Abstract
Objectives
Diabetic retinopathy (DR) is associated with long-term diabetes and is a leading cause of blindness if it is not diagnosed early. The rapid growth of deep learning eases the clinicians’ DR diagnosing procedure. It automatically extracts the features and performs the grading. However, training the image toward the majority of background pixels can impact the accuracy and efficiency of grading tasks. This paper proposes an auto-thresholding algorithm that reduces the negative impact of considering the background pixels for feature extraction which highly affects the grading process.
Methods
The PSO-based thresholding algorithm for retinal segmentation is proposed in this paper, and its efficacy is evaluated against the Otsu, histogram-based sigma, and entropy algorithms. In addition, the importance of retinal segmentation is analyzed using Explainable AI (XAI) to understand how each feature impacts the model’s performance. For evaluating the accuracy of the grading, ResNet50 was employed.
Results
The experiments were conducted using the IDRiD fundus dataset. Despite the limited data, the retinal segmentation approach provides significant accuracy than the non-segmented approach, with a substantial accuracy of 83.70 % on unseen data.
Conclusions
The result shows that the proposed PSO-based approach helps automatically determine the threshold value and improves the model’s accuracy.
Introduction
According to the International Diabetes Federation (IDF), half a billion people worldwide have diabetes. According to estimations, 783.2 million adults worldwide will have diabetes in 2045 [1]. The primary cause of DR is a consequence of diabetes. DR leads to vision loss if it is not treated early. DR is primarily classified as either Non-Proliferative (NPDR or early DR) and Proliferative (PDR or late DR) [2]. The International Clinical Diabetic Retinopathy (ICDR) severity scale is a comprehensive tool for assessing the development of DR using fundus images. The inputted fundus images are classified into five categories according to the international protocol: Non-Diabetic Retinopathy (No DR (R0)), Mild Non-Proliferative Diabetic Retinopathy (NPDR (R1)), Moderate NPDR (R2), Severe NPDR (R3), and Proliferative DR (R4).
Based on severity level and referral requirements, the classification can also be divided into non-referable DR (No DR and Mild NPDR) and referable DR (Moderate NPDR, Severe NPDR, and PDR [3], 4]. Lesions evident on fundus imaging help distinguish between background DR and more advanced stages of the medical condition. The lesion feature generalization is difficult in Machine learning systems. Due to the growth in the high number of patients with DR, deep learning-based automatic screening of eye fundus images is the solution to screen a larger population of diabetes patients and it overcomes some limitations of automatic feature extraction and classification of the machine learning approach. However, the quality of the images affects the performance in the deep learning system [5]. To obtain a promising result, this study proposes Particle Swarm Optimization (PSO)-based auto thresholding algorithm for segment the retina from the background. There is limited work using retinal segmentation in DR Grading and Lesion Detection [6]. In addition, the importance of retinal segmentation is not shown in the prior work.
The primary objective of the paper is to propose PSO-based auto thresholding algorithms for retinal segmentation and to demonstrate the impact of the retinal segmentation on the performance of the DR Grading System through the empirical assessment. The related objectives are described as follows:
Applying the proposed PSO-based thresholding algorithms on fundus images to segment the retina.
Comparing the effectiveness of proposed algorithms with the state-of-the-art Otsu, histogram-based sigma thresholding, and entropy-based thresholding algorithms.
Implementing the pre-trained model ResNet50 to assess the impact of the non-segmented and retinal-segmented approaches in diabetic retinopathy grading.
The proposed work suggests PSO-based auto thresholding for retinal segmentation of fundus images. Then the retinal-segmented image is trained using transfer learning. Finally, the results are assessed to understand how the inclusion and exclusion of the segmentation impact the grading’s performance. In this paper, the PSO-based thresholding algorithm is proposed to segment the foreground without manually setting the threshold, using entropy as an objective function. The PSO with local search and Entropy values are utilized to determine the positions of thresholds automatically.
The rest of the paper is structured as follows: In Section 2, relevant work on deep learning is reviewed. The dataset collection and proposed methods are covered in Section 3. Section 4 discusses the experimental outcomes. Section 5 presents the conclusion and future work.
Related work
An overview of the many methods utilized for DR Grading is given in this section. Early studies focused on improving the image quality using a standard approach of normalization, resizing, color conversion, and image augmentation.
The study [5] demonstrates the effectiveness of preprocessing in improving the performance of deep learning models for DR classification. The study, which concentrated on image augmentation and resizing to improve image quality, suggests that future research will include more preprocessing techniques. Feng et al. [7] combined Convolutional Neural Networks (CNN) and Graph Neural Networks (GNN) to extract the features and capture the complex relationships between data points, respectively. However, these models failed to focus on improving the quality of various dataset images.
To change the different sources images into a uniform format Gao et al. [8] utilized the resizing approach using fixed dimensions, discarded the black borders by a selected threshold value, shape normalization to avoid the small notch on the edge of the image circle, normalized the image color to provide the more stable input range to the learning model, and image augmentation to overcome the limitations of data. Since the study utilized manual threshold setting to segment the retinal area. The study by Pao et al. [9] presents a Bi-channel CNN system by combining the features from both gray-level entropy images and green component entropy images to improve the detection of referable DR. To improve the image quality resizing, unsharp masking (UM), and augmentation is applied before computation without considering the additional preprocess approach.
Al-Turk et al. [10] described an approach based on feature-based grading to enhance transparency and interpretability in DR screening with the use of a simple pre-process approach of color averaging and augmentation. However, a lack of pre-processing techniques will affect the model performance and feature detection. In references [11], [12], [13], [14], conventional pre-processing techniques are utilized to improve DR detection and grading. According to the study by Zhu et al. [15], the early studies focused on the primary preprocess of i) Image enhancement to overcome inhomogeneities of contrast using Contrast-limited Adaptive Histogram Equalization (CLAHE), AHE, and Modified CLAHE, ii) Denoising to remove the noise using the mean filter, gaussian filter, and wiener filter, iii) Normalization to reduce the feature bias, and iv) Augmentation to improve data size and diversity by applying geometric and color transformation.
Butt et al. [16] described an approach based on a hybrid method to extract features from the fundus images to improve the model performance in DR detection. In reference [17], custom training data were utilized to address varying image quality and class imbalance. The author introduced the pseudo-binary classification scheme to improve prediction reliability and provide more information, and also designed energy-efficient hardware, enabling deployment on edge devices. The study [18], evaluates the efficacy of two pre-trained models, Faster R-CNN and Mask R-CNN, for identifying hemorrhages and models were tested on an image database containing expert-labeled ground truth. Al-Hazaimeh et al. [19] proposed a novel method that combines artificial intelligence and image processing to enhance the identification of DR. The method aims to address issues with specificity, sensitivity, and accuracy in current techniques and successfully detects several forms of DR, including exudates, microaneurysms, and hemorrhages. The summary of the related work using deep learning approaches is shown in Table 1.
Summary of related work using deep learning approaches.
| References | Dataset | Method | Foreground retinal segmentation | Contributions |
|---|---|---|---|---|
| [5] | EyePACS | Image resize, and data augmentation | Not performed | To eliminate the overfitting, effective pre-processing and augmentation are carried out |
| [7] | APTOS2019, Messidor-2 | Pre-process is not described | Not performed | Graph convolution network is introduced to learn the deep features of an image and its class information |
| [8] | Clinical dataset | Size, shape, and color normalization, data augmentation, and background cropping by manual threshold setting | Manual threshold setting is used | Proposed a novel dataset for clinical practice |
| [9] | Kaggle diabetic retinopathy | Data augmentation, resize, color component extraction | Not performed | The features of the gray-level entropy images and green component entropy images are combined using bi-channel CNN to enhance referable DR detection |
| [10] | Both clinical and public dataset | Color averaging, augmentation | Not described | An end-to-end feature-based DR grading and progression system is implemented |
| [11] | MESSIDOR, IDRiD | Size and color normalization, denoising, histogram equalization using CLAHE, augmentation | Not performed | Quadrant ensemble InceptionResnet V-2 framework is proposed for DR grading accuracy |
| [12] | IDRiD | Denoising, resize, augmentation, normalization | Fixed cropping is applied | A customized EfficientNet model is presented to utilize small datasets |
| [13] | DIARETDB0, DIARETDB1, Messidor, HEI-MED, ODIR | Resizing, normalization, augmentation | Not performed | The multi-classification deep learning model was proposed for diagnose different eye diseases |
| [14] | IDRiD, Messidor, DDR | Resize, augmentation | Not described | A cross-lesion attention network is proposed for DR grading |
| [16] | APTOS | Image resize, normalization | Not performed | The hybrid model of Google Net and ResNet18 is presented for binary classification and grading |
| [17] | EyePACS, DDR, APTOS | Filter out corrupted images and undersampled the majority classes | Not described | Pseudo binary classification and energy efficient DR screening hardware device is designed for deployment |
| [18] | DIARETDB1 | Methodology is not described | Not described | Detectron2 framework is utilized for providing the segmentation mask on hemorrhage |
| [19] | EyePACS | Denoised using non-linear wiener filter, OD is segmented using CHT for effective detection of exudates, the BCSPNFCM algorithm is introduced for blood vessel segmentation, and morphological operation is applied to make hemorrhage detection efficient | Not described | A new approach was introduced to increase the accuracy of DR detection by segmenting the features, and detection with the selection of lesions |
The literature studies have proposed approaches to grading diabetic retinopathy (DR) in digital fundus images, but few considered retinal segmentation as another component that can enhance image quality. The current study addresses this gap by introducing a retinal segmentation technique based on Particle Swarm Optimisation (PSO) that aims to improve image quality and, consequently, the precision and robustness of automated DR grading systems. The general pipeline of the study is shown in Figure 1.
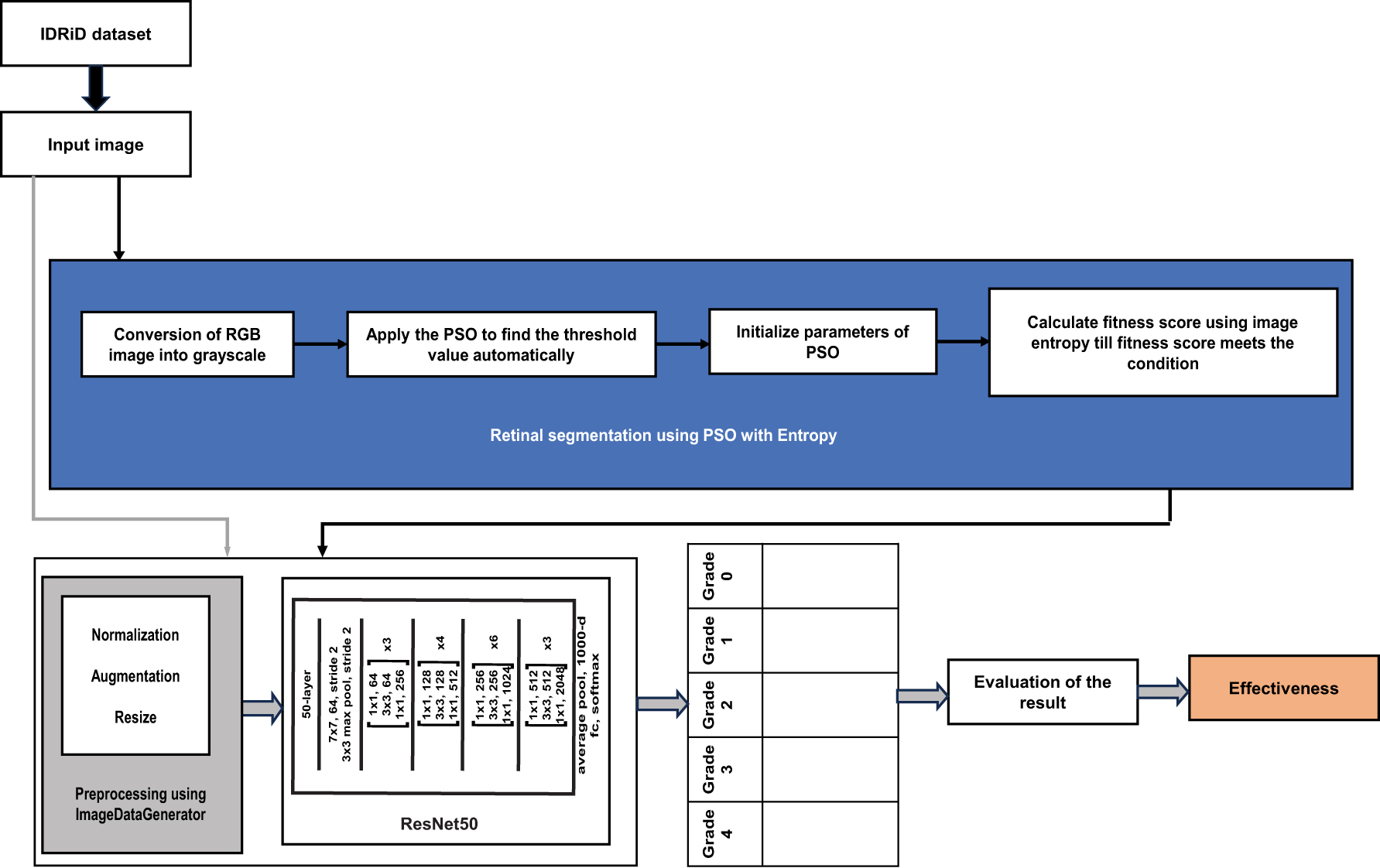
The general pipeline of the study.
Materials and proposed methodology
Dataset collection and description
The current work utilized the Indian Diabetic Retinopathy Image Dataset (IDRiD) [20]. The IDRiD Dataset is a publicly accessible benchmark dataset. The fundus images were captured by a retinal specialist using a Kowa VX-10 alpha digital fundus camera and all are centered near to the macula. The resolution of an image is 4,288 × 2,848 pixels. This dataset incorporates pixel-level annotated data and image-level grading data with binary mask ground-truth images to evaluate the performance of individual lesion segmentation techniques and grading classification.
Image pre-processing
The input data and the architecture of the networks play an important role in building an effective deep-leaning-based classification system. Pre-processing is an important step in improving the quality of the input image [21]. Improvement in preprocessing can help in eliminating artifacts [6]. This study has utilized normalization, augmentation, and resizing for preprocessing.
The image pixel values were originally in the range 0–255. These can lead to exploding or vanishing problems during the training process. Normalizing images to the range 0–1 helps to improve the image data, training stability, and performance of the model [22]. There are many normalization approaches for scaling the attribute values [23]. For this study, the rescale parameter from Keras ImageDataGenerator, a specific case of min-max normalization has used.
Image augmentation increases the size and diversity of the dataset artificially to improve the model’s generalizability. The horizontal and vertical flipping is applied on the training image to augment the image using the ImageDataGenerator. The ImageDataGenerator avoids overfitting because it will not add the transformed images to the range of original images [24]. The image resize is used in preprocessing to transform different dimensional images to a consistent scale [8]. The image resize helps to minimize the running time and required memory of the model to load and train the images [25]. In this work, the fundus image is resized to downscale the image size by passing a fixed dimension value.
Proposed PSO-based retinal segmentation
Prior studies have made significant contributions to DR classification by enhancing image quality and facilitating feature analysis using various approaches including CLAHE, Color extraction, Feature extraction, Blood vessel segmentation, and Optic Disc segmentation. The artifacts may potentially cause the model to concentrate on inappropriate information. It is illustrated in the following Figure 2a using Explainable AI SHAP values tools. SHAP helps to understand how each feature impacts the model’s prediction. SHAP gives an important value on each feature for a prediction that has been identified [26]. As emphasized in the figure, the model focuses on undesired black background information, impacting its accuracy and leading to misdiagnoses. This study suggests a retinal segmentation to segment the retinal area from the background area. This approach reduces background information to further enhance the model’s accuracy and reduces the size of the image for more efficient processing. For foreground segmentation, thresholding is one of the most popular approaches for converting an image into a binary image. The optimal threshold value setting is the primary challenge in thresholding approaches, which is determined by the user. If the user is unable to choose an ideal threshold setting, it may cause to miss certain valuable areas of an image [27].
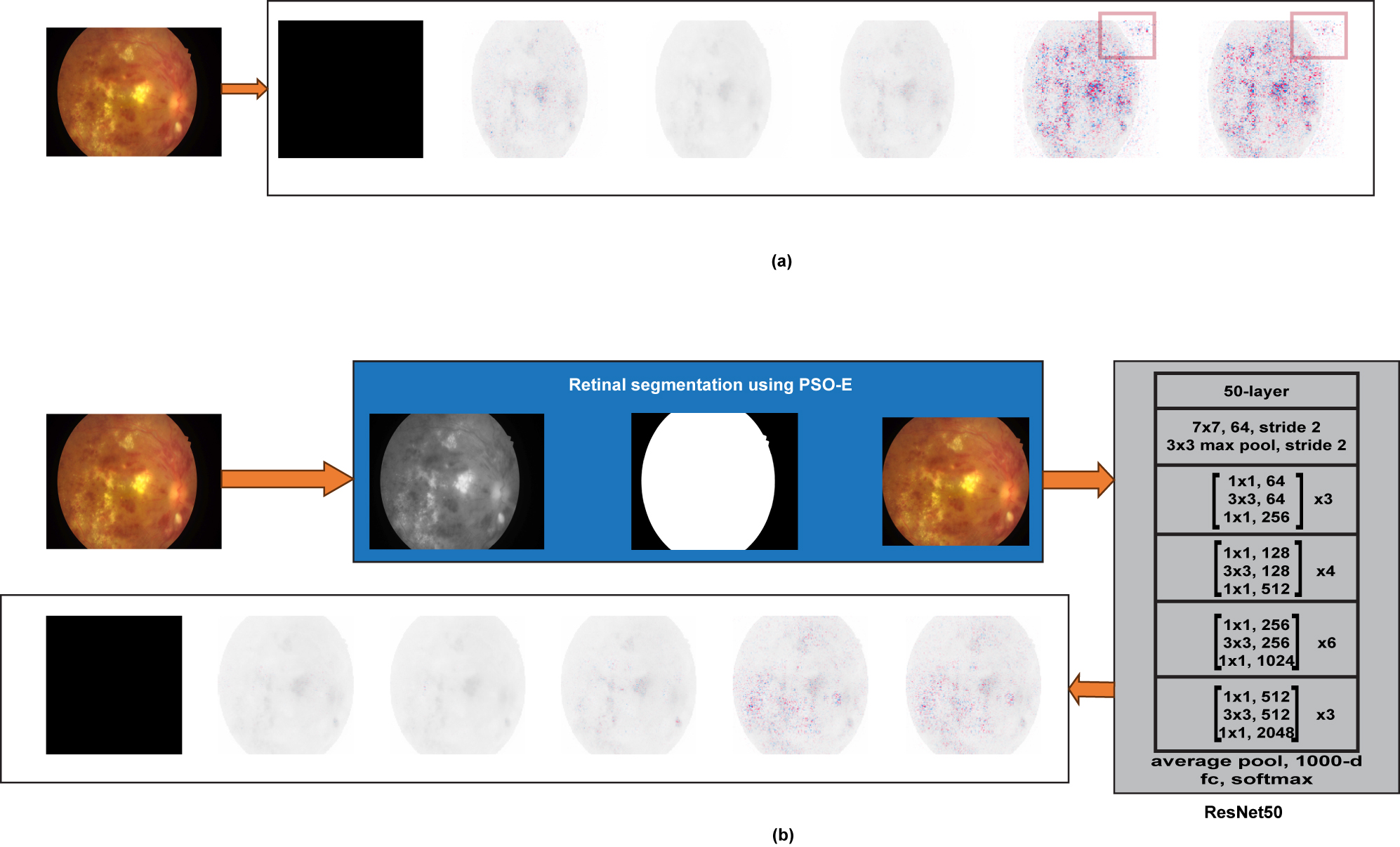
Result of SHAP interpretation on (a) non-segmented images and (b) segmented images using PSO-E.
Thresholding techniques include local and global thresholding algorithms. Global thresholding uses a single threshold to divide the image into foreground and background pixels and this study focuses on global threshold approaches. Otsu’s method is a popular state-of-the-art global thresholding method, though it may struggle with certain images since it expects that the foreground and background have similar Gaussian distributions, which is a highly complex order that real-world images are unable to satisfy [28]. Otsu may not perform well with images that have more than two peaks in the histograms.
In recent studies, bio-inspired optimization algorithms have been utilized to tackle the problems of traditional optimization algorithms [29]. The bio-inspired PSO algorithm has the potential to produce good results while addressing the limitations of Otsu’s technique, such as establishing an ideal threshold for accurate image segmentation and the images having a large intraclass variance difference and uneven illumination respectively. In 1990, Kennedy and Eberhart introduced the PSO [30]. PSO is a population and random-based approach inspired by the collective behavior of bird flocks or fish schools. The ideal strategy for the birds is to follow the one closest to the food. PSO treats each potential solution as a particle within the search space, much like a bird. Each particle has a fitness value, which is defined by a fitness function that requires optimization, as well as a velocity that regulates its movement. Particles move through the problem space by seeking the particle in the best-known position [31].
For segmenting the retinal area from the unwanted background information, the PSO algorithm is used in this study due to its ease of implementation, low parameter needs, fast convergence, and scalability [32]. The purpose of using the PSO algorithm is to determine the threshold value automatically for the binary conversion of an image to segment the retinal region from images. In order to get the best threshold value, the entropy approach is used as an objective function with the PSO. The PSO with Entropy (PSO-E)-based optimization algorithm is implemented for auto threshold value determination and the detected threshold value is applied on the image to segment the retinal region. As shown in Figure 2b, the majority of background pixels are removed using the PSO-E algorithm, and the SHAP tool is applied to interpret the result. The SHAP shows the model is focused on the appropriate information after retinal segmentation. The general steps for the PSO-E algorithm for thresholding are as follows:
Step 1: Input the grayscale image, set the number of particles to 0, maximum iterations to 20, PSO parameters: inertia weight (w) to 0.5, cognitive and social components (c1, c2) are to1.5.
Step 2: Define the fitness function based on image entropy. The entropy of the binary image is used as the fitness to obtain the threshold value.
where p j is the probability of foreground pixel value in the binary image.
Step 4: Iteration: begin;
Calculate the fitness of each particle.
Adjust the velocity and position of each particle.
where
Update personal and global best positions based on fitness values.
where
Check the convergence criteria. Stop if the global fitness reaches the desired entropy of 1.0.
Step 5: Get the output of the binarized image using the global best threshold value.
Multi-class classification using ResNet50
The non-segmented and PSO-based retinal-segmented images are inputted separately into the ResNet50 transfer learning model [33] to evaluate the segmentation and grading performance. The Residual Neural Network is a Convolutional Neural Network (CNN) that integrates residual connections to preserve network connectivity and prevent gradient vanishing by adding the normalization layers. These connections work as shortcuts, allowing information to jump to certain levels and proceed directly to the output. This technique enables the network to learn residual functions and make modest parameter changes, resulting in faster convergence and better performance. ResNet enhances overall performance by prioritizing residual functions over complex input-output mappings.
The 50-layer ResNet (Residual Neural Network) architecture extracts a feature from the input image, and then the Global Average Pooling (GAP) [34] layer reduces the high dimensional feature maps from ResNet50 into lower-dimension vectors, and finally fully connected (dense) layers perform classification for grading the fundus images. This procedure aids in customizing the underlying model’s higher-level features to the particular task at hand [35]. ResNet50 residual networks keep consistent computational complexity by doubling filters when halving feature map size and using the same filter count based on the output feature map size with bottleneck design to reduce the number of parameters and matrix multiplications. Grading results are analyzed to provide insights into the significance of retinal segmentation.
Experimental results
Experimental dataset distribution
The experimental investigation of the proposed PSO-based retinal segmentation was executed on the images acquired from the IDRiD dataset. In total 516 images, for thresholding analysis, 516 images were employed, and for assessing the grading accuracy 330 images were allocated for training, 83 images for validation, and 103 images for testing. All computation, training, testing, and validations were performed in Google Colab environments using GPU runtime, equipped with an Intel i5 -1035G1 CPU @ 1.00 GHz processor, and 8 GB of physical memory hardware.
Performance metrics
To measure the performance of the retinal segmentation, kappa score, mean accuracy, multi-class confusion matrix, and SHAP XAI, are employed. In a multi-class confusion matrix rows reflect actual classes, and columns indicate expected classes. The kappa score is determined from the multi-class confusion matrix. It is more reliable than basic accuracy since it shows how much better the segmentation results are than probability. The segmentation model’s mean accuracy provides an overview of how well it works across all classes, rather than concentrating on just one. The multi-class confusion matrix makes it possible to analyse errors for each class in greater detail and shows how frequently the model confuses one class with another. SHAP can explain which features are most crucial for predicting particular outcomes. The model’s accuracy, efficiency, and reliability in retinal segmentation are evaluated using these metrics.
Experimental analysis of thresholding
Figure 3 presents the visualization results obtained from the state-of-the-art Otsu thresholding, histogram-based sigma thresholding, Entropy thresholding, and proposed PSO-E thresholding techniques.
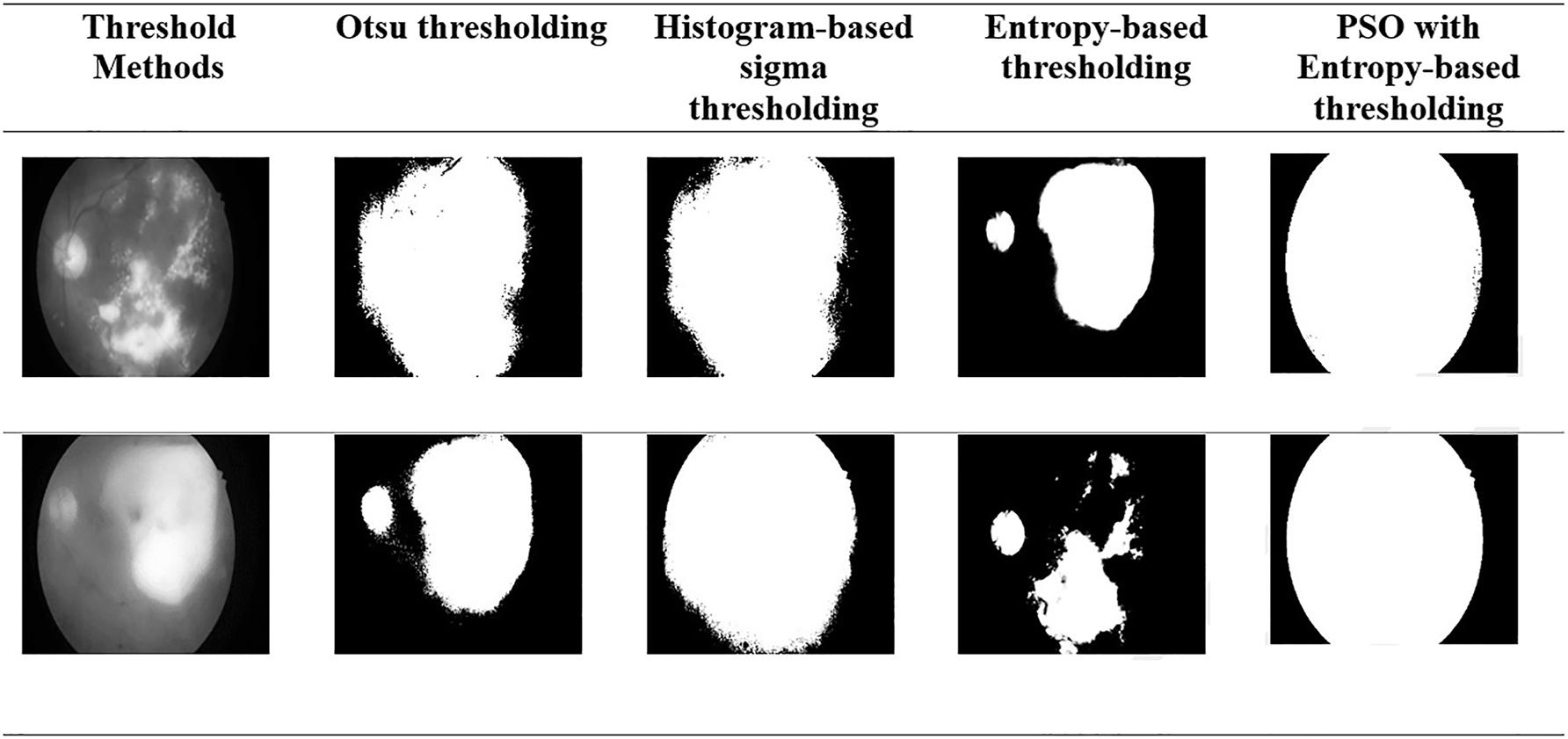
Comparative performance of the thresholding with various methods.
The various thresholding algorithms such as Otsu’s algorithm, the histogram-based sigma approach, the entropy-based thresholding algorithm, and the PSO optimization are effective for automatic thresholding methods. However, Otsu, histogram-based sigma, and entropy methods lead to incorrect segmentation due to large intraclass variance differences and uneven illumination in images. In contrast, the proposed PSO algorithm, which uses entropy-based optimization, works well with both unimodal and multimodal histogram distributions. It also eliminates manual threshold setting, resulting in a more robust and efficient solution.
Experimental analysis of grading
The effectiveness of retinal segmentation is assessed using ResNet50 architecture. The various threshold techniques are applied to segment the retinal area. Once the retinal area was segmented, the segmented images were trained and validated using the classification model. The performance of different retinal segmentation methods was evaluated using training accuracy, validation accuracy and the mean kappa score, as shown in Table 2.
Comparative result on retinal segmentation.
| Methods | Training | Validation | Mean kappa score |
|---|---|---|---|
| Retinal-segmented images using Otsu | 0.973 | 0.721 | 0.920 |
| Retinal-segmented images using sigma-based | 0.923 | 0.722 | 0.882 |
| Retinal-segmented images using entropy-based | 0.639 | 0.502 | 0.611 |
| Retinal-segmented images using PSO with entropy | 0.976 | 0.787 | 0.939 |
Based on the retinal segmentation table results, Otsu’s method, while effective in training, suffered from overfitting, the histogram-based approach had lower overall performance but a better balance between training and validation results, and the Entropy-based approach performed worse than Otsu or sigma-based approach, however, PSO-E-based thresholding indicates the highest agreement and showing good generalization on unseen data.
Ablation studies
To separately evaluate the impact of retinal segmentation, the deep learning model is trained using non-segmented images and PSO-based retinal-segmented images. Then the result is obtained separately using the ResNet-50 baseline model. The confusion matrix is a table that compares the grading performance of the two approaches, shown in Figure 4.
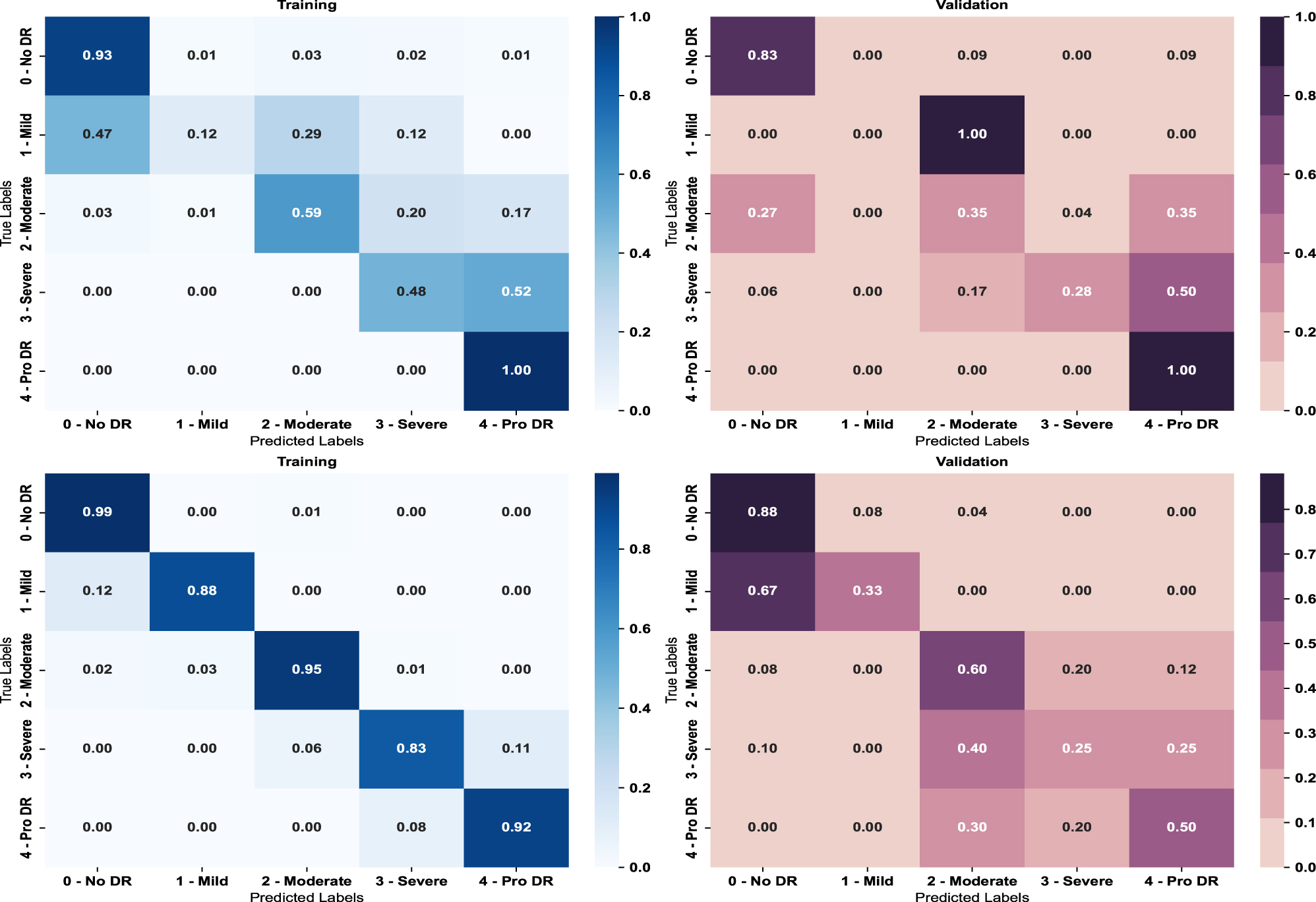
Result of multi-class confusion matrix on (top) non-segmented images (bottom) segmented images.
The figure shows retinal segmentation can significantly improve the accuracy of grading in deep learning. The findings suggest that retinal segmentation can help a deep learning model focus on the most important regions of an image, decreasing complexity and distractions. This can help the model learn crucial features and patterns for the task at hand. Table 3 (top) shows the class-wise performance.
Class-wise and overall accuracy results.
| Class-wise accuracy results | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Approaches | Class 0 | Class 1 | Class 2 | Class 3 | Class 4 | Kappa score | Mean kappa score | ||||||
| Training | Validation | Training | Validation | Training | Validation | Training | Validation | Training | Validation | Training | Validation | ||
| Non-segmented images | 0.89 | 0.96 | 0.80 | 0.79 | 0.83 | 0.61 | 0.85 | 0.74 | 0.91 | 0.85 | 93.6 | 69.5 | 88.6 |
| Retinal-segmented images | 1.00 | 0.95 | 0.99 | 0.86 | 0.98 | 0.66 | 0.98 | 0.72 | 0.98 | 0.86 | 97.6 | 78.7 | 93.9 |
| Overall accuracy | |||
|---|---|---|---|
| Approaches | Training | Validation | Testing |
| Non-segmented images | 0.879 | 0.817 | 0.817 |
| Retinal-segmented images | 0.975 | 0.831 | 0.837 |
-
Bolded values represent the highest values in each column, highlighting the best-performing metrics.
From the above table, training with segmentation images yields superior results than non-segmentation images across all classes. Non-segmented images perform slightly better in validation in certain Class 0 and Class 3 cases. However, Segmentation approach provides a significant speed improvement for specific classes, such as Class 1 and Class 2. It is evident that performance is generally improved by retinal-segmented images. For training, validation, and overall performance of a task, the mean kappa score, a measure of agreement between expected and actual outcomes is assessed.
The model with the proposed retinal segmentation image accuracy is compared with the non-segmented image approach. The model trained using the proposed segmentation images gave an accuracy of 93.9 % using Kappa’s score. The model with the non-segmented images obtained an accuracy of 88.6 %. Table 3 (bottom) indicates that the retinal-segmented approach performs much better than the non-segmented approach.
Compared to non-segmented images, the model performs much better when retinal-segmented images are used, especially when testing and validation stages are used to reflect real-world events. In order to improve generalization, the segmentation procedure aids the model in concentrating on important aspects inside the images. Figure 5 illustrates how test predictions increased for all classes when employing retinal-segmented images; nevertheless, the non-segmented approach was unable to meet this accuracy, especially for Grade 1, which is indicated by a red bounding box.
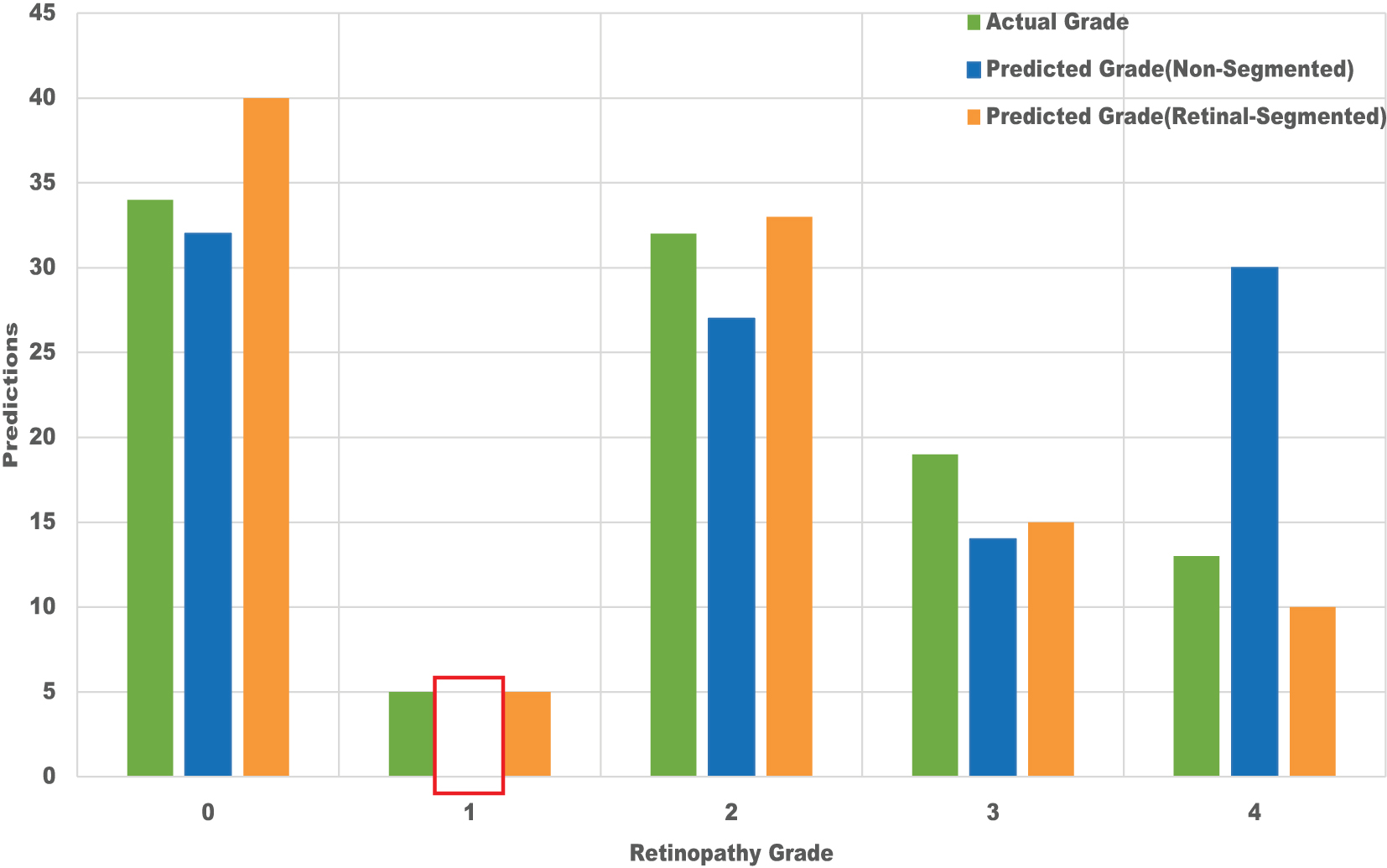
Prediction result on the test dataset.
Finally, the experimental results show that the majority of background pixels affect the DR grading. Therefore, retinal segmentation using a PSO-based thresholding approach is proposed to segment the foreground retinal region from the background. Hence, the retinal segmented images not only reduce the complexity but also obtain a modest improvement in the performance of the model and grading accuracy.
Conclusions
Recently, deep learning has been utilized in medical images. It can uncover complex patterns and segment medical images efficiently. It saves time and cost on feature engineering in medical categorization to identify medical pictures. It has the greatest accuracy among all image prediction algorithms. However, early processing is important to achieve the best result in deep learning. In this paper, PSO-based auto thresholding proposed to segment the retina from the background, and the impact of the retinal segmentation in DR grading is analyzed. The proposed PSO-thresholding approach does not require any threshold value setting. The algorithm automatically determines the threshold value to segment the foreground retinal area and background unwanted information. The entropy method is used with PSO to determine the threshold value for segmentation. To evaluate the ability of the proposed PSO segmentation, the image using PSO thresholding and state-of-the-art thresholding is inputted and tested using the ResNet50 architecture. The confusion matrix, kappa’s scores, and various performance metrics are considered for evaluation. The proposed approach improves the model’s accuracy. Both approaches present a promising result, with the retinal segmentation approach yielding a higher precision than the non-segmented approach. To obtain better outcomes, future research work will concentrate on the development of the detection of optic disc and multi-level lesions of the DR signs with the largest number of images.
Acknowledgments
This work has been supported by the Centre for Machine Learning and Intelligence (CMLI), ISO Certified (ISO/IEC 20000-1:2018) funded by the Department of Science and Technology (DST-CURIE).
-
Research ethics: As we used a public dataset, not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors has accepted responsibility for the entire content of this manuscript and approved its submission.
-
Conflict of interests: The authors state no conflict of interest.
-
Research funding: This research study did not receive external funding.
-
Data availability: Publicly available datasets have been used in this study.
-
Declaration of AI and AI-assisted technologies in the writing process: During the preparation of this work, the authors utilized QuillBot to paraphrase and Grammarly to grammar. After using these tools, the authors reviewed and edited the content as needed and took full responsibility for the final publication.
References
1. Sun, H, Saeedi, P, Karuranga, S, Pinkepank, M, Ogurtsova, K, Duncan, BB, et al.. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. https://doi.org/10.1016/j.diabres.2021.109119.Search in Google Scholar PubMed PubMed Central
2. Raudonis, V, Kairys, A, Verkauskiene, R, Sokolovska, J, Petrovski, G, Balciuniene, VJ, et al.. Automatic detection of microaneurysms in fundus images using an ensemble-based segmentation method. Sensors 2023;23:3431. https://doi.org/10.3390/s23073431.Search in Google Scholar PubMed PubMed Central
3. Raman, R, Ramasamy, K, Rajalakshmi, R, Sivaprasad, S, Natarajan, S. Diabetic retinopathy screening guidelines in India: All India Ophthalmological Society diabetic retinopathy task force and Vitreoretinal Society of India consensus statement. Indian J Ophthalmol 2021;69:678–88. https://doi.org/10.4103/ijo.ijo_667_20.Search in Google Scholar
4. UK Government. Your guide to diabetic retinopathy [Internet]; 2022. Available from: https://www.gov.uk/government/publications/diabetic-retinopathy-description-in-brief/your-guide-to-diabetic-retinopathy.Search in Google Scholar
5. Incir, R, Bozkurt, F. A study on effective data preprocessing and augmentation method in diabetic retinopathy classification using pre-trained deep learning approaches. Multimed Tools Appl 2024;83:12185–208. https://doi.org/10.1007/s11042-023-15754-7.Search in Google Scholar
6. Jabbar, A, Liaqat, HB, Akram, A, Sana, MU, Azpíroz, ID, Diez, IDLT, et al.. A lesion-based diabetic retinopathy detection through hybrid deep learning model. IEEE Access 2024;12:40019–36. https://doi.org/10.1109/access.2024.3373467.Search in Google Scholar
7. Feng, M, Wang, J, Wen, K, Sun, J. Grading of diabetic retinopathy images based on graph neural network. IEEE Access 2023;11:98391–401. https://doi.org/10.1109/access.2023.3312709.Search in Google Scholar
8. Gao, Z, Li, J, Guo, J, Chen, Y, Yi, Z, Zhong, J. Diagnosis of diabetic retinopathy using deep neural networks. IEEE Access 2018;7:3360–70. https://doi.org/10.1109/access.2018.2888639.Search in Google Scholar
9. Pao, SI, Lin, HZ, Chien, KH, Tai, MC, Chen, JT, Lin, GM, et al.. Detection of diabetic retinopathy using bichannel convolutional neural network. J Ophthalmol 2020;2020:9139713. https://doi.org/10.1155/2020/9139713.Search in Google Scholar PubMed PubMed Central
10. Al-Turk, L, Wawrzynski, J, Wang, S, Krause, P, Saleh, GM, Alsawadi, H, et al.. Automated feature-based grading and progression analysis of diabetic retinopathy. Eye 2022;36:524–32. https://doi.org/10.1038/s41433-021-01415-2.Search in Google Scholar PubMed PubMed Central
11. Bhardwaj, C, Jain, S, Sood, M. Deep learning–based diabetic retinopathy severity grading system employing quadrant ensemble model. J Digit Imaging 2021;34:440–57. https://doi.org/10.1007/s10278-021-00418-5.Search in Google Scholar PubMed PubMed Central
12. Abdelmaksoud, E, Barakat, S, Elmogy, M. Diabetic retinopathy grading system based on transfer learning. Int J Adv Comput Res 2021;11:1–12. https://doi.org/10.19101/IJACR.2020.1048117.Search in Google Scholar
13. Albelaihi, A, Ibrahim, DM. DeepDiabetic: an identification system of diabetic eye diseases using deep neural networks. IEEE Access 2024;12:10769–89. https://doi.org/10.1109/access.2024.3354854.Search in Google Scholar
14. Liu, X, Chi, W. A cross-lesion attention network for accurate diabetic retinopathy grading with fundus images. IEEE Trans Instrum Meas 2023;72:1–12. https://doi.org/10.1109/tim.2023.3322497.Search in Google Scholar
15. Zhu, S, Xiong, C, Zhong, Q, Yao, Y. Diabetic retinopathy classification with deep learning via fundus images: a short survey. IEEE Access 2024;12:20540–58. https://doi.org/10.1109/access.2024.3361944.Search in Google Scholar
16. Butt, MM, Iskandar, DNFA, Abdelhamid, SE, Latif, G, Alghazo, R. Diabetic retinopathy detection from fundus images of the eye using hybrid deep learning features. Diagnostics 2022;12:1607. https://doi.org/10.3390/diagnostics12071607.Search in Google Scholar PubMed PubMed Central
17. Diware, S, Chilakala, K, Joshi, RV, Hamdioui, S, Bishnoi, R. Reliable and energy-efficient diabetic retinopathy screening using memristor-based neural networks. IEEE Access 2024;12:47469–82. https://doi.org/10.1109/access.2024.3383014.Search in Google Scholar
18. Chincholi, F, Koestler, H. Detectron2 for lesion detection in diabetic retinopathy. Algorithms 2023;16:147. https://doi.org/10.3390/a16030147.Search in Google Scholar
19. Al-Hazaimeh, OM, Abu-Ein, AA, Tahat, NM, Al-Smadi, MA, Al-Nawashi, MM. Combining artificial intelligence and image processing for diagnosing diabetic retinopathy in retinal fundus images. Int J Online Biomed Eng 2022;18:131–51. https://doi.org/10.3991/ijoe.v18i13.33985.Search in Google Scholar
20. Porwal, P, Pachade, S, Kamble, R, Kokare, M, Deshmukh, G, Sahasrabuddhe, V, et al.. Indian diabetic retinopathy image dataset (IDRiD): a database for diabetic retinopathy screening research. Data 2018;3:25. https://doi.org/10.3390/data3030025.Search in Google Scholar
21. Asia, AO, Zhu, CZ, Althubiti, SA, Al-Alimi, D, Xiao, YL, Ouyang, PB, et al.. Detection of diabetic retinopathy in retinal fundus images using CNN classification models. Electronics (Basel) 2022;11:2740. https://doi.org/10.3390/electronics11172740.Search in Google Scholar
22. Gavali, P, Banu, JS. Deep convolutional neural network for image classification on CUDA platform. In: Deep learning and parallel computing environment for bioengineering systems. Cambridge, MA: Academia Press; 2019:99–122 pp.10.1016/B978-0-12-816718-2.00013-0Search in Google Scholar
23. Patro, SG, Sahu, KK. Normalization: a preprocessing stage. Int Adv Res J Sci Eng Technol 2015;2:20–2. https://doi.org/10.17148/IARJSET.2015.2305.Search in Google Scholar
24. Sarki, R, Ahmed, K, Wang, H, Zhang, Y, Ma, J, Wang, K. Image preprocessing in classification and identification of diabetic eye diseases. Data Sci Eng 2021;6:455–71. https://doi.org/10.1007/s41019-021-00167-z.Search in Google Scholar PubMed PubMed Central
25. Abd Aziz, N, Sulaiman, MAH, Mohd Yassin, AI, Megat Ali, MSA, Abu Hassan, H, M Shafie, S, et al.. Preprocessing of fundus images for detection of diabetic retinopathy. J Electric Electron Syst Res 2021;19:149–56. https://doi.org/10.24191/jeesr.v19i1.020.Search in Google Scholar
26. Lundberg, SM, Lee, SI. A unified approach to interpreting model predictions. Adv Neural Inf Process Syst 2017;30:4768–77.Search in Google Scholar
27. Rahkar Farshi, T, Demirci, R. Multilevel image thresholding with multimodal optimization. Multimed Tools Appl 2021;80:15273–89. https://doi.org/10.1007/s11042-020-10432-4.Search in Google Scholar
28. Zhou, D, Xia, Z. An improved Otsu threshold segmentation algorithm. J China Univ Metrol 2016;27:319–23.Search in Google Scholar
29. Darwish, A. Bio-inspired computing: algorithms review, deep analysis, and the scope of applications. Future Comput Inform J 2018;3:231–46. https://doi.org/10.1016/j.fcij.2018.06.001.Search in Google Scholar
30. Kennedy, J, Eberhart, R. Particle swarm optimization. Proceedings of ICNN’95. In: International Conference on Neural Networks. Perth, WA, Australia: IEEE; 1995, 4:1942–8 pp.10.1109/ICNN.1995.488968Search in Google Scholar
31. Bhandari, S, Rambola, R, Kumari, R. Swarm intelligence and evolutionary algorithms for diabetic retinopathy detection. In: Swarm intelligence and evolutionary algorithms in healthcare and drug development. New York: Chapman and Hall/CRC; 2019:65–92 pp.10.1201/9780429289675-4Search in Google Scholar
32. Shanthi, MB, Meenakshi, DK, Ramesh, PK. Particle swarm optimization. In: Advances in swarm intelligence for optimizing problems in computer science. New York: Chapman and Hall/CRC; 2018:115–44 pp.10.1201/9780429445927-5Search in Google Scholar
33. Bichri, H, Chergui, A, Hain, M. Image classification with transfer learning using a custom dataset: comparative study. Procedia Comput Sci 2023;220:48–54. https://doi.org/10.1016/j.procs.2023.03.009.Search in Google Scholar
34. Lin, M, Chen, Q, Yan, S. Network in Network. In: 2nd International Conference on Learning Representations, ICLR 2014, Banff, AB, 14–16 April; 2014. http://arxiv.org/abs/1312.4400 Search in Google Scholar
35. TensorFlow. Transfer learning and fine-tuning; 2024. Available from: https://www.tensorflow.org/tutorials/images/transfer_learning.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Research Articles
- An exploratory study of pilot EEG features during the climb and descent phases of flight
- Gesture recognition from surface electromyography signals based on the SE-DenseNet network
- Empirical analysis on retinal segmentation using PSO-based thresholding in diabetic retinopathy grading
- Prediction of remaining surgery duration based on machine learning methods and laparoscopic annotation data
- Caries-segnet: multi-scale cascaded hybrid spatial channel attention encoder-decoder for semantic segmentation of dental caries
- An analysis of initial force and moment delivery of different aligner materials
- Influence of microstructure on metal-ceramic bonding in SLM-manufactured titanium alloy crowns and bridges
- Design and evaluation of powered lumbar exoskeleton based on human biomechanics
- Evaluation of mesoporous polyaniline for glucose sensor under different pH electrolyte conditions
Articles in the same Issue
- Frontmatter
- Research Articles
- An exploratory study of pilot EEG features during the climb and descent phases of flight
- Gesture recognition from surface electromyography signals based on the SE-DenseNet network
- Empirical analysis on retinal segmentation using PSO-based thresholding in diabetic retinopathy grading
- Prediction of remaining surgery duration based on machine learning methods and laparoscopic annotation data
- Caries-segnet: multi-scale cascaded hybrid spatial channel attention encoder-decoder for semantic segmentation of dental caries
- An analysis of initial force and moment delivery of different aligner materials
- Influence of microstructure on metal-ceramic bonding in SLM-manufactured titanium alloy crowns and bridges
- Design and evaluation of powered lumbar exoskeleton based on human biomechanics
- Evaluation of mesoporous polyaniline for glucose sensor under different pH electrolyte conditions

