Abstract
The corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment has been systematically studied. Results showed that a layer of protective corrosion products can be formed quickly and become defective over time. During the periodic service, the HP (high hydrostatic pressure) promotes the anodic dissolution of the base and the generation of (Cu, Zn)2(OH)3Cl, which causes expansion of the corrosion products; the AP (alternating pressure) facilitates the wetting process during dry to wet stage, and the alternating force caused by AP leads to the cracks, peeling off of the corrosion products. Severe intergranular corrosion takes place, which initiates at the β phase and is accelerated by the combination of defective corrosion products and the drying stage.
1 Introduction
With the rapidly increasing demand for various resources by global industries and the depletion of terrestrial resources, the utilization of resources from the deep sea has become an important strategy for long-term economic development (Petersen et al. 2016). Compared with the shallow sea, the deep regions remain less explored due to significant safety concerns caused by the increasing HP (which increases by approximately 1 MPa per 100 m of depth) (Li et al. 2021). One of the main challenges for exploring deep sea environments is the corrosion-induced failure of underwater devices. Many studies have been carried out to request great anticorrosion performance for structural materials by both in situ (Dexter 1980; Ding et al. 2018; Luciano et al. 2013; Sawant et al. 1993; Venkatesan et al. 2002) or laboratory methods (Beccaria and Poggi 1985; Zhang et al. 2009) in deep sea environments. However, most research studies have been conducted under the assumption of static conditions in deep sea environments, which only apply to scenarios where materials remain stationary throughout their service life. In fact, for most detection equipment, such as submarines or underwater robots, a periodic working state involving diving and ascending is required (Hu et al. 2019; Meng et al. 2015), which subjects structural materials to cyclic exposure between atmospheric and deep sea environments, demanding consideration of a more complicated corrosion scenardio.
On one hand, materials operating in the deep sea environment are subjected to HP from the seawater. The role of HP on the corrosion of various alloys has been studied by laboratory experiments. Beccaria and coworkers studied the influence of HP on the corrosion of aluminum and its alloys, nickel, and stainless steels (Beccaria et al. 1991, 1993, 1994, 1995). They found that HP had a marked effect on the property of oxide formed at the metal surface. The pitting susceptibility of Al and 6061 T6 Al alloy increased with HP due to the increase in SO4 2− and Cl− in the oxidation layer of Al and the formation of more compact film with lower self-repairing power for 6061 alloy (Beccaria et al. 1994). The nickel corrosion resistance decreased with increasing HP in concentrated NaCl solution due to the decrease in percentage of wet nickel oxides and then the increase in the chemisorption of Cl− (Beccaria et al. 1993). Furthermore, they stated that both the localized and generalized corrosion resistance of stainless steels in seawater depended on the variation of passive film composition formed at different HP (Beccaria et al. 1995). Sun et al. compared the corrosion process of low alloy steels and Al-Zn-In-Mg-Ti alloy at HP and atmosphere pressure (Sun et al. 2013a,b), also found that these materials corroded more quickly at high HP with the similar effect on the corrosion process. Based on these findings, some research studies have attempted to unveil the basic mechanism on why HP affects the corrosion process. Yang et al. verified that the stress concentration caused by the growth of pits under HP can accelerate the corrosion rate (Yang et al. 2013). Ma et al. proved that HP affected the activity of species in the electrolyte, such as oxygen and Cl−, through mathematic calculation methods (Ma et al. 2019). Liu et al. (2021a) thought that the decreased thickness of the Helmholtz layer may be a contributing factor to high HP promoting the anodic dissolution process. Recent years, the corrosion behavior of new materials such as the nanocrystalline 304 under HP (Ma et al. 2024) and the effect of working conditions closer to the actual deep sea such as HP with water velocity (Liu et al. 2024) or mechanical tensile stress (Liu et al. 2024) on the corrosion process of some materials have been kept focusing.
On the other hand, AP typically experienced by devices moving vertically in marine environments may also influence the corrosion process of structural materials. Until now, the majority of studies (Cao et al. 2020; Liu et al. 2021b; Meng et al. 2015; Tian et al. 2014; Wang et al. 2019) concerning AP have focused on the coating deterioration process. The AP was found to decrease the protective properties of coatings via a “push-and-pull” effect, which can promote water transportation into the coatings, and deteriorate the interface structures of the coating/steel system. Such effects of AP should also be considered when examining the corrosion of naturally filmed metallic materials such as stainless steels (Duan et al. 2019) or copper alloys (Ma et al. 2015). It is noteworthy that several studies (Hu et al. 2011; Sun 2013; Sun et al. 2013b) continue to focus on the influence of AP on the corrosion behavior of structural materials. Hu (2012) investigated the performance of an Al–Zn–In sacrificial anode in a cyclic service between surface and deep sea environment and reported that the AP lead the anodic potential shifting toward the positive direction due to the deposition of corrosion product and decreased the circuit current of the CP system, consequently reducing the discharge efficiency of the anode. Sun (2013) focused the corrosion behavior of 10Ni5CrMoV low-alloy steel in 3.5 % NaCl solution under the influence of AP and found that the corrosion rate under AP is greater than that under normal pressure and static HP. On the one hand, the AP causes the internal rust layer to exhibit large-scale flaking through alternating tensile and compressive actions, facilitating the penetration of Cl− to the substrate. The presence of a large amount of Cl− induces the formation of β-FeOOH phase, which further deteriorates the protective performance of the product film, accelerating the corrosion of the substrate.
Furthermore, the surfaces of marine devices are subjected to alternating wet and dry states due to frequent exposure to seawater. During the drying period, the liquid film on the material surface gradually evaporates and becomes thinner, resulting in an increased concentration of Cl− in the liquid film, which can accelerate the electrochemical corrosion of materials (De et al. 2016; De La Fuente et al. 2011).
Brass, due to its high formability, good corrosion resistance, and electrical and thermal conductivity, is widely used in components such as valves, pipes, and radiators for marine engineering applications, including desalination plants and steamships (Damej et al. 2020; Dridi et al. 2020). The degradation process of brass in different seawater environments has been extensively studied, including various conditions: temperature (Rezakhani 2011), pH (Badawy and Al-Kharafi 1999), [Cl−] (Alfatazi et al. 2009; Ateya et al. 2009), inhibitor presence (Damej et al. 2020; Zhang et al. 2016), etc. Also, the corrosion behavior of brass in the environment related with deep sea is continuously focused by various researchers and organization (Traverso and Canepa 2014). However, when brass is used in applications such as submarines or underwater robots, the periodic conditions mentioned above are likely to affect the corrosion process. To the best of our knowledge, limited studies have addressed this specific type of corrosion issue.
In this study, we investigated the corrosion of Cu–40Zn brass subjected to a periodic transitioning between simulated atmospheric and deep-sea environments with a combination of gravimetric, SEM/EDS, electrochemical methods, etc. The effect of HP, AP, and the drying condition of the periodic service on the corrosion process have been discussed.
2 Materials and methods
2.1 Materials
The samples used in the tests were cut from sheet material with dimensions of 500 × 200 × 20 mm3, which was manufactured through casting at 1,150 °C and annealing at 780 °C. The chemical composition of Cu–40Zn is as follows (wt%): Zn 39, Fe 0.17, Ni 0.06, Mn 0.08, Cu balance. The matrix of the alloy consists of α (Cu-rich) and β (Zn-rich) phases (seeing the Supplementary Figure 1). EDS analysis indicated that the α phase contained 37–39 wt% Zn and 61–63 wt% Cu, whereas the β phase contained 53–56 wt% Zn and 44–47 wt% Cu.
2.2 Experimental settings
The corrosion tests were carried out using a high-pressure vessel as described in previous study (Hu et al. 2019) and presented in Supplementary Figure 2. In a single experimental cycle (T), as shown in Figure 1, specimens were first placed into the vessel and subjected to a pressurizing process for 0.5 h to simulate the diving period; after the pressure reached 6.3 MPa, the specimens were then exposed to this condition for 23 h to simulate the working period in the deep sea environment; a depressurizing process at the same velocity as the pressurizing process was then applied to simulate the ascending period; finally, samples were placed in an oven with constant conditions of 20 ± 1 °C and 24.7 % RH (relative humidity) for 24 h to simulate the working period in the atmosphere environment. For the contrast experiment, another set of specimens was immersed in the same vessel at common pressure (0.1 MPa) for 24 h and then kept in the oven under the same conditions for a subsequent 24 h. The electrolyte used in the vessel was neutral 3.5 % NaCl (wt%) solution and kept at a temperature of 20 ± 1 °C. The whole test duration lasted for 25 T (1,200 h).
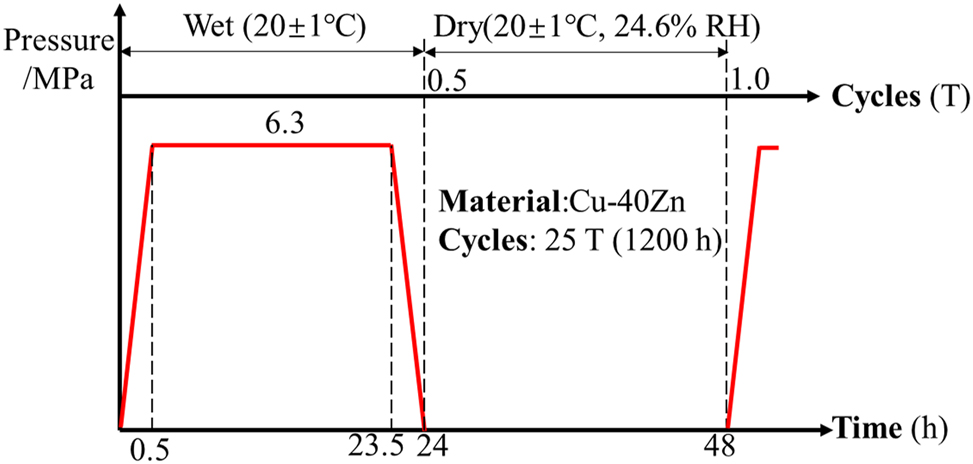
The periodic experimental settings in the study.
For simplicity, in this paper, 6.3 MPa denotes experiments simulating the period service between marine atmospheric and deep sea environments, while 0.1 MPa denotes the contrast experiment.
2.3 Weight loss experiments
Specimens were cut to dimensions of 30 × 15 × 4 mm3 using wire-electrode cutting. Five specimens were used for each experiment to ensure reproducible and reliable results. Before immersion, the specimens were wet ground to 1,000 grit step by step using SiC paper, cleaned with acetone, washed with deionized water, dried in laboratory air, and stored in a desiccator. The specimens were weighed by means of an analytical balance (Sartorius CF225D) with a precision of 10−5 g for the original weight. After each immersion test, the corrosion products on the specimens were removed by the ultrasonic cleaning in 6 mol/L hydrochloric acid solution. Afterward, the specimens were washed with deionized water, dried in laboratory air, and weighed again.
2.4 Analysis of the corrosion products
The surface and cross-sectional morphologies of the corrosion products were examined by SEM/EDS (S-400). For cross-sectional observation, three coupons after corroding for 25 T under each condition were sectioned and mounted with epoxy resin and then slowly ground to 3,000 grit and mildly polished with 1.0 μm diamond paste.
The XRD method (Panalytical X’Pert PRO, Cu Ka radiation at 40 kV, PA Analytical, Almelo, Holland) was used to analyze the phase components of corrosion products with a 2.0°/min scanning speed and a range of 10°–90°. Before the XRD tests, corrosion products were carefully scraped from the corroded specimens and ground to a fine powder in an agate mortar. The obtained diffraction spectra were analyzed by the commercial software JADE.5.
2.5 Electrochemical measurements
The specimens with a dimension of 10 × 10 × 4 mm3 were cut from the sheet material to make electrodes. Before the electrochemical test, the electrodes were ground to 2,000 grit, cleaned with alcohol, and dried in cold wind. The Autolab PGSTAT303 Electrochemical Measurement System in a conventional three-electrode cell was used to carry out electrochemical tests. A platinum plate (20 × 10 mm2) was used as the counter electrode and an Ag/AgCl electrode saturated with 3 M KCl as the reference electrode.
3 Results
3.1 Weight loss
The changes in weight loss with the number of corrosion cycles under the two experimental conditions are given in Figure 2. The corrosion rate is obtained as the weight loss divided by the immersion time. Figure 2a shows that both the weight loss increased with increasing corroding cycles. Compared with 0.1 MPa, the weight loss at 6.3 MPa is lower before 4 T, while become higher when the time exceeding 5 T. The corrosion rates at the two conditions in Figure 2b exhibit different characteristics: At 0.1 MPa, the corrosion rate decreases quickly during initial experimental cycles (before 5 T) and then continues decreasing at a slower rate; at 6.3 MPa, the corrosion rate also lives through a decreasing period (before 10 T) at a slower rate than that for 0.1 MPa, after which the corrosion rate becomes relatively stable. Generally, the corrosion kinetics results reveal that the conditions at 6.3 MPa decrease the corrosion rate during the initial period (before 5 T) but can accelerate the corrosion rate for prolonged exposure periods.

(a) The variation trend of weight loss and (b) corrosion rate of Cu–40Zn corroding at the two experimental conditions.
3.2 Corrosion morphology observation
The surface morphologies of the corrosion products formed at different experimental cycles are presented in Figure 3, with a combination of EDS analysis. At 0.1 MPa, a complete layer of corrosion products had been formed after corroding for 3 T (Figure 3a). At the surface of the corrosion products, some flocculent particles, formed by the reprecipitation process of Cu species, are present (Alfatazi et al. 2009; Bianchi and Longhi 1973). When the corroding time was prolonged to 25 T, the corrosion product layer became denser and a layer of precipitated particles also formed (Figure 3c). The corrosion morphologies at 6.3 MPa are different from those at 0.1 MPa. After corroding for 3 T, the corrosion product layer is thicker and began to generate cracks (Figure 3b). After 25 T, the corrosion product layer exhibits characteristics of fracturing in both the reprecipitated layer and the inner dense layer (Figure 3d). The EDS results show that the content of Cl in the corrosion products formed at 6.3 MPa is generally higher than that formed at 0.1 MPa after corroding for the same time. From Figure 4d, it can be seen that the outer precipitated layer contains more Cl than the inner dense layer.

Corrosion products morphologies of Cu–40Zn and EDS detecting results after corroding for 3 T: (a) 0.1 MPa, (b) 6.3 MPa and 25 T: (c) 0.1 MPa, (d) 6.3 MPa.

Cross-sectional morphologies and the EDS analysis of the corrosion products formed on the Cu–40Zn after corroding for 25 T: (a) 0.1 MPa and (b) 6.3 MPa.
The typical cross-sectional morphologies of the corrosion product layer formed after 25 T under the two experimental conditions are given in Figure 4. The result for 0.1 MPa shows that the corrosion layer consists of an outer loose layer and an inner relatively compact layer. The outer loose layer corresponds to the precipitated layer, as shown in Figure 3c. The corrosion product layer formed at 6.3 MPa only appears to consist only of the compact layer, potentially because the loose layer may have peeled off within the field of view (Figure 3d). Perforative cracks are found to exist in the compact layer at 6.3 MPa. The thickness of the compact layer is 3.4 μm at 0.1 MPa and 2.8 μm at 6.3 MP. Considering that the corrosion process at 6.3 MPa is faster than that at 0.1 MPa during the later experimental cycles (Figure 2). Thus, the compact layer formed at 6.3 MPa is likely to have undergone significant exfoliation. Intergranular corrosion occurs under both experimental conditions. Several cross sections of the corrosion product layer after corroding for 25 T were observed and the depth of a couple of intergranular corrosion pits, hence, was obtained and gone through statistical analysis of cumulative probability (Shibata and Ameer 1992), which can be found in the Supplementary Figure 3. Results show that the average depths of the intergranular corrosion observed are 3.3 μm at 0.1 MPa and 7.9 μm at 6.3 MPa, referring that the condition at 6.3 MPa significantly promotes the intergranular corrosion process.
EDS analysis was performed at different parts of the cross section for better understanding of the composition of the corrosion products. The relative ratio of Cu content (WCu/WCu + WZn) at different parts was calculated, and the results are shown in Table 1. The results show that the content of Cl decreases with increasing depth of the cross section for both experimental conditions. At the same part of the cross section, the content of Cl at 6.3 MPa (b1–b4) is higher than that at 0.1 MPa (a2–a3), implying that the diffusion of Cl during corrosion is accelerated by the condition at 6.3 MPa. In the initial regions affected by intergranular corrosion (a3, a4, and b2), the relative content ratios of Cu are also lower than the average content of the base. As reported (Song et al. 2017; Zhang et al. 2016), the β phase in the base has a lower electrochemical potential and is thus corroded preferentially. Thus, the initial regions of intergranular corrosion correspond to the β phase in the base.
Chemical composition of marked areas on the cross-sectional area in Figure 4.
| Locations/wt% | O | Cl | Cu | Zn | WCu/(WCu + WZn) |
|---|---|---|---|---|---|
| a1 | 14.61 | 17.69 | 53.10 | 14.60 | 78.43 |
| a2 | 30.48 | 11.31 | 20.49 | 37.72 | 35.20 |
| a3 | 18.27 | 1.32 | 37.15 | 43.26 | 46.20 |
| a4 | 24.11 | 1.21 | 35.73 | 38.95 | 47.84 |
| b1 | 27.79 | 12.20 | 25.63 | 34.38 | 42.71 |
| b2 | 25.95 | 6.64 | 21.53 | 45.89 | 31.93 |
| b3 | 16.11 | 2.88 | 58.50 | 22.52 | 72.20 |
| b4 | 13.94 | 1.80 | 70.13 | 14.13 | 83.23 |
3.3 X-ray diffractometry
The XRD patterns for the corrosion products formed after corroding for 25 T at the two experimental conditions are presented in Figure 5. Some characteristic peaks are marked to indicate the presence of (Cu, Zn)2(OH)3Cl, Cu2O, and ZnO in the corrosion product film formed at the two conditions. The relative amounts of phases have been semi-quantitatively analyzed by the K-value method (Sun et al. 2013a) and shown in Table 2. It can be found that (Cu, Zn)2(OH)3Cl takes the majority of the corrosion product, and the content of Cu2O in the corrosion products is higher than that of ZnO at both conditions. Comparing the two conditions, the main difference is that the content of (Cu, Zn)2(OH)3Cl at 6.3 MPa is much higher than that at 0.1 MPa. As reported (Song et al. 2017), the protective ability of the corrosion product layer formed on brass depends on the Cu2O and ZnO phases. Hence, the corrosion product layer formed at 6.3 MPa should be less protective.

XRD analysis of the corrosion products formed on Cu–40Zn after corroding for 25 T at the two experimental conditions.
The relative amounts of each component of corrosion products formed after corroding for 25 T at the two experimental conditions.
| Conditions/Phase (%) | (Cu,Zn)2(OH)3Cl | Cu2O | ZnO |
|---|---|---|---|
| 0.1 MPa | 57 | 30 | 13 |
| 6.3 MPa | 69 | 24 | 7 |
3.4 EIS analysis
The EIS measurements were carried out at 0.5 h, 3 T, and 25 T, respectively, and the results are displayed in Figure 6. The shape and diameter of the Nyquist plot can reflect the property of the corrosion process on the sample surface. The larger the diameter of the semicircle, the higher resistance for the corrosion process (Fuller et al. 2007). It can be seen from Figure 7a that the impedance spectrum after corroding for 0.5 h consists of a semicircle and an oblique line, indicating that the corrosion mechanism was jointly controlled by the charge transfer process and the diffusion process (Qin et al. 2020; Szczygiel and Kolodziej 2005) at the initial experimental time. With the increase in corroding cycles, the oblique line disappears, and the diameter of the semicircle enlarges (Figure 6a, c, e), which suggests the possible formation and thickening of a protective corrosion layer at the surface, as evidenced in Figure 4.

EIS results of Cu–40Zn after corroding at the two experimental conditions for different cycles: (a, b) 0.5 h; (c, d) 3 T; (e, f) 25 T. Solid lines represent the fitted results.

The equivalent circuit used to fit the EIS data: (a) equivalent circuit for the specimens after corroding for 0.5 h; (b) equivalent circuit for the specimens after corroding for 3 T and 25 T.
The equivalent circuits shown in Figure 7 were used to fit the impedance spectra in Figure 6. Considering the different surface state, Figure 7a was used to fit the impedance spectra at 0.5 h and Figure 7b was used to fit the impedance spectra at 3 T and 25 T. In the model, constant phase elements (CPE) were used instead of an ideal capacitance due to local inhomogeneities (roughness or porosity) of the electrode surface (Cao and Zhang 2002). In Figure 7a, Rs represents the solution resistance; the parallel pair of CPE1 (the double electric layer, Qdl), Rct (the charge transfer resistance) describe the electric properties of the charge transfer process, and W refers to the Warburg impedance, representing the ion diffusion through the corrosion product layer (Lv et al. 2022). In Figure 7b, besides the pair of CPE1 and Rct, another parallel pair of CPE2 (Q1) and R1 was used to represent the electric properties of the corrosion product layer. The fitting results are shown with solid lines in Figure 6 and are in good agreement with the experimental data.
As shown in Table 3, the Rct at 6.3 MPa is smaller than that at 0.1 MPa for the initial corroding time. In general, a lower Rct reflects a higher corrosion rate of the process since the exchange current is directly associated with the electrochemical process of corrosion (Hong et al. 2002; Sobral et al. 2001). Hence, the initial corrosion reaction rate at 6.3 MPa should be faster than that at 0.1 MPa. However, the data show that the weight loss rate at 6.3 MPa is less than that at 0.1 MPa prior to 5 T (Figure 3). As reported (Ma et al. 2015), a protective corrosion product film could be established on the surface of copper alloys within a few hours’ exposure to seawater. The lower corrosion rate at 6.3 MPa during the early corrosion cycles could be due to a faster formation and thickening process for the corrosion product layer, which can be verified by the result that the resistance of the product film formed after corroding for 3 T at 6.3 MPa is almost twice as big as that at 0.1 MPa. In addition, the Rf is much bigger than Rct at the same condition; hence, the protectiveness of the corrosion product film dominated the corrosion process of the base once it came into being. After corroding for 25 T, the lower Rf at 6.3 MPa indicates that the protectiveness of the corrosion product layer formed at 6.3 MPa is weaker than that at 0.1 MPa, which may be caused by the exploitation process at 6.3 MPa as shown in Figure 3b and d, in accordance with the weight loss result in Figure 2.
EIS fitting parameters of the corrosion products formed after corroding for different circles at the two experimental conditions.
| Conditions | Rs
Ω cm2 |
Qdl × 10−5
F cm−2 |
ndl | Rct
Ω cm2 |
W × 10−3
Ω cm2 |
Qf × 10−5
F cm−2 |
nf | Rf
Ω cm2 |
Σχ2
× 10−3 |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 h–0.1 MPa | 10.04 | 41.83 | 0.74 | 2,346 | 1.79 | 0.692 | |||
| 0.5 h–6.3 MPa | 9.04 | 48.32 | 0.74 | 943.9 | 2.05 | 2.11 | |||
| 3 T–0.1 MPa | 9.74 | 4.71 | 0.83 | 197.9 | – | 19.73 | 0.57 | 3,400 | 1.85 |
| 3 T–6.3 MPa | 10.98 | 4.36 | 0.80 | 232.2 | – | 12.95 | 0.60 | 6,403 | 1.07 |
| 25 T–0.1 MPa | 13.33 | 5.76 | 0.65 | 927.9 | – | 7.27 | 0.54 | 1.874 × 104 | 3.84 |
| 25 T–6.3 MPa | 13.67 | 4.97 | 0.65 | 365.7 | – | 10.13 | 0.41 | 1.609 × 104 | 3.97 |
4 Discussion
4.1 Effect of HP on the corrosion process
According to the experimental settings, the time suffering from the static HP condition takes the main part of the corroding cycles for the alloy. The HP condition is also the main difference between the two experiments. The obtained results clearly show that the corrosion process was closely related to the influence of HP. To further unveil the effect of HP on the corrosion process, the polarization curves of fresh Cu–40Zn during immersion at two pressures were obtained and are given in Figure 8. It is clearly seen that the curves at both pressures exhibited typical passive feature. Similar polarization characteristics of Cu–Zn alloy were also reported by the previous researches (Lv et al. 2022; Ren et al. 2023). Compared to the measurements at 0.1 MPa, the anodic branch of the polarization curves at 6.3 MPa shifted toward more negative potentials, which shows that HP mainly promotes the anodic dissolution reaction of the alloy and hence increases the corrosion current density. Moreover, it can find that the passive current density becomes bigger and the breakdown potential becomes smaller at 6.3 MPa, indicating that HP weakens the passive ability of the alloy.

Potentiodynamic polarization curves of Cu–40Zn alloy during initial immersion at the two pressures.
4.2 Effect of AP on the corrosion process
During the changing of pressure, a direct impact on the surface of metal is the alternating force, which exhibits compressive stress during the pressurizing period (devices rising up) and tensile stress during the depressurizing period (devices sinking). Therefore, the available researches regarding the influence of AP mainly focus on the outcome of AP on structural properties of the corrosion product film, such as cracking, peeling off, etc. This kind of effect should also be at work as it found that the structural defects in the corrosion product film of Cu–40Zn are more prevalent at 6.3 MPa than those at 0.1 MPa, as illustrated in Figures 3 and 4. However, as the HP makes an obvious influence on the corrosion reactions, consideration on such effect of the AP should also be taken into account. In addition, when studied the effect of AP, the existing researches used the sudden change of pressure (such as from HP to common pressure in several seconds) to simulate the sinking and rising process (Hu et al. 2011; Sun 2013), which surely deviates from the actual condition.
In this work, we adopted a gradual change in pressure by controlling the pressurization and depressurization speed during each experimental cycle (as shown in Figure 1). OCP is related to both the anodic and cathodic reaction states (Cao 2008) and is always used to study the dynamic state of the corrosion process (Abd et al. 2013; Haleem et al. 2010). To study the influence of AP, the OCPs of the filmed sample after corroding for 10 T during the dry to wet process were obtained, as shown in Figure 9a. The variation trend of the OCPs at the two experimental conditions both experienced two distinct stages. For the condition at 0.1 MPa, the OCP decreased quickly between 0 and 824 s and then kept relatively stable. For the condition at 6.3 MPa, the OCP also decreased quickly between 0 and 425 s and then kept decreasing at a relatively lower speed. Zhang et al. (2016) reported that the formation of corrosion product layer on Cu–40Zn can make the surface hydrophobic. Muster et al. (2004) deemed that the ability of a dried surface to wet is controlled by the roughness and porosities. Therefore, in this work, it is believed that it took time for the filmed sample to get totally wet during the wet to dry period. The relatively wetting rates (Rw) during the dry to wet period were measured, which could be found in Supplementary Figure 4. It found that Rw firstly decreased quickly and then stayed relatively stable at the two pressures, in consistent with the OCP. Hence, the rapid decrease in the OCPs should owe to the wetting process. After the corrosion product getting wet, a relatively stable electrochemical state achieved and the OCP at 0.1 MPa didn’t change much with time prolonging. However, for the condition at 6.3 MPa, the influence of the increasing pressure on the anodic reaction process (Figure 8) made the OCP keep decreasing. During the wetting period, the corrosion process is controlled by the oxygen diffusing to cathodic sites (Muster et al. 2004). After wetting, the corrosion process should be controlled by the charge transfer process as the EIS results illustrated (Figure 6). Hence, the variation trend of the polarization curves during the dry to wet process at the two experimental conditions can be schematically described as shown in Figure 9c.

(a) The variation trend of OCPs during the dry to wet period after corroding for 10 T at the two experimental conditions; (b) the schematic diagram to illustrate the variation trend of polarization curves during the dry to wet period: “QD” refers to “quick decreasing stage” and “GD” refers to “gradual decreasing stage.”
4.3 Effect of the drying stage on the corrosion process
To evaluate the influence of the drying stage on the corrosion process, the variation trend of the concentration of the residual solution on the samples after corroding for 10 T was obtained during the drying stage. The wet samples were placed on the electronic balance (Sartorius CF225D) in the drying environment (with the temperature of 20 °C and relative humidity of 24.7 %), and the total weight of samples was continuously monitored. The concentration of the residual solution was then calculated as:
where m 0 is the initial weight of the wet sample, m ∞ is the weight of the wet sample after getting totally dry, and m t is the weight of the wet sample with a drying time of t. As the evaporation of water mainly depends on the environmental condition, the variation trend of the concentration of the residual solution for the samples corroding at the two experimental conditions makes little difference, and the result is presented in Figure 10. Generally, the transition from wet to dry conditions occurred in two stages: during the initial 0–2,750 s, the concentration increased slowly; after then, the concentration increased quickly and reached 100 % at 4,400 s.

The variation trend of the concentration of the residual solution on the filmed sample after corroding for 10 T during the drying stage.
Before the residual solution could cover the surface of samples completely, the liquid film became thinner and the concentration increased slowly as the water evaporated gradually. On one hand, the thin liquid layer affects the diffusion rate of dissolved oxygen. The thinner the mass-transfer diffusion layer, the easier it is for oxygen to reach the matrix. On the other hand, the increased salinity improves the conductivity of the liquid film, and more importantly, the activity of the Cl− is also greatly promoted. Hence, the transfer rate of the substances required for the electrochemical reaction is accelerated, and the electrochemical reaction processes are promoted. As the drying time increased, the residual solution could no longer cover the entire surface of the corroded sample and gather in the cracks and defects where is easy to get wet (Muster et al. 2004; Zhang et al. 2016). The large Cl− concentration gradient in the residual solution provides the driving force for its diffusion into the cracks. The accumulation of Cl− in the cracks is favorable for the hydrolysis of Cu ions (Kamimura et al. 2005), which leads to acidification in cracks and promotes the propagation of the intergranular corrosion.
4.4 Combined effect of HP, AP, and drying stage on the corrosion process
The weight loss experiments show that the Cu–40Zn alloy is susceptible to both uniform corrosion and intergranular corrosion under the alternating dry/wet conditions, and the susceptibility is significantly increased by factors related to the periodic service between atmospheric and deep sea environment. According to the results of XRD (Figure 5), the surface corrosion products film mainly consists of (Cu, Zn)2(OH)3Cl, Cu2O, and ZnO, which was the consequence of the electrochemical reaction of the major elements in the base (Cu and Zn). Generally, the cathodic reaction of copper and its alloy in neutral NaCl solution is the reduction of oxygen as seen in Ref (Metikos-Hukovic et al. 2010):
The anodic reaction of Cu component in copper and its alloy in neutral chloride media has been depicted by the sequence as (Kear et al. 2004):
The Cu2O then can come into being through a hydrolysis reaction of CuCl2 − according to (Campbell et al. 2002):
Furthermore, Cu(I) oxidizes to Cu(II) according to Refs (Campbell et al. 2002; Ma et al. 2015):
It can be found that Cl− plays a key role on the dissolution process of Cu. In the presence of sodium chloride, Zn2+ could take part in the formation process of paratacamite (Equation (6)); hence, the Cu2(OH)3Cl transferred to (Cu, Zn)2(OH)3Cl. With respect to Zn, the anodic reaction of Zn can be described as follows (Alfatazi et al. 2009; Mouanga et al. 2010):
In the early corrosion cycles, it is believed that uniform corrosion plays a leading role of the electrochemical process. During this period, the corrosion product film comes into being and become protective, which can be schematically described by Figure 11a for 0.1 MPa and by Figure 11d for 6.3 MPa. Based on the measured potentiodynamic polarization curves, HP made an obvious effect on the corrosion behavior of the Cu–40Zn alloy. As found in the previous studies (Hu et al. 2019, 2020), HP mainly enhanced the adsorption of Cl− ion on the metal surface and increased its activity in the corrosion process of Cu alloys. In addition, the electrical conductivity of seawater and gas diffusion coefficient were also reported to increase with the increasing HP (Horne and Frysinger 1963). Therefore, the electrochemical reactions concerning the corrosion process were all accelerated during static immersion at HP and the AP period. For the concentration of Cl− in the electrolyte is much higher than OH−, the reactions depicted in Equations (3)–(6) are likely to be more significantly affected by HP compared to other reactions, which was verified by the relatively higher Cu components at 6.3 MPa than that at 0.1 MPa (Table 2). The accelerated electrochemical reactions at 6.3 MPa resulted in the faster generation of the corrosion product film. This is the reason why the corrosion rate at 6.3 MPa is relatively slower than at 0.1 MPa before reaching the 5 T (Figure 2).
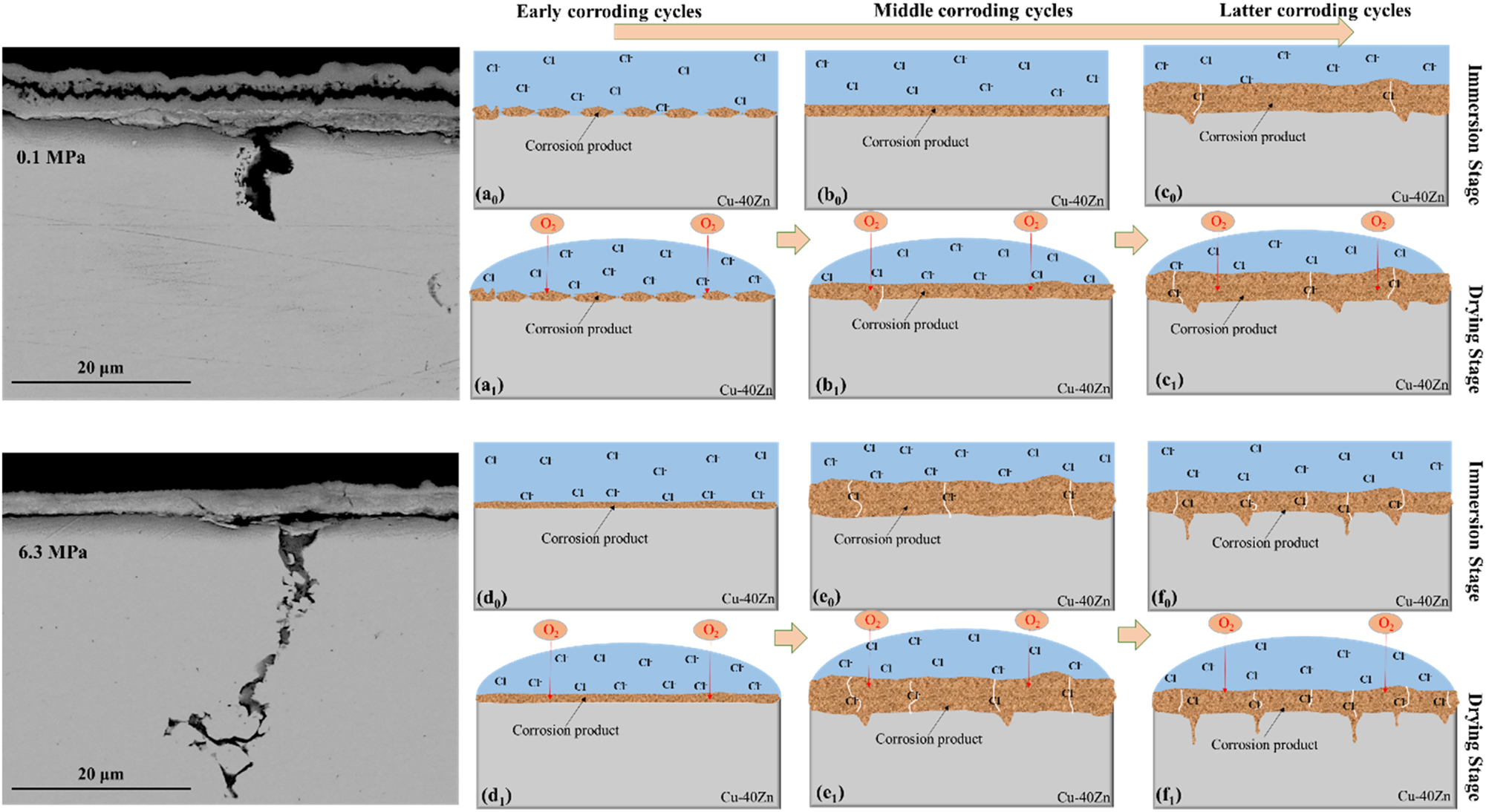
Schematic diagram for variation trend of the corrosion products film on the Cu–40Zn alloy with the corroding cycles increasing under the two experimental conditions: (a–c) 0.1 MPa; (d–f) 6.3 MPa.
In the middle corroding cycles, the corrosion product films at the two experimental conditions gradually became thicker and its protectiveness began to dominate the corrosion process, which can be schematically described in Figure 11b for 0.1 MPa and Figure 11e for 6.3 MPa. From the aspects of phase component, Cu2O and ZnO with dense structure are the main phases that afford protective function (Lv et al. 2022). During immersion time, the increased activity of Cl− by HP and AP could promote the redox transformation rate of Cu2O through equation (6). In addition, a more fractured structure of corrosion products at 6.3 MPa could facilitate the transport of Cl− and O2 during the drying period, which also shift equation (6) to the right. Therefore, the content of Cu2O and ZnO in the corrosion products (Table 2) decreased as a result of a significant increase in the content of paratacamite at 6.3 MPa. From the aspects of physical structure, the HP and AP both provided exerted compressive mechanical stress on the corrosion products. The transformation process of paratacamite (Equation (6)) and the thickening of the dense phase can cause the obvious volume expansion (Zhang et al. 2014), which produced huge internal stress. Hence, defects such as cracking and exfoliation of the corrosion products should generate much faster at 6.3 MPa under the synergistic effect of internal and external stresses (Figures 3 and 4). As a result, the protective ability of the corrosion products formed at 6.3 MPa decreased quickly, leading to a relatively higher uniform corrosion rate than that at 0.1 MPa after corroding for 5 T (Figure 2).
In the later corroding cycles, the variation trend of the corrosion product film can be schematically described in Figure 11c for 0.1 MPa and Figure 11f for 6.3 MPa. During this period, large penetrating cracks in the corrosion products have formed. The corrosive media could get to the metal base directly, which leads to the formation of pits at β phase for its lower potential (Figure 4). During the immersion state at 6.3 MPa, HP and AP promoted Cl− diffusion into the rust layer and the dissolution of the metal base, resulting in the accumulation of ions in the pits. The hydrolysis of Cu2+ and Zn2+ then should cause acidification in the pits, which promotes the cathodic hydrogen evolution reaction (Gong et al. 2020; Li et al. 2008). Under the application of mechanical stress generated at HP and the penetration of hydrogen into the metal, microcracks initiated at the pit bottoms, which contributed to the intergranular corrosion. As the corrosion products formed at 6.3 MPa have more cracks and defects, the accumulation of Cl− in the residual solution during the drying stage should be more severe and, hence, also contribute to the intergranular corrosion of the alloy.
Considering the accelerated uniform corrosion rate and especially the intergranular corrosion rate during the periodic service between atmospheric and deep sea environment, the use of Cu–40Zn alloy in such case should be avoided or in some kind of protection.
5 Conclusions
The corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment was investigated through comparing experiments. From the results, the following conclusions can be drawn:
A layer of protective corrosion products can be formed quickly and decrease the corrosion rate during the initial corroding period; with time prolonging, the corrosion products become defective and its protective capability decreases.
During periodic service, the HP and AP both affect the electrochemical reactions of the corrosion process and the structure of the corrosion product; the HP mainly promotes the anodic dissolution of the base and the formation of (Cu, Zn)2(OH)3Cl, which leads to volumetric expansion of the corrosion products; the AP facilitates the wetting process during dry to wet period and the alternating force caused by AP leads to the cracks, peeling off of the corrosion products.
Severe intergranular corrosion takes place during the periodic service, which initiates at the β phase and is accelerated by the combination of defective corrosion products and drying stage.
Funding source: Hubei Provincial Natural Science Joint-Foundation for Innovative Development
Award Identifier / Grant number: 2024AFD107
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 52271052
Funding source: Research Project of Hubei Provincial Department of Education
Award Identifier / Grant number: Q20221806
Funding source: PhD Research Startup Foundation of Hubei University of Automotive Technology
Award Identifier / Grant number: BK202105
Funding source: Hubei Provincial Natural Science Foundation of China
Award Identifier / Grant number: 2022CFB922
-
Research ethics: Not applicable.
-
Author contributions: Shengbo Hu: formal analysis, investigation, writing-original draft; Zhong Liu: resources, investigation; Xuwen Yuan: supervision, project administration; Fandi Meng: writing-review & editing, visualization; Luhai Liao: formal analysis; Guo Rui: writing-review & editing; Wei Yang: investigation; Fengguang Li: resources.
-
Competing interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
-
Research funding: The investigation was financially supported by the National Natural Science Foundation of China (No. 52271052), Hubei Provincial Natural Science Foundation of China (No. 2022CFB922) and Hubei Provincial Natural Science Joint-Foundation for Innovative Development (No. 2024AFD107), Research Project of Hubei Provincial Department of Education (No. Q20221806), PhD Research Startup Foundation of Hubei University of Automotive Technology (No. BK202105).
-
Data availability: The raw data can be obtained on request from the corresponding authors.
References
Abd, E.H.S.M., Abd, E.W.S., and Bahga, A. (2013). Environmental factors affecting the corrosion behaviour of reinforcing steel. V. Role of chloride and sulphate ions in the corrosion of reinforcing steel in saturated Ca(OH)2 solutions. Corros. Sci. 75: 1–15.10.1016/j.corsci.2013.04.049Suche in Google Scholar
Alfatazi, A.M., Ahmed, T.M., and Tromans, D. (2009). Corrosion behavior of copper alloys in chloride media. Mater. Des. 30: 2425–2430, https://doi.org/10.1016/j.matdes.2008.10.015.Suche in Google Scholar
Ateya, B.G., Al-kharafi, F.M., and Ghaya, I.M. (2009). Dezincification of brass in hot, concentrated salt water. Corrosion 65: 419–426, https://doi.org/10.5006/1.3319146.Suche in Google Scholar
Badawy, W. and Al-kharafi, F. (1999). Corrosion behavior of brass alloys in aqueous solutions of different pH. Corrosion 55: 268–277, https://doi.org/10.5006/1.3283987.Suche in Google Scholar
Beccaria, A., Ltraverso, P., Poggi, G., and Lorenzett, M. (1991). Effect of hydrostatic pressure on corrosion behaviour of 5086 Al-alloy in sea water. High Pressure Res. 7–8: 347–349, https://doi.org/10.1080/08957959108245588.Suche in Google Scholar
Beccaria, A., Poggi, G., Gingaud, D., and Castello, P. (1994). Effect of hydrostatic pressure on passivating power of corrosion layers formed on 6061 T6 aluminium alloy in sea water. Br. Corros. J. 29: 65–69, https://doi.org/10.1179/000705994798267962.Suche in Google Scholar
Beccaria, A.M. and Poggi, G. (1985). Influence of hydrostatic pressure on pitting of aluminium in sea water. Br. Corros. J.: 183–186, https://doi.org/10.1179/000705985798272632.Suche in Google Scholar
Beccaria, A.M., Poggi, G., Arfelli, M., and Mattogno, G. (1993). The effect of salt concentration on nickel corrosion behavior in slightly alkaline-solutions at different hydrostatic pressuers. Corros. Sci. 34: 989–1005, https://doi.org/10.1016/0010-938x(93)90075-r.Suche in Google Scholar
Beccaria, A.M., Poggi, G., and Castello, G. (1995). Influence of passive film composition and sea water pressure on resistance to localised corrosion of some stainless steels in sea water. Br. Corros. J. 30: 283–287, https://doi.org/10.1179/bcj.1995.30.4.283.Suche in Google Scholar
Bianchi, G. and Longhi, P. (1973). Copper in sea-water, potential-pH diagrams. Corros. Sci. 13: 853–864, https://doi.org/10.1016/s0010-938x(73)80067-8.Suche in Google Scholar
Campbell, S.A., Radford, G.J.W., Tuck, C.D.S., and Barker, B.D. (2002). Corrosion and galvanic compatibility studies of a high-strength copper-nickel alloy. Corrosion 58: 57–71, https://doi.org/10.5006/1.3277305.Suche in Google Scholar
Cao, C. (2008). Principles of electrochemistry of corrosion. Chemical Industrial Press, Beijing.Suche in Google Scholar
Cao, C. and Zhang, J. (2002). An introduction of electrochemical impedance spectroscopy science. Science Press, Beijing.Suche in Google Scholar
Cao, J., Wang, Z., Li, L., Meng, F., Liu, L., and Wang, F. (2020). Failure mechanism of organic coating with modified graphene under simulated deep-sea alternating hydrostatic pressure. J. Chin. Soc. Corros. Prot. 40: 139–145.Suche in Google Scholar
Damej, M., Chebabe, D., Abbout, S., Erramli, H., Oubair, A., and Hajjaji, N. (2020). Corrosion inhibition of brass 60Cu–40Zn in 3% NaCl solution by 3-amino-1,2,4-triazole-5-thiol. Heliyon 6: https://doi.org/10.1016/j.heliyon.2020.e04026.Suche in Google Scholar PubMed PubMed Central
De, L., Díaz, I., Alcántara, J., Chico, B., Simancas, J., Llorente, I., García-Delgado, A., Jiménez, J., Adeva, P., and Morcillo, M. (2016). Corrosion mechanisms of mild steel in chloride-rich atmospheres. Mater. Corros. 67: https://doi.org/10.1002/maco.201508488.Suche in Google Scholar
De La Fuente, D., Díaz, I., Simancas, J., Chico, B., and Morcillo, M. (2011). Long-term atmospheric corrosion of mild steel. Corros. Sci. 53: 604–617, https://doi.org/10.1016/j.corsci.2010.10.007.Suche in Google Scholar
Dexter, S.C. (1980). Effect of variations in sea water upon the corrosion of aluminum. Corrosion 36: 423–432, https://doi.org/10.5006/0010-9312-36.8.423.Suche in Google Scholar
Ding, K., Guo, W., Qiu, R., Hou, J., Fan, L., and Xu, L. (2018). Corrosion behavior of Q235 steel exposed in deepwater of South China Sea. J. Mater. Eng. Perform. 27: 4489–4496, https://doi.org/10.1007/s11665-018-3553-x.Suche in Google Scholar
Dridi, A., Dhouibi, L., Hihn, J.Y., Bercot, P., Rezrazi, E., Sassi, W., and Rouge, N. (2020). Analytical study of CuZn 30 and CuZn 39 brass surfaces in 3% NaCl solution under polarization. Chem. Afr. J. Tunis. Chem. Soc. 3: 735–747, https://doi.org/10.1007/s42250-020-00182-z.Suche in Google Scholar
Duan, T., Peng, W., Ding, K., Guo, W., Hou, J., Cheng, W., Liu, S., and Xu, L. (2019). Long-term field exposure corrosion behavior investigation of 316L stainless steel in the deep sea environment. Ocean Eng. 189: 106405, https://doi.org/10.1016/j.oceaneng.2019.106405.Suche in Google Scholar
Fuller, M.D., Swaminathan, S., Zhilyaev, A.P., and Mcnelley, T.R. (2007). Microstructural transformations and mechanical properties of cast NiAl bronze: effects of fusion welding and friction stir processing. Mater. Sci. Eng. A 463: 128–137, https://doi.org/10.1016/j.msea.2006.07.157.Suche in Google Scholar
Gong, K., Wu, M., Xie, F., Liu, G., and Sun, D. (2020). Effect of dry/wet ratio and pH on the stress corrosion cracking behavior of rusted X100 steel in an alternating dry/wet environment. Constr. Build. Mater. 260: 120478, https://doi.org/10.1016/j.conbuildmat.2020.120478.Suche in Google Scholar
Haleem, S.M.A.E., Wanees, S.A.E., Aal, E.E.A.E., and Diab, A. (2010). Environmental factors affecting the corrosion behavior of reinforcing steel. IV. Variation in the pitting corrosion current in relation to the concentration of the aggressive and the inhibitive anions. Corros. Sci. 52: 1675–1683.10.1016/j.corsci.2010.01.021Suche in Google Scholar
Hong, T., Sun, Y.H., and Jepson, W.P. (2002). Study on corrosion inhibitor in large pipelines under multiphase flow using EIS. Corros. Sci. 44: 101–112, https://doi.org/10.1016/s0010-938x(01)00052-x.Suche in Google Scholar
Horne, R.A. and Frysinger, G.R. (1963). The effect of pressure on the electrical conductivity of sea water. J. Geophys. Res. 68: 1967–1973, https://doi.org/10.1029/jz068i007p01967.Suche in Google Scholar
Hu, S. (2012). Research on property of Al-Zn-In sacrificial anode under simulate deep sea water. Harbin Engineering University, Harbin City, Heilongjiang Province of China.Suche in Google Scholar
Hu, S., Liu, L., Cui, Y., Li, Y., and Wang, F. (2019). Influence of hydrostatic pressure on the corrosion behavior of 90/10 copper-nickel alloy tube under alternating dry and wet condition. Corros. Sci. 146: 202–212, https://doi.org/10.1016/j.corsci.2018.10.036.Suche in Google Scholar
Hu, S., Liu, R., Liu, L., Cui, Y., Oguzie, E.E., and Wang, F. (2020). Effect of hydrostatic pressure on the galvanic corrosion of 90/10 Cu–Ni alloy coupled to Ti6Al4V alloy. Corros. Sci. 163: 108242, https://doi.org/10.1016/j.corsci.2019.108242.Suche in Google Scholar
Hu, S.N., Zhang, T., Shao, Y.W., Meng, G.Z., and Wanga, F.H. (2011). Effect of cyclic hydrostatic pressure on the sacrificial anode cathodic protection. Anti-Corros. Met. Mater. 58: 238–244, https://doi.org/10.1108/00035591111167703.Suche in Google Scholar
Kamimura, T., Nasu, S., Segi, T., Tazaki, T., Miyuki, H., Morimoto, S., and Kudo, T. (2005). Influence of cations and anions on the formation of β-FeOOH. Corros. Sci. 47: 2531–2542, https://doi.org/10.1016/j.corsci.2004.10.014.Suche in Google Scholar
Kear, G., Barker, B., Stokes, K., and Walsh, F. (2004). Electrochemical corrosion behaviour of 90-10 Cu-Ni alloy in chloride-based electrolytes. J. Appl. Electrochem. 34: 659–669, https://doi.org/10.1023/b:jach.0000031164.32520.58.10.1023/B:JACH.0000031164.32520.58Suche in Google Scholar
Li, G., Chen, X., Zhou, F., Liang, Y., Xiao, Y., Cao, X., Zhang, Z., Zhang, M., Wu, B., Yin, S., et al.. (2021). Self-powered soft robot in the Mariana trench. Nature 591: 66–71, https://doi.org/10.1038/s41586-020-03153-z.Suche in Google Scholar PubMed
Li, Q.X., Wang, Z.Y., Han, W., and Han, E.H. (2008). Characterization of the rust formed on weathering steel exposed to Qinghai salt lake atmosphere. Corros. Sci. 50: 365–371, https://doi.org/10.1016/j.corsci.2007.06.020.Suche in Google Scholar
Liu, R., Cui, Y., Liu, L., and Wang, F. (2021a). Study on the mechanism of hydrostatic pressure promoting electrochemical corrosion of pure iron in 3.5% NaCl solution. Acta Mater. 203: 116467, https://doi.org/10.1016/j.actamat.2020.11.009.Suche in Google Scholar
Liu, R., Liu, L., Tian, W.L., Cui, Y., and Wang, F.H. (2021b). Finite element analysis of effect of interfacial bubbles on performance of epoxy coatings under alternating hydrostatic pressure. J. Mater. Sci. Technol. 64: 233–240, https://doi.org/10.1016/j.jmst.2019.10.008.Suche in Google Scholar
Liu, R., Zhang, R., Cui, Y., Wang, A., Meng, F., Liu, L., and Wang, F. (2024). Failure mechanism of Al-Zn-In sacrificial anode under the synergic action of water pressure and fluid in the extreme deep-sea environment. Corros. Commun. 14: 39–48, https://doi.org/10.1016/j.corcom.2023.07.002.Suche in Google Scholar
Luciano, G., Letardi, R., Traverso, P., and Belsanti, L. (2013). Corrosion behaviour of Al, Cu, and Fe alloys in deep sea environment. Metall. Ital. 21–29.Suche in Google Scholar
Lv, Y.T., Lang, X.W., Zhang, Q., Liu, W.T., and Liu, Y.J. (2022). Study on corrosion behavior of (CuZnMnNi)(100-x)Sn-x high-entropy brass alloy in 5 wt% NaCl solution. J. Alloys Compd. 921: 166051, https://doi.org/10.1016/j.jallcom.2022.166051.Suche in Google Scholar
Ma, A., Jiang, S., Zheng, Y., and Ke, W. (2015). Corrosion product film formed on the 90/10 copper–nickel tube in natural seawater: composition/structure and formation mechanism. Corros. Sci. 91: 245–261, https://doi.org/10.1016/j.corsci.2014.11.028.Suche in Google Scholar
Ma, H., Yang, N., Cui, Y., Liu, R., Wang, F., and Liu, L. (2024). Investigation of the passive film of nanocrystalline 304 stainless steel in 3.5 wt% NaCl solution under hydrostatic pressure. Electrochim. Acta 481: 143981, https://doi.org/10.1016/j.electacta.2024.143981.Suche in Google Scholar
Ma, R.Y., Zhao, L., Wang, C.G., Mu, X., Wei, X., and Dong, J.H. (2019). Influence of hydrostatic pressure on the thermodynamicsand kinetics of metal corrosion. Acta Metall. Sin. 55: 281–290.Suche in Google Scholar
Meng, F.D., Liu, L., Tian, W.L., Wu, H., Li, Y., Zhang, T., and Wang, F.H. (2015). The influence of the chemically bonded interface between fillers and binder on the failure behaviour of an epoxy coating under marine alternating hydrostatic pressure. Corros. Sci. 101: 139–154, https://doi.org/10.1016/j.corsci.2015.09.011.Suche in Google Scholar
Metikos-Hukovic, M., Škugor, I., Grubac, Z., and Babic, R. (2010). Complexities of corrosion behaviour of copper–nickel alloys under liquid impingement conditions in saline water. Electrochim. Acta 55: 3123–3129, https://doi.org/10.1016/j.electacta.2010.01.066.Suche in Google Scholar
Mouanga, M., Berçot, P., and Rauch, J.Y. (2010). Comparison of corrosion behaviour of zinc in NaCl and in NaOH solutions. Part I: corrosion layer characterization. Corros. Sci. 52: 3984–3992, https://doi.org/10.1016/j.corsci.2010.08.003.Suche in Google Scholar
Muster, T.H., Neufeld, A.K., and Cole, I.S. (2004). The protective nature of passivation films on zinc: wetting and surface energy. Corros. Sci. 46: 2337–2354, https://doi.org/10.1016/j.corsci.2004.01.001.Suche in Google Scholar
Petersen, S., Krätschell, A., Augustin, N., Jamieson, J., Hein, J.R., and Hannington, M.D. (2016). News from the seabed – geological characteristics and resource potential of deep-sea mineral resources. Mar. Policy 70: 175–187, https://doi.org/10.1016/j.marpol.2016.03.012.Suche in Google Scholar
Qin, Z., Xia, D.-H., Zhang, Y., Wu, Z., Liu, L., Lv, Y., Liu, Y., and Hu, W. (2020). Microstructure modification and improving corrosion resistance of laser surface quenched nickel–aluminum bronze alloy. Corros. Sci. 174: 108744, https://doi.org/10.1016/j.corsci.2020.108744.Suche in Google Scholar
Ren, P.W., Meng, H.M., Xia, Q.J., Cui, A.L., Zhu, Z.Z., and He, M.T. (2023). Study on the tribocorrosion behavior of Cu-Ni-Zn alloy in deep-sea environment by in-situ electrochemical method. Wear: 514–515.10.1016/j.wear.2022.204594Suche in Google Scholar
Rezakhani, D. (2011). The effects of temperature, dissolved oxygen and the velocity of seawater, on the corrosion behavior of condenser alloys. Anti-Corros. Met. Mater. 58: 90–94, https://doi.org/10.1108/00035591111120191.Suche in Google Scholar
Sawant, S., Venkat, K., and Wagh, A. (1993). Corrosion of metals and alloys in the coastal and deep waters of the Arabian Sea and the Bay of Bengal. Indian J. Technol. 31: 862–866.Suche in Google Scholar
Shibata, T. and Ameer, M.A.M. (1992). Stochastic processes of pit generation on zirconium with an anodic oxide film. Corros. Sci. 33: 1633–1643, https://doi.org/10.1016/0010-938x(92)90039-6.Suche in Google Scholar
Sobral, A.V.C., Ristow, W., Azambuja, D.S., Costa, I., and Franco, C.V. (2001). Potentiodynamic tests and electrochemical impedance spectroscopy of injection molded 316L steel in NaCl solution. Corros. Sci. 43: 1019–1030, https://doi.org/10.1016/s0010-938x(00)00140-2.Suche in Google Scholar
Song, Q.N., Xu, N., Bao, Y.F., Jiang, Y.F., Gu, W., Yang, Z., Zheng, Y.G., and Qiao, Y.X. (2017). Corrosion behavior of Cu40Zn in sulfide-polluted 3.5% NaCl solution. J. Mater. Eng. Perform. 26: 4822–4830, https://doi.org/10.1007/s11665-017-2940-z.Suche in Google Scholar
Sun, H. (2013). Study on the corrosion behavior of low alloy steel and cathodic protection properties of sacrificial anode in deep sea environment. University of Chinese Academy of Sciences, Shenyang City, Liaoning Province of China.Suche in Google Scholar
Sun, H., Liu, L., Li, Y., and Wang, F. (2013a). Effect of hydrostatic pressure on the corrosion behavior of a low alloy steel. J. Electrochem. Soc. 160: C89–C96, https://doi.org/10.1149/2.040303jes.Suche in Google Scholar
Sun, H.J., Liu, L., Li, Y., Ma, L., and Yan, Y.G. (2013b). The performance of Al-Zn-In-Mg-Ti sacrificial anode in simulated deep water environment. Corros. Sci. 77: 77–87, https://doi.org/10.1016/j.corsci.2013.07.029.Suche in Google Scholar
Szczygiel, B. and Kolodziej, M. (2005). Composite Ni/Al2O3 coatings and their corrosion resistance. Electrochim. Acta 50: 4188–4195, https://doi.org/10.1016/j.electacta.2005.01.040.Suche in Google Scholar
Tian, W., Liu, L., Meng, F., Liu, Y., Li, Y., and Wang, F. (2014). The failure behaviour of an epoxy glass flake coating/steel system under marine alternating hydrostatic pressure. Corros. Sci. 86: 81–92, https://doi.org/10.1016/j.corsci.2014.04.038.Suche in Google Scholar
Traverso, P. and Canepa, E. (2014). A review of studies on corrosion of metals and alloys in deep-sea environment. Ocean Eng. 87: 10–15, https://doi.org/10.1016/j.oceaneng.2014.05.003.Suche in Google Scholar
Venkatesan, R., Venkatasamy, M.A., Bhaskaran, T.A., Dwarakadasa, E.S., and Ravindran, M. (2002). Corrosion of ferrous alloys in deep sea environments. Br. Corros. J. 37: 257–266, https://doi.org/10.1179/000705902225006633.Suche in Google Scholar
Wang, W., Wang, H.L., Zhao, J., Wang, X., Xiong, C.S., Song, L.Y., Ding, R., Han, P., and Li, W.H. (2019). Self-healing performance and corrosion resistance of graphene oxide-mesoporous silicon layer-nanosphere structure coating under marine alternating hydrostatic pressure. Chem. Eng. J. 361: 792–804, https://doi.org/10.1016/j.cej.2018.12.124.Suche in Google Scholar
Yang, Y.G., Zhang, T., Shao, Y.W., Meng, G.Z., and Wang, F.H. (2013). New understanding of the effect of hydrostatic pressure on the corrosion of Ni-Cr-Mo-V high strength steel. Corros. Sci. 73: 250–261, https://doi.org/10.1016/j.corsci.2013.04.013.Suche in Google Scholar
Zhang, T., Yang, Y., Shao, Y., Meng, G., and Wang, F. (2009). A stochastic analysis of the effect of hydrostatic pressure on the pit corrosion of Fe–20Cr alloy. Electrochim. Acta 54: 3915–3922, https://doi.org/10.1016/j.electacta.2009.02.010.Suche in Google Scholar
Zhang, X., Liu, X., Wallinder, I.O., and Leygraf, C.J.C.S. (2016). The protective role of hydrozincite during initial corrosion of a Cu40Zn alloy in chloride-containing laboratory atmosphere. Corros. Sci. 103: 20–29.10.1016/j.corsci.2015.10.027Suche in Google Scholar
Zhang, X., Wallinder, I.O., and Leygraf, C. (2014). Mechanistic studies of corrosion product flaking on copper and copper-based alloys in marine environments. Corros. Sci. 85: 15–25.10.1016/j.corsci.2014.03.028Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/corrrev-2023-0153).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Anticorrosion properties of flavonoids for rust-free building materials: a review
- Optimization strategies for graphene-based protection coatings: a review
- Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
- Efficient and reliable corrosion control for subsea assets: challenges in the design and testing of corrosion probes in aggressive marine environments
- Original Articles
- Corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment
- Model construction of corrosion resistance of alloying elements for low alloy steel in marine atmospheric corrosive environment based on machine learning
- Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 42 (2024)
Artikel in diesem Heft
- Frontmatter
- Reviews
- Anticorrosion properties of flavonoids for rust-free building materials: a review
- Optimization strategies for graphene-based protection coatings: a review
- Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
- Efficient and reliable corrosion control for subsea assets: challenges in the design and testing of corrosion probes in aggressive marine environments
- Original Articles
- Corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment
- Model construction of corrosion resistance of alloying elements for low alloy steel in marine atmospheric corrosive environment based on machine learning
- Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 42 (2024)

