Fractionating microplastics by density gradient centrifugation: a novel approach using LuerLock syringes in a low-cost density gradient maker
Abstract
Microplastics are now ubiquitous in the environment and are even considered “technofossils” of the Anthropocene. Given their omnipresence and potential impact, identifying and analyzing these particles becomes increasingly crucial. Novel approaches suggest density gradient centrifugation for simultaneous extraction and fractionation of microplastic particles based on their plastic-specific densities. In this article we describe a cheap and harmless experimental setting to fractionate microplastic particles by density gradient centrifugation. An innovative low-cost Do-It-Yourself (DIY) gradient maker using Luer-Lock syringes is presented. With this gradient maker it is possible to produce density gradients with water and sucrose solutions, covering a density range of 1.00–1.32 g/cm3, as well as with water and saturated potassium carbonate solutions, covering a density range of 1.06–1.53 g/cm3. The separation performance was tested with the most broadly used plastics polyamide, polyurethane, polycarbonate, polyethylene terephthalate and polyvinylchloride. Both density gradients show centrifugation stability and clear banding patterns after centrifugation. Due to its cheap and easy-to-build-easy-to-use nature, this experimental setting for microplastic fractionation by density gradient centrifugation offers an approach for schools not only to address the microplastic problems, but also to integrate new methods of microplastic analysis in upper secondary school laboratories.
1 Introduction
Plastic products are ubiquitous in our everyday lives, and with their use it is no surprise that plastic production is still increasing (Plastics Europe, 2022). As a result of human activities, macroplastics and microplastics (plastic particles smaller than 5 mm), are lost to the environment at different points across the plastic life cycle. For example, in 2015 approximately 6.2 Mt of macroplastics and 3.0 Mt of microplastics were lost to the environment (Ryberg et al., 2019). Macroplastic products that have entered the environment are usually of no use to nature. Instead, they are broken down into microplastic particles by external environmental influences, such as photooxidation and mechanical degradation (Andrady, 2015). Sediments are thought to be a major sink for these microplastic particles. It is therefore not surprising that microplastics have been detected in sediments worldwide, even in the deep sea (Van Cauwenberghe et al., 2013, 2015). The particles were shown to be enriched and preserved there. First plastiglomerates, molten and hardened plastic fused with other sediment, shells or other inorganic compounds were already found (Corcoran et al., 2014). Thus, plastic particles of all forms are more and more often proposed as “technofossils” of the Anthropocene for determining the stratigraphic age of core layers (Chen et al., 2022; Zalasiewicz et al., 2016). They have the potential being the “golden spike” besides radionuclides that emerged in the atmosphere since 1945 as a result of nuclear bomb tests. This makes the analysis of microplastic particles in sediments even more important for the future.
Nowadays density-based extractions with saturated salt solutions are often used to separate microplastic particles from sediments. They are based on the difference in density between plastics (0.8 g/cm3–1.6 g/cm3) and sediments (∼2.7 g/cm3). For density separation saturated high-density salt solutions, such as sodium chloride (NaCl), sodium bromide (NaBr), sodium iodide (NaI) and zinc bromide (ZnBr2) are often used (Prata et al., 2019; Quinn et al., 2017). A new density medium that is also suited for a school context is a saturated potassium carbonate solution (Gohla et al., 2021). For most protocols, the sediment sample is transferred to a flotation medium and shaken or stirred. After a while, the higher-density sediment grains sink to the bottom, while the microplastic particles float at the surface of the solution, from where they are removed and filtered. One limitation of this method is that it does not allow the identification of the plastic type(s) floating atop the medium. Subsequent identification is usually done only visually or by IR, Raman or GC-MS (Prata et al., 2019).
New approaches in research use density gradient centrifugation to identify different polymer types according to their specific density, but they also use highly hazardous density media such as caesium chloride and the methods usually require expensive materials (Jakobs et al., 2023; Jing et al., 2022).
In this publication we want to present an affordable and less harmful method for fractioning microplastic particles with density gradient centrifugation, suitable for educational settings. Furthermore, by replacing problematic substances, this method might serve as an example of green chemistry and can even be used by students. First, we introduce the low-cost density gradient maker. Second, cheap and harmless density media that are suitable for school use are discussed. Third, the separation performance of the gradient for selected microplastic particles is evaluated.
As this project is an example of a successful collaborative research and development approach between students, teachers and researchers, the following chapter will briefly describe the collaboration.
2 From school to university and vice versa
Originating from the course “marine sciences-oceanography” in the upper secondary level at Lise-Meitner Gymnasium in Willich-Anrath (Germany), two students took on the challenging task of microplastic analysis. Intrigued by the topic, the two students together with their teacher continued the project in a voluntary special course. Based on the principle of density separation, they optimized the method of density centrifugation for school use to identify different polymer types. The aim was to fractionate microplastic particles according to their specific density. Using only cheap materials and harmless chemicals, they wanted to develop a method that can easily be used by other students at the upper secondary or freshman level or researchers in citizen science projects. With this aim in mind, a literature review revealed that gradient makers are either heavily expensive when purchased, or there are self-built devices that cannot be easily made anymore. This is because materials are no longer manufactured or the device would need up-to-date modifications (Flurkey, 2000; Marks, 1998; Salo & Kouns, 1965).
First experiments were conducted with plastic tubes glued to a perspex disc and connected to each other. This horizontal setup was ruled out due to reoccurring mechanical issues like leakage. Afterwards, a more robust and easy-to-use vertical design was built using Luer-Lock components with minor modifications.
Subsequently first tests of density gradients using a 66 % sucrose solution were done by the two students. Microplastic particles were added for some experiments. Their promising initial results marked the start of the cooperation with the University of Graz and further development steps were discussed between students and researchers. The researchers tested the reproducibility of the gradient maker, as well as further cheap and harmless density media that are suitable for school use. Coming from school with a newly developed device, the university validated and enhanced the method with equipment that is not available to schools, and then brought the device back to school in a broadly usable way. Furthermore, the method has already been used and disseminated in teacher workshops to once again bridge the gap between research and school.
3 Materials and methods
3.1 Luer-Lock low-cost density gradient maker
The setup of the low-cost density gradient maker is simple and based on the Luer-Lock-system. This ISO-standardized medical system offers a simple option for leak-free connections through their special and accurate fittings. Normally used for medical equipment, it is increasingly used in laboratories due to its user-friendly design and compatibility with various products such as syringes, needles and tubing. To reduce costs, it can be helpful to ask hospitals or doctors for expired Luer-Lock items, which are often provided for free.
The only modification needed is piercing a stopper with a needle. All other parts used in the gradient maker are standard issue lab materials (see Figure 1).
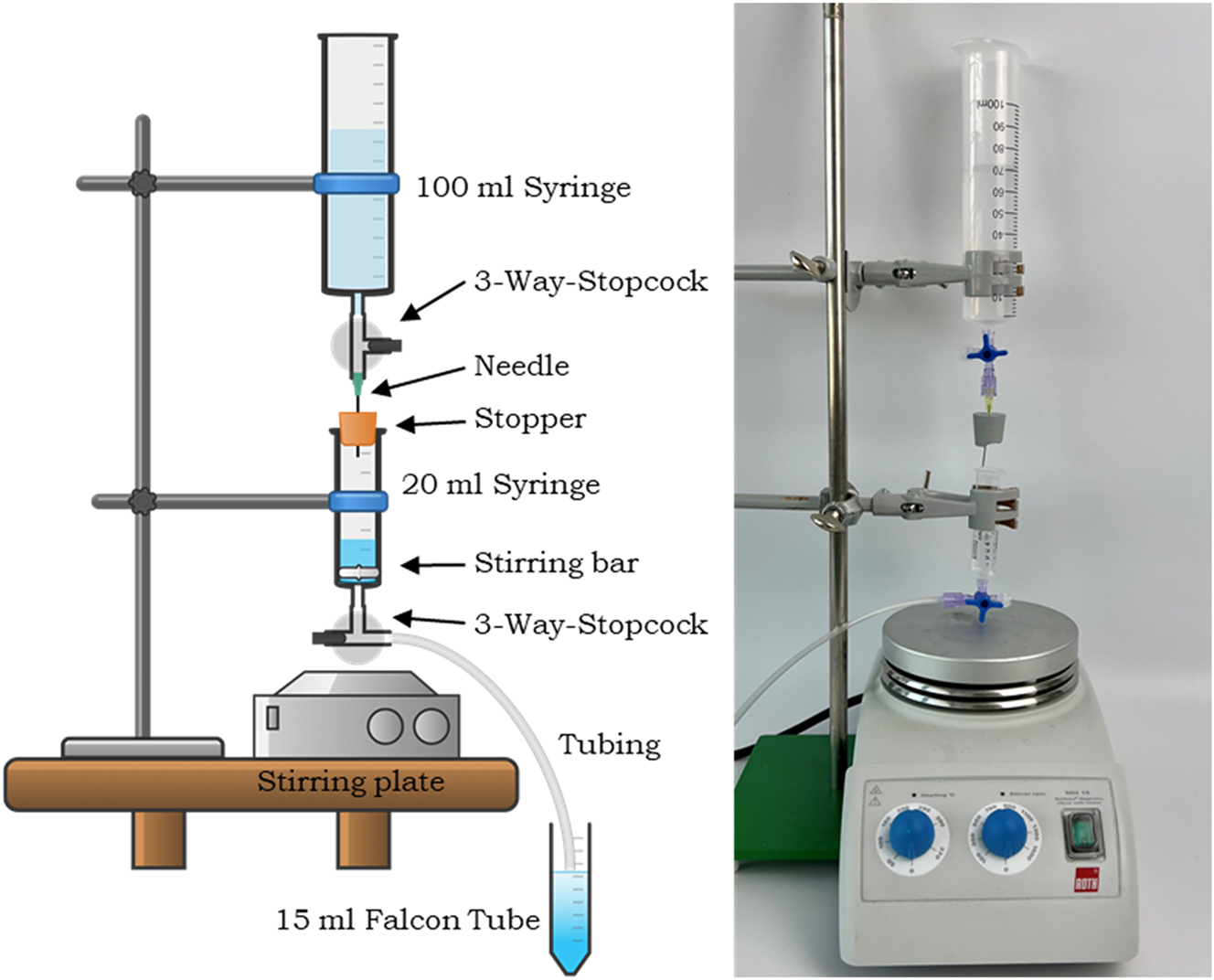
Setup of the Luer-Lock low-cost density gradient maker.
The Luer-Lock low-cost density gradient maker is assembled vertically as shown in Figure 1. The lower syringe acts as a mixing chamber and is filled with a high-density medium. The upper syringe, filled with a low-density medium, serves as the reservoir. Due to the airtight system, the maker can produce the gradients based on the principle of pressure equalization.
For every drop leaving the lower syringe through the tube, a drop from the upper syringe enters the lower syringe via the needle, diluting the solution. Thus, the gradient maker can ensure a continuous dilution of the lower solution that leads to a continuous density distribution.
To make a gradient, the apparatus has to be prepared with all three-way-stopcocks closed. The lower syringe is filled with 5 mL of the higher density medium (e.g. a saturated potassium carbonate or sucrose solution in water). Next, the upper (bigger) syringe reservoir is filled with the lower density medium, e.g. water. A fitting stirring bar or a neodymium magnet is placed in the lower syringe, so that the liquid in the lower syringe is in motion during use and can ensure permanent mixing and consequentially continuous dilution. Then it is crucial to connect the upper and lower syringe with the pierced stopper and the three-way-stopcock so that the connection is airtight.
To make a density gradient both three-way stopcocks need to be opened, allowing the media to flow through the apparatus. It is essential that this flow begins only after both three-way stopcocks have been opened. If there is any flow before this step, it indicates a lack of airtightness, making the apparatus unsuitable for use. To prevent the mixing of the gradient in the Falcon tube, it is important to raise the end of the tubing along the level of the liquid. We recommend filling the Falcon Tube with 12–13 mL of density medium so that there is still room in the tube for the sample to be analyzed.
3.2 Testing sucrose and potassium-carbonate solutions as harmless and functional density media
After it was shown that there was no leakage, the stability and reproducibility of the gradients were tested with different density media. At first a solution of 66 % sucrose in water as dense medium and water as the dilutant was tested. Thereafter, the density of each milliliter of the water-sugar gradient was determined using refractive indices and UV–VIS spectroscopy. This used methylene blue added to the sucrose solution, which was then used indirectly to determine the sucrose concentration (see Figure 3).
For further experiments, sucrose was ruled out due to insufficient maximum density (1.32 g/cm³) for separation of high-density plastics like PET and PVC. Hence a saturated potassium carbonate solution was used as a novel high-density medium (1.53 g/cm3) as suggested recently (Gohla et al., 2021). A big advantage of this setup is that the potassium carbonate solution can act as both the extraction medium for sample preparation as well as the separation medium in gradient centrifugation.
Continuity and reproducibility of the potassium-carbonate-and-water-gradient was measured 6-fold using a “DMA46” flexural vibrating meter from Anton Paar K.G. (0–3 g/cm3, accuracy ± 1 × 10−4 g/cm3). The gradients were collected in a 15 mL Falcon tube and centrifuged in a SBS-LZ-400B benchtop centrifuge from Steinberg Systems, with an angular rotor at 5000 rpm for 30 min. For validation the gradients were fractionated before and after centrifugation using a pipette to take 1 mL fractions from the top of the gradient to the bottom. Then, the density of each fraction was determined.
3.3 Fractionation of microplastic particles by density gradient centrifugation
Finally, we tested the separation efficiency of gradient centrifugation to fractionate microplastic particles. We only tested plastic types with a density higher than seawater (>1.02 g/cm3), since they will sink and therefore accumulate in sediments (see Table 1) (Van Cauwenberghe et al., 2015). Plastic types with a lower density, such as low-density polyethylen (LD-PE), polypropylen (PP), high-density polyethylen (HD-PE) and expanded polystyrol (EPS) would float on seawater, hence they also float atop of the gradient and fractionation is therefore not possible with the density media used here.
Tested common plastic types and their specific density (Pacher, 2021; Quinn et al., 2017) and examples for their source.
| Plastic-Type | Density [g/cm3] | Source |
|---|---|---|
| Polyamide (PA) | 1.13–1.15 | Tights, dowels, cable ties |
| Polyurethane (PU) | 1.05–1.28 | Rolls, mattresses |
| Polycarbonate (PC) | 1.2 | CDs, glasses of safety goggles |
| Polyethylene terephthalate (PET) | 1.38 | Bottles |
| Plasticised polyvinyl chloride (PVC) | 1.10–1.35 | Cables, tubes |
| Unplasticised polyvinyl chloride (PVC) | 1.35–1.45 | Pipes, flooring |
The tested microplastic particles were produced by cutting, chopping or grating of everyday items.
The density gradients were then prepared as described previously. The fractionation was repeated 10-fold where each time 3–5 particles of each plastic type were placed on the density gradient. The density gradients were then centrifuged at 5000 rpm for 30 min. Afterwards, the microplastic particles were identified visually based on their shape or color.
In Figure 2, a summary of the process is shown that can also be used in schools. First, the gradient maker is used to produce a centrifugation-stable gradient. Setting up the gradient maker requires 15 min, making the gradient takes around 10 min. Second, microplastic particles are placed in the Falcon tube. After 30 min of centrifugation, the microplastic particles are fractionated and can assigned to a density range.
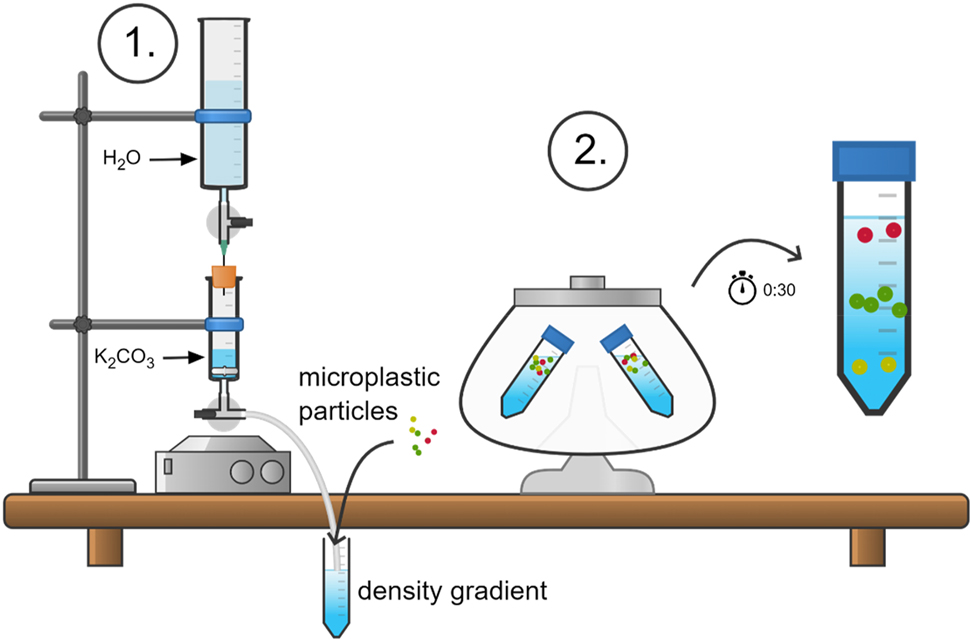
Overview of the two-step fractionation process of microplastic particles.
4 Results and discussion
4.1 Luer-Lock low-cost density gradient maker is suitable to produce continuous density gradients
With the Luer-Lock low-cost density gradient maker, continuous and reproducible density gradients can be produced both with a sucrose solution and water (see Figure 3) and with a saturated potassium carbonate solution and water (see Figure 4). A characteristic of a continuous gradient is its exponential decay, as can be seen in Figure 3.
![Figure 3:
The sucrose – water gradient achieved by the low-cost gradient maker, separated milliliter by milliliter. (A) Methylene blue was added to the sucrose solution for visualisation (B) the refractive index for each fraction is shown as a mean of 10 measurements. The black line shows a regression for exponential decay [f = a*exp (−b*x)].](/document/doi/10.1515/cti-2023-0079/asset/graphic/j_cti-2023-0079_fig_003.jpg)
The sucrose – water gradient achieved by the low-cost gradient maker, separated milliliter by milliliter. (A) Methylene blue was added to the sucrose solution for visualisation (B) the refractive index for each fraction is shown as a mean of 10 measurements. The black line shows a regression for exponential decay [f = a*exp (−b*x)].
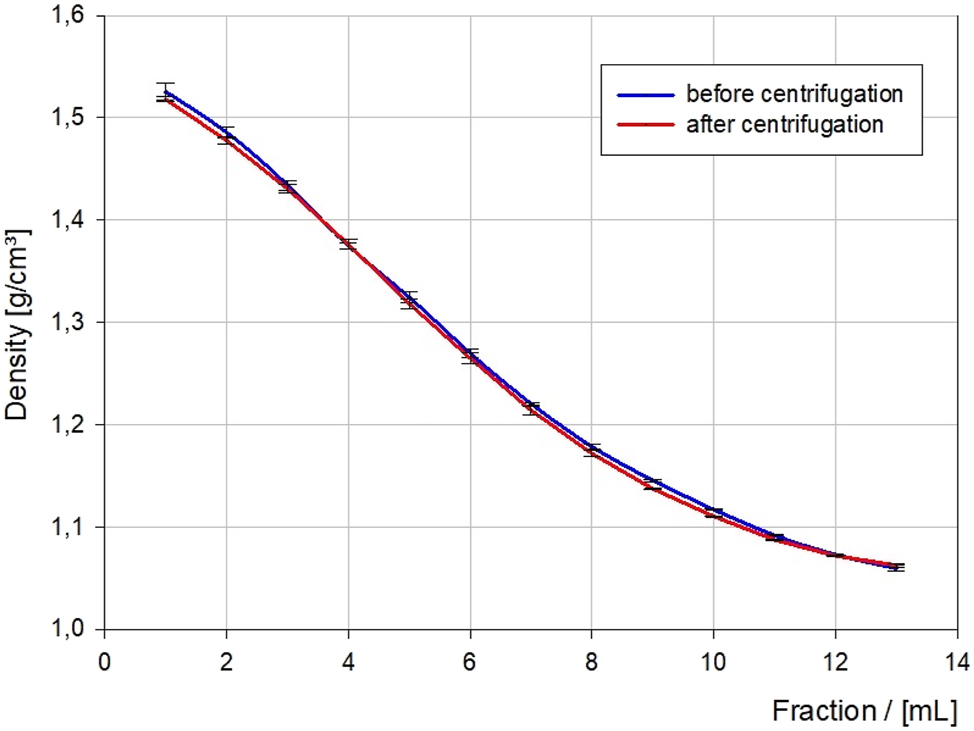
Densities of each fraction of the potassium-carbonate-and-water gradient before (blue) and after (red) centrifugation.
As shown in Figure 4 there are no significant changes in the gradient before and after centrifugation. Generally, the gradients are characterized by their high stability. Therefore, the handling of the gradients is also suitable for students handling the gradients with little caution.
The initial phase of exponential density decay can be approximated by a linear decay, especially in fractions 2–9, allowing for reliable separation of microplastic particles in this density range.
4.2 Fractionation of microplastic particles by density gradient centrifugation
Preliminary results with the sucrose gradient were promising separating plastic types polypropylene (PP), polystyrol (PS), polyamide (PA), polycarbonate (PC) and polyethylene terephthalate (PET) (see Figure 5) as confirmed by ATR-IR spectroscopy (see Supplementary Information).
A picture of the sucrose gradient with microplastic particles of the plastic types PP, PS, PA, PC and PET after centrifugation showing clear bands for each plastic type as confirmed by ATR-IR spectroscopy.
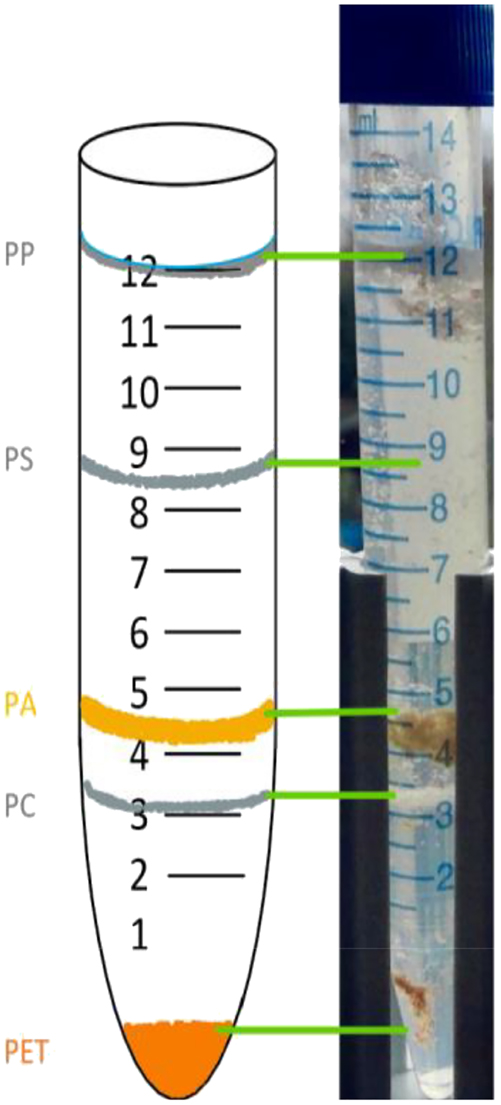
Furthermore, using the potassium carbonate gradient for fractionation makes the separation of PA, PC, PU, PET and PVC easily accessible. After centrifugation, the tested microplastic particles were separated into five well-defined bands (see Figure 6). The bands were in the same place in all 10 samples tested.
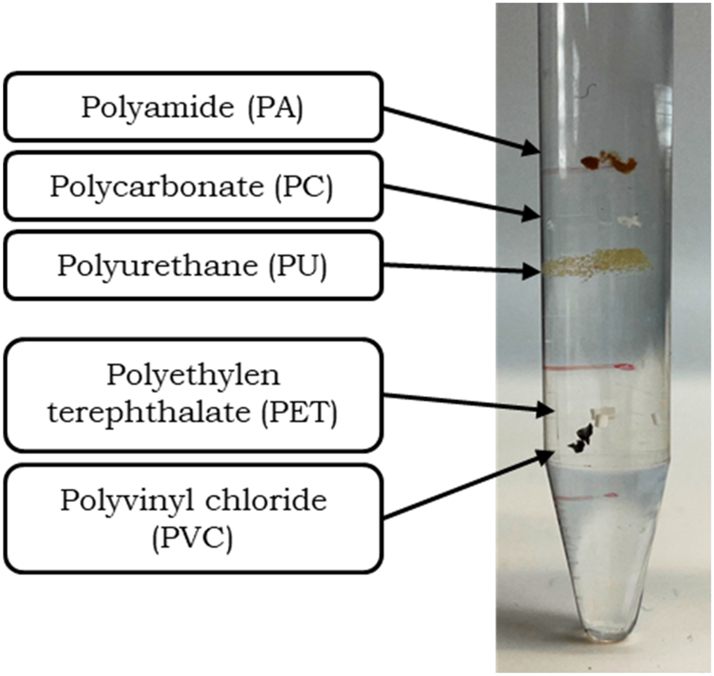
Picture of fractioned microplastic particles after centrifugation in a saturated-potassium-carbonate-and-water density gradient showing clear bands for each plastic type, attributed to the density of each fraction.
Particles were then identified visually, and the density of the fraction was compared to the specific density of the plastic types. All five polymers formed a band in their specific density range, showing this method is viable for separation of multiple microplastic particles by plastic type. Separation efficiency is similar to other protocols using caesium chloride solution (Jakobs et al., 2023; Jing et al., 2022), making a saturated potassium carbonate solution suitable and favourable as a non-hazardous and cost-effective alternative for school context. It should be noted that the separation does not allow for a clear identification due to the partially overlapping density ranges of some plastic types. Density can also vary due to the use of additives (such as plasticizers, flame retardants, slip additives and thermostabilizers) or non-polymeric fillers (Andrady, 2015; Hale et al., 2020). Nevertheless, density separation by gradient centrifugation allows an approximate estimation of the type of the microplastic particles.
4.3 Workshop for teachers
In order to transfer the knowledge created by the students from school and extended by further development at university back to school, a hands-on experience working with this device has already been integrated in workshops for teachers. In addition to theoretical information on the topic of microplastics, the teachers also received an introduction to the analysis of microplastics in the workshop. Afterwards, the teachers had enough time to practice the methods actively and independently with the help of instruction sheets that were developed to be used with students (see Supplementary Information). A subsequent plenum discussion showed potentials for integration of the device into upper secondary chemistry classes. The workshop received a lot of positive feedback. Following the workshops, teachers reported that they had already successfully used the gradient maker and method in their laboratory lessons with minor, class-specific modifications and plan to implement it on a more regular basis.
5 Conclusion and outlook
Using the novel Luer-Lock low-cost density gradient maker it is possible to easily produce stable and reproducible density gradients in a school and university context. Both a sucrose solution and a saturated potassium carbonate solution were shown to be suitable, low-cost, easy-to-handle and non-hazardous alternatives to a caesium chloride solution, which is state of the art in current research. We have also shown that with our approach density gradient centrifugation can be used to fractionate microplastic particles of the types PA, PUR, PC, PET and PVC based on their specific particle densities. Lower density polymers such as PE, PP and EPS might be fractionated in an ethanol-water gradient. First tests have shown promising results.
This simple yet powerful method can be used not only to address the environmental microplastic problem in classes, but also to show some limitations as well as challenges of microplastic analysis. In addition, it might contribute to career guidance towards analytical chemistry. Furthermore, the experimental setting can be used to quickly visualize density using a specific example from a real-world-context. Another fruitful field of application may be Citizen Science projects for identification of microplastic particles in real-world samples. First tests at a marine biology station in Croatia are already showing promising results.
Supplementary information
ATR-IR-Spectra of microplastic particles in the sucrose gradient; further information and a picture of a non-functional prototype gradient maker; statements on hazards and precautionary measures.
Acknowledgments
We like to thank Prof. Dr. Jürgen Schram, Fachhochschule Niederrhein, Instrumentelle and Chemische Analytik for the IR-Spektra. The authors acknowledge the financial support for open access publication by the University of Graz.
-
Ethical approval: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. J. G. had the idea for vertical design of the gradient maker.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Raw data can be obtained on request from the corresponding authors.
References
Andrady, A. L. (2015). Persistence of plastic litter in the ocean. In Marine Anthropogenic Litter (pp. 57–72). https://doi.org/10.1007/978-3-319-16510-3 Suche in Google Scholar
Chen, H., Zou, X., Ding, Y., Wang, Y., Fu, G., & Yuan, F. (2022). Are microplastics the ‘technofossils’ of the Anthropocene? Anthropocene Coasts, 5(8), 1–11. https://doi.org/10.1007/s44218-022-00007-1.Suche in Google Scholar
Corcoran, P. L., Moore, C. J., & Jazvac, K. (2014). An anthropogenic marker horizon in the future rock record. Geological Society of America Today, 24(6), 4–8. https://doi.org/10.1130/GSAT-G198A.1 Suche in Google Scholar
Flurkey, W. H. (2000). An inexpensive gradient maker for the biochemistry laboratory. Journal of Chemical Education, 77(8), 1041. https://doi.org/10.1021/ed077p1041 Suche in Google Scholar
Gohla, J., Bračun, S., Gretschel, G., Koblmüller, S., Wagner, M., & Pacher, C. (2021). Potassium carbonate (K2CO3) – A cheap, non-toxic and high-density floating solution for microplastic isolation from beach sediments. Marine Pollution Bulletin, 170. https://doi.org/10.1016/j.marpolbul.2021.112618.Suche in Google Scholar PubMed
Hale, R. C., Seeley, M. E., La Guardia, M. J., Mai, L., & Zeng, E. Y. (2020). A global perspective on microplastics. Journal of Geophysical Research: Oceans, 125. https://doi.org/10.1029/2018JC014719 Suche in Google Scholar
Jakobs, A., Gürkal, E., Möller, J. N., Löder, M. G. J., Laforsch, C., & Lueders, T. (2023). A novel approach to extract, purify, and fractionate microplastics from environmental matrices by isopycnic ultracentrifugation. Science of the Total Environment, 857, 159610. https://doi.org/10.1016/j.scitotenv.2022.159610 Suche in Google Scholar PubMed
Jing, S., Huang, Y., Chen, Y., He, X., Chen, Z., Lu, X., Wu, M., & Wanger, T. C. (2022). Non-destructive extraction and separation of nano- and microplastics from environmental samples by density gradient ultracentrifugation. Analytical Chemistry, 94(44), 15280–15287. https://doi.org/10.1021/acs.analchem.2c02543 Suche in Google Scholar PubMed
Marks, M. S. (1998). Determination of molecular size by zonal sedimentation analysis on sucrose density gradients. Current Protocols in Cell Biology. https://doi.org/10.1002/0471143030.cb0503s00 Suche in Google Scholar PubMed
Pacher, C. (2021). Microplastics in shore sediments – Development of a suitable extraction and purification technique for citizen science projects [Masterthesis]. University of Graz, Graz.Suche in Google Scholar
Plastics Europe. (2022). Plastics – the Facts 2022. Brussels: Plastics Europe AISBL. https://plasticseurope.org/wp-content/uploads/2023/03/PE-PLASTICS-THE-FACTS_FINAL_DIGITAL-1.pdf [Accessed 02 April 2023].Suche in Google Scholar
Prata, J. C., da Costa, J. P., Duarte, A. C., & Rocha-Santos, T. (2019). Methods for sampling and detection of microplastics in water and sediment: A critical review. TrAC, Trends in Analytical Chemistry, 110, 150–159. https://doi.org/10.1016/j.trac.2018.10.029 Suche in Google Scholar
Quinn, B., Murphy, F., & Ewins, C. (2017). Validation of density separation for the rapid recovery of microplastics from sediment. Analytical Methods, 9, 1491–1498. https://doi.org/10.1039/c6ay02542k Suche in Google Scholar
Ryberg, M. W., Hauschild, M. Z., Wang, F., Averous-Monnery, S., & Laurent, A. (2019). Global environmental losses of plastics across their value chains. Resources, Conservation and Recycling, 151, 104459. https://doi.org/10.1016/j.resconrec.2019.104459 Suche in Google Scholar
Salo, T., & Kouns, D. M. (1965). An improved gradient-making device for density gradient centrifugation. Analytical Biochemistry, 13, 74–79. https://doi.org/10.1016/0003-2697(65)90119-3 Suche in Google Scholar
Van Cauwenberghe, L., Devriese, L., Galgani, F., Robbens, J., & Janssen, C. R. (2015). Microplastics in sediments: A review of techniques, occurrence and effects. Marine Environmental Research, 111, 5–17. https://doi.org/10.1016/j.marenvres.2015.06.007 Suche in Google Scholar PubMed
Van Cauwenberghe, L., Vanreusel, A., Mees, J., & Janssen, C. R. (2013). Microplastic pollution in deep-sea sediments. Environmental Pollution, 182, 495–499. https://doi.org/10.1016/j.envpol.2013.08.013 Suche in Google Scholar PubMed
Zalasiewicz, J., Waters, C. N., Ivar do Sul, J. A., Corcoran, P. L., Barnosky, A. D., Cearreta, A., Edgeworth, M., Gałuszka, A., Jeandel, C., Leinfelder, R., McNeill, J. R., Steffen, W., Summerhayes, C., Wagreich, M., Williams, M., Wolfe, A. P., & Yonan, Y. (2016). The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene Vol. 13. (pp. 4–17). Elsevier Ltd. https://doi.org/10.1016/j.ancene.2016.01.002 Suche in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/cti-2023-0079).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Artikel in diesem Heft
- Frontmatter
- Review Article
- Teaching hydrogen bridges: it is not FON anymore!
- Research Articles
- Exploring the implementation of stepwise inquiry-based learning in higher education
- Ambassadors of professional development in teaching and learning in STEM higher education
- Investigating the influence of temperature on salt solubility in water: a STEM approach with pre-university chemistry students
- Analysis of undergraduate chemistry students’ responses to substitution reaction mechanisms: a road to mastery
- Development of augmented reality as a learning tool to improve student ability in comprehending chemical properties of the elements
- Fractionating microplastics by density gradient centrifugation: a novel approach using LuerLock syringes in a low-cost density gradient maker
- Elucidating atomic emission and molecular absorption spectra using a basic CD spectrometer: a pedagogical approach for secondary-level students
- Students’ perceptions towards the use of computer simulations in teaching and learning of chemistry in lower secondary schools
- International teacher survey on green and sustainable chemistry (GSC) practical activities: design and implementation
- Good Practice Reports
- Building words from chemical elements: a fun and inclusive approach to introduce the periodic table
- Design of Jacob’s ladder-based teaching aids for illustrating the dualities of benzene derivatives
- Learning with NanoKid: line-angle formula, chemical formula, molecular weight, and elemental analysis
Artikel in diesem Heft
- Frontmatter
- Review Article
- Teaching hydrogen bridges: it is not FON anymore!
- Research Articles
- Exploring the implementation of stepwise inquiry-based learning in higher education
- Ambassadors of professional development in teaching and learning in STEM higher education
- Investigating the influence of temperature on salt solubility in water: a STEM approach with pre-university chemistry students
- Analysis of undergraduate chemistry students’ responses to substitution reaction mechanisms: a road to mastery
- Development of augmented reality as a learning tool to improve student ability in comprehending chemical properties of the elements
- Fractionating microplastics by density gradient centrifugation: a novel approach using LuerLock syringes in a low-cost density gradient maker
- Elucidating atomic emission and molecular absorption spectra using a basic CD spectrometer: a pedagogical approach for secondary-level students
- Students’ perceptions towards the use of computer simulations in teaching and learning of chemistry in lower secondary schools
- International teacher survey on green and sustainable chemistry (GSC) practical activities: design and implementation
- Good Practice Reports
- Building words from chemical elements: a fun and inclusive approach to introduce the periodic table
- Design of Jacob’s ladder-based teaching aids for illustrating the dualities of benzene derivatives
- Learning with NanoKid: line-angle formula, chemical formula, molecular weight, and elemental analysis

