Abstract
Introduction
The correlation between serum angiopoietin-2 levels and acute kidney injury (AKI) is a topic of significant clinical interest. This meta-analysis aims to provide a comprehensive evaluation of this relationship.
Content
A systematic search was conducted in PubMed, Embase, Web of Science, and Cochrane databases up to October 11, 2023. The included studies were evaluated using the Newcastle-Ottawa Scale (NOS) and Methodological Index for Non-Randomized Studies (MINORS). Weighted mean differences (WMD) and odds ratios (OR) were calculated using random-effects models. Sensitivity analysis, funnel plots, and Egger’s test were used to assess the robustness and publication bias of the findings. Subgroup analyses were performed to explore potential variations between adults and children.
Summary
Eighteen studies encompassing a total of 7,453 participants were included. The analysis revealed a significant elevation in serum angiopoietin-2 levels in patients with AKI compared to those without (WMD: 4.85; 95 % CI: 0.75 to 0.27; I²=93.2 %, p<0.001). Subgroup analysis indicated significantly higher angiopoietin-2 levels in adults with AKI (WMD: 5.17; 95 % CI: 3.51 to 6.83; I²=82.6 %, p<0.001), but not in children. Additionally, high serum angiopoietin-2 levels were associated with an increased risk of AKI (OR: 1.58; 95 % CI: 1.39 to 1.8; I²=89.1 %, p<0.001). Sensitivity analysis validated the robustness of these results, showing no substantial change in the overall effect size upon the exclusion of individual studies.
Outlook
This meta-analysis supports a significant association between elevated serum angiopoietin-2 levels and increased risk of AKI. The observed differential association between adults and children highlights the need for further targeted research to understand these age-specific variations.
Background
Acute kidney injury (AKI) is emerging as a burgeoning burden on global health. Globally, one out of every five adults and one out of every three children encounter AKI during a hospital admission, accompanied by a high comorbidity rate, elevated mortality, and an extended hospital stay [1, 2]. AKI impacts 40–60 % of individuals admitted to the intensive care unit (ICU) [3], leading to unfavorable short- and long-term results [4]. In the ICU, AKI manifests as a sudden drop in renal function, indicated by elevated serum creatinine and/or diminished urine output. The occurrence of AKI in septic individuals prolongs ICU stays, escalates hospitalization costs, and leads to substantial long-term morbidity and mortality, particularly in cases of severe AKI. AKI is associated with poor outcomes; even in non-ICU hospitalized patients with AKI, mortality is typically 10–20 % [5, 6]. Timely identification and proactive intervention are crucial to avert unfavorable consequences. Thus, the identification of outcome-specific biomarkers in this patient population would be of great value for ICU physicians [7, 8].
Angiopoietin-2 is a protein involved in vascular formation and endothelial function regulation. Elevated levels of serum angiopoietin-2 are associated with the occurrence and severity of AKI. Angiopoietin-2 is believed to play a role in endothelial barrier disruption, inflammation, and impaired renal blood flow, all of which are key factors in the pathogenesis of AKI [9]. Angiopoietin-2, situated within the angiopoietin-Tie-2 signaling axis, is acknowledged for its dynamic contribution to the modulation of vascular stability and inflammation [10]. The maintenance of endothelial quiescence relies on the coordinated efforts of angiopoietin-1 and angiopoietin-2 in physiological conditions. In pathological conditions, such as inflammation or hypoxia, angiopoietin-2 disrupts the delicate balance, driving endothelial activation and resultant vascular leakage [11]. Early evidence from experimental studies in animal models revealed an upsurge in angiopoietin-2 expression in renal tissues during ischemic and nephrotoxic AKI [12]. A seminal study by Roviezzo et al. [11] demonstrated a notable upregulation of angiopoietin-2 expression within renal tissues during ischemic AKI. Experimental studies conducted by Fiedler et al. [13] revealed that elevated angiopoietin-2 levels were associated with increased vascular permeability and inflammation in the renal microenvironment following nephrotoxic insults. Robinson-Cohen et al. [14] reported plasma levels of endothelial biomarkers, especially angiopoietin-2, were significantly increased in critically ill patients with AKI, independent of inflammation, and angiopoietin-2 remained associated with the development of later onset AKI. These discoveries suggested a potential connection between angiopoietin-2 and the vascular disruptions observed in AKI. Over the course of research development, the focus transitioned from isolated assessments of angiopoietin-2 in AKI to a more holistic understanding of its clinical significance. Allegretti et al. [15] showed that angiopoietin-2 was associated with mortality and other clinically relevant outcomes in the patients with AKI. Liu et al. [16] also found that the levels of angiopoietin-2 may play an important role in development of AKI in patients with acute myocardial infarction. However, the intricate relationship between angiopoietin-2 levels and AKI development remains incompletely understood, necessitating a comprehensive investigation. This meta-analysis aims to bridge this gap by systematically synthesizing existing evidence to evaluate the correlation between serum angiopoietin-2 levels and the risk of AKI. By doing so, we seek to contribute valuable insights that can inform clinical practice and guide future research endeavors.
Methods
The systematic review and meta-analysis were executed following the guidelines set by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [17]. The protocol was registered in the International Prospective Register of Systematic Reviews with registration number CRD42023471523.
Search strategy
A search encompassing four major online databases, namely PubMed, Embase, Web of Science, and Cochrane, was undertaken to identify studies that met the eligibility criteria. The detailed search strategies used in the study’s comprehensive search are outlined in Additional file 1. The last date for retrieving data was October 11, 2023, and there were no limitations on language during this process. To enhance completeness and prevent oversights, we included a manual search of the reference lists of full-length articles in our methodology.
Eligibility criteria
The inclusion criteria were as follows: (1) populations: all patients were diagnosed as acute kidney injury. AKI was determined for each study day using the Kidney Disease: Improving Global Outcomes (KDIGO) serum creatinine criteria during the study period [18]. Briefly, Acute kidney injury (AKI) was diagnosed as an increase in serum creatinine (SCr) by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h, or an increase in SCr to ≥1.5 times baseline within the prior seven days, or urine volume <0.5 mL/kg/h for 6 h; (2) study designs: prospective or retrospective study; (3) interventions: a description of the association between AKI and angiopoietin-2 exists in the included studies; (4) comparators: comparing AKI patients to non-AKI patients or comparing patients with high angiopoietin-2 levels to patients with low angiopoietin-2 levels. The criteria for exclusion were established as follows: (1) conference abstracts, conference proceedings, expert opinions, editorials, letters, and case reports; (2) studies involving subjects other than humans; (3) instances where the full text was obtainable but lacked the necessary data.
Data extraction and quality assessment
To ensure consistency, a standardized form was created for the extraction of all available data, and two trained researchers independently conducted the data extraction. The following data were extracted from each eligible literature: first author, publication year, country of correspondence author, sample size, age, gender, participants, intervention, control, group, outcome and type of study. A third researcher resolved any disagreements arising during the pairing process through negotiation and arbitration. The Newcastle-Ottawa Scale (NOS) [19] was employed to assess the quality of the observational studies included. The assessment of non-randomized comparative studies (NRCSs) utilized the Methodological Index for Non-Randomized Studies (MINORS) [20].
Data analysis
Stata 14.0 was used for statistical analysis. Mean±standard deviation was used to describe continuous variables, and frequencies and percentages were employed for the description of categorical variables. We present data using forest plots and present odds ratios (ORs) and mean differences (MDs) with 95 % confidence intervals (CIs) for binary and continuous outcomes, respectively. Utilizing the I2 test, heterogeneity was calculated to measure the extent of variability among articles attributable to heterogeneity rather than random chance. Significance testing employed the fixed-effects model in cases of no heterogeneity and the random-effects model in instances of high heterogeneity [21]. Egger’s tests were employed for the assessment of publication bias, with Trim and Fill analysis applied in cases of bias. Sensitivity analyses were utilized to determine the influence of individual studies on the overall risk assessment. The estimated effect was regarded as significant where p<0.05.
Results
Study selection
Initial retrieval through electronic search yielded 380 papers, with contributions from PubMed (80), Embase (155), Web of Science (129), and Cochrane Library (16). After deduplication, 276 studies persisted, and subsequent assessment of titles and abstracts led to the exclusion of 187 studies. A total of 71 studies were excluded after a careful assessment of the full texts due to non-compliance with eligibility criteria. Finally, 18 articles [14], [15], [16, 22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36] were deemed suitable for inclusion in the meta-analysis (Figure 1).
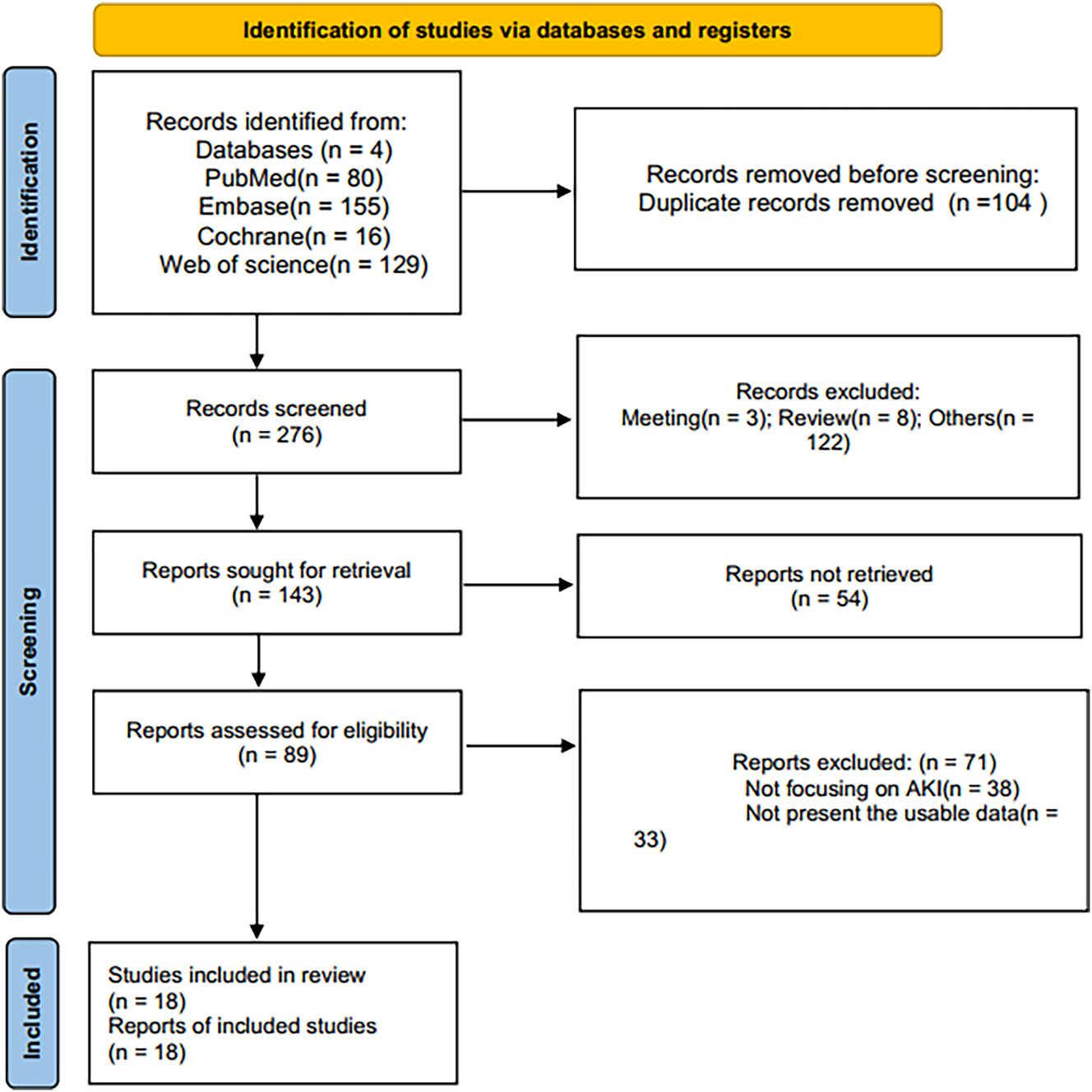
PRISMA flow chart of literature selection.
Study characteristics and quality assessment
The characteristics of the included studies are summarized in Table 1. The studies included in this analysis spanned the years 2010–2023. The studies were conducted in nine different countries, including USA (n=6), China (n=3), Netherlands (n=2), Australia (n=2), Canada (n=1), Germany (n=1), Finland (n=1), Poland (n=1), and Brazil (n=1). The study encompassed a total of 7,453 individuals. Literature quality assessment, based on the 9-star NOS, revealed that all studies achieved a score of ≥7 (Table S1). Quality assessment scores for the four non-randomized comparative studies (NRCSs) were 14, as determined by the MINORS tool (Table S2).
Characteristics of the studies included in this meta-analysis.
| Author | Year | Country | Sample size | Age, years | Female, % | Participants | Intervetion | Comparison | Outcomes assessed | Type of study |
|---|---|---|---|---|---|---|---|---|---|---|
| Yu [21] | 2021 | China | 228 | 55.62 | 48.68 | Severe sepsis and respiratory failure | Severe AKI | No severe AKI | Concentration of angiopietin-2, angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Wollborn [22] | 2023 | USA | 393 | 63 | 25 | Cardiac surgery patients | Postoperative acute kidney injury | \ | Angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Sporek [23] | 2016 | Poland | 65 | 62 | 48 | Acute pancreatitis | AKI (24, 48, 72 h) | \ | Angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Robinson-Cohen [14] | 2016 | USA | 948 | 55 | 36 | Critically ill patients in ICU | AKI | No AKI | Concentration of angiopietin-2, angiopoietin-2 (OR): occurrence of AKI | Retrospective study |
| Mansour [24] | 2022 | Brazil | 265 | 51.35 | 49.43 | Critically ill patients in ICU | Severe AKI | No-severe AKI | Angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Lin [25] | 2022 | USA | 1,503 | 65.8 | 37 | Patients with CKD progression and heart failure | AKI | No AKI | Angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Liu [16] | 2014 | China | 132 | 64 | 16.4 | Patients with acute heart failure | AKI | \ | Concentration of angiopietin-2 | Prospective study |
| Jongman [26] | 2015 | Netherlands | 541 | 66 | 26 | Patients underwent cardiac surgery | AKI | \ | Concentration of angiopietin-2 | Prospective study |
| Inkinen [27] | 2019 | China | 132 | 59 | 10.6 | “Patients with acute myocardial” | AKI | \ | Concentration of angiopietin-2, angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Conroy [28] | 2022 | USA | 999 | 1.67 | 44.4 | “Children hospitalized with an acute febrile illness” | Severe AKI | No-severe AKI | Concentration of angiopietin-2, angiopoietin-2 (OR): occurrence of death | Prospective study |
| Barber [29] | 2018 | Australia | 252 | 55 | 27 | “Severe plasmodium knowlesi malaria” | AKI | \ | Angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Atreya [30] | 2023 | Netherlands | 42 | 69.5 | 40.48 | Cardiac surgery patients | AKI | \ | Angiopoietin-2 (OR): occurrence of AKI | Prospective study |
| Kwong [31] | 2020 | Finland | 619 | 66 | 36 | Septic critical care patients | Severe AKI | No-severe AKI | Concentration of angiopietin-2 | Prospective study |
| Ouma [32] | 2020 | USA | 999 | 1.67 | 44.4 | “Children hospitalized with an acute febrile” | AKI | \ | Concentration of angiopietin-2 | Prospective study |
| Fisher [33] | 2016 | Canada | 341 | 59.5 | 46.3 | Septic shock | AKI stage 1/2/3 | No AKI | Concentration of angiopietin-2 | Retrospective study |
| Allegretti [15] | 2019 | Australia | 252 | 41.87 | 24.21 | “Severe” | AKI | No AKI | Concentration of angiopietin-2, angiopoietin-2 (OR): occurrence of death | Prospective study |
| Poss [34] | 2015 | Germany | 189 | 71 | 32 | “Acute myocardial infarction complicated by cardiogenic shock” | AKI stage 1/2/3 | No AKI | Angiopoietin-2 (OR): occurrence of death | Prospective study |
| Kumpers [35] | 2010 | USA | 414 | 3.53 | 45.89 | Pediatric septic shock | AKI stage 1/2/3 | No AKI | Concentration of angiopietin-2, angiopoietin-2 (OR): occurrence of death | Prospective study |
-
AKI, acute kidney injury; OR, odds ratios.
Comparison of angiopoietin-2 levels between patients with and without AKI
There were 11 studies investigating the relationship between angiopoietin-2 levels and acute kidney injury. Significant heterogeneity (I2=93.2 %, p<0.001) is observed and a random effects model is applied. Serum angiopoietin-2 levels were significantly different between patients with and without AKI (WMD: 4.85; 95 % CI: 0.75–0.27) (Figure 2A). In subgroup analysis, serum angiopoietin-2 levels were significantly higher in adults with AKI (WMD: 5.17; 95 % CI: 3.51–6.83; I2=82.6 %, p<0.001) (Figure 2B), but not children with AKI. The results of subgroup analysis based on study type (Supplemental Figure S1) were completely consistent with the overall analysis results.
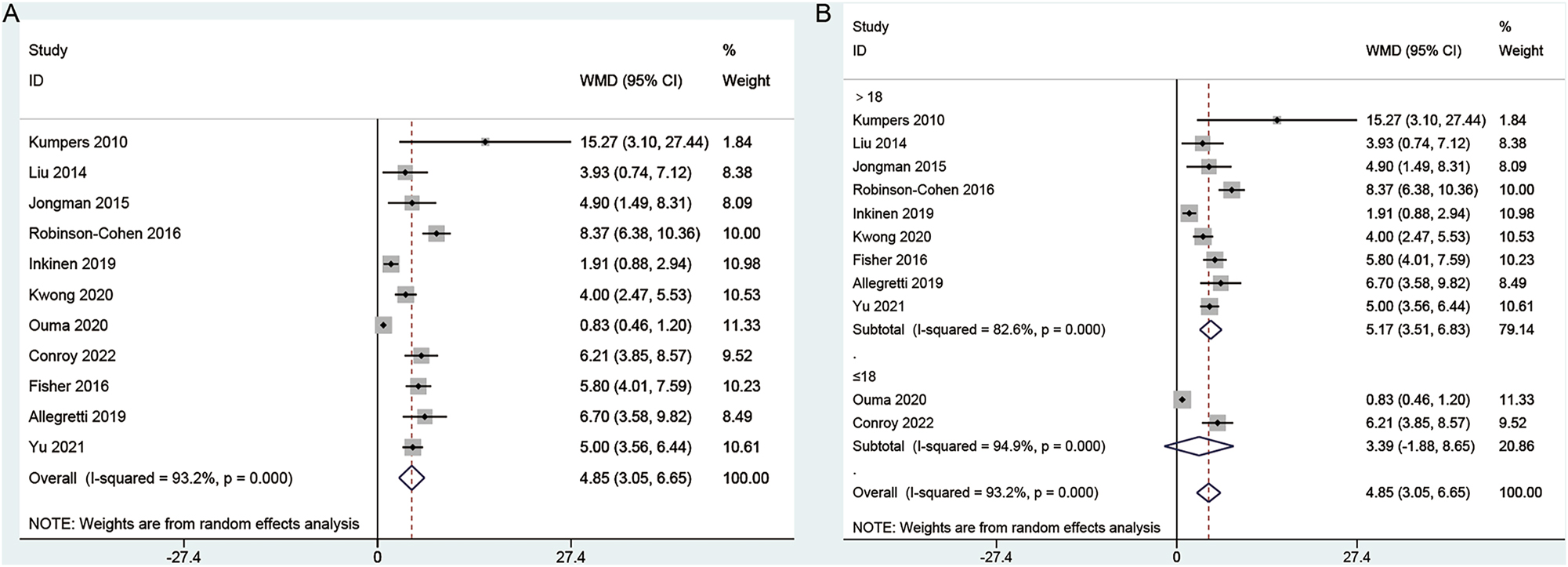
Forest plots of angiopoietin-2 levels between patients with and without AKI. (A) Overall comparison; (B) subgroup analysis.
Association between angiopoietin-2 level and risk of AKI
Thirteen studies provided sufficient information about the correlation of angiopoietin-2 levels and risk of AKI. Significant heterogeneity (I2=89.1 %, p<0.001) is observed and a random effects model is applied. In this meta-analysis, high angiopoietin-2 levels are associated with an increased risk of AKI (OR: 1.58; 95 % CI: 1.39–1.8) (Figure 3A). The results of subgroup analysis based on study type (Figure 3B), endpoint (Figure 3C) and age (Figure 3D) were completely consistent with the overall analysis results.
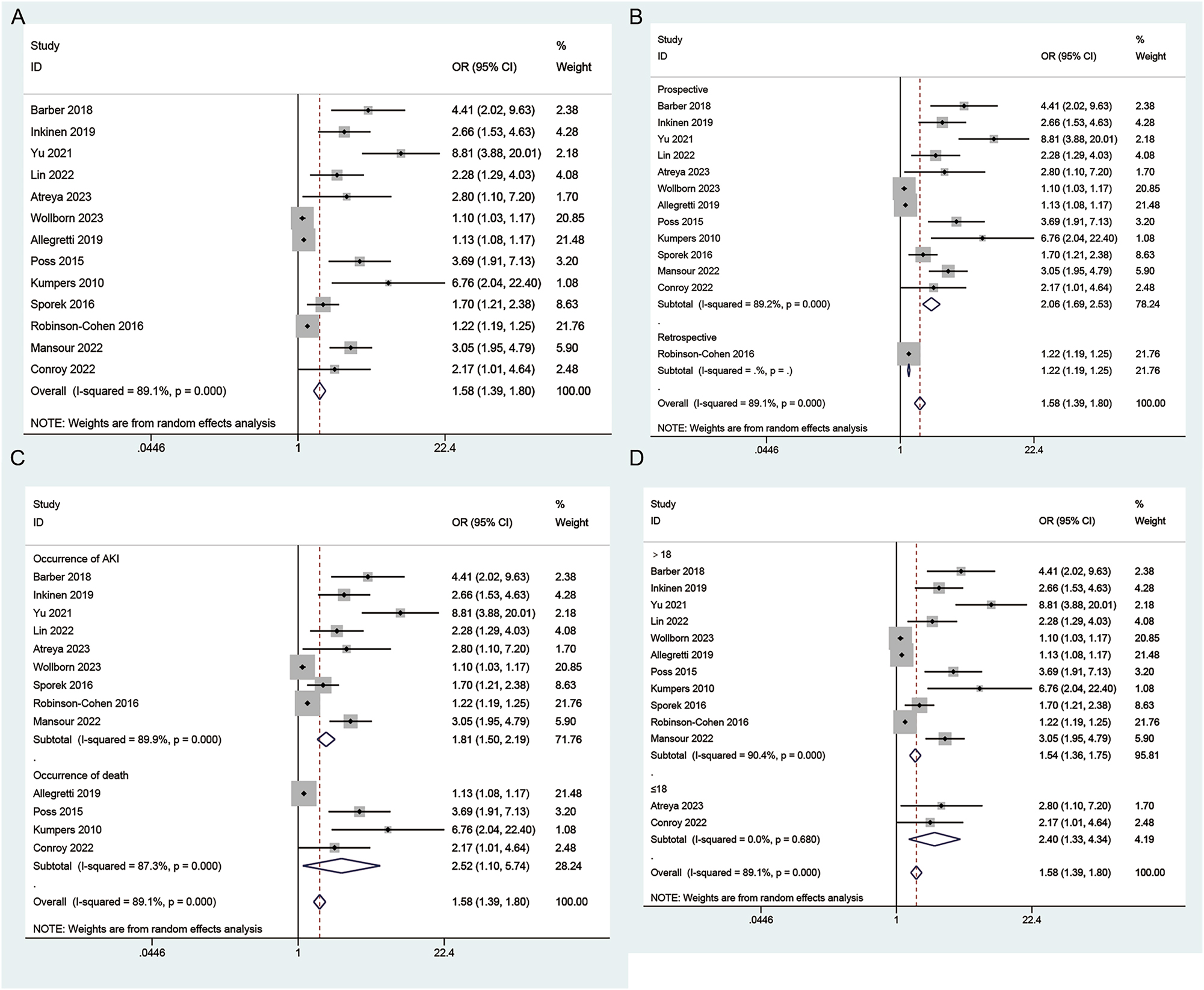
Forest plots of angiopoietin-2 level and risk of AKI. (A) Overall comparison; (B) subgroup analysis based on study type; (C) subgroup analysis based on endpoint; (D) subgroup analysis based on age.
Sensitivity analysis
In the sensitivity analysis, it was observed that excluding individual studies had negligible effects on the overall effect size (Figure 4).
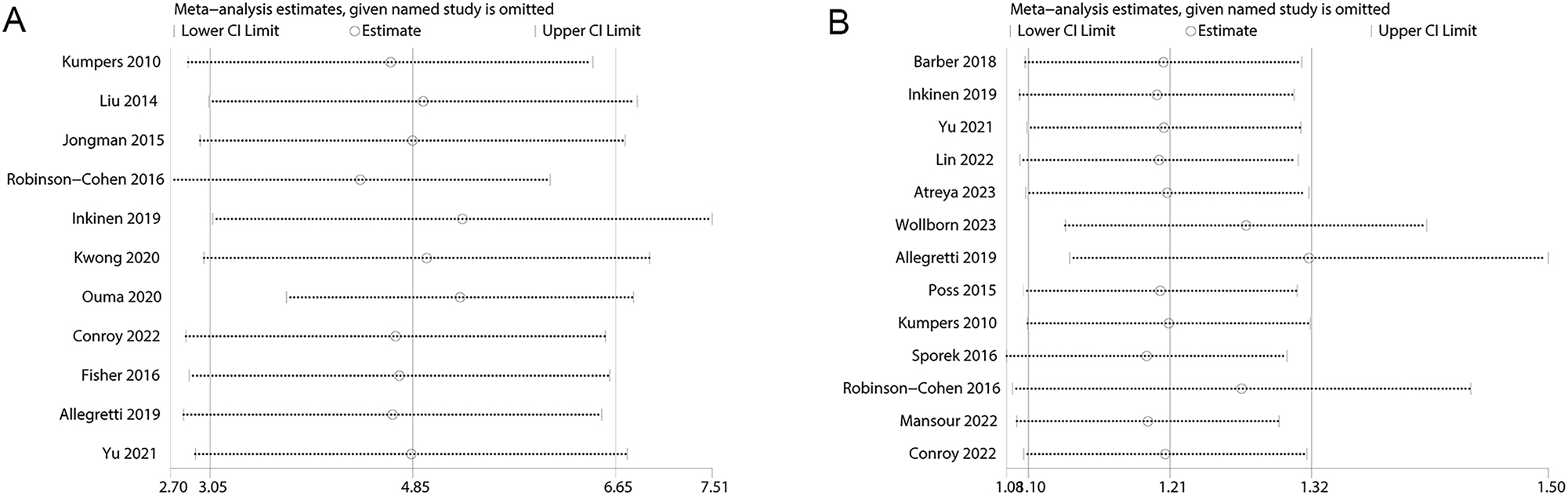
Sensitivity analysis examining the influence of individual studies on pooled results. (A) Angiopoietin-2 levels between patients with and without AKI; (B) angiopoietin-2 level and risk of AKI.
Publication bias
Publication bias was assessed through visual inspection of the funnel plot and Egger’s test. There was obvious asymmetry in the results of the funnel plot analysis for publication bias in the figure, as shown below (Figure 5). Egger’s test revealed evidence of publication bias for angiopoietin-2 levels between patients with and without TKI (Egger’s test: p<0.001) (Figure 5A) and angiopoietin-2 levels and the risk of AKI (Egger’s test: p=0.033) (Figure 5B). The ‘trim and fill’ analysis for angiopoietin-2 levels between patients with and without TKI indicated an estimated three missing studies, with the corresponding pooled mean difference estimate being 5.775 (95 % CI: 4.248, 7.851) (Supplemental Figure S2). For the ‘trim and fill’ analysis concerning angiopoietin-2 level and the risk of AKI, six missing studies were estimated, yielding a pooled odds ratio estimate of 1.189 (95 % CI: 1.165, 1.212) (Supplemental Figure S3).
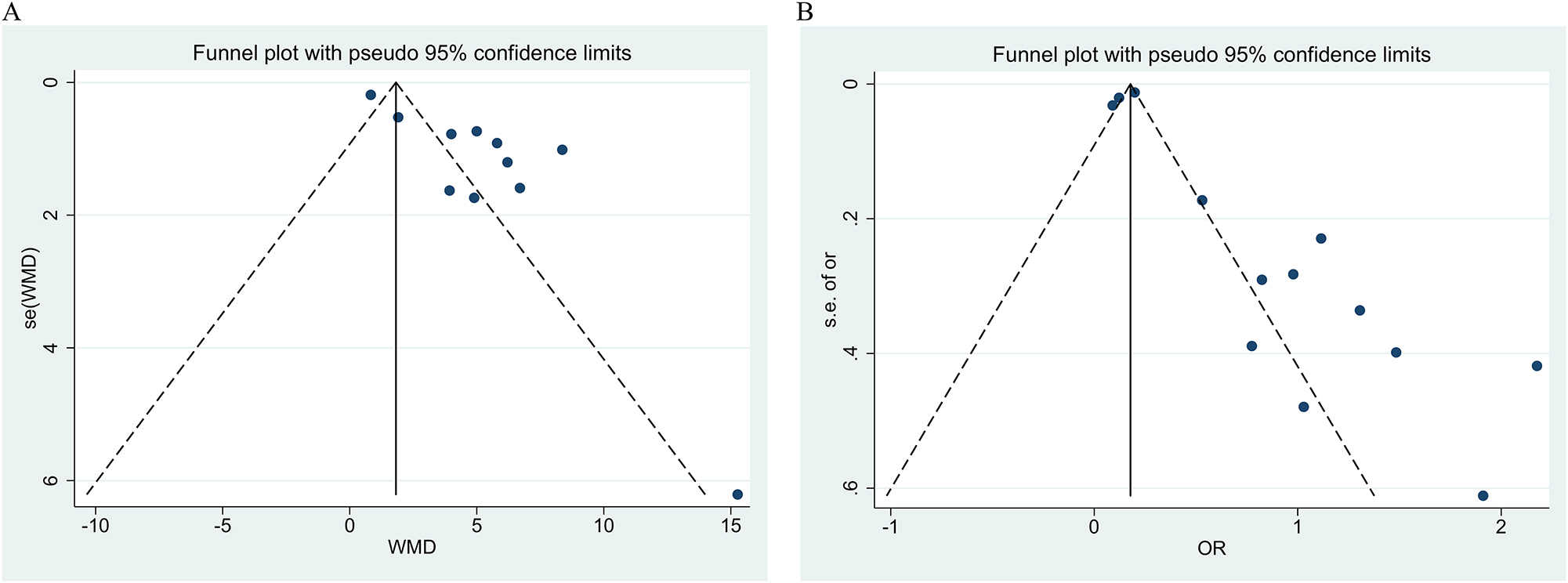
Funnel plot for publication bias. (A) Angiopoietin-2 levels between patients with and without AKI; (B) angiopoietin-2 level and risk of AKI.
Discussion
This meta-analysis focused on the correlation between angiopoietin-2 and acute kidney injury (AKI). The results revealed a significant difference in serum angiopoietin-2 levels between patients with and without AKI. Subgroup analysis highlighted elevated angiopoietin-2 levels in adults with AKI, but not in children. Furthermore, high angiopoietin-2 levels were associated with an increased risk of AKI. The association between endothelial dysfunction and AKI is multifaceted, reflecting the intricate interplay between vascular dynamics and renal function [37]. The endothelium, a key regulator of vascular homeostasis, plays a crucial role in maintaining renal perfusion and glomerular filtration [38]. Endothelial injury, as evidenced by elevated levels of von Willebrand factor (vWF) and soluble thrombomodulin (sTM), has been implicated in the pathogenesis of AKI [26]. Our meta-analysis focuses on angiopoietin-2, a vascular modulator with a dual role in maintaining endothelial quiescence and promoting vascular leakage under pathological conditions [10]. The observed elevation in serum angiopoietin-2 levels among individuals with AKI suggests its potential involvement in the endothelial dysfunction observed in AKI. This aligns with experimental evidence demonstrating increased angiopoietin-2 expression in renal tissues during ischemic and nephrotoxic AKI [11]. The observed elevation in serum angiopoietin-2 levels aligns with its recognized role as a key regulator of endothelial function. Angiopoietin-2 is intricately involved in the regulation of vascular permeability and inflammation, both pivotal aspects of endothelial dysfunction [39]. The consistent findings across clinical and experimental domains underscore the molecular basis of angiopoietin-2 in mediating endothelial responses during AKI. The connection between angiopoietin-2 and endothelial dysfunction is particularly relevant in the context of AKI, where disruption of renal microvascular integrity plays a central role in disease progression [14]. Angiopoietin-2’s ability to sensitize endothelial cells to inflammatory stimuli contributes to the dysregulation of vascular homeostasis observed in AKI [40]. The consistent elevation in angiopoietin-2 levels reinforces the notion that endothelial dysfunction is a central player in AKI pathogenesis. While these findings support the link between endothelial dysfunction and AKI, the specific mechanisms underlying angiopoietin-2-mediated effects warrant further exploration.
In endothelial cells, angiopoietin-2 is generated and stored within the Weibel-Palade bodies, where it coexists with von Willebrand factor (vWF). According to Kümpers et al., the glomerular endothelium of patients with lupus nephritis exhibited prominent protein expression of angiopoietin-2 in contrast to healthy subjects. Furthermore, they established a significant correlation between serum levels of angiopoietin-2 and the severity of systemic lupus erythematosus [41]. In ANCA-associated vasculitis with renal involvement, serum levels of angiopoietin-2 were significantly higher compared to patients with active limited granulomatous disease restricted to the respiratory tract or healthy subjects [42]. Taken together, these results suggest that the release of angiopoietin-2 from the renal endothelium is associated with inflammatory injury to the kidneys. In the context of sepsis, angiopoietin-2 is released into the circulation, leading to autocrine effects such as the disruption of the endothelial barrier and an elevation in microvascular permeability [43]. AKI stands as one of the prevailing organ dysfunctions associated with sepsis [12, 44]. According to Robinson-Cohen et al., plasma levels of endothelial biomarkers, especially angiopoietin-2, were significantly elevated in critically ill patients with AKI, independent of inflammation. Moreover, angiopoietin-2 retained its association with the subsequent onset of AKI 24 h later [14]. The observed elevation of serum angiopoietin-2 levels in patients with AKI suggests its potential as a clinically relevant biomarker for diagnostic and prognostic purposes. Our findings align with these efforts, emphasizing the clinical significance of angiopoietin-2 in identifying individuals at heightened risk for AKI. Moreover, the differential association between adults and children warrants attention, indicating that angiopoietin-2 may have age-specific implications that merit further investigation for refined clinical application. The renal response to injury varies across age groups, influenced by developmental factors, immune system maturation, and differences in nephron number and function. These age-specific dynamics may contribute to variations in the expression and response to angiopoietin-2 in the context of AKI [45]. Additionally, the underlying etiologies of AKI in adults and children often differ, with ischemic events and sepsis being more prevalent in adults, while congenital anomalies and infections are common in the pediatric population [46]. These distinct etiologies may further contribute to the observed differences in angiopoietin-2 levels in AKI.
Hypertension is a widespread medical condition with a high global prevalence [47]. The Renin-Angiotensin-Aldosterone System (RAAS), particularly the involvement of angiopoietin-2, holds a central role in the regulation of hypertension, emphasizing the intricate interrelationships between these elements [48]. The study by Lin et al. [26] delves into the relationship between hypertension, angiopoietin-2, and the development of AKI through regression analysis. Their findings illuminate the complex interplay between these factors, suggesting a potential contributory role of angiopoietin-2 in the pathogenesis of AKI, particularly in the context of hypertension (OR=3.79, 95 % CI 0.81–17.79). Additionally, the work of Liu et al. [16] further supports this association, revealing a significantly higher incidence of hypertension in patients with AKI compared to those without (84.6 vs. 44.5 %, p=0.008). Considering the intricate connections between RAAS, angiopoietin-2, hypertension, and AKI, our research serves as a stepping stone to unravel the underlying complexities of these relationships. However, it is crucial to acknowledge the limitations of the current statistical approach due to the scarcity of original research in this domain. While our study provides a foundation, there exists a critical need for more extensive original investigations employing robust statistical methodologies to further elucidate and quantify the associations between RAAS, angiopoietin-2, hypertension, and AKI.
While our meta-analysis provides valuable insights, it is imperative to acknowledge its inherent limitations. Firstly, this meta-analysis included studies with diverse designs, patient populations, and clinical contexts, introducing inherent heterogeneity. Variability in AKI etiologies, severity, and patient demographics may limit the generalizability of the findings. Secondly, the analysis may be susceptible to publication bias, as studies with statistically significant results are more likely to be published. Thirdly, variability in the methods used to measure angiopoietin-2 levels across studies, including differences in assay sensitivity, specificity, and standardization, introduces a potential source of bias. Fourthly, the included studies may employ different criteria for defining AKI, such as variations in serum creatinine thresholds or urine output criteria. This heterogeneity in AKI definitions could impact the accuracy and reliability of the meta-analysis results. Finally, the study is limited because of including data from one or more small studies. However, smaller studies are more likely to present superior findings. Because this phenomenon has been thoroughly documented in other fields [49].
Conclusions
In conclusion, our meta-analysis provides robust evidence supporting the correlation between elevated angiopoietin-2 levels and the risk of AKI. The clinical implications of this association are profound, offering potential avenues for early intervention and risk stratification. However, the complexity of AKI pathophysiology demands a nuanced understanding of angiopoietin-2’s role, considering its multifaceted involvement in angiogenesis, inflammation, and endothelial function. Future research should delve into the mechanistic aspects, exploring how angiopoietin-2 interacts with key pathways in renal injury. Additionally, prospective studies are warranted to validate angiopoietin-2 as a prognostic biomarker and assess its utility in guiding therapeutic interventions.
Funding source: Fujian Provincial Department of Science and Technology
Award Identifier / Grant number: 2021Y201020028
-
Research ethics: Not applicable. The manuscript we submitted is a meta-analysis, which is known as one of the secondary research. It does not involve human participants, so it does not need the approval of the ethics body.
-
Informed consent: Not applicable.
-
Author contributions: (I) Conception and design: JC Z, ZJ H, JY H. (II) Administrative support: JC Z, ZJ H, FL Z. (III) Provision of study materials or patients: JC Z, Q L, WP H, HB Z. (IV) Collection and assembly of data: JC Z, ZJ H, Q L, WP H, HB Z. (V) Data analysis and interpretation: JC Z, ZJ H, Q L, WP H, HB Z, (VI) Manuscript writing: All authors. (VII) Final approval of manuscript: All authors.
-
Competing interests: All authors state no conflict of interest.
-
Research funding: This work was financially supported by the Fujian Provincial Department of Science and Technology (2021Y201020028).
-
Data availability: Not applicable.
-
Registration: The study protocol was registered in the International Prospective Register of Systematic Reviews with registration number CRD42023471523.
References
1. Susantitaphong, P, Cruz, DN, Cerda, J, Abulfaraj, M, Alqahtani, F, Koulouridis, I, et al.. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol: CJASN 2013;8:1482. https://doi.org/10.2215/cjn.00710113.Search in Google Scholar PubMed PubMed Central
2. Hsu, C-N, Chen, H-L, Tain, Y-L. Epidemiology and outcomes of community-acquired and hospital-acquired acute kidney injury in children and adolescents. Pediatr Res 2018;83:622–9. https://doi.org/10.1038/pr.2017.262.Search in Google Scholar PubMed
3. Clermont, G, Acker, CG, Angus, DC, Sirio, CA, Pinsky, MR, Johnson, JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 2002;62:986–96. https://doi.org/10.1046/j.1523-1755.2002.00509.x.Search in Google Scholar PubMed
4. Chawla, LS, Eggers, PW, Star, RA, Kimmel, PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 2014;371:58–66. https://doi.org/10.1056/nejmra1214243.Search in Google Scholar PubMed PubMed Central
5. Chertow, GM, Burdick, E, Honour, M, Bonventre, JV, Bates, DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. https://doi.org/10.1681/asn.2004090740.Search in Google Scholar
6. Hoste, EA, Kellum, JA, Selby, NM, Zarbock, A, Palevsky, PM, Bagshaw, SM, et al.. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018;14:607–25. https://doi.org/10.1038/s41581-018-0052-0.Search in Google Scholar PubMed
7. El-Achkar, TM, Dagher, PC. Tubular cross talk in acute kidney injury: a story of sense and sensibility. Am J Physiol Ren Physiol 2015;308:F1317–23. https://doi.org/10.1152/ajprenal.00030.2015.Search in Google Scholar PubMed PubMed Central
8. Gomez, H, Ince, C, De Backer, D, Pickkers, P, Payen, D, Hotchkiss, J, et al.. A unified theory of sepsis-induced acute kidney injury: inflammation, microcirculatory dysfunction, bioenergetics and the tubular cell adaptation to injury. Shock (Augusta, Ga.) 2014;41:3. https://doi.org/10.1097/shk.0000000000000052.Search in Google Scholar PubMed PubMed Central
9. Fiedler, U, Augustin, HG. Angiopoietins: a link between angiogenesis and inflammation. Trends Immunol 2006;27:552–8. https://doi.org/10.1016/j.it.2006.10.004.Search in Google Scholar PubMed
10. Maisonpierre, PC, Suri, C, Jones, PF, Bartunkova, S, Wiegand, SJ, Radziejewski, C, et al.. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997;277:55–60. https://doi.org/10.1126/science.277.5322.55.Search in Google Scholar PubMed
11. Roviezzo, F, Tsigkos, S, Kotanidou, A, Bucci, M, Brancaleone, V, Cirino, G, et al.. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Therapeut 2005;314:738–44. https://doi.org/10.1124/jpet.105.086553.Search in Google Scholar PubMed
12. Peerapornratana, S, Manrique-Caballero, CL, Gómez, H, Kellum, JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int 2019;96:1083–99. https://doi.org/10.1016/j.kint.2019.05.026.Search in Google Scholar PubMed PubMed Central
13. Fiedler, U, Reiss, Y, Scharpfenecker, M, Grunow, V, Koidl, S, Thurston, G, et al.. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat Med 2006;12:235–9. https://doi.org/10.1038/nm1351.Search in Google Scholar PubMed
14. Robinson-Cohen, C, Katz, R, Price, BL, Harju-Baker, S, Mikacenic, C, Himmelfarb, J, et al.. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit Care 2016;20:1–8. https://doi.org/10.1186/s13054-016-1385-3.Search in Google Scholar PubMed PubMed Central
15. Allegretti, AS, Parada, XV, Ortiz, GA, Long, J, Krinsky, S, Zhao, S, et al.. Serum angiopoietin-2 predicts mortality and kidney outcomes in decompensated cirrhosis. Hepatology 2019;69:729–41. https://doi.org/10.1002/hep.30230.Search in Google Scholar PubMed PubMed Central
16. Liu, K-L, Lee, K-T, Chang, C-H, Chen, Y-C, Lin, S-M, Chu, P-H. Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit Care 2014;18:1–8. https://doi.org/10.1186/cc13876.Search in Google Scholar PubMed PubMed Central
17. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021;88:105906. https://doi.org/10.1016/j.ijsu.2021.105906.Search in Google Scholar PubMed
18. Kellum, JA, Lameire, N, Aspelin, P, Barsoum, RS, Burdmann, EA, Goldstein, SL, et al.. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138.Search in Google Scholar
19. Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. https://doi.org/10.1007/s10654-010-9491-z.Search in Google Scholar PubMed
20. Slim, K, Nini, E, Forestier, D, Kwiatkowski, F, Panis, Y, Chipponi, J. Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg 2003;73:712–16. https://doi.org/10.1046/j.1445-2197.2003.02748.x.Search in Google Scholar PubMed
21. Higgins, JP, Thompson, SG, Deeks, JJ, Altman, DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. https://doi.org/10.1136/bmj.327.7414.557.Search in Google Scholar PubMed PubMed Central
22. Yu, W-K, McNeil, JB, Wickersham, NE, Shaver, CM, Bastarache, JA, Ware, LB. Angiopoietin-2 outperforms other endothelial biomarkers associated with severe acute kidney injury in patients with severe sepsis and respiratory failure. Crit Care 2021;25:1–12. https://doi.org/10.1186/s13054-021-03474-z.Search in Google Scholar PubMed PubMed Central
23. Wollborn, J, Zhang, Z, Gaa, J, Gentner, M, Hausmann, C, Saenger, F, et al.. Angiopoietin-2 is associated with capillary leak and predicts complications after cardiac surgery. Ann Intensive Care 2023;13:70. https://doi.org/10.1186/s13613-023-01165-2.Search in Google Scholar PubMed PubMed Central
24. Sporek, M, Dumnicka, P, Gala-Bladzinska, A, Ceranowicz, P, Warzecha, Z, Dembinski, A, et al.. Angiopoietin-2 is an early indicator of acute pancreatic-renal syndrome in patients with acute pancreatitis. Mediat Inflamm 2016;2016. https://doi.org/10.1155/2016/5780903.Search in Google Scholar PubMed PubMed Central
25. Mansour, SG, Bhatraju, PK, Coca, SG, Obeid, W, Wilson, FP, Stanaway, IB, et al.. Angiopoietins as prognostic markers for future kidney disease and heart failure events after acute kidney injury. J Am Soc Nephrol 2022;33:613–27. https://doi.org/10.1681/asn.2021060757.Search in Google Scholar
26. Lin, S-M, Chang, C-H, Lin, T-Y, Huang, AC-C, Lin, C-H, Chen, Y-C, et al.. Plasma thrombomodulin levels are associated with acute kidney injury in patients with acute heart failure. Ann Med 2022;54:3168–75. https://doi.org/10.1080/07853890.2022.2142660.Search in Google Scholar PubMed PubMed Central
27. Jongman, RM, van Klarenbosch, J, Molema, G, Zijlstra, JG, de Vries, AJ, van Meurs, M. Angiopoietin/Tie2 dysbalance is associated with acute kidney injury after cardiac surgery assisted by cardiopulmonary bypass. PLoS One 2015;10:e0136205. https://doi.org/10.1371/journal.pone.0136205.Search in Google Scholar PubMed PubMed Central
28. Inkinen, N, Pettilä, V, Lakkisto, P, Kuitunen, A, Jukarainen, S, Bendel, S, et al.. Association of endothelial and glycocalyx injury biomarkers with fluid administration, development of acute kidney injury, and 90-day mortality: data from the FINNAKI observational study. Ann Intensive Care 2019;9:1–11. https://doi.org/10.1186/s13613-019-0575-y.Search in Google Scholar PubMed PubMed Central
29. Conroy, AL, Hawkes, MT, Leligdowicz, A, Mufumba, I, Starr, MC, Zhong, K, et al.. Blackwater fever and acute kidney injury in children hospitalized with an acute febrile illness: pathophysiology and prognostic significance. BMC Med 2022;20:1–11. https://doi.org/10.1186/s12916-022-02410-4.Search in Google Scholar PubMed PubMed Central
30. Barber, BE, Grigg, MJ, Piera, KA, William, T, Cooper, DJ, Plewes, K, et al.. Intravascular haemolysis in severe Plasmodium knowlesi malaria: association with endothelial activation, microvascular dysfunction, and acute kidney injury. Emerg Microb Infect 2018;7:1–10. https://doi.org/10.1038/s41426-018-0105-2.Search in Google Scholar PubMed PubMed Central
31. Atreya, MR, Cvijanovich, NZ, Fitzgerald, JC, Weiss, SL, Bigham, MT, Jain, PN, et al.. Prognostic and predictive value of endothelial dysfunction biomarkers in sepsis-associated acute kidney injury: risk-stratified analysis from a prospective observational cohort of pediatric septic shock. Crit Care 2023;27:260. https://doi.org/10.1186/s13054-023-04554-y.Search in Google Scholar PubMed PubMed Central
32. Kwong, YD, Mehta, KM, Miaskowski, C, Zhuo, H, Yee, K, Jauregui, A, et al.. Using best subset regression to identify clinical characteristics and biomarkers associated with sepsis-associated acute kidney injury. Am J Physiol Ren Physiol 2020;319:F979–87. https://doi.org/10.1152/ajprenal.00281.2020.Search in Google Scholar PubMed PubMed Central
33. Ouma, BJ, Ssenkusu, JM, Shabani, E, Datta, D, Opoka, RO, Idro, R, et al.. Endothelial activation, acute kidney injury, and cognitive impairment in pediatric severe malaria. Crit Care Med 2020;48:e734. https://doi.org/10.1097/ccm.0000000000004469.Search in Google Scholar PubMed PubMed Central
34. Fisher, J, Douglas, JJ, Linder, A, Boyd, JH, Walley, KR, Russell, JA. Elevated plasma angiopoietin-2 levels are associated with fluid overload, organ dysfunction, and mortality in human septic shock. Crit Care Med 2016;44:2018–27. https://doi.org/10.1097/ccm.0000000000001853.Search in Google Scholar
35. Pöss, J, Fuernau, G, Denks, D, Desch, S, Eitel, I, De Waha, S, et al.. Angiopoietin-2 in acute myocardial infarction complicated by cardiogenic shock—a biomarker substudy of the IABP-SHOCK II-Trial. Eur J Heart Fail 2015;17:1152–60. https://doi.org/10.1002/ejhf.342.Search in Google Scholar PubMed
36. Kümpers, P, Hafer, C, David, S, Hecker, H, Lukasz, A, Fliser, D, et al.. Angiopoietin-2 in patients requiring renal replacement therapy in the ICU: relation to acute kidney injury, multiple organ dysfunction syndrome and outcome. Intensive Care Med 2010;36:462–70. https://doi.org/10.1007/s00134-009-1726-7.Search in Google Scholar PubMed
37. Basile, DP, Bonventre, JV, Mehta, R, Nangaku, M, Unwin, R, Rosner, MH, et al.. Progression after AKI: understanding maladaptive repair processes to predict and identify therapeutic treatments. J Am Soc Nephrol: JASN 2016;27:687. https://doi.org/10.1681/asn.2015030309.Search in Google Scholar
38. Rabelink, TJ, De Zeeuw, D. The glycocalyx – linking albuminuria with renal and cardiovascular disease. Nat Rev Nephrol 2015;11:667–76. https://doi.org/10.1038/nrneph.2015.162.Search in Google Scholar PubMed
39. Isidori, A, Venneri, M, Fiore, D. Angiopoietin-1 and Angiopoietin-2 in metabolic disorders: therapeutic strategies to restore the highs and lows of angiogenesis in diabetes. J Endocrinol Invest 2016;39:1235–46. https://doi.org/10.1007/s40618-016-0502-0.Search in Google Scholar PubMed
40. Parikh, SM. Dysregulation of the angiopoietin–Tie-2 axis in sepsis and ARDS. Virulence 2013;4:517–24. https://doi.org/10.4161/viru.24906.Search in Google Scholar PubMed PubMed Central
41. Kümpers, P, David, S, Haubitz, M, Hellpap, J, Horn, R, Bröcker, V, et al.. The Tie2 receptor antagonist angiopoietin 2 facilitates vascular inflammation in systemic lupus erythematosus. Ann Rheum Dis 2009;68:1638–43. https://doi.org/10.1136/ard.2008.094664.Search in Google Scholar PubMed
42. Kümpers, P, Hellpap, J, David, S, Horn, R, Leitolf, H, Haller, H, et al.. Circulating angiopoietin-2 is a marker and potential mediator of endothelial cell detachment in ANCA-associated vasculitis with renal involvement. Nephrol Dial Transplant 2009;24:1845–50. https://doi.org/10.1093/ndt/gfn755.Search in Google Scholar PubMed
43. Akwii, RG, Sajib, MS, Zahra, FT, Mikelis, CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells 2019;8:471. https://doi.org/10.3390/cells8050471.Search in Google Scholar PubMed PubMed Central
44. Peters, E, Antonelli, M, Wittebole, X, Nanchal, R, François, B, Sakr, Y, et al.. A worldwide multicentre evaluation of the influence of deterioration or improvement of acute kidney injury on clinical outcome in critically ill patients with and without sepsis at ICU admission: results from The Intensive Care Over Nations audit. Crit Care 2018;22:1–11. https://doi.org/10.1186/s13054-018-2112-z.Search in Google Scholar PubMed PubMed Central
45. Basile, DP, Anderson, MD, Sutton, TA. Pathophysiology of acute kidney injury. Compr Physiol 2012;2:1303. https://doi.org/10.1002/cphy.c110041.Search in Google Scholar PubMed PubMed Central
46. Andreoli, SP. Acute kidney injury in children. Pediatr Nephrol 2009;24:253–63. https://doi.org/10.1007/s00467-008-1074-9.Search in Google Scholar PubMed PubMed Central
47. Mills, KT, Stefanescu, A, He, J. The global epidemiology of hypertension. Nat Rev Nephrol 2020;16:223–37. https://doi.org/10.1038/s41581-019-0244-2.Search in Google Scholar PubMed PubMed Central
48. Laghlam, D, Jozwiak, M, Nguyen, LS. Renin–angiotensin–aldosterone system and immunomodulation: a state-of-the-art review. Cells 2021;10:1767. https://doi.org/10.3390/cells10071767.Search in Google Scholar PubMed PubMed Central
49. Turner, RM, Bird, SM, Higgins, JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One 2013;8:e59202. https://doi.org/10.1371/journal.pone.0059202.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cclm-2024-0365).
© 2024 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- CD34+ progenitor cells meet metrology
- Reviews
- Venous blood collection systems using evacuated tubes: a systematic review focusing on safety, efficacy and economic implications of integrated vs. combined systems
- The correlation between serum angiopoietin-2 levels and acute kidney injury (AKI): a meta-analysis
- Opinion Papers
- Advancing value-based laboratory medicine
- Clostebol and sport: about controversies involving contamination vs. doping offence
- Direct-to-consumer testing as consumer initiated testing: compromises to the testing process and opportunities for quality improvement
- Perspectives
- An improved implementation of metrological traceability concepts is needed to benefit from standardization of laboratory results
- Genetics and Molecular Diagnostics
- Comparative analysis of BCR::ABL1 p210 mRNA transcript quantification and ratio to ABL1 control gene converted to the International Scale by chip digital PCR and droplet digital PCR for monitoring patients with chronic myeloid leukemia
- General Clinical Chemistry and Laboratory Medicine
- IVDCheckR – simplifying documentation for laboratory developed tests according to IVDR requirements by introducing a new digital tool
- Analytical performance specifications for trace elements in biological fluids derived from six countries federated external quality assessment schemes over 10 years
- The effects of drone transportation on routine laboratory, immunohematology, flow cytometry and molecular analyses
- Accurate non-ceruloplasmin bound copper: a new biomarker for the assessment and monitoring of Wilson disease patients using HPLC coupled to ICP-MS/MS
- Construction of platelet count-optical method reflex test rules using Micro-RBC#, Macro-RBC%, “PLT clumps?” flag, and “PLT abnormal histogram” flag on the Mindray BC-6800plus hematology analyzer in clinical practice
- Evaluation of serum NFL, T-tau, p-tau181, p-tau217, Aβ40 and Aβ42 for the diagnosis of neurodegenerative diseases
- An immuno-DOT diagnostic assay for autoimmune nodopathy
- Evaluation of biochemical algorithms to screen dysbetalipoproteinemia in ε2ε2 and rare APOE variants carriers
- Reference Values and Biological Variations
- Allowable total error in CD34 cell analysis by flow cytometry based on state of the art using Spanish EQAS data
- Clinical utility of personalized reference intervals for CEA in the early detection of oncologic disease
- Agreement of lymphocyte subsets detection permits reference intervals transference between flow cytometry systems: direct validation using established reference intervals
- Cancer Diagnostics
- Atypical cells in urine sediment: a novel biomarker for early detection of bladder cancer
- External quality assessment-based tumor marker harmonization simulation; insights in achievable harmonization for CA 15-3 and CEA
- Cardiovascular Diseases
- Evaluation of the analytical and clinical performance of a high-sensitivity troponin I point-of-care assay in the Mersey Acute Coronary Syndrome Rule Out Study (MACROS-2)
- Analytical verification of the Atellica VTLi point of care high sensitivity troponin I assay
- Infectious Diseases
- Synovial fluid D-lactate – a pathogen-specific biomarker for septic arthritis: a prospective multicenter study
- Targeted MRM-analysis of plasma proteins in frozen whole blood samples from patients with COVID-19: a retrospective study
- Letters to the Editor
- Generative artificial intelligence (AI) for reporting the performance of laboratory biomarkers: not ready for prime time
- Urgent need to adopt age-specific TSH upper reference limit for the elderly – a position statement of the Belgian thyroid club
- Sigma metric is more correlated with analytical imprecision than bias
- Utility and limitations of monitoring kidney transplants using capillary sampling
- Simple flow cytometry method using a myeloma panel that easily reveals clonal proliferation of mature B-cells
- Is sweat conductivity still a relevant screening test for cystic fibrosis? Participation over 10 years
- Hb D-Iran interference on HbA1c measurement
Articles in the same Issue
- Frontmatter
- Editorial
- CD34+ progenitor cells meet metrology
- Reviews
- Venous blood collection systems using evacuated tubes: a systematic review focusing on safety, efficacy and economic implications of integrated vs. combined systems
- The correlation between serum angiopoietin-2 levels and acute kidney injury (AKI): a meta-analysis
- Opinion Papers
- Advancing value-based laboratory medicine
- Clostebol and sport: about controversies involving contamination vs. doping offence
- Direct-to-consumer testing as consumer initiated testing: compromises to the testing process and opportunities for quality improvement
- Perspectives
- An improved implementation of metrological traceability concepts is needed to benefit from standardization of laboratory results
- Genetics and Molecular Diagnostics
- Comparative analysis of BCR::ABL1 p210 mRNA transcript quantification and ratio to ABL1 control gene converted to the International Scale by chip digital PCR and droplet digital PCR for monitoring patients with chronic myeloid leukemia
- General Clinical Chemistry and Laboratory Medicine
- IVDCheckR – simplifying documentation for laboratory developed tests according to IVDR requirements by introducing a new digital tool
- Analytical performance specifications for trace elements in biological fluids derived from six countries federated external quality assessment schemes over 10 years
- The effects of drone transportation on routine laboratory, immunohematology, flow cytometry and molecular analyses
- Accurate non-ceruloplasmin bound copper: a new biomarker for the assessment and monitoring of Wilson disease patients using HPLC coupled to ICP-MS/MS
- Construction of platelet count-optical method reflex test rules using Micro-RBC#, Macro-RBC%, “PLT clumps?” flag, and “PLT abnormal histogram” flag on the Mindray BC-6800plus hematology analyzer in clinical practice
- Evaluation of serum NFL, T-tau, p-tau181, p-tau217, Aβ40 and Aβ42 for the diagnosis of neurodegenerative diseases
- An immuno-DOT diagnostic assay for autoimmune nodopathy
- Evaluation of biochemical algorithms to screen dysbetalipoproteinemia in ε2ε2 and rare APOE variants carriers
- Reference Values and Biological Variations
- Allowable total error in CD34 cell analysis by flow cytometry based on state of the art using Spanish EQAS data
- Clinical utility of personalized reference intervals for CEA in the early detection of oncologic disease
- Agreement of lymphocyte subsets detection permits reference intervals transference between flow cytometry systems: direct validation using established reference intervals
- Cancer Diagnostics
- Atypical cells in urine sediment: a novel biomarker for early detection of bladder cancer
- External quality assessment-based tumor marker harmonization simulation; insights in achievable harmonization for CA 15-3 and CEA
- Cardiovascular Diseases
- Evaluation of the analytical and clinical performance of a high-sensitivity troponin I point-of-care assay in the Mersey Acute Coronary Syndrome Rule Out Study (MACROS-2)
- Analytical verification of the Atellica VTLi point of care high sensitivity troponin I assay
- Infectious Diseases
- Synovial fluid D-lactate – a pathogen-specific biomarker for septic arthritis: a prospective multicenter study
- Targeted MRM-analysis of plasma proteins in frozen whole blood samples from patients with COVID-19: a retrospective study
- Letters to the Editor
- Generative artificial intelligence (AI) for reporting the performance of laboratory biomarkers: not ready for prime time
- Urgent need to adopt age-specific TSH upper reference limit for the elderly – a position statement of the Belgian thyroid club
- Sigma metric is more correlated with analytical imprecision than bias
- Utility and limitations of monitoring kidney transplants using capillary sampling
- Simple flow cytometry method using a myeloma panel that easily reveals clonal proliferation of mature B-cells
- Is sweat conductivity still a relevant screening test for cystic fibrosis? Participation over 10 years
- Hb D-Iran interference on HbA1c measurement

