Abstract
The Sakeji horseshoe bat Rhinolophus sakejiensis is an endemic to south-central Africa. Until recently, it remained known only from the type series of three specimens collected in Nchila Wildlife Reserve, north-western Zambia. The phylogenetic position of R. sakejiensis was evaluated based on morphologic traits and it was suggested that it represents a part of the ‘clivosus-complex’ within the Rhinolophus ferrumequinum clade of the genus. In this study, we provide an insight into the phylogenetic position of R. sakejiensis employing molecular data, using a newly discovered specimen that originates from the identical area as the type series. We assessed the genetic relationships of R. sakejiensis using the mitochondrial cytochrome b gene. This analysis revealed this species to be phylogenetically distinct from morphologically similar congeners of the capensis, fumigatus, and ferrumequinum species groups. In fact, it does not belong to any recognised species group but rather represents a distinct evolutionary lineage – along with another African relic bat, Rhinolophus horaceki. The divergence time of R. sakejiensis from R. horaceki, its closest relative, was estimated to have occurred in the Middle Miocene, roughly at 15.3 Ma, a period of dynamic environmental changes that may have led to widely separated occurrences of various relic bat lineages.
1 Introduction
The Sakeji horseshoe bat Rhinolophus sakejiensis Cotterill, 2002 is a species endemic to south-central Africa, specifically known only from a very limited area of Ikelenge Pedicle in north-western Zambia. This region lies between the most upper parts of the Lwakela and Zambezi rivers and is located on the watershed of the Zambezi and Congo river systems that is partly demarcated by the international borders between Zambia and the Democratic Republic of the Congo (DRC). This bat species was described based on three male specimens collected at Kavunda (11°17′S, 24°21′E; 1,388 m a.s.l) in Nchila Wildlife Reserve on 11 October 1990 (Cotterill 2002). Until recently, it was known solely from this type series (Burgin 2019; Monadjem et al. 2020; Simmons 2005). However, a new male specimen of this bat was discovered in the collection of the National Museum, Prague, Czech Republic (NMP). This fourth known specimen of R. sakejiensis was collected at Sakeji (11°16′S, 24°19′E), Nchila WR, by the expedition of the University of South Bohemia, Czech Republic, in April 2009 (Benda et al. 2022a), just 4 km WNW of the type locality (Benda et al. 2022a; Cotterill 2002).
Cotterill (2002: 167–168) precisely described the morphological traits of R. sakejiensis, clearly distinguishing it from other African Rhinolophus species. It differs from representatives of the fumigatus group by differences in the shape of the noseleaf and rostrum, and from members of the ferrumequinum and capensis groups in both body and skull sizes (definition of groups followed that by Bogdanowicz 1992). According to the description by Cotterill (2002), the bat most similar to R. sakejiensis in body size and noseleaf shape is R. hillorum Koopman, 1989, a large-sized horseshoe bat of West African forests. However, R. hillorum still differs from R. sakejiensis in the skull shape. Cotterill (2002: 175) evaluated all the morphological evidence and discussed the phylogenetic position of R. sakejiensis and concluded that it belongs to the ferrumequinum clade (sensu Bogdanowicz 1992). Along with two other Afrotropical bats, R. clivosus [ = R. acrotis von Heuglin, 1861, see Uvizl et al. (2024)] and R. hillorum, they form the ‘clivosus-complex’ within this clade. Csorba et al. (2003) accepted this conclusion, and both Simmons (2005) and Burgin (2019) included R. sakejiensis in the ‘ferrumequinum species group’. However, Burgin (2019) stressed that this position is based solely on morphology, leaving the exact phylogenetic relationship of R. sakejiensis uncertain.
In this study, we provide the first insight into the phylogenetic position of R. sakejiensis employing molecular data using the newly discovered NMP specimen. Specifically, we analysed the mitochondrial cytochrome b gene to assess the genetic relationship between R. sakejiensis and other members of the Rhinolophus genus. This comparsion revealed that R. sakejiensis is phylogenetically distinct from the morphologically similar capensis, fumigatus, and ferrumequinum species groups. Additionally, morphometrical examination of the NMP specimen, when compared with the type series, confirmed the shared diagnostic traits that distinguish R. sakejiensis from its congeners. Based on molecular data, we estimate that R. sakejiensis diverged from other species approximately 15 million years ago (Ma), suggesting that this species has evolved in isolation within its restricted range.
2 Materials and methods
For the morphological and molecular genetic examinations, we used the male specimen housed in the collection of the National Museum, Prague (NMP 97587 [field number RS 796]; alcoholic specimen with skull extracted). It was collected at Sakeji, Zambia, by J. Šklíba, M. Lövy, V. Mazoch, and R. Šumbera, on 28 April 2009 (see above and Benda et al. 2022a). Standard linear measurements were taken with the use of mechanical callipers. Horizontal dental measurements were taken on cingulum margins.
For the molecular genetic analysis, DNA was extracted from the pectoral muscle tissue of the NMP specimen. This sample was supplemented with additional sequences of the genus Rhinolophus previously published and stored in the GenBank (Benda and Vallo 2012; Benda et al. 2022a, b, 2024; Demos et al. 2019; Dool et al. 2016; Jacobs et al. 2013; Uvizl et al. 2024). GenBank sequences of three Hipposideros species from the sister family Hipposideridae were included as an outgroup (Foley et al. 2015; Teeling et al. 2005; for details, see Supplementary Table S1).
The genomic DNA was extracted from the alcohol-preserved tissue samples using the Geneaid Genomic DNA Mini Kit, targeting the mitochondrial gene for cytochrome b. We employed the primers specifically designed for the order Chiroptera that demonstrated reliable amplification in previous studies (see e.g., Demos et al. 2019; Dool et al. 2016; Jacobs et al. 2013; Uvizl et al. 2024). The sequences were amplified using the primers mtDNA-R3-F (5′-TGGCATGAAAAATCACCGTTGT-3′; Puechmaille et al. 2011) and CytB-H (5′-CTTTTCTGGTTTACAAGACCAG-3; Weyeneth et al. 2008). The PCR amplifications followed the methodology described by Uvizl et al. (2024), and Sanger sequencing was performed in both directions by Macrogen, Inc. (Amsterdam, the Netherlands), using the PCR primers.
The sequences were edited and aligned using the MAFFT plugin (Katoh and Standley 2013) in Geneious 11.0.5 (https://www.geneious.com) and were subsequently manually edited and trimmed using Gblocks (Castresana 2000). The sequences of protein-coding markers were translated to amino acids to check for the presence of stop codons, which would indicate the presence of pseudogenes.
Phylogenetic analyses were conducted using maximum likelihood (ML) based on the cytochrome b dataset. The most appropriate nucleotide substitution model (TIM2 + F + R4) was selected using the Bayesian information criterion (BIC) in ModelFinder (Kalyaanamoorthy et al. 2017). The ML tree was inferred with the selected substitution model using IQ-TREE (Nguyen et al. 2015). The best-scoring ML was identified through the use of the ultrafast bootstrap (UFBoot; Hoang et al. 2018), employing 1,000 bootstrap replicates and 1,000 topology replicates. The ML analysis was carried out on the IQtree web server (Trifinopoulos et al. 2016).
For molecular dating analyses, the dataset was reduced to one to four samples per species. Divergence time was estimated using BEAST v2.5.0 (Bouckaert et al. 2014; Drummond et al. 2006), applying relaxed lognormal molecular clocks and the Yule speciation process. The family Rhinolophidae root age of 31.24 Ma was used as a calibration point sensu Álvarez-Carretero et al. (2021). BEAST was run three times for 10 million generations, saving trees every 1,000 generations. Tracer v1.6 confirmed adequate mixing of MCMC chains (ESS > 200). LogCombiner merged tree files, and TreeAnnotator identified the maximum clade credibility tree. All analyses were done using MetaCentrum (metacentrum.cz).
3 Results and discussion
3.1 Morphology and identification
The NMP specimen is a large horseshoe bat with the forearm length (LAt) 56.6 mm, the greatest length of skull (including the praemaxilla; LCr) 25.47 mm, condylocanine length (LCc) 21.86 mm, zygomatic width (LaZ) 13.39 mm, and the length of the upper tooth-row (CM3) 9.61 mm (for other skull dimensions see Benda et al. 2022a). The size of skull of the NMP bat conforms to those mentioned by Cotterill (2002) for the type series, its dimensions fall within the ranges of the type series: LCr 24.6–25.6 mm; LCc 21.7–22.3 mm; LaZ 12.9–13.5 mm; CM3 9.4–9.7 mm (Cotterill 2002: 169, Table 3). In two skull width dimensions, the value ranges of the type specimens lie slightly below the values of the NMP specimen (mastoidal width 11.56 mm, vs. 11.0–11.5 mm; the rostrum width across the third upper molars 9.89 mm, vs. 9.2–9.5 mm). However, these differences are very small and most likely insignificant, regarding the small sample size of the type series and a possibility of personal bias in measuring between authors. Similarly, not all dimensions of one of the paratypes that is housed in the Harrison Zoological Museum, Sevenoaks, UK (HZM), which were taken by Csorba et al. (2003), fall within the ranges of the type series given by Cotterill (2002). The forearm length of the NMP specimen exceeds the range of the type series (52.5–55.2 mm) reported by Cotterill (2002), likely due to his slightly unusual way of the dimension taking (see Cotterill 2002: 166).
The general colouration of the NMP bat is pale (considering the alcohol fixed body), the pelage is beige to very pale brown, the wing membranes are pale brown, and the noseleaf and ears are creamish. Its noseleaf is broad over the horseshoe (it was not measured due to certain deformations caused by the fixation and skull extraction), the lancet is broad and short, the connecting process of sella is blunt and medium-high, the tip of sella is low and rounded (Figure 1). Generally, the noseleaf structure corresponds with that described by Cotterill (2002) and Csorba et al. (2003), only the height of connecting process is somewhat smaller in the NMP specimen than in the type series. The skull structure of the NMP specimen corresponds with that of the HZM paratype of R. sakejiensis as depicted by Csorba et al. (2003: 132). In the NMP bat, the skull is heavily built, the sagittal crest is very high, the infraorbital bridge is narrow and short (Figure 2); the nasal swellings are very low, the posterior swellings are flattened, the anterior swellings only slightly bulged, and the frontal depression is very shallow (Figure 2). In the upper tooth-row, only one premolar (P4) is present (while the small premolar, P2, is absent), and the canine is high and narrow, with its distal cingulum in contact with that of the premolar (P4). In the lower tooth-row, only two large premolars are present and in full contact, while the small (middle) premolar is absent (Figure 3).
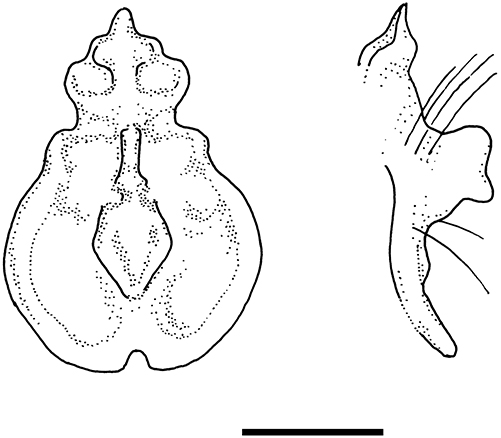
Frontal view (partial reconstruction) of the noseleaf of Rhinolophus sakejiensis (NMP 97587). Scale bar: 5 mm.
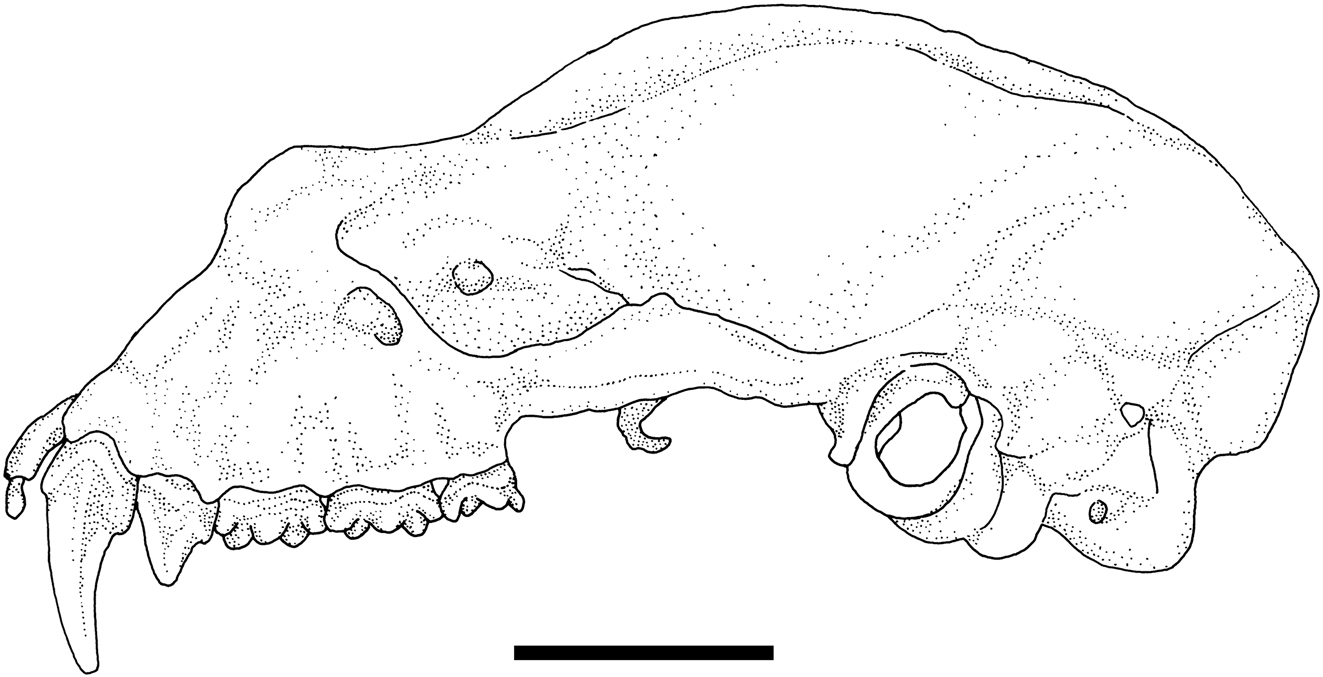
Skull in lateral view of Rhinolophus sakejiensis (NMP 97587). Scale bar: 5 mm.
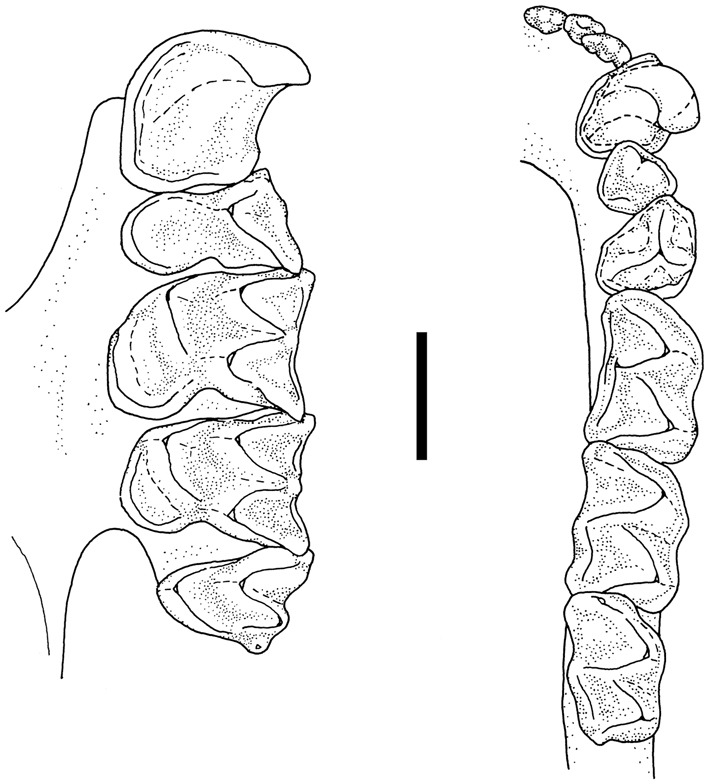
Occlusal views of the left upper tooth-row (C–M3) and the right lower toothrow (I1–M3) of Rhinolophus sakejiensis (NMP 97587). Scale bar: 2 mm.
In southern Africa, where 21–23 species of the genus Rhinolophus occur, only six horseshoe bat species of similar size to the NMP specimen are known (Monadjem et al. 2020: 200; Benda et al. 2024: 241), namely R. acrotis (LAt 48–58 mm), R. alcyone Temminck, 1852 (49–58 mm), R. cervenyi Benda, Uvizl, Eiseb et Avenant, 2024 (49–56 mm), R. deckenii Peters, 1868 (48–56 mm), R. eloquens Andersen, 1905 (53–59 mm), and R. fumigatus Rüppell, 1842 (48–59 mm). However, all these species differ from the NMP specimen by the presence of small premolars in both jaws. Additionally, in R. alcyone, the upper small premolar is placed within the tooth-row and the connecting process and tip of the sella are very prominent (but low in the NMP specimen); in R. alcyone, R. eloquens, and R. fumigatus, the nasal swellings of the skull are very developed and prominent (but low and undeveloped in the NMP bat); in R. eloquens and R. fumigatus, the connecting process of the sella is undeveloped, and the sella profile is rounded (but the connecting process is blunt but developed in the NMP bat); in R. deckenii, the noseleaf (in frontal view) is of a triangular shape (but narrow oval in the NMP bat); and in R. acrotis and R. cervenyi, the tip of the sella is pointed (but blunt in the NMP bat), and the canines are more massive and relatively low (but narrow and high in the NMP bat). As a result of revision of morphological traits and of employing of the identification keys by Csorba et al. (2003) and Monadjem et al. (2020), the identification of the NMP specimen as R. sakejiensis seems to be indisputable, as it shares all essential characters mentioned by these authors.
3.2 Phylogenetic position
The newly generated cytochrome b sequence of the NMP specimen of R. sakejiensis (GenBank Accession Number OQ224762) was supplemented with two new sequences of R. fumigatus, along with additional GenBank sequences, resulting in a final dataset comprising 132 sequences. The 1,057 bp-long dataset contained 574 parsimony informative positions (54.3 % of the total length). Attempts to further examine the phylogenetic relationships of R. sakejiensis using nuclear genetic markers were unsuccessful, likely due to degradation of the nuclear DNA, which led to failed amplification. Despite this restraint, the mitochondrial marker comparison allows an evaluation of phylogenetic position. However, it is necessary to consider it with certain limits, since the incongruity between mitochondrial and nuclear genetic evidence is relatively frequent in bats and even in the horseshoe bats of southern Africa (see e.g. Benda et al. 2024; Taylor et al. 2018). However, in the case of deep divergences like that discovered here, the phylogenetic positions based on both marker types are rather congruent in the horseshoe bats (see e.g., Benda et al. 2022b, Uvizl et al. 2024).
The phylogenetic tree generated by ML analysis of the cytochrome b dataset (Figure 4) positioned the NMP specimen of R. sakejiensis within the Afro-Palaearctic clade of the genus Rhinolophus, in a sister position to R. horaceki Benda and Vallo, (2012), and outside any recognised species group. Rhinolophus horaceki is a Palaearctic African bat with a relic distribution range in the Mediterranean arboreal zone of Cyrenaica, north-eastern Libya (Benda and Vallo 2012). Together, these two species form a sister lineage to two species-rich lineages: the R. fumigatus group (comprising numerous Afrotropical species) and the Rhinolophus ferrumequinum group (which includes both Afrotropical and Palaearctic species). This phylogenetic arrangement suggests that R. sakejiensis, like R. horaceki, does not belong to any recognised species group, but rather represents a distinct evolutionary lineage. This interpretation contrasts with the view presented by Demos et al. (2019) and also with the original views by Cotterill (2002) and Csorba et al. (2003), based solely on morphological comparison.
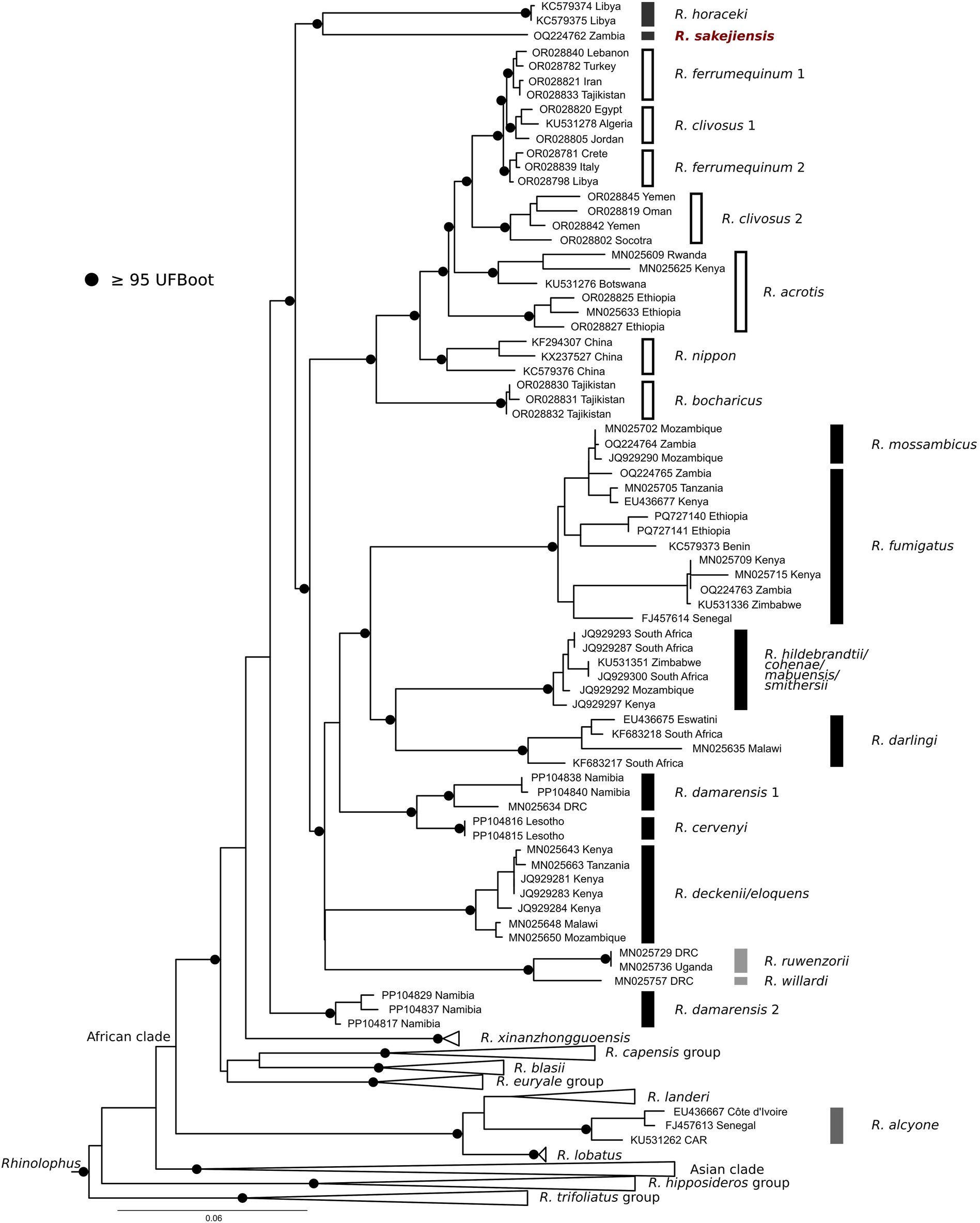
Maximum likelihood tree of reconstructed phylogenetic relationships of Rhinolophus sakejiensis (NMP 97587) with species of the ferrumequinum and fumigatus groups and other Rhinolophus groups/clades based on the cytochrome b dataset. Branch support values ≥95 % UFBoot are shown by black dots on the nodes. The numbering of lineages follows those used by Uvizl et al. (2024) (in R. ferrumequinum and R. clivosus) and Benda et al. (2024) (in R. damarensis).
The divergence time estimation tree revealed wide confidence intervals (95 % highest posterior density [HPD]), likely due to relatively high mutation rates and the short length of the mitochondrial dataset. Despite this, the divergence of R. sakejiensis from R. horaceki was estimated to have occurred around 15.3 Ma (HPD: 10.0–17.5 Ma; Figure 5). The group composed of R. sakejiensis, R. horaceki, and additionally R. eloquens, another African small-range species, was estimated to have diverged from the lineage that comprised the ferrumequinum and fumigatus groups around 20.4 Ma (HPD: 16.2–24.9 Ma; Figure 5). These periods of the Lower and Middle Miocene were characterised by an increasing prevalence of grasslands and fluctuating forest distributions in Africa (Jacobs 2004), environmental changes that may have influenced bat speciation due to the fragmentation of their populations in suitable humid versus arid habitats.
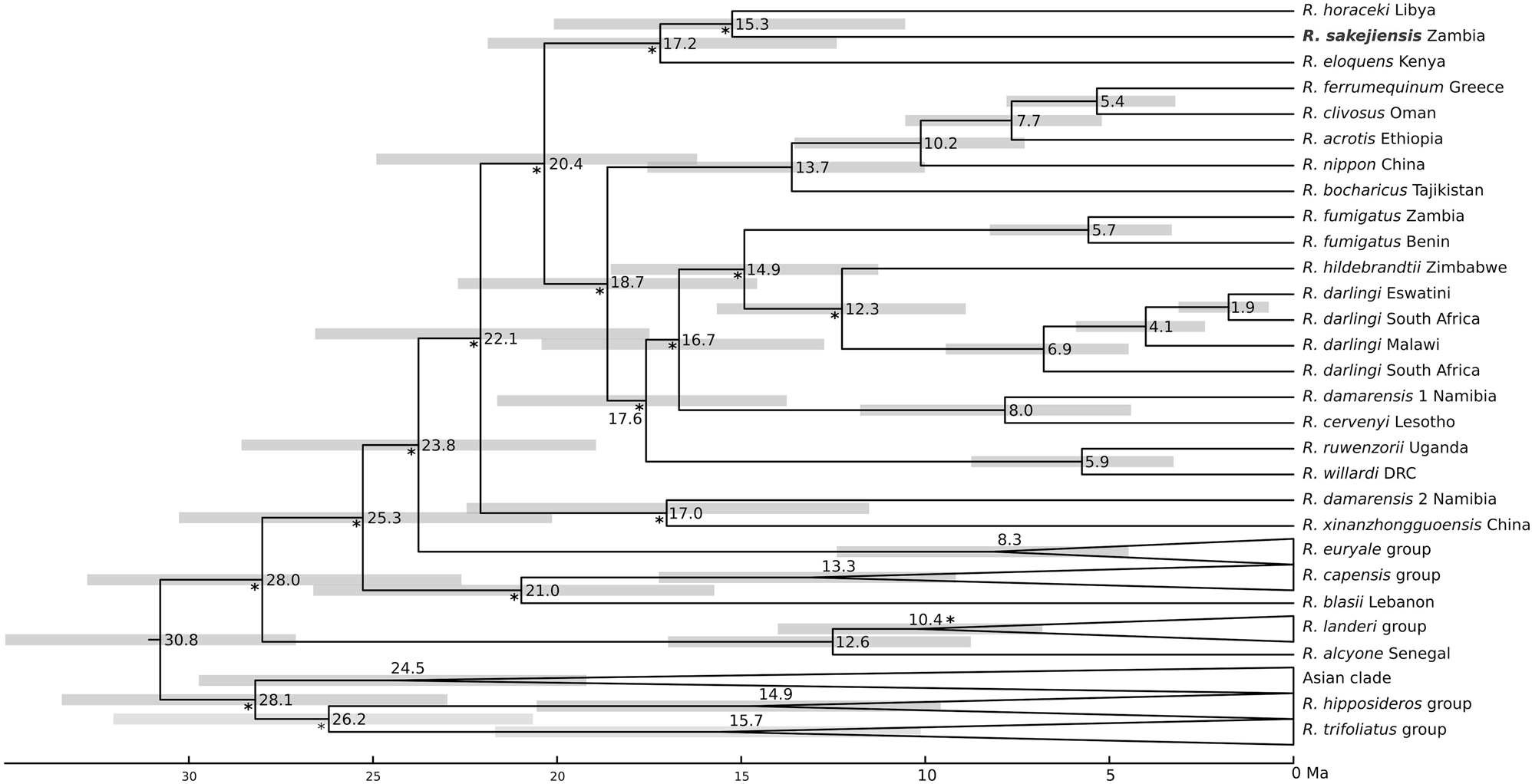
Chronogram of the family Rhinolophidae based on Bayesian inference of the mitochondrial dataset (following to the model by Álvarez-Carretero et al. 2021). Numbers at nodes indicate mean divergence time estimates (Ma) and pale grey horizontal boxes indicate the 95 % highest posterior density intervals of these estimates. The asterisk (*) indicates nodes with low branch support, while the rest of the nodes were supported (PP ≥ 0.95).
Overall, the divergence estimates suggest that R. sakejiensis represents a relic of an early radiation of horseshoe bats that took place in the African continent (Demos et al. 2019). This evolutionary radiation was likely followed by the emergence of the R. landeri, R. capensis, and R. euryale groups, which subsequently gave rise to the geographically widespread R. fumigatus and R. ferrumequinum groups (the groups definition after Burgin 2019). Alongside with species such as R. damarensis and R. horaceki in southern and northern Africa, respectively, and R. xinanzhogguoensis Zhou, Guillén-Servent, Lim, Eger, Wang et Jiang, 2009 in eastern Asia, R. sakejiensis remains as a relic of these ancient radiations, indicating a complex and fragmented evolutionary history of the Afro-Palaearctic lineage of the genus Rhinolophus.
Funding source: Ministry of Culture of the Czech Republic
Award Identifier / Grant number: DKRVO 2024–2028/6.I.b, National Museum, 00023272
Acknowledgments
Computational resources were provided by the e-INFRA CZ project (# 90254), supported by the Ministry of Education, Youth and Sports of the Czech Republic.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Both authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interests: The authors declare no conflict of interests regarding this article.
-
Research funding: The preparation of this contribution was supported by the Ministry of Culture of the Czech Republic (# DKRVO 2024–2028/6.I.b, National Museum, 00023272).
-
Data availability: Not applicable.
References
Álvarez-Carretero, S., Tamuri, A.U., Battini, M., Nascimento, F.F., Carlisle, E., Asher, R.J., Yang, Z., Donoghue, P.C.J., and dos Reis, M. (2021). A species-level timeline of mammal evolution integrating phylogenomic data. Nature 602: 263–267, https://doi.org/10.1038/s41586-021-04341-1.Suche in Google Scholar PubMed
Benda, P. and Vallo, P. (2012). New look on the geographical variation in Rhinolophus clivosus with description of a new horseshoe bat species from Cyrenaica, Libya. Vespertilio 16: 69–96.Suche in Google Scholar
Benda, P., Uvizl, M., Šklíba, J., Mazoch, V., and Červený, J. (2022a). African bats in the collection of the national Museum, Prague (Chiroptera). I. Bats from Zambia. Lynx, n. s. 53: 291–332, https://doi.org/10.37520/lynx.2022.021.Suche in Google Scholar
Benda, P., Uvizl, M., Vallo, P., Reiter, A., and Uhrin, M. (2022b). A revision of the Rhinolophus hipposideros group (Chiroptera: Rhinolophidae) with definition of an additional species from the Middle East. Acta Chiropterol. 24: 269–298, https://doi.org/10.3161/15081109acc2022.24.2.001.Suche in Google Scholar
Benda, P., Uvizl, M., Eiseb, S.J., and Avenant, N.L. (2024). On the systematic position of the horseshoe bats (Mammalia: Chiroptera) from Lesotho. Mammalia 88: 239–258, https://doi.org/10.1515/mammalia-2023-0119.Suche in Google Scholar
Bogdanowicz, W. (1992). Phenetic relationships among bats of the family Rhinolophidae. Acta Theriol. 37: 213–240, https://doi.org/10.4098/at.arch.92-22.Suche in Google Scholar
Bouckaert, R.R., Heled, J., Kuehnert, D., Vaughan, T.G., Wu, C.-H., Xie, D., Suchard, M.A., Rambaut, A., and Drummond, A.J. (2014). Beast 2: a software platform for Bayesian evolutionary analysis. Publ. Lib. Sci. Comp. Biol. 10: 1–6.10.1371/journal.pcbi.1003537Suche in Google Scholar PubMed PubMed Central
Burgin, C.J. (2019) Genus Rhinolophus Lacepède, 1799. In: Wilson, D.E., and Mittermeier, R.A. (Eds.). Handbook of the mammals of the world. 9. Bats. Lynx Edicions, Barcelona, pp. 280–332.Suche in Google Scholar
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17: 540–552, https://doi.org/10.1093/oxfordjournals.molbev.a026334.Suche in Google Scholar PubMed
Cotterill, F.P.D. (2002). A new species of horseshoe bat (Microchiroptera: Rhinolophidae) from south-central Africa: with comments on its affinities and evolution, and the characterization of rhinolophid species. J. Zool. Lond. 256: 165–179, https://doi.org/10.1017/s0952836902000201.Suche in Google Scholar
Csorba, G., Ujhelyi, P., and Thomas, N. (2003). Horseshoe bats of the world (Chiroptera: Rhinolophidae). Bishop’s Castle, Alana Books, pp. xxxii+160.Suche in Google Scholar
Demos, T.C., Webala, P.W., Goodman, S.M., Kerbis Peterhans, J.C., Bartonjo, M., and Patterson, B.D. (2019). Molecular phylogenetics of the African horseshoe bats (Chiroptera: Rhinolophidae): expanded geographic and taxonomic sampling of the Afrotropics. BioMedCent Evol. Biol. 19: 1–14, https://doi.org/10.1186/s12862-019-1485-1.Suche in Google Scholar PubMed PubMed Central
Dool, S.E., Puechmaille, S.J., Foley, N.M., Allegrini, B., Bastian, A., Mutumi, G.L., Maluleke, T.G., Odendaal, L.J., Teeling, E.C., and Jacobs, D.S. (2016). Nuclear introns outperform mitochondrial DNA in inter-specific phylogenetic reconstruction: lessons from horseshoe bats (Rhinolophidae: Chiroptera). Mol. Phylogenet. Evol. 97: 196–212, https://doi.org/10.1016/j.ympev.2016.01.003.Suche in Google Scholar PubMed
Drummond, A.J., Ho, S.Y.W., Phillips, M.J., and Rambaut, A. (2006). Relaxed phylogenetics and dating with confidence. Publ. Lib. Sci. Biol. 4: 699–710, https://doi.org/10.1371/journal.pbio.0040088.Suche in Google Scholar PubMed PubMed Central
Foley, N.M., Thong, V.D., Soisook, P., Goodman, S.M., Armstrong, K.N., Jacobs, D.S., Puechmaille, S.J., and Teeling, E.C. (2015). How and why overcome the impediments to resolution: lessons from rhinolophid and hipposiderid bats. Mol. Biol. Evol. 32: 313–333, https://doi.org/10.1093/molbev/msu329.Suche in Google Scholar PubMed PubMed Central
Hoang, D.T., Chernomor, O., von Haeseler, A., Minh, B.Q., and Vinh, L.S. (2018). UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35: 518–522, https://doi.org/10.1093/molbev/msx281.Suche in Google Scholar PubMed PubMed Central
Jacobs, B.F. (2004). Palaeobotanical studies from tropical Africa: relevance to the evolution of forest, woodland, and savannah biomes. Phil. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 359: 1573–1583, https://doi.org/10.1098/rstb.2004.1533.Suche in Google Scholar PubMed PubMed Central
Jacobs, D.S., Babiker, H., Bastian, A., Kearney, T., van Eeden, R., and Bishop, J.M. (2013). Phenotypic convergence in genetically distinct lineages of a Rhinolophus species complex (Mammalia, Chiroptera). Publ. Lib. Sci. One 8: 1–16, https://doi.org/10.1371/journal.pone.0082614.Suche in Google Scholar PubMed PubMed Central
Kalyaanamoorthy, S., Minh, B.Q., Wong, T.K.F., von Haeseler, A., and Jermiin, L.S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Meth. 14: 587–589, https://doi.org/10.1038/nmeth.4285.Suche in Google Scholar PubMed PubMed Central
Katoh, K. and Standley, D.M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30: 772–780, https://doi.org/10.1093/molbev/mst010.Suche in Google Scholar PubMed PubMed Central
Koopman, K.F. (1989). Systematic notes on Liberian bats. Amer. Mus. Novit. 2946: 1–11.Suche in Google Scholar
Monadjem, A., Taylor, P.J., Cotterill, F.P.D.(W), and Schoeman, M.C. (2020). Bats of southern and central Africa. A biogeographic and taxonomical synthesis, 2nd ed. Wits University Press, Johannesburg, pp. xiv+714.10.18772/22020085829Suche in Google Scholar
Nguyen, L., Schmidt, H.A., von Haesler, A., and Minh, B.Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 32: 268–274, https://doi.org/10.1093/molbev/msu300.Suche in Google Scholar PubMed PubMed Central
Puechmaille, S.J., Gouilh, M.A., Piyapan, P., Yokubol, M., Mie, K.M., Bates, P.J., Teeling, E.C., Nwe, T., Bu, S.S.H., Mackie, I.J., et al.. (2011). The evolution of sensory divergence in the context of limited gene flow in the bumblebee bat. Nature Comm. 2: 1–9, https://doi.org/10.1038/ncomms1582.Suche in Google Scholar PubMed PubMed Central
Simmons, N.B. (2005) Order Chiroptera. In: Wilson, D.E., and Reeder, D.M. (Eds.). Mammal species of the world. A taxonomic and geographic reference, 3rd ed., Vol. 1. The John Hopkins University Press, Baltimore, pp. 312–529.Suche in Google Scholar
Taylor, P.J., Macdonald, A., Goodman, S.M., Kearney, T., Cotterill, F.P., Stoffberg, S., Monadjem, A., Schoeman, M.C., Guyton, J., Naskrecki, P., et al.. (2018). Integrative taxonomy resolves three new cryptic species of small southern African horseshoe bats (Rhinolophus). Zool. J. Linn. Soc. 184: 1249–1276, https://doi.org/10.1093/zoolinnean/zly024.Suche in Google Scholar
Teeling, E.C., Springer, M.S., Madsen, O., Bates, P.J.J., Brien, S.J.O., and Murphy, W.J. (2005). A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307: 580–584, https://doi.org/10.1126/science.1105113.Suche in Google Scholar PubMed
Trifinopoulos, J., Nguyen, L.-T., von Haeseler, A., and Minh, B.Q. (2016). W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucl. Acids Res. 44: W232–W235, https://doi.org/10.1093/nar/gkw256.Suche in Google Scholar PubMed PubMed Central
Uvizl, M., Kotyková Varadínová, Z., and Benda, P. (2024). Phylogenetic relationships among horseshoe bats within the Rhinolophus ferrumequinum group (Mammalia: Chiroptera). Zool. Scr. 53: 249–266.10.1111/zsc.12650Suche in Google Scholar
Weyeneth, N., Goodman, S.M., Stanley, W.T., and Ruedi, M. (2008). The biogeography of Miniopterus bats (Chiroptera: miniopteridae) from the Comoro Archipelago inferred from mitochondrial DNA. Mol. Ecol. 17: 5205–5219, https://doi.org/10.1111/j.1365-294x.2008.03994.x.Suche in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/mammalia-2024-0147).
© 2025 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Conservation
- Tickell’s bat, Hesperoptenus tickelli (Blyth, 1851), in Sri Lanka with new records after 58 years and roosting ecology notes
- Ecology
- New ecological aspects of the pacarana (Dinomys branickii) in southeastern Peru
- Variation in mammal ecological patterns in response to seasonality in a Brazilian tropical dry forest
- Niche partitioning between two marsupials inhabiting the Yungas of Northwestern Argentina: overlapping diets in non-overlapping lifestyles?
- Characteristics of tree hollows used by Nilgiri marten Martes gwatkinsii in the Western Ghats, India
- How much do we know about wild canid (Carnivora: Canidae) ectoparasites in Mexico? Current state of knowledge
- First and new records of albinism and leucism in Jaculus orientalis and Jaculus jaculus (Rodentia, Dipodidae)
- Biogeography
- The first record of the long-eared hedgehog (Hemiechinus auritus Gmelin, 1770) in Lebanon
- First record of Andersen’s leaf-nosed bat, Hipposideros gentilis, and hairy-faced myotis, Myotis annectans from Bangladesh
- Ethology
- Temperature and pups influence daytime roosting behavior of the great fruit-eating bat, Artibeus lituratus, in an urban southern Brazilian habitat
- Taxonomy/Phylogeny
- On the phylogenetic position of Rhinolophus sakejiensis (Chiroptera: Rhinolophidae)
- New geographical records of Phyllostomidae (Chiroptera) for the Brazilian Caatinga, with taxonomic notes
- Complete mitogenome of Prionailurus bengalensis alleni and taxonomic revisions of leopard cat subspecies
- Karyotype of the lesser gymnure Hylomys maxi and comparison with its Vietnamese congeners (Mammalia: Eulipotyphla: Erinaceidae)
Artikel in diesem Heft
- Frontmatter
- Conservation
- Tickell’s bat, Hesperoptenus tickelli (Blyth, 1851), in Sri Lanka with new records after 58 years and roosting ecology notes
- Ecology
- New ecological aspects of the pacarana (Dinomys branickii) in southeastern Peru
- Variation in mammal ecological patterns in response to seasonality in a Brazilian tropical dry forest
- Niche partitioning between two marsupials inhabiting the Yungas of Northwestern Argentina: overlapping diets in non-overlapping lifestyles?
- Characteristics of tree hollows used by Nilgiri marten Martes gwatkinsii in the Western Ghats, India
- How much do we know about wild canid (Carnivora: Canidae) ectoparasites in Mexico? Current state of knowledge
- First and new records of albinism and leucism in Jaculus orientalis and Jaculus jaculus (Rodentia, Dipodidae)
- Biogeography
- The first record of the long-eared hedgehog (Hemiechinus auritus Gmelin, 1770) in Lebanon
- First record of Andersen’s leaf-nosed bat, Hipposideros gentilis, and hairy-faced myotis, Myotis annectans from Bangladesh
- Ethology
- Temperature and pups influence daytime roosting behavior of the great fruit-eating bat, Artibeus lituratus, in an urban southern Brazilian habitat
- Taxonomy/Phylogeny
- On the phylogenetic position of Rhinolophus sakejiensis (Chiroptera: Rhinolophidae)
- New geographical records of Phyllostomidae (Chiroptera) for the Brazilian Caatinga, with taxonomic notes
- Complete mitogenome of Prionailurus bengalensis alleni and taxonomic revisions of leopard cat subspecies
- Karyotype of the lesser gymnure Hylomys maxi and comparison with its Vietnamese congeners (Mammalia: Eulipotyphla: Erinaceidae)


