Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
Abstract
The field of corrosion has recently been considered a productive field for scientific research. With the increasing use of metals in several industrial fields, such as metal construction, the construction of arches and the automobile industry, the problem of corrosion is an important issue. To solve the corrosion problem of metal materials, several methods have been discovered to combat this phenomenon, such as process control, cathodic protection, organic and inorganic coatings. Nonetheless, the presentation of corrosion inhibitors, particularly organic inhibitors, stays the least expensive and simplest technique for the insurance of metals against consumption in acidic media. In this work, it was summed up the strategies for amalgamation and portrayal of newly innovative heterocyclic complexes from 8-hydroxyquinoline, their inhibition performances for M-steel, C40E steel and C35E steel in acidic conditions, for example, hydrochloric acid, sulfuric acid, etc.
1 Introduction
Corrosion of metallic materials is a significant issue in the chemical industry, leading to loss of material properties, economic losses, and environmental risks, with an estimated 3–4 % of global GDP spent annually to combat these issues (Groysman 2017a; Koch et al. 2005). This corrosion occurs as both a chemical and physical process on metal surfaces in various environments, including aqueous, gaseous, and atmospheric, leading to reactions that form corrosion products like rust on metals such as iron, aluminum, magnesium, copper, and zinc (Obot and Gasem 2014; Groysman 2010; Cramer and Covino 2005; Quadri et al. 2022). To mitigate this, corrosion inhibitors are widely used, especially in aqueous environments, where they form a protective film on metal surfaces through water-soluble electrochemical interactions, effectively preventing corrosion (Aslam et al. 2022; Douche et al. 2020; Umoren and Solomon 2014a,b). These inhibitors include diverse substances like ionic liquids, phytochemicals, plant extracts, and graphite-based nanomaterials, each offering unique benefits in terms of adsorption, efficiency, and environmental impact, with some being notably cost-effective and eco-friendly (Leonel et al. 2021).
Amongst the most common and flexible chemical molecules, 8-hydroxyquinoline is a crystalline organic substance containing 2 rings: a phenyl ring is bonded to another circle of pyridine. It is indicated that the melting point of 8-hydroxyquinoline is 73.6 °C and its boiling point is 265.6 °C. 8-Hydroxyquinoline is soluble in acetone, ethanol, chloroform, in most organic solvents and insoluble in water. 8-Hydroxyquinoline and its derivatives were widely used in many areas such as the pharmaceutical, agronomic, pharmacological, biological, analytical and electrochemical industries (Al-Farhan et al. 2021; Cipurković et al. 2021; Rbaa et al. 2022). Therefore, the synthesis of 8-hydroxyquinoline (8-HQ) derivatives is more interesting in modern corrosion protection as an effective corrosion inhibitor. Moreover, 8-HQ exhibits typical phenolic properties because of the presence of the phenolic group, showing that 8-HQ is comfortable for many structural changes and chemical reactions, such as molecular rearrangements, diazonium coupling and electrophilic aromatic substitution. Also, 8-HQ makes good monoprotic bidentate chelating agents with a different metal ions, such as Ni2 +, Fe3+, Al3+, Cd2+, Mn2 +, Mg2 +, Bi2 +, Mn2+ and Cu2 +. This is due to their proximity to heterocyclic nitrogen. Currently, the 8-HQ modification with a various range of substitutions is interestingly used for the corrosion protection of steel in the acidic environment (Jiang et al. 2020; Martins et al. 2004; Pytlakowska 2016; Tian et al. 2015).
This research is motivated by the significant impact of corrosion on metallic materials in the chemical industry, resulting in material property loss, economic losses, and environmental risks.
In this work, it was summed up the strategies for amalgamation and portrayal of newly innovative heterocyclic complexes from 8-hydroxyquinoline, their inhibition performances for M-steel, C40E steel and C35E steel in acidic conditions, for example, hydrochloric acid, sulfuric acid, etc. The data included in this review are studies that were recently published in scientific journals last year (2020). This research work is a breakthrough for the literature in the field of corrosion as it targets scientific researchers, academics, and professionals in the corrosion field.
2 Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline
Rouifi et al. (2020) synthesized two new heterocyclic compounds based on a reaction between 5-chloromethyl-8-hydroxyquinoline hydrochloride (5-CMH) and bi-nucleophiles in the presence of NaHCO 3 dissolved in (THF) for 24 h; the reaction procedure was indicated in Figure 1. Thin-layer chromatography tracks the reaction and cleanses the solid obtained through silica column chromatography (dichloromethane/hexane 85:15) to detect two heterocyclic compounds derived from 8-hydroxyquinoline 5-(aminooxy)methyl)quinoline-8-ol ( AMQN ) and 5-(hydrazinylmethyl)quinoline-8-olol ( HMQN ). IR and NMR spectroscopy were used to prove the molecular structure of obtained products.
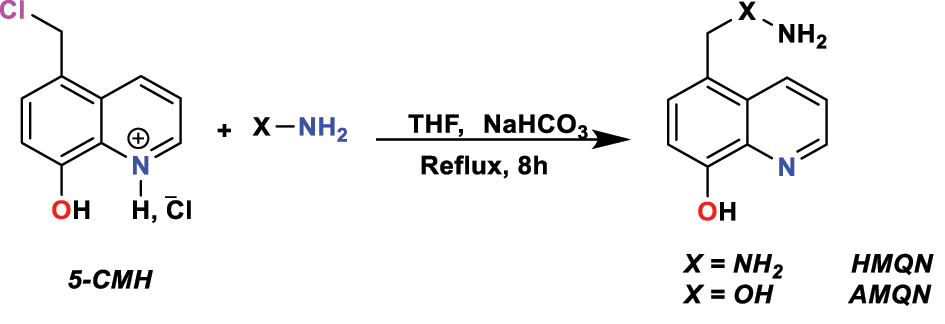
Synthases procedure of HMQN and AMQN .
Rbaa et al. (2020c) formulated new families of 8-hydroxyquinoline-based heterocyclic compounds, namely 5-((2-bromoethoxy)methyl)quinoline-8-ol ( QC2-Br ) and 5-(3-bromopropoxy)methyl)quinoline-8-ol ( QC3-Br ); it was also studied their corrosion inhibition performance for mild steel in aggressive hydrochloric acid medium. The researchers synthesized these derivatives in the presence of triethylamine ( Et3N ) throughout tetrahydrofuran ( THF ) at 8-h reflux (Figure 2) and condensing 5-hydroxymethyl-8-hydroxyquinoline with the equivalent of the compounds bearing nucleophilic groups. To characterize these products, the following methods have been used: IR, NMR, and AE.
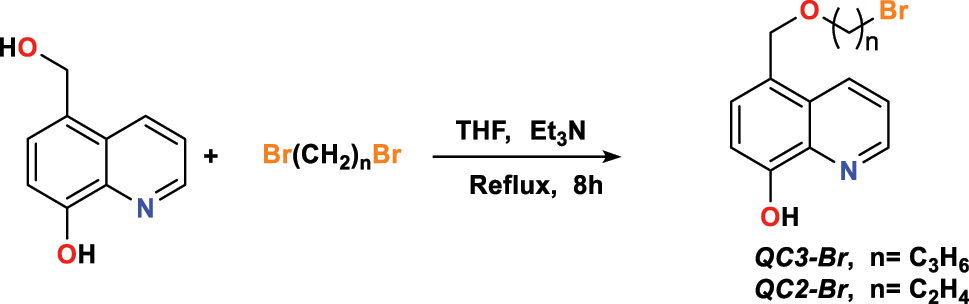
Compounds’ synthetic path: QC3-Br and QC2-Br .
Douche et al. (2020) (Douche et al. 2020) synthesized the new cyclic compounds, from the substrates (1a or 1b), paraformaldehyde and morpholine (or piperidine) in EtOH for 4 h in a controlled atmosphere (N2). The solvent was evaporated at low pressure and the resulting solid was cooled, filtered and vacuum-dried in cold ether (Figure 3). Then, three new heterocyclic compounds, which are 5-(azidomethyl)-7-(morpholinomethyl)quinolin-8-ol ( HM1 ), 2-(8-hydroxy-7-(morpholinomethyl)quinolin-5-yl)acetonitrile ( HM2 ) and 5-(azidomethyl)-7-(pipéridin-1-ylméthyl)quinolin-8-ol ( HM3 ) have been identified by the spectroscopic methods.
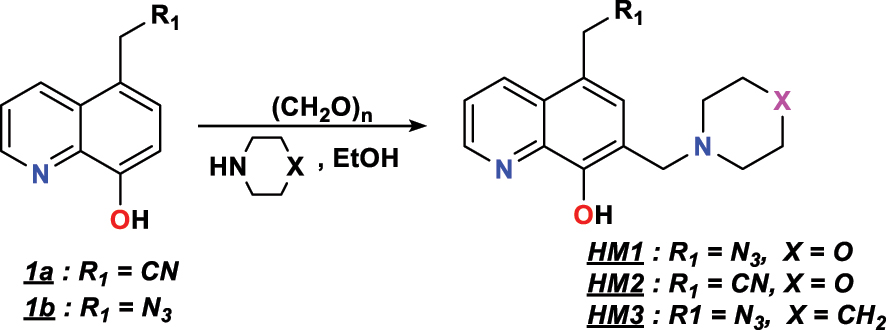
Synthetic scheme of HM 1 , HM 2 and HM 3 .
Rbaa and Lakhrissi (2019) discovered and develop new heterocyclic compounds. Rbaa and his colleagues extracted a new sequence of oxathiolane and triazole by condensing 2-(8-hydroxyquinoline-5-yl) acetonitrile (( 5-CNMHQ ) and bi-nucleophilic compounds in ethanol ( EtOH ) for 8 h via the presence of ( NaOH ) throughout reflux (Figure 4). The compounds obtained by silica column chromatography (acetone/hexane 85:15) were purified and accompanied by recrystallization in absolute ethanol to obtain the compounds 5-(1,3-oxathiolan-2-yl)methyl)quinolin-8-ol ( Q-Ox ) and 5-(4,5-dihydrothiazol-2-yl)methyl)quinolin-8-ol ( Q-T ), then they were characterized by the traditional spectral methods.
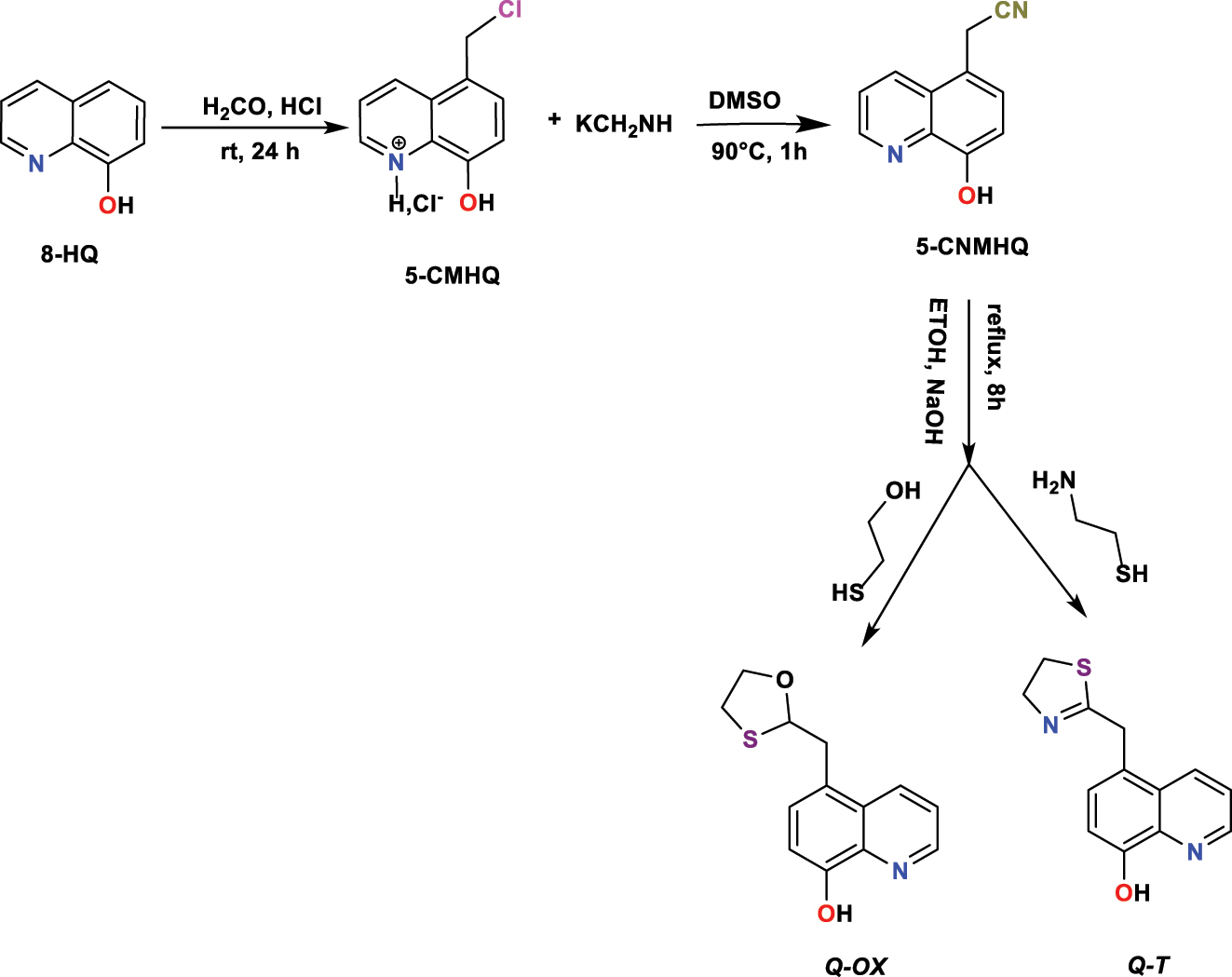
Syntheses procedure of Q-OX and Q-T .
El Faydy et al. (2020b) developed the heterocyclic compounds by introducing two compounds with the following names: 7-((4-(4-chloro phenyl)piperazin-1-yl) methyl) quinolin-8-ol ( CPQ ) and 7-((4-methyl piperazin-1-yl) methyl)quinolin-8-ol ( MPQ ) (Figure 5). The above new products have been synthesized by a typical Mannich reaction from quinolin-8-ol, paraformaldehyde, and piperazine derivatives, for 3 h reflux. To extract chemical compounds from impurities with excellent yields, it was used silica gel column chromatography (eluent, petroleum ether: acetone = 2:8). NMR spectroscopy, IR, and elemental analysis have been performed to confirm the chemical structure of studied compounds.
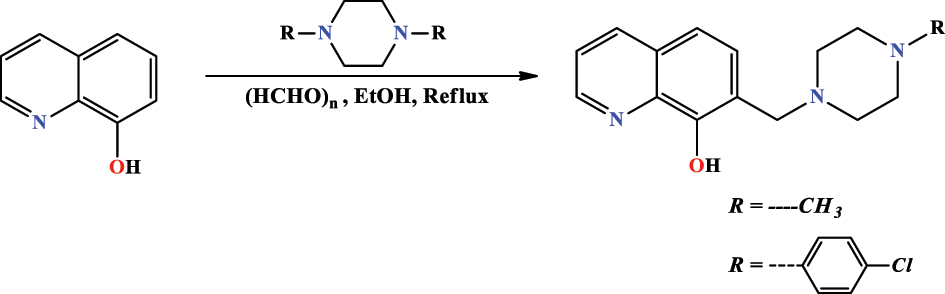
Synthetic pathway of CPQ and MPQ .
Fardioui et al. (2021) researched the reaction between alginate and 5-chloromethyl-8-hydroxyquinoline in distilled water to achieve the chemical 8-hydroxyquinoline-g-Alginateis ( HQ-g-Alg ) (Figure 6). To determine the final structure of this product, the experts used recognized techniques FT-IR and (1H,13C NMR).
Structures of alginate and modified alginate.
Abbout et al. (2021) investigated two additional 8-hydroxyquinoline conjugates, called N-((8-hydroxyquinoline-5-yl)methyl)-4-methylpiperazine-1-carbothioamide ( HQMC ) and N-((8-hydroxyquinoline-5-yl)methyl)-4-phenylpiperazine-1-carbothioamide ( HQPC ), which were produced through 5-thiocyanthyl-8-hydroxyquinoline and triethylamine reaction in absolute THF at 12 h reflux (Figure 7). To extract these new substances with a fair yield equal to 73 % for both, the authors resorted to silica gel column chromatography (hexane/CH2Cl2 9:1 to 4:6) to obtain pure products without impurities stuck. It was used the NMR and EA to identify the molecular structure.
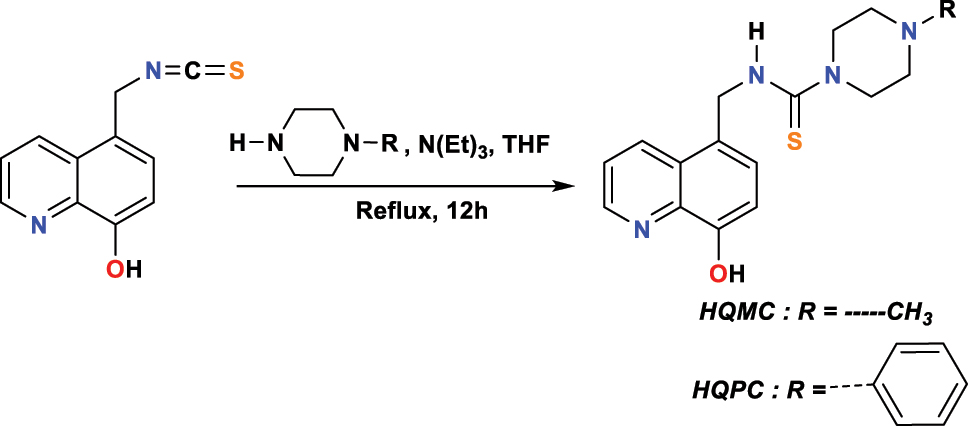
Synthesis of HQMC and HQPC .
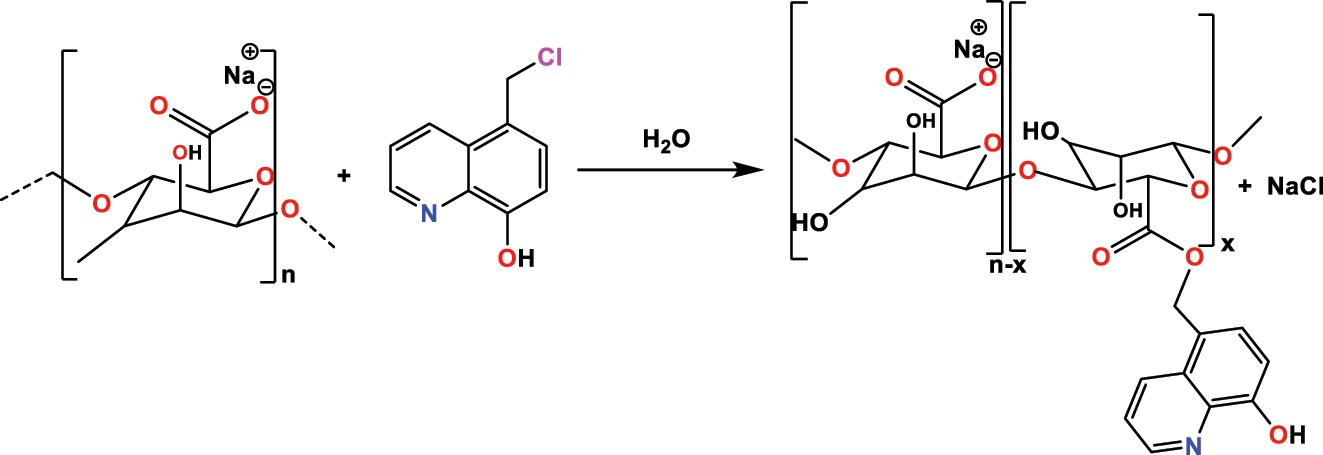
Rbaa et al. (2020b) have made the combination between 5- chloromethyl-8-hydroxyquinoline ( 5-CMHQ ) and chitosan ( CH ) in water to create another compound with some interesting properties. The condensation process was performed for 72 h at room temperature (Figure 8). The target product (5-chloromethyl-8-hydroxyquinoline modified with chitosan ( CH - HQ )) at a good yield equal to 98 % and the obtained structure was characterized by spectroscopic methods of 1H NMR and FT-IR.
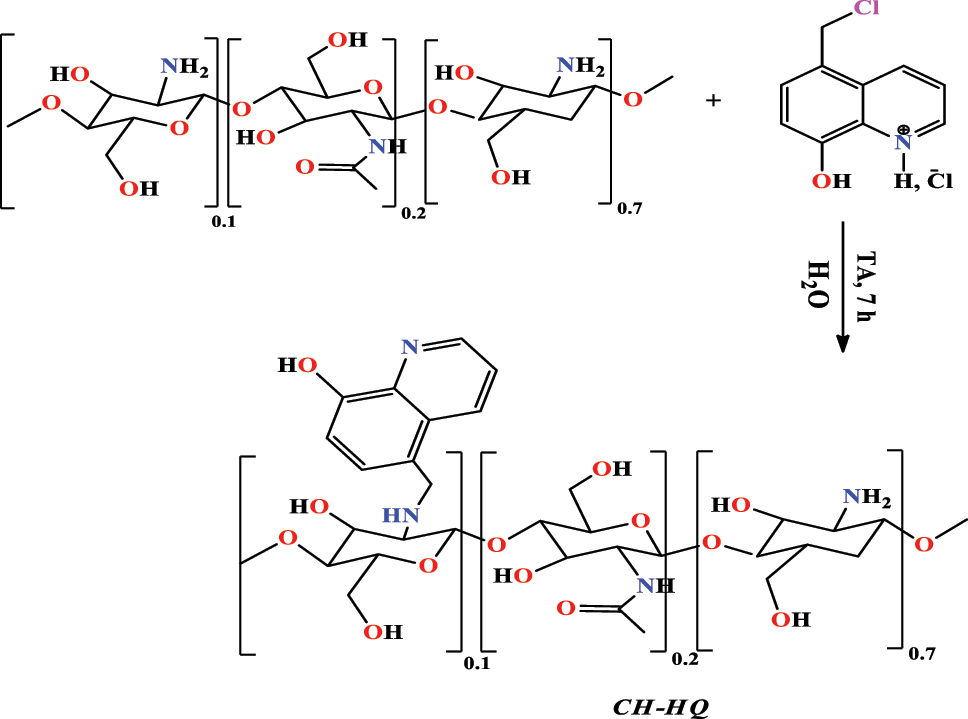
The synthetic route of CH-HQ .
El Faydy et al. (2020a) have extracted 2 new salts based on 5-alkythio-8-hydroxyquinoline to be tested as anticorrosive agents of C22E steel in a solution of hydrogen chloride (Figure 9). The salts were formulated by an alkylation reaction of 8,8′-dihydroxy-5,5′-diquinolyl disulfide with alkyl bromide ( R-Br ) alcoholic solution in ethanol ( EtOH ) in the presence of sodium hydroxide (NaOH) to get 86 % and 82 %, respectively, of the compounds sodium 5-methylthio-8-hydroxyquinolinolate ( SMQ ) and sodium 5-propylthio-8-hydroxyquinolinolate ( SPQ ). (1H, 13C) NMR and EA determined the structures of the synthesized compounds.
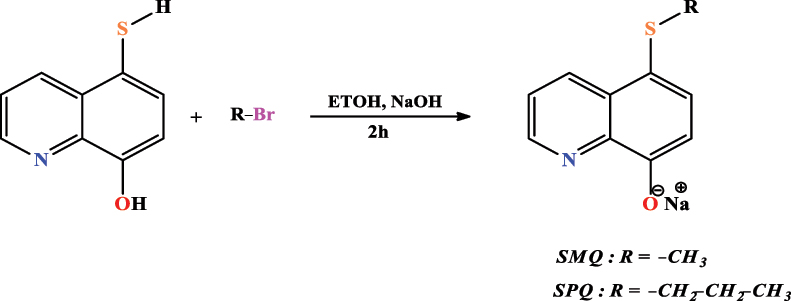
Synthetic path of 5-alkylthio-8-hydroxyquinolinolates.
Fakhry et al. (2021) introduced another quinoline by condensing 4-(dimethylamino) benzaldehyde with 5-aminomethyl-8-hydroxyquinoline ( 5-AMHQ ) into triethylamine N(Et) 3 and ethyl acetic acid derivative ACOEt at reflux for 12 h (Figure 10). The resulting residue was treated using chromatography of the hexane/acetone column (8:3 to 1:9 v/v) to attain an eventual product in the form of a white solid and with a 63 % yield. The molecular structure of this compound called 5-(((4-(dimethylamino)benzylidene)amino)methyl)quinoline-8-ol ( MABQ ) was detected by the normal spectral methods (NMR and EA) analysis.
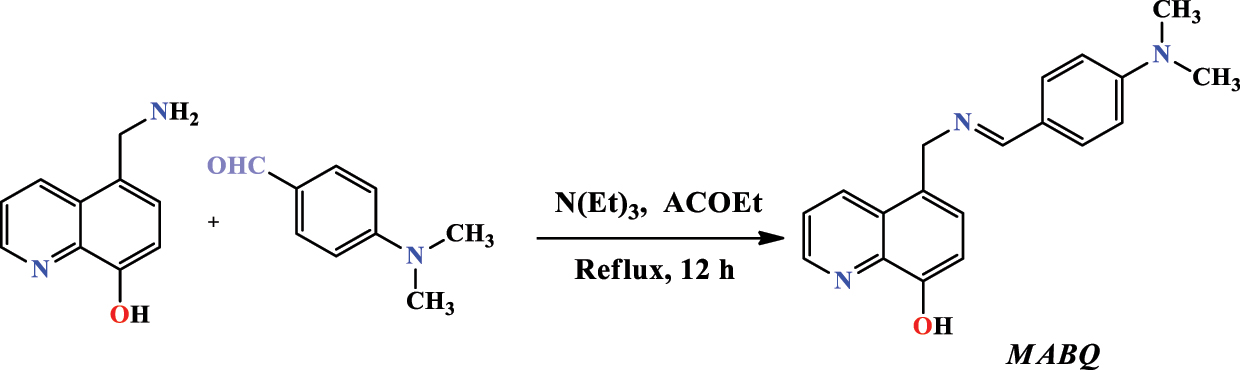
Structure of MABQ compound.
Hassan et al. (2020) indicated a simple strategy with a respectable yield in other quinoline syntheses. This method involves condensing triethylenetetramine ( TETA ) methyl quinoline-2-carboxylate into dry dioxane under reflux for 5 h to acquire the ideal compound, namely: N,N′-((ethane-1,2-diylbis(azanediyl))bis(ethane-2,1-diyl))bis(ethane-2,1-diyl) (quinoline-2-carboxamide). ( QATETA ) (Figure 11). After the synthesis stage, Hassan and colleagues recognized this product via the standard spectral methods (NMR and FT-IR).
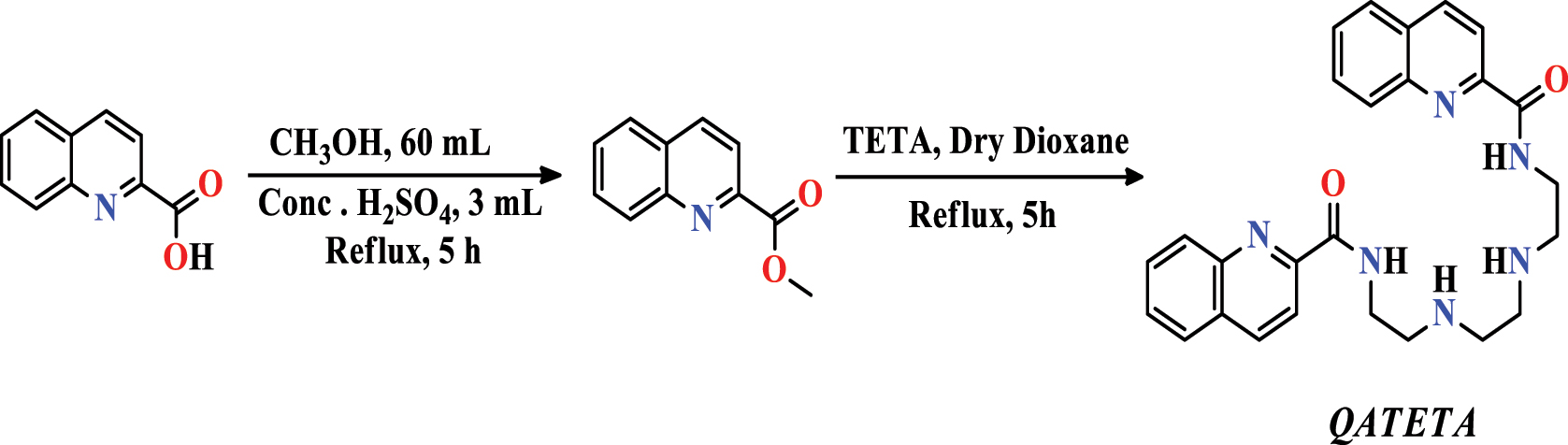
Formation of QATETA .
Rbaa et al. (2020a) introduced the new glucose derivatives based on 8-hydroxyquinoline, which have been synthesized and applied to the field of corrosion (Figure 12). It was synthesized these derivatives by condensing epoxy-glucose derivatives with 5-hydroxymethyl-8-hydroxyquinoline ( 5-HMHQ ) in the presence of Et 3 N in acetonitrile at reflux for 48 h to obtain the chemicals ( GQ-6 ) and ( GQ-12 ) with yields of 85 % and 82 %. After this, these products were subsequently characterized by the usual methods (IR and 1H, 13C NMR).
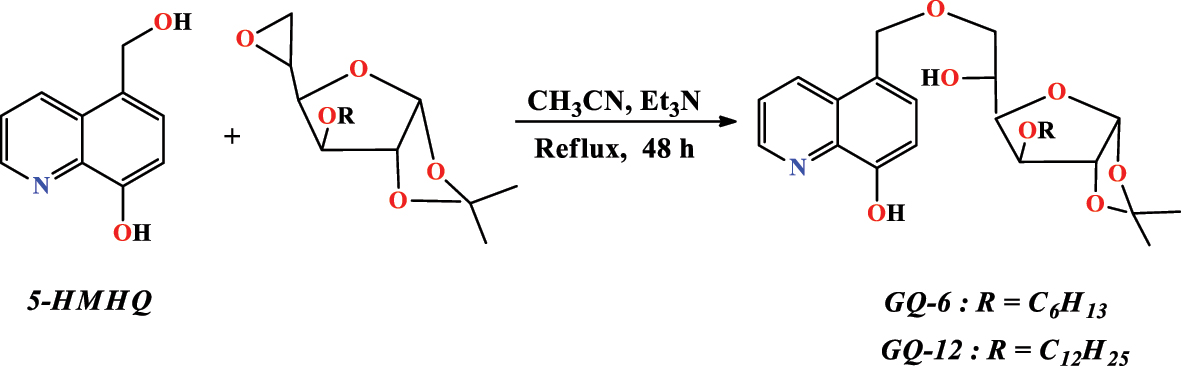
Compound synthesis ( GQ-6 ) and ( GQ-12 ).
3 Critical analysis in synthesis methods and description for new derivatives associated with 8-hydroxyquinoline
When critically evaluating the synthesis methods and descriptions for new derivatives associated with 8-hydroxyquinoline, several key aspects emerge:
Variety in synthesis approaches: the research conducted by different groups in 2020 demonstrates a wide array of synthesis methods, from Mannich reactions to reflux processes using various solvents and reactants. This diversity showcases the chemical versatility of 8-hydroxyquinoline and its derivatives (Alamshany and Ganash 2019; Pivarcsik et al. 2022; Rohini et al. 2020). However, it also indicates a lack of standardization in synthesis methods, which could pose challenges in comparing results and replicating studies.
Complexity and efficiency: some methods, like those used by Douche et al. and Fardioui et al. involve multiple steps and specific conditions (e.g., controlled atmosphere, precise temperature control), indicating a high level of complexity. While this complexity might be necessary for the synthesis of certain derivatives, it raises questions about the scalability and efficiency of these processes for practical applications (Assad and Kumar 2021; Baitule et al. 2021; Balaskas et al. 2015; Sharma et al. 2021).
Purity and yield: the use of chromatography for purification, as noted in many studies, is a standard and effective technique. However, the yield percentages and the purity of the final products are not always mentioned. High yields and purity are crucial for the commercial viability of these compounds, especially in pharmaceutical and industrial applications (Kaczerewska et al. 2018; Liu et al. 2019; Wu et al. 2018).
Characterization techniques: the widespread use of NMR and IR spectroscopy for characterizing the molecular structure of the synthesized compounds is a strength, as these are reliable and well-established methods. However, there’s a lack of information on the functional properties of these new derivatives, which is critical for understanding their potential applications (Alamshany and Ganash 2019; Li et al. 2003; Patel and Patel 2017; Rajasekaran et al. 2010).
Application focus: while some studies, like those by Rbaa et al. and El Faydy et al. focus on the potential applications of the derivatives (e.g., corrosion inhibition), others primarily concentrate on the synthesis process (Oliveri and Vecchio 2016; Yin et al. 2011; Rbaa et al. 2019). A more application-oriented approach could provide a better understanding of the practical utility of these compounds.
Environmental and safety considerations: the use of various chemicals and solvents in these synthesis processes raises concerns about environmental and safety implications. There’s limited discussion on the environmental impact and safety profiles of these synthesis methods, which is crucial in the context of sustainable chemistry practices (Julien et al. 2017; Cseri et al. 2018; Connolly et al. 2018; Inamuddin and Asiri 2020; Cioc et al. 2014).
In summary, while the research in 2020 on 8-hydroxyquinoline derivatives showcases significant progress and chemical diversity, there are areas for improvement in terms of standardization, efficiency, application focus, and environmental considerations (Gupta et al. 2021). Understanding these aspects is vital for advancing the practical applications of these compounds in various fields.
3.1 Comparison analysis
The comparison of various synthesis methods for 8-hydroxyquinoline (8-HQ) derivatives as acidic corrosion inhibitors reveals significant insights into the evolving landscape of corrosion protection technology (Table 1). It was found the following main points:
Nine synthesis methods yield derivatives with specialized applications. For instance, derivatives from sonication show enhanced performance in HCl solutions, whereas those from irradiation are more suited for H2SO4 environments. This specificity underlines the potential for tailor-made solutions in corrosion inhibition (Galai et al. 2021; Gerengi et al. 2016a; Obot et al. 2017; Oubaaqa et al. 2022b).
Techniques like irradiation and hydrothermal synthesis produce derivatives with improved chemical stability and reactivity. These modifications enhance the interaction with metal ions, crucial for effective corrosion inhibition in more aggressive environments (Groysman 2017b).
Green synthesis and biosynthesis methods emphasize environmental compatibility and reduced toxicity. The use of natural extracts and biologically synthesized derivatives aligns with the growing demand for eco-friendly corrosion inhibitors (Bakhshipour et al. 2022; Bennamara and Abourriche 2020; Jones 1963; Pippi et al. 2019).
The application of cutting-edge techniques like nanostructuring (sol-gel process) and electronic modification (electrochemical synthesis) introduces derivatives with superior properties like higher surface area interaction and targeted electron transfer, broadening the scope of 8-HQ in modern industrial applications (Berdimurodov et al. 2022; Mastouri et al. 2014; Dahaghin et al. 2017).
Derivatives from methods like chemical vapor deposition and chemical reduction offer enhanced durability and thermal stability, making them suitable for extreme conditions. This robustness is critical in industrial settings where long-term protection is essential (Kumar et al. 2008).
With an increasing focus on environmental protection, synthesis methods yielding biodegradable and less toxic derivatives are gaining prominence. This approach not only ensures effective corrosion inhibition but also minimizes environmental impact (Rahman et al. 2021).
Each synthesis method produces derivatives with unique properties, indicating a shift towards customized solutions for diverse corrosion challenges. For instance, nanostructured 8-HQ can tackle micro-corrosive environments, while polymeric integration addresses extreme pH conditions (Boztepe et al. 2021; Brandel et al. 2009).
Comparison analysis in synthesis methods: basic and main features.
| Synthesis method | Derivative description | Effects on corrosion inhibition | Basic characteristics | References |
|---|---|---|---|---|
| Sonication | 8-HQ with R group | Enhanced inhibition in HCl solution | High solubility, increased adsorption | (Mazkour et al. 2021) |
| Irradiation | 8-HQ with halogen modification | Improved protection in H2SO4 | Greater chemical stability, reactive with metal ions | (Musiol et al. 2010) |
| Hydrothermal synthesis | 8-HQ with organic framework | Broad-spectrum efficacy in various acids | Robust film formation, environmental compatibility | (Nguyen et al. 2024) |
| Green synthesis | 8-HQ combined with natural extracts | Enhanced biodegradability, effective in mild acidic conditions | Eco-friendly, lower toxicity | (Oliveri and Vecchio 2016; Obot and Gasem 2014) |
| Chemical vapor deposition | 8-HQ with polymeric integration | High efficacy in extreme pH conditions | Durable protective layer, resistant to thermal degradation | (Oubaaqa et al. 2022a) |
| Chemical reduction | 8-HQ with metal ion complexation | Effective in concentrated acid solutions | Enhanced metal interaction, increased density of active sites | (Ramos et al. 2013; Nixon and Ross 2016) |
| Sol-gel process | Nanostructured 8-HQ | Superior inhibition in micro-corrosive environments | Nanoscale interaction, high surface area | (Boztepe et al. 2021) |
| Electrochemical synthesis | Electronically modified 8-HQ | Enhanced electron transfer properties for corrosion prevention | Electrical conductivity, targeted action at corrosion sites | (Zborowski et al. 2013) |
| Biosynthesis | Biologically synthesized 8-HQ | Effective in bio-corrosive environments | Biocompatibility, reduced environmental impact | (Chan et al. 2013) |
In conclusion, the diversity in synthesis methods for 8-HQ derivatives reflects an advanced understanding of corrosion mechanisms and a move towards more targeted, sustainable, and effective corrosion inhibition strategies. This variety ensures that there are options available for a wide range of industrial applications, each with its unique set of challenges.
3.2 Critical analysis in synthesis comparison of 8-HQ
The differentiation in synthesis methods leading to derivatives with specialized applications is a double-edged sword. While it enables tailor-made solutions for specific environments (e.g., sonication for HCl solutions, irradiation for H2SO4), it also implies a limitation in the versatility of each derivative. This specialization might restrict the broad applicability of a single derivative across various corrosion environments. The use of advanced synthesis techniques like irradiation and hydrothermal synthesis, which enhance chemical stability and reactivity, is commendable (Yin et al. 2011). However, these methods often require sophisticated equipment and controlled conditions, which may increase the cost and complexity of production. The emphasis on green synthesis and biosynthesis methods aligns with the global trend towards environmental sustainability. While these methods offer reduced toxicity and environmental compatibility, there might be trade-offs in terms of the efficiency and stability of the corrosion inhibitors compared to more conventional methods. Techniques like nanostructuring and electronic modification introduce derivatives with superior properties. However, the long-term effects and potential environmental impact of nanoparticles and electronically modified compounds in real-world applications remain areas for further research (Royzen and Canary 2013). Methods like chemical vapor deposition and chemical reduction that offer enhanced durability and thermal stability are crucial for industrial applications. Nonetheless, there’s a need to balance this durability with cost-effectiveness and ease of application. The shift towards synthesis methods yielding biodegradable and less toxic derivatives is a positive development. However, ensuring these eco-friendly derivatives are as effective as traditional inhibitors is a significant challenge. The move towards customized solutions for diverse corrosion challenges is a significant advancement. It highlights the potential for targeted corrosion inhibition strategies (Seifzadeh et al. 2013). However, this customization requires a deep understanding of the specific corrosion environment and may lead to increased costs and complexity in inhibitor development. In conclusion, while the diversity in synthesis methods for 8-HQ derivatives demonstrates progress in corrosion inhibition technology, it also brings forth challenges such as cost, complexity, environmental impact, and the need for a balance between specialization and versatility. Future research should focus on optimizing these methods for practical, cost-effective, and environmentally sustainable applications in industrial settings.
4 Effect of solution type, metal type and temperature on the protection function of the 8-hydroxyquinoline based inhibitors
AMQN and HMQN (Rouifi et al. 2020) were tested as carbon steel corrosion inhibitors in 1.0 M HCl by the electrochemical techniques: (PDP, EIS) and gravimetric (ML) methods. As can be seen from the obtained results, Rouifi and his colleagues noted that the inhibition activity depends on the change in concentration and temperature, whereas the inhibition efficiency of these compounds decreased due to the temperature rise. The values in thermodynamic parameters indicated that the two species are absorbed by the Langmuir isotherm on the metallic substrate. The results in SEM images revealed that after the addition of the two substances, the surface morphology was well cured.
QC2-Br and QC3-Br (Rbaa et al. 2020c) were investigated the inhibition properties of selected compounds for mild steel in an acidic environment by the PDP, EIS and WL methods. The results in theoretical, experimental and surface characterization indicated that two compounds have a strong acid corrosion inhibitor. On the other hand, the thermodynamic data have proven that these inhibitors adsorb on the metal substrate by the formation of chemical bonds (chemisorptions) according to the Langmuir isotherm; these results confirmed that these inhibitors are mixed type. The obtained results were correlated with MD measurements and MC simulation. Figure 13 shows the Tafel plots of QC2-Br and QC3-Br . It is clear from the obtained results that the Tafel plots are cited in higher corrosion density regions at high temperatures. However, these values are nearly similar to the lower temperatures. These results confirmed that the QC2-Br and QC3-Br inhibitors are more efficient at higher temperatures.
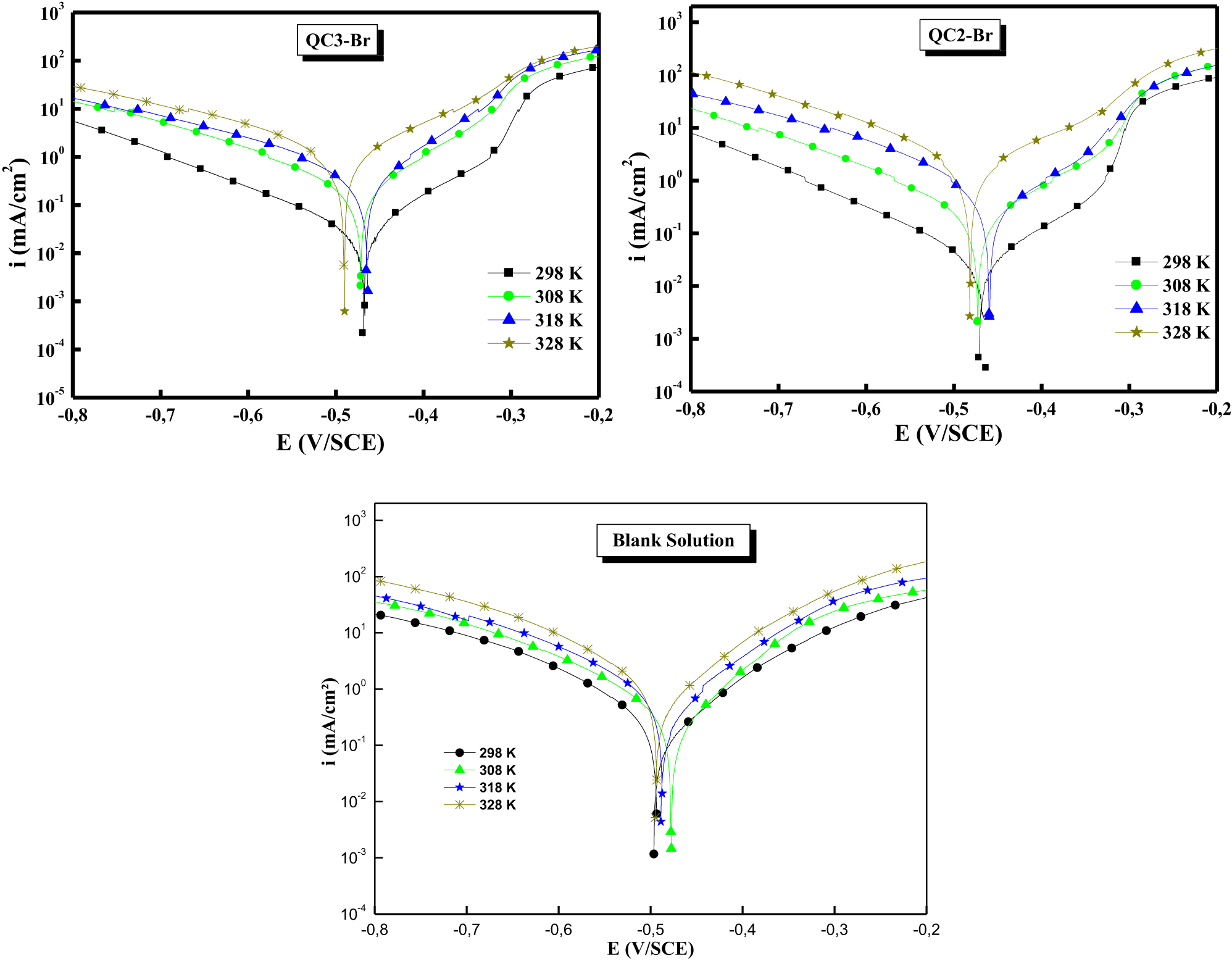
The Tafel plots of QC2-Br and QC3-Br .
Douche et al. (2020) subsequently tested these new molecules ( HM1, HM2 and HM3 ) as mild steel corrosion inhibitors in an acidic (1.0 M HCl) environment by gravimetric and electrochemical methods. Results in PDP analysis suggested that all synthesized compounds are cathode type inhibitors, excluding HM 3 , which shows a major mixed-type effect. These products have shown remarkable inhibitory efficiency with an increase in concentrations. Finally, their adsorption characteristics were obeyed with Langmuir adsorption-isotherm, showing the spontaneous chemical–physical adsorption of the selected inhibitor on the mild steel surface. These compounds are adsorbed on the metal surface by the active functional groups.
Using modern and effective electrochemical techniques (PP and EIS), the authors (Feng et al. 2023) examined the anticorrosive properties of the heterocyclic molecules based on 8-hydroxyquinoline in acidic HCl solution with a concentration of 1 M for mild steel. The authors also concluded that the inhibition mechanism was often based on the mixed behaviour in 8-hydroxyquinoline derivative samples. The experimental findings were confirmed by numerical simulations (DFT and MC).
Next, the compounds ( DEMQ and BMQ ) were subsequently tested as M-steel corrosion inhibitors in 1 mol of hydrogen chloride using the following methods: electrochemical process (EIS, PDP) and theoretical investigates (DFT, MC). The electrochemical findings demonstrated that both products are highly resistant to electrochemical polarization. The results of the PDP analysis indicated that these inhibitors are mixed types. On the other hand, the results in thermodynamic parameters suggest that the 8-hydroxyquinoline derivatives adsorbed on the metal surface by the formation of chemical bonds following the Langmuir isotherm; it is also demonstrated that the strong corrosion efficiency has occurred at elevated temperatures. The experimental results were supported by the results from theoretical approaches.
The corrosion inhibition performance of selected compounds ( MPQ and CPQ ) was investigated using modern and effective techniques, which are as follows: weight loss, PP, EIS, MC, MD, and DFT calculations. It was also noted that two inhibitors have strong anticorrosive properties for C35E steel in an acidic solution of hydrogen chloride and they were mixed types, which means that the rate of the cathodic and anodic reactions was effectively reduced.
After a good synthesis, Fardioui and her colleagues evaluated the anticorrosive properties of HQ-g-Alg molecule using a slice of mild steel in an acidic solution of hydrogen chloride with a concentration of 1 M. This evaluation was done using the usual and well-known methods: (PDP, EIS, DFT calculations, and MDS simulations). The scanning electronic microscopy (SEM) was used to analyze the surface morphology before and after the corrosion and inhibition. Electrochemical assessments demonstrated that HQ-g-Alg is a mixed-type inhibitor. It was also noted that the polarization resistance rose with a rise in the quantity of the inhibitor. It was also observed that this inhibitor showed greater adsorption and greater efficiency at all concentrations. Finally, the micrographs of SEM presented that the mild steel was protected from dissolution after adding the inhibitor HQ-g-Alg .
The corrosion inhibition performance of the compounds ( HQMC and HQPC )) for C22E steel in one mol of hydrogen chloride (HCl) was investigated through electrochemical and gravimetric approaches. It was found that both complexes are effective anti-corrosive agents for C22E steel in an acid environment and the inhibition performance increased with increasing doses, following the sequence: HQMC > HQPCC. As observed in adsorption studies and Langmuir isotherm, the selected corrosion inhibitors were adsorbed on the metal surface by the electron-rich functional groups. Finally, it was concluded that HQMC is an anodic inhibitor, while the compound ( HQPC ) is a mixed type inhibitor with anodic dominant characteristics.
It was studied the anticorrosive efficiency of CH - HQ in an acid solution of hydrogen chloride using theoretical and electrochemical approaches. Data from electrochemical measurements (PDP and EIS) indicated that the CH-HQ product efficient inhibited M-steel corrosion and that the inhibitory efficiency rose with raising inhibitor concentrations. The outcomes of the PDP studies demonstrated that CH-HQ was a mixed-type on the temperature intervals (298 K ± 1–328 K ± 1). It was also found that this compound uses donor-acceptor interactions to interact with a mild steel surface. The results in the MC simulation showed that CH-HQ uses flat or horizontal adsorption orientations on the metal surface.
Researchers (El Faydy et al. 2020a) evaluated two compounds ( SMQ and SPQ ) in a mole HCl through electrochemical (PDP, EIS), gravimetric (WL), and theoretical measurement (DFT, MDS) studies. It was observed that these derivatives exhibit a remarkable anticorrosive effect at a temperature of 298 K. The adsorption thermodynamic analysis was investigated by Langmuir adsorption isotherm. SEM results confirmed that the studied inhibitors adsorbed on the metal surface. Finally, DFT and MD approaches show good alignment with the experimental evidence.
QATETA chemical (Hassan et al. 2020) was empirically examined as an anti-corrosion agent for M-steel in aqueous sodium chloride (3.5 %) using the following methods: gravimetric (ML), electrochemical (PDP & EIS) methods. To support the experimental results, some theoretical methods such as DFT and MC were used. The results showed that QATETA has a good ability to inhibit corrosion and this activity is related to concentration. As can be viewed in Tafel polarization results, both the evolution of hydrogen and the dissolution of the metal are reduced by the presence of corrosion inhibitors. Thermodynamic parameters (Δ G° ads ) have verified that QATETA adsorption on the metal surface is a spontaneous mechanism obeying Langmuir isothermal model. Finally, this substance adsorbs on the surface of the metal (M-steel) by physical and chemical adsorption mechanisms.
An anti-corrosion performance of MABQ inhibitor (Fakhry et al. 2021) for mild steel (1 M HCl) was tested in 1 M hydrochloric acid by the electrochemical (PDP, EIS) and theoretical study (DFT, MD). The obtained results demonstrated that this inhibitor is a mixed type. It was also found that this inhibitor is an excellent corrosion resistance inhibitor. Lastly, the thermodynamic parameters indicate that the substance of MABQ absorbed by the Langmuir isothermal model on the mild steel surface in an acid environment.
It is tested (Rbaa et al. 2020a) as anti-corrosion agents of ( GQ-6 ) and ( GQ-12 ) for M-steel in a solution of hydrogen chloride (1.0 M) utilizing gravimetric (ML), electrochemical (PDP & EIS) and some theoretical approaches such as DFT and MC. Next, the surface morphology of the steel after the corrosion and inhibition processes was determined using SEM spectroscopy, together with energy dispersive (EDS). In terms of results, the authors note that the inhibitory efficacy increases with the rise in the concentration of an inhibitor at 298 K. Furthermore, tests revealed that these items are effective in inhibiting M-steel corrosion and their inhibition efficiency depends on the inhibitor molecular structure. Thermodynamic parameters (ΔH a *, ΔS a *, and ΔG a *) suggested that two substances ( GQ-6 & GQ-12 ) are adsorbed with chemical bonds on the front of the metal surface (chemisorption).
The effectiveness of 8-hydroxyquinoline is well described in the changes on mild steel or metallic surface in the corrosion solution without and with corrosion inhibitors. In absence of a corrosion inhibitor medium, the metal surface was seriously destructed. Corrosion is a chemical and physical process; it has occurred on the surface of the metal in aqueous, gas and atmospheric environments. According to the aqueous phase, corrosion processes occurred through the electrochemical and physical interaction between the metal surface and solvent molecules, resulting in the metal atoms are rapidly reacted with the solution ions to form the various corrosion products. The corrosion solutions may be acidic, (hydrochloric, sulfate acids) saline (sodium chloride) and gas saturated solutions (oxygen, hydrosulphide and carbon dioxide gases are saturated in the aqueous phase). The metal surface may be iron, aluminium, magnesium, copper and zinc, which are more active metals. The corrosive ions are easily reacted with the above metal to form the metal salt, hydroxide and various compounds. They are covered on the metal surface. As a result, the metal materials are rusty.
On the other hand, the metal surface is well protected with the presence of a corrosion inhibitor. The corrosion inhibitor electrochemically interacted with the metal surface by the inhibition centres, which contained benzoyl rings, functional groups and heteroatoms. Then, the inhibitor formed rigid covalent bonds with the metal surface. As a result, a protective film was formed on the metal surface. The metal surface was effectively insulated from the chemical attacks of corrosive ions.
5 Critical part in effect of solution type, metal type and temperature on the protection function of the 8-hydroxyquinoline based inhibitors
The studies demonstrate that the efficiency of inhibitors like AMQN, HMQN, QC2-Br, and QC3-Br varies with changes in concentration and temperature. This variability underlines the need to understand the specific environmental conditions under which these inhibitors are most effective. For instance, the decrease in inhibition efficiency with rising temperature is a critical factor that limits the applicability of these inhibitors in high-temperature industrial environments. The Langmuir isotherm adsorption noted in several studies suggests a strong and uniform adsorption of inhibitors on metal surfaces. However, the balance between chemisorption and physisorption is crucial, as it impacts the strength and durability of the protective layer formed on the metal (Shen et al. 2013). A deeper understanding of these adsorption dynamics can help in designing more efficient inhibitors. The studies indicate that inhibitors like HM1, HM2, and HM3 have varying effects on different types of corrosion processes (cathodic, anodic, or mixed). This specificity (Nam et al. 2018) underscores the importance of matching the inhibitor type to the specific corrosion mechanism encountered in practice. The use of modern electrochemical techniques and theoretical approaches (like DFT and MC simulations) provides a comprehensive understanding of the corrosion inhibition process. However, the complexity of these techniques may limit their practicality in routine industrial applications. The effectiveness of inhibitors on different metals (like carbon steel and C35E steel) in various corrosive solutions (like HCl and NaCl) highlights the need for tailored inhibitor formulations. The interaction between the inhibitor, metal type, and solution composition is complex and requires careful consideration in inhibitor design. The studies show a general trend of increasing inhibitor efficiency with concentration, but also a dependence on temperature. The challenge lies in developing inhibitors that maintain their efficiency across a wide range of temperatures, particularly in industrial applications where extreme conditions are common. SEM analysis in several studies reveals how the surface morphology of metals is altered upon inhibitor addition, leading to improved corrosion protection. Understanding these morphological changes is crucial for assessing the long-term protective capabilities of inhibitors. While the effectiveness of these inhibitors is evident, there is less focus on their environmental impact. Future research should also consider the ecological footprint of these inhibitors, especially in terms of biodegradability and toxicity. In summary, while 8-hydroxyquinoline-based inhibitors show promise in corrosion protection, their effectiveness is influenced by a range of factors including solution type, metal type, temperature, and concentration. Future research and development should focus on enhancing the efficiency of these inhibitors under varied environmental conditions, understanding their adsorption mechanisms in greater detail, and ensuring their environmental sustainability.
6 8-Hydroxyquinoline is the good anti-polarizing agent in various mediums
8-Hydroxyquinoline (8-HQ) is recognized as an effective anti-polarizing agent in various mediums, owing to its unique chemical properties and the ability to form stable chelates with metal ions. This compound demonstrates remarkable efficacy in different environments, making it a versatile agent in corrosion inhibition and other chemical processes. The effectiveness of 8-HQ as an anti-polarizing agent is primarily attributed to its strong coordination and chelation behaviors. This allows it to form stable complexes with metal ions, which can be crucial in mitigating corrosion processes. In the context of corrosion inhibition, 8-HQ acts by forming a protective layer on the metal surface, thereby reducing the polarization effects that lead to corrosion (Marcelin and Pébère 2015). 8-HQ’s ability to function effectively in various mediums, including acidic, alkaline, and saline environments, makes it a highly versatile agent. Its performance in these diverse conditions is a testament to its robust molecular structure and its ability to adapt to different chemical interactions. Another advantage of 8-HQ is its relative environmental friendliness compared to other traditional corrosion inhibitors. This aspect is increasingly important in modern industrial applications where environmental impact is a critical consideration. 8-HQ’s role as an anti-polarizing agent extends beyond corrosion inhibition. It is also utilized in various industrial and chemical processes, including metal extraction, wastewater treatment, and as a stabilizing agent in certain chemical reactions (Ahmed et al. 2023). 8-Hydroxyquinoline’s role as an anti-polarizing agent is well-established in various mediums, primarily due to its strong coordination and chelation properties. Its versatility, effectiveness in diverse environments, and environmental compatibility make it a valuable compound in numerous industrial applications.
8-Hydroxyquinoline (8-HQ) exhibits distinct and effective properties when used in acidic solutions, such as hydrochloric acid (HCl), sulfuric acid (H2SO4), and other acidic environments. Its behavior in these settings is particularly significant in the context of corrosion inhibition and metal ion complexation. In acidic solutions, 8-HQ is a potent corrosion inhibitor, especially for metals like steel, aluminum, and copper. The acidic environment often accelerates metal corrosion, but the presence of 8-HQ can significantly mitigate this effect. It adsorbs onto the metal surface, forming a protective film that impedes the electrochemical processes responsible for corrosion (Truc et al. 2019; Chen et al. 2022). This protective layer is crucial in environments where metals are exposed to harsh acidic conditions. 8-HQ’s ability to chelate metal ions is enhanced in acidic solutions. It forms stable complexes with various metal ions, which is beneficial in processes like metal extraction and purification. The chelation properties of 8-HQ are utilized in analytical chemistry as well, particularly in the qualitative and quantitative determination of metal ions. 8-HQ maintains structural integrity and chemical stability in acidic presence environments. This stability is crucial for its effectiveness in these media, ensuring that its protective and complex-forming capabilities are retained even in highly acidic conditions. In acidic solutions, the protonation of 8-HQ can occur, influencing its mode of interaction with metal surfaces. The protonated form of 8-HQ can engage differently with the metal surface compared to its neutral form, which can impact the mechanism of corrosion inhibition. The use of 8-HQ in acidic environments finds practical applications in industries such as petrochemicals, metal processing, and wastewater treatment. Its efficacy in preventing corrosion in acidic mediums is particularly valued in industries where metals are regularly exposed to such conditions. In conclusion, 8-hydroxyquinoline is a highly effective compound in acidic solutions, offering significant benefits in corrosion inhibition and metal ion complexation. Its stability and effectiveness in such environments make it a valuable asset in various industrial and analytical applications.
7 Anticorrosion performance of 8-hydroxyquinoline for steel
The anticorrosion performance of 8-hydroxyquinoline (8-HQ) for steel in various environments, particularly in acidic conditions, is noteworthy due to its remarkable ability to inhibit corrosion. 8-HQ acts primarily by adsorbing onto the steel surface, forming a protective barrier that shields the metal from corrosive agents. This film significantly reduces the direct contact between the steel and the corrosive environment, thereby slowing down or preventing the electrochemical reactions that lead to corrosion. In acidic solutions, such as those containing hydrochloric acid (HCl) or sulfuric acid (H2SO4), 8-HQ shows a particularly high level of effectiveness. The acidic environment, which typically accelerates corrosion, is counteracted by 8-HQ’s ability to form a stable, protective film on the steel surface. Another important aspect of 8-HQ’s anticorrosion properties is its ability to chelate metal ions. This chelation can stabilize the steel surface by complexing with metal ions that may otherwise contribute to corrosion processes. The protective layer formed by 8-HQ is resilient and can withstand a range of environmental conditions, providing long-term protection against corrosion (Truc et al. 2019). This durability is crucial for applications in harsh industrial environments. Given its efficacy, 8-HQ is widely used in industries where steel is regularly exposed to corrosive substances. This includes the petrochemical industry, pipeline construction, and marine applications where steel structures are susceptible to corrosion. 8-HQ can be used in various formulations, either as a standalone inhibitor or in combination with other inhibitors to enhance its protective properties. Its versatility allows for tailored applications depending on the specific requirements of the steel structure and the corrosive environment. While 8-HQ is effective in corrosion inhibition, its environmental impact and safety profile are also considered. In general, 8-HQ is regarded as a safer alternative compared to some other corrosion inhibitors, though its handling and disposal must still adhere to environmental regulations. In summary, the anticorrosion performance of 8-hydroxyquinoline for steel is marked by its effective protective action in acidic environments, its ability to form stable complexes with metal ions, and its durability under various environmental conditions. These properties make it a valuable asset in protecting steel structures from corrosion.
8 Anticorrosion performance of 8-hydroxyquinoline for copper
8-Hydroxyquinoline (8-HQ) is also recognized for its effective anticorrosion performance on copper, a metal widely used in various industries due to its excellent electrical and thermal conductivity, as well as its aesthetic appeal. The anticorrosion characteristics of 8-HQ on copper, particularly in environments conducive to corrosion, are as follows (Gerengi et al. 2016b):
Protective film formation: similar to its action on steel, 8-HQ forms a protective film on the surface of copper. This film acts as a barrier, significantly reducing the interaction between copper and corrosive agents. It’s especially effective in preventing oxidation, which is a common form of corrosion for copper.
Chelation with copper ions: One of the key mechanisms by which 8-HQ protects copper is through its ability to chelate with copper ions. This chelation stabilizes the copper surface and inhibits further corrosion, as the complex formed is less reactive than the free metal ions.
Efficacy in various media: 8-HQ shows a high level of effectiveness in diverse environments, including in the presence of oxygen, moisture, and various corrosive agents. Its ability to provide protection in both acidic and neutral pH conditions makes it a versatile choice for different industrial applications.
Pitting corrosion prevention: copper is particularly susceptible to pitting corrosion, especially in chloride-containing environments. 8-HQ is effective in mitigating this form of localized corrosion, thus preserving the integrity and appearance of copper surfaces.
Electrochemical impedance: 8-hydroxyquinoline increases the electrochemical impedance on copper surfaces, which is a measure of the resistance against electron flow that causes corrosion. By increasing impedance, 8-HQ effectively slows down the corrosion process, providing long-term protection to copper structures.
Adsorption mechanism: the adsorption of 8-HQ on copper surfaces is a critical aspect of its anticorrosion capabilities. It involves the attachment of 8-HQ molecules to the copper surface, forming a thin protective layer that shields the metal from corrosive substances. This adsorption is influenced by the molecular structure of 8-HQ, particularly its ability to donate electrons to copper atoms, forming a stable layer.
Environmental resistance: In addition to its effectiveness in controlled environments, 8-HQ also demonstrates strong resistance to environmental factors such as humidity, temperature fluctuations, and exposure to different pH levels. This makes it suitable for outdoor copper structures and components exposed to varying weather conditions.
Synergistic effects with other inhibitors: 8-HQ can be used in combination with other corrosion inhibitors to enhance its protective effect on copper. This synergistic approach can lead to improved protection, especially in highly corrosive environments.
Minimal impact on copper properties: importantly, the use of 8-HQ as a corrosion inhibitor does not significantly alter the inherent properties of copper, such as conductivity or mechanical strength. This makes it an ideal choice for applications where maintaining the native characteristics of copper is essential.
Application in various industries: the anticorrosion properties of 8-HQ on copper have practical implications in various industries, including electronics, plumbing, marine applications, and architectural elements, where copper’s aesthetic and functional properties are crucial.
In conclusion, 8-hydroxyquinoline offers substantial protection to copper against corrosion, making it a valuable agent in preserving the integrity and functionality of copper-based materials and components in various industrial and environmental settings.
9 Anticorrosion performance of 8-hydroxyquinoline for copper
8-Hydroxyquinoline (8-HQ) is not only effective for steel and copper, but also exhibits significant anticorrosion performance for a variety of other metals. This includes common industrial metals such as aluminum, magnesium, and their alloys. 8-HQ is particularly effective in protecting aluminum alloys, which are widely used in aerospace, automotive, and construction industries. It forms a protective film on the surface, which acts as a barrier against moisture and other corrosive elements. This is crucial for aluminum, as it is susceptible to corrosion in the presence of salts and acidic environments. Magnesium and its alloys, known for their lightweight and high strength-to-weight ratio, are prone to corrosion, especially in saline and humid environments. 8-HQ helps in forming a passive layer on magnesium surfaces, significantly reducing the rate of corrosion. This makes it valuable in automotive and aerospace applications where magnesium alloys are common. The effectiveness of 8-HQ extends across various corrosive environments, including acidic, saline, and alkaline conditions. This broad-spectrum efficacy is attributed to its ability to form stable chelates with metal ions, preventing the typical electrochemical reactions that lead to corrosion (Gerengi et al. 2016a).
Similar to its use with copper and steel, 8-HQ can be combined with other corrosion inhibitors to enhance its protective action on other metals. This synergy can lead to better protection, especially in harsh industrial environments. The use of 8-HQ generally does not adversely affect the physical and mechanical properties of the metals it protects. This makes it an attractive option in industries where maintaining the integrity of the metal is crucial. As an organic compound, 8-HQ is relatively benign from an environmental standpoint, especially when compared to some traditional corrosion inhibitors that can be more hazardous. Given its versatility, 8-HQ finds applications in various industries, including marine, automotive, aerospace, and electronics, where it helps in prolonging the life and maintaining the integrity of metal components. In summary, 8-hydroxyquinoline is a versatile and effective anticorrosion agent for a variety of metals, providing robust protection in diverse environments while maintaining the essential properties of the metals. Its application is particularly valuable in industries where metal longevity and performance are critical.
10 Challenges and outlooks in the development and application of 8-hydroxyquinoline derivatives as corrosion inhibitors
10.1 Challenges
Complexity in synthesis: the diverse synthesis methods of 8-hydroxyquinoline derivatives, while beneficial, also present complexities in achieving consistent quality and properties, especially on a large industrial scale.
Environmental impact: despite strides towards eco-friendly derivatives, the complete environmental impact, including the production process and degradation products of these inhibitors, needs thorough evaluation.
Cost-effectiveness: balancing the cost of advanced synthesis techniques with the efficiency of the inhibitors remains a challenge, particularly for widespread industrial application.
Adaptability to varied conditions: customizing inhibitors to different corrosive environments, metals, and temperatures requires extensive research and testing, which can be resource-intensive.
Long-term stability and efficacy: ensuring long-term stability and consistent corrosion protection in diverse and extreme environments is a significant challenge.
Regulatory compliance: meeting increasingly stringent environmental and safety regulations requires continuous adaptation and innovation in the synthesis and application of these inhibitors.
10.2 Outlook
Advancement in green chemistry: continued development in green synthesis methods will likely lead to more environmentally sustainable and less toxic corrosion inhibitors.
Nanotechnology integration: the use of nanotechnology in the synthesis of 8-hydroxyquinoline derivatives can lead to enhanced performance, particularly in micro-corrosive environments.
Tailored corrosion inhibition solutions: future research is expected to focus on creating more tailor-made solutions that cater to specific industrial needs and conditions.
Enhanced durability and performance: ongoing innovation will likely yield derivatives with improved durability and effectiveness, especially in extreme conditions.
Integration with other corrosion protection methods: combining 8-hydroxyquinoline derivatives with other corrosion protection strategies (e.g., coatings, cathodic protection) might offer more comprehensive solutions.
Economic optimization: research into cost-effective synthesis and application methods will remain pivotal to make these inhibitors more accessible for wide-scale industrial use.
Broader industrial application: as research progresses, the application of these inhibitors is expected to expand into more industries, offering broader corrosion protection solutions.
Increased computational modeling: the use of computational modeling and simulations in predicting the efficacy of new derivatives will streamline the development process.
Global regulatory alignment: harmonizing global standards and regulations regarding corrosion inhibitors can foster wider acceptance and utilization of these derivatives.
Collaborative research and development: partnerships between academia, industry, and government agencies can accelerate the development and implementation of advanced corrosion inhibitors.
In conclusion, while challenges exist in the development and application of 8-hydroxyquinoline derivatives as corrosion inhibitors, the outlook is promising. Ongoing research and innovation are expected to overcome these challenges, leading to more effective, sustainable, and economically viable corrosion protection solutions.
11 Conclusions
Corrosion has become very important with the developing technology. Corrosion inhibitors are used in many industries. For this reason, designing and synthesizing more effective and more active molecules has gained importance in chemistry. In the previous studies, the corrosion inhibitory properties of new innovative heterocyclic complexes from 8-hydroxyquinoline were discussed. It was found the following main conclusions:
Significant economic impact of corrosion: the review underscores the severe economic burden of metal corrosion, emphasizing the necessity of effective corrosion management strategies in the chemical industry.
Effectiveness of organic inhibitors: organic corrosion inhibitors, particularly those derived from 8-hydroxyquinoline, are highlighted as cost-effective and efficient solutions for protecting metals in acidic environments.
Advancements in 8-hydroxyquinoline derivatives: the synthesis and characterization of innovative heterocyclic complexes from 8-hydroxyquinoline represent a significant advancement in corrosion inhibition technology.
Diverse synthesis methods: various synthesis methods, such as reactions with 5-chloromethyl-8-hydroxyquinoline and morpholine, produce derivatives with unique properties and enhanced corrosion inhibition capabilities.
Impact of solution type and metal: the effectiveness of the inhibitors is shown to depend on the type of acidic solution and the nature of the metal, underlining the need for tailored corrosion protection strategies.
Temperature effects on inhibition: the review indicates that the efficiency of corrosion inhibitors can vary with temperature, highlighting the importance of considering environmental conditions in corrosion protection.
Innovative derivatives with improved properties: new derivatives of 8-hydroxyquinoline exhibit improved corrosion inhibition, increased solubility, and enhanced chemical stability.
Environmentally friendly approaches: the synthesis of eco-friendly derivatives through green synthesis methods marks a shift towards sustainable corrosion inhibition strategies.
Customized solutions for corrosion challenges: the review shows that specific synthesis methods yield derivatives suited for particular corrosion environments, allowing for customized solutions.
Enhanced durability and thermal stability: some derivatives, particularly those from chemical vapor deposition and reduction methods, offer robust and thermally stable corrosion protection.
Shift towards biodegradable inhibitors: there is an increasing emphasis on biodegradable and less toxic derivatives, reflecting a growing concern for environmental impact in corrosion inhibition.
Advanced techniques for superior properties: cutting-edge synthesis methods like nanostructuring and electronic modification introduce derivatives with superior properties for modern industrial applications.
Tailored inhibition for micro-corrosive environments: nanostructured 8-HQ derivatives provide superior inhibition in micro-corrosive environments due to their high surface area interaction.
Comprehensive analysis of inhibitor effectiveness: the study conducts an in-depth analysis of various inhibitors, assessing their effectiveness based on factors like concentration, inhibitor molecular structure, and environmental conditions.
Conclusion on 8-hydroxyquinoline derivatives: the review concludes that derivatives of 8-hydroxyquinoline hold significant promise in the field of corrosion protection, emphasizing the importance of ongoing research to further enhance their effectiveness.
These conclusions highlight the importance of 8-hydroxyquinoline derivatives in the field of corrosion inhibition and the need for continued research and development to optimize their use in various industrial applications.
-
Research ethics: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
About, H., El Faydy, M., Benhiba, F., Kerroum, Y., Kaichouh, G., Oudda, H., Guenbour, A., Lakhrissi, B., Warad, I., and Zarrouk, A. (2021). Experimental and empirical assessment of two new 8-hydroxyquinoline analogs as effective corrosion inhibitor for C22E steel in 1 M HCl. J. Mol. Liq. 325: 114644, https://doi.org/10.1016/j.molliq.2020.114644.Suche in Google Scholar
Ahmed, G.H.G., El-Naggar, G.A., Nasr, F., and Matter, E.A. (2023). N-doped and N, Si-doped carbon dots for enhanced corrosion inhibition of mild steel in acidic environment. Diam. Relat. Mater. 136: 109979, https://doi.org/10.1016/j.diamond.2023.109979.Suche in Google Scholar
Alamshany, Z.M. and Ganash, A.A. (2019). Synthesis, characterization, and anti-corrosion properties of an 8-hydroxyquinoline derivative. Heliyon 5: e02895, https://doi.org/10.1016/j.heliyon.2019.e02895.Suche in Google Scholar PubMed PubMed Central
Al-Farhan, B.S., Basha, M.T., Abdel Rahman, L.H., El-Saghier, A.M.M., Abou El-Ezz, D., Marzouk, A.A., Shehata, M.R., and Abdalla, E.M. (2021). Synthesis, DFT calculations, antiproliferative, bactericidal activity and molecular docking of novel mixed-ligand salen/8-hydroxyquinoline metal complexes. Molecules 26: 4725, https://doi.org/10.3390/molecules26164725.Suche in Google Scholar PubMed PubMed Central
Aslam, R., Mobin, M., Zehra, S., and Aslam, J. (2022). A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J. Mol. Liq. 364: 119992, https://doi.org/10.1016/j.molliq.2022.119992.Suche in Google Scholar
Assad, H. and Kumar, A. (2021). Understanding functional group effect on corrosion inhibition efficiency of selected organic compounds. J. Mol. Liq. 344: 117755, https://doi.org/10.1016/j.molliq.2021.117755.Suche in Google Scholar
Baitule, P.K., Victoria, S.N., and Manivannan, R. (2021). Review on assessment of corrosion of mild steel in alkaline environment by using plant extract. IOP Conf. Ser.: Mater. Sci. Eng. 1057: 012012, https://doi.org/10.1088/1757-899X/1057/1/012012.Suche in Google Scholar
Bakhshipour, S., Shahedi, Z., Mirahmadi, F., Fereidonnejad, R., and Hesani, M. (2022). Effect of different in situ temperatures on the crystallinity and optical properties of green synthesized 8-hydroxyquinoline zinc by saffron extract. Opt. Continuum 1: 1401, https://doi.org/10.1364/OPTCON.459222.Suche in Google Scholar
Balaskas, A.C., Curioni, M., and Thompson, G.E. (2015). Effectiveness of 2‐mercaptobenzothiazole, 8‐hydroxyquinoline and benzotriazole as corrosion inhibitors on AA 2024‐T3 assessed by electrochemical methods. Surf. Interface Anal. 47: 1029–1039, https://doi.org/10.1002/sia.5810.Suche in Google Scholar
Bennamara, A. and Abourriche, A. (2020). Alkaloids 8-Hydroxyquinoline derivatives: synthesis and biological activities. Journal of Anal. Sci. Appl. Biotechnol. 2: 57–62, https://doi.org/10.48402/IMIST.PRSM/JASAB-V2I1.21491.Suche in Google Scholar
Berdimurodov, E., Verma, C., Berdimuradov, K., Quraishi, M.A., Kholikov, A., Akbarov, K., Umirov, N., and Borikhonov, B. (2022). 8–Hydroxyquinoline is key to the development of corrosion inhibitors: an advanced review. Inorg. Chem. Commun. 144: 109839, https://doi.org/10.1016/j.inoche.2022.109839.Suche in Google Scholar
Bozoklu, G., Marchal, C., Pécaut, J., Imbert, D., and Mazzanti, M. (2010). Structural and photophysical properties of trianionic nine-coordinated near-IR emitting 8-hydroxyquinoline-based complexes. Dalton Trans. 39: 9112, https://doi.org/10.1039/c0dt00225a.Suche in Google Scholar PubMed
Boztepe, T., Scioli-Montoto, S., Ruiz, M.E., Alvarez, V.A., Castro, G.R., and León, I.E. (2021). 8-Hydroxyquinoline platinum( ii ) loaded nanostructured lipid carriers: synthesis, physicochemical characterization and evaluation of antitumor activity. New J. Chem. 45: 821–830, https://doi.org/10.1039/D0NJ03940C.Suche in Google Scholar
Brandel, J., Torelli, S., Gellon, G., Serratrice, G., Putaux, J., and Pierre, J. (2009). From molecular to nanostructured iron complexes of amphiphilic chelators based on 8‐hydroxyquinoline subunits – evidence of self‐assembled edifices mimicking siderophores from marine bacteria. Eur. J. Inorg. Chem. 2009: 86–92, https://doi.org/10.1002/ejic.200800741.Suche in Google Scholar
Chan, S.H., Chui, C.H., Chan, S.W., Kok, S.H.L., Chan, D., Tsoi, M.Y.T., Leung, P.H.M., Lam, A.K.Y., Chan, A.S.C., Lam, K.H., and Tang, J.C.O. (2013). Synthesis of 8-hydroxyquinoline derivatives as novel antitumor agents. ACS Med. Chem. Lett. 4: 170–174, https://doi.org/10.1021/ml300238z.Suche in Google Scholar PubMed PubMed Central
Chen, Yanning, Wu, L., Yao, W., Chen, Yonghua, Zhong, Z., Ci, W., Wu, J., Xie, Z., Yuan, Y., and Pan, F. (2022). A self-healing corrosion protection coating with graphene oxide carrying 8-hydroxyquinoline doped in layered double hydroxide on a micro-arc oxidation coating. Corros. Sci. 194: 109941, https://doi.org/10.1016/j.corsci.2021.109941.Suche in Google Scholar
Cioc, R.C., Ruijter, E., and Orru, R.V.A. (2014). Multicomponent reactions: advanced tools for sustainable organic synthesis. Green Chem. 16: 2958–2975, https://doi.org/10.1039/C4GC00013G.Suche in Google Scholar
Cipurković, A., Horozić, E., Marić, S., Mekić, L., and Junuzović, H. (2021). Metal complexes with 8-hydroxyquinoline: synthesis and in vitro antimicrobial activity. OJAppS 11: 1–10, https://doi.org/10.4236/ojapps.2021.111001.Suche in Google Scholar
Connolly, B.M., Mehta, J.P., Moghadam, P.Z., Wheatley, A.E.H., and Fairen-Jimenez, D. (2018). From synthesis to applications: metal–organic frameworks for an environmentally sustainable future. Curr. Opin. Green Sustain. Chem. 12: 47–56, https://doi.org/10.1016/j.cogsc.2018.06.012.Suche in Google Scholar
Cramer, S.D., and Covino, B.S. (Eds.) (2005). Corrosion of zinc and zinc alloys. Corrosion: materials. ASM International, US, pp. 402–417.10.31399/asm.hb.v13b.a0003830Suche in Google Scholar
Cseri, L., Razali, M., Pogany, P., and Szekely, G. (2018) Organic solvents in sustainable synthesis and engineering. In: Green chemistry. Elsevier, Amsterdam, Netherlands, pp. 513–553.10.1016/B978-0-12-809270-5.00020-0Suche in Google Scholar
Dahaghin, Z., Mousavi, H.Z., and Sajjadi, S.M. (2017). Synthesis and application of magnetic graphene oxide modified with 8‐hydroxyquinoline for extraction and preconcentration of trace heavy metal ions. ChemistrySelect 2: 1282–1289, https://doi.org/10.1002/slct.201601765.Suche in Google Scholar
Douche, D., Elmsellem, H., Anouar, E.H., Guo, L., Hafez, B., Tüzün, B., El Louzi, A., Bougrin, K., Karrouchi, K., and Himmi, B. (2020). Anti-corrosion performance of 8-hydroxyquinoline derivatives for mild steel in acidic medium: gravimetric, electrochemical, DFT and molecular dynamics simulation investigations. J. Mol. Liq. 308: 113042, https://doi.org/10.1016/j.molliq.2020.113042.Suche in Google Scholar
El Faydy, M., About, H., Benhiba, F., Lakhrissi, B., Guenbour, A., Bentiss, F., Warad, I., Ebenso, E.E., and Zarrouk, A. (2020a). The inhibitory effect of two 5-alkylthio-8-hydroxyquinoline salts on steel C22E in a molar electrolyte of hydrochloric acid: experimental and theoretical studies. Surf. Interfaces 20: 100575, https://doi.org/10.1016/j.surfin.2020.100575.Suche in Google Scholar
El Faydy, M., Benhiba, F., Berisha, A., Kerroum, Y., Jama, C., Lakhrissi, B., Guenbour, A., Warad, I., and Zarrouk, A. (2020b). An experimental-coupled empirical investigation on the corrosion inhibitory action of 7-alkyl-8-hydroxyquinolines on C35E steel in HCl electrolyte. J. Mol. Liq. 317: 113973, https://doi.org/10.1016/j.molliq.2020.113973.Suche in Google Scholar
Fakhry, H., El Faydy, M., Benhiba, F., Laabaissi, T., Bouassiria, M., Allali, M., Lakhrissi, B., Oudda, H., Guenbour, A., Warad, I., and Zarrouk, A. (2021). A newly synthesized quinoline derivative as corrosion inhibitor for mild steel in molar acid medium: characterization (SEM/EDS), experimental and theoretical approach. Colloids Surf. A: Physicochem. Eng. Asp. 610: 125746, https://doi.org/10.1016/j.colsurfa.2020.125746.Suche in Google Scholar
Fardioui, M., Rbaa, M., Benhiba, F., Galai, M., Guedira, T., Lakhrissi, B., Warad, I., and Zarrouk, A. (2021). Bio-active corrosion inhibitor based on 8-hydroxyquinoline-grafted-alginate: experimental and computational approaches. J. Mol. Liq. 323: 114615, https://doi.org/10.1016/j.molliq.2020.114615.Suche in Google Scholar
Feng, X.-J., Yan, N., Wang, Y., Mei, P., Chen, W., Lu, L., and Lai, L. (2023). Corrosion inhibition studies of 8-hydroxyquinoline derivatives for N80 steel in a 1.0 M HCl solution: experimental, computational chemistry, and quantitative structure–activity relationship studies. Langmuir 39: 519–532, https://doi.org/10.1021/acs.langmuir.2c02807.Suche in Google Scholar PubMed
Galai, M., Rbaa, M., Ouakki, M., Dahmani, K., Kaya, S., Arrousse, N., Dkhireche, N., Briche, S., Lakhrissi, B., and Ebn Touhami, M. (2021). Functionalization effect on the corrosion inhibition of novel eco-friendly compounds based on 8-hydroxyquinoline derivatives: experimental, theoretical and surface treatment. Chem. Phys. Lett. 776: 138700, https://doi.org/10.1016/j.cplett.2021.138700.Suche in Google Scholar
Gerengi, H., Mielniczek, M., Gece, G., and Solomon, M.M. (2016a). Experimental and quantum chemical evaluation of 8-hydroxyquinoline as a corrosion inhibitor for copper in 0.1 M HCl. Ind. Eng. Chem. Res. 55: 9614–9624, https://doi.org/10.1021/acs.iecr.6b02414.Suche in Google Scholar
Gerengi, H., Uygur, I., Solomon, M., Yildiz, M., and Goksu, H. (2016b). Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain. Chem. Pharm. 4: 57–66, https://doi.org/10.1016/j.scp.2016.10.003.Suche in Google Scholar
Groysman, A. (2010). Corrosion mechanism and corrosion factors. In: Corrosion for everybody. Springer, Netherlands, Dordrecht, pp. 1–51.10.1007/978-90-481-3477-9_1Suche in Google Scholar
Groysman, A. (2017a). Corrosion problems and solutions in oil, gas, refining and petrochemical industry. Koroze ochr. mater. 61: 100–117, https://doi.org/10.1515/kom-2017-0013.Suche in Google Scholar
Groysman, A. (2017b). Corrosion problems and solutions in oil, gas, refining and petrochemical industry. Koroze ochr. mater. 61: 100–117, https://doi.org/10.1515/kom-2017-0013.Suche in Google Scholar
Gupta, R., Luxami, V., and Paul, K. (2021). Insights of 8-hydroxyquinolines: a novel target in medicinal chemistry. Bioorg. Chem. 108: 104633, https://doi.org/10.1016/j.bioorg.2021.104633.Suche in Google Scholar PubMed
Hassan, N., Ramadan, A.M., Khalil, S., Ghany, N.A.A., Asiri, A.M., and El-Shishtawy, R.M. (2020). Experimental and computational investigations of a novel quinoline derivative as a corrosion inhibitor for mild steel in salty water. Colloids Surf. A: Physicochem. Eng. Asp. 607: 125454, https://doi.org/10.1016/j.colsurfa.2020.125454.Suche in Google Scholar
Inamuddin, and Asiri, A.M. (Eds.) (2020). Applications of nanotechnology for green synthesis, nanotechnology in the life sciences. Springer International Publishing, Cham.10.1007/978-3-030-44176-0Suche in Google Scholar
Jiang, H., Tang, D., Li, Z., Li, J., Liu, H., Meng, Q., Han, Q., and Liu, X. (2020). A dual-channel chemosensor based on 8-hydroxyquinoline for fluorescent detection of Hg2+ and colorimetric recognition of Cu2+. Spectrochim. Acta A Mol. Biomol. Spectrosc. 243: 118784, https://doi.org/10.1016/j.saa.2020.118784.Suche in Google Scholar PubMed
Jones, O. (1963). The inhibition of bacteriochlorophyll biosynthesis in rhodopseudomonas spheroides by 8-hydroxyquinoline. Biochem. J. 88: 335–343, https://doi.org/10.1042/bj0880335.Suche in Google Scholar PubMed PubMed Central
Julien, P.A., Mottillo, C., and Friščić, T. (2017). Metal–organic frameworks meet scalable and sustainable synthesis. Green Chem. 19: 2729–2747, https://doi.org/10.1039/C7GC01078H.Suche in Google Scholar
Kaczerewska, O., Leiva-Garcia, R., Akid, R., Brycki, B., Kowalczyk, I., and Pospieszny, T. (2018). Effectiveness of O – bridged cationic gemini surfactants as corrosion inhibitors for stainless steel in 3 M HCl: experimental and theoretical studies. J. Mol. Liq. 249: 1113–1124, https://doi.org/10.1016/j.molliq.2017.11.142.Suche in Google Scholar
Koch, G.H., Brongers, M.P.H., Thompson, N.G., Virmani, Y.P., and Payer, J.H. (2005). Cost of corrosion in the United States. In: Handbook of environmental degradation of materials. Elsevier, Amsterdam, Netherlands, pp. 3–24.10.1016/B978-081551500-5.50003-3Suche in Google Scholar
Kumar, P., Misra, A., Bhardwaj, R., Kamalasanan, M.N., Jain, S.C., Chand, S., and Tandon, R.P. (2008). Synthesis and characterization of some 5-coordinated aluminum-8-hydroxyquinoline derivatives for OLED applications. Displays 29: 351–357, https://doi.org/10.1016/j.displa.2007.10.006.Suche in Google Scholar
Leonel, A.G., Mansur, A.A.P., and Mansur, H.S. (2021). Advanced functional nanostructures based on magnetic iron oxide nanomaterials for water remediation: a review. Water Res. 190: 116693, https://doi.org/10.1016/j.watres.2020.116693.Suche in Google Scholar PubMed
Li, H., Zhang, F., Wang, Y., and Zheng, D. (2003). Synthesis and characterization of tris-(8-hydroxyquinoline)aluminum. Mater. Sci. Eng. B 100: 40–46, https://doi.org/10.1016/S0921-5107(03)00067-9.Suche in Google Scholar
Liu, J., Zhong, X., Wu, S., Li, Y., Xu, Y., and Zeng, H. (2019). Green synthesis and characterization for 8-hydroxyquinoline magnesium. Mater. Res. Express 6: 055101, https://doi.org/10.1088/2053-1591/ab01a5.Suche in Google Scholar
Marcelin, S. and Pébère, N. (2015). Synergistic effect between 8-hydroxyquinoline and benzotriazole for the corrosion protection of 2024 aluminium alloy: a local electrochemical impedance approach. Corros. Sci. 101: 66–74, https://doi.org/10.1016/j.corsci.2015.09.002.Suche in Google Scholar
Martins, A.O., Da Silva, E.L., Carasek, E., Gonçalves, N.S., Laranjeira, M.C.M., and De Fávere, V.T. (2004). Chelating resin from functionalization of chitosan with complexing agent 8-hydroxyquinoline: application for metal ions on line preconcentration system. Anal. Chim. Acta 521: 157–162, https://doi.org/10.1016/j.aca.2004.06.033.Suche in Google Scholar
Mastouri, A., Peulon, S., Bellakhal, N., and Chaussé, A. (2014). M(II) transfer across a liquid-liquid microinterface facilitated by a complex formation with 8-Hydroxyquinoline: application to quantification of Pb(II), Cd(II) and Zn(II) alone or in mixture in effluents. Electrochim. Acta 130: 818–825, https://doi.org/10.1016/j.electacta.2014.03.073.Suche in Google Scholar
Mazkour, A., El Hajjaji, S., Labjar, N., Lotfi, E.M., and El Mahi, M., 2021. Investigation of corrosion protection of austenitic stainless steel in 5.5 M polluted phosphoric acid using 5-azidomethyl-7-morpholinomethyl-8-hydroxyquinoline as an ecofriendly inhibitor. Int. J. Corros. 1–15. https://doi.org/10.1155/2021/6666811 Suche in Google Scholar
Musiol, R., Serda, M., Hensel-Bielowka, S., and Polanski, J. (2010). Quinoline-based antifungals. CMC 17: 1960–1973, https://doi.org/10.2174/092986710791163966.Suche in Google Scholar PubMed
Nam, N.D., Hien, P.V., Hoai, N.T., and Thu, V.T.H. (2018). A study on the mixed corrosion inhibitor with a dominant cathodic inhibitor for mild steel in aqueous chloride solution. J. Taiwan Inst. Chem. Eng. 91: 556–569, https://doi.org/10.1016/j.jtice.2018.06.007.Suche in Google Scholar
Nguyen, L.H.T., Tran, K.D.H., Le, T.M.T., Nguyen, V.P., Vu, G.B.N., Tran, P.H., Ung, T.D.T., Pham, A.T.T., Tran, N.Q., Le Minh, T., and Doan, T.L.H. (2024). Enhancing antimicrobial efficacy: 8-hydroxyquinoline incorporation into metal-organic frameworks with iron ion coupling. Mater. Chem. Phys. 319: 129346, https://doi.org/10.1016/j.matchemphys.2024.129346.Suche in Google Scholar
Nixon, R.L. and Ross, A.R.S. (2016). Evaluation of immobilized metal-ion affinity chromatography and electrospray ionization tandem mass spectrometry for recovery and identification of copper(II)-binding ligands in seawater using the model ligand 8-hydroxyquinoline. Front. Mar. Sci. 3, https://doi.org/10.3389/fmars.2016.00246.Suche in Google Scholar
Obot, I.B., Ankah, N.K., Sorour, A.A., Gasem, Z.M., and Haruna, K. (2017). 8-Hydroxyquinoline as an alternative green and sustainable acidizing oilfield corrosion inhibitor. Sustain. Mater. Technol. 14: 1–10, https://doi.org/10.1016/j.susmat.2017.09.001.Suche in Google Scholar
Obot, I.B. and Gasem, Z.M. (2014). Theoretical evaluation of corrosion inhibition performance of some pyrazine derivatives. Corros. Sci. 83: 359–366, https://doi.org/10.1016/j.corsci.2014.03.008.Suche in Google Scholar
Oliveri, V. and Vecchio, G. (2016). 8-Hydroxyquinolines in medicinal chemistry: a structural perspective. Eur. J. Med. Chem. 120: 252–274, https://doi.org/10.1016/j.ejmech.2016.05.007.Suche in Google Scholar PubMed
Oubaaqa, M., Ouakki, M., Rbaa, M., Benhiba, F., Galai, M., Idouhli, R., Maatallah, M., Jarid, A., Warad, I., Lakhrissi, B., Zarrouk, A., and Ebn Touhami, M. (2022a). Experimental and theoretical investigation of corrosion inhibition effect of two 8-hydroxyquinoline carbonitrile derivatives on mild steel in 1 M HCl solution. J. Phys. Chem. Solids 169: 110866, https://doi.org/10.1016/j.jpcs.2022.110866.Suche in Google Scholar
Oubaaqa, M., Rbaa, M., Ouakki, M., Idouhli, R., Maatallah, M., Jarid, A., Warad, I., Abousalem, A.S., Lakhrissi, B., Zarrouk, A., and Ebn Touhami, M. (2022b). Novel triphenyl imidazole based on 8-hydroxyquinoline as corrosion inhibitor for mild steel in molar hydrochloric acid: experimental and theoretical investigations. J. Appl. Electrochem. 52: 413–433, https://doi.org/10.1007/s10800-021-01632-3.Suche in Google Scholar
Patel, K.D. and Patel, H.S. (2017). Synthesis, spectroscopic characterization and thermal studies of some divalent transition metal complexes of 8-hydroxyquinoline. Arab. J. Chem. 10: S1328–S1335, https://doi.org/10.1016/j.arabjc.2013.03.019.Suche in Google Scholar
Pippi, B., Lopes, W., Reginatto, P., Silva, F.É.K., Joaquim, A.R., Alves, R.J., Silveira, G.P., Vainstein, M.H., Andrade, S.F., and Fuentefria, A.M. (2019). New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 27: 41–48, https://doi.org/10.1016/j.jsps.2018.07.017.Suche in Google Scholar PubMed PubMed Central
Pivarcsik, T., Pósa, V., Kovács, H., May, N.V., Spengler, G., Pósa, S.P., Tóth, S., Nezafat Yazdi, Z., Özvegy-Laczka, C., Ugrai, I., Szatmári, I., Szakács, G., and Enyedy, É.A. (2022). Metal complexes of a 5-nitro-8-hydroxyquinoline-proline hybrid with enhanced water solubility targeting multidrug resistant cancer cells. IJMS 24: 593, https://doi.org/10.3390/ijms24010593.Suche in Google Scholar PubMed PubMed Central
Pytlakowska, K. (2016). Preconcentration of Zn, Cu, and Ni ions from coffee infusions via 8-hydroxyquinoline complexes on graphene prior to energy dispersive X-ray fluorescence spectrometry determination. Appl. Spectrosc. 70: 1891–1899, https://doi.org/10.1177/0003702816644758.Suche in Google Scholar PubMed
Quadri, T.W., Akpan, E.D., Olasunkanmi, L.O., Fayemi, O.E., and Ebenso, E.E. (2022) Fundamentals of corrosion chemistry. In: Environmentally sustainable corrosion inhibitors. Elsevier, Amsterdam, Netherlands, pp. 25–45.10.1016/B978-0-323-85405-4.00019-7Suche in Google Scholar
Rahman, Md.M., Haque, T.Md.A., Sourav, N.S., Rahman, S., Yesmin, S., Mia, R., Al Noman, A., and Begum, K. (2021). Synthesis and investigation of dyeing properties of 8-hydroxyquinoline-based azo dyes. J. Iran. Chem. Soc. 18: 817–826, https://doi.org/10.1007/s13738-020-02070-2.Suche in Google Scholar
Rajasekaran, M., Anbusrinivasan, P., and Mojumdar, S.C. (2010). Growth, spectral and thermal characterization of 8-hydroxyquinoline. J. Therm. Anal. Calorim. 100: 827–830, https://doi.org/10.1007/s10973-010-0761-5.Suche in Google Scholar
Ramos, M.L., Justino, L.L.G., Branco, A., Fonseca, S.M., and Burrows, H.D. (2013). Theoretical and experimental insights into the complexation of 8-hydroxyquinoline-5-sulfonate with divalent ions of Group 12 metals. Polyhedron 52: 743–749, https://doi.org/10.1016/j.poly.2012.07.074.Suche in Google Scholar
Rbaa, M., Abousalem, A.S., Rouifi, Z., Lakhrissi, L., Galai, M., Zarrouk, A., Lakhrissi, B., and Lakhrissi, Y. (2020a). Selective synthesis of new sugars based on 8-hydroxyquinoline as corrosion inhibitors for mild steel in HCl solution-effect of the saturated hydrocarbon chain: theoretical and experimental studies. Inorg. Chem. Commun. 118: 108019, https://doi.org/10.1016/j.inoche.2020.108019.Suche in Google Scholar
Rbaa, M., Fardioui, M., Verma, C., Abousalem, A.S., Galai, M., Ebenso, E.E., Guedira, T., Lakhrissi, B., Warad, I., and Zarrouk, A. (2020b). 8-Hydroxyquinoline based chitosan derived carbohydrate polymer as biodegradable and sustainable acid corrosion inhibitor for mild steel: experimental and computational analyses. Int. J. Biol. Macromol. 155: 645–655, https://doi.org/10.1016/j.ijbiomac.2020.03.200.Suche in Google Scholar PubMed
Rbaa, M. and Lakhrissi, B. (2019). Novel oxazole and imidazole based on 8-hydroxyquinoline as a corrosion inhibition of mild steel in HCl solution: insights from experimental and computational studies. Surf. Interfaces 15: 43–59, https://doi.org/10.1016/j.surfin.2019.01.010.Suche in Google Scholar
Rbaa, M., Mequedade, M., Berkiks, I., Lakhrissi, Y., Mague, J., El Hessni, A., Hadda, T.B., Warad, I., Lakhrissi, B., and Zarrouk, A. (2022). Toxicological and pharmacological studies of a crystal structure derivative of 8-hydroxyquinoline. Arab. J. Sci. Eng. 47: 6889–6900, https://doi.org/10.1007/s13369-021-06007-6.Suche in Google Scholar
Rbaa, M., Ouakki, M., Galai, M., Berisha, A., Lakhrissi, B., Jama, C., Warad, I., and Zarrouk, A. (2020c). Simple preparation and characterization of novel 8-hydroxyquinoline derivatives as effective acid corrosion inhibitor for mild steel: Experimental and theoretical studies. Colloids Surf. A: Physicochem. Eng. Asp. 602: 125094, https://doi.org/10.1016/j.colsurfa.2020.125094.Suche in Google Scholar
Rbaa, M., Oubihi, A., Anouar, E.H., Ouhssine, M., Almalki, F., Hadda, T.B., Zarrouk, A., and Lakhrissi, B. (2019). Synthesis of new heterocyclic systems oxazino derivatives of 8-hydroxyquinoline: drug design and POM analyses of substituent effects on their potential antibacterial properties. Chem. Data Coll. 24: 100306, https://doi.org/10.1016/j.cdc.2019.100306.Suche in Google Scholar
Rohini, Paul, K., and Luxami, V. (2020). 8‐Hydroxyquinoline fluorophore for sensing of metal ions and anions. Chem. Rec. 20: 1430–1473, https://doi.org/10.1002/tcr.202000082.Suche in Google Scholar PubMed
Rouifi, Z., Rbaa, M., Benhiba, F., Laabaissi, T., Oudda, H., Lakhrissi, B., Guenbour, A., Warad, I., and Zarrouk, A. (2020). Preparation and anti-corrosion activity of novel 8-hydroxyquinoline derivative for carbon steel corrosion in HCl molar: computational and experimental analyses. J. Mol. Liq. 307: 112923, https://doi.org/10.1016/j.molliq.2020.112923.Suche in Google Scholar
Royzen, M. and Canary, J.W. (2013). Structural parameters of Zn(II) complexes of 8-hydroxyquinoline-based tripodal ligands affect fluorescence quantum yield. Polyhedron 58: 85–91, https://doi.org/10.1016/j.poly.2012.11.027.Suche in Google Scholar
Seifzadeh, D., Basharnavaz, H., and Bezaatpour, A. (2013). A Schiff base compound as effective corrosion inhibitor for magnesium in acidic media. Mater. Chem. Phys. 138: 794–802, https://doi.org/10.1016/j.matchemphys.2012.12.063.Suche in Google Scholar
Sharma, J., Kumar Bhardwaj, V., Singh, R., Rajendran, V., Purohit, R., and Kumar, S. (2021). An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chem. 346: 128933, https://doi.org/10.1016/j.foodchem.2020.128933.Suche in Google Scholar PubMed PubMed Central
Shen, W., Ritzwoller, M.H., and Schulte‐Pelkum, V. (2013). A 3‐D model of the crust and uppermost mantle beneath the Central and Western US by joint inversion of receiver functions and surface wave dispersion. JGR Solid Earth 118: 262–276, https://doi.org/10.1029/2012JB009602.Suche in Google Scholar
Tian, Z., Shi, H., Liu, F., Xu, S., and Han, E.-H. (2015). Inhibiting effect of 8-hydroxyquinoline on the corrosion of silane-based sol–gel coatings on AA 2024-T3. Prog. Org. Coat. 82: 81–90, https://doi.org/10.1016/j.porgcoat.2015.01.018.Suche in Google Scholar
Truc, T.A., Thuy, T.T., Oanh, V.K., Hang, T.T.X., Nguyen, A.S., Caussé, N., and Pébère, N. (2019). 8-hydroxyquinoline-modified clay incorporated in an epoxy coating for the corrosion protection of carbon steel. Surf. Interfaces 14: 26–33, https://doi.org/10.1016/j.surfin.2018.10.007.Suche in Google Scholar
Umoren, S.A. and Solomon, M.M. (2014a). Recent developments on the use of polymers as corrosion inhibitors – a review. TOMSJ 8: 39–54, https://doi.org/10.2174/1874088X01408010039.Suche in Google Scholar
Umoren, S.A. and Solomon, M.M. (2014b). Recent developments on the use of polymers as corrosion inhibitors – a review. TOMSJ 8: 39–54, https://doi.org/10.2174/1874088X01408010039.Suche in Google Scholar
Wu, S., Zhong, X., Zeng, H., You, W., and Zhou, W. (2018). Study on green synthesis and properties of luminescent material bis(8-hydroxyquinoline) calcium (CaQ 2 ). J. Lumin. 195: 120–125, https://doi.org/10.1016/j.jlumin.2017.11.029.Suche in Google Scholar
Yin, Z., Wang, B., Chen, G., and Zhan, M. (2011). One-dimensional 8-hydroxyquinoline metal complex nanomaterials: synthesis, optoelectronic properties, and applications. J. Mater. Sci. 46: 2397–2409, https://doi.org/10.1007/s10853-011-5264-7.Suche in Google Scholar
Zborowski, K., Solá, M., Poater, J., and Proniewicz, L. (2013). Aromatic properties of 8-hydroxyquinoline and its metal complexes. Open Chem. 11: 655–663, https://doi.org/10.2478/s11532-013-0215-6.Suche in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Reviews
- Anticorrosion properties of flavonoids for rust-free building materials: a review
- Optimization strategies for graphene-based protection coatings: a review
- Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
- Efficient and reliable corrosion control for subsea assets: challenges in the design and testing of corrosion probes in aggressive marine environments
- Original Articles
- Corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment
- Model construction of corrosion resistance of alloying elements for low alloy steel in marine atmospheric corrosive environment based on machine learning
- Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 42 (2024)
Artikel in diesem Heft
- Frontmatter
- Reviews
- Anticorrosion properties of flavonoids for rust-free building materials: a review
- Optimization strategies for graphene-based protection coatings: a review
- Synthesis methods and description for new derivatives associated with 8-hydroxyquinoline and their use as acidic corrosion inhibitors for steel and other alloys: a review
- Efficient and reliable corrosion control for subsea assets: challenges in the design and testing of corrosion probes in aggressive marine environments
- Original Articles
- Corrosion behavior of Cu–40Zn alloy in a periodic service between simulating atmospheric and deep sea environment
- Model construction of corrosion resistance of alloying elements for low alloy steel in marine atmospheric corrosive environment based on machine learning
- Reviewer Acknowledgement
- Reviewer acknowledgement Corrosion Reviews volume 42 (2024)

