measures for corrosion, encompassing considerations such as material selection, design practices, operational guidelines, and the implementation of cathodic and anodic protection. | Faes et al. (2019) |
| The high failure corrosion rate caused by low temperature in the Chinese water heater market. | Gas water heating | 1. Development of a new phase transition heat exchanger for gas water heaters. | Tan et al. (2018) |
Ghayad et al. (2015) conducted an industrial-level diagnosis of the corrosion issue in a heat exchanger used to warm up cold basin water in Sharm El Sheikh, Egypt. They found that the problem was related to the high concentration of chloride. As a result, they recommended the use of corrosion-resistant materials, water treatment, and/or protective coatings as mentioned in Table 2. Similarly, Kuźnicka (2009) identified the same cause of corrosion in hard water, which was attributed to high chloride concentration and turbulent water flow. They proposed modifying the system’s design to minimize areas of disturbed water flow (which is responsible for the erosion corrosion), implementing water treatment, and applying protective coatings as potential solutions (see Table 2). Ranjbar (2010) investigated the relationship between water flow velocity and corrosion-erosion in a tubular copper heat exchanger failure. His study suggests that maintaining an optimal flow rate can mitigate these issues. A minimum velocity of 0.5 m/s was recommended to minimize sediment buildup and reduce corrosion risk, while remaining below the threshold for significant erosion.
Several researchers (Cozzarini et al. 2020; Peltola and Lindgren 2015) demonstrated that the corrosion failure in heat exchangers is susceptible to formicary, or ant-nest, corrosion, which is related to the presence of organic acid species. To mitigate ant-nest corrosion, it is crucial to minimize the presence of corrosive organic species on the surface of copper tubes. Therefore, they provided some recommendations, including the implementation of routine checks and maintenance to detect early signs of corrosion and take necessary action as referred in Table 2. Additionally, they emphasized the importance of controlling the source of corrosive organic species and using materials that are resistant to corrosion by organic species. Table 2 presents a summary of heat exchanger corrosion problems and the recommendations discussed below. From this data, it becomes evident that many authors have identified the cause of copper heat exchanger corrosion to be related to organic acids or high chloride concentration.
Based on the provided literature overview, it should be underscored the valuable starting point for understanding and addressing corrosion problems in heat exchangers. By carefully considering material selection, water treatment options, protective coatings, and the potential for addressing the root cause of the corrosion, we can develop effective strategies to ensure the longevity and performance of these critical components. However, it’s important to remember that a comprehensive approach is necessary, and ongoing research into innovative solutions holds promise for the future. The reference to a new phase transition heat exchanger for gas water heaters in the context of low-temperature corrosion suggests ongoing research into innovative solutions. Further exploration of these advancements and their applicability across different scenarios could be beneficial.
6 Conclusions
This review paper provides valuable insights into understanding the mechanisms of copper corrosion in water circuits of heat exchangers. By examining the key factors influencing copper corrosion, it offers relevant tools for developing effective corrosion mitigation strategies in copper corrosion in water-based systems.
The following main conclusions are drawn as follows:
The corrosion mechanisms commonly encountered in copper water circuits, including pitting, erosion–corrosion, stress corrosion cracking, and uniform corrosion are reported.
Copper corrosion in heat exchangers is a significant challenge that hinders performance and lifespan. While copper is widely used in heat exchangers due to its excellent thermal properties, there is a limited understanding of copper corrosion within these systems. Therefore, corrosion assessment is crucial for maintaining heat exchanger integrity.
Reviewed studies indicated the influence of water chemistry, temperature, and flow velocity on the pitting corrosion and erosion corrosion, as they are the main responsible mechanisms for copper corrosion in water circuits of heat exchangers.
Corrosion rates are accelerated by higher temperature and flow velocity. Maintaining optimal value of water flow velocity is essential to preventing sediment buildup and reducing corrosion and erosion risks.
Water chemistry plays a complex role in copper pitting corrosion. While some elements like sulfate can activate corrosion and others like bicarbonate can buffer it, the overall impact depends on the interplay between factors like pH, dissolved gases. This complex interplay determines the formation and properties of corrosion products, ultimately influencing the rate and severity of copper corrosion.
Mitigation strategies include the use of protective coatings, such as tin coating, residual-free carbon, and Ni–Cu–P organic coatings, which have shown resistance against pitting corrosion in copper heat exchangers.
Microorganisms can significantly contribute to copper pitting corrosion in water-based systems by promoting biofilm formation and potentially converting sulfates to damaging sulfides. Factors like stagnant water and residual organic films on pipes can further exacerbate this microbially influenced corrosion.
7 Outlook
To develop next-generation heat exchangers with superior corrosion resistance, a deeper understanding of copper corrosion’s fundamental aspects is crucial. Unveiling the intricacies of reactions, surface chemistry, and atomic-level interactions holds the key to tailor-made materials with superior corrosion resistance.
Several promising avenues exist for exploring novel prevention and mitigation strategies. Coatings and self-healing materials that adapt to their environment, along with advancements in surface treatments with enhanced adhesion properties, offer exciting possibilities. Synergistic approaches integrating coatings, cathodic protection, and corrosion inhibitors warrant investigation for comprehensive protection.
Furthermore, sophisticated monitoring tools like real-time corrosion sensors and predictive modeling can help early detection and mitigation strategies. Tailoring research methodologies to specific heat exchanger environments and fostering interdisciplinary collaboration are essential for innovation.
An alternative approach could involve modifying water circuit designs within heat exchangers to either tolerate corrosion or facilitate the easy replacement of corroded parts. These advancements will significantly impact the durability and performance of copper heat exchangers.
Funding source: Fundaçao para Ciencia e Tecnologia
Award Identifier / Grant number: UID/EMS/00285/2020
Funding source: Projeto apoiado pelo PRR-Plano de Recuperação e Resiliência e pelos Fundos Europeus Next Generation EU
Award Identifier / Grant number: 02/C05-i01/2022
Funding source: ARISE
Award Identifier / Grant number: LA/P/0112/2020
Funding source: Componente 5 – Capitalização e Inovação Empresarial – Agendas Mobilizadores para a Inovação Empresarial
Funding source: 7225-ILLIANCE High Performing Energy
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This research was funded through 7225-ILLIANCE High Performing Energy. Projeto apoiado pelo PRR-Plano de Recuperação e Resiliência e pelos Fundos Europeus Next Generation EU, no sequência do AVISO N.° 02/C05-i01/2022, Componente 5 – Capitalização e Inovação Empresarial – Agendas Mobilizadores para a Inovação Empresarial, and FCT- Fundação para a Ciência e a Tecnologia, under the project UID/EMS/00285/2020, ARISE -LA/P/0112/2020.
-
Data availability: Not applicable.
References
Akkaya, M. and Ambrose, J.R. (1985). Effect of ammonium chloride and fluid velocity on the corrosion behavior of copper in sodium bicarbonate solutions. Corrosion 41: 707–714, https://doi.org/10.5006/1.3583007.Search in Google Scholar
Akpanyung, K. and Loto, R. (2019). Pitting corrosion evaluation: a review. J. Phys. Conf. Ser. 1378: 022088, https://doi.org/10.1088/1742-6596/1378/2/022088.Search in Google Scholar
Amendola, R. and Acharjee, A. (2022). Microbiologically influenced corrosion of copper and its alloys in anaerobic aqueous environments: a review. Front. Microbiol. 13: 1–10, https://doi.org/10.3389/fmicb.2022.806688.Search in Google Scholar PubMed PubMed Central
Arrousse, N., Fernine, Y., Haldhar, R., Berdimurodov, E., Ichou, H., Al-Zaqri, N., Koudad, M., Kim, S.C., and Taleb, M. (2023). Corrosion protection studies of different alloys in 1 M HCl by benzimidazole derivative: combined molecular dynamic simulations/DFT. J. Environ. Chem. Eng. 11: 109642, https://doi.org/10.1016/j.jece.2023.109642.Search in Google Scholar
Arshad, N., Imran, M., Akram, M., and Altaf, F. (2022). Graphene oxide-aryl substituted triazole thin hybrid corrosion resistant coating for copper. Port. Electrochim. Acta 40: 193–207, https://doi.org/10.4152/pea.2022400304.Search in Google Scholar
Baba, H., Kodama, T., Fujii, T., Hisamatsu, Y., and Ishikawa, Y. (1981). Measurements of pitting potential of copper tubes in hot water. Corros. Eng. 30: 113–118, https://doi.org/10.3323/jcorr1974.30.2_113.Search in Google Scholar
Bastidas, D.M., Cayuela, I., and Bastidas, J.M. (2006). Ant-nest corrosion of copper tubing in air-conditioning units. Rev. Metal. 42: 367–381, https://doi.org/10.3989/revmetalm.2006.v42.i5.34.Search in Google Scholar
Bi, H., Burstein, G.T., Rodriguez, B.B., and Kawaley, G. (2016). Some aspects of the role of inhibitors in the corrosion of copper in tap water as observed by cyclic voltammetry. Corros. Sci. 102: 510–516, https://doi.org/10.1016/j.corsci.2015.11.005.Search in Google Scholar
Božinović, S. (2017). Influence of inorganic inhibitor on copper corrosion in acidic medium. Innovations 94: 92–94.Search in Google Scholar
Bremer, P.J., Webster, B.J., and Brett Wells, D. (2001). Biocorrosion of copper in potable water. J. /Am. Water Work. Assoc. 93: 82–91, https://doi.org/10.1002/j.1551-8833.2001.tb09269.x.Search in Google Scholar
Broo, A.E., Berghult, B., and Hedberg, T. (1998). Copper corrosion in water distribution systems-The influence of natural organic matter (NOM) on the solubility of copper corrosion products. Corros. Sci. 40: 1479–1489, https://doi.org/10.1016/s0010-938x(98)00059-6.Search in Google Scholar
Burleigh, T.D., Gierke, C.G., Fredj, N., and Boston, P.J. (2014). Copper tube pitting in santa fe municipal water caused by microbial induced corrosion. Materials 7: 4321–4334, https://doi.org/10.3390/ma7064321.Search in Google Scholar PubMed PubMed Central
Burstein, G.T., Liu, C., Souto, R.M., and Vines, S.P. (2004). Origins of pitting corrosion. Corros. Eng. Sci. Technol. 39: 25–30, https://doi.org/10.1179/147842204225016859.Search in Google Scholar
Campbell, H.S. (1950). Pitting corrosion in copper water pipes caused by films of carbonaceous material produced during manufacture. J. Inst. Metals. 77: 345.Search in Google Scholar
Chae, H., Wang, H., Hong, M., Kim, W.C., Kim, J.G., Kim, H., and Lee, S.Y. (2020). Stress corrosion cracking of a copper pipe in a heating water supply system. Met. Mater. Int. 26: 989–997, https://doi.org/10.1007/s12540-019-00386-0.Search in Google Scholar
Chaitanya Kumar, K. and Appa Rao, B.V. (2016). Mitigation of microbially influenced corrosion of Cu–Ni (90/10) alloy in a seawater environment. Res. Chem. Intermed. 42: 5807–5823, https://doi.org/10.1007/s11164-015-2405-7.Search in Google Scholar
Chandra, K., Kain, V., Shetty, P.S., and Kishan, R. (2014). Failure analysis of copper tube used in a refrigerating plant. Eng. Fail. Anal. 37: 1–11, https://doi.org/10.1016/j.engfailanal.2013.11.014.Search in Google Scholar
Chaubey, N., Savita, Qurashi, A., Chauhan, D.S., and Quraishi, M.A. (2021). Frontiers and advances in green and sustainable inhibitors for corrosion applications: a critical review. J. Mol. Liq. 321: 114385, https://doi.org/10.1016/j.molliq.2020.114385.Search in Google Scholar
Churchill, D.M., Mavinic, D.S., Neden, D.G., and MacQuarrie, D.M. (2000). The effect of zinc orthophosphate and pH-alkalinity adjustment on metal levels leached into drinking water. Can. J. Civ. Eng. 27: 33–43, https://doi.org/10.1139/cjce-27-1-33.Search in Google Scholar
Corbett, R.A. and Elliott, P. (2002). Ant-nest corrosion – digging the tunnels. Corros. Rev. 20: 51–68, https://doi.org/10.1515/corrrev.2002.20.1-2.51.Search in Google Scholar
Cornwell, F.J., Wildsmith, G., and Gilbert, P.T. (1973). Pitting corrosion in copper tubes in cold water service. Br. Corros. J. 8: 202–209, https://doi.org/10.1179/000705973798321973.Search in Google Scholar
Coyne, J.M. (2009). Flow induced failures of copper drinking water tube, PhD thesis. Blacksburg, VA, Virginia Polytechnic Institute and State University.Search in Google Scholar
Cozzarini, L., Marsich, L., and Schmid, C. (2020). Ant-nest corrosion failure of heat exchangers copper pipes. Eng. Fail. Anal. 109: 104387, https://doi.org/10.1016/j.engfailanal.2020.104387.Search in Google Scholar
Critchley, M.M., Cromar, N.J., McClure, N.C., and Fallowfield, H.J. (2003). The influence of the chemical composition of drinking water on cuprosolvency by biofilm bacteria. J. Appl. Microbiol. 94: 501–507, https://doi.org/10.1046/j.1365-2672.2003.01857.x.Search in Google Scholar PubMed
Custalow, B.D. (2009). Influences of water chemistry and flow conditions on non-uniform corrosion in copper tube, PhD thesis, Blacksburg, VA. Virginia Polytechnic Institute and State University.Search in Google Scholar
Dagdag, O., Gouri, M.El, Touhami, M.E., and Harfi, A.El (2017). Evaluation of corrosion protection of epoxy coatings on copper during exposure to an aerated 3 % NaCl solution. Mor. J. Chem. 5: 120–130.Search in Google Scholar
Dodrill, D.M. and Edwards, M. (1995). Corrosion control on the basis of utility experience. J. /Am. Water Works Assoc. 87: 74–85, https://doi.org/10.1002/j.1551-8833.1995.tb06395.x.Search in Google Scholar
Drogowska, M., Brossard, L., and Menard, H. (1987). Anodic copper dissolution in the presence of CI-ions at pH 12. Corrosion 43: 549–552, https://doi.org/10.5006/1.3583899.Search in Google Scholar
Duthil, J.P., Mankowski, G., and Giusti, A. (1996). The synergetic effect of chloride and sulphate on pitting corrosion of copper. Corros. Sci. 38: 1839–1849, https://doi.org/10.1016/s0010-938x(96)88250-3.Search in Google Scholar
Edwards, M., Ferguson, J.F., and Reiber, S.H. (1994a). On the pitting corrosion of copper. J. /Am. Water Work. Assoc. 86: 74–90, https://doi.org/10.1002/j.1551-8833.1994.tb06226.x.Search in Google Scholar
Edwards, M., Meyer, T., and Rehring, J. (1994b). Effect of selected anions on copper corrosion rates. J. Am. Water Work. Assoc. 86: 73–81, https://doi.org/10.1002/j.1551-8833.1994.tb06287.x.Search in Google Scholar
Edwards, M., Hidmi, L., and Gladwell, D. (2002). Phosphate inhibition of soluble copper corrosion by-product release. Corros. Sci. 44: 1057–1071, https://doi.org/10.1016/s0010-938x(01)00112-3.Search in Google Scholar
Efird, K.D. (1977). Effect of fluid dynamics on the corrosion of copper-base alloys in sea water. Corrosion 33: 3–8, https://doi.org/10.5006/0010-9312-33.1.3.Search in Google Scholar
Fabjan, E.Š., Kosec, T., Kuhar, V., and Legat, A. (2011). Corrosion stability of different bronzes in simulated urban rain. Mater. Technol. 45: 585–591.Search in Google Scholar
Faes, W., Lecompte, S., Ahmed, Z.Y., Van Bael, J., Salenbien, R., Verbeken, K., and De Paepe, M. (2019). Corrosion and corrosion prevention in heat exchangers. Corros. Rev. 37: 131–155, https://doi.org/10.1515/corrrev-2018-0054.Search in Google Scholar
Farooq, S.A., Raina, A., Ul Haq, M.I., and Anand, A. (2022). Corrosion behaviour of engineering materials: a review of mitigation methodologies for different environments. J. Inst. Eng. Ser. D 103: 639–661, https://doi.org/10.1007/s40033-022-00367-5.Search in Google Scholar
Fontana, M. (1987). Corrosion engineering, 3rd ed McGraw-Hill, Singapore.Search in Google Scholar
Fouda, A.S., El-desoky, A.M., and Nabih, A. (2013). Inhibitive, adsorption, synergistic studies on copper corrosion in nitric acid solutions by some organic derivatives. Adv. Mater. Corros. 2: 1–15.Search in Google Scholar
Frankel, G.S. (1998). Pitting corrosion of metals: a review of the critical factors. J. Electrochem. Soc. 145: 2186–2198, https://doi.org/10.1149/1.1838615.Search in Google Scholar
Ghayad, I., Abdel-Hamid, Z., and Gomaa, N. (2015). A case study: corrosion failure of tube heat exchanger. J. Metall. Eng. 4: 57, https://doi.org/10.14355/me.2015.04.007.Search in Google Scholar
Ghelichkhah, Z., Sharifi-Asl, S., Farhadi, K., Banisaied, S., Ahmadi, S., and Macdonald, D.D. (2015). L-cysteine/polydopamine nanoparticle-coatings for copper corrosion protection. Corros. Sci. 91: 129–139, https://doi.org/10.1016/j.corsci.2014.11.011.Search in Google Scholar
Gibson, J. and Karney, B. (2021). A 30-year review of copper pitting corrosion and pinhole leaks: achievements and research gaps. AWWA Water Sci. 3: 1–9, https://doi.org/10.1002/aws2.1221.Search in Google Scholar
Gopi, D., Govindaraju, K.M., Collins Arun Prakash, V., Angeline Sakila, D.M., and Kavitha, L. (2009). A study on new benzotriazole derivatives as inhibitors on copper corrosion in ground water. Corros. Sci. 51: 2259–2265, https://doi.org/10.1016/j.corsci.2009.06.008.Search in Google Scholar
Guibadj, A. and Carlo, M. (2017). Studies of copper corrosion inhibition using iodate ions from potassium electrochemical, chemical and theoretical studies of copper corrosion inhibition using iodate ions from potassium iodate as an inorganic inhibitor in 0.5 M NaCl solution. Moroccan J. Chem. 4: 1144–1156.Search in Google Scholar
Gurrappa, I. (2005). Cathodic protection of cooling water systems and selection of appropriate materials. J. Mater. Process. Technol. 166: 256–267, https://doi.org/10.1016/j.jmatprotec.2004.09.074.Search in Google Scholar
Ha, H., Taxen, C., Williams, K., and Scully, J. (2011). Effects of selected water chemistry variables on copper pitting propagation in potable water. Electrochim. Acta 56: 6165–6183, https://doi.org/10.1016/j.electacta.2011.04.008.Search in Google Scholar
Harrison, D.B., Nicholas, D.M., and Evans, G.M. (2004). Pitting corrosion of copper tubes in soft drinking waters: corrosion mechanism. J./ Am. Water Work. Assoc. 96: 67–76, https://doi.org/10.1002/j.1551-8833.2004.tb10742.x.Search in Google Scholar
Hawsawi, H. (2022). Investigation of Solupred as a pharmaceutical drug as a corrosion inhibitor for copper corrosion in 1.0 M sulfamic acid solution. Chem. Pap. 76: 7745–7757, https://doi.org/10.1007/s11696-022-02430-7.Search in Google Scholar
Hedin, A., Johansson, A.J., Lilja, C., Boman, M., Berastegui, P., Berger, R., and Ottosson, M. (2018). Corrosion of copper in pure O2-free water. Corros. Sci. 137: 1–12, https://doi.org/10.1016/j.corsci.2018.02.008.Search in Google Scholar
Hong, M., Chae, H., Kim, W.C., Kim, J.G., Kim, H., and Lee, S.Y. (2019). Failure analysis of a water wall boiler tube for power generation in a district heating system. Met. Mater. Int. 25: 1191–1201, https://doi.org/10.1007/s12540-019-00267-6.Search in Google Scholar
Hoshi, Y., Ochi, K., Shitanda, I., Itagaki, M., Sekiguchi, K., Hirata, Y., and Hishinuma, T. (2016). Diagnosis of copper tube corrosion by water quality analysis of cooling water flowing through copper heat exchanger of air-conditioner. Zair. to Kankyo/Corros. Eng. 65: 88–94, https://doi.org/10.3323/jcorr.65.88.Search in Google Scholar
Hou, B., Li, X., Ma, X., Du, C., Zhang, D., Zheng, M., Xu, W., Lu, D., and Ma, F. (2017). The cost of corrosion in China. npj Mater. Degrad. 1: 4, https://doi.org/10.1038/s41529-017-0005-2.Search in Google Scholar
Huang, X., Sun, M., and Kang, Y. (2019). Fireside corrosion on heat exchanger surfaces and its effect on the performance of gas-fired instantaneous water heaters. Energies 12: 2583, https://doi.org/10.3390/en12132583.Search in Google Scholar
Huang, Y.J., Nakajima, M., Kurotaki, H., and Nozawa, T. (2022). The effect of dissolved oxygen respectively dissolved hydrogen on corrosion behavior of CuCrZr alloy in high temperature water. Nucl. Mater. Energy 31: 101190, https://doi.org/10.1016/j.nme.2022.101190.Search in Google Scholar
Iyasu, T., Kuratani, M., Ikeda, I., Tanaka, N., Yamada, Y., and Sakurada, O. (2020). A study of water treatment chemical effects on type I” pitting corrosion of copper tubes. Mater. Sci. Appl. 11: 494–504, https://doi.org/10.4236/msa.2020.117034.Search in Google Scholar
Jacobs, S. and Edwards, M. (2000). Sulfide scale catalysis of copper corrosion. Water Res. 34: 2798–2808, https://doi.org/10.1016/s0043-1354(00)00025-7.Search in Google Scholar
Jesus, A.C.N. (2008). Estudo dos parâmetros: teor de NaCl e acabamento superficial, Na resistência à corrosão por pite em tubos de cobre, PhD thesis. Instituto de Pesquisas Energéticas e Nucleares, Sao Paulo.Search in Google Scholar
Khan, A.H. (2019a). Corrosion of copper cooling-water tubing in a heat exchanger. ASM Fail. Anal. Case Hist. Power Gener. Equip. ASM International, OHIO.10.31399/asm.fach.power.c9001700Search in Google Scholar
Khan, A.H. (2019b). Uniform corrosion of copper piping caused by microbiological attack. ASM Fail. Anal. Case Hist. Fail. Modes Mech. ASM International, OHIO.Search in Google Scholar
King, F. (2010). Critical review of the literature on the corrosion of copper by water. Technical Report TR−10−69. Svensk Kärnbränslehantering AB, Sweden.Search in Google Scholar
King, F., Hall, D.S., and Keech, P.G. (2017). Nature of the near-field environment in a deep geological repository and the implications for the corrosion behaviour of the container. Corros. Eng. Sci. Technol. 52: 25–30, https://doi.org/10.1080/1478422x.2017.1330736.Search in Google Scholar
Knutsson, L., Mattsson, E., and Ramberg, B.E. (1972). Erosion corrosion in copper water tubing. Br. Corros. J. 7: 208–211, https://doi.org/10.1179/000705972798322856.Search in Google Scholar
Korshin, G.V., Ferguson, J.F., and Lancaster, A.N. (2005). Influence of natural organic matter on the morphology of corroding lead surfaces and behavior of lead-containing particles. Water Res. 39: 811–818, https://doi.org/10.1016/j.watres.2004.12.009.Search in Google Scholar PubMed
Kristiansen, H. (1977). Corrosion of copper by water of various temperatures and carbon dioxide contents. Werkstoffe und Korrosion 28: 743–748, https://doi.org/10.1002/maco.19770281102.Search in Google Scholar
Kuźnicka, B. (2009). Erosion-corrosion of heat exchanger tubes. Eng. Fail. Anal. 16: 2382–2387, https://doi.org/10.1016/j.engfailanal.2009.03.026.Search in Google Scholar
Lachowicz, M.M. (2020). A metallographic case study of formicary corrosion in heat exchanger copper tubes. Eng. Fail. Anal. 111: 104502, https://doi.org/10.1016/j.engfailanal.2020.104502.Search in Google Scholar
Lapeire, L., Martinez Lombardia, E., De Graeve, I., Terryn, H., and Verbeken, K. (2017). Influence of grain size on the electrochemical behavior of pure copper. J. Mater. Sci. 52: 1501–1510, https://doi.org/10.1007/s10853-016-0445-z.Search in Google Scholar
Lattyak, R. (2007). Non-uniform copper corrosion in potable water: theory and practice, PhD thesis. Blacksburg, VA, Virginia Polytechnic Institute and State University.Search in Google Scholar
Latva, M., Kaunisto, T., and Pelto-Huikko, A. (2017). Durability of the non-dezincification resistant CuZn40Pb2 brass in Scandinavian waters. Eng. Fail. Anal. 74: 133–141, https://doi.org/10.1016/j.engfailanal.2017.01.011.Search in Google Scholar
Lavanya, M. (2021). A brief insight into microbial corrosion and its mitigation with eco-friendly inhibitors. J. Bio- Tribo-Corros. 7: 1–9, https://doi.org/10.1007/s40735-021-00563-y.Search in Google Scholar
Lee, S.H., Kim, J.G., and Koo, J.Y. (2010). Investigation of pitting corrosion of a copper tube in a heating system. Eng. Fail. Anal. 17: 1424–1435, https://doi.org/10.1016/j.engfailanal.2010.05.002.Search in Google Scholar
Leygraf, C., Chang, T., Herting, G., and Odnevall Wallinder, I. (2019). The origin and evolution of copper patina colour. Corros. Sci. 157: 337–346, https://doi.org/10.1016/j.corsci.2019.05.025.Search in Google Scholar
Li, S., Teague, M.T., Doll, G.L., Schindelholz, E.J., and Cong, H. (2018). Interfacial corrosion of copper in concentrated chloride solution and the formation of copper hydroxychloride. Corros. Sci. 141: 243–254, https://doi.org/10.1016/j.corsci.2018.06.037.Search in Google Scholar
Li, X., Lou, H.H., Xiao, J., and Huang, Y. (2008). Surface coating thickness distribution in electrodeposition: a case study on copper plating of a tube inner surface. Plat. Surf. Finish. 95: 39–47.Search in Google Scholar
Liu, G., Zhang, X., and Talley, J.W. (2007). Effect of copper(II) on natural organic matter removal during drinking water coagulation using aluminum-based coagulants. Water Environ. Res. 79: 593–599, https://doi.org/10.2175/106143006x136829.Search in Google Scholar PubMed
Lu, X., Liu, Y., wei, Z., Tao, H., Pan, C., and Wang, Z.Y (2021). Corrosion behavior of copper in extremely harsh marine atmosphere in Nansha Islands, China. Trans. Nonferrous Met. Soc. China (English Ed.) 31: 703–714.10.1016/S1003-6326(21)65531-0Search in Google Scholar
Lucey, V.F. (1967). Mechanism of pitting corrosion of copper in supply waters. Br. Corros. J. 2: 175–185, https://doi.org/10.1179/000705967798326731.Search in Google Scholar
Lyman, W.S. and Cohen, A. (1972). Service experience with copper plumbing tube. Mater Prot. Perform. 11: 48–53.Search in Google Scholar
Lytle, D.A. and Nadagouda, M.N. (2010). A comprehensive investigation of copper pitting corrosion in a drinking water distribution system. Corros. Sci. 52: 1927–1938, https://doi.org/10.1016/j.corsci.2010.02.013.Search in Google Scholar
Lytle, D.A. and Schock, M.R. (2008). Pitting corrosion of copper in waters with high pH and low alkalinity. Am. Water Work. Assoc. 100: 115–129, https://doi.org/10.1002/j.1551-8833.2008.tb09586.x.Search in Google Scholar
Lytle, D.A. and White, C.P. (2014). The effect of phosphate on the properties of copper drinking water pipes experiencing localized corrosion. J. Fail. Anal. Prev. 14: 203–219, https://doi.org/10.1007/s11668-014-9786-6.Search in Google Scholar
Lytle, D.A., Payne, J., and Feldhaus, J. (2005). Copper pitting corrosion and pinhole leaks: a case study, June 13-17, 2005: Am. Water Work. Assoc. Annu. Conf. United States Environmental Protection Agency, San Francisco, CA.Search in Google Scholar
Ma, Y., Tian, X., Yin, J., Chen, J., and Jiang, J. (2019). The pitting corrosion behavior of copper with different grain size. Int. J. Electrochem. Sci. 14: 4047–4056, https://doi.org/10.20964/2019.05.11.Search in Google Scholar
Mahmood, M.H. and Suryanto and Al Hazza, M.H.F. (2017). The effects of water flow rate on copper corrosion. Key Eng. Mater. 748: 235–239, https://doi.org/10.4028/www.scientific.net/kem.748.235.Search in Google Scholar
Marshall, B. and Edwards, M. (2005). Copper pinhole leak development in the presence of AL(OH)3 and chlorine. AWWA 124th Annu. Conf. Expo. World’s Water Event, ACE, San Francisco, CA.Search in Google Scholar
Marshall, B.J. (2004). Initiation, propagation, and mitigation of aluminum and chlorine induced pitting corrosion, PhD thesis. Blacksburg, VA, Virginia Polytechnic Institute and State University.Search in Google Scholar
Martić, I., Maslarević, A., Mladenović, S., Lukić, U., and Budimir, S. (2015). Water deoxygenation using hollow fiber membrane module with nitrogen as inert gas. Desalin. Water Treat. 54: 1563–1567, https://doi.org/10.1080/19443994.2014.888677.Search in Google Scholar
Martinez-Lombardia, E., Gonzalez-Garcia, Y., Lapeire, L., De Graeve, I., Verbeken, K., Kestens, L., Mol, J.M.C., and Terryn, H. (2014). Scanning electrochemical microscopy to study the effect of crystallographic orientation on the electrochemical activity of pure copper. Electrochim. Acta 116: 89–96, https://doi.org/10.1016/j.electacta.2013.11.048.Search in Google Scholar
Martinović, I., Zlatić, G., Pilić, Z., Šušić, L., Kowalska, O., Petrović, D., Falak, F., and Mišković, J. (2019). Self assembled monolayers of alkanethiol as inhibitors against copper corrosion in synthetic acid rain. Int. J. Electrochem. Sci. 14: 4206–4215, https://doi.org/10.20964/2019.05.28.Search in Google Scholar
Mattsson, E. (1980). Corrosion of copper and brass: practical experience in relation to basic data. Br. Corros. J. 15: 6–13, https://doi.org/10.1179/000705980798318708.Search in Google Scholar
Metikoš-Huković, M., Babić, R., Škugor, I., and Grubač, Z. (2011). Copper-nickel alloys modified with thin surface films: corrosion behaviour in the presence of chloride ions. Corros. Sci. 53: 347–352, https://doi.org/10.1016/j.corsci.2010.09.041.Search in Google Scholar
Montes, J.C., Hamdani, F., Creus, J., Touzain, S., and Correc, O. (2014). Impact of chlorinated disinfection on copper corrosion in hot water systems. Appl. Surf. Sci. 314: 686–696, https://doi.org/10.1016/j.apsusc.2014.07.069.Search in Google Scholar
Murakami, M., Sugita, K., Yabuki, A., and Matsumura, M. (2003). Mechanism of so-called erosion-corrosion and flow velocity difference corrosion of pure copper. Zair. to Kankyo/ Corros. Eng. 52: 155–159, https://doi.org/10.3323/jcorr1991.52.155.Search in Google Scholar
Myers, J. and Cohen, A. (2005). Copper-tube corrosion in domestic water systems. HPAC Eng. 77: 22–31.Search in Google Scholar
Myers, J. and Obrecht, M. (1972). Potable water systems: recognition of cause vital to minimizing corrosion. Mater Prot. Perform 11: 41–46.Search in Google Scholar
Naseer, A. and Khan, A.Y. (2009). A study of growth and breakdown of passive film on copper surface by electrochemical impedance spectroscopy. Turkish J. Chem. 33: 739–750.10.3906/kim-0708-23Search in Google Scholar
Ngaotrakanwiwat, P., Heawphet, P., and Rangsunvigit, P. (2020). Enhancement of photoelectrochemical cathodic protection of copper in marine condition by Cu-doped TiO2. Catalysts 10: 146, https://doi.org/10.3390/catal10020146.Search in Google Scholar
Nishikata, A., Itagaki, M., Tsuru, T., and Haruyama, S. (1990). Passivation and its stability on copper in alkaline solutions containing carbonate and chloride ions. Corros. Sci. 31: 287–292, https://doi.org/10.1016/0010-938x(90)90121-k.Search in Google Scholar
Obrecht, M.F. and Quill, L.L. (1960). How temperature, treatment, and velocity of potable water affect corrosion of copper and its alloys: monitoring system reveals effects of different operating conditions. Heating, Piping, Air Cond. 32: 131–137.Search in Google Scholar
Otmacic Curkovic, H., Stupnisek-Lisac, E., and Takenouti, H. (2010). The influence of pH value on the efficiency of imidazole based corrosion inhibitors of copper. Corros. Sci. 52: 398–405, https://doi.org/10.1016/j.corsci.2009.09.026.Search in Google Scholar
Pasha, A., Khasim, S., Darwish, A.A.A., Hamdalla, T.A., and Al-Ghamdi, S.A. (2022). High performance organic coatings of polypyrrole embedded with manganese iron oxide nanoparticles for corrosion protection of conductive copper surface. J. Inorg. Organomet. Polym. Mater. 32: 499–512, https://doi.org/10.1007/s10904-021-02130-x.Search in Google Scholar
Patel, K.K. and Vashi, R.T. (2016). Fruit extract as a green inhibitor for copper corrosion in nitric acid solution. J. Corros. Sci. Eng. 19: 4545–4553.Search in Google Scholar
Pavissich, J.P., Vargas, I.T., González, B., Pastén, P.A., and Pizarro, G.E. (2010). Culture dependent and independent analyses of bacterial communities involved in copper plumbing corrosion. J. Appl. Microbiol. 109: 771–782, https://doi.org/10.1111/j.1365-2672.2010.04704.x.Search in Google Scholar PubMed
Pedeferri, P. (2018). Uniform corrosion in acidic and aerated solutions. In: Corrosion science and engineering. Springer International Publishing, Switzerland, pp. 145–167.10.1007/978-3-319-97625-9_8Search in Google Scholar
Pehkonen, S.O., Palit, A., and Zhang, X. (2002). Effect of specific water quality parameters on copper corrosion. Corrosion 58: 156–165, https://doi.org/10.5006/1.3277316.Search in Google Scholar
Peltola, H. and Lindgren, M. (2015). Failure analysis of a copper tube in a finned heat exchanger. Eng. Fail. Anal. 51: 83–97, https://doi.org/10.1016/j.engfailanal.2015.02.016.Search in Google Scholar
Pomberger, A., Jose, N., Walz, D., Meissner, J., Holze, C., Kopczynski, M., Müller-Bischof, P., and Lapkin, A.A. (2023). Automated pH adjustment driven by robotic workflows and active machine learning. Chem. Eng. J. 451: 139099, https://doi.org/10.1016/j.cej.2022.139099.Search in Google Scholar
Rahman, M.M. and Ahmed, S.R. (2020). Corrosion behavior of copper based heat exchanger tube in waters of Bangladesh region at varied temperature and flow velocity. MIST Int. J. Sci. Technol. 8: 15–23, https://doi.org/10.47981/j.mijst.08(02)2020.196(15-23).Search in Google Scholar
Ranjbar, K. (2010). Effect of flow induced corrosion and erosion on failure of a tubular heat exchanger. Mater. Des. 31: 613–619, https://doi.org/10.1016/j.matdes.2009.06.025.Search in Google Scholar
Reiber, S. (2013). Corrosion assessment of copper tubing from residences in the Cobb County − Marietta water authority (CCMWA) service area. Technical Memorandum. Bellevue, Washington.Search in Google Scholar
Rizvi, M., Gerengi, H., Kaya, S., Uygur, I., Yıldız, M., Sarıoglu, I., Cingiz, Z., Mielniczek, M., and El Ibrahimi, B. (2021). Sodium nitrite as a corrosion inhibitor of copper in simulated cooling water. Sci. Rep. 11: 8353, https://doi.org/10.1038/s41598-021-87858-9.Search in Google Scholar PubMed PubMed Central
Roy, S., Coyne, J.M., Novak, J.A., and Edwards, M.A. (2018). Flow-induced failure mechanisms of copper pipe in potable water systems. Corros. Rev. 36: 449–481, https://doi.org/10.1515/corrrev-2017-0120.Search in Google Scholar
Rushing, J.C. and Edwards, M. (2004). The role of temperature gradients in residential copper pipe corrosion. Corros. Sci. 46: 1883–1894, https://doi.org/10.1016/j.corsci.2003.11.001.Search in Google Scholar
Sakai, M. (2022). A new type of silica-induced ‘moundless’ pitting corrosion in copper observed in Japan. Heliyon 8: e10110, https://doi.org/10.1016/j.heliyon.2022.e10110.Search in Google Scholar PubMed PubMed Central
Sakamoto, A., Yamasaki, T., and Matsumura, M. (1995). Erosion-corrosion tests on copper alloys for water tap use. Wear 186–187: 548–554, https://doi.org/10.1016/0043-1648(95)07124-5.Search in Google Scholar
Sarver, E. and Edwards, M. (2012). Inhibition of copper pitting corrosion in aggressive potable waters. Int. J. Corros. 2012: 857823, https://doi.org/10.1155/2012/857823.Search in Google Scholar
Sato, S., Minamoto, T., Seki, K., Hiroshi, Y., Takizawa, Y., Okada, S., Yamauchi, S., Hisamatsu, Y., Suzuki, I., Fujii, T., et al.. (1982). Case studies on pitting corrosion failures of copper tubes in hot water. Boshoku gijutsu 31: 3–11, https://doi.org/10.3323/jcorr1974.31.1_3.Search in Google Scholar
Savita, Mourya, P., Chaubey, N., Singh, V.K., and Singh, M.M. (2016). Eco-friendly inhibitors for copper corrosion in nitric acid: experimental and theoretical evaluation. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 47: 47–57, https://doi.org/10.1007/s11663-015-0488-6.Search in Google Scholar
Scardina, Paolo, Edward, E., Darrell, B.J., G V, L., and Sharon, D.K. (2008). Assessment of non-uniform corrosion in copper piping. Technical Report AwwaRF 3015. Awwa Research Foundation, Washington D.C.Search in Google Scholar
Schindelholz, E.J., Cong, H., Jove-Colon, C.F., Li, S., Ohlhausen, J.A., and Moffat, H.K. (2018). Electrochemical aspects of copper atmospheric corrosion in the presence of sodium chloride. Electrochim. Acta 276: 194–206, https://doi.org/10.1016/j.electacta.2018.04.184.Search in Google Scholar
Schleich, W. (2004) Typical failures of cuni 90/10 seawater tubing systems and how to avoid them. In: EUROCORR 2004: long term prediction & modelling of corrosion, September 12–16, 2004, Copper Development Association Inc., Nice, France.Search in Google Scholar
Schmitt, G., Slavcheva, E., and Plagemann, P. (2001). ECN-measurements at copper in artificial tap water: investigation of anion-effects. Werkstoffe und Korrosion 52: 439–444, https://doi.org/10.1002/1521-4176(200106)52:6<439::aid-maco439>3.0.co;2-4.10.1002/1521-4176(200106)52:6<439::AID-MACO439>3.0.CO;2-4Search in Google Scholar
Schmitt, H.G. and Bakalli, M. (2010). Flow assisted corrosion. Shreir’s Corros. 2: 954–987, https://doi.org/10.1016/b978-044452787-5.00039-1.Search in Google Scholar
Schock, M.R., Lytle, D.A., and Clement, J.A. (1995). Effect of pH, DIC, orthophosphate and sulfate on drinking water cuprosolvency. Technical Report EPA/600/R-95/085, United States Environmental Protection Agency, Cincinnati, Ohio 45268.Search in Google Scholar
Seth, A.D. and Edyvean, R.G.J. (2006). The function of sulfate-reducing bacteria in corrosion of potable water mains. Int. Biodeterior. Biodegrad. 58: 108–111, https://doi.org/10.1016/j.ibiod.2006.10.005.Search in Google Scholar
Shalaby, H.M., Al-Kharafi, F.M., and Gouda, V.K. (1989). Morphological study of pitting corrosion of copper in soft tap water. Corrosion 45: 536–547, https://doi.org/10.5006/1.3577869.Search in Google Scholar
Singer, F., Deisenroth, D.C., Hymas, D.M., and Ohadi, M.M. (2017) Additively manufactured copper components and composite structures for thermal management applications.In: Proceedings of the 16th Intersociety Conference on thermal and thermomechanical phenomena in electronic systems (ITHERM), May 30−June 02, 2017, IEEE, Orlando, FL, USA.10.1109/ITHERM.2017.7992469Search in Google Scholar
Skrypnikova, E.A., Kaluzhina, S.A., and Agafonova, L.E. (2014). Inhibition of copper pitting corrosion in alkaline sulphate media by benzotriazole at elevated temperatures. Int. J. Corros. Scale Inhib. 3: 059–065, https://doi.org/10.17675/2305-6894-2014-3-1-059-065.Search in Google Scholar
Standards France (2016). Copper and copper alloys: seamless, round tubes for air conditioning and refrigeration. Part 2: tubes for equipment (EN 12735-2:2016).Search in Google Scholar
Sun, W., Wang, N., Li, J., Xu, S., Song, L., Liu, Y., and Wang, D. (2021). Humidity-resistant triboelectric nanogenerator and its applications in wind energy harvesting and self-powered cathodic protection. Electrochim. Acta 391: 138994, https://doi.org/10.1016/j.electacta.2021.138994.Search in Google Scholar
Suzuki, S., Yamada, Y., Kawano, K., and Atsumi, T. (2005). Pitting corrosion and its prevention of copper heat exchanger tubes for water heater. Zair. to Kankyo/Corros. Eng. 54: 20–24, https://doi.org/10.3323/jcorr1991.54.20.Search in Google Scholar
Syrett, B.C. (1976). Erosion-corrosion of copper-nickel alloys in sea water and other aqueous environments - a literature review. Corrosion 32: 242–252, https://doi.org/10.5006/0010-9312-32.6.242.Search in Google Scholar
Tamhane, D., Thalapil, J., Banerjee, S., and Tallur, S. (2021). Smart cathodic protection system for real-time quantitative assessment of corrosion of sacrificial anode based on electro-mechanical impedance (EMI). IEEE Access 9: 12230–12240, https://doi.org/10.1109/access.2021.3051953.Search in Google Scholar
Tan, Z., Yao, Y., Yao, C., Yang, J., Ruan, Y., and Wang, Q. (2018). A new phase transition heat exchanger for gas water heaters. Inventions 3: 3020037, https://doi.org/10.3390/inventions3020037.Search in Google Scholar
Tasić, Ž.Z., Petrović Mihajlović, M.B., Radovanović, M.B., and Antonijević, M.M. (2019). New trends in corrosion protection of copper. Chem. Pap. 73: 2103–2132, https://doi.org/10.1007/s11696-019-00774-1.Search in Google Scholar
Touzé, E. and Cougnon, C. (2018). Study of the air-formed oxide layer at the copper surface and its impact on the copper corrosion in an aggressive chloride medium. Electrochim. Acta 262: 206–213, https://doi.org/10.1016/j.electacta.2017.12.187.Search in Google Scholar
Vargas, I.T., Fischer, D.A., Alsina, M.A., Pavissich, J.P., Pablo, P., and Pizarro, G.E. (2017). Copper corrosion and biocorrosion events in premise plumbing. Materials 10: 1036, https://doi.org/10.3390/ma10091036.Search in Google Scholar PubMed PubMed Central
Vashi, R.T., Contractor, K.S., and Prajapati, N.I. (2021). Green corrosion inhibitors for copper in HNO3 and H3PO4 solution: a review. Int. J. Res. Appl. Sci. Eng. Technol. 10: 8404–8416.Search in Google Scholar
Vaštag, D., Apostolov, S., Hadžistević, M., and Sekulić, M. (2013). The possibility of copper corrosion protection in acidic media using a thiazole derivative. Mater. Tehnol. 47: 329–333.Search in Google Scholar
Viciano-Tudela, S., Parra, L., Sendra, S., and Lloret, J. (2023). A low-cost virtual sensor for underwater pH monitoring in coastal waters. Chemosensors 11: 11040215, https://doi.org/10.3390/chemosensors11040215.Search in Google Scholar
Vincente, G. (1996). Corrosão. 3rd editio. LTC-Livros Tecnicos e Cientificos, Editoria S.A.Search in Google Scholar
Wan, S., Miao, C.H., Wang, R.M., Zhang, Z.F., and Dong, Z.H. (2019). Enhanced corrosion resistance of copper by synergetic effects of silica and BTA codoped in polypyrrole film. Prog. Org. Coatings 129: 187–198, https://doi.org/10.1016/j.porgcoat.2019.01.014.Search in Google Scholar
Winston, R.R. (2011). Corrosion handbook. John Wiley & Sons, Inc, New Jersey.Search in Google Scholar
Winston, R.R. and Uhlig, H.H. (2008). Corrosion and corrosion control: an introduction to corrosion science and engineering. John Wiley Sons, INC., New Jersey.Search in Google Scholar
Wu, L., Ma, A., Zhang, L., and Zheng, Y. (2022). Intergranular erosion corrosion of pure copper tube in flowing NaCl solution. Corros. Sci. 201: 110304, https://doi.org/10.1016/j.corsci.2022.110304.Search in Google Scholar
Xiong, S., Si, J., Sun, J., Wu, H., Dong, H., and Zhang, C. (2020). Experimental and computational studies on heterocyclic organic compounds as corrosion inhibitors for copper immersed in O/W emulsion medium. Anti-Corros. Methods Mater. 67: 214–227, https://doi.org/10.1108/acmm-10-2019-2200.Search in Google Scholar
Xu, W., Hu, Y., Bao, W., Xie, X., Liu, Y., Song, A., and Hao, J. (2017). Superhydrophobic copper surfaces fabricated by fatty acid soaps in aqueous solution for excellent corrosion resistance. Appl. Surf. Sci. 399: 491–498, https://doi.org/10.1016/j.apsusc.2016.12.099.Search in Google Scholar
Xuejun, X., Zhang, Y., Wang, R., Zhang, Yu, and Ruan, M. (2018). Research on the effect of the pH value on corrosion and protection of copper in desalted water. Anti-Corros. Methods Mater. 65: 528–537, https://doi.org/10.1108/acmm-12-2016-1739.Search in Google Scholar
Yan, X., Sun, J., and Meng, Y. (2018). Experimental insight into the chemical corrosion mechanism of copper with an oil-in-water emulsion solution. RSC Adv. 8: 9833–9840, https://doi.org/10.1039/c8ra00432c.Search in Google Scholar PubMed PubMed Central
Yang, G.L., Ouyang, Y., Xie, Z.H., Liu, Y., Dai, W., and Wu, L. (2021a). Nickel interlayer enables indirect corrosion protection of magnesium alloy by photoelectrochemical cathodic protection. Appl. Surf. Sci. 558: 149840, https://doi.org/10.1016/j.apsusc.2021.149840.Search in Google Scholar
Yang, W., Liu, Z., and Huang, H. (2021b). Galvanic corrosion behavior between AZ91D magnesium alloy and copper in distilled water. Corros. Sci. 188: 109562, https://doi.org/10.1016/j.corsci.2021.109562.Search in Google Scholar
Zanotto, F., Grassi, V., Balbo, A., Monticelli, C., Melandri, C., and Zucchi, F. (2018). Effect of brief thermal aging on stress corrosion cracking susceptibility of LDSS 2101 in the presence of chloride and thiosulphate ions. Corros. Sci. 130: 22–30, https://doi.org/10.1016/j.corsci.2017.10.024.Search in Google Scholar
Zhang, H., Lin, Y.S., Zhang, K.L., Wei, Z.G., and Wang, W.W. (2013). Corrosion analysis of copper tubes in the exchangers with the assistance of atom absorption spectroscopy. Appl. Mech. Mater. 378: 275–279, https://doi.org/10.4028/www.scientific.net/amm.378.275.Search in Google Scholar
Zhang, Y., Wang, S., Zhang, W., Ding, Y., Cheng, M., Mu, L., and Zhao, X. (2022). Investigation of the condensation heat-transfer between the wet air and 3-D finned-tube heat exchanger surface with different anti-corrosion coatings. Exp. Heat Transf. 35: 399–418, https://doi.org/10.1080/08916152.2021.1877371.Search in Google Scholar
Zhao, P., Zhang, G., Sun, Y., and Xu, Y. (2017). A review of oxygen removal from oxygen-bearing coal-mine methane. Environ. Sci. Pollut. Res. 24: 15240–15253, https://doi.org/10.1007/s11356-017-8916-6.Search in Google Scholar PubMed
Zlatanović, L., van der Hoek, J.P., and Vreeburg, J.H.G. (2017). An experimental study on the influence of water stagnation and temperature change on water quality in a full-scale domestic drinking water system. Water Res. 123: 761–772, https://doi.org/10.1016/j.watres.2017.07.019.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Biomacromolecules as green corrosion inhibitors: a review based on mild steel corrosion in acidic media
- Copper corrosion mechanisms, influencing factors, and mitigation strategies for water circuits of heat exchangers: critical review and current advances
- Original Articles
- Influence of corrosion inhibitors on aging mechanism of epoxy resin coatings for copper 62 alloy in simulated marine environment
- High tribo-corrosion resistance of Ni-Cr-5Al2O3 thermal spray coating: a comparison of post processing techniques
- Understanding the role of S and Cl degrading the corrosion resistance of 12Cr1MoVG steel in a waste incineration boiler: on-site and laboratory testing
Articles in the same Issue
- Frontmatter
- Reviews
- Biomacromolecules as green corrosion inhibitors: a review based on mild steel corrosion in acidic media
- Copper corrosion mechanisms, influencing factors, and mitigation strategies for water circuits of heat exchangers: critical review and current advances
- Original Articles
- Influence of corrosion inhibitors on aging mechanism of epoxy resin coatings for copper 62 alloy in simulated marine environment
- High tribo-corrosion resistance of Ni-Cr-5Al2O3 thermal spray coating: a comparison of post processing techniques
- Understanding the role of S and Cl degrading the corrosion resistance of 12Cr1MoVG steel in a waste incineration boiler: on-site and laboratory testing

