Abstract
Two new organically templated borates, [(1,10-phen)(H3BO3)2] (1) and [2-EtpyH][(B5O6(OH)4] (2), were synthesized under mild solvothermal conditions and characterized by single-crystal X-ray diffraction, FT-IR, elemental analyses and thermogravimetry. Complex 1 is a co-crystal component in which B(OH)3 molecules form crinkled tapes via hydrogen bonds, which in turn are packed in a two-dimensional framework with 1,10-phen molecules sandwiched between the framework of crinkled chains. The structure of complex 2 is composed of 2-ethylpyridinium cations [2-EtpyH]+ and pentaborate anions [B5O6(OH)4]– which are linked together with hydrogen bonds to form a three-dimensional supramolecular framework. The templating [2-EtpyH]+ cations are situated in the cavities arising from the supramolecular pentaborate framework and interact also with the inorganic framework through hydrogen bonds.
1 Introduction
In recent decades, borate materials have been extensively studied for their rich structural chemistry and their wide range of applications [1–11]. From the structural chemistry point of view, the boron atoms are bound with oxygen atoms in both tetrahedral BO4 and triangular BO3 units. These basic structures may further be linked together via common oxygen atoms to create one-dimensional chains, two-dimensional sheets, and three-dimensional frameworks [12, 13]. So far, borates with alkali metal, alkaline earth metal, rare earth metal, and transition metal ions have been widely studied [14–16].
Recently, structures directed by effects of organic templates on polyborate anions have been observed, and most of the reported organic template borates are comprised of polyborate anions such as [B3O3(OH)4]– [17, 18], [B4O5(OH)4]2– [19], [B5O6(OH)4]– [19–21], [B5O8(OH)]2– [20], [B6O9(OH)2]2– [22], [B7O9(OH)5]2– [23, 24], [B7O10(OH)3]– [25], [B8O11(OH)4]2– [26], [B8O10(OH)6]2– [21], [B9O12(OH)6]3– [27], and [B14O20(OH)6]– [23]. Borate chemistry has been reviewed recently [28], A competent overview of recent solid-state borate chemistry is given in a wealth of further articles in: Z. Kristallogr. 2013, 228, issues 9 and 10. Understanding how various amine cations direct borate structural units in polyborate compounds is fundamental for the rational design of novel borates having useful properties. During our investigations of such borate materials, we have investigated the self-assembly of organic and inorganic moieties under mild solvothermal conditions. Herein, we describe the syntheses and crystal structures of two new organically templated borate compounds, [(1,10-phen)(H3BO3)2] (1) and [2-EtpyH][(B5O6(OH)4] (2). Complex 1 is a co-crystal, where B(OH)3 molecules are linked via hydrogen bonds to form crinkled tapes, which in turn, are packed in a two-dimensional framework so that 1,10-phen molecules are sandwiched between the crinkled chains. Complex 2 is built up of [2-EtpyH]+ cations and pentaborate anions, which are linked together by hydrogen bonds to form a three-dimensional supramolecular framework.
2 Experimental section
2.1 General
B(OH)3, 1,10-phenanthroline monohydrate (1,10-phen·H2O), pyridine (py), and 2-ethyl-pyridine (2-Etpy) were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China and used without further purification. FT-IR spectra were measured on a Nicolet FT 1703X spectrophotometer in the 4000–400 cm–1 region using KBr pellets (USA). The thermogravimetric analysis (TGA) was performed with a TA-SDT Q600 thermal analyzer under N2 atmosphere with a heating rate of 10 oC min–1 (USA). Elemental analyses were carried out using a Perkin-Elmer 2400 CHN analyzer (USA). The single-crystal X-ray diffraction was performed on SMART APEX II (Germany). The collected frames were processed with the software saint, Area Detector Control and Integration Software, Bruker Analytical X-ray Instruments Inc., Madison, Wisconsin (USA). An empirical absorption correction was applied using thesadabs program, Program for Empirical Absorption Correction of Area Detector Data, University of Gottingen, Gottingen, Germany. The structures were all solved by Direct Methods using the shelxs-97 program package. The structures were refined on F2 by full-matrix least-squares methods using the program shelxl-97, Software Reference Manual (version 5.1), Bruker Analytical X-ray Instruments Inc., Madison, Wisconsin (USA), also see G. M. Sheldrick, Acta Crystallogr. 2008, A64, 112.
2.2 Synthesis of [(1,10-phen)(H3BO3)2] (1)
A mixture of B(OH)3 (247.3 mg, 4.0 mmol), 1,10-phen anthroline monohydrate (198.2 mg, 1.0 mmol), pyridine (3955.2 mg, 50 mmol), and distilled water (900 mg, 50 mmol) was sealed in a 23 mL Teflon-lined stainless steel autoclave, heated to 160 °C under autogenous pressure for 3 days, and then cooled to room temperature at a rate of 5 °C/h. The large colorless prism-shaped crystals of 1 were filtered, washed with distilled water, and dried at ambient temperature in air (0.287 g, yield 79 % based on boron). – Anal. for C12H14B2N2O6: Calcd. C 47.43, H 4.64, N 7.12; found: C 47.40, H 4.63, N 7.14.
2.3 Synthesis of [2-EtpyH][(B5O6(OH)4](2)
A mixture of B(OH)3 (494.4 mg, 8.0 mmol), 2-ethylpyridine (2-Etpy) (932.3 mg, 8.7 mmol), and N,N′-dimethylformamide (DMF) (3655.2 mg, 50 mmol) in a 23 mL Teflon-lined stainless steel autoclave was heated to 180 °C under autogenous pressure for 3 days and then cooled to room temperature at a rate of 5 °C h–1. The colorless block-shaped crystals of 2 were filtered, washed with distilled water, and dried at ambient temperature (0.324 g, yield 62 % based on boron). – Anal. for C7H14B5NO10: Calcd. C 25.77, H 4.33, N 4.29; found: C 25.79, H 4.35, N, 4.26 %.
2.4 Determination of crystal structures
Single crystals of 1 (prism, dimensions 0.27 × 0.14 × 0.12 mm3) and 2 (block, dimensions 0.36 × 0.31 × 0.27 mm3) were carefully selected under an optical microscope and glued to thin glass fibers with epoxy resin. Single-crystal X-ray diffraction data were collected on a Siemens SMART CCD 2K diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073 Å) in an ω-scanning mode at 293 K. The collected frames were processed with the software saint. An empirical absorption correction was applied using the sadabs program. The structures were all solved by Direct Methods using the shelxs-97 program package. The structures were refined on F2 by full-matrix least-squares methods using the program shelxl-97. All the non-hydrogen atoms were refined anisotropically. The crystallographic data and experimental details are summarized in Table 1, while selected bond lengths and angles are given in the supplementary materials.
Crystallographic data and experimental details for [(1,10-phen)(H3BO3)2] (1) and [2-EtpyH][(B5O6(OH)4] (2).
| 1 | 2 | |
|---|---|---|
| Empirical formula | C12H14B2N2O6 | C7H14B5NO10 |
| Formula weight | 303.87 | 326.23 |
| Space group | P1̅ | P21/c |
| Z | 2 | 4 |
| a, Å | 7.163(5) | 9.2997(15) |
| b, Å | 9.644(5) | 16.251(3) |
| c, Å | 10.533(9) | 9.9497(17) |
| α, deg | 93.770(9) | 90 |
| β, deg | 101.760(7) | 91.938(2) |
| γ, deg | 90.725(5) | 90 |
| V (Å3) | 710.6(9) | 1502.8(4) |
| Dcalcd, g cm–3 | 1.42 | 1.44 |
| Temperature, K | 296(2) | 296(2) |
| F(000), e | 316 | 672 |
| µ(MoKα), mm–1 | 0.1 | 0.1 |
| Refl. total/unique/Rint | 4436/3127/0.0228 | 9075/3393/0.0277 |
| Ref. Parameters | 206 | 213 |
| R1a/wR2b (I > 2σ(I)) | 0.0432/0.1127 | 0.0458/0.1188 |
| R1a/wR2b (all data) | 0.0629/0.1258 | 0.0669/0.1323 |
| GoFc | 1.036 | 1.045 |
| Δρfin (max/min), e Å–3 | +0.18/–0.17 | +0.42/–0.30 |
aR1 = ∑||Fo|–|Fc||/∑|Fo|; bwR2 = [∑w(Fo2–Fc2)2/∑w(Fo2)2]1/2, w = [σ2(Fo2) + (AP)2+BP]–1, where P = (Max(Fo2, 0) + 2Fc2)/3; cGoF = [∑w(Fo2–Fc2)2/(nobs–nparam)]1/2.
CCDC 1029042 (1) and 1029043 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
3 Results and discussion
3.1 Synthesis and growth of crystals
Complex 1 was synthesized under mild conditions from the reaction of B(OH)3 and 1,10-phen monohydrate in the presence of pyridine and distilled water. Unlike most organic templates, 1,10-phen does not take up protons from water molecules because its capacity of accepting protons is lower than that of pyridine. B(OH)3 does not form polyborate anions through condensation (dehydration) reactions. Complex 2 was obtained from the similar reaction of B(OH)3 and 2-Etpy in the presence of DMF solvent. Obviously, 2-Etpy is protonated to form [2-EtpyH]+ cation as an organic template for the pentaborate [B5O6(OH)4]– anion.
3.2 Infrared spectra
The infrared (IR) spectra of 1 and 2 exhibit the typical bands for the components (Fig. 1). In the IR spectrum of 1, the strong bands at 1427 and 1272 cm–1 are consistent with the existence of trigonally coordinated boron. The stretching vibration of the O–H bond is observed at 3329 cm–1 as a broad band. In the IR spectrum of 2, the strong bands at 1336 and 1125 cm–1 are characteristic of trigonally coordinated boron, while the bands at 1032, 929, and 770 cm–1 are characteristic of tetrahedrally coordinated boron [29]. The stretching vibration of O–H is observed at 3330 cm–1. The broad band at 3200–2713 cm–1 is attributed to the stretching vibrations of the O–H bonds and the combination of CH2, CH3, and NH groups. The IR spectra of 1 and 2 show bands in two ranges of 1559–1574 cm–1 as the strong peaks and 1165–1200 cm–1as the medium peaks, which can be obviously assigned to the vC–N) of the pyridine ligands.
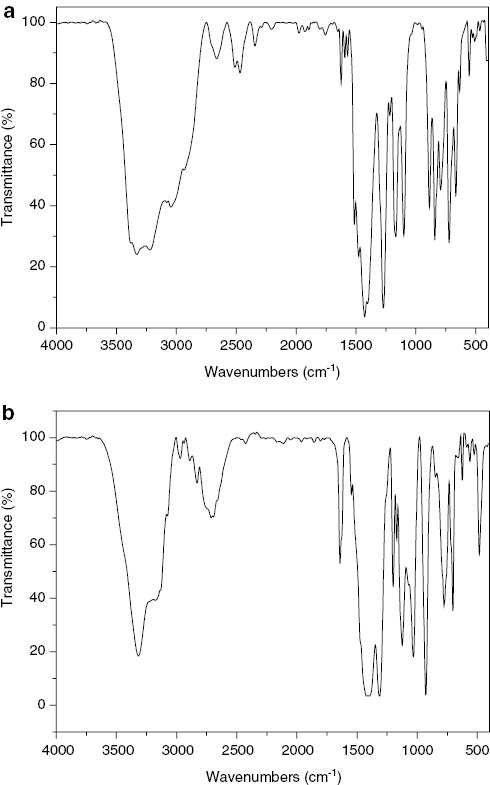
IR spectra of complexes 1 (a) and 2 (b).
3.3 Crystal structures
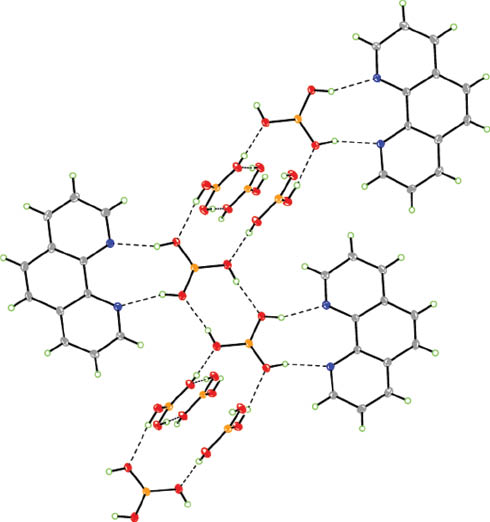
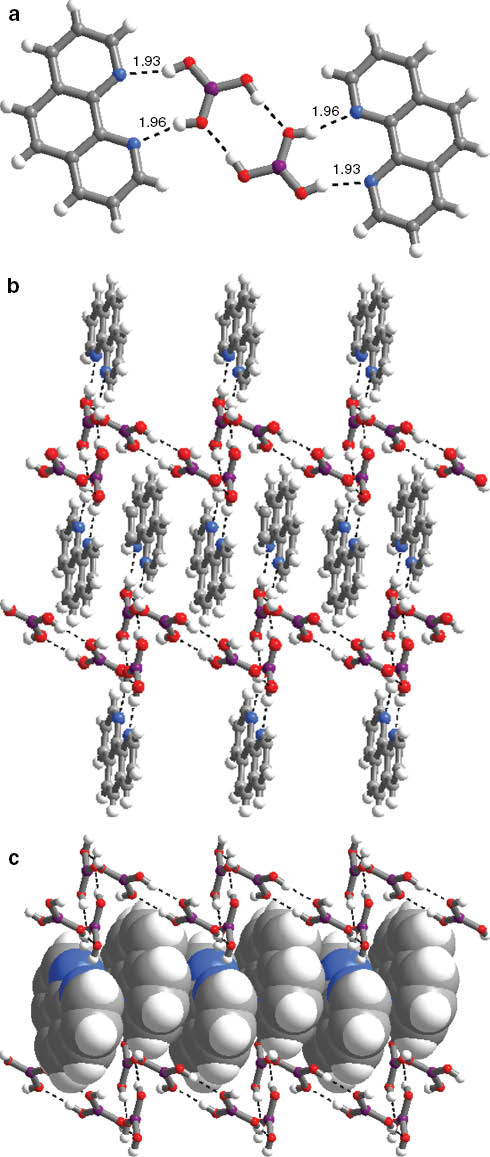
Complex 1 crystallizes in the triclinic space group P1̅ with Z = 2. The molecular structure is illustrated in Fig. 2. Complex 1 is a co-crystal that consists of two B(OH)3 molecules and one 1,10-phen molecule per formula unit. The B(OH)3 and 1,10-phen molecules interact with each other, forming O–H···N hydrogen bonds with H···N distances of 1.93–1.96 Å, as shown in Fig. 3a. Further, as observed for B(OH)3 in 1, the hydroxyl groups are involved in formation of the O–H···.O hydrogen bonds with the H···O distances of 1.90–2.0 Å. As shown in Fig. 3b, the hydrogen bonds involved in the aggregation of the ensembles leads to crinkled tapes, which, in turn, are packed into a two-dimensional hydrogen-bonding framework. 1,10-Phen molecules are sandwiched between the [B(OH)3]n chains (see Fig. 3c). The details of hydrogen bonds of 1 are given in Table 2.
ortep plot of the principal structure components of complex 1.
(a) Recognition pattern between the co-crystal formers boronic acid and 1,10-phenanthroline; (b) O–H···O hydrogen bonds formed by B(OH)3 molecules; (c) packing of molecules in the two-dimensional arrangement in the crystal structure of 1.
Hydrogen bond parameters for 1a.
| D–H···A | d(D–H) (Å) | d(H···A) (Å) | d(D···A) (Å) | ∠(D–H···A) (deg) |
|---|---|---|---|---|
| O1–H1A···O2#1 | 0.82 | 1.94 | 2.752(2) | 173.3 |
| O2–H2A···O6#2 | 0.82 | 1.93 | 2.735(2) | 166.0 |
| O3–H3A···O4#3 | 0.82 | 2.00 | 2.811(2) | 171.6 |
| O6–H6A···O5#4 | 0.82 | 1.90 | 2.716(2) | 172.3 |
| O4–H4···N2 | 0.82 | 1.93 | 2.725(2) | 162.0 |
| O5–H5···N1 | 0.82 | 1.96 | 2.746(2) | 160.1 |
aSymmetry transformations used to generate equivalent atoms: #1–x + 2, –y + 2, –z; #2 x, y + 1, z–1; #3 –x + 1, –y + 1, –z + 1; #4 –x + 1, –y + 1, –z + 2.
Complex 2 crystallizes in the monoclinic space group P21/c with Z = 4. Figure 4 displays an ortep plot of 2. Selected bond lengths and angles are given in Table 3. The formula unit of 2 consists of one protonated [2-EtpyH]+ cation and one pentaborate [B5O6(OH)4]– anion. As found in several known borates, such as hydrated A[B5O6(OH)4]·xH2O (A = Li+, Na+, K+, Cs+, [NH4]+; x = 2, 3) [30], [Zn(dien)2][B5O6(OH)4]2 (dien = diethylenetriamine) [31], [Me3NCH2CH2OH][B5O6(OH)4] [19], and [(4-MepyH)·(4-Mepy)][B5O6(OH)4] [19], the polyanion [B5O6(OH)4]– is composed of one BO4 tetrahedron and four BO2(OH) triangles, which form two B3O3 rings linked by the common BO4 tetrahedron. The trigonal boron centers have B–O distances in the range of 1.346(2)–1.383(2) Å (average of 1.363(2) Å), and the tetrahedral boron center has relatively longer B–O distances in the range of 1.462(2)–1.473(2) Å (average of 1.468(2) Å). These values are in good agreement with those of other borate compounds reported previously [19–21]. The O–B–O angles in the BO4 tetrahedron lie in the range of 108.52(13)–110.91(12)°, and those in the BO3 triangles span the range from 116.78(15)° to 122.74(15)°; the averages for the corresponding angles are very close to 109.47° and 120°, respectively.
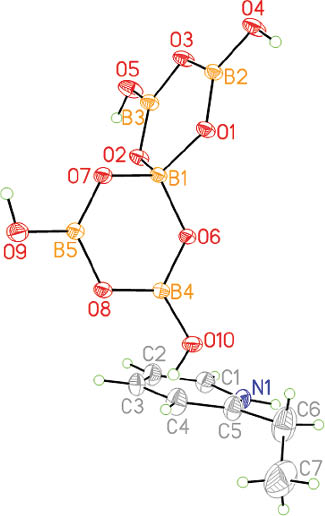
ortep plot of the components of complex 2.
Selected bond lengths (Å) and angles (deg) for complex 2.
| B(1)–O(1) | 1.473(2) | B(3)–O(3) | 1.382(2) |
| B(1)–O(2) | 1.471(2) | B(3)–O(5) | 1.346(2) |
| B(1)–O(6) | 1.462(2) | B(4)–O (6) | 1.366(2) |
| B(1)–O(7) | 1.464(2) | B(4)–O(8) | 1.372(2) |
| B(2)–O(1) | 1.356(2) | B(4)–O(10) | 1.348(2) |
| B(2)–O(3) | 1.378(2) | B(5)–O(7) | 1.358(2) |
| B(2)–O(4) | 1.352(2) | B(5)–O(8) | 1.383(2) |
| B(3)–O(2) | 1.361(2) | B(5)–O(9) | 1.353(2) |
| B(2)–O(1)–B(1) | 123.05(13) | O(4)–B(2)–O(1) | 121.83(15) |
| B(3)–O(2)–B(1) | 123.72(12) | O(4)–B(2)–O(3) | 117.08(14) |
| B(2)–O(3)–B(3) | 119.56(13) | O(1)–B(2)–O(3) | 121.06(14) |
| B(4)–O(6)–B(1) | 123.26(12) | O(5)–B(3)–O(2) | 122.74(15) |
| B(5)–O(7)–B(1) | 123.30(12) | O(5)–B(3)–O(3) | 116.99(14) |
| B(4)–O(8)–B(5) | 119.23(13) | O(2)–B(3)–O(3) | 120.26(15) |
| O(6)–B(1)–O(7) | 110.91(12) | O(10)–B(4)–O(6) | 117.19(14) |
| O(6)–B(1)–O(2) | 108.90(12) | O(10)–B(4)–O(8) | 121.87(14) |
| O(7)–B(1)–O(2) | 109.78(13) | O(6)–B(4)–O(8) | 120.94(14) |
| O(6)–B(1)–O(1) | 108.52(13) | O(9)–B(5)–O(7) | 122.33(15) |
| O(7)–B(1)–O(1) | 108.73(12) | O(9)–B(5)–O(8) | 116.78(15) |
| O(2)–B(1)–O(1) | 109.99(12) | O(7)–B(5)–O(8) | 120.89(15) |
The pentaborate anions [B5O6(OH)4]– are linked together by O–H···O hydrogen bonds to form a three-dimensional supramolecular framework, as shown in Figs. 5a and 5b [32–34]. The templating [2-EtpyH]+ cations are situated in the cavities arising from the supramolecular pentaborate framework and interact with the inorganic framework through the N–H···O hydrogen bonds (N1···O6 = 2.725(2) Å), as shown in Fig. 5c. Hydrogen bond data for complex 2 are summarized in Table 4.
![Fig. 5: (a) The pentaborate anions [B5O6(OH)4]– linked by O–H···O hydrogen bonds; (b) a view along the crystallographic a axis showing a unidirectional rectangle-like borate anion host lattice with 12-membered boron rings; (c) packing of molecules in the three-dimensional arrangement in 2.](/document/doi/10.1515/znb-2014-0277/asset/graphic/j_znb-2014-0277_fig_005.jpg)
(a) The pentaborate anions [B5O6(OH)4]– linked by O–H···O hydrogen bonds; (b) a view along the crystallographic a axis showing a unidirectional rectangle-like borate anion host lattice with 12-membered boron rings; (c) packing of molecules in the three-dimensional arrangement in 2.
Hydrogen bond parameters for 2a.
| D–H···A | d(D–H) (Å) | d(H···A) (Å) | d(D···A) (Å) | ∠(D–H···A) (deg) |
|---|---|---|---|---|
| O4–H4···O1#1 | 0.82 | 1.86 | 2.679(2) | 173.7 |
| O5–H5···O7#2 | 0.82 | 1.96 | 2.772(2) | 173.5 |
| O9–H9···O2#3 | 0.82 | 1.95 | 2.772(2) | 174.8 |
| O10–H10···O4#4 | 0.82 | 1.90 | 2.706(2) | 166.5 |
| N1–H1N···O6#5 | 0.86 | 1.87 | 2.725(2) | 171.3 |
aSymmetry transformations used to generate equivalent atoms: #1 –x, –y, –z; #2 x, –y + ½, z + ½; #3 x, –y + ½, z–½; #4 x + 1, y, z; #5 –x + 1, –y, –z + 1.
3.4 Thermal properties
TGA of complex 2 was carried out in nitrogen atmosphere from 40 to 800 °C with a heating rate of 10 °C min–1. As shown in Fig. 6, the TGA curve of complex 2 shows a continuous weight loss between 270 and 597 °C, which was attributed to the removal of the 2-Etpy (C7H9N) and the dehydration of the boric acids (found: 47.93 %, calcd.: 44.20 %).
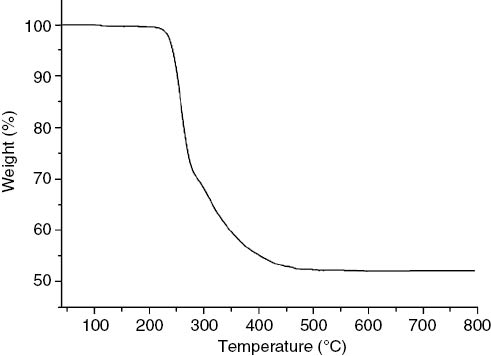
TGA curve of complex 2.
4 Conclusions
In summary, the syntheses and crystal structures, along with the infrared and TGA of two organically templated borates, [(1,10-phen)(H3BO3)2] (1) and [2-EtpyH][(B5O6(OH)4] (2), are reported. Complex 1 comprises two B(OH)3 molecules and one unprotonated 1,10-phen molecule which interact with each other forming O–H···N and O–H···O hydrogen bonds that lead to the aggregation in ensembles in the form of crinkled tapes, which, in turn, are packed in a three-dimensional framework. The 1,10-phen molecules are sandwiched between tapes of B(OH)3 molecules. Complex 2 consists of protonated [2-EtpyH]+ cations and pentaborate anions. The pentaborate [B5O6(OH)4]– anions are linked by hydrogen bonds to form a three-dimensional supramolecular framework. The templating [2-EtpyH]+ cations are situated in the cavities of this framework and interact with it through N–H···O hydrogen bonds.
Acknowledgments
This project was supported by the Natural Science Foundation of China (90922008).
References
[1] Y. C. Wu, T. Sasaki, A. Yokotani, H. G. Tang, C. T. Chen, Appl. Phys. Lett. 1993, 62, 2614.10.1063/1.109262Suche in Google Scholar
[2] S. L. Pan, Y. C. Wu, P. Z. Fu, G. C. Zhang, Z. H. Li, C. X. Du, C. T. Chen, Chem. Mater. 2003, 15, 2218.10.1021/cm020878kSuche in Google Scholar
[3] Y. J. Wang, S. L. Pan, X. L. Tian, Z. X. Zhou, G. Liu, J. D. Wang, D. Z. Jia, Inorg. Chem. 2009, 48, 7800.10.1021/ic900700uSuche in Google Scholar
[4] X. Y. Fan, S. L. Pan, X. L. Tian, Z. X. Zhou, G. Liu, J. D. Wang, Inorg. Chem. 2009, 48, 4806.Suche in Google Scholar
[5] C. D. McMillen, J. W. Kolis, Inorg. Chem. 2011, 50, 6809.Suche in Google Scholar
[6] S. C. Wang, N. Ye, J. Am. Chem. Soc. 2011, 133, 11458.10.1021/ja204179gSuche in Google Scholar
[7] T. Sasaki, Y. Mori, M. Yoshimura, Opt. Mater. 2004, 26, 421.Suche in Google Scholar
[8] F. Li, S. L. Pan, X. L. Hou, J. Yao, Cryst. Growth Des. 2009, 9, 4091.10.1021/cg900336rSuche in Google Scholar
[9] F. Li, X. L. Hou, S. L. Pan, X. A. Wang, Chem. Mater. 2009, 21, 2846.10.1021/cm900560xSuche in Google Scholar
[10] H. T. Zhou, X. L. He, W. N. Zhou, G. H. Zhang, C. L. Zhang, D. H. Huo, J. H. Wang, S. H. Qin, Y. B. Zuo, F. U. Lu, L. J. Liu, X. Y. Wang, C. Y. Liu, D. P. Li, H.X. Zhang, Y. X. Chen, J. Cryst. Growth 2011, 318, 613.10.1016/j.jcrysgro.2010.08.036Suche in Google Scholar
[11] C. McMillen, C. Heyward, H. Giesber, J. Kolis, J. Solid State Chem. 2011, 184, 2966.Suche in Google Scholar
[12] P. C. Burns, J. D. Grice, F. C. Hawthorne, Can. Mineral. 1995, 33, 1131.Suche in Google Scholar
[13] J. D. Grice, P. C. Burns, F. C. Hawthorne, Can. Mineral. 1999, 37, 731.Suche in Google Scholar
[14] A. K. Paul, K. Sachidananda, S. Natarajan, Cryst. Growth Des. 2010, 10, 456.10.1021/cg901054hSuche in Google Scholar
[15] K. Brown, P. E. Car, A. Vega, D. Venegas-Yazigi, V. Paredes-Garcia, M. G. F. Vaz, R. A. Allao, J.-Y. Pivan, E. Le Fur, E. Spodine, Inorg. Chim. Acta 2011, 367, 21.10.1016/j.ica.2010.11.045Suche in Google Scholar
[16] M. J. Saly, J. Li, M. J. Heeg, C. H. Winter, Inorg. Chem. 2011, 50, 7385.10.1021/ic2012815Suche in Google Scholar
[17] D. M. Schubert, M. Z. Visi, C. B. Knobler, Inorg. Chem. 2008, 47, 2017.10.1021/ic7020098Suche in Google Scholar
[18] M. A. Beckett, P. N. Horton, M. B. Hursthouse, J. L. Timmis, RSC Advances 2013, 3, 15185.10.1039/c3ra42387eSuche in Google Scholar
[19] M. A. Beckett, C. C. Bland, P. N. Horton, M. B. Hursthouse, K. S. Varma, J. Organomet. Chem. 2007, 692, 2832.Suche in Google Scholar
[20] G.-M. Wang, Y.-Q. Sun, G.-Y. Yang, J. Solid State Chem. 2004, 177, 4648.10.1016/j.jssc.2004.08.039Suche in Google Scholar
[21] M. Z. Visi, C. B. Knobler, J. J. Owen, M. I. Khan, D. M. Schubert, Cryst. Growth Des. 2006, 6, 538.10.1021/cg0504915Suche in Google Scholar
[22] M. Li, J. Chang, Z. Wang, H. Shi, J. Solid State Chem. 2006, 179, 3265.Suche in Google Scholar
[23] Z.-H. Liu, L.-Q. Li, W.-J. Zhang, Inorg. Chem. 2006, 45, 1430.Suche in Google Scholar
[24] D. M. Schubert, M. Z. Visi, S. Khan, C. B. Knobler, Inorg. Chem. 2008, 47, 4740.10.1021/ic800068tSuche in Google Scholar
[25] S. Yang, G. Li, S. Tian, F. Liao, M. Xiong, J. Lin, J. Solid State Chem. 2007, 180, 2225.Suche in Google Scholar
[26] C.-Y. Pan, G.-M. Wang, S.-T. Zheng, G.-Y. Yang, J. Solid State Chem. 2007, 180, 1553.Suche in Google Scholar
[27] D. M. Schubert, M. Z. Visi, C. B. Knobler, Inorg. Chem. 2000, 39, 2250.10.1021/ic000217uSuche in Google Scholar
[28] R. S. Bubnova, S. K. Filatov, Z. Kristallogr. 2013, 228, 395.10.1524/zkri.2013.1646Suche in Google Scholar
[29] M. A. Beckett, P. N. Horton, M. B. Hursthouse, J. L. Timmis, K. S. Varma, Dalton Trans. 2012, 41, 4396.10.1039/c2dt12310jSuche in Google Scholar
[30] P. Li, Z.-H. Liu, S. W. Ng, Inorg. Chem. Commun. 2008, 11, 893.Suche in Google Scholar
[31] G.-M. Wang, Y.-Q. Sun, G.-Y. Yang, J. Solid State Chem. 2005, 178, 729.10.1016/j.jssc.2004.11.016Suche in Google Scholar
[32] M. Wiebcke, C. C. Freyhardt, J. Felsche, G. Engelhardt, Z. Naturforsch. 1993, 48b, 978.10.1515/znb-1993-0722Suche in Google Scholar
[33] C. C. Freyhardt, M. Wiebcke, J. Felsche, G. Engelhardt, J. Inclusion Phenom. Mol. Recognit. Chem. 1994, 18, 161.Suche in Google Scholar
[34] M. A. Beckett, P. N. Horton, M. B. Hursthouse, D. A. Knox, J. L. Timmis, Dalton Trans. 2010, 39, 3944.10.1039/b927072hSuche in Google Scholar
©2015 by De Gruyter
Artikel in diesem Heft
- Frontmatter
- In this Issue
- EPR studies on carboxylic esters, 23 [1]. Preparation of new dialkyl azulenedicarboxylates and EPR-spectroscopic study of their radical anions
- Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
- A polyoxometalate-based inorganic–organic hybrid material: synthesis, characterization structure and photocatalytic study
- Hydrogen-bonded assemblies of two organically templated borates: syntheses and crystal structures of [(1,10-phen)(H3BO3)2] and [2-EtpyH][(B5O6(OH)4]
- Catalytic activity of the nanoporous MCM-41 surface for the Paal–Knorr pyrrole cyclocondensation
- A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation
- Structural and spectroscopic characterization of isotypic sodium, rubidium and cesium acesulfamates
- Nd39Ir10.98In36.02 – A complex intergrowth structure with CsCl- and AlB2-related slabs
- Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands
- Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems
- Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents
Artikel in diesem Heft
- Frontmatter
- In this Issue
- EPR studies on carboxylic esters, 23 [1]. Preparation of new dialkyl azulenedicarboxylates and EPR-spectroscopic study of their radical anions
- Synthesis and crystal structure of a 3D copper(II)–silver(I) coordination polymer assembled through hydrogen bonding, π–π stacking and metal–π interactions, {[Cu(phen)2(CN)][Ag(CN)2] · 3H2O}n (phen = 1,10-phenanthroline)
- A polyoxometalate-based inorganic–organic hybrid material: synthesis, characterization structure and photocatalytic study
- Hydrogen-bonded assemblies of two organically templated borates: syntheses and crystal structures of [(1,10-phen)(H3BO3)2] and [2-EtpyH][(B5O6(OH)4]
- Catalytic activity of the nanoporous MCM-41 surface for the Paal–Knorr pyrrole cyclocondensation
- A new route for the synthesis of 4-arylacetamido-2-aminothiazoles and their biological evaluation
- Structural and spectroscopic characterization of isotypic sodium, rubidium and cesium acesulfamates
- Nd39Ir10.98In36.02 – A complex intergrowth structure with CsCl- and AlB2-related slabs
- Syntheses, structures and magnetic properties of two mononuclear nickel(II) complexes based on bicarboxylate ligands
- Synthesis and characterization of some new fluoroquinolone-barbiturate hybrid systems
- Synthesis of pyrazoles containing benzofuran and trifluoromethyl moieties as possible anti-inflammatory and analgesic agents

