Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
-
Ahmed Hussain Jawhari
Abstract
A series of ruthenium(ii) complexes with N-heterocyclic carbene (NHC) ligands of the general type (arene)(NHC)Ru(ii)X2 (where X = halide) (3a–3d) were synthesized and characterized in order to compare their antibacterial activities with benzimidazolium salts 2. Our comparison revealed that ruthenium(ii) NHC complexes 3 were more active than benzimidazolium salts 2. Furthermore, the two complexes 3b and 3d had a potent inhibitory effect against acetylcholinesterase with an IC50 of 4.52 and 4.04 g·mL−1 and against tyrosinase with an IC50 of 20.77 and 25.84 g·mL−1, respectively. In addition, screening of benzimidazolium salts (2a–2d) and their ruthenium(ii) complexes (3a–3d) against colon carcinoma cell lines (HCT-116) and hepatocellular carcinoma cell lines (HepG-2) were studied. The obtained results revealed that complex 3a is the most active against vinblastines.
1 Introduction
In modern medicine, the use of inorganic and organometallic substances is widespread (Rosenberg et al., 1969; Medici et al., 2015). N-Heterocyclic carbene (NHC) complexes are the new promising members of organometallic complexes for drug formulation (Noffke et al., 2012; Arduengo et al., 1991). The first study on the biological activities of NHC complexes was reported by Çetinkaya (Herrmann, 2002; Barnard et al., 2004) and co-workers. Several research groups, including ours, have synthesized functionalized NHC complexes and explored their biological properties for this purpose (Garner et al., 2015; Oehninger et al., 2013). Some studies showed that the main target of palladium NHCs in cancer cells is DNA similar to cisplatin (Ray, 2007). In this regard, ruthenium(ii/iii) complexes have been extensively investigated as anticancer and antimicrobial drugs as well as DNA binding agents (Vuradi et al., 2020). In particular, in the design of innovative anticancer drugs, ruthenium tpy complexes have special consideration and have been evaluated against different cancer cell lines as promising alternates for the well-known Pd(ii) (cisplatin) and its derivatives (Ezhilarasu and Balasubramanian, 2018). Ruthenium possesses +2 and +3 oxidation states at physiological conditions and can bind to proteins, nucleic acids, sulfur, or oxygen-containing compounds in the cells (Jakupec et al., 2008; Keene et al., 2009). Furthermore, the interaction kinetics of ruthenium complexes with the cell components can be optimized depending on the nature of their ligand. This permits the ligand exchange rates of ruthenium complexes to be close to those of cellular processes, which makes them well-adapted for various medicinal applications. Therefore, compared with platinum-based drugs, ruthenium compounds might exhibit a higher cytostatic activity against various cancer cells and could be effective in the cells resistant to cisplatin. In addition, ruthenium complexes have emerged as a new and very interesting class of biologically active agents.
On several cancer cell lines, our research team is also examining metal complexes with anticancer properties (Slimani et al., 2020; Karaca et al., 2021). NHCs are easily synthesized, chemically modified, and exhibit superior properties ligands. The lipophilic end is essential in drug molecules, and to have this lipophilic end on NHC, it needs to be modified chemically. Thus, easy chemical modification of NHCs to have lipophilic ends in NHC-based drug molecules is significant. The NHCs can form a strong bond with the metal centers that lead to a more stable complex under moisture, heat, and air conditions. Due to these superior features, NHCs play an essential role in catalysis, biomedical applications, and functional material applications. Ruthenium-based complexes were used in medical applications due to low toxicity and are more capable of overcoming cancer cells’ resistance than Pt-based drugs. The benefits of Ru complexes in biological applications were reported by different groups (Meier-Menches et al., 2020). The most prominent feature of ruthenium in these studies is that it imitates iron elements in binding to biological molecules such as albumin and transferrin. Burgos et al. investigated antioxidant/prooxidant activities and toxicity of some of the ruthenium–arene complexes. They concluded that Ru(ii)–arene complexes behave as oxidants at low concentrations and as prooxidants at high concentrations. However, they reported that the ruthenium complexes could not negatively affect the Zebrafish embryos. Therefore, Ru(ii)–arene complexes can be considered nontoxic. Among the NHCs, benzimidazole-based silver, gold, and ruthenium–NHC complexes have been studied intensely due to the benzimidazole structure being a component of many biological structures. In our previous study, Ru–NHC complexes showed good antiparasitical and antiproliferative activities (Al Nasr et al., 2023). Encouraged by these results, we thought it would be helpful to examine the biological activities of some similar ruthenium complexes against different types of cancer cell lines and bacteria to determine the affinity between them. Herein, we synthesize and characterize a series of benzimidazole-containing Ru(ii)–NHC complexes. Several spectroscopic and analytical techniques were used to characterize the novel compounds. Then, studies of their various biological properties, including antibacterial, cytotoxic, acetylcholinesterase (AChE) and inhibition, and antiproliferative, were conducted.
2 Results
2.1 Preparation of benzimidazolium salts 2a–d
The benzimidazolium salts (2a–d) as NHC precursors were synthesized as previously described (Ciftci et al., 2011). The structures of benzimidazolium salts 2a–d were verified by 1H NMR, 13C NMR, FT-IR, and elemental analyses. Scheme 1 summarizes the synthesis of the 1,3-dialkylbenzimidazolium salts 2a–d.
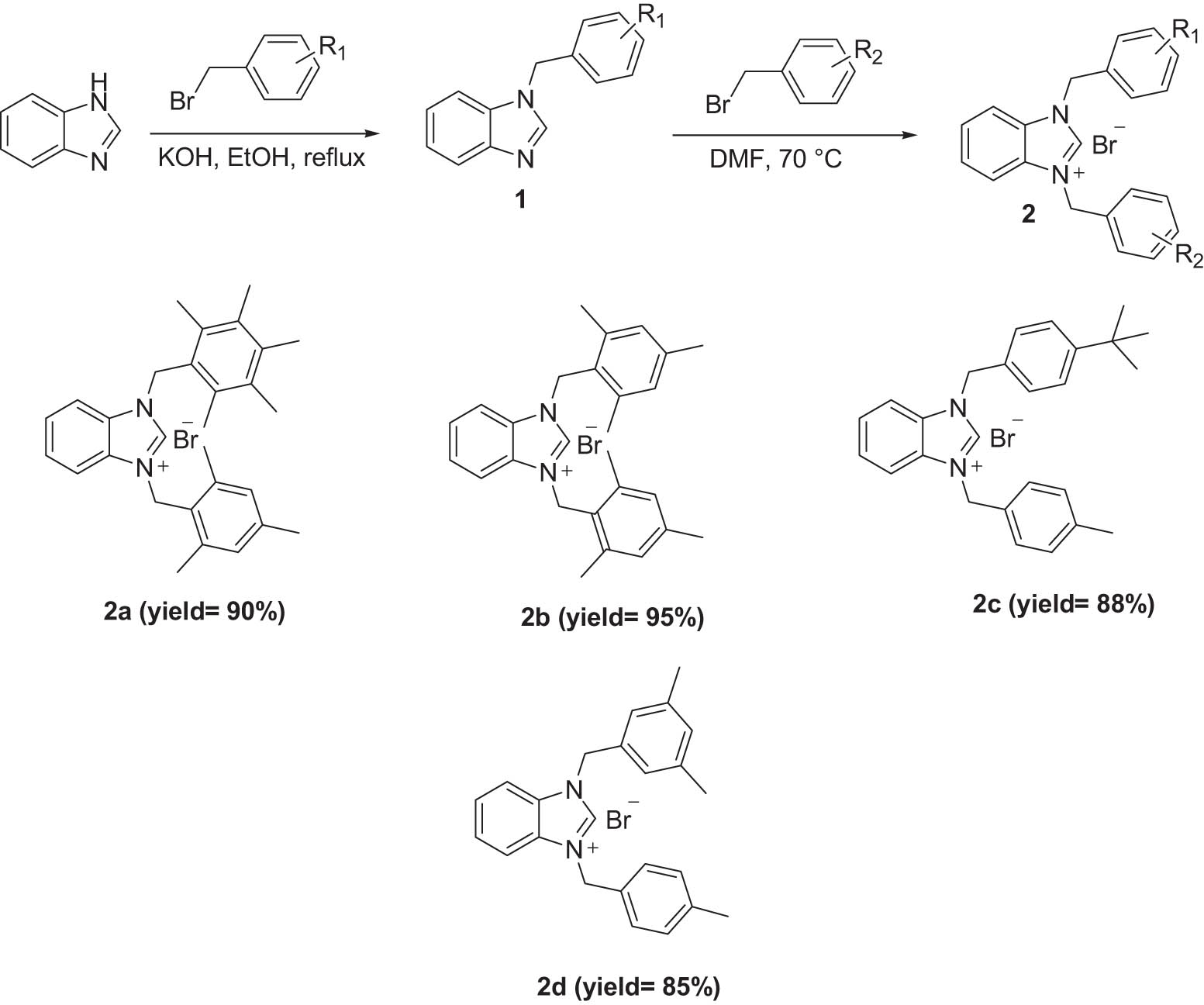
General preparation and structures of benzimidazolium salts (2a–2d).
Due to the positive charge of the molecule, salts 2a–2d show a low-field shift in the 1H NMR spectra at 9.85–11.83 ppm characterizing the NCHN proton range. The 1H NMR spectra showed resonance for the proton C(2)–H position of benzimidazolium salts 2a–2d as sharp singlets at 10.85, 10.35, 11.62, and 11.75 ppm, respectively. In the 13C NMR spectrum, the imino carbon occurs as typical singlet forms in the 1H decoupled mode at 141.3, 143.0, 143.1, and 152.8 ppm, respectively, for benzimidazolium 2a–d. Other signals include singlets at 20.76–35.18 ppm, attributable to the resonance of aliphatic carbon nuclei. The aromatic region shows a spectrum of peaks in the range of 113.38–152.82 ppm. These values are in perfect agreement with previously reported results (Antonarakis and Emadi, 2010; Bilel et al., 2014; Briguglio et al., 2015; Çetinkaya et al., 1996; Ciurea et al., 2020; Crump et al., 2004; Hartinger et al., 2013; He et al., 2016; Keppler and Rupp, 1986; Pizarro-Cerdá and Cossart, 2019).
2.2 Preparation of ruthenium–carbene complexes 3a–3d
Various methods have been described for the preparation of Ru(ii)–NHC complexes (3a–d). In this regard, transmetallation with Ag–NHC complexes has proven to be the most useful used as a carbene transfer agent in order to avoid complicated working difficulties (Moore and Flaws, 2011).
The synthesis of (p-cymene) NHC Ru(ii)Cl2 was achievable through a well-known two-step procedure (Scheme 2). Benzimidazoles 1a–1d were alkylated with benzyl bromide in the first step, resulting in the synthesis of the benzimidazolium bromides 2a–2d. Then, the activation of salts 2a–2d with silver oxide and their subsequent transmetallation with [(p-Cym)RuCl2] gave the target complexes [RuCl2(p-cymene) (NHC)] (3a–3d) with a good yield (85–95%).
![Scheme 2
Synthesis of NHC (p-Cym)Ru(ii)Cl2 complexes (3a–3d): (a) Ag2O and (b) [(p-Cym)RuCl2]2.](/document/doi/10.1515/mgmc-2023-0008/asset/graphic/j_mgmc-2023-0008_fig_006.jpg)
Synthesis of NHC (p-Cym)Ru(ii)Cl2 complexes (3a–3d): (a) Ag2O and (b) [(p-Cym)RuCl2]2.
The methylic protons of complexes 3a–3d appeared between 0.81–1.21 and 2.18–2.41 ppm as singlets, but the aromatic protons resonated between 6.35–6.88 and 7.25–7.73 ppm as a multiplet. In all complexes (3a–3d), –CH-protons of the p-cymene group were seen as heptets between 2.32 and 2.65 ppm range. NCH2 resonated as a doublet in the range of 4.32–4.89 and 5.18–5.41 ppm in the 1H-NMR spectra of 3a–3d. The obtained complexes (3a–3d) display carbene carbon 13C chemical shifts at 187.1, 186.6, 187.7, and 189.2 ppm, respectively. The results are comparable to those seen in other Ru–NHC complexes. Moreover, the Ru complexes 3 were also further characterized by CHN analysis. The results demonstrate good agreement with the calculated percentages of these complexes, with an acceptable range of 0.4% in all cases. The results are comparable to those seen in other Ru–NHC complexes (Molero et al., 1998).
2.3 Biological evaluation
2.3.1 Antimicrobial activity
The obtained compounds were tested in vitro for their antibacterial activities using the agar well diffusion method in accordance with earlier studies (Lionetto et al., 2013; Ahmad et al., 2003, and Zolghadri et al., 2019). The minimum inhibition concentration (MIC), which is the lowest concentration of the test sample that completely inhibits the growth of microorganisms, was determined. CMI against Escherichia coli (ATCC 25988), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumoniae (ATCC 700603), Staphylococcus aureus (ATCC 29213), and methicillin-resistant Staphylococcus aureus were measured at a concentration of 100 L. The MIC values of compounds 2–3 against all bacterial strains are shown in Figure 1.
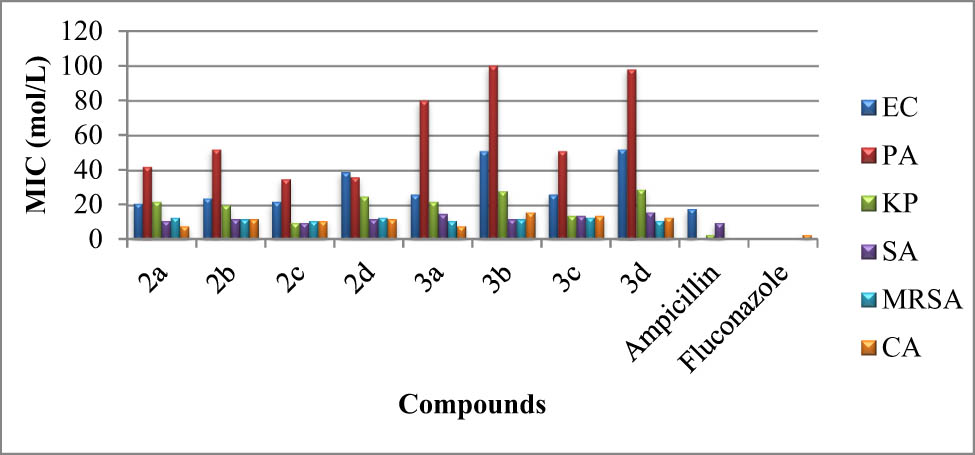
MIC (μmol·L−1) values of salts 2 and their ruthenium(ii) complexes (3a–3d) against bacterial and fungal strains. (a) EC: E. coli (ATCC 25988). PA: P. aeruginosa (ATCC 27853). KP: K. pneumonia (ATCC 700603). SA: S. aureus (ATCC 29213). MRSA: Methicillin-resistant S. (ATCC 43300). (b) CA: Candida albicans (ATCC 14053).
From Figure 1, complexes 3a and 3c did not affect the resistance of E. coli (ATCC 25988). The 3b and 3d complexes were the least effective against the bacteria P. aeruginosa (ATCC 27853). Whereas ruthenium(ii) complexes (3a–3d) demonstrated promising activity comparable to standard, and NHC salts 2 had relatively higher MIC values. The difference in the activity of ligands and complexes may be due to the synergistic effect of ruthenium–ligand bonding that causes the enhancement in lipophilicity of these complexes.
2.3.2 AChE and tyrosinase (TyrE) inhibitory activities
According to previous studies (Zolghadri et al., 2019), the inhibitory activities of ruthenium(ii) complexes (3a–3d) were studied. The obtained results are represented in IC50 (g·mL−1) units. The most potent inhibitors of TyrE and AchE, according to Figure 2, were complexes 3b and 3d, with IC50 values of 19.88 and 24.95 g·mL−1 and 2.52 and 5.06 g·mL−1 respectively (Figure 2).
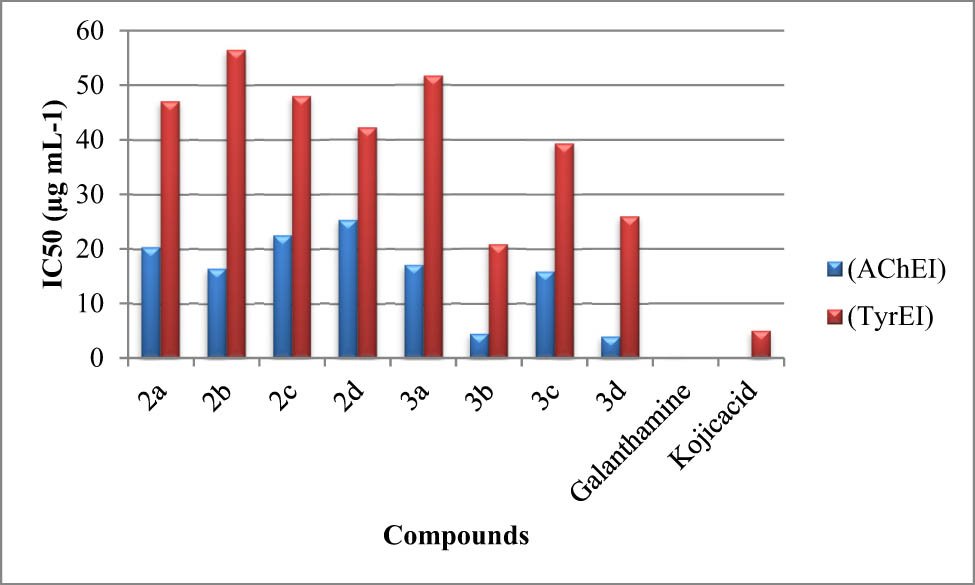
Anti-AChE (AChEI and anti-TyrE (TyrEI) inhibitory activities of the synthesized (NHC) ligands (2a–2d) and their respective ruthenium(ii) complexes (3a–3d) presented by their IC50 (µg·mL−1) values.
2.3.3 Antioxidant activity
Figure 3 illustrates the strong antioxidant activity of complex 3d, which is quite similar to BHT. The IC50 values for the other complexes were, in order, 41.78, 32.05, 45.04, and 17.41 g·mL−1 (Figure 3).
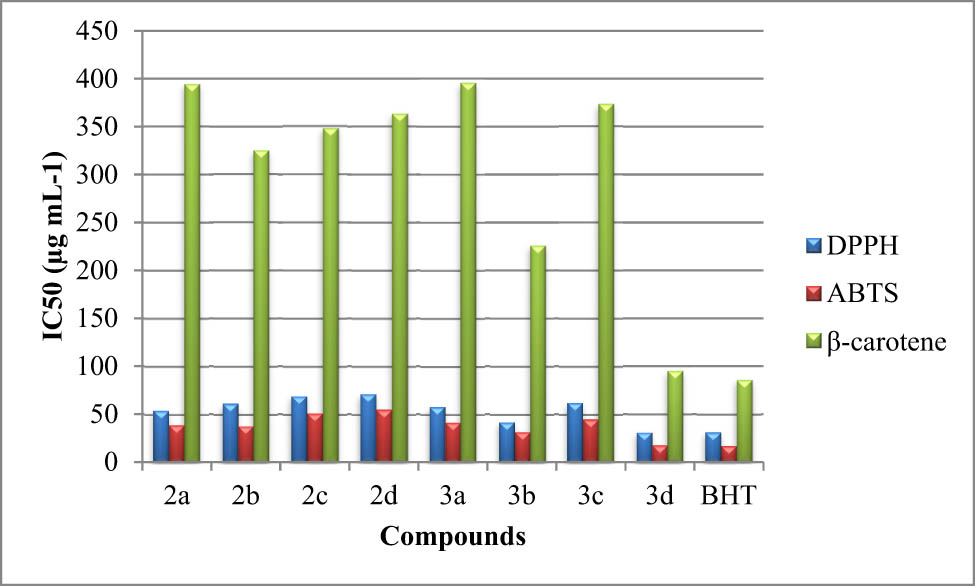
Antioxidant activities of the synthesized NHC ligands (2a–2d) and their respective ruthenium(ii) complexes (3a–3d) assessed by DPPH, ABTS antiradical scavenging power, and β-carotene bleaching test presented by their IC50 values.
2.3.4 Antiproliferative activity
The anticancer activities of the synthesized NHC ligands (2a–2d) and their corresponding ruthenium(ii) complexes (3a–3d) were studied using the methods for colon carcinoma cell lines (HCT-116) and hepatocellular carcinoma. Compounds 2a, 2b, and 3c had IC50 values from 10.45 to 16.45 g in hepatocellular carcinoma cell lines and from 6.65 to 13.45 g in human colon carcinoma cancer cell lines. Moreover, compounds 2c, 2d, and 3b had weak cytotoxic action, with IC50 values ranging from 12.27 to 17.22 g in human colon carcinoma cancer cell lines and from 13.25 to 18.32 g in hepatocellular carcinoma cell lines. The results are summarized in Figure 4.
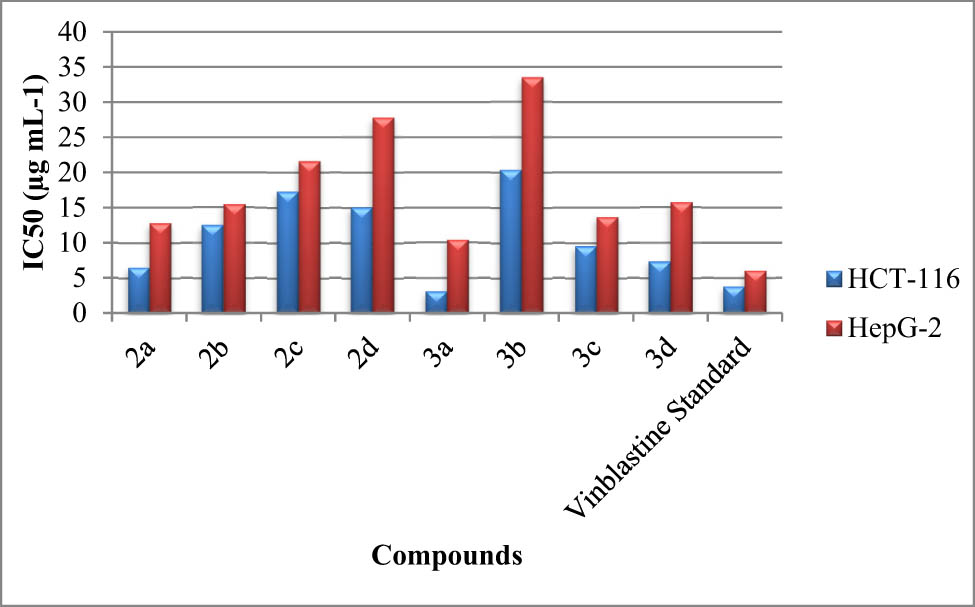
IC50 (µg) values for the synthesized NHC ligands (2a–2d) and their respective ruthenium(ii) complexes (3a–3d) and vinblastine against colon carcinoma cells HCT-116 and HepG-2.
These observations point out that (a) the modification of the steric and electronic properties of NHCs by N-substituents is crucial, (b) the arene type and metal center genus have a significant influence on the antiproliferative activity of complexes, and (c) complexes have properties that facilitate their cellular uptake into cells.
3 Conclusions
The target complexes [RuCl2(p-cymene)NHC] (3a–3d) were isolated rapidly with good yields following the activation of salts 2a–2d with silver oxide and then their transmetallation with [(p-Cym)RuCl2]2 at room temperature and in dichloromethane. The structures of Ru(ii)–NHC complexes 3a–d and benzimidazolium salts (2a–2d) were identified via elemental analysis and from their 1H and 13C NMR spectra. Enzyme inhibition studies of AChE and TyrE revealed that both complexes 3b and 3d have the potent inhibition against AchE and TyrE, respectively. In order to evaluate the antioxidant activity of the synthesized NHC ligands (2a tyrosinase 2d) and their corresponding ruthenium(ii) complexes (3a–3d) three different methods were used. DPPH (2,2 diphenyl-1-picrylhydrazyl (DPPH)), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), and β-carotene linoleic acid bleaching revealed that the 3d complex showed significant antioxidant activity. As for cancer cell lines (HCT-116) and hepatocellular carcinoma (HepG-2), NHC ligands (2a–2d) and their corresponding ruthenium(ii) complexes (3a–3d) revealed that ruthenium(ii) complex 3a have cytotoxic activity comparable to that of standard vinblastine.
Experimental
General methods
All manipulations were carried out under argon using standard Schlenk line techniques in accordance with our earlier work (Karaca et al., 2021) and (Slimani et al., 2020). Chemicals and solvents were purchased from Sigma-Aldrich Co. (Poole, Dorset, UK). The solvents used were purified by distillation over the drying agents indicated and were transferred under argon. Melting points were measured in open capillary tubes with an Electrothermal-9200 melting point apparatus. IR spectra were recorded on an ATR unit in the range of 400–4,000 cm−1 with PerkinElmer Spectrum 100 Spectrophotometer. 1H NMR and 13C NMR spectra were recorded using Bruker Avance AMX and Bruker Avance III spectrometer operating at 400 MHz (1H NMR) and at 100 MHz (13C NMR) in CDCl3 with TMS added. NMR multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, hept = heptet, and m = multiplet signal. The NMR studies were carried out in high-quality 5 mm NMR tubes. The chemical shifts (d) are reported in ppm relative to CDCl3. Coupling constants (J values) are given in hertz. Elemental analyses were performed by LECO CHNS-932 elementary chemical analyzer
Synthesis of ligands (2a–d)
During a period of 2–3 days, benzimidazolium salt 1 (1 g) and an appropriate amount of benzyl bromide (1 eq) in DMF (2 ml) were agitated at 70°C. Then, the white solid that had formed was agitated for a couple of hours and rinsed with diethyl ether (20 ml). After filtering the reaction mixture through paper, the white solid was dried under vacuum and crystallized with DCM–ether (1:3) for further purification.
1,3-bis(2,3,4,5,6-Pentamethylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (2a)
M.p. = 285°C, yield: 90%, ν(CN) = 1,441 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.12(s, 9H, CH3), 2.25(s, 9H, CH3), 2.20(s, 3H, CH3), 6.75–7.45(m, HAr). 10.85(s, 1H, H2). 13CNMR (CDCl3, 101 MHz) δ (ppm): 16.01(2CH3), 19.12(CH3), 16.9(CH3), 21.01(CH3), 21.2(CH3), 53.1(C1), 50.2(C1′), 127.7–135.2 (CAr), 143. 2(C2). Anal. calcd for C29H35BrN2 : C, 70.8%; H, 7.1%; N, 5.7%. Found: C, 70.9; H, 7.2; N, 5.8%.
1,3-bis(2,4,6-Trimethylbenzyl)-1H-benzo[d]imidazol-3-ium bromide (2b)
M.p. = 275°C, yield: 95%, ν(CN) = 1,456 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.15(s, 6H, CH3), 2.23(s, 9H, CH3), 2.21(s, 3H, CH3), 4.40(s, 2H, CH2), 4.45(s, 2H, CH2), 6.74–7.45(m, HAr). 10.35 (s, 1H, H2). 13CNMR (CDCl3, 101 MHz) δ (ppm): 15.9(CH3), 18.14(CH3), 16.3(CH3), 53.2(C1), 50.3(C1′), 127.5–135.4 (CAr), 145. 1(C2). Anal. Calcd for C27H31BrN2: C, 69.9%; H, 6.7%; N, 6.0%. Found: C, 69.9; H, 6.8; N, 6.1%.
1-(4-(tert-butyl)Benzyl)-3-(4-methylbenzyl)-1H-benzo[d]imidazole-3-ium bromide (2c)
M.P. = 285°C, yield: 88%, ν(CN) = 1,445 cm−1. 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.12(s, 6H, 2CH3), 2.11(s, 9H, CH3), 2.32(m, 1H, CH), 4.56(s, 2H, CH2) , 4.47(s, 2H, CH2), 6.42–7.57(m, HAr). 11.62 (s, 1H, H2). 13CNMR(CDCl3, 101 MHz) δ (ppm): 15.5(2CH3), 18.15(CH3), 53.1(C1), 50.4(C1′), 127.6–135.5 (CAr), 144. 2(C2). Anal. calcd for C26H29BrN2 : C, 69.4%; H, 6.5%; N, 6.2%. Found: C, 69.5; H, 6.5; N, 6.3%.
Dimethyl-1,3-(3,5)-dimethyl-4-methylbenzyl)-2,3-dihydro-1H-benzo[d]imidazolium bromide (2d)
Yield: 85%; m.p. = 287°C; FT-IR (KBr) ν(CN)(cm−1): 1,567; 1H NMR (CDCl3, 400 MHz) δ (ppm) 2.12(s, 6H, CH3), 2.21(s, 3H, CH3), 4.45(s, 2H, CH2), 4.48(s, 2H, CH2), 6.35–7.47(m, HAr). 11.75 (s, 1H, H2). 13CNMR(CDCl3, 101 MHz) δ (ppm): 15.8(CH3), 17.12(CH3), 53.1(C1), 50.4(C1′), 125.5–136.4 (CAr), 144. 7(C2). Anal. calcd for C24H25BrN2: C, 68.4%; H, 5.9%; N, 6.6%. Found: C, 68.5; H, 5.9; N, 6.7%.
Synthesis of ruthenium NHC complexes (3a–3d)
The ruthenium–NHC complexes were synthesized through transmetalation via silver–NHC complexes (2a–d) without isolation. We reacted 1 eq. of in situ silver–NHC complexes and 0.5 eq. of [RuCl2(p-cymene)]2 in anhydrous dichloromethane (30 ml). The reaction mixture was stirred for 4 days at room temperature in the dark. Then, the solution was filtered through celite. and the filtrate was concentrated to dryness in a vacuum. The red solid obtained was recrystallized with DCM-diethyl ether or DCM/pentane. The structure of Ru–NHC complexes were determined by IR and NMR analysis.
Dichloro[1-(2,3,4,5,6-pentamethylbenzyl)-3-(2,4,6-trimethylbenzyl)benzimidazol-2-ylidene](p-cymene)ruthenium(ii) (3a)
Yield: 85%; m.p. = 224°C. FT-IR(KBr)ν(CN)(cm−1) = 1,410. 1HNMR (300 MHz, CDCl3, δ (ppm)): 1.12 (d, 6H,2CH3), 2.17 ((s, 3H, CH3), 1.21 (d, 6H, CH3), 2.24 (m, 1H, CH3), 2.13 (s, 3H, CH3), 2.17 (s, 3H, CH3), 4.65 (s, 2H, CH2), 4.52 (s, 2H, CH2), 6.35–7.35 (m, HAr). 13C NMR (CDCl3, 75 MHz) (δ (ppm)): 18.3 (4CH3), 22.1 (CH3), 19.5 (CH3), 20.8 (CH); 22.3(CH3), 51.7(C1′, CH2); 47.8(C1, CH2), 126.3–134.5(CAr). 177.6 (C2). Anal. calcd for C39H48Cl2N2Ru: C, 65.3%; H, 6.7%; N, 3.9%. Found: C, 65.4%; H, 6.8%; N, 3.9%.
Dichloro[1-(2,4,6-trimethylbenzyl)-3-(2,4,6-trimethylbenzyl)benzimidazol-2-ylidene](p-cymene)ruthenium(ii) (3b)
Yield: 90%; m.p. = 230°C. FT-IR(KBr)ν(CN)(cm−1) = 1,418; 1HNMR (300 MHz, CDCl3, δ (ppm)): 2.12 (s, 3H, CH3), 2.24 ((s, 6H, CH3), 2.11 (s, 3H, CH3), 1.12 (d, 6H, CH3), 2.26 (s, 6H, CH3), 2.18 (s, 6H, CH3), 4.5 (s, 2H, CH2), 4.7 (s, 2H, CH2), 6.35–7.37 (m, HAr).13C NMR (CDCl3, 75 MHz) (δ (ppm)): 19.1(3CH3), 20.1(CH3), 21.1 (CH3), 21.7 (CH), 22.2 (2CH3), 21.7 (CH3), 50.8 (C1′, CH2); 47.7 (C1, CH2), 126.2–135.8 (CAr). 189.17 (C2). Anal. calcd for C37H44Cl2N2Ru : C, 64.5%; H, 6.4%; N, 4.0%. Found: C, 64.6%; H, 6.5%; N, 4.1%.
Dichloro[1-(4-tert-butyl) 3-(4-methylbenzyl)benzimidazol-2-ylidene](p-cymene)ruthenium(ii) (3c)
Yield: 92%; m.p. = 245°C. FT-IR(KBr)ν(CN)(cm−1) = 1,609. 1HNMR (300 MHz, CDCl3, δ (ppm)): 1.14 (s, 6H, CH3), 1.26 (s, 9H, CH3), 2.23 ((s, 3H, CH3), 2.10(s, 3H, CH3), 2.63 (d, 1H, CH), 4.61 (s, 2H, CH2), 4.61 (s, 2H, CH2), 6.37–7.42 (m, HAr).13C NMR (CDCl3, 75 MHz) (δ (ppm)): 31.2 (3CH3), 22.2 (2CH3), 21.3 (2CH3), 32.2 (CH), 51.7 (C1′, CH2); 52.4 (C1, CH2), 127.2–143.4 (CAr). 188.95 (C2). Anal. calcd for C38H46Cl2N2Ru: C, 64.9%; H, 6.6%; N, 3.9%. Found: C, 65.1%; H, 6.7%; N, 4.1%.
Dichloro[1-(3,5-dimethylbenzyl)-3-(4-methylbenzyl)benzimidazol-2-ylidene](p-cymene)ruthenium(ii) (3d)
Yield: 95%; m.p. = 220°C. FT-IR(KBr)ν(CN)(cm−1) = 1,409. 1HNMR (300 MHz, CDCl3, δ (ppm)): 1.23 (s, 6H, CH3), 2.12 (s, 6H,), 2.25 ((s, 3H, CH3), 2.25 (s, 3H, CH3), 2.85 (d, 1H, CH), 4.62 (s, 2H, CH2), 4.65 (s, 2H, CH2), 6.39–7.36 (m, HAr). 13C NMR (CDCl3, 75 MHz) (δ (ppm)): 21.2 (3CH3), 22.2 (2CH3), 21.3 (CH3), 33.3 (CH), 50.4 (C1′, CH2); 52.5 (C1, CH2), 126.5–144.6 (CAr). 182.34 (C2). Anal. Calcd cor C34H38Cl2N2Ru: C, 63.1%; H, 5.9%; N, 4.3%. Found: C, 63.2%; H, 5.9%; N, 4.4%.
Biological activities
Microorganisms, media, and growth conditions, agar well diffusion method, and MIC were performed according to the literature work (Vatanabe et al., 2017; Ellman et al., 1961). AChEI activity was measured according to Ellman. The tyrosinase inhibitory activity was determined spectrophotometrically according to the literature. DPPH radical scavenging activity was determined according to Re et al. (1999). ABTS radical scavenging activity was carried out according to Re et al. (1999) protocol. β-Carotene bleaching test was performed according to Pratt’s method (Karataş et al., 2016).
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project (number RSP2023R75), King Saud University, Riyadh, Saudi Arabia.
-
Funding information: Authors state no funding involved.
-
Author contributions: Ahmed Hussain Jawhari, Nasser Amri, Rafik Gatri: methodology, experimental analysis; Waleed S. Koko and Lamjed Mansour, done the biological part, Ismail Özdemir, Nevin Gürbüz and Naceur Hamdi: writing – original draft, experimental analysis; Naceur Hamdi: writing, resources, project administration.
-
Conflict of interest: Authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmad W., Ahmad B., Ahmad M., Iqbal Z., Nisar M., Ahmad M., In vitro inhibition of acetylcholinesterase, butyrylcholinesterase and lipoxygenase by crude extract of Myricaria elegans Royle. J. Biol. Sci., 2003, 11, 1046–1049. 10.3923/jbs.2003.1046.1049.Suche in Google Scholar
Al Nasr I.S., Koko W.S., Khan T.A., Gürbüz N., Özdemir I., Hamdi N., Evaluation of Ruthenium(ii) N-heterocyclic carbene complexes as enzymatic inhibitory agents with antioxidant, antimicrobial, antiparasitical and antiproliferative activity. Molecules, 2023, 28, 1359. 10.3390/molecules28031359.Suche in Google Scholar PubMed PubMed Central
Antonarakis E.S., Emadi A., Ruthenium-based chemotherapeutics: are they ready for prime time?. Cancer Chemother. Pharmacol., 2010, 66(1), 1–9. 10.1007/s00280-010-1293-1.Suche in Google Scholar PubMed PubMed Central
Arduengo III A.J., Harlow R.L., Kline M., A stable crystalline carbine. J. Am. Chem. Soc., 1991, 113(1), 361–363; 10.1021/ja00001a054.Suche in Google Scholar
Barnard P.J., Baker M.V., Berners-Price S.J., Day D.A., Mitochondrial permeability transition induced by dinuclear gold(i)–carbene complexes: potential new antimitochondrial antitumour agents. J. Inorg. Biochem., 2004, 1, 98(10), 1642–1647. 10.1016/j.jinorgbio.2004.05.011.Suche in Google Scholar PubMed
Bilel H., Hamdi N., Zagrouba F., Fischmeister C., Bruneau C., Terminal conjugated dienes via a ruthenium-catalyzed cross-metathesis/elimination sequence: application to renewable resources. Catal. Sci. Technol., 2014, 4(7), 2064–2071.10.1039/C4CY00315BSuche in Google Scholar
Briguglio I., Piras S., Corona P., Gavini E., Nieddu M., Boatto G., et al., Benzotriazole: An overview on its versatile biological behavior. Eur. J. Med. Chem., 2015, 97, 612–648. 10.1016/j.ejmech.2014.09.089.Suche in Google Scholar PubMed PubMed Central
Çetinkaya B., Çetinkaya E., Küçükbay H., Durmaz R., Antimicrobial activity of carbene complexes of rhodium(i) and ruthenium(ii). Arzneim.-Forsch./Drug Res., 1996, 46(8), 821–823.Suche in Google Scholar
Ciftci O., Ozdemir I., Cakir O., Demir S., The determination of oxidative damage in heart tissue of rats caused by ruthenium(ii) and gold(i) N-heterocyclic carbene complexes. Toxicol. Ind. Health., 2011, 27(8), 735–741. 10.1177/0748233710395993.Suche in Google Scholar PubMed
Ciurea C.N., Kosovski I.B., Mare A.D., Toma F., Pintea-Simon I.A., Man A., Candida and candidiasis – opportunism versus pathogenicity: a review of the virulence traits. Microorganisms, 2020 Jun, 8(6), 857. 10.3390/microorganisms8060857.Suche in Google Scholar PubMed PubMed Central
Crump J.A., Luby S.P., Mintz E.D., The global burden of typhoid fever. Bull. World Health Organ., 2004, 82, 346–353. https://www.scielosp.org/article/bwho/2004.v82n5/346-353/.Suche in Google Scholar
Ellman G.L., Courtney K.D., Andres Jr V., Featherstone R.M., A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol., 1961, 7(2), 88–95. 10.1016/0006-2952(61)90145-9.Suche in Google Scholar PubMed
Ezhilarasu T., Balasubramanian S., Synthesis, characterization, photophysical and electrochemical studies of Ruthenium(ii) complexes with 4′‐substituted terpyridine ligands and their biological applications. ChemistrySelect, 2018, 3(43), 12039–12049. 10.1002/slct.201801624.Suche in Google Scholar
Garner M.E., Niu W., Chen X., Ghiviriga I., Abboud K.A., Tan W., Veige A.S. N-heterocyclic carbene gold(i) and silver(ii) complexes bearing functional groups for bio-conjugation. Dalton. Trans., 2015;44(4). 1914–1923. 10.1039/C4DT02850C.Suche in Google Scholar
Hartinger C.G., Groessl M., Meier S.M., Casini A., Dyson P.J., Application of mass spectrometric techniques to delineate the modes-of-action of anticancer metallodrugs. Chem. Soc. Rev., 2013, 42(14), 6186–6199. 10.1039/C3CS35532B.Suche in Google Scholar
He Z., Zhang S.F., Xue J.R., Liang Y., Zhang X., Jing L.H., et al., Verstile silver(i) and nickel(ii) NHC complexes bearing benzotriazole-function: Synthesis, fluorescence and catalytic property. J. Organomet., 2016, 808, 12–22. 10.1016/j.jorganchem.2016.02.005.Suche in Google Scholar
Herrmann W.A., N‐heterocyclic carbenes: a new concept in organometallic catalysis. Angew. Chem. Int. Ed., 2002, 41(8), 1290–1309. 10.1002/1521-3773(20020415)41:8<1290::AID-ANIE1290>3.0.CO;2-Y.Suche in Google Scholar
Jakupec M.A., Galanski M., Arion V.B., Hartinger C.G., Keppler B.K., Antitumour metal compounds: more than theme and variations. Dalton Trans., 2008, (2), 183–194. 10.1039/B712656P.Suche in Google Scholar
Karaca E.Ö., Dehimat Z.I., Yaşar S., Gürbüz N., Tebbani D., Çetinkaya B., et al., Ru(ii)-NHC catalysed N-Alkylation of amines with alcohols under solvent-free conditions. Inorganica Chim. Acta., 2021, 520, 120294. 10.1016/j.ica.2021.120294.Suche in Google Scholar
Karataş M.O., Olgundeniz B., Günal S., Özdemir İ., Alıcı B., Çetinkaya E., Synthesis, characterization and antimicrobial activities of novel silver(i) complexes with coumarin substituted N-heterocyclic carbene ligands. Bioorg. Med. Chem., 2016, 24(4), 643–650. 10.1016/j.bmc.2015.12.032.Suche in Google Scholar
Keene F.R., Smith J.A., Collins J.G. Metal complexes as structure-selective binding agents for nucleic acids. Coord. Chem. Rev., 2009, 253(15–16), 2021–2035. 10.1016/j.ccr.2009.01.004.Suche in Google Scholar
Keppler B.K., Rupp W., Antitumor activity of imidazolium-bisimidazole-tetrachlororuthenate(iii). A representative of a new class of inorganic antitumor agents. J. Cancer Res. Clin. Oncol., 1986, 111(2), 166–168. 10.1007/bf00400758.Suche in Google Scholar
Lionetto M.G., Caricato R., Calisi A., Giordano M.E., Schettino T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. BioMed. Res. Int., 2013, 2013. 10.1155/2013/321213.Suche in Google Scholar PubMed PubMed Central
Medici S., Peana M., Nurchi V.M., Lachowicz J.I., Crisponi G., Zoroddu M.A., Noble metals in medicine: Latest advances. Coord. Chem. Rev., 2015, 284, 329–350. 10.1016/j.ccr.2014.08.002.Suche in Google Scholar
Meier-Menches SM, Neuditschko B, Zappe K, Schaier M, Gerner MC, Schmetterer KG, et al. An organometallic gold(I) bis-n-heterocyclic carbene complex with multimodal activity in ovarian cancer cells. Chem. Eur. J., 2020, 26(67), 15528–15537. 10.1002/chem.202003495.Suche in Google Scholar PubMed PubMed Central
Molero G., Diez-Orejas R., Navarro-Garcia F., Monteoliva L., Pla J., Gil C., et al., Candida albicans: genetics, dimorphism and pathogenicity. Int. Microbiol., 1998, 1(2), 95–106.Suche in Google Scholar
Moore N.M., Flaws M.L., Epidemiology and pathogenesis of Pseudomonas aeruginosa infections. Clin. Lab., 2011, 24(1), 43–46. http://clsjournal.ascls.org/content/ascls/24/1/43.full.pdf.10.29074/ascls.24.1.43Suche in Google Scholar
Noffke A.L., Habtemariam A., Pizarro A.M., Sadler P.J. Designing organometallic compounds for catalysis and therapy. Chem. Commun., 2012, 48(43), 5219–5246. 10.1039/C2CC30678F.Suche in Google Scholar
Oehninger L., Stefanopoulou M., Alborzinia H., Schur J., Ludewig S., Namikawa K., et al., Evaluation of arene ruthenium(ii) N-heterocyclic carbene complexes as organometallics interacting with thiol and selenol containing biomolecules. Dalton. Trans., 2013, 42(5), 1657–1666. 10.1039/C2DT32319B.Suche in Google Scholar PubMed
Pizarro-Cerdá J., Cossart P., Microbe Profile: Listeria monocytogenes: a paradigm among intracellular bacterial pathogens. Microbiology, 2019, 165(7), 719–721. 10.1099/mic.0.000800.Suche in Google Scholar PubMed
Ray S., Mohan R., Singh J.K., Samantaray M.K., Shaikh M.M., Panda D., et al., Anticancer and antimicrobial metallopharmaceutical agents based on palladium, gold, and silver N-heterocyclic carbene complexes. J. Am. Chem. Soc., 2007, 129(48), 15042–15053. 10.1021/ja075889z.Suche in Google Scholar PubMed
Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med., 1999, 26(9–10), 1231–1237. 10.1016/S0891-5849(98)00315-3.Suche in Google Scholar
Rosenberg B., Vancamp L., Trosko J.E., Mansour V.H., Platinum compounds: a new class of potent antitumour agents. Nature, 1969, 222(5191), 385–386. 10.1038/222385a0.Suche in Google Scholar PubMed
Slimani I., Chakchouk-Mtibaa A., Mansour L., Mellouli L., Özdemir I., Gürbüzd N., et al., Synthesis, characterization, biological determination and catalytic evaluation of ruthenium(ii) complexes bearing benzimidazole-based NHC ligands in transfer hydrogenation catalysis. N. J. Chem., 2020, 44(14), 5309–5323. 10.1039/D0NJ00311E.Suche in Google Scholar
Vatanabe I.P., Rodrigues C.N., Buzinari T.C., Moraes T.F., Silva R.S., Rodrigues G.J., Ruthenium complex improves the endothelial function in aortic rings from hypertensive rats. Arq. Bras. Cardiol., 2017, 109, 124–131. 10.5935/abc.20170090 Suche in Google Scholar PubMed PubMed Central
Vuradi R.K., Nambigari N., Pendyala P., Gopu S., Kotha L.R., Sirasani S., Study of Anti‐Apoptotic mechanism of Ruthenium(ii) Polypyridyl Complexes via RT‐PCR and DNA binding. Appl. Organometal. Chem., 2020, 34(3), e5332. 10.1002/aoc.5332.Suche in Google Scholar
Zolghadri S., Bahrami A., Hassan Khan M.T., Munoz-Munoz J., Garcia-Molina F., Garcia-Canovas F., et al., A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem., 2019, 34(1), 279–309. 10.1080/14756366.2018.1545767.Suche in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”
Artikel in diesem Heft
- Research Articles
- Two new zinc(ii) coordination complexes constructed by phenanthroline derivate: Synthesis and structure
- Lithium fluoroarylsilylamides and their structural features
- On computation of neighbourhood degree sum-based topological indices for zinc-based metal–organic frameworks
- Two novel lead(ii) coordination complexes incorporating phenanthroline derivate: Synthesis and characterization
- Thermodynamics of transamination reactions with aminotrimethylsilanes and diaminodimethylsilanes
- From synthesis to biological impact of palladium bis(benzimidazol-2-ylidene) complexes: Preparation, characterization, and antimicrobial and scavenging activity
- Novel ruthenium(ii) N-heterocyclic carbene complexes: Synthesis, characterization, and evaluation of their biological activities
- Entropy measures of the metal–organic network via topological descriptors
- Rapid Communication
- Synthesis and crystal structure of an ionic phenyltin(iv) complex of N-salicylidene-valine
- Special Issue: Theoretical and computational aspects of graph-theoretic methods in modern-day chemistry (Guest Editors: Muhammad Imran and Muhammad Javaid)
- Topological indices for random spider trees
- On distance-based indices of regular dendrimers using automorphism group action
- Retraction
- Retraction to “Aluminium(iii), Fe(ii) Complexes and Dyeing Properties of Apigenin(5,7,4′-trihydroxy flavone)”

