Abstract
Triethylammonium dichloro[N-[(2-oxyphenyl)methylidene]valinato]-phenyl-tin(iv) was prepared and characterized by spectroscopic methods and single crystal X-ray crystallography. The compound consists of a triethylammonium cation and a tin complex as anion. The tin complex is composed of a tridentate O,N,O-Schiff base ligand coordinated to a SnCl2Ph unit. The tin atom is in distorted octahedral coordination geometry. NMR spectroscopic studies have shown that the unusual hexa-coordinated tin complex was formed as a kinetically controlled product. Storage of the solid product for several years led to a transformation into a thermodynamically stable penta-coordinated tin complex.
Organotin complexes of Schiff bases are a fast-developing field of research due to their various possible applications which were covered in a review until 2011 (Nath and Saini, 2011). These complexes can exhibit anti-tumor, anti-microbial, anti-nematicidal, anti-insecticidal, anti-inflammatory properties (Amin et al., 2019; Bhatra et al., 2016; Kobakhidze et al., 2010; Shah et al., 2020; Sharma et al., 2016; Yao et al., 2017; Wang et al., 2022), and might be useful as highly selective sensor molecules for hydrogen sulfide (Basu Baul et al., 2020). We are interested in chiral complexes of group 14 elements. For that purpose, we have explored several chiral ligand types (Böhme et al., 2006; Böhme and Fels, 2013; Schwarzer et al., 2015, 2018; Warncke et al., 2012, 2016).
The present work describes the synthesis and crystal structure of the title compound triethylammonium dichloro[N-[(2-oxyphenyl)methylidene]valinato]-phenyl-tin(iv) (1 in Scheme 1). Several related dialkyltin complexes of N-salicylidene-valine have been already prepared and were structurally characterized (2 in Scheme 1). These were derivatives with R = Me (Tian et al., 2020), Ph (Beltran et al., 2003; Yin and Wang, 2004a), n-Bu (Beltran et al., 2003; Smith et al., 1992; Yin et al., 2004b), 2-fluorobenzyl (Ding et al., 2007), cyclohexyl (Shi et al., 2016), and tert-butyl (Ding et al., 2006). These contain the same chelate ligand, but different substituents of tin. None of these complexes crystallize as anion like the title compound.
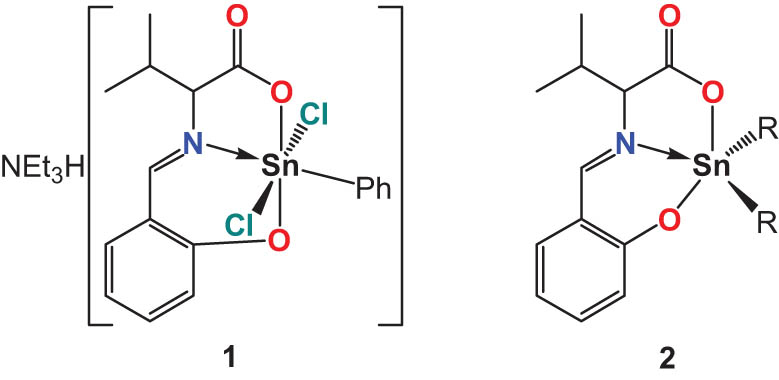
Title compound 1 (left) and dialkyltin complexes of N-salicylidene-valine (2, right).
Compound 1 crystallizes in the monoclinic space group Cc with cation and anion in the asymmetric unit. The space group Cc (containing a glide plane) indicates that both enantiomers of the title compound are present in the crystal (Suh et al., 1997). The value of optical rotation of the batch product was determined to be [α]20 D = 0°, which confirms the presence of the racemate. Initially the S-isomer of the ligand molecule, (S)-N-[(2-hydroxyphen-1-yl)methylidene]valine-methylester, was used for the synthesis. Apparently, a racemization of the ligand system took place during the formation of the complex. This is associated with cleavage of the carboxylic acid ester, as has already been observed in other cases (Belokon et al., 1981; Warncke et al., 2012). Another special feature of the title compound is its crystallization as an ionic compound composed of a triethylammonium cation (NEt3H+) and an organostannate anion (tin complex). This leads to a six-fold coordination of the tin complex (Figure 1). The tin atom is in a distorted octahedral coordination geometry (see bond angles in Table 1).

Diagram of the title molecule showing the atom-labeling scheme. Atomic displacement parameters are at the 50% probability level.
Selected bond lengths (Å) and angles (°) for compound 1
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Sn1–O3 | 2.050 (4) | Sn1–N1 | 2.224 (5) |
| Sn1–O1 | 2.134 (4) | Sn1–Cl2 | 2.4620 (13) |
| Sn1–C13 | 2.178 (6) | Sn1–Cl1 | 2.5132 (16) |
| O3–Sn1–O1 | 161.28 (13) | C13–Sn1–Cl2 | 95.48 (16) |
| O3–Sn1–C13 | 100.49 (19) | N1–Sn1–Cl2 | 87.88 (13) |
| O1–Sn1–C13 | 98.11 (18) | O3–Sn1–Cl1 | 91.39 (12) |
| O3–Sn1–N1 | 85.42 (15) | O1–Sn1–Cl1 | 85.33 (12) |
| O1–Sn1–N1 | 75.89 (15) | C13–Sn1–Cl1 | 93.48 (17) |
| C13–Sn1–N1 | 173.2 (2) | N1–Sn1–Cl1 | 82.88 (11) |
| O3–Sn1–Cl2 | 90.17 (12) | Cl2–Sn1–Cl1 | 170.47 (5) |
| O1–Sn1–Cl2 | 90.19 (12) |
The tridentate Schiff-base ligand is coordinated meridionally to the central atom. The chlorine atoms are in trans position to each other. The phenyl group C13 to C18 is in trans position to the Schiff-base nitrogen atom N1. The O1–Sn1–O3 angle of 161.3 (1)° deviates strongly from 180°. The Cl1–Sn1–Cl2 and C13–Sn1–N1 angles are 170.47 (5)° and 173.2 (2)°, respectively. The interatomic Sn–O and Sn–N distances are similar as in comparable hexa-coordinated tin complexes (Paul et al., 2014; Schwarzer et al. 2018). The Sn–Cl1 and Sn–Cl2 distances are 2.513 (2) and 2.462 (1) Å, respectively. These are longer than the sum of the covalent radii of Sn and Cl with 2.39 Å (Pauling, 1962). However, anionic tin complexes with the same coordination geometry at tin containing a tridentate O,N,O-ligand, two chlorine atoms, and one alkyl or aryl substituent show similar Sn–Cl distances. The Sn–Cl bond lengths in such complexes range from 2.429 to 2.600 Å (Böhme and Dittrich, 2020; Hong et al., 2010; Jimenez-Perez et al., 2000). The elongation of the Sn–Cl distances is caused by the six-fold coordination.
A bifurcated intermolecular N–H⋯O interaction is observed at N2–H2 with O1 and O2 (Table 2 and Figure 2). Furthermore, there are short contacts between the chlorine atom Cl1 and C–H bonds from the neighboring molecules (C2–H2⋯Cl1 and C6–H6⋯Cl1).
H-bonding geometry parameters (Å and °) for compound 1
| D–H⋯A | D–H | H⋯A | D⋯A | D–H⋯A |
|---|---|---|---|---|
| N2–H2A⋯O1i | 1.05(6) | 2.55(6) | 3.326(6) | 130(5) |
| N2–H2A⋯O2i | 1.05(6) | 1.80(6) | 2.840(6) | 170(5) |
| C2–H2⋯Cl1ii | 1.00 | 2.80 | 3.700(5) | 150 |
| C6–H6⋯Cl1ii | 0.95 | 2.81 | 3.713(5) | 160 |
Symmetry codes: (i) x + 1/2, −y + 1/2, z + 1/2; (ii) x, −y, z − 1/2.

Relevant intermolecular interactions.
A 119Sn NMR spectrum at room temperature of complex 1 in CDCl3 solution showed two resonances at δ −337.1 (35.6%) and −507.2 ppm (64.4%), respectively, which are typical for penta- and hexa-coordinated monoorganotin compounds (Schwarzer et al., 2018). Both 1H and 13C NMR spectra of the same solution revealed two sets of signals (NMR spectra are presented in Appendix). These data suggest that complex 1 might be involved in an equilibrium. However, this is not the case. The product was stored as a solid for 6 years. A renewed examination of a solution of this solid in chloroform showed that the hexa-coordinated compound (1) was only present in the sample in a proportion of approx. 7%, whereas 93% of the sample was present as penta-coordinated compound (2a) (Scheme 2).

Transformation of the hexa-coordinated into the penta-coordinated tin complex.
Triethylammonium chloride in chloroform was added to this NMR sample. However, this did not change the ratio of the two compounds. After 68 h, the two compounds were still present in the same ratio (see Appendix). From this we conclude with caution that initially the hexa-coordinated compound crystallized out as a kinetically controlled product. During the long storage time, this transformed into the penta-coordinated compound.
In summary, an unusual ionic tin complex was obtained. The crystal structure analysis proved the formation of the hexa-coordinated complex. Storage of the solid product for several years led to a transformation into the thermodynamically stable penta-coordinated complex. This also explains why such an unusual ionic tin complex has not been described in the literature so far.
Experimental
Synthesis of 1
The synthesis of the title compound is shown in Scheme 3. Dichlorodiphenylstannane was used as a starting material for the synthesis. Surprisingly, the crystal structure proved the formation of a product containing just one phenyl group at the tin atom. Therefore, a redistribution reaction as shown in Scheme 3 must be assumed taking place during the reaction. This redistribution reaction of organic groups is commonly known in tin(iv) compounds and is used for the preparation of different organotin halides in industrial scale (Davies and Smith, 1982; Schwarzer et al., 2013). The used starting material was checked with 119Sn NMR. It contains only Ph2SnCl2 with 119Sn NMR shift of δ = −26.4 ppm (Casas et al., 1996).

Reaction scheme for the synthesis of the title compound.
(S)-N-[(2-hydroxyphen-1-yl)methylidene]valine-methylester (1.0 g, 4.2 mmol) was dissolved in 40 mL tetrahydrofuran and triethylamine (0.86 g, 8.4 mmol) was added via syringe. The yellow solution was then cooled down to 273 K. Dichlorodiphenylstannane (1.46 g, 4.2 mmol) was added to the solution. Immediately, a white precipitate of triethylamine hydrochloride was formed. After 30 min the mixture was warmed up to room temperature and stirred for 72 h at room temperature. Triethylamine hydrochloride was collected via suction filtration, washed with 10 mL tetrahydrofuran, and the solvent was removed completely under reduced pressure. The residue was dissolved in chloroform. For crystallization, n-hexane was added, the crystals collected via suction filtration and dried in vacuo.
Yield: 0.9 g (37%); m.p. 475 K. NMR (CDCl3, TMS, δ, ppm); 119Sn: −337.1 (35.6%, penta-coordinated Sn), −507.2 (64.4%, hexa-coordinated Sn).
1H: 0.77 (d, CH(CH 3)2, 3 J HH = 5 Hz), 0.86 (d, CH(CH 3)2, 3 J HH = 5 Hz), 1.07 (t, CH3, Et3NH+, 3 J HH = 7.5 Hz), 2.17 (m, CH(CH3)2), 2.81 (q, CH2, Et3NH+, 3 J HH = 7.5 Hz), 3.66 (d, CH–COO, 3 J HH = 5 Hz), 3.86 (d, CHCOO, 3 J HH = 5 Hz), 6.66–8.0 (mm, HPh), 8.1 (s, HC═N), 8.23 (s, HC═N).
13C: 7.6 (CH3, Et3NH+), 17.9, 19.7 (2CH3), 32.2, 33.9 (CH(CH3)2), 45.0 (CH2, Et3NH+), 72.2, 73.0 (CH–COO), 116.3, 116.6, 117.0, 121.9, 122.3, 127.9, 128.2, 129.6, 129.8, 134.3, 134.5, 135.0, 135.2, 135.5, 135.6, 137.1 (CPh), 168.5, 166.9 (C═N), 176.1, 172.0 (COO).
IR (KBr pellets, cm–1): 1,670 ν(C(O)O)asym, 1,614 ν(C═N), 1,538 ν(C(O)O)sym. UV–Vis (c = 1.72 mmol‧L−1 in CHCl3): λ max (ε, L‧mol−1‧cm−1) = 379 (5,116), 285 (13,799) nm, [α]20 D = 0°.
X-ray crystallography
Single-crystal X-ray diffraction data was obtained using a STOE IPDS 2T diffractometer equipped with an image plate detector with graphite monochromated MoKα radiation (λ = 0.71073 Å). Software for data collection: X-AREA, cell refinement: X-AREA, and data reduction: X-RED (Stoe, 2009). The structure was solved by direct methods using SHELXT (Sheldrick, 2015a) and refined by full matrix least squares with SHELXL2018 (Sheldrick, 2015b), refining on F 2. Molecule graphics was generated with ORTEP-3 (Farrugia, 1997). Hydrogen atoms bonded to C were positioned geometrically and allowed to ride on their parent atoms. The hydrogen atom at N2 was localized from residual electron density maps and was freely refined. The absolute structure was determined as absolute structure parameter x = −0.047(6), also denoted as Flack-parameter (Parsons et al., 2013). Detailed crystal data and structure refinement are listed in Table 3. Crystallographic data has been deposited with the Cambridge Crystallographic Centre as supplementary publication number CCDC-2234038.
Crystal data and structure refinement of 1
| Parameter | Value |
|---|---|
| Chemical formula | C18H18Cl2NO3Sn·C6H16N |
| Formula weight | 588.12 |
| Crystal dimensions (mm) | 0.16 × 0.22 × 0.45 |
| Crystal system | Monoclinic |
| Space group | Cc |
| a (Å) | 16.8326 (6) |
| b (Å) | 16.2397 (8) |
| c (Å) | 9.7225 (3) |
| β (°) | 97.651 (3) |
| V (Å3) | 2,634.05 (18) |
| Z | 4 |
| D calc (g‧cm−3) | 1.483 |
| µ (mm−1) | 1.20 |
| F(000) | 1,200 |
| T (K) | 153 |
| λ (Å) | Mo Kα (0.71073) |
| θ range (°) | 2.9–28.0 |
| Measured reflections | 29,165 |
| Unique reflections | 6,077 |
| Observed reflections [I > 2σ(I)] | 5,787 |
| No. of parameters refined | 297 |
| R 1, wR 2 [I > 2σ(I)] | 0.028, 0.0591 |
| R 1, wR 2 [all data] | 0.032, 0.0618 |
| Goodness-of-fit on F 2 | 1.27 |
| Largest peak and hole (e‧Å−3) | 2.38–0.84 |
Acknowledgements
The authors thank TU Bergakademie Freiberg (Freiberg, Germany) for financial support. Beate Kutzner and Betty Günther are acknowledged for spectroscopic measurements and analyses.
-
Funding information: Open Access Funding by the Publication Fund of the TU Bergakademie Freiberg.
-
Author contributions: Uwe Böhme: writing – original draft, writing – review and editing, formal analysis, visualization, project administration, investigation, and data curation; Gisela Weling: methodology, formal analysis, investigation, and data curation.
-
Conflict of interest: The authors state no conflict of interest.
-
Data availability statement: NMR spectra are available in Appendix.
Appendix

119Sn NMR spectrum – initial sample after synthesis (2016).

13C NMR spectrum – initial sample after synthesis (2016).

1H NMR spectrum – initial sample after synthesis (2016).

119Sn NMR spectrum – after 6 years (2023).

119Sn NMR spectrum – after 6 years (2023) with added HNEt3Cl and standing for 68 h.
References
Amin N.A.M., Hussen R.S.D., Lee S.M., Sim K.S., Navanesan S., Synthesis, structural characterization, cytotoxicity and encapsulation studies of N,Nʹ-(1,2-dicyano-1,2-vinylene)-bis(4-hydroxysalicylideneaminato) di(p-chlorobenzyl)tin as potential anticancer drug. Main Group Met. Chem., 2019, 42(1), 94–101. 10.1515/mgmc-2019-0010.Suche in Google Scholar
Basu Baul T.S., Chaurasiya A., Rabha M., Khatua S., Lyčka A., Schollmeyer D., et al., Diorganotin compounds containing α-aminoacidato schiff base ligands derived from functionalized 2-hydroxy-5-(aryldiazenyl)benzaldehyde. Syntheses, structures and sensing of hydrogen sulfide. Eur. J. Inorg. Chem. 2020, 2020, 1803–1813. 10.1002/ejic.202000177.Suche in Google Scholar
Belokon Y.N., Melikyan A.S., Bakhmutov V.I., Vitt S.V., Belikov V.M., Kinetics and stereochemistry of deuterium exchange of the α-hydrogen of an amino acid moiety in metal complexes of amino acid Schiff bases with ortho-hydroxyacetophenone. Inorg. Chim. Acta, 1981, 55, 117–124. 10.1016/S0020-1693(00)90792-8.Suche in Google Scholar
Beltran H.I., Zamudio-Rivera L.S., Mancilla T., Santillan R., Farfan N., One-step preparation, structural assignment, and X-ray study of 2,2-di-n-butyl- and 2,2-diphenyl-6-aza-1,3-dioxa-2-stannabenzocyclononen-4-ones derived from amino acids, Chem. Eur. J., 2003, 9, 2291–2306. 10.1002/chem.200204260.Suche in Google Scholar PubMed
Bhatra P., Sharma J., Sharma R.A., Singh Y., Synthesis, characterization and antimicrobial activity of diorganotin(IV) derivatives of some bioactive bifunctional tridentate Schiff base ligands. Main Group Met. Chem., 2016, 39(1–2), 1–8. 10.1515/mgmc-2015-0022.Suche in Google Scholar
Böhme U., Dittrich N., CCDC 2017029: Experimental Crystal Structure Determination, 2020. 10.5517/ccdc.csd.cc25pwg7.Suche in Google Scholar
Böhme U., Fels S., A new type of chiral pentacoordinated silicon compounds with azomethine ligands made from acetylacetone and amino acids. Inorg. Chim. Acta, 2013, 406, 251–255. 10.1016/j.ica.2013.04.045.Suche in Google Scholar
Böhme U., Wiesner S., Günther B., Easy access to chiral penta- and hexacoordinate silicon compounds. Inorg. Chem. Commun., 2006, 9, 806–809. 10.1016/j.inoche.2006.05.002.Suche in Google Scholar
Casas J.S., Castellano E.E., Barros F.J.G., Sánchez A., González A.S., Sordo J., et al., Imidazole and pyrazole complexes of phenyltin(IV) halides: the crystal structures of dichlorodiphenyl bis(pyrazole)tin(IV) and trichlorophenylbis(pyrazole)tin(IV). J. Organomet. Chem., 1996, 519(1), 209–216. 10.1016/S0022-328X(96)06246-8.Suche in Google Scholar
Davies A.G., Smith P.J., Comprehensive organometallic chemistry. In E. W. Abel, F. G. A. Stone, G. Wilkinson (Eds.), Pergamon Press, Oxford, 1982, Vol. 2, p. 548.Suche in Google Scholar
Ding R., Zhu J., Zhang D., Ling A., Yin H., Bis(2-fluorobenzyl)(N-salicylidenevalinato-κ3N,O,O’)tin(IV). Acta Crystallogr., 2007, E63, m1242. 10.1107/S1600536807013840 Suche in Google Scholar
Ding R.-F. Zhu J., Zhang D.-D., Yin H.-D., Di-tert-butyl[3-methyl-2-(2-oxidobenzylideneamino)butanoato-κ3N,O,O’]tin(IV). Acta Crystallogr., 2006, E62, m3158–m3159. 10.1107/S1600536806044333.Suche in Google Scholar
Farrugia L.J., ORTEP-3 for Windows – a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst., 1997, 30, 565–565. 10.1107/S0021889897003117.Suche in Google Scholar
Hong M., Yin H.-D., Chen S.-W., Wang D.-Q., Synthesis and structural characterization of organotin(IV) compounds derived from the self-assembly of hydrazone Schiff base series and various alkyltin salts. J. Organomet. Chem., 2010, 695, 653–662. 10.1016/j.jorganchem.2009.11.035.Suche in Google Scholar
Jimenez-Perez V.M., Camacho-Camacho C., Guizado-Rodriguez M., Nöth H., Contreras R., New hexacyclic binuclear tin complexes derived from bis-(3,5-di-tert-butyl-2-phenol)oxamide. J. Organomet. Chem., 2000, 614, 283–293. 10.1016/S0022-328X(00)00689-6.Suche in Google Scholar
Kobakhidze N., Farfán N., Romero M., Méndez-Stivalet J.M., Ballinas-López M.G., García-Ortega H., et al., New pentacoordinated Schiff-base diorganotin(IV) complexes derived from nonpolar side chain α-amino acids. J. Organomet. Chem., 2010, 695(8), 1189–1199. 10.1016/j.jorganchem.2010.01.024.Suche in Google Scholar
Nath M., Saini P.K., Chemistry and applications of organotin(IV) complexes of Schiff bases. Dalton Trans, 2011, 40(27), 7077–7121. 10.1039/C0DT01426E.Suche in Google Scholar
Parsons S., Flack H.D., Wagner T., Use of intensity quotients and differences in absolute structure refinement. Acta Cryst., 2013, B69, 249–259. 10.1107/S2052519213010014.Suche in Google Scholar PubMed PubMed Central
Paul L.E.H., Foehn I.C., Schwarzer A., Brendler E., Böhme U., Salicylaldehyde-(2-hydroxyethyl)imine – a flexible ligand for group 13 and 14 elements. Inorg. Chim. Acta, 2014, 423, 268–280. 10.1016/j.ica.2014.08.026.Suche in Google Scholar
Pauling L. Die Natur der chemischen Bindung, Verlag Chemie, Weinheim, 1962, p. 213.Suche in Google Scholar
Schwarzer A., Paul L.E.H., Böhme U., Enantiotropic phase transition in a binuclear tin complex with an O,N,O’-tridentate ligand. Acta Cryst., 2013, C69, 1336–1339. 10.1107/S0108270113027194.Suche in Google Scholar PubMed
Schwarzer A., Fels S., Böhme U., Two reversible enantiotropic phase transitions in a pentacoordinate silicon complex with an O,N,O’-tridentate valinate ligand. Acta Cryst., 2015, C71, 511–516. 10.1107/S2053229615009778.Suche in Google Scholar PubMed
Schwarzer S., Böhme U., Fels S., Günther B., Brendler E., (S)-N-[(2-hydroxynaphthalen-1-yl)methylidene]valine – a valuable ligand for the preparation of chiral complexes. Inorg. Chim. Acta, 2018, 483, 136–147. 10.1016/j.ica.2018.08.011.Suche in Google Scholar
Shah S.S., Shah D., Khan I., Ahmad S., Ali U., ur Rahman A., Synthesis and antioxidant activities of schiff bases and their complexes: an updated review. Biointerface Res. Appl. Chem., 2020, 10(6), 6936–6963. 10.33263/BRIAC106.69366963.Suche in Google Scholar
Sharma S., Meena R., Singh R.V., Fahmi N., Synthesis, characterization, antimicrobial, and DNA cleavage evaluation of some organotin(IV) complexes derived from ligands containing the 1H-indole-2,3-dione moiety. Main Group Met. Chem., 2016, 39(1–2), 31–40. 10.1515/mgmc-2015-0030.Suche in Google Scholar
Sheldrick G.M., SHELXT – Integrated space-group and crystal structure determination. Acta Cryst., 2015a, A71, 3–8. 10.1107/S2053273314026370.Suche in Google Scholar PubMed PubMed Central
Sheldrick G.M., Crystal structure refinement with SHELXL. Acta Cryst., 2015b, C71, 3–8. 10.1107/S2053229614024218.Suche in Google Scholar PubMed PubMed Central
Shi X., Kong G., Liu L., Gao H., Gao X., Tian L., Synthesis, characterization and antibacterial activity of cyclohexyltin N-(salicylidene)valinates. Commun. Inorg. Synth., 2016, 4, 12–16. 10.21060/cis.2016.421.Suche in Google Scholar
Smith F.E., Hynes R.C., Ang T.T., Khoo L.E., Eng G., The synthesis and structural characterization of a series of pentacoordinate diorganotin(IV) N-arylidene-α-amino acid complexes. Can. J. Chem., 1992, 70, 1114–1120. 10.1139/v92-147.Suche in Google Scholar
Stoe. X-RED (Version 1.53) and X-AREA (Version 1.55), Stoe & Cie GmbH, Darmstadt, Germany, 2009.Suche in Google Scholar
Suh I.-H., Park K.H., Jensen W.P., Lewis D.E., Molecules, crystals, and chirality. J. Chem. Educ., 1997, 74(7), 800. 10.1021/ed074p80.Suche in Google Scholar
Tian L., Wang R., Zhang J., Zhong F., Qiu Y., Synthesis and structural characterization of dialkyltin complexes of N-salicylidene-L-valine. Main Group Met. Chem., 2020, 43(1), 138–146. 10.1515/mgmc-2020-0017.Suche in Google Scholar
Wang R., Zhang J., Cui G., Tian L., Synthesis, structure, and cytotoxicity of some triorganotin(IV) complexes of 3-aminobenzoic acid-based Schiff bases. Main Group Met. Chem., 2022, 45, 242–254. 10.1515/mgmc-2022-0026.Suche in Google Scholar
Warncke G., Böhme U., Günther B., Kronstein M., Racemization versus retention of chiral information during the formation of silicon and tin complexes with chiral Schiff base ligands. Polyhedron, 2012, 47, 46–52. 10.1016/j.poly.2012.08.027.Suche in Google Scholar
Warncke G., Fels S., Brendler E., Böhme U., Tautomerism in N-(2-hydroxy-1-naphthylidene)amino acids and the search for an answer to the difficult question about where the proton belongs. J. Mol. Struc., 2016, 1117, 37–48. 10.1016/j.molstruc.2016.03.040.Suche in Google Scholar
Yao Y., Yang M., Zheng X., Tian L., Synthesis, characterization, and cytotoxic activity of triphenyltin complexes of N-(5-bromosalicylidene)-α-amino acids. Main Group Met. Chem., 2017, 40(3–4), 93–99. 10.1515/mgmc-2017-0015.Suche in Google Scholar
Yin H.D., Wang Q.B., Crystallographic report: diphenyltin(IV) N-salicylidenevalinate. Appl. Organomet. Chem., 2004a, 18, 493. 10.1002/aoc.689.Suche in Google Scholar
Yin H.-D., Wang Q.-B., Xue S.-C., Synthesis and structural characterization of diorganotin(IV) esters of salicylidene-amino acids. J. Organomet. Chem., 2004b, 689, 2480–2485. 10.1016/j.jorganchem.2004.05.004.Suche in Google Scholar
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”
Artikel in diesem Heft
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”

