Abstract
Two lead(ii) coordination complexes with 2-(4-fluoro-phenyl)-1H-imidazo[4,5-f][1,10]phenanthroline formulated as [Pb(L)2(tlba)2)] (1) and [Pb(L)(dpea)]2 (2) (HTLBA = 2,3,4,5-tetrachlorobenzoic acid, H2dpea = diphenic acid) were synthesized under hydrothermal conditions. In 1, the neighboring [Pb(L)2(tlba)2)] molecules formed into a two-dimensional (2D) layer structure with C–Cl⋯π interactions and N–H⋯O hydrogen bond interactions. For 2, Pb1 and Pb1i ions are connected by four carboxylate groups from two dpea anions to yield a binuclear unit. Two L ligands are situated in two flanks of the dimer. The L ligands from dimers in the vicinity pile up by two π–π interactions to form a 2D supramolecular structure. Moreover, PXRD of 2 was also studied.
1 Introduction
Coordination complexes are crystalline solids that can be self-assembled from metal centers and organic ligands and have attracted much attention from chemical researcher for their promising applications in catalysis (Sahoo and Sarma, 2022; Zheng et al., 2021), magnetism (Zhong et al., 2022), luminescence (Islam et al., 2021; Xu et al., 2022), biomedical applications (Chen and Wu, 2018), etc. Because the architecture of coordination complexes would be easily impacted by some conditions such as metal centers (Koksharova et al., 2023), nitrogen-containing ligands, carboxylate ligands (Song et al., 2020), and reaction conditions (Kharlamova et al., 2019). Therefore, selecting an appropriate organic ligand and adopting a befitting construction method can help us to obtain expectant coordination complexes (Wang et al., 2018). Nitrogen-containing ligands have shown outstanding coordination properties (Hou et al., 2014; Zhao et al., 2019) and multiple coordination modes (Leng et al., 2016) in past experiments. For example, 2-(4-fluoro-phenyl)-1H-imidazo[4,5-f][1,10]phenanthroline is a rigid ligand that can readily form hydrogen-bonding interactions and is an excellent composition for making up supramolecular architectures (Wang et al., 2019). Besides, carboxylate ligands play a significant role in the design and construction of various coordination complexes, and different carboxylate ligands can make the coordination complexes show different structures (Song et al., 2021; Su et al., 2022; Wang et al., 2016).
In this article, the coordination complexes were [Pb(L)2(tlba)2)] (1) and [Pb(L)(dpea)]2 (2) (Htlba = 2,3,4,5-tetrachlorobenzoic acid, H2dpea = diphenic acid) and have been synthesized by hydrothermal methods and characterized.
2 Results and discussion
2.1 Structural analysis
Complex 1 crystallizes in the monoclinic space group C2/c with an independent Pb(ii) ion, two L ligands, and two tlba anions. Each Pb(ii) is mainly coordinated by four N atoms (from two chelating L ligands) and two oxygen atoms (O(1) and O(1i)) from two tlba anions (Pb(1)–O(1) = 2.613(5) Å) in a twisted [PbN4O2] octahedral geometry (Figure 1 and Table 1).

Coordination environment of the Pb(ii) atom of 1 (symmetric code: i –x, y, –z + 1/2).
Selected bond lengths (Å) and angles (°) for complexes 1 and 2
| 1 | |||
|---|---|---|---|
| Pb(1)–N(1) | 2.560(5) | Pb(1)–O(1) | 2.613(5) |
| Pb(1)–N(2) | 2.572(5) | ||
| N(1)–Pb(1)–N(1)ⅰ | 84.3(2) | N(1)–Pb(1)–N(2)ⅰ | 85.36(16) |
| N(1)ⅰⅰ–Pb(1)–N(2)ⅰ | 64.40(16) | N(1)–Pb(1)–N(2) | 64.40(16) |
| N(1)ⅰ–Pb(1)–N(2) | 85.36(16) | N(2)ⅰ–Pb(1)–N(2) | 139.5(2) |
| N(1)–Pb(1)–O(1) | 144.56(19) | N(1)ⅰ–Pb(1)–O(1) | 78.69(17) |
| N(2)ⅰ–Pb(1)–O(1) | 113.93(14) | N(2)–Pb(1)–O(1) | 83.28(16) |
| N(1)–Pb(1)–O(1)ⅰ | 78.69(17) | N(1)ⅰ–Pb(1)–O(1)ⅰ | 144.56(19) |
| N(2)ⅰ–Pb(1)–O(1)ⅰ | 83.28(16) | N(2)–Pb(1)–O(1)ⅰ | 113.94(14) |
| O(1)–Pb(1)–O(1)ⅰ | 130.7(3) | ||
| 2 | |||
|---|---|---|---|
| Pb(1)–N(1) | 2.665(5) | Pb(1)–O(2) | 2.289(4) |
| Pb(1)–N(2) | 2.525(4) | Pb(1)–O(3)ⅰ | 2.666(4) |
| Pb(1)–O(4)ⅰ | 2.347(4) | ||
| O(2)–Pb(1)–O(4)ⅰ | 87.25(15) | O(2)–Pb(1)–N(2) | 73.29(13) |
| O(4)ⅰ–Pb(1)–N(2) | 79.89(13) | O(2)–Pb(1)–N(1) | 85.70(15) |
| O(4)ⅰ–Pb(1)–N(1) | 143.25(12) | N(2)–Pb(1)–N(1) | 63.54(13) |
| O(2)–Pb(1)–O(3)ⅰ | 82.43(16) | O(4)ⅰ–Pb(1)–O(3)ⅰ | 51.72(12) |
| N(2)–Pb(1)–O(3)ⅰ | 126.68(14) | N(1)–Pb(1)–O(3)ⅰ | 160.40(14) |
Symmetry codes: 1: i −x, y, −z + 1/2; 2: i −x + 1, −y + 1, −z + 1.
The tlba anion chelates one Pb(ii) with μ 1 -η 1 mode. It is noted that both the chlorine atoms involved in stacking lead to the formation of C(22)–Cl(1)⋯π1 (3.473(3) Å; π1: ring centroid of quinoline) and C(23)–Cl(2)⋯π2 (3.393(3) Å; π2: ring centroid of imidazole) interactions (Figure 2 and Table 2). And these C–Cl⋯π interactions make the adjacent [Pb(L)2(tlba)2)] molecules to be formed into a one-dimensional supramolecular chain (Figure 3). Interestingly, there are N(4)–H(4)⋯O(2)iii hydrogen bonds (symmetric code: iii −x + 1/2, y + 1/2, −z + 1/2), which extend the adjacent one-dimensional chains to extend into a two-dimensional (2D) supramolecular structure (Figure 4).

View of the C–Cl⋯π interactions between tlba anions and L ligands.
The chlorine interactions (Å, °) in complex 1
| Y–X⋯π | X⋯π (Å) | Y⋯π (Å) | γ (°) | Y–X⋯π (°) | Symmetry code# |
|---|---|---|---|---|---|
| C(22)–Cl(1)⋯π1 | 3.473(3) | 4.204(6) | 13.07 | 102.36(18) | −x, y − 1, −z + ½ |
| C(23)–Cl(2)⋯π2 | 3.393(3) | 3.726(7) | 7.14 | 87.1(2) | −x, y − 1, −z + ½ |
π1: ring centroids of quinoline (N(1)/C(1)–C(6)/C(10)–C(11)); π2: ring centroids of imidazole (C(11)–C(12)/N(3)/C(13)/N(4)).
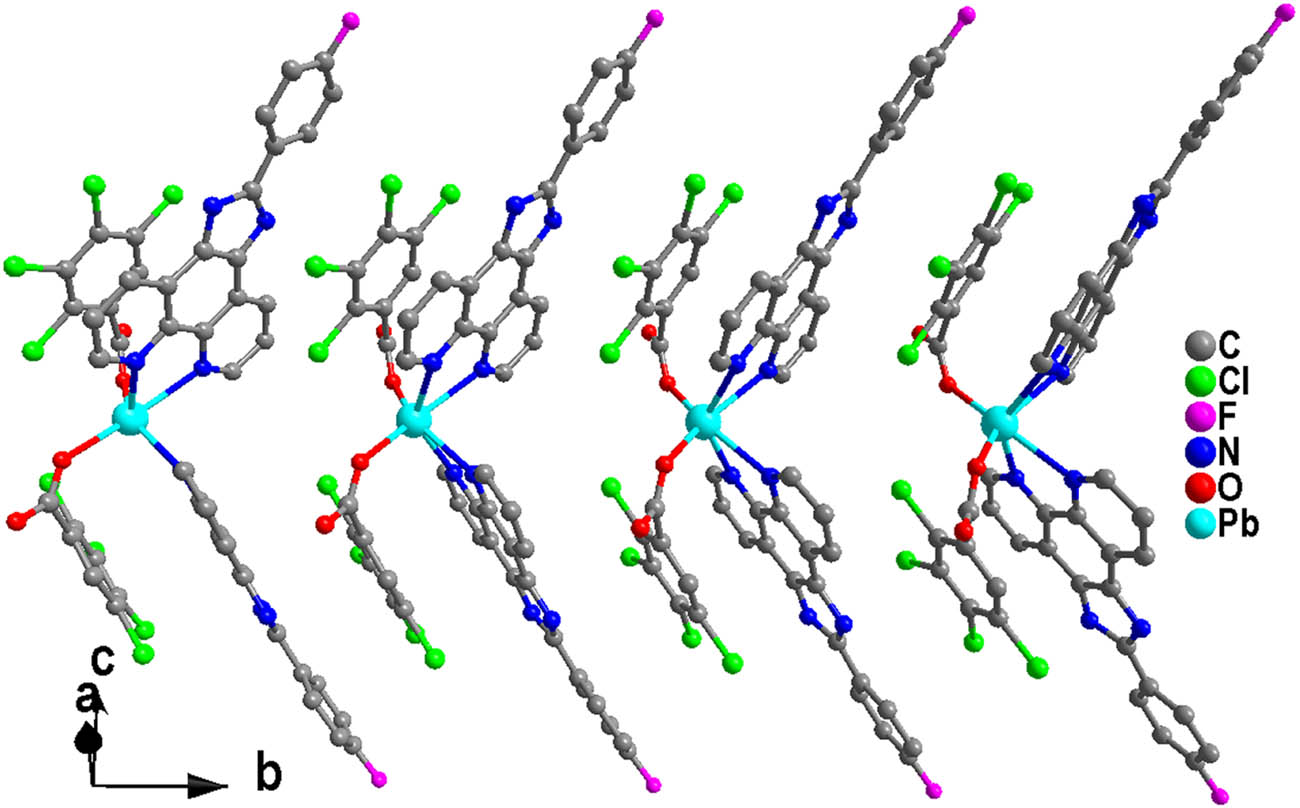
View of the 1D supramolecular chain of 1.
View of the 2D supramolecular layer structure of 1.
The asymmetric unit of 2 incorporates an independent 2Pb(ii) ion and one L ligand, as well as one dpea anion. As shown in Figure 5, the Pb atom features a distorted [:PbN2O3] octahedral geometry consisting of two N atoms (N(1), N(2)) from an L ligand and three oxygen atoms (O(2), O(3i), and O(4i)) from two dpea anions. The Pb–O distances are from 2.525(4) to 2.665(5) Å, and the Pb–N bond lengths are 2.289(4) and 2.666(4) Å. The basal plane of octahedral geometry is made up of two N and two O atoms, and the axial positions are comprised of the remaining O atom (O(2)) and the lone pair of electrons. Pb1 and Pb1i atoms are linked with four carboxylate groups from two μ 2 -dpea anions to generate a [Pb(L)(dpea)]2 binuclear molecule. The Pb⋯Pb separation by two μ 2 -dpea anions is 6.701 Å. Two L ligands are situated in two flanks of the dimer (Figure 5).

View of the dimeric structure of 2 (symmetric code: i –x + 1, –y + 1, –z + 1).
The most remarkable feature of compound 2 is that there exist two types of π–π stacking interactions.
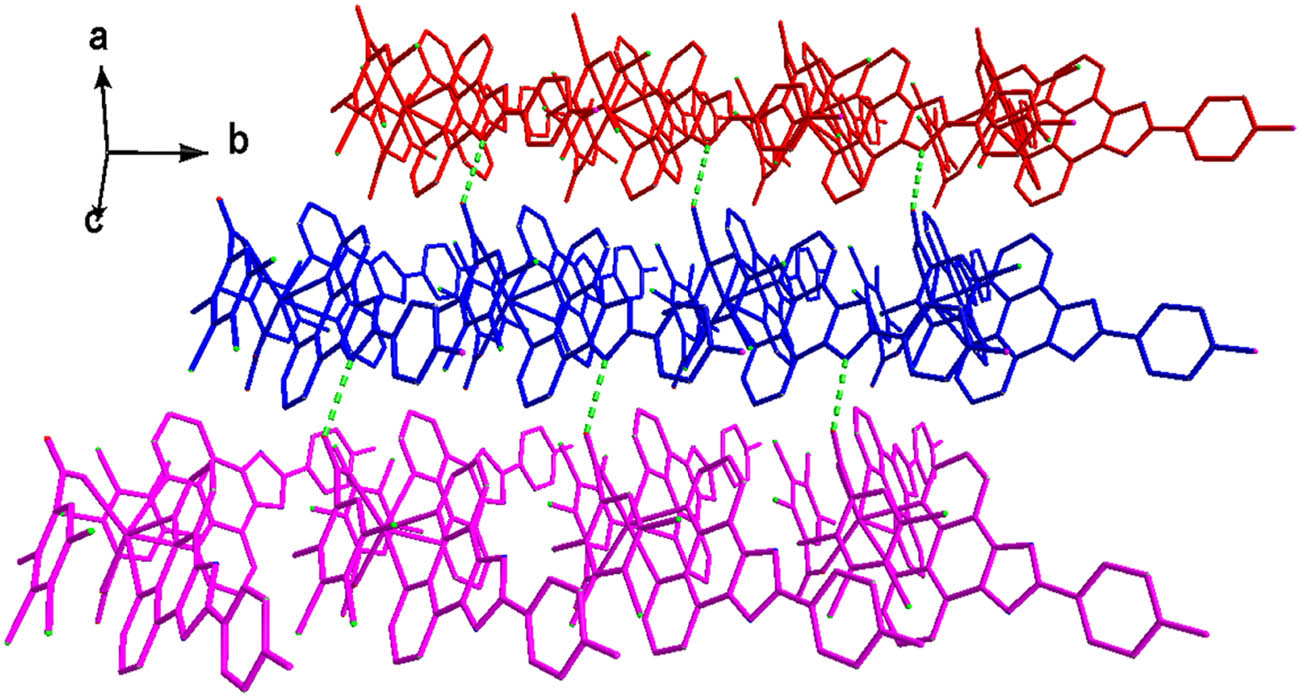
As depicted in Figure 6, the L ligands from dimers in the vicinity are paired through one type of strong π–π interactions (distance between ring centroids being ca. 3.401(2) Å, and dihedral angle of 0.00(11)°) to grow up to a one-dimensional supramolecular chain (Figure 7). More interestingly, neighboring 1D supramolecular chains are expanded to 2D supramolecular layer by the other types of π–π interactions between the two benzene rings of L ligands with the distance between ring centroids of 3.830(4) Å, a slippage distance of 1.866 Å, and a dihedral angle of 0.0(3)° (two benzene rings are composed of C(14)–C(19) and C(14)–C(19) at −1 − x, −y, −z; respectively) (Figure 8).

View of the π–π interactions between two L ligands of neighboring dimers.

View of the 1D supramolecular chain of 2.

View of the 2D supramolecular layer of 2.
2.2 PXRD patterns
As seen in Figure 9, the diffraction peaks of 2 well correspond to the simulated powder X-ray diffraction (PXRD) from single crystal diffraction data, illustrating the phase purity of the yellow block crystals.
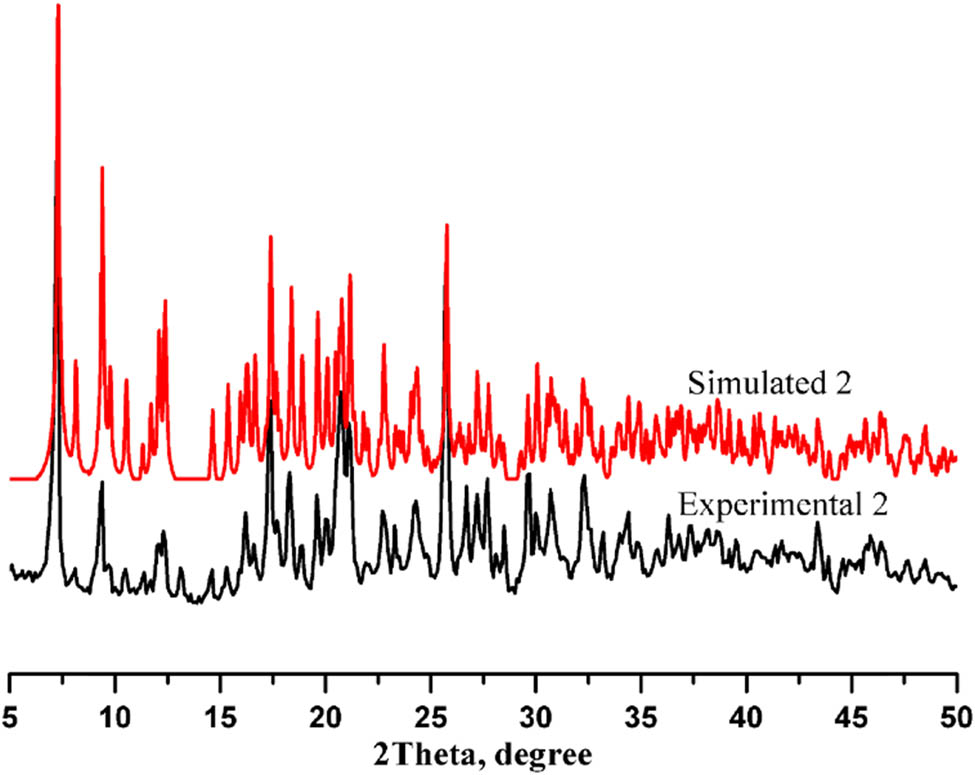
Experimental and simulated PXRD patterns of 2.
3 Conclusions
In conclusion, we have synthesized two novel coordination complexes based on Pb((ii) via applying 2-(4-fuoro-phenyl)-1H-imidazo[4,5-f][1,10]phenanthroline and their structures were characterized. In 1, the neighboring molecules are associated into a 2D supramolecular structure through the C–Cl⋯π interactions as well as N–H⋯O hydrogen bonds. In 2, the sideways L ligands from adjacent dipolymers are twined through two π–π stacking interactions to yield a 2D supramolecular layer. The different structures of the two complexes explain that carboxylic acid ligands play an essential part in the process of complex construction.
Experimental
The L ligand was compounded according to the procedures recounted in the document (Kong et al., 2015). All other chemicals with the quality of reagent grade were purchased from the mercantile sources (Cangzhou Shengqiang Chemical Technology Co., Ltd and Wuhan Canrong Biological Technology Co., Ltd, China). The C, H, and N elemental analyses were carried out on a Perkin-Elmer 2400 elemental analyzer (Perkin-Elmer, North Waltham, USA). All PXRD analyses were recorded with a Rigaku Dmax 2000 X-ray diffractometer (Rigaku, Japan).
Preparation of [Pb(L)2(tlba)2] (1)
Pb(NO3)2 (132 mg, 0.4 mmol), L (125 mg, 0.4 mmol), and HTLBA (208 mg, 0.8 mmol) were dissolved in a mixture of solvents (8 mL of deionized water and 2 mL of anhydrous ethanol), and the mixed solvent was agitated for 0.5 h at indoor temperature. Then adjusted the pH value to 9.45 with NaOH. The mixed solvent was sealed up in a 20 mL Teflon-lined stainless steel autoclave and kept at 185℃ for 96 h. The brown rodlike crystals were gained in 37% yield (based on Pb). Analytical found for C52H24Cl8F2N8O4Pb, calculated %: C, 46.14; H, 1.79; N, 8.28; found %: C, 45.61; H, 1.77; N, 8.19.
Preparation of [Pb(L)(dpea)]2 (2)
Pb(NO3)2 (331 mg, 1.0 mmol), L (151 mg, 0.48 mmol) and H2dpea (242 mg, 1.0 mmol) were dissolved in a mixture of solvents (7 mL of deionized water and 3 mL of anhydrous ethanol), and the mixed solvent was agitated for 0.5 h at indoor temperature. Then adjusted the pH value to 5.69 with NaOH. The mixed solvent was sealed up in a 20 mL Teflon-lined stainless steel autoclave and kept at 140℃ for 144 h. Yellow block crystals were gained in 40% yield (based on Pb). Analytical found for C66H38F2N8O8Pb2, calculated %: C, 52.04; H, 2.51; N, 7.36; found %: C, 51.61; H, 2.47; N, 7.25.
X-ray crystallography
The single-crystal data 1–2 were measured on an in-house Bruker P4 diffractometer equipped with a SMART CCD at 296(2) K, with graphite-monochromatized Mo-Kα radiation (λ = 0.71073 Å) at room temperature. All the structures used SIR2014 to solve by direct methods (Burla et al., 2015) and ameliorated on F 2 by full-matrix least-squares techniques using the SHELXL2018/3 program (Sheldrick, 2015). The hydrogen atoms were geometrically placed in the computed positons and deemed to be riding. The non-hydrogen atoms were refined anisotropically and located. Crystallographic data for both complexes are presented in Table 3. Table 1 is the selected bond lengths and angles for complexes 1 and 2. Crystallographic data for the crystalline material have been deposited with the Cambridge Crystallographic Data Centre (CCDC), and the CCDC numbers are 2232961 and 2232962.
Crystalline data and refinement parameters for complexes 1 and 2
| Complex | 1 | 2 |
|---|---|---|
| Empirical formula | C52H24Cl8F2N8O4Pb | C66H38F2N8O8Pb2 |
| Formula weight | 1,353.58 | 1,523.42 |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | P̄1 |
| a (Å) | 18.177(6) | 10.183(2) |
| b (Å) | 8.5114(17) | 11.790(2) |
| c (Å) | 32.630(9) | 12.137(2) |
| α (°) | 90 | 93.70(3) |
| β (°) | 105.34(3) | 90.32(3) |
| γ (°) | 90 | 112.58(3) |
| Volume (Å3) | 4,868(2) | 1,341.8(5) |
| Z | 4 | 1 |
| D c (g·cm−3) | 1.847 | 1.885 |
| µ (mm−1) | 3.971 | 6.342 |
| F (000) | 2,640 | 736 |
| θ range (°) | 2.987–25.009 | 3.366–25.009 |
| Crystal size (mm) | 0.281 × 0.145 × 0.102 | 0.257 × 0.185 × 0.151 |
| Tot. reflections | 4,248 | 4,681 |
| Uniq. reflections, R int | 18,207, 0.1334 | 10,549, 0.0472 |
| GOF on F 2 | 1.050 | 1.031 |
| R 1 indices [I > 2σ(I)] | 0.0577 | 0.0332 |
| wR 2 indices (all data) | 0.0899 | 0.0747 |
| ∆ρ min, ∆ρ max (e·Å–3) | −1.873, 1.438 | −0.665, 1.486 |
| CCDC No. | 2232961 | 2232962 |
-
Funding information: The authors state that no funding is involved.
-
Author contributions: Jingdong Feng: writing – original draft, experimental work; Ziru Li: writing – original draft, conceptualization; Xiuyan Wang: software; data curation, experimental work.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: All data generated or analyzed during this study are included in this published article.
References
Burla M.C., Caliandro R., Carrozzini B., Cascarano G. L., Cuocci C., Giacovazzo C., et al., Crystal structure determination and refinement via SIR2014. J. Appl. Cryst., 2015, 48, 306–309. 10.1107/S1600576715001132.Suche in Google Scholar
Chen W., Wu C.S., Synthesis, functionalization, and applications of metal–organic frameworks in biomedicine. Dalton Trans., 2018, 47(7), 2114–2133. 10.1039/c7dt04116k.Suche in Google Scholar PubMed
Hou S.S., Tan J.B., Lian Z.Y., Zeng D.W., Huang T.L., Huang B.R., et al., Construction of three new mixed-ligand Zn(II) coordination polymers based on nitrogen-containing heterotopic ligands and carboxylate co-ligands. Inorg. Chem. Commun., 2014, 47, 112–118. 10.1016/j.inoche.2014.07.002.Suche in Google Scholar
Islam M.J., Kitagawa Y., Tsurui M., Hasegawa Y., Strong circularly polarized luminescence of mixed lanthanide coordination polymers with control of 4f electronic structures. Dalton Trans., 2021, 50(16), 5433–5436. 10.1039/d1dt00519g.Suche in Google Scholar PubMed
Koksharova T., Slyvka Y., Savchenko O., Mandzii T., Smola S., 5-Sulfosalicylato Cu(II), Zn(II) and Ni(II) coordination compounds with benzohydrazide: Synthesis, structure and luminescent properties. J. Mol. Struct., 2023, 1271, 133980. 10.1016/j.molstruc.2022.133980.Suche in Google Scholar
Kharlamova A.D., Abel A.S., Averin A.D., Beletskaya I.P., N,N-di(pyridin-2-yl)quinolin-6-amine: synthesis and coordination properties. Russ. Chem. B., 2019, 68(3), 597–600. 10.1007/s11172-019-2460-0.Suche in Google Scholar
Kong Z.G., Wang W., Zhang S.Q., Zhao F.W., Wang X.Y., Synthesis, crystal structure, physical properties and theoretical calculations of a new one-dimensional Ni(II) coordination polymer constructed by 1,10-phenanthroline derivative ligand and sulfate. J. Inorg. Organomet. P., 2015, 25(6), 1441–1447. 10.1007/s10904-015-0257-7.Suche in Google Scholar
Leng W.G., Peng Y.S., Zhang J.Q., Lu H., Feng X., Ge R.L., et al., Sophisticated design of covalent organic frameworks with controllable bimetallic docking for a cascade reaction. Chem Eur J., 2016, 22(27), 9087–9091. 10.1002/chem.201601334.Suche in Google Scholar PubMed
Sheldrick G.M., Crystal structure refinement with SHELXL. Acta Cryst., 2015, C71, 3–8. 10.1107/S2053229614024218.Suche in Google Scholar PubMed PubMed Central
Sahoo S., Sarma D., Synthesis, structure, and heterogeneous catalysis of a series of structurally diverse coordination polymers based on 5-nitroisophthalate. Cryst. Growth Des., 2022, 22(9), 5645–5657. 10.1021/acs.cgd.2c00737.Suche in Google Scholar
Song J., Duan B.F., Lu J.F., Ge H.G., Three new lanthanide coordination polymers constructed from 2,6-Bis(pyrazin-2-yl)pyridine-4-carboxylate: syntheses, structures and luminescence. Chin. J. Struct Chem., 2020, 39(4), 793–800. 10.14102/j.cnki.0254-5861.2011-2393.Suche in Google Scholar
Song Y., Yan Y., Zhang H., Wang X.Y., Synthesis and structural characterization of a novel 2D supramolecular lead coordination polymer with phenanthroline derivate and adipic acid. Main. Group. Met. Chem., 2021, 44, 239–242. 10.1515/mgmc-2021-0025.Suche in Google Scholar
Su B.H., Shi Y.H., Peng X.H., Kong Z.G., Chang L.M., A new cadmium(II) coordination polymer with 1,4-cyclohexanedicarboxylate acid and phenanthroline derivate: synthesis and crystal structure. Main. Group. Met. Chem., 2022, 45, 208–212. 10.1515/mgmc-2022-0020.Suche in Google Scholar
Wang X.C., Chen Y., Yuan H., Yang Q., Zeng X.S., Qiu H.J., et al., Coordination polymers with 2D-3D interdigitated arrays based on 5-(4-(1H-1,2,4-Triazol-1-yl)phenyl)-1H-tetrazole: syntheses, structures, and properties. Z. Anorg. Allg. Chem., 2016, 642(11–12), 724–729. 10.1002/zaac.201600120.Suche in Google Scholar
Wang X.Y., Li C., Zou C.K., Kan R.F., Zhang X.X., Xu Z.L., A new Co(Ⅱ) coordination polymer based on a flexible 1,4-cyclohexanedicarboxylic acid: synthesis, structure and thermal behavior. Chin. J. Struct Chem., 2019, 38(12), 2155–2160. 10.14102/j.cnki.0254-5861.2011-2482.Suche in Google Scholar
Wang S.P., Liu J., Zhao H.M., Guo Z.F., Xing H.Z., Gao Y., Electrically conductive coordination polymer for highly selective chemiresistive sensing of volatile amines. Inorg. Chem., 2018, 57(2), 541–544. 10.1021/acs.inorgchem.7b02464.Suche in Google Scholar PubMed
Xu M.M., Lu H.J., Wang C.H., Qiu J., Zheng Z.F., Guo X.F., et al., Enhancing photosensitivity via the assembly of a uranyl coordination polymer. Chem. Commun., 2022, 58(67), 9389–9392. 10.1039/d2cc02985e.Suche in Google Scholar PubMed
Zhao L., Liu X., Zhao C.J., Meng L.S., Synthesis and properties of interspersed structure complexes prepared from 4,4′-(phenylazanediyl)-dibenzoic acid with rigid and semi-rigid nitrogen-containing ligands. J. Mol. Struct., 2019, 1180, 547–555. 10.1016/j.molstruc.2018.12.026.Suche in Google Scholar
Zhong X., Hu J.J., Yao S.L., Zhang R.J., Wang J.J., Cai D.G., et al., Gd(Ⅲ)-Based inorganic polymers, metal-organic frameworks and coordination polymers for magnetic refrigeration. CrystengComm., 2022, 24(13), 2370–2382. 10.1039/d1ce01633d.Suche in Google Scholar
Zheng S.J., Pan J.P., Wang J.H., Liu S., Zhou T.T., Wang L., et al., Ag(I) pyridine-amidoxime complex as the catalysis activity domain for the rapid hydrolysis of organothiophosphate-based nerve agents: mechanistic evaluation and application. Acs Appl. Mater. Interface., 2021, 13(29), 34428–34437. 10.1021/acsami.1c09003.Suche in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”
Artikel in diesem Heft
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”

