Abstract
An efficient method for the synthesis of the title compounds by reactions of divinyl ketones with thiourea is described. This protocol has the advantages of high yields, mild reaction conditions and simple work-up procedure.
Introduction
Pyrimidine-2-thiols are widely used in medicinal chemistry because of their broad spectrum of biological activities [1], [2]. They are precursors to important organic compounds [3], [4], [5] and organometallic complexes [6], [7], [8]. In addition, they can also be utilized in the determination of many metal ions (Pb, Ru, Bi, Pd, Pt) as selective and sensitive ligands in analytical chemistry [9], [10], [11], [12], [13], [14].
The traditional method for the synthesis of unsubstituted pyrimidine-2-thiol is based on the reaction of 1,1,3,3-tetraethoxypropane with thiourea in a strong acidic medium [15]. Synthesis of substituted pyrimidine-2-thiols involves the reactions of β-enaminoamides with thiourea under basic conditions [16] and the reactions of chalcones with thiourea [17], [18], [19], [20]. In this paper, we report a facile preparation of 4-arylethyl-6-arylpyrimidine-2-thiols by reactions of divinyl ketones with thiourea through aza-Michael addition/nucleophilic addition/aromatization tandem processes.
Results and discussion
Initially, (1E,4E)-1,5-diphenylpenta-1,4-dien-3-one (1a) was allowed to react with thiourea in ethanol in the presence of potassium hydroxide as a base (Scheme 1). The isolated product was 4-phenethyl-6-phenylpyrimidine-2-thiol (2a), the structure of which was confirmed by spectral methods and elemental analysis. The reactions conducted in MeCN, N,N-dimethylformamide, dimethyl sulfoxide and n-PrOH were inefficient, and a moderate yield of 42% was obtained in MeOH. However, the best yield of 86% was obtained using EtOH as solvent. Inorganic bases, such as Na2CO3, K2CO3 and Cs2CO3, and organic bases, such as 1,8-diazabicyclo[5.4.0]undec-7-ene, 1,4-diazabicyclo[2.2.2]octane, 4-dimethylaminopyridine and Et3N, had little or no effect on the reaction. However, high yields of 2a in the range of 79–86% were obtained for the reaction conducted in the presence of strong bases including NaOH, KOH and KOBut. The highest yield of 86% was obtained in the presence of KOH. This optimal yield was observed by using 2 equiv of KOH based on 1a at 80°C for 8 h.
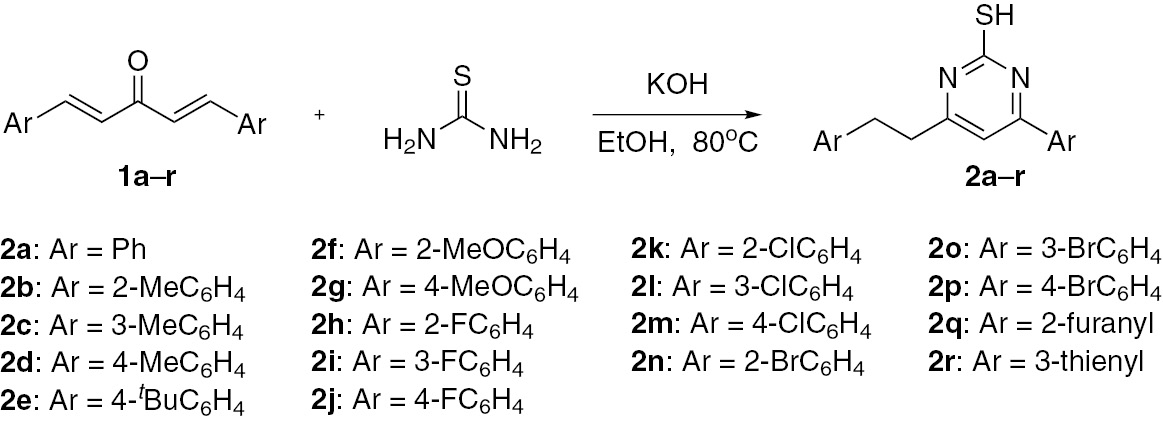
Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols.
The reactions of other substrates containing a wide range of functional groups including electron-donating groups (Me and MeO) and electron-withdrawing groups (F, Cl and Br) on aromatic rings of divinyl ketones 1a–p furnished the corresponding products 2a–p in good to high yields. In addition, divinyl ketones containing heterocycles, such as 2-furanyl and 3-thienyl, were also transformed into the corresponding products 2q–r in good yields. Unfortunately, the reactions of divinyl ketones bearing nitro group on aromatic rings and aliphatic divinyl ketones were not successful. All products 2a–r were characterized by proton nuclear magnetic resonance (1H NMR), carbon-13 nuclear magnetic resonance (13C NMR), infrared (IR) and elemental analysis.
A plausible mechanism is illustrated for the synthesis of 2a in Scheme 2 [16], [18], [20]. As can be seen, the initial aza-Michael addition reaction generates the intermediate product A. Compound A undergoes intramolecular cyclization, which is followed by dehydration to afford intermediate product B. The intermediate product B is a direct precursor to the observed final product 2a.
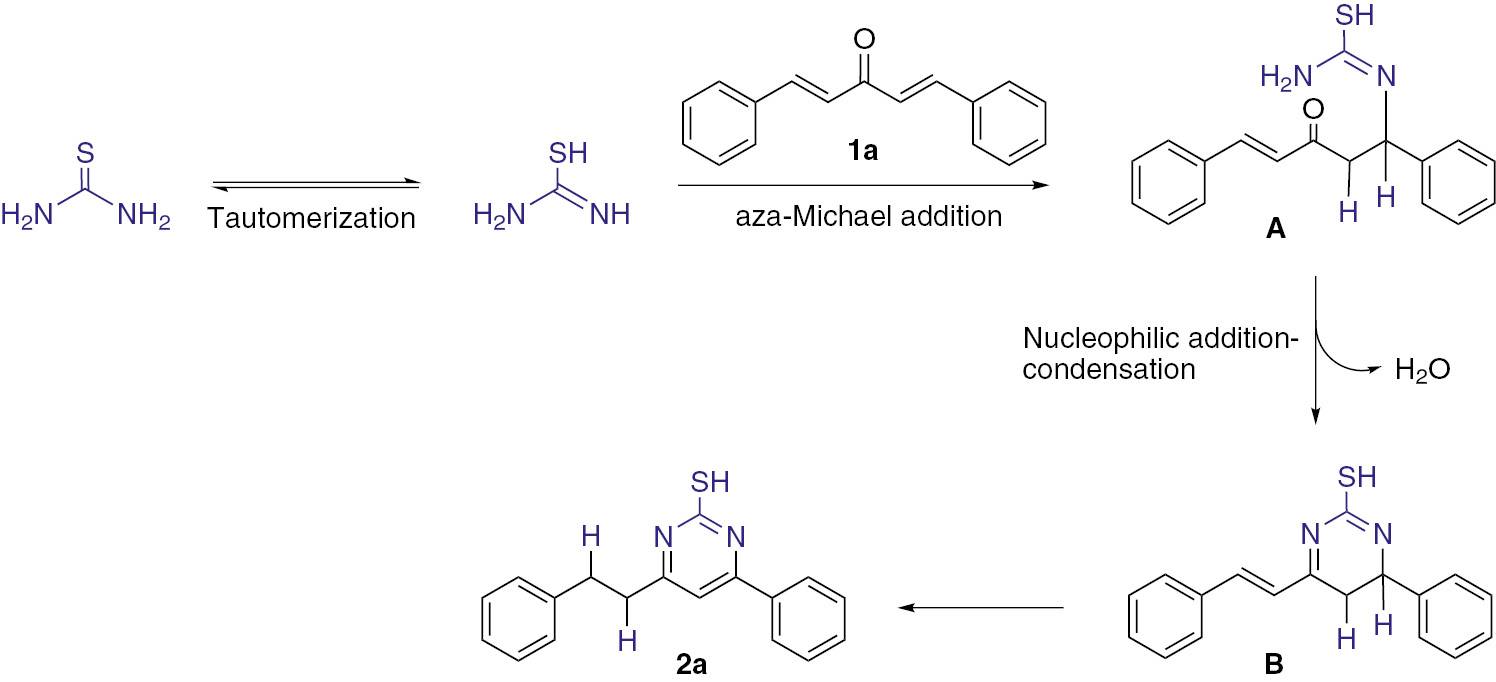
Proposed mechanism for the reaction 1a with thiourea.
Conclusion
An efficient method for the synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols by reactions of divinyl ketones with thiourea through aza-Michael addition/nucleophilic addition/aromatization tandem processes was developed.
Experimental
1H NMR (600 MHz) and 13C NMR (150 MHz) spectra were obtained with a Mercury-600B instrument using deuterated chloroform (CDCl3) as solvent and tetramethylsilane (Me4Si) as the internal standard. IR spectra were recorded as KBr pellets. Elemental analyses were performed on a Vario El Elemental Analysis instrument. Melting points were observed on an Electrothermal melting point apparatus. Divinyl ketones were synthesized according to the literature procedure [21], [22].
General procedure for synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols (2a–r)
A mixture of divinyl ketone (1, 1 mmol), thiourea (1.5 mmol) and potassium hydroxide (2 mmol) in ethanol (5 mL) was stirred at 80°C for 8 h. The reaction progress was monitored by thin-layer chromatography (TLC). After the completion of the reaction, the mixture was extracted with ethyl acetate (3×10 mL). The extract was washed with saturated brine (3×10 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue of 2a–r was purified by column chromatography eluting with a mixture of petroleum ether and ethyl acetate (10:1).
4-Phenethyl-6-phenylpyrimidine-2-thiol (2a)
Yellow oil; yield 86%; 1H NMR: δ 7.93 (d, J=6.8 Hz, 2H), 7.43–7.33 (m, 4H), 7.17–7.13 (m, 4H), 7.07 (s, 1H), 3.02 (t, J=6.0 Hz, 2H), 2.97 (t, J=6.0 Hz, 2H), SH signal was not observed; 13C NMR: δ 171.1, 169.6, 164.2, 140.7, 136.2, 131.0, 128.8, 128.5, 128.4, 127.2, 126.1, 112.5, 39.6, 34.5; IR: ν 2921, 2849, 1515, 1229, 829 cm−1. Anal. Calcd for C18H16N2S: C, 73.94; H, 5.52; N, 9.58. Found: C, 74.03; H, 5.50; N, 9.54.
4-(2-Methylphenethyl)-6-(o-tolyl)pyrimidine-2-thiol (2b)
Yellow oil; yield 74%; 1H NMR: δ 7.31–7.27 (m, 2H), 7.21 (t, J=7.3 Hz, 2H), 7.13–7.04 (m, 4H), 6.89 (s, 1H), 3.00 (s, 4H), 2.26 (s, 3H), 2.23 (s, 3H), SH signal was not observed; 13C NMR: δ 170.8, 169.1, 167.9, 138.9, 137.2, 136.7, 135.9, 131.2, 130.3, 129.6, 128.8, 126.3, 126.0, 116.7, 38.2, 32.0, 20.4, 19.2; IR: ν 2930, 2850, 1563, 1517, 1237, 698 cm−1. Anal. Calcd for C20H20N2S: C, 74.96; H, 6.29; N, 8.74. Found: C, 74.88; H, 6.32; N, 8.77.
4-(3-Methylphenethyl)-6-(m-tolyl)pyrimidine-2-thiol (2c)
Yellow oil; yield 88%; 1H NMR: δ 7.71 (s, 2H), 7.26−7.22 (m, 2H), 7.15 (s, 1H), 7.07 (t, J=6.9 Hz, 1H), 6.98–6.93 (m, 2H), 6.89 (d, J=5.6 Hz, 1H), 3.01 (t, J=7.9 Hz, 2H), 2.90 (t, J=7.9 Hz, 2H), 2.32 (s, 3H), 2.26 (s, 3H), SH signal was not observed; 13C NMR: δ 171.1, 169.5, 164.4, 140.8, 138.4, 138.0, 136.2, 131.7, 129.2, 128.6, 128.3, 127.8, 126.8, 125.4, 124.3, 112.5, 39.6, 34.5, 21.4, 21.3. IR: ν 2938, 2850, 1563 cm−1, 1514, 1254, 689 cm−1. Anal. Calcd for C20H20N2S: C, 74.96; H, 6.29; N, 8.74. Found: C, 75.07; H, 6.27; N, 8.72.
4-(4-Methylphenethyl)-6-(p-tolyl)pyrimidine-2-thiol (2d)
Yellow solid; mp 120−122°C; yield 75%; 1H NMR: δ 7.83 (d, J=8.0 Hz, 2H), 7.17 (d, J=8.0 Hz, 2H), 7.09 (s, 1H), 6.97 (s, 4H), 2.99 (t, J=7.5 Hz, 2H), 2.92 (t, J=7.5 Hz, 2H), 2.36 (s, 3H), 2.26 (s, 3H), SH signal was not observed; 13C NMR: δ 171.0, 169.4, 164.1, 141.4, 137.8, 135.5, 133.5, 129.5, 129.0, 128.3, 127.1, 112.1, 39.7, 34.1, 21.4, 20.9. IR: ν 2921, 2856, 1563. 1506, 1237, 820 cm−1. Anal. Calcd for C20H20N2S: C, 74.96; H, 6.29; N, 8.74. Found: C, 74.90; H, 6.30; N, 8.72.
4-(4-tert-Butylphenethyl)-6-(4-tert-butylphenyl)pyrimidine-2-thiol (2e)
Yellow solid; mp 113−115°C; yield 81%; 1H NMR: δ 7.87 (d, J=8.3 Hz, 2H), 7.38 (d, J=8.3 Hz, 2H), 7.18 (d, J=8.1 Hz, 2H), 7.07 (s, 1H), 7.00 (d, J=8.1 Hz, 2H), 3.02 (t, J=7.6 Hz, 2H), 2.93 (t, J=7.6 Hz, 2H), 1.29 (s, 9H), 1.25 (s, 9H), SH signal was not observed; 13C NMR: δ 171.0, 169.6, 164.1, 154.4, 148.8, 137.7, 133.5, 128.1, 127.0, 125.7, 125.2, 112.4, 39.6, 34.8, 34.3, 34.1, 31.3, 31.1; IR: ν 2917, 2910, 2851, 1568, 1516, 1217, 827 cm−1. Anal. Calcd for C26H32N2S: C, 77.18; H, 7.97; N, 6.92. Found: C, 77.23; H, 8.00; N, 6.89.
4-(2-Methoxyphenethyl)-6-(2-methoxyphenyl)pyrimidine-2-thiol (2f)
Yellow oil; yield 73%; 1H NMR: δ 7.85 (d, J=6.0 Hz, 1H), 7.53 (s, 1H), 7.34 (t, J=7.8 Hz, 1H), 7.13 (t, J=8.6 Hz, 1H), 7.03 (d, J=7.3 Hz, 1H), 6.94−6.91 (m, 2H), 6.80–6.74 (m, 2H), 3.79 (s, 3H), 3.75 (s, 3H), 3.02–2.98 (m, 4H), SH signal was not observed; 13C NMR: δ 170.9, 168.7, 162.7, 158.1, 157.4, 131.5, 131.4, 129.9, 129.4, 127.2, 125.8, 121.1, 120.3, 117.3, 111.4, 110.1, 55.5, 55.1, 37.8, 29.4; IR: ν 2930, 2849, 1563, 1506, 1237, 759 cm−1. Anal. Calcd for C20H20N2O2S: C, 68.16; H, 5.72; N, 7.95. Found: C, 68.09; H, 5.74; N, 7.99.
4-(4-Methoxyphenethyl)-6-(4-methoxyphenyl)pyrimidine-2-thiol (2g)
Brown oil; yield 63%; 1H NMR: δ 7.93 (d, J=8.8 Hz, 2H), 7.03 (s, 1H), 6.96 (d, J=8.4 Hz, 2H), 6.87 (d, J=8.8 Hz, 2H), 6.67 (d, J=8.4 Hz, 2H), 3.80 (s, 3H), 3.70 (s, 3H), 2.98 (t, J=7.4 Hz, 2H), 2.91 (t, J=7.4 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.8, 169.4, 163.7, 162.1, 157.9, 130.0, 129.3, 128.8, 128.7, 114.1, 113.7, 111.7, 55.3, 55.1, 39.8, 33.6; IR: ν 2938, 2850, 1563, 1514, 1237, 838 cm−1. Anal. Calcd for C20H20N2O2S: C, 68.16; H, 5.72; N, 7.95. Found: C, 68.21; H, 5.71; N, 7.92.
4-(2-Fluorophenethyl)-6-(2-fluorophenyl)pyrimidine-2-thiol (2h)
Yellow solid; mp 108–110°C; yield 91%; 1H NMR: δ 8.02 (t, J=7.0 Hz, 1H), 7.38 (s, 2H), 7.16–7.04 (m, 4H), 6.98–6.94 (t, J=9.2 Hz, 1H), 6.91 (t, J=7.5 Hz, 1H), 3.07–3.01 (m, 4H), SH signal was not observed; 13C NMR: δ 171.0, 169.3, 162.7, 161.9, 160.6, 160.4, 160.3, 132.3, 130.9, 130.6, 127.9, 124.6, 123.9, 116.8, 116.4, 115.3, 37.8, 27.7; IR: ν 2921, 2850, 1569, 1840, 1237, 751 cm−1. Anal. Calcd for C18H14F2N2S: C, 65.84; H, 4.30; N, 8.53. Found: C, 65.96; H, 4.28; N, 8.49.
4-(3-Fluorophenethyl)-6-(3-fluorophenyl)pyrimidine-2-thiol (2i)
Yellow solid; mp 122−124°C; yield 96%; 1H NMR: δ 7.85 (s, 1H), 7.80 (d, J=7.8 Hz, 1H), 7.39 (d, J=8.0 Hz, 1H), 7.32 (t, J=7.9 Hz, 1H), 7.18–7.05 (m, 4H), 6.94 (d, J=7.4 Hz, 1H), 3.04 (t, J=7.8 Hz, 2H), 2.98–2.95 (t, J=7.8 Hz, 2H), SH signal was not observed; 13C NMR: δ 171.0, 169.8, 163.0, 142.6, 137.9, 135.0, 134.2, 131.0, 130.1, 129.7, 128.5, 127.3, 126.5, 126.4, 125.2, 112.7, 39.0, 33.8; IR: ν 2921, 2853, 1568, 1514, 1237, 759 cm−1. Anal. Calcd for C18H14F2N2S: C, 65.84; H, 4.30; N, 8.53. Found: C, 65.77; H, 4.31; N, 8.57.
4-(4-Fluorophenethyl)-6-(4-fluorophenyl)pyrimidine-2-thiol (2j)
Yellow solid; mp 134−136°C; yield 90%; 1H NMR: δ 7.96 (d, J=5.4 Hz, 1H), 7.95 (d, J=5.4 Hz, 1H), 7.08 (s, 1H), 7.06 (d, J=8.6 Hz, 2H), 7.02−6.99 (m, 2H), 6.82 (t, J=8.6 Hz, 2H), 3.00 (t, J=7.0 Hz, 2H), 2.95 (t, J=7.0 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.9, 169.7, 165.5, 163.9, 163.2, 162.1, 160.5, 136.2, 132.2, 129.7, 129.2, 115.9, 115.1, 112.2, 39.5, 33.4; IR: ν 2921, 2856, 1573, 1224, 846 cm−1. Anal. Calcd for C18H14F2N2S: C, 65.84; H, 4.30; N, 8.53. Found: C, 65.80; H, 4.32; N, 8.55.
4-(2-Chlorophenethyl)-6-(2-chlorophenyl)pyrimidine-2-thiol (2k)
Orange soild; mp 119−121°C; yield 87%; 1H NMR: δ 7.47 (d, J=7.5 Hz, 1H), 7.40 (d, J=8.1 Hz, 1H), 7.33–7.26 (m, 3H), 7.25 (s, 1H), 7.11−7.05 (m, 3H), 3.10−3.06 (m, 4H), SH signal was not observed; 13C NMR: δ 170.2, 169.4, 164.3, 138.2, 136.2, 133.9, 132.3, 131.6, 130.8, 130.5, 130.4, 129.5, 127.7, 127.1, 126.8, 117.6, 37.4, 32.3. IR: ν 2935, 2861, 1565, 1514, 1228, 759 cm−1. Anal. Calcd for C18H14Cl2N2S: C, 59.84; H, 3.91; N, 7.75. Found: C, 59.77; H, 3.92; N, 7.78.
4-(3-Chlorophenethyl)-6-(3-chlorophenyl)pyrimidine-2-thiol (2l)
Yellow solid; mp 121−122°C; yield 90%; 1H NMR: δ 7.85 (s, 1H), 7.80 (d, J=7.8 Hz, 1H), 7.40 (d, J=7.8 Hz, 1H), 7.32 (t, J=7.2 Hz, 1H), 7.16 (s, 1H), 7.13–7.07 (m, 3H), 6.94 (d, J=7.4 Hz, 1H), 3.04 (t, J=7.6 Hz, 2H), 2.96 (t, J=7.6 Hz, 2H), SH signal was not observed; 13C NMR: δ 171.0, 169.8, 163.0, 142.6, 137.9, 135.0, 134.2, 131.0, 130.1, 129.7, 128.5, 127.3, 126.5, 126.4, 125.2, 112.7, 39.0, 33.8; IR: ν 2921, 2850, 1560, 1514, 1217, 749 cm−1. Anal. Calcd for C18H14Cl2N2S: C, 59.84; H, 3.91; N, 7.75. Found: C, 59.90; H, 3.89; N, 7.72.
4-(4-Chlorophenethyl)-6-(4-chlorophenyl)pyrimidine-2-thiol (2m)
White solid; mp 115−117°C; yield 88%; 1H NMR: δ 7.87 (d, J=8.5 Hz, 2H), 7.35 (d, J=8.5 Hz, 2H), 7.09 (d, J=8.3 Hz, 3H), 6.96 (d, J=8.3 Hz, 2H), 2.99 (t, J=7.2 Hz, 2H), 2.94 (t, J=7.2 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.9, 169.8, 163.1, 139.0, 137.5, 134.4, 131.9, 129.7, 129.1, 128.5, 128.4, 112.3, 39.1, 33.4; IR: ν 2930, 2856, 1579, 1560, 1514, 828 cm−1. Anal. Calcd for C18H14Cl2N2S: C, 59.84; H, 3.91; N, 7.75. Found: C, 59.89; H, 3.90; N, 7.73.
4-(2-Bromophenethyl)-6-(2-bromophenyl)pyrimidine-2-thiol (2n)
Orange solid; mp 117−119°C; yield 88%; 1H NMR: δ 7.60 (d, J=7.9 Hz, 1H), 7.49 (d, J=6.8 Hz, 1H), 7.40 (d, J=7.6 Hz, 1H), 7.32 (t, J=7.4 Hz, 1H), 7.24 (t, J=7.4 Hz, 1H), 7.21 (s, 1H), 7.13–7.08 (m, 2H), 7.02 (t, J=6.6 Hz, 1H), 3.10 (t, J=7.6 Hz, 2H), 3.06 (t, J=7.6 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.0, 169.4, 165.7, 139.9, 138.3, 133.6, 132.8, 131.5, 130.8, 130.6, 127.9, 127.6, 127.5, 124.4, 121.4, 117.6, 37.6, 34.8. IR: ν 2921, 2856, 1573, 1506, 1219, 751 cm−1. Anal. Calcd for C18H14Br2N2S: C, 48.02; H, 3.13; N, 6.22. Found: C, 47.92; H, 3.15; N, 6.25.
4-(3-Bromophenethyl)-6-(3-bromophenyl)pyrimidine-2-thiol (2o)
Orange oil; yield 91%; 1H NMR: δ 8.00 (s, 1H), 7.85 (d, J=8.0 Hz, 1H), 7.55 (d, J=8.0 Hz, 1H), 7.28–7.27 (m, 2H), 7.25 (d, J=5.9 Hz, 1H), 7.15 (s, 1H), 7.04–6.97 (m, 2H), 3.04 (t, J=7.7 Hz, 2H), 2.95 (t, J=7.7 Hz, 2H), SH signal was not observed. 13C NMR: δ 170.9, 169.8, 162.9, 142.9, 138.1, 133.9, 131.4, 130.3, 130.2, 130.0, 129.3, 127.0, 125.6, 123.1, 122.5, 112.7, 39.1, 33.8. IR: ν 2921, 2850, 1575, 1514, 1237, 751 cm−1. Anal. Calcd for C18H14Br2N2S: C, 48.02; H, 3.13; N, 6.22. Found: C, 48.09; H, 3.13; N, 6.20.
4-(4-Bromophenethyl)-6-(4-bromophenyl)pyrimidine-2-thiol (2p)
Yellow solid; mp 70−72°C; yield 89%; 1H NMR: δ 7.80 (d, J=8.6 Hz, 2H), 7.51 (d, J=8.6 Hz, 2H), 7.24 (d, J=8.3 Hz, 2H), 7.08 (s, 1H), 6.91 (d, J=8.3 Hz, 2H), 3.00 (t, J=7.7 Hz, 2H), 2.93 (t, J=7.7 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.9, 169.8, 163.2, 139.5, 134.9, 132.1, 131.4, 130.1, 128.6, 125.9, 119.9, 112.3, 39.0, 33.4. IR: ν 2921, 2850, 1573, 1514, 1237 828 cm−1. Anal. Calcd for C18H14Br2N2S: C, 48.02; H, 3.13; N, 6.22. Found: C, 48.11; H, 3.11; N, 6.19.
4-(Furan-2-yl)-6-(2-(furan-2-yl)ethyl)pyrimidine-2-thiol (2q)
Yellow solid; mp 64–66°C; yield 74%; 1H NMR: δ 7.64 (d, J=3.7 Hz, 1H), 7.41 (d, J=5.0 Hz, 1H), 7.07–7.02 (m, 3H), 6.80–6.78 (m, 1H), 6.67 (s, 1H), 3.20 (t, J=7.6 Hz, 2H), 3.06 (t, J=7.6 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.0, 169.4, 159.3, 143.3, 142.0, 130.3, 128.2, 127.7, 126.7, 124.7, 123.3, 110.9, 39.4, 28.2. IR: ν 2921, 2849, 1573, 1428, 689 cm−1. Anal. Calcd for C14H12N2O2S: C, 61.75; H, 4.44; N, 10.29. Found: C, 61.81; H, 4.42; N, 10.25.
4-(Thiophen-3-yl)-6-(2-(thiophen-3-yl)ethyl)pyrimidine-2-thiol (2r)
Black solid; mp 92−94°C; yield 63%; 1H NMR: δ 7.53 (s, 1H), 7.23 (s, 1H), 7.16 (m, 1H), 7.12 (s, 1H), 6.49 (s, 1H), 6.16 (s, 1H), 5.86 (s, 1H), 3.02 (t, J=6.8 Hz, 2H), 2.98 (t, J=6.8 Hz, 2H), SH signal was not observed; 13C NMR: δ 170.6, 169.4, 155.7, 154.4, 151.5, 145.0, 141.0, 113.0, 112.5, 110.2, 110.0, 105.4, 35.9, 26.3. IR: ν 2921, 2845, 1600, 1262, 1011, 724 cm−1. Anal. Calcd for C14H12N2S3: C, 55.23; H, 3.97; N, 9.20. Found: C, 55.16; H, 3.99; N, 9.23.
Acknowledgments
The authors thank the National Natural Science Foundation of China (grants 21462038 and 21362034) and Key Laboratory of Eco-Environment-Related Polymer Materials of Ministry of Education for financial support of this work.
References
[1] Bansal, V.; Verma, P.; Walia, R. Pyrimidine analogue 1-(4-nitrophenyl)-4,4,6-trimethyl(1H,4H)pyrimidine-2-thiol: a potential new antiepileptic. Indian J. Pharmacol.2008, 40, 117.Suche in Google Scholar
[2] Porter, C. C.; Torchiana, M. L. 4,4,6-Trimethyl-3,4-dihydropyrimidine-2-thiol, an effective inhibitor of dopamine-β-hydroxylation in-vivo. Biochem. Pharmacol.1971, 20, 183−191.10.1016/0006-2952(71)90484-9Suche in Google Scholar
[3] Wright, S. W.; Hallstrom, K. N. A convenient preparation of heteroaryl sulfonamides and sulfonyl fluorides from heteroaryl thiols. J. Org. Chem.2006, 71, 1080−1084.10.1021/jo052164+Suche in Google Scholar
[4] Nugent, R. A.; Schlachter, S. T.; Murphy, M. J.; Cleek, G. J.; Poel, T. J.; Wishka, D. G.; Graber, D. R.; Yagi, Y.; Keiser, B. J.; Olmsted, R. A.; et al. Pyrimidine thioethers: A novel class of HIV-1 reverse transcriptase inhibitors with activity against BHAP-resistant HIV. J. Med. Chem.1998, 41, 3793−3803.10.1021/jm9800806Suche in Google Scholar
[5] Fotouhi, L.; Behrozi, L.; Heravi, M. M.; Nematollahi, D. Electrochemical oxidation of catechols in the presence of pyrimidine-2-thiol: application to electrosynthesis. Phosphorus Sulfur Silicon Relat. Elem.2009, 184, 2749−2757.10.1080/10426500802591614Suche in Google Scholar
[6] Ahmad, M. F.; Sarker, J. C.; Azam, K. A.; Kabir, S. E.; Ghosh, S.; Hogarth, G.; Siddiquee, T. A.; Richmond, M. G. Re2(CO)6(μ-thpymS)2 (thpymSH=pyrimidine-2-thiol) as a versatile precursor to mono- and polynuclear complexes: X-ray crystal structures of fac-Re(CO)3(PPh3)(k2-thpymS) and two isomers of ReRu3(CO)13 (μ3-thpymS). J. Organomet. Chem.2013, 728, 30−37.10.1016/j.jorganchem.2012.12.030Suche in Google Scholar
[7] Latham, I. A.; Leigh, G. J.; Pickett, C. J.; Huttner, G.; Jibrill, I.; Zubieta, J. The anion of pyrimidine-2-thiol as a ligand to molybdenum, tungsten, and iron: Preparation of complexes, their structure and reactivity. J. Chem. Soc. Dalton Trans.1986, 1181−1187.10.1039/dt9860001181Suche in Google Scholar
[8] Nath, D.; Katyal, M.; Singh, R. P. Cu(I), Ag(I), Tl(I), Zn(II), Cd(II) and Hg(II) complexes of 1-amino-4,4,6-trimethyl-1H,4H-pyrimidine-2-thiol. J. Indian Chem. Soc.1982, 59, 625−626.Suche in Google Scholar
[9] Ghaedi, M. Pyrimidine-2-thiol as selective and sensitive ligand for preconcentration and determination of Pb2+. Chem. Anal. (Warsaw)2006, 51, 593−602.Suche in Google Scholar
[10] Lokhande, T.; Kolekar, G. B.; Anuse, M. A.; Chavan, M. B. Extraction of ruthenium(IV) from hydrochloric acid medium with N-octylaniline and its determination spectrophotometrically with pyrimidine-2-thiol. Sep. Sci. Technol.2000, 35, 153−168.10.1081/SS-100100149Suche in Google Scholar
[11] Kolekar, G. B.; Lokhande, T. N.; Bhosale, P. N.; Anuse, M. A. Extraction, separation and spectrophotometric determination of bismuth(III) using 1-(4′-bromophenyl)-4,4,6-trimethyl (1H,4H)-pyrimidine-2-thiol. Anal. Lett.1998, 31, 2241−2254.10.1080/00032719808005299Suche in Google Scholar
[12] Sahu, R.; Sondhi, S. M.; Gupta, B. Extractive spectrophotometric determination of Pd(II) with 1-(2′-aminoaryl)-4,4,6-trimethyl-1,4,5,6-tetrahydro-6-hydroxy pyrimidine-2-thiol. Indian J. Chem. Sect. A1998, 37, 1140−1143.Suche in Google Scholar
[13] Roy, B.; Singh, R. P.; Singh, A. K. Spectrophotometric determination of platinum with substituted pyrimidine-2-thiol. Microchem. J. 1985, 31, 326−328.10.1016/0026-265X(85)90122-5Suche in Google Scholar
[14] Wasey, A.; Bansal, R. K.; Puri, B. K.; Satake, M. Solid-liquid separation after liquid-liquid-extraction–spectrophotometric determination of copper by extraction of its 1-phenyl-4,4,6-trimethyl-(1H,4H)-pyrimidine-2-thiol into molten naphthalene. Analyst1983, 108, 515−520.10.1039/an9830800515Suche in Google Scholar
[15] Crosby, D. G.; Berthold, R. V.; Johnson, H. E. 2-Mercaptopyrimidine. Org. Synth.1963, 43, 68.10.1002/0471264180.os043.21Suche in Google Scholar
[16] Qomi, H. R.; Habibi, A. Synthesis of a novel functionalized tricyclic pyrimidine-fused 1,5-benzodiazepine library. Tetrahedron2017, 73, 2991−3001.10.1016/j.tet.2017.03.079Suche in Google Scholar
[17] Khunt, R. C.; Khedkar, V. M.; Chawda, R. S.; Chauhan, N. A.; Parikh, A. R.; Coutinho, E. C. Synthesis, antitubercular evaluation and 3D-QSAR study of N-phenyl-3-(4-fluorophenyl)-4-substituted pyrazole derivatives. Bioorg. Med. Chem. Lett.2012, 22, 666–678.10.1016/j.bmcl.2011.10.059Suche in Google Scholar PubMed
[18] Munawar, M. A.; Azad, M.; Siddiqui, H. L.; Nasim, F. H. Synthesis and antimicrobial studies of some quinolinylpyrimidine derivatives. J. Chin. Chem. Soc.2008, 55, 394−400.10.1002/jccs.200800058Suche in Google Scholar
[19] Wendelin, W.; Schramm, H. W.; Blasi-Rabassa, A. Über die reaktionen von guanidin bzw. Thioharnstoff mit α,β,γ,δ-ungesättigten ketonen. Monatsh. Chem.1985, 116, 385−400.10.1007/BF00799973Suche in Google Scholar
[20] Shao, Z. Y.; Pan, Q. H.; Chen, J.; Yu, Y. P.; Zhang, G. L. Synthesis of polysubstituted 5-aminopyrimidine-2(1H)-thiones from vinyl azides and thiourea. Tetrahedron2012, 68, 6565−6568.10.1016/j.tet.2012.05.057Suche in Google Scholar
[21] Motiur Rahman, A. F. M.; Ali, R.; Jahng, Y.; Kadi, A. A. A facile solvent free Claisen-Schmidt reaction: synthesis of α,α′-bis-(substituted-benzylidene)cycloalkanones and α,α′-bis-(substituted-alkylidene)cycloalkanones. Molecules2012, 17, 571−583.10.3390/molecules17010571Suche in Google Scholar PubMed PubMed Central
[22] Hazarkhani, H.; Kumar, P.; Kondiram, K. S.; Gadwal I. M. S. Highly selective Claisen–Schmidt condensation catalyzed by silica chloride under solvent-free reaction conditions. Synth. Commun. 2010, 40, 2887−2896.10.1080/00397910903340637Suche in Google Scholar
©2018 Walter de Gruyter GmbH, Berlin/Boston
Artikel in diesem Heft
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives
Artikel in diesem Heft
- Frontmatter
- Review
- Research progress in quinazoline derivatives as multi-target tyrosine kinase inhibitors
- Research Articles
- A bio-inspired approach to proline-derived 2,4-disubstituted oxazoles
- Solvent-free preparation of 3-aryl-2-[(aryl)(arylamino)]methyl-4H-furo[3,2-c]chromen-4-one derivatives using ZnO-ZnAl2O4 nanocomposite as a heterogeneous catalyst
- Synthesis of 4-arylethyl-6-arylpyrimidine-2-thiols through aza-Michael addition/nucleophilic addition/aromatization tandem reactions
- 3-Carboxy-1-sulfopyridin-1-ium chloride ([CPySO3H]+Cl−): an efficient catalyst for one-pot synthesis of hexahydroquinoline-3-carboxamides
- Synthesis of new 3H-chromeno[2,3-d]pyrimidine-4,6(5H,7H)-diones via the tandem intramolecular Pinner/Dimroth rearrangement
- A new synthesis of pyrrolo[3,2-d]pyrimidine derivatives by a one-pot, three-component reaction in the presence of L-proline as an organocatalyst
- Diversity-oriented synthesis of amide derivatives of tricyclic thieno[2,3-d]pyrimidin-4(3H)-ones and evaluation of their influence on melanin synthesis in murine B16 cells
- Synthesis, crystal structure, molecular docking studies and bio-evaluation of some N4-benzyl-substituted isatin- 3-thiosemicarbazones as urease and glycation inhibitors
- Ultrasound-assisted synthesis and antimicrobial activity of tetrazole-based pyrazole and pyrimidine derivatives

