Abstract
Background
10–30% of chronic abdominal pain originates in the abdominal wall. A common cause for chronic abdominal wall pain is the Anterior Cutaneous Nerve Entrapment Syndrome (ACNES), in which an intercostal nerve branch is entrapped in the abdominal rectus sheath. Treatment consists of local anaesthetics and neurectomy, and is ineffective in 25% of cases for yet unknown reasons.
In some conditions, chronic pain is the result of altered pain processing. This so-called sensitization can manifest as segmental or even generalized hyperalgesia, and is generally difficult to treat.
Objective
The aim of this study was to assess pain processing in ACNES patients responsive and refractory to treatment by using Quantitative Sensory Testing, in order to explore whether signs of altered central pain processing are present in ACNES and are a possible explanation for poor treatment outcomes.
Methods
50 patients treated for ACNES with locally orientated treatment were included. They were allocated to a responsive or refractory group based on their response to treatment. Patients showing an improvement of the Visual Analogue Scale (VAS) pain score combined with a current absolute VAS of <40 mm were scored as responsive.
Sensation and pain thresholds to pressure and electric skin stimulation were determined in the paravertebral bilateral ACNES dermatomes and at four control areas on the non-dominant side of the body, i.e. the musculus trapezius pars medialis, musculus rectus femoris, musculus abductor hallucis and the thenar. The ACNES dermatomes were chosen to signal segmental hyperalgesia and the sum of the control areas together as a reflection of generalized hyperalgesia. Lower thresholds were interpreted as signs of sensitized pain processing. To test for alterations in endogenous pain inhibition, a conditioned pain modulation (CPM) response to a cold pressor task was determined. Also, patients filled in three pain-related questionnaires, to evaluate possible influence of psychological characteristics on the experienced pain.
Results
Patients refractory to treatment showed significantly lower pressure pain thresholds in the ACNES dermatomes and for the sum of as well as in two individual control areas. No differences were found between groups for electric thresholds or CPM response. Duration of complaints before diagnosis and treatment was significantly longer in the refractory compared to the responsive group, and refractory patients scored higher on the pain-related psychological surveys.
Conclusion and Implications
In this hypothesis-generating exploratory study, ACNES patients refractory to treatment showed more signs of sensitized segmental and central pain processing. A longer duration of complaints before diagnosis and treatment may be related to these alterations in pain processing, and both findings could be associated with less effective locally orientated treatment. In order to validate these hypotheses further research is needed.
Registration number
NCT01920880 (Clinical Trials Register; http://www.clinicaltrials.gov).
1 Introduction
Chronic abdominal pain is commonly seen in medical practice, caused in 10–30% of patients by an abdominal wall problem [1, 2]. Often, abdominal wall pain is caused by the entrapment of an intercostal nerve in the abdominal rectus sheath [3, 4]. This so-called Anterior Cutaneous Nerve Entrapment Syndrome (ACNES) results in a localized pain that can be indicated with the tip of a finger [3]. Primary treatment consists of consecutive injections with local anaesthetics combined with corticosteroids. If such treatment is only temporarily effective, the intercostal segments of the nerve are surgically removed. Conservative local treatment is successful in one-third of patients, and surgical neurectomy is effective in 70% of the transient responders [4, 5, 6].
Why the remaining 20–25% of patients is refractory to both forms of local treatment is little understood.
As in other chronic pain syndromes, understanding the underlying mechanism of pain is of great importance for adequate treatment. From conditions such as fibromyalgia, temporomandibular disorder and osteoarthritis, it is known that the ongoing nociceptive input can lead to alterations in central pain processing [7]. This central sensitization is manifest as spreading hyperalgesia, increasing a patient’s susceptibility to pain [8, 9, 10]. In some cases, pain can even become independent of peripheral nociceptive input [11]. Local treatment directed at the nociceptive source can be expected to be more ineffective in the presence of central sensitization [12]. The detection of central sensitization may thus aid in understanding and predicting the failure of locally directed treatment approaches.
Quantitative Sensory Testing (QST) is a tool for assessing alterations in pain processing at the peripheral and central levels of the nervous system. It is a validated instrument to identify neuroplasticity by evaluating responses to external stimuli of controlled intensity, such as mechanical and electrical stimuli [13, 14].
The purpose of this retrospective exploratory study was to document possible changes in central pain processing in ACNES using Quantitative Sensory Testing, with the goal of better understanding treatment failure after locally directed treatment. We hypothesized that refractory patients would show lower pain thresholds as a sign of altered central pain processing.
2 Methods
This study was conducted at the Radboud university medical centre, Nijmegen. Patients were recruited from the Radboud university medical centre and from two teaching hospitals specialized in diagnosing and treating chronic abdominal wall pain: SolviMáx, subdepartment of the Máxima Medical Centre, Veldhoven and Maasziekenhuis Pantein, Boxmeer.
The study was conducted according to the principles of the Declaration of Helsinki, and in accordance with the International Conference on Harmonization guidelines of Good Clinical Practice.
The regional Medical Ethics Committee approved the study protocol and all subjects provided oral and written consent before conduct of any protocol-related procedures. The study was registered in the Clinical Trials Register of the U.S. National institutes of Health (NCT01920880).
2.1 Study population
All patients of 18 years of age or older were eligible for study participation if they met the following inclusion criteria: (1) Patient (had) suffered from abdominal complaints matching ACNES with a constant superficially located site of tenderness, a small (<2cm2) area of maximal tenderness, and increased tenderness by abdominal muscle tensing while palpating the trigger point [1, 2, 4] (Carnett’s test positive) [15]. (2) Patients had been treated for this condition with injection therapy or surgery at least 3 and at most 12 months before.
Exclusion criteria were: (1) a history of a chronic pain syndrome that possibly interfered with the interpretation of QST results, e.g. fibromyalgia, (2) pre-existing affected sensory input (e.g. neuropathy due to diabetes mellitus) and (3) pain localized in a surgical scar.
2.2 Allocation
The pain state before and after treatment was documented using the Visual Analogue Scale (VAS). Patients were asked to mark their pain on a 100 mm line, with the extreme left representing no pain and the extreme right the worst imaginable pain [16].
Patients were allocated to either the responsive or refractory treatment group based on their response to treatment. They were scored ‘responsive’ if they showed an improvement of the VAS score after treatment resulting in an absolute pain score of <40. As a VAS of 40 or higher indicates moderate to severe and clinically relevant pain, we chose this as our cut-off point [17, 18]. The satisfaction of treatment result was also evaluated.
Baseline characteristics comprised age, sex, aetiology, Body Mass Index (BMI), abdominal medical history, use of analgesics, duration of pain symptoms until diagnosis and local sensory dysfunction before treatment.
2.3 Study procedures
Quantitative Sensory Testing was performed according to the Nijmegen Aalborg QST Screening protocol (NASQ) [19, 20]. Mechanical, i.e. pressure, and electric stimuli were applied. All measurements were performed by the same investigator, in the same calm and climate controlled room, specifically equipped for QST measurements. The complete protocol took approximately 45 min.
After initial QST training, pressure pain detection thresholds (pPDT, stimulation just becomes painful) were obtained using a handheld pressure algometer with a 1.0 cm2 probe (Wagner Instruments, Greenwich CT, USA). Pressure was increased at a rate of 5N/s. Each of the following areas on the non-dominant body side was stimulated: musculus trapezius pars medialis, musculus rectus femoris, musculus abductor hallucis, the thenar and bilaterally the paravertebral dermatome affected by ACNES.
Three electric thresholds were measured: electric sensation threshold (eST, stimulation just felt), electric pain detection threshold (ePDT, stimulation just becomes painful), and electric pain tolerance threshold (ePTT, painfulness of stimulation just becomes intolerable).
Thresholds to electric constant current skin stimulation (Digistim; Biometer A/S, Copenhagen, Denmark: titanic stimulation 100 Hz; square waves; self-adhesive electrodes, 3 cm apart) were measured on the musculus trapezius pars medialis, musculus rectus femoris and bilaterally on the abdominal dermatome of the ACNES-affected area.
The sum of the individual dermatomal non-ACNES thresholds was used as a measure of generalized hyperalgesia and the sum of the bilateral dermatomal ACNES thresholds as a measure of segmental hyperalgesia.
The ability to generate descending central inhibitory pain modulation was tested using a Conditioned Pain Modulation (CPM) paradigm, in which we measured the relative change (%) in pain thresholds (pPDT and ePTT) before and after a cold pressor task (CPT). The cold pressor task comprised the immersion of the subjects’ non-dominant hand in ice-chilled water during 2 min, or less if the pain was considered intolerable.
To ensure the validity and reproducibility of the threshold measures, patients were thoroughly instructed and trained. Every threshold was measured twice for pressure testing and three times for electric testing. For all elements of QST measurements VAS scores were documented to verify whether patients understood the instructions correctly and were consistent in indicating their thresholds. The CPM response was tested last, as it may influence the other measurements. All concomitant medication was continued.
2.4 Psychosocial assessment
To assess the psychosocial dimension of pain in ACNES, subjects were asked to complete three questionnaires before QST measurements. The Pain Anxiety Symptom Scale (PASS) measures four aspects of pain related anxiety: (1) fear of pain, (2) cognitive anxiety, (3) flight or avoidance behaviour and (4) physiological symptoms of pain. Higher scores reflect a higher degree of pain-related anxiety [21, 22]. The Pain Catastrophizing Scale (PCS) assesses pain-related ruminating, magnifying and helplessness, a score of more than 24 indicates a high level of catastrophizing [23]. The Hospital Anxiety and Depression Scale (HADS) is a screening instrument for possible signs of depression and anxiety. Scores for each subscale (anxiety and depression) can range from 0 to 21 with normal (0–7), mild (8–10), moderate (11–14), severe (15–21) [24, 25].
2.5 Statistical analysis
This was an exploratory study for which no sample size calculation was performed. We pragmatically chose a sample size of 50 subjects. The primary effect parameter for comparison between groups was the difference in sum of the individual dermatomal non-ACNES thresholds. Only the second measurements were compared to decrease bias due to unmodulated first responses, using a Mann–Whitney U test. Secondary outcome measures were differences in sum of electric thresholds, differences in individual dermatomal thresholds and differences in CPM response and questionnaire results. For determination of correlations we used Kendall’s Tau test. Data analysis was performed using the Statistical Package for the Social Sciences (SPSS version 20, SPSS Inc., Chicago, IL, USA), and all statistical tests were two-tailed. The chance for type one errors was set at 5%. Given the non-Gaussian data distribution purely non-parametric analysis was performed and all baseline characteristics and test results are presented as medians with interquartile ranges (IQR). Missing data were left out of analysis.
3 Results
Between July 2013 and February 2014, 50 patients were enrolled (Fig. 1). Patients declined to participate for a variety of reasons, e.g. time-consuming effort and no personal benefit. The study was completed without any adverse events. All of the included patients (15 men, 35 women) were analyzed. 35 patients were allocated to the responsive group and 15 to the refractory group based on pre- and post-treatment VAS scores. Two patients did not complete all measurements according to protocol due to technical issues. One patient in the refractory group missed the electric testing, and one patient in the responsive group did not complete the pressure algometry. Therefore, CPM data was also not available for these patients.

Flowchart.
The duration of complaints until diagnosis and treatment was significantly longer in the refractory group (p = 0.020). Duration in months correlated with post-treatment VAS scores as well as with success (Kendall’s Tau: r 0.22, p0.039 and r 0.25, p0.039, respectively). More spontaneous aetiology was present in the responsive group, compared to more ACNES related to pregnancy and recent abdominal surgery in the refractory group.
Patients in the refractory group were using analgesics ranging from acetaminophen combined with NSAIDs to strong opioids, and in six cases supplemented with centrally acting analgesics such as gapapentinoids. Most responsive patients did not use any analgesics (n = 27) at time of measurements. The remainder used acetaminophen, occasionally combined with NSAIDs. All of the responsive patients had used analgesics during complaints, ranging from acetaminophen to strong opioidsand centrally acting analgesics.
Two patients allocated to the responsive group were unsatisfied with the result of treatment. Both patients exhibited a VAS improvement of more than 50% and had absolute scores of <40 mm post treatment (Table 1).
Demographics and clinical characteristics.
| Responsive (n = 35) | Refractory (n = 15) | |
|---|---|---|
| Sex, M:F | 11:24 | 4:11 |
| Age, y | 27 (24–52) | 34 (23–58) |
| BMI, kg/m2 | 26.0 (22.6–27.8) | 22.2 (21.1–30.0) |
| Abdominal medical history | ||
| Surgery | 8 (23%) | 4 (27%) |
| Other | 3 (9%) | 1 (7%) |
| Aetiology | ||
| Spontaneous | 29 (83%)[*] | 8 (53%) |
| Pregnancy | 0 | 3 (20%) |
| Exercise-related | 1 (3%) | 0 |
| Trauma-related | 3 (8%) | 0 |
| Recent abdominal surgery | 2 (6%) | 4 (27%)[*] |
| Duration, months | 6 (2–12) | 18 (4–36)[*] |
| Final treatment | ||
| Injections | 10 (29%)[*] | 0 (0%) |
| Surgery | 25 (77%) | 15 (100%) |
| Number of surgeries | ||
| 0 | 10 (29%)[*] | 0 (0%) |
| 1 | 19 (54%) | 6 (40%) |
| 2 | 4 (11%) | 7 (47%)[*] |
| >2 | 2 (6%) | 2 (13%) |
| Visual Analogue Scale score, mm | ||
| Before treatment | 80 (67–89) | 68 (63–81) |
| Aftertreatment | 1 (0–11) | 63 (51–68)[*] |
| Location | ||
| Right upper quadrant | 4 (11%) | 2 (13%) |
| Right lowerquadrant | 19 (54%) | 9 (60%) |
| Left upper quadrant | 2 (6%) | 1 (7%) |
| Left lower quadrant | 10 (29%) | 3 (20%) |
| Local sensory dysfunction, n | 16 (46%) | 8 (53%) |
-
If applicable, data are presented in medians with interquartile ranges (IQR). BMI indicates Body Mass Index.
Local sensory dysfunction contains hyper- and hypoesthesia of skin surrounding the ACNES area before treatment.
3.1 Pressure pain thresholds
Refractory ACNES patients showed a significantly lower sum of the non-ACNES as well as the ACNES dermatomal pressure pain thresholds compared to the responsive group as shown in Figs. 2 and 3. Pressure pain detection thresholds also differed according to individual site of measurement. In the refractory group significantly lower thresholds were found for the musculus trapezius, musculus abductor hallucis and both corresponding paravertebral ACNES dermatomes (Fig. 4).
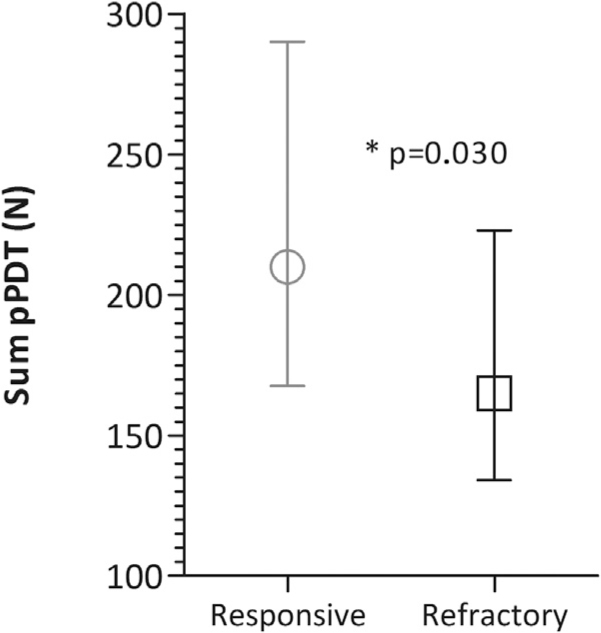
Sum of non-ACNES dermatomes pressure Pain Detection Thresholds (pPDT) in responsive (n = 15) and refractory (n = 34) ACNES patients, presented as medians with IQR.

Sum of ACNES dermatomes pressure Pain Detection Thresholds (pPDT) in responsive (n = 15) and refractory (n = 34) ACNES patients, presented as medians with IQR.
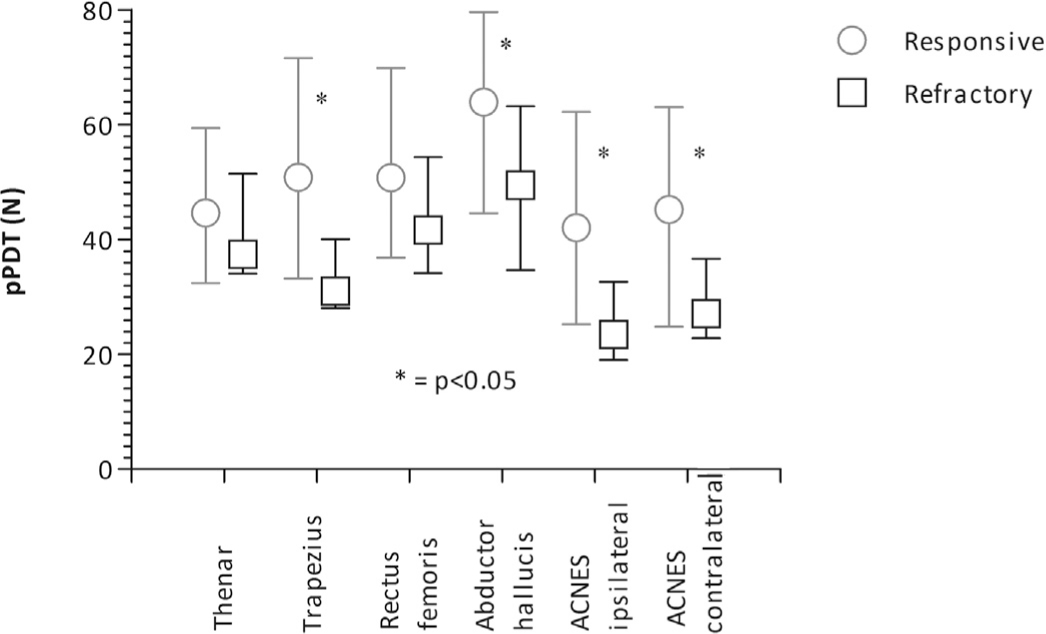
Individual pressure Pain Detection Thresholds (pPDT) in responsive (n = 15) and refractory (n = 34) ACNES patients, presented as medians with IQR.
3.2 Electric pain thresholds
Sums of electric non-ACNES and ACNES dermatomes thresholds did not differ between groups (Table 2). No differences in individual thresholds were found (data not shown)
Sums of electric (mA) thresholds.
| Responsive (n = 34) | Refractory (n = 15) | p-Value | |
|---|---|---|---|
| Non-ACNES dermatomes | |||
| Sum electric sensation thresholds | 15.9 (13.8–19.5) | 16.3 (12.9–21.3) | 0.745 |
| Sum electric pain detection thresholds | 15.5 (l2.8–24.2) | 16.7 (14.1–19.5) | 0.820 |
| Sum electric pain tolerance thresholds | 112.2 (89.7–130.4) | 98.1 (72.2–118) | 0.329 |
| ACNES dermatomes | |||
| Sum electric sensation thresholds | 5.7 (4.3–7.4) | 6.9 (5.4–9.4) | 0.184 |
| Sum electric pain detection thresholds | 17.2 (12.0–22.8) | 16.3 (10.6–25.0) | 0.928 |
| Sum electric pain tolerance thresholds | 31.1 (22.4–42.8) | 30.2 (19.3–42.5) | 0.700 |
-
Sums of electric thresholds are measured in milliampéres, presented as medians with (IQR).
3.3 Conditioned pain modulation
CPM response was similar in both groups for latency of cold pressor task (CPT), VAS score during CPT and percentage change in pressure and electric thresholds after CPT (Table 3).
Conditioned pain modulation response.
| Responsive (n = 14) | Refractory (n = 34) | p-Value | |
|---|---|---|---|
| Duration CPT (s) | 120 (65–120) | 120 (43–120) | 0.172 |
| Change in pPDT (%) | 19.4 (9.4–42.5) | 23.9 (2.8–39.7) | 0.946 |
| Change in ePTT (%) | 12.2 (4.9–28.7) | 8.8 (0.6–26.4) | 0.352 |
| VAS during CPT (mm) | 66 (21–81) | 73 (42–85) | 0.467 |
-
Conditioned pain modulation response is measured in relative change (%) of pressure and electric pain detection thresholds (pPDT)and (ePDT) before and after CPT (cold pressor test). Duration CPT is measured in seconds. VAS: Visual Analogue Scale in millimetres. Data are presented as medians with (IQR).
3.4 Psychological assessments
Patients refractory to treatment scored significantly higher on the PASS and both subscales of the HADS questionnaire. For the PCS only a trend towards higher scores was seen in treatment-refractory patients (Table 4).
Pain-related questionnaire scores of responsive and refractory ACNES patients.
| Responsive (n = 15) | Refractory (n = 35) | p-Value | |
|---|---|---|---|
| PASS | 47.0 (23.0–62.0) | 75 (28.8–93.5) | 0.042 |
| PCS | 13.0 (6.0–24.0) | 26.0 (18.0–30.0) | 0.080 |
| HADS fear | 2.0 (0.0–4.0) | 6.0 (3.0–7.0) | 0.01 |
| HADS depression | 1.0 (0.0–2.0) | 7.0 (2.0–11.0) | <0.001 |
-
Scores on pain-related questionnaires. PASS: Pain Anxiety Symptom Scale, PCS: Pain Catastrophizing Scale, HADS: Hospital Anxiety and Depression Scale. Bold values represent significant differences between groups.
4 Discussion
4.1 Findings
This is the first study investigating central pain processing in ACNES. The refractory group showed significantly lower pressure pain thresholds, sum and individual-dermatomal, compared to the responsive group, despite the use of more and stronger analgesics in the refractory group.
Lower pressure pain detection thresholds in the affected ACNES dermatome in refractory patients are to be expected, as these patients are currently experiencing pain. Interestingly, pressure pain thresholds are also decreased in areas remote from the ACNES dermatome, despite the very localized character of ACNES.
Based on our results, we hypothesize that the lower pressure pain detection thresholds in the ipsi- and contralateral ACNES dermatomes are a representation of segmental hyperalgesia. The higher sum and individual pain thresholds in the areas remote from the ACNES dermatomes may be a reflection of generalized hyperalgesia. In other words, sensitization of both spinal and supraspinal central pain processing might be present in some patients suffering from ACNES. Given the localized treatment, one could imagine poorer outcomes in these patients.
There was a significantly longer duration of complaints until diagnosis and treatment in the refractory group, which also correlated with treatment outcome. As persistent nociceptive input is associated with the development of spreading hyperalgesia and central sensitization, this finding is compatible with our hypothesis of altered central pain processing in ACNES.
The distribution of age and gender, which are known to influence thresholds, was equal between groups [26, 27]. Therefore, we assume they have not altered the results.
No differences were found in any of the electric thresholds. A possible explanation for this outcome can be found in the different targets of the different QST modalities. Electric testing is predominantly targeted at cutaneous Aβ-fibres and normally does not penetrate beyond cutaneous tissue [28, 29]. Pressure algometry is directed at the more deeply situated tissue, such as muscles and bones [29]. The absence of difference in electric testing could thus reflect the pathophysiology of ACNES, in which the problem is located in the more deeply situated tissues, i.e. at the level of the rectus abdominis muscle [3].
The CPM results were similar between groups. The used paradigm for testing met the methodological criteria advised in a recent review on CPM [30]. Based on these results we have no indications that impaired endogenous analgesia plays a role in persisting pain in ACNES, However, the lack of difference could be due to the small sample size.
Refractory patients scored higher on the psychological surveys, which is in line with a higher prevalence of depression and anxiety in chronic pain patients [31]. Whether these characteristics are determinants for, or consequences of persistent pain cannot be answered by the current study design. Given the bidirectional association between chronic pain and factors such as depression and anxiety described in literature, baseline and follow up measurements would be needed [32]. It would be very interesting to see whether the presence of these factors will fluctuate with intensity and duration of pain. Though refractory patients scored higher on the HADS questionnaire, their scores remained in the normal range.
4.2 Methodological aspects
A major limitation of this study is the retrospective data collection of patient demographics and pain scores, which has a considerable risk of recall bias. Considering treatment effects, recall bias often leads to an overestimation of the degree of effect [33, 34]. Group allocation was based on improvement after treatment resulting in a current VAS score of <40, but not on the degree of effect. Therefore we believe this has not significantly influenced the allocation procedure and consequently our results.
Although satisfaction is an important success parameter, we allocated two unsatisfied patients to the responsive group. Thirty-three to 50% or 11–30 mm improvements on a Visual Analogue Scale are generally accepted as clinically relevant changes in pain [35, 36, 37], and VAS scores of <40 indicate only mild pain [17, 18]. As both criteria applied to these two unsatisfied patients, we judge this allocation as correct.
Quantitative Sensory Testing is a validated and commonly used tool for exploring the central nociceptive processing of a group of patients [13]. Apart from pure diagnostic value, QST has shown to be able to monitor disease progression [38], predict treatment effects [11, 39] and contribute to choice of treatment [14].
These characteristics of QST could be particularly useful in ACNES, given the relatively high percentage of patients refractory to standard treatment.
Unfortunately, we only performed a single post-treatment QST measurement. Therefore, we do not know whether the differences in central pain processing between groups were present before treatment. Nor do we know whether treatment has influenced pain processing, in either direction. For instance, in osteoarthritis of the hip successful treatment has led to normalization of pain thresholds [40]. We find it less likely that the lower pain thresholds in refractory patients are a consequence of treatment, as both groups received equal treatment.
To validate the aforementioned utilities of QST in ACNES and our hypotheses that (1) altered central pain processing can play a role in ACNES and (2) local treatment failure may be related to these alterations, longitudinal research is needed. We suggest a prospective repeated measures cohort study, with thorough baseline measurements and multiple follow up moments during and after treatment, up to at least one year post-treatment [41, 42]. The good intra- and interobserver and test-retest reliability of QST makes it an appropriate tool for this study design [43, 44].
An additional weakness in the study design is the collection and analysis of data by the same investigator, who was not blinded to group allocation during measurements, which increased the risk of bias. Furthermore there is a chance of type II error given the relatively small sample size.
4.3 Clinical and scientific implications
The experimental protocol presented in this exploratory study provides a non-invasive technique to identify ACNES patients with abnormalities in central pain processing. Hypothethically, these patients suffer from an increased risk of failure of locally directed treatments or the development of chronic pain. In such patients, it would seem logical to consider treatment with drugs targeting central pain processing, e.g. gabapentinoids or tricyclic antidepressants, instead of instituting local deafferenting treatments such as local anaesthestic injection or neurectomy [45]. To validate these hypotheses additional research is needed.
5 Conclusion
The results of this exploratory hypothesis generating study suggest the presence of altered central pain processing in ACNES, as a possible explanation for the failure of locally orientated treatments such as nerve block or neurectomy. In patients exhibiting these alterations in pain processing, there may be a role for centrally acting analgesics. Early diagnosis and treatment seem desirable to aid in the prevention of developing generalized hyperalgesia. However, as this study was merely hypothesis generating, further research is mandated before founded conclusions can be drawn.
Highlights
Chronic pain in ACNES may be more than justa localized problem.
ACNES patients refractory to treatment show signs of generalized hyperalgesia.
Refractory ACNES patients suffered from a longer duration of pain before treatment.
Local treatment failure may be related to sensitized central pain processing.
DOI of refers to article: http://dx.doi.org/10.1016/j.sjpain.2016.11.019.
-
Ethical issues: The regional Medical Ethics Committee approved the protocol of this study and all subjects provided oral and written consent before conduct of any protocol-related procedures. The study was registered in the Clinical Trials Register of the U.S. National institutes of Health (NCT01920880).
-
Conflict of interest: The authors declare no conflict of interest. No funding was obtained for the preparation of this manuscript.
References
[1] Srinivasan R, Greenbaum DS. Chronic abdominal wall pain: a frequently over-looked problem. Practical approach to diagnosis and management. Am J Gastroenterol 2002;97:824–30.Search in Google Scholar
[2] Lindsetmo RO, Stulberg J. Chronic abdominal wall pain – a diagnostic challenge for the surgeon. Am J Surg 2009;198:129–34.Search in Google Scholar
[3] Applegate WV. Abdominal cutaneous nerve entrapment syndrome. Am Fam Physician 1973;8:132–3.Search in Google Scholar
[4] Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Management of anterior cutaneous nerve entrapment syndrome in a cohort of 139 patients. Ann Surg 2011;254:1054–8.Search in Google Scholar
[5] Boelens OB, Scheltinga MR, Houterman S, Roumen RM. Randomized clinical trial of trigger point infiltration with lidocaine to diagnose anterior cutaneous nerve entrapment syndrome. Br J Surg 2013;100:217–21.Search in Google Scholar
[6] Boelens OB, van Assen T, Houterman S, Scheltinga MR, Roumen RM. A double-blind, randomized, controlled trial on surgery for chronic abdominal pain due to anterior cutaneous nerve entrapment syndrome. Ann Surg 2013;257:845–9.Search in Google Scholar
[7] Cervero F. Visceral pain-central sensitisation. Gut 2000;47(Suppl. 4):iv56–7, discussion iv58.Search in Google Scholar
[8] Woolf CJ. Pain. Neurobiol Dis 2000;7:504–10.Search in Google Scholar
[9] Wilder-Smith OH, Tassonyi E, Arendt-Nielsen L. Preoperative back pain is associated with diverse manifestations of central neuroplasticity. Pain 2002;97:189–94.Search in Google Scholar
[10] Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011;152(3 Suppl.):S2–15.Search in Google Scholar
[11] Bouwense SA, Buscher HC, van Goor H, Wilder-Smith OH. Has central sensitization become independent of nociceptive input in chronic pancreatitis patients who fail thoracoscopic splanchnicectomy? Reg Anesth Pain Med 2011;36:531–6.Search in Google Scholar
[12] Bouwense SA, Ahmed Ali U, ten Broek RP, Issa Y, van Eijck CH, Wilder-Smith OH, van Goor H. Altered central pain processing after pancreatic surgery for chronic pancreatitis. Br J Surg 2013;100:1797–804.Search in Google Scholar
[13] Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am 2003;14:261–86.Search in Google Scholar
[14] Olesen SS, van Goor H, Bouwense SA, Wilder-Smith OH, Drewes AM. Reliability of static and dynamic quantitative sensory testing in patients with painful chronic pancreatitis. Reg Anesth Pain Med 2012;37:530–6.Search in Google Scholar
[15] Carnett JB, Bates W. The treatment of intercostal neuralgia of the abdominal wall. Ann Surg 1933;98:820–9.Search in Google Scholar
[16] McCormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med 1988;18:1007–19.Search in Google Scholar
[17] Li KK, Harris K, Hadi S, Chow E. What should be the optimal cut points for mild, moderate, and severe pain? J Palliat Med 2007;10:1338–46.Search in Google Scholar
[18] Rice AS. Clinical pain management. 2nd ed. Taylor and Francis Group LLC; 2008.Search in Google Scholar
[19] Timmerman HWO, van Weel C, Wolff AP, Vissers KCP. Detecting the neuropathic pain component in the clinical setting: a study protocol for validation of screening instruments for the presence of a neuropathic pain component. BMC Neurol 2014;14:94.Search in Google Scholar
[20] Wilder-Smith O. A paradigm-shift in pain medicine: implementing a systematic approach to altered pain processing in everyday clinical practice based on quantitative sensory testing. Aalborg University; 2013.Search in Google Scholar
[21] McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain 1992;50:67–73.Search in Google Scholar
[22] Roelofs J, McCracken L, Peters ML, Crombez G, van Breukelen G, Vlaeyen JW. Psychometric evaluation of the Pain Anxiety Symptoms Scale (PASS) in chronic pain patients. J Behav Med 2004;27:167–83.Search in Google Scholar
[23] Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain 2002;96:319–24.Search in Google Scholar
[24] Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med 1997;27:363–70.Search in Google Scholar
[25] Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69–77.Search in Google Scholar
[26] Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 2010;151:598–605.Search in Google Scholar
[27] Petrini L, Matthiesen ST, Arendt-Nielsen L. The effect of age and gender on pressure pain thresholds and suprathreshold stimuli. Perception 2015;44:587–96.Search in Google Scholar
[28] Rollman GB, Harris G. The detectability, discriminability, and perceived magnitude of painful electrical shock. Percept Psychophys 1987;42:257–68.Search in Google Scholar
[29] Stawowy M, Rossel P, Bluhme C, Funch-Jensen P, Arendt-Nielsen L, Drewes AM. Somatosensory changes in the referred pain area following acute inflammation of the appendix. Eur J Gastroenterol Hepatol 2002;14:1079–84.Search in Google Scholar
[30] Nir RR, Yarnitsky D. Conditioned pain modulation. Curr Opin Support Palliat Care 2015;9:131–7.Search in Google Scholar
[31] de Heer EW, Gerrits MM, Beekman AT, Dekker J, van Marwijk HW, de Waal MW, Spinhoven P, Penninx BW, van der Feltz-Cornelis CM. The association of depression and anxiety with pain: a study from NESDA. PLOS ONE 2014;9:e106907.Search in Google Scholar
[32] Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med 2003;163:2433–45.Search in Google Scholar
[33] Pellise F, Vidal X, Hernandez A, Cedraschi C, Bago J, Villanueva C. Reliability of retrospective clinical data to evaluate the effectiveness of lumbar fusion in chronic low back pain. Spine (Phila Pa 1976) 2005;30:365–8.Search in Google Scholar
[34] Bitzer EM, Petrucci M, Lorenz C, Hussein R, Dorning H, Trojan A, Nickel S. A comparison of conventional and retrospective measures of change in symptoms after elective surgery. Health Qual Life Outcomes 2011;9:23.Search in Google Scholar
[35] Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med 1996;27:485–9.Search in Google Scholar
[36] Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain 2000;88:287–94.Search in Google Scholar
[37] Giraudeau B, Rozenberg S, Valat JP. Assessment of the clinically relevant change in pain for patients with sciatica. Ann Rheum Dis 2004;63:1180–1.Search in Google Scholar
[38] Bouwense SA, Olesen SS, Drewes AM, Frokjaer JB, van Goor H, Wilder-Smith OH. Is altered central pain processing related to disease stage in chronic pancreatitis patients with pain? An exploratory study. PLoS ONE 2013;8:e55460.Search in Google Scholar
[39] Olesen SS, Graversen C, Bouwense SA, van Goor H, Wilder-Smith OH, Drewes AM. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS ONE 2013;8:e57963.Search in Google Scholar
[40] Staud R. Abnormal endogenous pain modulation is a shared characteristic of many chronic pain conditions. Expert Rev Neurother 2012;12:577–85.Search in Google Scholar
[41] Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain 2008;138:22–8.Search in Google Scholar
[42] Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: a pilot study. J Pain Palliat Care Pharmacother 2010;24:119–28.Search in Google Scholar
[43] Delaney GA, McKee AC. Inter- and intra-rater reliability of the pressure threshold meter in measurement of myofascial trigger point sensitivity. Am J Phys Med Rehabil 1993;72:136–9.Search in Google Scholar
[44] Geber C, Klein T, Azad S, Birklein F, Gierthmuhlen J, Huge V, Lauchart M, Nitzsche D, Stengel M, Valet M, Baron R, Maier C, Tolle T, Treede RD. Test–retest and inter-observer reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain 2011;152:548–56.Search in Google Scholar
[45] Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain 2005;118: 289–305.Search in Google Scholar
© 2016 Scandinavian Association for the Study of Pain
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Patients with chronic neck-pain after trauma do not differ in type of symptoms and signs, but suffer more than patients with chronic neck pain without a traumatic onset
- Observational study
- Chronic neck pain patients with traumatic or non-traumatic onset: Differences in characteristics. A cross-sectional study
- Editorial Comment
- Re-enforcing therapeutic effect by positive expectations of pain-relief from our interventions
- Original experimental
- Effect of expectation on pain assessment of lower- and higher-intensity stimuli
- Editorial comment
- Objective methods for the assessment of the spinal and supraspinal effects of opioids
- Topical review
- Objective methods for the assessment of the spinal and supraspinal effects of opioids
- Editorial Comment
- Multi-target treatment of bone cancer pain using synergistic combinations of pharmacological compounds in experimental animals
- Original experimental
- Synergistic combinations of the dual enkephalinase inhibitor PL265 given orally with various analgesic compounds acting on different targets, in a murine model of cancer-induced bone pain
- Editorial comment
- Terminal cancer pain intractable by conventional pain management can be effectively relieved by intrathecal administration of a local anaesthetic plus an opioid and an alfa2-agonist into the cerebro-spinal-fluid
- Observational study
- Multimodal intrathecal analgesia in refractory cancer pain
- Editorial comment
- Treatment success in neck pain: The added predictive value of psychosocial variables in addition to clinical variables
- Observational study
- Treatment success in neck pain: The added predictive value of psychosocial variables in addition to clinical variables
- Editorial comment
- Why are some patients with chronic pain from anterior abdominal nerve entrapment syndrome (ACNES) refractory to peripheral treatment with neurectomy?
- Clinical pain research
- Treatment response and central pain processing in Anterior Cutaneous Nerve Entrapment Syndrome: An explorative study
- Editorial comment
- Gain in functions before pain reduction during intensive multidisciplinary paediatric pain rehabilitation programme
- Clinical pain research
- Physical and occupational therapy outcomes: Adolescents’ change in functional abilities using objective measures and self-report
- Editorial comment
- Complex Regional Pain Syndrome (CRPS): High risk of CRPS after trauma in another limb in patients who already have CRPS in one hand or foot: Lasting changes in neural pain modulating systems?
- Clinical pain research
- The risk of pain syndrome affecting a previously non-painful limb following trauma or surgery in patients with a history of complex regional pain syndrome
- Editorial Comment
- Positive affect could reduce the impact of pain
- Original experimental
- The buffering role of positive affect on the association between pain intensity and pain related outcomes
- Editorial comment
- The meaning and consequences of amputation and mastectomy from the perspective of pain and suffering – Lessons to be learned and relearned
- Clinical pain research
- The meaning and consequences of amputation and mastectomy from the perspective of pain and suffering
- Editorial comment
- Invasive intervention for “intractable” Complex Regional Pain Syndromes (CRPS)?
- Educational case report
- Intrathecal management of complex regional pain syndrome: A case report and literature
- Observational study
- Item response theory analysis of the Pain Self-Efficacy Questionnaire
- Announcement
- Scandinavian Association for the Study of Pain (SASP): Annual Meeting 2017
Articles in the same Issue
- Scandinavian Journal of Pain
- Editorial comment
- Patients with chronic neck-pain after trauma do not differ in type of symptoms and signs, but suffer more than patients with chronic neck pain without a traumatic onset
- Observational study
- Chronic neck pain patients with traumatic or non-traumatic onset: Differences in characteristics. A cross-sectional study
- Editorial Comment
- Re-enforcing therapeutic effect by positive expectations of pain-relief from our interventions
- Original experimental
- Effect of expectation on pain assessment of lower- and higher-intensity stimuli
- Editorial comment
- Objective methods for the assessment of the spinal and supraspinal effects of opioids
- Topical review
- Objective methods for the assessment of the spinal and supraspinal effects of opioids
- Editorial Comment
- Multi-target treatment of bone cancer pain using synergistic combinations of pharmacological compounds in experimental animals
- Original experimental
- Synergistic combinations of the dual enkephalinase inhibitor PL265 given orally with various analgesic compounds acting on different targets, in a murine model of cancer-induced bone pain
- Editorial comment
- Terminal cancer pain intractable by conventional pain management can be effectively relieved by intrathecal administration of a local anaesthetic plus an opioid and an alfa2-agonist into the cerebro-spinal-fluid
- Observational study
- Multimodal intrathecal analgesia in refractory cancer pain
- Editorial comment
- Treatment success in neck pain: The added predictive value of psychosocial variables in addition to clinical variables
- Observational study
- Treatment success in neck pain: The added predictive value of psychosocial variables in addition to clinical variables
- Editorial comment
- Why are some patients with chronic pain from anterior abdominal nerve entrapment syndrome (ACNES) refractory to peripheral treatment with neurectomy?
- Clinical pain research
- Treatment response and central pain processing in Anterior Cutaneous Nerve Entrapment Syndrome: An explorative study
- Editorial comment
- Gain in functions before pain reduction during intensive multidisciplinary paediatric pain rehabilitation programme
- Clinical pain research
- Physical and occupational therapy outcomes: Adolescents’ change in functional abilities using objective measures and self-report
- Editorial comment
- Complex Regional Pain Syndrome (CRPS): High risk of CRPS after trauma in another limb in patients who already have CRPS in one hand or foot: Lasting changes in neural pain modulating systems?
- Clinical pain research
- The risk of pain syndrome affecting a previously non-painful limb following trauma or surgery in patients with a history of complex regional pain syndrome
- Editorial Comment
- Positive affect could reduce the impact of pain
- Original experimental
- The buffering role of positive affect on the association between pain intensity and pain related outcomes
- Editorial comment
- The meaning and consequences of amputation and mastectomy from the perspective of pain and suffering – Lessons to be learned and relearned
- Clinical pain research
- The meaning and consequences of amputation and mastectomy from the perspective of pain and suffering
- Editorial comment
- Invasive intervention for “intractable” Complex Regional Pain Syndromes (CRPS)?
- Educational case report
- Intrathecal management of complex regional pain syndrome: A case report and literature
- Observational study
- Item response theory analysis of the Pain Self-Efficacy Questionnaire
- Announcement
- Scandinavian Association for the Study of Pain (SASP): Annual Meeting 2017


