Abstract
Graphene has become an emerging and promising option in the field of protection coating for anti-corrosion due to its specific properties in chemical inertia and physical impermeability. It can be applied to metal protection coating in forms of either atomically thin films or composite materials, known, respectively, as pure chemical vapour deposition (CVD) graphene coatings and graphene composite coatings (GCCs). Nonetheless, various structure defects, synthesis imperfections and graphene’s positive potential to metals would make graphene-based protective coatings tend to exhibit corrosion promotion by arousing micro-galvanic corrosion, largely undermining its anti-corrosion efficiency. Based on this, many optimization strategies and methods have been conceived and applied to the graphene-based protection coatings in these two aspects for improving its anti-corrosion efficiency. For example, a good dispersion and orderly arrangement of graphene derivatives in the GCCs can largely optimize its anti-corrosion performance. Here, this paper separately reviews detailed optimization strategies, corresponding mechanisms and key factors for the use of representative graphene-based materials in these two aspects, with the aim of providing comprehensive knowledge and a roadmap of developing cheap, powerful and effective barrier technologies. Finally, perspectives on opportunities and challenges in improving the barrier coating efficiency of graphene-based materials are discussed.
1 Introduction
Most metals and alloys are subjected to spontaneous corrosion initiated by water and oxygen in the atmosphere due to their thermodynamic instability. The rate at which corrosion occurs depends on multi factors such as the type of metal, the chemical environment, temperature, mechanical forces and so forth. According to a survey (Chang et al. 2012), the corrosion of metals has caused significant financial and material damage, and almost a quarter of the world’s annual steel production is damaged by corrosion. In addition to steel, several other materials, including polymers and ceramics, are not immune to corrosion (Landolt 2007). Corrosion would degrade the good properties typical of the surfaces of some reactive metals commonly used in the industry. So, corrosion resistance is decisive factor in the longevity of metal materials. It becomes imperative that adequate defensive measures are taken to effectively control the rate of corrosion so that the metals can achieve their full effectiveness throughout their lifetime.
The protective coating is commonly used as a barrier to isolate the underlying metal from the outside environment, thus reducing the risk of corrosion to the metal, which is an effective and economical method of corrosion protection. Various protective coatings including inert metals (Pushpavanam et al. 1981), organic polymers (Stratmann et al. 1994), conductive polymers (Redondo and Breslin 2007), silanes (Zhu and van Ooij 2004), oxide layers (Mittal et al. 2009) and even thiol-based monolayers (Lusk and Jennings 2001) have been applied as metal protection coatings. Among them, the ability of forming a barrier between the metal and the outside environment is the key to the corrosion resistance of these coatings, and any flaking or cracking of the coating will affect its corrosion resistance (Sai Pavan and Ramanan 2016). In addition, there are a number of factors that would limit their anti-corrosion efficiency. For example, many protective coatings would change the electrical, thermal and optical properties of the substrate material due to their non-negligible thickness. Therefore, the ideal anti-corrosion surface barrier coating should be lightweight, thin, impermeable, chemically inert and abrasion resistant. Therefore, developing thin protective materials with minimal impact on the substrate material is highly demanded (Prasai et al. 2012).
1.1 Barrier properties of graphene
Graphene is assembled by arrangement of sp 2-hybridized carbon atoms, showing uniquely potential in corrosion protection because it can protect metals without altering their inherent properties in a way that is not possible with three-dimensional protective coatings, polymers or oxides. It has been demonstrated that graphene is the thinnest material and that a single layer of graphene can keep an atomic distance between a metal surface and its external environment (Geim and Novoselov 2007; Novoselov et al. 2005). In addition, graphene possesses many distinguished advantages in terms of mechanical toughness, physical impermeability, chemical inertness and optical transparency, which allow graphene to be taken for granted as the most promising alternative for the protective coating. Figure 1a shows graphene’s structure with a very dense grid of hexagonal carbon atoms, and this remarkable two-dimensional physical structure shows exceptional resistance to gases, liquids, salts and acids (Berry 2013). The graphene lattice has a hexagonal pore size of 0.246 nm and a C–C bond length of 0.14 nm (Gass et al. 2008). Taking into account the van der Waals radius of the carbon atom, the pore size of the graphene lattice is reduced to 0.064 nm, through which even an atom as small as helium cannot pass (Bunch et al. 2008). In addition, graphene possesses a delocalized dense electron cloud of π-conjugated carbon networks (Sreeprasad and Berry 2013), which not only blocks voids within the aromatic rings but also provides a repulsive field to reactive atoms or molecules, allowing physical isolation between the metal surface and the environment. The energetics and energy barriers involved in the transfer of oxygen atom and H2O molecule from one side of graphene to the other were calculated in a bare graphene super cell (Figure 1b–c) (Seethamraju et al. 2016; Topsakal et al. 2012). If an oxygen atom traverses a fixed vertical path along the hole in the centre of the graphene hexagonal lattice from the top to the bottom of the graphene, a barrier of 16.34 eV needs to be overcome. This prevents the oxygen atom from passing through. For the H2O molecule, Figure 1c plots the potential energy barrier as a function of distance for water molecules passing through the hexagonal centre of a graphene film. Density functional theory (DFT) calculations show that the energy barrier encountered by a single water molecule passing through a graphene layer with 170 carbon atoms is 42.8 eV. Such a high energy barrier effectively limits the penetration of water molecules. All of the above simulations and calculations show that the resistance of graphene to permeation is attributed to the high potential energy barrier encountered in penetrating the film. This excellent resistance to permeation also has been proved experimentally through a graphene-sealed microchamber (Figure 1d). By applying a pressure difference (∆P = P int−P ext) across the graphene membrane in this way (Figure 1e), the mass transport of graphene can be measured at either ∆P > 0 (Figure 1f) or ∆P < 0 cases (Figure 1g). Figure 1h shows the results of experiments performed when the thickness of the graphene film varies and when different gases are present in the micro-chamber. As seen, helium leaks two orders of magnitude faster than air and argon. However, this leakage rate is not related to the thickness of the graphene films, indicating that the gas leakage is relatively stable and may be caused by the micro-chamber sealed interface between graphene and silica, instead of graphene film itself. This leads to the conclusion that the graphene film is a perfect barrier for gas permeability. However, it has recently been shown that the nanoscale non-flatness of two-dimensional membranes greatly facilitates proton transport (Wahab et al. 2023). The spatial distribution of proton currents visualized by scanning electrochemical cell microscopy (SECCM) reveals significant inhomogeneities correlated with nanoscale wrinkles and other features where strain is accumulated. From Figure 1i–l, it can be seen that the positions of the wrinkles in the graphene devices correlate with some of the most conductive regions in the SECCM maps. Other regions of high proton conductivity appear around the apertures’ rims. Even though, the graphene film is likely to be the ‘thinnest known anti-corrosion coating’ (Ambrosi and Pumera 2015; Prasai et al. 2012).
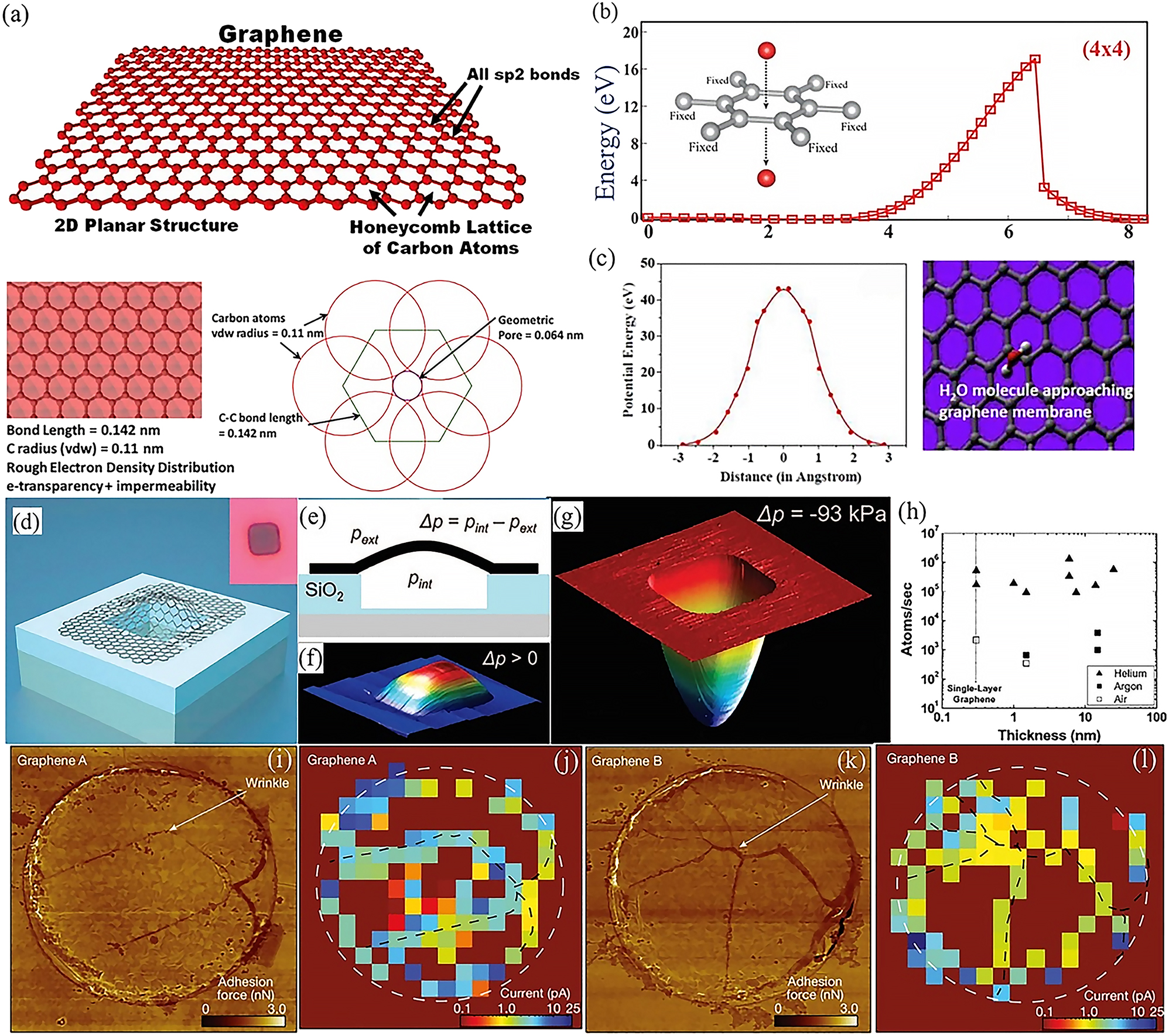
The structrue and barrier properties of graphene. (a) Graphene lattice structure: sp 2 hybridized carbon atoms arranged in a two-dimensional honeycomb lattice. Bottom: molecular structure with a rough electron density distribution (Berry 2013). Reprinted with permission from Elsevier. (b) Energy barrier for an oxygen atom to pass from the top to the bottom of a suspended graphene (Topsakal et al. 2012). Reprinted with permission from American Physical Society. (c) Simulations to determine the potential energy barrier required to cross a graphene membrane: potential energy barrier versus distance for H2O molecules crossing a monolayer of graphene (left); snapshots of DFT simulations when calculating the potential energy barrier for H2O molecules crossing a graphene membrane (right) (Seethamraju et al. 2016). Reprinted with permission from American Chemistry Society. (d) Schematic diagram of graphene sealed microchamber. Inset: optical image of a single atomic layer of graphene drumhead on silica at 440 nm. The dimensions of the microchamber are 4.75 µm × 4.75 µm × 380 nm. (e) Schematic side view of a graphene-sealed microchamber. (f) Tapping mode atomic force microscopy (AFM) image of a 9 nm thick multi-layer graphene drumhead with ∆P > 0. The square microchamber has dimensions of 4.75 µm × 4.75 µm. The upward offset of the film centre is z = 90 nm. (g) AFM image of the graphene sealed microchamber of Figure 1d with ∆P = −93 kPa across it. (h) Gas leakage rate versus thickness of graphene film for helium (▲), argon (■) and air (□) (Bunch et al. 2008). Reprinted with permission from American Chemical Society. (i, k) AFM force maps of graphene for the proton transport detection. (j, l) SECCM maps for two graphene devices. The white dashed circles mark the rim of the 2-μm diameter apertures in SiN x (Wahab et al. 2023). Reprinted with permission from Nature.
1.2 Graphene-based anti-corrosion coatings and their optimization
Despite these exceptional highlights of features, the application of graphene as a corrosion barrier coating is not as simple as it seems (Camilli et al. 2019). Firstly, the corrosion resistance of graphene coating highly depends on whether it can prevent the vertical penetration of oxidative species. In principle, as discussed above, continuous and perfect graphene layer would isolate metal surface from any oxidative species. It is thus critical to make prefect graphene coating with high quality and deposit it onto metal surface somehow in a compatible way. Secondly, the negative polarity of graphene to metals would induce galvanic corrosion of metals over time (Schriver et al. 2013; Zhou et al. 2013), which is the notably named corrosion-promotion activity of graphene (Dong et al. 2014; Keil et al. 2010; Lee and Berman 2018). Based on these points, as well as the experimental facts that graphene-based protective coatings are not as effective as we anticipate, numerous efforts have been devoted to improve its protective efficiency for anti-corrosion of metals in recent years. In short, research on graphene-based barrier coating for anti-corrosion applications is currently divided into two types of strategy, that is, using pure graphene anti-corrosive barrier coating (which is usually correlated with the CVD production process) and using GCC (which incorporates graphene into the matrix of a polymer coating). In a sense, GCC is an extended application of pure graphene coating, which gets the research of graphene-based coating started in the field of metal coatings. Therefore, GCC has more advantages in the perspective of mass production in a low-coast manner than pure graphene coating on metals. The differences in the comparison between them are listed in Figure 2. Whether it is pure CVD graphene coating or GCC, great progresses have been achieved among them on optimization strategies for improving their anti-corrosive efficiency of metals by numerous excellent studies. Although a few reviews on various graphene production, anti-corrosion mechanisms and its typical applications for metal protection (Chen et al. 2011; Dlubak et al. 2012; Krishnamurthy et al. 2013; Mohanty et al. 2011), the systematic introduction and summary for the optimization strategies of efficient graphene-based coatings based on their anti-corrosion issues is still lacking in the literatures.
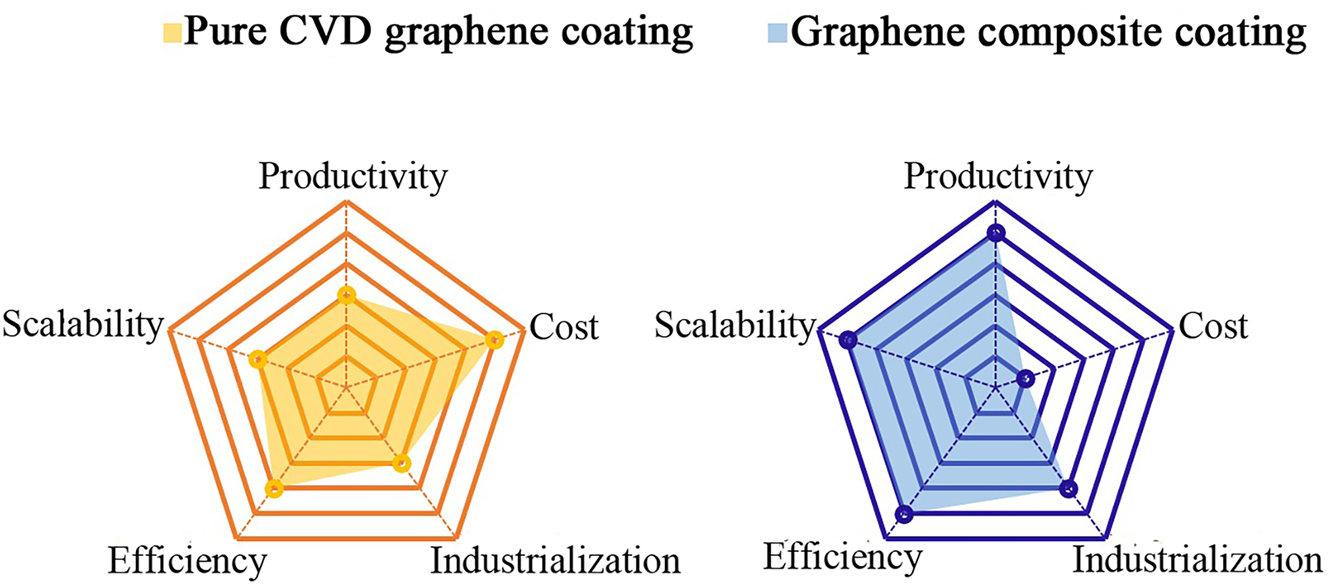
Comparison between pure CVD graphene coating and GCC from the perspective of anti-corrosion efficiency, the industrialization, the production cost, the productivity and the scalability of coating. (a) Pure CVD graphene coating. (b) GCC.
Herein, we carefully review various optimization strategies involving preparation methods, as well as the corresponding optimization mechanisms for graphene-based anti-corrosion barrier coatings in both cases of pure CVD graphene coatings and GCCs (Figure 3). As seen from Figure 3, for the pure CVD graphene coating, optimization strategies including post-healing of defects, CVD optimization and conductivity modification are proposed based on the analysis of the issues that a pure graphene coating cannot effectively provide the long-term protection of metals. For graphene composites coating, optimization strategies involving functionalization of graphene, polymer-hybrids and cathodic protection are included. Many detailed factors, features and drawbacks are analysed between each other to disclose the influence in anti-corrosion properties of graphene-based coatings. Finally, we provide an outlook for the opportunities and challenges in improving the corrosion barrier of graphene-based coatings, aiming to seek a roadmap for developing cheap, powerful and effective graphene-based corrosion barrier technologies.
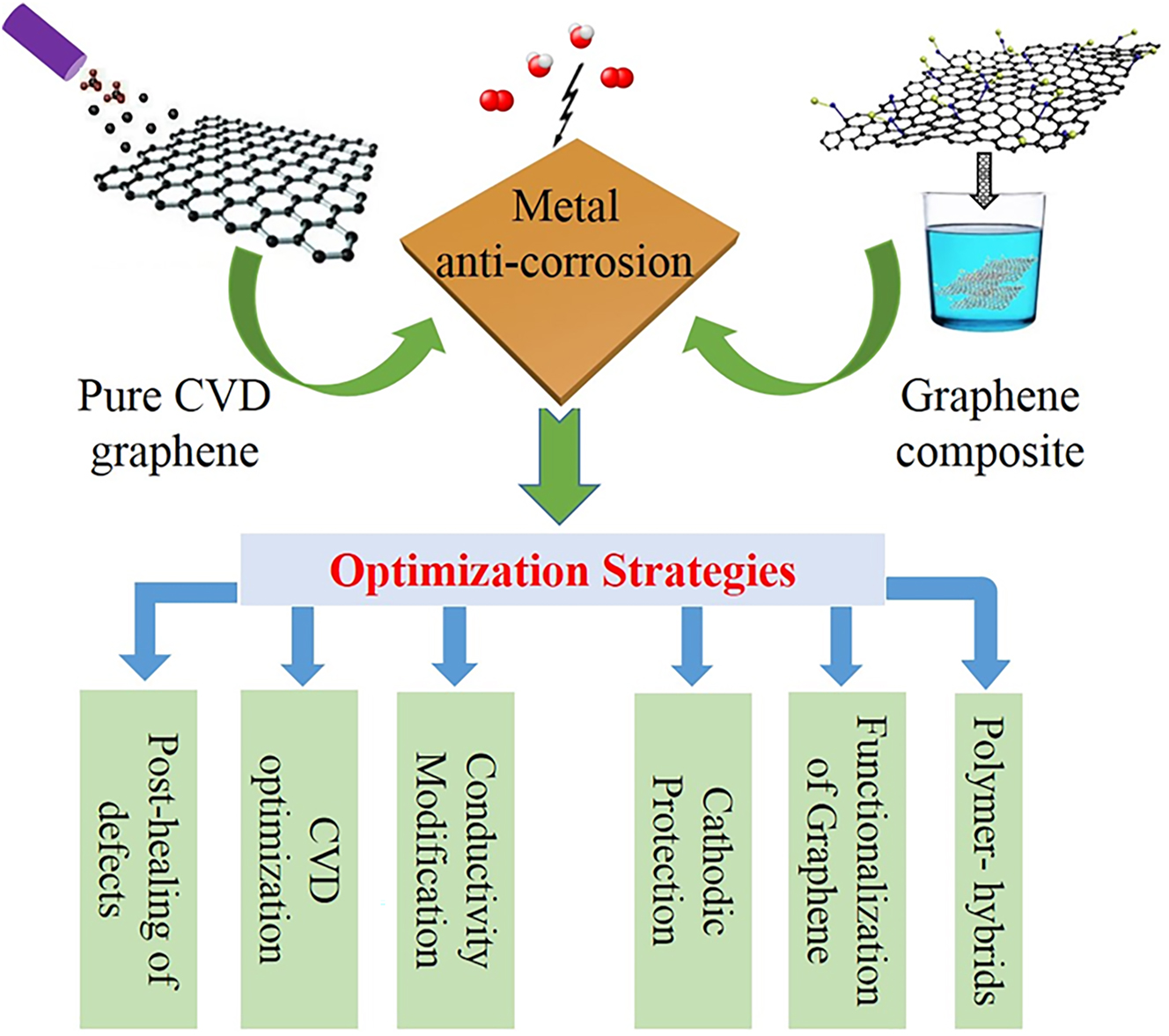
Representative optimization strategies for graphene-based protection coatings in the cases of pure CVD graphene coatings and graphene composites coatings.
2 Pure CVD graphene coatings and the optimization strategies
As discussed above, pure graphene films, which are normally prepared by CVD method for the large-scale coverage, stand out from the crowds of protective coatings due to their two main advantages of impermeability and chemical stability and are expected to be a favourable material for a wide range of research areas from biology to electronics. As a protective barrier, CVD graphene grown on metal substrates blocks mass transfer between the environment and the metal, thus protecting it from corrosive degradation. Moreover, the experiment demonstrates that graphene as a passivation layer can retard microbially induced electrochemical corrosion (MIC) of metals (Krishnamurthy et al. 2013). In addition, it can provide protection for unstable surfaces in fields as diverse as spintronics (Dlubak et al. 2012) and biology (Mohanty et al. 2011). However, with the development of CVD graphene, there are conflicting opinions about the protective properties of CVD graphene films. Researchers have found that in short-term protection, graphene films do provide protection for the underlying metals from corrosion. However, once the timeline is extended, the graphene conversely promotes the corrosion of the protected metals due to a galvanic corrosion effect. Further, various graphene-related causes of corrosion and the corresponding inhibition strategies have been revealed. As an important part of the graphene-based coatings, the CVD graphene grown on metals for corrosion barrier coating have aroused a wide research interest in developing graphene protective coatings.
2.1 CVD graphene for short-term and long-term anti-corrosion
The representative studies that aroused a wide interest in developing CVD graphene as a metal protective coating can date back to 2011. Chen et al. pioneered the ability of CVD-grown graphene films for protecting the surface of the growth metal substrates (Cu or Cu/Ni alloys) from air oxidation, both in air at high-temperature and hydrogen peroxide solutions (Chen et al. 2011). The basic principle for such a protection is that the presence of graphene films can isolate the metal surfaces from external oxidative species. Indeed, they observed that graphene provided nearly perfect protection for metal substrates in aggressive environments, such as high-temperature annealing and oxidizing liquid solutions. By exposure to H2O2 solution, the half graphene-coated coin retained its original metallic colour in contrast to the seriously oxidative colour in the other uncoated half. This experiment clearly demonstrated how graphene can be used as a passivation layer to protect metal substrates. In addition, Prasai et al. (Prasai et al. 2012) further confirmed that through growing graphene films on Cu surface and then mechanically transferring it onto bare Ni or Cu surface with different layers was another effective method for increasing the corrosion resistance of Ni or Cu. Electrochemical corrosion test results show that copper sheets coated with transferred CVD graphene have a corrosion rate that is seven times slower than that of bare copper. Moreover, with the increase of transferred graphene layers, the corrosion rate of Ni with thicker CVD graphene would have lower corrosion rate. Overall, these examples show that pure CVD graphene films can protect the underlying metals from corrosion in the short term whether in high-temperature air or aggressive solutions, thanks to their impermeability and chemical stability.
Indeed, it is impressive that the CVD graphene coating prevents corrosive substances from coming into direct contact with the metal surface, protecting the metal surface from corrosion in the short term. But over time, however, it promotes the corrosion of the protected metal surfaces due to galvanic corrosion effect. The long-term protective performance of the graphene coating was investigated by placing bare copper and graphene-covered copper samples at an ambient temperature of 25 °C for ∼2 years and inspecting them optically at regular intervals. The results show that the Cu substrate with graphene was already heavier oxidized than bare Cu substrate and the oxidation phase detected was mainly cuprous oxide. It was then concluded that the conductivity of graphene is responsible for such a promotion of corrosion. It would favour the corrosion by creating a galvanic circuit between graphene and the underlying metal (Schriver et al. 2013). Figure 4a and b shows a schematic representation of the graphene promotion mechanism of galvanic corrosion (Zhou et al. 2013). In the absence of a graphene, copper oxidation occurs at the interface between Cu and Cu2O. In particular, electron migrates through the Cu2O film to the interface between Cu2O and air and reacts with oxygen to produce O2−, which, on the other hand, diffuses into the Cu2O lattice and further combines with Cu+ to form Cu2O. In contrast, in the presence of a graphene coating, free electrons generated at the Cu/Cu2O interface can quickly migrate to the Cu2O/graphene/air interface through the transport of graphene for oxygen reduction, while oxygen can also diffuse through defects and cracks in the graphene layer to the surface of Cu2O. Although the oxidation of copper is not spatially homogeneous, electrons can rapidly migrate to graphene and accelerate corrosion as long as electrical contact between copper and graphene in maintained (Figure 4b). Based on this mechanism, CVD graphene’s anti-corrosion action has been controversial for nearly a decade, with many studies suggesting that graphene accelerates the corrosion of copper. Recently, Lee et al. (Lee et al. 2020) have shown that the CVD grown graphene–Cu2O hybrid structure, which forms after a long period as normal, significantly slows down the oxidation of copper in the longer term, as compared to bare copper (Figure 4c). They observed that over a longer time scale, the CVD graphene–Cu2O hybrid structure became a protective layer against corrosion. At the same time, the unprotected copper area was observed heavily oxidized with CuO, with a far deeper depth compared to the corrosion beneath the graphene shield. Even though, graphene does trigger galvanic corrosion, especially preferential initialling at some defects.
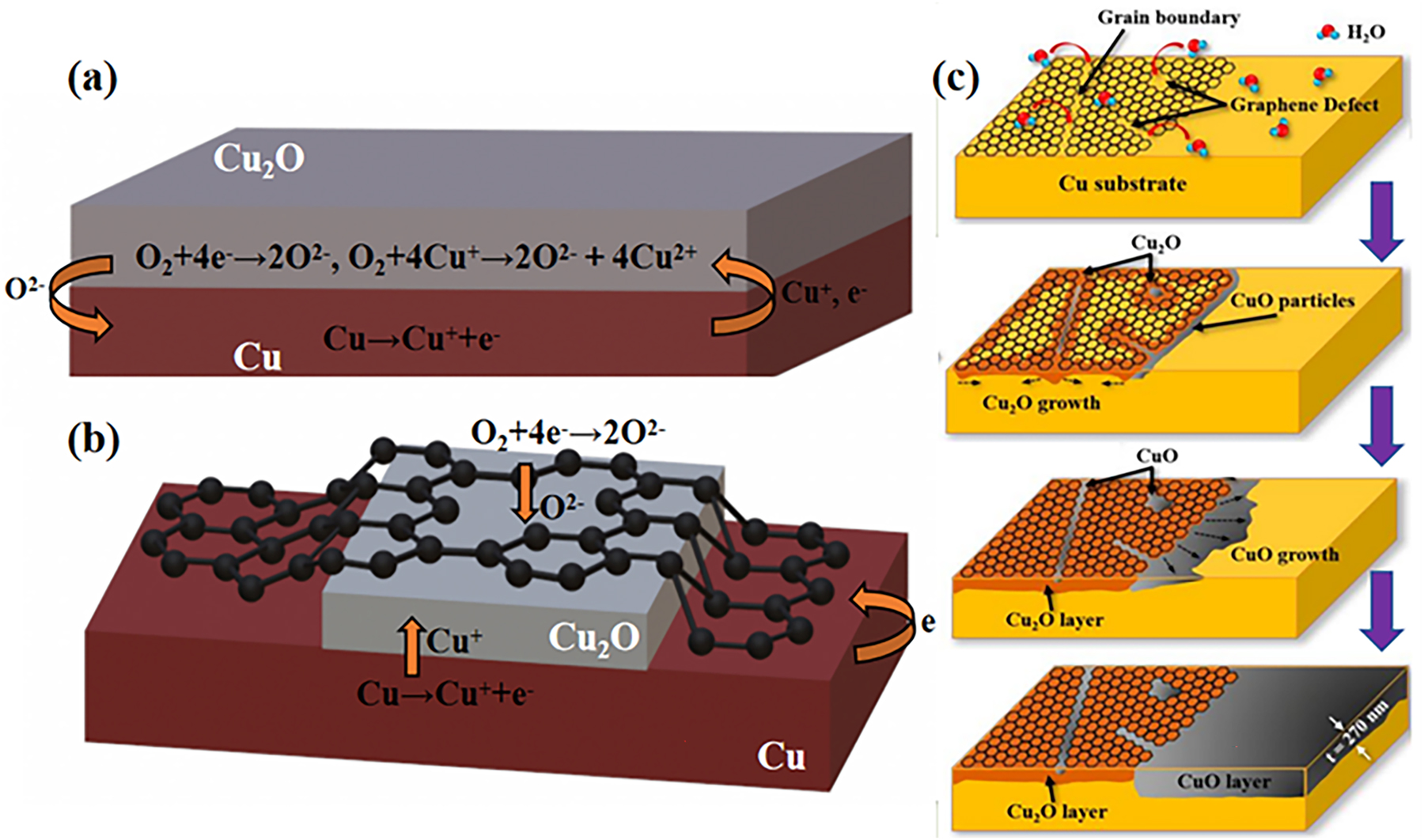
Schematic representation of the corrosion-promoting mechanism of graphene. (a) Electrochemical oxidation of copper in the absence of graphene and (b) the presence of graphene coverage. (c) Schematic diagram of CVD graphene–Cu2O hybrid layer as a protective layer for copper in the long term (Lee et al. 2020). Reprinted with permission from American Chemistry Society.
2.2 Corrosion originators of pure CVD graphene coating
In principle, continuous and perfect graphene with tight coupling to the metal substrate would block the diffusion of oxygen and water molecules between the interfaces, thus preventing the initialization of galvanic corrosion (Yoon et al. 2012). However, CVD graphene films are always subject to various defects such as grain boundaries (Bets et al. 2021), vacancies, S–W defects and cracks (Zhang et al. 2021), sometimes accompanied by folds, wrinkles (Song et al. 2022) and surface contamination (Lin et al. 2019), as seen in Figure 5a–d. These structural defects and/or growth imperfections would eventually facilitate the mass transport of oxidative elements as a pathway in the long term (Luo et al. 2016, 2020; Yuan et al. 2020a). At the same time, the presence of conductive graphene promotes the continuation of electrochemical reactions in the system through delivering electrons (Cui et al. 2019). On this basis, these sites of structural defects and/or growth imperfections as the corrosion originators will further cause more cracks of graphene due to the mechanical stresses of the formed oxides layer and thus opening up new corrosion paths. Singh Raman et al. have shown that if a graphene coating does not provide proper coverage on a metal surface, it can lead to poor corrosion resistance due to the highly cathodic nature of graphene and that the content of defects is the main cause why graphene coatings have varying degrees of corrosion resistance (Singh Raman et al. 2012). Prasai et al. also experimentally confirmed that corrosion starts in the cracks of the graphene film (Prasai et al. 2012). Hsieh et al. suggest that especially the grain boundaries of graphene are responsible for its incomplete passivation (Hsieh et al. 2014). Other types of defects in the graphene coating can also provide a path for corrosion molecules to penetrate and even trap chlorine atoms, undermining the protection of the graphene film to the substrate (Lee and Berman 2018).
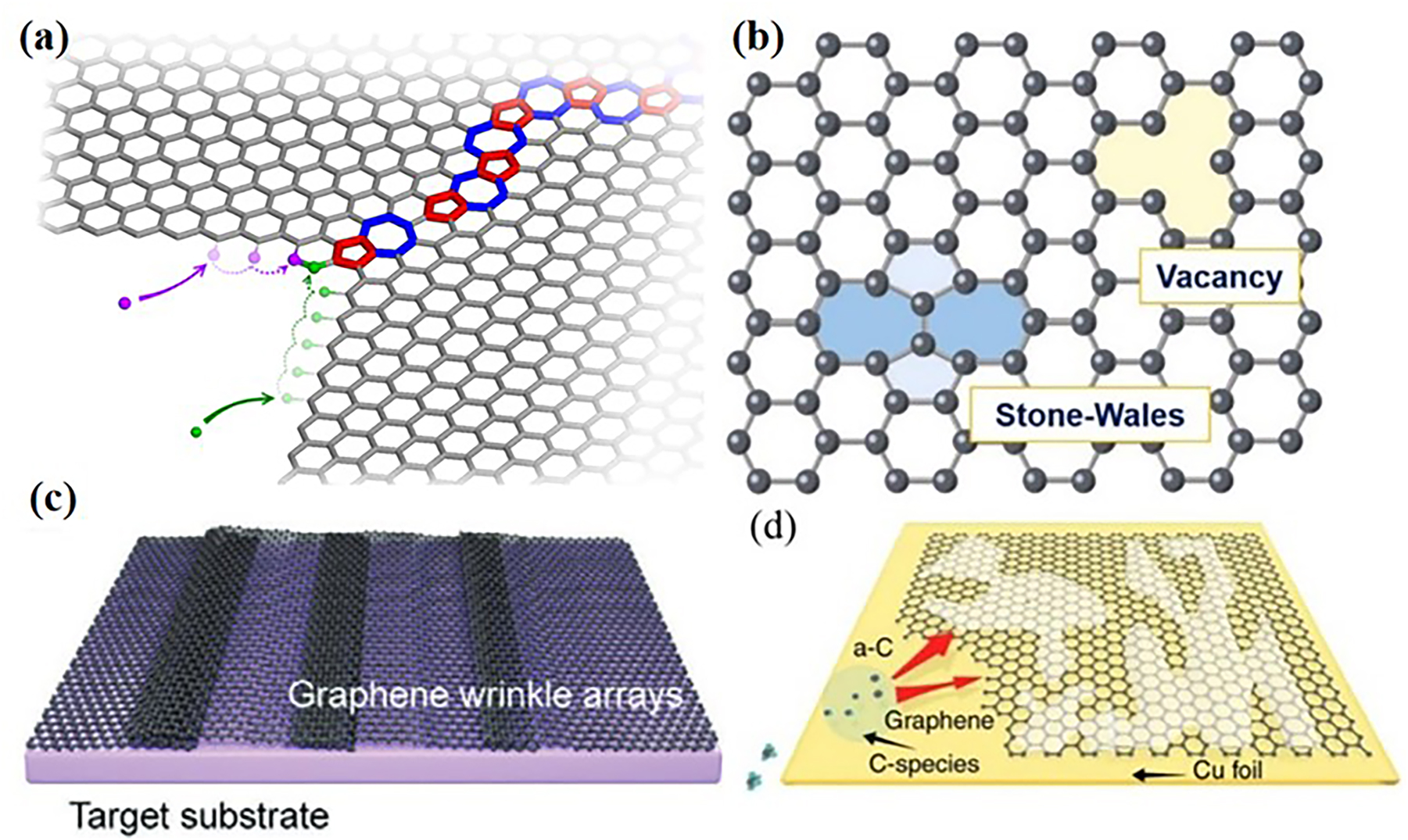
Corrosion originators of CVD graphene coating. (a) Graphene grain boundaries (Bets et al. 2021). Reprinted with permission from American Chemistry Society. (b) Vacancies and S–W defects (Zhang et al. 2021). Reprinted with permission from Elsevier. (c) Graphene wrinkle arrays (Song et al. 2022). Reprinted with permission from Wiley Online Library. (d) Surface contamination (Lin et al. 2019). Reprinted with permission from Nature.
Therefore, in order to improve the anti-corrosion efficiency of CVD graphene, extensive efforts have been investigated to inhibit the galvanic corrosion related with various corrosion originators. Many optimization strategies that can enhance the barrier resistance of CVD graphene coatings, as well as their inhibition mechanism related with galvanic corrosion are revealed. It includes post-healing of defects, doping modification of graphene and improving the graphene growth quality by reducing various defects correlated with CVD processes et al., which are demonstrated as follow.
2.3 Post-healing of defects strategies for pure CVD graphene coating
During the CVD growth or post-growth processing of graphene, structural defects like vacancy, disorder and dislocation defects are normally introduced. Besides of this, due to the randomly distributed graphene grains in CVD process, conventional graphene film is a polycrystalline structure composed of many grain boundaries. Here, the galvanic corrosion of the metal beneath would originate. For this issue, several optimization measures by post-healing of defects have been taken to repair the structure of CVD graphene and obtain high quality graphene coatings that provide effective long-term protection of the substrate material.
2.3.1 Atomic layer deposition for defects healing
Post-healing for various defects is the most direct method that could improve the anti-corrosion efficiency of CVD graphene. Kim et al. selectively deposited metal nanoparticles (Pt) on linear defects in CVD graphene by atomic layer deposition (ALD) (Kim et al. 2014), as schematically seen in Figure 6a. The metal nanoparticles are selectively deposited on the one-dimensional defect sites of the graphene, particularly at the grain boundaries. From this point, a graphene–metal hybrid structure was obtained by deposition of metal nanoparticles, which just repair defective sites of the graphene. Experimentally, both scanning electron microscopy (SEM) and transmission electron microscopy (TEM) have demonstrated that the deposition sites of most metal particles are highly compatible with the linear defects of graphene. So, such an ALD technique enables the repairment of the local graphene defects and the enhancement of its corrosion barrier as a protective coating.
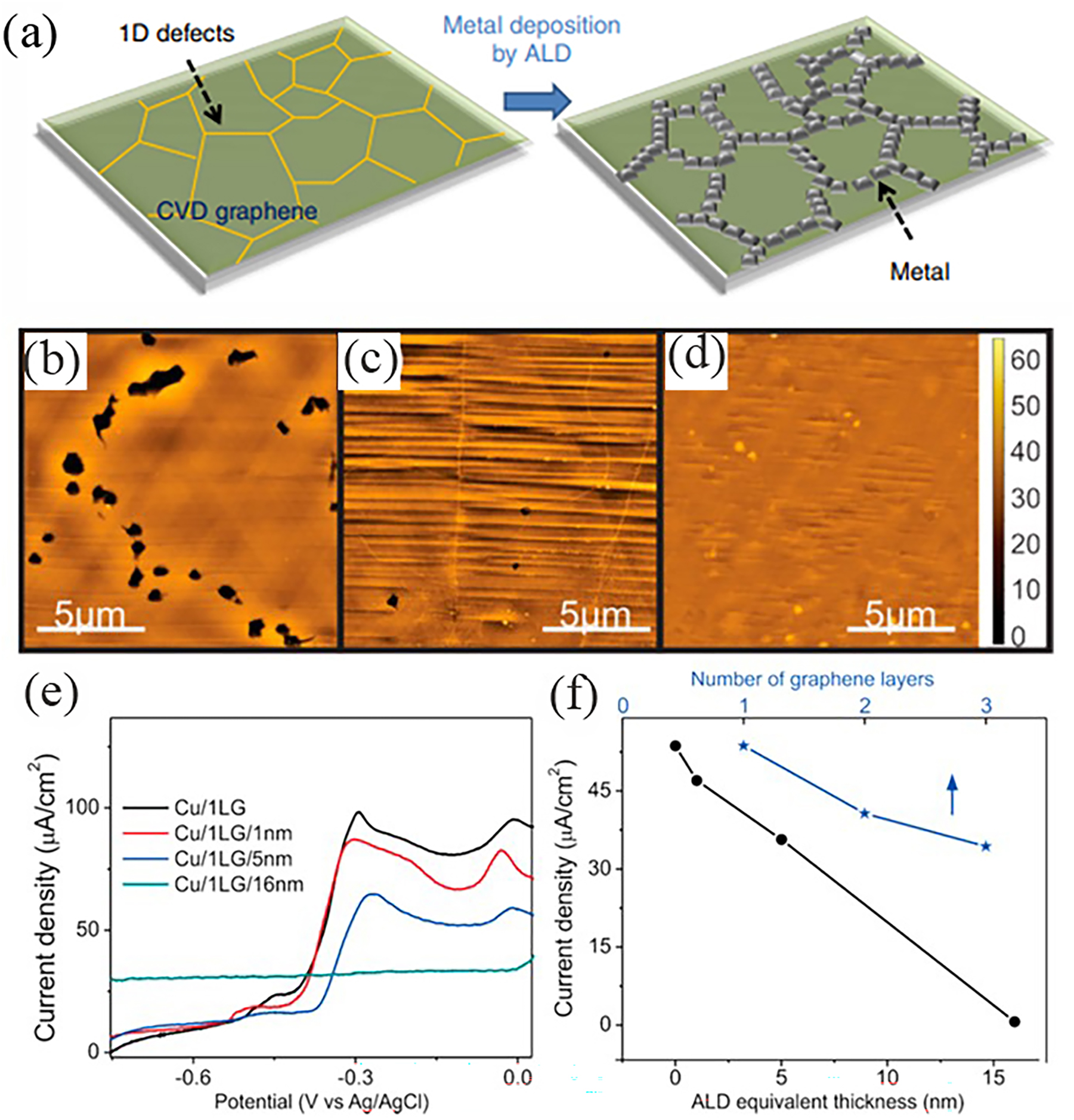
ALD healing of graphene defects for optimizing anti-corrosion properties. (a) Schematic diagram of the selective growth of platinum on one-dimensional defects in CVD graphene, where various linear defects such as grain boundaries, cracks and folded structures are present. By using atomic layer deposition, metals can be selectively deposited on one-dimensional defect sites in graphene. A graphene–metal hybrid structure can be obtained by this process (Kim et al. 2014). Reprinted with permission from Nature. (b–d) Representative AFM height images of etched graphene: (b) none, (c) ALD film with 5 nm and (d) ALD film with 16 nm. (e) CV spectra of Cu/graphene/ALD samples with different ALD thicknesses. (f) CuO peak currents of Cu/graphene/ALD samples with different ALD thicknesses and comparison with peak currents of Cu/graphene samples with 1–3 layers of graphene (Hsieh et al. 2014). Reprinted with permission from American Chemistry Society.
For example, it has been shown that due to the sensitivity of ALD to dangling bonds, atomic layers of alumina (Al2O3) can preferentially be deposited at lattice defects in graphene, producing separate clusters rather than continuous films on graphene. Moreover, the basic properties of graphene are not much deteriorated by passivation of ALD (Wang et al. 2008, Mazza et al. 2022). By doing this, the anti-corrosion efficiency can be improved accordingly as the thickness of the ALD alumina film increases (Hsieh et al. 2014). Figure 6b–d shows representative AFM height images of etched graphene with and without an ALD deposited film after corrosion. It can be observed that the density of etched pits on the graphene surface decreases as the ALD film thickness increases. Cyclo-voltammetry (CV) plots (Figure 6e) on the Cu/graphene/ALD film system with different ALD thicknesses also show that the corrosion current of the whole system shows a significant decrease as the ALD deposited film increases. Figure 6f shows the variation of current density for the Cu/graphene/ALD film system with different ALD thicknesses, showing that the passivation of the surface of the sample after 50 ALD cycles is better than that of the sample covered with a triple graphene film. In addition, the corrosion rate of the sample after 160 ALD cycles (corresponding to a 16 nm equivalent film thickness) is 1.6 × 10−15 m/s, a reduction of 99 and 87 %, compared to bare copper and the single layer graphene coating, respectively. Electrochemical impedance spectroscopy (EIS) also shows that the pore resistance associated with this healed graphene coating increases by a factor of 65 after 160 ALD cycles. These values are comparable to even the best copper corrosion inhibitors. Overall, inhibiting the passage of corrosive agents through post-ALD healing of structural defects in graphene improves the performance of graphene as a corrosion barrier and opens up the possibility for CVD graphene-based passivation layer applications.
2.3.2 Polymer self-assembly for defects healing
In addition to ALD metal nanoparticles/oxide, polymers can also be used to accurately repair defects of graphene thus improving its barrier resistance property. Wu et al. reported the self-assembly of a hydrophobic perfluorooctyltriethoxysilane (PTES) film, a healing agent selectively chemically grafting onto a copper substrate exposed to a graphene defect site, can repair the defect without sacrificing the electronic properties of the graphene (Wu et al. 2019a). The reaction mechanism for polymer repair is driven by the in situ formation of polysiloxanes, followed by a polymerization reaction (Lee et al. 2016) in which defective sites on graphene are covered by chemical grafting between copper and PTES. Further to improving the healing efficiency, they also proposed a rapid treatment method capable of accurately healing different types and sizes of structural defects on CVD graphene within 15 min by self-assembling hydrophobic 1H,1H,2H,2H-perfluorooctanethiol (PFOT) molecules into the defect sites (Wu et al. 2021b) (Figure 7a). PFOT molecules can accurately graft onto the Cu substrate at the defect sites through Cu–S bonding and the hydrophobic groups of CF3−CF2− can minimize the wetting of corrosive solutions, both of which result in the enhanced anti-corrosion action of graphene coating on Cu substrate. The bonding mechanism and self-assembly behaviour of PFOT molecules was further explained in detail by DFT calculations. Figure 7b shows the six possible adsorption conformations (C1–C6) of PFOT molecules. For C1 and C3, the PFOT molecules are physically adsorbed on the surface of the pristine graphene with a large adsorption distance and low adsorption energy (ΔE ads) (0.37–0.4 eV). The low ΔE ads values for these pristine sites ensure that the physisorbed PFOT molecules can be easily removed by the ethanol rising as shown by in situ self-assembly process in Figure 7a. The higher ΔE ads values in C5 and C6 indicate that the healed PFOT molecules are capable of strong chemical bonding to any part of the copper substrate exposed to the graphene defect region. The relatively low ΔE ads in the C4 conformation can capture PFOT molecules to the defect edge of the graphene and allow C4 to continue to transition to the more stable C5 and C6 states. It is only when the exposed copper substrate is completely filled with PFOT molecules that the C atoms at the edges of the defects begin to be saturated with PFOT molecules. The conformation C4–C6, which enables complete passivation of structural defects, provides a good barrier to the intrusion of corrosion factors. On this basis, galvanic corrosion in the system is significantly suppressed. Figure 7c shows optical images of graphene-coated copper with and without PFOT healing exposed to ambient conditions for 30 and 60 days. After 60 days, as expected, galvanic corrosion (Wu et al. 2016) occurred between the untreated graphene coating and copper, and the copper substrate in the defected area was severely corroded. In the defected areas of the healed graphene, the accumulation of hydrophobic PFOT molecules successfully prevents the wetting of the surface by corrosive media, and some corrosive media such as H2O and O2 are unable to penetrate the graphene coating. Indeed, this potential healing method can significantly improve the corrosion resistance of graphene without affecting its structural and electronic properties.
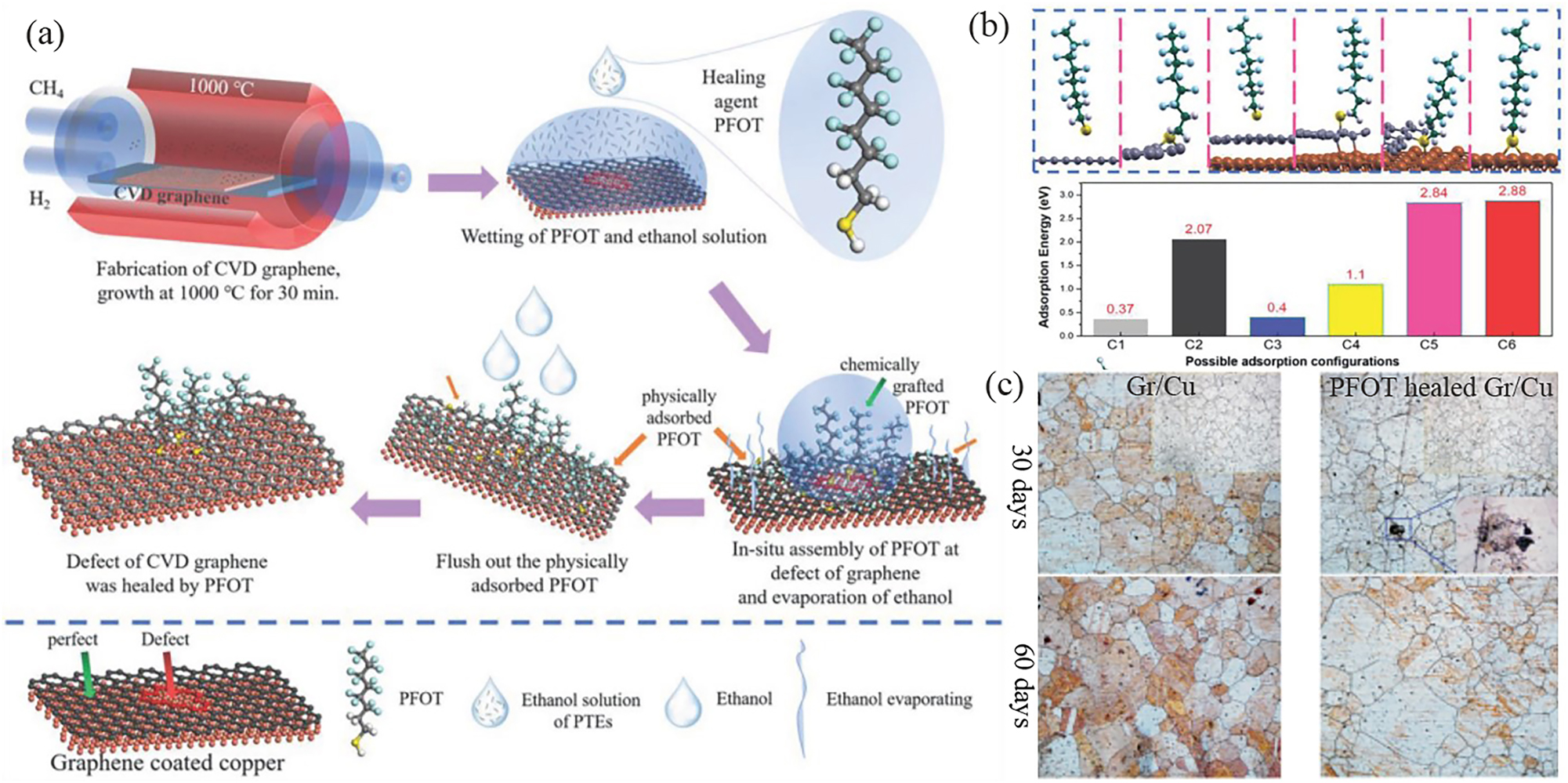
Polymer self-assembly healing of graphene defects for optimizing anti-corrosion properties. (a) Fast healing strategies of the defects in CVD graphene coating. (b) The adsorption configurations (C1–C6), energies (ΔE ads) of PFOT molecule on pristine/defective graphene coating, where both free-standing and Cu-supported graphene are compared to reveal the effect of Cu substrate. (c) The optical images of pristine graphene-coated Cu and PFOT-healed graphene-coated Cu surface after the exposure to ambient condition of 20–25 °C with a relative humidity at 50–60 % for 30 (upper panels) and 60 (lower panels) days (Wu et al. 2021b). Reprinted with permission from Wiley Library.
2.4 CVD growth optimization strategies
The above-mentioned post-healing strategies are based on the remediation way, which is carried out after the graphene has been grown, depending on the type of defects. Considering that the long-term barrier resistance of graphene is highly correlated with its growth quality, the CVD synthesis history is of significant importance. How to grow graphene films directly from the beginning with good corrosion barrier resistance or even defects-free is also one of the challenges that researchers are concerned about. Various factors such as layers number, initial nucleation, the interface alignment between graphene and the underlying metal surface are revealed to be critical for the inhibition of galvanic corrosion of CVD graphene–metal system and improving its anti-corrosion efficiency.
2.4.1 Multi-layer strategy
Firstly, the preparation of multi-layer graphene films opens a new door to improving the corrosion barrier resistance of CVD graphene. And the basic idea is that structural defects in adjacent graphene layer do not overlap with each other, eventually forming a spatial resistance that effectively prevents the penetration of corrosive media. Figure 8a-b schematically shows the spatial diffusion paths of the corrosion agents in the cases of mono-layer and multi-layer graphene films (Stoot et al. 2015). Comparing to single layer systems, corrosion agents face far more obstacles in passing through multi-layer graphene films. H2O and O2 molecules need to find lattice defects on the film surface to penetrate to the next layer, crossing multiple complex tortuous paths between layers and finally reaching the metal surface. Based on this optimization mechanism, it was confirmed that CVD grown multi-layer graphene would provide a superior long-term corrosion protection for stainless steel relevant as bipolar plates in polymer electrolyte fuel cells (Yu et al. 2016). In addition, Zhao et al. recently grew polycrystalline few-layer graphene (FLG) films on copper to investigate its long-term corrosion protection (Zhao et al. 2021). They compared copper covered with single and few-layer graphene films for 6 years ambient oxidation, showing a clear superiority of the FLG with respect to the single-layer graphene (SLG). Spatial structure configuration of the FLG (Figure 8c–d) was then verified through an isotope-labelled CVD process, demonstrating the similar spatial barrier resistance with complex diffusion paths. Figure 8e–h further shows the results of an atomic-scale simulation of corrosion molecules passing through defects in graphene to corrode the base metal for different cases. As seen in Figure 8e, the energy required for water molecules to pass through the defects (two-adjacent-carbon-atoms vacancy) in the SLG is approximately 0.06 eV and that a small increase in ambient temperature can assist the diffusion of water molecules to corrode the underlying metal (Figure 8f). However, molecular dynamics (MD) and DFT calculations revealed that the energy required for water molecules to diffuse into the van der Waals gap between the bilayer graphene films is as high as 2.5 eV from MD and 2.27 eV from DFT (Figure 8g). Further theoretical calculations revealed that energy of approximately 0.37 eV is required to move water molecules between the FLG layers, which is 14 times greater than the room temperature thermal activation energy and, therefore, unlikely to lead to the penetration of corrosion molecules as shown in Figure 8h. In short, the high diffusion energy barriers between graphene layers, combined with grain boundary misalignment, make polycrystalline FLG films a promising material for protective coatings in the field of metal corrosion protection. Specially, with such advantages from a multi-layered graphene coating, it can also effectively mitigate hydrogen embrittlement of the protected pipe steel through a strong dangling bonds adsorption effect on hydrogen atoms. Which can produce hydrogen traps to capture hydrogen atoms, enhancing the internal barrier effects (Shi et al. 2022) (see Figure 8i).
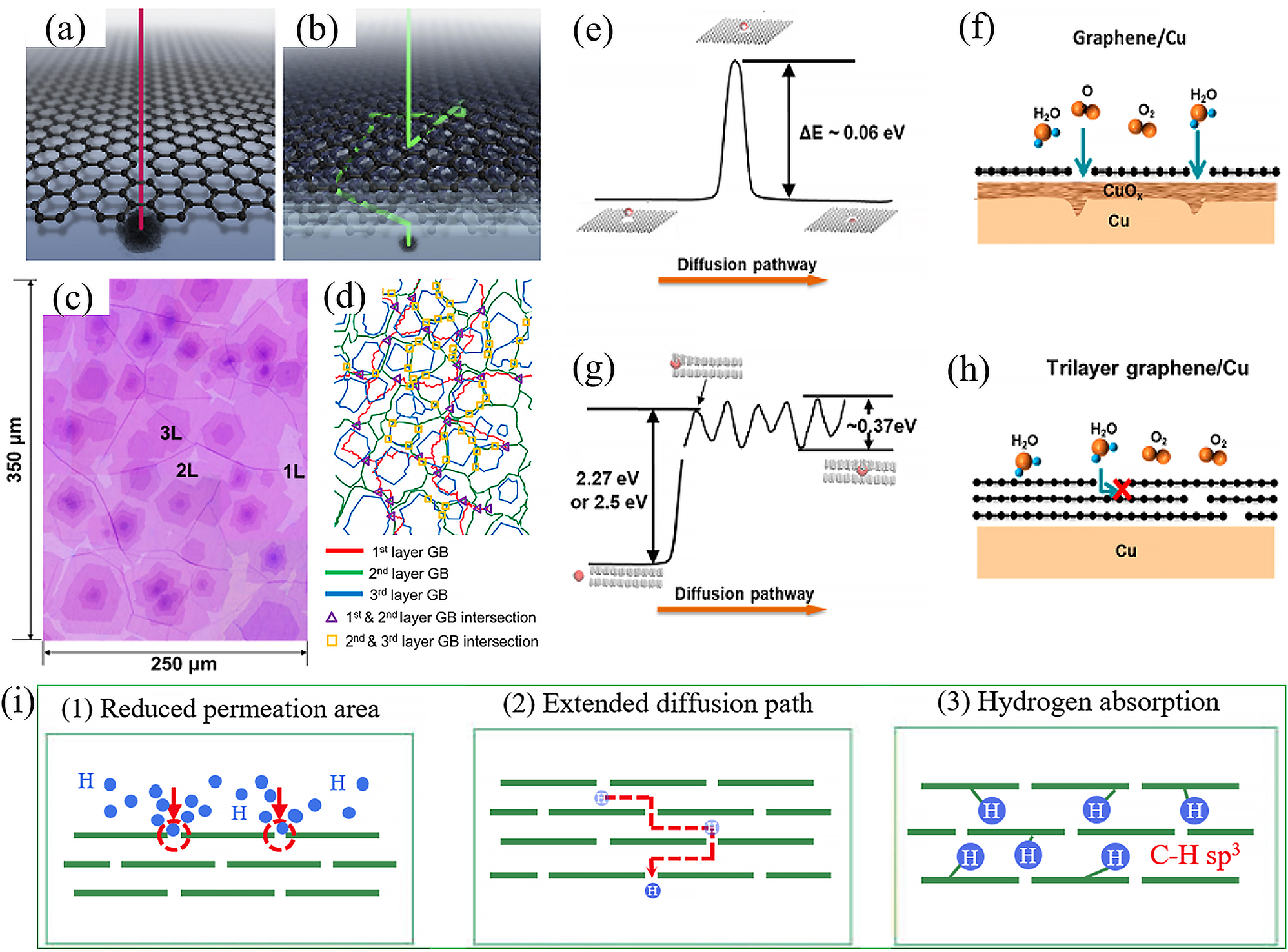
Anti-corrosion optimization mechanisms of multi-layer graphene. (a, b) Schematic diagrams of the diffusion paths of single-layer (a) and multi-layer (b) graphene. In the case of multi-layer films, the diffusion paths for oxygen and water molecules are longer (Stoot et al. 2015). Reprinted with permission from Elsevier. (c) Optical image of a large area of FLG film on a SiO2/Si substrate and (d) corresponding GB lines, with the intersection of the different layers marked in the diagram. (e) Schematic diagram of water molecules diffusing through the defective SLG and the calculated energy barrier. (f) Schematic showing the easy diffusion of reactive species such as oxygen and water molecules through the SLG and oxidized copper surface. (g) Schematic and calculated energy barrier for the diffusion of water molecules through the defective BLG. (h) Schematic showing the difficulty of diffusion of oxygen and water molecules through polycrystalline trilayer graphene and into contact with the underlying copper surface, even though the trilayer graphene contains multiple GB defects (Zhao et al. 2021). Reprinted with permission from American Chemical Society. (i) Internal hydrogen resistance mechanism of the MLG coating (Shi et al. 2022). Reprinted with permission from Elsevier.
Zhang et al. investigated the oxidation behaviour of graphene-coated copper in atomic oxygen (AO) environment, both theoretically and experimentally (Zhang et al. 2018). The experimental results show that the multi-layer graphene coating has better resistance to oxidation than the single-layer graphene coating after AO irradiation. In multi-layer graphene coatings, the diffusion of oxygen atoms in the vertical direction is largely inhibited due to the misalignment of defect positions and the formation of steric hindrances. The interlayer potential limits the diffusion of oxygen atoms in the horizontal direction, so that the oxidation process proceeds very slowly in multi-layer graphene systems. Sanjid et al. found through electrochemical characterization that the multi-layer graphene films were able to circumvent their defects and provide a complete covering surface, significantly improving the corrosion resistance of the alloy (Sanjid et al. 2019). Prasai et al. found experimentally that the mechanical transfer of multi-layer graphene films onto a target substrate was able to significantly reduce the corrosion rate of the metal (Prasai et al. 2012).
2.4.2 Interface engineering strategy
The coupling of the interaction between a graphene coating and the underlying metal is also critical in determining the barrier action for corrosion protection. The strong interaction between graphene and the substrate prevents the lateral diffusion of corrosive species at the interface, thereby inhibiting the oxidation of the metal substrate. Researchers have, therefore, been looking for ways to enhance the interaction between graphene and the substrate. Weatherup et al. grew continuous monolayer graphene films on the surface of various polycrystalline transition metal catalysts such as nickel, cobalt and platinum by a CVD process and studied their oxidation at room temperature when exposed to humid air at different time durations (Weatherup et al. 2015). The results showed that single-layer graphene films grown by the CVD process can protect polycrystalline nickel surfaces from oxidation for more than 18 months, due to the strong coupling between graphene and nickel. Graphene grown on copper or platinum substrates would have relatively poor interactions and tend to decouple from the substrates, thereby facilitating the diffusion and invasion of corrosive species at the interface. On the other side, graphene grown on nickel, cobalt and iron substrates would interact strongly, showing much better corrosion barrier resistance. The strong CVD graphene–metal interactions/coupling can prevent the lateral diffusion of aggressive species along with the graphene–substrate interface. These apparent differences in the long-term passivation ability of graphene grown on different metals are highly dependent on the alignment and lattice match between graphene and the substrate. This effective suppression of surface oxidation is critical for many applications such as ferromagnetic spin jets (Martin et al. 2014, 2015), non-precious plasmonic materials (Kravets et al. 2014), etc., which are very sensitive to even minor surface oxidation.
In addition to exploring the interactions with graphene from the perspective of the type of substrate, it is also important to seek how to engineer the interface of CVD graphene on metal substrate (e.g. Cu), which don’t always have strong coupling/interactions with CVD grown graphene. It was pointed out that the lattice alignment and mismatch between CVD graphene and the Cu growth surface play significant role in determining the coupling strength of the interface, then defining their corrosion barrier resistance (Xu et al. 2018). The typical experiment is the Cu facet-dependent anti-corrosion of CVD graphene coating. As shown in Figure 9a–b, the CVD grown single-crystal graphene grain on Cu(111) and Cu(100) surfaces shows disparate oxidation of the Cu surface under graphene in the long-term exposure to the ambient environment. It can be seen that after 1 year in air, Cu surface under graphene was heavily oxidized in the case of CVD graphene–Cu(100) interface (Figure 9a). In contrast, CVD graphene grown on Cu(111) can well protect then underlying Cu surface from oxidation for as long as 2.5 years (Figure 9b). It was revealed that only 4 % of the graphene and Cu(111) surfaces are lattice mismatched so that graphene can be epitaxially grown on its surface to form a commensurate system (Figure 9c). On the other hand, in the graphene–Cu(100) system, a small relative rotation is always observed, demonstrating that the graphene lattice is not perfectly aligned with the lattice orientation of Cu(100) facet (Figure 9d). In addition, Luo et al. systematically investigated the relationship between the crystal structure of CVD-grown graphene and that of the copper substrate and its effect on the oxidation of the substrate metal (Luo et al. 2019). The experimental results demonstrate that the oxidation of copper covered with graphene is highly dependent on the lattice arrangement and crystal orientation of copper and graphene. Figure 9e shows optical images of graphene bi-crystalline domains on Cu substrates with different degrees of oxidation. The corresponding electron backscatter diffraction (EBSD) characterization (Figure 9f) shows that the graphene domains are covered by a homogeneous Cu(111) surface, which implies that the crystal orientation of the graphene bi-crystal and the coupling between graphene and Cu(111) lead to different oxidation levels of the substrate. Recently, Zhao et al. reported a Janus-doped bilayer graphene coating, which provides protection for Cu substrates for more than 5 years at room temperature and 1,000 h at 200 °C (Zhao et al. 2023). This excellent anti-corrosion is attributed to the fact that the heavily doped bottom layer forms a strong interaction with Cu limiting the interfacial diffusion, while the nearly charge neutral top layer behaves inertly to alleviate the galvanic coupling corrosion. All of the above reveals that the anti-corrosive behaviour of the passivation surface is closely related to the differences in interfacial coupling in the graphene–Cu system, which is crucial for the development of efficient anti-corrosive technologies and materials and offers new prospect and opportunities for graphene to achieve ultra-high passivation of metals through precise interface engineering strategy.
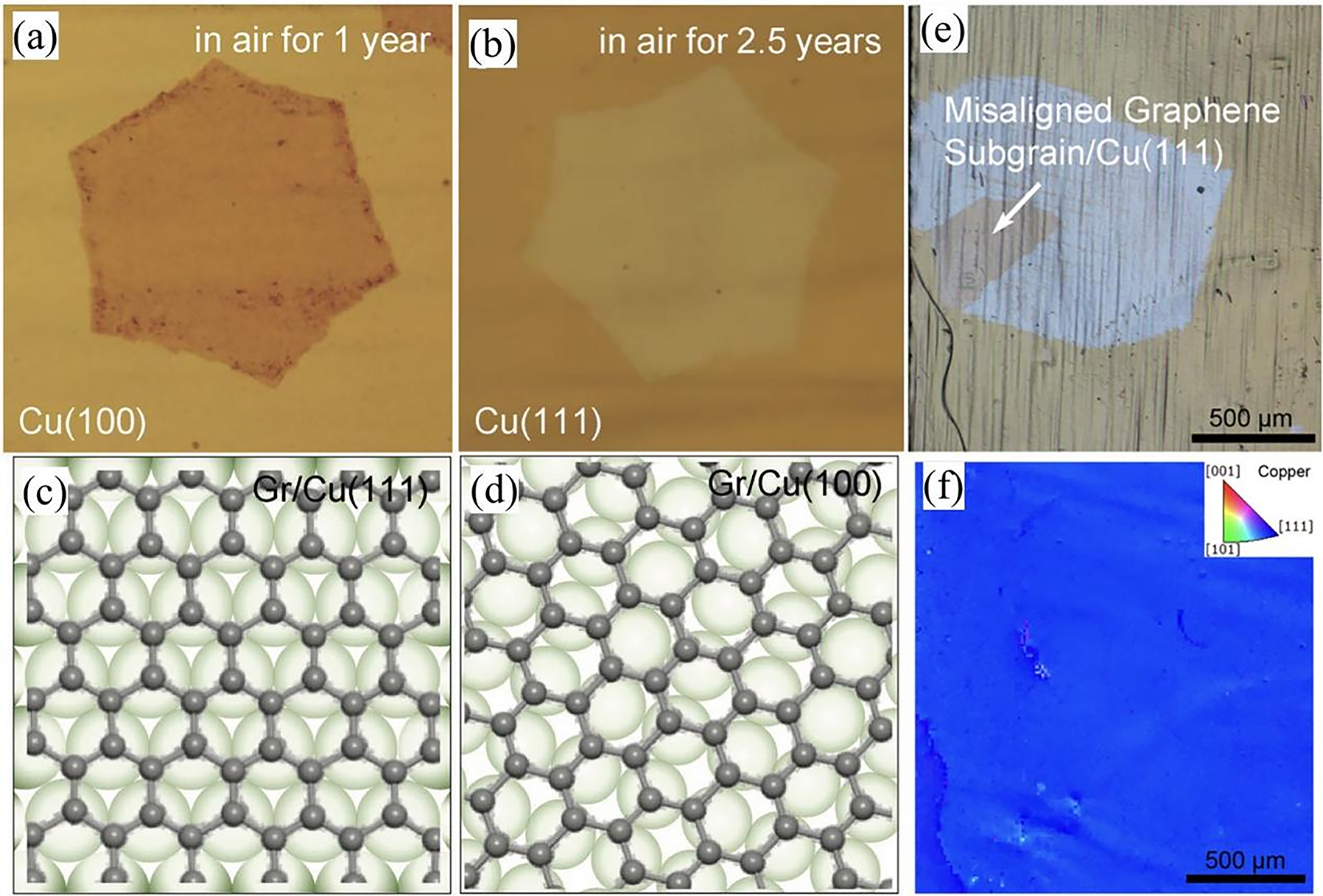
Interface coupling of CVD graphene growth and its anti-corrosion properties. (a) Optical image of graphene-coated copper (100) after 1 year of oxidation under ambient conditions. (b) Optical image of graphene-coated copper (111) after 2.5 years of oxidation under ambient conditions. (c) Schematic representation of the graphene lattice on Cu(111). (d) Schematic representation of the graphene lattice on Cu(100). Carbon atoms cannot be matched to Cu. The grey (green) spheres indicate the carbon (Cu) atoms in (c) and (d) (Xu et al. 2018). Reprinted with permission from Wiley Online Library. (e) Optical image and (f) corresponding EBSD orientation map of a graphene bi-crystal domain on a single Cu facet with different degrees of oxidation (Luo et al. 2019). Reprinted with permission from American Chemical Society.
Besides, wrinkles and contamination, which greatly impair the corrosion barrier properties of graphene coating, are inevitably associated with interfacial coupling of graphene–Cu system. For example, wrinkles on the graphene surface can lead to local decoupling and then reduce the interfacial strength of the overall coupled graphene–Cu system. Similarly, contamination can lead to a decrease in graphene’s quality. Therefore, the production of flat and clean graphene shows attractive prospects for optimization of large-area highly tight interface of graphene–Cu system, which is important for suppressing the initialization of galvanic corrosion originated from interfacial coupling. Some CVD growth methods have been exploited for the growth of wrinkles- and contamination-free graphene layer (Choi et al. 2015; Lee et al. 2014; Mun et al. 2014). Especially, as shown in Figure 10, it shows several optimization growth techniques for large-scale production of wrinkles- and contamination-free graphene on Cu substrate.
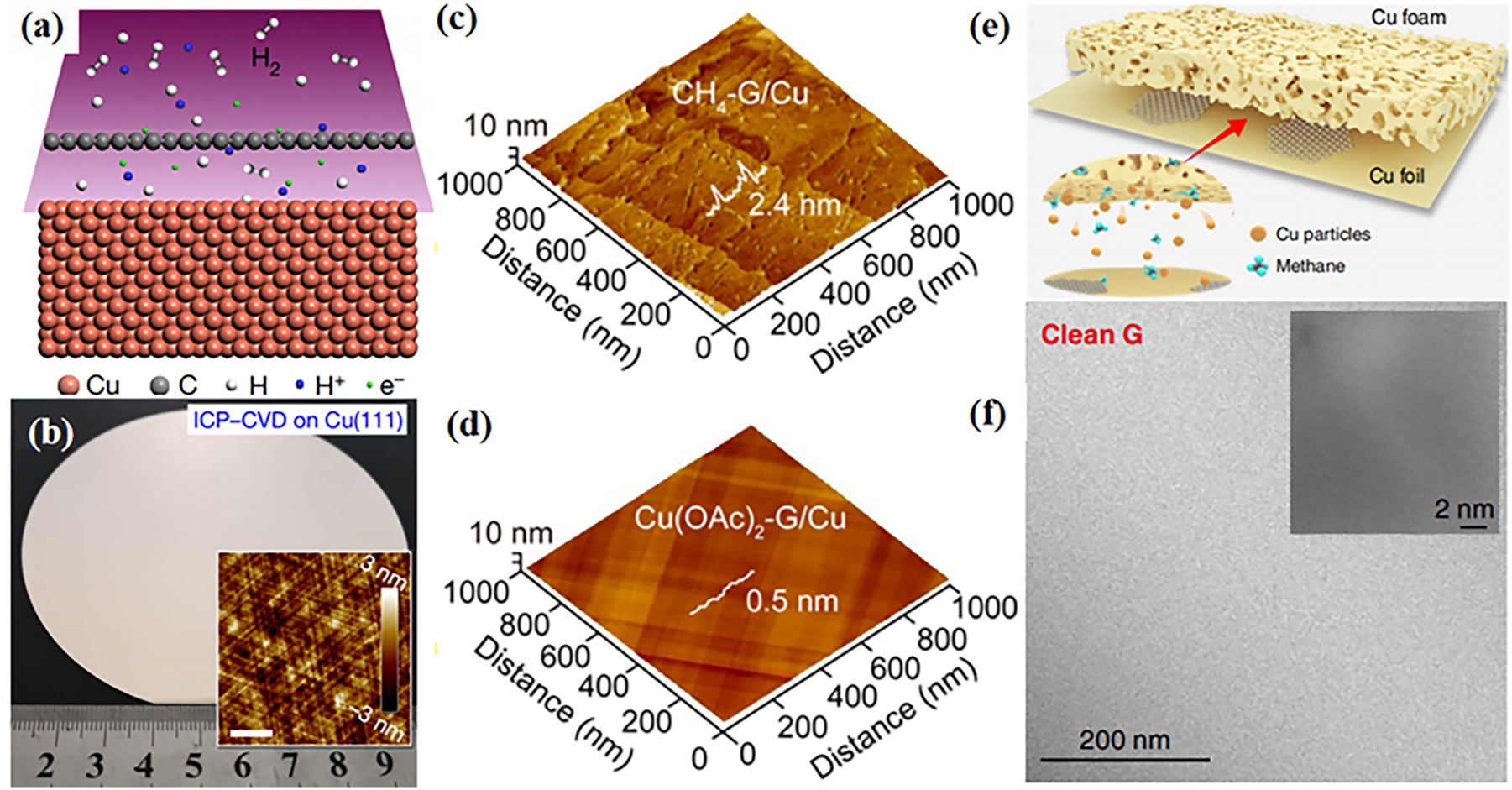
Synthesis and characterization of ultra-flat and ultra-clean graphene. (a) Illustration of protons penetrating through the as-grown graphene film during ICP treatment. (b) Photo of 4-inch graphene film grown on Cu(111). Inset, typical AFM image (Yuan et al. 2020b). Reprinted with permission from Nature. (c, d) AFM of clean graphene by CH4 (c) and Cu(OAc)2 (d) (Jia et al. 2019). Reprinted with permission from American Chemical Society. (e) Schematics of the experimental design. (f) TEM image of the super-clean graphene membrane. Inset: HRTEM image of the graphene lattice (Lin et al. 2019). Reprinted with permission from Nature.
Firstly, Figure 10a and b shows that wrinkle-free, ultra-flat graphene films could be grown on Cu film using proton-assisted CVD method. A proton penetration as well as recombinant hydrogen method effectively reduces the wrinkles formed in graphene during the conventional CVD process (Yuan et al. 2020b). In addition, Wang et al. investigated the wrinkling process of graphene films grown on single-crystal Cu–Ni foils using ethylene as a precursor (Wang et al. 2021). By limiting the initial growth temperature to between 1,000 and 1,030 k, it is possible to grow high-quality, wrinkle-free single-crystal monolayer graphene films that possess highly uniform transport properties, as for the contamination-free production of graphene.
Jia et al. successfully prepared ultraclean graphene by using a novel carbon source (Jia et al. 2019). Compared with methane, using Cu(OAc)2 as the carbon source increases the participation of copper in the reaction and promotes the timely decomposition of carbon clusters, achieving a clean graphene surface. Figure 10c and d shows AFM images of graphene grown with CH4 and Cu(OAc)2 as carbon sources. The surface of graphene grown with Cu(OAc)2 as the carbon source is visibly clean and almost free of impurities. Similarly, Lin et al. used alternately stacked copper foils and copper foam substrates to provide more copper catalysts, greatly inhibiting the formation of amorphous carbon (Lin et al. 2019). Copper foam is a porous structure that grows graphene with a very clean surface (Figure 10e and f). At the same time, the growth rate of graphene on the copper foam is lower than that on the copper foil, ensuring a continuous supply of copper during the growth process. These CVD growth strategies can produce highly tight interface of graphene–Cu system, largely improving their anti-corrosion protection for the underneath Cu substrates.
2.5 Graphene conductivity modification strategy
As we know, the semi-metal conductivity of graphene is one of the key elements responsible for the galvanic corrosion of graphene coating in the long-term protection for metals. Thus, modifying graphene’s properties by reducing its conductivity is a strategy for improving its corrosion protection efficiency. Typically, hexagonal boron nitride (h-BN), known as ‘white graphene’ with graphene-like lattice structure (Dean et al. 2010), excellent thermal conductivity (Golberg et al. 2010), high impermeability (Cai et al. 2015; Husain et al. 2013; Liu et al. 2013; Yi et al. 2014) and air stability (Chen et al. 2004; Li et al. 2014), has been considered to offer better corrosion protection than graphene mainly due to its insulating properties, which prevent the occurring of electrochemical reactions in the long term (Galbiati et al. 2017). Shen et al. prepared h-BN coatings by CVD, which exhibited corrosion resistance far beyond of pure graphene after exposure to air for up to 160 days (Shen et al. 2016). Lee et al. reported a method of synthesizing wafer-scale single-crystalline hBN(SC-hBN) monolayer films by CVD (Lee et al. 2018). Such wafer-scale SC-hBN film can serve as a protective layer against metal oxidation and a gas-diffusion barrier for water vapour transport. The Cu surface covered by SC-hBN film is not clearly changed after the oxidation test at 300 ℃ in air, whereas both polycrystalline hBN (PC-hBN)-covered and bare Cu surfaces are severely oxidized (Figure 11a, c, e and 11b, d, f). Water vapour transmission rate (WVTR) measurements of the hBN films (Figure 11g–i) showed that the SC-hBN monolayer films outperformed the PC-hBN monolayer films by about a factor of two, obtaining WVTR values comparable to those of single-layer polycrystalline graphene films. Such wafer-scale SC-hBN films do not have any grain boundaries, thus forming a water vapour barrier and complete protection against Cu oxidation. Structural defects and/or growth imperfections in the CVD graphene and hBN allow unprotected areas of copper to react with oxygen and produce copper oxides, and the corrosion reaction could have been inhibited as the oxides grow. However, the high electrical conductivity of graphene provides a new pathway for electron transport, transporting electrons to the oxygen atoms and allowing the corrosion area to expand in both horizontal and vertical directions, as we have shown above. In contrast, due to the insulating properties of hBN, electron transport is effectively inhibited in the horizontal direction, and then the circuit of the electrochemical reaction is cut off, finally resulting in a slower corrosion rate. Therefore, the conductivity reduction manipulation of graphene down to that of hBN has been a viable strategy to their anti-corrosion protection for metals (Jiang et al. 2017; Khan et al. 2017; Li and Chen 2016).
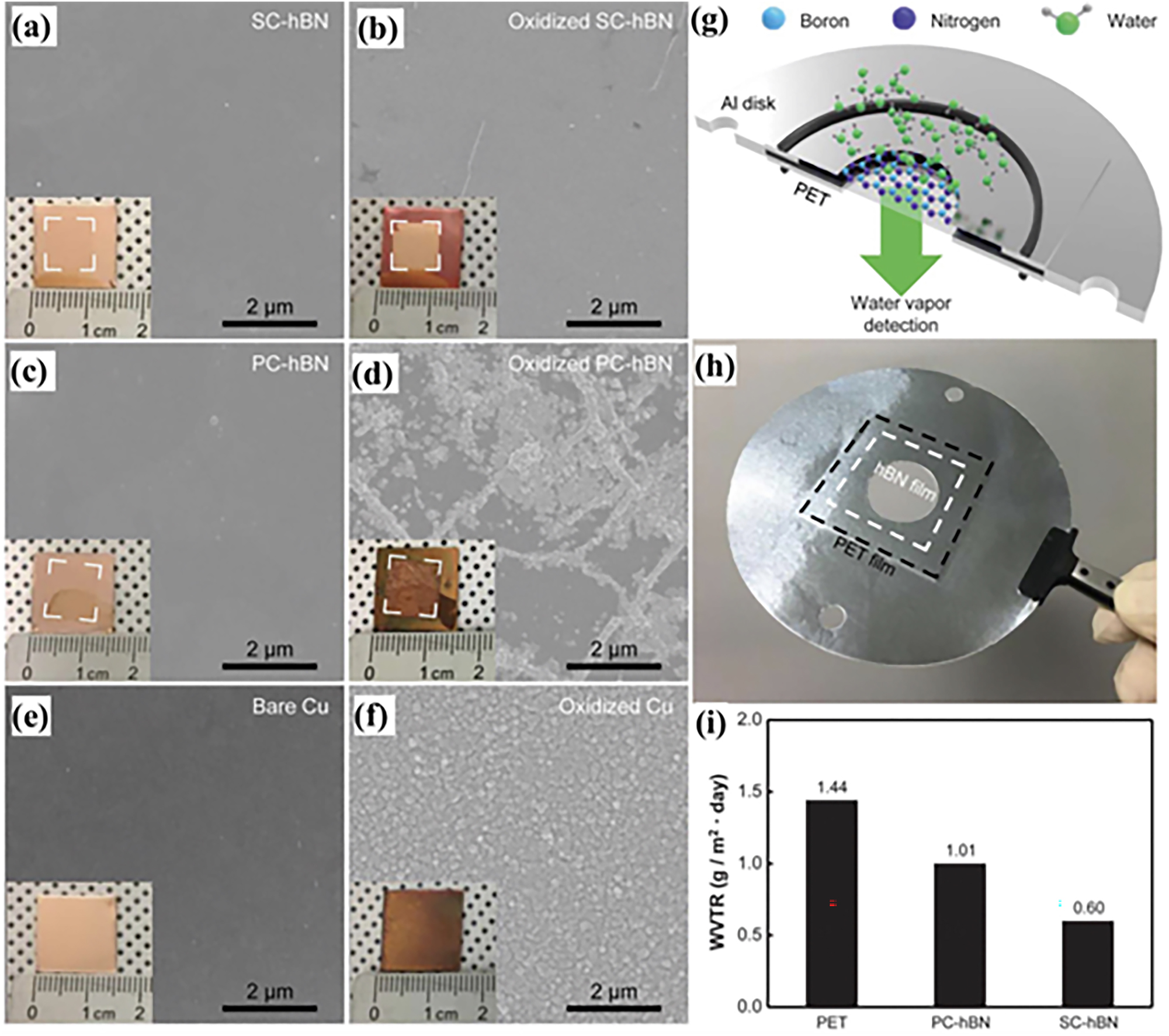
Anti-corrosion properties of h-BN. (a–f) SEM images of SC-hBN-, PC-hBN-covered and bare Cu foils before (a, c and e) and after (b, d and f) oxidation in air at 300 °C for 1 h. (g, h) Schematic and photograph for the WVTR. (i) WVTR values of PET, PC-hBN and SC-hBN samples (Lee et al. 2018). Reprinted with permission from Science.
2.5.1 Boron or/and nitrogen doped graphene for anti-corrosion
The most common strategy for reducing the conductivity of graphene is including doping atoms into the atomic lattice of graphene. Numerous studies have found that doping graphene with heteroatoms (such as boron, nitrogen, phosphorus and oxygen) can alter the electrochemical properties of graphene by modulating its local electron density and improving its capacitive performance (Bonanni et al. 2012; Lv and Terrones 2012; Poh et al. 2013; Wu et al. 2012; Yang et al. 2011b). The doping of boron atoms into the atomic lattice of graphene is a very attractive corrosion protection strategy, as their atomic sizes are very close to each other, thus avoiding the formation of structural defects (Banhart et al. 2011). Indeed, the stability between boron-doped graphene and copper interfaces was investigated (Boukhvalov et al. 2018), showing that boron-doped graphene coatings can provide high-strength corrosion protection for copper substrates.
In recent years, researchers have also paid much attention to nitrogen-doped graphene (NG) (Han et al. 2021; Li et al. 2017; Kong et al. 2014; Wang et al. 2010), as nitrogen atoms have similar atomic radii to carbon atoms and are more electronegative than carbon atoms (Cervantes-Sodi et al. 2008; Xie et al. 2015; Yang et al. 2012), which make N-doped graphene considerably less conductive compared to pristine graphene (PG) (Guo et al. 2010; Jin et al. 2011; Liu et al. 2011; Wei et al. 2009). Therefore, the preparation of high-quality NG sheets with desirable chemical properties is key to future corrosion protection applications. Ren al et. used NH3 and CH4 as the source of nitrogen and carbon, respectively, and synthesized NG flakes with different concentrations (increased as NG1, NG2 and NG3) by CVD (Ren et al. 2018). As the environmental oxidation experiment shows in Figure 12a–i, NG could provide better corrosion protection than PG over larger period of time. On the other hand, it is worth considering that excessive atomic doping inevitably introduces additional defects in the graphene lattice, which would conversely increase the physical corrosion on such defects. As a result, proper nitrogen doping (for instance, NG2) enables the film to have good quality and corrosion protection properties as demonstrated in the longer-term corrosion protection (Figure 12k). In general, nitrogen-doped graphene can provide longer corrosion protection than pure graphene. As shown in Figure 12m, during long-term exposure at room temperature, corrosion starts at defects in the PG film and accelerates under the subsequent galvanic corrosion effect (Cubides et al. 2016; Jalili et al. 2015), resulting in a horizontal corrosion reaction gradually. On the other side, nitrogen doping and topological defects reduce the electrical conductivity of graphene, thereby inhibiting corrosion of the copper substrate (Figure 12n) by reducing the transport of electrons. In addition, the good catalysis ability to the absorbed oxygen atoms on NG surface, specifically catalysing the bonding of adsorbed O into O2 to release from NG surface and avoiding the damage of NG film, is another factor that makes the better oxidation resistance of NG than PG (Ren et al. 2019).
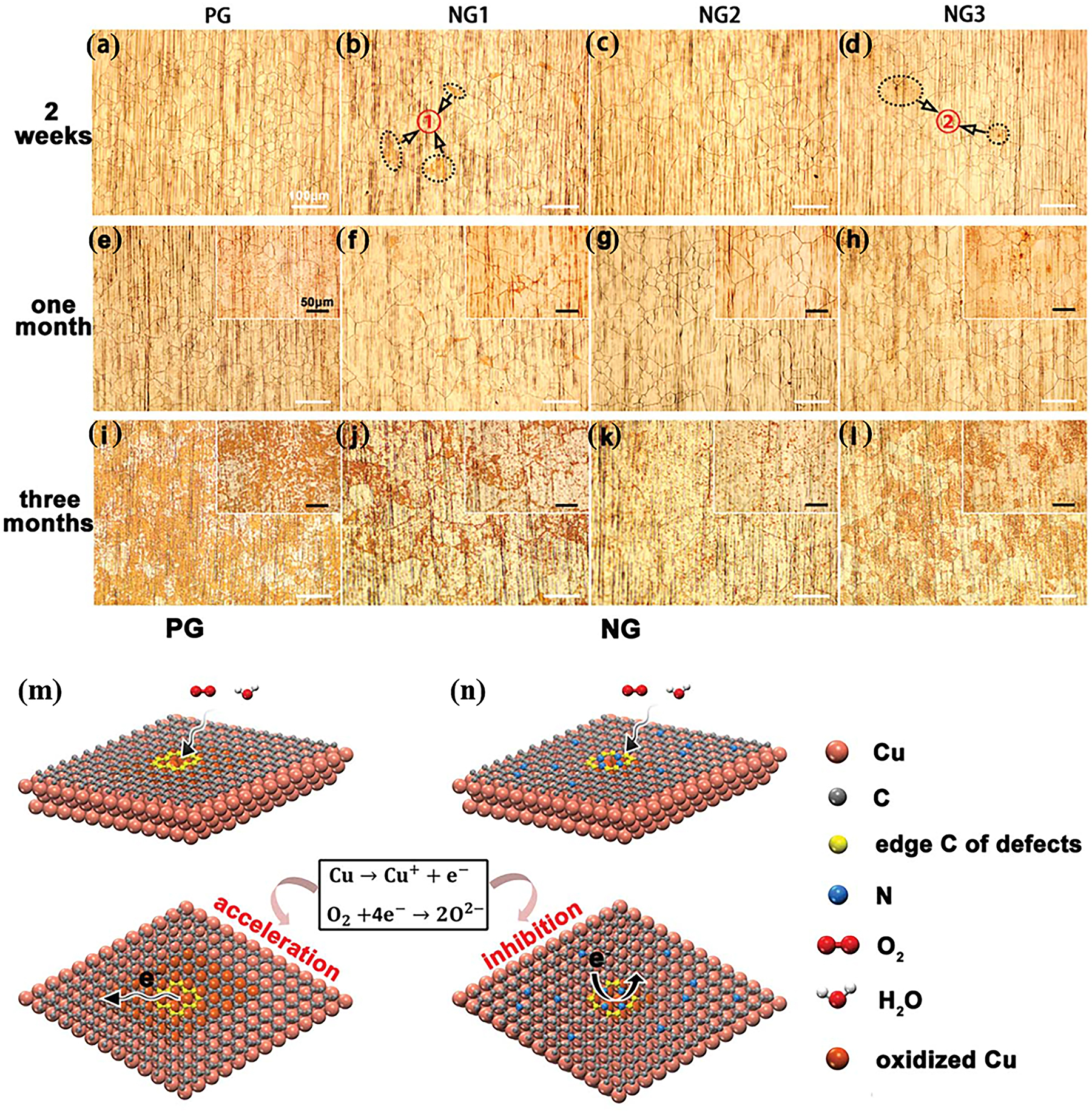
Optical micrographs of PG- and NG-coated copper foils exposed to air for (a–d) 2 weeks, (e–h) 1 month and (i–l) 3 months, respectively; scale bars are 100 mm. Areas 1 and 2 marked with ellipses in (b) and (d) are dark yellow areas of corrosion. The insets in (e–l) show corresponding enlarged optical images of PG- and NG-coated copper foils exposed to air for 1 and 3 months; scale bars are 50 mm. (m) Schematic illustration of the corrosion mechanism of (m) PG- and (n) NG-coated copper foils (Ren et al. 2018). Reprinted with permission from Royal Society of Chemistry.
In addition to the single heteroatom doping of graphene, the co-doping of boron and nitrogen can be another way to reducing the conductivity of graphene and promoting its anti-corrosion behaviour. This new material, termed hexagonal boron-carbon nitrogen (BCN), is composed of alternating boron, carbon and nitrogen atoms in a two-dimensional honeycomb lattice. However, the nanostructures of BCN are diverse due to the different bonding forms such as C–N bonds, B–N bonds and B–C bonds (Lei et al. 2011). Different bonding structures can have a profound effect on the properties and chemical activity of BCN materials, so the magnetic, optical and electrochemical properties of BCN materials can be further tuned by adjusting the elemental composition of the boron–carbon–nitrogen triad (Wang et al. 2017). Similarly, their anti-corrosion properties also largely depend on the elemental composition of BCN. The corrosion behaviour of B x C y N z films in acidic, neutral and alkaline solutions was investigated by measuring the dissolution rates using an ellipsometer (Byon et al. 2004). The dissolution rate of the B x C y N z films varied with the pH of the solution, specifically NaOH > NaCl > HCl. At the same time, the effect of different compositions on the dissolution rate of the films gradually increases as the carbon content decreases. This means that the doping of B/N in graphene with the composition of the film closer to that of BN would have better corrosion resistance. In fact, this strategy by using BCN films through doping of B/N in graphene for the corrosion protection has been already reported elsewhere (ArunKumar et al. 2017, 2018, Duan et al. 2022).
2.5.2 Fluorinated graphene film for anti-corrosion
As another case of doping graphene for anti-corrosion purposes, it is worth mentioning fluorinated graphene (FG), which exhibits good physical barrier properties, excellent electrical insulation and thermal stability with low surface energy (Ci et al. 2019; Gong et al. 2018; Lazar et al. 2015). Wu et al. performed vapour phase fluorination of graphene at different temperature (FG1 – room temperature, FG2 – 100 °C, FG3 – 200 °C) and measured their anti-corrosion effects comparing with PG (Wu et al. 2021c). Figure 13a shows optical micrographs of these four samples exposed to ambient conditions for 10, 30 and 90 days. As shown, with the air exposure time increased from 10 to 90 days, FG2 (100 °C) demonstrates the best anti-corrosion effects for copper substrate due to the appropriate fluorination temperature (100 °C), which lead to fluorinated graphene with more intact structure and, therefore, more resistant to the attach from corrosive species. In contrast, FG3 (200 °C) has a poor anti-corrosion resistance. This is because the relative high temperature (200 °C) disrupted the structural integrity of the graphene film. On this basis, the corrosion species could easily reach the copper substrate through the disrupted structure and initiate corrosion. However, the anti-corrosion barrier of FG1 (room temperature) doesn’t show much differences from that of PG mainly due to the low fluorination degree of graphene. Based on these experimental results, Figure 13b–c shows a schematic diagram of the corrosion protection mechanisms of the PG and FG coatings on copper. In the case of PG, the unavoidable defects provide a direct channel for the corrosion molecules to diffuse between the film and the substrate. In addition, the high electrical conductivity of graphene accelerates the local electrochemical corrosion at the graphene–copper interface (Figure 13b). Compared to PG, FG presents good corrosion resistance, especially in the case of FG2. The fluorine atoms bond with the carbon atoms at the edges of the vacancy defects, which greatly increase the physical barrier between corrosion species and the copper substrate. Moreover, the fluorination treatment reduces the electrical conductivity of graphene, effectively inhibiting electrochemical corrosion (Figure 13c). The FG coating is, therefore, able to provide long-term effective corrosion protection for the copper substrate, and even for some special applications such as triboelectric nanogenerators (Jiang et al. 2021).
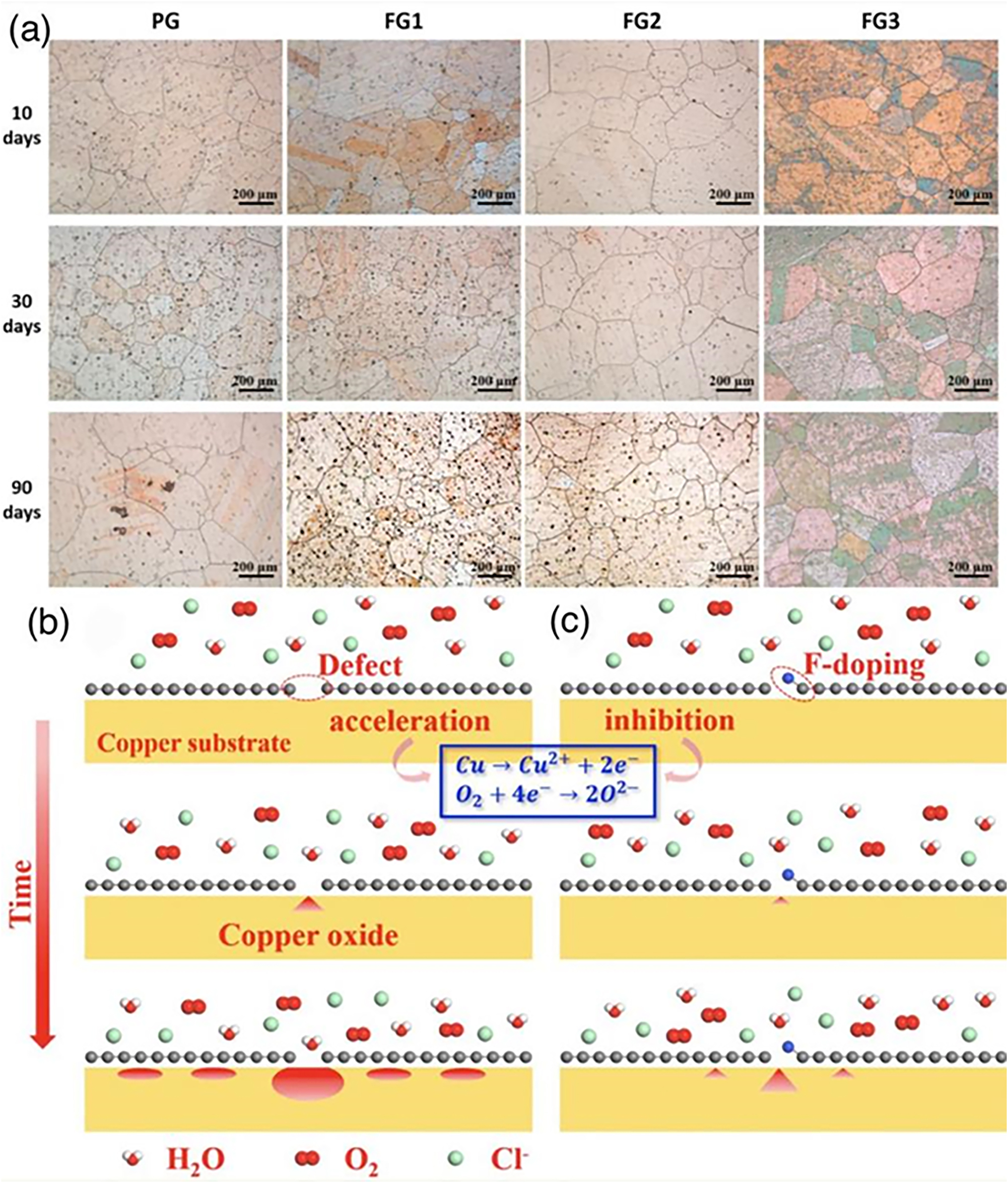
Fluorinated graphene for optimizing anti-corrosion properties. (a) Optical micrographs of PG- and FG-coated copper exposed to air for different times. (b) Schematic diagram of the corrosion protection mechanism of PG and (c) FG (Wu et al. 2021c). Reprinted with permission from Elsevier.
In all, the anti-corrosion effects of CVD graphene coatings are influenced by both the various defects of graphene itself and its intrinsic semimetal conductivity, wherein defects including structural defects and growth defects are mainly related with the CVD preparation processes on metal surfaces, while the semimetal conductivity of graphene would cause micro-galvanic corrosion in the long-term protection of metals. Therefore, controlling the quality of CVD graphene on metals is of great importance for the anti-corrosion action related with physical barrier. It can be optimized through controllable growth of graphene on metals, including multi-layer growth of graphene, interface engineering and even wrinkles/grain boundaries-free graphene (Chen et al. 2013; Wang et al. 2021). Meanwhile, post-healing approach exhibits high flexibility and compatibility to prepare large area CVD graphene coatings for metal anti-corrosion. On the other hand, conductivity modification of graphene can reduce the conductivity of graphene through doping graphene with heteroatoms, then preventing the micro-galvanic corrosion to some extent in the long term. With such a strategy, the doping process is also highly correlated with CVD processes. Thus, improving the quality and productivity of CVD graphene techniques on protective metals is still the key point for their anti-corrosion application as a metal coating. The representative works reporting the optimization strategies for anti-corrosion properties of CVD graphene coatings are summarized in Table 1 based on their mechanisms, with the main advantages extracted.
Comparison between the three anti-corrosion strategies of pure CVD graphene coatings.
| Strategies | Mechanism | Advantages | References | |
|---|---|---|---|---|
| Defects-healing | Selective deposition/chemical grafting of metal particles/polymers onto linear defects in CVD graphene, thereby repairing defective sites in graphene | Visualization of defect locations and extrapolation of notch sizes allows precise healing of defects without introducing additional defects; the binding of metal particles/polymers to defects does not spread to other regions of the graphene and does not affect the electronic properties of the original graphene |
Kim et al. (2014)
Hsieh et al. (2014) Wu et al. (2021b) |
|
| CVD growth | Multi-layer optimization | Structural defects in adjacent graphene layer do not overlap with each other, thus forming a tortuous path for corrosive species penetration of corrosive media | Provides years of protection for the substrate metal without altering the properties of the substrate |
Stoot et al. (2015)
Zhao et al. (2021) Shi et al. (2022) |
| Interface engineering | The strong interaction between graphene and the substrate metal prevents the lateral diffusion of corrosive species in interface, thereby inhibiting the oxidation of the metal substrate |
Xu et al. (2018)
Luo et al. (2019) Yuan et al. (2020b) Zhao et al. (2023) |
||
| Heteroatomic modification | The introduction of foreign atoms into the graphene lattice can significantly reduce the electrical conductivity of graphene, thus reducing electrochemical corrosion at the graphene–metal interface | Heteroatomic doping can modify the electrochemical properties of graphene by adjusting its local electron density and improving its capacitive properties. Foreign atoms (e.g. boron, nitrogen, fluorine) have similar atomic radii to carbon atoms, thus avoiding the formation of structural defects. The modified graphene after doping has a lattice structure similar to that of pure graphene, as well as good physical barrier properties, excellent electrical insulation and thermal stability |
Lee et al. (2018)
Ren et al. (2018) Wu et al. (2021c) |
|
3 Graphene composite coatings and the optimization strategies
CVD graphene coatings have many excellent properties and show a unique advantage in the corrosion protection of metals, but there are many limitations when it comes to industrial applications (Hui et al. 2014; Lanza et al. 2013; Wu et al. 2019b; Zhang et al. 2014a,b). Indeed, the process is difficult to scale up and industrialize under current technological conditions, and the cost of preparation is relatively high. On the other hand, graphene composites show great potential in the field of corrosion protection (Assad et al. 2023; Ding et al. 2023; Jena and Philip 2022; Qi et al. 2015; Qureshi et al. 2022). By physically or chemically mixing graphene or its derivative in forms of nanosheets with polymer matrices such as polyethylene (PE), poly(vinyl alcohol) (PVA), polystyrene (PS), polyacrylonitrile (PAN), etc., different kinds of GCCs are obtained (Yoo et al. 2014). GCCs combine the strong adhesion properties of graphene with the film-forming properties of the coating substrate, greatly improving the overall performance of the final coating. The preparation and application roadmap of GCC is shown in Figure 14. Covering metals with such composite coatings can effectively resist the intrusion of corrosion agents and provide long-term corrosion protection for metal substrates. In addition, compared to pure polymers, GCCs have additional properties, such as electrical conductivity, which could be useful in particular applications, thermal conductivity, thermal stability and mechanical properties (reinforcement action of graphene), showing great promise as multifunctional protective coatings (Das and Harimkar 2014; Kim et al. 2019; Shi and Zhu 2020; Wang et al. 2015; Yang et al. 2019).
Conceptual illustration of graphene composite anti-corrosive coatings on metal.
The corrosion protection mechanism of GCCs lies in their ‘labyrinth effect’. In the case of pure polymer coatings, once the corrosive medium penetrates the interface and reaches the metal substrate, corrosion reaction occurs almost unimpeded. The special physical structure of graphene makes it more resistant to penetration. Randomly dispersed in the coating matrix, graphene nanosheets hinder the penetration of corrosive agents by extending their diffusion path (Figure 15). As a result, a good dispersion of graphene derivatives in the coating is crucial for its shielding protection. Only when the graphene nanosheets are uniformly dispersed and stable in the coating can the composite coating achieve higher barrier properties (Papageorgiou et al. 2017; Pierleoni et al. 2016; Sekhavat Pour and Ghaemy 2016; Tan and Thomas 2016). The pure graphene surface has a high aspect ratio, van der Waals interactions and no functional groups, so it can easily agglomerate in aqueous solutions or organic matter, and this low dispersion severely reduces the anti-corrosion properties of the GCC. Therefore, improving the dispersion of graphene in the coating matrix is essential to improve the corrosion protection properties of the composite coating (Wang et al. 2019). In addition, graphene can play a key role in improving the toughness and elasticity of the coating, thus reducing the penetration of corrosion species by effectively hindering the expansion of cracks or tears in the coating.
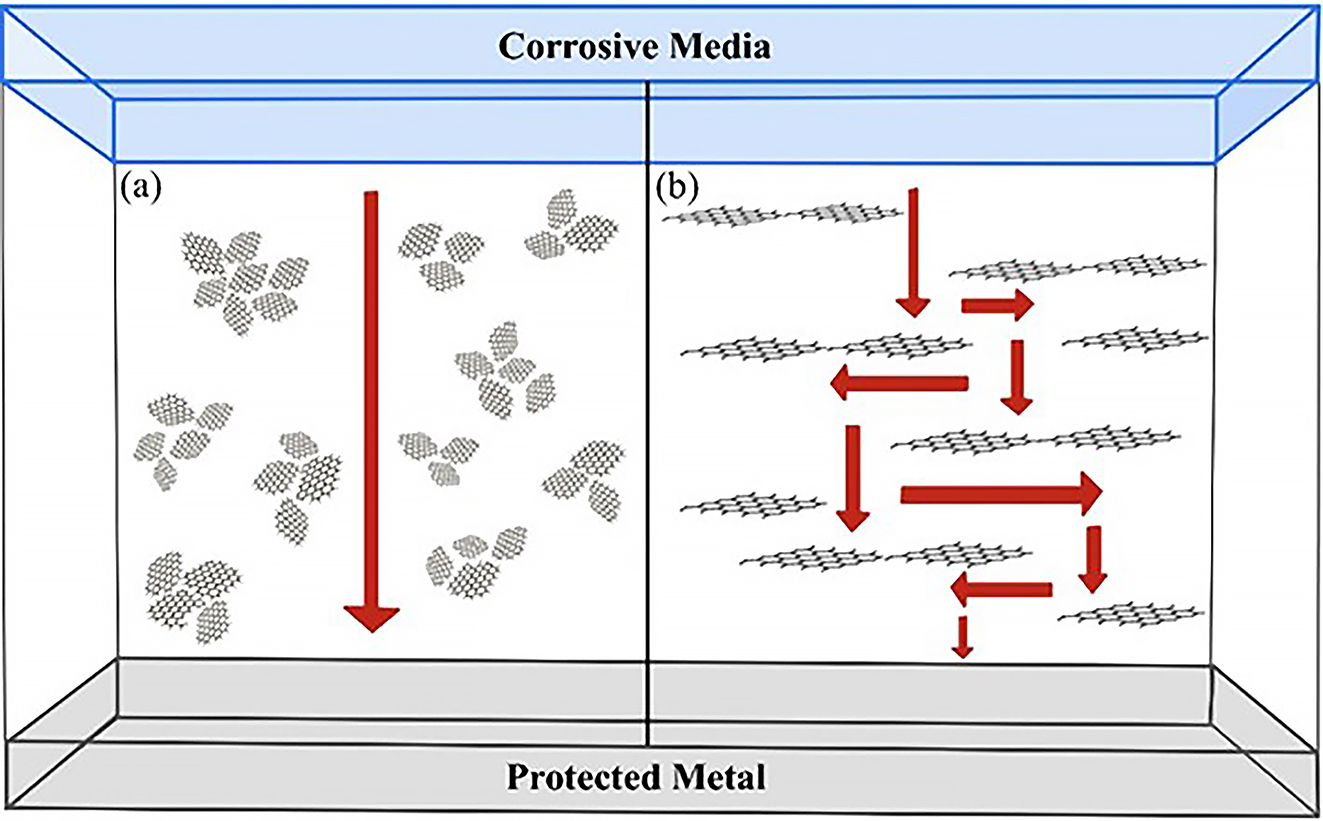
The corrosion protection mechanism of GCCs. (a) A poorly dispersed coating has a short permeation path. (b) Well-dispersed graphene prolongs the permeation path of the corrosive materials.
The preparation method and film-forming process of GCCs are based on the traditional coating preparation process and show good operability and controllability in industrial synthesis and application. It is believed that GCCs will become a main force in the production of new anti-corrosion coating materials (Chai et al. 2022; Cui et al. 2017; Huang et al. 2020, 2023; Rajabi et al. 2014). This section lists several optimization strategies for GCCs. By exploiting the impermeability and manipulating the electrical conductivity of graphene, the anti-corrosion performance of GCCs can be significantly improved.
3.1 Strategy of functionalization of graphene and its derivatives
It is well known that the dispersion of graphene in a polymer matrix can be significantly improved by graphene functionalization. Depending on the type of chemical bonding between graphene and other materials, functionalization can be classified as covalent and non-covalent functionalization. Covalent functionalization is the strong binding of small molecules, polymer chains or nanoparticles onto graphene (Liu et al. 2018a; Ma et al. 2020; Parhizkar et al. 2018; Wu et al. 2021a; Zhao et al. 2022; Zhang et al. 2022, 2023), while the non-covalent functionalization of graphene occurs through weaker interactions, such as hydrogen bonds, and π-π bonds and electrostatic interactions (Cui et al. 2018; Ding et al. 2018a; Liu et al. 2015; Qiu et al. 2018; Yuan et al. 2023; Zheng et al. 2021). Covalent functionalization is beneficial in maintaining the mechanical properties and chemical stability of graphene. Silane coupling agents are one of the most common chemical modifiers. After functionalization, the surface or edge of the graphene is grafted with a long polymer chain, which improves the compatibility of the graphene in the coating matrix. Parhizkar et al. used 3-(triethoxysilyl) propyl isocyanate (TEPI, IGO nano-fillers) and 3-aminopropyltriethoxysilane (APTES, AGO nano-fillers) for the covalent functionalization of GO, the resulting IGO and AGO were able to be stably dispersed in silane solutions. The experimental results show that the corrosion resistance and adhesion properties of the epoxy coating are significantly improved due to the better compatibility of IGO and AGO with the silane matrix and the formation of covalent bonds with the top epoxy coating (Parhizkar et al. 2018).
Liu et al. successfully prepared ionic liquid-graphene oxide (IL-GO) hybrid nanomaterials by covalently grafting imidazole ionic liquids onto the surface of graphene oxide nanosheets (Liu et al. 2018a). Scanning vibrating electrode technique (SVET) results show in the case of the IL-GO/epoxy composite coating a decreased trend in current density relative to the pure epoxy and rGO 0.5 % coating, exhibiting the lowest current density values after 30 h of immersion (Figure 16a–c). This effectively demonstrates that the IL-GO mixture performs its protective function by impeding the penetration of corrosive media and inhibiting anodic dissolution. The corrosion protection properties of the composite coating were further verified by salt spray testing, and Figure 16d–f shows digital photographs of the samples at various times. After 100 h of exposure, red rust stains appeared on the surface of both the pure epoxy and the rGO epoxy composite coating, with rust accumulating at the scratches and gradually spreading across the surface as the exposure time increased. However, the IL-GO composite coating showed no visible signs of corrosion even after 300 h of exposure, as the well-dispersed graphene nanosheets effectively hindered the penetration of the corrosive medium and, in addition, the ionic liquid prevented the oxidation of the steel in the scratches. The micro-pores and cracks that form during coating formation and applications are the channels through which the corrosive medium penetrates the coating and initiates the corrosion reaction of the metal. Figure 16g shows a schematic diagram of the protection mechanism of pure epoxy and IL-GO composite coating. The pure epoxy resin coating has an inferior barrier effect and corrosion products can reach the metal surface and initiate corrosion in a short period of time. As for the IL-GO hybrid epoxy composite coating, the well-dispersed graphene nanoflakes impede the straightforward penetration of corrosive media and the covalently grafted imidazole ionic liquid imparts corrosion inhibition to the hybrid material, allowing the composite coating to exhibit significant corrosion resistance. Besides, Wu et al. synthesized a novel bio-based cardanol epoxy–modified graphene oxide (GODN) nanomaterial through the rapid phase transfer process and applied it to epoxy coatings, then explored the anti-corrosion performance of the GODN/EP composite coating (Wu et al. 2021a). DN chains were successfully attached to the surface of GO without damaging the sp2 structure of graphene. In addition, DN also promoted the formation of chemical bonds between GO and EP resin and improved the interface interaction between GO and EP resin. EIS results showed that the addition of GODN had significantly enhanced the anti-corrosion performance of the coating. The schematic diagram of corrosion protection mechanism of the GODN/EP composite coating is shown in Figure 16h. Based on such covalent functionalization of graphene, Zhao et al. recently prepared an EP/GF/A-GO composite coating with a dual physical barrier by incorporating 3-(2-aminoethylamino) propyldimethoxymethylsilane–modified graphene oxide (A-GO) and glass fibre (GF) into an epoxy matrix. Figure 16i shows a schematic diagram of the preparation of the covalently modified A-GO. The modified GO has better compatibility with the epoxy resin, which enhances the crosslink density of the composite coating and reduces defects of coating. In addition, the amine terminal groups of AAPDS can react with the epoxy groups of the epoxy resin and these significantly enhance the barrier performance of the composite coating. The EIS results show that the EP/GF/A-GO composite coating has the highest impedance modulus of the different coatings. After 45 days of immersion, the value was still above 3.9 × 107 Ω cm2 (Zhao et al. 2022).
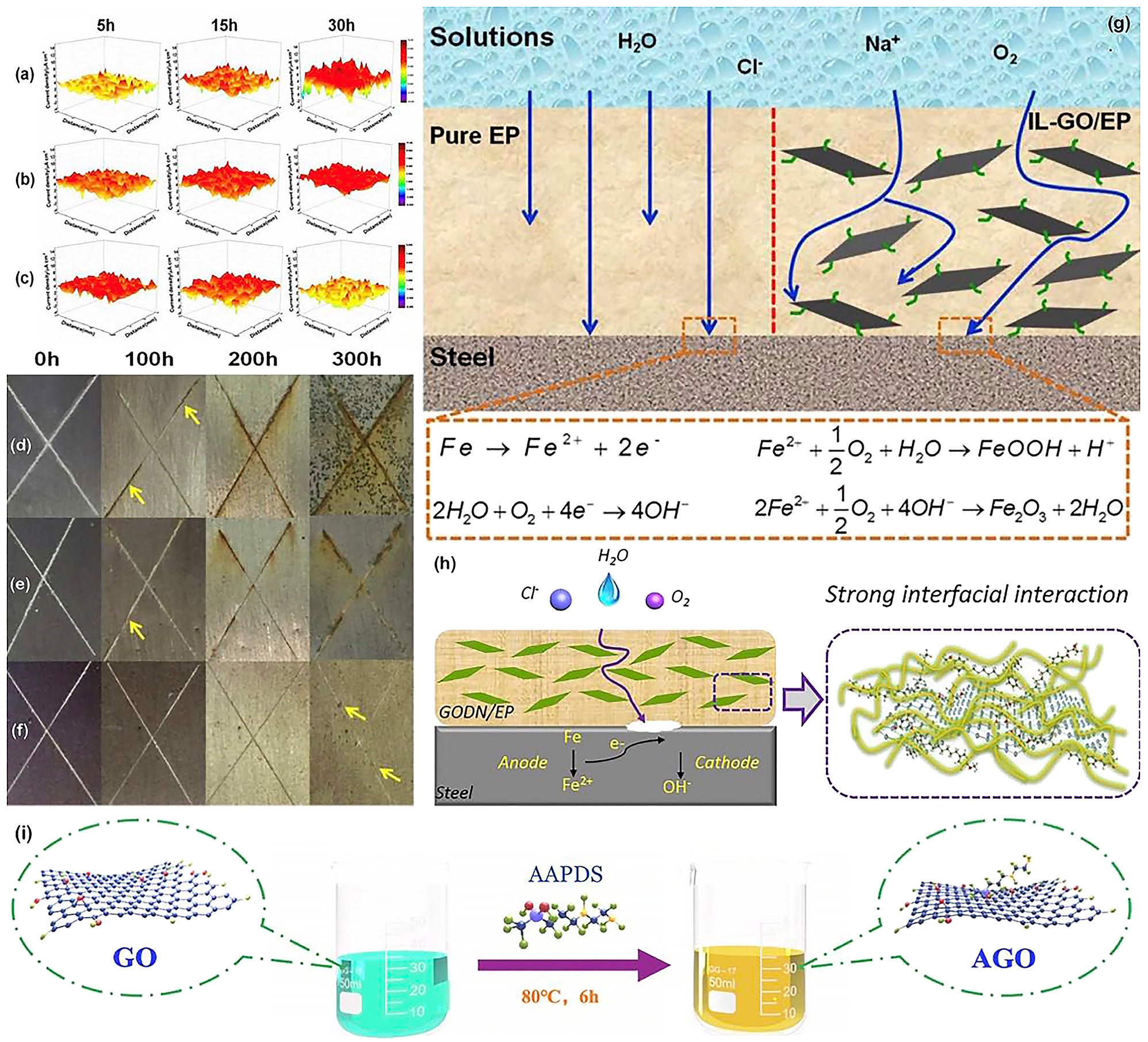
Covalent functionalization of GO for optimizing anti-corrosion properties. (a) SVET maps of the current density for steel electrodes coated with pure epoxy, (b) rGO0.5 % and (c) IL-GO0.5 % coatings immersed in 3.5 wt% NaCl solution. (d) Digital images of the salt spray tested steel substrates covered with pure epoxy, (e) rGO0.5 % and (f) IL-GO0.5 % coatings. (g) Illustration of protective mechanism for pure epoxy and IL-GO hybrids coatings (Liu et al. 2018a). Reprinted with permission from Royal Society of Chemistry. (h) Schematic diagram of corrosion protection mechanism of GODN/EP coatings in 3.5 wt% NaCl solution (Wu et al. 2021a). Reprinted with permission from Elsevier. (i) Schematic illustration for preparation of A-GO (Zhao et al. 2022). Reprinted with permission from Elsevier.
Non-covalent functionalization helps to maintain excellent electrical conductivity and ultra-high specific surface area of graphene (Imani et al. 2018). In addition, the non-covalent functionalization of graphene is easier to manipulate and does not require complex chemical reactions compared to covalent functionalization. For example, embedding non-covalent functionalized graphene flakes in aqueous epoxy resin (EP) coatings led to substantial improve of the corrosion resistance of EP coating (Liu et al. 2015). Yuan et al. prepared a unique bi-layer composite coating using a two-step high-speed spinning method, which consisting of primer thermoplastic polyurethane (TPU) layer and top phenylenediamine (PPD) non-covalent modified orientated graphene (Gr-PPD) incorporated epoxy resin layer. The orientated Gr-PPD nanosheets effectively extend the penetration pathway of the corrosive media. In addition, the interfacial compatibility between Gr-PPD and EP is enhanced by involvement of amino groups on surface of Gr in epoxy curing reaction, which reduces the free volume spaces in composites and thus lowering gas permeability. The experimental results showed that 0.5Gr-PPD-EP/TPU coating had the highest impedance value after 90 days of immersion in NaCl solution and the strongest toleration to continuous salt spray attack for 60 days, making it ideal for industrial applications (Yuan et al. 2023). Ding et al. synthesized a novel hydroxy epoxy phosphate monomer (PGHEP) as an effective dispersant for graphene, which greatly improved the compatibility of graphene in epoxy resins (Ding et al. 2018a). A schematic diagram of the dispersion process of PGHEP functionalized graphene in water is shown in Figure 17a. Raman spectroscopy, ultraviolet-visible spectroscopy (UV–vis) and X-ray photoelectron spectroscopy (XPS) studies confirmed the efficient π-π interaction between PGHEP and graphene sheets. The results of EIS and salt spray tests show that the composite coatings offer greater corrosion resistance, higher contact angles (thus are more hydrophobic) and lower water absorption compared to pure epoxy resin. This excellent corrosion protection is mainly attributed to the good dispersion of the functionalized graphene nanosheets in the coating matrix. In addition, Zheng et al. synthesized polydopamine (pDA)-graphene (pDA-GR) composites on Cu substrate, and found through experiments that pDA can heal the inherent defects of GR through π-π interaction, thus effectively improving the corrosion resistance of the coating (Zheng et al. 2021), as shown in Figure 17b.
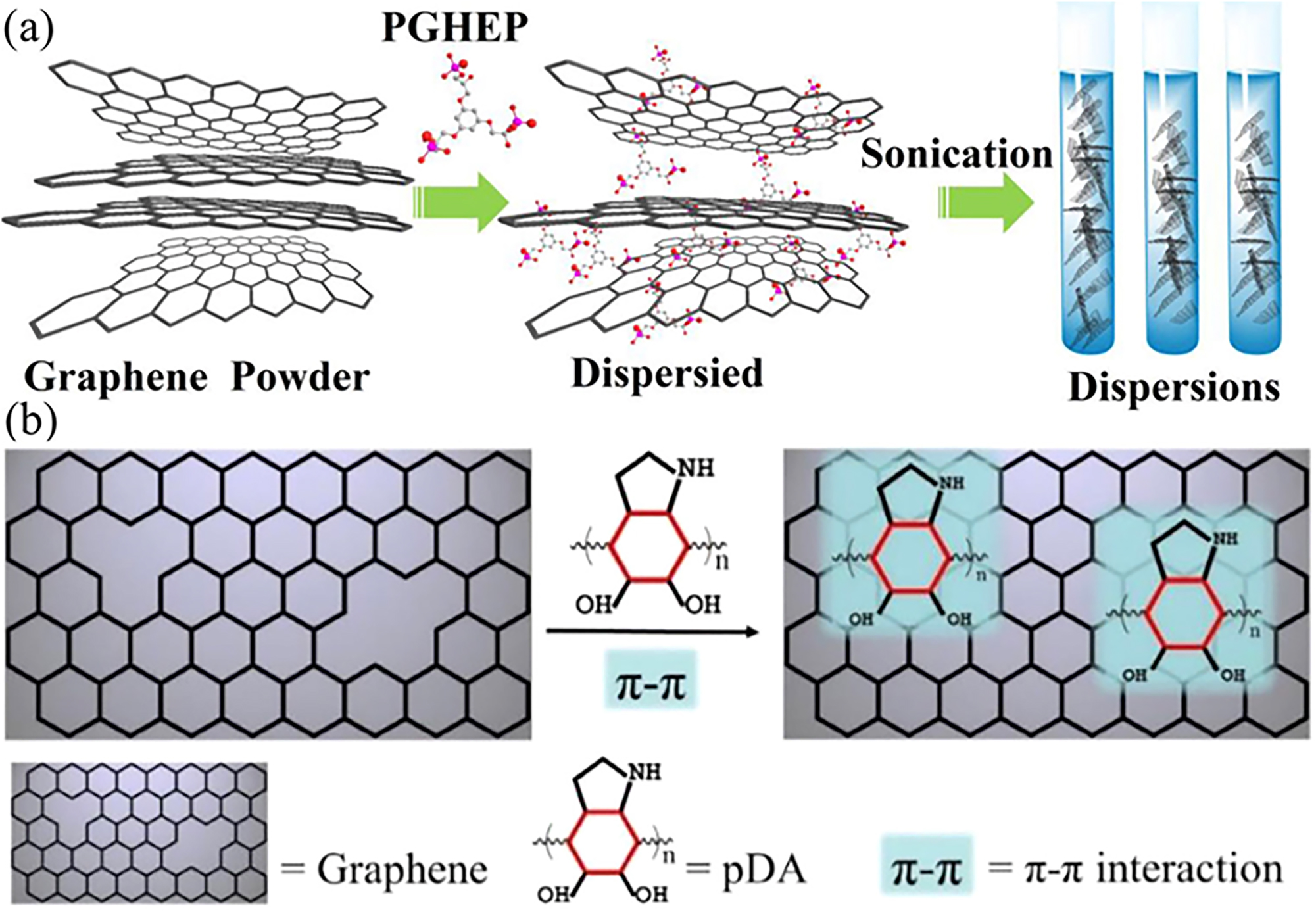
Non-covalent functionalization of graphene for optimizing anti-corrosion properties. (a) The dispersion process of PGHEP functionalized graphene in water (Ding et al. 2018a). Reprinted with permission from Elsevier. (b) Schematic representation of pDA healing structure defects of GR via π-π interaction (Zheng et al. 2021). Reprinted with permission from Elsevier.
It is important to note that both covalent and non-covalent functionalized graphene could be incorporated into the coating matrix at the same time and the different types of graphene can synergistically enhance corrosion resistance. Zhan et al. investigated the synergistic functionalization of bio-inspired graphene oxide/Fe3O4 hybrids and their synergistic effect on the corrosion protection properties of epoxy coatings (Zhan et al. 2018). The graphene oxide/Fe3O4 hybrid was functionalized by self-polymerization between dopamine and KH550, with dopamine adsorbed on the surface of the graphene in the form of π-π interactions and KH550 covalently functionalizing the graphene. The experimental results showed that the bio-inspired synergistic functionalization significantly improved the dispersion of the graphene oxide/Fe3O4 hybrid in the epoxy resin and enhanced the interfacial adhesion between the nanofiller and the epoxy coating through chemical cross-linking reactions. The EIS test results showed that the corrosion resistance of the epoxy coating with the addition of 0.5 wt% of the modified bio-inspired functionalized GO/Fe3O4 blend was significantly improved compared to the pure epoxy and other nano-filler composite coatings (Figure 18a–d). The presence of Fe3O4 significantly improved the dispersion of GO, which together with the co-functionalization of KH550 and dopamine further improved the distribution and interfacial adhesion of the GO/Fe3O4 mixture, resulting in an overall significant increase in the anti-corrosion performance of the composite coating. The dispersion of the nanoparticles and their interfacial adhesion to the epoxy resin matrix are two key elements in considering the corrosion protection performance of the coating. Figure 18e–g shows a schematic diagram of the corrosion protection mechanism of the epoxy resin composite coating. H2O and O2 are the main factors contributing to the corrosion of steel. For pure epoxy resin, the corrosion factors can easily penetrate the coating through its inherent micropores (Figure 18e). In contrast, with the addition of 0.5 wt% GO-Fe3O4 mixture, the high aspect ratio of GO and the synergistic effect of GO and Fe3O4 nanoparticles resulted in a significant increase in the corrosion protection performance of the composite coating, as shown in Figure 18f. The modified bio-inspired functionalization of the GO/Fe3O4 hybrid was achieved through self-polymerization between dopamine and KH550. The synergistic functionalization allowed the hybrid to be well dispersed in the epoxy resin and further enhanced the interfacial adhesion between the nanoparticles and the epoxy resin matrix, as shown in Figure 18g. This bio-inspired synergistic functionalization is very different from conventional surface functionalization. After the functionalization, the corrosion protection properties of the epoxy composite coatings were significantly improved, in addition to their micro-mechanical properties, which also provided a new idea for the preparation of epoxy-based composites with excellent corrosion protection properties.
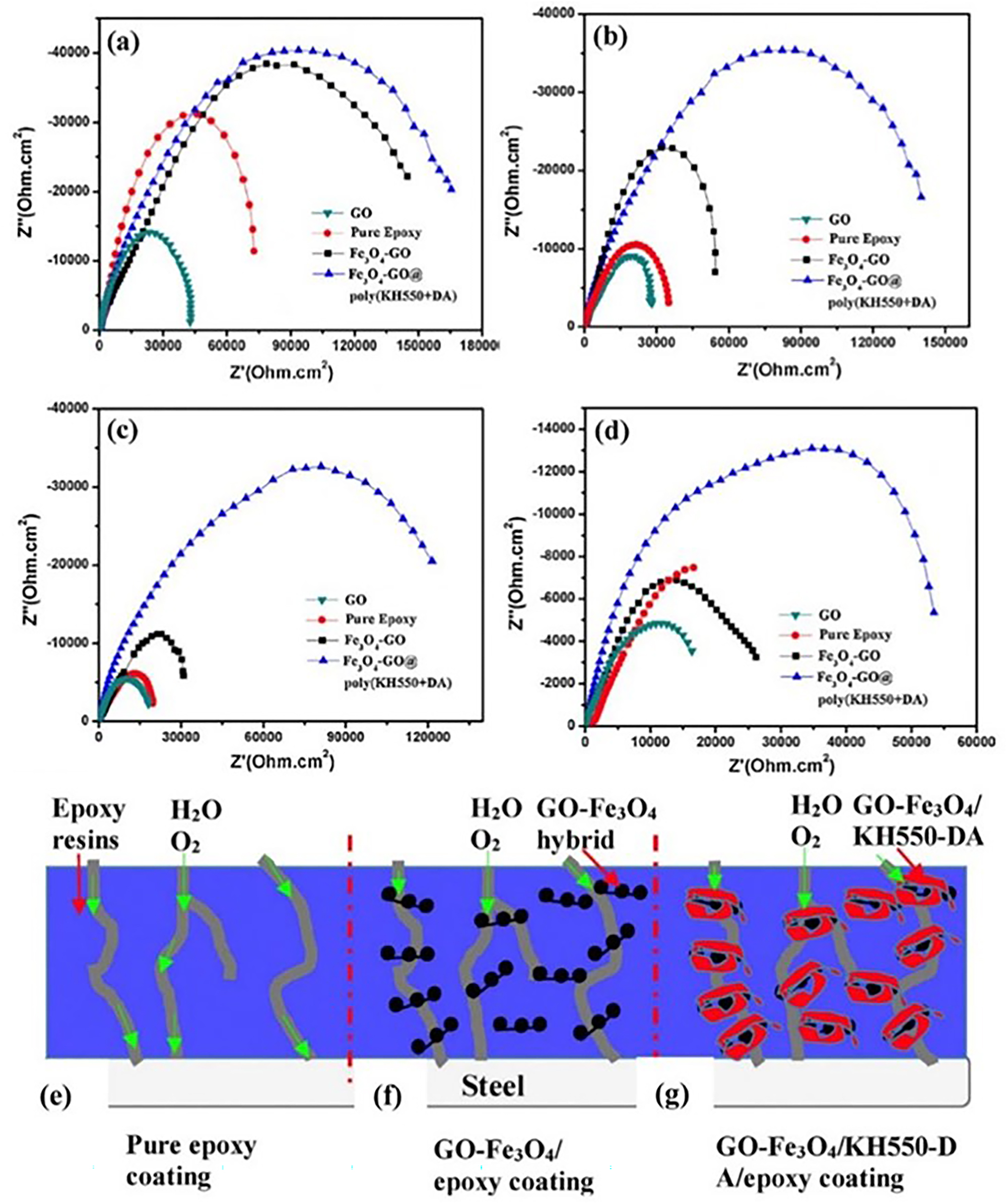
Impedance spectra of various epoxy composite coatings (with 0.5 wt %) after soaking for different times: (a) 12 h, (b) 24 h, (c) 72 h and (d) 144 h. (e) Anti-corrosion mechanism of pure epoxy coating, (f) GO-Fe3O4/epoxy coating and (g) GO-Fe3O4@poly (KH550 + DA) hybrid/epoxy coating (Zhan et al. 2018). Reprinted with permission from Elsevier.
Functional groups functionalized on the surface of graphene can prevent agglomeration by forming covalent bonds with certain groups in the polymer to anchor graphene to polymer chains. However, covalent functionalization often results in the breaking of covalent bonds or ring-opening reactions, which reduces the barrier effect of graphene, leading to the penetration of corrosive media such as water, oxygen and chloride ions (Yang et al. 2011a). In contrast, non-covalent functionalization has no bond breakage or ring opening reactions, thus ensuring the structural integrity of graphene (Imani et al. 2018). In addition, special attention needs to be paid to the fact that the degree of chemical functionalization can have a significant impact on the corrosion protection properties of GCCs. A high degree of functionalization not only helps to improve the dispersion of graphene and the overall performance of the composite coating but can also greatly reduce the electrical conductivity of graphene and inhibit galvanic corrosion referring to graphene composites. In contrast, a low degree of functionalization can greatly improve the dispersion of graphene while slightly reducing the electrical conductivity of graphene, which may lead to incomplete inhibition of galvanic corrosion of GCCs. In addition, the concentration of functionalized graphene can significantly affect the shielding effect of the coating. A right amount of functionalized graphene can be evenly and stably dispersed in the coating matrix, whereas adding too much can lead to the accumulation of graphene in the coating, thus creating pores that reduce the corrosion resistance of the coating (Liu et al. 2016; Zhu et al. 2020).
3.2 Strategy of polymer-hybrids
In addition to the chemically modified strategies described in the previous section, composite coatings formed by alternate stacking of graphene films with other films (e.g. polymers) in a layer-by-layer fashion can also provide effective long-term corrosion protection for metal substrates.
Zhu et al. used plasma-enhanced chemical vapour deposition (PECVD) technique to grow fluorocarbon polymer films on monolayer graphene films and the resulting hybrid coatings exhibited much improved corrosion protection action (Zhu et al. 2019). Fluorocarbon polymer films provide effective physical barrier of corrosive media due to their low surface free energy and hydrophobicity (Abadjieva et al. 2012; Drummond et al. 1997). Figure 19a–d shows schematic diagrams of the growth and corrosion resistance mechanisms of the fluorocarbon polymer–graphene hybrid films. The fluorocarbon polymers first nucleated in the defective areas of the graphene and then cross-linked with each other with increasing deposition time. And finally, a uniform and complete fluorocarbon polymer film tightly covered the graphene. The hydrophobic fluorocarbon polymer film covered the defects in the graphene film, inhibiting direct corrosion at the defect site and preventing galvanic coupling between the graphene and the Cu substrate. In addition, the impermeability of graphene inhibited the penetration of corrosive media into the metal substrate, resulting in the layer-by-layer hybrid coating exhibiting superior corrosion protection. It also has been proposed a layer-by-layer composite coating to develop highly efficient microwave absorbers with optimized corrosion resistance (Xu et al. 2021). Three-dimensional NiAl-layered double hydroxide/graphene (NiAl-LDH/G) composites were synthesized by atomic layer deposition–assisted in situ growth (Figure 19e). Combining the excellent impermeability of graphene with the ability of NiAl-LDH to trap chloride ions, the composite coating exhibited long-term corrosion resistance on carbon steel surfaces.
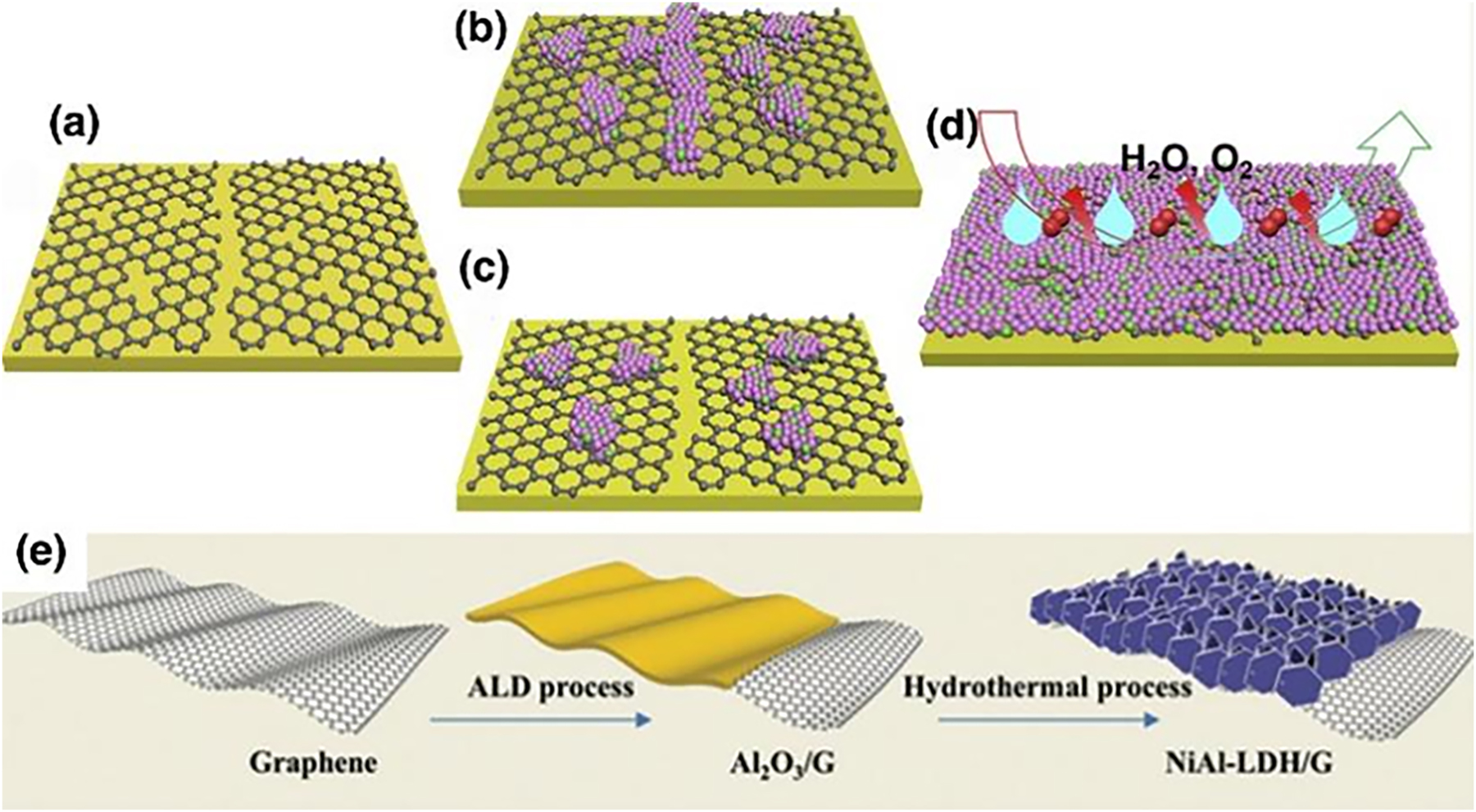
Corrosion resistance mechanisms of polymer-graphene hybrids coatings. (a–d) The diagrammatic sketch of the grow process and anti-corrosion mechanism of fluorocarbon polymer/SLG hybrid film (Zhu et al. 2019). Reprinted with permission from Elsevier. (e) Schematic illustration of the preparation process of NiAl-LDH/G (Xu et al. 2021). Reprinted with permission from Wiley Online Library.
In addition to bilayer configuration of graphene and polymers, multi-layer assembly has also been developed. For example, Yu et al. developed a graphene–polymer hybrid coating that sandwiched two CVD graphene single layers between three polyvinyl butyral (PVB) polymer films. Such a hybrid coating was able to protect metal substrates for up to 4 months in simulated seawater, showing superior corrosion protection to other coatings (Yu et al. 2018). Figure 20a–h shows the corrosion patterns of the three samples after immersion in the NaCl solution for a period of time. For aluminium alloy coated with one graphene single layer sandwiched by two PVB films (AA-P-G-P) and aluminium alloy coated only by PVB (AA-P-P-P), extensive corrosion marks appeared on the surface after 30 days of immersion (Figure 20a, b and e–f). For the aluminium alloy coated with two graphene single layers each sandwiched by two PVB films (AA-P-G-P-G-P) samples, no visible signs of corrosion were observed after optical inspection after 30 and 120 days of immersion (Figure 20c–d). From the SEM images, the surface of the samples was uniformly smooth, whether immersed for 30 or 120 days (Figure 20g–h). This demonstrates the limited corrosion resistance of the P-G-P and P-P-P coatings, while highlighting the excellent performance of the P-G-P-G-P multi-layer hybrid coatings even over a long period of time. The EIS test results showed that the AA-P-G-P-G-P samples still had a |Z|0.01Hz value in the range of 109 Ω cm2 even after 120 days of immersion, indicating excellent barrier properties and outstanding resistance to environmental degradation. Figure 20i shows the open circuit potential (OCP) and the relationship between corrosion current density and low-frequency impedance for different tested samples. Both samples in the first group were not in direct contact with the substrate and were characterized by small |Z|0.01 Hz values and high corrosion current densities. The second group of coatings, despite providing some initial protection, still did not prevent the attack of the corrosion species on the substrate, with OCP values comparable to those of the bare substrate. The third group of samples had OCP values approximately 1.5 V higher than the bare substrate and had the highest |Z|0.01 Hz values and the lowest corrosion current density. Figure 20j-m shows a schematic diagram of the corrosion protection mechanism for a graphene–polymer hybrid coating. Graphene films are insufficient to resist penetration of corrosion species due to their high electrical conductivity and various defects (Figure 20j). Pure polymer coatings, although insulating and thicker, do not have the impermeability that graphene has (Figure 20k). A heterogeneous coating with a thin layer of graphene added provides short-term protection to the substrate. However, over time, corrosion species absorbed by the top polymer film could penetrate through cracks and defects in the single graphene layer and reach the bottom polymer film, eventually diffusing to the metal surface and triggering corrosion reactions (Figure 20l). By alternating two graphene films with three polymer films, such a multi-layer configuration of graphene and polymer has an optimizing combination of the adhesive and insulating properties of polymer films and the impermeability of graphene, which effectively retards the spread of corrosive species (e.g. P-G-P-G-P structure and P-P-G-G-P structure) (Figure 20m). This simple and convenient strategy for preparing high-performance anti-corrosion coatings can also be extended to other 2D materials and polymers for the long-term protection of metals and alloys.
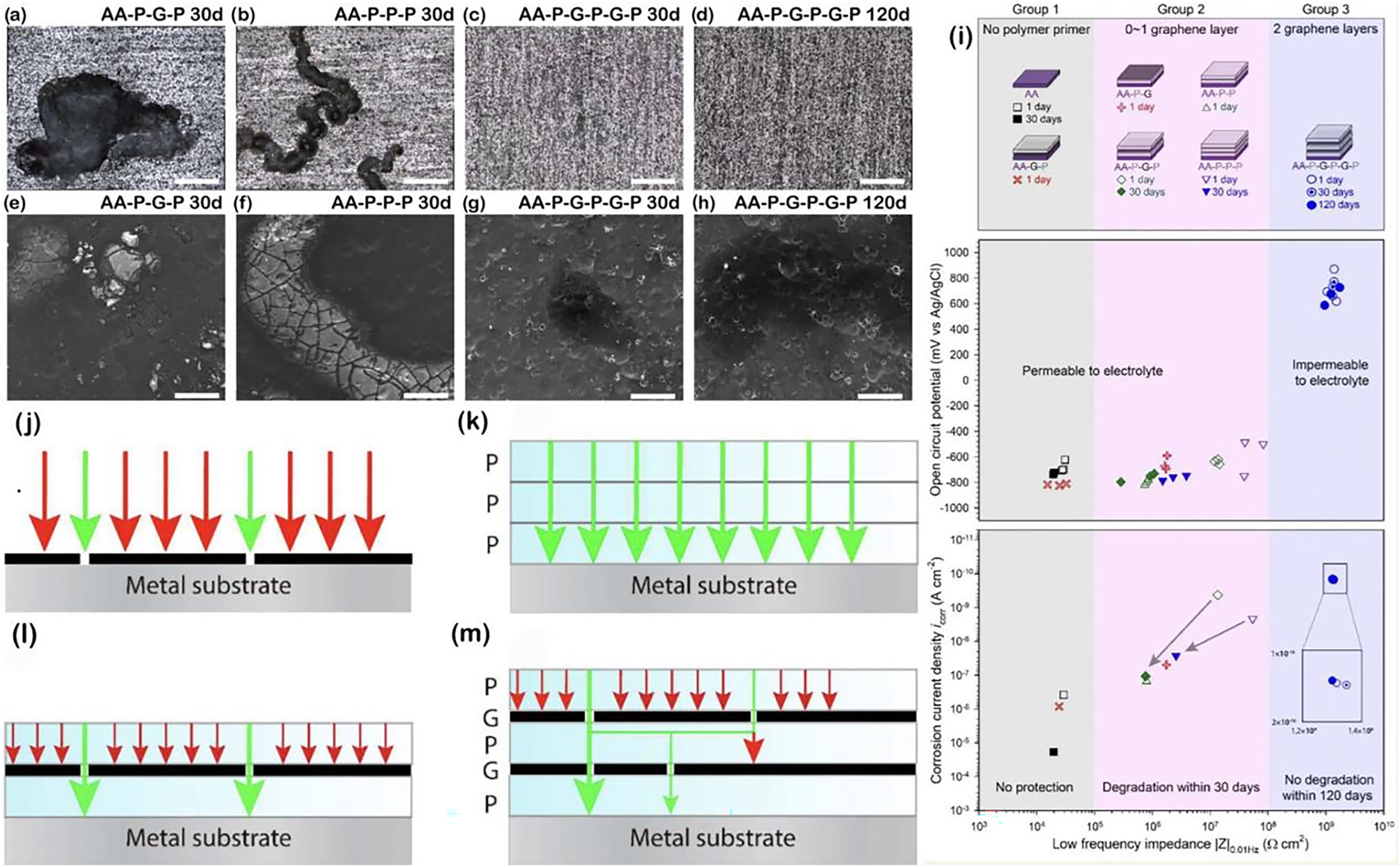
Optical images (a, b) and SEM images (e, f) of AA-P-G-P and AA-P-P-P after 30 days of immersion in 3.5 wt% NaCl solution; optical images (c, d) and SEM images (g, h) of AA-P-G-P-G-P after 30 and 120 days of immersion in 3.5 wt% NaCl solution. (i) Grouping of all samples tested and summary of the results of the electrochemical tests. (j–m) Schematic representation of the corrosion protection mechanism of (j) ungrown graphene (G), (k) bare polymer (P), (l) P-G-P and (m) P-G-P-G-P coatings on metal substrates (Yu et al. 2018). Reprinted with permission from Elsevier.
3.3 Strategy of graphene-improved cathodic protection
In some anti-corrosion coatings, cathodic protection is usually employed by adding into coating matrix active metals (e.g. Zn, Al), whose potential is negative compared to that of protected substrates. Typically, zinc-rich epoxy coatings (ZREs) are often used to protect metal substrates in harsh environments, where the zinc powder particles are sacrificed as the anode and the substrate is protected as the cathode (Jalili et al. 2015; Lei et al. 2020; Marchebois et al. 2004; Shreepathi et al. 2010). In addition, the corrosion products produced by the zinc powder particles plug the voids in the coating, thus reinforcing the barrier effect of the coating. Over time, however, these non-conductive corrosion products produced by zinc accumulate in large quantities, impeding the contact between the zinc powder and the substrate, and compromising the cathodic protection of the zinc powder. To ensure contact between the zinc powder and the substrate, a high zinc content of over 80 wt% is usually required (Akbarinezhad et al. 2011; Cubides et al. 2016; Langer et al. 2019). However, the excessive addition of zinc powder is not fully utilized (Gergely et al. 2013) and instead causes environmental pollution and waste of resources.
To this end, the addition of graphene to zinc-rich coatings is an excellent strategy for optimizing the corrosion protection of the composite coatings. On the one hand, the addition of graphene provides a bridge for the transfer of electrons between zinc powder particles and then the metal substrate thanks to its excellent electrical conductivity, thus further improving the cathodic protection of the zinc-rich coating (Ding et al. 2017). On the other hand, graphene-enhanced ‘labyrinth effect’ is another optimized factor that provides the enhanced protection performance of graphene-reinforced zinc-rich coatings. Ding et al. investigated the microscopic mechanism of graphene to improve the cathodic protection of zinc-rich coatings (Ding et al. 2019). The electrons lost by the sacrifice of zinc as an anode needed to cross two types of potential barriers during their migration to the protected substrate, the metal–graphene barrier and the graphene–graphene barrier (Figure 21). Analysis based on physical models and mathematical calculations indicated that the electron penetration of the latter was less difficult than the former and that graphene facilitated the electron transfer between metals. Generally, graphene presents a dual effect of impermeability and electrical conductivity in the coating. The impermeability of graphene reduces the rate of diffusion of corrosive media in the coating, while the electrical conductivity of graphene increases the electrical contact between the zinc particles and the substrate, which improves the utilization of the zinc powder and enhances the cathodic protection of the coating.
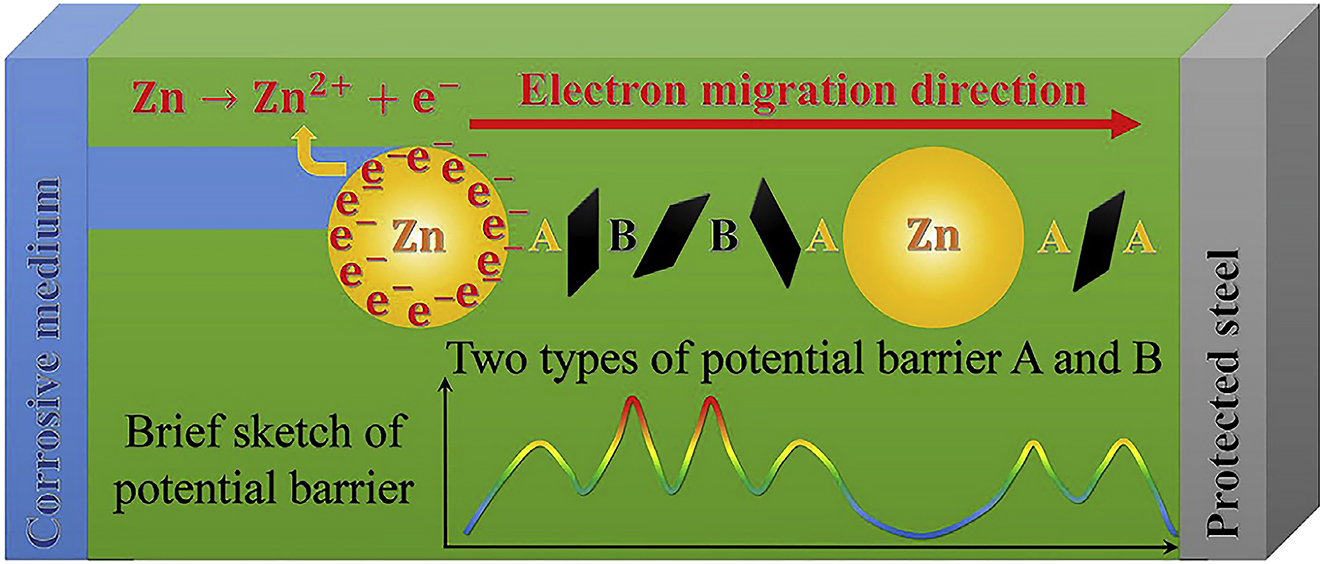
Two kinds of potential barrier that electrons lost by anode sacrifice of zinc need to cross in the conduction process in zinc-rich coatings: (A) metal–graphene potential barrier and (B) graphene–graphene potential barrier (Ding et al. 2019). Reprinted with permission from Elsevier.
Many studies have shown that the incorporation of graphene and its derivatives (e.g. graphene oxide) can improve the corrosion resistance of ZREs (Ding et al. 2018b; Hayatdavoudi and Rahsepar 2017; Teng et al. 2018). Zhou et al. incorporated rGO and/or GO nanosheets into ZREs and obtained composite coatings with much different cathodic protection duration (Zhou et al. 2019). As seen, Figure 22a–d shows SEM images of several samples without and with incorporation of rGO and/or GO after SVET testing. The ZRE surface was covered with large particles and spheres, and the oxide layer completely filled in the indentation points, indicating that the samples were severely corroded (Figure 22a). Some obvious corrosion marks were also observed on the surfaces of ZRE-GO and ZRE-rGO/GO (Figure 22c–d). In contrast, the surface of the ZRE-rGO sample was very smooth with only a few tiny particles present (Figure 22b), suggesting that the addition of rGO was effective in improving the corrosion resistance of the ZRE coating. Figure 22e shows the charge transfer resistance (R ct) of the four samples as a function of immersion time. ZRE-rGO showed the highest R ct values after 57 days of immersion, indicating that its protection was optimal. Figure 22f shows the variation of OCP values for the four samples as a function of immersion time. After 45 days of immersion, the cathodic protection of ZRE disappeared and ZRE-rGO exhibited lower OCP values than the other two samples, indicating its effective cathodic protection behaviour. Figure 22g shows the corrosion resistance mechanisms of ZRE, ZRE-rGO and ZRE-GO. Pure ZRE coatings are unable to provide long-lasting corrosion protection for steel due to their large number of cracks and pores. GO nanosheets have very poor electrical conductivity and, therefore, rely mainly on the barrier effect for protection. In contrast, rGO nanosheets play a dual role in the corrosion protection process with high electrical conductivity and impermeability, which can comprehensively enhance the cathodic protection of zinc-rich coatings.
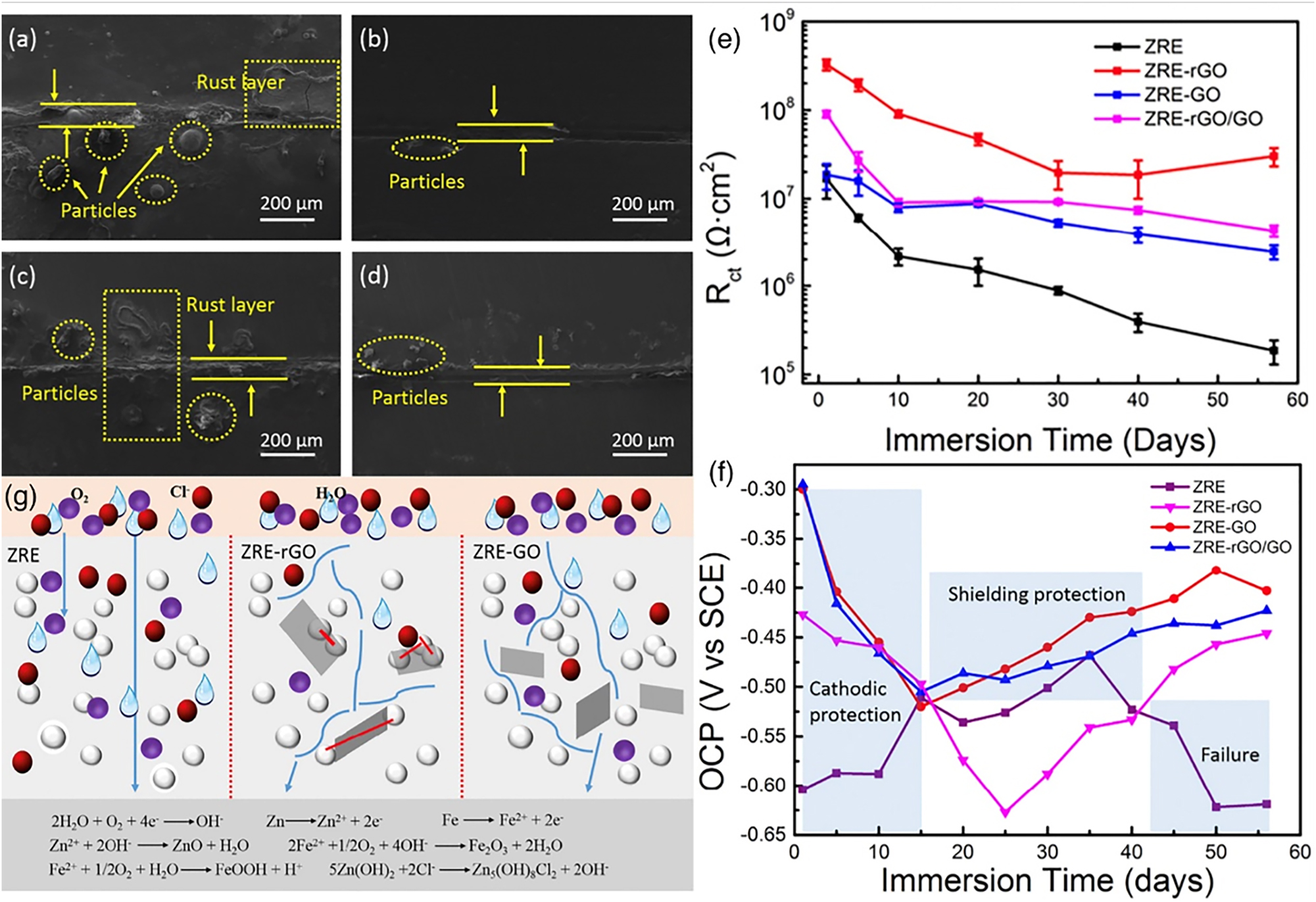
SEM micrographs of (a) ZRE, (b) ZRE-rGO, (c) ZRE-GO and (d) ZRE-rGO/GO after SVET testing. (e) R ct of prepared coatings immersed in 3.5 wt% NaCl solution. (f) Variation of OCP values for different samples at successive immersion times. (g) Illustration of the corrosion protection mechanism of the different coatings (Zhou et al. 2019). Reprinted with permission from Elsevier.
In addition, the amount of graphene added has also a significant impact on the cathodic protection performance of the coating (Chen et al. 2022; Hayatdavoudi and Rahsepar 2017; Liu et al. 2018b). Bai et al. investigated ZREs with different graphene contents and demonstrated that there was an optimum amount to achieve the cathodic protection and barrier effect of ZREs (Bai et al. 2021). Figure 23a shows the pore diameter distribution of ZRE without and with graphene contents. The calculation of porosity and average pore diameter reveals that when the graphene content is below 0.3 wt%, an increase in graphene content leads to a decrease in the coating porosity and average pore diameter. On the other hand, when the graphene content exceeds 0.3 wt%, a further increase in graphene content does not lead to a change in the pore size and average pore diameter of the coating. Figure 23b shows the OCP evolution of the different samples as a function of immersion time in NaCl solution. The ZRE without graphene had OCP values above the threshold potential after 168 h of immersion, whereas the ZRE with graphene required a longer soaking time for the OCP values to be above the threshold potential. The ZRE with 0.3 wt% graphene requires the longest soaking time. Figure 23c shows the charge transfer resistance R ct versus immersion time for several samples. The R ct of ZRE with graphene was higher than that of ZRE without graphene. In addition, the amount of graphene affected the variation of R ct. R ct increased with increasing graphene content when the graphene content was between 0 and 0.3 wt% and decreased with increasing graphene content when the graphene content was between 0.3 and 1.2 wt%. To further investigate the mechanism, the corrosion rate of zinc was measured using the H2 evolution technique. The results showed that the ZRE with graphene all had more residual zinc content than the ZRE without graphene. Furthermore, the residual zinc content increased with increasing graphene content when the graphene content was below 0.3 wt%, and the opposite was true for graphene content above 0.3 wt% (Figure 23d). Figure 23e shows a schematic diagram of the effect of graphene content on the cathodic protection time of the coated samples. When the addition of graphene is below 0.3 wt%, the effect of electrochemical corrosion is negligible and the density of the coating is the key factor affecting the cathodic protection time. The addition of graphene reduces the porosity of the coating and slows down the corrosion rate of the zinc powder, leaving the electrochemical corrosion potential at a lower value and the cathodic protection time of the coating extended. And when the addition of graphene exceeds 0.3 wt%, the effect of graphene on electrochemical corrosion becomes a key factor; electrochemical corrosion between graphene and zinc powder increases the corrosion rate of zinc powder and reduces the duration of cathodic protection.
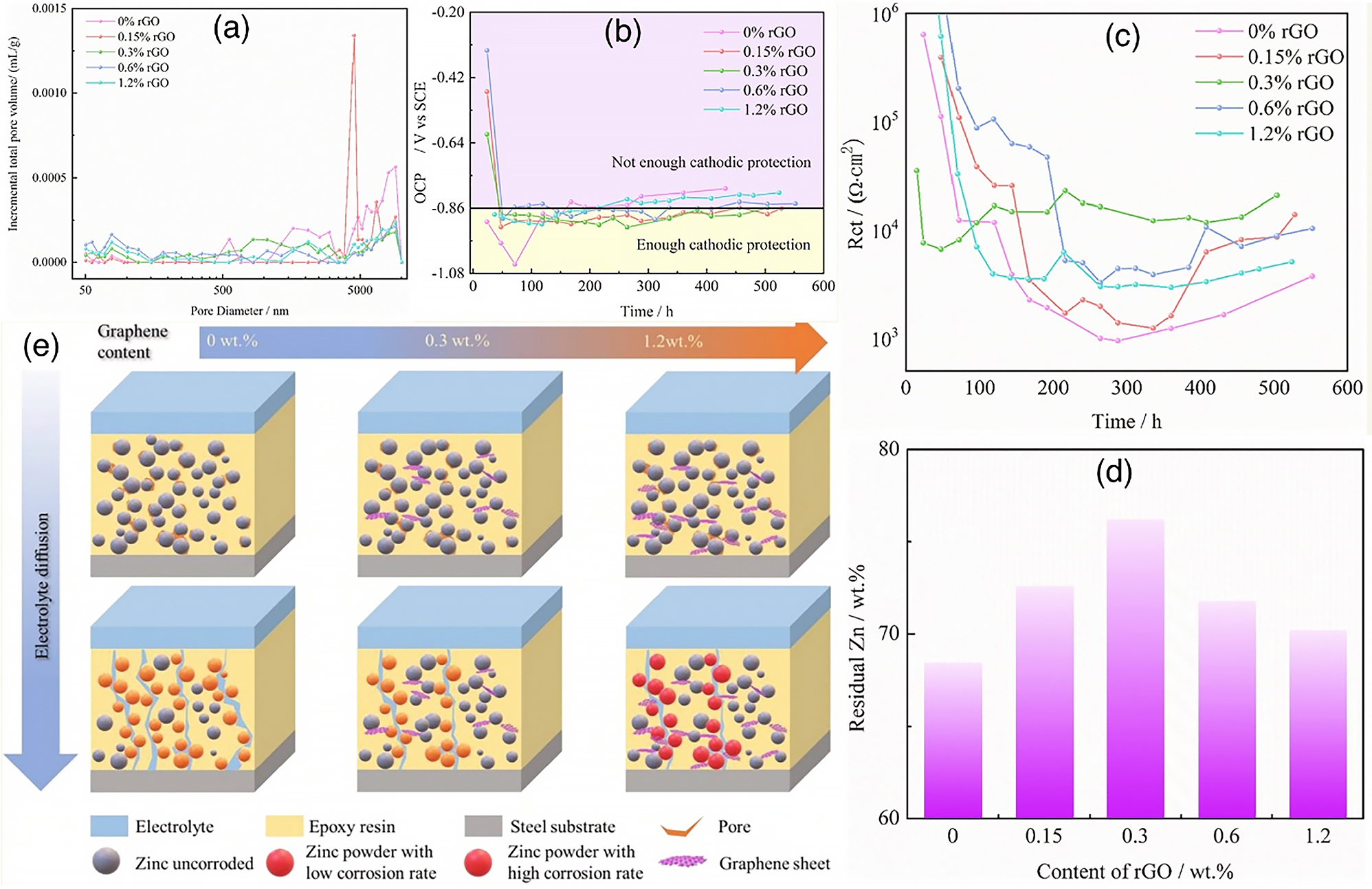
The cathodic protection and barrier effect of ZREs. (a) Distribution of the pore diameter for ZRE without graphene and ZREs with different graphene contents. (b) Evolution of OCP (vs. SCE) for ZRE without graphene and ZREs with different graphene contents as a function of time while immersed in 3.5 wt% NaCl solution. (c) Charge transfer resistance R ct for ZRE without graphene and ZREs with different graphene contents as a function of the immersion time in 3.5 wt% NaCl solution. (d) Evolution of remain zinc contents of ZRE after 360 h immersion time in 3.5 wt% NaCl solution as a function of graphene content. (e) Schematic representation for the influence of galvanic corrosion on the cathodic protection duration of ZREs with graphene (Bai et al. 2021). Reprinted with permission from Elsevier.
Table 2 briefly summarizes the comparison of representative works of different strategies for the above discussion regarding GCCs. Generally, the anti-corrosion properties of GCCs are mainly compromised to the interactions and mutual configuration between graphene and polymers. Whether through chemical modification of graphene or formation of hybrid films by alternating stacks of graphene films with, for example polymer films, this strategy is aimed at optimizing the mutual configuration between graphene and polymers in compatibility. Nevertheless, it is necessary to point out that the temperature resistance of GCCs for applications should be largely dependent on the carrier of polymer matrices (e.g. epoxy) instead of the graphene-based fillers. As far as the cathodic protection is concerned, it is important to optimize the interactions between graphene and polymers by changing their potential relationships.
Comparison between the three anti-corrosion strategies of GCCs.
| Strategies | Features | Advantages | References | |
|---|---|---|---|---|
| Chemical functionalization | Covalent functionalization | Graphene in combination with small molecules, polymer chains or nanoparticles | Helps to maintain the mechanical properties and chemical stability of graphene |
Liu et al. (2018a) Parhizkar et al. (2018)
Zhao et al. (2022) Zhang et al. (2023) Qiu et al. (2018) Ding et al. (2018a) Cui et al. (2018) Yuan et al. (2023) Liu et al. (2015) Zheng et al. (2021) |
| Non-covalent functionalization | Occurs through hydrogen bonds, electrostatic interactions and π–π bonding interactions | Helps maintain graphene’s excellent electrical conductivity and ultra-high specific surface area and is easy to handle without complex chemical reactions | ||
| Polymers-hybrids | Graphene films are alternately stacked with other types of films, each layer having its own specific function | The impermeability of graphene impedes the penetration of corrosive agents and the adhesion, insulating and hydrophobic properties of the polymer synergistically enhance the corrosion resistance of the composite coating |
Zhu et al. (2019)
Xu et al. (2021) Yu et al. (2018) |
|
| Cathodic protection | The addition of graphene to zinc-rich coatings serves two purposes: (1) to act as a cathode and (2) to conduct electricity for the zinc powder particles | (1) Acts as a cathode for the electrochemical reaction with zinc powder, producing insoluble corrosion products that fill the penetration channels and impede further penetration of the electrolyte. (2) Promotes electrical coupling between the zinc powder and the metal matrix, further improving the cathodic protection performance of the zinc-rich coating |
Ding et al. (2019)
Zhou et al. (2019) Bai et al. (2021) |
|
4 Conclusion and outlook
This paper presents an in-depth review of the strategies so far used for optimizing the anti-corrosion action of graphene-based coatings, covering the most recent and significant results for the cases of pure CVD graphene coatings and graphene composites coatings. In addition, the corresponding mechanisms, key factors, features and drawbacks are hereby analysed for a deeper understanding on graphene-based protection coatings from the perspective of optimization or enhancement of the anti-corrosion ability of graphene-based coatings.
In the past decade, graphene-based coatings have been widely studied due to graphene’s excellent water/gas impermeability. Comparing with traditional coatings, the addition of graphene changes the diffusion path of corrosive media to metal surfaces. In addition, graphene-based coatings have many other advantages such as non-toxicity and high cost-effectiveness. Nevertheless, there is also a balance issue between materials preparation and practical applications. Despite the emergence of various optimization strategies as we summarized here for bridging the gap between materials preparation and anti-corrosion applications, more efforts are still needed to enhance the corrosion barrier properties of graphene-based coatings.
For pure CVD graphene coating, although the existing CVD techniques can already achieve large-area and large-scale preparation, it is still relatively high cost. Furthermore, the defects in CVD graphene are an unavoidable issue. Defects are the main cause of graphene-coating failure degradation, as oxidation or corrosion of the metal substrate usually occurs below defects in the graphene. Moreover, defects can seriously affect the mechanical, electronic and chemical properties of graphene. Therefore, the reduction or even elimination of defects is still a major direction for the future optimization strategy for pure CVD graphene coating. Much work is still required to obtain ‘perfect CVD graphene coating’. At the same time, the atomic doping strategy of CVD graphene coating for property modification is also largely dependent on the CVD growth techniques. It is still facing some challenges that cannot be ignored. For example, the formation of ternary bonds in CVD-modified graphene like B x C y N z is difficult to precisely control and modulate. Overall, CVD growth techniques are the basic core for the optimization of anti-corrosion properties of pure CVD graphene. In addition to growing graphene films directly on the target metal substrate, alternative transferring the grown graphene films to the target metal will be another mainstream strategy for pure CVD graphene coatings in the future, with the development of transferring techniques of CVD graphene. Such an alternative transferring approach has advantages in some aspects, like scalability and multi-layer constructions for CVD graphene coatings. However, it should be noted that the passivation properties of graphene transferred to the target substrate are to some degree poorer than those of graphene grown directly on the substrate, due to the weaker interactions between the transferred graphene film and the substrate. This, in turn, highlights a key advantage of the direct CVD techniques route, namely that the in-built interactions between the substrate and the 2D material in its growth process, which allows the 2D material to be integrated directly into the substrate structure to provide long-term passivation protection for the substrate.
Pure CVD graphene coatings indeed offer many advantages, but there are many limitations in industrial applications. In addition, the relatively high cost of preparing pure CVD graphene films makes it difficult to achieve large-scale industrial production. Comparatively, GCC has lower cost and higher productivity as we have shown earlier in Figure 2, thanks to the relative variety of preparation strategies for graphene composite and easy-to-use coating methods. At present, the wildly used preparation method for graphene composite is the chemical stripping of graphene powder or the reduction of graphene oxides (rGO), which has mass industrial scalability. However, there are still some problems with controllability and cost of preparation. For example, it is difficult to balance the quality of graphene with the demand for mass industrial production. Furthermore, during the mass production of graphene powder and chemical modification of graphene, some strong oxidants and organic solvents are needed. From the perspective of environmental protection, most of the strong oxidants and organic solvents currently used for graphene modification are harmful to the environment. Thus, there is a need to investigate green solvents and methods to achieve efficient and high-quality modification. In addition, graphene-based coating has not yet established a comprehensive system in the field of metal anti-corrosion, and some mechanisms for inhibiting corrosion are still to be elaborated. At the same time, there is a lack of comprehensive testing of anti-corrosion properties for graphene-based protection coatings, as well as the evaluation of its stability in actual service conditions.
In sum, the graphene-based protection coating will be developed towards the green, high-quality, multi-functional integration and large-scale preparation of graphene in the future and continue to be widely used in industry, new energy and other fields.
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: Tianjin Postgraduate Research Innovation Project (2022SKYZ154), Tianjin Normal University Postgraduate Research Innovation Project (2022KYCX098Y) and Natural Science Foundation of Tianjin (20JCYBJC00350).
-
Data availability: Not applicable.
References
Abadjieva, E., Van Der Heijden, A.E.D.M., Creyghton, Y.L.M., and Van Ommen, J.R. (2012). Fluorocarbon coatings deposited on micron-sized particles by atmospheric PECVD. Plasma Processes Polym. 9: 217–224, https://doi.org/10.1002/ppap.201100044.Suche in Google Scholar
Akbarinezhad, E., Ebrahimi, M., Sharif, F., Attar, M.M., and Faridi, H.R. (2011). Synthesis and evaluating corrosion protection effects of emeraldine base PAni/clay nanocomposite as a barrier pigment in zinc-rich ethyl silicate primer. Prog. Org. Coat. 70: 39–44, https://doi.org/10.1016/j.porgcoat.2010.09.016.Suche in Google Scholar
Ambrosi, A. and Pumera, M. (2015). The structural stability of graphene anticorrosion coating materials is compromised at low potentials. Chemistry 21: 7896–7901, https://doi.org/10.1002/chem.201406238.Suche in Google Scholar PubMed
Arunkumar, S., Jegathish, V., Soundharya, R., Jesyalka, M., Arul, C., Sathyanarayanan, S., and Mayavan, S. (2017). Evaluating the performance of MoS2 based materials for corrosion protection of mild steel in an aggressive chloride environment. RSC Adv. 7: 17332–17335, https://doi.org/10.1039/c7ra01372h.Suche in Google Scholar
Arunkumar, S., Jegatheesh, V., Soundharya, R., Jesy Alka, M., and Mayavan, S. (2018). BCN based oil coatings for mild steel under aggressive chloride ion environment. Appl. Surf. Sci. 449: 287–294, https://doi.org/10.1016/j.apsusc.2018.01.030.Suche in Google Scholar
Assad, H., Fatma, I., and Kumar, A. (2023). An overview of the application of graphene-based materials in anticorrosive coatings. Mater. Lett. 330: 133287, https://doi.org/10.1016/j.matlet.2022.133287.Suche in Google Scholar
Bai, W., Ma, Y., Meng, M., and Li, Y. (2021). The influence of graphene on the cathodic protection performance of zinc-rich epoxy coatings. Prog. Org. Coat. 161: 106456, https://doi.org/10.1016/j.porgcoat.2021.106456.Suche in Google Scholar
Banhart, F., Kotakoski, J., and Krasheninnikov, A.V. (2011). Structural defects in graphene. ACS Nano 5: 26–41, https://doi.org/10.1021/nn102598m.Suche in Google Scholar PubMed
Berry, V. (2013). Impermeability of graphene and its applications. Carbon 62: 1–10, https://doi.org/10.1016/j.carbon.2013.05.052.Suche in Google Scholar
Bets, K.V., Artyukhov, V.I., and Yakobson, B.I. (2021). Kinetically determined shapes of grain boundaries in graphene. ACS Nano 15: 4893–4900, https://doi.org/10.1021/acsnano.0c09696.Suche in Google Scholar PubMed
Bonanni, A., Ambrosi, A., and Pumera, M. (2012). On oxygen-containing groups in chemically modified graphenes. Chemistry 18: 4541–4548, https://doi.org/10.1002/chem.201104003.Suche in Google Scholar PubMed
Boukhvalov, D.W., Zhidkov, I.S., Kukharenko, A.I., Slesarev, A.I., Zatsepin, A.F., Cholakh, S.O., and Kurmaev, E.Z. (2018). Stability of boron-doped graphene/copper interface: DFT, XPS and OSEE studies. Appl. Surf. Sci. 441: 978–983, https://doi.org/10.1016/j.apsusc.2018.02.074.Suche in Google Scholar
Bunch, J.S., Verbridge, S.S., Alden, J.S., Van Der Zande, A.M., Parpia, J.M., Craighead, H.G., and Mceuen, P.L. (2008). Impermeable atomic membranes from graphene sheets. Nano Lett. 8: 2458–2462, https://doi.org/10.1021/nl801457b.Suche in Google Scholar PubMed
Byon, E., Son, M., Hara, N., and Sugimoto, K. (2004). Corrosion behavior of boron-carbon-nitride films grown by magnetron sputtering. Thin Solid Films 447–448: 197–200, https://doi.org/10.1016/s0040-6090(03)01056-3.Suche in Google Scholar
Cai, Q., Li, L.H., Yu, Y., Liu, Y., Huang, S., Chen, Y., Watanabe, K., and Taniguchi, T. (2015). Boron nitride nanosheets as improved and reusable substrates for gold nanoparticles enabled surface enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 17: 7761–7766, https://doi.org/10.1039/c5cp00532a.Suche in Google Scholar PubMed
Camilli, L., Yu, F., Cassidy, A., Hornekær, L., and Bøggild, P. (2019). Challenges for continuous graphene as a corrosion barrier. 2D Materials 6: 022002, https://doi.org/10.1088/2053-1583/ab04d4.Suche in Google Scholar
Cervantes-Sodi, F., Csányi, G., Piscanec, S., and Ferrari, A.C. (2008). Edge-functionalized and substitutionally doped graphene nanoribbons: electronic and spin properties. Phys. Rev. B 77: 165427, https://doi.org/10.1103/physrevb.77.165427.Suche in Google Scholar
Chai, Z.-L., Chen, Y.-X., Zhou, D., Zhang, M., and Liu, J.-K. (2022). Excellent corrosion resistance of FGO/Zn2SiO4 composite material in epoxy coatings. Prog. Org. Coat. 170: 106992, https://doi.org/10.1016/j.porgcoat.2022.106992.Suche in Google Scholar
Chang, C.-H., Huang, T.-C., Peng, C.-W., Yeh, T.-C., Lu, H.-I., Hung, W.-I., Weng, C.-J., Yang, T.-I., and Yeh, J.-M. (2012). Novel anticorrosion coatings prepared from polyaniline/graphene composites. Carbon 50: 5044–5051, https://doi.org/10.1016/j.carbon.2012.06.043.Suche in Google Scholar
Chen, Y., Zou, J., Campbell, S.J., and Le Caer, G. (2004). Boron nitride nanotubes: pronounced resistance to oxidation. Appl. Phys. Lett. 84: 2430–2432, https://doi.org/10.1063/1.1667278.Suche in Google Scholar
Chen, S., Brown, L., Levendorf, M., Cai, W., Ju, S.Y., Edgeworth, J., Li, X., Magnuson, C.W., Velamakanni, A., Piner, R.D., et al.. (2011). Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 5: 1321–1327, https://doi.org/10.1021/nn103028d.Suche in Google Scholar PubMed
Chen, J., Shi, T., Cai, T., Xu, T., Sun, L., Wu, X., and Yu, D. (2013). Self healing of defected graphene. Appl. Phys. Lett. 102: 103107, https://doi.org/10.1063/1.4795292.Suche in Google Scholar
Chen, Z., Xu, X., Liu, H., Han, F., and Liu, S. (2022). Graphene modified phosphate-based metal/ceramic composite coating for corrosion protection in the high-temperature marine environment. Ceram. Int. 48: 25858–25871, https://doi.org/10.1016/j.ceramint.2022.05.262.Suche in Google Scholar
Choi, J.K., Kwak, J., Park, S.D., Yun, H.D., Kim, S.Y., Jung, M., Kim, S.Y., Park, K., Kang, S., Kim, S.D., et al.. (2015). Growth of wrinkle-free graphene on texture-controlled platinum films and thermal-assisted transfer of large-scale patterned graphene. ACS Nano 9: 679–686, https://doi.org/10.1021/nn5060909.Suche in Google Scholar PubMed
Ci, X., Zhao, W., Luo, J., Wu, Y., Ge, T., Shen, L., Gao, X., and Fang, Z. (2019). Revealing the lubrication mechanism of fluorographene nanosheets enhanced GTL-8 based nanolubricant oil. Tribol. Int. 138: 174–183, https://doi.org/10.1016/j.triboint.2019.05.044.Suche in Google Scholar
Cubides, Y., Su, S.S., and Castaneda, H. (2016). Influence of zinc content and chloride concentration on the corrosion protection performance of zinc-rich epoxy coatings containing carbon nanotubes on carbon steel in simulated concrete pore Eenvironments. Corrosion 72: 1397–1423, https://doi.org/10.5006/2104.Suche in Google Scholar
Cui, C., Lim, A.T.O., and Huang, J. (2017). A cautionary note on graphene anti-corrosion coatings. Nat. Nanotechnol. 12: 834–835, https://doi.org/10.1038/nnano.2017.187.Suche in Google Scholar PubMed
Cui, M., Ren, S., Zhao, H., Xue, Q., and Wang, L. (2018). Polydopamine coated graphene oxide for anticorrosive reinforcement of water-borne epoxy coating. Chem. Eng. J. 335: 255–266, https://doi.org/10.1016/j.cej.2017.10.172.Suche in Google Scholar
Cui, G., Bi, Z., Zhang, R., Liu, J., Yu, X., and Li, Z. (2019). A comprehensive review on graphene-based anti-corrosive coatings. Chem. Eng. J. 373: 104–121, https://doi.org/10.1016/j.cej.2019.05.034.Suche in Google Scholar
Das, A. and Harimkar, S.P. (2014). Effect of graphene nanoplate and silicon carbide nanoparticle reinforcement on mechanical and tribological properties of spark plasma sintered magnesium matrix composites. J. Mater. Sci. Technol. 30: 1059–1070, https://doi.org/10.1016/j.jmst.2014.08.002.Suche in Google Scholar
Dean, C.R., Young, A.F., Meric, I., Lee, C., Wang, L., Sorgenfrei, S., Watanabe, K., Taniguchi, T., Kim, P., Shepard, K.L., et al.. (2010). Boron nitride substrates for high-quality graphene electronics. Nat. Nanotechnol. 5: 722–726, https://doi.org/10.1038/nnano.2010.172.Suche in Google Scholar PubMed
Ding, R., Wang, X., Jiang, J., Gui, T., and Li, W. (2017). Study on evolution of coating state and role of graphene in graphene-modified low-zinc waterborne epoxy anticorrosion coating by electrochemical impedance spectroscopy. J. Mater. Eng. Perform. 26: 3319–3335, https://doi.org/10.1007/s11665-017-2790-8.Suche in Google Scholar
Ding, J., Rahman, O.U., Peng, W., Dou, H., and Yu, H. (2018a). A novel hydroxyl epoxy phosphate monomer enhancing the anticorrosive performance of waterborne graphene/epoxy coatings. Appl. Surf. Sci. 427: 981–991, https://doi.org/10.1016/j.apsusc.2017.08.224.Suche in Google Scholar
Ding, R., Zheng, Y., Yu, H., Li, W., Wang, X., and Gui, T. (2018b). Study of water permeation dynamics and anti-corrosion mechanism of graphene/zinc coatings. J. Alloys Compd. 748: 481–495, https://doi.org/10.1016/j.jallcom.2018.03.160.Suche in Google Scholar
Ding, R., Chen, S., Zhou, N., Zheng, Y., Li, B.-J., Gui, T.-J., Wang, X., Li, W.-H., Yu, H.-B., and Tian, H.-W. (2019). The diffusion-dynamical and electrochemical effect mechanism of oriented magnetic graphene on zinc-rich coatings and the electrodynamics and quantum mechanics mechanism of electron conduction in graphene zinc-rich coatings. J. Alloys Compd. 784: 756–768, https://doi.org/10.1016/j.jallcom.2019.01.070.Suche in Google Scholar
Ding, J., Zhao, H., Wang, G., Wang, J., and Zhu, J. (2023). Interface modulations of high-performance graphene anticorrosion coatings. Prog. Org. Coat. 178, https://doi.org/10.1016/j.porgcoat.2023.107463.Suche in Google Scholar
Dlubak, B., Martin, M.B., Weatherup, R.S., Yang, H., Deranlot, C., Blume, R., Schloegl, R., Fert, A., Anane, A., Hofmann, S., et al.. (2012). Graphene-passivated nickel as an oxidation-resistant electrode for spintronics. ACS Nano 6: 10930–10934, https://doi.org/10.1021/nn304424x.Suche in Google Scholar PubMed
Dong, Y., Liu, Q., and Zhou, Q. (2014). Corrosion behavior of Cu during graphene growth by CVD. Corros. Sci. 89: 214–219, https://doi.org/10.1016/j.corsci.2014.08.026.Suche in Google Scholar
Drummond, C.J., Vasic, Z.R., Geddes, N., Jurich, M.C., Chatelier, R.C., Gengenbach, T.R., and Griesser, H.J. (1997). Hydrophobic radiofrequency plasma-deposited polymer films: dielectric properties and surface forces. Colloids Surf., A: Physicochem. Eng. Aspects 129-130: 117–129, https://doi.org/10.1016/s0927-7757(97)00030-7.Suche in Google Scholar
Duan, C., Li, X., Ji, Y., He, L., Qian, J., and Zhao, Z. (2022). In-situ catalytic preparation of two-dimensional BCN/graphene composite for anti-corrosion application. Catalysts 12: 1618, https://doi.org/10.3390/catal12121618.Suche in Google Scholar
Galbiati, M., Stoot, A.C., Mackenzie, D.M., Boggild, P., and Camilli, L. (2017). Real-time oxide evolution of copper protected by graphene and boron nitride barriers. Sci. Rep. 7: 39770, https://doi.org/10.1038/srep39770.Suche in Google Scholar PubMed PubMed Central
Gass, M.H., Bangert, U., Bleloch, A.L., Wang, P., Nair, R.R., and Geim, A.K. (2008). Free-standing graphene at atomic resolution. Nat. Nanotechnol. 3: 676–681, https://doi.org/10.1038/nnano.2008.280.Suche in Google Scholar PubMed
Geim, A.K. and Novoselov, K.S. (2007). The rise of graphene. Nat. Mater. 6: 183–191, https://doi.org/10.1038/nmat1849.Suche in Google Scholar PubMed
Gergely, A., Bertóti, I., Török, T., Pfeifer, É., and Kálmán, E. (2013). Corrosion protection with zinc-rich epoxy paint coatings embedded with various amounts of highly dispersed polypyrrole-deposited alumina monohydrate particles. Prog. Org. Coat. 76: 17–32, https://doi.org/10.1016/j.porgcoat.2012.08.005.Suche in Google Scholar
Golberg, D., Bando, Y., Huang, Y., Terao, T., Mitome, M., Tang, C., and Zhi, C. (2010). Boron nitride nanotubes and nanosheets. ACS Nano 4: 2979–2993, https://doi.org/10.1021/nn1006495.Suche in Google Scholar PubMed
Gong, P., Ji, S., Wang, J., Dai, D., Wang, F., Tian, M., Zhang, L., Guo, F., and Liu, Z. (2018). Fluorescence-switchable ultrasmall fluorinated graphene oxide with high near-infrared absorption for controlled and targeted drug delivery. Chem. Eng. J. 348: 438–446, https://doi.org/10.1016/j.cej.2018.04.193.Suche in Google Scholar
Guo, B., Liu, Q., Chen, E., Zhu, H., Fang, L., and Gong, J.R. (2010). Controllable N-doping of graphene. Nano Lett. 10: 4975–4980, https://doi.org/10.1021/nl103079j.Suche in Google Scholar PubMed
Han, L., Dong, L., Chen, H., Yang, S., Yuan, A., Guan, R., Yan, H., Wu, J., Zhang, B., Li, D., et al.. (2021). Chemical vapor deposition of N-doped graphene through pre-implantation of nitrogen ions for long-term protection of copper. Materials (Basel) 14: 3751, https://doi.org/10.3390/ma14133751.Suche in Google Scholar PubMed PubMed Central
Hayatdavoudi, H. and Rahsepar, M. (2017). A mechanistic study of the enhanced cathodic protection performance of graphene-reinforced zinc rich nanocomposite coating for corrosion protection of carbon steel substrate. J. Alloys Compd. 727: 1148–1156, https://doi.org/10.1016/j.jallcom.2017.08.250.Suche in Google Scholar
Hsieh, Y.P., Hofmann, M., Chang, K.W., Jhu, J.G., Li, Y.Y., Chen, K.Y., Yang, C.C., Chang, W.S., and Chen, L.C. (2014). Complete corrosion inhibition through graphene defect passivation. ACS Nano 8: 443–448, https://doi.org/10.1021/nn404756q.Suche in Google Scholar PubMed
Huang, H., Sheng, X., Tian, Y., Zhang, L., Chen, Y., and Zhang, X. (2020). Two-dimensional nanomaterials for anticorrosive polymeric coatings: a review. Ind. Eng. Chem. Res. 59: 15424–15446, https://doi.org/10.1021/acs.iecr.0c02876.Suche in Google Scholar
Huang, W.-Q., Chai, Z.-L., Zhao, S.-R., Liu, J.-K., Liu, J.-C., Ma, Y.-S., and Cai, Y.-H. (2023). Design synthesis and excellent anti-corrosion property of GO/Mn-Zn2SiO4 composite materials. Colloids Surf., A: Physicochem. Eng. Aspects 656: 130281, https://doi.org/10.1016/j.colsurfa.2022.130281.Suche in Google Scholar
Hui, F., Shi, Y., Ji, Y., Lanza, M., and Duan, H. (2014). Mechanical properties of locally oxidized graphene electrodes. Arch. Appl. Mech. 85: 339–345, https://doi.org/10.1007/s00419-014-0957-4.Suche in Google Scholar
Husain, E., Narayanan, T.N., Taha-Tijerina, J.J., Vinod, S., Vajtai, R., and Ajayan, P.M. (2013). Marine corrosion protective coatings of hexagonal boron nitride thin films on stainless steel. ACS Appl. Mater. Interfaces 5: 4129–4135, https://doi.org/10.1021/am400016y.Suche in Google Scholar PubMed
Imani, R., Mohabatpour, F., and Mostafavi, F. (2018). Graphene-based nano-carrier modifications for gene delivery applications. Carbon 140: 569–591, https://doi.org/10.1016/j.carbon.2018.09.019.Suche in Google Scholar
Jalili, M., Rostami, M., and Ramezanzadeh, B. (2015). An investigation of the electrochemical action of the epoxy zinc-rich coatings containing surface modified aluminum nanoparticle. Appl. Surf. Sci. 328: 95–108, https://doi.org/10.1016/j.apsusc.2014.12.034.Suche in Google Scholar
Jena, G. and Philip, J. (2022). A review on recent advances in graphene oxide-based composite coatings for anticorrosion applications. Prog. Org. Coat. 173: 107208, https://doi.org/10.1016/j.porgcoat.2022.107208.Suche in Google Scholar
Jia, K., Zhang, J., Lin, L., Li, Z., Gao, J., Sun, L., Xue, R., Li, J., Kang, N., Luo, Z., et al.. (2019). Copper-containing carbon feedstock for growing superclean graphene. J. Am. Chem. Soc. 141: 7670–7674, https://doi.org/10.1021/jacs.9b02068.Suche in Google Scholar PubMed
Jiang, L., Xiao, N., Wang, B., Grustan-Gutierrez, E., Jing, X., Babor, P., Kolíbal, M., Lu, G., Wu, T., Wang, H., et al.. (2017). High-resolution characterization of hexagonal boron nitride coatings exposed to aqueous and air oxidative environments. Nano Res. 10: 2046–2055, https://doi.org/10.1007/s12274-016-1393-2.Suche in Google Scholar
Jiang, C., Li, X., Ying, Y., and Ping, J. (2021). Fluorinated graphene-enabled durable triboelectric coating for water energy harvesting. Small 17: e2007805, https://doi.org/10.1002/smll.202007805.Suche in Google Scholar PubMed
Jin, Z., Yao, J., Kittrell, C., and Tour, J.M. (2011). Large-scale growth and characterizations of nitrogen-doped monolayer graphene sheets. ACS Nano 5: 4112–4117, https://doi.org/10.1021/nn200766e.Suche in Google Scholar PubMed
Keil, P., Frahm, R., and Lützenkirchen-Hecht, D. (2010). Native oxidation of sputter deposited polycrystalline copper thin films during short and long exposure times: comparative investigation by specular and non-specular grazing incidence X-ray absorption spectroscopy. Corros. Sci. 52: 1305–1316, https://doi.org/10.1016/j.corsci.2009.12.012.Suche in Google Scholar
Khan, M.H., Jamali, S.S., Lyalin, A., Molino, P.J., Jiang, L., Liu, H.K., Taketsugu, T., and Huang, Z. (2017). Atomically thin hexagonal boron nitride nanofilm for Cu protection: the importance of film perfection. Adv. Mater. 29: 1603937, https://doi.org/10.1002/adma.201603937.Suche in Google Scholar PubMed
Kim, K., Lee, H.B., Johnson, R.W., Tanskanen, J.T., Liu, N., Kim, M.G., Pang, C., Ahn, C., Bent, S.F., and Bao, Z. (2014). Selective metal deposition at graphene line defects by atomic layer deposition. Nat. Commun. 5: 4781, https://doi.org/10.1038/ncomms5781.Suche in Google Scholar PubMed
Kim, H., Lee, H., Lim, H.-R., Cho, H.-B., and Choa, Y.-H. (2019). Electrically conductive and anti-corrosive coating on copper foil assisted by polymer-nanocomposites embedded with graphene. Appl. Surf. Sci. 476: 123–127, https://doi.org/10.1016/j.apsusc.2019.01.066.Suche in Google Scholar
Kong, X.K., Chen, C.L., and Chen, Q.W. (2014). Doped graphene for metal-free catalysis. Chem. Soc. Rev. 43: 2841–2857, https://doi.org/10.1039/c3cs60401b.Suche in Google Scholar PubMed
Kravets, V.G., Jalil, R., Kim, Y.J., Ansell, D., Aznakayeva, D.E., Thackray, B., Britnell, L., Belle, B.D., Withers, F., Radko, I.P., et al.. (2014). Graphene-protected copper and silver plasmonics. Sci. Rep. 4: 5517, https://doi.org/10.1038/srep05517.Suche in Google Scholar PubMed PubMed Central
Krishnamurthy, A., Gadhamshetty, V., Mukherjee, R., Chen, Z., Ren, W., Cheng, H.M., and Koratkar, N. (2013). Passivation of microbial corrosion using a graphene coating. Carbon 56: 45–49, https://doi.org/10.1016/j.carbon.2012.12.060.Suche in Google Scholar
Landolt, D. (2007). Corrosion and surface chemistry of metals. EPFL Press, Lausanne.10.1201/9781439807880Suche in Google Scholar
Langer, E., Zubielewicz, M., Kuczyńska, H., Królikowska, A., and Komorowski, L. (2019). Anticorrosive effectiveness of coatings with reduced content of Zn pigments in comparison with zinc-rich primers. Corros. Eng., Sci. Technol. 54: 627–635, https://doi.org/10.1080/1478422x.2019.1652428.Suche in Google Scholar
Lanza, M., Wang, Y., Gao, T., Bayerl, A., Porti, M., Nafria, M., Zhou, Y., Jing, G., Zhang, Y., Liu, Z., et al.. (2013). Electrical and mechanical performance of graphene sheets exposed to oxidative environments. Nano Res. 6: 485–495, https://doi.org/10.1007/s12274-013-0326-6.Suche in Google Scholar
Lazar, P., Otyepková, E., Karlický, F., Čépe, K., and Otyepka, M. (2015). The surface and structural properties of graphite fluoride. Carbon 94: 804–809, https://doi.org/10.1016/j.carbon.2015.07.064.Suche in Google Scholar
Lee, J. and Berman, D. (2018). Inhibitor or promoter: insights on the corrosion evolution in a graphene protected surface. Carbon 126: 225–231, https://doi.org/10.1016/j.carbon.2017.10.022.Suche in Google Scholar
Lee, J.H., Lee, E.K., Joo, W.J., Jang, Y., Kim, B.S., Lim, J.Y., Choi, S.H., Ahn, S.J., Ahn, J.R., Park, M.H., et al.. (2014). Wafer-scale growth of single-crystal monolayer graphene on reusable hydrogen-terminated germanium. Science 344: 286–289, https://doi.org/10.1126/science.1252268.Suche in Google Scholar PubMed
Lee, E.-J., An, A.K., He, T., Woo, Y.C., and Shon, H.K. (2016). Electrospun nanofiber membranes incorporating fluorosilane-coated TiO2 nanocomposite for direct contact membrane distillation. J. Membr. Sci. 520: 145–154, https://doi.org/10.1016/j.memsci.2016.07.019.Suche in Google Scholar
Lee, J.S., Choi, S.H., Yun, S.J., Kim, Y.I., Boandoh, S., Park, J.H., Shin, B.G., Ko, H., Lee, S.H., Kim, Y.M., et al.. (2018). Wafer-scale single-crystal hexagonal boron nitride film via self-collimated grain formation. Science 362: 817–821, https://doi.org/10.1126/science.aau2132.Suche in Google Scholar PubMed
Lee, U., Han, Y., Lee, S., Kim, J.S., Lee, Y.H., Kim, U.J., and Son, H. (2020). Time evolution studies on strain and doping of graphene grown on a copper substrate using Raman spectroscopy. ACS Nano 14: 919–926, https://doi.org/10.1021/acsnano.9b08205.Suche in Google Scholar PubMed
Lei, W., Portehault, D., Dimova, R., and Antonietti, M. (2011). Boron carbon nitride nanostructures from salt melts: tunable water-soluble phosphors. J. Am. Chem. Soc. 133: 7121–7127, https://doi.org/10.1021/ja200838c.Suche in Google Scholar PubMed
Lei, Y., Qiu, Z., Tan, N., Du, H., Li, D., Liu, J., Liu, T., Zhang, W., and Chang, X. (2020). Polyaniline/CeO2 nanocomposites as corrosion inhibitors for improving the corrosive performance of epoxy coating on carbon steel in 3.5% NaCl solution. Prog. Org. Coat. 139: 105430, https://doi.org/10.1016/j.porgcoat.2019.105430.Suche in Google Scholar
Li, L.H. and Chen, Y. (2016). Atomically thin boron nitride: unique properties and applications. Adv. Funct. Mater. 26: 2594–2608, https://doi.org/10.1002/adfm.201504606.Suche in Google Scholar
Li, L.H., Cervenka, J., Watanabe, K., Taniguchi, T., and Chen, Y. (2014). Strong oxidation resistance of atomically thin boron nitride nanosheets. ACS Nano 8: 1457–1462, https://doi.org/10.1021/nn500059s.Suche in Google Scholar PubMed
Li, D., Duan, X., Sun, H., Kang, J., Zhang, H., Tade, M.O., and Wang, S. (2017). Facile synthesis of nitrogen-doped graphene via low-temperature pyrolysis: the effects of precursors and annealing ambience on metal-free catalytic oxidation. Carbon 115: 649–658, https://doi.org/10.1016/j.carbon.2017.01.058.Suche in Google Scholar
Lin, L., Zhang, J., Su, H., Li, J., Sun, L., Wang, Z., Xu, F., Liu, C., Lopatin, S., Zhu, Y., et al.. (2019). Towards super-clean graphene. Nat. Commun. 10: 1912, https://doi.org/10.1038/s41467-019-09565-4.Suche in Google Scholar PubMed PubMed Central
Liu, H., Liu, Y., and Zhu, D. (2011). Chemical doping of graphene. J. Mater. Chem. 21: 3335–3345, https://doi.org/10.1039/c0jm02922j.Suche in Google Scholar
Liu, Z., Gong, Y., Zhou, W., Ma, L., Yu, J., Idrobo, J.C., Jung, J., Macdonald, A.H., Vajtai, R., Lou, J., et al.. (2013). Ultrathin high-temperature oxidation-resistant coatings of hexagonal boron nitride. Nat. Commun. 4: 2541, https://doi.org/10.1038/ncomms3541.Suche in Google Scholar PubMed
Liu, X., Shao, Y., Zhang, Y., Meng, G., Zhang, T., and Wang, F. (2015). Using high-temperature mechanochemistry treatment to modify iron oxide and improve the corrosion performance of epoxy coating – I. High-temperature ball milling treatment. Corros. Sci. 90: 451–462, https://doi.org/10.1016/j.corsci.2014.04.015.Suche in Google Scholar
Liu, D., Zhao, W., Liu, S., Cen, Q., and Xue, Q. (2016). Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 286: 354–364, https://doi.org/10.1016/j.surfcoat.2015.12.056.Suche in Google Scholar
Liu, C., Qiu, S., Du, P., Zhao, H., and Wang, L. (2018a). An ionic liquid-graphene oxide hybrid nanomaterial: synthesis and anticorrosive applications. Nanoscale 10: 8115–8124, https://doi.org/10.1039/c8nr01890a.Suche in Google Scholar PubMed
Liu, J., Liu, T., Guo, Z., Guo, N., Lei, Y., Chang, X., and Yin, Y. (2018b). Promoting barrier performance and cathodic protection of zinc-rich epoxy primer via single-layer graphene. Polymers (Basel) 10: 591, https://doi.org/10.3390/polym10060591.Suche in Google Scholar PubMed PubMed Central
Luo, B., Whelan, P.R., Shivayogimath, A., Mackenzie, D.M.A., Bøggild, P., and Booth, T.J. (2016). Copper oxidation through nucleation sites of chemical vapor deposited graphene. Chem. Mater. 28: 3789–3795, https://doi.org/10.1021/acs.chemmater.6b00752.Suche in Google Scholar
Luo, B., Koleini, M., Whelan, P.R., Shivayogimath, A., Brandbyge, M., Boggild, P., and Booth, T.J. (2019). Graphene-subgrain-defined oxidation of copper. ACS Appl. Mater. Interfaces 11: 48518–48524, https://doi.org/10.1021/acsami.9b15931.Suche in Google Scholar PubMed
Luo, B., Yang, S., Yuan, A., Zhang, B., Li, D., Boggild, P., and Booth, T.J. (2020). Selective area oxidation of copper derived from chemical vapor deposited graphene microstructure. Nanotechnology 31: 485603, https://doi.org/10.1088/1361-6528/abb26d.Suche in Google Scholar PubMed
Lusk, A.T. and Jennings, G.K. (2001). Characterization of self-assembled monolayers formed from sodium S-alkyl thiosulfates on copper. Langmuir 17: 7830–7836, https://doi.org/10.1021/la010816t.Suche in Google Scholar
Lv, R. and Terrones, M. (2012). Towards new graphene materials: doped graphene sheets and nanoribbons. Mater. Lett. 78: 209–218, https://doi.org/10.1016/j.matlet.2012.04.033.Suche in Google Scholar
Ma, Y., Ye, Y., Wan, H., Chen, L., Zhou, H., and Chen, J. (2020). Chemical modification of graphene oxide to reinforce the corrosion protection performance of UV-curable polyurethane acrylate coating. Prog. Org. Coat. 141: 105547, https://doi.org/10.1016/j.porgcoat.2020.105547.Suche in Google Scholar
Marchebois, H., Savall, C., Bernard, J., and Touzain, S. (2004). Electrochemical behavior of zinc-rich powder coatings in artificial sea water. Electrochim. Acta 49: 2945–2954, https://doi.org/10.1016/j.electacta.2004.01.053.Suche in Google Scholar
Martin, M.B., Dlubak, B., Weatherup, R.S., Yang, H., Deranlot, C., Bouzehouane, K., Petroff, F., Anane, A., Hofmann, S., Robertson, J., et al.. (2014). Sub-nanometer atomic layer deposition for spintronics in magnetic tunnel junctions based on graphene spin-filtering membranes. ACS Nano 8: 7890–7895, https://doi.org/10.1021/nn5017549.Suche in Google Scholar PubMed PubMed Central
Martin, M.B., Dlubak, B., Weatherup, R.S., Piquemal-Banci, M., Yang, H., Blume, R., Schloegl, R., Collin, S., Petroff, F., Hofmann, S., et al.. (2015). Protecting nickel with graphene spin-filtering membranes: a single layer is enough. Appl. Phys. Lett. 107: 012408, https://doi.org/10.1063/1.4923401.Suche in Google Scholar
Mazza, M.F., Caban-Acevedo, M., Fu, H.J., Meier, M.C., Thompson, A.C., Ifkovits, Z.P., Carim, A.I., and Lewis, N.S. (2022). Selective-area, water-free atomic layer deposition of metal oxides on graphene defects. ACS Mater. Au. 2: 74–78, https://doi.org/10.1021/acsmaterialsau.1c00049.Suche in Google Scholar PubMed PubMed Central
Mittal, V.K., Bera, S., Saravanan, T., Sumathi, S., Krishnan, R., Rangarajan, S., Velmurugan, S., and Narasimhan, S.V. (2009). Formation and characterization of bi-layer oxide coating on carbon-steel for improving corrosion resistance. Thin Solid Films 517: 1672–1676, https://doi.org/10.1016/j.tsf.2008.09.094.Suche in Google Scholar
Mohanty, N., Fahrenholtz, M., Nagaraja, A., Boyle, D., and Berry, V. (2011). Impermeable graphenic encasement of bacteria. Nano Lett. 11: 1270–1275, https://doi.org/10.1021/nl104292k.Suche in Google Scholar PubMed
Mun, J.H., Oh, J.G., Bong, J.H., Xu, H., Loh, K.P., and Cho, B.J. (2014). Wrinkle-free graphene with spatially uniform electrical properties grown on hot-pressed copper. Nano Res. 8: 1075–1080, https://doi.org/10.1007/s12274-014-0585-x.Suche in Google Scholar
Novoselov, K.S., Jiang, D., Schedin, F., Booth, T.J., Khotkevich, V.V., Morozov, S.V., and Geim, A.K. (2005). Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. U. S. A. 102: 10451–10453, https://doi.org/10.1073/pnas.0502848102.Suche in Google Scholar PubMed PubMed Central
Papageorgiou, D.G., Kinloch, I.A., and Young, R.J. (2017). Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 90: 75–127, https://doi.org/10.1016/j.pmatsci.2017.07.004.Suche in Google Scholar
Parhizkar, N., Ramezanzadeh, B., and Shahrabi, T. (2018). Corrosion protection and adhesion properties of the epoxy coating applied on the steel substrate pre-treated by a sol-gel based silane coating filled with amino and isocyanate silane functionalized graphene oxide nanosheets. Appl. Surf. Sci. 439: 45–59, https://doi.org/10.1016/j.apsusc.2017.12.240.Suche in Google Scholar
Pierleoni, D., Xia, Z.Y., Christian, M., Ligi, S., Minelli, M., Morandi, V., Doghieri, F., and Palermo, V. (2016). Graphene-based coatings on polymer films for gas barrier applications. Carbon 96: 503–512, https://doi.org/10.1016/j.carbon.2015.09.090.Suche in Google Scholar
Poh, H.L., Šimek, P., Sofer, Z., Tomandl, I., and Pumera, M. (2013). Boron and nitrogen doping of graphene via thermal exfoliation of graphite oxide in a BF3 or NH3 atmosphere: contrasting properties. J. Mater. Chem. A 1: 13146–13153, https://doi.org/10.1039/c3ta12460f.Suche in Google Scholar
Prasai, D., Tuberquia, J.C., Harl, R.R., Jennings, G.K., Rogers, B.R., and Bolotin, K.I. (2012). Graphene: corrosion-inhibiting coating. ACS Nano 6: 1102–1108, https://doi.org/10.1021/nn203507y.Suche in Google Scholar PubMed
Pushpavanam, M., Raman, V., and Shenoi, B.A. (1981). Rhodium – electrodeposition and applications. Surf. Technol. 12: 351–360, https://doi.org/10.1016/0376-4583(81)90029-7.Suche in Google Scholar
Qi, K., Sun, Y., Duan, H., and Guo, X. (2015). A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 98: 500–506, https://doi.org/10.1016/j.corsci.2015.05.056.Suche in Google Scholar
Qiu, S., Liu, G., Li, W., Zhao, H., and Wang, L. (2018). Noncovalent exfoliation of graphene and its multifunctional composite coating with enhanced anticorrosion and tribological performance. J. Alloys Compd. 747: 60–70, https://doi.org/10.1016/j.jallcom.2018.03.007.Suche in Google Scholar
Qureshi, T., Wang, G., Mukherjee, S., Akibul Islam, M., Filleter, T., Singh, C.V., and Panesar, D.K. (2022). Graphene-based anti-corrosive coating on steel for reinforced concrete infrastructure applications: challenges and potential. Constr. Build. Mater. 351: 128947, https://doi.org/10.1016/j.conbuildmat.2022.128947.Suche in Google Scholar
Rajabi, M., Rashed, G.R., and Zaarei, D. (2014). Assessment of graphene oxide/epoxy nanocomposite as corrosion resistance coating on carbon steel. Corros. Eng., Sci. Technol. 50: 509–516, https://doi.org/10.1179/1743278214y.0000000232.Suche in Google Scholar
Redondo, M.I. and Breslin, C.B. (2007). Polypyrrole electrodeposited on copper from an aqueous phosphate solution: corrosion protection properties. Corros. Sci. 49: 1765–1776, https://doi.org/10.1016/j.corsci.2006.10.014.Suche in Google Scholar
Ren, S., Cui, M., Li, W., Pu, J., Xue, Q., and Wang, L. (2018). N-doping of graphene: toward long-term corrosion protection of Cu. J. Mater. Chem. A 6: 24136–24148, https://doi.org/10.1039/c8ta05421e.Suche in Google Scholar
Ren, S., Cui, M., Li, Q., Li, W., Pu, J., Xue, Q., and Wang, L. (2019). Barrier mechanism of nitrogen-doped graphene against atomic oxygen irradiation. Appl. Surf. Sci. 479: 669–678, https://doi.org/10.1016/j.apsusc.2019.02.137.Suche in Google Scholar
Sai Pavan, A.S. and Ramanan, S.R. (2016). A study on corrosion resistant graphene films on low alloy steel. Appl. Nanosci. 6: 1175–1181, https://doi.org/10.1007/s13204-016-0530-2.Suche in Google Scholar
Sanjid, A., Banerjee, P.C., and Raman, R.K.S. (2019). Multi-layer graphene coating for corrosion resistance of Monel 400 alloy in chloride environment. Surf. Coat. Technol. 370: 227–234, https://doi.org/10.1016/j.surfcoat.2019.04.077.Suche in Google Scholar
Schriver, M., Regan, W., Gannett, W.J., Zaniewski, A.M., Crommie, M.F., and Zettl, A. (2013). Graphene as a long-term metal oxidation barrier: worse than nothing. ACS Nano 7: 5763–5768, https://doi.org/10.1021/nn4014356.Suche in Google Scholar PubMed
Seethamraju, S., Kumar, S., B, K.B., Madras, G., Raghavan, S., and Ramamurthy, P.C. (2016). Million-fold decrease in polymer moisture permeability by a graphene monolayer. ACS Nano 10: 6501–6509, https://doi.org/10.1021/acsnano.6b02588.Suche in Google Scholar PubMed
Sekhavat Pour, Z. and Ghaemy, M. (2016). Polymer grafted graphene oxide: for improved dispersion in epoxy resin and enhancement of mechanical properties of nanocomposite. Compos. Sci. Technol. 136: 145–157, https://doi.org/10.1016/j.compscitech.2016.10.014.Suche in Google Scholar
Shen, L., Zhao, Y., Wang, Y., Song, R., Yao, Q., Chen, S., and Chai, Y. (2016). A long-term corrosion barrier with an insulating boron nitride monolayer. J. Mater. Chem. A 4: 5044–5050, https://doi.org/10.1039/c6ta01604a.Suche in Google Scholar
Shi, Q. and Zhu, A. (2020). Interface regulation of graphene/carbon nanotube on the thermal conductivity and anticorrosion performance of their nanocomposite. Prog. Org. Coat. 140: 105480, https://doi.org/10.1016/j.porgcoat.2019.105480.Suche in Google Scholar
Shi, K., Xiao, S., Ruan, Q., Wu, H., Chen, G., Zhou, C., Jiang, S., Xi, K., He, M., and Chu, P.K. (2022). Hydrogen permeation behavior and mechanism of multi-layered graphene coatings and mitigation of hydrogen embrittlement of pipe steel. Appl. Surf. Sci. 573: 151529, https://doi.org/10.1016/j.apsusc.2021.151529.Suche in Google Scholar
Shreepathi, S., Bajaj, P., and Mallik, B.P. (2010). Electrochemical impedance spectroscopy investigations of epoxy zinc rich coatings: role of Zn content on corrosion protection mechanism. Electrochim. Acta 55: 5129–5134, https://doi.org/10.1016/j.electacta.2010.04.018.Suche in Google Scholar
Singh Raman, R.K., Chakraborty Banerjee, P., Lobo, D.E., Gullapalli, H., Sumandasa, M., Kumar, A., Choudhary, L., Tkacz, R., Ajayan, P.M., and Majumder, M. (2012). Protecting copper from electrochemical degradation by graphene coating. Carbon 50: 4040–4045, https://doi.org/10.1016/j.carbon.2012.04.048.Suche in Google Scholar
Song, Y., Gao, Y., Liu, X., Ma, J., Chen, B., Xie, Q., Gao, X., Zheng, L., Zhang, Y., Ding, Q., et al.. (2022). Transfer-enabled fabrication of graphene wrinkle arrays for epitaxial growth of AlN Films. Adv. Mater. 34: e2105851, https://doi.org/10.1002/adma.202105851.Suche in Google Scholar PubMed
Sreeprasad, T.S. and Berry, V. (2013). How do the electrical properties of graphene change with its functionalization? Small 9: 341–350, https://doi.org/10.1002/smll.201202196.Suche in Google Scholar PubMed
Stoot, A.C., Camilli, L., Spiegelhauer, S.-A., Yu, F., and Bøggild, P. (2015). Multilayer graphene for long-term corrosion protection of stainless steel bipolar plates for polymer electrolyte membrane fuel cell. J. Power Sources 293: 846–851, https://doi.org/10.1016/j.jpowsour.2015.06.009.Suche in Google Scholar
Stratmann, M., Feser, R., and Leng, A. (1994). Corrosion protection by organic films. Electrochim. Acta 39: 1207–1214, https://doi.org/10.1016/0013-4686(94)e0038-2.Suche in Google Scholar
Tan, B. and Thomas, N.L. (2016). A review of the water barrier properties of polymer/clay and polymer/graphene nanocomposites. J. Membr. Sci. 514: 595–612, https://doi.org/10.1016/j.memsci.2016.05.026.Suche in Google Scholar
Teng, S., Gao, Y., Cao, F., Kong, D., Zheng, X., Ma, X., and Zhi, L. (2018). Zinc-reduced graphene oxide for enhanced corrosion protection of zinc-rich epoxy coatings. Prog. Org. Coat. 123: 185–189, https://doi.org/10.1016/j.porgcoat.2018.07.012.Suche in Google Scholar
Topsakal, M., Şahin, H., and Ciraci, S. (2012). Graphene coatings: an efficient protection from oxidation. Phys. Rev. B 85, https://doi.org/10.1103/physrevb.85.155445.Suche in Google Scholar
Wahab, O.J., Daviddi, E., Xin, B., Sun, P.Z., Griffin, E., Colburn, A.W., Barry, D., Yagmurcukardes, M., Peeters, F.M., Geim, A.K., et al.. (2023). Proton transport through nanoscale corrugations in two-dimensional crystals. Nature 620: 782–786, https://doi.org/10.1038/s41586-023-06247-6.Suche in Google Scholar PubMed PubMed Central
Wang, X., Tabakman, S.M., and Dai, H. (2008). Atomic layer deposition of metal oxides on pristine and functionalized graphene. J. Am. Chem. Soc. 130: 8152–8153, https://doi.org/10.1021/ja8023059.Suche in Google Scholar PubMed
Wang, Y., Shao, Y., Matson, D.W., Li, J., and Lin, Y. (2010). Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4: 1790–1798, https://doi.org/10.1021/nn100315s.Suche in Google Scholar PubMed
Wang, H., Xie, G., Ying, Z., Tong, Y., and Zeng, Y. (2015). Enhanced mechanical properties of multi-layer graphene filled poly(vinyl chloride) composite films. J. Mater. Sci. Technol. 31: 340–344, https://doi.org/10.1016/j.jmst.2014.09.009.Suche in Google Scholar
Wang, J., Hao, J., Liu, D., Qin, S., Portehault, D., Li, Y., Chen, Y., and Lei, W. (2017). Porous boron carbon nitride nanosheets as efficient metal-free catalysts for the oxygen reduction reaction in both alkaline and acidic solutions. ACS Energy Lett. 2: 306–312, https://doi.org/10.1021/acsenergylett.6b00602.Suche in Google Scholar
Wang, J., Jin, X., Li, C., Wang, W., Wu, H., and Guo, S. (2019). Graphene and graphene derivatives toughening polymers: toward high toughness and strength. Chem. Eng. J. 370: 831–854, https://doi.org/10.1016/j.cej.2019.03.229.Suche in Google Scholar
Wang, M., Huang, M., Luo, D., Li, Y., Choe, M., Seong, W.K., Kim, M., Jin, S., Wang, M., Chatterjee, S., et al.. (2021). Single-crystal, large-area, fold-free monolayer graphene. Nature 596: 519–524, https://doi.org/10.1038/s41586-021-03753-3.Suche in Google Scholar PubMed
Weatherup, R.S., Darsie, L., Cabrero-Vilatela, A., Caneva, S., Blume, R., Robertson, J., Schloegl, R., and Hofmann, S. (2015). Long-term passivation of strongly interacting metals with single-layer graphene. J. Am. Chem. Soc. 137: 14358–14366, https://doi.org/10.1021/jacs.5b08729.Suche in Google Scholar PubMed PubMed Central
Wei, D., Liu, Y., Wang, Y., Zhang, H., Huang, L., and Yu, G. (2009). Synthesis of N-doped graphene by chemical vapor deposition and its electrical properties. Nano Lett. 9: 1752–1758, https://doi.org/10.1021/nl803279t.Suche in Google Scholar PubMed
Wu, Z.S., Winter, A., Chen, L., Sun, Y., Turchanin, A., Feng, X., and Mullen, K. (2012). Three-dimensional nitrogen and boron co-doped graphene for high-performance all-solid-state supercapacitors. Adv. Mater. 24: 5130–5135, https://doi.org/10.1002/adma.201201948.Suche in Google Scholar PubMed
Wu, R., Gan, L., Ou, X., Zhang, Q., and Luo, Z. (2016). Detaching graphene from copper substrate by oxidation-assisted water intercalation. Carbon 98: 138–143, https://doi.org/10.1016/j.carbon.2015.11.002.Suche in Google Scholar
Wu, Y., Zhao, W., Zhu, X., and Xue, Q. (2019a). Improving the corrosion resistance of graphene-coated copper via accurate defect healing without sacrificing electronic conductivity. Carbon 153: 95–99, https://doi.org/10.1016/j.carbon.2019.07.006.Suche in Google Scholar
Wu, Y., Zhu, X., Zhao, W., Wang, Y., Wang, C., and Xue, Q. (2019b). Corrosion mechanism of graphene coating with different defect levels. J. Alloys Compd. 777: 135–144, https://doi.org/10.1016/j.jallcom.2018.10.260.Suche in Google Scholar
Wu, H., Cheng, L., Liu, C., Lan, X., and Zhao, H. (2021a). Engineering the interface in graphene oxide/epoxy composites using bio-based epoxy-graphene oxide nanomaterial to achieve superior anticorrosion performance. J. Colloid Interface Sci. 587: 755–766, https://doi.org/10.1016/j.jcis.2020.11.035.Suche in Google Scholar PubMed
Wu, Y., Sun, T.Y., Ge, T., Zhao, W., and Huang, L.F. (2021b). Eliminating the galvanic corrosion effect of graphene coating by an accurate and rapid self-assembling defect healing approach. Adv. Funct. Mater. 32: 2110264, https://doi.org/10.1002/adfm.202110264.Suche in Google Scholar
Wu, Y., Zhao, W., Lu, Z., and Wang, L. (2021c). Fluorinated graphene film for corrosion control on copper: experimental and theoretical studies. Carbon 179: 445–457, https://doi.org/10.1016/j.carbon.2021.04.040.Suche in Google Scholar
Xie, X., Su, D., Zhang, J., Chen, S., Mondal, A.K., and Wang, G. (2015). A comparative investigation on the effects of nitrogen-doping into graphene on enhancing the electrochemical performance of SnO2/graphene for sodium-ion batteries. Nanoscale 7: 3164–3172, https://doi.org/10.1039/c4nr07054b.Suche in Google Scholar PubMed
Xu, X., Yi, D., Wang, Z., Yu, J., Zhang, Z., Qiao, R., Sun, Z., Hu, Z., Gao, P., Peng, H., et al.. (2018). Greatly enhanced anticorrosion of Cu by commensurate graphene coating. Adv. Mater. 30: 1702944, https://doi.org/10.1002/adma.201702944.Suche in Google Scholar PubMed
Xu, X., Shi, S., Tang, Y., Wang, G., Zhou, M., Zhao, G., Zhou, X., Lin, S., and Meng, F. (2021). Growth of NiAl-layered double hydroxide on graphene toward excellent anticorrosive microwave absorption application. Adv. Sci. (Weinh.) 8: 2002658, https://doi.org/10.1002/advs.202002658.Suche in Google Scholar PubMed PubMed Central
Yang, S.-Y., Lin, W.-N., Huang, Y.-L., Tien, H.-W., Wang, J.-Y., Ma, C.-C.M., Li, S.-M., and Wang, Y.-S. (2011a). Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 49: 793–803, https://doi.org/10.1016/j.carbon.2010.10.014.Suche in Google Scholar
Yang, Z.H.I., Nie, H., Zhou, X., Yao, Z., Huang, S., and Chen, X. (2011b). Investigation of homologous series as precursory hydrocarbons for aligned carbon nanotube formation by the spray pyrolysis Method. Nano 6: 205–213, https://doi.org/10.1142/s1793292011002536.Suche in Google Scholar
Yang, S., Zhi, L., Tang, K., Feng, X., Maier, J., and Müllen, K. (2012). Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv. Funct. Mater. 22: 3634–3640, https://doi.org/10.1002/adfm.201200186.Suche in Google Scholar
Yang, M., Liu, B., Xia, J., Liu, Y., Shi, Z., and Lv, X. (2019). Study on the properties of a novel electrostatic conductive and anti-corrosive composite coating improved by graphene nanosheets. Prog. Org. Coat. 136: 105244, https://doi.org/10.1016/j.porgcoat.2019.105244.Suche in Google Scholar
Yi, M., Shen, Z., Zhao, X., Liang, S., and Liu, L. (2014). Boron nitride nanosheets as oxygen-atom corrosion protective coatings. Appl. Phys. Lett. 104: 143101, https://doi.org/10.1063/1.4870530.Suche in Google Scholar
Yoo, B.M., Shin, H.J., Yoon, H.W., and Park, H.B. (2014). Graphene and graphene oxide and their uses in barrier polymers. J. Appl. Polym. Sci. 131: 39628, https://doi.org/10.1002/app.39628.Suche in Google Scholar
Yoon, T., Shin, W.C., Kim, T.Y., Mun, J.H., Kim, T.S., and Cho, B.J. (2012). Direct measurement of adhesion energy of monolayer graphene as-grown on copper and its application to renewable transfer process. Nano Lett. 12: 1448–1452, https://doi.org/10.1021/nl204123h.Suche in Google Scholar PubMed
Yu, F., Stoot, A.C., Bøggild, P., and Camilli, L. (2016). Failure of multi-layer graphene coatings in acidic media. RSC Adv. 6: 21497–21502, https://doi.org/10.1039/c6ra01556e.Suche in Google Scholar
Yu, F., Camilli, L., Wang, T., Mackenzie, D.M.A., Curioni, M., Akid, R., and Bøggild, P. (2018). Complete long-term corrosion protection with chemical vapor deposited graphene. Carbon 132: 78–84, https://doi.org/10.1016/j.carbon.2018.02.035.Suche in Google Scholar
Yuan, A., Guan, R., and Luo, B. (2020a). Oxidative originators of graphene barrier coating grown on surfaces. Chem. Nano. Mat 6: 1285–1297, https://doi.org/10.1002/cnma.202000269.Suche in Google Scholar
Yuan, G., Lin, D., Wang, Y., Huang, X., Chen, W., Xie, X., Zong, J., Yuan, Q.Q., Zheng, H., Wang, D., et al.. (2020b). Proton-assisted growth of ultra-flat graphene films. Nature 577: 204–208, https://doi.org/10.1038/s41586-019-1870-3.Suche in Google Scholar PubMed
Yuan, S., Sun, Y., Cong, C., Liu, Y., Lin, D., Pei, L., Zhu, Y., and Wang, H. (2023). A bi-layer orientated and functionalized graphene-based composite coating with unique hydrogen gas barrier and long-term anti-corrosion performance. Carbon 205: 54–68, https://doi.org/10.1016/j.carbon.2023.01.027.Suche in Google Scholar
Zhan, Y., Zhang, J., Wan, X., Long, Z., He, S., and He, Y. (2018). Epoxy composites coating with Fe3O4 decorated graphene oxide: modified bio-inspired surface chemistry, synergistic effect and improved anti-corrosion performance. Appl. Surf. Sci. 436: 756–767, https://doi.org/10.1016/j.apsusc.2017.12.095.Suche in Google Scholar
Zhang, Y.H., Wang, B., Zhang, H.R., Chen, Z.Y., Zhang, Y.Q., Wang, B., Sui, Y.P., Li, X.L., Xie, X.M., Yu, G.H., et al.. (2014a). The distribution of wrinkles and their effects on the oxidation resistance of chemical vapor deposition graphene. Carbon 70: 81–86, https://doi.org/10.1016/j.carbon.2013.12.075.Suche in Google Scholar
Zhang, Y.H., Zhang, H.R., Wang, B., Chen, Z.Y., Zhang, Y.Q., Wang, B., Sui, Y.P., Zhu, B., Tang, C.M., Li, X.L., et al.. (2014b). Role of wrinkles in the corrosion of graphene domain-coated Cu surfaces. Appl. Phys. Lett. 104: 143110, https://doi.org/10.1063/1.4871000.Suche in Google Scholar
Zhang, H., Ren, S., Pu, J., and Xue, Q. (2018). Barrier mechanism of multilayers graphene coated copper against atomic oxygen irradiation. Appl. Surf. Sci. 444: 28–35, https://doi.org/10.1016/j.apsusc.2018.03.026.Suche in Google Scholar
Zhang, R., Yu, X., Yang, Q., Cui, G., and Li, Z. (2021). The role of graphene in anti-corrosion coatings: a review. Constr. Build. Mater. 294: 123613, https://doi.org/10.1016/j.conbuildmat.2021.123613.Suche in Google Scholar
Zhang, C., Li, W., Liu, C., Zhang, C., Cao, L., Kong, D., Wang, W., and Chen, S. (2022). Effect of covalent organic framework modified graphene oxide on anticorrosion and self-healing properties of epoxy resin coatings. J. Colloid Interface Sci. 608: 1025–1039, https://doi.org/10.1016/j.jcis.2021.10.024.Suche in Google Scholar PubMed
Zhang, X., Li, B., Chen, T., Ke, X., and Xiao, R. (2023). Study on CePO4 modified PANI/RGO composites to enhance the anti-corrosion property of epoxy resin. Prog. Org. Coat. 178: 107472, https://doi.org/10.1016/j.porgcoat.2023.107472.Suche in Google Scholar
Zhao, Z., Hou, T., Wu, N., Jiao, S., Zhou, K., Yin, J., Suk, J.W., Cui, X., Zhang, M., Li, S., et al.. (2021). Polycrystalline few-layer graphene as a durable anticorrosion film for copper. Nano Lett. 21: 1161–1168, https://doi.org/10.1021/acs.nanolett.0c04724.Suche in Google Scholar PubMed
Zhao, Z., Zhou, M., Zhao, W., Hu, J., and Fu, H. (2022). Anti-corrosion epoxy/modified graphene oxide/glass fiber composite coating with dual physical barrier network. Prog. Org. Coat. 167: 106823, https://doi.org/10.1016/j.porgcoat.2022.106823.Suche in Google Scholar
Zhao, M., Zhang, Z., Shi, W., Li, Y., Xue, C., Hu, Y., Ding, M., Zhang, Z., Liu, Z., Fu, Y., et al.. (2023). Enhanced copper anticorrosion from Janus-doped bilayer graphene. Nat. Commun. 14: 7447, https://doi.org/10.1038/s41467-023-43357-1.Suche in Google Scholar PubMed PubMed Central
Zheng, Z., Xiao, L., Huang, P., and Wang, F. (2021). Polydopamine improved anticorrosion of graphene on copper: inhibiting galvanic corrosion and healing structure defects. Appl. Mater. Today 24: 101069, https://doi.org/10.1016/j.apmt.2021.101069.Suche in Google Scholar
Zhou, F., Li, Z., Shenoy, G.J., Li, L., and Liu, H. (2013). Enhanced room-temperature corrosion of copper in the presence of graphene. ACS Nano 7: 6939–6947, https://doi.org/10.1021/nn402150t.Suche in Google Scholar PubMed
Zhou, S., Wu, Y., Zhao, W., Yu, J., Jiang, F., Wu, Y., and Ma, L. (2019). Designing reduced graphene oxide/zinc rich epoxy composite coatings for improving the anticorrosion performance of carbon steel substrate. Mater. Des. 169: 107694, https://doi.org/10.1016/j.matdes.2019.107694.Suche in Google Scholar
Zhu, D. and Van Ooij, W.J. (2004). Corrosion protection of metals by water-based silane mixtures of bis-[trimethoxysilylpropyl]amine and vinyltriacetoxysilane. Prog. Org. Coat. 49: 42–53, https://doi.org/10.1016/j.porgcoat.2003.08.009.Suche in Google Scholar
Zhu, X., Yinghao, W., Zhao, W., Pu, J., Yang, D., and Xue, Q. (2019). Large-area preparation of defect-repaired fluorocarbon polymer coatings on graphene for long-term corrosion resistance. Prog. Org. Coat. 134: 234–243, https://doi.org/10.1016/j.porgcoat.2019.05.003.Suche in Google Scholar
Zhu, X., Yan, Q., Cheng, L., Wu, H., Zhao, H., and Wang, L. (2020). Self-alignment of cationic graphene oxide nanosheets for anticorrosive reinforcement of epoxy coatings. Chem. Eng. J. 389: 124435, https://doi.org/10.1016/j.cej.2020.124435.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Anticorrosion properties of flavonoids for rust-free building materials: a review
- Optimization strategies for graphene-based protection coatings: a review
- Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
- Efficient and reliable corrosion control for subsea assets: challenges in the design and testing of corrosion probes in aggressive marine environments
- Original Articles
- Corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment
- Model construction of corrosion resistance of alloying elements for low alloy steel in marine atmospheric corrosive environment based on machine learning
- Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 42 (2024)
Artikel in diesem Heft
- Frontmatter
- Reviews
- Anticorrosion properties of flavonoids for rust-free building materials: a review
- Optimization strategies for graphene-based protection coatings: a review
- Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
- Efficient and reliable corrosion control for subsea assets: challenges in the design and testing of corrosion probes in aggressive marine environments
- Original Articles
- Corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment
- Model construction of corrosion resistance of alloying elements for low alloy steel in marine atmospheric corrosive environment based on machine learning
- Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 42 (2024)

