Abstract
Pharmaceuticals are widely used for treating human and animal diseases. Naproxen [(S) 6-methoxy-α-methyl-2-naphthalene acetic acid] and its sodium salt are members of the α-arylpropionic acid group of nonsteroidal anti-inflammatory drugs. Due to excessive usage of naproxen, this drug has been determined even in drinking water. In this study, four fungal strains Phanerochaete chrysosporium, Funalia trogii, Aspergillus niger, and Yarrowia lipolytica were investigated in terms of naproxen removal abilities. According to LC/MS data, A. niger was found the most efficient strain with 98% removal rate. Two main by-products of fungal transformation, O-desmethylnaproxen and 7-hydroxynaproxen, were identified by using LC/MS, 1HNMR, and 13CNMR. Our results showed that O-demethylation and hydroxylation of naproxen is catalyzed by cytochrome P450 enzyme system.
1 Introduction
Pollution of aquatic environments by micropollutans such as pharmaceuticals, pesticides, and industrial chemicals, is a worldwide issue [1]. Pharmaceuticals are widely used for treatment human and animal diseases. These active compounds and their metabolites can enter aquatic environments via urine, feces, discharge from production facilities, hospitals, and disposal of unused or expired drugs [2], [3]. If they are not eliminated during sewage treatment, pharmacologically active compounds and their metabolites can be detected in surface water, ground water, and waste water even in drinking water [4], [5].
Naproxen [(S) 6-methoxy-α-methyl-2-naphthalene acetic acid] and its sodium salt are members of the α-aryl propionic acid group of non-steroidal anti-inflammatory drugs, which is widely used worldwide to reduce pain, inflammation, and fever. Due to excessive usage of naproxen, this drug has been detected in aquatic environments and even in drinking water up to several μg L−1 [6]. Consequently, the presence of pharmaceuticals in aquatic environments has received more attention recent years because of their potential long-term toxic effects [5], [7].
Several studies demonstrated elimination of naproxen with physicochemical methods such as oxidation of naproxen with α-MnO2 nanostructures, photolysis, ozonation, chlorination, photo-electrochemical degradation, adsorptive removal, and ultrasonic degradation [8], [9], [10], [11], [12], [13], [14].
Physicochemical methods are used to remove micropollutant naproxen, which have various disadvantages, such as causing formation of secondary pollutants and having high operational costs [15]. On the other hand, biological methods are eco-friendly and provide low-cost option to remove pharmaceutical pollutants [16].
Beside chemical processes, many researchers achieved removal of naproxen through biological methods [6], [15], [17], [18]. Phanerochaete chrysosporium and Funalia trogii are the members of white-rot fungi. White-rot fungi are able to degrade wide variety environmental pollutants such as pesticides, polyaromatic hydrocarbons, polychlorinated biphenyls, and other various xenobiotic compounds [19]. Yarrowia lipolytica is a nonpathogenic aerobic yeast widely used in industrial applications including citric acid production, peach flavor, and enzyme production [20], [21], [22]. Previous studies showed that Yarrowia lipolytica could degrade TNT (2, 4, 6-trinitrotoluene), azo dye Reactive Black 5, and halogenated alkanes [23], [24], [25]. Aspergillus niger is a filamentous fungus. A. niger is used for production of citric acid and extracellular enzymes such as pectinase, protease, lipase, and amyloglucosidase in industrial area [26], [27]. In addition, some studies demonstrated that A. niger is a good candidate for degradation of environmental pollutants [28], [29].
The principal aim of this research was assessment of naproxen degradation capabilities of four fungal strains, include two white-rot fungi (Phanerochaete chrysosporium, Funalia trogii), yeast Yarrowia lipolytica, filamentous fungus Aspergillus niger and identification metabolites of naproxen.
2 Materials and methods
2.1 Chemicals
Naproxen-Na (CAS NO: 26159-34-2) and 1-aminobenzotriazole were obtained from Sigma-Aldrich.
2.2 Microorganisms
Phanerochaete chrysosporium ME446, Funalia trogii ATCC 200800, Aspergillus niger NRRL 328, and Yarrowia lipolytica NBRC1658 were used to compare removal capabilities of naproxen sodium salt. Fungal strains were kept as slopes on potato dextrose agar for up to 20 days at 4°C.
2.3 Culture medium
Two different culture mediums were used in this study. Modified Vogel’s medium was used for Funalia trogii, Aspergillus niger, and Yarrowia lipolytica, which is composed of per liter: 20 g glucose, 10 mL Modified Vogel’s medium and 100 μL trace elements solution. Modified Vogel’s medium is composed of (L−1): 150 g Na3 citrate 5·H2O, 250 g KH2PO4, 100 g NH4NO3, 10 g MgSO4·7H2O, 5 g CaCl2·2H2O. Trace elements solution contains (100 mL): 5 g citric acid·H2O, 5 g ZnSO4·7H2O, 1 g Fe(NH4)2(SO4)2·6H2O, 0.25 g CuSO4·5H2O, 0.05 g MnSO4·H2O, 0.05 g H3BO3, and 0.05 g Na2MoO4·2H2O [30]. Nitrogen-limited Vogel’s medium for Phanerochaete chrysosporium, contained 50 mg L−1 NH4NO3 as a nitrogen source, all other components of culture medium were same as stated above. All culture mediums pH were adjusted to 4.7.
2.4 Removal experiments
One milliliter suspended mycelia of Funalia trogii (approximately 0.1 g, wet weight) and one milliliter spore suspension of Phanerochaete chrysosporium (1 mL; 0.5 absorbance unit at 650 nm) were inoculated into 250-mL Erlenmeyer flasks. Cultures of these fungi were precultured for 7 days at 30°C (150 rpm).
One milliliter Yarrowia lipolytica cell suspension (1 mL: 1 absorbance unit at 650 nm) and one milliliter Aspergillus niger spore suspension (1 mL; 1.2×106 spore) were inoculated into 250-mL Erlenmeyer flasks and then cultures precultured for 3 days at 30°C (150 rpm). All experiments were conducted using 99-mL culture medium.
After preincubation periods, 1-mL stock solution of naproxen in methanol was added into flasks to give desired final concentration of pharmaceutical (50 mg L−1). In order to determine most efficient fungal strain, all cultures incubated with 50 mg L−1 naproxen on rotary shaker (30°C, 150 rpm) for 48 h.
To avoid photo transformation reactions, all the experiments were carried out in the dark. Each experiment was prepared triplicate, control groups included abiotic and heat killed organisms.
2.5 Experiments with cytochrome P450 inhibitor
1-Aminobenzotriazole (ABT) is a suicide inhibitor of cytochrome P450 and chloroperoxidases. Before addition of naproxen, 2 mM ABT was added to the culture medium, in order to reveal the role of cytochrome P450.
2.6 Isolation of metabolites
After biotransformation of naproxen, the media was extracted with the mixture of CH2Cl2:MeOH (93:7), evaporated and directly transferred to an open column with silica gel 60 (0.04–0.063 mm) and eluted with first only CH2Cl2, then the mixture of CH2Cl2:MeOH (100:5) with gradient elution.
2.7 LC/MS analysis
The LC/MS spectra were taken on a Waters Micromass ZQ connected with Waters Alliance HPLC, using ESI(-) method, with C-18 column. The HPLC of LC/MS was carried out on a column XTerra®MS C-18 (4.6 mm×250 mm, 5 μm) with WATER:MeOH:% 0.1 HCOOH in acetonitrile (37:53:10) as mobile phase. The flow rate was 0.55 mL/min, the injection volume was 4 μL and the running time 20 min. The eluate was monitored by a photo-diode array detector at 254 nm. The analytical condition of mass was as follows: capillary voltage: 3.71 kV, cone voltage: 29 V, source temperature: 100°C:desolvation temperature: 350°C.
2.8 NMR analysis
For the structural elucidation of isolated metabolites, low-resolution LC/MS and NMR spectroscopy have been used. 1H (400 MHz) and 13C (100 MHz) NMR spectra were recorded employing a VARIAN MERCURY AGILENT 400 MHz FT spectrometer, with CD3OD as solvent. Chemical shifts (δ) are in ppm relative to TMS.
2.9 Mycelial dry weights
Mycelial weight was determined by filtering the content of the flasks through preweighed filter paper (Whatman) and then filter paper was left to dry at 40°C for 48 h.
3 Results and discussion
3.1 Selecting most efficient strain
Aspergillus niger, Funalia trogii, Phanerochaete chrysosporium, and Yarrowia lipolytica were assessed in terms of naproxen degradation capabilities. According to LC–MS data, A. niger was found to be most efficient fungal source with 98% naproxen removal rate within 48 h (Figure 1).
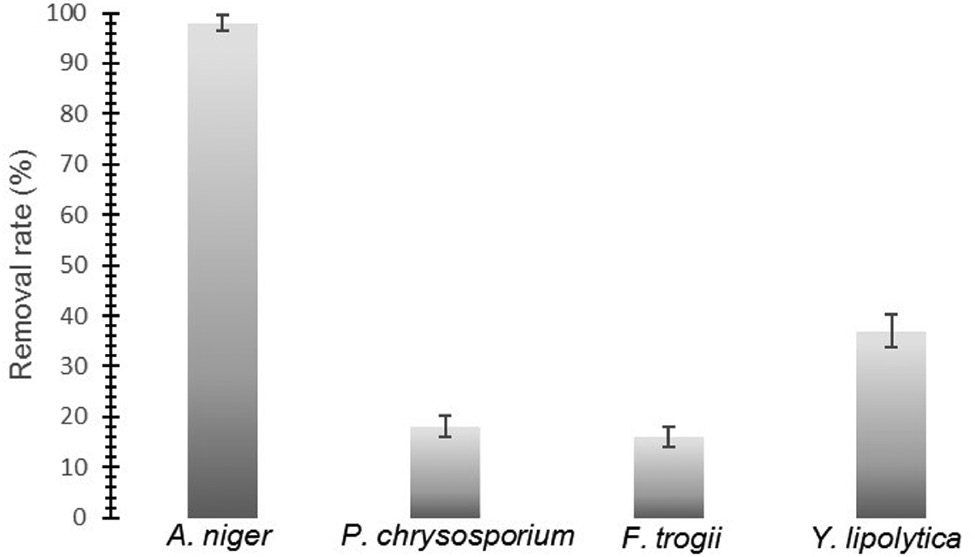
Naproxen removal capabilities of A. niger, P. chrysosporium, F. trogii, and Y. lipolytica (After 48 h incubation).
While living Aspergillus niger (0.74 g dry weight) cells were able to remove naproxen in high percentage (98%), heat killed Aspergillus niger cells (0.69 g dry weight) reached barely to a 19% removal rate in 48 h (Figure 2). Therefore, A. niger removes naproxen through biodegradation rather than adsorption.
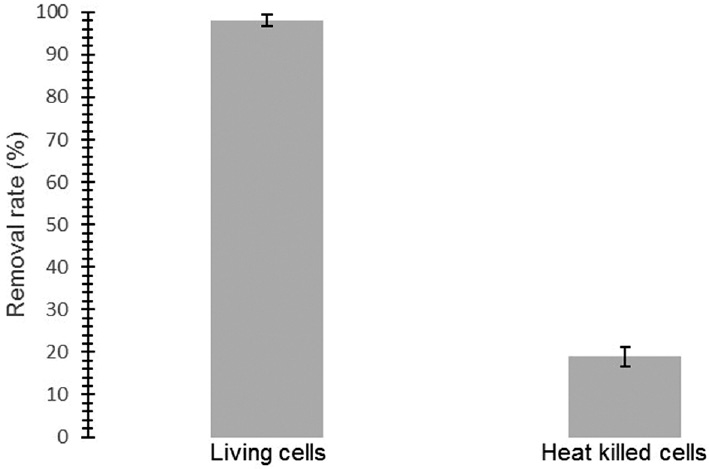
Naproxen removal rates for living and heat-killed Apergillus niger cells.
White-rot fungi are good candidate for biodegradation of environmental pollutants and many studies reported that these fungi are able to degrade wide variety of pollutants by means of their nonspecific ligninolytic enzymes [31]. A previous study showed that white-rot fungus Trametes versicolor almost completely degrade naproxen (10 mg L−1) in 24 h [6]. In addition, another study demonstrated that Trametes versicolor remove significant amount of naproxen (0.1 mg L−1) in the sterile soil environment in 24 h and the authors suggested that T. versicolor could be used for soil-bioaugmentation processes [17]. Rodarte-Morales et al. reported that 1 mg L−1 naproxen was totally degraded by P. chrysosporium at day 4th [32]. Immobilized Phanerochaete chrysosporium cells almost completely degrade 1 mg L−1 naproxen in 7 days [33]. Eibes et al. achieved 80% removal of naproxen using versatile peroxidase which was obtained from Bjerkandera adusta [34]. While, in our study, two white-rot fungal strains Funalia trogii and Phanerochaete chrysosporium showed lowest removal percentage in 48 h (Figure 1). Consequently, A. niger was chosen as the most efficient fungus in terms of naproxen degradation capability. A. niger is a filamentous aerobic fungus. A. niger is able to grow in the range of various pH (1.4–9.8) and temperature (6–47°C) [26]. A. niger could degrade environmental pollutants such as herbicide chlorimuron-ethyl, chlorsulfuron, and metsulfuron-methyl [28], [35]. Due to A. niger’s growth ability in a wide range of pH and temperature, we think that A. niger is a good candidate for naproxen removal applications. In addition, in our study, we showed that A. niger could remove much higher concentrations of naproxen (50 mg L−1) than previous studies.
3.2 Identification of degradation products
In order to identify the degradation by-products of naproxen, LC/MS, 1HNMR, and 13CNMR analyses were performed. Metabolite 1 (tR 6.99 min), metabolite 2 (tR 8.35 min), and untransformed naproxen (Figure 3A) were detected by LC/MS (ESI-) technique of the direct extraction of medium. Naproxen gave a molecular ion of [M–H] at m/z 229 and the adduct ion [2M–H] at 459 m/z (Figure 3B).
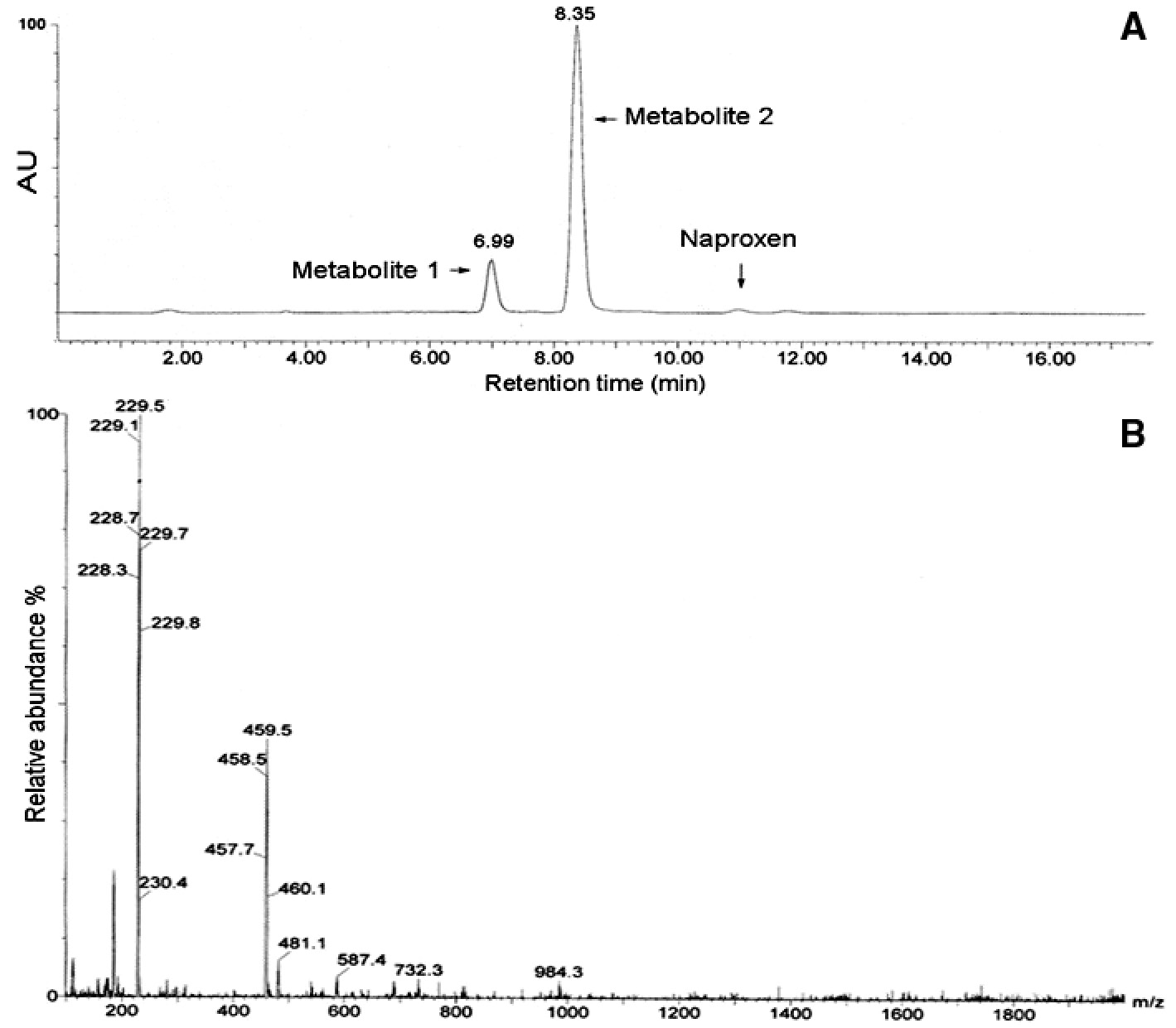
(A) LC chromatogram relevant to naproxen after 48-h incubation with Aspergillus niger, (B) Full scan ESI (–) mass spectrum for untransformed naproxen.
Metabolite 1 (M1) (yield 14%) exhibits three major ions at m/z 215 (100%), 431 (19%), and 171 (22%), which are corresponded to molecular ion [M–H], to the adduct ion [2M–H] and ion [M-COOH], respectively (Figure 4). Major ion at m/z 215, 14 mass units lower than naproxen which was identified as O-desmethylnaproxen (C13H12O3). This compound was identified as a product of fungal transformation reactions by several studies [6], [36], [37]. 1H-NMR and 13C-NMR results confirmed chemical structure of M1 (Table 1).
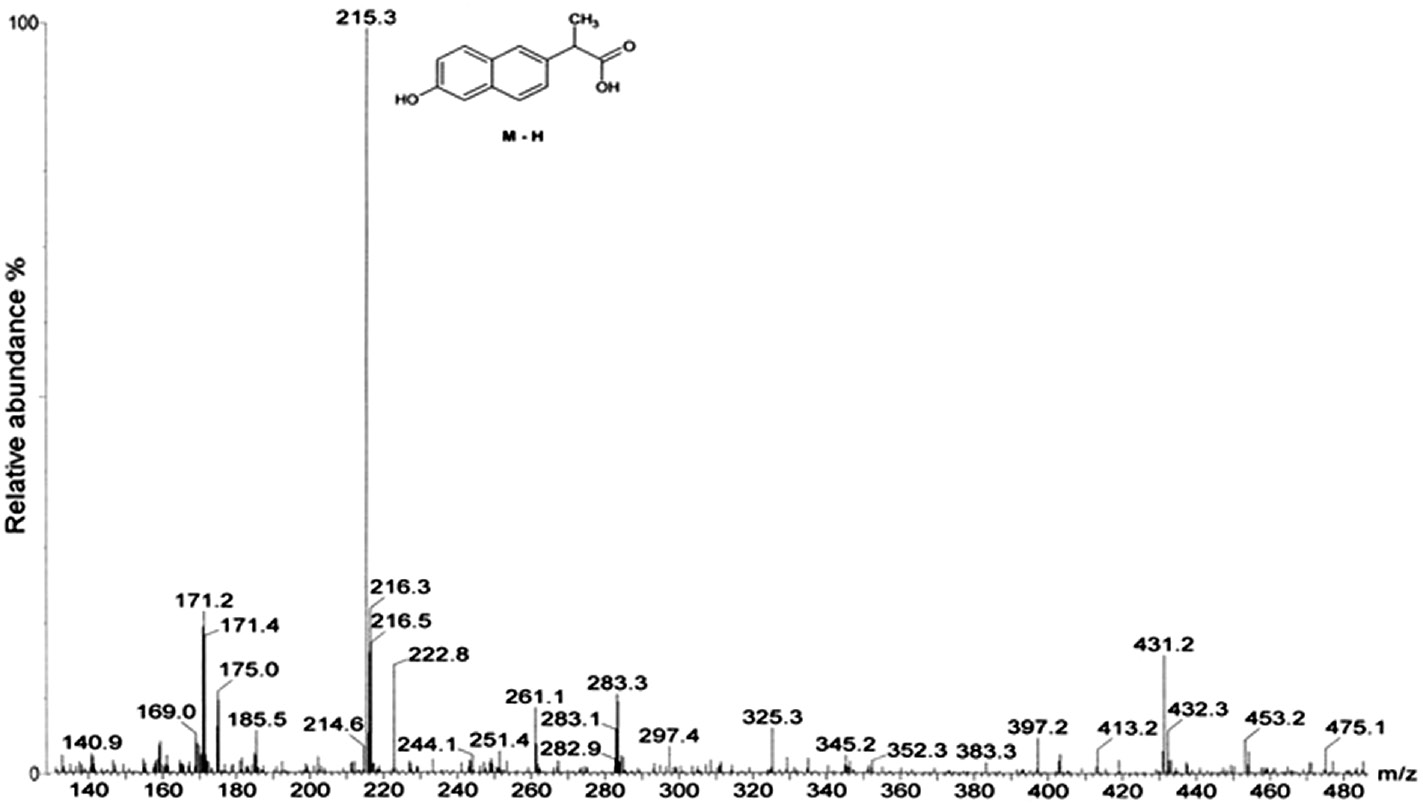
Full scan ESI (–) mass spectrum for metabolite 1 (O-desmethylnaproxen).
Chemical structures and 1H-NMR and 13C-NMR data for fungal transformation metabolites of naproxen.
| Metabolite | 1H-NMR (CD3OD) 400 MHz δ ppm | 13C-NMR (CD3OD) 100 MHz δ ppm |
|---|---|---|
M1 (O-desmethylnaproxen)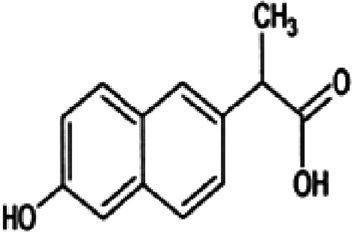 | 1.51 (d, 3H, J=7.2Hz, -CH3) 3.79 (q, 1H, J=7.2Hz, -CH-) 7.02 (dd, 1H, J=8,6 & 2.4Hz, H-7) 7.06 (d, 1H, J=2.4Hz, H-5) 7.35 (dd, 1H, J=8,4 & 1.6Hz, H-3) 7.58 (d, 1H, J=8.4Hz, H-4) 7.63 (s, 1H, H-1) 7.66 (d, 1H, J=8.4Hz, H-8) | 179.3, 156.6, 137.3, 135.6, 130.5, 130.1, 127.7, 127.3, 127.1, 119.6, 109.9, 47.1, 19.3 |
M2 (7-hydroxynaproxen)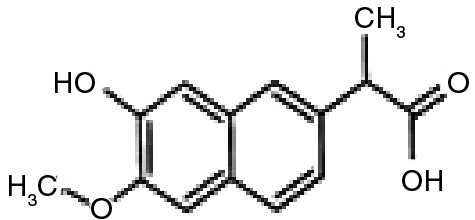 | 1.48 (d, 3H, J=7.2Hz, -CH3) 3.72 (q, 1H, J=7.2Hz, -CH-) 3.88 (s, 3H, -OCH3) 6.81 (d, 1H, J=1.6Hz, H-5), 7.06 (dd, 1H, J=9,2 & 2.8Hz, H-3) 7.18 (s, 1H, H-8), 7.46 (d, 1H, J=2.8Hz, H-1), 7.62 (d, 1H, J=9.2Hz, H-4) | 178.9, 158.6, 153.9, 138.1, 131.8, 130.2, 126.5, 120.1, 118.5, 109.3, 101.5, 55.8, 46.9, 19.2 |
Metabolite 2 (M2) (yield 84%) exhibits two major ions at m/z 245 (29%) and 201 (100%), which are corresponded to molecular ions [M–H] and [M–COOH], respectively (Figure 5). Molecular ion at m/z 245 [M–H] is 16 mass unit higher than naproxen. This ion should be formed by addition of one hydroxyl group to the aromatic moiety of naproxen molecule. Actually, the NMR data of M2 confirmed that hydroxylation has been occurred at 7 position of naproxen (Table 1). Thus, metabolite 2 was identified as 7-hydroxynaproxen (C14H14O4), which was previously reported as a fungal metabolite of naproxen [37].
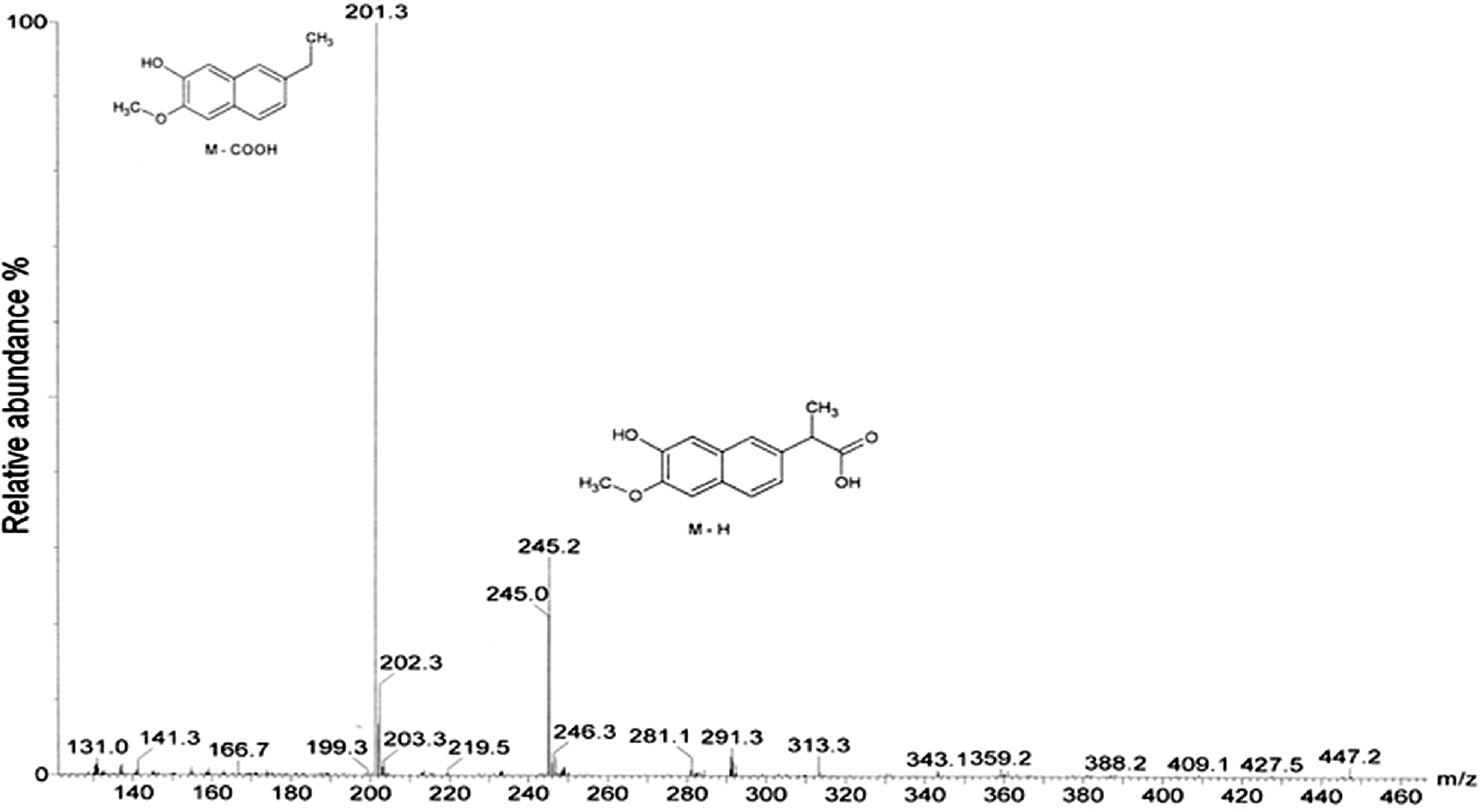
Full scan ESI (–) mass spectrum for metabolite 2 (7-hydroxynaproxen).
3.3 Removal mechanism of naproxen
In the first part of this study, Aspergillus niger was the efficient strain in terms of naproxen removal potential and two fungal transformation products of naproxen, O-desmethylnaproxen and 7-hydroxynaproxen were identified using LC/MS and NMR analyses.
First of all, we had to demonstrate localization of enzymes/enzyme which were responsible of transformation reactions. In order to show whether extracellular enzymes responsible of formation of metabolites, culture supernatant was used for extracellular enzyme source.
We incubated naproxen with A. nigerculture supernatant for 48 h, and we could not determine formation of any metabolites. These results indicate that intracellular enzymes or enzyme have a role biotransformation of naproxen. Cytochrome P450 (CYP) isoforms CYP2C9 and CYP1A2 are responsible for O-demethylation of naproxen in the human liver [38]. Fungal cytochrome 450 catalyzes many transformation reactions including hydroxylation, epoxidation, and O-demethylation. CYP53A1 of A. niger catalyze O-demethylation of 3-methoxybenzoate derivative [39]. Moreover, previous studies showed that fungal cytochrome P450 enzyme system have an important role on transformation of naproxen [36], [37]. To ascertain the role of cytochrome P450, CYP450 inhibitor 2 mM 1-aminobenzotriazole was added in culture medium before addition of naproxen.
After 48 h incubation, we observed that 1-aminobenzotriazole completely inhibited formation of O-desmethylnaproxen and 7-hydroxynaproxen. A previous study reported that Cunninghamella species transformed naproxen into desmethylnaproxen and desmethylnaproxen-6-O-sulfate via mammalian analog cytochrome P450 enzyme system [36]. Cytochrome enzymes are usually involved in various hydroxylation reactions. Aspergillus nidulans can catalyze hydroxylation of phenylacetate by means of cytochrome P450 monooxygenase [40]. Thus, we suggest that A. niger can perform biotransformation of naproxen by CYP450 enzyme system (Figure 6).
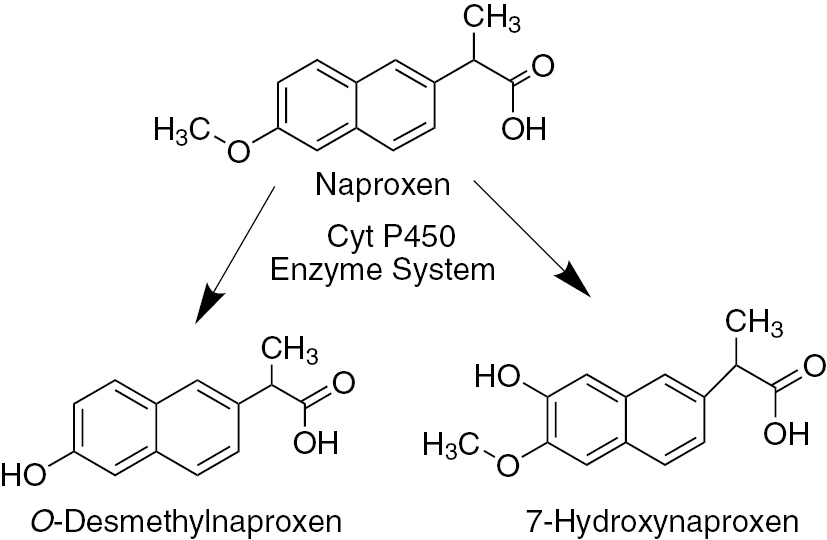
Proposed pathway of naproxen transformation.
4 Conclusion
In this study, Phanerochaete chrysosporium, Funalia trogii, Aspergillus niger and Yarrowia lipolytica were tested in order to select most efficient fungal strain in terms of naproxen removal potential. Among the tested strains, Aspergillus niger was found the most efficient fungal source with 98% naproxen removal capability. Two main metabolites, O-desmethylnaproxen and 7-hydroxynaproxen, were identified using LC/MS and NMR analyses in Aspergillus niger culture medium. Our observations suggest that O-demethylation and hydroxylation of naproxen catalyze via fungal P450 enzyme system. Consequently, we report that Aspergillus niger is a good candidate for removal of micro pollutant naproxen.
Acknowledgments
This study was supported by Hacettepe University Scientific Research Projects Coordination Unit (Project No: 013D05601011).
References
1. Luo Y, Guo W, Ngo HH, Nghiem LD, Hai FI, Zhang J, et al. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci Total Environ 2014;473–474:619–41.10.1016/j.scitotenv.2013.12.065Suche in Google Scholar
2. Kosjek T, Heath E, Krbavcic A. Determination of Non-steroidal Anti-inflammatory Drug (NSAIDs) residues in water samples. Environ Int 2005;31:679–85.10.1016/j.envint.2004.12.001Suche in Google Scholar
3. Martín J, Camacho-Muñoz D, Santos JL, Aparicio I, Alonso E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. J Hazard Mater 2012;239–240:40–7.10.1016/j.jhazmat.2012.04.068Suche in Google Scholar
4. Kümmerer K. Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources–a review. Chemosphere 2001;45:957–69.10.1016/S0045-6535(01)00144-8Suche in Google Scholar
5. Fent K, Weston AA, Caminada D. Ecotoxicology of human pharmaceuticals. Aquat Toxicol 2006;76:122–59.10.1016/j.aquatox.2005.09.009Suche in Google Scholar PubMed
6. Marco-Urrea E, Pérez-Trujillo M, Blánquez P, Vicent T, Caminal G. Biodegradation of the analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DAD-MS and NMR. Bioresour Technol 2010;101:2159–66.10.1016/j.biortech.2009.11.019Suche in Google Scholar PubMed
7. Isidori M, Lavorgna M, Nardelli A, Parrella A, Previtera L, Rubino M. Ecotoxicity of naproxen and its phototransformation products. Sci Total Environ 2005;348:93–101).10.1016/j.scitotenv.2004.12.068Suche in Google Scholar PubMed
8. Zhang Y, Yang Y, Zhang Y, Zhang T, Ye M. Heterogeneous oxidation of naproxen in the presence of α-MnO2 nanostructures with different morphologies. Appl Catal B Environ 2012;127:182–9.10.1016/j.apcatb.2012.08.014Suche in Google Scholar
9. Arany E, Szabó RK, Apáti L, Alapi T, Ilisz I, Mazellier P, et al. Degradation of naproxen by UV, VUV photolysis and their combination. J Hazard Mater 2013;262:151–7.10.1016/j.jhazmat.2013.08.003Suche in Google Scholar PubMed
10. Nakada N, Shinohara H, Murata A, Kiri K, Managaki S, Sato N, et al. Removal of selected pharmaceuticals and personal care products (PPCPs) and endocrine-disrupting chemicals (EDCs) during sand filtration and ozonation at a municipal sewage treatment plant. Water Res 2007:41:4373–82.10.1016/j.watres.2007.06.038Suche in Google Scholar PubMed
11. Zhao X, Qu J, Liu H, Qiang Z, Liu R, Hu C. Photoelectrochemical degradation of anti-inflammatory pharmaceuticals at Bi2MoO6-Boron-Doped diamond hybrid electrode under visible light irradiation, Appl Catal B Environ 2009;91:539–45.10.1016/j.apcatb.2009.06.025Suche in Google Scholar
12. Hasan Z, Jeon J, Jhung SH. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J Hazard Mater 2012;209–210:151–7.10.1016/j.jhazmat.2012.01.005Suche in Google Scholar PubMed
13. Im JK, Heo J, Boateng LK, Her N, Flora JR V, Yoon J, et al. Ultrasonic degradation of acetaminophen and naproxen in the presence of single-walled carbon nanotubes. J Hazard Mater 2013;254–255:284–92.10.1016/j.jhazmat.2013.04.001Suche in Google Scholar PubMed
14. Boyd GR, Zhang S, Grimm DA. Naproxen removal from water by chlorination and biofilm processes. Water Res 2005;39:668–76.10.1016/j.watres.2004.11.013Suche in Google Scholar PubMed
15. Domaradzka D, Guzik U, Hupert-Kocurek K, Wojcieszyńska D. Cometabolic degradation of naproxen by Planococcus sp. strain S5. Water Air Soil Pollut 2015;226:297.10.1007/s11270-015-2564-6Suche in Google Scholar PubMed PubMed Central
16. Zhang L, Hu J, Zhu R, Zhou Q, Chen J. Degradation of paracetamol by pure bacterial cultures and their microbial consortium. Appl Microbiol Biotechnol 92013;7:3687–98.10.1007/s00253-012-4170-5Suche in Google Scholar PubMed
17. Borrás E, Llorens-Blanch G, Rodríguez-Rodríguez CE, Sarrá M, Caminal G. Soil colonization by Trametes versicolor grown on lignocellulosic materials: substrate selection and naproxen degradation. Int Biodeterior Biodegrad 2011;65:846–852.10.1016/j.ibiod.2011.06.005Suche in Google Scholar
18. Yu T-H, Lin AY-C, Panchangam SC, Hong P-KA, Yang P-Y, Lin C-F. Biodegradation and bio-sorption of antibiotics and non-steroidal anti-inflammatory drugs using immobilized cell process. Chemosphere 2011;84:1216–22.10.1016/j.chemosphere.2011.05.045Suche in Google Scholar PubMed
19. Gao D, Du L, Yang J, Wu W-M, Liang H. A critical review of the application of white rot fungus to environmental pollution control. Crit Rev Biotechnol 2010;30:70–7.10.3109/07388550903427272Suche in Google Scholar PubMed
20. Rymowicz W, Fatykhova AR, Kamzolova SV, Rywińska A, Morgunov IG. Citric acid production from glycerol-containing waste of biodiesel industry by Yarrowia lipolytica in batch, repeated batch, and cell recycle regimes. Appl Microbiol Biotechnol 2010;87:971–9.10.1007/s00253-010-2561-zSuche in Google Scholar PubMed
21. Destain J, Roblain D, Thonart P. Improvement of lipase production from Yarrowia lipolytica. Biotechnol Lett 1997;19:105–7.10.1023/A:1018339709368Suche in Google Scholar
22. Schrader J, Etschmann MM, Sell D, Hilmer JM, Rabenhorst J. Applied biocatalysis for the synthesis of natural flavour compounds – current industrial processes and future prospects. Biotechnol Lett 2004;26:463–72.10.1023/B:BILE.0000019576.80594.0eSuche in Google Scholar
23. Jain MR, Zinjarde SS, Deobagkar DD. Deobagkar DN, 2,4,6-trinitrotoluene transformation by a tropical marine yeast, Yarrowia lipolytica NCIM 3589. Mar Pollut Bull 2004;49:783–8.10.1016/j.marpolbul.2004.06.007Suche in Google Scholar
24. Aracagök YD, Cihangir N. Decolorization of reactive black 5 by Yarrowia lipolytica NBRC 1658. Am J Microbiol Res 2013;1:16–20.10.12691/ajmr-1-2-1Suche in Google Scholar
25. Murphy GL, Perry JJ. Assimilation of chlorinated alkanes by hydrocarbon-utilizing fungi. J Bacteriol 1984;160:1171–4.10.1128/jb.160.3.1171-1174.1984Suche in Google Scholar
26. Schuster E, Dunn-Coleman N, Frisvad JC, Van Dijck PW. On the safety of Aspergillus niger – a review. Appl Microbiol Biotechnol 2002;59:426–35.10.1007/s00253-002-1032-6Suche in Google Scholar
27. Cihangir N, Sarikaya E. Investigation of lipase production by a new isolate of Aspergillus sp. World J Microbiol Biotechnol 2004;20:193–7.10.1023/B:WIBI.0000021781.61031.3aSuche in Google Scholar
28. Sharma S, Banerjee K, Choudhury PP. Degradation of chlorimuron-ethyl by Aspergillus niger isolated from agricultural soil. FEMS Microbiol Lett 2012;337:18–24.10.1111/1574-6968.12006Suche in Google Scholar
29. Bhalerao TS, Puranik PR. Biodegradation of organochlorine pesticide, endosulfan, by a fungal soil isolate, Aspergillus niger. Int Biodeterior Biodegrad 2007;59:315–21.10.1016/j.ibiod.2006.09.002Suche in Google Scholar
30. Aktaş N, Çiçek H, Taşpnar Ünal A, Kibarer G, Kolankaya N, Tanyolaç A. Reaction kinetics for laccase-catalyzed polymerization of 1-naphthol. Bioresour Technol 2001;80:29–36.10.1016/S0960-8524(01)00063-3Suche in Google Scholar
31. Editors S, Murphy RJ, Rulkens WH. Microbial degradation of xenobiotics. In: Singh SN, editor. Environmental science and engineering. Berlin, Heidelberg: Springer, 2011.Suche in Google Scholar
32. Rodarte-Morales AI, Feijoo G, Moreira MT, Lema JM. Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J Microbiol Biotechnol 2011;27:1839–46.10.1007/s11274-010-0642-xSuche in Google Scholar
33. Li X, de Toledo RA, Wang S, Shim H. Removal of carbamazepine and naproxen by immobilized phanerochaete chrysosporium under non-sterile condition. N Biotechnol 2015;32:282–9.10.1016/j.nbt.2015.01.003Suche in Google Scholar
34. Eibes G, Debernardi G, Feijoo G, Moreira MT, Lema JM. Oxidation of pharmaceutically active compounds by a ligninolytic fungal peroxidase. Biodegradation 2011;22:539–50.10.1007/s10532-010-9426-0Suche in Google Scholar
35. Boschin G, D’Agostina A, Arnoldi A, Marotta E, Zanardini E, Negri M, et al. Biodegradation of chlorsulfuron and metsulfuron-methyl by Aspergillus niger in laboratory conditions. J Environ Sci Health B 2003;38:737–46.10.1081/PFC-120025557Suche in Google Scholar
36. Zhong D-F, Sun L, Liu L, Huang H-H. Microbial transformation of naproxen by Cunninghamella species. Acta Pharmacol Sin 2003;24:442–7.Suche in Google Scholar
37. He A, Rosazza JP. Microbial transformations of S-naproxen by Aspergillus niger ATCC 9142. Pharmazie 2003;58:420–2.10.1002/chin.200339085Suche in Google Scholar
38. Miners JO, Coulter S, Tukey RH, Veronese ME, Birkett DJ. Cytochromes P450, 1A2, and 2C9 are responsible for the human hepatic O’demethylation of R- and S-naproxen. Biochem Pharmacol 1996;51:1003–8.10.1016/0006-2952(96)85085-4Suche in Google Scholar
39. Črešnar B, Petrič Š. Cytochrome P450 enzymes in the fungal Kingdom. Biochim Biophys Acta 2011;1814:29–35.10.1016/j.bbapap.2010.06.020Suche in Google Scholar PubMed
40. Mingot JM, Peñalva MA, Fernández-Cañón JM Disruption of phacA, an Aspergillus nidulans gene encoding a novel cytochrome P450 monooxygenase catalyzing phenylacetate 2-hydroxylation, results in penicillin overproduction. J Biol Chem 1999;274:14545–50.10.1074/jbc.274.21.14545Suche in Google Scholar PubMed
Supplemental Material:
The online version of this article (DOI: https://doi.org/10.1515/znc-2016-0162) offers supplementary material, available to authorized users.
©2017 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol
Artikel in diesem Heft
- Frontmatter
- Aspernolides L and M, new butyrolactones from the endophytic fungus Aspergillus versicolor
- Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position
- Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites
- A comparative proteomic analysis for adventitious root formation in lotus root (Nelumbo nucifera Gaertn)
- Curviflorside and curviflorin, new naphthalene glycoside and flavanol from Plicosepalus curviflorus
- Termiglaucescin, a new polyhydroxy triterpene glucoside from Terminalia glaucescens with antioxidant and anti-inflammatory potential
- Influence of structure of lactones with the methylcyclohexene and dimethylcyclohexene ring on their biotransformation and antimicrobial activity
- Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: in vitro evaluations
- Bioenergetic strategy of microalgae for the biodegradation of tyrosol and hydroxytyrosol


